Abstract
Tumor diseases are unfortunately quick spreading, even though numerous studies are under way to improve early diagnosis and targeted treatments that take into account both the different characteristics associated with the various tumor types and the conditions of individual patients. In recent years, studies have focused on the role of ion channels in tumor development, as these proteins are involved in several cellular processes relevant to neoplastic transformation. Among all ion channels, many studies have focused on the superfamily of Transient Receptor Potential (TRP) channels, which are non-selective cation channels mediating extracellular Ca2+ influx. In this review, we examined the role of different endothelial TRP channel isoforms in tumor vessel formation, a process that is essential in tumor growth and metastasis.
Keywords: transient receptor potential, tumor vascularization, endothelial cells, endothelial colony-forming cells, Ca2+ signaling
1. Introduction
Like all tissues, tumors depend on blood vessels for the supply of oxygen (O2) and nutrients, as well as for the removal of metabolic catabolites; blood vessels also make it unavoidable for metastatic cancer cells to spread and invade different districts [1].
Physiologically, endothelial cells (ECs) play a crucial role in the development and maintenance of the vascular network, a process that requires the interaction of three distinct mechanisms: vasculogenesis, angiogenesis, and arteriogenesis [2].
Vasculogenesis is the de novo formation of a vascular network during embryonic development, angiogenesis is a regulated process in which the blood vessel network is formed from pre-existing capillaries, and arteriogenesis refers to the enlargement of pre-existing arterial connections into completely developed and functional arteries. Every day, approximately 100 quiescent ECs undergo turnover in response to pro-angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF).
Several processes contribute to neovessel formation in growing tumors and distant metastases: sprouting angiogenesis (SA) which often results in non-productive angiogenesis with blood vessels that are abnormal in structure and function [3,4]; intussusception angiogenesis (IA), which is often activated because it is less energy-intensive [5]; non-angiogenic processes, such as vascular co-option, where the tumor surrounds an already existing vascular network [6]; vasculogenesis, which is mediated by the recruitment of multiple subtypes of endothelial progenitor cells (EPCs), including endothelial colony-forming cells (ECFCs) [7].
The recruitment of many Ca2+-sensitive decoders in response to pro-angiogenic cues including VEGF, bFGF, stromal derived factor-1 (SDF-1), and angiopoietins has long been known to take place during angiogenesis. In addition, it has recently been demonstrated that intracellular Ca2+ signaling also promotes the proliferation, tube development, and neovessel formation in circulating ECFCs [8,9,10,11]. A crucial role in this process has been attributed to endothelial TRP channels, which are sensitive to pro-angiogenic factors and can regulate both angiogenesis and vasculogenesis [7].
Endogenous Ca2+ release through inositol-1,4,5-trisphosphate receptors (InsP3R) and store-operated Ca2+ entry (SOCE) through Orai1 channels are the main drivers of the endothelial Ca2+ response to pro-angiogenic cues, which can take on a variety of waveforms, including Ca2+ transients, biphasic Ca2+ signals, and repetitive Ca2+ oscillations. Lysosomal Ca2+ release via nicotinic acid adenine dinucleotide phosphate (NAADP)-gated two-pore channels is a critical pro-angiogenic route that supports intracellular Ca2+ mobilization [12]. Therefore, understanding how TRP-mediated endothelial Ca2+ signaling regulates neovessel formation could shed light on alternative strategies to interfere with the aberrant vascularization in cancer.
2. Vasculogenesis, Angiogenesis, and Arteriogenesis in Neovessel Formation
Vasculogenesis is the process through which mesoderm-derived progenitors (angioblasts) differentiate into ECs, which then assemble and unite to produce blood islands and the major capillary plexus. Angiogenesis, which can occur by SA or by IA, is primarily responsible for the subsequent extension and remodeling of this primitive network (2). SA is triggered by a tipping of the balance between pro- and anti-angiogenic factors in favor of pro-angiogenic cues. EC activation is induced in response to a gradient of VEGF, which stimulates endothelial tip cells to elongate actin-rich filopodia that migrate toward the source of VEGF, thereby conferring directionality to the sprout [13]. Digestion of the extracellular matrix and basement membrane by specific proteases also occurs during this migration. Behind the tip cells, stalk cells proliferate, allowing the nascent vessel to elongate [13]; this immature vessels is then stabilized by the recruitment of mural cells (i.e., smooth muscle cells and pericytes). IA is a microvascular growth process that tears an existing vessel in two. A cellular pillar is inserted into the arterial lumen, and the whole process seems to depend less on cell migration and more on cell proliferation and reorganization [14]. Finally, arteriogenesis involves pre-existing arterioles that undergo growth and remodeling under the induction of PDGF, and subsequent stabilization through the recruitment of smooth muscle cells (SMCs) or pericytes [13]; the result is the formation of larger vessels [15], as more widely illustrated in the following section.
3. Neovessel Formation in the Tumor
Tumor vessels turn out to be highly immature [3], surrounded by only a few pericytes and lacking the adhesion molecule VE-cadherin (vascular endothelial cadherin) with a weakening of the intercellular endothelial junctions. This results in the formation of an endothelium that leads to an increase in interstitial fluid, accumulation of solutes, and transmigration of tumor cells [1,16]. The tumor vasculature is extremely scattered and chaotically distributed, and blood frequently travels via the same channel in multiple directions [17]. Pro-angiogenic factors, such as VEGF, FGF, placental growth factor (PlGF), and angiopoietins, are stimulated even more when there are arteries that are not fully functional, and this results in a continuous cycle of unproductive angiogenesis [4].
In addition to SA, IA is a mechanism that is frequently activated in tumor formation [5] because, from a metabolic standpoint, IA may be less difficult than SA given the low proliferation and migration of ECs along with the quicker rate at which ECM and perivascular cells promote the rapid generation of new vessels. It is known that blocking Notch signaling in the existing vascular bed encourages pericyte detachment and mononuclear cell overflow, which causes rapid vascular expansion via IA, while blocking Notch signaling in the anterior margins of the developing vessel causes SA. The exact molecular mechanisms of IA are still largely unknown [18]. There may be additional non-angiogenic processes for tumor vascularization. One of them is vascular co-option, in which cancer cells take over already-existing, dormant vessels from the surrounding parenchymal tissue and incorporate them into the mass of the tumor. The proliferation of ECs in co-opted vasculature is smaller than that of angiogenic ones, and they exhibit the normal angiogenic markers at low levels. Vessel co-option has been seen in primary and metastatic cancers, particularly in lung, brain, and liver tumors, and is a resistance mechanism to anti-angiogenic therapy. It is associated with a poor prognosis in patients [6].
A crucial contribution to the angiogenic switch that turns a dormant lesion into a growing tumor is provided by the recruitment of myeloid angiogenic cells and ECFCs, which are released from the bone marrow and vascular stem cell niches, respectively. Indeed, several studies have shown that circulating ECFCs contribute to the tumor vasculature in several malignancies, including breast carcinoma [19,20], renal cell carcinoma [19], and melanoma [21], and thus represent a cellular target for dismantling the tumor vasculature [16,22].
4. ECFCs in Tumor Vascularization
Tumor vascularization is a far more complicated process than was initially thought. During the development of tumors and the spread of metastatic disease, angiogenic alteration is fueled by the coordinated interaction of local ECs and circulating EPCs [23,24,25]. The cross-talk with the surrounding microenvironment, which is characterized by hypoxia, low pH, disorganized basement membrane, high interstitial fluid pressure, enrichment of growth factors and cytokines, and the different vascular beds of origin, determines the heterogeneity of tumor-endothelial cells (T-ECs) [26].
Tumor-cell-derived VEGF and SDF-1 mobilize EPCs from bone marrow and vascular wall stem cell habitats. Once these mediators are released into circulation, they create a concentration gradient that facilitates EPC recruitment to both the primary tumor lesion and the more remote pre-metastatic niches. Here, EPCs may both physically engraft within tumor vasculature and emit angiogenic factors to drive local angiogenesis, causing the transformation from a latent undetected lesion to a fatal metastatic cancer [25,27,28]. The higher frequency of circulating EPCs corresponds with enhanced angiogenesis and tumor volume and is related with a lower rate of patient survival, which is consistent with the role they play in cancer neovascularization. However, it should be noted that the level of EPC integration within tumor vasculature varies greatly, from 0% to more than 90%. A variety of overlapping factors may be used to account for this heterogeneity [3,23,24]. The kind, stage, and location of the tumor all affect the neovascularization caused by EPC. Mice heterozygous for the tumor suppressor Pten (phosphatase and tensin homolog deleted on chromosome 10) (Pten+/), which can display a variety of malignant neoplasms including uterine carcinomas and lymph hyperplasia, have been used to reveal this trait. In the former, EPC-induced angiogenesis was observed, but not in the latter [29]. EPCs are found in developing pulmonary metastases before vascular formation, implanted LLC (Lewis lung carcinoma), B6RV2 (human lymphoma cells), and melanoma, as well as spontaneous breast cancer occurring in MMTV-PyMT mice (mouse mammary tumor virus-polyoma middle tumor-antigen) [3,23]. They are thereby diminished by both local endothelial cells and hematopoietic cells coming from bone marrow, a characteristic that may understate their contribution at a later stage of tumor growth [24,27]. Last but not least, and maybe the most significant source of variation in the assessment of their role in the angiogenic switch, the EPC phenotype is seldom known [30,31]. The term “EPC” refers to at least two distinct subsets that can be categorized as “hematopoietic” and “non-hematopoietic”, respectively, rather than a single cell population with distinctive surface markers that make it simple to detect and quantify in vivo [31].
The ability of hematopoietic progenitor and stem cells to support vessel growth through the paracrine secretion of growth factors and cytokines, as well as the absence of a combination of markers and receptors selective for truly endothelial EPCs, have both contributed to the confusion surrounding the definition of EPC [28,30]. Colony-forming unit-endothelial cells (CFU-ECs) and circulating angiogenic cells (CACs) make up the hematopoietic EPCs, whereas so-called ECFCs make up the non-hematopoietic EPCs. Despite being drawn to the expanding tumor, CFU-ECs and CACs do not immediately integrate into neovessels because they are located perivascularly and promote malignant growth in a paracrine way. In contrast, ECFCs are devoted to differentiating into mature endothelial cells, form capillary-like structures in vitro, and patent vasculature in vivo [28,30,31]. They also have a remarkable capacity for cloning. They are now recognized as genuine EPCs that have the potential to physically engraft within cancer vasculature. Recent research has proven that human ECFCs, which really come from the endothelium lineage, may be directly injected into the bloodstream of multiple mouse models of human malignancies and then recruited to areas of tumor angiogenesis. More specifically, subcutaneously implanted glioma [32] and breast cancer [33] xenografts, as well as LLC metastases [34], actively reside in DiI-labeled ECFCs. It turns out that ECFC is the best subset to use when examining the molecular pathways causing EPC integration into tumor vasculature and, as a result, when figuring out which targets are most useful in slowing down unfavorable metastatic growth.
