Abstract
Breast cancer has been the most common cancer in women worldwide, and metastasis is the leading cause of death from breast cancer. Even though the study of breast cancer metastasis has been extensively carried out, the molecular mechanism is still not fully understood, and diagnosis and prognosis need to be improved. Breast cancer metastasis is a complicated process involving multiple physiological changes, and lung, brain, bone and liver are the main metastatic targets. Exosomes are membrane-bound extracellular vesicles that contain secreted cellular constitutes. The biogenesis and functions of exosomes in cancer have been intensively studied, and mounting studies have indicated that exosomes play a crucial role in cancer metastasis. In this review, we summarize recent findings on the role of breast cancer-derived exosomes in metastasis organotropism and discuss the potential promising clinical applications of targeting exosomes as novel strategies for breast cancer diagnosis and therapy.
Keywords: breast cancer, metastasis, exosome, extracellular vesicles, organotropism
1. Introduction
Breast cancer is a common frequently occurring malignancy among women worldwide, and it has surpassed lung cancer to become the most diagnosed cancer all over the world with around 2.3 million new cases, accounting for 11.7% of all cancer cases and 24.5% of female cancers [1]. With recent advances in early diagnosis and therapeutic strategies including neoadjuvant therapy, endocrine therapy, molecular targeted therapy, and immunotherapy [2,3], the prognosis of breast cancer has greatly improved. However, breast cancer patients with distant metastasis have worse outcomes, and the five-year survival rate was less than 30% [4]. With approximately 685,000 deaths in 2020, it remains the first leading cause of cancer death in women [1]. Therefore, there is an urgent need to understand the molecular mechanisms underlying breast cancer metastasis for developing novel therapeutic strategies. Exosomes, as one type of extracellular vesicle (EVs), have been reported to play a crucial role in cancer metastasis, namely, contributing to form pre-metastatic niches, influence the tumor microenvironment, and identify specific organotropic metastasis. Here, we endeavor to highlight the role of tumor-derived exosomes in breast cancer metastasis, elucidate the underlying mechanism of metastasis organotropism mediated by exosomes, and prospect the potential application of exosomes in breast cancer therapeutics.
1.1. Breast Cancer Classification
Breast cancer develops from epithelial cells in the terminal duct lobular units and can be classified into two subtypes histologically, including ductal carcinoma in situ (DCIS) and invasive ductal carcinoma (IDC) [5,6]. According to the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 labelling index (Ki-67) which reflects the proliferation [7], breast cancer has four primary molecular subtypes, namely luminal A, luminal B, HER2-positive, and triple-negative breast cancer (TNBC) [5,8]. The luminal A subtype is ER/PR-positive, with lower levels of Ki-67 (<14), accounting for about 60–70% of diagnostic breast cancers. The luminal B subtype is ER-positive combined with HER-2 positive or Ki-67 high (≥14), accounting for about 10–20%. Both luminal A and luminal B breast cancer are likely to benefit from endocrine therapy, and patients with luminal A breast cancer have a better prognosis compared to luminal B. HER2-positive subtype is ER/PR-negative and HER2-positive, with a diagnostic rate of approximately 13−15%. This subtype can benefit from treatment targeted to HER2 and chemotherapy with good prognosis. TNBC is characterized by ER, PR, and HER2 negativity in 10−15% of cases. Breast cancer susceptibility gene 1 (BRCA1) is a major breast cancer suppressor gene that encodes a protein critical for maintaining genome stability; its mutation predisposes women to TNBC [9]. As BRCA1 mutation impairs homologous recombination repair, poly (ADP-ribose) polymerase (PARP) inhibitors have been approved as target therapy for metastatic TNBC [10]. However, due to its highly aggressive clinical properties, TNBC still has a poorer prognosis compared to other breast cancer subtypes [2,5,11,12]. Although the five-year survival rate for women diagnosed with breast cancer exceeds 90%, all breast cancer subtypes have the potential to exhibit adverse clinic features, such as high invasiveness and recurrence, mainly caused by metastasis [5].
1.2. Breast Cancer Metastasis
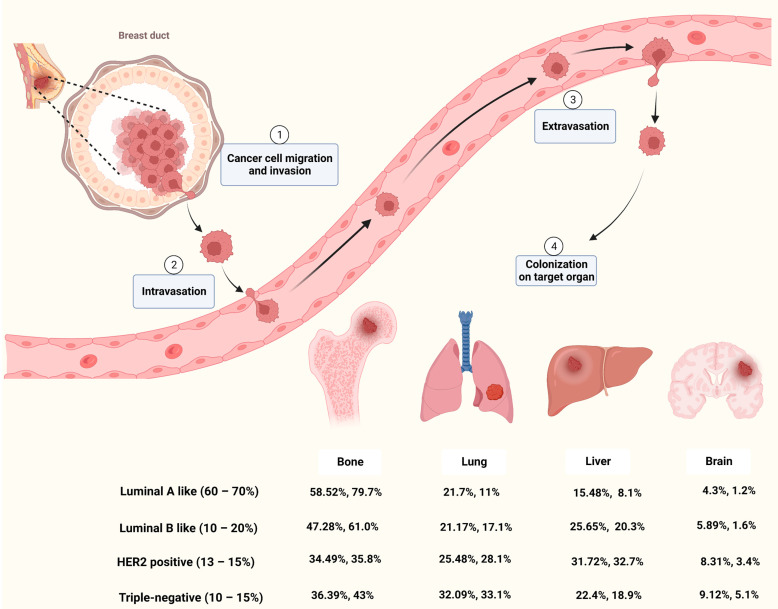
Metastasis occurs frequently and accounts for as much as 90% of cancer-related deaths [13]. Breast cancer metastasis is also frequently diagnosed and exhibits organ tropism to lung, brain, bone and liver, which is highly heterogeneous and affects treatment outcomes and patient prognosis [14]. As shown in Figure 1, breast cancer patients are most prone to bone metastasis, accounting for 50.7−68.8% of all metastatic cases with different molecular subtypes. The lung and liver were similar, with 16.0−23.9% and 13.3−19.7% of breast cancer metastasis occurring in the lung or liver, respectively. The incidence of brain metastasis is approximately 1.9−5.7% of all metastatic cases. Based on molecular subtypes, lung metastasis most commonly occurs in TNBC, accounting for about 32% of patients with metastasis, while the HER2-positive subtype is likely to have liver metastasis [15,16].
Figure 1.
Breast cancer metastasis to bone, lung, liver and brain [5,15,16]. Figure is created by BioRender.
Cancer metastasis is a complicated process that goes through multiple steps such as invasion, intravasation, extravasation and colonization on target organ (Figure 1). Metastasis of tumor cells to distant organs requires not only tumor cell invasiveness, but also a microenvironment that is conducive to tumor survival and proliferation in secondary organs [17]. Although the molecular mechanisms of breast cancer metastasis are not fully understood, mounting studies have shown that primary cancer cells could secrete factors to remodel the microenvironment of the target organ and prime it into a site favorable for cancer cell proliferation, known as pre-metastatic niche (PMN) [18,19,20]. Among these factors, EVs secreted by cancer cells play important roles in microenvironment remodeling and metastatic organotropism [21].
1.3. EVs and Exosome
EVs are membrane-bound vesicles secreted by various cells which play critical roles in cell-cell communication under physiological and pathological conditions [22]. In general, EVs could be divided into three types based on their morphology: exosomes (30–150 nm), micro-vesicles (150–1000 nm) and apoptotic bodies (500–2000 nm) [23]. Nowadays, EVs are considered non-negligible factors in cellular homeostasis and mediators of cancer metastasis [24]. Exosomes are small EVs with phospholipid bilayers whose heterogeneous “cargoes”, such as protein, lipids, RNA and DNA, vary from different types of cells [25,26]. These cargoes are located inside or on the surface of exosomes and mediate the communication between original cells and recipient cells [26].
