Abstract
Background
This meta-analysis evaluated the real-world effectiveness of onabotulinumtoxinA (BOTOX®), the first preventive treatment FDA-approved specifically for chronic migraine in 2010.
Methods
We systematically reviewed onabotulinumtoxinA observational data in chronic migraine published between 1 January 2010 and 31 March 2021. Random-effects models evaluated available data for primary and secondary endpoints defined in onabotulinumtoxinA pivotal trials at approximately 24 weeks and 52 weeks.
Results
Of the 44 full-text eligible studies (29 prospective; 13 retrospective; 2 other), seven evaluated change from baseline (mean[confidence interval]) at ∼24 weeks and ∼52 weeks, respectively, for onabotulinumtoxinA in: number of headache days/month: (–10.64 [–12.31, –8.97]; –10.32 [−14.92, –5.73]); number of days of acute headache pain medication intake per month (–7.40 [–13.04, –1.77]; overlapping CIs at 52 weeks); total Headache Impact Test-6 score (–11.70 [–13.86, –9.54]); –11.80 [14.70, –8.90]); and Migraine-Specific Quality-of-Life v2.1 score (MSQ; 23.60 [CI: 21.56, 25.64]; 30.90 [CI: 28.29, 33.51]). At ∼24 weeks onabotulinumtoxinA showed total Migraine Disability Assessment score of 44.74 [28.50, 60.99] and ≥50% reduction in migraine days response rate of 46.57% [29.50%, 63.65%]. A sensitivity analysis at study-end suggested durability of onabotulinumtoxinA effectiveness on MSQ.
Conclusion
The meta-analysis reflecting real-world practice broadly corroborated with evidence from pivotal and long-term open-label studies of onabotulinumtoxinA in chronic migraine preventive treatment.
Keywords: Meta-analysis, effectiveness, onabotulinumtoxinA, BOTOX®, chronic migraine, prophylaxis, preventive, real-world evidence, real-world data
Introduction
Migraine is characterised by both painful symptoms, including attacks of intense throbbing headache that are often accompanied by nausea, vomiting and sensitivity to light and sound, and other sensory symptoms, such as tiredness, numbness, and allodynia (1,2). The symptoms of chronic migraine cause a level of functional and emotional impact in people with migraine (3) that ranks the disease second among worldwide causes of disability (4).
When headache occurs on 15 or more days per month beyond a period of three months and has the features of migraine occurring at least eight days per month, the presentation is termed chronic migraine (5), a complex, progressive, neurological disease (6) that prevails in 1–2% of the global population (7). Chronic migraine is associated with considerable disease burden; people with chronic migraine experience important impairments to daily functioning (8), health-related quality of life (HRQoL), and productivity (9,10). The disease also poses substantial direct costs to healthcare systems (11,12) and indirect costs to society (8).
Preventive therapeutic strategies aim to reduce frequency, duration, and intensity of migraine attacks (13–15). Among oral medications used and studied in the prevention of migraine, only topiramate 100 mg/day has clearly demonstrated efficacy in the prophylaxis of chronic migraine (16,17), but no treatments are licensed for preventive treatment of chronic migraine specifically. Moreover, poor efficacy and tolerability of oral preventive medications has led to low treatment persistence (14% at 12 months), and overuse of acute prescription medication for migraine (18–26). Calcitonin gene-related peptide (CGRP) monoclonal antibodies and gepants, which act on the CGRP pathway, have been more recently licensed by the US Food and Drug Administration (FDA) as preventive treatment options for migraine indications, including atogepant (27,28) and rimegepant (29,30) for episodic migraine; and eptinezumab (31,32), erenumab (33,34), fremanezumab (35,36) and galcanezumab (37,38) for both episodic and chronic migraine.
OnabotulinumtoxinA (BOTOX®) is an intramuscularly-injected acetylcholine release inhibitor and neuromuscular blocking agent that was the first prophylactic treatment specifically indicated for chronic migraine; onabotulinumtoxinA was approved in 2010 by the FDA (39) and by some national medicines agencies in Europe (40,41). Since its approval in the chronic migraine indication, the effectiveness of onabotulinumtoxinA has been captured within a large body of evidence from the real-world collected from the open-label prospective studies (42,43) (such as FORWARD, COMPEL) in addition to observational studies of treatment effectiveness. This real-world evidence (RWE) has complemented gold-standard evidence (44) from the onabotulinumtoxinA Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy (PREEMPT 1 [NCT00156910] and PREEMPT 2 [NCT00168428]) pivotal randomised controlled trial (RCT) programme (6,45) in the chronic migraine population.
