Abstract
Subtractive hybridization was employed to isolate specific genes from virulent Porphyromonas gingivalis strains that are possibly related to abscess formation. The genomic DNA from the virulent strain P. gingivalis W83 was subtracted with DNA from the avirulent strain ATCC 33277. Three clones unique to strain W83 were isolated and sequenced. The cloned DNA fragments were 885, 369, and 132 bp and had slight homology with only Bacillus stearothermophilus IS5377, which is a putative transposase. The regions flanking the cloned DNA fragments were isolated and sequenced, and the gene structure around the clones was revealed. These three clones were located side-by-side in a gene reported as an outer membrane protein. The three clones interrupt the open reading frame of the outer membrane protein gene. This inserted DNA, consisting of three isolated clones, was designated IS1598, which was 1,396 bp (i.e., a 1,158-bp open reading frame) in length and was flanked by 16-bp terminal inverted repeats and a 9-bp duplicated target sequence. IS1598 was detected in P. gingivalis W83, W50, and FDC 381 by Southern hybridization. All three P. gingivalis strains have been shown to possess abscess-forming ability in animal models. However, IS1598 was not detected in avirulent strains of P. gingivalis, including ATCC 33277. The IS1598 may interrupt the synthesis of the outer membrane protein, resulting in changes in the structure of the bacterial outer membrane. The IS1598 isolated in this study is a novel insertion element which might be a specific marker for virulent P. gingivalis strains.
Porphyromonas gingivalis, a potent pathogen of periodontal disease, has been classified into virulent and avirulent strains based on its ability to form necrotic abscesses (AFNA) in animal models (5, 8, 13, 23). It has been suggested that the AFNA of virulent P. gingivalis plays an important role in the initiation and progression of periodontal disease (5, 23). In fact, it has been reported that virulent strains of P. gingivalis were frequently isolated from severe periodontal lesions, whereas avirulent strains were detected more commonly in healthy subjects (8). There is currently great interest in virulence factors involved in necrotic abscess formation. Several attempts have been made to identify the virulence factors associated with abscess formation. However, definitive factors specific to virulent strains or factors involved in abscess formation have not yet been isolated. Biochemical profiles of potential virulence factors such as lipopolysaccharides, proteinases, etc., are almost the same in both virulent and avirulent strains (23). In recent years, the search for virulence factors of microorganisms has been greatly facilitated by the use of molecular genetics (20, 21, 24, 25). Analysis of genetic elements is crucial to understanding the properties and roles of virulence factors. The subtractive hybridization (SH) technique has great potential in the comparison of genomic sequences between related bacterial strains that differ in virulence (1). The purpose of the current study was to identify a gene for the virulence factor of P. gingivalis related to the AFNA. The SH technique was used in this study, and a unique gene was detected in the virulent strain P. gingivalis W83 by comparing its chromosomal DNA with the DNA from the avirulent strain P. gingivalis ATCC 33277.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
P. gingivalis W83 and ATCC 33277 were used for this study as the virulent and avirulent strains, respectively. Other bacterial strains used in this study included P. gingivalis W50, SU63, SUNY 1021, FDC 381, and ESO 89; Porphyromonas asaccharolytica ATCC 25260; Prevotella loescheii ATCC 15930; Porphyromonas endodontalis ATCC 35406; Prevotella intermedia ATCC 25611; Capnocytophaga ochracea S3; Fusobacterium nucleatum ATCC 25586; Actinobacillus actinomycetemcomitans Y4; and Campylobacter rectus ATCC 33238. Each Porphyromonas, Prevotella, Capnocytophaga, and Fusobacterium strain was cultured in GAM broth (Nissui Seiyaku, Tokyo, Japan) supplemented with 0.0005% hemin and 0.0001% vitamin K3 at 37°C for 24 to 48 h in an anaerobic box (model ANX-1; Hirasawa Works, Tokyo, Japan) containing 80% N2, 10% H2, and 10% CO2. A. actinomycetemcomitans Y4 and C. rectus ATCC 33238 were grown as previously described (11, 14, 16). The bacterial cells were harvested by centrifugation at 10,000 × g for 20 min at 4°C and were stored at −80°C until use. Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) was used for cloning and was cultured aerobically in Luria-Bertani (LB) medium. LB broth and agar were supplemented with ampicillin (Sigma Chemical Co., St. Louis, Mo.) at 50 μg/ml when bacteria carrying plasmids were cultured. The pUC118 and pUC19 plasmids (Takara Shuzo, Otsu, Shiga, Japan) served as vectors for cloning, subcloning, and sequencing.