Exosomes are generated originally from early endosomes by fusion of endocytic vesicles with plasma membranes. Early endosomes subsequently mature into late endosomes and form the multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs). MVBs could either fuse with plasma membrane for exocytosis of contained ILVs (namely exosomes) into the extracellular space, or with lysosomes or autophagosomes for degradation [27,28]. ILV formation is largely dependent on the endosomal sorting complex required for transport (ESCRT) function. ESCRT is an intricate machinery which is made up of five complexes, which include the ESCRT-0, -Ⅰ, -Ⅱ, -Ⅲ and Vps4-Vta1 complexes [27,29]. Beside the ESCRT-dependent pathway, an alternative pathway could also be involved in exosome biogenesis. Proteolipid proteins (PLP) could be incorporated into ILVs in a sphingolipid ceramide-dependent manner. Then, ceramide induces raft-based microdomains formation and coalescence and promotes the budding of ILVs [30]. Moreover, tetraspanins, such as CD63, CD81, CD9, could regulate ESCRT-independent sorting [27]. Therefore, ESCRT dependent and independent mechanisms co-exist and function synergistically in exosome formation.
In recent years, studies have found that cancer-derived exosome could promote the progression of cancer, including cancer metastasis and drug resistance [31]. Exosomes have been proved to be involved in various processes of cancer metastasis, such as vascular leakiness, epithelial-mesenchymal transition (EMT) induction, immune escape and PMN formation [24,32,33]. Integrins are cell surface adhesion molecules that could mediate cell signaling by interacting with components of the extracellular matrix [34]. It has been demonstrated that integrins on exosomes could guide them into different organs, thereby inducing PMN formation and promoting cancer metastasis. Exosomes with integrins α6β4 and α6β1 tend to accumulate in the lung, while integrin αvβ5 preferably drives exosomes into the liver [35]. In addition, comparative proteomics of exosomes derived from different breast cancer cells demonstrated that their exosomal proteins are heterogenous, which is associated with different cancer cell metastatic properties [36]. These studies suggest that exosomes play a crucial role in causing metastasis organotropism through various mechanisms. In the following section, we will summarize the contribution of exosomes to the tropism of breast cancer metastasis to different organs, namely bone, lung, liver and brain, and the metastatic models used in these findings (Table 1), which will help to comprehensively decipher how exosomes are involve in breast cancer metastasis.
Table 1.
Breast cancer models used to study the mechanism of exosomes promoting metastasis.
| Metastatic Organs | Exosomal Molecules | Cell Lines | Metastasis Mouse Model | Reference |
|---|---|---|---|---|
| Bone | miR-940 | MDA-MB-231 | Calvaria implantation | [37] |
| miR-218 | MDA-MB-231, MCF-7 | Tail vein injection of EVs | [38] | |
| miR-20a-5p | MDA-MB-231, MCF-7 | In vitro | [39] | |
| miR-21 | MDA-MB-231 | Orthotopic model and Caudal artery injection | [40] | |
| miR-19a, IBSP | MDA-MB-231, MCF7, T47D | Intra-cardiac model, intratibial implantation and orthotopic model | [41] | |
| L-plastin | MDA-MB-231 | Intratibial implantation | [42] | |
| CDH11, ITGA5 | MDA-MB-231, 4T1, MCF7 |
Intra-cardiac model and orthotopic model | [43] | |
| lung | miR-122 | MDA-MB-231, MCF10DCIS.com | Intra-cardiac model and orthotopic model | [44] |
| miR-138-5p | 4T1 | Tail vein injection | [45] | |
| miR-183-5p | 4T1 | Orthotopic model | [46] | |
| miR-200b-3p | 4T1 | Orthotopic model | [47] | |
| Let-7 | 4TO7 | Orthotopic model, tail vein injection and intra-cardiac model | [48] | |
| circPSMA1 | MDA-MB-231 | Orthotopic model | [49] | |
| CCL2 | EO771 | Tail vein injection | [50] | |
| TβRII | MDA-MB-231, 4T1, 4T07 | Intra-cardiac model, tail vein injection and orthotopic model | [51] | |
| Myosin-9 | MDA-MB-231 | Subcutaneous xenograft, orthotopic model | [52] | |
| MMPs | MDA-MB-231 | Tail vein injection, orthotopic model | [53] | |
| MMP-1 | MDA-MB-231 | Tail vein injection | [54] | |
| NDPK | MDA-MB-231 | Tail vein injection | [55] | |
| Annexin II | MDA-MB-231, MDA-MB-831, MDA-MB-4175 | Tail vein injection and intra-cardiac model | [56] | |
| Liver | miR-4443 | MCF-7, MDA-MB-231 | Orthotopic model | [57] |
| miR-197 | MBA-MB-231 or SUM149PT | Subcutaneous injection and tail vein injection | [58] | |
| E-cadherin, p120-catenin | MTPa | Orthotopic model (Rat) | [59] | |
| TGFβ1 | MCF7, MDA-MB-231 | In vitro | [60] | |
| Brain | miR-181c | MDA-MB-231 | Intra-cardiac model | [61] |
| lnc GS1-600G8.5 | MDA-MB-231 | Intra-cardiac model | [62] | |
| CEMIP | MDA-MB-231 | Intra-cardiac model, intracranial injection and orthotopic model | [63] | |
| miR-503 | MCF7, ZR75-1, SKBR3, MDA-MB-231 |
Intra-cardiac model and intracranial injection | [64] | |
| miR-301a-3p | MDA-MB-231 | Retro-orbital injection of EVs | [65] |
2. Exosomes and Breast Cancer Metastasis Organotropism
2.1. Exosomes Mediate Breast Cancer Metastasis to Bone
Bone is the most likely site for all types of breast cancer to metastasize. Patients with bone metastasis are often accompanied by other serious complications, such as severe bone pain, fractures, serious hypercalcemia, and nerve compression syndromes, which seriously affect the patients’ life expectancy and quality of life [66]. Bone metastasis involves a complicated interaction between cancer cells and bone microenvironments. Under normal circumstances, bone undergoes a dynamic balance of bone resorption and bone formation, mediated by osteoclasts and osteoblasts, respectively, while bone metastasis often exhibits a disordered balance of this process [67]. Therefore, there are mainly two types of bone metastasis, osteolytic and osteoblastic, depending on which cells are overactive [68]. Osteolytic bone metastasis frequently occurs in breast cancer, mainly due to the activation of the RANK-RANKL signaling pathway that mediates osteoclastogenesis. Breast cancer stimulates RANKL expression in osteoblasts by producing parathyroid hormone-related protein (PTHrP), which in turn leads to excessive osteolysis and promotes bone metastasis by activating RANK-RANKL signaling [66,67,69]. Besides PTHrP, many other factors such as calcium-sensing receptor (CaSR), TNF-α, and interleukins also affect bone metastasis [67].
