Abstract
Systemic treatment with hedgehog inhibitors (HHis) is available to treat basal cell carcinomas but their utility is limited by adverse effects. Topical delivery methods may reduce adverse effects, but successful topical treatment depends on sufficient skin uptake, biological response, and time in tumor tissue. The aim of this review was to evaluate the current status of topical HHi delivery for BCCs and discuss barriers for translating systemic HHis into topical treatments. A literature search identified 16 preclinical studies and 7 clinical trials on the topical delivery of 12 HHis that have been clinically tested on BCCs. Preclinical studies on drug uptake demonstrated that novel formulations, and delivery- and pre-treatment techniques enhanced topical HHi delivery. Murine studies showed that the topical delivery of sonidegib, itraconazole, vitamin D₃ and CUR-61414 led to biological responses and tumor remission. In clinical trials, only topical patidegib and sonidegib led to at least a partial response in 26/86 BCCs and 30/34 patients, respectively. However, histological clearance was not observed in the samples analyzed. In conclusion, the incomplete clinical response could be due to poor HHi uptake, biodistribution or biological response over time. Novel topical delivery techniques may improve HHi delivery, but additional research on cutaneous pharmacokinetics and biological response is needed.
Keywords: keratinocyte carcinoma, basal cell carcinoma, hedgehog inhibitors, smoothened inhibitors, vismodegib, sonidegib, topical delivery
1. Introduction
Keratinocyte carcinomas are the most common human malignancies and include cancers that develop in both the squamous and basal cell layers of the skin [1]. Among the keratinocyte cancers, basal cell carcinoma (BCC) is the most prevalent form, entailing roughly 5 million new cases annually in the US alone [2]. A major risk factor for BCCs is exposure to ultraviolet radiation, which leads to genetic mutations. In virtually all BCCs, these mutations cause dysregulation and increased activity of the hedgehog signaling pathway, which plays a pivotal role in BCC oncogenesis [3]. Hedgehog inhibitors (HHis) target the hedgehog pathway to decrease the expression of various proteins such as GLI family zinc finger 1 (GLI1), cyclins and MYC, which leads to reduced tumor cell survival, increased immune infiltration, and tumor remission [4,5,6,7,8,9] (see Figure 1). Of these three proteins, GLI1 is the most potent effector and mRNA levels of GLI1 are often used to estimate biological response following HHi treatment [10].
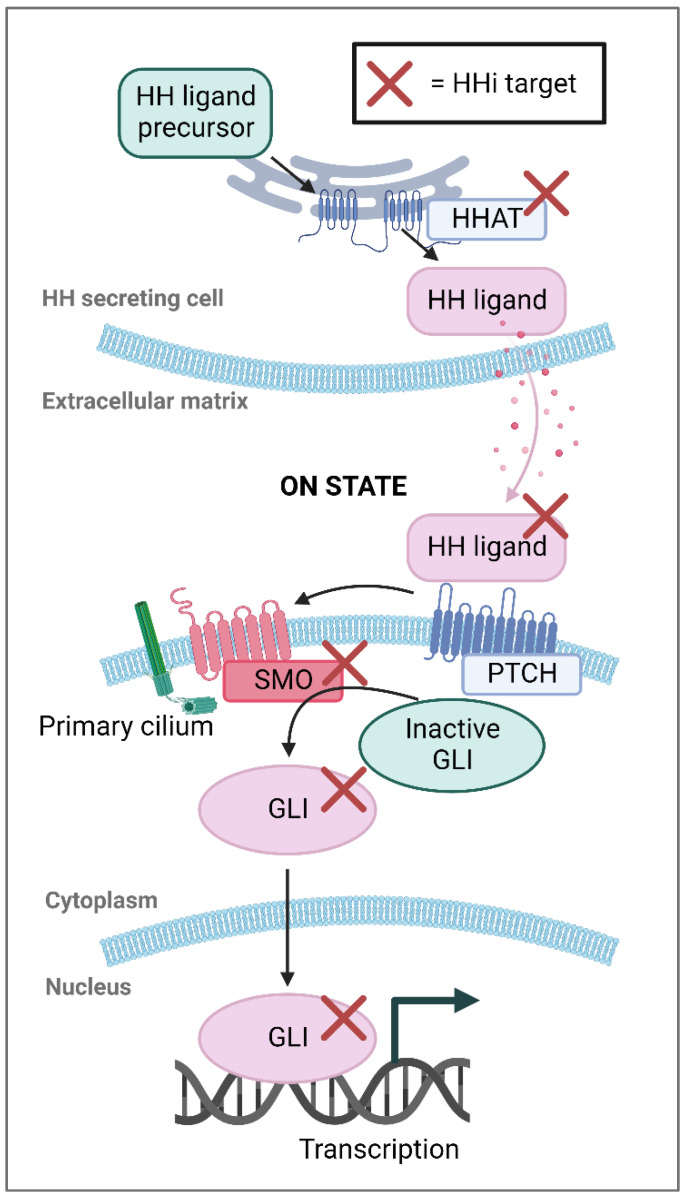
Figure 1.
The main components of the hedgehog pathway. Membrane proteins are either blue or red, inactive proteins are green and active proteins are colored pink. The red crosses indicate target proteins for hedgehog inhibitors (HHis). In canonical hedgehog (HH) signaling, HH ligands are modified by hedgehog acyltransferase (HHAT) and released from HH-secreting cells. HH ligands then bind to the cell membrane protein patched (PTCH), which leads to release of smoothened (SMO). SMO moves to the primary cilium where it prevents breakdown of GLI family zinc finger proteins (GLI) allowing them to translocate to the nucleus and promote expression of HH-signaling target genes. In basal cell carcinomas (BCCs), inactivating PTCH1 mutations are most common (70–90%) followed by activating SMO mutations (10–20%). Created with BioRender.com.
Two smoothened (SMO) inhibitors, vismodegib and sonidegib, have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for systemic treatment of advanced and metastatic BCCs [11]. The efficacy of vismodegib and sonidegib after treatment of locally advanced BCCs for 18–21 months is 47.6% (30/63) and 60.6% (40/66), respectively [12]. However, during treatment, most patients experience adverse effects such as muscle spasms, alopecia, dysgeusia and weight loss, which are caused by systemic SMO inhibition, and lead to treatment termination and tumor regrowth [12,13,14,15,16]. To improve HHi treatment, topical delivery methods that reduce systemic HHi exposure have been explored. Clinical trials show that topical HHi treatments are associated with fewer adverse effects allowing for new treatment opportunities [17,18]. For example, topical HHis could potentially be used for life-long treatment in patients with multiple BCCs, for combination therapy with other treatments, for prophylactic treatment of sun-exposed patients, and for adjuvant treatment of normal BCCs before excision [19].
An overview of potential candidates for topical HHi treatment is shown in Table 1. The size of HHis ranges from small molecules (0.1–1 kDa) to antibodies (150 kDa), where SMO inhibitors represent the most used HHis. HHis inhibit the hedgehog pathway in two ways, either directly by reducing the activity of hedgehog proteins like vismodegib [20], or indirectly by inhibiting cross-talk with other pathways such as imiquimod [21].
Table 1.
