Abstract
To better understand the role of tumor necrosis factor (TNF) during Trypanosoma cruzi infection in BALB/c mice, we have investigated the kinetics of circulating tumor necrosis factor (TNF), soluble TNF receptor 1 (sTNR1), and sTNFR2 levels, as well as the interactions between such factors, in relation to parasitemia, cachexia, and mortality of acutely infected animals. Our data show that the parasitemic phase of T. cruzi infection in mice is associated with high levels of circulating TNF and sTNFR2, resulting in the formation of cytokine-receptor complexes and some degree of neutralization of TNF bioactivity. Although sTNR2 levels always exceeded TNF levels, low sTNFR/TNF circulating ratios were associated with cachexia in all infected mice, whereas the lowest ratios were observed in dying animals harboring the highest parasitemia. We also studied the modulation of sTNFR/TNF ratios induced by anti-TNF antibodies administered to infected animals and their consequences on the outcome of the infection. The injection of anti-TNF monoclonal antibody (MAb) TN3 into infected mice resulted in a paradoxical overproduction of TNF (associated with a higher parasitemia), lowered the sTNFR/TNF circulating ratios, and considerably worsened cachexia and mortality of animals. Another anti-TNF MAb (1F3F3) decreased the in vivo availability of TNF as well as parasite levels and reduced cachexia. Altogether, such results highlight that, besides playing a beneficial role early in infection, TNF also triggers harmful effects in the parasitemic phase, which are limited by the in vivo simultaneous endogenous production of soluble receptors.
Tumor necrosis factor (TNF) includes two related molecules, termed TNF-α (found in membrane and soluble forms) and lymphotoxin alpha (produced only in soluble form), which transduce their activities through two membrane TNF receptors (TNFRs) with apparent molecular masses of 55 kDa (TNFR1, CD120a) and 75 kDa (TNFR2, CD120b) (2, 56). The extracellular domains of these receptors are released in the circulation of healthy individuals by proteolytic cleavage (4, 26, 41). Such soluble TNFRs (sTNFR) retain the ability to bind TNF, acting either as antagonist or agonist of TNF bioactivity (18, 39, 42). Among its numerous biological activities, TNF is involved in the killing of tumor cells and in the control of intracellular pathogen multiplication (19, 20, 46), and it limits the extent and duration of inflammatory processes (37). Besides these beneficial effects, it induces cachexia associated with cancer and various infectious diseases (38) and is involved in the pathogenesis and lethality of septic shock (2, 56) and cerebral malaria (20, 36).
Trypanosoma cruzi is the protozoan parasite causing Chagas' disease, a highly prevalent infection in Latin America. In vitro infection of human and murine cells with T. cruzi increases TNF mRNA levels and TNF release (10, 51, 55). This cytokine has been detected in situ and in the supernatants of splenic cells as well as in the blood of some infected mice (28, 31, 49, 51, 58). Studies using sTNFR1-deficient mice (12), transgenic mice expressing high levels of sTNFR1-Fcγ3 fusion protein (33), or mice in which TNF-specific antibodies (Abs) were injected in vivo (1, 28, 48) suggested a beneficial role of TNF in the control of the acute T. cruzi infection in mice. However, in vivo reduction of TNF levels, which would support such conclusions, was not demonstrated in these latter studies. Moreover, no information is available on the production of sTNFR during T. cruzi infection. Though the ability of TNF to enhance the in vitro NO-dependent trypanocidal activity of gamma interferon (IFN-γ)- or lipopolysaccharide (LPS)-activated macrophages has been clearly demonstrated (8, 21, 40, 48, 57), we have shown TNF to mediate a harmful effect by inducing cachexia associated with murine T. cruzi acute infection (54). In addition, in vivo administration of exogenous TNF (8) or of potent TNF inducers such as LPS (30) or anti-CD3 Abs (29) resulted in higher mortality in animals acutely infected with T. cruzi.
To better define the role of TNF during T. cruzi infection in mice and considering that sTNFR can considerably modulate the bioactivity of TNF, we have investigated the kinetics of circulating TNF, sTNFR1, and sTNFR2 levels, as well as the interactions between such factors, in relation to parasitemia, cachexia, and mortality of acutely infected animals. We also investigated the modulation of sTNFR/TNF ratios induced by anti-TNF antibodies administered to infected animals and their consequences on the outcome of the infection.
MATERIALS AND METHODS
Mice, T. cruzi infection, and blood processing.
Two-month-old male BALB/c mice were purchased from B&K Universal (Hull, United Kingdom). Mice were infected by intraperitoneal (i.p.) inoculation of 100 blood trypomastigotes of the Tehuantepec strain of T. cruzi maintained in our laboratory. Parasitemia was determined in tail blood every 3 to 4 days, as previously described (11). Mortality and weight of mice were regularly recorded. The body weight changes were expressed as (weight on experimental day − weight on day 0) × 100/weight on day 0. Blood was obtained from tail or by cardiac puncture (in mice anesthetized by ether), using special precautions to avoid cytokine proteolysis and unexpected release: after being collected on heparin with LPS-free material, blood was immediately kept on ice, mixed with 1 volume of 13 mM sodium citrate containing protease inhibitors (1 mM TCLK [N-p-tosyl-l-lysine-chloromethyl ketone] hypochloride [Sigma, St. Louis, Mo.] and 1,000 KIU of aprotinin [Boehringer Mannheim, Mannheim, Germany] per ml), and centrifuged. Plasma samples were stored at −70°C until use.
Treatment of mice with TNF-specific MAbs.
Two kinds of monoclonal Abs (MAbs) recognizing specifically mouse TNF were administered to mice: a chimeric construction of F(ab′)2 from hamster MAb TN3 (clone 19.12) with mouse Fcγ1 (47, 50) (kindly provided by M. Bodmer, Celltech, Slough, United Kingdom) and MAb 1F3F3 (a rat IgM [35]), used as ascites fluid. Purified unrelated mouse monoclonal immunoglobulin G1 (IgG1) and ascites fluid containing an irrelevant rat monoclonal IgM (IR968) (both kindly provided by H. Bazin, Unit of Experimental Immunology, University of Leuven, Brussels, Belgium) were used as controls. The material to be injected to mice contained less than 10 pg of endotoxin per injection, as measured with the Limulus amoebocyte lysate assay (detection limit, 1 pg/ml; Coatest endotoxin; Chromogenix, Mölndal, Sweden). T. cruzi-infected mice received i.p. injections of TN3-Fcγ1 (200 μg), 1F3F3 (11 μg), or control MAb (at the same amount) in phosphate-buffered saline twice a week during 2 weeks, the first injection being given the day before T. cruzi inoculation. The choice of Ab amount to be injected was based on the previously described capacity of the Ab to bind TNF and to neutralize its biological activity (35, 47, 50).