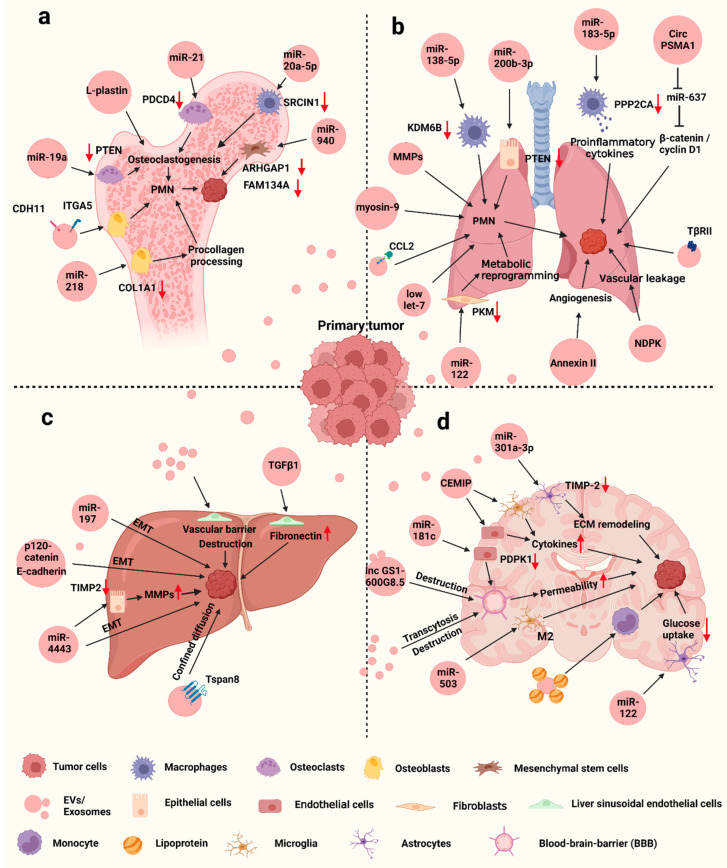
Furthermore, recent studies have shown that EVs, especially exosomes, play an important role in bone metastasis of breast cancer (Figure 2a). The main role is that exosomes can bring osteoclastogenesis-enhancing miRNA into bone, thereby promoting osteolytic metastasis. As an osteolytic phenotype-inducing cell, MDA-MB-231 could facilitate the osteogenic differentiation of mesenchymal stem cells via exosomal miR-940 targeting Rho GTPase activating protein 1 (ARHGAP1) and FAM134A [37]. MDA-MB-231 also secreted exosomal miR-218 to impede procollagen processing during osteoblast differentiation and tip the balance toward osteolysis to form a metastatic bone niche [38]. Guo et al. found that MDA-MB-231-derived exosomes could transfer miR-20a-5p to bone marrow macrophages, which then enhanced osteoclast proliferation and differentiation by targeting SRC Kinase Signaling Inhibitor 1 (SRCIN1) [39]. It is also reported that exosomal miR-21 produced from MDA-MB-231 derived cells with high bone metastatic ability could contribute to bone lesion and PMN formation via targeting programmed cell death 4 (PDCD4), which has an inhibitory function on osteoclast differentiation [40]. Unlike MDA-MB-231 cells, ER+ bone-tropic breast cancer cells could produce exosomal miR-19a, which promotes osteoclastogenesis and bone metastasis by suppressing PTEN expression and inducing NF-κB and AKT pathways [41].
Figure 2.
Exosomes mediate breast cancer metastasis to bone (a), lung (b), liver (c) and brain (d). Figure is created by BioRender.
Besides miRNAs, exosomes can also carry other “cargoes” to facilitate bone metastasis. L-plastin was found to be a soluble factor secreted from MDA-MB-231 cells, belonging to the actin-binding proteins family. L-plastin could be encapsulated in exosomes, which stimulated osteoclastogenesis and further promoted bone metastasis [42]. Breast cancer cells with high runt-related transcription factor 2 (RUNX2) expression could secrete EVs with high levels of cadherin 11 (CDH11) and integrin α5 (ITGA5), which synergistically promote osteogenic PMN formation. Mechanistically, CDH11 mediated tumor-derived EVs uptake by osteoblasts and ITGA5 was responsible for the formation of PMN that facilitated cancer cell colonization in bone [43]. Integrin-binding Sialoprotein (IBSP) secreted by ER+ bone-tropic breast cancer cells could bind to αvβ3 integrin and attract osteoclasts, assisting the delivery of exosomal miR-19a to osteoclast to induce bone metastatic lesions [41].
2.2. Exosomes Mediate Breast Cancer Metastasis to Lung
Lung is another target of frequent breast cancer metastasis. Pulmonary capillaries are composed of endothelial cells surrounded by a based membrane and adjacent alveolar cells. Tumor cells need to adhere to this endothelial membrane and extravasate into lung parenchyma to establish metastatic tumors [70]. The process of lung metastasis is affected by many factors, among which exosomes play a critical role in remodeling the immune microenvironment and inducing EMT (Figure 2b).
Tumor-derived exosomes promote lung metastasis via non-coding RNAs that mediate relevant signaling. Exosomal miR-122 was secreted by high metastatic breast cancer cells and increased nutrient availability in lung metastatic cancer cells by downregulating glycolytic enzyme pyruvate kinase (PKM) in lung fibroblasts [44]. Recent study further found that exosomal miR-122 could also target PKM in pancreatic β-cells to suppress insulin secretion and disrupt systemic glucose homeostasis to promote cancer progression [71]. Besides metabolism reprogramming in metastatic organ, exosome also mediated miRNA transfer from cancer cells to other cells for microenvironment remodeling and promoting the pre-metastatic niche formation in the lung. Exosomal miR-138-5p and miR-183-5p secreted by cancer cells could modulate tumor-associated macrophages’ (TAMs) activity to enhance lung metastasis via targeting KDM6B and PPP2CA, respectively [45,46]. miR-200b-3p was enriched in tumor-derived exosomes and transferred to lung, thereby increasing the expression of C-C motif chemokine ligand 2 (CCL2) by targeting PTEN. CCL2 could further recruit myeloid-derived suppressor cells (MDSC) and contribute to the establishment of an immunosuppressive microenvironment for metastasis [47]. However, exosomes that could enhance metastasis are not always enriched with miRNA. Lin28 is an RNA-binding protein that regulates the expression of miRNA let-7 family members and acts as a modulator for self-renewal of embryonic stem cells [72,73]. Lin28B is mainly expressed in TNBC and could promote breast cancer progression [74]. Recent studies indicate that Lin28B could increase breast cancer stem cell population, a major source of low let-7 exosomes. Moreover, these exosomes contribute to neutrophil N2 transformation and induce immunosuppressive PMN, promoting lung metastasis [48]. In addition to miRNA, tumor-derived exosomal long non-coding RNA (lncRNA) and circular RNA (CircRNA) also contribute to lung metastasis of breast cancer. Abnormal expression of lncRNAs in exosomes facilitated the formation of a lung metastatic microenvironment [75]. Exosomal circPSMA1 could act as a “sponge” to neutralize miR-637, thereby upregulating the expression of Akt1 and affecting downstream genes such as β-catenin and cyclin D1. The circPAMA1/miR637/Akt1/β-catenin (cyclin D1) axis promoted not only tumorigenesis, but also TNBC metastasis to the lung [49].
Some protein factors could also be the cargo in exosomes and promote metastasis. Recent studies found that cytokines in a tumor microenvironment can regulate the organotropism of metastasis via exosome secretion. CCL2 could directly bind to tumor-derived exosomes through the glycosaminoglycan side chains of proteoglycans. Furthermore, these CCL2-decorated exosomes were directed to CCR2-expressing cells, especially in the lung, leading to the lung metastasis burden [50]. Another chemokine, CCL5 expressed by tumor cells not only induces macrophage recruitment, but also promotes the secretion of more EVs by tumor cells, whereby EVs educated macrophages into TAMs and enhanced metastasis in the lung [76]. Besides cytokines, tumor-derived exosomes carry other proteins to contribute to lung metastasis. TGF-β type II receptor (TβRII) could be transferred by exosomes from malignant cells and activate TGF-β signaling in recipient cells. On the one hand, exosomal TβRII could induce TGF-β activation to initiate EMT in low-grade cancer cells, thus enhancing cancer stemness and metastasis. On the other hand, exosomal TβRII could also induce CD8+ T cell exhaustion by activating SMAD3, thereby leading to immunosuppression [51]. Feng et al. found that macrophage infiltration was positively correlated with the levels of signal-induced proliferation-associated 1(SIPA1) in invasive breast ductal carcinoma. SIPA1 could increase the level of myosin-9 in exosomes, which enhances macrophage infiltration and lung metastasis [52]. Aspartate β-hydroxylase (ASPH) could induce Notch signaling activation in breast cancers and orchestrate pro-oncogenic/pro-invasive cargoes into tumor-derived exosomes, especially matrix metalloproteinases (MMPs). These exosomal cargoes could enhance breast cancer metastasis in distant organs, especially the lung [53]. Furthermore, exosomal MMP-1 generated by high metastatic cells could mediate EMT in low metastatic cells by interacting with membrane G protein receptor protease activated receptor 1 (PAR1) and enhance their migration and invasion [54]. Exosome could also carry nucleoside diphosphate kinase A and B (NDPK) and Annexin II, thereby enhancing lung metastasis by promoting the vascular leakage and angiogenesis in the lung, respectively [55,56].
