Abstract
Schistosomiasis is a neglected tropical disease affecting 40 million women of childbearing age worldwide. Its global disease prevalence among pregnant women is still unknown. This meta-analysis determined the pooled prevalence of schistosomiasis among pregnant women globally. Additionally, this study also determined the pooled prevalence based on infection intensity based on eggs per gram. Observational studies on the prevalence of schistosomiasis among pregnant patients were obtained from Medline, Scopus, and CINAHL from January 2001 until August 2020. A review of titles and abstracts was done independently by six reviewers. The quality of the included studies was assessed using the Newcastle–Ottawa Scale for case–control, cohort, and cross-sectional studies. A total of 27 studies were included in the meta-analysis and meta-regression. The pooled prevalence of S. haematobium was 13.44 (CI: 8.90–19.80) per 100 observations, while the pooled prevalence of S. mansoni was 12.18 (CI: 4.47–29.12) per 100 observations. The prevalence of S. japonicum infection in one study was 53.54 (CI: 43.23–63.62) per 100 observations. Our results showed a prevailing health problem of schistosomiasis during pregnancy in various countries worldwide. This strengthens the need to conduct more schistosomiasis research, prevention, and control programs in pregnant women.
Keywords: parasitic infection, pregnancy, prevalence, Schistosoma haematobium, Schistosoma japonicum, Schistosoma mansoni
1. Introduction
Schistosomiasis is a neglected tropical disease caused by parasitic infection of the genus Schistosoma with the most common disease-causing species being Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum [1]. In 2018, it was estimated to affect at least 290.8 million people, claiming 24,068 lives globally [1,2]. It is also highly debilitating, leading to an estimated loss of 1.43 million all-age disability-adjusted life years [3]. The infection is widespread in the tropics and subtropics, with considerable morbidity in parts of the Middle East, South America, Southeast Asia, and sub-Saharan Africa. It is also linked to poverty, leading to chronic ill health [4].
Despite these, its disease burden remains underestimated and has resulted in insufficient resource allocation to schistosomiasis research and control [4]. One population group with a scarcity of data on Schistosoma infections is the population of pregnant women. Currently, schistosomes are estimated to infect 40 million women of childbearing age, but little is known about the prevalence and schistosome-associated morbidity in pregnant women and their newborn [5]. The effects of the disease in pregnant women are thought to be greater as infection will affect the mother and the growing fetus [4]. Although it is not well understood due to the paucity of studies and conflicting results, the proposed mechanisms of schistosomiasis-mediated adverse birth outcomes are placental inflammation, maternal and fetal iron deficiency due to extracorporeal iron loss, anemia of inflammation, and anorexia due to proinflammatory cytokines. These may lead to problems such as intrauterine growth restriction [5]. Additionally, pregnancy is thought to exacerbate Schistosoma-induced pathology due to the immunological shift to Th2 during pregnancy because of progesterone and placental products [6,7]. It has also been hypothesized that maternal and childhood infections reduce the effectiveness of childhood immunizations and increase susceptibility to viral and bacterial diseases [4]. However, these need to be verified and studied more thoroughly.
Initially, there was a lack of adequate and well-controlled studies that demonstrated the safety of using praziquantel as a treatment for schistosomiasis in pregnancy. This became a significant challenge for implementing this treatment program among pregnant women. Although the WHO published a report in 2003 stating that all schistosome-infected pregnant and breastfeeding women be considered high-risk groups and be offered treatment with praziquantel either individually or during treatment campaigns, the lack of sufficient safety data from controlled trials made many countries reluctant to follow this recommendation [8]. This has been improving slowly since 2006, when positive results of two randomized clinical trials encouraged more countries to follow the WHO recommendation [9,10]. In 2011, the World Health Organization advocated for the disease control of schistosomiasis by 2020 and its elimination as a global health problem by 2025. It defines morbidity control as having a prevalence of heavy-intensity infection (PHI) that is below 5% in all sentinel sites and elimination as a public health problem as having a prevalence of heavy-intensity infection below 1% in all sentinel sites [11]. The intensity of infection is the number of schistosomes infecting an individual and is expressed by the number of helminth eggs excreted per gram of feces and by the number of eggs seen per 10 mL of urine. This is used as a marker for morbidity as chronic sequelae are suggested to be associated with cumulative exposure to schistosomes [12]. This may be because eggs excreted by adult schistosome worms need to leave the body in via the fecal route else they become trapped in nearby tissues. This induces a distinct immune-mediated granulomatous response that leads to a range of problems not limited to anemia, impaired cognition, severe hepatosplenism, periportal fibrosis, and urogenital inflammation. However, there is a lack of studies associating the infection intensity with disease burden in pregnant women [13].
As of 2020, some countries still have not yet adopted the mass drug administration policy [12]. Additionally, even in countries that adopt this policy, pregnant women are still excluded because implementers are unaware of the changes. Such exclusion causes millions of women of reproductive age to possibly miss treatment for many years during repeated cycles of pregnancy and lactation [14]. Therefore, it is imperative to advocate for more research in maternal schistosomiasis to help provide policymakers with adequate information for better control strategies in pregnant women to prevent these outcomes and lessen the overall prevalence of schistosomiasis [4]. Identifying and quantifying the effect of infection on the pregnant population may also play a part in improving birth outcomes in endemic areas [14].
2. Materials and Methods
2.1. Study Selection
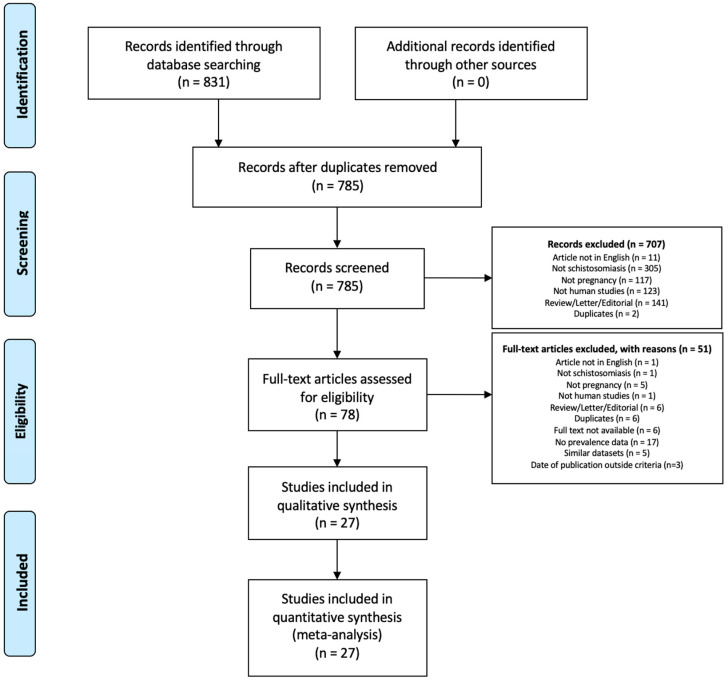
The systematic review and meta-analysis were conducted based on the PRISMA flow diagram (Figure 1). The protocol was registered in PROSPERO (CRD42020212181). A systematic review of the literature was performed in three electronic databases: OVID Medline, Scopus, and CINAHL, from January 2001 to August 2020. Search strings were provided in the supplementary material (Supplementary File S1). Articles included in the study were restricted to those written in English. The most recent or complete study was used for articles with overlapping data and by the same authors. Review articles, letters to the editor, comments, case reports, and preclinical studies were excluded.