ELISA for murine TNF, sTNFR, and TNF-sTNFR complexes.
Enzyme-linked immunosorbent assay (ELISA) for murine TNF was performed as described elsewhere (16). Briefly, 96-well Immunomaxisorp plates (Nunc, Roskskilde, Denmark) were coated with rabbit polyclonal IgG directed against murine TNF-α. After blocking nonspecific binding with 1% bovine serum albumin and washing, 50-μl aliquots of plasma samples (half-diluted in protease inhibitor solution as described above) or of serial dilutions of recombinant murine TNF-α (Boehringer Mannheim) were added to each well. After washing, the plates were incubated successively with a polyclonal rabbit IgG anti-mouse TNF-α, peroxidase-conjugated donkey F(ab′)2 anti-rabbit IgG (Jackson Immunoresearch, West Grove, Pa.), and H2O2 and 3,3′, 5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) as substrate and chromogen, respectively. The reaction was stopped after 15 min before absorbances at 450 nm were read. Such ELISA detected free TNF as well as TNF bound to sTNFR1 and -2 (or MAb TN3), with a detection threshold of 20 pg of TNF per ml.
In some studies, MAb TN3 was used as capture Ab instead of the anti-TNF polyclonal Ab (the other ELISA steps remaining identical), since it was verified that the TNF-TN3 Ab complexes were not detected with such ELISAs. The differences between the levels of TNF obtained in both ELISAs allowed to appreciate the amounts of TNF-TN3 complexes.
ELISAs for sTNFR were performed as described earlier (3). Soluble receptors were captured from plasma samples (diluted 1/15 and 1/25 for sTNFR1 and R2, respectively) with polyclonal rabbit IgG anti-murine sTNFR1 or -R2 and revealed with the same biotinylated antibodies and streptavidin-peroxidase (Dako, Glostrup, Denmark). Saturation, washings, incubation times, and substrate were as for TNF ELISA. Standard titration curves were obtained by making serial dilutions of recombinant murine sTNFR1 or R2. These sTNFR ELISAs recognized both unbound receptors and receptor-ligand complexes. The detection thresholds were 40 and 390 pg/ml, respectively for sTNFR1 and -R2.
Another ELISA was developed for the detection of complexes between TNF and its soluble receptors. The complexes, captured by rabbit polyclonal IgG against murine TNF-α from mouse plasma samples (diluted twofold), were detected by successive incubation with biotinylated polyclonal rabbit IgG anti-murine sTNFR1 or R2 and streptavidin-peroxidase. Incubations, saturation, washings, and substrate were as described above. Results are expressed as absorbances at 450 nm.
sTNFR/TNF molar ratios were calculated as C1 × M2/C2 × M1, where C1 and C2 are the concentrations in nanograms per milliliter of sTNFR and TNF, respectively, and M1 and M2 are the molecular masses of sTNFR (sTNFR1, 30,000; sTNR2, 40,000) and monomeric TNF (17,000), respectively.
Bioassay for TNF-neutralizing activity.
The assay measured the effect of mouse plasma samples on the bioactivity of standard TNF, using the previously described TNF bioassay (17). Briefly, 5 pg of standard murine recombinant TNF TNF (Boehringer Mannheim), the amount able to lyse 50% of 3 × 104 WEHI cells, was incubated for 1 h at 37°C with mouse plasma samples (1/4 diluted) before being added to WEHI cells (3 × 104 cells/well in a final volume of 100 μl). After incubation for 20 h, cell viability was assessed by a colorimetric method (incubation with 12 mM MTT (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide; Sigma) during 3 h followed by solubilization of crystals with acid isopropanol containing 1% Triton X-100). The TNF-neutralizing activity was estimated by the decrease of the percentage of lysed cells. It was verified that the protease inhibitors added into the blood samples did not interfere with the bioassay.
ELISA for murine interleukin-6 (IL-6) and IFN-γ.
The cytokines were detected in mouse plasma samples (fourfold diluted), using commercially available assays (Intertests; Genzyme, Cambridge, Mass.) as described by the manufacturer. The detection limit was 5 pg/ml for both ELISAs.
RESULTS
The acute T. cruzi infection resulted in high circulating levels of both TNF and sTNFR.
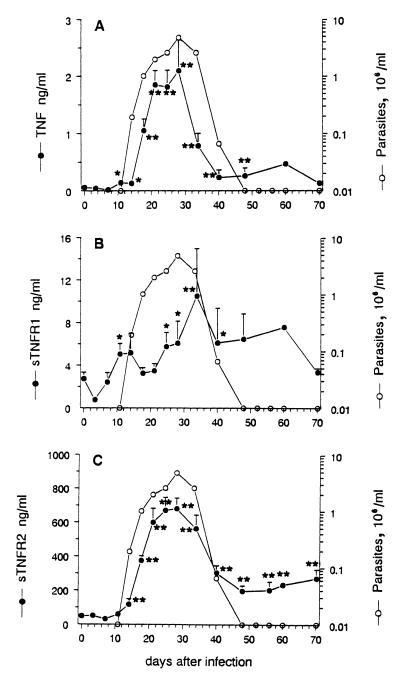
As shown in Fig. 1A, TNF was not found in the blood of noninfected mice, whereas it became detectable in T. cruzi-infected mice from day 11 postinfection (p.i.). Mean levels peaked on day 28 p.i. (mean ± standard error of the mean [SEM], 2.1 ± 0.5 ng/ml), as did parasitemia, before decreasing and remaining detectable in the circulation of 53% of mice in the chronic phase of infection, when circulating parasites were undetectable (from day 48 p.i.), at levels ranging between 0.2 and 0.5 ng/ml.
FIG. 1.
Parasite, TNF, and sTNR circulating levels in T. cruzi-infected mice. BALB/c mice were inoculated with 100 parasites at day 0 (n = 28); 52% died during the parasitemic phase (mean survival time, 24 ± 0.7 days). Parasitemia is expressed as geometric means. TNF and sTNFR levels, detected by ELISA, are given as arithmetic means ± SEM. Asterisks indicate that TNF or sTNFR levels are significantly different from initial levels before infection (∗, p < 0.05; ∗∗, p < 0.005; Mann-Whitney-Wilcoxon U test).
As shown in Fig. 1B and C, uninfected mice displayed basal levels of sTNFR2 about 20 times higher than those of sTNFR1 (mean ± SEM, 49.3 ± 2.8 versus 2.8 ± 0.6 ng/ml). T. cruzi infection induced a moderate increase of circulating amounts of TNFR1, which reached maximal mean value around four times above the basal level on day 34 p.i. By contrast, sTNFR2 exhibited a major increase during the acute phase of infection, peaking at day 28 p.i. with levels 14 times above the basal mean level (681.7 ± 63.7 ng/ml). Its kinetics closely paralleled those of TNF and parasitemia. During the chronic phase of the infection (from day 48 p.i.), the levels stabilized between 4 and 8 ng/ml for sTNFR1 and 200 to 300 ng/ml for sTNFR2.