Overview of different hedgehog inhibitors (HHis) and their molecular target. We selected HHis by first determining whether the HHis have been tested on BCCs in a clinical trial followed by investigation of whether the HHi has a direct effect on the HH pathway. Empty cells indicate that the drug was excluded in previous steps.
| Target | Drug | Drug Aliases | Tested on BCC in Clinical Trial? | Direct Effect on HH Pathway? | Included in the Review? | Reference |
|---|---|---|---|---|---|---|
| SMO | Vismodegib | GDC-0449 | Yes | Yes | Yes | [5,22,23] |
| SMO | Sonidegib | Erismodegib, LDE225 | Yes | Yes | Yes | [5,22,23] |
| SMO | Itraconazole | Yes | Yes | Yes | [22,23] | |
| SMO | Patidegib | Saridegib, IPI-926 | Yes | Yes | Yes | [5,22,23] |
| SMO | Vitamin D₃ | Cholecalciferol, Calcitriol | Yes | Yes | Yes | [22] |
| SMO | CUR-61414 | Yes | Yes | Yes | [5,23] | |
| SMO | BMS-833923 | XL-139 | Yes | Yes | Yes | [5,23] |
| SMO | LEQ506 | Yes | Yes | Yes | [22,23] | |
| SMO | TAK-441 | Yes | Yes | Yes | [5,22,23] | |
| SMO | Taladegib | LY2940680 | Yes | Yes | Yes | [5,23] |
| SMO | ZSP1602 | Yes | Yes | Yes | [24] | |
| GLI | Arsenic Trioxide | Yes | Yes | Yes | [5,23] | |
| GLI | Imiquimod | Yes | No | [5] | ||
| SMO | Tazarotene | Yes | No | [22] | ||
| SMO | Acitretin | Yes | No | [22] | ||
| GLI | 4SC-202 | Domatinostat | No | [23] | ||
| GLI | GANT58 | No | [5] | |||
| GLI | GANT61 | No | [5,23] | |||
| GLI | Glabrescione B | No | [5,23] | |||
| GLI | NanoHHI (HPI-1) | No | [25] | |||
| GLI | Nanoquinacrine | No | [5] | |||
| GLI | Pirfenidone | No | [5,23] | |||
| GLI | Pyrvinium | No | [5] | |||
| GLI | HPI 1–4 | No | [5] | |||
| HH ligand | 3H8 | MEDI-5304 | No | [23] | ||
| HH ligand | 5E1 antibody | No | [5] | |||
| HH ligand | Robotnikinin | No | [5] | |||
| HHAT | RU-SKI 41 | No | [23] | |||
| HHAT | RU-SKI 43 | No | [23] | |||
| SMO | ALLO-1 | No | [23] | |||
| SMO | AZD8542 | No | [23] | |||
| SMO | Cyclopamine | No | [5,23] | |||
| SMO | DCBCO1303 | No | [26] | |||
| SMO | DHCEO | No | [23] | |||
| SMO | DY131 | No | [23] | |||
| SMO | Glasdegib | PF-04449913 | No | [5,23] | ||
| SMO | Jervine | No | [5] | |||
| SMO | MK-4101 | No | [23] | |||
| SMO | MRT-83 | No | [23] | |||
| SMO | PF403 | CAT3 | No | [23] | ||
| SMO | PF-5274857 | No | [23] | |||
| SMO | Posaconazole | Noxafil, SCH56592 | No | [23] | ||
| SMO | SANT-1 | No | [23] | |||
| SMO | SEN450 | No | [23] | |||
| SMO | Tretinoin | No | [22] |
Abbreviations: BCC, basal cell carcinoma; GLI, GLI family zinc finger 1; HH, hedgehog; HHAT, hedgehog acyltransferase; SMO, smoothened.
This review focuses on 12 HHis that directly target the hedgehog pathway and have been tested in clinical trials on BCCs (Table 1 and Table 2). These HHis include established HHis such as the FDA-approved vismodegib and sonidegib [11], experimental HHis such as patidegib and taladegib, and atypical HHis with other mechanisms of action such as itraconazole, which was originally developed as an antifungal [27], and vitamin D₃, whose role in BCC oncogenesis and treatment is complex [28]. The 12 HHis are advantageous drug candidates for topical delivery due to their lipophilicity and molecular weight of roughly 0.5 kDa [29,30], and sonidegib, patidegib, itraconazole, vitamin D₃ and CUR-61414 have all been tested in clinical trials for topical treatment of BCCs. Of these HHis, patidegib has reached the highest drug development stage by completing a phase III clinical trial in December 2020, but so far, no topical HHi has been approved for the treatment of BCCs.
Table 2.
HHi drug structures and molecular properties. Information on ZSP1602 and arsenic trioxide was not found. An increased clogP value corresponds to increased lipophilicity.
| HHi | MW [g/mol] | cLogP | Drug Development Stage | Molecular Structure | Reference |
|---|---|---|---|---|---|
| Vismodegib | 421.3 | 3.8 | FDA approval, oral treatment Indication: laBCC, mBCC No topical clinical trials |
|
[11] |
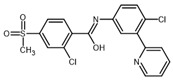
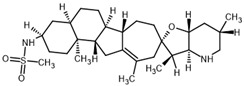
| Sonidegib | 485.5 | 5.8 | FDA approval, oral treatment Indication: laBCC Phase II clinical trial, topical treatment of BCC |
|
[11] and NLM, NCT00961896 |
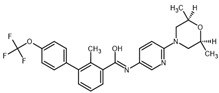
| Patidegib | 504.8 | 4.6 | Phase III clinical trial, topical treatment of BCC |
|
NLM, NCT03703310 |
| Itraconazole | 705.6 | 5.7 | Phase II clinical trial, topical treatment of BCC |
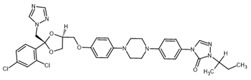
|
[31] and NLM, NCT02735356 |
| Vitamin D₃ | 384.6 | 7.9 | Phase II clinical trial, topical treatment of BCC |
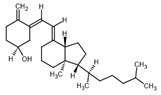
|
NLM, NCT01358045 |
| CUR-61414 | 550.7 | 3.3 | Phase I clinical trial, topical treatment of BCC |
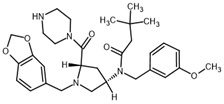
|
[32] |
| BMS-833923 | 473.6 | 5.7 | Phase I clinical trial, oral treatment of BCC |
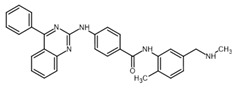
|
NLM, NCT00670189 |
| LEQ506 | 432.6 | 2.9 | Phase I clinical trial, oral treatment of BCC |
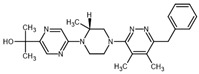
|
NLM, NCT01106508 |
| TAK-441 | 576.6 | 2.6 | Phase I clinical trial, oral treatment of BCC |
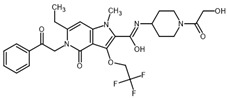
|
NLM, NCT01204073 |
| Taladegib | 512.5 | 4.3 | Phase I clinical trial, oral treatment of BCC |
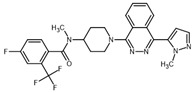
|
NLM, NCT01226485 |
| ZSP1602 | -- | -- | Phase I clinical trial, oral treatment of BCC | -- | NLM, NCT03734913 |
| Arsenic trioxide | 197.8 | -- | Phase II clinical trial, IV injection treatment of mBCC | -- | NLM, NCT01791894 |
Abbreviations: FDA, Food and Drug Administration (US); laBCC, locally advanced BCC; mBCC, metastatic BCC; MW, molecular weight; NLM, National Library of Medicine. MW and cLogP data have been retrieved from PubChem.
To achieve successful topical delivery, three primary barriers must be overcome. First, sufficient intra-tumoral HHi concentrations need to be achieved, second, HHi treatment must lead to a biological response, and third, the biological response must persist long enough to produce a clinical response. The aim of this review was to evaluate the current status of topical HHi delivery for BCCs and discuss the barriers for translating systemic HHi treatment into topical treatment.
2. Results
2.1. Preclinical Studies
From our search, we identified 16 preclinical studies reporting on the effects of topical HHi application using either ex vivo models (n = 5), in vivo models (n = 5), or both in combination (n = 6). An overview of the studies is presented in Table 3. Vismodegib was investigated in the largest number of studies (n = 8), itraconazole in five studies, sonidegib and CUR-61414 in two studies and vitamin D3 in one study. LEQ506, BMS-833923, taladegib and TAK-441 were only included once in a study comparing multiple HHis [33]. Topical treatment in combination with skin pre-treatments such as microneedles (n = 3) and ablative fractional laser (AFL, n = 2) was also investigated.
Table 3.