RWE is of increasing interest for both initial and ongoing regulatory and payer assessments in healthcare markets across the world (46–48) and such evidence on onabotulinumtoxinA effectiveness and safety representing real-world practice is particularly crucial for optimising chronic migraine treatment pathways given the heterogeneity of the patient population (49) (not reflected in RCTs) and complex patient management in the real world (50,51). Hence, given the ten-year experience with onabotulinumtoxinA since the treatment was licensed in chronic migraine, a systematic identification and evaluation of observational data around its real-world effectiveness in reducing headache symptoms, headache-related disability, and improving HRQoL in patients with chronic migraine is warranted for informing payer and clinical decision-making.
Objectives
The purpose of this study was to conduct a meta-analysis to evaluate the real-world effectiveness of onabotulinumtoxinA for the treatment of chronic migraine and to discuss these results in comparison with those obtained in the onabotulinumtoxinA PREEMPT pivotal trials and long-term open-label studies, where feasible.
Methods
The meta-analysis followed methodological guidelines (52–56) and was reported in accordance with the Meta-analyses Of Observational Studies in Epidemiology (MOOSE) Checklist (57).
Data collection
We conducted a systematic literature review (SLR) to inform the meta-analysis; the SLR was performed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) reporting guidelines (58,59) and included studies published between 1 January 2010 and 31 March 2021. We formulated the research questions and PICOS (Population, Intervention, Comparison, Outcomes, and Setting) framework based on the specific chronic migraine disease area, disease-modifying factors, interventions, and study types, which in turn informed the search strategies (Supplementary Table 1A).
Our searches spanned Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (Embase), and the Cochrane Library/Cochrane Central Register of Controlled Trials to identify relevant published real-world data. The searches applied controlled vocabulary and keywords, limited to articles involving human participants and published in English. Both MeSH and Emtree (Embase subject heading) terms were used in the search strategy (Supplementary Table 1B). MeSH terms included migraine, migraine disorders. Keywords for the condition were combined with those used for study design; examples of MeSH for study design were: retrospective studies, health survey, observational study, etc. Finally, we applied a filter to exclude publications in the form of letters, editorials or notes.
We also conducted bibliographic searches and gray literature searches of published systematic reviews and narrative reviews, and conducted manual searches within PubMed, Google, and Google Scholar. To retrieve the latest data from conference proceedings, manual searching was conducted for the previous 3 years (January 2017 to March 2020 and during the update from March 2020 to January 2021).This search identified relevant abstracts and presentations made at relevant scientific congresses, including: International Headache Society (5), Migraine Trust (60), American Headache Society (61), European Headache Federation (62), American Academy of Neurology (63) and International Society for Pharmacoeconomics and Outcomes Research (64). In addition, trial registry searches (Clinicaltrials.gov and the EU Clinical Trials Register [65]) retrieved ongoing and planned real-world observational studies for onabotulinumtoxinA in the target population of adult patients with chronic and unspecified migraine.