2.3. Exosomes Mediate Breast Cancer Metastasis to Liver
As mentioned above, liver metastasis is also an important subgroup of breast cancer metastasis diagnosed, often occurring in HER2-positive breast cancer with poor prognosis [15,16]. The microenvironment in both primary tumors and liver tissues undergoes great changes during the establishment of secondary metastatic sites. Many factors could be involved in breast cancer liver metastasis, such as inflammatory factors, chemokines and the related receptors, and cell adhesion molecules, etc., which could promote the establishment of a pro-inflammatory environment and EMT in tumors. In addition, there are some factors related to liver microenvironment that affect metastasis, such as angiogenesis-related factors, hypoxia-regulated genes, liver metabolic status, and the interaction between sinusoidal capillaries and cancer cells [77,78]. Recent studies indicate that exosomes are also extensively involved in liver metastasis (Figure 2c). Similarly, exosomes could contribute to liver metastasis by carrying miRNAs against the relevant targets. Highly invasive breast cancer cells secreted exosomes with high level of miR-4443 against tissue inhibitors of metalloproteinase 2 (TIMP2); the exosomes mainly accumulated in liver to upregulate MMP-2 and induced liver metastasis [57]. Exosomal miR-197 was produced by enriched breast cancer stem cells and downregulated the expression of PPARγ, thereby activating EMT in cancer cells and facilitating liver metastasis [58]. Tetraspanins are transmembrane proteins associated with cell membrane compartmentalization [79]. Tspan8, a member of tetraspanins, enhanced breast cancer metastasis to the liver. Mechanistically, Tspan8 promoted the secretion of EVs carrying high levels of E-cadherin and p120-catenin, thereby increasing liver metastases by modulating the EMT-MET programme [59]. One recent study indicated that Tspan8 not only enhances exosome secretion, but also promotes the uptake of exosome in some tissues, including liver, through confined diffusion, thus promoting tumor progression [80]. With the development of new technologies, more molecular mechanisms by which exosomes are involved in liver metastasis have been discovered. Based on a three-dimensional microfluidic liver chip, Kim et al. found that breast cancer-derived exosomes activated liver sinusoidal endothelial cells (LSECs), leading to endothelial-to-mesenchymal transition and disruption of the vascular barrier. Furthermore, exosomes upregulated fibronectin on LSECs through delivering TGFβ1, which facilitates cancer cell attachment to the liver microenvironment [60].
2.4. Exosome Mediate Breast Cancer Metastasis to Brain
Unlike other tissues, brain metastasis is a heterogeneous process involving the interaction between the tumor cell and the blood-brain barrier (BBB). The BBB is mainly composed of astrocytes, pericytes and endothelial cells, which make up the neurovascular unit. Endothelial cells are the first to crosstalk with circulating tumor cells [81]. Under normal conditions, BBB controls the supply of essential nutrients to brain cells, protects the brain from toxic compounds in the blood, and filters the harmful factors into the blood, which plays an important role in maintaining central nervous system homeostasis [82]. Although BBB also acts as a barrier against circulating tumor cell (CTCs) infiltration, many studies have revealed that the changes in molecular and cellular signal pathways are involved in this process to disrupt the BBB and establish the PMN for brain metastasis [83]. Meanwhile, exosomes also participate in the process through multiple mechanisms (Figure 2d). According to single cell force spectroscopy by atomic force microscopy, exosomes from cancer cells reduced brain endothelial adhesion when in direct contact with breast cancer cells, suggesting that exosomes may modulate the adhesiveness of brain endothelium and affect their permeability [84]. Tubulin tyrosine ligase-like 4 (TTLL4) is a cytoskeleton-associated protein, and its expression was positively correlated with brain metastasis. Mechanistically, upregulated TTLL4 increased β-tubulin glutamylation and MVB trafficking, which increased adhesion of breast cancer cells to BBB endothelial cells as well as permeability of these endothelial cells by altering exosome signatures [85]. It suggests that cancer-derived exosomes could enhance brain metastasis by disrupting the BBB through certain factors. miR-181c could be enriched in brain metastatic breast cancer cell-derived exosomes and target 3-phosphoinositide-dependent protein kinase-1(PDPK1) in endothelial cells. Downregulation of PDPK1 further reduced phosphorylation of cofilin and led to abnormal actin filament organization, thereby destroying BBB and promoting brain metastasis [61]. Cancer-derived exosomes also transferred lncRNA GS1-600G8.5 to endothelial cells and increased the permeability of BBB to enhance the passage of cancer cells through the BBB. However, the downstream targets of this lncRNA are unclear [62].
Recent studies have shown that exosome could also remodel a brain microenvironment that favors cancer cell colonization and proliferation. Cell migration-inducing and hyaluronan-binding protein (CEMIP), a Wnt-signaling associated protein, was identified as a dominant exosomal protein in brain metastatic cells, with low or undetectable levels in exosomes from lung and bone metastatic cells. CEMIP-positive exosomes were taken up by brain endothelial and microglial cells and contribute to the establishment of PMN by causing the cerebral vascular remodeling through upregulation of pro-inflammatory cytokines [63]. LncRNA X-inactive-specific transcript (XIST) was found to be significantly downregulated in brain metastatic tumors, and its expression was negatively correlated with brain metastasis. Besides enhancing aggressiveness of cancer cells by induction of EMT and c-Met signaling, loss of XIST could increase exosomal miR-503 secretion, which triggered M2 polarization in microglia and suppressed T cell proliferation to form PMN [64]. In addition, miR-301a-3p enriched exosomes were taken up by astrocytes via non-canonical Cdc42-dependent endocytosis, and these exosomes resulted in extracellular matrix remodeling by suppressing TIMP-2 expression in preparation for a metastasis microenvironment [65]. To enhance the effect of tumor-derived exosomes on brain metastasis, exosomes can manipulate the brain endothelial cells to facilitate their transfer into brain parenchyma by downregulating the expression of Rab7 [86] and can also bind with low-density lipoprotein (LDL) during circulation, causing the LDL aggregation and promoting monocyte uptake [87].
In conclusion, tumor-derived exosomes contribute to breast cancer metastasis to bone, lung, liver and brain through multiple mechanisms, which provide potential therapeutic targets for diagnosis of and therapy for this deadly disease.