Overview of included preclinical studies.
| HHi | Formulation & Pre-Tx | Study Design | Delivery Method | Measurement Time | Effects | Reference |
|---|---|---|---|---|---|---|
| Vismodegib | Nanoformulation No pre-treatment |
Ex vivo: Human skin | SPM | 1 h, 4 h, 8 h | Human viable epidermis + dermis, 8 h: [8.4 µg/mL] | [34,35] |
| In vitro: Human cell culture In vivo: Zebrafish larvae |
Added to medium | 4 h, 24 h, 48 h, 72 h | Tumor cell viability ↓ Larvae toxicity ↓ |
|||
| Binary ethosomes No pre-treatment |
Ex vivo: Rat skin | Frz.C. | Running measure, 0 h to 24 h | Rat skin, 24 h: 40% of initial vismodegib permeated. Permeation flux: [3.22 ± 0.02 μg/cm2/h] |
[36] | |
| In vivo: Mouse tumor skin | Topical app. 3 tx/week |
Maybe 16 w | Tumor viability ↓ | |||
| Polymeric micelle nanocarriers No pre-treatment |
Ex vivo: Porcine skin, human skin |
Frz.C. | 6 h, 12 h, 24 h | Human skin, 120–200 µm depth, 12 h: [6.4 ± 3.3 µg/mL] | [37] | |
| Propylene glycol Microneedles (500, 1200, 1500 µm) |
Ex vivo: Porcine skin | Frz.C. | Running measure, 0 h to 24 h | Increased needle length and needle app. time leads to enhanced penetration of vismodegib | [38] | |
| Oil/water microemulsion Ablative fractional laser |
Ex vivo: Porcine skin | Frz.C. | 0.5 h, 4 h, 24 h | Pig skin +AFL, 0–300 µm, 4 h: [85 µg/mL] Pig skin +AFL, 600–900 µm, 4 h: [35 µg/mL] Pig skin -−AFL, 0–300 µm, 4 h: [66 µg/mL] Pig skin -−AFL, 600–900 µm, 4 h: [37 µg/mL] |
[39] | |
| Oil/water microemulsion Ablative fractional laser |
In vivo: Porcine skin | Topical app. 1 tx |
4 h, 2 d, 5 d, 9 d | Pig skin +AFL, 0–300 µm, 4 h: [131 µg/mL] Pig skin +AFL, 600–900 µm, 4 h: [30 µg/mL] Pig skin -−AFL, 0–300 µm, 4 h: [16 µg/mL] Pig skin -−AFL, 600–900 µm, 4 h: [6 µg/mL] |
[40] | |
| Sonidegib | Propylene glycol + ethanol No pre-treatment |
Ex vivo: Murine basaloids | Added to medium | 8 d | 4 x fewer basaloid lesions | [18] |
| In vivo: Porcine skin | Topical app. 1 tx |
1 h to 8 h | Pig skin sonidegib concentration between 1 h and 8 h: [1–1.5 µg/g tissue] | |||
| In vivo: Murine hair regrowth | 1 tx/d | 15 d | Hair growth inhibited for 15 days | |||
| In vivo: Depilated murine skin | 1 tx/d | 8 d | Skin Gli1 mRNA level ≈ −95% Skin Gli2 mRNA level ≈ −87% |
|||
| Itraconazole | Nonionic surfactant vesicles No pre-treatment |
Ex vivo: Rat skin | Frz.C. | 1 h, 2 h, 3 h, 4 h, 6 h | Rat skin, flux: [1.88 ± 0.24 mg/cm2/h] | [41] |
| In vivo: Tinea Pedis rat model | Topical app. 1 tx/d |
14 d | Tinea Pedis infection is cured by both formulation and control itraconazole cream | |||
| DMSO + PEG Polyglycolic acid microneedles |
In vivo: Human BCC regenerated in mice | Topical app. 1 tx/d |
14 d | BCC formation seen in control group not present in treated mice | [42] | |
| Lipid nanocapsules No pre-treatment |
Ex vivo: Human skin | Frz.C. | 6 h | Itraconazole skin retention at 6 h: 66.3 ± 2.5% | [43] | |
| In vivo: Cutaneous candidiasis, rat skin | Topical app. 2 tx/d |
14 d | Both novel and control treatments cure candidiasis infection | |||
| Nanoemulsion No pre-treatment |
Ex vivo: Mouse skin | Frz.C. | 6 h | 27.6 ± 4.4% of itraconazole permeated after 6 h. 72.9% was present in skin or permeated at this time point | [44] | |
| Nanocrystals Microneedles |
Ex vivo: Porcine skin | Frz.C. | 0.5 h, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 12 h, 24 h, 48 h, 72 h | Highest concentrations reached in dermis after 3 h [1.97 ± 0.32 mg/cm3]. Drug diffuses deeper than needle length. |
[45] | |
| Ex vivo: Candidiasis infection, porcine skin | Skin sustained in a Frz.C. | 12 h, 24 h, 48 h, 72 h | Microneedle treatment cure candidiasis infection after 48 h, control cream only shows limited effect | |||
| Vitamin D₃ | Acetone No pre-treatment |
In vivo: Murine BCCs | Topical app. 1 tx/d |
30 d | Lower proliferation of treated BCCs but no cell death. | [46] |
| In vivo: Murine BCC Gli1 mRNA | 4 d | BCC Gli1 mRNA level ≈ −66% | ||||
| CUR-61414 | Topical formulation No pre-treatment |
In vivo: Depilated murine skin Gli1 mRNA | Topical app. 1–2 tx/d |
3 d | 2 tx/d, skin Gli1 mRNA level ≈ −85% 1 tx/d, skin Gli1 mRNA level ≈ −62% |
[32] |
| In vivo: Murine BCC Gli1 mRNA | 10 tx/w | 21 d | BCC Gli1 mRNA level ≈ −60–65% | |||
| Multiple | Propylene glycol + DMSO or Propylene glycol + DMSO + ethanol No pre-treatment |
In vivo: Depilated murine skin | Topical app. 1 tx |
8 h | Highest topical inhibition by LY-2940680, Gli1 mRNA: −85% Vismodegib, Gli1 mRNA: −40% Sonidegib, Gli1 mRNA: −60% |
[33] |
Abbreviations: app., application; BCC, basal cell carcinoma; DMSO, dimethyl sulfoxide; Frz.C., Franz cell; PEG, polyethylene glycol SPM, Saarbrücken penetration model; tx, treatment(s).
In four of the preclinical studies, pre-treatment of the skin was included before topical application of HHi [38,39,40,45]. Olesen et al. tested AFL as a pre-treatment before application of vismodegib formulation in both ex vivo and in vivo pig skin. AFL treatment creates microscopic channels in the skin and was found to enhance vismodegib concentration after 24 h in ex vivo skin when compared to no pre-treatment [39]. In in vivo skin, AFL boosted vismodegib concentrations as early as 4 h after treatment with the highest increase observed after 5 days [40]. Similarly, microneedles also create channels in the skin before topical application. One study demonstrated that increased microneedle length and microneedle application time enhanced vismodegib penetration of the skin [38], and another study showed increased drug uptake following treatment with itraconazole containing dissolving microneedles [45]. In the latter study, itraconazole uptake was measured at multiple timepoints with the highest itraconazole concentration detected after 2 h in epidermis and 3 h in dermis. Furthermore, the study showed that itraconazole remained in the skin at least 72 h after treatment, especially in dermis [45]. Overall, pre-treatment with AFL and microneedles enhanced skin uptake of HHis.
2.1.1. Drug Concentration in Skin
Eleven of the preclinical studies focused on drug uptake in the skin following topical treatment. Franz cell and Saarbrücken penetration model setups, which use ex vivo skin to simulate an in vivo skin barrier, were most common (n = 10). However, direct comparison between publications was challenging due to inconsistent reporting of experimental results. In three directly comparable studies, skin concentrations were measured in ex vivo skin samples after treatment with vismodegib in specialized formulations including microemulsion, nanoformulation, and polymeric micelle nanocarriers. The highest skin concentration of vismodegib was achieved by Olesen et al. in pig skin (66 µg/mL) [39], while the other studies measured six to eight times lower concentrations in human skin (6.4–8.4 µg/mL) [34,35,37]. However, this may be explained by Olesen et al. using a substantially higher vismodegib dosing (>500 µg/cm2 versus 86 and 12 µg/cm2). According to Graham et al., the plasma concentration of vismodegib is between 2–24 µg/mL during oral treatment of humans [47], which is similar to the range of concentrations achieved in the studies. In the remaining studies, vismodegib or itraconazole skin uptake was reported as a percentage of total drug permeated, as a skin retention percentage, or as flux through the skin [36,41,43,44]. All studies concluded that novel HHi formulations improve skin uptake or permeation.