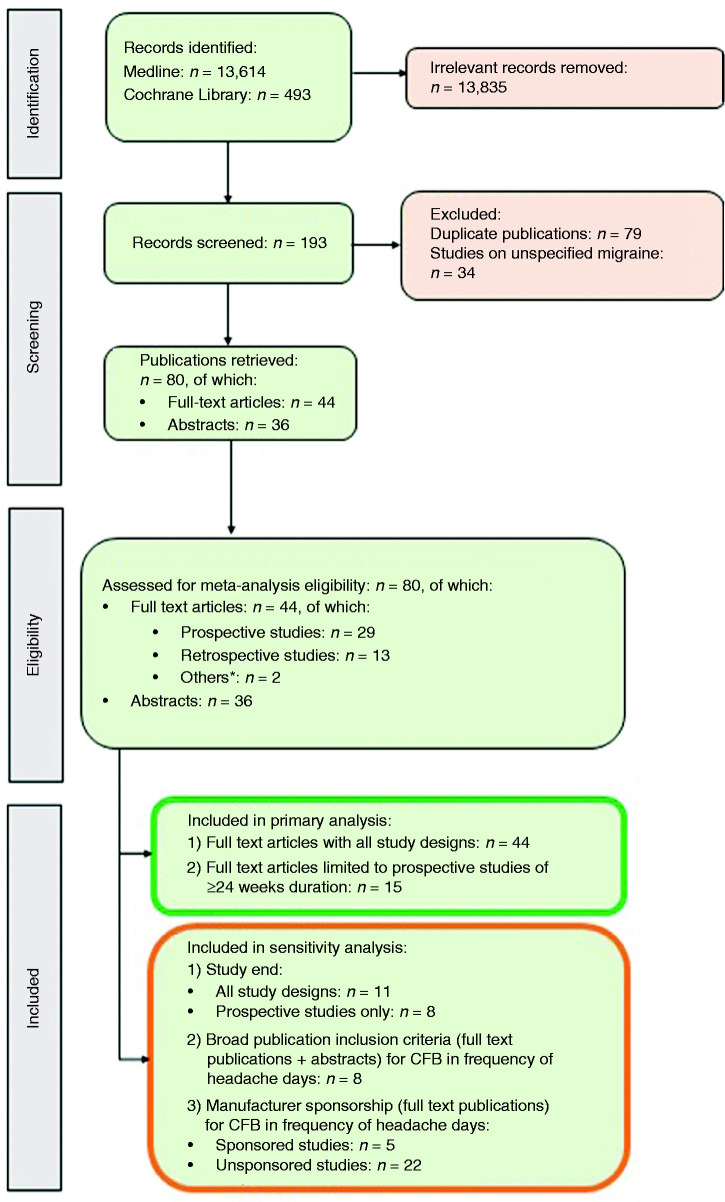
Research analysts with master’s degree in a pharmacy qualification conducted the study selection, initially reviewing the abstracts and titles of each citation and identifying a list of potentially relevant studies following a screening flowchart (Figure 1). Only those studies that met all pre-defined criteria for inclusion (Supplementary Table 1A) were considered for final extraction, while excluded citations were given a code justifying the reasons for exclusion. The lists obtained from the two reviewers were combined; a third reviewer (EO) then resolved any discrepancy. Any article that could not be clearly excluded based on the abstract was progressed for full-text review. Full-text versions of relevant studies were examined in more detail to determine a final list of included studies based on the Newcastle–Ottawa Scale (NOS) (66); a score of 4 (out of a maximum of 9) was the minimum cut-off point we applied for inclusion of full journal publications only (66,67). Assessment criteria varied according to study type (cohort studies or case–control studies; Supplementary Table 2). For meta-analysis, we selected studies of patients with chronic migraine specifically.
Figure 1.
PRISMA flowchart. CFB: change from baseline; NOS: Newcastle–Ottawa Scale. *1 case–control study (82), 1 subgroup analysis (116).
Analysis
The meta-analysis considered the key endpoints examined in the onabotulinumtoxinA PREEMPT RCT programme in patients with chronic migraine (6,45,67). Both PREEMPT 1 and 2 comprised a 24-week, randomised, double-blind phase followed by a 32-week, open-label phase. The two PREEMPT RCTs were identical in design in terms of inclusion/exclusion criteria, randomisation, visits, double-blind phase, open-label phase, safety assessments, and treatment; in both studies, eligible patients were randomised (1:1) to onabotulinumtoxinA (155–195 U) or placebo injections every 12 weeks and assessed every four weeks. The primary study endpoint of both RCTs was Week 24 change from baseline (CFB) in number of headache days per month (a headache day was defined as any headache lasting at least 30 minutes; a month was defined as a 28-day period). Secondary efficacy variables included: CFB in number of migraine days per month, moderate/severe headache days per month, number of cumulative hours of headache on days when headache was experienced, proportion of patients scoring severe (≥60 points) on the Headache Impact Test (HIT)-6, number of headaches per month, number of migraine attacks (meeting ICHD-2 criteria 1.1 migraine without aura or 1.2 migraine with aura according to the patient's diary) per month, and number of days of acute headache pain medication intake per month. The definition used for the two headache/migraine attack frequency outcomes was a patient-reported headache/migraine with a start and end time indicating that the pain lasted four or more continuous hours. A further response analysis for PREEMPT included the rate of patients with ≥50% reduction in the number of headache days per month (6,67,68).