As shown in Table 1, calculation of molar ratios between circulating levels of sTNFR and TNF highlights the large excess of sTNFR2 over TNF during the course of infection, with mean ratios ranging from 135 to 1,700, whereas sTNFR1 barely (1.1- to 32-fold) exceeded TNF. Interestingly, both sTNFR/TNF ratios were particularly reduced during the ascending phase of parasitemia (days 18 to 28 p.i.). Altogether, these data suggest in vivo interactions between sTNFR and TNF that may modulate circulating TNF bioactivity during the acute phase of infection.
TABLE 1.
Molar ratios between sTNFR and TNF circulating levels during the course of T. cruzi infection in micea
| Day p.i. | sTNFR1/TNF | sTNFR2/TNF |
|---|---|---|
| 11 | 32.2 ± 11.5 | 251 ± 105 |
| 14 | 22.6 ± 4.6 | 373 ± 39 |
| 18 | 5.0 ± 3.7 | 203 ± 29 |
| 21 | 1.1 ± 0.2 | 135 ± 11 |
| 25 | 2.0 ± 0.4 | 192 ± 35 |
| 28 | 2.3 ± 0.6 | 303 ± 81 |
| 34 | 5.2 ± 1.3 | 475 ± 89 |
| 40 | 17.2 ± 9.0 | 660 ± 196 |
| 48 | 17.7 ± 3.7 | 1,122 ± 573 |
| 58 | 14.8 ± 5.7 | 1,714 ± 1,501 |
Mice were inoculated with 100 parasites on day 0. Results are expressed as arithmetic means ± SEM of individual molar ratios calculated from TNF and sTNFR levels measured by ELISA (n = 8 to 12).
TNF-sTNFR complexes and TNF-neutralizing activity are detectable in blood of mice acutely infected with T. cruzi.
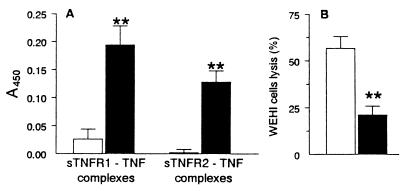
As shown in Fig. 2A, complexes between TNF and both soluble receptors could be detected in plasma of acutely infected mice at day 28 p.i. but not in those of noninfected animals. Moreover, plasma of infected mice displayed a TNF-neutralizing activity (Fig. 2B), since they could significantly (by 63%) inhibit the cytotoxic activity of standard TNF on WEHI cells. Such neutralizing activity was significantly correlated with the levels of sTNFR2 but not with those of sTNFR1 (Spearman correlation coefficients, 0.664 [P < 0.001] and 0.173 [P > 0.05], respectively; n = 15). These results strongly argues for the involvement of sTNFR2 (rather than another unknown factor) in the neutralizing activity found in plasma from infected mice.
FIG. 2.
Detection of TNF-sTNFR complexes (A) and TNF-neutralizing activity (B) in the plasma of T. cruzi-infected (■) or noninfected (□) mice. (A) TNF-sTNFR complexes were detected by ELISA. Results are expressed as absorbances at 450 nm (n = 11 noninfected and 35 infected mice, respectively, at day 28 p.i.). Asterisks indicate significant differences between noninfected and infected groups (P < 0.005; Mann-Whitney-Wilcoxon U test). (B) TNF-neutralizing activity was measured by testing the biological activity of exogenous TNF on TNF-sensitive cells (at a concentration able to lyse 50% of WEHI cells), in the presence of plasma from noninfected or infected (day 28 p.i.) mice. Results are expressed as the percentage of WEHI cells lysed after overnight incubation with TNF plus mouse plasma (n = 15 in each mouse group). A reduction of this value denotes an inhibition of TNF bioactivity.
Mortality of T. cruzi-infected mice is associated with low sTNFR/TNF ratios.
Table 2 compares the data for surviving and nonsurviving mice. The results indicate that on day 21 p.i., mice that died (mean survival time, 24 ± 0.7 days p.i.) displayed significantly (3.3-fold) higher circulating mean levels of TNF and of both sTNFR (1.7- and × 1.5-fold for sTNFR1 and -R2, respectively), but lower sTNFR/TNF molar ratios, than surviving mice.
TABLE 2.
Circulating levels of parasites, TNF, and sTNR and sTNFR/TNF ratios in surviving and dying micea
| Mouse group | n | Parasites (106/ml) | Circulating level (ng/ml)
|
Molar ratio
|
|||
|---|---|---|---|---|---|---|---|
| TNF | sTNFR1 | sTNFR2 | sTNFR1/TNF | sTNFR2/TNF | |||
| Surviving | 5 | 1.33 ± 0.23 | 1.01 ± 0.25 | 1.54 ± 0.33 | 472 ± 52 | 1.18 ± 0.12 | 285 ± 24 |
| Dying | 12 | 3.41 ± 0.44* | 3.38 ± 0.67* | 2.56 ± 0.29* | 714 ± 130* | 0.55 ± 0.05* | 126 ± 18** |
Mice were inoculated with 100 parasites on day 0. Molar ratios were calculated from TNF and sTNFR levels detected by ELISA at day 21 p.i. Results are expressed as arithmetic means ± SEM. Asterisks indicate significant differences between dying and surviving mice (∗, P < 0.05; ∗∗, P < 0.005; Student t test).
A more detailed analysis of data obtained for mice 1 or 2 days before or on the day of death (n = 6) shows that nonsurviving mice always exhibited circulating TNF levels above 2.8 ± 0.31 ng/ml, associated with sTNFR1 and -R2/TNF ratios below 0.81 ± 0.19 and 170 ± 37, respectively. Such profiles were never observed in surviving mice. These data suggest that the endogenous balance between sTNFR and TNF, as well as a threshold level of TNF, determines the outcome of infection.
Moreover, nonsurviving mice always displayed higher parasitemia than surviving mice, indicating that the high TNF levels and subsequent low sTNFR/TNF ratios were not related to the control of infection, at least during the ascending phase of parasitemia (between days 14 and 28 p.i.).
Cachexia in T. cruzi-infected mice is also associated with low sTNFR/TNF ratios.
