Abstract
Background
Nivolumab is an anti-PD-1 antibody approved for treating metastatic melanoma (MM), for which still limited evidence is available on the correlation between drug exposure and patient outcomes.
Methods
In this observational retrospective study, we assessed whether nivolumab concentration is associated with treatment response in 88 patients with MM and if the patient’s genetic profile plays a role in this association.
Results
We observed a statistically significant correlation between nivolumab serum concentration and clinical outcomes, measured as overall and progression-free survival. Moreover, patients who achieved a clinical or partial response tended to have higher levels of nivolumab than those who reached stable disease or had disease progression. However, the difference was not statistically significant. In particular, patients who reached a clinical response had a significantly higher concentration of nivolumab and presented a distinct genetic signature, with more marked activation of ICOS and other genes involved in effector T-cells mediated proinflammatory pathways.
Conclusions
In conclusion, these preliminary results show that in patients with MM, nivolumab concentration correlates with clinical outcomes and is associated with an increased expression of ICOS and other genes involved in the activation of T effectors cells.
Keywords: Melanoma, Immunotherapy, Tumor Biomarkers
What is already known on this topic
Nivolumab is effective in treating metastatic melanoma. Still there is limited evidence on the correlation between drug exposure, patient’s pretreatment immune constitution and response.
What this study adds
The serum concentration of nivolumab correlates with patients’ outcomes both in terms of survival and tumor response, yet is independent of nivolumab treatment regimen and might depend on the patient’s genetic immune constitution.
How this study might affect research, practice or policy
Identifying appropriate biomarkers such as patients’ gene profiling and measurement of nivolumab serum concentrations might help individualize and further optimize the treatment of patients with metastatic melanoma.
Introduction
Metastatic melanoma (MM) is one of the deadliest forms of cutaneous neoplasms, with an increasing incidence worldwide, and an estimated survival rate at 5 years of 16%.1 2
Surgery is the first treatment option in the case of resectable melanoma; however, an advanced disease with either lymph node involvement (stage III) or distant metastasis (stage IV) requires additional systemic treatments. Chemotherapy has been the mainstay of treatment for MM for many years, despite not providing a remarkable survival prolongation.1 Starting from 2010, new therapies have been developed, such as immunotherapies, targeted therapies, vaccines, and small molecules, which have progressively changed the prognosis for patients with MM.3
Nivolumab is a fully human, monoclonal IgG4 antibody that belongs to the immune-checkpoint inhibitor (ICI) class and acts by binding the programmed death-1 (PD-1) receptor, which is involved in immune tolerance and downregulation and inhibition of T cells activation. An aberrant expression of PD-1 ligands in the tumor microenvironment allows tumor cells to escape immune recognition and elimination, thus favoring tumor growth; conversely, in the presence of nivolumab, the PD-1 function is inhibited, and immune cells can overcome the pathological immune suppression, recognizing and killing tumor cells.4
A randomized controlled phase III clinical trial showed that nivolumab is effective and superior to chemotherapy in terms of overall survival (OS), progression-free survival (PFS) and ORR in patients with stage IIIC or IV MM.5 Moreover, nivolumab showed superior results compared with ipilimumab in terms of efficacy (decreased recurrence-free survival and distant metastasis-free survival)6 7 and in terms of tolerability.8 Currently, nivolumab is indicated as monotherapy or in combination with ipilimumab in patients with unresectable melanoma or MM, or as adjuvant therapy in case of lymph node involvement or metastatic disease after complete resection.9
Despite the positive results obtained by nivolumab, and more in general by ICI, in the treatment of MM and other types of cancer, limited evidence is available on the pharmacokinetic and pharmacodynamics profile of these agents, including variations in treatment exposure and exposure–response relationships in relation to clinical outcomes. Understanding these aspects is essential to further optimize and individualize treatment, together with the identification of appropriate biomarkers to support treatment improvement and patients’ selection.10 For example, Topalian et al assessed the pharmacokinetic and pharmacodynamic profile of an anti-PD-1 antibody (nivolumab) in patients with different types of cancer.11 The peak serum concentration of the anti-PD-1 agent, administered at a dose of 0.1 to 10.0 mg/kg every 2 weeks, was 1–4 hours after the start of infusion and the pharmacokinetic profile was linear, with a dose-proportional increase in the peak concentration and area under the curve (AUC) from day 1 to day 14. In terms of pharmacodynamics, in 65 patients with melanoma treated with one cycle of anti-PD-1, the median PD-1 receptor occupancy of circulating CD3 +T cells was 64%–70%. Moreover, the authors observed that an objective response was obtained by 36% of the patients with PD-L1-positive tumors versus none of those with PD-L1-negative tumors, thus suggesting the expression of PD-L1 on the surface of tumor cells before treatment may be useful in predicting patient’s response to treatment.11
In this study, we aimed to assess whether, in patients with MM treated with nivolumab, the serum concentration of the anti-PD-1 agent was associated with the patient’s survival and response to treatment and whether the patient’s genetic profile influenced this correlation.