The patient-reported outcomes in PREEMPT included functioning, and HRQoL, as measured through CFB in total HIT-6 and Migraine-Specific Quality of Life v2.1 (MSQ) scores. HIT-6 evaluates the impact of headache on patient general well-being and daily activity, with scores ranging from 36 (no impact) to 78 (maximum impact); ≥60 is considered a major impact on general well-being and daily activity (69). The MSQ 14-item questionnaire measures the impact of migraine on the respondent’s physical and emotional functioning based on three domains: 1) role-function restrictive (limitations to daily social and work-related activities); 2) role-function preventive (how migraine prevents these activities); and 3) emotional function (migraine-associated emotional functioning) (70). All item scores are reverse-coded, and both MSQ domain and total score (evaluated in the present meta-analysis) are scaled 0–100, with higher score indicating better migraine-related quality of life (70).
We also conducted a meta-analysis on the Migraine Disability Assessment (MIDAS) score, if available in the selected studies. The MIDAS assesses headache-related disability, whereby the total score is graded across four levels: Grade I (0–5; minimal or infrequent), Grade II (6–10; mild or infrequent); Grade III (11–20, moderate), and Grade IV (≥21; severe disability) (71). Although MIDAS was not administered in the PREEMPT clinical programme, we evaluated the endpoint in meta-analysis as it has been deemed an important outcome by the International Headache Society for assessing the impact of preventive migraine treatments in clinical practice (72).
We conducted primary meta-analyses on PREEMPT endpoints for which there were enough studies available, on assessments ‘close to 24 weeks’ (∼24 weeks; up to six months) and ‘close to 52 weeks’ (∼52 weeks; up to 13 months), and a sensitivity analysis at ‘study-end’, whatever the duration of follow-up (Table 1). We applied both fixed-effects (inverse variance) and random-effects approaches. For the latter approach, we followed the DerSimonian and Laird method (73), adjusting standard errors of the study-specific estimates to incorporate a measure of study heterogeneity (i.e., Tau-squared [τ2]). However, given this high heterogeneity (both clinical and statistical) across studies, the random-effects approach was considered to be much more appropriate for interpretation of results and as such, reported in this study (74). In those studies, for which the input data in the required format were incomplete, we imputed missing values, estimating means and standard deviations from median, interquartile range and number of patients, based on the method described by Wan et al. (2014) for n > 200 (75):
Table 1.
Primary and sensitivity analyses.
| Analysis | Endpoints | Study criteria | Assessment period |
||
|---|---|---|---|---|---|
| ∼24 weeks | ∼52 weeks | Study end | |||
| Primary analysis Full publications | Change from baseline in number of headache days | All studies | ✓ | ✓ | |
| Prospective studies* | ✓ | ✓ | |||
| Change from baseline in number of days of acute headache pain medication intake | All studies | ✓ | ✓ | ||
| Prospective studies* | N/A | N/A | |||
| Rate of ≥50% reduction in migraine days | All studies | ✓ | N/A | ||
| Prospective studies* | ✓ | N/A | |||
| Change from baseline in total HIT-6 score | All studies | ✓ | ✓ | ||
| Prospective studies* | N/A | N/A | |||
| Change from baseline in MSQ score | All studies | ✓ | ✓ | ||
| Prospective studies* | |||||
| MIDAS score | All studies | ✓ | N/A | ||
| Prospective studies* | ✓ | N/A | |||
| Sensitivity analysis 1Full publications | Change from baseline in number of headache days | All studies | ✓ | ||
| Prospective studies* | ✓ | ||||
| Change from baseline in number of days of acute headache pain medication intake | All studies | ✓ | |||
| Prospective studies* | ✓ | ||||
| Change from baseline in total HIT-6 score | All studies | ✓ | |||
| Prospective studies* | ✓ | ||||
| Change from baseline in MSQ score | All studies | ✓ | |||
| Prospective studies* | |||||
| MIDAS score | All studies | ✓ | |||
| Prospective studies* | ✓ | ||||
| Sensitivity analysis 2Full publications + congress abstracts/presentations | Change from baseline in number of headache days | All studies | (Same as primary analysis) | ✓ | |
| Sensitivity analysis 3Full publications | Change from baseline in number of headache days | All studies: sponsored vs unsponsored | ✓ | – | |
*With durations of least 24 weeks; N/A: not applicable,
The primary analysis on available data for PREEMPT endpoints and MIDAS score included populations from all studies/all designs (i.e., prospective and retrospective) to allow for comprehensiveness and large sample sizes, and full, peer-reviewed publications only (Table 1; Figure 1). Data from conference abstracts were not included in this analysis as the granularity of information published in abstracts did not allow for assessing the quality of the study using the NOS score. To minimise the heterogeneity observed across studies, we then narrowed the primary analysis to prospective studies that had durations of at least 24 weeks. (Table 1; Figure 1).
