Abstract
The most prominent gamma interferon (IFN-γ)-induced antimicrobial effector mechanisms are the induction of nitric oxide (NO) synthase (NOS) and of indoleamine 2,3-dioxygenase (IDO) activity. We have recently found that human glioblastoma cells and human macrophages inhibit the growth of group B streptococci after stimulation with IFN-γ. In this report, we show that in addition, human RT4 (uroepithelial) cells can inhibit the growth of enterococci. Murine macrophages (RAW cells) are unable to inhibit bacterial growth after IFN-γ stimulation. Stimulation of human glioblastoma cells, macrophages, and RT4 cells with human IFN-γ results in a strong expression of IDO activity; however, NO production remains undetectable. In strong contrast, murine RAW cells produce large amounts of NO when stimulated with murine IFN-γ and IDO activity is not detectable. Interleukin-1 (IL-1) induces NO synthase in human RT4 cells when the cells are costimulated with IFN-γ. We found that IL-1 inhibits IFN-γ-stimulated IDO activity and antimicrobial effects in RT4 cells, while in human glioblastoma cells, which lack detectable NO synthase activity, neither of these effects was altered by costimulation with IFN-γ and IL-1. The IL-1-mediated inhibition of IDO activity and of subsequent antibacterial effect is due to the production of NO. This conclusion was supported by evidence that NG-monomethyl-l-arginine, a competitive inhibitor of inducible NOS activity, is able to block the inhibitory action of IL-1 on IFN-γ-induced bacteriostasis. We therefore conclude that NO production does not inhibit the growth of enterococci but might be involved in the regulation of IDO activity in some human cells.
Streptococci are spherical microorganisms growing in chains, which were first classified by their capacity to hemolyze erythrocytes. In the early 1930s the beta-hemolytic streptococci were differentiated into a number of groups based on immunologically reactive surface characteristics. Many human diseases are mediated by beta-hemolytic streptococci group A and B. Among them, local diseases such as impetigo and erysipelas and systemic infections like puerperal sepsis and meningitis are well described. The infections with beta-hemolytic streptococci are in most cases easy to control with antimicrobial drugs like penicillin and erythromycin (19).
Besides beta-hemolytic streptococci, humans are colonized with many of different nonhemolytic streptococci. Within the gastrointestinal tract, enterococci are the most frequent nonhemolytic streptococci. These bacteria are exceptionally hardy microorganisms, are relatively resistant to heat and hyperosmolar solutions, and more important in clinical practice, can be highly resistant to antimicrobial agents. Enterococci frequently cause urinary tract infections as well as peritonitis and wound infections. Furthermore enterococci can enter the bloodstream and cause septicemia and are an important cause of endocarditis. Due to the high resistance of many enterococci to many antimicrobial agents, these bacteria are frequently isolated from patients on intensive care units (21). Recently vancomycin resistent strains have been increasingly isolated both from hospitalized patients and from the gastrointestinal tracts of healthy persons. These strains are often resistant to all available antibiotics (21).
Local host defense mechanisms are usually efficient in eliminating microorganisms, and the pH, chemical content, and flushing mechanisms of urine helps to eliminate the bacteria from the urogenital tract. In addition, granulocytes are very effective in eliminating bacteria from the urinary tract. Despite this, bacteria occasionally enter the bloodstream and cause systemic infections. We were interested in analyzing the antibacterial effector mechanisms active in the local defense of the urinary tract. We therefore investigated antibacterial effects inducible in human macrophages as well as in human nonprofessional phagocytes. We have recently described that gamma interferon (IFN-γ)-activated human cord blood macrophages are able to inhibit the growth of group B streptococci (18). The antimicrobial effector mechanism active in the macrophages was the induction of indoleamine 2,3-dioxygenase (IDO) activity, resulting in a degradation of l-tryptophan, which is an essential amino acid for streptococci. We also described the same effector mechanism in human glioblastoma cells (17). The question arose as to whether human uroepithelial cells as nonprofessional phagocytes are able to restrict the growth of a urinary tract pathogen such as enterococci. In addition, we sought to determine the role of nitric oxide (NO) in the defense against enterococci. Thomas et al. (30) first described that synthetic NO generators inhibit IDO activity in IFN-γ-primed mononuclear phagocytes. We were therefore wished to investigate whether NO produced by a human cell line might influence the antibacterial effect of IDO activity expressed by the same cell.