Figure 1.
Search strategy and study selection for studies on the prevalence of schistosomiasis in pregnant patients.
2.2. Inclusion and Exclusion Criteria
Eligible studies were included based on the following inclusion criteria: (i) Cross-sectional and cohort studies that determined the prevalence of schistosomiasis (S. japonicum, S. haematobium, and S. mansoni) in pregnant women; (ii) schistosomiasis was diagnosed using molecular, parasitological, rapid diagnostic test (urine CCA), serological, and ultrasonographic methods; (iii) sufficient data to calculate the prevalence of schistosomiasis. The exclusion criteria included schistosomiasis diagnosed by clinical questionnaires without confirmation through standard diagnostic methods for schistosomiasis.
2.3. Data Extraction
A review of titles and abstracts was done independently by six reviewers. Full-text articles were retrieved for all the eligible studies. Full-text articles were evaluated independently by six reviewers (OAT, IKT, MG, LFC, JC, and GAP). All irrelevant articles were excluded, with recordkeeping of the reasons for exclusion. The following data were collected from each included study: first author, year of publication, country of origin, study design, sample size, prevalence or incidence of schistosomiasis, detection methods, and source of specimens.
2.4. Assessment of Study Quality
The quality of the included studies was assessed using the Newcastle–Ottawa Scale for case–control, cohort, and cross-sectional studies. The scale is composed of eight questions covering three domains: (1) selection of study groups (four points for case–control and cohort studies, and five points for cross-sectional studies); (2) comparability of groups (two points); and (3) ascertainment of exposure and outcomes (three points). The scale assigns a maximum score of nine for case–control and cohort studies and a maximum score of ten for cross-sectional studies which represent a high-quality study [15,16].
2.5. Statistical Analysis
All analyses were done in R v.4.1,1 (R Foundation for Statistical Computing, Vienna, Austria) using the ‘meta’ package (version 4.19-0) [17]. The ‘metaprop’ function was used to conduct the meta-analysis of single proportions to obtain the pooled prevalence per species of Schistosoma. A random-effects model was employed through generalized linear mixed models (GLMM) with logit transformation for pooling studies. GLMM, particularly a random intercept logistic regression model, is a one-step approach in pooling proportions, which is generally less biased compared to two-step approaches [17,18]. The 95% confidence intervals for the individual studies were calculated using the Clopper–Pearson method, also known as the exact binomial method, which provides more conservative estimates [19]. Heterogeneity was evaluated using the Cochran’s Q test, the T2, and the I2 statistics. A statistically significant Cochran’s Q test at α = 0.05 indicates evidence of true heterogeneity of effect sizes between studies. The T2 statistic is the estimate of between-studies variance obtained in this study through the maximum likelihood method. The I2 statistic describes the percentage of total variability due to between-study variability, wherein an I2 value of 75% or higher shall be considered to indicate substantial heterogeneity.
Potential sources of heterogeneity were explored through meta-regression using the ‘metareg’ function. Univariable meta-regression was performed on species with at least ten studies [20]. The covariates considered for meta-regression were study design, sample size, specimen for diagnosis, and timing of study relative to approval of praziquantel mass drug administration for pregnant women, all measured as dichotomous variables. The regression coefficient estimates the difference in logit prevalence compared to the reference group and was considered statistically significant at α = 0.05. Publication bias was evaluated through the Egger’s test, wherein a statistically significant intercept at α = 0.05 indicated evidence of funnel plot asymmetry.
3. Results
3.1. Study Selection
In this study, 831 records were selected from three databases of published literature. After the removal of duplicates, 785 unique records remained. After the selection process, 27 studies were included in the qualitative synthesis, meta-analysis, and meta-regression (Figure 1).
3.2. Study Characteristics
Most studies (26/27) were from African countries and only one was from the Philippines (Supplementary Table S1). Seven (7) were cohort studies and 20 cross-sectional. A steady increase was noted from 2005 until 2020. The most common species of Schistosoma diagnosed among pregnant patients was S. haematobium (20/27), followed by S. mansoni (8/27), and S. japonicum (1/27). Four (4) studies detected both S. haematobium and S. mansoni (Supplementary Table S1).
The most used sample for diagnosing schistosomiasis among pregnant patients was urine sample alone (15/27), followed by stool sample alone (6/27). Several studies used both stool and urine samples (6/27). In terms of diagnosing schistosomiasis, the most used was urine filtration, centrifugation, and microscopy (17/27) and stool Kato Katz (8/27) (Supplementary Table S1).
Most cross-sectional studies were assessed to be of good quality with an average score of 7.80 based on the Newcastle–Ottawa Scale (Supplementary Table S2). The cohort studies were similarly assessed as good quality with an average score of 6.86 (Supplementary Table S3).
3.3. Synthesis of Meta-Analysis Results
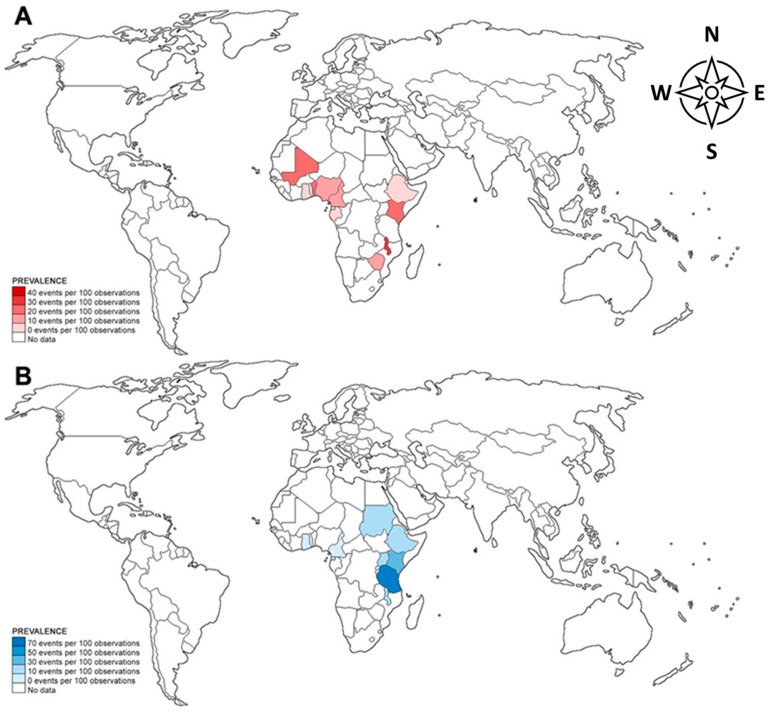
In terms of geographic distribution, the prevalence of S. haematobium and S. mansoni was highest among pregnant women in African countries while S. japonicum was only reported in pregnant women from the Philippines. S. haematobium infection among pregnant women was highest in Malawi (32.31%), while S. mansoni infection was highest in Tanzania (63.48%) (Figure 2).