5. Pro-Angiogenic Ca2+ Signals in Vascular Endothelial Cells and ECFCs
Three Ca2+ transport systems work together to set the [Ca2+]i in vascular endothelial cells between 100 and 200 nM. These systems either extrude Ca2+ across the plasma membrane, such as the plasma membrane Ca2+-ATPase and the Na+/Ca2+ exchanger (NCX), or sequester cytosolic Ca2+ in the endoplasmic reticulum (ER), the largest intracellular Ca2+ reservoir (Sarco-Endoplasmic Reticulum Ca2+-ATPase (SERCA)) [35,36]. Neovessel formation depends on an increase in intracellular Ca2+ concentration ([Ca2+]i) in both vascular endothelial cells [11,12,37] and circulating ECFCs. In accord, pro-angiogenic Ca2+ signals can be elicited in vascular ECs by a plethora of growth factors, such as VEGF [12], epidermal growth factor [38], and basic fibroblast growth factor, as well as by vasoactive and inflammatory mediators, such as thrombin [39], ATP [40,41], ADP [42], acetylcholine [43], and pleiotropic hormones, including erythropoietin [44]. Pro-angiogenic Ca2+ signals can also be delivered to vascular endothelial cells by mechanical stimuli, such as laminar shear stress, which increases during collateral blood vessel growth and stimulates arteriogenesis [38], and endothelial denudation, which stimulates wound repair and endothelial regrowth [45,46]. Furthermore, an increase in [Ca2+]i also stimulates proliferation, migration, tube formation and neovessel formation in circulating ECFCs [15,37,47], which represent the only EPC subtype truly belonging to the endothelial lineage [48,49]. For instance, pro-angiogenic Ca2+ signals can be induced in circulating ECFCs by VEGF [50], SDF-1α [51], and the human amniotic fluid stem cell secretome [52].
An increase in endothelial [Ca2+]i can recruit a number of downstream Ca2+-dependent pro-angiogenic decoders, such as the transcription factors nuclear factor of activated T-cells (NFAT), nuclear factor-kappaB (NF-kB), cAMP responsive element binding protein (CREB), myosin light chain kinase (MLCK), myosin 2, endothelial nitric oxide synthase (eNOS), extracellular signal-regulated kinases 1/2 (ERK 1/2), and serine/threonine kinase (Akt) [12].
The endothelial Ca2+ response to pro-angiogenic signals can occur as recurrent Ca2+ spikes or a transitory or biphasic increase in [Ca2+]i. Phospholipase Cγ (PLCγ), which cleaves phosphatidylinositol-4,5-bisphosphate (PIP2) to generate the second messengers inositol-1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG), is brought about by the binding of VEGF to its specific receptor, VEGFR-2, on the plasma membrane. The latter triggers the proto-oncogene, serine/threonine kinase (RAF1)-MEK-ERK1/2 cascade by activating the DAG-sensitive protein-kinase C (PKC). InsP3 then activates the InsP3R on the ER membrane, thereby causing Ca2+ to be released from the ER. The following decrease in ER Ca2+ levels induces stromal interaction molecule 1 (Stim1), an ER Ca2+ sensor, to oligomerize and translocate from perinuclear to peripheral ER cisternae, where it interacts with the Ca2+-permeable channel, Orai1, at ER-plasma membrane junctions known as puncta (10–25 nm). The influx of Ca2+ through Orai1 channels has been termed store-operated Ca2+ entry (SOCE) and maintains the Ca2+ response to VEGF in both vascular endothelial cells [8,12,53] and circulating ECFCs [54]. Concurrent changes in the endothelial membrane potential (Vm), brought about by the recruitment of Ca2+-dependent conductances, have the capacity to modify the pro-angiogenic Ca2+ signal [55]. Ca2+ entry is regulated by the Ca2+ driving force, i.e., the difference between the Vm and the equilibrium potential for Ca2+ (ECa). The influence of the membrane potential is determined by the expression pattern of various ion channels. K+ channels, including Ca2+-activated K+ channels, inwardly rectifying K+ channels, and probably also voltage-dependent K+ channels, are the main class of ion channels in the regulation of membrane potential. Furthermore, Ca2+ entry into EC requires the presence of extracellular Cl− to maintain a polarized membrane [56]. An important role is attributed to the TRPV4 channel, which is responsible for almost half of the ability to increase [Ca2+]i in response to Vm ≥ −60 mV. The remaining [Ca2+]i response depends on TRPC1,3,4,5,6/TRPV1,3/TRPA1 [57], which assemble homomeric or heteromeric [58,59].
Several studies have shown that endothelial Ca2+ signals may be potentially responsible for aberrant angiogenesis and tumor proliferation [60,61]. The modification of the Ca2+ machinery in malignant cells, which contributes to the distinctive hallmarks of cancer [62], is a well-established tenet of neoplastic transformation [63,64]. T-ECs and tumor-derived ECFCs (T-ECFCs) exhibit a significant dysregulation of their Ca2+ signaling toolkit [19,65].
6. TRP Channels
TRP channels are a large family of cation channels, with sequence homology with the Drosophila TRP channel protein. They are divided into non-selective channels and highly selective cation channels. In mammals, using sequence homology, the 28 members of the TRP channel superfamily of non-selective cation channels can be classified into six subfamilies: TRP Canonical (TRPC1–7), TRP Vanilloid (TRPV1-6), TRP Melastatin (TRPM1–8), TRP Ankyrin1 (TRPA1), TRP Mucolipin (TRPM1-3), and TRP Polycystin (TRPP) [66,67,68,69]. There are eight members of the TRPP subfamily, although only TRPP2, TRPP3, and TRPP5 exhibit the structure and functionality of an ion channel [67]. In addition, TRPC2, which is essential for the mouse acrosomal reaction and pheromone detection, only exists as a pseudo-gene in people [67]. TRP channels can assemble into homomeric or heteromeric channels, combining with subunits belonging to the same or different subfamilies [70,71]. Heteromeric TRP channels, which differ from their homomeric counterparts in terms of biophysical fingerprints and regulation processes, have been extensively documented in naive cells, such as vascular endothelial cells, and heterologous expression systems. The TRPC subfamily has been the subject of in-depth research on subunit heteromerization. For instance, TRPC1 and TRPC3, TRPC4, or TRPC5 [69] may come together to form functional heteromeric channels, but TRPC3, TRPC6, and TRPC7 may also come together to form functional heteromeric channels in both naive tissues [72] and heterologous expression systems [73]. Heterotetramers made up of TRPV subunits are another possibility. Multiple studies have shown that TRPV5/TRPV6 subunits assemble into heteromeric channel complexes [74], as TRPV1-4 subunits. These heteromeric channel complexes are only found on the plasma membrane [58]. Additionally, functional heterotetramers made up of TRP channel subunits from several subfamilies have been widely documented. Examples of these heterotetramers include TRPC1/TRPP2 [70], TRPC1/TRPV4 [59], TRPC1/TRPV6 [75], TRPV4/TRPC6 [76], and TRPML3/TRPV5 [77].
TRP channels are multimodal cellular sensors that can be triggered by a wide range of chemical and physical stimuli, such as intracellular second messengers including diacylglycerol (DAG), arachidonic acid (AA), adenosine diphosphate ribose (ADPr), and hydrogen peroxide (H2O2), and intracellular ions, such as an increase in cytosolic HC and Ca2+, and a decrease in cytosolic Mg2+. Other possible triggers include dietary agonists, such as capsaicin, menthol, and allyl isothiocyanate (AITC), synthetic ligands, such as 4α-phorbol-12,13-didecanoate (4αPDD), gasotransmitters, such as nitric oxide (NO), proteins, such as G proteins, mechanical perturbation, such as membrane stretch, osmotic swelling, and laminar shear stress, and temperature fluctuations [67,68,78,79].
Although the relative permeability to Ca2+ and Na+ (PCa/PNa) might vary significantly between the various subunits, TRP channels are permeable to monovalent (Na+ and K+) and divalent (Ca2+ and Mg2+) cations. TRP channels transmit inward Na+ (and Ca2+ or Mg2+) currents in response to external stimulation, which have a significant effect on intracellular Ca2+ dynamics. In light of this, Ca2+ entry via Ca2+-permeable TRP channels may directly increase [Ca2+]i, whereas Na+ influx causes membrane depolarization, activating voltage-gated Ca2+ channels in excitable cells and altering the driving-force for Ca2+ entry in non-excitable cells [67]. Therefore, the “fractional Ca2+ current” of Ca2+-permeable TRP channels, which has not been measured for all TRP channels, determines the functional effect they have on intracellular Ca2+ homeostasis. For instance, TRPA1 and TRPM3 display fractional Ca2+ currents of 20%, whereas TRPV1 exhibits a fractional Ca2+ current of 5%, in contrast to TRPV5 and TRPV6, which mediate actual Ca2+ currents [67,68]. Some TRP channels can cause a significant rise in local Ca2+ concentration, which may be spatially restricted to the submembrane space or spread to the bulk cytosol through the Ca2+-dependent recruitment of InsP3Rs and ryanodine receptors (RyRs) [66,68].
6.1. The Role of TRP Channels in Endothelial Cells
ECs line the inner lumen of blood arteries and are consequently exposed to a variety of chemical and physical stimuli (growth factors and chemokines) that must be properly interpreted in order to maintain tissue homeostasis [12,80]. Due to the adaptability of their gating mechanisms, TRP channels provide the most ideal signal transduction system by which vascular ECs integrate such a wide variety of external inputs. Due to their sensitivity to many signaling pathways, endothelial TRP channels, with the exception of TRPC3, TRPC6, and TRPM4, serve as polymodal cellular sensors [66,68].
Vascular ECs have been found to contain the majority of mammalian TRP isoforms (TRPC1-7, TRPV1-4, TRPA1, TRPP1-2 and TRPM1-8) with the exception of TRPM5 [66,68,69], however TRP channel distribution patterns may change across the vascular tree and in various animal species. TRPC1 is present in mouse but not rat aortic ECs [81], whereas TRPC3 is broadly expressed in human but not bovine pulmonary artery ECs. Mouse brain microvascular ECs specifically express TRPC1-6 channels, but not human ones [82,83]; this may be affected by cell culture conditions and expression detection techniques. Naive TRP channels may include heteromeric subunits in vascular endothelial cells, as has been observed in other cell types. The following endothelial TRP channel complexes were reported: TRPC1-TRPC4 [84], TRPC1-TRPV4 [59], TRPC3-TRPC4 [85], TRPV1-TRPV4 [86], TRPP2-TRPC1 [70], and TRPC1-TRPP2-TRPV4 [87].