Subtractive hybridization.
The chromosomal DNAs were purified from P. gingivalis W83 (target) and P. gingivalis ATCC 33277 (subtracter) by the method of Stauffer et al. (29) and were digested with endonucleases Sau3AI and SmaI, respectively. The SmaI-digested DNA fragments of P. gingivalis ATCC 33277 were labeled with Photoprobe Biotin (Vector Laboratories, Burlingame, Calif.) according to the manufacturer's instructions.
SH was carried out according to the method described by Bjourson et al. (1). Briefly, 20 μg of Sau3AI-digested target DNA fragments from P. gingivalis W83 and 200 μg of biotin-labeled SmaI-digested subtracter DNA fragments from P. gingivalis ATCC 33277 were mixed with 50 μl of hybridization solution containing 50 mM HEPES (pH 7.5), 500 mM NaCl, 1 mM EDTA, and 0.1% sodium dodecyl sulfate (SDS). The mixture was overlaid with 2 drops of mineral oil, heated at 99°C for 10 min, rapidly cooled on ice, and then incubated at 64°C for 48 h. A volume of the mixture was adjusted to 550 μl by adding a solution containing 50 mM HEPES (pH 7.5), 500 mM NaCl, and 1 mM EDTA, and the mixture below the mineral oil was pipetted out. To remove the biotin-labeled DNA fragments (subtracter), 50 μl (1 mg/ml) of streptavidin was added to the mixture, and phenol-chloroform (50:50 [vol/vol]) extraction was performed, by which the denatured biotin-streptavidin complex appeared in an interlayer phase with the conjugated DNA fragments. The aqueous phase was recovered, and SDS solution was added for a final concentration of 0.1%. After additional phenol-chloroform extraction, the DNA fragments in the aqueous phase were precipitated in ethanol and then ligated to the bacterial alkaline phosphate-treated BamHI site of pUC118 (Takara Shuzo). Competent E. coli XL1-Blue cells were transformed with the ligated plasmids. By blue-white selection, positive colonies were isolated on LB agar plates supplemented with 50 μg of ampicillin per ml, isopropyl-β-d-thiogalactopyranoside, and 5-bromo-4-chloro-3-indolyl-β-d-galactoside and then transferred to new LB agar plates and cultured at 37°C overnight.
Colony hybridization.
Colony hybridization was performed to isolate genes unique to strain W83 according to the method of Maniatis et al. (19). The white colonies on the plates were transferred to nylon membranes (Hybond-N+; Amersham, Arlington Heights, Ill.), which were probed with the Sau3AI-digested DNA fragments (whole genomic DNA) from P. gingivalis ATCC 3277 labeled with [α-32P]dCTP (Amersham) by using the Random Primer Labeling kit (Stratagene). The clones that did not react with the probe were selected as candidates specific for P. gingivalis W83 and were subjected to further characterization.
Southern hybridization.
Southern hybridization was performed as described by Southern (28), with slight modifications (22). Genomic DNAs from various anaerobic bacteria were digested with endonuclease PstI and electrophoresed on 1.0% agarose gel (SeaKem GTG Agarose; FMC BioProducts, Rockland, Maine). The separated DNA fragments were transferred onto nylon membranes (Hybond-N+) under alkaline conditions (0.4 M NaOH transfer buffer). The DNA fragments, which were isolated by SH, were labeled with [α-32P]dCTP as described above and was used as a probe. Hybridization was performed in a hybridization buffer described Church and Gilbert (3) at 65°C overnight with 2 × 106 cpm/ml of labeled probe. After hybridization, membrane was washed at 65°C for 1 h with a washing buffer containing 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.5% SDS and then washed twice at 65°C for 1 h with a washing buffer containing 0.2× SSC and 0.1% SDS. Autoradiography was performed with an intensifying screen at −70°C for 6 h.
