Abstract
The SARS-CoV-2 pandemic continues to spread worldwide, generating a high impact on healthcare systems. The aim of the study was to examine the epidemiological burden of SARS-CoV-2 reinfections and to identify potential related risk factors. A retrospective observational study was conducted in Liguria Region, combining data from National Vaccines Registry and Regional Chronic Condition Data Warehouse. In the study period (September 2021 to May 2022), 335,117 cases of SARS-CoV-2 infection were recorded in Liguria, of which 15,715 were reinfected once. During the Omicron phase (which predominated from 3 January 2022), the risk of reinfection was 4.89 times higher (p < 0.001) than during the Delta phase. Unvaccinated and vaccinated individuals with at least one dose for more than 120 days were at increased risk of reinfection compared with vaccinated individuals with at least one dose for ≤120 days, respectively (odds ratio (OR) of 1.26, p < 0.001; OR of 1.18, p < 0.001). Healthcare workers were more than twice as likely to be reinfected than non-healthcare workers (OR of 2.38, p < 0.001). Lower ORs were seen among people aged 60 to 79 years. Two doses or more of vaccination were found to be protective against the risk of reinfection rather than a single dose (mRNA vaccines: OR of 0.06, p < 0.0001, and OR of 0.1, p < 0.0001; vector vaccines: OR of 0.05, p < 0.0001). Patients with chronic renal failure, cardiovascular disease, bronchopneumopathy, neuropathy and autoimmune diseases were at increased risk of reinfection (OR of 1.38, p = 0.0003; OR of 1.09, p < 0.0296; OR of 1.14, p = 0.0056; OR of 1.78, p < 0.0001; OR of 1.18, p = 0.0205). Estimating the epidemiological burden of SARS-CoV-2 reinfections and the role played by risk factors in reinfections is relevant for identifying risk-based preventive strategies in a pandemic context characterized by a high circulation of the virus and a high rate of pathogen mutations.
Keywords: COVID-19, SARS-CoV-2, epidemiological burden, comorbidities, risk factor, vaccination, vaccine
1. Introduction
The COVID-19 pandemic continues to spread worldwide and has caused 532,887,351 confirmed cases, including 6,307,021 deaths, as of 7 June 2022 [1].
SARS-CoV-2, an ever-mutating ribonucleic acid (RNA), has been reported to have a heterogeneous genetic composition in different geographic locations [2], increasing the risk of reinfection. The first case of COVID-19 reinfection was reported in Hong Kong in August 2020 [3]. Since then, the emergence of re-infected cases has been reported in many other countries, including the United States [4] and Italy [5].
In particular, in Italy, since August 2021, several cases of COVID-19 reinfections have been recorded more than 90 days (or more than 60 days in cases with genotyping results indicated different variants) after the previous laboratory-confirmed infection with molecular or antigen testing, according to the case definition of the Ministry of Health [6].
In addition, during the same period, Italy experienced a predominant circulation of two different SARS-CoV-2 variants of concern (VOCs) [7]: from 24 August 2021 to 5 December 2021, the Delta variant predominated, and from 3 January 2022, the Omicron variant became prevalent, with an intermediate period in which the epidemic variants shifted from Delta to Omicron (from 6 December 2021 to 2 January 2022) [8].
According to the latest report from the Italian National Institute of Health (Istituto Superiore di Sanità), 519,603 cases of reinfection were reported in Italy from 24 August 2021 to 5 June 2022 (4.0% of all notified cases). However, the incidence of reinfection, which remained stable at around 1% until 6 December 2021 (the reference date for the start of the Omicron variant), increased sharply to 3% in early January 2022, remained stable until the end of March 2022, and further increased between 6 April and 25 May 2022, reaching an incidence of 4.1% and 6.5%, respectively [9]. In particular, the risk of reinfection starting from 3 January 2022 (starting point of the Omicron variant) highlighted an increase in the adjusted relative risk of reinfection (values significantly greater than 1) compared with the previous phases [9]. Omicron variants are characterized by higher contagiousness and an increased incidence of breakthrough infection and reinfection due to enhanced escape mechanisms of neutralizing antibodies.
Meanwhile, despite the concerns about the infectiousness of Omicron variants, such as the latest BA.5, the effectiveness of the COVID-19 vaccine in preventing severe disease remains high, reaching approximately 68% among those vaccinated with a complete cycle for less than 90 days and 87% among booster-vaccinated [9].
In light of the large number of infected and reinfected individuals, and high vaccination coverage, the healthcare burden and deaths are limited; to the best of our knowledge, the scientific literature about the characteristics of the reinfected individuals and associated potential risk factors is limited.
Knowing the frequency and natural course of reinfection is important to develop control/mitigation strategies against SARS-CoV-2 and to better address the preventive measures and the vaccination strategies. According to the latest report from the National Institute of Health in Italy (Istituto Superiore di Sanità), a higher risk was described in women, younger persons (12–49 years), healthcare workers, unvaccinated people and those who have been vaccinated for more than 120 days [9].
Therefore, the objective of this study is to examine the epidemiological burden of SARS-CoV-2 reinfection in Liguria Region from September 2021 to May 2022, analyzing the potential risk factors of reinfected patients.
2. Materials and Methods
2.1. Study Design and Timeline
A retrospective observational study was conducted to assess the epidemiological burden of SARS-CoV-2 reinfections and potential associated risk factors in Liguria Region (Italy). First-time infections and reinfections from September 2021 to May 2022 were included in the analysis with data on infections extracted on 1 June 2022.
2.2. Study Population
Patients were captured through the regional administrative flows selecting reinfections according to the Ministry of Health case definition [6]. Specifically, the inclusion criteria considered individuals with a single episode of reinfection, excluding those who had reported more than one. Data were collected taking into account the age group (0–19, 20–39, 40–59, 60–79 and ≥80 years), vaccination status, VOC predominant phase (Delta phase, Transition phase, Omicron phase), gender (male/female), healthcare worker status (yes/no), nationality (Italian/non-Italian) and comorbidities. Individuals with an interval of 14 days after vaccination were considered as fully vaccinated; those who tested positive for SARS-CoV-2 within 14 days after vaccination were considered as unvaccinated; those who tested positive within 14 days of the second vaccination were classified as having received a single dose. Participants were classified as unvaccinated (never vaccinated or 0–14 days from first dose), vaccinated (at least one dose) within 120 days or vaccinated (at least one dose) for more than 120 days. Regarding the analysis of the vaccination status, vaccine formulations were classified into four groups: mRNA vaccines, which included BNT162b2 and mRNA-1273 vaccines; viral vector vaccines (ChAdOx1 and Ad26.COV2.S); protein vaccines (NVX-CoV2373); and mixed vaccines, which included the administration of a first dose with a viral vector vaccine and subsequent doses with an mRNA vaccine. Vaccinated individuals were divided into single-dose vaccinated, primary-cycle vaccinated (administration of two doses) and vaccinated with booster, additional or second booster doses. Number of days between participants’ study initiation (1 September 2021) and positive SARS-CoV-2 PCR/antigen test results were used to calculate person–time and days to infection and reinfection [10].