The majority of ECs functions are regulated by TRP channels, which either create a spatially constrained Ca2+ domain around the cytosolic mouth of the channel pore or an increase in [Ca2+]i globally [66]; they mediate Ca2+ entry in vascular ECs subjected to a variety of acetates, hormones, and mechanical stimuli, such as pulsatile stretch and laminar shear stress [66,68,69]. Additionally, TRP channels influence endothelial VM by conducting a depolarizing inward current carried by Na+ and Ca2+, resulting in a positive shift in VM that may be amplified by the Ca2+-dependent recruitment of TRPM4 (yet to be shown] [66,68,69]. Endothelial TRP channels may either support long-lasting processes, such as gene expression, proliferation, and migration, or short-term responses, such as vasodilation or an increase in vascular permeability. They could also serve as sensors of oxidative stress and local temperature changes; H2O2 induces an aberrant increase in [Ca2+]i by activating TRPM2 in pulmonary artery ECs after ischemic and reperfusion injury in mouse cerebral microcirculation [88]. In mouse aortic ECs, TRPV4 can detect a small increase in temperature (from 19 to 38 °C] but TRPV1 causes Ca2+ entry when heating from room temperature to over 40 °C [89]. This sensitivity has been linked to changes in vascular tone and endothelial permeability caused by temperature-dependent changes in NO release [89,90].
6.2. The Role of Endothelial TRP Channels in Physiological Angiogenesis
Endothelial TRP channels may facilitate vascular growth by promoting proliferation, migration, and tube formation in response to external growth factors, such as VEGF and bFGF, that are released in peripheral circulation after an ischemic shock or vascular injury [7,12,91]. Alternately, they can stimulate angiogenesis or arteriogenesis, respectively, as a consequence of cellular stress, such as a rise in intracellular ROS or a fall in cytosolic Mg2+ levels, or of an increase in laminar shear stress [7,91]. It is also known that angiogenesis is initiated in the hypoxic environment; oxygen concentration is a key regulator of angiogenesis, along with the heterodimeric hypoxia-inducible factor (HIF] protein, which includes HIF1α, degraded under normal conditions, and HIF1β [92].
6.2.1. Role of TRPCs
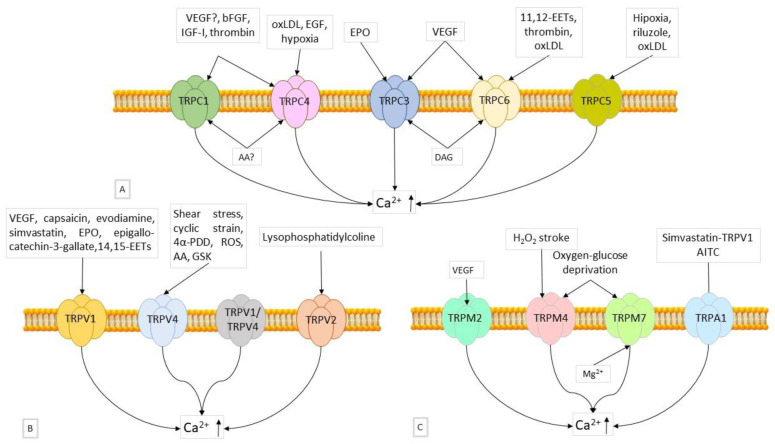
When DAG is produced in human microvascular endothelial cells (HUVECs) downstream of VEGFR, TRPC3 is activated. This causes Na+ to inflow by activating the Na+/Ca2+ exchanger in a reversal mode, which aids in angiogenesis [93]. In contrast, when there is inhibition of TRPC3 or its silencing with siRNA, VEGF activation of ERK1/2 phosphorylation and stimulation of [Ca2+]i transients is attenuated; endothelial tube formation is also suppressed [93]. In EPCs, molecular and pharmacological inhibition of TRPC3 abrogated the Ca2+ response induced by VEGF and thus blocked proliferation [94]. Silencing the expression of TRPC3, TRPC4 or TRPC5 also blocked spontaneous [Ca2+]i oscillations and inhibited tube formation in HUVEC-derived EA.hy926 cells and in HUVECs [95]. Specifically, TRPC4 silencing attenuated oxLDL-induced proliferation and migration of human coronary ECs and angiogenesis tube formation in vitro on matrigel [96]. TRPC6 also appears to be crucial in human microvascular ECs and HUVECs. Studies using a dominant-negative mutant of TRPC6, with three pore-region alterations, showed decreased EC migration, proliferation, and sprouting in the matrigel experiment [97]. Similarly, in HUVECs, a dominant-negative version of TRPC6 reduced VEGF-evoked capillary formation and cation current, as well as cell growth and proliferation [98]. In EPCs, TRPC1 regulates cell proliferation and tubulogenesis; indeed, an in vivo matrigel experiment showed that EPCs derived from TRPC1 mutant mice had significantly reduced functional activity, including migration and tube formation [99]. The Figure 1A shows the main factors involved in the activation of TRPCs (Figure 1A). Table 1 shows the angiogenic processes regulated by the different subunits [7].
Figure 1.
(A) TRPC channels in angiogenesis. Representation of the main TRPC channels involved in angiogenesis. The factors that stimulate the expression of the different components are shown. VEGF, IGF, bFGF, and thrombin induce expression of TRPC1; EGF, oxLDL, and hypoxic condition induce expression of TRPC4; erythropoietin (EPO) and VEGF induce expression of TRPC3; oxLDL, thrombin, and 14,15-EETs induce expression of TRPC6; hypoxic condition, riluzole, and oxLDL induce expression of TRPC5. (B) TRPV channels in angiogenesis. TRPV1, TRPV2, and TRPV4 are the main TRPV isoforms that mediate angiogenesis. VEGF, capsicin, evodiamine, simvastatin, EPO, epigallo-catechin-3-gallate, and 14,15-EETs induce TRPV1 activation; Shear stress, cyclic strain, 4α-PDD, ROS, AA, and GSK activate TRPV4; lysophosphatidylcholine can induce TRPV2 stimulation. (C) TRPM and TRPA1 channels in angiogenesis. It has been shown that certain TRPM isoforms are involved in angiogenesis. VEGF stimulates TRPM2; H2O2, pathological conditions such as stroke, oxygen, and glucose deprivation induce TRPM4; conditions of reduced intracellular Mg2+ concentration, oxygen, and glucose deprivation induce TRPM7 activation; AITC and simvastatin-dependent TRPV1 activation induces TRPA1.
Table 1.
Angiogenic processes regulated by TRPC subunits.
| TRPC Subunit | Angiogenic Processes Regulated |
|---|---|
| TRPC1/TRPC4/TRPC3/TRPC5/TRPC6 | Proliferation, migration, in vitro tubulogenesis. |
| TRPC1 | Filipodia extension, motility in vivo (sprouting angiogenesis); ECFC and MAC proliferation and tube formation. |
| TRPC4 | Retinal neovascularization. |
| TRPC6 | Wound closure in vitro and carotid artery regeneration in vivo. |
| TRPC5 | Neovascularization in hypoxic retina and in mouse hindlimb ischemia. Negative modulation of migration and wound closure in vitro and arterial regeneration in vivo. |
6.2.2. Role of TRPVs
According to a recent study, TRPV4 has long been recognized to control angiogenesis and neovascularization by promoting EC migration and proliferation [60]. TRPV4 is crucial for cytoskeletal remodeling and alterations in cell adhesion, which regulate EC motility and proliferation through mechanotransduction [100,101]. In mutant mice, the lack of TRPV4 was linked to an increase in baseline Rho/Rho kinase activity, a significant increase in EC migration and proliferation, and aberrant tube formation in vitro [101]. Intriguingly, a subsequent investigation from the same team verified that over-expressing or pharmacologically activating TRPV4 with GSK1016790 corrected the aberrant ECs’ abnormal tube formation in the matrigel experiment and restored their mechanosensitivity [100]. TRPV1 has been discovered to promote angiogenesis. Intraperitoneal injection of the TRPV1 ligand evodiamine, promoted vascularization in matrigel plugs used in vivo in wild type mice [102]; in contrast, TRPV1 knockout animals showed a significant reduction in induced angiogenesis. Furthermore, in human microvascular ECs [103], TRPV1 activation is dependent on simvastatin-activated Ca2+ influx, which induces activation of CaMKII signaling and enhances TRPV1-eNOS complex formation, leading to NO production and angiogenic tube formation in vitro [104].
The Figure 1B shows the main factors involved in the activation of TRPVs (Figure 1B). Table 2 shows the angiogenic processes regulated of the different subunits [7].
Table 2.
Angiogenic processes regulated by TRPV subunits.
| TRP Subunit | Angiogenic Processes Regulated |
|---|---|
| TRPV1 | Proliferation, migration and tube formation in vitro, angiogenesis in vivo. |
| TRPV4 | Proliferation, migration, tube formation in vitro, angiogenesis and arteriogenesis in vivo, ECFC proliferation. |
6.2.3. Role of TRPMs
It has also been discovered that TRPM2, TRPM4, and TRPM7 are involved in angiogenesis [105]. VEGF has been shown to stimulate EC migration and induce ROS-dependent Ca2+ entry through TRPM2 activation. Furthermore, matrigel plugs supplemented with VEGF injected subcutaneously into TRPM2 knockout mice show significantly reduced blood vessel formation compared to wild type mice [106]. In response to hypoxia/ischemia, TRPM4 is increased in vascular endothelium both in vitro and in vivo, as well as in HUVECs after oxygen-glucose deprivation. Enhancing tube formation on matrigel and improving capillary integrity in vivo were the results of pharmacologically inhibiting TRPM4 or silencing it using siRNA [107]. An earlier study showed that silencing TRPM7 replicates the effect of Mg2+ deficit on the development and migration of microvascular ECs, suggesting that magnesium and TRPM7 are regulators of angiogenesis [108]. Figure 1C shows the main factors involved in the activation of TRPMs and TRPA1 (Figure 1C). Table 3 shows the angiogenic processes regulated of the different subunits [7].
Table 3.