Patients and methods
This observational retrospective study was conducted at the Melanoma, Cancer Immunotherapy and Innovative Therapies Unit of the Istituto Nazionale Tumori – Fondazione ‘G. Pascale’, Naples (Italy).
We collected data on patients with MM treated with nivolumab (Opdivo) according to clinical practice, namely via intravenous infusion at the dose of 240 mg or 3 mg/kg every 2 weeks or 480 mg every 4 weeks. Patients’ demographic and clinical characteristics before and after treatment were also collected, including diagnosis, BRAF status, body mass index (BMI), lactate dehydrogenase levels, treatment regimen and outcomes.
Nivolumab serum concentration and antibodies to nivolumab were measured 12 weeks after treatment start (at the time of the first assessment) and before nivolumab dosing. As the same time point (12 weeks) was used for both patients in the every 2 weeks and every 4 weeks regimen, serum samples were taken 2 weeks after the last nivolumab dose for patients in the every 2 weeks regimen and 4 weeks after the last nivolumab dose for patients in the every 4 weeks regimen. We used the enzyme immunoassays ELISA SHIKARI Q-NIVO kit12 and the SHIKARI S-ATN kit,13 which allow for high specificity quantitative determination of respectively free nivolumab and antibodies to nivolumab in serum or plasma. We assessed whether the concentration levels of nivolumab were associated with treatment outcomes, namely OS, PFS and response to treatment, defined as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD), but also disease control rate (defined as the sum of CR, PR, and SD >1 year) and objective response rate (ORR; defined as the sum of CR and PR) We also conducted a univariate and multivariate analysis to assess the effects of other variables on patients' PFS and OS. Finally, we investigated the association between nivolumab concentrations and patients’ characteristics, such as BMI, presence of colitis, and renal and hepatic function.
Gene expression analysis
Blood samples from 37 naïve patients were collected at the same time as serum collection to conduct gene expression analysis. RNA was extracted from peripheral blood mononuclear cells (PBMCs) using RNA blood mini-Kit (Qiagen). Purified RNA was used for hybridization and subjected to gene expression analysis on NanoString nCounter through PanCancer IO 360 panel, characterized by 770 human genes involved in the interplay between tumor microenvironment and immune response. Gene data were normalized using nSolver Version 4.0 Software; NanoString. Counts were normalized to internal ERCC (External RNA Control Consortium) technical controls and 30 housekeeping genes. Statistical analysis was performed via Benjamini-Hochberg.
Flow cytometry analysis
PBMCs from 47 melanoma patients were also collected at baseline and 9 months post-treatment. Subpopulations of PBMCs were analyzed using the following antibodies: CD3-V500, CD8-APC Clone BW135/80, PD-1-PE Clone PD1.3.1.3 (all from MiltenyiBiotecS.r.l.) and CD73 PE-Cy7 Clone AD2 (BioLegend). Samples data were acquired using a FACSAria II (Becton-Dickinson, USA). Cell viability was assessed by 7-AAD staining, and dead cells were excluded by selecting only 7-AAD-negative cells. The population of lymphocytes was identified using a morphological gating on forward/side light scatters (FSC-A and SSC-A, respectively) and further gated by the expression of CD3 and CD8. The expression of CD73 and/or PD-1 was determined on the population of interest (CD3 +CD8+T cells). Data were analyzed using FACS DIVA software (Beckman Coulter).
Statistical analysis
Demographic and clinical data were reported descriptively as medians and ranges or numbers and percentages. Differences in mean concentrations according to subgroups were evaluated using the Student’s t-test for unpaired data or one-way analysis of variance when the subgroups were more than two. PFS was calculated from the start of treatment with nivolumab to the evidence of PD or death, whichever occurred first; OS was calculated from the start of treatment with nivolumab to death or censored at the last follow-up. Survival times were analyzed using the Kaplan-Meier method, and the log-rank test assessed differences among curves. A Cox regression model estimated HR and their 95% CIs. The correlation between nivolumab concentration and patients/treatment characteristics was calculated using Spearman’s r non-parametric correlation coefficient. This coefficient was also used to assess the strength and direction of the correlation between gene expression and drug concentrations. P values less than 0.05 were considered statistically significant.