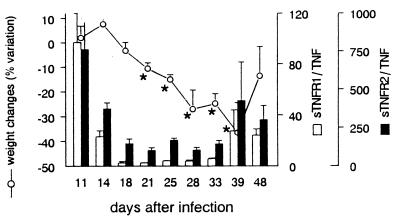
The features of cachexia occurring in mice acutely infected with T. cruzi have been detailed in a previous study (54). As shown in Fig. 3, such cachexia, observed in all acutely infected mice from days 18 to 39 p.i., was associated with reduced sTNFR/TNF ratios (between 2.5 and 5.8 for sTNFR1/TNF and 150 and 170 for sTNFR2/TNF). A time lag of roughly 1 week was noted between weight and ratio variations.
FIG. 3.
Circulating sTNFR/TNF molar ratios and cachexia in T. cruzi-infected mice. Results are from four representative mice inoculated on day 0 with 100 parasites and expressed as arithmetic means ± SEM. Molar ratios sTNFR/TNF (bars) are calculated from circulating TNF and sTNFR levels measured by ELISA. Body weight changes (line) are expressed as the percent variation of mouse weight at each experimental point compared to the weight on day 0 of infection. Asterisks indicate that the weight is significantly different from the weight at day 11 p.i. (P < 0.05; Student t test).
Interestingly, the reduction in molar ratios were not so drastic as those observed in dying mice (Table 2), suggesting that an increasing concentration of bioactive TNF accounts for the evolution from nonlethal cachexia to mortality.
Administration of anti-TNF MAb TN3 to T. cruzi-infected mice increased TNF levels, cachexia, and mortality through decreasing the sTNFR/TNF ratios.
To better study the dynamic relationship between sTNFR/TNF ratios, mortality and cachexia, MAbs documented to be able to neutralize TNF in vivo were administered to infected mice.
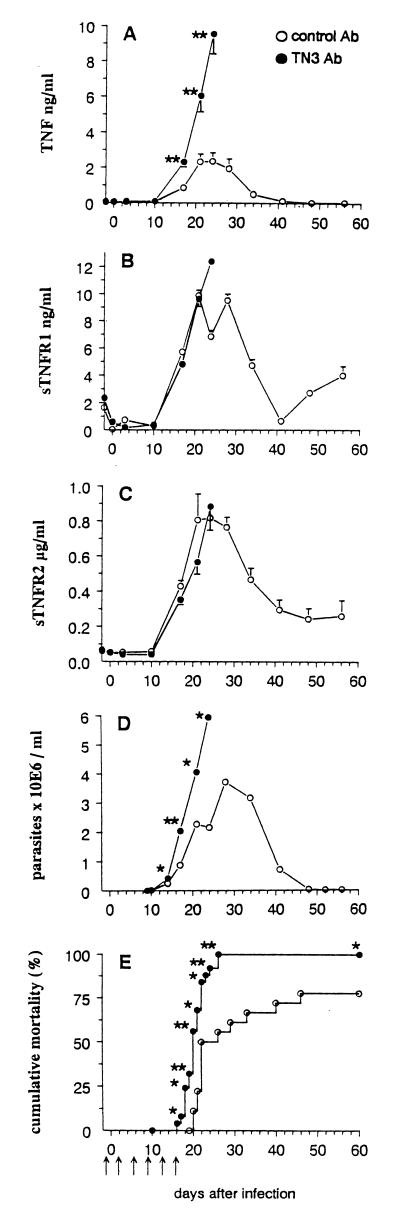
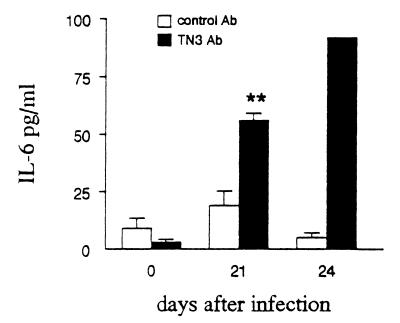
Mice infected with T. cruzi received MAb TN3 or an unrelated control MAb by the i.p. route on days −1, 2, 6, 9, 13, and 16 relative to parasite inoculation. The two experiments performed (with 18 and 11 mice per group) gave similar results. As shown in Fig. 4A (displaying results obtained from one representative experiment), TNF levels increased drastically in the blood of TN3-treated mice compared to control animals. This could not be due to a direct effect of MAb TN3 since previous work showed it did not induce TNF release in noninfected mice (5). An effect related to endotoxin contamination of injected reagents may also be ruled out since (i) only traces of LPS were found in the injected material and (ii) the control Ab, which contained similar traces of LPS, did not trigger TNF overrelease in T. cruzi-infected mice (comparison between Fig. 1A and 4A). The TNF increment could also result from the prolongation of TNF half-life in the circulation by its complexation with the injected MAb TN3, as previously observed with other anticytokine MAbs (6, 53). Since the TNF ELISA used, based on an anti-TNF polyclonal Ab, does not discriminate between free and TN3-complexed TNF, we constructed TNF ELISA using MAb TN3 as capture Ab, that detects only free TNF. As shown in Table 3, if TNF-TN3 complexes could be detected in the plasma at day 17 p.i., their amounts were considerably reduced later in the infection, on days 21 and 24 p.i., indicating the presence of higher amounts of free TNF, resulting from a higher endogenous production in TN3-treated mice. Since TNF is known to stimulate the production of IL-6, a cytokine previously documented to be present in the blood of T. cruzi-infected mice (53), levels of this cytokine were determined in blood of TN3-treated infected mice as a potential marker of TNF bioactivity. As shown in Fig. 5, IL-6 production at days 21 and 24 p.i. was higher in TN3-treated mice than in control animals, suggesting that the TNF detected in the former group of animals is more bioactive.
FIG. 4.
TNF, sTNFR, and parasite blood levels and mortality rates in T. cruzi-infected mice treated with anti-TNF MAb TN3. Mice were inoculated with 100 parasites on day 0 and received either TN3 (n = 25 for parasitemia and mortality determinations; n = 10 for TNF and sTNFR determinations) or unrelated Ab (n = 18 for parasitemia and mortality determinations; n = 9 for TNF and sTNFR determinations). Arrows indicate time points of Ab injections. Asterisks indicate time points at which differences between groups were statistically significant (∗, P < 0.05; ∗∗, P < 0.005; Student t test for TNF and sTNFR levels, Mann-Whitney-Wilcoxon U test for parasitemia, and Yates corrected chi-square test for mortality rates).
TABLE 3.
Circulating levels of total and TN3-complexed TNF in T. cruzi-infected mice treated with anti-TNF TN3 or control Aba
| Day p.i. | Total TNF (ng/ml) | TN3-complexed TNF
|
|
|---|---|---|---|
| ng/ml | % | ||
| 17 | 2.43 ± 0.28 | 1.86 ± 0.16 | 79.7 ± 5.0 |
| 21 | 5.99 ± 1.16 | 1.07 ± 0.60 | 20.1 ± 9.8 |
| 24 | 9.57 ± 1.13 | 1.45 ± 1.02 | 14.1 ± 8.9 |
Mice were inoculated with 100 parasites on day 0 and treated as described for Fig. 4. TNF levels were detected by ELISA using either a polyclonal anti-TNF Ab (total TNF) or MAb TN3 as capture Ab. Differences between the two assays represent the levels of TN3-complexed TNF. Results are expressed as arithmetic means ± SEM (n = 7).