2.3. Data Sources and Statistical Analysis
Different data sources were used: National Vaccine Registry and regional administrative flows, such as Chronic Condition Data Warehouse and regional Data Warehouse for vaccination status, comorbidities, demographics and subjects positive to SARS-CoV-2, respectively. Odds ratios (ORs) for SARS-CoV-2 reinfections were estimated with the corresponding 95% confidence intervals (CIs). Multivariate logistic regression was used to calculate odds ratios to evaluate the potential risk factors for reinfection compared with the first infection. Data were analyzed using JMP version 13.0.0 software (SAS Institute, Cary, NC, USA). A value of p < 0.05 was considered statistically significant.
3. Results
Epidemiological and Clinical Burden
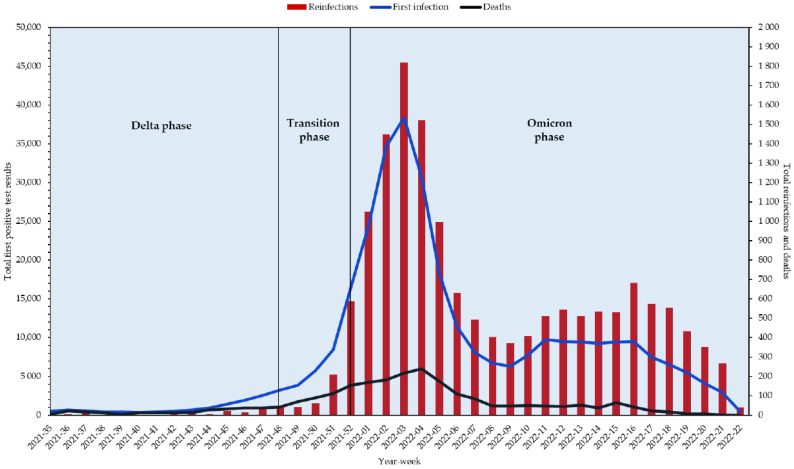
During the study period, on a total Ligurian population of 1,545,174 individuals, 335,117 cases of SARS-CoV-2 infection were recorded in Liguria, of which were 15,795 reinfections (15,715 individuals with one reinfection), indicating an overall reinfection rate of 4.95 per hundred first infections. The first recorded SARS-CoV-2 reinfection case was in early September 2021 (Week 35). Since then, from Week 2021-51, the number of SARS-CoV-2 reinfections markedly increased, probably due to both the increase in the number of first infections and in accordance with the emergence of the more infectious Omicron variant, reaching a peak in Week 2022-03. Thereafter, a rapid decline in first infections, reinfections and deaths until Week 2022-10, when there was a gradual increase in reinfections and conversely a decrease in early infections and deaths, was observed (Figure 1).
Figure 1.
Weekly number of SARS-CoV-2 reinfections, primary infection and deaths in Liguria Region.
In particular, the risk of reinfection during the Omicron phase was 4.89 times higher (95% CI of 4.19–5.72, p < 0.001) than during the Delta phase.
Regardless of the predominant variant, the risk of reinfection was 1.26 (95% CI of 1.22–1.31, p < 0.001) times higher in unvaccinated individuals and 1.18 (95% CI of 1.13–1.23, p < 0.001) times higher in individuals vaccinated with at least one dose for more than 120 days than in individuals vaccinated with at least one dose for ≤120 days.
The median age of COVID-19-reinfected individuals was 41 years (interquartile range: 23–53). Furthermore, female individuals showed a 17% higher risk of reinfection than the male gender (OR of 1.17, 95% CI of 1.13–1.21, p < 0.0001). Non-Italian individuals had a 15% higher risk of reinfection than Italian residents (OR of 1.15, 95% CI of 1.09–1.21, p < 0.001); however, after data adjustment, the results showed no associations with a higher risk of reinfection. Healthcare workers were more than twice as likely to be reinfected than non-healthcare workers (OR of 2.38, 95% CI of 2.25–2.52, p < 0.001). Individuals aged between 60 and 79 years old had significantly lower odds of becoming reinfected than the other age groups (Table 1).
Table 1.
Main characteristics and odds ratios of SARS-CoV-2-reinfected individuals in Liguria.
| Variable | Reinfection (15,715) | PDs (53,891,505) | Incidence Per 10,000 PD | Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Epidemic phase | Delta | 161 | 958,178 | 1.68 | Reference | Reference | ||
| Transition | 903 | 4,013,487 | 2.25 | 2.36 (1.99–2.79) | <0.001 | 2.40 (2.03–2.84) | <0.001 | |
| Omicron | 14,651 | 48,919,840 | 2.99 | 4.89 (4.19–5.72) | <0.001 | 5.27 (4.51–6.17) | <0.001 | |
| Vaccination Status | Vaccinated with at least one dose for ≤120 days | 5350 | 22,330,172 | 2.40 | Reference | Reference | ||
| Vaccinated with at least one dose for >120 days | 4581 | 14,764,645 | 3.10 | 1.18 (1.13–1.23) | <0.001 | 1.29 (1.25–1.35) | <0.001 | |
| Unvaccinated | 5784 | 16,796,688 | 3.44 | 1.26 (1.22–1.31) | <0.001 | 1.62 (1.55–1.69) | <0.001 | |
| Gender | Male | 6812 | 25,089,342 | 2.72 | Reference | Reference | ||
| Female | 8903 | 28,802,163 | 3.09 | 1.17 (1.13–1.21) | <0.001 | 1.11 (1.08–1.15) | <0.001 | |
| Age-group | 0–19 | 3235 | 12,177,126 | 2.66 | 1.37 (1.29–1.46) | <0.001 | 1.22 (1.15–1.30) | <0.001 |
| 20–39 | 4293 | 12,222,708 | 3.51 | 1.84 (1.74–1.96) | <0.001 | 1.76 (1.66–1.87) | <0.001 | |
| 40–59 | 5804 | 17,582,644 | 3.30 | 1.77 (1.68–1.88) | <0.001 | 1.66 (1.57–1.76) | <0.001 | |
| 60–79 | 1588 | 8,776,040 | 1.81 | Reference | Reference | |||
| ≥80 | 795 | 3,132,987 | 2.54 | 1.46 (1.34–1.60) | <0.001 | 1.52 (1.39–1.66) | <0.001 | |
| Nationality | Italian | 14,080 | 304,189 | 462.87 | Reference | Reference | ||
| Non-Italian | 1635 | 30,928 | 528.65 | 1.15 (1.09–1.21) | <0.001 | 1.04 (0.99–1.10) | 0.1016 | |
| Healthcare worker | No | 14,265 | 51,391,133 | 2.78 | Reference | Reference | ||
| Yes | 1450 | 2,500,372 | 5.80 | 2.38 (2.25–2.52) | <0.001 | 2.43 (2.29–2.58) | <0.001 | |
CI, confidence interval; OR, odds ratio; PDs, person days. Adjusted ORs include all factors in the table, mutually adjusted for each other.