The human uroepithelial cell line RT4 can produce NO after stimulation with IFN-γ and interleukin-1 (IL-1). In this report, we show that IFN-γ induces IDO activity in RT4 cells, resulting in an efficient inhibition of enterococcal growth. A simultaneous activation of NO in these RT4 cells results in an inhibition of IDO activity and blocks the IFN-γ-induced antimicrobial effect.
MATERIALS AND METHODS
Media, chemicals, and cytokines.
Iscove's modified Dulbecco's medium and RPMI 1640 (Gibco, Grand Island, N.Y.), with and without l-tryptophan, supplemented with 2 mM l-glutamine and 5% heat-inactivated fetal calf serum were used as culture medium for all cell lines. Kynurenine, l-tryptophan, Ehrlich's reagent, and Griess reagent (naphthylethylenediamine dihydrochloride [0.3%] and sulfanilamide [1%] in 1.2 N HCl) were obtained from Sigma (Deisenhofen, Germany), and acetic acid was obtained from Merck (Darmstadt, Germany). Human recombinant IFN-γ (rIFN-γ) was a gift from M. Augst (Dr. Karl Thomae GmbH, Bieberach an der Riss, Germany). Human rIL-1β and murine rIFN-γ were obtained from Genzyme (Cambridge, Mass.); NG-monomethyl-l-arginine (NGMMA) was obtained from Calbiochem (Bad Soden, Germany).
Cells and bacteria.
The human uroepithelial carcinoma cell line RT4 and the murine macrophage line RAW 264.7 were obtained from the American Type Culture Collection (Rockville, Md.). The human glioblastoma cell line 86HG39 was characterized by immunocytochemical and immunohistological criteria and was a kind gift from T. Bilzer (Institut für Neuropathologie, Heinrich-Heine-Universität, Düsseldorf, Germany) (1). Cells were grown in culture medium in tissue culture flasks (Costar, Cambridge, Mass.) and divided weekly, using trypsin-EDTA (Gibco) to harvest the strongly adherent cells. All bacterial strains were isolated from clinical specimens (blood, wound swabs, urine, and feces). The 30 Enterococcus faecalis strains were identified by colony morphology and agglutination with a Strep Plus diagnostic kit (Oxoid, Basingstoke, Hampshire, England) and in addition confirmed biochemically by using a commercial system (API-20strep; bioMerieux, Lyon, France). Results are shown for a representative strain isolated from the urine of a patient with symptomatic uncomplicated urinary tract infection. Similar data were obtained with all of the enterococcal strains tested.
All bacteria were grown on brain heart infusion agar (Difco, Hamburg, Germany) containing 5% sheep blood and incubated at 37°C in 5% CO2-enriched atmosphere. For use in experiments a 24-h-old single bacterial colony was picked and resuspended in RPMI 1640 without l-tryptophan. Bacteria were serially diluted in RPMI 1640 without l-tryptophan, and CFU in each dilution were calculated by plating two 10-μl aliquots of each suspension onto agar plates.
Determination of nitrite accumulation.
Nitrite accumulation in the supernatant of cultured cells was used as an indicator of NO production and was determined by the Griess reaction (detection limit, 1 μM) with sodium nitrite as standard as previously described (11). We are aware that this method is not a direct measurement of NO and underestimates total NO synthesis.
RT4, RAW, or 86HG39 cells were incubated in culture medium (Iscove's medium or RPMI 1640 medium with 5% fetal calf serum) in 96-well, flat-bottom culture plates (Greiner, Nürtingen, Germany) at 1 × 104 to 3 × 104 cells/well and were stimulated with IFN-γ (0 to 400 U/ml) and/or IL-1 (0 to 200 U/ml). After 3 days of incubation, the culture supernatant was harvested for the determination of nitrite.
Detection of IDO activity.
The tumor cells were stimulated with IL-1β and IFN-γ as described above in culture medium supplemented with 50 to 100 μg of l-tryptophan per ml. After 3 days of incubation, the supernatant was harvested and IDO activity was measured by determining kynurenine content in the cell supernatant as we have previously described (9). In brief, 160 μl of the cell supernatant was removed from each well and transferred to a corresponding well of a 96-well V-bottom culture plate. After addition of 10 μl of 30% (vol/vol) trichloroacetic acid to each well, the plates were incubated for 30 min at 50°C to hydrolyze N-formylkynurenine to kynurenine. After centrifugation for 10 min, 100 μl of supernatant was transferred to wells of a 96-well flat-bottom plate and mixed with 100 μl of freshly prepared Ehrlich's reagent. The absorbance was read with a microplate reader at 492 nm. A blanking procedure was performed by using a cell control without IFN-γ.