2.1.2. Biological Response to Topical HHi Application
Biological response to HHi treatment is often assessed by investigating the expression of GLI1 to estimate the activity of the hedgehog signaling pathway [10]. Four of the preclinical studies measured murine Gli1 mRNA levels, but only one study compared mRNA reduction with skin drug concentrations. In this study, mice were depilated to activate the hedgehog pathway and increase Gli1 transcription [33]. These mice were then used as a model to evaluate different HHis in terms of drug concentration in skin and Gli1 inhibition. The authors found that even though some HHis had comparable IC₅₀ values in cell inhibition studies, HHi skin uptake and Gli1 reduction varied widely in the in vivo setting [33]. In similar murine skin-depilation experiments, Skvara et al. showed that Gli1 mRNA levels were reduced by 95% after 8 days of single topical sonidegib applications [18], and Tang et al. reported a 62% reduction in Gli1 mRNA levels after 3 days of single topical CUR-61414 applications and by 85% after 3 days of two applications [32]. Overall, this indicates that the type of HHi as well as the frequency and duration of applications correlate with biological response to topical HHi treatment.
The most complex models in the preclinical studies used experimentally induced murine BCCs to investigate biological and tumor responses to topical HHi treatment. Topical application of CUR-61414 and vitamin D3 was tested in the same murine BCC model. Twenty-one days of topical CUR-61414 treatment resulted in reduced Gli1 expression and significant tumor remission [32], and four days of vitamin D3 treatment led to reductions in Gli1 expression and tumor proliferation as measured by Ki67 protein levels [46]. Topical application of sonidegib and itraconazole also demonstrated effects in murine BCC models. Topical sonidegib blocked the formation of basaloids in ex vivo murine tissue [18], and topical itraconazole with microneedle pre-treatment prevented human BCCs from forming in nude mice [42].
2.2. Clinical Trials
We identified seven clinical trials consisting of the following: two patidegib phase II trials and one itraconazole phase I trial from 2016; one vitamin D3 phase II trial from 2011; two sonidegib phase II trials from 2009; one CUR-61414 phase I trial from 2005. An overview of the clinical trials is presented in Table 4. None of the clinical trials reported on the skin concentration of HHi, whereas the biological response (GLI1 mRNA) was explored in four trials, and the clinical tumor response was investigated in all seven trials.
Table 4.
Overview of included clinical trials.
| HHi | Formulation & Pre-Tx | Study Design | Delivery Method | Measurement Time | Effect(s) | Reference |
|---|---|---|---|---|---|---|
| Sonidegib | Topical formulation No pre-treatment |
Clinical trial: Phase II Superficial or nodular BCCs. n = 24 BCCs |
Topical app. 2 tx/d |
6 w | 0.75%, complete regression: 3/16 0.75%, partial regression: 9/16 0.75%, no reaction: 4/16 |
NCT01033019 |
| Topical formulation No pre-treatment |
Clinical trial: Phase II BCNS patients, n = 61 BCCs |
Topical app. 2 tx/d |
4 w, 6 w, 9 w | Tumor volume ± SD: 4 w, 0.75%: −53,4 ± 30.85% 6 w, 0.25%: −35.2 ± 37.99% 9 w, 0.75%: −61.3 ± 31.18% |
NCT00961896, [18] | |
| Itraconazole | Topical formulation No pre-treatment |
Clinical trial: Early phase I BCNS patients and high frequency BCCs. n = 79 BCCs |
Topical app. 2 tx/d |
4 w, 12 w | No effect on BCC (GLI1 mRNA levels and tumor size) Intra-tumoral drug concentration: 4 w: [133 μg/g]; 12 w: [96 μg/g] |
NCT02735356, [31] |
| Patidegib | Topical formulation No pre-treatment |
Clinical trial: Phase II BCNS patients. n = 85 BCCs. 5–6 patients per group with multiple treated tumors |
Topical app. 2 tx/d |
26 w |
GLI1 mRNA levels ± SD: Patidegib 2%: [−54 ± 27%]; 4%: [−21 ± 35%] Tumor size ± SD: Patidegib 2%: [−51 ± 42%]; 4%: [−27 ± 41%] |
NCT02762084 |
| Topical formulation No pre-treatment |
Clinical trial: Phase II Nodular BCCs. n = 38 BCCs. 6 patients per treated group, multiple tumors per patient |
Topical app. 1–2 tx/d |
12 w |
GLI1 mRNA levels ± SD: 1 tx/d, 2%: [−56 ± 99%]; 4%: [−3 ± 69%] 2 tx/d, 2%: [−43 ± 56%]; 4%: [−29 ± 46%] Tumor size (±SD): 1 tx/d, 2%: [+56 ± 48%]; 4%: [+9 ± 47%] 2 tx/d, 2%: [+17 ± 37%]; 4%: [+18 ± 61%] |
NCT02828111 | |
| Vitamin D₃ & diclofenac | Topical formulation No pre-treatment |
Clinical trial: Phase II Superficial or nodular BCCs. n = 64 |
Topical app. 2 tx/d |
8 w | No effect on BCC (clinical response) | NCT01358045, [48] |
| CUR-61414 | Topical formulation No pre-treatment |
Clinical trial: Phase I Superficial or nodular BCCs. n = 42 |
Topical app. 2 tx/d |
4 d | No effect on BCC (GLI1 mRNA levels) | [32] |
Abbreviations: app., application; BCC, basal cell carcinoma; BCNS, basal cell nevus syndrome (also Gorlin syndrome); SD, standard deviation; tx, treatment(s).
Biological and Clinical Response
As in the preclinical studies, biological response is estimated by GLI1 mRNA expression, whereas clinical response is based on both objective and subjective measures e.g., changes in tumor volume, versus visually determined tumor clearance. In trials investigating CUR-61414 and itraconazole, no significant change in GLI1 mRNA levels was reported, which corresponded with an observed lack of clinical response to treatment [31,32]. Topical treatment with vitamin D3 also had no clinical effect, but GLI1 mRNA levels were not investigated ([48] and National Library of Medicine (NLM), NCT01358045). In trials on topical treatment with 2% or 4% patidegib, one to two daily applications over 12–26 weeks led to clearance of palpable tumor tissue with only visible residual macular erythema in 26/86 (30.2%) tumors, whereas placebo led to an equal response in 9/37 (24.3%) tumors (NLM, NCT02762084 and NCT02828111). Similarly, two daily topical applications of sonidegib over 4–9 weeks resulted in a partial response of at least a single tumor in 30/34 (88.2%) of patients, while placebo led to partial response in 6/16 (37.5%) of patients ([18] and NLM, NCT01033019 and NCT00961896). However, in one of the sonidegib clinical trials, subsequent histological examinations revealed that tumor nests were still present in all partial (n = 5) and all complete responders (n = 3) [18]. Biological response to treatment was also investigated demonstrating that both sonidegib and patidegib treatment reduced GLI1 expression. It is worth noting that in both the sonidegib and patidegib trials, only a few patients were included. This led to a considerable impact of outliers, in part because of slow BCC regression [18] and the spontaneous response of placebo-treated BCCs (NLM, NCT02762084 and NCT02828111). Pre-treatment of the skin in combination with HHis has not been tested in a clinical setting.
3. Methods
In June 2022, a literature search was conducted to identify publications on the topical delivery of HHis in both in- and ex vivo preclinical studies as well as clinical trials. The search included PubMed and ClinicalTrials.gov databases with no time limit on publication date. The full search queries are listed in Table 5. For the PubMed search, we included the HHis from Table 2, and search terms covering basal cell carcinoma and topical application. For the ClinicalTrials.gov search, we removed topical application from the terms, because relevant clinical trials were excluded. The searches returned a total of 287 PubMed entries and 57 clinicaltrials.gov entries, which were screened to identify 16 preclinical studies and 12 clinical trials fit for inclusion. However, five of the clinical trials have not published their findings; thus, we could only include seven clinical trials.
Table 5.