Angiogenic processes regulated by TRPM subunits and TRPA1.
| TRP Subunit | Angiogenic Processes Regulated |
|---|---|
| TRPM2 | Migration in vitro, neovascularization in vivo. |
| TRPM4 | Negative regulation of in vitro tubulogenesis and in vivo angiogenesis; supports H2O2-induced migration. |
| TRPM7 | Negative regulation of HUVEC proliferation, adhesion, and migration in vitro and tubulogenesis in vivo; positive regulation of HMEC proliferation and migration. |
| TRPA1 | Tube formation in vitro and neovascularization upon corneal cauterization in vivo. |
A preliminary report showed that only TRPC1 and TRPC4 were expressed by human ECFCs generated from peripheral blood (PB-ECFCs), although TRPC3 was also present in ECFCs produced from umbilical cord blood [109]. Following genetic silencing using specific siRNAs, it was discovered that TRPC1 interacts with STIM1 and Orai1 to produce SOCE in ECFCs [110,111]. It is unclear if Orai1 and TRPC1, which are both gated by STIM1, produce independent Ca2+-permeable channels or whether they come together to form a supermolecular complex that may also include TRPC4. SOCE can be recruited by SDF-1α, to increase ECFC migration in vitro and neovessel development in vivo [10], as well as by VEGF to promote ECFC proliferation and tube formation [54,112]. Specifically, whereas the pro-angiogenic response to VEGF requires the Ca2+-sensitive transcription factor NF-kB, SOCE activates the ERK1/2 and PI3K/Akt signaling pathways to promote SDF-1α-induced ECFC motility [10,52]. STIM1, TRPC1, and Orai1 interact to drive SOCE and facilitate in vitro angiogenesis (proliferation, motility, and formation) in rodent MACs [113], just as they do in human ECFCs. Furthermore, CaM-dependent e NOS activation was impaired by genetic TRPC1 knockdown, which hindered neovessel formation in Matrigel plugs in vivo [99]. TRPC3 is only produced in umbilical cord blood (UCB)-derived ECFCs, where it is physiologically gated by DAG and causes intracellular Ca2+ oscillations generated by VEGF [94]. Genetic (using certain siRNAs) and pharmacological (using Pyr3) treatments demonstrated that TRPC3-mediated extracellular Ca2+ entry causes the dynamic interaction between InsP3Rs and SOCE to alter the spiking Ca2+ response to VEGF, increasing the proliferation of UCB-ECFC [94]. The increased frequency of VEGF-induced intracellular Ca2+ oscillations in UCB-ECFCs, which is related to their higher proliferative capability, has been postulated to be caused by TRPC3 participation [114]. This finding gave rise to the theory that exogenous TRPC3 insertion could rejuvenate the reparative phenotype of senescent/aging UCB-ECFCs and increase the effectiveness of autologous cell-based therapy in ischemic patients [114].
The two most significant endothelial TRPV isoforms involved in angiogenesis and arteriogenesis are TRPV1 and TRPV4. Multiple independent studies revealed that human ECFCs also express TRPM7 [108], TRPV1 [115], and TRPV4 [116]. Pharmacological stimulation of TRPV4 with the endogenous agonist, arachidonic acid, stimulated PB-ECFC proliferation in a NO-dependent manner [117], while genetic silencing of TRPM7 with a specific siRNA had no impact on the rate of ECFC proliferation [108]. The activation of TRPV1 could also be sufficient to cause ECFC proliferation [91,118]. Previous work has shown that TRPV1 promoted proliferation of UCB-ECFC cells, as well as of the HUVEC-derived EA.hy926 cells, by mediating anandamide uptake independently of Ca2+ entry [119]. Subsequently, Lodola et al. (2019) [120] found that optical excitation of PB-ECFCs plated on the photosensitive polymer Poly(3-hexyl-thiophene) (P3HT) stimulated proliferation and tube formation by stimulating the nuclear translocation of p65 NF-kB [120]. A recent investigation showed that TRPV1 activation depends on the local increase in ROS levels at the interface between P3HT thin layers and the cell surface [115].
7. The Role of Endothelial TRP Channels in Tumor Vascularization
In a growing number of cancers, recent data have suggested that deregulation of the endothelial Ca2+ mechanism is essential for neovascularization and resistance to anti-angiogenic and chemotherapeutic treatments [41,60,61]. A relevant role has been attributed to abnormal expression and activity of TRP channels in T-ECs and T-ECFCs, which can boost tumor neovascularization.
7.1. Breast Cancer
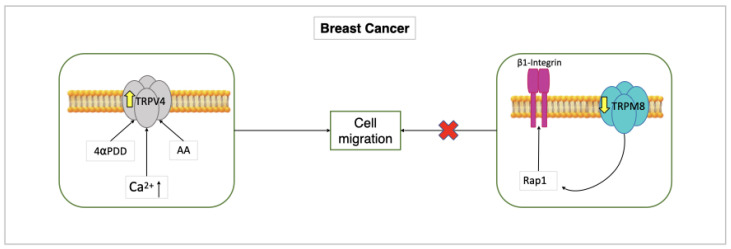
TRPV4 has been the first endothelial TRP channel directly linked to malignant angiogenesis. In line with this, TRPV4 expression was noticeably increased in breast-tumor-derived endothelial cells (B-TECs), and TRPV4 activation with AA or 4αPDD stimulated migration in B-TECs, but not in the control human microvascular endothelial cells. On the other hand, when cells were transfected with a short hairpin RNA that selectively targets TRPV4 (shTRPV4), AA-induced B-TEC migration was stopped [121]. Notably, a brief (10 min) pre-incubation with AA alone dramatically raised the cell surface expression of TRPV4, thereby increasing AA-induced extracellular Ca2+ entry in migrating B-TECs compared to non-migrating cells. Furthermore, AA has been shown to stimulate extracellular Ca2+ entry in B-TECs during the first stages of the tubulogenic process, but not when a capillary-like network was already developed. These findings clearly suggest that the early stages of breast cancer angiogenesis are characterized by the over-expression of endothelial TRPV4 channels [122] (Figure 2).
Figure 2.
Role of TRPV4 and TRPM8 in B-TECs. AA or 4αPDD induce increased expression of TRPV4 in B-TECs, resulting in increased migration of these and increased intracellular Ca2+ entry. This shows that over-expression of TRPV4 induces the early stages of tumor angiogenesis.
TRPM8 was also found to control breast cancer angiogenesis, but in a Ca2+-independent manner. In contrast to HMECs and HUVECs, in which it was mostly found in the ER, TRPM8 was substantially down-regulated in B-TECs [123]. Intriguingly, in normal endothelial cells, TRPM8 prevented migration and tube formation by trapping Rap1 intracellularly and blocking its movement towards the plasma membrane, which is essential to trigger β1-integrin signaling. As a consequence, TRPM8 down-regulation in B-TECs is likely to accelerate vascular growth; this feature suggests that TRPM8 activation by icilin or menthol could represent an efficient strategy to treat breast cancer [123] (Figure 2).
TRPM8 is down-regulated in B-TEC, Rap1 isn’t trapped, β1-integrin isn’t inhibited, migration and tube formation are blocked.
7.2. Prostate Cancer
Prostate cancer (PCa) involves a complex remodeling of endothelial TRP channels. TRPA1, TRPV2, and TRPC3 were shown to be up-regulated in three different endothelial cell lines established from PCa patients as well as in endothelial cells lining tumor capillaries in vivo [124]. In PCa-derived endothelial cells, it has been reported that: (1) TRPA1 supports migration; (2) TRPC3 supports chemoattraction towards tumor microenvironment; and (3) TRPV2 induces capillary-like formation in vitro. Furthermore, TRPV2 activation stimulates vascular development in a mouse model of postnatal retina in vivo [124]. When compared to non-tumor endothelium, the pro-angiogenic effect of TRP channels on PCa-derived endothelial cells was accompanied by a significant increase in intracellular Ca2+ activity. As a result, PCa patients could benefit from the pharmacological blockage of these specific endothelial TRP channel isoforms [124].
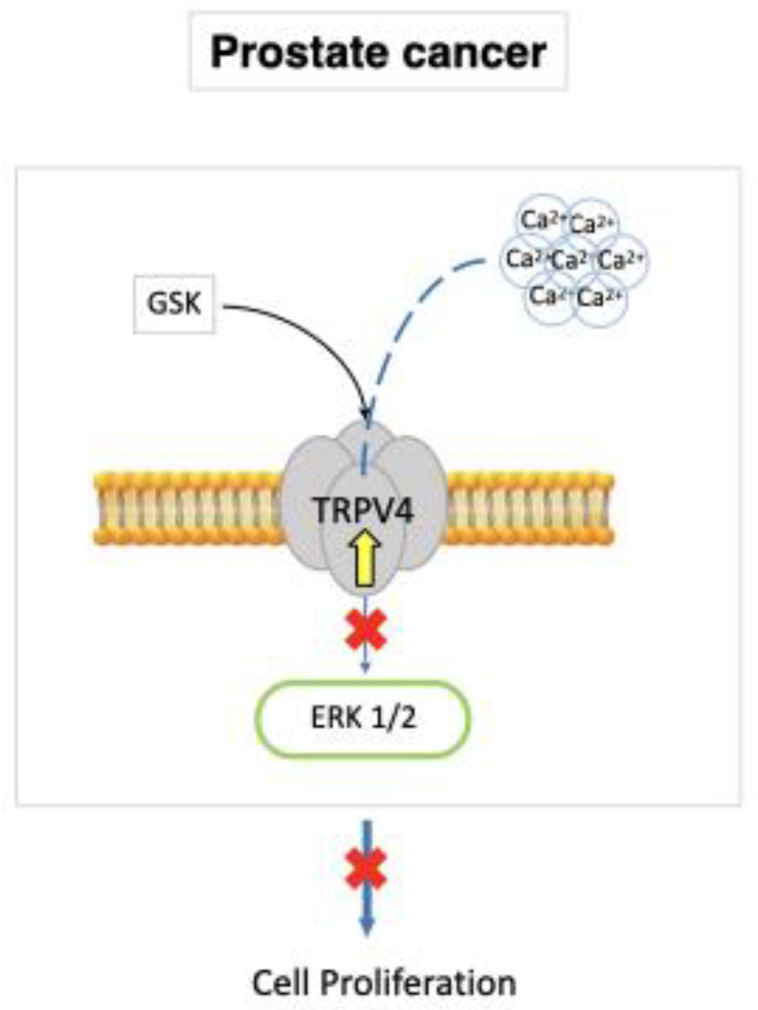
A parallel investigation showed that TRPV4 was down-regulated in prostate adenocarcinoma-derived endothelial cells (A-TECs), thereby reducing their mechanosensitivity to extracellular matrix (ECM) rigidity. This, in turn, favored A-TEC motility. The tumor vasculature in TRPV4 KO mice was characterized by an increased proportion of hyperpermeable, pericyte-free and dilated microvessels, which are known to moderate the therapeutic effect of anticancer treatments [100]. A subsequent report found that TRPV4 down-regulation significantly reduced the VE-cadherin expression at cell–cell contacts, thereby further increasing vascular leakage [125]. To rescue the phenotype of aberrant capillary tubules in vitro, overexpression or pharmacological stimulation of TRPV4 with GSK was sufficient to restore their mechanosensitivity to ECM rigidity by blocking basal Rho activity [126]. Furthermore, TRPV4-mediated extracellular Ca2+ influx blocked the ERK1/2 pathway, reducing the rate of A-TEC proliferation (Figure 3). These results imply that TRPV4 inhibits the development of malignant vasculature in the adenocarcinoma of the prostate [100,126].