Figure 2.
Pooled prevalence of S. haematobium (A) and S. mansoni (B) infection among pregnant patients in different countries. Borders of countries on the map do not imply any political statement.
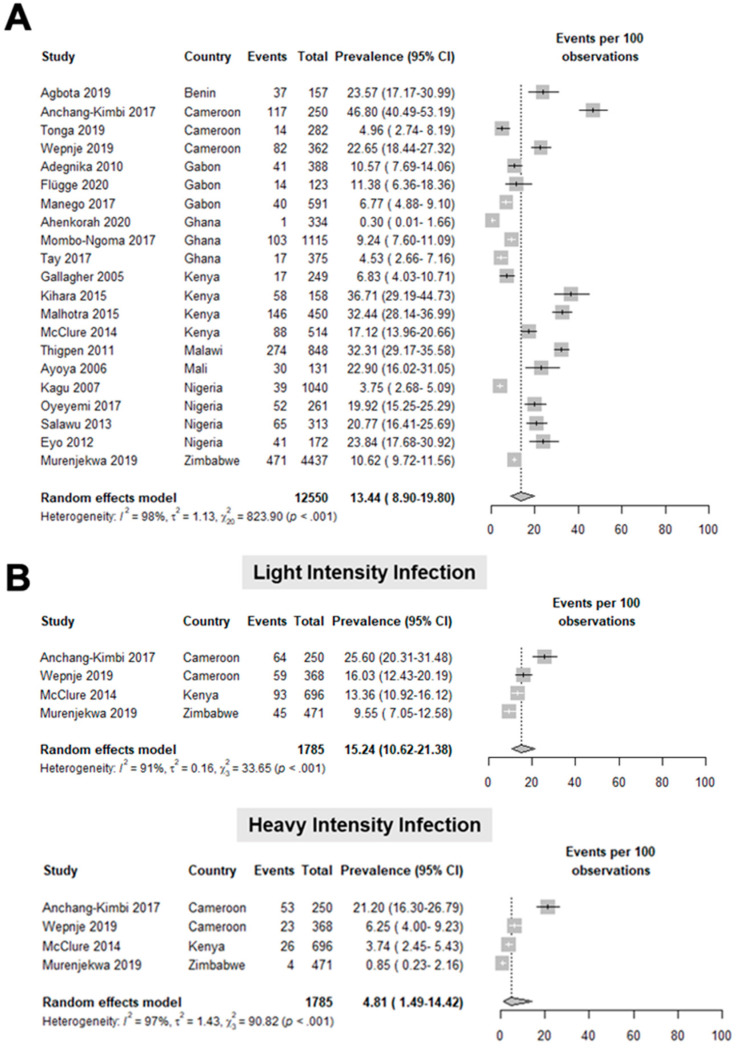
Based on 21 studies [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39] involving 12,550 pregnant women, the pooled prevalence of S. haematobium infection was 13.44 (8.90–19.80) per 100 observations (Figure 3A). The studies included in this meta-analysis had a high heterogeneity, as shown by a T2 value of 1.13, an I2 value of 98%, and a statistically significant Q test (p < 0.001). Upon classifying the intensity of S. haematobium infections, it was noted that light-intensity infection had a pooled prevalence of 15.24 (10.62–21.38) per 100 observations, while the heavy-intensity infection was 4.81 (1.49–14.42) per 100 observations (Figure 3B; Supplementary Table S4).
Figure 3.
Forest plot for the (A) general prevalence (events per 100 observations) and (B) prevalence per intensity of infection of S. haematobium in pregnant patients worldwide [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Based on one study [40] with 99 pregnant women, the prevalence of S. japonicum among pregnant women in the Philippines was 53.54%. We then performed a subgroup analysis to determine the prevalence of S. japonicum infection based on the intensity of infection. The prevalence of light-intensity S. japonicum infection was 42.42 (32.55–52.77) per 100 observations, while the moderate-intensity infection was 11.11 (5.68–19.01) per 100 observations (Supplementary Table S4).
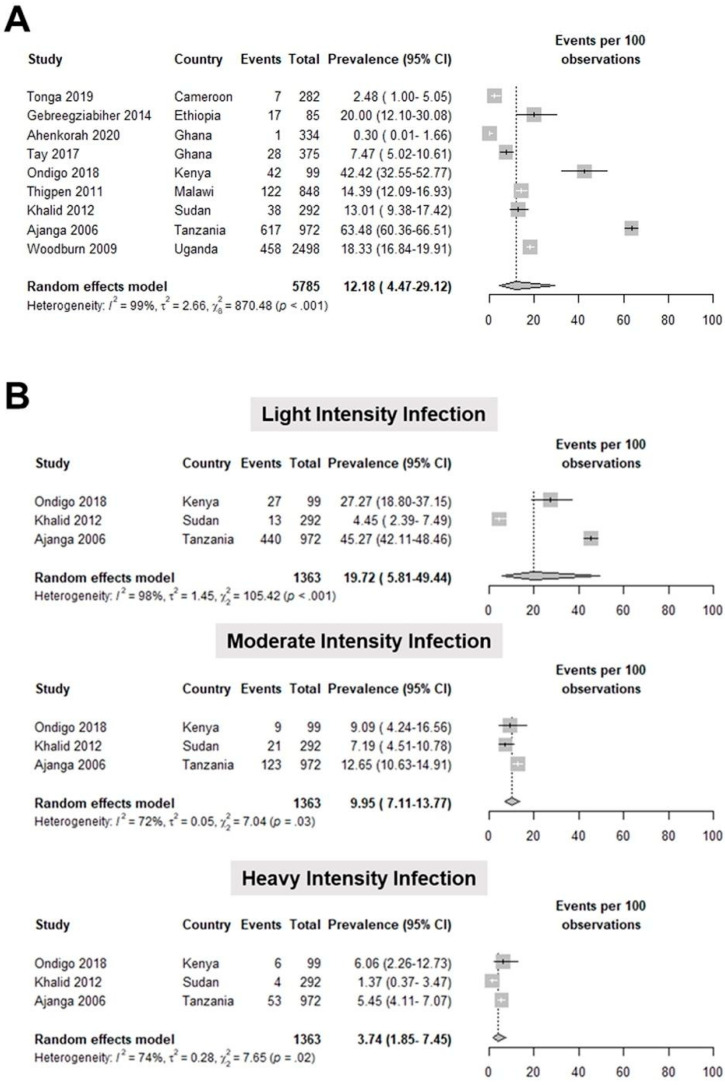
Meta-analysis of nine studies [28,29,30,32,41,42,43,44,45] involving 5785 pregnant women showed that the pooled prevalence of S. mansoni infection was 12.18 (4.47–29.12) per 100 observations (Figure 4A). The studies included in this meta-analysis had a high heterogeneity, as shown by a T2 value of 2.66, an I2 value of 99%, and a statistically significant Q test (p < 0.001). We further classified the intensity of S. mansoni infection and determined the prevalence of light-intensity infection to be 19.72 (5.81–49.44) per 100 observations. The pooled prevalence of moderate-intensity infection was 9.95 (7.11–13.37) per 100 observations, while heavy intensity-infection was 3.74 (1.85–7.45) per 100 observations (Figure 4B; Supplementary Table S4).