With regard to vaccination status, the incidence rate of SARS-CoV-2 infection in vaccinated individuals with a single dose was 66.64 per 10,000 person days (PDs), 69.82 in the primary-cycle vaccinated and 49.07 in the booster/additional/second booster group. For reinfected individuals, an incidence rate of reinfection equal to 20.35 (per 10,000 PDs) was detected in vaccinated individuals with a single dose compared with 1.96 and 1.87 (per 10,000 PDs) in vaccinated individuals with primary cycle and subsequent doses, respectively. Furthermore, primary cycle and subsequent doses resulted protective factors in preventing reinfections rather than a single-dose vaccination (mRNA vaccines: OR of 0.06, 95% CI of 0.06–0.07, p < 0.0001; and OR of 0.1, 95% CI of 0.09–0.11, p < 0.0001; vector vaccines: OR of 0.05, 95% CI of 0.04-0.07, p < 0.0001) (Table 2). For mixed vaccines, at least three doses resulted more protective against reinfection rather than only two doses. The small number of subjects vaccinated with protein vaccines did not allow us to have reliable results, showing no statistically significant differences in the number of doses administered.
Table 2.
Vaccinated individuals and odds ratios of SARS-CoV-2-reinfected individuals in Liguria.
| Variable * | Overall (335,037) | Not Reinfected (319,322) |
Reinfected (15,715) | PDs (37,094,817) | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
| All vaccines | Single dose | 10,216 (66.64) | 7096 (46.29) | 3120 (20.35) | 1,533,000 | Reference | |
| Primary cycle | 118,547 (69.82) | 115,218 (67.86) | 3329 (1.96) | 16,979,202 | 0.07 (0.06–0.07) | <0.0001 | |
| Booster/additional dose/second booster | 94,675 (49.07) | 91,193 (49.07) | 3482(1.87) | 18,582,615 | 0.09 (0.08–0.09) | <0.0001 | |
| mRNA | Single dose | 9930 (66.58) | 6886 (46.17) | 3044 (20.41) | 1,491,509 | Reference | |
| Primary cycle | 103,560 (68.72) | 100,680 (66.81) | 2880 (1.91) | 15,070,369 | 0.06 (0.06–0.07) | <0.0001 | |
| Booster/additional dose/second booster | 75,953 (50.82) | 72,715 (48.65) | 3238 (2.17) | 14,946,436 | 0.1 (0.09–0.11) | <0.0001 | |
| Vector | Single dose | 243 (75.38) | 170 (52.74) | 73 (22.65) | 32,235 | Reference | |
| Primary cycle | 9123 (79.74) | 8922 (77.98) | 201 (1.76) | 1,144,134 | 0.05 (0.04–0.07) | <0.0001 | |
| Protein | Single dose | 43 (46.46) | 40 (43.22) | 3 (3.24) | 9256 | Reference | |
| Primary cycle | 32 (41.31) | 31 (40.02) | 1 (1.29) | 7747 | 0.43 (0.04–4.34) | 0.4743 | |
| Mixed | Primary cycle | 5832 (77.05) | 5585 (73.78) | 247 (3.26) | 756,952 | Reference | |
| Booster/additional dose/second booster | 18,722 (51.49) | 18,478 (50.82) | 244 (0.67) | 3636,179 | 0.29 (0.25–0.36) | <0.0001 | |
* Expressed as number and incidence rate per 10,000 PDs. CI, confidence interval; OR, odds ratio; PDs, person days.
Relatively to the clinical status detected in May 2022, among the 15,715 reinfected individuals, 12,778 (81.31%) were recovered; a total of 1237 (7.87%) were asymptomatic, and 1172 (7.46%) were pauci-symptomatic; a total of 425 (2.70%) had a mild clinical status, and 14 (0.09%) had a severe or critic clinical status, while 89 (0.57%) were dead.
Most of reinfected individuals (12,843; 81.72%) were healthy, while 2872 (18.28%) had at least one comorbidity. Among reinfected individuals, persons aged 60 years and older had a seven-fold higher risk to have at least one underlying chronic disease than other age groups (OR of 7.39, 95% CI of 6.73–8.14, p < 0.0001); chronic renal failure (OR of 1.38, 95% CI of 1.16–1.65, p = 0.0003), cardiovascular diseases (OR of 1.09, 95% CI of 1.01–1.19, p = 0.0296), bronchopneumopathy (OR of 1.14, 95% CI of 1.04–1.26, p = 0.0056), neuropathy (OR of 1.78, 95% CI of 1.58–2.01, p < 0.0001) and autoimmune diseases (OR of 1.18, 95% CI of 1.03 -1.37, p = 0.0205) were the most implicated comorbidities in patients with reinfection compared with non-reinfected individuals (Table 3).
Table 3.
Comorbidities implicated in reinfected SARS-CoV-2 patients from September 2021 to May 2022.
| Comorbidity | OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| 02—Transplant | 1.06 | 0.68 | 1.67 | 0.7872 |
| 03—Chronic renal failure | 1.38 | 1.16 | 1.65 | 0.0003 |
| 04—HIV/AIDS | 0.52 | 0.27 | 1.00 | 0.0503 |
| 05—Cancer | 0.95 | 0.88 | 1.04 | 0.2785 |
| 06—Diabetes | 0.91 | 0.81 | 1.01 | 0.0877 |
| 07—Cardiovascular disease | 1.09 | 1.01 | 1.19 | 0.0296 |
| 08—Bronchopneumopathy | 1.14 | 1.04 | 1.26 | 0.0056 |
| 09—Gastroenteropathy | 1.09 | 0.98 | 1.22 | 0.0941 |
| 10—Neuropathy | 1.78 | 1.58 | 2.01 | <0.0001 |
| 11—Autoimmune disease | 1.18 | 1.03 | 1.37 | 0.0205 |
| 12—Endocrine and metabolic disease | 0.89 | 0.82 | 0.97 | 0.0103 |
| 13—Rare disease | 1.05 | 0.89 | 1.24 | 0.5391 |
| 14—Psychosis | 1.28 | 1.04 | 1.59 | 0.0215 |
In addition, an in-depth analysis of comorbidities revealed that among patients with chronic renal failure, those undergoing dialysis had an almost 3 times higher risk of reinfection (OR of 2.77, 95% CI of 1.76–4.38, p < 0.0001). Among the chronic cardiovascular diseases, heart failure and cerebral vasculopathy were the most involved in the risk of reinfection, with 1.24 (95% CI of 1.04–1.47, p = 0.0184) and 1.49 (95% CI of 1.29–1.71, p < 0.0001) times higher risk than non-reinfected individuals.
Patients with asthma and respiratory failure/oxygen therapy showed 1.17-fold (95% CI of 1.05–1.33, p = 0.0070) and 1.67-fold (95% CI of 1.23–2.25, p = 0.0009) increased risk of reinfection, respectively, compared with non-reinfected individuals.
