Abstract
The lungs are the remote organ most commonly affected in human peritonitis. The major goals of this study were to define the dose- and time-dependent relationship between graded septic peritonitis and systemic and pulmonary inflammatory responses in mice. BALB/c mice were treated with intraperitoneal polymicrobial inoculi and sacrificed at 3, 12, and 24 h. The treatment protocol resulted in distinct groups of animals with respect to mortality rate, kinetics, and concentrations of a broad spectrum of pro- and anti-inflammatory endogenous mediators, intrapulmonary bacterial accumulation, and static lung compliance. In sublethally infected mice, pulmonary bacterial proliferation was controlled. Levels of monocyte chemoattractant protein-1 (MCP-1), interleukin-10, interleukin-6, granulocyte colony-stimulating factor (G-CSF), and tumor necrosis factor (TNF) in plasma were elevated 3 h after infection exclusively. At 3 h, MCP-1, gamma interferon, and TNF were detected in extracts of pulmonary tissue or in bronchoalveolar lavage (BAL) fluid. Static lung compliance (Cst) was transiently decreased at 12 h. In contrast, in lethally infected mice pulmonary bacterial proliferation was not contained. Concentrations of MCP-1, G-CSF, and TNF in plasma were maximal at 24 h, as were pulmonary MCP-1 levels. Lung myeloperoxidase activity was increased at 3, 12, and 24 h. Cst was reduced after 3 h and did not reach control values at 24 h. Pulmonary cyclooxygenase-2 mRNA and eicosanoids in BAL fluid and plasma were elevated at 3 and 24 h. This study shows that polymicrobial peritonitis in mice leads to dose-dependent systemic and pulmonary inflammation accompanied by a decrease in lung compliance.
In human peritonitis, which is the leading cause of multisystem organ failure (22, 26), the lungs are the remote organ most frequently affected (41) and are either the first organ to fail or are one of several organ systems to fail simultaneously. In order to investigate alterations in pulmonary homeostasis secondary to septic peritoneal infection, the cecal ligation and puncture (CLP) model in sheep (8, 17, 18), mice (1, 15, 24, 28, 29, 41), and rats (13, 21, 27, 31) has been used most frequently. Secondary pulmonary injury has also been investigated in septic peritonitis models induced by intraperitoneal application of bacteria (40). Using the CLP model in mice (37), both mortality and the extent and timing of systemic proinflammatory cytokine release have been shown to vary with cecal-puncture diameter (with 60% mortality in the large-puncture-diameter group and 0% mortality in the small-puncture-diameter group at 24 h). The outcome in the CLP model may largely depend on the efficacy with which the puncture can be closed, a factor which cannot be controlled very well experimentally. As a consequence, mortality in this model varies considerably, i.e., with an 18-gauge puncture needle 3-day survival rates in mice of 20 (37), 55 (15), and 68% (23) were reported. Thus, in this model, mortality cannot be predicted accurately and dose (hole)-response relationships are difficult to interpret and are not exactly reproducible.
Another focus-defined rodent model that is related to human abdominal sepsis utilizes a quantitatively and qualitatively defined intraperitoneal bacterial challenge and was introduced by Lorenz and coworkers (20). The advantages of this model include good reproducibility (3) and the possibility to study dose-response relationships both systemically and in remote organ systems.
The major goal of this descriptive study was to investigate the dose- and time-dependent relationship between graded polymicrobial septic peritonitis and pulmonary responses within the first 24 h after peritonitis induction in mice. Kinetics of proinflammatory (tumor necrosis factor [TNF], gamma interferon [IFN-γ], and interleukin-6 [IL-6]) and anti-inflammatory (IL-10 and monocyte chemoattractant protein-1 [MCP-1]) endogenous mediators, as well as growth factors (granulocyte colony-stimulating factor [G-CSF] and granulocyte-macrophage [GM]-CSF), which are potentially involved in the systemic and pulmonary inflammatory response to peritonitis were determined in serum, bronchoalveolar lavage (BAL) fluid, and lung tissue and related to pulmonary inflammatory cell recruitment and lung bacterial clearance. Static lung compliance was determined, since this parameter of lung function has been reported to decrease before the onset of lung permeability injury in a nonpulmonary induced septic porcine lung injury model (6). In addition, there is some evidence that the endogenous surfactant system is compromised in CLP-treated rats (21). Increased concentrations of cyclooxygenase (COX) pathway products are thought to contribute to the acute alterations in pulmonary hemodynamics and inflammation found after induction of bacterial sepsis. Therefore, pulmonary gene expression of COX isoenzymes I and II and thromboxane synthase (TXS), as well as the concentrations of thromboxane A2 and prostacyclin in serum, BAL fluid, and lung tissue, was determined. Since pulmonary inducible nitric oxide synthase (iNOS) expression has been shown to be upregulated after intraperitoneal lipopolysaccharide (LPS) application (7), we also measured iNOS mRNA in our model.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free, 6- to 8-week-old, male in-house-bred BALB/c mice (26 to 28 g) were used in this study. All animals received humane care in accordance with the National Institutes of Health guidelines and the legal requirements in Germany. Mouse maintenance included a temperature of 24°C, 55% humidity, 12-h light-dark cycles, and mouse chow (Altromin C 1310), with water provided ad libitum.
Animal experiments.
The mice received intraperitoneal stool suspensions of 0.125 (group A), 0.25 (group B), 0.5 (group C), and 1.0 (group D) ml/kg of body weight in a volume of 0.3 ml per mouse or saline (controls). The injected stool suspension contained about 40 different aerobic and anaerobic bacterial species (≈107 CFU), with Escherichia coli, Enterococcus, and Staphylococcus predominating among the aerobic bacteria. A detailed characterization of the suspension has been published elsewhere (20). Aliquots were stored at −70°C, thawed on the morning of the experiment, and diluted in oxygen-free Ringer's solution. Infection was initiated by intraperitoneal injection of the diluted stool suspension with a 1 ml syringe and a 22-gauge needle. The mice were euthanized with an overdose of pentobarbital-heparin at 3, 12, and 24 h after infection. Heparinized blood was obtained by heart puncture. The thorax was opened rapidly, a tracheotomy tube was placed into the upper third of the trachea, and specific lung compliance was measured as described below. Finally, the lungs were lavaged and removed. Lung lavage cells were counted, and tissue supernatants of part of the lungs were used for microbiological examinations. Plasma, BAL fluid, and supernatants of homogenized lungs were analyzed for the following cytokines and chemokines: TNF, IL-6, IL-10, G-CSF, GM-CSF, IFN-γ, and MCP-1. In addition, the eicosanoids thromboxane (TXB2) and prostacyclin (6-keto-PGF1α) were measured in the three compartments. Parts of the lungs were used for the determination of COX-1, COX-2, TXS, and iNOS mRNA by a reverse transcriptase PCR technique.
Pressure-volume analysis.