Figure 3.
TRPV4 is up-regulated in A-TECs. Pharmacological GSK stimulation of TRPV4 restores mechanosensitivity to ECM rigidity, blocks the ERK1/2 pathway, and reduces the rate of A-TEC proliferation.
7.3. Renal Cellular Carcinoma
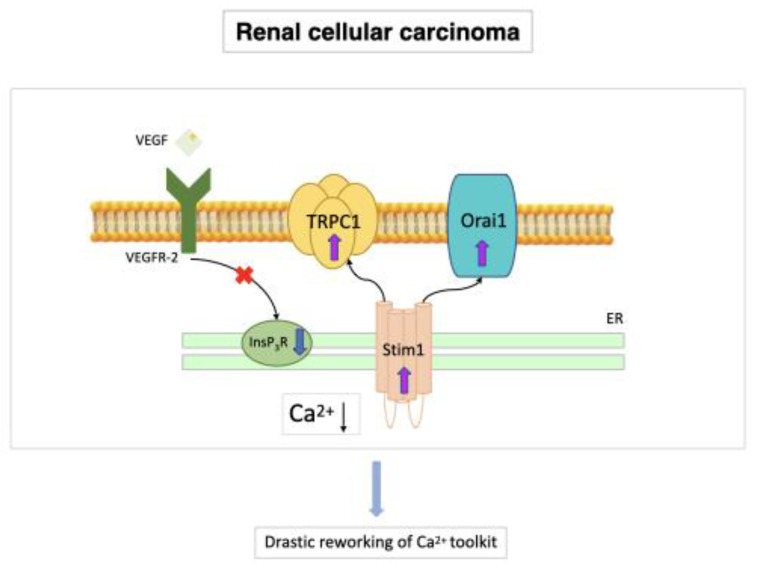
The most common form of kidney cancer in adults is renal cellular carcinoma (RCC) and there is substantial evidence that ECFCs may play a primary role in RCC neovascularization [25,127,128,129,130,131]. All research carried out on the subject has supported each other in demonstrating that ECFCs need functional VEGFR-2 to maintain malignant transformation, although they reached this conclusion by using normal cells rather than tumor cells [16,65]. RCC has recently been linked to a significant deregulation of the Ca2+ signaling machinery; in accord, primary tumor samples express more Orai1 and TRPC6 channel proteins than normal renal tissues [132,133]. In contrast, two separate human kidney cancer cell lines exhibited TRPC4 down-regulation [134]. These changes might be crucial to the neoplastic transformation of a healthy kidney. While Orai1 controls RCC cell proliferation and migration [133] and TRPC6 up-regulation favors the transition through the G2/M phase [132], the loss of TRPC4 results in a decreased release of the endogenous inhibitor thrombospondin-1, which favors the angiogenic switch [134]. In addition, ECFCs derived from naive RCC patients (RCC-ECFCs) exhibit a striking decrease in ER Ca2+ concentration and InsP3R expression, while they displayed a remarkable up-regulation of Stim1, Orai1, and TRPC1. These changes result in a remarkable rewiring of their Ca2+ toolkit, which render RCC-ECFCs less responsive to VEGF [111]. However, the pharmacological blockade of SOCE has been shown to interfere with RCC-ECFC proliferation and tube formation [25] (Figure 4).
Figure 4.
Stim1, Orai1, and TRPC1 (SOCE) in RCC-ECFCs. RCC-ECFCs show a significant decrease in ER Ca2+ concentration and InsP3R expression, while they present higher expression levels of Stim1, Orai1, and TRPC1 compared to control ECFCs. This results in a dramatic rewiring of their Ca2+ signaling toolkit. The higher amplitude of SOCE in RCC-ECFCs could underlie the increase in frequency reported in peripheral blood of RCC patients in response to hypoxic cues released by the tumor microenvironment [19,65].
8. Conclusions
Endothelial TRP channels play a crucial role in vascular remodeling, regulating angiogenesis, arteriogenesis and vasculogenesis. These channels are highly heterogeneous in their gating mechanisms, with the propensity of some isoforms to form heteromeric complexes, making them the most versatile Ca2+ entry pathway in ECs, EPCs, and ECFCs. However, they can also block angiogenesis, as reported for TRPM4 in HUVECs and TRPM7 in cerebral microvascular endothelial cells. An important aspect of endothelial TRP signaling that deserves much attention is its involvement in the abnormal vascularization that characterizes cancer. A crucial contribution to the angiogenic switch that transforms a dormant lesion into a growing tumor is provided by the recruitment of myeloid angiogenic cells and ECFCs, which are released from vascular stem cell niches, respectively. The abnormal expression and activity of TRP channels in T-ECs and T-ECFCs, may promote tumor neovascularization, however, several aspects are still not entirely clear, e.g., which endothelial TRP isoforms are dysregulated, and therefore further studies are needed.
Acknowledgments
The Authors would like to thank Pia Furno and Rosalina Perna for editorial assistance. Eleonora Hay is supported by Ph.D program of University of Campania “Luigi Vanvitelli”, Naples, Italy. Supporters of the work were the University of Campania “Luigi Vanvitelli” and the University of Molise, who we thank.
Author Contributions
Conceptualisation, A.P. and C.S.; resources, P.D.B. and C.S.; data curation, K.K. and E.H.; writing—original draft preparation A.P. and A.L.; writing—review and editing F.M. and A.R.; supervision, F.M.; project administration, G.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca C.G., Barbacena P., Franco C.A. Endothelial cells on the move: Dynamics in vascular morphogenesis and disease. Vasc Biol. 2020;2:H29–H43. doi: 10.1530/VB-20-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao D., Nolan D.J., Mellick A.S., Bambino K., McDonnell K., Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 4.García-Caballero M., Sokol L., Cuypers A., Carmeliet P. Metabolic Reprogramming in Tumor Endothelial Cells. Int. J. Mol. Sci. 2022;23:11052. doi: 10.3390/ijms231911052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saravanan S., Vimalraj S., Pavani K., Nikarika R., Sumantran V.N. Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci. 2020;252:117670. doi: 10.1016/j.lfs.2020.117670. [DOI] [PubMed] [Google Scholar]
- 6.Kuczynski E.A., Vermeulen P.B., Pezzella F., Kerbel R.S., Reynolds A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 7.Negri S., Faris P., Berra-Romani R., Guerra G., Moccia F. Endothelial Transient Receptor Potential Channels and Vascular Remodeling: Extracellular Ca(2+) Entry for Angiogenesis, Arteriogenesis and Vasculogenesis. Front. Physiol. 2019;10:1618. doi: 10.3389/fphys.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota Y., Nakajima H., Wakayama Y., Muto A., Kawakami K., Fukuhara S., Mochizuki N. Endothelial Ca 2+ oscillations reflect VEGFR signaling-regulated angiogenic capacity in vivo. elife. 2015;4:e08817. doi: 10.7554/eLife.08817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noren D.P., Chou W.H., Lee S.H., Qutub A.A., Warmflash A., Wagner D.S., Popel A.S., Levchenko A. Endothelial cells decode VEGF-mediated Ca2+ signaling patterns to produce distinct functional responses. Sci. Signal. 2016;9:ra20. doi: 10.1126/scisignal.aad3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuccolo E., Kheder D.A., Lim D., Perna A., Di Nezza F., Botta L., Scarpellino G., Negri S., Martinotti S., Soda T., et al. Glutamate triggers intracellular Ca. J. Cell Physiol. 2018;234:3538–3554. doi: 10.1002/jcp.26953. [DOI] [PubMed] [Google Scholar]
- 11.Savage A.M., Kurusamy S., Chen Y., Jiang Z., Chhabria K., Macdonald R.B., Kim H.R., Wilson H.L., Van Eeden F.J.M., Armesilla A.L., et al. tmem33 is essential for VEGF-mediated endothelial calcium oscillations and angiogenesis. Nat. Commun. 2019;10:732. doi: 10.1038/s41467-019-08590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moccia F., Negri S., Shekha M., Faris P., Guerra G. Endothelial Ca(2+) Signaling, Angiogenesis and Vasculogenesis: Just What It Takes to Make a Blood Vessel. Int. J. Mol. Sci. 2019;20:3962. doi: 10.3390/ijms20163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potente M., Gerhardt H., Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Mentzer S.J., Konerding M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis. 2014;17:499–509. doi: 10.1007/s10456-014-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moccia F. Calcium Signaling in Endothelial Colony Forming Cells in Health and Disease. Adv. Exp. Med. Biol. 2020;1131:1013–1030. doi: 10.1007/978-3-030-12457-1_40. [DOI] [PubMed] [Google Scholar]
- 16.Moccia F., Zuccolo E., Poletto V., Cinelli M., Bonetti E., Guerra G., Rosti V. Endothelial progenitor cells support tumour growth and metastatisation: Implications for the resistance to anti-angiogenic therapy. Tumour Biol. 2015;36:6603–6614. doi: 10.1007/s13277-015-3823-2. [DOI] [PubMed] [Google Scholar]
- 17.Hida K., Maishi N., Torii C., Hida Y. Tumor angiogenesis--characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016;21:206–212. doi: 10.1007/s10147-016-0957-1. [DOI] [PubMed] [Google Scholar]
- 18.Dimova I., Hlushchuk R., Makanya A., Styp-Rekowska B., Ceausu A., Flueckiger S., Lang S., Semela D., Le Noble F., Chatterjee S., et al. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16:921–937. doi: 10.1007/s10456-013-9366-5. [DOI] [PubMed] [Google Scholar]
- 19.Moccia F., Fotia V., Tancredi R., Della Porta M.G., Rosti V., Bonetti E., Poletto V., Marchini S., Beltrame L., Gallizzi G., et al. Breast and renal cancer-Derived endothelial colony forming cells share a common gene signature. Eur. J. Cancer. 2017;77:155–164. doi: 10.1016/j.ejca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Porro C., Soleti R., Benameur T., Maffione A., Andriantsitohaina R., Martínez M. Sonic Hedgehog Pathway as a Target for Therapy in Angiogenesis-Related Diseases. Curr. Signal Transduct. Ther. 2009;4:31–45. doi: 10.2174/157436209787048694. [DOI] [Google Scholar]
- 21.Margheri G., Zoppi A., Olmi R., Trigari S., Traversi R., Severi M., Bani D., Bianchini F., Torre E., Margheri F., et al. Tumor-tropic endothelial colony forming cells (ECFCs) loaded with near-infrared sensitive Au nanoparticles: A “cellular stove” approach to the photoablation of melanoma. Oncotarget. 2016;7:39846–39860. doi: 10.18632/oncotarget.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poletto V., Rosti V., Biggiogera M., Guerra G., Moccia F., Porta C. The role of endothelial colony forming cells in kidney cancer’s pathogenesis, and in resistance to anti-VEGFR agents and mTOR inhibitors: A speculative review. Crit. Rev. Oncol. Hematol. 2018;132:89–99. doi: 10.1016/j.critrevonc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Nolan D.J., Ciarrocchi A., Mellick A.S., Jaggi J.S., Bambino K., Gupta S., Heikamp E., McDevitt M.R., Scheinberg D.A., Benezra R., et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao D., Nolan D., McDonnell K., Vahdat L., Benezra R., Altorki N., Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim. Biophys. Acta Rev. Cancer. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moccia F., Dragoni S., Poletto V., Rosti V., Tanzi F., Ganini C., Porta C. Orai1 and Transient Receptor Potential Channels as Novel Molecular Targets to Impair Tumor Neovascularization in Renal Cell Carcinoma and other Malignancies. Anti-Cancer Agents Med. Chem. 2014;14:296–312. doi: 10.2174/18715206113139990315. [DOI] [PubMed] [Google Scholar]