Figure 4.
Forest plot for the (A) general prevalence (events per 100 observations) and (B) prevalence per intensity of infection of S. mansoni in pregnant patients worldwide [28,29,30,32,41,42,43,44,45].
3.4. Meta-Regression
Meta-regression was performed to investigate potential contributing factors to the heterogeneity of studies on the logit prevalence of S. haematobium (Table 1) among pregnant women, namely, study design, publication of the study before or after the approval of praziquantel mass drug administration for pregnant women, sample size, and specimen used for diagnosing schistosomiasis. Univariable analysis for S. haematobium infection revealed insufficient evidence of a linear relationship between the tested covariates and the logit prevalence (Table 1). The number of studies for S. haematobium was inadequate to conduct multivariable meta-regression with all covariates included in the model.
Table 1.
Results of univariable meta-regression analysis exploring the source of heterogeneity for studies on the prevalence of S. haematobium in pregnant women.
| Variable | Number of Studies Included in Analysis | β (95% CI) | p-Value | I2 (%) | T2 |
|---|---|---|---|---|---|
| Study Design | |||||
| Cohort (ref: Cross-Sectional) | 21 | −0.01 (−1.09, 1.07) | 0.9840 | 98.47 | 1.13 |
| Study Conducted after Praziquantel MDA for Pregnant Women | |||||
| Yes (ref: No) | 20 | 0.13 (−0.98, 1.24) | 0.8187 | 98.50 | 1.17 |
| Sample Size | |||||
| ≥500 (ref: <500) | 21 | −0.34 (−1.34, 0.66) | 0.5076 | 98.38 | 1.09 |
| Specimen for Diagnosis | |||||
| Urine (ref: others) | 21 | −0.21 (−1.23, 0.81) | 0.6836 | 98.40 | 1.12 |
3.5. Publication Bias
Results of classical tests of asymmetry, particularly the Egger’s test, revealed no sufficient evidence of publication bias or small-study effects for S. haematobium (p = 0.76) and S. mansoni (p = 0.40) (Supplementary Table S5).
4. Discussion
A total of 27 studies were included for the qualitative synthesis, meta-analysis, and meta-regression for the prevalence of schistosomiasis in pregnancy. Only studies done from 2001 were included because it was then that the WHO promoted praziquantel for mass drug administration through the Fifty-fourth World Health Assembly in resolution WHA54.19 [46]. Data before this year may not reflect the current global efforts against schistosomiasis and may skew the current prevalence.
The most common species diagnosed among pregnant patients was S. hematobium with a pooled prevalence of 13.44%. This was followed by S. mansoni with a pooled prevalence of 12.18%; finally, S. japonicum, found only in one study, has a prevalence of 53.54%. A similar meta-analysis of schistosomiasis in pregnancy, which included 32 studies in Africa, had a pooled prevalence estimate of 13.2% [47]. The availability of schistosomiasis prevalence classified according to infection intensity based on eggs per gram (EPG) of stool for S. mansoni and eggs per 10 mL urine for S. haematobium in several studies allowed further analysis on this parameter [25,31,35,40,41,43,44,48]. According to WHO, S. haematobium infection intensity is classified as light infection if with 1–49 eggs per 10 mL of urine and heavy infection if with ≥50 eggs per 10 mL of urine. For S. mansoni, it is classified according to the number of eggs per gram of stool: 1–99 EPG for light infection, 100–399 EPG for moderate infection, and ≥400 EPG for heavy infection [49]. It can be observed that there is a decreasing trend in both S. mansoni and S. haematobium in prevalence as the intensity increases. The analysis for S. haematobium showed high heterogeneity, but none of the potential sources included in the analysis showed sufficient evidence of a linear relationship to the logit prevalence.
There are limited studies comparing the prevalence of schistosomiasis in pregnant and non-pregnant women. One study in Kenya reported that the prevalence of S. haematobium in non-pregnant women was 30% compared to 36.94% in pregnant women [39]. The non-pregnant women had an intensity of 40.88 eggs/10 mL urine compared to 51.21 eggs/10 mL urine in pregnant women. However, the prevalence and intensity of infection between the two populations are not statistically different. In addition, several other studies reported the prevalence of schistosomiasis in women of reproductive age, although not specifying the exclusion of pregnant women in their studies. These ranged from 4.5% in Tanzania to 62% in Uganda [50,51,52,53,54]. With these limited findings, it may be possible to suspect that maternal schistosomiasis is either only carried over from infection before their pregnancy or that the immunologic changes of pregnancy may have increased the susceptibility of pregnant patients to schistosomiasis [55].
Pregnancy causes significant changes in the body’s immune response. Generally, pregnancy causes a bias toward an anti-inflammatory environment to avoid fetal rejection—affecting disease pathogenesis during pregnancy. Several proteins and hormones are altered during pregnancy to favor the anti-inflammatory milieu. For example, the activity of steroids that suppress the transcriptional regulation of inflammatory cytokines such as IFN-γ, which has essential anti-viral and anti-parasitic properties, is suppressed. This contributes to significantly worse outcomes from infectious diseases during pregnancy [56]. Specifically, schistosomiasis infection during pregnancy is exacerbated due to an immunologic shift to Th2, probably through the up-regulation of parasite-specific IL-4 due to progesterone and placental products [6].