FIG. 5.
Circulating IL-6 levels in T. cruzi-infected mice treated with the TN3 anti-TNF MAb TN3. IL-6 was detected by ELISA in plasma of infected mice treated with TN3 or control MAb (n = 5 per group). Levels are given as arithmetic means ± SEM. Asterisks indicate significant differences between TN3-treated and control mice (P < 0.05, Student t test).
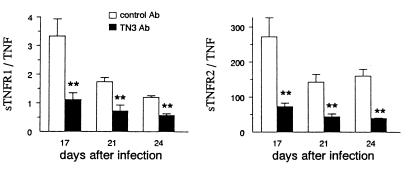
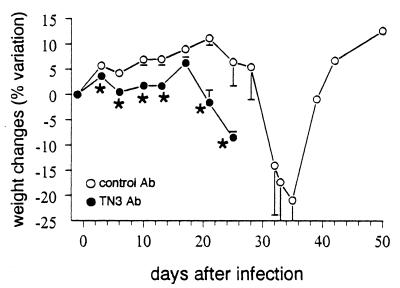
However, the treatment of mice with MAb TN3 did not affect the circulating levels of sTNFR1 and -R2 (Fig. 4B and C), which underwent variations similar to those described for untreated T. cruzi-infected mice (Fig. 1). Subsequently, the molar ratios sTNFR/TNF were significantly decreased on days 17, 21, and 24 p.i. in TN3-treated mice in comparison to animals receiving the control Ig (Fig. 6). Such reduction of sTNFR/TNF ratios was associated with considerably worsened cachexia, which started earlier (Fig. 7), as well as with significantly shortened survival times (20.5 ± 2.5 versus 26.1 ± 8.1 days, P < 0.005) and enhanced mortality rates, since all TN3-treated mice died (Fig. 4D), harboring extremely high parasitaemia (Fig. 4E).
FIG. 6.
Molar ratios between circulating levels of sTNFR and TNF in T. cruzi-infected mice treated with the anti-TNF MAb TN3. Mice were inoculated with 100 parasites on day 0 and treated with the anti-TNF TN3 or control MAb during the 2 first weeks of infection (Fig. 4). Results are expressed as arithmetic means ± SEM (n = 5 to 9). Asterisks indicate significant differences between groups (P < 0.05, Student t test).
FIG. 7.
Cachexia in T. cruzi-infected mice treated with the anti-TNF MAb TN3. T. cruzi-infected mice were treated as for Fig. 4 with anti-TNF TN3 or control MAb. Changes in body weight of 10 mice per group are expressed as percent variation of mouse weight at each experimental point compared to the weight on day 0 of infection (arithmetic means ± SEM). Asterisks indicate significant differences between the two groups (P < 0.05, Student t test).
Administration of anti-TNF MAb 1F3F3 to T. cruzi-infected mice reduced cachexia but did not affect survival of animals, through slightly increasing the sTNFR/TNF ratios.
The two experiments performed (with 11 and 7 mice per group) gave similar results. As shown in Table 4 (results obtained from one representative experiment), TNF as well as IL-6 levels were roughly reduced by half when MAb 1F3F3 was administered to infected mice on days −1, 2, 5, 8, 11, and 14 relative to parasite inoculation, indicating lower bioavailability of TNF in blood of these mice. As for TN3 MAb, 1F3F3 treatment did not modify the levels of sTNFR compared to animals receiving the control Ab; consequently, the sTNFR/TNF ratios were increased slightly, 1.5- to 2-fold. Although parasitemia was reduced by half during the experiments, the mortality rates remained similar in the two groups of mice (mean survival time ± SEM, 22.0 ± 0.9 versus 23.1 ± 1.1), suggesting that the induced increases of sTNFR/TNF ratios were not sufficient enough to improve survival. However, as previously published (29), mice treated with MAb 1F3F3 underwent a significant reduction of cachexia.
TABLE 4.
Circulating levels of TNF and sTNFR/TNF ratios in T. cruzi-infected mice treated with 1F3F3 anti-TNF or control MAbsa
| Day p.i. | Mouse treatment | TNF (ng/ml) | sTNFR1/TNF | sTNFR2/TNF | IL-6 (pg/ml) | Parasites (106/ml) | % variation in wt |
|---|---|---|---|---|---|---|---|
| 15 | Anti-TNF | 0.12 ± 0.12* | 11.5 ± 2.9 | 334 ± 46* | NDb | 0.273 | 4.5 ± 3.8 |
| control | 0.56 ± 0.11 | 6.4 ± 1.6 | 179 ± 30 | ND | 0.417 | 3.2 ± 2.3 | |
| 18 | Anti-TNF | 0.75 ± 0.30 | 2.64 ± 0.40 | 303 ± 18* | 93 ± 3* | 1.437 | 6.2 ± 5.4 |
| control | 1.05 ± 0.24 | 2.04 ± 0.49 | 159 ± 33 | 166 ± 4 | 1.702 | 4.1 ± 5.8 | |
| 25 | Anti-TNF | 1.17 ± 0.65 | 1.99 ± 0.53 | 248 ± 27 | ND | 2.134* | 4.4 ± 2.4 |
| control | 2.79 ± 0.89 | 1.09 ± 0.19 | 149 ± 50 | ND | 4.775 | −5.7 ± 7.5 |
Mice were inoculated with 100 parasites on day 0 and treated with anti-TNF 1F3F3 or control MAb during the first 2 weeks of infection as mentioned in the text. Results are expressed as arithmetic means ± SEM, except for parasitemia, expressed as geometric means (n = 5 to 9). Asterisks represent significant differences between the two groups of mice (P < 0.05 [Student t test] except for parasitemia [analysis of variance]).
ND, not determined.
DISCUSSION
Our data show that the parasitemic phase of T. cruzi infection in mice is associated with high levels of circulating TNF and sTNFR2, resulting in the formation of cytokine-receptor complexes and some degree of neutralization of TNF bioactivity. Low sTNFR/TNF circulating ratios were associated with cachexia in all infected mice, and the lowest ratios were observed only in dying animals harboring the highest parasitemia. The administration of anti-TNF MAb TN3 to infected mice lowered the sTNFR/TNF circulating ratios (through a paradoxical overproduction of TNF) and considerably worsened cachexia and mortality in the animals while increasing parasite levels. Another anti-TNF MAb (1F3F3) reduced blood amounts of TNF and parasites, as well as cachexia. Collectively, these results indicate that during the parasitemic phase of infection, TNF triggers harmful effects (contrasting with its beneficial role earlier in the infection), which are limited by the simultaneous in vivo production of endogenous soluble receptors.