Following primary analysis, we conducted sensitivity analysis 1 at study end, whatever the study duration, to provide higher analytical power and to allow evaluating the durability of treatment effect beyond ∼52 weeks on all available PREEMPT endpoints and MIDAS score (Table 1; Figure 1). Two further sensitivity analyses were conducted on Change from baseline in number of headache days per month. Sensitivity analysis 2 first considered the totality of full publications and abstracts on this outcome at 52 weeks; then sensitivity analysis 3 examined manufacturer-sponsored versus unsponsored studies to assess potential ‘sponsored bias’ at ∼24 weeks (Table 1; Figure 1).
All analyses were conducted on R statistical software (version 4.0.4).
Results
In total, the SLR identified 193 real-world studies in migraine, among which 157 were published as full papers and 36 as conference abstracts. After removing duplicate publications and studies in unspecified migraine, 44 full-text publications and 36 abstracts on chronic migraine passed screening and eligibility assessment and were considered for inclusion in the primary meta-analysis (Table 2; Figure 1). Of the 44 studies in full-text form, 29 were prospective studies (of which 15 had durations of 24 weeks or longer and were thus included in the primary analysis restricted to prospective studies); 13 were retrospective studies; two were classified as “other”. Geographically, most of the included studies were conducted in Italy (n = 15) and Spain (n = 11), with other countries across Europe, North America and Australia (Supplementary Figure 1).
Table 2.
Characteristics of studies included in meta-analysis.
| Study name | Study design | Publication type | Data source (Data collection period) | Country | Treatment/ comparator | Sample size | FU (months) | Effectiveness | Safety | Tolerability | Industry sponsorship | NOS score‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed 2015 (Hull migraine study) (83) | PCS | JP | Primary data (2010–2019) | UK | OnabotA | 972 | 96 | Yes | Yes | Yes | No | 6/9 |
| Ahmed 2019 (REPOSE) (86) | PCS | JP | Primary data (2012–2016) | Multi-national* | OnabotA | 633 | 24 | Yes | Yes | Yes | Yes; Allergan | 6/9 |
| Alessiani 2018 (103) | PCS | CA | Primary data (N/R) | Italy | OnabotA | 54 | 9 | Yes | No | Yes | No | N/A |
| Alpuente 2019 (109) | PCS | JP | Primary data (2014–2018) | Spain | OnabotA | N/R | 24 | Yes | Yes | Yes | No | 5/9 |
| Andreou 2018 (110) | PCS | JP | Primary data (2013–2016) | UK | OnabotA | 200 | ∼36 | Yes | Yes | Yes | Yes; Allergan | 5/9 |
| Aydinlar 2017 (76) | PCS | JP | Primary data (2012–2016) | Turkey | OnabotA | 190 | 11 | Yes | Yes | Yes | No | 5/9 |
| Belvís 2018 (111) | PCS | CA | Primary data (N/R) | Spain | OnabotA | 90 | 3.7 | Yes | No | Yes | No | N/A |
| Boudreau 2020 (112,113) (PREDICT) | PCS | CA | Primary data (N/R) | Canada | OnabotA | 184 | 24 | Yes | No | No | Yes; Allergan | N/A |
| Butera 2016 (89) | PCS | JP | Primary data (2013–2015) | Italy | OnabotA | 44 | 8.3 | Yes | Yes | Yes | No | 5/9 |
| Caronna 2018 (99) | RCS | JP | Medical records (2014–2017) | N/R | OnabotA | 139 | 6 | Yes | No | No | No | 6/9 |
| Corbelli 2019 (104) | PCS | CA | Primary data (N/R) | Italy | OnabotA | 195 | 24 | Yes | Yes | Yes | No | N/A |