Among patients with neuropathy, the risk of reinfection was almost twice as high for those suffering from epilepsy (OR of 1.47, 95% CI of 1.18–1.83, p = 0.0006), Parkinson’s and Parkinsonism disease (OR of 1.54, 95% CI of 1.16–2.04, p = 0.0029) and Alzheimer’s disease (OR of 1.44, 95% CI of 1.07–1.93, p = 0.0159). The risk was about four times higher for people with dementia (OR of 3.71, 95% CI of 3.04–4.52, p < 0.0001). Finally, in individuals suffering from autoimmune disease, Hashimoto’s thyroiditis was the most implicated disease in reinfected individuals (OR of 1.19, 95% CI of 1.00–1.44, p = 0.0479) (Table 4).
Table 4.
Detail of the main comorbidities implicated in reinfected SARS-CoV-2 patients from September 2021 to May 2022.
| Comorbidity | OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|
| 03—Chronic renal failure | ||||
| 03A—Chronic renal failure—dialysis | 2.77 | 1.76 | 4.38 | <0.0001 |
| 03B—Chronic renal failure—no dialysis | 1.27 | 1.05 | 1.54 | 0.0143 |
| 07—Cardiovascular disease | ||||
| 07S1—Arterial hypertension | 0.75 | 0.70 | 0.79 | <0.0001 |
| 07S2—Ischemic heart disease | 0.97 | 0.84 | 1.12 | 0.6772 |
| 07S3—Valvular heart disease | 0.99 | 0.80 | 1.24 | 0.9882 |
| 07S4—Arrhythmic myocardiopathy | 1.03 | 0.89 | 1.19 | 0.6850 |
| 07S5—Non-arrhythmic myocardiopathy | 1.08 | 0.89 | 1.31 | 0.4125 |
| 07S6—Heart failure | 1.24 | 1.04 | 1.47 | 0.0184 |
| 07V1—Arterial vasculopathy | 1.19 | 0.94 | 1.51 | 0.1575 |
| 07V2—Venous vasculopathy | 0.99 | 0.60 | 1.63 | 0.9630 |
| 07V3—Cerebral vasculopathy | 1.49 | 1.29 | 1.71 | <0.0001 |
| 08—Bronchopneumopathy | ||||
| 08A—Asthma | 1.17 | 1.05 | 1.33 | 0.0070 |
| 08B—Chronic obstructive pulmonary disease | 1.09 | 0.94 | 1.25 | 0.2636 |
| 08C—Respiratory failure/oxygen therapy | 1.67 | 1.23 | 2.25 | 0.0009 |
| 10—Neuropathy | ||||
| 10A—Epilepsy | 1.47 | 1.18 | 1.83 | 0.0006 |
| 10B—Parkinson’s and Parkinsonisms disease | 1.54 | 1.16 | 2.04 | 0.0029 |
| 10C—Alzheimer’s disease | 1.44 | 1.07 | 1.93 | 0.0159 |
| 10D—Multiple sclerosis | 1.02 | 0.68 | 1.54 | 0.9237 |
| 10E—Optic neuromyelitis | - | - | - | - |
| 10F—Dementia | 3.71 | 3.04 | 4.52 | <0.0001 |
| 11—Autoimmune disease | ||||
| 11A—Rheumatoid arthritis | 1.39 | 0.97 | 1.97 | 0.0698 |
| 11B—Systemic lupus erythematosus | 0.77 | 0.29 | 2.10 | 0.6151 |
| 11C—Systemic sclerosis | 1.42 | 0.66 | 3.06 | 0.3674 |
| 11D—Sjogren’s disease | 1.45 | 0.63 | 3.32 | 0.3780 |
| 11E—Ankylosing spondylitis | 0.85 | 0.31 | 2.30 | 0.7442 |
| 11F—Myasthenia gravis | 1.12 | 0.35 | 3.61 | 0.8381 |
| 11G—Hashimoto’s thyroiditis | 1.19 | 1.00 | 1.44 | 0.0479 |
| 11H—Immune hemolytic anemias | 0.70 | 0.09 | 5.14 | 0.7265 |
| 11I—Psoriasis and psoriatic arthropathy | 1.14 | 0.73 | 1.80 | 0.5561 |
Overall, the death rate was 7.14 per 1000 first infections (2280 dead individuals without reinfection) and 5.66 per 1000 reinfections (89 dead individuals with reinfection).
Among individuals who died of SARS-CoV-2, reinfected individuals had a two-fold higher risk of having neuropathy than non-reinfected individuals (OR of 2.31, 95% CI of 1.43–3.75, p = 0.0007), with a four times higher risk for people with dementia (OR of 3.57, 95% CI of 2.02–6.30, p < 0.0001). After adjusting data by age and gender, neuropathy and dementia resulted not to be associated with a higher risk of reinfection (Table 5).
Table 5.
Comorbidities implicated in dead reinfected SARS-CoV-2 patients from September 2021 to May 2022.
| Comorbidity | Unadjusted OR | Lower 95% CI | Upper 95% CI | p-Value | Adjusted OR | Lower 95% CI | Upper 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|
| 02—Transplant | - | - | - | - | - | - | - | - |
| 03—Chronic renal failure | 1.23 | 0.65 | 2.35 | 0.5247 | 1.17 | 0.58 | 2.35 | 0.6662 |
| 04—HIV/AIDS | - | - | - | - | - | - | - | - |
| 05—Cancer | 0.80 | 0.46 | 1.38 | 0.4230 | 0.79 | 0.45 | 1.40 | 0.4320 |
| 06—Diabetes | 0.93 | 0.53 | 1.63 | 0.7935 | 0.85 | 0.47 | 1.54 | 0.5899 |
| 07—Cardiovascular disease | 1.44 | 0.94 | 2.21 | 0.0938 | 1.43 | 0.88 | 2.33 | 0.1515 |
| 08—Bronchopneumopathy | 1.15 | 0.62 | 2.14 | 0.6583 | 1.13 | 0.59 | 2.16 | 0.7085 |
| 09—Gastroenteropathy | 0.85 | 0.44 | 1.61 | 0.6079 | 0.79 | 0.41 | 1.56 | 0.5105 |
| 10—Neuropathy | 2.31 | 1.43 | 3.75 | 0.0007 | 1.47 | 0.41 | 5.18 | 0.5524 |
| 10A—Epilepsy | 1.36 | 0.32 | 5.71 | 0.6778 | 0.75 | 0.15 | 3.84 | 0.7306 |
| 10B—Parkinson’s And Parkinsonisms | 1.82 | 0.77 | 4.29 | 0.1694 | 1.28 | 0.38 | 4.34 | 0.6968 |
| 10C—Alzheimer’s | 1.27 | 0.50 | 3.20 | 0.6109 | 0.77 | 0.24 | 2.51 | 0.6686 |
| 10D—Multiple sclerosis | - | - | - | - | - | - | - | - |
| 10E—Optic neuromyelitis | - | - | - | - | - | - | - | - |
| 10F—Dementia | 3.57 | 2.02 | 6.30 | <0.0001 | 2.49 | 0.78 | 7.99 | 0.1221 |
| 11—Autoimmune disease | 1.09 | 0.26 | 4.57 | 0.9039 | 0.91 | 0.21 | 3.99 | 0.9042 |
| 12—Endocrine and metabolic disease | 0.93 | 0.53 | 1.64 | 0.8015 | 0.89 | 0.49 | 1.61 | 0.6983 |
| 13—Rare disease | 1.51 | 0.19 | 11.49 | 0.6891 | 1.65 | 0.21 | 13.09 | 0.6359 |
| 14—Psychosis | 1.43 | 0.34 | 6.05 | 0.6244 | 0.82 | 0.18 | 3.68 | 0.7988 |
Adjusted ORs include all factors in the table adjusted for age and gender.