The cannulated trachea was connected to an airtight syringe and a pressure transducer via a three-way connector. After the chest was opened, the lungs were inflated with 1 ml of air and deflated in 100-μl steps. Specific lung compliance was determined from the slope of the deflation portion of the pressure-volume curve at deflation pressures of <10 cm of H2O divided by the lung volume at 30 cm of H2O.
BAL.
The lungs and trachea were surgically exposed. The trachea was cannulated, and a silk ligature was fastened around it to secure the cannula. Lung lavage fluid was obtained by instilling and withdrawing lavage solution (1.0 ml of Ca2+- and Mg2+-free phosphate-buffered saline with 0.2 mM EDTA) three times via the tracheal cannula before finally transferring it to the syringe. The recovery ranged from 70 to 90% of the instilled fluid. There were no differences in the recoveries of the groups. Lavage samples were centrifuged at 150 × g for 10 min at 4°C; the cells were counted, and cytocentrifuge preparations were stained with May-Grünwald and Giemsa stain.
Reverse transcriptase PCR technique.
Part of the lungs of each mouse was snap frozen in liquid nitrogen and stored at −80°C. RNA was isolated by using RNAclean (AGS, Heidelberg, Germany). RNA (2 μg) was used for target-specific reverse transcription (RT). The wobble primer PCOXR1 [5′-A(G/C)AGCTCAGT(G/T)GA(A/G)CG(C/T)CT-3′] complementary to a homologous 3′ part of COX-1 and -2 was used for simultaneous RT of the mRNA of both COXs. For TXS, the primer was PTXSMR1 (5′-GCGTGACACAATCTTGATGTAGACTCC-3′). PCR was performed with the cDNA template with the nested primer pairs described elsewhere (34). For analysis of iNOS RT was performed with PINOSR1 (5′-AACGTTTCTGGCTCTTGAGC TGGA-3′) and PCR was performed with the primers PINOSR2 (5′-GCTTCTTCAAAGTGGTAGCCA-3′) and PINOSF1 (5′-CCCTTCCGAAGTTTCTGGCA GCA-3′). The reactions were cycled 32 times as follows: 30 s at 94°C, 30 s at 56°C, and 30 s at 72°C after 5 min of denaturating at 95°C. The amplification products were analyzed by 1.8% agarose gel electrophoresis and ethidium bromide staining. No amplification products were found when RT was performed without the specific primer or when the PCR was done without a template. The proportion of PCR products was estimated relative to the control fragment of β-actin by measuring the intensity of ethidium bromide luminescence by a charge-coupled device image sensor in combination with the BIOPROFIL program (LFT, Wasserburg, Germany).
Mediator determinations.
Endogenous mediators were measured in plasma, BAL supernatants, and supernatants of lung tissue samples. IL-10 was measured with the Quantikine mouse immunoassay (detection limit, 4 pg/ml) from R&D Systems (Minneapolis, Minn.). For the MCP-1, IL-6, and IFN-γ enzyme-linked immunosorbent assays (ELISAs), antibodies and standards were purchased from Pharmingen (San Diego, Calif.). The detection limits were 45, 15, and 15 pg/ml for MCP-1, IL-6, and IFN-γ, respectively. GM-CSF was measured with a GM-CSF minikit (detection limit, 5 pg/ml) purchased from Endogen (Cambridge, Mass.). For the TNF ELISA, a purified anti-mouse TNF capture polyclonal antibody (immunoglobulin G protein solution, 20 mg/ml [in-house preparation]) and a biotinylated anti-mouse TNF antibody from Pharmingen were used (detection limit, 10 pg/ml). Streptavidin-peroxidase was purchased from Jackson Immuno Research (West Grove, Pa.), and peroxidase substrate BM blue was purchased from Boehringer Mannheim (Mannheim, Germany). The antibodies and standards required for G-CSF ELISA (detection limit, 15 pg/ml) were kindly provided by Amgen (Thousand Oaks, Calif.). Thromboxane A2 and prostacyclin were assessed by an enzyme immunoassay from Cayman (Ann Arbor, Mich.) as the stable by-products thromboxane B2 (TXB2) and 6-keto PGF1α, respectively (detection limit, 5 pg/ml).
Lung lavage protein determination.
BAL fluid protein content was analyzed by using the Microprotein-PR kit from Sigma (Deisenhofen, Germany).
Lung MPO assay.
MPO activity was estimated according to the method of Schneider and Issekutz (30). The lungs were removed, washed, weighed (0.14 to 0.23 g), and freeze-dried. For enzyme extraction, samples were homogenized in 2 ml of 50 mM HEPES, pH 8.0 (12 passages with a pestle homogenizer). Then, samples were centrifuged at 10,000 × g for 30 min at 4°C, and the supernatant was discarded. The pellet was resuspended in 2 ml of 0.5% cetyltrimethylammonium chloride (Sigma) in distilled water, rehomogenized (three passages), and centrifuged as before. This resulted in a pellet with a clear supernatant and a thin lipid layer on the top. The supernatants were diluted 1/5 in 0.5% cetyltrimethylammonium chloride. Aliquots of 75 μl of each sample were pipetted into four wells of a 96-well tissue culture plate. Cold stop solution (4 N H2SO4) was added to two wells (150 μl/well) to stop the reaction at t = 0 s (background optical density). The MPO substrate solution was 3,3′,5,5′-tetramethyl-benzidine (ready-to-use liquid substrate system; Sigma). Substrate solution (75 μl) was added to each well, the reaction was stopped after 2 min with 150 μl of cold stop solution, and the optical density at 450 nm was determined. A standard curve was assayed with MPO from human leukocytes (Sigma). The MPO activity was expressed in MPO U/ml per g (dry weight) of lung.
Microbiological examinations.
After being weighed, specimens of lungs were passed through nylon cell strainers (Falcon; Becton Dickinson, Heidelberg, Germany) with 10 ml of phosphate-buffered saline. Aerobic CFU of gram-negative and gram-positive bacteria were determined on blood agar plates (Heipha; BioTest, Heidelberg, Germany) after overnight incubation at 37°C of serial dilutions (100 μl/plate) of organ supernatants. CFU per gram of lung tissue was calculated.
Statistics.
Data in the figures are given as mean ± standard error of the mean (SEM); data in the tables and in the text are given as mean ± standard deviation (SD). The data were analyzed by analysis of variance and subsequently by Dunnett's test. Correlation coefficients were calculated by Kendal's rank correlation test (Unistat 4.5; Unistat, Ltd., London, United Kingdom).
RESULTS
Dose-response relationship.