The close relationship between the kinetics of blood parasites, TNF levels, and sTNFR2 levels during the parasitemic phase of T. cruzi infection argues for a direct effect of parasites on the release of TNF and sTNFR2. This may be due to the action of membrane glycoproteins of T. cruzi, which have recently been shown to trigger TNF production in vitro (10). Since TNF and sTNFR2 are cleaved from cell membrane by the same protease (43), these parasitic glycoproteins might at the same time account for the TNF and sTNFR2 release. TNF itself, known to trigger its own release (2) as well as that of sTNFR2 (4, 32), and other TNF-inducing cytokines produced during T. cruzi infection such as IFN-γ and IL-12 (45, 52), may also enhance the parasitic effect. In addition, the renal lesions occurring during T. cruzi infection (14) might increase the blood levels of TNF and sTNFR2 further by reducing their clearance (3).
Plasma from acutely infected mice displayed some capacity to neutralize TNF, associated with the presence of cytokine-receptor complexes involving mainly sTNFR2 (present at much higher amounts than sTNFR1). On the other hand, our results suggest that variations of sTNFR/TNF ratios modified the amount of bioactive TNF. Indeed, mice harboring reduced sTNFR/TNF ratios (treated with MAb TN3) displayed increased levels of IL-6, a cytokine previously detected in mouse T. cruzi infection (53) and that may be considered a functional marker of TNF bioactivity (27). Conversely, animals presenting higher sTNFR/TNF ratios (treated with MAb 1F3F3) lowered their IL-6 amounts. These observations strongly suggest that during murine T. cruzi infection, TNF bioactivity is regulated by sTNFR.
We have previously established the role of TNF in the cachexia associated with T. cruzi acute murine infection (54). Indeed, infected mice exhibited a severe weight loss that could be reduced by the administration of MAb 1F3F3. In the present work, we confirm our previous results by showing that MAb 1F3F3 limits the in vivo TNF bioactivity and that, conversely, the increase of TNF levels (observed in the TN3-treated mice) exacerbated the weight loss of infected animals. A relationship was established between sTNFR/TNF ratios and the intensity of cachexia during the course of the parasitemic phase of infection, indicating the capacity of sTNFR to limit the TNF effects on weight loss. Moreover, the occurrence of cachexia in all mice acutely infected with T. cruzi 1 week after the changes in sTNFR/TNF ratios indicates that only low levels of bioactive endogenous TNF are required during a prolonged time to induce such harmful effects, in full agreement with previous studies (13).
The present results also indicate that high levels of bioactive TNF are lethal in acutely infected mice and that a massive production of TNF, as paradoxically induced by the administration of MAb TN3, kills all mice. Though not currently studied in T. cruzi infection, the mechanisms of TNF-dependent mortality have been described for two models: the low-dose endotoxin septic shock (24) and the generalized Shwartzman reaction (22). Both are mediated through TNFR1 and require sensitizing factors (d-galactosamine and an initial injection of a low dose of LPS, respectively). Acutely T. cruzi-infected mice are known to be sensitized to the lethal action of TNF, since administration of exogenous TNF (at concentrations not lethal for control mice) kills them (8). Such sensitization in T. cruzi infection might be related to an overexpression of TNFR1 on host cell membrane, since they are released only at low levels in the circulation. A role for IFN-γ should not be excluded since this cytokine was documented to trigger lethality during septic shock (24) and Shwartzman reactions (22) in association with TNF and was increased in blood of our acutely infected mice (data not shown).
The harmful effects of TNF (cachexia and mortality), observed during the parasitemic phase of the infection, are associated with parasite levels. This observation completes previous studies highlighting a protective role of TNF in T. cruzi infection (12, 33, 48). In addition, different in vitro studies clearly showed a protective effect of TNF in the control of cell infection with parasites (8, 21, 40, 48, 57). This apparent contradiction can be resolved by considering TNF as differently involved in two evolutive steps of the acute phase of infection: the incubation period (roughly the first week after parasite inoculation), with undetectable levels of parasites in the blood, and the parasitemic phase (around 1 to 6 weeks p.i.). The TNF initially produced in the first days of infection in response to T. cruzi inoculation could contribute to initiate the control of parasite multiplication, in synergy with IFN-γ produced under IL-12 stimulation (9). Parasites having escaped this initial control continue to multiply actively in the vertebrate tissues till being detected in blood (parasitemic phase). At this step, TNF production, highly stimulated by the circulating parasites, may reach dangerous concentrations. The simultaneous release of a large excess of sTNFR2 in the circulation, also induced by infection, might partially inhibit the harmful effects of TNF, allowing the survival of some animals, though enough bioactive TNF remains available to trigger cachexia. Surviving mice develop specific immune responses, improving the control of infection and thereby ensuring the continuation of the parasite cycle. This view is strengthened by the results obtained when the TNF-neutralizing MAb TN3 was administered into mice before and early after infection (between days −1 and 16 p.i.). This Ab might efficiently neutralize the low levels of TNF produced in vivo during this early step of infection, resulting in a more intense tissue parasite multiplication and ultimately extremely high parasitemia and overproduction of TNF (this should explain the paradoxical TNF increase observed in these animals). These high cytokine amounts may overcome the neutralization capacities of soluble receptors, causing extreme cachexia and death of all mice.
Why another MAb such as 1F3F3 has an inverse effect remains unclear, whereas its capacity to neutralize TNF has clearly been demonstrated in vitro and in vivo (35). Besides isotype-related differences (IgM for 1F3F3 versus IgG1 for TN3) in their pharmacokinetics and capacities to bind FcR and C1q, 1F3F3 displays a higher avidity than TN3 and neutralizes TNF in another way. Indeed, 1F3F3 binds to a TNF sequence situated outside the site responsible for its recognition by the receptors (34), whereas TN3 interacts with an amino acid sequence located within this region (47). Therefore, MAb 1F3F3 might neutralize only soluble TNF (since it might be more difficult for a conformational change of membrane-anchored TNF to occur), whereas MAb TN3 would inhibit the activity of both forms. As membrane and soluble TNF differ in the ability to bind to TNFR1 and -R2 (23, 44) and are differently involved in the control of other infections with intracellular pathogens (7, 15), we might further hypothesize that the use of MAb 1F3F3 rather than TN3 has also a different impact on T. cruzi infection. Moreover, 1F3F3, as an IgM MAb, might bridge several molecules of TNF on host cell membranes (the TNF sequence recognized by 1F3F3 is extracellular), thereby triggering a better control of parasite multiplication through higher IFN-γ production, as observed in human T-cell leukemia virus type 1-infected T cells treated with an anti-TNF MAb (25).