Cloning of the entire gene containing the DNA fragments isolated by SH.
For further characterization of the DNA fragments isolated by SH, the flanking regions were isolated based on sized DNA fragments found to be positive by Southern hybridization. P. gingivalis W83 chromosomal DNA was digested with endonuclease PstI and separated by agarose gel electrophoresis (SeaKem GTG Agarose). DNA fragments between 1.8 and 6.0 kb which hybridized with the DNA fragments isolated by SH (see Results) were excised from the gel and purified with a Qiaex II Gel Extraction kit (Qiagen GmbH, Hilden, Germany). The purified DNA fragments were ligated to the PstI-site of pUC118, and a size-restricted library was constructed. The library was screened with the DNA fragment isolated by SH.
Analyses of nucleotide sequences and amino acid sequences.
DNA sequencing was performed by using an ABI Prism DNA Sequencing kit (Perkin-Elmer, Foster City, Calif.), based on the dideoxy chain termination method, and a 373A automated DNA sequencer (Applied Biosystems, Foster City, Calif.). The revealed sequence data were analyzed with a Genetyx sequence analysis program (version 8.0; Software Development Co., Tokyo, Japan) and subjected to a homology search by using the Genetyx homology search program at the GenBank database, the BLAST sequence homology search system, and the SWISS-PROT protein database at GenomeNet.
Northern hybridization.
Total RNA was isolated from P. gingivalis W83 and ATCC 33277 strains by using TRIzol reagent (GIBCO-BRL Life Technologies, Grand Island, N.Y.) according to the method described by Chomczynski and Sacchi (2). RNA (10 μg for each lane) was electrophoresed in a formaldehyde gel (final concentration, 6.3% formaldehyde and 1.0% agarose) and was transferred to a nylon membrane (Hybond-N+) with alkaline transfer buffer (0.05 M NaOH) for 3 h. The DNA fragment isolated by SH and pga67 (the gene encoding the 67-kDa outer membrane protein [OMP] in reference 10) were labeled with [α-32P]dCTP by using the Random Primer Labeling kit (Stratagene). Hybridization and washing were performed under the same conditions as described above for Southern hybridization. Autoradiography was performed with an intensifying screen at −70°C for a week.
Nucleotide sequence accession number.
The nucleotide sequences of the cloned gene described here have been assigned the following GenBank accession numbers: KIS-1, AB003149; KIS-2, AB003150; KIS-3, AB011547; and IS1598, AB009361.
RESULTS
Isolation of DNA fragments unique to P. gingivalis W83.
SH was performed to distinguish the virulent P. gingivalis W83 strain from the avirulent P. gingivalis ATCC 33277 strain at the molecular level and to identify genes specific to the P. gingivalis virulent strain. After SH, 192 colonies were obtained by blue-white selection. Colony hybridization was performed to isolate genes unique to the W83 strain by using a probe prepared from the whole genomic DNA (SmaI-digested fragments) of P. gingivalis ATCC 33277. Twenty clones did not hybridize with the probe and were selected as DNA unique to the W83 strain. Restriction fragment length polymorphism (RFLP) analysis revealed that there were three different clones among the 20. The insert sizes of each clone were 885, 369, and 132 bp. They were designated KIS-1, KIS-2, and KIS-3 respectively, and were used for further characterization.
Characterization of the DNA fragments unique to the W83 strain.
The nucleotide sequences of the three isolated clones were determined (Fig. 1 and 2) and subjected to a homology search. Amino acid sequences deduced from the nucleotide sequences of KIS-1 and KIS-2 showed homology (29.6 and 35.2%) only to Bacillus stearothermophilus IS5377 (32), which is a putative transposase. The KIS-3 clone did not show significant homology to any registered sequence.