Accounting for the anti-inflammatory shift during pregnancy and the poorly understood sequelae of maternal schistosomiasis in the offspring, it is crucial to decrease the disease burden in pregnant women. In addition to these, schistosomiasis infection may also be a risk factor for several diseases, including human immunodeficiency syndrome (HIV) and human papillomavirus (HPV) infection [57,58]. Praziquantel (PZQ) is the drug of choice against all schistosome infections with its reliable therapeutic effectiveness, even on severe morbidity in endemic areas [14]. A randomized clinical trial on PZQ in pregnant women with low-intensity infection showed that the treatment effectively reduced maternal infection. Results show a significantly higher proportion of women with more than 90% reduction in eggs per gram compared to placebo and a cure rate of 83.7% [10]. Another study showed a decrease in S. mansoni infection from 16.7% to 4.8% at delivery with praziquantel treatment [9]. These support the efficacy of PZQ in reducing the schistosomiasis burden in pregnant women, despite the suppression of antibody levels against schistosomes and PZQ-induced boosts in antibody levels [59]. Before the treatment of schistosomiasis in pregnancy was endorsed by the WHO, PZQ treatment was avoided during pregnancy and lactation due to the lack of safety studies [8]. This served as a significant barrier to treating schistosomiasis in pregnant women. Despite the WHO recommendation to include pregnant women in PZQ treatment, there are still countries that have not adopted this due to the lack of relevant information and a failure to carry out appropriate risk: benefit analyses about the use of anthelminthic drugs during pregnancy and lactation; and for the countries that have, the changes may not have been properly endorsed to the implementers, leading to a lack of awareness regarding the changes in maternal schistosomiasis treatment [14,60].
The WHO had a goal of morbidity control, a prevalence of heavy-intensity infection below 5% in all sentinel sites by 2020 [11]. This brings the question: has the goal of disease control been reached among pregnant women? As the WHO guidelines did not include 95% confidence intervals, the pooled prevalence of S. haematobium and S. mansoni suggests that disease control has been achieved among pregnant women. However, an upper limit of 95% CI surpassing 5% and the paucity of studies in other endemic countries do not prove this achievement further. It is not easy to compare whether our results mirror the global initiative for schistosomiasis control. In African countries, routine monitoring and evaluation of schistosomiasis disease control are limited to school-age children [61]. This selectivity in monitoring may fail to capture unintentional reservoirs, such as adults, particularly pregnant women.
In 2022, the WHO strongly recommended including pregnant women after the first trimester and lactating women in the treatment coverage in endemic communities where the infection is ≥10%. The annual preventive chemotherapy in this group, aside from those two years and above, includes a single dose of praziquantel at ≥75% treatment coverage to control schistosomiasis morbidity and move forward to achieve the elimination of schistosomiasis as a public health problem. A specific systematic review commissioned by the Guideline Development Group presented data to prove the safety of praziquantel for preventive chemotherapy and treatment in children aged ≥2 years, adults, pregnant women after the first trimester, and lactating women with moderate certainty of evidence [12]. In the same WHO Guideline on Control and Elimination of Human Schistosomiasis in 2022, the WHO proposes that health facilities offer praziquantel treatment to all people positive for schistosomiasis without regard to age, including pregnant women who are infected (but excluding those in their first trimester), lactating women and pre-SAC aged <2 years. To implement this, pregnancy status should be evaluated by subtly asking the woman. If she is unsure of her status, there should be a negative urine-based test before treatment is given.
Schistosomiasis is a highly debilitating but treatable and preventable neglected tropical disease [1]. However, despite the disease burden, there are still insufficient studies and resource allocation to schistosomiasis research and control. One of the barriers to the diagnosis and treatment of schistosomiasis in pregnancy includes poor sensitivity of the Kato–Katz technique in diagnosing schistosomiasis infection. These problems are encompassed by the lack of maternal health care access, research, and policies in the affected countries. More studies need to be conducted to help us determine the actual global burden of schistosomiasis among pregnant women. For example, several African countries, including Liberia, Cote d’Ivoire, Sierra Leone, and Guinea, have a high pediatric schistosomiasis endemicity but have no data for the prevalence of maternal schistosomiasis [4]. There are also no prevalence data of schistosomiasis infection in the Middle East, Indonesia, and China, countries that are also endemic to the infection [62]. There are also limited studies on the maternal and neonatal adverse outcomes caused by schistosomiasis. This data is vital in the clinical management of pregnant patients afflicted with schistosomiasis. This data will also inform health policymakers in improving antenatal care for these patients. Improving the information on schistosomiasis burden among pregnant patients will require standardizing the specimen, time and frequency of collection, and diagnostic tests used to detect schistosomiasis among pregnant patients.
This study provided the most recent and comprehensive global prevalence of schistosomiasis among pregnant patients. This study also showed the prevalence of schistosomiasis in pregnant women based on the intensity of the infection. Only studies that used the WHO classification were included in the analysis [63]. However, the results presented in this meta-analysis and meta-regression may be subject to several limitations. While we tried to identify studies published before and after the approval of praziquantel MDA for pregnant women, most studies did not report whether the population included in their studies received praziquantel. This may have affected the result of prevalence studies. Multivariable meta-regression, which may evaluate the relationship of a single covariate to logit prevalence while adjusting for other covariates, was not performed due to a lack of studies. Egger’s test for asymmetry showed that there was not sufficient evidence of publication bias or small study effects for S. haematobium and S. mansoni; however, it is essential to note that these methods may be unreliable, especially in cases with studies having low or high prevalence outcomes [64]. Results may also be unreliable in the case of S. mansoni where there are fewer than ten studies, thus decreasing the statistical power of the test. While it is unlikely that studies reporting single proportions or prevalence decide against publishing results compared to comparative studies, publication bias may arise from the exclusion of studies published in other languages, and, given the databases searched, exclusion of non-published data possibly due to poorly designed studies, which can either attenuate or accentuate the pooled prevalence.
5. Conclusions
The meta-analysis results show a prevailing health problem of schistosomiasis infection during pregnancy in various countries worldwide. It also emphasizes that schistosomiasis disease control has yet to be achieved. Additionally, it highlights the need for integrated prevention and control strategies and sufficient resource allocation for maternal schistosomiasis research to improve its diagnosis and treatment. Furthermore, antenatal care should be implemented especially in areas endemic to schistosomiasis, to understand better the lasting impacts of schistosomiasis infection on pregnant women and their offspring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed7110354/s1, File S1: Search Strategy; Table S1: Qualitative synthesis of schistosomiasis studies included in the meta-analyses; Table S2: Quality assessment of cross-sectional studies using the Newcastle–Ottawa Scale; Table S3: Quality assessment of cohort studies using the Newcastle–Ottawa Scale; Table S4: Prevalence of schistosomiasis in pregnant women based on the intensity of infection; Table S5: Egger’s test of publication bias.