Search strategy. All search queries used for our searches. Cutane* covers all words that start with “cutane” e.g., cutaneous, and cutaneously. The latest search was performed 10 June 2022.
| PUBMED | ||
|---|---|---|
| Search | Query | Hits |
| 1 | (BMS-833923 OR XL-139) OR (“CUR 61414”) OR (Itraconazole) OR (LEQ506) OR (Patidegib OR Saridegib OR IPI-926) OR (Sonidegib OR Erismodegib OR LDE225) OR TAK-441 OR (Vismodegib OR GDC-0449 OR HhAntag691) OR (“Vitamin D3” OR Cholecalciferol OR Calcitriol) | 52,838 |
| 2 | #1 AND (“basal cell carcinoma” OR BCC OR (“Skin/abnormalities” [Mesh] OR “Skin/adverse effects” [Mesh] OR “Skin/cytology” [Mesh] OR “Skin/drug effects” [Mesh] OR “Skin/organization and administration” [Mesh] OR “Skin/pharmacology” [Mesh] OR “Skin/surgery” [Mesh] OR “Skin/therapeutic use” [Mesh] OR “Skin/therapy” [Mesh])) | 1115 |
| 3 | #2 AND (topical OR “Administration, Topical” [Mesh] OR cutane* OR “transdermal”) | 287 |
| CLINICALTRIALS.GOV | ||
| Search | Query | Hits |
| 1 | Condition or disease: BCC OR basal cell carcinoma Other terms: (BMS-833923 OR XL-139) OR (“CUR 61414”) OR (Itraconazole) OR (LEQ506) OR (Patidegib OR Saridegib OR IPI-926) OR (Sonidegib OR Erismodegib OR LDE225) OR TAK-441 OR (Vismodegib OR GDC-0449) OR (“Vitamin D3” OR Cholecalciferol OR Calcitriol) |
57 |
4. Discussion
Topical treatment of BCCs with HHis holds great potential. HHis are potent molecules that can be formulated to cross the skin barrier, and topical HHi treatments have been shown to significantly reduce activity of the hedgehog signaling pathway in both murine skin and BCC models. However, when these topical HHi treatments are translated into the clinic, the observed outcome is less efficacious, which is reflected by the fact that no topical HHis are currently approved for treatment of BCCs. The main barriers that prevent effective topical HHi treatment of human BCCs appear to be the insufficient penetration of tumor tissue, lack of biological response, and poor biodistribution or too short intra-tumoral HHi presence during topical BCC treatment.
Insufficient tissue penetration results in drug concentrations too low to affect the target tissue. Preclinical studies showed that advanced formulations and pre-treatments could significantly increase topical uptake in both porcine and human skin. However, intra-tumoral HHi concentrations following either topical or systemic HHi treatment have never been measured, and it is unclear whether topical treatments penetrate tumor tissue as efficiently as they penetrate skin tissue. Notably, larger clinical studies showed that topical BCC treatment with diclofenac, imiquimod, or a combination of 5-fluorouracil and cisplatin was more effective against superficial BCCs than nodular BCCs [48,49,50,51]. This could be a result of insufficient drug penetration in the nodular subtype due to morphological differences that increase tumor depth [52,53], or a result of genetic variation between the subtypes [54,55]. Similarly, topical HHi treatments likely face the same challenges, and future studies on HHis may benefit by addressing these challenges. For example, knowledge of intra-tumoral HHi concentrations would allow future studies to verify HHi penetration of the tumor tissue and improve our knowledge of HHi cutaneous pharmacokinetics. However, currently only plasma and excrement concentrations have been measured clinically following oral treatment with the FDA-approved SMO inhibitors sonidegib and vismodegib [47,56,57].
Sufficient drug uptake is linked to biological response, but in some cases, a preclinical biological response is not reflected in clinical studies. The preclinical studies showed that both depilation models and BCC tissue respond to HHi treatment, and that the frequency and duration of applications affected this response. However, in most cases, these preclinical results did not translate well into clinical trials. For example, topical CUR-61414, vitamin D₃ and itraconazole reduced Gli1 levels and decreased tumor size in murine preclinical studies, but when the drugs went into clinical trials, none of the patients responded to treatment ([31,32,48] and NLM, NCT02735356 and NCT01358045). Because of the complex nature of cancers, this poor translatability could be due to differences in the immune system [58,59], skin structures [60,61] and the vascularization and extracellular matrix of skin and tumor tissues [62,63]. For example, studies have shown that vismodegib binds with high affinity to α-1-acid glycoprotein—a protein present in blood and interstitial fluid—which results in early steady-state levels of vismodegib during oral treatment [47,64]. In topical treatments, α-1-acid glycoprotein could potentially affect the biodistribution and cutaneous pharmacokinetics of vismodegib by decreasing levels of unbound drug and increasing vismodegib wash-out from skin or tumor tissue. Because α-1-acid glycoprotein is present in both murine and human tissues, and studies have shown that murine α-1-acid glycoprotein levels change with age and inflammation status [65], α-1-acid glycoprotein could affect translatability of vismodegib studies. Similarly, other HHis might be affected by factors that change drug wash-out or biological response. For example, in ex vivo human percutaneous absorption studies, CUR-61414 concentrations far exceeded the IC50 levels, but when it was tested in clinical trials, no downregulation of GLI1 mRNA was observed [32]. While drug potencies from in vitro experiments rarely translate directly into in vivo experiments, intra-tumoral CUR-61414 was not measured, which means that whether they achieved sufficient intra-tumoral concentration of CUR-61414 is unknown.
Apart from insufficient intra-tumoral HHi concentrations, a poor translation of biological response into clinical trials could also be associated with differences in tumor immune infiltration. Studies have shown that hedgehog inhibition leads to increased immune infiltration in BCCs [7,8,9], and that HHis can reduce the activity of regulatory T-cells [66], which are abundantly present in and around BCCs [67]. Currently, it is not known whether this immune regulation requires systemic hedgehog inhibition, e.g., in tumor-draining lymph nodes. This could explain why topical HHi treatments perform better in mice, in which a relatively large skin area is treated, potentially leading to some degree of systemic HHi distribution. Future studies should address HHi-induced immune regulation for topical treatments.
After a biological response to HHi treatment is established, it must persist for long enough to induce a clinical response. Trials on oral vismodegib show that the median time to response for advanced BCCs is around 15–20 weeks [13]. Comparably, the median length of the included topical HHi clinical trials was 7 weeks (IQR 4 to 12 weeks), while the longest trial was 26 weeks. Therefore, the included trials might not last long enough to achieve a clinical response. However, some patients showed a clinical response to topical sonidegib already after 6 weeks (NLM, NCT01033019), which suggests that factors other than time-on-target alter the BCC response. These factors could include drug resistance of some tumor cells, intermittently insufficient drug penetration, or a combination of the two. If intra-tumoral HHi concentration drops too low between topical applications, HHi biodistribution might suffer, which could explain why tumor nests remained in patients where treatment appeared successful [18]. On the other hand, even though HHi concentration is sustained at steady state during oral treatment of advanced BCCs [47], stable disease or tumor regrowth after treatment termination is commonly observed [14]. Future clinical studies on topical HHis will have to investigate whether HHi resistance is common in non-advanced BCCs, which in turn will help decide whether topical HHi treatment is best suited for monotherapy, adjuvant therapy to surgery, or combination therapy with other established topical BCC therapies.
The preclinical studies showed that skin pre-treatments improve the cutaneous uptake of HHis. Thus, future topical HHis treatments might benefit from the inclusion of pre-treatments. However, as HHis need extended time-on-target, potential pre-treatments must be repeatable without significant adverse effects to maintain sufficient HHi concentrations during treatment. Since repeated pre-treatments were not investigated in preclinical studies and the clinical trials did not include pre-treatments at all, there is a knowledge gap of whether these topical delivery methods can be applied at sufficient frequency to improve HHi delivery. Inclusion of pre-treatments may also reduce patients’ ease-of-use and raise treatment costs if the pre-treatment needs to be applied by a physician. Overall, the ideal topical HHi treatment would be able to sustain long-term concentrations of HHi in BCCs without significant increases to cost, treatment time and the number of medical checkups.