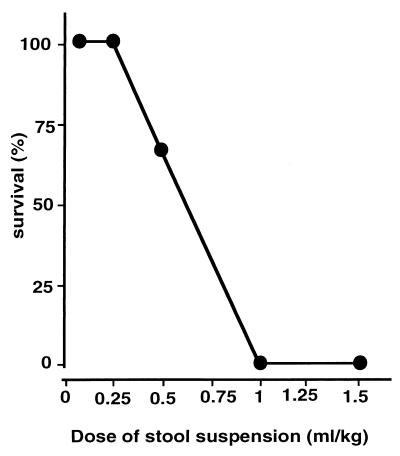
Figure 1 shows the dose-response relationship between the dosage of fecal suspension and mortality within 5 days of monitoring; death occurred between 26 and 53 h after challenge. During the first hours after infection, mice of groups C and D (high dose) developed the following clinical symptoms of sepsis: lethargy, diarrhea, and tachypnoe. They also demonstrated piloerection, reduced mobility, and absence of congregation with other mice. In sublethally infected mice, multiple abscesses within the peritoneal cavity were found at the time of autopsy. Of importance, these results with a newly prepared fecal suspension reproduce the dose-response data obtained by our group in a previous study (3).
FIG. 1.
Survival of mice subjected to polymicrobial peritoneal infection: dose-response. Polymicrobial suspension was injected intraperitoneally at the doses indicated, and survival was monitored over 5 days. The data with doses of 1.5, 1, 0.5, 0.25, and 0.125 ml/kg are based on n = 3 animals per treatment group from one experiment.
Kinetics of cytokine concentrations.
Table 1 shows the complete set of data for G-CSF concentrations in the three compartments investigated. Similar protocols were used for all of the cytokines studied, i.e., TNF, IL-10, IL-6, MCP-1, IFN-γ, and GM-CSF. G-CSF plasma concentrations were strikingly increased, in contrast to low G-CSF levels in lung lavage fluid and lung tissue. Analysis of the data in Table 1 indicates that cytokine spillover from the circulation resulted in pulmonary cytokine concentrations of no more than 2% of that found in plasma. Therefore, lung tissue or lung lavage cytokine concentrations are only shown if they exceeded 2% of the corresponding plasma levels.
TABLE 1.
G-CSF concentrations in plasma, BAL fluid, and lung homogenates in mice 3, 12, and 24 h after infection with 0.125, 0.25, 0.5, and 1.0 ml of stool suspension/kga
| Dose (ml/kg) (n = 4) | G-CSF concn (ng/ml) at:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 h
|
12 h
|
24 h
|
|||||||
| Plasma | BAL fluid | Homogenized lung | Plasma | BAL fluid | Homogenized lung | Plasma | BAL fluid | Homogenized lung | |
| Controls | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| 0.125 | 54 ± 8§ | 0.2 ± 0.1† | 0.8 ± 0.1§ | 46 ± 14‡ | 0.2 ± 0.1† | 0.4 ± 0.1† | 4.2 ± 4.1 | 0.1 ± 0.1 | 0.3 ± 0.1† |
| 0.25 | 57 ± 10§ | 0.1 ± 0.1 | 0.9 ± 0.3† | 113 ± 17§ | 0.3 ± 0.1 | 0.7 ± 0.1§ | 19 ± 6.5† | 0.1 ± 0.1 | 0.3 ± 0.1‡ |
| 0.5 | 35 ± 20* | 0.1 ± 0.1 | 0.2 ± 0.1† | 598 ± 145‡ | 0.8 ± 0.1§ | 1.5 ± 1.0* | 296 ± 280 | 0.6 ± 0.4* | 1.2 ± 0.9* |
| 1.0 | 73 ± 23‡ | 0.2 ± 0.1† | 1.0 ± 0.1§ | 960 ± 870 | 1.3 ± 0.1§ | 1.1 ± 0.2§ | 1,240 ± 490† | 4.8 ± 2.4† | 6.1 ± 3.7* |
Data are means ± SD. *, P < 0.05 versus controls; †, P < 0.01 versus controls; ‡, P < 0.001 versus controls; §, P < 0.0001 versus controls.
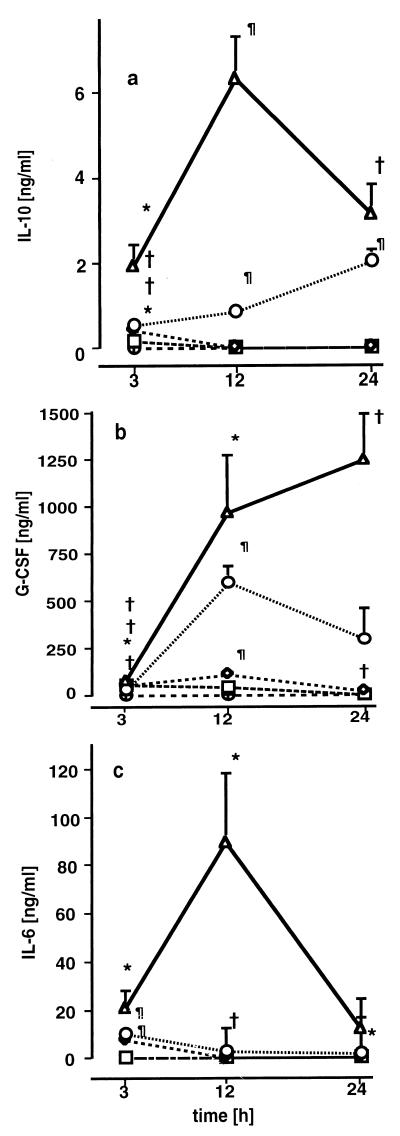
Elevated concentrations of IL-10 (Fig. 2a), G-CSF (Fig. 2b), and IL-6 (Fig. 2c) were found in the plasma but not in lung tissue or in BAL fluid (data not shown). Plasma IL-10, G-CSF, and IL-6 concentrations were dose-dependently increased 3 h after infection in all treatment groups. In groups A and B, plasma IL-10 levels were elevated 3 h after infection exclusively, whereas in groups C and D, IL-10 concentrations were elevated during the whole observation period. Plasma IL-10 concentrations were strongly related to MCP-1 concentrations in plasma (r = 0.72; P < 0.001), in lung homogenates (r = 0.80; P < 0.001), and in BAL fluid (r = 0.51; P = 0.007). In groups C and D, dose-dependent increases in plasma G-CSF concentrations as high as 300 to 1,200 ng/ml were observed 12 and 24 h after infection. IL-6 values had returned to baseline in all groups after 12 h, except in group D, where IL-6 concentrations remained elevated for 24 h.
FIG. 2.
Plasma IL-10 (a), G-CSF (b), and IL-6 (c) concentrations 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg, □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001 versus controls.