Determination of bacterial growth in cultures of cytokine-treated cells.
Tumor cells were stimulated with murine IFN-γ, human IFN-γ, and/or human IL-1β as described above. After 3 days of incubation, enterococci (5 to 50 CFU/well) were added in RPMI 1640 with or without additional l-tryptophan. Bacterial growth was monitored after a further incubation period of 10 to 16 h by using a microplate photometer (SLT Labinstruments, Grailsheim, Germany), measuring optical density at 620 nm (OD620) as described previously (17, 18).
RESULTS
Inducible antienterococcal effects in human and murine cells.
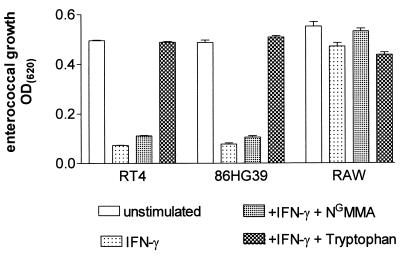
In murine cells, the most prominent antimicrobial effector mechanism is the expression of inducible NO synthase (iNOS), while in human cells the induction of IDO is responsible for many antimicrobial effects. To analyze the IFN-γ-inducible effect against enterococci, we stimulated the murine macrophage cell line RAW 264.7 with murine IFN-γ. As a control, human glioblastoma cells and a human uroepithelial cell line stimulated with human IFN-γ were used. As shown in Fig. 1, the human cell lines 86HG39 and RT4 were able to restrict the growth of enterococci, while the murine RAW cells failed to do so. The same results were obtained with 30 different strains of enterococci, including three vancomycin-resistant strains. Figure 1 also shows that the antienterococcal effect mediated by the IFN-γ-activated human cells is completely blocked by the addition of excess amounts of l-tryptophan, indicating that IFN-γ induced IDO activity, resulting in the degradation of the essential amino acid l-tryptophan, is responsible for the detected antibacterial effect. In contrast, NGMMA, an inhibitor of NO synthesis, did not influence IFN-γ-induced bacteriostasis.
FIG. 1.
Enterococcal growth in IFN-γ-activated human and murine cells. A total of 3 × 104 RT4, 86HG39, or RAW cells were stimulated with IFN-γ (100 U/ml) for 3 days. Thereafter, enterococci were added (50 CFU/well) with or without NGMMA or l-tryptophan (100 μg/ml). Bacterial growth was determined 16 h later by measuring OD620. Data are given as mean OD ± SE of triplicate cultures.
In addition we analyzed the induction of IDO activity as well as the production of NO by the IFN-γ-stimulated cells. We found that RAW cells produce large amounts of NO after IFN-γ stimulation (30.6 μM ± 6.9 = mean ± standard error [SE] of five independent experiments), but we were unable to detect NO in the supernatant of the human cells (<1 μM). In contrast, both human cell lines exhibited a strong IDO response to IFN-γ, which was not detected in the murine RAW cells.
NO production by RT4 cells results in an inhibition of IDO activity.