5. Conclusions
Preclinical studies focused on HHi uptake in pig and human skin and biological response in murine models. The studies demonstrated that topical delivery of HHis can be improved and that topical HHi treatment leads to biological response of the hedgehog pathway in murine skin and tumor models. However, when the topical treatments were translated into a clinical setting, they had little or no effect on BCCs. We find that the main barriers that prevent clinical response to topical HHi treatment include insufficient drug penetration and a lack of biological response due to the poor translatability of preclinical studies. Furthermore, partially successful clinical trials are limited by incomplete understanding of cutaneous pharmacokinetics, HHi biodistribution and biological response over time. Overall, novel topical delivery techniques could have the potential to improve HHi delivery, but additional knowledge of cutaneous pharmacokinetics and biological response of BCCs is necessary to guide further development.
Acknowledgments
The authors thank Katrine Togsverd-Bo for her help with conceptualization and data curation. This work was executed as a part of the Danish Research Center for Skin Cancer as well as the Skin Cancer Innovation Clinical Academic Group (SCIN CAG), Greater Copenhagen Health Science Partners (GCHSP).
Author Contributions
Conceptualization, K.K.P., M.H.H.-H., M.H. and U.H.O.; investigation, retrieval of main articles used in the review and data curation K.K.P.; writing—original draft preparation, K.K.P.; writing—review and editing, K.K.P., M.H.H.-H., M.H., T.L. and U.H.O.; supervision, U.H.O.; visualization K.K.P. All authors contributed to manuscript revision, read, and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
M.H. is supported by a grant from Leo Pharma.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nehal K.S., Bichakjian C.K. Update on Keratinocyte Carcinomas. N. Engl. J. Med. 2018;379:363–374. doi: 10.1056/NEJMra1708701. [DOI] [PubMed] [Google Scholar]
- 2.Wu S., Han J., Li W.-Q., Li T., Qureshi A.A. Basal-Cell Carcinoma Incidence and Associated Risk Factors in US Women and Men. Am. J. Epidemiol. 2013;178:890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein E.H. Basal Cell Carcinomas: Attack of the Hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber W., Hutzinger M., Elmer D.P., Parigger T., Sternberg C., Cegielkowski L., Zaja M., Leban J., Michel S., Hamm S., et al. DYRK1B as Therapeutic Target in Hedgehog/GLI-Dependent Cancer Cells with Smoothened Inhibitor Resistance. Oncotarget. 2016;7:7134–7148. doi: 10.18632/oncotarget.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Didiasova M., Schaefer L., Wygrecka M. Targeting GLI Transcription Factors in Cancer. Molecules. 2018;23:1003. doi: 10.3390/molecules23051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reifenberger J., Wolter M., Knobbe C.B., Köhler B., Schönicke A., Scharwächter C., Kumar K., Blaschke B., Ruzicka T., Reifenberger G. Somatic Mutations in the PTCH, SMOH, SUFUH and TP53 Genes in Sporadic Basal Cell Carcinomas. Br. J. Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 7.Gambini D., Passoni E., Nazzaro G., Beltramini G., Tomasello G., Ghidini M., Kuhn E., Garrone O. Basal Cell Carcinoma and Hedgehog Pathway Inhibitors: Focus on Immune Response. Front. Med. 2022;9:893063. doi: 10.3389/fmed.2022.893063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grund-Gröschke S., Ortner D., Szenes-Nagy A.B., Zaborsky N., Weiss R., Neureiter D., Wipplinger M., Risch A., Hammerl P., Greil R., et al. Epidermal Activation of Hedgehog Signaling Establishes an Immunosuppressive Microenvironment in Basal Cell Carcinoma by Modulating Skin Immunity. Mol. Oncol. 2020;14:1930–1946. doi: 10.1002/1878-0261.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otsuka A., Dreier J., Cheng P.F., Nägeli M., Lehmann H., Felderer L., Frew I.J., Matsushita S., Levesque M.P., Dummer R. Hedgehog Pathway Inhibitors Promote Adaptive Immune Responses in Basal Cell Carcinoma. Clin. Cancer Res. 2015;21:1289–1297. doi: 10.1158/1078-0432.CCR-14-2110. [DOI] [PubMed] [Google Scholar]
- 10.Pietrobono S., Stecca B. Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors. Cells. 2018;7:272. doi: 10.3390/cells7120272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peris K., Fargnoli M.C., Garbe C., Kaufmann R., Bastholt L., Seguin N.B., Bataille V., del Marmol V., Dummer R., Harwood C.A., et al. Diagnosis and Treatment of Basal Cell Carcinoma: European Consensus–Based Interdisciplinary Guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Dummer R., Ascierto P.A., Basset-Seguin N., Dréno B., Garbe C., Gutzmer R., Hauschild A., Krattinger R., Lear J.T., Malvehy J., et al. Sonidegib and Vismodegib in the Treatment of Patients with Locally Advanced Basal Cell Carcinoma: A Joint Expert Opinion. J. Eur. Acad. Dermatol. Venereol. 2020;34:1944–1956. doi: 10.1111/jdv.16230. [DOI] [PubMed] [Google Scholar]
- 13.Frampton J.E., Basset-Séguin N. Vismodegib: A Review in Advanced Basal Cell Carcinoma. Drugs. 2018;78:1145–1156. doi: 10.1007/s40265-018-0948-9. [DOI] [PubMed] [Google Scholar]
- 14.Sekulic A., Migden M.R., Oro A.E., Dirix L., Lewis K.D., Hainsworth J.D., Solomon J.A., Yoo S., Arron S.T., Friedlander P.A., et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacouture M.E., Dréno B., Ascierto P.A., Dummer R., Basset-Seguin N., Fife K., Ernst S., Licitra L., Neves R.I., Peris K., et al. Characterization and Management of Hedgehog Pathway Inhibitor-Related Adverse Events in Patients With Advanced Basal Cell Carcinoma. Oncologist. 2016;21:1218–1229. doi: 10.1634/theoncologist.2016-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verkouteren B.J.A., Wakkee M., Reyners A.K.L., Nelemans P., Aarts M.J.B., Rácz E., Terra J.B., Devriese L.A., Alers R.-J., Kapiteijn E., et al. Eight Years of Experience with Vismodegib for Advanced and Multiple Basal Cell Carcinoma Patients in The Netherlands: A Retrospective Cohort Study. Br. J. Cancer. 2021;124:1199–1206. doi: 10.1038/s41416-020-01220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epstein E.H., Lear J., Saldanha G., Tang J.Y., Harwood C. Hedgehog Pathway Inhibition by Topical Patidegib to Reduce BCC Burden in Patients with Basal Cell Nevus (Gorlin) Syndrome. JCO. 2018;36:e21626. doi: 10.1200/JCO.2018.36.15_suppl.e21626. [DOI] [Google Scholar]
- 18.Skvara H., Kalthoff F., Meingassner J.G., Wolff-Winiski B., Aschauer H., Kelleher J.F., Wu X., Pan S., Mickel L., Schuster C., et al. Topical Treatment of Basal Cell Carcinomas in Nevoid Basal Cell Carcinoma Syndrome with a Smoothened Inhibitor. J. Investig. Dermatol. 2011;131:1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H., Lewis D.J. Topical Hedgehog Inhibitors for Basal Cell Carcinoma: How Far Away Are We? Expert Opin. Pharmacother. 2022;23:739–740. doi: 10.1080/14656566.2022.2050215. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe H.J., Wang W., Hannoush R.N., de Sauvage F.J. Regulation of the Oncoprotein Smoothened by Small Molecules. Nat. Chem. Biol. 2015;11:246–255. doi: 10.1038/nchembio.1776. [DOI] [PubMed] [Google Scholar]