Author Contributions
Conceptualization, O.A.G.T.; methodology, L.F.T.C., G.A.S.P., O.A.G.T., M.D.D., J.A.C., M.J.G.G. and I.K.B.T.; validation, L.F.T.C., G.A.S.P., O.A.G.T. and M.D.D.; formal analysis, L.F.T.C., G.A.S.P., O.A.G.T., M.D.D., J.A.C., M.J.G.G. and I.K.B.T.; data curation, L.F.T.C., G.A.S.P., O.A.G.T. and M.D.D.; writing—original draft preparation, L.F.T.C., G.A.S.P., O.A.G.T. and M.D.D.; writing—review and editing, O.A.G.T., L.R.L. and I.K.B.T.; visualization, L.F.T.C. and M.D.D.; supervision, L.R.L. and I.K.B.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting reported results can be found in the Supplementary File.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McManus D.P., Dunne D.W., Sacko M., Utzinger J., Vennervald B.J., Zhou X.-N., World Health Organization. Friedman J.F., Olveda R.M., Mirochnick M.H., et al. Schistosomiasis. Trends Parasitol. 2018;21:29. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO Methods and Data Sources for Country-Level Causes of Death 2000–2016. World Health Organization; Geneva, Switzerland: 2018. [Google Scholar]
- 3.Kyu H.H., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salawu O.T., Odaibo A.B. Maternal Schistosomiasis: A Growing Concern in Sub-Saharan Africa. Pathog. Glob. Health. 2014;108:263–270. doi: 10.1179/2047773214Y.0000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman J.F., Mital P., Kanzaria H.K., Olds G.R., Kurtis J.D. Schistosomiasis and Pregnancy. Trends Parasitol. 2007;23:159–164. doi: 10.1016/j.pt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Farah I.O., Langoi D., Nyaundi J., Hau J. Schistosome-Induced Pathology Is Exacerbated and Th2 Polarization Is Enhanced During Pregnancy. In Vivo. 2007;21:599–602. [PubMed] [Google Scholar]
- 7.Kelly R.W., Critchley H.O.D. A T-Helper-2 Bias in Decidua: The Prostaglandin Contribution of the Macrophage and Trophoblast. J. Reprod. Immunol. 1997;33:181–187. doi: 10.1016/S0165-0378(97)00021-1. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Report of the WHO Informal Consultation on the Use of Praziquantel during Pregnancy/Lactation and Albendazole/Mebendazole in Children under 24 Months. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 9.Ndibazza J., Muhangi L., Akishule D., Kiggundu M., Ameke C., Oweka J., Kizindo R., Duong T., Kleinschmidt I., Muwanga M., et al. Effects of Deworming during Pregnancy on Maternal and Perinatal Outcomes in Entebbe, Uganda: A Randomized Controlled Trial. Clin. Infect. Dis. 2010;50:531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olveda R.M., Acosta L.P., Tallo V., Baltazar P.I., Lesiguez J.L.S., Estanislao G.G., Ayaso E.B., Monterde D.B.S., Ida A., Watson N., et al. Efficacy and Safety of Praziquantel for the Treatment of Human Schistosomiasis during Pregnancy: A Phase 2, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2016;16:199–208. doi: 10.1016/S1473-3099(15)00345-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Expert Consultation to Accelerate Elimination of Asian Schistosomiasis, Shanghai, China, 22–23 May 2017: Meeting Report. World Health Organization; Geneva, Switzerland: 2017. Regional Office for the Western Pacific. [Google Scholar]
- 12.World Health Organization . WHO Guideline on Control and Elimination of Human Schistosomiasis. World Health Organization; Geneva, Switzerland: 2022. [PubMed] [Google Scholar]
- 13.Colley D.G., Bustinduy A.L., Secor W.E., King C.H. Human Schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman J.F., Olveda R.M., Mirochnick M.H., Bustinduy A.L., Elliott A.M. Praziquantel for the Treatment of Schistosomiasis during Human Pregnancy. Bull. World Health Organ. 2018;96:59–65. doi: 10.2471/BLT.17.198879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: Comparing Reviewers’ to Authors’ Assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 1 January 2022)]. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Balduzzi S., Rücker G., Schwarzer G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based. Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L., Chu H. Meta-Analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology. 2020;31:713–717. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agresti A., Coull B.A. Approximate Is Better than “Exact” for Interval Estimation of Binomial Proportions. Am. Stat. 1998;52:119–126. doi: 10.1080/00031305.1998.10480550. [DOI] [Google Scholar]
- 20.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons; Hoboken, NJ, USA: 2021. [Google Scholar]
- 21.Adegnika A.A., Ramharter M., Agnandji S.T., Ateba Ngoa U., Issifou S., Yazdanbahksh M., Kremsner P.G. Epidemiology of Parasitic Co-Infections during Pregnancy in Lambaréné, Gabon. Trop. Med. Int. Health. 2010;15:1204–1209. doi: 10.1111/j.1365-3156.2010.02598.x. [DOI] [PubMed] [Google Scholar]
- 22.Agbota G., Polman K., Wieringa F.T., Campos-Ponce M., Accrombessi M., Yovo E., Roucher C., Ezinmègnon S., Marcos J.Y., Vachot L., et al. Maternal Malaria but Not Schistosomiasis Is Associated with a Higher Risk of Febrile Infection in Infant during the First 3 Months of Life: A Mother-Child Cohort in Benin. PLoS ONE. 2019;14:e0222864. doi: 10.1371/journal.pone.0222864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra I., McKibben M., Mungai P., McKibben E., Wang X., Sutherland L.J., Muchiri E.M., King C.H., King C.L., LaBeaud A.D. Effect of Antenatal Parasitic Infections on Anti-Vaccine IgG Levels in Children: A Prospective Birth Cohort Study in Kenya. PLoS Negl. Trop. Dis. 2015;9:e0003466. doi: 10.1371/journal.pntd.0003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mombo-Ngoma G., Honkpehedji J., Basra A., Mackanga J.R., Zoleko R.M., Zinsou J., Agobe J.C.D., Lell B., Matsiegui P.-B., Gonzales R., et al. Urogenital Schistosomiasis during Pregnancy Is Associated with Low Birth Weight Delivery: Analysis of a Prospective Cohort of Pregnant Women and Their Offspring in Gabon. Int. J. Parasitol. 2017;47:69–74. doi: 10.1016/j.ijpara.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Murenjekwa W., Makasi R., Ntozini R., Chasekwa B., Mutasa K., Moulton L.H., Tielsch J.M., Humphrey J.H., Smith L.E., Prendergast A.J., et al. Determinants of Urogenital Schistosomiasis among Pregnant Women and Its Association with Pregnancy Outcomes, Neonatal Deaths and Child Growth. J. Infect. Dis. 2019. in press . [DOI] [PMC free article] [PubMed]