4. Discussion
The impact of the immune system memory developed after SARS-CoV-2 infection and reinfection is an object of interest for the scientific community. Immune memory to SARS-CoV-2 can be generated by natural infection, vaccination or hybrid immunity (combination of infection-induced immunity and vaccine-induced immunity) and can provide sustained protection against disease by means T-cell memory, B-cell memory and long-lasting antibody responses [11]. Evidence from large observational studies in healthcare workers and the general population suggests that SARS-CoV2-immunity post-infection confers a level of protection against COVID-19 [12,13,14].
The risk of reinfection depends on immune status, infection severity, cross-immunity, age and other immunological factors such as T-cell and B-cell memory or lack of antibody neutralizing capacity [15].
Our study results showed that SARS-CoV-2 reinfection had a similar trend to that observed for primary infection, showing an increasing trend with the spread of the Omicron variant. In particular, the risk of reinfection during the Omicron phase was found to be 4.89 times higher compared with during the Delta phase. Despite the marked increase in the reinfection rates during the Omicron wave, the deaths decreased and remained close to zero values. Our findings are consistent with other studies suggesting that the Omicron variant may evade prior immunity through infection or vaccination but less frequently causes severe clinical outcomes [16,17,18,19]. Indeed, the effect of vaccination on the reduction in the death risk was found in several studies during the Omicron wave [20,21].
Reinfection appears to occur more frequently among women. As some authors reported, the highest risk among women seems to be due to their greater exposure to the infectious disease in work activities, especially in occupations that require proximity to other individuals, such as in the educational or medical fields [22,23].
According to our findings, healthcare workers are at an increased risk of SARS-CoV-2 reinfection, which could be plausible given the high risk of front-line work exposure to COVID-19 among these individuals [24,25,26,27,28]. Indeed, healthcare workers are heavily exposed to SARS-CoV-2 during their work. Although personal protective equipment such as masks protect healthcare workers from viral infections, they may not always protect them due to improper donning and the removal of previously contaminated masks if proper hand hygiene and disinfection are not performed [29]. Thus, the lack of adequate infection control measures represents another potential reason for protective failures and nosocomial transmission. Personal protective equipment and hand hygiene have been shown to be essential in the protection from respiratory viruses, and hand hygiene is the primary recommended measure by the WHO to control cross-infection [30,31], but the frequency of emergency procedures performed in healthcare facilities during the COVID-19 pandemic was high, causing a minimized use of these infection control measures and decreased risk perception [29]. The current COVID-19 pandemic has strained healthcare workers around the world affecting their daily work. Healthcare workers, especially nurses, are typically the professional group that spends the most time in direct contact with patients compared with other healthcare workers, and it is essential to maintain the quality of their working lives in order for quality care and good patient outcomes to be achieved [32].
Thus, national health authorities should support or increase manpower, especially by recruiting new healthcare workers, providing appropriate personal protective equipment kits during treatment or exposure to infected patients, minimizing work pressure, highly motivating healthcare workers and recognizing the responsibilities of different healthcare professions [33].
Immunization has shown a protective effect against reinfection, especially among those vaccinated for less than 120 days. This effect can be explained by the high vaccination coverage achieved in Italy and by the effectiveness of the vaccine in preventing the onset of SARS-CoV-2 infection and in preventing cases of severe disease [34].
Furthermore, the risk of COVID-19 reinfection was found to be significantly lower in those vaccinated after the primary cycle or subsequent doses than in those vaccinated with a single dose, confirming the need for a complete vaccination cycle whenever possible, also taking into account the duration of immunity against COVID-19 infection, an important aspect that is not yet fully understood [35], raising the risk of COVID-19 re-infection.
Our data confirm the robustness of the applied vaccination strategies that recommend that one of the objectives of COVID-19 vaccination campaigns continues to be to protect health systems, reducing COVID-19 hospitalization, severe disease and death. Improving vaccine uptake in eligible individuals who are yet to receive them remains a priority, especially for population groups at higher risk of severe outcomes [36].
The evaluation of the risk of reinfection by age group showed a lower reinfection risk in individuals aged 60–79 years; this is probably linked to their behaviors. Indeed, the association between age and the adoption of preventive measures has been examined in other studies, and it has shown inconsistencies across different countries and during different pandemics [37,38,39,40]. However, some authors have attributed hygiene practices and social distancing to greater compliance with government regulations and greater fear of infection due to greater vulnerability among those aged 65 years and older than among younger people aged 18–34 years [37,41]. The current study also revealed that 18.28% of the individuals had one or more comorbidities and that the most implicated in reinfection were chronic renal failure, cardiovascular diseases, bronchopneumopathy, neuropathy and autoimmune diseases, especially in individuals on dialysis and with heart failure, cerebrovascular disease, asthma, respiratory failure/oxygen therapy, epilepsy, Parkinson’s and Parkinsonism disease, Alzheimer’s disease, dementia and Hashimoto’s thyroiditis. It is worth noting that dialysis patients, being dependent on regular treatment sessions, often using public transportation, expose themselves to the risk of community-transmitted infection and have contacts with healthcare workers, potential sources of transmission. These patients are at a higher risk of worsened prognoses with COVID-19, especially those with additional comorbidities, and are particularly susceptible to SARS-CoV-2 infection, as treatment necessitates frequent visits to outpatient dialysis units [42,43].
Although several scientific studies have reported an association between certain comorbidities and the development of severe forms of SARS-CoV-2 infection [44,45,46,47,48,49,50], literature studies on the association between comorbidities and the risk of reinfection are limited.
A systematic review published in 2022, reporting data from 1 December 2019 to 1 September 2021, described that hypertension and obesity were the most common among reinfected patients, followed by end-stage renal failure, asthma, chronic obstructive pulmonary disease (COPD), dementia, dyslipidemia and type 2 diabetes [51].
Other studies have also reported a higher risk of reinfection in individuals with end-stage renal failure, hypertension, diabetes, chronic respiratory disease, liver disease and a history of cardiovascular disease [52,53,54,55].