- 26.Dudley A.C. Tumor endothelial cells. Cold Spring Harb. Perspect Med. 2012;2:a006536. doi: 10.1101/cshperspect.a006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patenaude A., Parker J., Karsan A. Involvement of endothelial progenitor cells in tumor vascularization. Microvasc. Res. 2010;79:217–223. doi: 10.1016/j.mvr.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Moccia F., Lodola F., Dragoni S., Bonetti E., Bottino C., Guerra G., Laforenza U., Rosti V., Tanzi F. Ca<sup>2+</sup> Signalling in Endothelial Progenitor Cells: A Novel Means to Improve Cell-Based Therapy and Impair Tumour Vascularisation. Curr. Vasc. Pharmacol. 2014;12:87–105. doi: 10.2174/157016111201140327162858. [DOI] [PubMed] [Google Scholar]
- 29.Ruzinova M.B., Schoer R.A., Gerald W., Egan J.E., Pandolfi P.P., Rafii S., Manova K., Mittal V., Benezra R. Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell. 2003;4:277–289. doi: 10.1016/S1535-6108(03)00240-X. [DOI] [PubMed] [Google Scholar]
- 30.Yoder M.C. Human endothelial progenitor cells. Cold Spring Harb. Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basile D.P., Yoder M.C. Circulating and tissue resident endothelial progenitor cells. J. Cell. Physiol. 2014;229:10–16. doi: 10.1002/jcp.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bieback K., Vinci M., Elvers-Hornung S., Bartol A., Gloe T., Czabanka M., Klüter H., Augustin H., Vajkoczy P. Recruitment of human cord blood-derived endothelial colony-forming cells to sites of tumor angiogenesis. Cytotherapy. 2013;15:726–739. doi: 10.1016/j.jcyt.2013.01.215. [DOI] [PubMed] [Google Scholar]
- 33.Smadja D.M., D’Audigier C., Weiswald L.-B., Badoual C., Dangles-Marie V., Mauge L., Evrard S., Laurendeau I., Lallemand F., Germain S., et al. The Wnt antagonist Dickkopf-1 increases endothelial progenitor cell angiogenic potential. Arterioscler Thromb. Vasc Biol. 2010;30:2544–2552. doi: 10.1161/ATVBAHA.110.213751. [DOI] [PubMed] [Google Scholar]
- 34.Wei J., Jarmy G., Genuneit J., Debatin K.-M., Beltinger C. Human blood late outgrowth endothelial cells for gene therapy of cancer: Determinants of efficacy. Gene Ther. 2007;14:344–356. doi: 10.1038/sj.gt.3302860. [DOI] [PubMed] [Google Scholar]
- 35.Pászty K., Caride A.J., Bajzer Z., Offord C.P., Padányi R., Hegedűs L., Varga K., Strehler E.E., Enyedi A. Plasma membrane Ca2+-ATPases can shape the pattern of Ca2+ transients induced by store-operated Ca2+ entry. Sci. Signal. 2015;8:ra19. doi: 10.1126/scisignal.2005672. [DOI] [PubMed] [Google Scholar]
- 36.Zuccolo E., Lim D., Kheder D.A., Perna A., Catarsi P., Botta L., Rosti V., Riboni L., Sancini G., Tanzi F., et al. Acetylcholine induces intracellular Ca. Cell Calcium. 2017;66:33–47. doi: 10.1016/j.ceca.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Moccia F., Guerra G. Ca(2+) Signalling in Endothelial Progenitor Cells: Friend or Foe? J. Cell. Physiol. 2016;231:314–327. doi: 10.1002/jcp.25126. [DOI] [PubMed] [Google Scholar]
- 38.Moccia F., Berra-Romani R., Tritto S., Signorelli S., Taglietti V., Tanzi F. Epidermal growth factor induces intracellular Ca2+ oscillations in microvascular endothelial cells. J. Cell. Physiol. 2003;194:139–150. doi: 10.1002/jcp.10198. [DOI] [PubMed] [Google Scholar]
- 39.Andrikopoulos P., Kieswich J., Harwood S.M., Baba A., Matsuda T., Barbeau O., Jones K., Eccles S.A., Yaqoob M.M. Endothelial Angiogenesis and Barrier Function in Response to Thrombin Require Ca2+ Influx through the Na+/Ca2+ Exchanger. J. Biol. Chem. 2015;290:18412–18428. doi: 10.1074/jbc.M114.628156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avanzato D., Genova T., Fiorio Pla A., Bernardini M., Bianco S., Bussolati B., Mancardi D., Giraudo E., Maione F., Cassoni P., et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016;6:32602. doi: 10.1038/srep32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarpellino G., Genova T., Avanzato D., Bernardini M., Bianco S., Petrillo S., Tolosano E., de Almeida Vieira J.R., Bussolati B., Fiorio Pla A., et al. Purinergic Calcium Signals in Tumor-Derived Endothelium. Cancers. 2019;11:766. doi: 10.3390/cancers11060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyubchenko T., Woodward H., Veo K.D., Burns N., Nijmeh H., Liubchenko G.A., Stenmark K.R., Gerasimovskaya E.V. P2Y1 and P2Y13 purinergic receptors mediate Ca2++ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am. J. Physiol. Cell. Physiol. 2011;300:C266–C275. doi: 10.1152/ajpcell.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egleton R.D., Brown K.C., Dasgupta P. Angiogenic activity of nicotinic acetylcholine receptors: Implications in tobacco-related vascular diseases. Pharmacol. Ther. 2009;121:205–223. doi: 10.1016/j.pharmthera.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y.-B., Su K.-H., Kou Y.R., Guo B.-C., Lee K.-I., Wei J., Lee T.-S. Role of transient receptor potential vanilloid 1 in regulating erythropoietin-induced activation of endothelial nitric oxide synthase. Acta Physiol. (Oxf.) 2017;219:465–477. doi: 10.1111/apha.12723. [DOI] [PubMed] [Google Scholar]
- 45.Berra-Romani R., Raqeeb A., Avelino-Cruz J.E., Moccia F., Oldani A., Speroni F., Taglietti V., Tanzi F. Ca2+ signaling in injured in situ endothelium of rat aorta. Cell Calcium. 2008;44:298–309. doi: 10.1016/j.ceca.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Berra-Romani R., Raqeeb A., Torres-Jácome J., Guzman-Silva A., Guerra G., Tanzi F., Moccia F. The mechanism of injury-induced intracellular calcium concentration oscillations in the endothelium of excised rat aorta. J. Vasc. Res. 2012;49:65–76. doi: 10.1159/000329618. [DOI] [PubMed] [Google Scholar]
- 47.Balbi C., Lodder K., Costa A., Moimas S., Moccia F., Van Herwaarden T., Rosti V., Campagnoli F., Palmeri A., De Biasio P., et al. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int. J. Cardiol. 2019;287:87–95. doi: 10.1016/j.ijcard.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Medina R.J., Barber C.L., Sabatier F., Dignat-George F., Melero-Martin J.M., Khosrotehrani K., Ohneda O., Randi A.M., Chan J.K.Y., Yamaguchi T., et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017;6:1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faris P., Negri S., Perna A., Rosti V., Guerra G., Moccia F. Therapeutic Potential of Endothelial Colony-Forming Cells in Ischemic Disease: Strategies to Improve their Regenerative Efficacy. Int. J. Mol. Sci. 2020;21:7406. doi: 10.3390/ijms21197406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodola F., Laforenza U., Cattaneo F., Ruffinatti F.A., Poletto V., Massa M., Tancredi R., Zuccolo E., Khdar D.A., Riccardi A., et al. VEGF-induced intracellular Ca(2+) oscillations are down-regulated and do not stimulate angiogenesis in breast cancer-derived endothelial colony forming cells. Oncotarget. 2017;8:95223–95246. doi: 10.18632/oncotarget.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuccolo E., Di Buduo C., Lodola F., Orecchioni S., Scarpellino G., Kheder D.A., Poletto V., Guerra G., Bertolini F., Balduini A., et al. Stromal Cell-Derived Factor-1α Promotes Endothelial Colony-Forming Cell Migration Through the Ca(2+)-Dependent Activation of the Extracellular Signal-Regulated Kinase 1/2 and Phosphoinositide 3-Kinase/AKT Pathways. Stem Cells Dev. 2018;27:23–34. doi: 10.1089/scd.2017.0114. [DOI] [PubMed] [Google Scholar]
- 52.Balducci V., Faris P., Balbi C., Costa A., Negri S., Rosti V., Bollini S., Moccia F. The human amniotic fluid stem cell secretome triggers intracellular Ca(2+) oscillations, NF-κB nuclear translocation and tube formation in human endothelial colony-forming cells. J. Cell Mol. Med. 2021;25:8074–8086. doi: 10.1111/jcmm.16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galeano-Otero I., Del-Toro R., Khatib A.-M., Rosado J.A., Ordóñez-Fernández A., Smani T. Corrigendum: SARAF and Orai1 Contribute to Endothelial Cell Activation and Angiogenesis. Front. Cell Dev. Biol. 2021;9:683097. doi: 10.3389/fcell.2021.683097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dragoni S., Laforenza U., Bonetti E., Lodola F., Bottino C., Berra-Romani R., Carlo Bongio G., Cinelli M.P., Guerra G., Pedrazzoli P., et al. Vascular Endothelial Growth Factor Stimulates Endothelial Colony Forming Cells Proliferation and Tubulogenesis by Inducing Oscillations in Intracellular Ca2+ Concentration. Stem Cells. 2011;29:1898–1907. doi: 10.1002/stem.734. [DOI] [PubMed] [Google Scholar]
- 55.Faehling M., Koch E.D., Raithel J., Trischler G., Waltenberger J. Vascular endothelial growth factor-A activates Ca2+ -activated K+ channels in human endothelial cells in culture. Int. J. Biochem. Cell Biol. 2001;33:337–346. doi: 10.1016/S1357-2725(01)00021-8. [DOI] [PubMed] [Google Scholar]
- 56.Nilius B., Droogmans G. Ion Channels and Their Functional Role in Vascular Endothelium. Physiol. Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 57.Yue Z., Xie J., Yu A.S., Stock J., Du J., Yue L. Role of TRP channels in the cardiovascular system. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng W., Yang F., Takanishi C.L., Zheng J. Thermosensitive TRPV Channel Subunits Coassemble into Heteromeric Channels with Intermediate Conductance and Gating Properties. J. Gen. Physiol. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma X., Cheng K.-T., Wong C.-O., O’Neil R.G., Birnbaumer L., Ambudkar I.S., Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca2++ entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moccia F. Endothelial Ca(2+) Signaling and the Resistance to Anticancer Treatments: Partners in Crime. Int. J. Mol. Sci. 2018;19:217. doi: 10.3390/ijms19010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scarpellino G., Munaron L., Cantelmo A.R., Pla A.F. Calcium-Permeable Channels in Tumor Vascularization: Peculiar Sensors of Microenvironmental Chemical and Physical Cues. Rev. Physiol. Biochem. Pharmacol. 2022;182:111–137. doi: 10.1007/112_2020_32. [DOI] [PubMed] [Google Scholar]