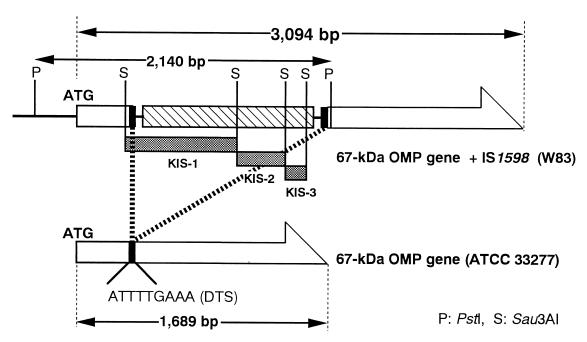
FIG. 1.
Restriction map of the 2.1-kb PstI-digested DNA fragment containing IS1598. KIS-1, KIS-2, and KIS-3 are located in tandem on the 2.1-kb cloned DNA fragment (shaded bars). The 67-kDa OMP gene (pga67 [10]) is shown as a large open arrow. The pga67 is interrupted by the insertion of IS1598 at the DTS site and is shown as solid bars. The hatched bar indicates the ORF in IS1598.
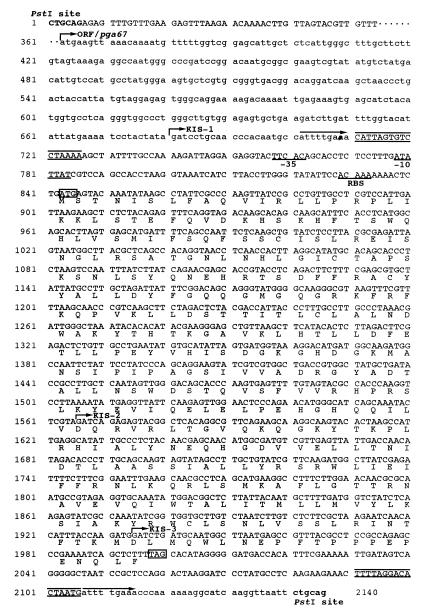
FIG. 2.
Nucleotide sequences and structure of the 2.1-kb neighbor region of IS fragment. Nucleotide numbers are indicated to the left. PstI sites at the both ends of the cloned 2.1-kb DNA fragment are shown in boldface. The locations of KIS-1, KIS-2, and KIS-3 are shown by indicating the 5′-end Sau3AI site of each fragment (vertical bar with arrow). pga67 is shown in lowercase letters (the start codon is shown by an arrow with ORF/pga67). The nucleotide sequence of the 5′-flanking region of the pga67 is partially omitted (indicated by dots). A DTS indicated by arrows above the sequence is found at both ends of IS1598, and the TIR indicated by lines above and below the sequence are found inside of each DTS. The ATG start codon and the TGA stop codon of the IS1598 are indicated by boxes. Putative translational products of IS1598 are given below the nucleotide sequence by a single uppercase letter. Upstream regulatory sequences are underlined and represent the −35 and −10 sequences and RBS (ribosome-binding site).
We focused on KIS-2 since it was the most likely among the three to be a homologue of the transposase. For isolation of the flanking region, KIS-2 was labeled with [α-32P]dCTP and used as a probe for Southern hybridization and colony hybridization. Southern hybridization revealed that the multiple PstI-digested DNA fragments of the chromosomal DNA from the W83 strain hybridized with KIS-2 of 1.8 to 6.0 kb (Fig. 3, lane 1). Thus, the size-restricted library (1.8 to 6.0-kb PstI-digested fragments in pUC118) was screened to obtain the flanking regions of KIS-2 by colony hybridization. Three clones were isolated from 104 colonies. RFLP analysis revealed that these three clones had the same 2.1-kb PstI-digested DNA fragment. Nucleotide sequence analysis of the 2.1-kb DNA fragment showed the following characteristics. (i) Three fragments, KIS-1, KIS-2, and KIS-3, were found to be tandem aligned in the 2.1-kb PstI-digested DNA fragment (Fig. 1 and 2). (ii) A single open reading frame (ORF) consisting of 1,158 nucleotides, which encodes a putative polypeptide of 386 amino acids, was found in the 2.1-kb fragment (Fig. 2). The deduced amino acid sequence of the ORF showed a 28.3% homology to B. stearothermophilus IS5377 on the SWISS-PROT protein database. (iii) The sequence also contained 16-bp terminal inverted repeats (TIR), and 9-bp duplicated target sequences (DTS; ATTTTGAAA) were present outside each TIR (Fig. 2). (iv) At both ends of the 2.1-kb fragment, a gene encoding the 67-kDa OMP (10) of P. gingivalis was found (Fig. 1 and 2). The 67-kDa OMP gene (pga67) was interrupted by an insertion of the sequences containing the ORF, TIR, and DTS.