Results
Eighty-eight patients were enrolled in the study; their clinical and demographic characteristics are summarized in table 1. Most patients were affected by stage IV MM (96%), while only 3% and 1% were diagnosed with stage IIIC or IIIB cancer, respectively. Fifty-eight patients (66%) received nivolumab at the dose of 3 mg/kg every 2 weeks, 11 (12%) received a flat dose of 240 mg every 2 weeks and 19 (22%) a flat dose of 480 mg every 4 weeks. Nivolumab was the first-line therapy for most patients (62.5%), while 25%, 11.4% and 1.1% of the patients used it as second-line therapy, third-line therapy and fourth-line therapy, respectively. At the first assessment, 7% of the patients achieved a CR, 18% had PR, 34% had SD and 41% experienced PD.
Table 1.
Patient’s characteristics
| Patients’ characteristics | N=88 |
| Median age, years (range) | 60 (27–91) |
| Gender: female/male, n (%) | 45 (51)/43(49) |
| Melanoma AJCC stage | |
| Stage IV, n (%) | 84 (96) |
| Stage IIIC, n (%) | 3 (3) |
| Stage IIIB, n (%) | 1 (1) |
| CNS metastases at baseline, n (%) | 25 (22) |
| BRAF status | |
| Wilde type, n (%) | 57 (65) |
| Mutation, n (%) | 26 (29) |
| NA, n (%) | 5 (6) |
| BMI | |
| Normal weight (18.5<BMI≤24.9), n (%) | 24 (27) |
| Overweight (25<BMI≤29.9), n (%) | 36 (41) |
| Obese (BMI ≥30), n (%) | 28 (32) |
| Line of treatment | |
| First-line treatment, n (%) | 55 (62,5) |
| Second-line treatment, n (%) | 22 (25) |
| Third-line treatment, n (%) | 10 (11,4) |
| Fourth-line treatment, n (%) | 1 (1,1) |
| Response rate at 1° assessment | |
| Complete response, n (%) | 6 (7) |
| Partial response, n (%) | 16 (18) |
| Stable disease, n (%) | 30 (34) |
| Progression disease, n (%) | 36 (41) |
| DCR | 44 (50) |
| ORR | 22 (25) |
| Nivolumab dosage | |
| Flat dose 240 mg, n (%) | 11 (12) |
| Flat dose 480 mg, n (%) | 19 (22) |
| Dose 3 mg/kg, n (%) | 58 (66) |
| LDH level | |
| High | 30 (34) |
| Normal | 33 (38) |
| NA | 25 (28) |
AJCC, American Joint Commission on Cancer; BMI, body mass index; CNS, central nervous system; DCR, disease control rate; LDH, lactate dehydrogenase; NA, not available; ORR, overall response rate.