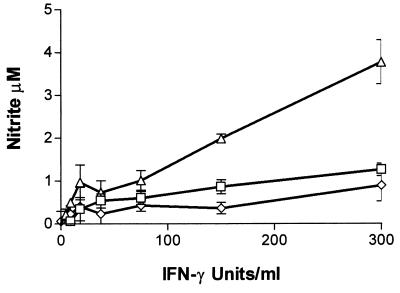
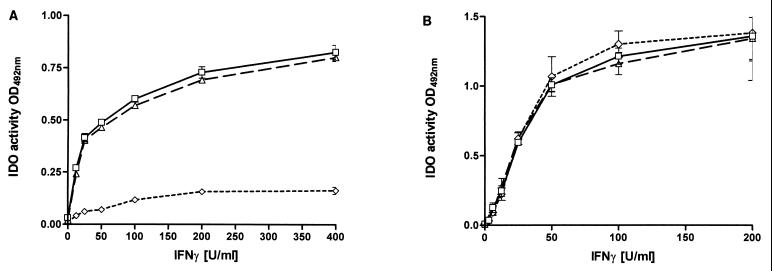
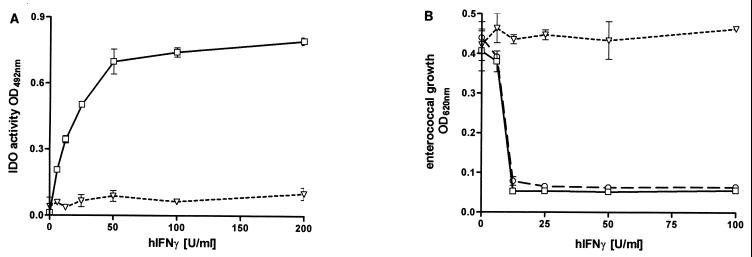
The human cell line RT4 is one of the few human cell lines capable of producing clearly measurable amounts of NO. Having demonstrated in Fig. 1 that the antienterococci effect inducible in RT4 cells is the induction of IDO, we analyzed whether NO produced by RT4 cells after stimulation with IFN-γ and IL-1β influences IFN-γ-induced IDO activity. RT4 cells were stimulated with IFN-γ in the presence or absence of additional IL-1β, and nitrite accumulation and IDO activity were determined by measuring nitrite concentration and kynurenine content in the culture supernatants after 3 days of incubation (Fig. 2 and 3). We used 86HG39 cells, which do not express detectable iNOS activation after IFN-γ–IL-1 stimulation, as a control. The failure of 86HG39 cells to produce NO after stimulation with IL-1 is not simply due to a lack of the IL-1 receptor since we found that these cells respond to IL-1 by secreting IL-6 (data not shown). The results shown in Fig. 3 clearly demonstrate that IL-1 mediates a significant inhibition of IDO activity in RT4 cells (t test, P < 0.05), while IDO activity inducible in 86HG39 cells is not by inhibited IL-1. In addition, Fig. 2 and 3 demonstrate that NGMMA blocks nitrite production in RT4 cells stimulated with IL-1 and IFN-γ and that NGMMA antagonizes the inhibitory effect of IL-1 on IFN-γ-induced bacteriostasis.
FIG. 2.
Nitrite accumulation in the supernatant of RT4 cells stimulated with IFN-γ and IL-1. A total of 3 × 104 RT4 cells were stimulated with IFN-γ (0 to 300 U/ml) alone (□) or in the presence of IL-1 (100 U/ml) (▵) or IL-1 and NGMMA (100 μg/ml) (◊) in culture medium. After 3 days of incubation, nitrite accumulation was determined by the Griess reagent as described in Materials and Methods. Data are given as mean ± SE of triplicate cultures.
FIG. 3.
Inhibitory effect of IL-1 on IFN-γ-induced IDO activity in RT4 cells. A total of 3 × 104 RT4 (A) or 86HG39 (B) cells were stimulated with IFN-γ alone (▵) or in the presence of IL-1 (100 U/ml) (◊) or IL-1 and NGMMA (100 μg/ml) (▵) in medium supplemented with l-tryptophan (50 μg/ml). After 3 days of incubation, IDO activity was determined by measuring the kynurenine content in the culture supernatant. Data are given as mean ± SE of triplicate cultures.
IL-1 inhibits IFN-γ-induced antibacterial effects in RT4 cells.