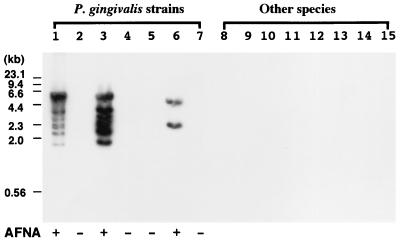
FIG. 3.
Distribution of IS1598 in several gram-negative bacteria. Southern hybridization of PstI-digested genomic DNA from each strain was carried out with KIS-2 as a probe. Lanes: 1, P. gingivalis W83; 2, P. gingivalis ATCC 33277; 3, P. gingivalis W50; 4, P. gingivalis SU63; 5, P. gingivalis SUNY 1021; 6, P. gingivalis FDC 381; 7, P. gingivalis ESO 89; 8, P. asaccharolytica ATCC 25260; 9, P. loescheii ATCC 15930; 10, P. endodontalis ATCC 35406; 11, P. intermedia ATCC 25611; 12, C. ochracea S3; 13, F. nucleatum ATCC 25586; 14, A. actinomycetemcomitans Y4; 15, C. rectus ATCC 33238. P. gingivalis strains were classified based on AFNA and are indicated as “+” (AFNA positive) or “−” (AFNA negative) at the bottom of the panel.
The sequence of the 2.1-kb PstI-digested DNA fragment showed characteristic properties of bacterial insertion sequence (IS) elements. The novel IS-like gene isolated from P. gingivalis W83 in this study was registered in the Plasmid Reference Center (17) and was designated IS1598.
Prevalence of IS1598 in oral gram-negative bacteria.
We investigated the distribution of IS1598 in 14 strains of oral gram-negative bacteria by Southern hybridization with KIS-2 as a probe (Fig. 3). The PstI-digested chromosomal DNA from virulent strains W83, W50, and FDC 381 had positive hybridization signals. The KIS-2 probe did not hybridize with DNA from avirulent strains or from other bacteria that were tested. Multiple hybridized bands, at least six of them distinguishable, were detected in P. gingivalis W83 and W50 between the sizes of 1.8 and 6.0 kb. Two hybridized bands were present in the PstI-digested DNA from the FDC 381 strain at the sizes of 2.3 and 5.0 kb.
Expression of IS1598 in P. gingivalis.
Northern hybridization was performed in order to determine whether IS1598 is transcribed in W83 by using KIS-2 as a probe. The expression of pga67 was examined as an internal control. pga67 was transcribed in both W83 and ATCC 33277 (shown by an asterisk in Fig. 4), but the sizes of transcripts were different. The pga67 transcript detected in W83 strain (3.3 kb) was larger than that of ATCC 33277 (1.9 kb). The strong signals of IS1598 transcripts were detected in W83 strain with various sizes around the pga67 transcript. On the other hand, no signal was detected in the ATCC 33277 strain.
FIG. 4.
Transcripts from IS1598 and pga67. Total RNAs (10 μg/lane) isolated from W83 and ATCC 33277 strains were probed with IS1598 and pga67 by Northern hybridization. An asterisk shows a size difference of the hybridization signals between the two strains. Hybridization signal with the pga67 was detected at the 1.8-kb length in ATCC 33277 strain and was detected at the 3.0-kb length in W83. The hybridization signals in both strains corresponded well with the expected size (Fig. 1). Transcripts of IS1598 were detected only in W83 strain but varied in size.