- 62.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 63.Monteith G.R., Prevarskaya N., Roberts-Thomson S.J. The calcium-cancer signalling nexus. Nat. Rev. Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 64.Prevarskaya N., Skryma R., Shuba Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018;98:559–621. doi: 10.1152/physrev.00044.2016. [DOI] [PubMed] [Google Scholar]
- 65.Moccia F., Poletto V. May the remodeling of the Ca2+ toolkit in endothelial progenitor cells derived from cancer patients suggest alternative targets for anti-angiogenic treatment? Biochim. Biophys. Acta. 2015;1853:1958–1973. doi: 10.1016/j.bbamcr.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 66.Thakore P., Earley S. Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling. Compr. Physiol. 2019;9:1249–1277. doi: 10.1002/cphy.c180034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gees M., Colsoul B., Nilius B. The role of transient receptor potential cation channels in Ca2++ signaling. Cold Spring Harb. Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Earley S., Brayden J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smani T., Gómez L.J., Regodon S., Woodard G.E., Siegfried G., Khatib A.-M., Rosado J.A. TRP Channels in Angiogenesis and Other Endothelial Functions. Front. Physiol. 2018;9:1731. doi: 10.3389/fphys.2018.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berrout J., Jin M., O’Neil R.G. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood–brain barrier endothelial cells. Brain Res. 2012;1436:1–12. doi: 10.1016/j.brainres.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 71.Storch U., Forst A.-L., Philipp M., Gudermann T., Schnitzler M.M.Y. Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J. Biol. Chem. 2012;287:3530–3540. doi: 10.1074/jbc.M111.283218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goel M., Sinkins W.G., Schilling W.P. Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 73.Hofmann T., Schaefer M., Schultz G., Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng W., Sun C., Zheng J. Heteromerization of TRP channel subunits: Extending functional diversity. Protein Cell. 2010;1:802–810. doi: 10.1007/s13238-010-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schindl R., Fritsch R., Jardin I., Frischauf I., Kahr H., Muik M., Riedl M.C., Groschner K., Romanin C. Canonical Transient Receptor Potential (TRPC) 1 Acts as a Negative Regulator for Vanilloid TRPV6-mediated Ca2+ Influx *. J. Biol. Chem. 2012;287:35612–35620. doi: 10.1074/jbc.M112.400952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldenberg N.M., Wang L., Ranke H., Liedtke W., Tabuchi A., Kuebler W.M. TRPV4 Is Required for Hypoxic Pulmonary Vasoconstriction. Anesthesiology. 2015;122:1338–1348. doi: 10.1097/ALN.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 77.Guo Z., Grimm C., Becker L., Ricci A.J., Heller S. A Novel Ion Channel Formed by Interaction of TRPML3 with TRPV5. PLoS ONE. 2013;8:e58174. doi: 10.1371/journal.pone.0058174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venkatachalam K., Montell C. TRP Channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guerra G., Lucariello A., Perna A., Botta L., De Luca A., Moccia F. The Role of Endothelial Ca. Int. J. Mol. Sci. 2018;19:938. doi: 10.3390/ijms19040938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moccia F., Dragoni S., Lodola F., Bonetti E., Bottino C., Guerra G., Laforenza U., Rosti V., Tanzi F. Store-dependent Ca(2+) entry in endothelial progenitor cells as a perspective tool to enhance cell-based therapy and adverse tumour vascularization. Curr. Med. Chem. 2012;19:5802–5818. doi: 10.2174/092986712804143240. [DOI] [PubMed] [Google Scholar]
- 81.Moccia F., Berra-Romani R., Tanzi F. Update on vascular endothelial Ca(2+) signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012;3:127–158. doi: 10.4331/wjbc.v3.i7.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown R.C., Wu L., Hicks K., O’Neil R.G. Regulation of Blood-Brain Barrier Permeability by Transient Receptor Potential Type C and Type V Calcium-Permeable Channels. Microcirculation. 2008;15:359–371. doi: 10.1080/10739680701762656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuccolo E., Laforenza U., Negri S., Botta L., Berra-Romani R., Faris P., Scarpellino G., Forcaia G., Pellavio G., Sancini G., et al. Muscarinic M5 receptors trigger acetylcholine-induced Ca2++ signals and nitric oxide release in human brain microvascular endothelial cells. J. Cell. Physiol. 2019;234:4540–4562. doi: 10.1002/jcp.27234. [DOI] [PubMed] [Google Scholar]
- 84.Greenberg H.Z., Carlton-Carew S.R., Khan D.M., Zargaran A., Jahan K.S., Ho W., Albert A. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vasc. Pharmacol. 2017;96–98:53–62. doi: 10.1016/j.vph.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 Associate to Form a Redox-sensitive Cation Channel: Evidence for expression of native trpc3-trpc4 heteromeric channels in endothelial cells *. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 86.O’Leary C., McGahon M.K., Ashraf S., McNaughten J., Friedel T., Cincolà P., Barabas P., Fernandez J.A., Stitt A.W., McGeown J.G., et al. Involvement of TRPV1 and TRPV4 Channels in Retinal Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2019;60:3297–3309. doi: 10.1167/iovs.18-26344. [DOI] [PubMed] [Google Scholar]
- 87.Du J., Ma X., Shen B., Huang Y., Birnbaumer L., Yao X. TRPV4, TRPC1, and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 2014;28:4677–4685. doi: 10.1096/fj.14-251652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pires P.W., Earley S. Redox regulation of transient receptor potential channels in the endothelium. Microcirculation. 2017;24:e12329. doi: 10.1111/micc.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mergler S., Valtink M., Coulson-Thomas V.J., Lindemann D., Reinach P.S., Engelmann K., Pleyer U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp. Eye Res. 2010;90:758–770. doi: 10.1016/j.exer.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Watanabe H., Vriens J., Suh S.H., Benham C.D., Droogmans G., Nilius B. Heat-evoked Activation of TRPV4 Channels in a HEK293 Cell Expression System and in Native Mouse Aorta Endothelial Cells*. J. Biol. Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 91.Negri S., Faris P., Rosti V., Antognazza M.R., Lodola F., Moccia F. Endothelial TRPV1 as an Emerging Molecular Target to Promote Therapeutic Angiogenesis. Cells. 2020;9:1341. doi: 10.3390/cells9061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Armani G., Pozzi E., Pagani A., Porta C., Rizzo M., Cicognini D., Rovati B., Moccia F., Pedrazzoli P., Ferraris E. The heterogeneity of cancer endothelium: The relevance of angiogenesis and endothelial progenitor cells in cancer microenvironment. Microvasc. Res. 2021;138:104189. doi: 10.1016/j.mvr.2021.104189. [DOI] [PubMed] [Google Scholar]
- 93.Andrikopoulos P., Eccles S.A., Yaqoob M.M. Coupling between the TRPC3 ion channel and the NCX1 transporter contributed to VEGF-induced ERK1/2 activation and angiogenesis in human primary endothelial cells. Cell Signal. 2017;37:12–30. doi: 10.1016/j.cellsig.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 94.Dragoni S., Laforenza U., Bonetti E., Lodola F., Bottino C., Guerra G., Borghesi A., Stronati M., Rosti V., Tanzi F., et al. Canonical transient receptor potential 3 channel triggers vascular endothelial growth factor-induced intracellular Ca2++ oscillations in endothelial progenitor cells isolated from umbilical cord blood. Stem Cells Dev. 2013;22:2561–2580. doi: 10.1089/scd.2013.0032. [DOI] [PubMed] [Google Scholar]
- 95.Antigny F., Girardin N., Frieden M. Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J. Biol. Chem. 2012;287:5917–5927. doi: 10.1074/jbc.M111.295733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song H.B., Jun H.-O., Kim J.H., Fruttiger M., Kim J.H. Suppression of transient receptor potential canonical channel 4 inhibits vascular endothelial growth factor-induced retinal neovascularization. Cell Calcium. 2015;57:101–108. doi: 10.1016/j.ceca.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Zadeh M.H., Glass C.A., Magnussen A., Hancox J.C., Bates D.O. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation. 2008;15:605–614. doi: 10.1080/10739680802220323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ge R., Tai Y., Sun Y., Zhou K., Yang S., Cheng T., Zou Q., Shen F., Wang Y. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett. 2009;283:43–51. doi: 10.1016/j.canlet.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 99.Du L.-L., Shen Z., Li Z., Ye X., Wu M., Hong L., Zhao Y. TRPC1 Deficiency Impairs the Endothelial Progenitor Cell Function via Inhibition of Calmodulin/eNOS Pathway. J. Cardiovasc. Transl. Res. 2018;11:339–345. doi: 10.1007/s12265-018-9798-9. [DOI] [PubMed] [Google Scholar]
- 100.Adapala R.K., Thoppil R.J., Ghosh K., Cappelli H.C., Dudley A.C., Paruchuri S., Keshamouni V., Klagsbrun M., Meszaros J.G., Chilian W.M., et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. 2016;35:314–322. doi: 10.1038/onc.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thoppil R.J., Cappelli H.C., Adapala R.K., Kanugula A.K., Paruchuri S., Thodeti C.K. TRPV4 channels regulate tumor angiogenesis via modulation of Rho/Rho kinase pathway. Oncotarget. 2016;7:25849–25861. doi: 10.18632/oncotarget.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ching L.-C., Kou Y.R., Shyue S.-K., Su K.-H., Wei J., Cheng L.-C., Yu Y.-B., Pan C.-C., Lee T.-S. Molecular mechanisms of activation of endothelial nitric oxide synthase mediated by transient receptor potential vanilloid type 1. Cardiovasc. Res. 2011;91:492–501. doi: 10.1093/cvr/cvr104. [DOI] [PubMed] [Google Scholar]