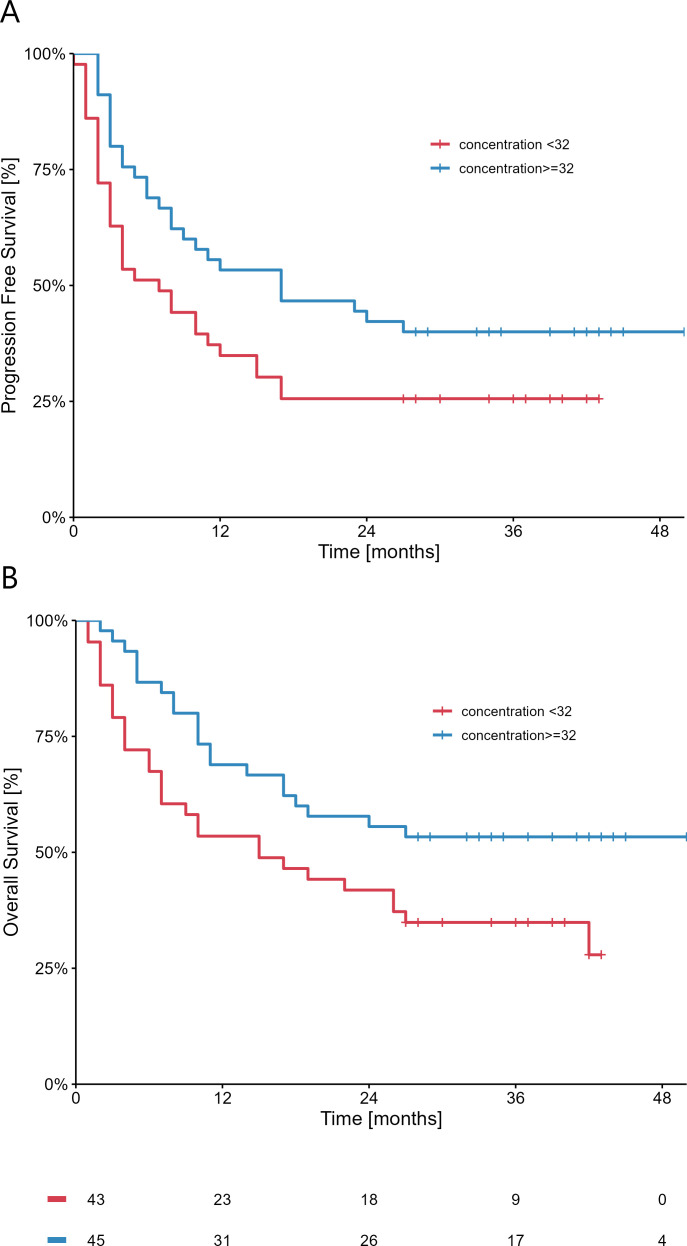
Nivolumab median concentration in the entire cohort was 32 µg/mL, with no statistically significant difference between those who stopped nivolumab prematurely (n=25) and those who completed the treatment course (n=63). A statistically significant difference was measured both in terms of OS (p=0.01; HR 0.97 (95% CI 0.95 to 0.99)) and PFS (p=0.007; HR 0.97 (95% CI 0.94 to 0.99) when considering serum concentrations of nivolumab as a continuum; according to the median value, concentrations were divided in higher (≥32 µg/mL) vs lower (<32 µg/mL) and plots are reported in figure 1. No other variable except nivolumab concentration was associated with PFS and OS, as showed by the univariate and multivariate analyses (online supplemental tables 1 and 2). Moreover, we observed that patients who achieved a CR had significantly higher serum concentrations of nivolumab compared with those who did not reach a CR (median 38.9 µg/mL (IQR: 37.5–42.1) vs 31.8 µg/mL (IQR: 22.0–37.4); p=0.02). In terms of response, median serum concentrations were higher in the subgroup of patients achieving a CR or PR (33.8 µg/mL (IQR: 30.3–38.6) compared with patients with SD or PD (29.7 µg/mL (IQR: 21.9–37.5). However, no statistical difference was reached (p=0.14) (online supplemental figure 1). Interestingly, no antidrug antibodies (ADAs) were detected by ELISA in none of the samples analyzed (data not shown). Finally, in order to exclude the influence of previous therapies on the analysis, we compared nivolumab concentration in patients treated with nivolumab in the first line versus other lines, reporting no statistically significant difference in the two groups (median 31.8 µg/mL (IQR: 23.9–37.5)) vs (33.8 µg/mL (IQR: 21.1–37.7); p=0.99).
Figure 1.
Relationship between nivolumab serum concentration and overall survival (A) or progression free survival (B).
jitc-2022-005132supp001.pdf (72.5KB, pdf)
A trend was established between the occurrence of colitis and nivolumab concentration, which was higher among patients with no signs of colitis (median 33.0 µg/mL; IQR: 26.0–37.7; n=64) and remarkably, although not statistically, lower among those with signs of colitis (median 24.6 µg/mL; IQR: 16.3–36.3; n=16; p=0.057). No correlation was found between nivolumab serum concentration and nivolumab treatment regimen; nivolumab concentrations ranged from 27.1 to 31.5 µg/mL regardless of the dosage/frequency of administration (p=0.32). In particular, the median concentration of nivolumab was 33.7 µg/mL (IQR: 22.1–38.3) for patients receiving 240 mg every 2 weeks vs 23.9 (IQR: 19.1–36.4) for patients receiving 480 mg every 4 weeks (p=0.47). No significant difference was observed in serum concentrations of nivolumab depending on the patients BMI (p=0.07) (table 2). Lastly, no significant changes were detected in terms of eGFR (Estimated Glomerular Filtration Rate), creatinine, AUC (area under the free nivolumab concentration versus time curve), albumin, ALT (alanine aminotransferase), AST (aspartate aminotransferase), and GGT (Gamma-glutamyl Transferase).