DISCUSSION
Virulent and avirulent strains of P. gingivalis have been classified in animal models based on the AFNA (8). Biochemical approaches to compare the growth requirements and enzyme activities of P. gingivalis have failed to identify differences between the virulent and avirulent strains (23). Recently, the SH technique has been developed as a useful tool to identify genetic differences between closely related bacterial strains or strains associated with virulence or genomic mutation (1).
Three DNA fragments, KIS-1, KIS-2, and KIS-3, which are unique to P. gingivalis W83 were isolated in this study. Based on the homology analyses of the deduced amino acid sequences, two of them (KIS-1 and KIS-2) were found to be homologous to B. stearothermophilus IS5377, a putative transposase (32). It has been reported that bacterial IS often causes mutations resulting in a number of genetic defects, including disruption of gene function, polarity effects, and activation of nearby genes or DNA rearrangements, all of which may modify of bacterial characteristics (4). It is possible that the bacterial transposon and/or IS have been incorporated into P. gingivalis virulent strains and affect virulence related to abscess forming ability. This possibility led us to isolate the entire IS-like gene from the P. gingivalis virulent strain W83.
The entire IS-like gene was isolated from a size-restricted genomic DNA library of P. gingivalis W83 and was designated IS1598. Nucleotide sequence analysis revealed that IS1598 is 1,396 bp long and is flanked by a characteristic 16-bp TIR and a 9-bp DTS. In general, the size of IS elements varies from 0.7 to 7.1 kb, including the characteristic TIR and DTS (12). IS1598 contained a single ORF (1,158 bp) encoding a putative polypeptide of 386 amino acids. The GenBank homology search indicated that IS1598 does not exhibit significant similarity to any known IS elements except for in B. stearothermophilus IS5377. The G+C content of IS1598 was 44.7% and was lower than that of P. gingivalis chromosomal DNA, which is approximately 46 to 48% (26). Shea et al. (27) reported that the reduced G+C content has been linked to the presence of pathogenicity islands, which are large stretches of DNA associated with virulence and thought to originate from other species. The low G+C content is thought to be related to gene transfer. Sequence data and the evidence of previous studies suggest that IS1598 is a novel IS element which might be unique to P. gingivalis W83.
Southern hybridization analysis revealed that the IS1598 was present in three P. gingivalis strains, W83, W50, and FDC 381, all of which are known to be capable of forming abscess in animal models (8). The IS1598 was not detected in avirulent strains, including ATCC 33277, or in related anaerobic bacteria as far as examined. Previously, two novel IS elements, IS1126 and PGIS2, were isolated and characterized from P. gingivalis W83 and A7436, respectively (18, 31). However, these two IS elements were present in both virulent and avirulent strains of P. gingivalis (30). The IS1598 isolated in this study was specifically present in P. gingivalis virulent strains. Therefore, it can be a good marker for virulent P. gingivalis and may be related to abscess-forming ability.
Genco et al. (6, 7) described the development of an efficient transpositional mutagenesis system by using the virulent P. gingivalis strain A7436 and a transposon (Tn4351) from Bacteroides fragilis. The Tn4351-generated mutants showed more infectious and virulent properties than the parent strain in mouse models. Furthermore, these authors reported morphological and structural alteration of the cell surface and an overproduction of membrane vesicles in these mutants as seen by electron microscopic observation. Genco et al. (7) suggested that Tn4351 may cause the mutation in structural genes of OMPs and modify the virulence of P. gingivalis. Bacterial OMPs were thought to be important virulence factors in the pathogenicity of periodontopathic bacteria (9). We have shown that IS1598 was found in the structural gene for the 67-kDa OMP of P. gingivalis. This pga67 was isolated from the avirulent ATCC 33277 strain, and its product was reported as an immunodominant antigen (10). On the other hand, it should be noticed that the 67-kDa OMP was not detected in the virulent strains (15). Transcripts of IS1598 were detected in the W83 strain but not in ATCC 33277 by Northern hybridization. Although the transcript of pga67 was detected in both strains W83 and ATCC 33277, the size of the transcript in W83 strain was larger than that of the ATCC 33277 strain. The longer signal may be a complex of pga67 and IS1598 transcripts. Thus, the insertion of IS1598 may interrupt the translation of pga67, resulting in the lack of 67-kDa OMP in W83 strain.