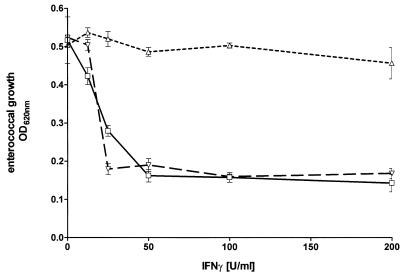
RT4 cells produce NO after stimulation with IFN-γ and IL-1, as shown in Fig. 2. We were interested in analyzing the NO-mediated effects in our in vitro culture system with human cells and enterococci. Therefore, RT4 cells, and as a control human 86HG39 cells, were stimulated with a combination of IFN-γ and IL-1, and after 3 days of incubation enterococci were added to the cell cultures. The results of a representative experiment are shown in Fig. 4. Human glioblastoma cells were activated by IFN-γ to inhibit enterococcal growth in a dose-dependent manner, and the addition of IL-1 did not affect this antibacterial effect. In contrast, in the human uroepithelial cell line RT4 the IFN-γ-induced antibacterial effect was significantly inhibited by the addition of IL-1 (P < 0.05). Since IFN-γ–IL-1-stimulated RT4 cells produce NO, we added NGMMA, a competitive inhibitor of NO production, to the culture system. As seen in Fig. 4, the inhibitory effect of IL-1 on IFN-γ-mediated bacteriostasis was antagonized by the presence of NGMMA. This was not due to a toxic effect of NGMMA since bacterial growth in cultures with untreated RT4 cells was not influenced by NGMMA, and NGMMA did not influence bacterial growth in cultures with IFN-γ-stimulated glioblastoma cells. The data suggest that the NO produced by the IFN-γ–IL-1-stimulated RT4 cells is responsible for the abrogation of the IFN-γ-induced bacteriostasis. We have thus shown that iNOS expression inhibits IDO-mediated bacteriostasis in cells in which both effector mechanisms can be activated simultaneously.
FIG. 4.
Inhibitory effect of IL-1 on IFN-γ-induced bacteriostasis in RT4 cells. A total of 3 × 104 RT4 cells were stimulated with IFN-γ alone (□) or in the presence of IL-1 (100 U/ml) (▵) or IL-1 and NGMMA (100 μg/ml) (▿). After 3 days of incubation, enterococci (50 CFU/well) were added. Bacterial growth was determined 16 h later by measuring OD620. Data are given as mean OD ± SE of triplicate cultures.
To determine if the induction of iNOS in neighboring cells has the same effect on IDO activation, we used a model in which glioblastoma cells (non-NO producing) were coincubated with RAW cells. As shown in Fig. 5, stimulation of RAW cells with human IFN-γ did not influence the bacteriostasis mediated in cocultured glioblastoma calls. In contrast, when RAW cells were stimulated with murine IFN-γ, IDO activity and bacteriostasis mediated by 86HG39 cells stimulated with human IFN-γ were significantly inhibited (P < 0.05). That this is due to the NO production by RAW cells is shown by the abrogation of this effect in the presence of the iNOS inhibitor NGMMA.
FIG. 5.
Inhibitory effect of activated RAW cells on antibacterial effects of 86HG39 cells. A total of 3 × 104 86HG39 cells were cocultured with 104 RAW cells. The mixed cell cultures were stimulated with human IFN-γ (hIFNγ; 0 to 200 U/ml) in the absence (□) or presence of murine IFN-γ (30 U/ml) alone (▿) and with NGMMA (100 μg/ml) (○). Three days after stimulation, IDO activity was determined as described in Materials and Methods (A). Thereafter, enterococci (50 CFU/well) were added, and bacterial growth was determined 16 h later by measuring OD at 620 nm (B). Data are given as mean ± SE of triplicate cultures.
DISCUSSION
Production of NO is a major defense mechanism against several intracellular parasites, including Toxoplasma and Leishmania species, in murine cells. Furthermore, NO is also involved in the defense against extracellular pathogens such as fungi (23). In this report, we show that NO produced by murine and human cells does not inhibit bacterial growth in our culture system. Comparable in vivo data have been obtained by Sriskandan et al., who showed that group A streptococci were able to induce iNOS in mice but that inhibition of NO production with NGMMA does not influence the course of streptococcal infection (29). We have shown here that human glioblastoma cells and more interestingly human uroepithelial cells, in contrast to murine RAW cells, are able to restrict the growth of enterococci. This antimicrobial effect is induced by IFN-γ and is mediated by the induction of IDO. Antimicrobial effects caused by the activation of IDO in human cells has been described for human fibroblasts (25), glioblastoma cells (10), and epithelial cells (22) against the intracellular pathogen Toxoplasma gondii. In addition, it has been reported that the growth of intracellular bacteria such as chlamydiae is also inhibited by activation of IDO activity (3). Previously we showed that growth of group B streptococci and some strains of Streptococcus pneumoniae also was inhibited by activation of IDO activity in professional and nonprofessional phagocytes (17, 18).