In conclusion, our data highlight the role of the endogenous balance between TNF and its soluble receptors and the subsequent harmful versus beneficial effects of TNF on the outcome of T. cruzi infection.
ACKNOWLEDGMENTS
We thank Francine Keruzore for diligent technical assistance, H. Collet for LPS determinations, M. Bodmer for providing the anti-TNF chimeric MAb TN3, and H. Bazin for providing an unrelated MAb.
This work was supported by grants from the Ministry of Scientific Policy and the Université Libre de Bruxelles, Brussels, Belgium (Concerted Research Actions).
REFERENCES
- 1.Abrahamsohn I A, Coffman R L. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B B, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 3.Bemelmans M H, Gouma D J, Buurman W A. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J Immunol. 1993;150:2007–2017. [PubMed] [Google Scholar]
- 4.Bemelmans M H, Gouma D J, Buurman W A. LPS-induced sTNF-receptor release in vivo in a murine model. Investigation of the role of tumor necrosis factor, IL-1, leukemia inhibiting factor, and IFN-gamma. J Immunol. 1993;151:5554–5562. [PubMed] [Google Scholar]
- 5.Bemelmans M H, Gouma D J, Greve J W, Buurman W A. Effect of antitumour necrosis factor treatment on circulating tumour necrosis factor levels and mortality after surgery in jaundiced mice. Br J Surg. 1993;80:1055–1058. doi: 10.1002/bjs.1800800845. [DOI] [PubMed] [Google Scholar]
- 6.Billiau A, Matthys P, Martens E, Heremans H. Effects of anti-interferon-gamma and anti-interleukin-6 antibodies in disease models in mice: antibodies as carriers of cytokines. J Interferon Res. 1994;14:277–279. doi: 10.1089/jir.1994.14.277. [DOI] [PubMed] [Google Scholar]
- 7.Birkland T P, Sypek J P, Wyler D J. Soluble TNF and membrane TNF expressed on CD4+ T lymphocytes differ in their ability to activate macrophage antileishmanial defense. J Leukoc Biol. 1992;51:296–299. doi: 10.1002/jlb.51.3.296. [DOI] [PubMed] [Google Scholar]
- 8.Black C M, Israelski D M, Suzuki Y, Remington J S. Effect of recombinant tumour necrosis factor on acute infection in mice with Toxoplasma gondii or Trypanosoma cruzi. Immunology. 1989;68:570–574. [PMC free article] [PubMed] [Google Scholar]
- 9.Brener Z, Gazzinelli R T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol. 1997;114:103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 10.Camargo M M, Almeida I C, Pereira M E, Ferguson M A, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 11.Carlier Y, Rivera M T, Truyens C, Goldman M, Lambert P, Flament J, Bauwens D, Vray B. Pregnancy and humoral immune response in mice chronically infected by Trypanosoma cruzi. Infect Immun. 1987;55:2496–2501. doi: 10.1128/iai.55.10.2496-2501.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castanos Velez E, Maerlan S, Osorio L M, Aberg F, Biberfeld P, Orn A, Rottenberg M E. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect Immun. 1998;66:2960–2968. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3–10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 14.Costa R S, Monteiro R C, Lehuen A, Joskowicz M, Noel L H, Droz D. Immune complex-mediated glomerulopathy in experimental Chagas' disease. Clin Immunol Immunopathol. 1991;58:102–114. doi: 10.1016/0090-1229(91)90152-z. [DOI] [PubMed] [Google Scholar]
- 15.Deckert-Schlüter M, Bluethmann H, Rang A, Hof H, Schlüter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 16.Dentener M A, Greve J W, Maessen J G, Buurman W A. Role of tumour necrosis factor in the enhanced sensitivity of mice to endotoxin after exposure to lead. Immunopharmacol Immunotoxicol. 1989;11:321–334. doi: 10.3109/08923978909005373. [DOI] [PubMed] [Google Scholar]
- 17.Espevik T, Nissen Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Botran R, Chilton P M, Ma Y. Soluble cytokine receptors: their roles in immunoregulation, disease, and therapy. Adv Immunol. 1996;63:269–336. doi: 10.1016/s0065-2776(08)60858-5. [DOI] [PubMed] [Google Scholar]
- 19.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 20.Garcia I, Miyazaki Y, Araki K, Araki M, Lucas R, Grau G E, Milon G, Belkaid Y, Montixi C, Lesslauer W, et al. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur J Immunol. 1995;25:2401–2407. doi: 10.1002/eji.1830250841. [DOI] [PubMed] [Google Scholar]
- 21.Golden J M, Tarleton R L. Trypanosoma cruzi: cytokine effects on macrophage trypanocidal activity. Exp Parasitol. 1991;72:391–402. doi: 10.1016/0014-4894(91)90085-b. [DOI] [PubMed] [Google Scholar]
- 22.Grau G E, Vesin C, De Groote D, Delacroix D, Gysler C, Piguet P F, Lambert P H. Prevention of human TNF-induced cutaneous Shwartzmann reaction and acute mortality in mice treated with anti-human TNF monoclonal antibodies. Clin Exp Immunol. 1991;84:411–414. [PMC free article] [PubMed] [Google Scholar]
- 23.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, Georgopoulos S, Lesslauer W, Kollias G, Pfizenmaier K, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez Ramos J C, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi M, Nagasawa K, Horiuchi T, Oike M, Ito Y, Yasukawa M, Niho Y. Membrane tumor necrosis factor-alpha (TNF-alpha) expressed on HTLV-I-infected T cells mediates a costimulatory signal for B cell activation—characterization of membrane TNF-alpha. Clin Immunol Immunopathol. 1997;82:133–140. doi: 10.1006/clin.1996.4291. [DOI] [PubMed] [Google Scholar]
- 26.Himmler A, Maurer Fogy I, Kronke M, Scheurich P, Pfizenmaier K, Lantz M, Olsson I, Hauptmann R, Stratowa C, Adolf G R. Molecular cloning and expression of human and rat tumor necrosis factor receptor chain (p60) and its soluble derivative, tumor necrosis factor-binding protein. DNA Cell Biol. 1990;9:705–715. doi: 10.1089/dna.1990.9.705. [DOI] [PubMed] [Google Scholar]
- 27.Huang D, Reittie J E, Stephens S, Hoffbrand A V, Brenner M K. Effects of anti-TNF monoclonal antibody infusion in patients with hairy cell leukaemia. Br J Haematol. 1992;81:231–234. doi: 10.1111/j.1365-2141.1992.tb08212.x. [DOI] [PubMed] [Google Scholar]
- 28.Hunter C A, Ellis Neyes L A, Slifer T, Kanaly S, Grunig G, Fort M, Rennick D, Araujo F G. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J Immunol. 1997;158:3311–3316. [PubMed] [Google Scholar]