IS1598 was found in genomic DNA from representative virulent P. gingivalis W83 and W50 strains as multiple copies and was found in P. gingivalis FDC381 at two sites. Corresponding to the result of Southern hybridization, the various sizes of IS1598 transcripts were detected in W83 strain, suggesting multiple insertion of IS1598 in structural genes other than pga67. P. gingivalis W83 and W50 are more virulent than the FDC 381 strain based on the abscess-formation ability in animal models (8). Thus, the degree of AFNA of P. gingivalis may be related to the IS1598 copy number. We have examined locations of IS1598 in whole P. gingivalis W83 genome through the TIGR (The Institute for Genomic Research) microbial database. IS1598 elements were found at 13 sites in the genome database, although most sites were in unknown area. Two insertion sites were revealed in addition to the pga67. One of them is located 100 bp downstream of the gene encoding the methyl malonyl coenzyme A mutase large subunit. Another location of IS1598 was revealed 1,388-bp downstream from the gene for 16S rRNA. Further characterization of the functions and the target sites of IS1598 in P. gingivalis W83, W50, and FDC 381 will allow us to understand the role of this gene in AFNA and in the pathogenicity of P. gingivalis in periodontal disease.
ACKNOWLEDGMENTS
We thank Charles F. Shuler (Director, Center for Craniofacial Molecular Biology, University of Southern California) for helpful discussions.
This study was supported by Grants-in-Aid for Scientific Research (C09671953, C11672082, and C11897023) and Grants-in-Aid for Encouragement of Young Scientists (B09922076 and B09922079) from the Ministry of Education, Science, Sports, and Culture of Japan.
ADDENDUM
The P. gingivalis insertion sequence ISPg4 transposase gene isolated from virulent strain W50 was submitted to GenBank (accession no. AF148127) by Jackson and Reynolds (13a) during the revision of our manuscript. This ISPg4 locates in the downstream of the gene encoding the methylmalonyl-CoA mutase large subunit and is completely identical to our IS1598 at the amino acid sequence level. At this point, there is no information on their ISPg4 in P. gingivalis W50.
REFERENCES
- 1.Bjourson A J, Stone C E, Cooper J E. Combined subtraction hybridization and polymerase chain reaction amplification procedure for isolation of strain-specific Rhizobium DNA sequences. Appl Environ Microbiol. 1992;58:2296–2301. doi: 10.1128/aem.58.7.2296-2301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 3.Church G M, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 5.Genco C A, Cutler C W, Kapczynski D, Maloney K, Arnold R R. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991;59:1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genco C A, Schifferle R E, Njoroge T, Forng R Y, Cutler C W. Resistance of a Tn4351-generated polysaccharide mutant of Porphyromonas gingivalis to polymorphonuclear leukocyte killing. Infect Immun. 1995;63:393–401. doi: 10.1128/iai.63.2.393-401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genco C A, Simpson W, Forng R Y, Egal M, Odusanya B M. Characterization of a Tn4351-generated hemin uptake mutant of Porphyromonas gingivalis: evidence for the coordinate regulation of virulence factors by hemin. Infect Immun. 1995;63:2459–2466. doi: 10.1128/iai.63.7.2459-2466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grenier D, Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987;25:738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt S C, Bramanti T E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- 10.Hongyo H, Kokeguchi S, Kurihara H, Miyamoto M, Maeda H, Takashiba S, Murayama Y. Comparative study of two outer membrane protein genes from Porphyromonas gingivalis. Microbios. 1998;95:91–100. [PubMed] [Google Scholar]
- 11.Hongyo H, Kurihara H, Kokeguchi S, Miyamoto M, Maeda H, Hayakawa M, Abiko Y, Takashiba S, Murayama Y. Molecular cloning and characterization of the gene encoding 53-kD outer membrane protein of Porphyromonas gingivalis. Microbios. 1997;92:47–57. [PubMed] [Google Scholar]
- 12.Iida S, Marcoli R, Bickle T A. Variant insertion element IS1 generates 8-base pair duplications of the target sequence. Nature. 1981;294:374–376. doi: 10.1038/294374a0. [DOI] [PubMed] [Google Scholar]
- 13.Isoshima O, Ohta H, Kurihara K, Kato K, Fukui K, Murayama K. Distribution of black-pigmented Prevotella and Porphyromonas species in the dentition of moderate periodontitis patients. Microb Ecol Health. 1995;8:159–169. [Google Scholar]
- 13a.Jackson, C. A., and E. C. Reynolds. Unpublished data.