To our knowledge, this is one of the first literature studies to assess risk factors in SARS-CoV-2-reinfected individuals considering a large subset; the epidemiological burden of SARS-CoV-2 reinfections and the role played by risk factors for reinfections have immediate implications for public health policy, and risk-based prevention strategy identification is an appropriate and necessary aspect to consider.
The present study has some limitations. Although healthcare workers, especially those in high-risk occupations, are highly responsible and routinely screened, and the reinfection rate may not be very high, the large number of asymptomatic, undetected primary infected patients may underestimate the reinfection rate.
Thus, reinfection is more difficult to document when asymptomatic cases of reinfections are included, and precisely for this reason, it is essential that countries continuously monitor rates and patterns of reinfection to guide policy choices to strengthen data management systems.
It is, therefore, crucial to maintain the microbiological and epidemiological surveillance of COVID-19 [56], which continuously and systematically collects, compares, and analyzes information on all cases of SARS-CoV-2 infection confirmed by molecular and antigen diagnosis at regional reference laboratories in Italy. This is a necessary and useful observational tool both to inform citizens about the impact and evolution of the epidemic and to support decision-making in the public health response of health authorities.
Furthermore, as the SARS-CoV-2 virus continues to evolve, VOCs may increase transmissibility and reduce the effectiveness of COVID-19 vaccines. Therefore, laboratories around the world need to continue genotyping SARS-CoV-2 variants.
To summarize the study results, recurrent and severe SARS-CoV-2 infections tend to occur more frequently in unvaccinated individuals, those with weak immunity, and those with pre-existing health conditions, who have reduced access to services and quality healthcare and who live and work in environments that increase their risk of infection. As COVID-19 revealed significant differences in outcomes among high-risk populations, pandemics also provide an opportunity for policymakers to take steps to reduce inequalities in the long term based on the latest findings. Furthermore, our findings suggest that health systems must focus on community interventions to prevent the spread of infection to vulnerable populations, especially high-risk groups, and the need to maintain epidemiological and virological surveillance for policymaking and response activities to prevent the impact on health services.
5. Conclusions
Considering the different epidemic phases, the study results showed a five times higher risk of reinfection during the Omicron phase compared with the circulation phase of the Delta variant. Regardless of the predominant variant, being unvaccinated was the most relevant risk factor for reinfection. Additionally, healthcare workers showed a two-fold higher risk of SARS-CoV-2 reinfection rather than non-healthcare workers.
It is important to underline the weight of some comorbidities in individuals with reinfection, such as chronic renal failure, cardiovascular diseases, bronchopneumopathy, gastroenteropathy, neuropathy and autoimmune diseases.
These results are relevant for identifying the best prevention strategies in a pandemic context characterized by a high circulation of the SARS-CoV-2 virus and a high rate of pathogen mutations.
Author Contributions
Conceptualization, F.A.; methodology, M.F.P., D.A. and F.A.; validation, M.F.P., D.A. and F.A.; formal analysis, M.F.P., D.A. and F.M.; data curation, M.F.P., D.A. and F.M.; writing—original draft preparation, M.F.P. and D.A.; writing—review and editing, M.F.P., D.A., F.M., C.P., M.A., F.G., A.B., C.S., R.L., G.B.A., G.I., A.O. and F.A.; supervision, F.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Data collected in the administrative healthcare data regional service are transmitted by Ligurian Local Health Units and hospitals to the regional health agency of Liguria, “Azienda Ligure Sanitaria” (A.Li.Sa.). Thus, ethical review and approval were waived for this study because the institutional activities of A.Li.Sa. include handling regional healthcare administrative data and conducting epidemiological studies, projects and research studies to support strategical choices in healthcare [57]. The current regulation on privacy allows professionals belonging to the regional A.Li.Sa. to access healthcare administrative data routinely transmitted by publicly funded LHUs and hospitals in Liguria Region. Data are anonymous and were evaluated in an aggregate manner. Finally, patients who have access to regional public health services give consent to the use of healthcare data also for scientific purposes.
Informed Consent Statement
Administrative healthcare data, or Data Warehouse, is a regional service that collects hospital discharge records (HDRs), the flow of outpatient visits and pharmaceutical consumption and was used as a data source. HDR data are recorded with the consent of the patient and can be used for scientific studies in the form of aggregated and de-identified data.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO WHO Coronavirus Disease (COVID-19) Situation Dashboard. [(accessed on 14 June 2022)]. Available online: https://covid19.who.int/
- 2.Islam M.R., Hoque M.N., Rahman M.S., Alam A., Akther M., Puspo J.A., Akter S., Sultana M., Crandall K.A., Hossain M.A. Genome-wide analysis of SARS-CoV-2 virus strains circulating worldwide implicates heterogeneity. Sci. Rep. 2020;10:14004. doi: 10.1038/s41598-020-70812-6. Erratum in Sci. Rep. 2021, 11, 20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To K.K., Hung I.F., Ip J.D., Chu A.W., Chan W.M., Tam A.R., Fong C.H., Yuan S., Tsoi H.W., Ng A.C., et al. Coronavirus Disease 2019 (COVID-19) Re-infection by a Phylogenetically Distinct Severe Acute Respiratory Syndrome Coronavirus 2 Strain Confirmed by Whole Genome Sequencing. Clin. Infect. Dis. 2021;73:e2946–e2951. doi: 10.1093/cid/ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., Laverdure C., Verma S.C., Rossetto C.C., Jackson D., et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgogna C., De Andrea M., Griffante G., Lai A., Bergna A., Galli M., Zehender G., Castello L., Ravanini P., Cattrini C., et al. SARS-CoV-2 reinfection in a cancer patient with a defective neutralizing humoral response. J. Med. Virol. 2021;93:6444–6446. doi: 10.1002/jmv.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Italian Ministry of Health [(accessed on 2 November 2022)];Circolare N. 0037911 of 20 August 2021. Flusso Dati Aggregati Ministero Della Salute/Protezione Civile: Aggiornamento Sulla Possibilità di Inserimento Delle Reinfezioni da SARS-CoV-2. Available online: https://www.seremi.it/sites/default/files/GR3917-000127.pdf.
- 7.Stefanelli P., Trentini F., Petrone D., Mammone A., Ambrosio L., Manica M., Guzzetta G., Andrea V.D., Marziano V., Zardini A., et al. Tracking the progressive spread of the SARS-CoV-2 Omicron variant in Italy, December 2021–January 2022. medRxiv. 2022 doi: 10.1101/2022.01.27.22269949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Istituto Superiore di Sanità (ISS) Estimation of the Prevalence of Variants of Concern (VOC) and Other Variants of SARS-CoV-2 in Italy (Survey Dated 4 Apr 2022) ISS; Rome, Italy: 2022. [(accessed on 14 June 2022)]. Available online: https://www.iss.it/documents/20126/0/Relazione+tecnica+Flash+4+Aprile+2022.pdf/f32164a2-2115-7b38-88aa-64a2f355b42e?t=1649933427680. [Google Scholar]
- 9.Report Esteso ISS COVID-19: Sorveglianza, Impatto Delle Infezioni ed Efficacia Vaccinale Aggiornamento Nazionale 25/05/2022—Ore 12:00. [(accessed on 22 August 2022)]. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_25-maggio-2022.pdf.