Streptococci and enterococci are essentially extracellular bacteria; however, it has recently been shown that streptococci can survive in human and murine phagocytes for more than 24 h (5, 31). Once inside the cell, the bacteria are no longer susceptible to antibodies and complement, and it has therefore been suggested that these phagocytes may carry the bacteria throughout the body. If this is so, we believe that an IFN-γ-mediated activation of these bacterium-carrying cells might result in a reduction of the bacterial load. Indeed, it was shown that activation with IFN-γ results in reduced survival of intracellular streptococci; however, the mechanism by which IFN-γ mediated this effect was not described (5).
In most cases of streptococcal and enterococcal infections, local defense mechanisms in which granulocytes and macrophages are the most important cells are sufficient to control bacterial growth and prevent dissemination. Occasionally, local defense mechanisms are insufficient to control infection and streptococci and enterococci enter the bloodstream. It has been shown that streptococci are able to induce cytokine secretion in several cell types (16), and bacterial antigens and superantigens produced by the bacteria result in the activation of T cells and possibly also NK cells, resulting in IFN-γ production (6, 27). In addition, it has been reported that in an in vivo model, IFN-γ treatment results in a reduction of mortality after a group B streptococcal infection (7). The induction of IDO activity in human cells is a potent antimicrobial effector mechanism which is inducible in macrophages (4) and more importantly also in many other cell types, including epithelial and endothelial cells, fibroblasts (25), and several brain cells (8). All of these cells therefore can contribute to the defense against streptococci, especially in the case of a disseminated infection. We suggest that IDO is active against enterococci in the second phase of defense, in which IFN-γ derived from activated T cells is responsible for IDO induction. In addition, we propose that IDO-mediated effects may also contribute to the initial local first-line defense by helping to prevent the proliferation and spread of bacteria from the local site of infection to the bloodstream and other body sites. In this case, IFN-γ is probably derived from NK cells. We believe that these in vitro data are relevant for the in vivo situation. The tryptophan concentration in our culture medium (RPMI 1640 and Iscove's modified Dulbecco's medium, 5 to 15 μg/ml) is comparable to that found in human serum (10 to 15 μg/ml). In addition, we demonstrate that 3 × 104 cells in a volume of 200 μl are sufficient to inhibit bacterial growth, whereas in vivo there are more than 100 times more cells in the same tissue volume. Furthermore, there are many reports in the literature that IFN-γ induces IDO activity in vivo and that the tryptophan concentration in different body fluids is reduced and the kynurenine concentration is increased in various clinical situations in patients with enhanced IFN-γ production (2, 15, 26). The decrease in the plasma tryptophan concentration is about −20% of normal, and with this concentration range no bacteriostatic effect could be observed in vitro. No data concerning the local tryptophan concentration at the side of infection are available, but we assume that, as discussed above, the local IDO-mediated tryptophan degradation is sufficient to inhibit bacterial growth.
The inhibition of IDO activity and of IDO-mediated bacteriostasis by NO is a very interesting effect. It is shown here that NO does not influence the growth of enterococci in our culture system but does inhibit IDO activation. This inhibitory effect was also found when exogenous NO was introduced to an IDO-positive cell as well as in cells in which both effector mechanisms were activated simultaneously by IFN-γ and IL-1. This might be of interest in vivo, since many bacteria are able to induce IL-1 secretion, one of the coinducers involved in iNOS activation in several cells, and this might represent a bacterial modification of the immune defense. Such bacterial antigens inducing regulatory effects have been termed modulins (14). Furthermore, regulation of IDO activity in human cells is necessary, since IDO induction is also an antiproliferative effector mechanism (24), and IDO-mediated tryptophan depletion also affects human cell proliferation. Human cells, however, are protected to some extent against tryptophan starvation by the expression of tryptophan tRNA synthase, an intracellular tryptophan depot, which is also induced by IFN-γ (12).
The interaction of NO with IDO is most likely a posttranslational effect, since IDO is a heme-containing enzyme and it is known that NO regulates the activity of many heme-containing enzymes (23). This was first shown by Thomas et al. (30), who found that NO gas inhibits IDO activity and that IDO activity could be detected in iNOS-positive murine cells after inhibition of NO synthesis. On the other hand, Mellilo et al. (20) described a positive interaction between iNOS and IDO in which picolinic acid, a late degradation product of tryptophan, enhances NO production. Besides iNOS- and IDO-mediated antibacterial effects, the production of toxic oxygen radicals is of importance in the defense against microorganisms. These oxygen radicals interact with NO produced by iNOS activation, resulting in the production of the toxic metabolites such as peroxynitrite (23). Furthermore, oxygen radicals produced by oxidative burst are required as cofactors for the degradation of tryptophan by IDO (13, 28).