Table 2.
Correlation between nivolumab serum concentration and patients’/treatment characteristics
| Parameter | N | Median nivolumab concentration (µg/mL) (IQR) | P value |
| Complete response | |||
| Yes | 6 | 38.9 (37.5–42.1) | 0.024 |
| No | 82 | 31.8 (22.0–37.4) | |
| Colitis | |||
| Yes | 16 | 24.6 (16.3–36.3) | 0.09 |
| No | 64 | 33.0 (26.0–37.7) | |
| Nivolumab regimen | |||
| 3 mg/kg | 58 | 32.6 (26.2–37.5) | 0.38 |
| 240 mg | 11 | 33.7 (22.1–38.3) | |
| 480 mg | 19 | 23.9 (19.1–36.4) | |
| BMI | |||
| Normal | 24 | 35.8 (29.5–37.7) | 0.07 |
| Overweight | 36 | 32.6 (27.6–36.8) | |
| Obesity | 28 | 24.6 (18.3–37.3) |
BMI, body mass index.
Gene expression analysis
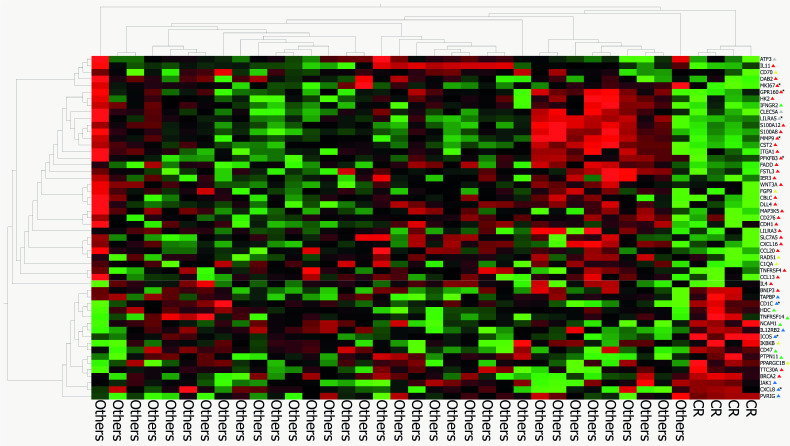
The results of the gene expression analysis are presented in figure 2. Among the 37 patients included in the analysis, 4 had a CR, 10 had a PR, 11 had an SD and 12 had a PD. The analysis showed a specific gene signature for patients who achieved a CR, who were also those with elevated concentrations of nivolumab. Some genes, such as ICOS, CXCL8 and CD1C were directly correlated with drug concentrations, whereas others, such as MKI67, GPR160, PFKFB3, MMP9, and CLEC5A were inversely correlated with drug concentrations. In particular, higher levels of nivolumab concentration were associated with increased expression of the ICOS (Inducible T Cell Costimulator) gene (r=0.34, p=0.04), that is related with the genes expressed by activated effector T lymphocytes, such as CD40LG (r=0.64, p<0.0001), HLA_DRB1 (r=0.38, p=0.02), IRF4 (r=0.42, p=0.008), and IL21R (r=0.43, p=0.007). Moreover, patients with higher drug concentrations had a higher expression of HLA-DQA1, which encodes for a HLA class II histocompatibility antigen that contributes to the presentation of antigens to CD4 +T cells.
Figure 2.
Gene expression analysis of patients who reached a complete response versus other patients. The triangle shape indicate the following genes: protumor genes (red), immune suppressor pathway (gray), apoptosis pathway (yellow), antitumor genes (green), immune activation pathway (blue) and genes related with drug concentration (black). Genes marked with asterisk are related to drug concentration.
Flow cytometry
From the flow cytometry analysis performed on basal samples, we observed that drug concentration was inversely related to CD8 +PD-1+ (r=−0.45, p=0.002) and CD8 +CD73+PD-1+ (r=−0.38, p=0.010). In order to exclude any possible influence of prior therapies on CD8 +PD-1+expression, we repeated the analysis in a subgroup of patients (n=28) who received nivolumab as first-line therapy, confirming the inverse correlation (r=−0.389, p=0.041) (figure 3). No significant data were observed in the post-treatment samples.