- 10.Rivelli A., Fitzpatrick V., Blair C., Copeland K., Richards J. Incidence of COVID-19 reinfection among Midwestern healthcare employees. PLoS ONE. 2022;17:e0262164. doi: 10.1371/journal.pone.0262164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sette A., Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol. Rev. 2022;310:27–46. doi: 10.1111/imr.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vitale J., Mumoli N., Clerici P., De Paschale M., Evangelista I., Cei M., Mazzone A. Assessment of SARS-CoV-2 Reinfection 1 Year After Primary Infection in a Population in Lombardy, Italy. JAMA Intern. Med. 2021;181:1407–1408. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumley S.F., O’Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., Marsden B.D., Cox S., James T., Warren F., et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N. Engl. J. Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. Erratum in Lancet 2021, 397, 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend J.P., Hassler H.B., Wang Z., Miura S., Singh J., Kumar S., Ruddle N.H., Galvani A.P., Dornburg A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe. 2021;2:e666–e675. doi: 10.1016/S2666-5247(21)00219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feikin D.R., Abu-Raddad L.J., Andrews N., Davies M.A., Higdon M.M., Orenstein W.A., Patel M.K. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40:3516–3527. doi: 10.1016/j.vaccine.2022.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauring A.S., Tenforde M.W., Chappell J.D., Gaglani M., Ginde A.A., McNeal T., Ghamande S., Douin D.J., Talbot H.K., Casey J.D., et al. Clinical Severity and mRNA Vaccine Effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: A Prospective Observational Study. medRxiv. 2022 doi: 10.1101/2022.02.06.22270558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600:367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- 19.Auvigne V., Vaux S., Strat Y.L., Schaeffer J., Fournier L., Tamandjou C., Montagnat C., Coignard B., Levy-Bruhl D., Parent du Châtelet I. Severe hospital events following symptomatic infection with SARS-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: A retrospective, population-based, matched cohort study. eClinicalMedicine. 2022;48:101455. doi: 10.1016/j.eclinm.2022.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva-Valencia J., Soto-Becerra P., Escobar-Agreda S., Fernandez-Navarro M., Elorreaga O.A., Mayta-Tristán P., Mezones-Holguin E., Solari L. Relative vaccine effectiveness of the booster dose of COVID-19 vaccine for preventing death in individuals with a primary regimen based on the BBIBP-CorV, ChAdOx1-S, or BNT162b2 vaccines during the Omicron wave in Peru: A nested case-control study using national population data. Vaccine. 2022;40:6512–6519. doi: 10.1016/j.vaccine.2022.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson A.G., Amin A.B., Ali A.R., Hoots B., Cadwell B.L., Arora S., Avoundjian T., Awofeso A.O., Barnes J., Bayoumi N.S., et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71:132–138. doi: 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballering A.V., Oertelt-Prigione S., Olde Hartman T.C., Rosmalen J.G.M. Sex and gender-related differences in COVID-19 diagnoses and SARS-CoV-2 testing practices during the first wave of the pandemic: The Dutch lifelines COVID-19 cohort study. J. Womens Health. 2021;30:1686–1692. doi: 10.1089/jwh.2021.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marconi M. Gender differences in COVID-19: The importance of sex-disaggregated data. Ital. J. Gend.-Specif. Med. 2021;7:4–6. [Google Scholar]
- 24.Bielicki J.A., Duval X., Gobat N., Goossens H., Koopmans M., Tacconelli E., van der Werf S. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect. Dis. 2020;20:e261–e267. doi: 10.1016/S1473-3099(20)30458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veronica F., Anne R., Christopher B., Kenneth C., Jon R. Incidence of COVID-19 recurrence among large cohort of healthcare employees. Ann. Epidemiol. 2021;60:8–14. doi: 10.1016/j.annepidem.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett E.S., Horton D.B., Roy J., Xia W., Greenberg P., Andrews T., Gennaro M.L., Parmar V., Russell W.D., Reilly N., et al. Risk Factors for Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Hospital Workers: Results from a Screening Study in New Jersey, United States in Spring 2020. Open Forum Infect. Dis. 2020;7:ofaa534. doi: 10.1093/ofid/ofaa534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen L.H., Drew D.A., Graham M.S., Joshi A.D., Guo C.G., Ma W., Mehta R.S., Warner E.T., Sikavi D.R., Lo C.H., et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health. 2020;5:e475–e483. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murillo-Zamora E., Trujillo X., Huerta M., Ríos-Silva M., Aguilar-Sollano F., Mendoza-Cano O. Symptomatic SARS-CoV-2 reinfection: Healthcare workers and immunosuppressed individuals at high risk. BMC Infect. Dis. 2021;21:923. doi: 10.1186/s12879-021-06643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brainard J.S., Jones N., Lake I., Hooper L., Hunter P. Facemasks and similar barriers to prevent respiratory illness such as COVID-19: A rapid systematic review. medRxiv. 2020 doi: 10.1101/2020.04.01.20049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Infection Prevention and Control during Health Care When Novel Coronavirus (nCoV) Infection Is Suspected. n.d. [(accessed on 14 June 2022)]. Available online: https://www.who.int/publications/i/item/10665-331495.
- 31.Musu M., Lai A., Mereu N.M., Galletta M., Campagna M., Tidore M., Piazza M.F., Spada L., Massidda M.V., Colombo S., et al. Assessing hand hygiene compliance among healthcare workers in six Intensive Care Units. J. Prev. Med. Hyg. 2017;58:E231–E237. [PMC free article] [PubMed] [Google Scholar]
- 32.Galletta M., Portoghese I., Pili S., Piazza M.F., Campagna M. The effect of work motivation on a sample of nurses in an Italian healthcare setting. Work. 2016;54:451–460. doi: 10.3233/WOR-162327. [DOI] [PubMed] [Google Scholar]
- 33.Kandula U.R., Wake A.D. Assessment of Quality of Life Among Health Professionals During COVID-19: Review. J. Multidiscip. Healthc. 2021;14:3571–3585. doi: 10.2147/JMDH.S344055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Report Vaccini Anti COVID-19. [(accessed on 14 June 2022)]; Available online: https://www.governo.it/it/cscovid19/report-vaccini/
- 35.Cohen J.I., Burbelo P.D. Reinfection with SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis. 2020;18:ciaa1866. doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Centre for Disease Prevention and Control Preliminary Public Health Considerations for COVID-19 Vaccination Strategies in the Second Half of 2022. [(accessed on 14 September 2022)]. Available online: https://www.ecdc.europa.eu/en/publications-data/preliminary-public-health-considerations-covid-19-vaccination-strategies-second.