Several bacteria are able to induce an oxidative burst in granulocytes and macrophages, therefore providing sufficient amounts of superoxides for the IDO-mediated cleavage of the tryptophan ring. In addition, the consumption of superoxide by IDO-mediated tryptophan cleavage is one of the rare pathways in which toxic oxygen radicals are eliminated while simultaneously activating another mechanism.
We therefore conclude that in human cells IFN-γ-induced IDO activity is a major antibacterial effector mechanism. We suggest that IFN-γ-induced IDO-mediated effects in vivo may be important as a local second-line defense, behind the phagocytic effects mediated by granulocytes and macrophages. We suggest that IDO-mediated bacteriostasis is one of the effects preventing the dissemination of bacteria from the site of the local infection. NO production by human RT4 cells is not an antibacterial effector mechanism, but may function as a regulator of IDO activity. Furthermore, bacterial stimulation of NO production could inhibit IDO in neighboring cells, therefore permitting bacteria such as enterococci and group B streptococci to disseminate in the body.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (grants SFB194 and TPB8) and by the Forschungsförderung der Heinrich-Heine-Universität Düsseldorf.
We thank M. Augst (Dr. Karl Thomae GmbH, Biberach, Germany) for the generous gift of human rIFN-γ. We also thank Claudia Oberdörfer for expert technical assistance.
REFERENCES
- 1.Bilzer T, Stavrou D, Dahme E, Keiditsch E, Dürring K F, Anzil A P, Wechsler W. Morphological-immunocytochemical and growth characteristics of three human glioblastomas established in vitro. Virchows Arch A. 1991;418:281–293. doi: 10.1007/BF01600156. [DOI] [PubMed] [Google Scholar]
- 2.Byrne G I, Lehmann L K, Kirschbaum J G, Borden E C, Lee C M, Brown R R. Induction of tryptophan degradation in vitro and in vivo: a γ-interferon-stimulated activity. J Interferon Res. 1986;6:389–396. doi: 10.1089/jir.1986.6.389. [DOI] [PubMed] [Google Scholar]
- 3.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma interferon mediated inhibition of intracellular Chlamydia psitacci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlin J M, Borden E C, Sondel P M, Byrne G I. Biologic response modifier-induced indolamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987;139:2414–2418. [PubMed] [Google Scholar]
- 5.Cornacchione P, Scaringi L, Fettucciari K, Rosati E, Sabatini R, Orefici G, von Hunolstein C, Modesti A, Modica A, Minelli F, Marconi P. Group B streptococci persist inside macrophages. Immunology. 1998;93:86–95. doi: 10.1046/j.1365-2567.1998.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham M W, Antone S M, Smart M, Liu R, Kosanke S. Molecular analysis of human cardiac myosin-cross-reactive B- and T-cell epitopes of the group A streptococcal M5 protein. Infect Immun. 1997;65:3913–3923. doi: 10.1128/iai.65.9.3913-3923.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cusumano V, Mancuso G, Genovese F, Demetrio D, Beninati C, Losi E, Teti G. Role of gamma interferon in a neonatal mouse model of group B streptococcal disease. Infect Immun. 1996;64:2941–2944. doi: 10.1128/iai.64.8.2941-2944.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Däubener W, Hadding U. Cellular immune reactions directed against Toxoplasma gondii with special emphasis on the central nervous system. Med Microbiol Immunol. 1997;185:195–206. doi: 10.1007/s004300050031. [DOI] [PubMed] [Google Scholar]
- 9.Däubener W, Wanagat N, Pilz K, Seghrouchni S, Fischer H G, Hadding U. A new, simple, bioassay for human IFN-γ. J Immunol Methods. 1994;168:39–47. doi: 10.1016/0022-1759(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 10.Däubener W, Pilz K, Seghrouchni-Zennati S, Bilzer T, Fischer H G, Hadding U. Induction of toxoplasmostasis in a human glioblastoma by interferon-γ. J Neuroimmunol. 1993;43:31–38. doi: 10.1016/0165-5728(93)90072-7. [DOI] [PubMed] [Google Scholar]