Figure 3.
Flow cytometry analysis.
Discussion
The results of this study show that in patients with MM treated with nivolumab, the serum concentration of the anti-PD-1 agent correlates with patients’ outcomes, both in terms of survival and tumor response. Indeed, patients with higher levels of nivolumab experienced better survival rates (OS and PFS), and all the patients who achieved a CR had higher blood levels of nivolumab compared with those who achieved a PR or had stable or PD. Overall, nivolumab concentrations were higher in patients with a better response (CR or PR vs SD or PD), although this difference did not reach a statistical significance.
Interestingly, nivolumab serum concentrations were independent of the nivolumab treatment regimen; these results align with previous dose-escalation studies that showed that patients’ ORR is not associated with nivolumab dosage but is correlated with nivolumab clearance.14 15 These findings suggest that the most important parameter in predicting patients’ outcomes is not nivolumab initial dosage but its clearance: the lower the clearance, the higher the concentration of the agent in the blood, which translates to higher bioavailability and possibly in a superior therapeutic effect.
In our study, we reported no correlation between nivolumab concentrations and patients’ BMI, renal and hepatic functions, while a trend was observed towards a decreased occurrence of colitis in patients with higher levels of nivolumab. These results partially differ from those reported by Bajaj et al, according to whom patient-specific characteristics such as baseline performance status, body weight, eGFR, race, and sex account for 30% of the variability in nivolumab clearance.16 Moreover, contrary to Bajaj, that showed the development of post-treatment ADAs in 11.2% of the patients treated with nivolumab, with a consequent increase in nivolumab clearance by 14% on average,16 no post-treatment ADAs were detected in our cohort.
Furthermore, the genetic analysis showed that patients who achieved a CR had a different genetic profile compared with other patients, with more marked activation of ICOS and other genes involved in T-cells mediated proinflammatory pathways.
ICOS is an inducible T cell costimulator expressed on activated T cells, which exerts diverse effects depending on the T cell subpopulation involved. On the one hand, the ICOS signal can activate the immune response, exerting an anti-tumor activity through activating T effector (T eff) cells, such as CD4 +and CD8+. On the other hand, sustained activation of the ICOS pathway can potentiate immunosuppression, mediated by the regulatory T cells (T regs) and Th2 response in the tumor microenvironment.17
A significant increase in ICOS expression has been observed after treatment with anti-PD-1 or anti-CTLA4 agents. An in vitro study on mice showed that PD-1 blockade on CD4 +and B cells leads to the upregulation of ICOS expression on CD4 +T cells, activating ERK signaling, enhancing humoral activation.18 Preliminary results in patients exposed to ipilimumab also showed a remarkable increase in the frequency of CD4 +ICOShi and CD8 +ICOShi T cells in the bloodstream and tumor tissues after exposure to the anti-CTLA-4 agent. These results were observed in particular in patients treated with higher doses of ipilimumab (10 mg/kg/dose vs 3 mg/kg/dose), who also presented increased rates of infiltrating cells into blood vessels. Patients with a higher frequency of CD4 +ICOShi T cells, sustained for 12 weeks, also had a higher chance of clinical benefit and OS, thus suggesting that CD4 +ICOShi T cells may serve as a biomarker of treatment outcome in patients treated with anti-CTLA-4.19
The important role of ICOS in regulating the immune response has led to the hypothesis that a combinational approach targeting both ICOS and other regulatory pathways, such as CTLA-4 and PD-1, could be useful to overcome resistance to cancer immunotherapy or to potentiate its activity and effectiveness further. Preliminary evidence suggests that this approach has a good safety profile and promising anti-tumor activity indeed, especially when anti-ICOS antibodies are combined with anti-PD-1 agents (pembrolizumab and nivolumab).17 20
This study further confirms the association between ICOS and anti-PD-1 nivolumab. We observed that elevated ICOS expression is associated with genes involved in the activity of effector T lymphocytes, such as CD40LG, HLA_DRB1, IRF4, and IL21R, thus suggesting that higher levels of nivolumab in the bloodstream might induce the activation of ICOS-mediated immune response. Moreover, serum nivolumab concentration positively correlated with higher expression of CXCL8 (IL-8), that is, a chemotactic factor that attracts neutrophils, basophils, and T-cells, interleukin 1821 and CD1C, which encodes for an antigen-presenting protein that binds self and non-self lipid and glycolipid antigens and presents them to T-cell receptors on natural killer T-cells.22