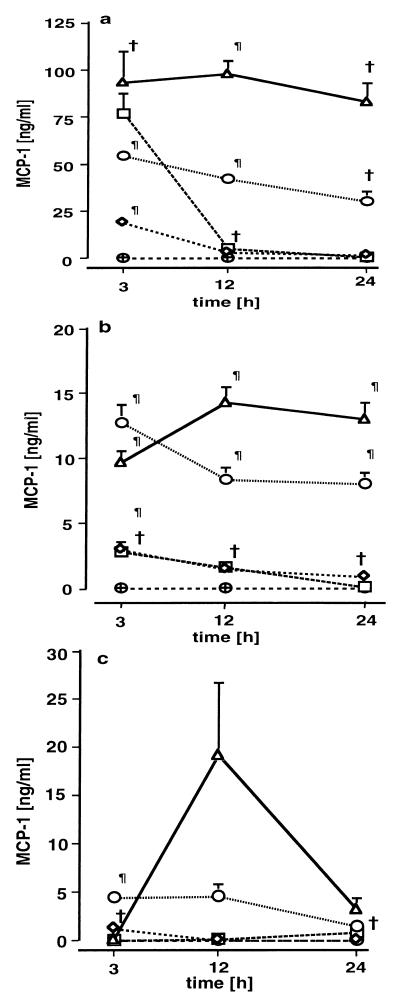
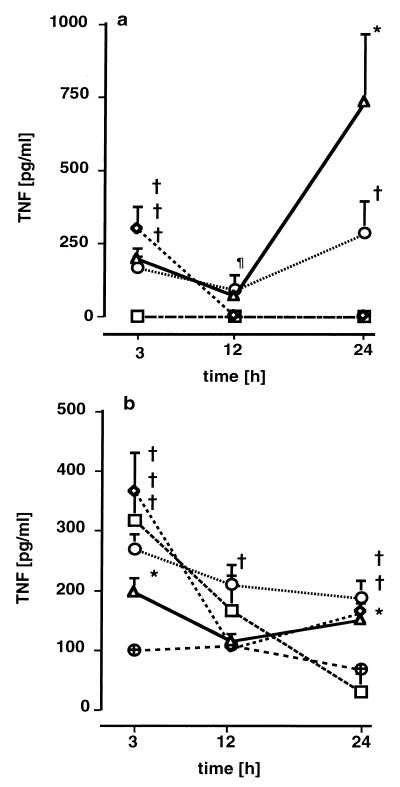
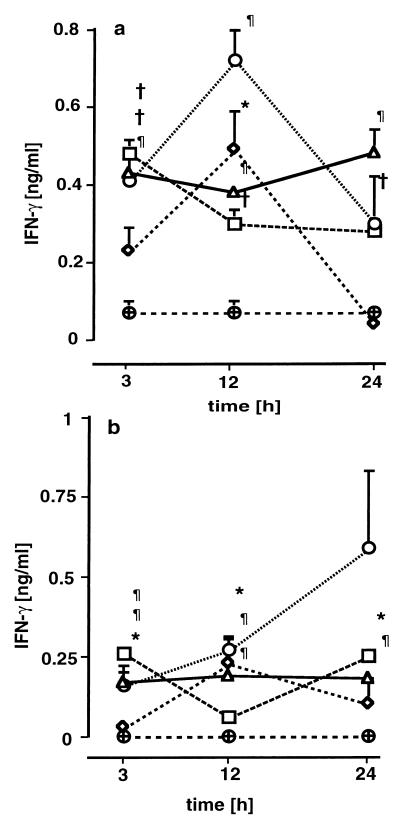
MCP-1, TNF, and IFN-γ were found to be elevated in plasma, lung tissue, and lung lavage fluid. MCP-1 concentrations were increased in plasma (Fig. 3a) and lung tissue (Fig. 3b) 3 h after infection. In groups C and D, plasma and lung tissue MCP-1 concentrations remained significantly elevated 12 and 24 h after infection. In groups A and B, plasma and lung tissue MCP-1 levels were initially elevated but decreased after 12 h. With the exception of group A, MCP-1 levels were enhanced dose dependently. Elevated MCP-1 levels in the lavage fluid were only noted in groups C and D (Fig. 3c). The lung tissue MCP-1 concentrations were strongly related to CFU of gram-negative (0.58; P = 0.002) and gram-positive (0.68; P < 0.001) bacteria. Plasma TNF concentrations (Fig. 4a) were significantly increased, though not strictly in a dose-dependent manner, in groups B, C, and D 3 h after infection. In groups C and D, plasma TNF concentrations followed a biphasic kinetic, with a second peak after 24 h. Similarly to the plasma levels, lung tissue TNF levels (Fig. 4b) were increased after 3 h but tended to decrease during the remainder of the experiment. TNF levels in lavage fluid were slightly elevated in all treatment groups but were not dose dependent. The BAL fluid TNF levels in the treatment groups were in a range from 100 to 250 pg/ml, while the controls ranged between 40 and 100 pg/ml. Plasma IFN-γ concentrations were below the detection limit in all groups except group D, where plasma levels were slightly elevated, i.e., 0.17 ± 0.35, 0.03 ± 0.07, and 0.19 ± 0.16 ng/ml after 3, 12, and 24 h, respectively, compared to undetectable levels in control animals. In lung tissue (Fig. 5a) and BAL fluid (Fig. 5b), IFN-γ levels were increased in all groups, although not in a dose-dependent manner. Systemic levels of GM-CSF were not elevated in any of the groups (data not shown). Slightly increased amounts of GM-CSF in supernatants of lung tissues were found at various time points without dose dependency (data not shown).
FIG. 3.
MCP-1 concentrations in plasma (a), lung tissue (b), and lung lavage (c) 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg; □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001 versus controls.
FIG. 4.
TNF concentrations in plasma (a) and lung tissue (b) 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg; □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001 versus controls.
FIG. 5.
IFN-γ concentrations in lung tissue (a) and BAL fluid (b) 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg; □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals. ∗, P < 0.05; †, P < 0.01; ¶, P < 0.001 versus controls.
Kinetics of eicosanoid concentrations.
6-keto-PGF1α, the stable metabolite of prostacyclin, was measured in those samples in which we also analyzed the gene expression of the related enzymes (see below), i.e., in animals of group D. In these animals, plasma 6-keto-PGF1α levels were elevated at all time points, i.e., 660 ± 70, 740 ± 140, and 530 ± 140 pg/ml after 3, 12, and 24 h, respectively, compared to 110 ± 40 pg/ml (range, 67 to 165) in control animals. Lung tissue and BAL fluid 6-keto-PGF1α levels were not different from those of controls. The level of plasma TXB2, the stable by-product of thromboxane A2, was 650 ± 360, 490 ± 320, and 350 ± 70 pg/ml after 3, 12, and 24 h, respectively, compared to 140 ± 90 pg/ml in control animals. BAL fluid TXB2 concentrations were elevated at all time points, i.e., 140 ± 40, 180 ± 30, and 650 ± 220 pg/ml after 3, 12, and 24 h, respectively, compared to 30 ± 10 pg/ml (range, 21 to 48) in control animals.
Gene expression.