- 26.Oyeyemi O.T., Odaibo A.B. Maternal Urogenital Schistosomiasis; Monitoring Disease Morbidity by Simple Reagent Strips. PLoS ONE. 2017;12:e0187433. doi: 10.1371/journal.pone.0187433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salawu O.T., Odaibo A.B. Schistosomiasis among Pregnant Women in Rural Communities in Nigeria. Int. J. Gynaecol. Obstet. 2013;122:1–4. doi: 10.1016/j.ijgo.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Tay S.C.K., Nani E.A., Walana W. Parasitic Infections and Maternal Anaemia among Expectant Mothers in the Dangme East District of Ghana. BMC Res. Notes. 2017;10:3. doi: 10.1186/s13104-016-2327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thigpen M.C., Filler S.J., Kazembe P.N., Parise M.E., Macheso A., Campbell C.H., Newman R.D., Steketee R.W., Hamel M. Associations between Peripheral Plasmodium Falciparum Malaria Parasitemia, Human Immunodeficiency Virus, and Concurrent Helminthic Infection among Pregnant Women in Malawi. Am. J. Trop. Med. Hyg. 2011;84:379–385. doi: 10.4269/ajtmh.2011.10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonga C., Ngo Bayoi C., Tchanga F.C., Yengue J.F., Wepnje G.B., Nyabeyeu Nyabeyeu H., Kangam L., Koudjip Nono L., Akono Ntonga P., Lehman L.G. Schistosomiasis among Pregnant Women in Njombe-Penja Health District, Cameroon. J. Infect. Dev. Ctries. 2019;13:1150–1158. doi: 10.3855/jidc.11767. [DOI] [PubMed] [Google Scholar]
- 31.Wepnje G.B., Anchang-Kimbi J.K., Ndassi V.D., Lehman L.G., Kimbi H.K. Schistosoma Haematobium Infection Status and Its Associated Risk Factors among Pregnant Women in Munyenge, South West Region, Cameroon Following Scale-up of Communal Piped Water Sources from 2014 to 2017: A Cross-Sectional Study. BMC Public Health. 2019;19:392. doi: 10.1186/s12889-019-6659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahenkorah B., Nsiah K., Baffoe P., Ofosu W., Gyasi C., Owiredu E.-W. Parasitic Infections among Pregnant Women at First Antenatal Care Visit in Northern Ghana: A Study of Prevalence and Associated Factors. PLoS ONE. 2020;15:e0236514. doi: 10.1371/journal.pone.0236514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anchang-Kimbi J.K., Elad D.M., Sotoing G.T., Achidi E.A. Coinfection with Schistosoma Haematobium and Plasmodium Falciparum and Anaemia Severity among Pregnant Women in Munyenge, Mount Cameroon Area: A Cross-Sectional Study. J. Parasitol. Res. 2017;2017:6173465. doi: 10.1155/2017/6173465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoya M.A., Spiekermann-Brouwer G.M., Traoré A.K., Stoltzfus R.J., Garza C. Determinants of Anemia among Pregnant Women in Mali. Food Nutr. Bull. 2006;27:3–11. doi: 10.1177/156482650602700101. [DOI] [PubMed] [Google Scholar]
- 35.Eyo J.E., Onyishi G.C., Okafor F.C. Urinary Schistosomiasis among Pregnant Women in Some Endemic Tropical Semi-Urban Communities of Anambra State, Nigeria. Trop. Biomed. 2012;29:575–579. [PubMed] [Google Scholar]
- 36.Flügge J., Adegnika A.A., Honkpehedji Y.J., Sandri T.L., Askani E., Manouana G.P., Massinga Loembe M., Brückner S., Duali M., Strunk J., et al. Impact of Helminth Infections during Pregnancy on Vaccine Immunogenicity in Gabonese Infants. Vaccines. 2020;8:381. doi: 10.3390/vaccines8030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher M., Malhotra I., Mungai P.L., Wamachi A.N., Kioko J.M., Ouma J.H., Muchiri E., King C.L. The Effects of Maternal Helminth and Malaria Infections on Mother-to-Child HIV Transmission. AIDS. 2005;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 38.Kagu M.B., Kawuwa M.B., Gadzama G.B. Anaemia in Pregnancy: A Cross-Sectional Study of Pregnant Women in a Sahelian Tertiary Hospital in Northeastern Nigeria. J. Obstet. Gynaecol. 2007;27:676–679. doi: 10.1080/01443610701612144. [DOI] [PubMed] [Google Scholar]
- 39.Kihara J.H., Kutima H.L., Ouma J., Churcher T.S., Changoma J.M., Mwalisetso M.A., French M.D., Mwandawiro C.S. Urogenital Schistosomiasis in Women of Reproductive Age and Pregnant Mothers in Kwale County, Kenya. J. Helminthol. 2015;89:105–111. doi: 10.1017/S0022149X13000643. [DOI] [PubMed] [Google Scholar]
- 40.Kurtis J.D., Higashi A., Wu H.-W., Gundogan F., McDonald E.A., Sharma S., PondTor S., Jarilla B., Sagliba M.J., Gonzal A., et al. Maternal Schistosomiasis Japonica Is Associated with Maternal, Placental, and Fetal Inflammation. Infect. Immun. 2011;79:1254–1261. doi: 10.1128/IAI.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ajanga A., Lwambo N.J.S., Blair L., Nyandindi U., Fenwick A., Brooker S. Schistosoma Mansoni in Pregnancy and Associations with Anaemia in Northwest Tanzania. Trans. R. Soc. Trop. Med. Hyg. 2006;100:59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Gebreegziabiher D., Desta K., Howe R., Abebe M. Helminth Infection Increases the Probability of Indeterminate QuantiFERON Gold in Tube Results in Pregnant Women. Biomed Res. Int. 2014;2014:364137. doi: 10.1155/2014/364137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalid A., Abdelgadir M.A., Ashmaig A., Ibrahim A.M., Ahmed A.-A.M., Adam I. Schistosoma Mansoni Infection among Prenatal Attendees at a Secondary-Care Hospital in Central Sudan. Int. J. Gynaecol. Obstet. 2012;116:10–12. doi: 10.1016/j.ijgo.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Ondigo B.N., Muok E.M.O., Oguso J.K., Njenga S.M., Kanyi H.M., Ndombi E.M., Priest J.W., Kittur N., Secor W.E., Karanja D.M.S., et al. Impact of Mothers’ Schistosomiasis Status During Gestation on Children’s IgG Antibody Responses to Routine Vaccines 2 Years Later and Anti-Schistosome and Anti-Malarial Responses by Neonates in Western Kenya. Front. Immunol. 2018;9:1402. doi: 10.3389/fimmu.2018.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodburn P.W., Muhangi L., Hillier S., Ndibazza J., Namujju P.B., Kizza M., Ameke C., Omoding N.E., Booth M., Elliott A.M. Risk Factors for Helminth, Malaria, and HIV Infection in Pregnancy in Entebbe, Uganda. PLoS Negl. Trop. Dis. 2009;3:e473. doi: 10.1371/journal.pntd.0000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Assembly . Fifty-Fourth World Health Assembly, Geneva, 14–22 May 2001: Resolutions and Decisions. World Health Organization; Geneva, Switzerland: 2001. [Google Scholar]