| d’Onofrio 2020 (81) | PCS | JP | Primary data, self-reported scale, interview (N/R) | Italy | OnabotA | 40 | 6 | Yes | No | No | None | 3/9 |
| de Tommaso 2019 (114) | PCS | JP | Primary data (2015–2016) | Italy | OnabotA | 99 | 24 | Yes | Yes | Yes | No | 5/9 |
| DiCola 2019 (95) | PCS | JP | Primary data (2015–2018) | Italy | OnabotA | 84 | 12 | Yes | Yes | Yes | No | 5/9 |
| Dikmen 2018 (82) | RCS | JP | Survey (2012–2017) | Turkey | OnabotA | 180 | 60 | Yes | Yes | Yes | No | 5/9 |
| Dominguez 2018 (88) | PCS | JP | Primary data (2014–2015) | Spain | OnabotA | 725 | 12 | Yes | Yes | Yes | No | 8/9 |
| Eren 2020 (100) | PCS | JP | Primary data (N/R) | Germany | OnabotA | 49 | 3 | Yes | No | No | No | 5/9 |
| Gandolfi 2019 (79) | PCS | JP | Primary data | Italy | OnabotA | 40 | 12 | Yes | No | No | No | 4/9 |
| Garcia-Azorín 2018 (115) | PCS | CA | Primary data (N/R) | Spain | OnabotA | 49 | 1 | Yes | No | No | No | N/A |
| Gonzalez-Martinez 2020 (80) | RCS | JP | Medical records (Jan 2018–May 2018) | Spain | OnabotA | 112 | ∼8.3 | Yes | No | No | No | 5/9 |
| Grazzi 2015 (116) | PCS | JP | Primary data (N/R) | Italy | OnabotA | 66 | 12 | Yes | Yes | No | No | 4/9 |
| Grazzi 2016 (117) | PCS | CA | Primary data (N/R) | N/R | OnabotA | 53 | 12 | Yes | No | No | No | N/A |
| Guerzoni 2017 (118) | RCS | JP | Medical records (2013–2017) | Italy | OnabotA | 90 | 36 | Yes | Yes | Yes | Yes; Allergan | 5/9 |
| Kennedy 2017 (119) | PCS | CA | Primary data (2013–2017) | UK | OnabotA | 120 | 6 | Yes | Yes | No | No | N/A |
| Lee 2016 (101) | PCS | JP | Primary data (2013–2015) | Korea | OnabotA | 70 | 1 | Yes | No | No | No | 6/9 |
| Lin 2014 (102) | RCS | JP | Medical records (2008–2012) | Taiwan | OnabotA | 94 | 3 | Yes | Yes | No | No | 5/9 |
| Navarrete Perez 2017 (120) | PCS | CA | Primary data (N/R) | Spain | OnabotA | 117 | 3 | Yes | No | No | No | N/A |
| Negro 2016 (90) | PCS | JP | Primary data (2012–2013) | Italy | OnabotA 195 U/OnabotA 155 U | 275 | 24 | Yes | Yes | Yes | No | 6/9 |
| Ornello 2020 (96) | PCS | JP | Primary data (2015–2018) | Italy | OnabotA | 115 | 15 | Yes | Yes | Yes | Yes; Allergan | 5/9 |
| Pedraza 2015 (121) | PCS | JP | Primary data (2012–2014) | Spain | OnabotA | 52 | 3 | Yes | No | Yes | No | 4/9 |
| Quintas 2019 (122) | RCS | JP | Primary data (2012–2017) | Spain | OnabotA | 193 | 6 | Yes | Yes | Yes | No | 2/9 |
| Ranoux 2017 (97) | RCS | JP | Primary data (2008–2015) | France | OnabotA | 63 | 7 | Yes | Yes | Yes | No | 4/9 |
| Romoli 2017 (123) | PCS | CA | Primary data (N/R) | Italy | OnabotA | 56 | 12 | Yes | No | No | No | N/A |
| Russo 2016 (91) | PCS | JP | Primary data (2014–2015) | Italy | OnabotA | 52 | 9 | Yes | Yes | Yes | No | 4/9 |
| Santoro 2020 (98) | RCS | JP | EMRs and interviews (2013–2019) | Italy | OnabotA | 109 | 48 | Yes | Yes | Yes | No | 6/9 |
| Sanz 2018 (92) | PCS | JP | Primary data (2013–2015) | Spain | OnabotA | 69 | 16 | Yes | Yes | Yes | No | 5/9 |
| Sarchielli 2017 (85) | PCS | JP | Primary data (2014–2016) | Italy | OnabotA | 56 | 15 | Yes | Yes | No | Yes; Allergan | 5/9 |
| Stark 2019 (93) | RCS | JP | Chart review (2016–2017) | Australia | OnabotA | 211 | 13.8 | Yes | Yes | No | Yes; Allergan | 7/9 |