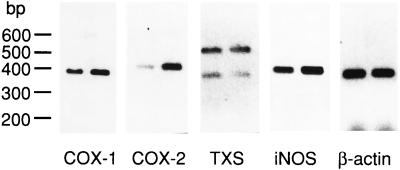
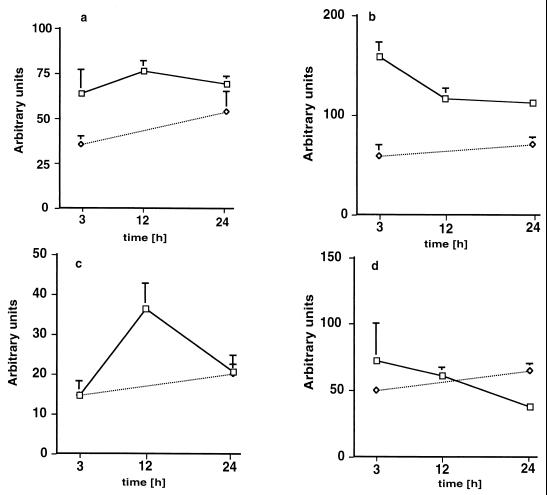
The expression of COX-1, COX-2, TXS, and iNOS was studied in group D exclusively (Fig. 6 and 7). Two TXS fragments were amplified by the primers used (Fig. 6). The fragment with a molecular size of 544 bp corresponds to active TXS, and the lower fragment of 351 bp corresponds to an alternatively spliced inactive TXS, which is missing a 163-bp exon (38). Three hours after infection, the pulmonary COX-2-specific mRNA content was increased compared to that of control lungs (Fig. 6). The mRNA/β-actin ratio for COX-1 and COX-2 (Fig. 7a and b) was about twofold elevated 3, 12, and 24 h after infection, was transiently increased for TXS after 12 h (Fig. 7c), and was not elevated for iNOS (Fig. 7d) compared to control values.
FIG. 6.
RT-PCR analysis of lung tissue obtained from mice infected at 1 ml/kg at 3 h after induction of polymicrobial peritonitis (right lane). Shown are COX-1, COX-2, TXS, iNOS, and β-actin message. The left lane corresponds to control mice.
FIG. 7.
Expression of mRNAs for COX-1 (a), COX-2 (b), TXS (c), and iNOS (d) in lungs 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. □ (group D), dosage of 1 ml/kg; ◊, controls. Each point in each panel represents the mean ± SEM of measurements in four animals. The data are expressed as described in Materials and Methods.
Microbiological examinations.
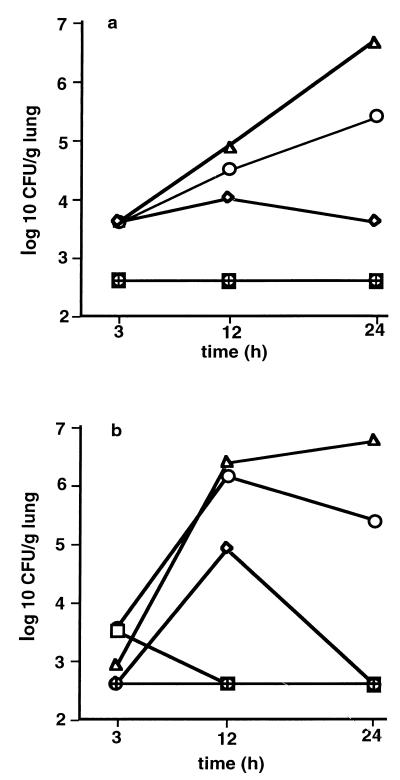
CFU counts of both gram-positive and gram-negative bacteria in the lungs of infected mice of groups C and D increased in a dose- and time-dependent manner beginning 12 h after challenge (Fig. 8). In groups A and B, bacterial CFU in the lungs were not different from those in controls 24 h after infection. Kendal's correlation coefficient between CFU of gram-positive and gram-negative bacteria was calculated as r = 0.69 (P = 0.001).
FIG. 8.
CFU (log 10/g of lung) of gram-positive (a) and gram-negative (b) bacteria 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg; □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals.
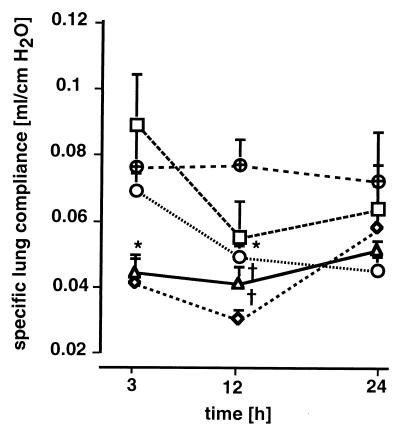
Specific lung compliance.
As early as 3 h after infection, specific lung compliance was significantly decreased in group D compared with that in controls (Fig. 9). Twelve hours after challenge, specific lung compliance was significantly decreased in all treatment groups except group A, which did not show significantly decreased compliance at any time. Lung compliance was inversely related to CFU of gram-positive (r = −0.52; P = 0.007) and gram-negative (r = −0.50; P = 0.007) bacteria and to levels of MCP-1 in the lungs (r = 0.40; P = 0.021).
FIG. 9.
Specific lung compliance (ml/cm of H2O) 3, 12, and 24 h after induction of polymicrobial peritonitis in mice. Specific lung compliance was measured as described in Materials and Methods. Dosages are indicated as follows: ▵ (D), 1 ml/kg; ○ (C), 0.5 ml/kg; ◊ (B), 0.25 ml/kg; □ (A), 0.125 ml/kg; ⊕, controls. Each point in each panel is the mean ± SEM of measurements in four animals. ∗, P < 0.05; †, P < 0.01 versus controls.
Bronchoalveolar cells and protein concentrations.
The total number of cells recovered by lung lavage was moderately increased 3 h after infection in all treatment groups. Notably, numbers of polymorphonuclear leukocytes (PMNs) in BAL fluid were not elevated (data not shown). A small, but insignificant, increase in the number of mononuclear cells was noticed (data not shown). To determine whether intra-alveolar protein leakage occurred in this model, we measured the BAL fluid protein content in lethally infected mice (group D). The BAL fluid protein concentrations were similar at all time points, i.e., 320 ± 100 μg/ml in controls and 300 ± 70, 390 ± 80, and 470 ± 190 μg/ml (mean ± SD) at 3, 12, and 24 h, respectively (n = 4 per group).
Lung MPO activity.
Lung MPO activity was only assessed in group D, where it was significantly increased, i.e., 7.2 ± 0.7, 4.9 ± 0.5, and 5.9 ± 1.3 U/g (wet weight) of lung (mean ± SD) at 3, 12, and 24 h, respectively, compared to 1.3 ± 1.1 U/g (wet weight) of lung in control animals. At the corresponding time points, less than 4% of cells recovered by BAL were neutrophils.