3. Clinical Application
3.1. Exosomes as Diagnostic Biomarkers for Breast Cancer
With the convenience of being non-invasive and highly efficient, liquid biopsy brings an opportunity for cancer diagnosis with detection through various body fluids such as blood or urine, rather than invasive methods to remove a piece of cancerous tissue [88]. Liquid biopsy has made some progress in establishing the diagnosis of various cancers by using certain molecules as biomarkers, including CTCs, circulating tumor DNA (ctDNA), tumor-educated platelet (TEP) and exosomes [89,90]. As mentioned above, exosomes contain specific “cargoes” derived from metastatic cancer cells, which not only play an important role in breast cancer progression and metastasis but may also have the potential to be biomarkers for diagnosing metastasis. To discover exosome-based biomarkers, Wang et al. established a comprehensive database—ExoBCD—by combining four high-throughput datasets, transcriptome of 1191 TCGA cases and manual mining of 950 studies. The database identified 306 valuable exosomal molecules, including 49 potential biomarkers and 257 biologically interesting molecules [91]. Since traditional detection methods, such as real-time PCR and Western Blotting analysis, are time-consuming and laborious and require exosome enrichment, which make them unsuitable for exosome-based diagnosis, it is necessary to develop alternative methods. A rapid, sensitive, and low-cost thermophoretic aptasensor (TAS) was developed for the analysis of cancer-associated protein profiles of plasma EVs. Based on this analysis, the EV protein signature was established and used to accurately monitor and predict metastatic breast cancer [92]. Kwizera et al. developed an inexpensive and highly efficient device based on the surface-enhanced Raman scattering (SERS) to detect exosomes and exosomal protein profiles. Using this device, they identified exosomal HER2 and EpCAM as biomarkers in the plasma of HER2-positive breast cancer patients [93]. Then, Lee et al. established another SERS-based platform to detect and quantify exosomal miRNAs in serum for breast cancer diagnosis [94]. In addition, a nano-sized fluorescent oligonucleotide probe-molecular beacon has also been developed for the measurement of miRNAs in blood exosomes with high sensitivity and specificity, such as miR-21 [95,96], miR-27a, miR-375 [96], and miR-1246 [97]. Recently, a microfluidic chip-based exosomal mRNA sensor was developed to directly detect exosomal ERBB2 in blood for the diagnosis of HER2-positive breast cancer [98]. Therefore, technological advances will enable exosomes to be used as biomarkers for breast cancer diagnosis in the future.
3.2. Engineered Exosomes for Therapeutics of Breast Cancer
The natural characteristics of exosomes, such as low toxicity, low immunogenicity, high-flexibility engineering, and inherent targeting and interaction with recipient cells, make them ideal drug carriers for breast cancer therapy [99,100,101,102]. First, exosomes can serve as delivery vesicles for chemotherapeutic drugs such as doxorubicin, with some engineering modification on their surface to improve their targeting efficiency and reduce side effects [103]. Hydrophobic drugs, such as Aspirin, could also be loaded into exosomes to increase their solubility and enhance their cytotoxicity against cancer cells [104]. In addition, to enhance the efficacy of PARP inhibitors, exosomes isolated from TNBC cells were loaded with Olaparib (PARP inhibitor) and superparamagnetic iron oxide (SPIO) nanoparticles, which could be tracked by magnetic particle imaging (MPI) and also effectively inhibited tumor growth [105]. Second, functional small RNAs, such as siRNA and miRNA, can be packaged into exosomes and delivered to cancer cells to downregulate target genes, thereby inhibiting cancer progression [106,107,108,109]. Third, engineered exosomes can be used as vaccines to stimulate an immune response against tumor cells. A novel exosome-like nanoparticles was developed from fibroblast activation protein-α (FAP) engineered cancer cells as a tumor vaccine, which induced robust and specific cytotoxic T lymphocyte immunity against tumor cells and reprogrammed the immunosuppressive microenvironment [110]. Exosomes from α-lactalbumin overexpressing breast cancer cells were packaged together with immunogenic cell death inducers—human neutrophil elastase (ELANE) and Hiltonol (a TLR3 agonist) to construct a vaccine, thereby priming dendritic cells in situ and improving subsequent tumor-reactive CD8+ T cell responses [111]. Exosome can also be engineered to display both anti-CD3 and anti-HER2 antibodies to mediate cytotoxic T cells that directly target HER2-positive breast cancer and improve immunotherapy [112]. Fourth, exosomes can be utilized as nanocarriers to provide cancer-targeted sonosensitizers for sonodynamic therapy (SDT), which employs reactive oxygen species (ROS) generated by ultrasonic excitation to kill cancer cells [113]. Indocyanine green-loaded exosomes were surface-modified with cancer-binding ligand to increase their target specificity, resulting in greater SDT against cancer cells [114]. Sinoporphyrin sodium (DVDMs) could also be loaded into tumor-derived exosomes to increase its efficiency in SDT [115]. Exosomes can be used not only for primary tumor therapy, but also be engineered to treat breast cancer metastasis. As exosomes derived from metastatic breast cancers have natural organotropism to lung [35] and brain [86], therapeutic drugs can be encapsulated with exosomes to target the relevant metastatic foci. Gold nanorods, a nanomaterial for photothermal therapy, were loaded into lung metastatic cancer cells-derived exosomes, exhibiting better therapeutic effects on lung metastases [116]. Finally, more engineered exosomes will be developed to improve precision therapy for breast cancers, especially with regard to metastasis.
4. Conclusions
Extensive studies have been conducted on the function of exosomes in breast cancer metastasis, and these studies have shown that exosomes can broadly affect its metastasis. By controlling the contents encapsulated in exosomes, cancer cells can remodel the microenvironment of their preferred distant organs through exosome secretion, further promoting the metastatic process of cancer and generating metastasis organotropism. Exosomes will become new cancer diagnostic markers in cancer therapy due to their own characteristics. Therefore, a comprehensive understanding of exosome functions in breast cancer metastasis could provide some new insights into their clinical applications.
Acknowledgments
This work is supported by National Natural Science Foundation of China (81672603 and 81401978), Startup Research Grant from University of Macau (SRG2021-00008-FHS), and Macao Science and Technology Development Fund grant (0058/2022/A1 and 0011/2019/AKP). The figures were created using BioRender.
Author Contributions
Writing—original draft preparation, S.H.; writing—review and editing, Q.C.; supervision, Q.C. and M.D.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by National Natural Science Foundation of China (81672603 and 81401978), Startup Research Grant from University of Macau (SRG2021-00008-FHS), and Macao Science and Technology Development Fund grant (0058/2022/A1 and 0011/2019/AKP).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.McDonald E.S., Clark A.S., Tchou J., Zhang P., Freedman G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. 2016;57((Suppl. 1)):9s–16s. doi: 10.2967/jnumed.115.157834. [DOI] [PubMed] [Google Scholar]