- 11.Ding A H, Nathan C F, Stuehr D J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 12.Flohr T, Bange C, von Euch A, Kiekenbeck M, Böttger E C. Depletion of tryptophan is not involved in expression of tryptophanyl-tRNA synthase mediated by interferon. Infect Immun. 1992;60:4418–4421. doi: 10.1128/iai.60.10.4418-4421.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayaishi O, Hirata F, Ohnishi T, Henry J P, Rosenthal I, Katoh A. Indoleamine 2,3-dioxygenase. J Biol Chem. 1977;252:3548–3550. [PubMed] [Google Scholar]
- 14.Henderson B, Wilson M. Modulins: a new class of cytokine-inducing, pro-inflammatory bacterial virulence factor. Inflamm Res. 1995;44:187–197. doi: 10.1007/BF01782257. [DOI] [PubMed] [Google Scholar]
- 15.Heyes M P, Brew B J, Saito K, Quearry B J, Price R W, Lee K, Bhalla R B, Der M, Markey S P. Inter-relationships between quinolonic acid, neuroactive kynurenines, neopterin and β2-microglobulin in cererospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992;40:71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- 16.Hunolstein C, Totolian A, Alfarone G, Mancuso G, Cusumano V, Teti G, Orefici G. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun. 1997;65:4017–4021. doi: 10.1128/iai.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacKenzie C R, Willberg C B, Däubener W. Inhibition of group B streptococcal growth by IFN-γ activated human glioblastoma cells. J Neuroimmunol. 1998;89:191–197. doi: 10.1016/s0165-5728(98)00138-6. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie C R, Hadding U, Däubener W. Interferon-γ-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J Infect Dis. 1998;178:875–878. doi: 10.1086/515347. [DOI] [PubMed] [Google Scholar]
- 19.McCarty M. Streptococci. In: Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, editors. Microbiology—1990. 4th ed. Philadelphia, Pa: J. B. Lippincott Company; 1990. pp. 525–538. [Google Scholar]
- 20.Mellilo G, Cox G W, Radzioch D, Varesio L. Picolinic acid, a catabolite of l-tryptophan, is a costimulus for the induction of reactive nitrogen intermediate production in murine macrophages. J Immunol. 1993;150:4031–4040. [PubMed] [Google Scholar]
- 21.Morris J G, Shay D K, Hebden J N, McCarter R T, Perdue B E, Jarvis W, Johnson J A, Dowling T C, Polish L B, Schwabe R S. Enterococci resistant to multiple antimicrobial agents, including vancomycin. Establishment of endemicity in a university medical center. Ann Intern Med. 1995;123:250–259. doi: 10.7326/0003-4819-123-4-199508150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Nagineni C N, Pardhasaradhi K, Martins M C, Detrick B, Hooks J J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 24.Ozaki Y, Edelstein M P, Duch D S. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon-γ. Proc Natl Acad Sci USA. 1988;85:1242–1246. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfefferkorn E R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanni L A, Thomas S R, Tattam B N, Moore D E, Chaudhri G, Stocker R, Hunt N H. Dramatic changes in oxidative tryptophan metabolism along the kynurenine pathway in experimental cerebral and noncerebral malaria. Am J Pathol. 1998;152:611–619. [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt K H, Gerlach D, Wollweber L, Reichardt W, Mann K, Ozegowski J H, Fleischer B. Mitogenicity of M5 protein extracted from Streptococcus pyogenes cells is due to streptococcal pyrogenic exotoxin C and mitogenic factor MF. Infect Immun. 1995;63:4569–4575. doi: 10.1128/iai.63.12.4569-4575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-Dioxygenase: purification and some properties. J Biol Chem. 1978;253:4700–4706. [PubMed] [Google Scholar]
- 29.Sriskandan S, Moyes D, Buttery L K, Wilkinson J, Evans T J, Polak J, Cohen J. The role of nitric oxide in experimental murine sepsis due to pyrogenic exotoxin A-producing Streptococcus pyogenes. Infect Immun. 1997;65:1767–1772. doi: 10.1128/iai.65.5.1767-1772.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas S R, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-γ primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–14464. [PubMed] [Google Scholar]
- 31.Valentin-Weigand P, Benkel P, Rohde M, Chhatwal G S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]