DISCUSSION
The present study evaluated the dose- and time-dependent relationships between graded polymicrobial septic peritonitis and pulmonary responses within the first 24 h after induction of peritonitis in mice. The treatment protocols resulted in three distinct response patterns (groups A plus B, C, and D) with respect to mortality rate, kinetics and extent of plasma and pulmonary cytokine release, intrapulmonary bacterial accumulation, and static lung compliance. In sublethally infected mice (groups A and B) with disseminated intra-abdominal abscesses at the time of autopsy, no pulmonary bacterial proliferation occurred in the lungs. In these animals, MCP-1, IL-10, IL-6, TNF, and G-CSF concentrations in plasma were elevated as early as 3 h after infection and returned to control levels after 24 h. Lung tissue TNF, MCP-1, and IFN-γ concentrations peaked early and decreased over time. Static pulmonary compliance was transiently decreased. Mice treated with higher bacterial doses (groups C and D) had bacterial growth in their lungs. The plasma MCP-1, IL-10, IL-6, and G-CSF concentrations were significantly and, with the exception of TNF, dose-dependently elevated at 3 h. The amounts and kinetics of cytokine release differed substantially between group C (30% mortality at 48 h) and group D (70% mortality at 48 h), with the highest MCP-1, G-CSF, and TNF concentrations in group D mice at 24 h. In group C animals, plasma IL-10 concentrations were highest at 24 h, while G-CSF and MCP-1 levels decreased at that time. Pulmonary TNF and IFN-γ concentrations were not dose-dependently elevated, whereas MCP-1 levels were increased in proportion to inoculum size at 24 h. Pulmonary static compliance was decreased in groups C and D.
Of the cytokines that were increased in the plasma, only MCP-1 and, to a lesser degree, TNF were increased in lung tissue. MCP-1 was additionally increased in the alveolar space. IFN-γ, which was not detectable in the circulation, was modestly increased in the lungs and in the alveolar space. Thus, as measured by cytokine responses, a limited pulmonary inflammatory response occurred in the early stage of septic peritonitis. The data suggest that the lungs are not a source of IL-6, IL-10, G-CSF, and TNF during the early stage of polymicrobial peritonitis. Furthermore, the spillover from the circulation to the pulmonary tissue appears to be quite small.
The observed pattern of cytokine expression of low levels of TNF and IFN-γ in conjunction with high levels of MCP-1 and IL-10 suggests a trend toward a reduced Th-1 response. Previous studies have reported that IL-10 and MCP-1 suppress TNF and IFN-γ production (12, 42) and prolong survival in sepsis models (35, 37, 42). Pulmonary and/or alveolar macrophage MCP-1 mRNA has been shown to be upregulated by LPS and inflammatory cytokines (4, 5) in mice challenged with endotoxin intraperitoneally (42) and in the CLP model (20-gauge needle) (29). In all of these models, as in the present study, an early increase in pulmonary MCP message or protein levels was noted. In line with this, we found a close correlation between intrapulmonary MCP-1 levels and CFU. Taken together, our data support the concept of Hogaboam et al. (16) that IL-10 and MCP-1 work in a complementary manner to exert beneficial effects in experimental peritonitis, and they possibly contribute to the suppression of TNF in our model. This conclusion is further corroborated by the close correlation between the IL-10 and MCP levels in the present study.
The TNF levels that can be induced by intraperitoneal bacterial challenge or CLP markedly differ from those that are found after intravenous challenge (with live bacteria or LPS). Though plasma TNF levels are elevated, they are significantly lower after focus-induced sepsis (15, 36) compared with models of non-focus-induced sepsis (2). In addition, anti-TNF treatment in various peritonitis models has no protective effects (2, 10) and may even enhance mortality (9).
Recently, we provided evidence (3) for the crucial role of endogenous G-CSF in controlling neutrophil-dependent defense against bacterial invasion at the onset of fecal peritonitis. Pretreatment of mice with G-CSF raised blood neutrophil counts fivefold and significantly protected animals against lethal peritonitis (3). Thus, the dramatically increased plasma G-CSF concentrations in mice prior to death in the present study may reflect a failure of this mechanism.
Another indication for the progress of lung inflammation is the expression of pulmonary COX-2 mRNA (19) and iNOS mRNA (7), which are both upregulated after intraperitoneal injection of LPS. In contrast to LPS, fecal peritonitis enhanced expression of only pulmonary COX-2 mRNA (in lethally infected mice) but not of iNOS mRNA. Of note, TXS and COX-1 mRNAs were also transiently increased. This observation is somewhat surprising, since neither gene has been shown to be regulated during inflammatory processes. The increased expression of pulmonary COX-2 and TXS mRNAs was reflected by elevated BAL fluid TXB2 concentrations. An increase in alveolar TXB2 concentration was also observed in the CLP model in rats (31). In that study, the authors observed a pulmonary edema that was abolished by COX inhibition. However, in their experiments the lungs from the CLP rats were subsequently extracorporally perfused and ventilated, and lavage concentrations of thromboxane and protein were determined after perfusion for 20 min. Due to these differences in experimental setup, their study and the present one cannot be readily compared and the role of thromboxane under our conditions remains unknown.
The most interesting single observation in the present study was the decrease in static lung compliance, which correlated well with the bacterial CFU and weakly with pulmonary MCP-1 levels. Static lung compliance was only transiently decreased in sublethally infected mice in which no pulmonary bacterial proliferation was found at 24 h. In contrast, in lethally infected mice in which intrapulmonary bacterial proliferation was not contained, static lung compliance was decreased from 3 to 24 h after infection. A decrease in static lung compliance could be caused by edema or alterations in the surfactant metabolism. The unaltered BAL fluid protein content suggests that alveolar (though not interstitial) edema is unlikely. In line with this, static lung compliance decreased well before the onset of pulmonary edema in a model of septic porcine lung injury (6). Therefore, we suggest that changes in the surfactant system remain as the most probable explanation for the reduced pulmonary compliance observed in our model. In support of this hypothesis, we observed the occurrence of giant lamellar bodies in pulmonary type II cells in group D mice (39). Giant lamellar bodies were also observed soon after endotoxin treatment in the perfused rat lung, i.e., independent of blood-derived leukocytes (33), probably because of disturbed surfactant secretion (11). Lewis et al. (18) found significant alterations of the surfactant system early in the course of lung injury in CLP-septic adult sheep. Malloy et al. (21) demonstrated alterations of the endogenous surfactant system in CLP rats that had septic manifestations but no evidence of lung injury. In addition, there is some clinical evidence of significant alterations in surfactant composition and function in septic patients at risk of developing acute respiratory distress syndrome (14, 32).