- 3.Emens L.A. Breast Cancer Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018;24:511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 5.Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F. Breast cancer. Nat. Rev. Dis. Prim. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 6.Makki J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015;8:23–31. doi: 10.4137/CPath.S31563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang A., Wang X., Fan C., Mao X. The Role of Ki67 in Evaluating Neoadjuvant Endocrine Therapy of Hormone Receptor-Positive Breast Cancer. Front. Endocrinol. 2021;12:687244. doi: 10.3389/fendo.2021.687244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang M.C., Martin M., Nielsen T.O., Prat A., Voduc D., Rodriguez-Lescure A., Ruiz A., Chia S., Shepherd L., Ruiz-Borrego M., et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015;20:474–482. doi: 10.1634/theoncologist.2014-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye F., He M., Huang L., Lang G., Hu X., Shao Z., Di G., Cao A. Insights Into the Impacts of BRCA Mutations on Clinicopathology and Management of Early-Onset Triple-Negative Breast Cancer. Front. Oncol. 2020;10:574813. doi: 10.3389/fonc.2020.574813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won K.A., Spruck C. Triple-negative breast cancer therapy: Current and future perspectives (Review) Int. J. Oncol. 2020;57:1245–1261. doi: 10.3892/ijo.2020.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J.J., Swain S.M. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist. 2018;23:556–565. doi: 10.1634/theoncologist.2017-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin L., Duan J.J., Bian X.W., Yu S.C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaffer C.L., Weinberg R.A. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y., Zhang H., Song X., Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Li J., Zhu S., Wu J., Chen C., Liu Q., Wei W., Zhang Y., Sun S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget. 2017;8:27990–27996. doi: 10.18632/oncotarget.15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Y., Liu Y.R., Ji P., Hu X., Shao Z.M. Impact of molecular subtypes on metastatic breast cancer patients: A SEER population-based study. Sci. Rep. 2017;7:45411. doi: 10.1038/srep45411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 18.Gupta G.P., Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Wen S.W., Sceneay J., Lima L.G., Wong C.S., Becker M., Krumeich S., Lobb R.J., Castillo V., Wong K.N., Ellis S., et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer-Derived Exosomes. Cancer Res. 2016;76:6816–6827. doi: 10.1158/0008-5472.CAN-16-0868. [DOI] [PubMed] [Google Scholar]
- 22.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell. Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 23.Dong M., Liu Q., Xu Y., Zhang Q. Extracellular Vesicles: The Landscape in the Progression, Diagnosis, and Treatment of Triple-Negative Breast Cancer. Front. Cell. Dev. Biol. 2022;10:842898. doi: 10.3389/fcell.2022.842898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegtel D.M., Gould S.J. Exosomes. Annu. Rev. Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 26.Mathivanan S., Ji H., Simpson R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Gurung S., Perocheau D., Touramanidou L., Baruteau J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell. Commun. Signal. 2021;19:47. doi: 10.1186/s12964-021-00730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gatta A.T., Carlton J.G. The ESCRT-machinery: Closing holes and expanding roles. Curr. Opin. Cell. Biol. 2019;59:121–132. doi: 10.1016/j.ceb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 31.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer. 2019;18:75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbichler T.B., Dudás J., Riechelmann H., Skvortsova I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017;44:170–181. doi: 10.1016/j.semcancer.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Syn N., Wang L., Sethi G., Thiery J.P., Goh B.C. Exosome-Mediated Metastasis: From Epithelial-Mesenchymal Transition to Escape from Immunosurveillance. Trends Pharmacol. Sci. 2016;37:606–617. doi: 10.1016/j.tips.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Radisky D., Muschler J., Bissell M.J. Order and disorder: The role of extracellular matrix in epithelial cancer. Cancer Investig. 2002;20:139–153. doi: 10.1081/CNV-120000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangoda L., Liem M., Ang C.S., Keerthikumar S., Adda C.G., Parker B.S., Mathivanan S. Proteomic Profiling of Exosomes Secreted by Breast Cancer Cells with Varying Metastatic Potential. Proteomics. 2017;17 doi: 10.1002/pmic.201600370. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K., Ochi H., Sunamura S., Kosaka N., Mabuchi Y., Fukuda T., Yao K., Kanda H., Ae K., Okawa A., et al. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. USA. 2018;115:2204–2209. doi: 10.1073/pnas.1717363115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Cao M., Palomares M., Wu X., Li A., Yan W., Fong M.Y., Chan W.C., Wang S.E. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res. 2018;20:127. doi: 10.1186/s13058-018-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo L., Zhu Y., Li L., Zhou S., Yin G., Yu G., Cui H. Breast cancer cell-derived exosomal miR-20a-5p promotes the proliferation and differentiation of osteoclasts by targeting SRCIN1. Cancer Med. 2019;8:5687–5701. doi: 10.1002/cam4.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan X., Qian N., Ling S., Li Y., Sun W., Li J., Du R., Zhong G., Liu C., Yu G., et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics. 2021;11:1429–1445. doi: 10.7150/thno.45351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu K., Feng J., Lyu F., Xing F., Sharma S., Liu Y., Wu S.Y., Zhao D., Tyagi A., Deshpande R.P., et al. Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nat. Commun. 2021;12:5196. doi: 10.1038/s41467-021-25473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiedemann K., Sadvakassova G., Mikolajewicz N., Juhas M., Sabirova Z., Tabariès S., Gettemans J., Siegel P.M., Komarova S.V. Exosomal Release of L-Plastin by Breast Cancer Cells Facilitates Metastatic Bone Osteolysis. Transl. Oncol. 2019;12:462–474. doi: 10.1016/j.tranon.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X.Q., Zhang R., Lu H., Yue X.M., Huang Y.F. Extracellular Vesicle-Packaged CDH11 and ITGA5 Induce the Premetastatic Niche for Bone Colonization of Breast Cancer Cells. Cancer Res. 2022;82:1560–1574. doi: 10.1158/0008-5472.CAN-21-1331. [DOI] [PubMed] [Google Scholar]
- 44.Fong M.Y., Zhou W., Liu L., Alontaga A.Y., Chandra M., Ashby J., Chow A., O’Connor S.T., Li S., Chin A.R., et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xun J., Du L., Gao R., Shen L., Wang D., Kang L., Chen C., Zhang Z., Zhang Y., Yue S., et al. Cancer-derived exosomal miR-138-5p modulates polarization of tumor-associated macrophages through inhibition of KDM6B. Theranostics. 2021;11:6847–6859. doi: 10.7150/thno.51864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo J., Duan Z., Zhang C., Wang W., He H., Liu Y., Wu P., Wang S., Song M., Chen H., et al. Mouse 4T1 Breast Cancer Cell-Derived Exosomes Induce Proinflammatory Cytokine Production in Macrophages via miR-183. J. Immunol. 2020;205:2916–2925. doi: 10.4049/jimmunol.1901104. [DOI] [PubMed] [Google Scholar]
- 47.Gu P., Sun M., Li L., Yang Y., Jiang Z., Ge Y., Wang W., Mu W., Wang H. Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis Through Regulating CCL2 Expression in Lung Epithelial Cells. Front. Cell Dev. Biol. 2021;9:657158. doi: 10.3389/fcell.2021.657158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi M., Xia Y., Wu Y., Zhang Z., Wang X., Lu L., Dai C., Song Y., Xu K., Ji W., et al. Lin28B-high breast cancer cells promote immune suppression in the lung pre-metastatic niche via exosomes and support cancer progression. Nat. Commun. 2022;13:897. doi: 10.1038/s41467-022-28438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S.J., Wang D.D., Zhong S.L., Chen W.Q., Wang F.L., Zhang J., Xu W.X., Xu D., Zhang Q., Li J., et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (cyclin D1) axis. Cell Death Dis. 2021;12:420. doi: 10.1038/s41419-021-03680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lima L.G., Ham S., Shin H., Chai E.P.Z., Lek E.S.H., Lobb R.J., Müller A.F., Mathivanan S., Yeo B., Choi Y., et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat. Commun. 2021;12:3543. doi: 10.1038/s41467-021-23946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie F., Zhou X., Su P., Li H., Tu Y., Du J., Pan C., Wei X., Zheng M., Jin K., et al. Breast cancer cell-derived extracellular vesicles promote CD8(+) T cell exhaustion via TGF-β type II receptor signaling. Nat. Commun. 2022;13:4461. doi: 10.1038/s41467-022-31250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng L., Weng J., Yao C., Wang R., Wang N., Zhang Y., Tanaka Y., Su L. Extracellular Vesicles Derived from SIPA1(high) Breast Cancer Cells Enhance Macrophage Infiltration and Cancer Metastasis through Myosin-9. Biology. 2022;11:543. doi: 10.3390/biology11040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Q., Chen X., Meng F., Ogawa K., Li M., Song R., Zhang S., Zhang Z., Kong X., Xu Q., et al. ASPH-notch Axis guided Exosomal delivery of Prometastatic Secretome renders breast Cancer multi-organ metastasis. Mol. Cancer. 2019;18:156. doi: 10.1186/s12943-019-1077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y., Tao Z., Chen Y., Lin S., Zhu M., Ji W., Liu X., Li T., Hu X. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res. Treat. 2022;193:65–81. doi: 10.1007/s10549-022-06514-6. [DOI] [PubMed] [Google Scholar]