A better understanding of which factors influence patients’ response to nivolumab, including the pharmacokinetics and pharmacodynamics properties of the drug, target-mediated drug disposition and time-varying drug clearance, as well as the association between drug concentrations and treatment outcomes, is essential to predict patient’s response to treatment, optimize treatment and individualize therapy. An interesting study published in 2020 presented a mathematical model to predict a patient’s response to immunotherapy, considering multiple aspects of the complex biological and physical interaction between the immune system and cancer cells. According to the model, the diffusion of immunotherapy drugs into the bloodstream, and their infiltration in the tumor microenvironment, activate the response of immune cells through drug binding, cell signaling pathways and chemotaxis, ultimately activating effector immune T cells for cancer cell killing.23 This hypothesis is supported by the results of our study, which suggests the importance of nivolumab concentration in the serum to predict patients’ responses. Higher levels of nivolumab seem to translate into greater inhibition of PD-1 receptor and higher activation of ICOS pathway on effector cells, which ultimately means an increased tumor response. This observation is further supported by the results of flow cytometry analysis (figure 3), where nivolumab concentration was negatively associated with cells expressing CD8+/PD-1+ (exhausted T cells),24 and CD8+/CD73+/PD-1+, T-cells, which are involved in the generation of immune suppression. Interestingly, a previous study showed a correlation between lower pre-treatment CD8+/CD73+/PD-1+lymphocytes and better outcomes to nivolumab treatment, thus suggesting a potential role of peripheral CD8 +lymphocytes positive to CD73 in predicting anti-PD-1 therapeutic response in melanoma patients.25 At present, there is no evidence on the mechanisms behind the negative correlation between nivolumab serum concentration and CD8+/PD-1+cells. We can speculate that these cells can somehow modulate serum drug concentration, and that a high level of CD8+/PD-1+cells is associated with less drug concentration, which is ultimately insufficient to inhibit its target, thus negatively influencing treatment response.
In conclusion, in patients with MM, elevated serum concentration of nivolumab correlates directly with patients’ CR and with the increased expression of ICOS and other genes involved in activating T effectors cells. These results suggest that strong inhibition of the PD-1 receptor potentiates the killing activity of the immune system mainly through ICOS pathway.
Acknowledgments
The authors wish to acknowledge Ambra Corti (Polistudium srl, Milan, Italy) for medical writing, Aashni Shah and Fabio Perversi (Polistudium srl, Milan, Italy) for editorial assistance, and Alessandro Manzoni for support in data analysis.
Footnotes
Twitter: @AlfredoBudillon, @PAscierto
Correction notice: This article has been corrected since it was first published online. Figure 1 was incorrectly published in black and white. This has now been amended.
Contributors: Study conception and design: DM, EC, and PAA; collection and interpretation of data: DM, MGV, GT, AE, MAI, GD'A, LF, VV, AW, and MB; statistical analysis: DM and DG; manuscript drafting: DM, MGV, and PAA; manuscript editing: DM, DG, MGV, GT, AE, MAI, GD'A, LF, VV, CT, AW, MB, SP, CC, PM, AB, SW, EC, and PAA; approval to submit: DM, DG, MGV, GD'A, GT, AE, MAI, GD'A, LF, VV, CT, AW, TDC, MB, SP, CC, AP, PM, AB, SW, EC, and PAA. Guarantor: PAA.
Funding: This work was supported by Grants from the Italian Ministry of Health (IT-MOH) through 'Ricerca Corrente'.
Competing interests: PAA has/had a consultant/advisory role for Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health. He also received research funding from Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi. SW is employee and stockholder in NanoString Technologies. All other authors have declared no conflicts of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study was approved by the Ethics Committee of Istituto Nazionale Tumori - IRCCS - Fondazione 'G. Pascale', Naples, Italy, protocol number 33/17 oss. All patients provided their written informed consent to participate in this study.