- 21.Wolff F., Loipetzberger A., Gruber W., Esterbauer H., Aberger F., Frischauf A.M. Imiquimod Directly Inhibits Hedgehog Signalling by Stimulating Adenosine Receptor/Protein Kinase A-Mediated GLI Phosphorylation. Oncogene. 2013;32:5574–5581. doi: 10.1038/onc.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Smaele E., Cucchi D., Occhione M.A., Gulino A. Hedgehog Signaling Pathway and Its Targets for Treatment in Basal Cell Carcinoma. JEP. 2012;4:173. doi: 10.2147/JEP.S28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh M. Genomic Testing, Tumor Microenvironment and Targeted Therapy of Hedgehog-Related Human Cancers. Clin. Sci. 2019;133:953–970. doi: 10.1042/CS20180845. [DOI] [PubMed] [Google Scholar]
- 24.Niebel D., Sirokay J., Hoffmann F., Fröhlich A., Bieber T., Landsberg J. Clinical Management of Locally Advanced Basal-Cell Carcinomas and Future Therapeutic Directions. Dermatol. Ther. 2020;10:835–846. doi: 10.1007/s13555-020-00382-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chenna V., Hu C., Pramanik D., Aftab B.T., Karikari C., Campbell N.R., Hong S.-M., Zhao M., Rudek M.A., Khan S.R., et al. A Polymeric Nanoparticle Encapsulated Small-Molecule Inhibitor of Hedgehog Signaling (NanoHHI) Bypasses Secondary Mutational Resistance to Smoothened Antagonists. Mol. Cancer Ther. 2012;11:165–173. doi: 10.1158/1535-7163.MCT-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y.-S.E., Kuo M.-Y., Tsai P.-Y., Liu C.W., Wei W.Y. Abstract C046: A Smo Inhibitor DCBCO1303 with Potent Hedgehog Signaling Pathway Antagonist Activity Is Highly Active in Animal Tumor Model of Medulloblastoma and Cholangiocarcinoma. Mol. Cancer Ther. 2019;18:C046. doi: 10.1158/1535-7163.TARG-19-C046. [DOI] [Google Scholar]
- 27.Boogaerts M., Maertens J. Clinical Experience with Itraconazole in Systemic Fungal Infections. Drugs. 2001;61:39–47. doi: 10.2165/00003495-200161001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Makarova A., Wang G., Dolorito J.A., Kc S., Libove E., Epstein E.H. Vitamin D3 Produced by Skin Exposure to UVR Inhibits Murine Basal Cell Carcinoma Carcinogenesis. J. Investig. Dermatol. 2017;137:2613–2619. doi: 10.1016/j.jid.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Wenande E., Anderson R.R., Haedersdal M. Fundamentals of Fractional Laser-Assisted Drug Delivery: An in-Depth Guide to Experimental Methodology and Data Interpretation. Adv. Drug Deliv. Rev. 2020;153:169–184. doi: 10.1016/j.addr.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Gorzelanny C., Mess C., Schneider S.W., Huck V., Brandner J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics. 2020;12:684. doi: 10.3390/pharmaceutics12070684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohn G.K., Kwon G.P., Bailey-Healy I., Mirza A., Sarin K., Oro A., Tang J.Y. Topical Itraconazole for the Treatment of Basal Cell Carcinoma in Patients With Basal Cell Nevus Syndrome or High-Frequency Basal Cell Carcinomas: A Phase 2, Open-Label, Placebo-Controlled Trial. JAMA Dermatol. 2019;155:1078. doi: 10.1001/jamadermatol.2019.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang T., Tang J.Y., Li D., Reich M., Callahan C.A., Fu L., Yauch R.L., Wang F., Kotkow K., Chang K.S., et al. Targeting Superficial or Nodular Basal Cell Carcinoma with Topically Formulated Small Molecule Inhibitor of Smoothened. Clin. Cancer Res. 2011;17:3378–3387. doi: 10.1158/1078-0432.CCR-10-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lauressergues E., Heusler P., Lestienne F., Troulier D., Rauly-Lestienne I., Tourette A., Ailhaud M., Cathala C., Tardif S., Denais-Laliève D., et al. Pharmacological Evaluation of a Series of Smoothened Antagonists in Signaling Pathways and after Topical Application in a Depilated Mouse Model. Pharm. Res. Perspect. 2016;4:e00214. doi: 10.1002/prp2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calienni M.N., Maza Vega D., Temprana C.F., Izquierdo M.C., Ybarra D.E., Bernabeu E., Moretton M., Alvira F.C., Chiappetta D., Alonso S. del V.; et al. The Topical Nanodelivery of Vismodegib Enhances Its Skin Penetration and Performance In Vitro While Reducing Its Toxicity In Vivo. Pharmaceutics. 2021;13:186. doi: 10.3390/pharmaceutics13020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calienni M.N., Febres-Molina C., Llovera R.E., Zevallos-Delgado C., Tuttolomondo M.E., Paolino D., Fresta M., Barazorda-Ccahuana H.L., Gómez B., Alonso S., et al. Nanoformulation for Potential Topical Delivery of Vismodegib in Skin Cancer Treatment. Int. J. Pharm. 2019;565:108–122. doi: 10.1016/j.ijpharm.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Gamal F A., Sayed O.M., Abo El-Ela F.I., Kharshoum R.M., Salem H.F. Treatment of Basal Cell Carcinoma Via Binary Ethosomes of Vismodegib: In Vitro and In Vivo Studies. AAPS PharmSciTech. 2020;21:51. doi: 10.1208/s12249-019-1574-x. [DOI] [PubMed] [Google Scholar]
- 37.Kandekar S.G., Singhal M., Sonaje K.B., Kalia Y.N. Polymeric Micelle Nanocarriers for Targeted Epidermal Delivery of the Hedgehog Pathway Inhibitor Vismodegib: Formulation Development and Cutaneous Biodistribution in Human Skin. Expert Opin. Drug Deliv. 2019;16:667–674. doi: 10.1080/17425247.2019.1609449. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen H.X., Banga A.K. Enhanced Skin Delivery of Vismodegib by Microneedle Treatment. Drug Deliv. Transl. Res. 2015;5:407–423. doi: 10.1007/s13346-015-0241-3. [DOI] [PubMed] [Google Scholar]
- 39.Olesen U.H., Clergeaud G., Lerche C.M., Andresen T.L., Haedersdal M. Topical Delivery of Vismodegib Using Ablative Fractional Laser and Micro-Emulsion Formulation in Vitro: TOPICAL VISMODEGIB AND LASER IN VITRO. Lasers Surg. Med. 2019;51:79–87. doi: 10.1002/lsm.23013. [DOI] [PubMed] [Google Scholar]
- 40.Olesen U.H., Clergeaud G., Hendel K.K., Yeung K., Lerche C.M., Andresen T.L., Haedersdal M. Enhanced and Sustained Cutaneous Delivery of Vismodegib by Ablative Fractional Laser and Microemulsion Formulation. J. Investig. Dermatol. 2020;140:2051–2059. doi: 10.1016/j.jid.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 41.Kumar N., Goindi S. Statistically Designed Nonionic Surfactant Vesicles for Dermal Delivery of Itraconazole: Characterization and in Vivo Evaluation Using a Standardized Tinea Pedis Infection Model. Int. J. Pharm. 2014;472:224–240. doi: 10.1016/j.ijpharm.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Wang Y., Jin J.Y., Degan S., Hall R.P., Boehm R.D., Jaipan P., Narayan R.J. Use of Drawing Lithography-Fabricated Polyglycolic Acid Microneedles for Transdermal Delivery of Itraconazole to a Human Basal Cell Carcinoma Model Regenerated on Mice. JOM. 2016;68:1128–1133. doi: 10.1007/s11837-016-1841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Sheridy N.A., Ramadan A.A., Eid A.A., El-Khordagui L.K. Itraconazole Lipid Nanocapsules Gel for Dermatological Applications: In Vitro Characteristics and Treatment of Induced Cutaneous Candidiasis. Colloids Surf. B Biointerfaces. 2019;181:623–631. doi: 10.1016/j.colsurfb.2019.05.057. [DOI] [PubMed] [Google Scholar]