- 14.Kokeguchi S, Kato K, Kurihara H, Murayama Y. Cell surface protein antigen from Wolinella recta ATCC 33238T. J Clin Microbiol. 1989;27:1210–1217. doi: 10.1128/jcm.27.6.1210-1217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokeguchi S, Kato K, Kurihara H, Nishimura F, Murayama Y. Purification and characterization of two major outer membrane proteins from Porphyromonas gingivalis. Dent Jpn. 1990;27:29–34. [PubMed] [Google Scholar]
- 16.Kokeguchi S, Kato K, Nishimura F, Kurihara H, Murayama Y. Isolation and partial characterization of a 39-kDa major outer membrane protein of Actinobacillus actinomycetemcomitans Y4. FEMS Microbiol Lett. 1991;61:85–89. doi: 10.1016/0378-1097(91)90018-6. [DOI] [PubMed] [Google Scholar]
- 17.Lederberg E M. Plasmid Reference Center Registry of transposon (Tn) and insertion sequence (IS) allocations through December 1986. Gene. 1987;51:115–118. doi: 10.1016/0378-1119(87)90299-x. [DOI] [PubMed] [Google Scholar]
- 18.Maley J, Roberts I S. Characterization of IS1126 from Porphyromonas gingivalis W83: a new member of the IS4 family of insertion sequence elements. FEMS Microbiol Lett. 1994;123:219–224. doi: 10.1111/j.1574-6968.1994.tb07225.x. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. pp. 123–124. [Google Scholar]
- 20.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 21.Miller V L, Bliska J B, Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990;172:1062–1029. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto M, Noji S, Kokeguchi S, Kato K, Kurihara H, Murayama Y, Taniguchi S. Molecular cloning and sequence analysis of antigen gene tdpA of Treponema denticola. Infect Immun. 1991;59:1941–1947. doi: 10.1128/iai.59.6.1941-1947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neiders M E, Chen P B, Suido H, Reynolds H S, Zambon J J, Shlossman M, Genco R J. Heterogeneity of virulence among strains of Bacteroides gingivalis. J Periodontal Res. 1989;24:192–198. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 24.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah H N, Collins M D. Proposal for reclassification of Bacteroides asaccharolyticus, Bacteroides gingivalis, and Bacteroides endodontalis in a new genus, Porphyromonas. Int J Syst Bacteriol. 1988;381:128–131. [Google Scholar]
- 27.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer G V, Plamann M D, Stauffer L T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981;14:63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Ikeda T, Noguchi T, Yoshimura F. Detection and prevalence of IS1126, an insertion sequence, Porphyromonas gingivalis by Southern blot analysis. Dent Jpn. 1997;33:111–115. [Google Scholar]
- 31.Wang C Y, Bond V C, Genco C A. Identification of a second endogenous Porphyromonas gingivalis insertion element. J Bacteriol. 1997;179:3808–3812. doi: 10.1128/jb.179.11.3808-3812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu K, He Z Q, Mao Y M, Sheng R Q, Sheng Z J. On two transposable elements from Bacillus stearothermophilus. Plasmid. 1993;29:1–9. doi: 10.1006/plas.1993.1001. [DOI] [PubMed] [Google Scholar]