- 29.Jacobs F, Dubois C, Carlier Y, Goldman M. Administration of anti-CD3 monoclonal antibody during experimental Chagas' disease induces CD8+ cell-dependent lethal shock. Clin Exp Immunol. 1996;103:233–238. doi: 10.1046/j.1365-2249.1996.d01-632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kierszenbaum F, Saavedra L E. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. J Protozool. 1972;19:655–657. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- 31.Krassner S M, Granger B, Morrow C, Granger G. In vitro release of lymphotoxin by spleen cells from C3H/HEJ and C57BL/6 mice infected with Trypanosoma cruzi. Am J Trop Med Hyg. 1982;31:1080–1089. doi: 10.4269/ajtmh.1982.31.1080. [DOI] [PubMed] [Google Scholar]
- 32.Lantz M, Malik S, Slevin M L, Olsson I. Infusion of tumor necrosis factor (TNF) causes an increase in circulating TNF-binding protein in humans. Cytokine. 1990;2:402–406. doi: 10.1016/1043-4666(90)90048-x. [DOI] [PubMed] [Google Scholar]
- 33.Lima E C, Garcia I, Vicentelli M H, Vassalli P, Minoprio P. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–465. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas R. Functional dissection of tumor necrosis factor. PhD thesis. Brussels, Belgium: Free University of Brussels; 1993. [Google Scholar]
- 35.Lucas R, Heirwegh K, Neirynck A, Remels L, Van Heuverswyn H, De Baetselier P. Generation and characterization of a neutralizing rat anti-rmTNF-alpha monoclonal antibody. Immunology. 1990;71:218–223. [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas R, Juillard P, Decoster E, Redard M, Burger D, Donati Y, Giroud C, Monso Hinard C, De Kesel T, Buurman W A, Moore M W, Dayer J M, Fiers W, Bluethmann H, Grau G E. Crucial role of tumor necrosis factor (TNF) receptor 2 and membrane-bound TNF in experimental cerebral malaria. Eur J Immunol. 1997;27:1719–1725. doi: 10.1002/eji.1830270719. [DOI] [PubMed] [Google Scholar]
- 37.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthys P, Billiau A. Cytokines and cachexia. Nutrition. 1997;13:763–770. doi: 10.1016/s0899-9007(97)00185-8. [DOI] [PubMed] [Google Scholar]
- 39.Mohler K M, Torrance D S, Smith C A, Goodwin R G, Stremler K E, Fung V P, Madani H, Widmer M B. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 40.Munoz Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 41.Nophar Y, Kemper O, Brakebusch C, Englemann H, Zwang R, Aderka D, Holtmann H, Wallach D. Soluble forms of tumor necrosis factor receptors (TNF-Rs). The cDNA for the type I TNF-R, cloned using amino acid sequence data of its soluble form, encodes both the cell surface and a soluble form of the receptor. EMBO J. 1990;9:3269–3278. doi: 10.1002/j.1460-2075.1990.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olsson I, Gatanaga T, Gullberg U, Lantz M, Granger G A. Tumour necrosis factor (TNF) binding proteins (soluble TNF receptor forms) with possible roles in inflammation and malignancy. Eur Cytokine Netw. 1993;4:169–180. [PubMed] [Google Scholar]
- 43.Peschon J J, Slack L, Reddy P, Stocking K L, Sunnarborg S W, Lee D C, Russel W E, Castner B J, Johnson R S, Fitzner J N, Boyce R W, Nelson N, Kozlosky C J, Wolfson M F, Rauch C T, Cerreti D P, Paxton R J, March C J, Black R A. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 44.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 45.Reed S G, Brownell C E, Russo D M, Silva J S, Grabstein K H, Morrissey P J. IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol. 1994;153:3135–3140. [PubMed] [Google Scholar]
- 46.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan K C, Ruddle N H, Schreiber R D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- 48.Silva J S, Vespa G N, Cardoso M A, Aliberti J C, Cunha F Q. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starobinas N, Russo M, Minoprio P, Hontebeyrie Joskowicz M. Is TNF alpha involved in early susceptibility of Trypanosoma cruzi-infected C3H/He mice? Res Immunol. 1991;142:117–122. doi: 10.1016/0923-2494(91)90019-f. [DOI] [PubMed] [Google Scholar]
- 50.Suitters A J, Foulkes R, Opal S M, Palardy J E, Emtage J S, Rolfe M, Stephens S, Morgan A, Holt A R, Chaplin L C, et al. Differential effect of isotype on efficacy of anti-tumor necrosis factor alpha chimeric antibodies in experimental septic shock. J Exp Med. 1994;179:849–856. doi: 10.1084/jem.179.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarleton R L. Tumour necrosis factor (cachectin) production during experimental Chagas' disease. Clin Exp Immunol. 1988;73:186–190. [PMC free article] [PubMed] [Google Scholar]
- 52.Torrico F, Heremans H, Rivera M T, Van Marck E, Billiau A, Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–3632. [PubMed] [Google Scholar]
- 53.Truyens C, Angelo Barrios A, Torrico F, Van Damme J, Heremans H, Carlier Y. Interleukin-6 (IL-6) production in mice infected with Trypanosoma cruzi: effect of its paradoxical increase by anti-IL-6 monoclonal antibody treatment on infection and acute-phase and humoral immune responses. Infect Immun. 1994;62:692–696. doi: 10.1128/iai.62.2.692-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Truyens C, Torrico F, Angelo Barrios A, Lucas R, Heremans H, De Baetselier P, Carlier Y. The cachexia associated with Trypanosoma cruzi acute infection in mice is attenuated by anti-TNF-alpha, but not by anti-IL-6 or anti-IFN-gamma antibodies. Parasite Immunol. 1995;17:561–568. doi: 10.1111/j.1365-3024.1995.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 55.Van Voorhis W C. Coculture of human peripheral blood mononuclear cells with Trypanosoma cruzi leads to proliferation of lymphocytes and cytokine production. J Immunol. 1992;148:239–248. [PubMed] [Google Scholar]
- 56.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 57.Wirth J J, Kierszenbaum F. Recombinant tumor necrosis factor enhances macrophage destruction of Trypanosoma cruzi in the presence of bacterial endotoxin. J Immunol. 1988;141:286–288. [PubMed] [Google Scholar]
- 58.Zhang L, Tarleton R L. Characterization of cytokine production in murine Trypanosoma cruzi infection by in situ immunocytochemistry: lack of association between susceptibility and type 2 cytokine production. Eur J Immunol. 1996;26:102–109. doi: 10.1002/eji.1830260116. [DOI] [PubMed] [Google Scholar]