- 47.Adam I., ALhabardi N.A., Al-Wutayd O., Khamis A.H. Prevalence of Schistosomiasis and Its Association with Anemia among Pregnant Women: A Systematic Review and Meta-Analysis. Parasit. Vectors. 2021;14:133. doi: 10.1186/s13071-021-04642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClure E.M., Meshnick S.R., Mungai P., Malhotra I., King C.L., Goldenberg R.L., Hudgens M.G., Siega-Riz A.M., Dent A.E. The Association of Parasitic Infections in Pregnancy and Maternal and Fetal Anemia: A Cohort Study in Coastal Kenya. PLoS Negl. Trop. Dis. 2014;8:e2724. doi: 10.1371/journal.pntd.0002724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wiegand R.E., Secor W.E., Fleming F.M., French M.D., King C.H., Montgomery S.P., Evans D., Utzinger J., Vounatsou P., de Vlas S.J. Open Forum Infectious Diseases. Volume 8. Oxford University Press; New York, NY, USA: 2021. Control and Elimination of Schistosomiasis as a Public Health Problem: Thresholds Fail to Differentiate Schistosomiasis Morbidity Prevalence in Children; p. ofab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Downs J.A., Mguta C., Kaatano G.M., Mitchell K.B., Bang H., Simplice H., Kalluvya S.E., Changalucha J.M., Johnson W.D.J., Fitzgerald D.W. Urogenital Schistosomiasis in Women of Reproductive Age in Tanzania’s Lake Victoria Region. Am. J. Trop. Med. Hyg. 2011;84:364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kjetland E.F., Kurewa E.N., Ndhlovu P.D., Midzi N., Gwanzura L., Mason P.R., Gomo E., Sandvik L., Mduluza T., Friis H., et al. Female Genital Schistosomiasis—A Differential Diagnosis to Sexually Transmitted Disease: Genital Itch and Vaginal Discharge as Indicators of Genital Schistosoma Haematobium Morbidity in a Cross-Sectional Study in Endemic Rural Zimbabwe. Trop. Med. Int. Health. 2008;13:1509–1517. doi: 10.1111/j.1365-3156.2008.02161.x. [DOI] [PubMed] [Google Scholar]
- 52.Poggensee G., Kiwelu I., Weger V., Göppner D., Diedrich T., Krantz I., Feldmeier H. Female Genital Schistosomiasis of the Lower Genital Tract: Prevalence and Disease-Associated Morbidity in Northern Tanzania. J. Infect. Dis. 2000;181:1210–1213. doi: 10.1086/315345. [DOI] [PubMed] [Google Scholar]
- 53.Rite E.E., Kapalata S.N., Munisi D.Z. Prevalence, Intensity, and Factors Associated with Urogenital Schistosomiasis among Women of Reproductive Age in Mbogwe District Council, Geita Region, Tanzania. Biomed Res. Int. 2020;2020:5923025. doi: 10.1155/2020/5923025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seto E.Y.W., Sousa-Figueiredo J.C., Betson M., Byalero C., Kabatereine N.B., Stothard J.R. Patterns of Intestinal Schistosomiasis among Mothers and Young Children from Lake Albert, Uganda: Water Contact and Social Networks Inferred from Wearable Global Positioning System Dataloggers. Geospat. Health. 2012;7:1–13. doi: 10.4081/gh.2012.99. [DOI] [PubMed] [Google Scholar]
- 55.Mor G., Cardenas I. The Immune System in Pregnancy: A Unique Complexity. Am. J. Reprod. Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson D.P., Klein S.L. Pregnancy and Pregnancy-Associated Hormones Alter Immune Responses and Disease Pathogenesis. Horm. Behav. 2012;62:263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Downs J.A., van Dam G.J., Changalucha J.M., Corstjens P.L.A.M., Peck R.N., de Dood C.J., Bang H., Andreasen A., Kalluvya S.E., van Lieshout L., et al. Association of Schistosomiasis and HIV Infection in Tanzania. Am. J. Trop. Med. Hyg. 2012;87:868–873. doi: 10.4269/ajtmh.2012.12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swai B., Poggensee G., Mtweve S., Krantz I. Female Genital Schistosomiasis as an Evidence of a Neglected Cause for Reproductive Ill-Health: A Retrospective Histopathological Study from Tanzania. BMC Infect. Dis. 2006;6:134. doi: 10.1186/1471-2334-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tweyongyere R., Mawa P.A., Emojong N.O., Mpairwe H., Jones F.M., Duong T., Dunne D.W., Vennervald B.J., Katunguka-Rwakishaya E., Elliott A.M. Effect of Praziquantel Treatment of Schistosoma Mansoni during Pregnancy on Intensity of Infection and Antibody Responses to Schistosome Antigens: Results of a Randomised, Placebo-Controlled Trial. BMC Infect. Dis. 2009;9:32. doi: 10.1186/1471-2334-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savioli L., Crompton D.W.T., Neira M. Use of Anthelminthic Drugs during Pregnancy. Am. J. Obstet. Gynecol. 2003;188:5–6. doi: 10.1067/mob.2003.78. [DOI] [PubMed] [Google Scholar]
- 61.Deol A.K., Fleming F.M., Calvo-Urbano B., Walker M., Bucumi V., Gnandou I., Tukahebwa E.M., Jemu S., Mwingira U.J., Alkohlani A., et al. Schistosomiasis—Assessing Progress toward the 2020 and 2025 Global Goals. N. Engl. J. Med. 2019;381:2519–2528. doi: 10.1056/NEJMoa1812165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunette G.W. CDC Yellow Book 2018: Health Information for International Travel. Oxford University Press; New York, NY, USA: 2017. [Google Scholar]
- 63.World Health Organization . Tropical Disease Research, TDR Strategic Direction: Schistosomiasis, WHO-TDR. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 64.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In Meta-Analyses of Proportion Studies, Funnel Plots Were Found to Be an Inaccurate Method of Assessing Publication Bias. J. Clin. Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting reported results can be found in the Supplementary File.