- 44.Botros S.R., Hussein A.K., Mansour H.F. A Novel Nanoemulsion Intermediate Gel as a Promising Approach for Delivery of Itraconazole: Design, In Vitro and Ex Vivo Appraisal. AAPS PharmSciTech. 2020;21:272. doi: 10.1208/s12249-020-01830-w. [DOI] [PubMed] [Google Scholar]
- 45.Permana A.D., Paredes A.J., Volpe-Zanutto F., Anjani Q.K., Utomo E., Donnelly R.F. Dissolving Microneedle-Mediated Dermal Delivery of Itraconazole Nanocrystals for Improved Treatment of Cutaneous Candidiasis. Eur. J. Pharm. Biopharm. 2020;154:50–61. doi: 10.1016/j.ejpb.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Tang J.Y., Xiao T.Z., Oda Y., Chang K.S., Shpall E., Wu A., So P.-L., Hebert J., Bikle D., Epstein E.H. Vitamin D3 Inhibits Hedgehog Signaling and Proliferation in Murine Basal Cell Carcinomas. Cancer Prev. Res. 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham R.A., Lum B.L., Cheeti S., Jin J.Y., Jorga K., Von Hoff D.D., Rudin C.M., Reddy J.C., Low J.A., LoRusso P.M. Pharmacokinetics of Hedgehog Pathway Inhibitor Vismodegib (GDC-0449) in Patients with Locally Advanced or Metastatic Solid Tumors: The Role of Alpha-1-Acid Glycoprotein Binding. Clin. Cancer Res. 2011;17:2512–2520. doi: 10.1158/1078-0432.CCR-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brinkhuizen T., Frencken K.J.A., Nelemans P.J., Hoff M.L.S., Kelleners-Smeets N.W.J., zur Hausen A., van der Horst M.P.J., Rennspiess D., Winnepenninckx V.J.L., van Steensel M.A.M., et al. The Effect of Topical Diclofenac 3% and Calcitriol 3 Μg/g on Superficial Basal Cell Carcinoma (SBCC) and Nodular Basal Cell Carcinoma (NBCC): A Phase II, Randomized Controlled Trial. J. Am. Acad. Dermatol. 2016;75:126–134. doi: 10.1016/j.jaad.2016.01.050. [DOI] [PubMed] [Google Scholar]
- 49.Fredman G., Wenande E., Hendel K., Togsverd-Bo K., Haedersdal M. Efficacy and Safety of Laser-assisted Combination Chemotherapy: A Follow-up Study of Treatment with 5-fluorouracil and Cisplatin for Basal Cell Carcinoma. Lasers Surg. Med. 2021;54:113–120. doi: 10.1002/lsm.23497. [DOI] [PubMed] [Google Scholar]
- 50.Huang C.M., Kirchhof M.G. Topical Imiquimod as a Treatment Option for Nodular Basal Cell Carcinoma: A Systematic Review. J. Cutan. Med. Surg. 2020;24:495–503. doi: 10.1177/1203475420931770. [DOI] [PubMed] [Google Scholar]
- 51.Roozeboom M.H., Arits A.H.H.M., Nelemans P.J., Kelleners-Smeets N.W.J. Overall Treatment Success after Treatment of Primary Superficial Basal Cell Carcinoma: A Systematic Review and Meta-Analysis of Randomized and Nonrandomized Trials: Overall Treatment Success in Primary Superficial Basal Cell Carcinoma. Br. J. Dermatol. 2012;167:733–756. doi: 10.1111/j.1365-2133.2012.11061.x. [DOI] [PubMed] [Google Scholar]
- 52.Rippey J.J. Why Classify Basal Cell Carcinomas? Histopathology. 1998;32:393–398. doi: 10.1046/j.1365-2559.1998.00431.x. [DOI] [PubMed] [Google Scholar]
- 53.Pyne J., Mint E., Barr E., Clark S., Hou R. Basal Cell Carcinoma: Variation in Invasion Depth by Subtype, Sex, and Anatomic Site in 4565 Cases. Dermatol. Pract. Concept. 2018;8:314–319. doi: 10.5826/dpc.0804a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Nardo L., Pellegrini C., Di Stefani A., Ricci F., Fossati B., Del Regno L., Carbone C., Piro G., Corbo V., Delfino P., et al. Molecular Alterations in Basal Cell Carcinoma Subtypes. Sci. Rep. 2021;11:13206. doi: 10.1038/s41598-021-92592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Do Carmo N.G., Sakamoto L.H.T., Pogue R., Do Couto Mascarenhas C., Passos S.K., Soares Felipe M.S., De Andrade R.V. Altered Expression of PRKX, WNT3 and WNT16 in Human Nodular Basal Cell Carcinoma. AR. 2016;36:4545–4552. doi: 10.21873/anticanres.11002. [DOI] [PubMed] [Google Scholar]
- 56.Graham R.A., Lum B.L., Morrison G., Chang I., Jorga K., Dean B., Shin Y.G., Yue Q., Mulder T., Malhi V., et al. A Single Dose Mass Balance Study of the Hedgehog Pathway Inhibitor Vismodegib (GDC-0449) in Humans Using Accelerator Mass Spectrometry. Drug. Metab. Dispos. 2011;39:1460–1467. doi: 10.1124/dmd.111.039339. [DOI] [PubMed] [Google Scholar]
- 57.Zollinger M., Lozac’h F., Hurh E., Emotte C., Bauly H., Swart P. Absorption, Distribution, Metabolism, and Excretion (ADME) of 14C-Sonidegib (LDE225) in Healthy Volunteers. Cancer Chemother. Pharm. 2014;74:63–75. doi: 10.1007/s00280-014-2468-y. [DOI] [PubMed] [Google Scholar]
- 58.Shay T., Jojic V., Zuk O., Rothamel K., Puyraimond-Zemmour D., Feng T., Wakamatsu E., Benoist C., Koller D., Regev A., et al. Conservation and Divergence in the Transcriptional Programs of the Human and Mouse Immune Systems. Proc. Natl. Acad. Sci. USA. 2013;110:2946–2951. doi: 10.1073/pnas.1222738110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X., Li L., Starr T.K., Subramanian S. Tumor Location Impacts Immune Response in Mouse Models of Colon Cancer. Oncotarget. 2017;8:54775–54787. doi: 10.18632/oncotarget.18423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh B., Reddy L.H., Kulkarni R.V., Khanam J. Comparison of Skin Permeability of Drugs in Mice and Human Cadaver Skin. Indian J. Exp. Biol. 2000;38:42–45. [PubMed] [Google Scholar]
- 61.Zomer H.D., Trentin A.G. Skin Wound Healing in Humans and Mice: Challenges in Translational Research. J. Dermatol. Sci. 2018;90:3–12. doi: 10.1016/j.jdermsci.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 62.Choi I.-K., Strauss R., Richter M., Yun C.-O., Lieber A. Strategies to Increase Drug Penetration in Solid Tumors. Front. Oncol. 2013;3:193. doi: 10.3389/fonc.2013.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viallard C., Larrivée B. Tumor Angiogenesis and Vascular Normalization: Alternative Therapeutic Targets. Angiogenesis. 2017;20:409–426. doi: 10.1007/s10456-017-9562-9. [DOI] [PubMed] [Google Scholar]
- 64.Clerc C., Pibouin M., Ruelland A., Legras B., Chevrant-Breton J., Cloarec L. Cutaneous Interstitial Fluid Protein Concentrations in the Inflammatory Syndrome: Pharmacological Consequences. Clin. Chim. Acta. 1990;189:181–189. doi: 10.1016/0009-8981(90)90090-F. [DOI] [PubMed] [Google Scholar]
- 65.Carter K.C., Post D.J., Papaconstantinou J. Differential Expression of the Mouse A1-Acid Glycoprotein Genes (AGP-1 and AGP-2) during Inflammation and Aging. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1991;1089:197–205. doi: 10.1016/0167-4781(91)90008-A. [DOI] [PubMed] [Google Scholar]
- 66.Papaioannou E., Yánez D.C., Ross S., Lau C.-I., Solanki A., Chawda M.M., Virasami A., Ranz I., Ono M., O’Shaughnessy R.F.L., et al. Sonic Hedgehog Signaling Limits Atopic Dermatitis via Gli2-Driven Immune Regulation. J. Clin. Investig. 2019;129:3153–3170. doi: 10.1172/JCI125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Omland S., Nielsen P., Gjerdrum L., Gniadecki R. Immunosuppressive Environment in Basal Cell Carcinoma: The Role of Regulatory T Cells. Acta Derm. Venerol. 2016;96:917–921. doi: 10.2340/00015555-2440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.