References
- 1. Villani A, Scalvenzi M, Fabbrocini G, et al. Looking into a better future: novel therapies for metastatic melanoma. Dermatol Ther 2021;11:751–67. 10.1007/s13555-021-00525-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forsea A-M. Melanoma epidemiology and early detection in Europe: diversity and disparities. Dermatol Pract Concept 2020;10:e2020033. 10.5826/dpc.1003a33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luther C, Swami U, Zhang J, et al. Advanced stage melanoma therapies: detailing the present and exploring the future. Crit Rev Oncol Hematol 2019;133:99–111. 10.1016/j.critrevonc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 4. Queirolo P, Boutros A, Tanda E, et al. Immune-checkpoint inhibitors for the treatment of metastatic melanoma: a model of cancer immunotherapy. Semin Cancer Biol 2019;59:290–7. 10.1016/j.semcancer.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 5. Larkin J, Minor D, D'Angelo S, et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol 2018;36:383–90. 10.1200/JCO.2016.71.8023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weber J, Mandala M, Del Vecchio M. CheckMate 238 Collaborators. N Engl J Med 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 7. Weber . Five-year outcomes with adjuvant nivolumab versus ipilimumab in resected stage IIIB–C or IV melanoma (CheckMate 238). Oral presentation at the 18th International Congress of the Society for Melanoma Research (virtual); 28–31 October 2021, 2021:28–31. [Google Scholar]
- 8. Ascierto PA, Del Vecchio M, Mandalá M, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:1465–77. 10.1016/S1470-2045(20)30494-0 [DOI] [PubMed] [Google Scholar]
- 9. Opdivo: EPAR -product information. Available: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf
- 10. Centanni M, Moes DJAR, Trocóniz IF, et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019;58:835–57. 10.1007/s40262-019-00748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. SHIKARI®Q-NIVO - Nivolumab (Opdivo®) (quantitative). Available: https://www.iwai-chem.net/elisa-kit/shikari-q-nivo-nivolumab-opdivo-quantitative/
- 13. SHIKARI® S-ATN ELISA kit. Available: https://www.iwai-chem.net/elisa-kit/shikari-s-atn-antibodies-to-nivolumab-opdivo-qualitative/
- 14. Agrawal S, Feng Y, Roy A, et al. Nivolumab dose selection: challenges, opportunities, and lessons learned for cancer immunotherapy. J Immunother Cancer 2016;4:72. 10.1186/s40425-016-0177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang X, Feng Y, Bajaj G, et al. Quantitative characterization of the exposure-response relationship for cancer immunotherapy: a case study of nivolumab in patients with advanced melanoma. CPT Pharmacometrics Syst Pharmacol 2017;6:40–8. 10.1002/psp4.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bajaj G, Wang X, Agrawal S, et al. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst. Pharmacol. 2017;6:58–66. 10.1002/psp4.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solinas C, Gu-Trantien C, Willard-Gallo K. The rationale behind targeting the ICOS-ICOS ligand costimulatory pathway in cancer immunotherapy. ESMO Open 2020;5:e000544. 10.1136/esmoopen-2019-000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang M, Xia L, Yang Y, et al. PD-1 blockade augments humoral immunity through ICOS-mediated CD4+ T cell instruction. Int Immunopharmacol 2019;66:127–38. 10.1016/j.intimp.2018.10.045 [DOI] [PubMed] [Google Scholar]
- 19. Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861–71. 10.1158/1078-0432.CCR-10-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Tourneau C, Rischin D, Groenland S, et al. 1O inducible T cell co-stimulatory (ICOS) receptor agonist, GSK3359609 (GSK609) alone and combination with pembrolizumab: preliminary results from INDUCE-1 expansion cohorts in head and neck squamous cell carcinoma (HNSCC). Annals of Oncology 2020;31:S1. 10.1016/j.annonc.2020.01.049 [DOI] [Google Scholar]
- 21. UniProtKB - P10145 (IL8_HUMAN). Available: https://www.uniprot.org/uniprot/P10145
- 22. UniProtKB - P29017 (CD1C_HUMAN). Available: https://www.uniprot.org/uniprot/P29017
- 23. Butner JD, Elganainy D, Wang CX, et al. Mathematical prediction of clinical outcomes in advanced cancer patients treated with checkpoint inhibitor immunotherapy. Sci Adv 2020;6:eaay6298. 10.1126/sciadv.aay6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haymaker C, Wu R, Bernatchez C, et al. PD-1 and BTLA and CD8(+) T-cell "exhaustion" in cancer: "Exercising" an alternative viewpoint. Oncoimmunology 2012;1:735–8. 10.4161/onci.20823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Capone M, Fratangelo F, Giannarelli D, et al. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J Transl Med 2020;18:121. 10.1186/s12967-020-02285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005132supp001.pdf (72.5KB, pdf)
Data Availability Statement
Data are available in a public, open access repository.