- 37.Atchison C.J., Bowman L., Vrinten C., Redd R., Pristera P., Eaton J.W., Ward H. Perceptions and behavioural responses of the general public during the COVID-19 pandemic: A cross-sectional survey of UK Adults. medRxiv. 2020 doi: 10.1101/2020.04.01.20050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.JTF L., Yang X., Tsui H., Kim J.H. Monitoring community responses to the SARS epidemic in Hong Kong: From day 10 to day 62. J. Epidemiol. Commun. Health. 2003;57:864–870. doi: 10.1136/jech.57.11.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leung G.M., Lam T.H., Ho L.M., Ho S.Y., Chan B.H.Y., Wong I.O.L., Hedley A.J. The impact of community psychological responses on outbreak control for severe acute respiratory syndrome in Hong Kong. J. Epidemiol. Commun. Health. 2003;57:857–863. doi: 10.1136/jech.57.11.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seale H., Heywood A.E., Leask J., Sheel M., Thomas S., Durrheim D.N., Bolsewicz K., Kaur R. COVID-19 is rapidly changing: Examining public perceptions and behaviors in response to this evolving pandemic. PLoS ONE. 2020;15:e0235112. doi: 10.1371/journal.pone.0235112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atchison C., Bowman L., Eaton J., Imai N., Redd R., Pristera P., Vrinten C., Ward H. Report 10: Public response to UK government recommendations on COVID-19: Population survey, 17–18 march 2020. 2020. [(accessed on 2 November 2022)]. Available online: http://hdl.handle.net/10044/1/77581.
- 42.Guidotti R., Pruijm M., Ambühl P.M. COVID-19 Pandemic in Dialysis Patients: The Swiss Experience. Front. Public Health. 2022;10:795701. doi: 10.3389/fpubh.2022.795701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salerno S., Messana J.M., Gremel G.W., Dahlerus C., Hirth R.A., Han P., Segal J.H., Xu T., Shaffer D., Jiao A., et al. COVID-19 Risk Factors and Mortality Outcomes Among Medicare Patients Receiving Long-term Dialysis. JAMA Netw. Open. 2021;4:e2135379. doi: 10.1001/jamanetworkopen.2021.35379. Erratum in JAMA Netw. Open 2022, 5, e2219305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murillo-Zamora E., Trujillo X., Huerta M., Ríos-Silva M., Mendoza-Cano O. Male gender and kidney illness are associated with an increased risk of severe laboratory-confirmed coronavirus disease. BMC Infect. Dis. 2020;20:674. doi: 10.1186/s12879-020-05408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen N.N., Houhamdi L., Hoang V.T., Delerce J., Delorme L., Colson P., Brouqui P., Fournier P.E., Raoult D., Gautret P. SARS-CoV-2 reinfection and COVID-19 severity. Emerg. Microbes Infect. 2022;11:894–901. doi: 10.1080/22221751.2022.2052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mensah A.A., Lacy J., Stowe J., Seghezzo G., Sachdeva R., Simmons R., Bukasa A., O’Boyle S., Andrews N., Ramsay M., et al. Disease severity during SARS-CoV-2 reinfection: A nationwide study. J. Infect. 2022;84:542–550. doi: 10.1016/j.jinf.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi J.H., Choi S.H., Yun K.W. Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J. Korean Med. Sci. 2022;37:e35. doi: 10.3346/jkms.2022.37.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachelard A., Sautereau A., Digumber M., Isernia V., Phung B., Lehur A.C., Gac S.L., Landman R., Yazdanpanah Y., Ghosn J. Risk Factors Associated with Severe/Critical COVID-19 in People Living with HIV-1. Int. J. Infect. Dis. 2022;122:152–154. doi: 10.1016/j.ijid.2022.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etemadifar M., Nouri H., Maracy M.R., Akhavan Sigari A., Salari M., Blanco Y., Sepúlveda M., Zabalza A., Mahdavi S., Baratian M., et al. Risk factors of severe COVID-19 in people with multiple sclerosis: A systematic review and meta-analysis. Rev. Neurol. 2022;178:121–128. doi: 10.1016/j.neurol.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozel A.S., Altunal L.N., Aydin M., Unal B., Cam G., Caglar Ozer M., Korten V. Clinical characteristics and risk factors associated with severe disease and outcome of patients with COVID-19. J. Infect. Dev. Ctries. 2022;16:435–444. doi: 10.3855/jidc.15411. [DOI] [PubMed] [Google Scholar]
- 51.Ren X., Zhou J., Guo J., Hao C., Zheng M., Zhang R., Huang Q., Yao X., Li R., Jin Y. Reinfection in patients with COVID-19: A systematic review. Glob. Health Res. Policy. 2022;7:12. doi: 10.1186/s41256-022-00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu H., Fu L., Jin Y., Shao J., Zhang S., Zheng N., Fan L., Yu Z., Ying J., Hu Y., et al. Clinical features of COVID-19 convalescent patients with re-positive nucleic acid detection. J. Clin. Lab. Anal. 2020;34:e23392. doi: 10.1002/jcla.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu J., Peng J., Xiong Q., Liu Z., Lin H., Tan X., Kang M., Yuan R., Zeng L., Zhou P., et al. immunological and virological characterization of COVID-19 patients that test re-positive for SARS-CoV-2 by RT-PCR. eBioMedicine. 2020;59:102960. doi: 10.1016/j.ebiom.2020.102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian M., Long Y., Hong Y., Zhang X., Zha Y. The treatment and follow-up of ‘recurrence’ with discharged COVID-19 patients: Data from Guizhou, China. Environ. Microbiol. 2020;22:3588–3592. doi: 10.1111/1462-2920.15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen S.L., Xu H., Feng H.Y., Sun J.F., Li X., Zhou L., Song W.L., Huang S.S., He J.L., Deng Y.Y., et al. Epidemiological and Clinical Findings of Short-Term Recurrence of Severe Acute Respiratory Syndrome Coronavirus 2 Ribonucleic Acid Polymerase Chain Reaction Positivity in 1282 Discharged Coronavirus Disease 2019 Cases: A Multicenter, Retrospective, Observational Study. Open Forum Infect. Dis. 2020;7:ofaa432. doi: 10.1093/ofid/ofaa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sistema di Sorveglianza Integrata COVID-19. [(accessed on 14 June 2022)]. Available online: https://www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza.
- 57.Regional Law 29/07/2016, N.17. Istituzione dell’Azienda Ligure Sanitaria della Regione Liguria (A.Li.Sa.) e Indirizzi per il Riordino Delle Disposizioni Regionali in Materia Sanitaria e Sociosanitaria. Bollettino Ufficiale N.15, 30/07/2016. [(accessed on 13 June 2022)]. Available online: http://lrv.regione.liguria.it/liguriass_prod/articolo?urndoc=urn:nir:regione.liguria:legge:2016-07-29;17&pr=idx,0;artic,%201;articparziale,0http://lrv.regione.liguria.it/liguriass_prod/articolo?urndoc=urn:nir:regione.liguria:legge:2016-07-29;17&pr=idx,0;artic,%201;articparziale,0.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.