- 55.Duan S., Nordmeier S., Byrnes A.E., Buxton I.L.O. Extracellular Vesicle-Mediated Purinergic Signaling Contributes to Host Microenvironment Plasticity and Metastasis in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021;22:597. doi: 10.3390/ijms22020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maji S., Chaudhary P., Akopova I., Nguyen P.M., Hare R.J., Gryczynski I., Vishwanatha J.K. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Mol. Cancer Res. 2017;15:93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Zhang Q., Wang D., Yang S., Zhou S., Xu H., Zhang H., Zhong S., Feng J. Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. J. Cell Physiol. 2020;235:5722–5735. doi: 10.1002/jcp.29507. [DOI] [PubMed] [Google Scholar]
- 58.Li L., Xiong Y., Wang N., Zhu M., Gu Y. Breast Cancer Stem Cells-derived Extracellular Vesicles Affect PPARG Expression by Delivering MicroRNA-197 in Breast Cancer Cells. Clin. Breast Cancer. 2022;22:478–490. doi: 10.1016/j.clbc.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Voglstaetter M., Thomsen A.R., Nouvel J., Koch A., Jank P., Navarro E.G., Gainey-Schleicher T., Khanduri R., Groß A., Rossner F., et al. Tspan8 is expressed in breast cancer and regulates E-cadherin/catenin signalling and metastasis accompanied by increased circulating extracellular vesicles. J. Pathol. 2019;248:421–437. doi: 10.1002/path.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J., Lee C., Kim I., Ro J., Kim J., Min Y., Park J., Sunkara V., Park Y.S., Michael I., et al. Three-Dimensional Human Liver-Chip Emulating Premetastatic Niche Formation by Breast Cancer-Derived Extracellular Vesicles. ACS Nano. 2020;14:14971–14988. doi: 10.1021/acsnano.0c04778. [DOI] [PubMed] [Google Scholar]
- 61.Tominaga N., Kosaka N., Ono M., Katsuda T., Yoshioka Y., Tamura K., Lötvall J., Nakagama H., Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y., Chen L., Li L., Cao Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying lncRNA GS1-600G8.5. Biomed Res. Int. 2020;2020:7461727. doi: 10.1155/2020/7461727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigues G., Hoshino A., Kenific C.M., Matei I.R., Steiner L., Freitas D., Kim H.S., Oxley P.R., Scandariato I., Casanova-Salas I., et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat. Cell Biol. 2019;21:1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xing F., Liu Y., Wu S.Y., Wu K., Sharma S., Mo Y.Y., Feng J., Sanders S., Jin G., Singh R., et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018;78:4316–4330. doi: 10.1158/0008-5472.CAN-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morad G., Daisy C.C., Otu H.H., Libermann T.A., Dillon S.T., Moses M.A. Cdc42-Dependent Transfer of mir301 from Breast Cancer-Derived Extracellular Vesicles Regulates the Matrix Modulating Ability of Astrocytes at the Blood-Brain Barrier. Int. J. Mol. Sci. 2020;21:3851. doi: 10.3390/ijms21113851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Theriault R.L., Theriault R.L. Biology of bone metastases. Cancer Control. 2012;19:92–101. doi: 10.1177/107327481201900203. [DOI] [PubMed] [Google Scholar]
- 67.Wang M., Xia F., Wei Y., Wei X. Molecular mechanisms and clinical management of cancer bone metastasis. Bone Res. 2020;8:30. doi: 10.1038/s41413-020-00105-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Browne G., Taipaleenmäki H., Stein G.S., Stein J.L., Lian J.B. MicroRNAs in the control of metastatic bone disease. Trends Endocrinol. Metab. 2014;25:320–327. doi: 10.1016/j.tem.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karaplis A.C., Goltzman D. PTH and PTHrP effects on the skeleton. Rev. Endocr. Metab. Disord. 2000;1:331–341. doi: 10.1023/A:1026526703898. [DOI] [PubMed] [Google Scholar]
- 70.Jamil A., Kasi A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Lung Metastasis. [Google Scholar]
- 71.Cao M., Isaac R., Yan W., Ruan X., Jiang L., Wan Y., Wang J., Wang E., Caron C., Neben S., et al. Cancer-cell-secreted extracellular vesicles suppress insulin secretion through miR-122 to impair systemic glucose homeostasis and contribute to tumour growth. Nat. Cell Biol. 2022;24:954–967. doi: 10.1038/s41556-022-00919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piskounova E., Polytarchou C., Thornton J.E., LaPierre R.J., Pothoulakis C., Hagan J.P., Iliopoulos D., Gregory R.I. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066–1079. doi: 10.1016/j.cell.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rehfeld F., Rohde A.M., Nguyen D.T., Wulczyn F.G. Lin28 and let-7: Ancient milestones on the road from pluripotency to neurogenesis. Cell Tissue Res. 2015;359:145–160. doi: 10.1007/s00441-014-1872-2. [DOI] [PubMed] [Google Scholar]
- 74.Chen C., Bai L., Cao F., Wang S., He H., Song M., Chen H., Liu Y., Guo J., Si Q., et al. Targeting LIN28B reprograms tumor glucose metabolism and acidic microenvironment to suppress cancer stemness and metastasis. Oncogene. 2019;38:4527–4539. doi: 10.1038/s41388-019-0735-4. [DOI] [PubMed] [Google Scholar]
- 75.Feng T., Zhang P., Sun Y., Wang Y., Tong J., Dai H., Hua Z. High throughput sequencing identifies breast cancer-secreted exosomal LncRNAs initiating pulmonary pre-metastatic niche formation. Gene. 2019;710:258–264. doi: 10.1016/j.gene.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 76.Rabe D.C., Walker N.D., Rustandy F.D., Wallace J., Lee J., Stott S.L., Rosner M.R. Tumor Extracellular Vesicles Regulate Macrophage-Driven Metastasis through CCL5. Cancers. 2021;13:3459. doi: 10.3390/cancers13143459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma R., Feng Y., Lin S., Chen J., Lin H., Liang X., Zheng H., Cai X. Mechanisms involved in breast cancer liver metastasis. J. Transl. Med. 2015;13:64. doi: 10.1186/s12967-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zuo Q., Park N.H., Lee J.K., Madak Erdogan Z. Liver Metastatic Breast Cancer: Epidemiology, Dietary Interventions, and Related Metabolism. Nutrients. 2022;14:2376. doi: 10.3390/nu14122376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Charrin S., Jouannet S., Boucheix C., Rubinstein E. Tetraspanins at a glance. J. Cell Sci. 2014;127:3641–3648. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- 80.Wang T., Wang X., Wang H., Li L., Zhang C., Xiang R., Tan X., Li Z., Jiang C., Zheng L., et al. High TSPAN8 expression in epithelial cancer cell-derived small extracellular vesicles promote confined diffusion and pronounced uptake. J. Extracell. Vesicles. 2021;10:e12167. doi: 10.1002/jev2.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burn L., Gutowski N., Whatmore J., Giamas G., Pranjol M.Z.I. The role of astrocytes in brain metastasis at the interface of circulating tumour cells and the blood brain barrier. Front. Biosci. (Landmark. Ed.) 2021;26:590–601. doi: 10.52586/4969. [DOI] [PubMed] [Google Scholar]