- 103.Su K.-H., Lee K.-I., Shyue S.-K., Chen H.-Y., Wei J., Lee T.-S. Implication of transient receptor potential vanilloid type 1 in 14,15-epoxyeicosatrienoic acid-induced angiogenesis. Int. J. Biol. Sci. 2014;10:990–996. doi: 10.7150/ijbs.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su K.-H., Lin S.-J., Wei J., Lee K.-I., Zhao J.-F., Shyue S.-K., Lee T.-S. The essential role of transient receptor potential vanilloid 1 in simvastatin-induced activation of endothelial nitric oxide synthase and angiogenesis. Acta Physiol. (Oxf.) 2014;212:191–204. doi: 10.1111/apha.12378. [DOI] [PubMed] [Google Scholar]
- 105.Zhou W., Guo S., Xiong Z., Liu M. Oncogenic role and therapeutic target of transient receptor potential melastatin 7 channel in malignancy. Expert Opin. Ther. Targets. 2014;18:1177–1196. doi: 10.1517/14728222.2014.940894. [DOI] [PubMed] [Google Scholar]
- 106.Mittal M., Urao N., Hecquet C.M., Zhang M., Sudhahar V., Gao X.-P., Komarova Y., Ushio-Fukai M., Malik A.B. Novel role of reactive oxygen species-activated Trp melastatin channel-2 in mediating angiogenesis and postischemic neovascularization. Arterioscler. Thromb. Vasc. Biol. 2015;35:877–887. doi: 10.1161/ATVBAHA.114.304802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loh K., Ng G., Yu C.Y., Fhu C.K., Yu D., Vennekens R., Nilius B., Soong T.W., Liao P. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. 2014;466:563–576. doi: 10.1007/s00424-013-1347-4. [DOI] [PubMed] [Google Scholar]
- 108.Baldoli E., Maier J.A.M. Silencing TRPM7 mimics the effects of magnesium deficiency in human microvascular endothelial cells. Angiogenesis. 2012;15:47–57. doi: 10.1007/s10456-011-9242-0. [DOI] [PubMed] [Google Scholar]
- 109.Sánchezn-Hernández Y., Laforenza U., Bonetti E., Fontana J., Dragoni S., Russo M., Avelino-Cruz J.E., Schinelli S., Testa D., Guerra G., et al. Store-Operated Ca2++ Entry Is Expressed in Human Endothelial Progenitor Cells. Stem Cells Dev. 2010;19:1967–1981. doi: 10.1089/scd.2010.0047. [DOI] [PubMed] [Google Scholar]
- 110.Li J., Cubbon R.M., Wilson L.A., Amer M.S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V.A., Taylor H., et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2++ entry and endothelial tube formation. Circ. Res. 2011;108:1190–1198. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lodola F., Laforenza U., Bonetti E., Lim D., Dragoni S., Bottino C., Ong H.L., Guerra G., Ganini C., Massa M., et al. Store-operated Ca2++ entry is remodelled and controls in vitro angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS ONE. 2012;7:e42541. doi: 10.1371/journal.pone.0042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dragoni S., Reforgiato M., Zuccolo E., Poletto V., Lodola F., Ruffinatti F.A., Bonetti E., Guerra G., Barosi G., Rosti V., et al. Dysregulation of VEGF-induced proangiogenic Ca2++ oscillations in primary myelofibrosis-derived endothelial colony-forming cells. Exp. Hematol. 2015;43:1019–1030.e3. doi: 10.1016/j.exphem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Wang R., Gurguis C.I., Gu W., Ko E.A., Lim I., Bang H., Zhou T., Ko J.-H. Ion channel gene expression predicts survival in glioma patients. Sci. Rep. 2015;5:11593. doi: 10.1038/srep11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moccia F., Berra-Romani R., Rosti V. Manipulating Intracellular Ca2++ Signals to Stimulate Therapeutic Angiogenesis in Cardiovascular Disorders. Curr. Pharm. Biotechnol. 2018;19:686–699. doi: 10.2174/1389201019666180808165309. [DOI] [PubMed] [Google Scholar]
- 115.Negri S., Faris P., Tullii G., Vismara M., Pellegata A.F., Lodola F., Guidetti G., Rosti V., Antognazza M.R., Moccia F. Conjugated polymers mediate intracellular Ca(2+) signals in circulating endothelial colony forming cells through the reactive oxygen species-dependent activation of Transient Receptor Potential Vanilloid 1 (TRPV1) Cell Calcium. 2022;101:102502. doi: 10.1016/j.ceca.2021.102502. [DOI] [PubMed] [Google Scholar]
- 116.Dragoni S., Guerra G., Pla A.F., Bertoni G., Rappa A., Poletto V., Bottino C., Aronica A., Lodola F., Cinelli M.P., et al. A functional transient receptor potential vanilloid 4 (TRPV4) channel is expressed in human endothelial progenitor cells. J. Cell Physiol. 2015;230:95–104. doi: 10.1002/jcp.24686. [DOI] [PubMed] [Google Scholar]
- 117.Zuccolo E., Dragoni S., Poletto V., Catarsi P., Guido D., Rappa A., Reforgiato M., Lodola F., Lim D., Rosti V., et al. Arachidonic acid-evoked Ca(2+) signals promote nitric oxide release and proliferation in human endothelial colony forming cells. Vascul. Pharmacol. 2016;87:159–171. doi: 10.1016/j.vph.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 118.Moccia F., Negri S., Faris P., Ronchi C., Lodola F. Optical excitation of organic semiconductors as a highly selective strategy to induce vascular regeneration and tissue repair. Vascul. Pharmacol. 2022;144:106998. doi: 10.1016/j.vph.2022.106998. [DOI] [PubMed] [Google Scholar]
- 119.Hofmann N.A., Barth S., Waldeck-Weiermair M., Klec C., Strunk D., Malli R., Graier W.F. TRPV1 mediates cellular uptake of anandamide and thus promotes endothelial cell proliferation and network-formation. Biol. Open. 2014;3:1164–1172. doi: 10.1242/bio.20149571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lodola F., Rosti V., Tullii G., Desii A., Tapella L., Catarsi P., Lim D., Moccia F., Antognazza M.R. Conjugated polymers optically regulate the fate of endothelial colony-forming cells. Sci. Adv. 2019;5:eaav4620. doi: 10.1126/sciadv.aav4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pla A.F., Ong H.L., Cheng K.T., Brossa A., Bussolati B., Lockwich T., Paria B., Munaron L., Ambudkar I.S. TRPV4 mediates tumor-derived endothelial cell migration via arachidonic acid-activated actin remodeling. Oncogene. 2012;31:200–212. doi: 10.1038/onc.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pla A.F., Grange C., Antoniotti S., Tomatis C., Merlino A., Bussolati B., Munaron L. Arachidonic acid-induced Ca2++ entry is involved in early steps of tumor angiogenesis. Mol. Cancer Res. 2008;6:535–545. doi: 10.1158/1541-7786.MCR-07-0271. [DOI] [PubMed] [Google Scholar]
- 123.Genova T., Grolez G.P., Camillo C., Bernardini M., Bokhobza A., Richard E., Scianna M., Lemonnier L., Valdembri D., Munaron L., et al. TRPM8 inhibits endothelial cell migration via a non-channel function by trapping the small GTPase Rap1. J. Cell Biol. 2017;216:2107–2130. doi: 10.1083/jcb.201506024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bernardini M., Brossa A., Chinigo G., Grolez G.P., Trimaglio G., Allart L., Hulot A., Marot G., Genova T., Joshi A., et al. Transient Receptor Potential Channel Expression Signatures in Tumor-Derived Endothelial Cells: Functional Roles in Prostate Cancer Angiogenesis. Cancers. 2019;11:956. doi: 10.3390/cancers11070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cappelli H.C., Kanugula A.K., Adapala R.K., Amin V., Sharma P., Midha P., Paruchuri S., Thodeti C.K. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019;442:15–20. doi: 10.1016/j.canlet.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thoppil R.J., Adapala R.K., Cappelli H.C., Kondeti V., Dudley A.C., Gary Meszaros J., Paruchuri S., Thodeti C.K. TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci. Rep. 2015;5:14257. doi: 10.1038/srep14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hill P.A. Recipient origin of vasculature in renal cell carcinoma in a kidney allograft. Pathology. 2010;42:479–480. doi: 10.3109/00313025.2010.494284. [DOI] [PubMed] [Google Scholar]
- 128.Matsuda K., Ohga N., Hida Y., Muraki C., Tsuchiya K., Kurosu T., Akino T., Shih S.-C., Totsuka Y., Klagsbrun M., et al. Isolated tumor endothelial cells maintain specific character during long-term culture. Biochem. Biophys. Res. Commun. 2010;394:947–954. doi: 10.1016/j.bbrc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 129.Bhatt R.S., Zurita A.J., O’Neill A., Norden-Zfoni A., Zhang L., Wu H.K., Wen P.Y., George D., Sukhatme V.P., Atkins M.B., et al. Increased mobilisation of circulating endothelial progenitors in von Hippel-Lindau disease and renal cell carcinoma. Br. J. Cancer. 2011;105:112–117. doi: 10.1038/bjc.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hernandez-Yanez M., Heymach J.V., Zurita A.J. Circulating Biomarkers in Advanced Renal Cell Carcinoma: Clinical Applications. Curr. Oncol. Rep. 2012;14:221–229. doi: 10.1007/s11912-012-0231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang L., Guan H., He J., Zeng L., Yuan Z., Xu M., Zhang W., Wu X., Guan J. VEGF increases the proliferative capacity and eNOS/NO levels of endothelial progenitor cells through the calcineurin/NFAT signalling pathway. Cell Biol. Int. 2012;36:21–27. doi: 10.1042/CBI20100670. [DOI] [PubMed] [Google Scholar]
- 132.Song J., Wang Y., Li X., Shen Y., Yin M., Guo Y., Diao L., Liu Y., Yue D. Critical role of TRPC6 channels in the development of human renal cell carcinoma. Mol. Biol. Rep. 2013;40:5115–5122. doi: 10.1007/s11033-013-2613-4. [DOI] [PubMed] [Google Scholar]
- 133.Kim J.-H., Lkhagvadorj S., Lee M.-R., Hwang K.-H., Chung H.C., Jung J.H., Cha S.-K., Eom M. Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem. Biophys. Res. Commun. 2014;448:76–82. doi: 10.1016/j.bbrc.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 134.Veliceasa D., Ivanovic M., Hoepfner F.T.-S., Thumbikat P., Volpert O.V., Smith N.D. Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J. 2007;274:6365–6377. doi: 10.1111/j.1742-4658.2007.06159.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study did not report any data.