It has been hypothesized that lung injury as a consequence of intra-abdominal infection is mediated primarily by PMNs. Sepsis following CLP in sheep (8, 17) has been shown to result in PMN migration into the pulmonary interstitium by 24 h. Transiently increased lung PMN content (measured by MPO activity) at 6 h after CLP in mice has been described by others (24, 27). However, the mechanisms responsible for PMN recruitment in the mouse lung during polymicrobial sepsis and the role of PMNs in mediating in lung tissue injury remain to be determined. In the present study, neither increased counts nor percentages of PMNs in lung lavage fluid of lethally infected mice were noted, whereas MPO activity in the lung tissue was increased approximately sevenfold compared with that in control mice at corresponding time points. Consistent with this observation is the recent finding that increased lung MPO activity after CLP in mice mainly reflects increased sequestration of neutrophils in the pulmonary vasculature (28).
Despite an increase in MPO activity, bacterial clearance in high-dose-challenged mice was impaired. Sequestration of PMNs at other sites of insult is a possible explanation for an inadequate PMN recruitment to the lungs. Previously (3), we have shown that during severe fecal peritonitis the percentage of PMNs in the peritoneal cavity dramatically increases (up to 80% compared to 3% in uninfected controls), suggesting a strong compartmentalization of the cells to the main infectious focus. In line with this, it has been shown that during pulmonary infection with Pseudomonas aeruginosa, mice with peritonitis recruit fewer PMNs into their lungs than mice without peritonitis (40). The only cellular alteration we observed in the lung lavage fluid was a slight increase in mononuclear cells, a finding which may be related to the increased pulmonary levels of MCP-1.
In summary, both the intensity and the kinetics of systemic and pulmonary inflammatory responses to polymicrobial peritonitis in mice substantially depend on the inoculum size. At low doses of bacteria, systemic and pulmonary responses occur early but transiently, while they become sustained at higher lethal doses. These changes are characterized by increased pulmonary neutrophil sequestration, impaired bacterial clearance, and decreased lung compliance. Systemically, the kinetics of plasma MCP-1, G-CSF, and IL-10 concentrations were found to be valuable indicators of septic progress. The finding that MCP-1 was markedly increased in the lungs suggests that MCP-1 may be an important factor governing the pulmonary response to peritonitis. We conclude that fecal peritonitis is a useful model for the study of extrapulmonary induced sepsis.
ACKNOWLEDGMENTS
This study was supported by Deutsche Forschungsgemeinschaft Grants AW 686/18-1 and UH 88/2-1.
We thank Arthur Bauhofer (University of Marburg) for providing the fecal stool suspension used in these studies. We thank Elisabeth Schmid and Margarete Ullmann (University of Konstanz) for excellent technical assistance.
REFERENCES
- 1.Ayala A, Perrin M M, Kisala J M, Ertel W, Chaudry I H. Polymicrobial sepsis selectively activates peritoneal but not alveolar macrophages to release inflammatory mediators (Interleukins-1 and -6 and tumor necrosis factor) Circ Shock. 1992;36:191–199. [PubMed] [Google Scholar]
- 2.Bagby G J, Plessala K J, Wilson L A, Thompson J J, Nelson S. Divergent efficacy of antibody to tumor necrosis factor-α in intravascular and peritonitis models of sepsis. J Infect Dis. 1991;163:83–88. doi: 10.1093/infdis/163.1.83. [DOI] [PubMed] [Google Scholar]
- 3.Barsig J, Bundschuh D S, Hartung T, Bauhofer A, Sauer A, Wendel A. Control of fecal peritoneal infection in mice by colony-stimulating factors. J Infect Dis. 1996;174:790–799. doi: 10.1093/infdis/174.4.790. [DOI] [PubMed] [Google Scholar]
- 4.Brieland J K, Flory C M, Jones M L, Miller G R, Remick D G, Warren J S, Fantone J C. Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-α, and interleukin-1 β. Am J Respir Cell Mol Biol. 1995;12:104–109. doi: 10.1165/ajrcmb.12.1.7811465. [DOI] [PubMed] [Google Scholar]
- 5.Brieland J K, Jones M L, Clarke S J, Baker J B, Warren J S, Fantone J C. Effect of acute inflammatory lung injury on the expression of monocyte chemoattractant protein-1 (MCP-1) in rat pulmonary alveolar macrophages. Am J Respir Cell Mol Biol. 1992;7:134–139. doi: 10.1165/ajrcmb/7.2.134. [DOI] [PubMed] [Google Scholar]
- 6.Byrne K, Cooper K R, Carey P D, Berlin A, Sielaff T D, Blocher C R, Jenkins J K, Fisher B J, Hirsch J I, Tatum J L, Fowler A A, Sugerman H J. Pulmonary compliance: early assessment of evolving lung injury after onset of sepsis. J Appl Physiol. 1990;69:2290–2295. doi: 10.1152/jappl.1990.69.6.2290. [DOI] [PubMed] [Google Scholar]
- 7.Cunha F Q, Assreuy J, Moss D W, Rees D, Leal L M C, Moncada S, Carrier M, O'Donnell C A, Liew F Y. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-α and IL-1-β. Immunology. 1994;81:211–215. [PMC free article] [PubMed] [Google Scholar]
- 8.Craig I, Judges D, Gnidec A, Lefcoe M, Paterson N, Finley R, Sibbald W. Pulmonary permeability edema in a large animal model of nonpulmonary sepsis. Am J Pathol. 1987;128:241–251. [PMC free article] [PubMed] [Google Scholar]
- 9.Echtenacher B, Falk W, Männel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 10.Eskandari M K, Bolgos G, Miller C, Nguyen T, DeForge L E, Remick D G. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol. 1992;148:2724–2730. [PubMed] [Google Scholar]
- 11.Fehrenbach H, Brasch F, Uhlig S, Weisser M, Stamme C, Wendel A, Richter J. Early alterations in intracellular and alveolar surfactant of the rat lung in response to endotoxin. Am J Respir Crit Care Med. 1998;157:1630–1639. doi: 10.1164/ajrccm.157.5.9611070. [DOI] [PubMed] [Google Scholar]
- 12.Gérard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goya T, Abe M, Shimura H, Torisu M. Characteristics of alveolar macrophages in experimental septic lung. J Leukoc Biol. 1992;52:236–243. doi: 10.1002/jlb.52.2.236. [DOI] [PubMed] [Google Scholar]