- 82.Persidsky Y., Ramirez S.H., Haorah J., Kanmogne G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune. Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 83.Hosonaga M., Saya H., Arima Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020;39:711–720. doi: 10.1007/s10555-020-09881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fazakas C., Kozma M., Molnár K., Kincses A., Dér A., Fejér A., Horváth B., Wilhelm I., Krizbai I.A., Végh A.G. Breast adenocarcinoma-derived exosomes lower first-contact de-adhesion strength of adenocarcinoma cells to brain endothelial layer. Colloids Surf. B Biointerfaces. 2021;204:111810. doi: 10.1016/j.colsurfb.2021.111810. [DOI] [PubMed] [Google Scholar]
- 85.Arnold J., Schattschneider J., Blechner C., Krisp C., Schlüter H., Schweizer M., Nalaskowski M., Oliveira-Ferrer L., Windhorst S. Tubulin Tyrosine Ligase Like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis. J. Exp. Clin. Cancer Res. 2020;39:205. doi: 10.1186/s13046-020-01712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morad G., Carman C.V., Hagedorn E.J., Perlin J.R., Zon L.I., Mustafaoglu N., Park T.E., Ingber D.E., Daisy C.C., Moses M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano. 2019;13:13853–13865. doi: 10.1021/acsnano.9b04397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Busatto S., Yang Y., Walker S.A., Davidovich I., Lin W.H., Lewis-Tuffin L., Anastasiadis P.Z., Sarkaria J., Talmon Y., Wurtz G., et al. Brain metastases-derived extracellular vesicles induce binding and aggregation of low-density lipoprotein. J. Nanobiotechnol. 2020;18:162. doi: 10.1186/s12951-020-00722-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poulet G., Massias J., Taly V. Liquid Biopsy: General Concepts. Acta Cytol. 2019;63:449–455. doi: 10.1159/000499337. [DOI] [PubMed] [Google Scholar]
- 89.Ye Q., Ling S., Zheng S., Xu X. Liquid biopsy in hepatocellular carcinoma: Circulating tumor cells and circulating tumor DNA. Mol. Cancer. 2019;18:114. doi: 10.1186/s12943-019-1043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou H., Zhu L., Song J., Wang G., Li P., Li W., Luo P., Sun X., Wu J., Liu Y., et al. Liquid biopsy at the frontier of detection, prognosis and progression monitoring in colorectal cancer. Mol. Cancer. 2022;21:86. doi: 10.1186/s12943-022-01556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X., Chai Z., Pan G., Hao Y., Li B., Ye T., Li Y., Long F., Xia L., Liu M. ExoBCD: A comprehensive database for exosomal biomarker discovery in breast cancer. Brief Bioinform. 2021;22:bbaa088. doi: 10.1093/bib/bbaa088. [DOI] [PubMed] [Google Scholar]
- 92.Tian F., Zhang S., Liu C., Han Z., Liu Y., Deng J., Li Y., Wu X., Cai L., Qin L., et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat. Commun. 2021;12:2536. doi: 10.1038/s41467-021-22913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwizera E.A., O’Connor R., Vinduska V., Williams M., Butch E.R., Snyder S.E., Chen X., Huang X. Molecular Detection and Analysis of Exosomes Using Surface-Enhanced Raman Scattering Gold Nanorods and a Miniaturized Device. Theranostics. 2018;8:2722–2738. doi: 10.7150/thno.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee J.U., Kim W.H., Lee H.S., Park K.H., Sim S.J. Quantitative and Specific Detection of Exosomal miRNAs for Accurate Diagnosis of Breast Cancer Using a Surface-Enhanced Raman Scattering Sensor Based on Plasmonic Head-Flocked Gold Nanopillars. Small. 2019;15:e1804968. doi: 10.1002/smll.201804968. [DOI] [PubMed] [Google Scholar]
- 95.Lee J.H., Kim J.A., Kwon M.H., Kang J.Y., Rhee W.J. In situ single step detection of exosome microRNA using molecular beacon. Biomaterials. 2015;54:116–125. doi: 10.1016/j.biomaterials.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 96.Wang H., He D., Wan K., Sheng X., Cheng H., Huang J., Zhou X., He X., Wang K. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst. 2020;145:3289–3296. doi: 10.1039/D0AN00393J. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y., Zhai L.Y., Zhang L.M., Ma X.S., Liu Z., Li M.M., Chen J.X., Duan W.J. Breast cancer plasma biopsy by in situ determination of exosomal microRNA-1246 with a molecular beacon. Analyst. 2021;146:2264–2276. doi: 10.1039/D0AN02224A. [DOI] [PubMed] [Google Scholar]
- 98.Lim J., Kang B., Son H.Y., Mun B., Huh Y.M., Rho H.W., Kang T., Moon J., Lee J.J., Seo S.B., et al. Microfluidic device for one-step detection of breast cancer-derived exosomal mRNA in blood using signal-amplifiable 3D nanostructure. Biosens. Bioelectron. 2022;197:113753. doi: 10.1016/j.bios.2021.113753. [DOI] [PubMed] [Google Scholar]
- 99.Liu Q., Zhang X., Zhang J. Exosome-Based Nanoplatforms: The Emerging Tools for Breast Cancer Therapy. Front. Oncol. 2022;12:898605. doi: 10.3389/fonc.2022.898605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wiklander O.P., Nordin J.Z., O’Loughlin A., Gustafsson Y., Corso G., Mäger I., Vader P., Lee Y., Sork H., Seow Y., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tian Y., Li S., Song J., Ji T., Zhu M., Anderson G.J., Wei J., Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 104.Tran P.H.L., Wang T., Yin W., Tran T.T.D., Barua H.T., Zhang Y., Midge S.B., Nguyen T.N.G., Lee B.J., Duan W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019;566:697–707. doi: 10.1016/j.ijpharm.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 105.Jung K.O., Jo H., Yu J.H., Gambhir S.S., Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139–148. doi: 10.1016/j.biomaterials.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Limoni S.K., Moghadam M.F., Moazzeni S.M., Gomari H., Salimi F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019;187:352–364. doi: 10.1007/s12010-018-2813-4. [DOI] [PubMed] [Google Scholar]
- 107.Naseri Z., Oskuee R.K., Jaafari M.R., Forouzandeh Moghadam M. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018;13:7727–7747. doi: 10.2147/IJN.S182384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sheykhhasan M., Kalhor N., Sheikholeslami A., Dolati M., Amini E., Fazaeli H. Exosomes of Mesenchymal Stem Cells as a Proper Vehicle for Transfecting miR-145 into the Breast Cancer Cell Line and Its Effect on Metastasis. Biomed Res. Int. 2021;2021:5516078. doi: 10.1155/2021/5516078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O’Brien K.P., Khan S., Gilligan K.E., Zafar H., Lalor P., Glynn C., O’Flatharta C., Ingoldsby H., Dockery P., De Bhulbh A., et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37:2137–2149. doi: 10.1038/s41388-017-0116-9. [DOI] [PubMed] [Google Scholar]
- 110.Hu S., Ma J., Su C., Chen Y., Shu Y., Qi Z., Zhang B., Shi G., Zhang Y., Zhang Y., et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–581. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Huang L., Rong Y., Tang X., Yi K., Qi P., Hou J., Liu W., He Y., Gao X., Yuan C., et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol. Cancer. 2022;21:45. doi: 10.1186/s12943-022-01515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shi X., Cheng Q., Hou T., Han M., Smbatyan G., Lang J.E., Epstein A.L., Lenz H.J., Zhang Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020;28:536–547. doi: 10.1016/j.ymthe.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan X., Wang H., Wang S., Sun X., Wang L., Wang W., Shen H., Liu H. Sonodynamic therapy (SDT): A novel strategy for cancer nanotheranostics. Sci. China Life Sci. 2018;61:415–426. doi: 10.1007/s11427-017-9262-x. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen Cao T.G., Kang J.H., You J.Y., Kang H.C., Rhee W.J., Ko Y.T., Shim M.S. Safe and Targeted Sonodynamic Cancer Therapy Using Biocompatible Exosome-Based Nanosonosensitizers. ACS Appl. Mater. Interfaces. 2021;13:25575–25588. doi: 10.1021/acsami.0c22883. [DOI] [PubMed] [Google Scholar]
- 115.Liu Y., Bai L., Guo K., Jia Y., Zhang K., Liu Q., Wang P., Wang X. Focused ultrasound-augmented targeting delivery of nanosonosensitizers from homogenous exosomes for enhanced sonodynamic cancer therapy. Theranostics. 2019;9:5261–5281. doi: 10.7150/thno.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang H., Shao L., Chen Y., Tang L., Liu T., Li J., Zhu H. Synergistic strategy with hyperthermia therapy based immunotherapy and engineered exosomes-liposomes targeted chemotherapy prevents tumor recurrence and metastasis in advanced breast cancer. Bioeng. Transl. Med. 2022;7:e10284. doi: 10.1002/btm2.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.