- 14.Gregory T J, Longmore W J, Moxley M A, Whitsett J A, Reed C R, Fowler A A, Hudson L D, Maunder R J, Crim C, Hyers T M. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Investig. 1991;88:1976–1981. doi: 10.1172/JCI115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadjiminas D J, McMasters K M, Peyton J C, Cook M D, Cheadle W G. Passive immunization against tumor necrosis factor and interleukin-1 fails to reduce lung neutrophil sequestration in chronic sepsis. Shock. 1994;2:376–380. doi: 10.1097/00024382-199411000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Hogaboam C M, Steinhauser M L, Schock H, Lukacs N, Strieter R M, Standiford T, Kunkel S L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect Immun. 1998;66:650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judges D, Sharkey P, Cheung H, Craig I, Driedger A A, Sibbald W J, Finley R. Pulmonary microvascular fluid flux in a large animal model of sepsis: evidence for increased pulmonary endothelial permeability accompanying surgically induced peritonitis in sheep. Surgery. 1986;99:222–234. [PubMed] [Google Scholar]
- 18.Lewis J F, Veldhuizen R, Possmayer F, Sibbald W, Whitsett J, Qanbar R, McCaig L. Altered alveolar surfactant is an early marker of acute lung injury in septic adult sheep. Am J Respir Crit Care Med. 1994;150:123–130. doi: 10.1164/ajrccm.150.1.8025737. [DOI] [PubMed] [Google Scholar]
- 19.Liu S F, Newton R, Evans T W, Barnes P J. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci. 1996;90:301–306. doi: 10.1042/cs0900301. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz W, Reimund K P, Weitzel F, Celik I, Kurnatowski M, Schneider C, Mannheim W, Heiske A, Neumann K, Sitter H, Rothmund M. Granulocyte colony-stimulating factor prophylaxis before operation protects against lethal consequences of postoperative peritonitis. Surgery. 1994;116:925–934. [PubMed] [Google Scholar]
- 21.Malloy J, McCaig L, Veldhuizen R, Yao L J, Joseph M, Whitsett J, Lewis J. Alterations of the endogenous surfactant system in septic adult rats. Am J Respir Crit Care Med. 1997;156:617–623. doi: 10.1164/ajrccm.156.2.9608009. [DOI] [PubMed] [Google Scholar]
- 22.McLauchlan G J, Anderson I D, Grant I S, Fearon K C. Outcome of patients with abdominal sepsis treated in an intensive care unit. Brit J Surg. 1995;82:524–529. doi: 10.1002/bjs.1800820429. [DOI] [PubMed] [Google Scholar]
- 23.McMasters K M, Peyton J C, Hadjiminas D J, Cheadle W G. Endotoxin and tumour necrosis factor do not cause mortality from caecal ligation and puncture. Cytokine. 1994;6:530–536. doi: 10.1016/1043-4666(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 24.Mercer-Jones M A, Hadjiminas D J, Heinzelmann M, Peyton J, Cook M, Cheadle W G. Continuous antibiotic treatment for experimental abdominal sepsis: effects on organ inflammatory cytokine expression and neutrophil sequestration. Br J Surg. 1998;85:385–389. doi: 10.1046/j.1365-2168.1998.00580.x. [DOI] [PubMed] [Google Scholar]
- 25.Mercer-Jones M A, Heinzelmann M, Peyton J C, Wickel D J, Cook M, Cheadle W G. The pulmonary inflammatory response to experimental fecal peritonitis: relative roles of tumor necrosis factor-α and endotoxin. Inflammation. 1997;21:401–417. doi: 10.1023/a:1027366403913. [DOI] [PubMed] [Google Scholar]
- 26.Merrell R C. The abdomen as source of sepsis in critically ill patients. Crit Care Clin. 1995;11:255–273. [PubMed] [Google Scholar]
- 27.Peralta J G, Barnard M L, Turrens J F. Characteristics of neutrophil influx in rat lungs following fecal peritonitis. Inflammation. 1993;17:263–271. doi: 10.1007/BF00918989. [DOI] [PubMed] [Google Scholar]
- 28.Que L G, Kang B H, Huang Y C T, Piantadosi C A, Chang L Y. Anti-intercellular adhesion molecule-1 antibody and intercellular adhesion molecule-1 gene deficiency do not prevent pulmonary neutrophil recruitment in polymicrobial sepsis. Shock. 1998;9:304–309. doi: 10.1097/00024382-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Salkowski C A, Detore G, Franks A, Falk M C, Vogel S N. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider T, Issekutz A C. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198:1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- 31.Schneidkraut M J, Carlson R W. Cecal ligation and puncture is associated with pulmonary injury in the rat: role of cyclooxygenase pathway products. Prostaglandins. 1993;45:323–334. doi: 10.1016/0090-6980(93)90110-s. [DOI] [PubMed] [Google Scholar]
- 32.Stamme C, Leuwer M, Lührs J, Ensink M, Tschorn H, Vangerow B, Rückoldt H, Schürmann W, Bernhard W, Piepenbrock S. Alterations in pulmonary surfactant during the course of sepsis-induced ARDS predisposition. Appl Cardiopulm Pathophysiol. 1997;6:223–232. [Google Scholar]
- 33.Uhlig S, Brasch F, Wollin L, Fehrenbach H, Richter J, Wendel A. Functional and fine structural changes in isolated rat lungs challenged with endotoxin ex vivo and in vitro. Am J Pathol. 1995;146:1235–1247. [PMC free article] [PubMed] [Google Scholar]
- 34.Uhlig S, Nüsing R, von Bethmann A, Featherstone R L, Klein T, Brasch F, Müller K M, Ullrich V, Wendel A. Cyclooxygenase-2-dependent bronchoconstriction in perfused rat lungs exposed to endotoxin. Mol Med. 1996;2:373–383. [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Poll T, Marchant A, Buurman W A, Berman L, Keogh C V, Lazarus D D, Nguyen L, Goldman M, Moldawer L L, Lowry S F. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 36.Villa P, Sartor G, Angelini M, Sironi M, Conni M, Gnocchi P, Isetta A M, Grau G, Buurman W, van Tits L J H, Ghezzi P. Pattern of cytokines and pharmacomodulation in sepsis induced by cecal ligation and puncture compared with that induced by endotoxin. Clin Diagn Lab Immunol. 1995;2:549–553. doi: 10.1128/cdli.2.5.549-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walley K R, Lukacs N W, Standiford T J, Strieter R M, Kunkel S L. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–4738. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L H, Tazawa R, Lang A Q, Wu K K. Alternate splicing of human thromboxane synthase mRNA. Arch Biochem Biophys. 1994;315:273–278. doi: 10.1006/abbi.1994.1500. [DOI] [PubMed] [Google Scholar]
- 39.Weisser M, Wendel A, Uhlig S. Two novel methods for the assessment of alterations in pulmonary surfactant in LPS-treated lungs. Am J Respir Crit Care Med. 1999;159:A892. [Google Scholar]
- 40.White J C, Nelson S, Winkelstein J A, Booth F V, Jakab G J. Impairment of antibacterial defense mechanisms of the lung by extrapulmonary infection. J Infect Dis. 1986;153:202–208. doi: 10.1093/infdis/153.2.202. [DOI] [PubMed] [Google Scholar]
- 41.Wickel D J, Cheadle W G, Mercer-Jones M A, Garrison R N. Poor outcome from peritonitis is caused by disease acuity and organ failure, not recurrent peritoneal infection. Ann Surg. 1997;225:744–756. doi: 10.1097/00000658-199706000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zisman D A, Kunkel S L, Strieter R M, Tsai W C, Bucknell K, Wilkowski J, Standiford T J. MCP-1 protects mice in lethal endotoxemia. J Clin Investig. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]