| Taddei-Allen 2019 (78) | RCS | CA | Questionnaire (Jan 2018–Dec 2018) | N/R | CGRP^/OnabotA | 42 | 12 | Yes | No | No | No | N/A |
| Torres-Ferrus 2020 (94) | PCS | JP | Primary data (2014–2019) | Spain | OnabotA | 395 | ≥4 | Yes | No | No | No | 7/9 |
| Velasco-Juanes 2018 (124) | PCS | JP | Primary data (2005–2015) | Spain | OnabotA | 70 | 3 | Yes | Yes | Yes | No | 4/9 |
| Vernieri 2019 (77) | RCS | JP | Medical records (2015–2017) | Italy | OnabotA | 115 | 12 | Yes | No | Yes | No | 7/9 |
CA: conference abstract; CGRP: calcitonin gene-related peptide; Dec: December; EMR: electronic medical record; FU: follow-up; Jan: January; JP: journal paper; N/A: not applicable; NOS: Newcastle–Ottawa Scale; N/R: not reported; onabotA: onabotulinumtoxinA; PCS: prospective cohort study; RCS: retrospective cohort study; UK: United Kingdom.
Note: *Germany, Italy, Norway, Russia, Sweden, Spain, and the United Kingdom. ^Erenumab, galcanezumab, fremanezumab. ‡NOS N/A for abstracts or secondary references
PREEMPT endpoints, assessed where sufficient data were available in eligible studies, included mean CFB in number of headache days per month, number of days of acute headache pain medication intake per month, total HIT-6 score, and MSQ score. Studies also evaluated incidence of ≥50% reduction in the number of headache days per month. In addition to PREEMPT endpoints, some included studies also assessed the absolute MIDAS score. However, not all outcomes were assessed across all studies (Table 3).
Table 3.
Outcomes assessed across studies (any time points).
| Study name | CFB monthly headache days | CFB monthly migraine days | CFB monthly moderate/severe headache days | CFB number of days acute headache pain med intake | CFB total HIT-6 score | Severe (≥60) HIT-6 score | Monthly migraine days | Migraine duration | Incidence of ≥50% reduction in migraine days | CFB MSQ score | MIDAS score | Treatment-related adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpuente 2019 (109) | No | No | No | No | No | No | Yes | No | No | No | Yes | No |
| Ahmed 2019 (86) (REPOSE) | Yes | No | No | No | No | No | No | No | No | Yes | No | No |
| Ahmed 2015 (83) (Hull migraine study) | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | No | No | No |
| Alessiani 2018 (103) | Yes | No | No | No | No | No | No | No | No | No | No | No |
| Andreou 2018 (110) | No | No | No | No | No | No | Yes | No | No | No | No | Yes |
| Aydinlar 2017 (76) | No | No | No | No | No | No | No | No | No | No | Yes | No |
| Boudreau 2020 (112,113) (PREDICT) | Yes | No | No | No | No | No | No | No | No | Yes* | No | Yes |
| Belvís 2018 (111) | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Butera 2016 (89) | No | No | No | No | No | No | Yes | No | No | No | Yes | No |
| Caronna 2018 (99) | No | No | No | No | No | No | Yes | No | Yes | No | Yes | No |
| Corbelli 2019 (104) | Yes | No | No | No | No | No | No | No | No | No | Yes | No |
| d’Onofrio 2020 (81) | No | No | No | No | No | No | No | No | No | No | Yes | No |
| DiCola 2019 (95) | Yes | No | Yes | Yes | No | Yes | No | No | No | No | No | No |
| Dikmen 2018 (82) | No | No | No | No | No | No | No | No | No | No | Yes | No |
| Dominguez 2018 (88) | No | No | No | No | No | No | Yes | Yes | No | No | No | No |
| Eren 2020 (100) | Yes | No | No | No | No | No | No | No | No | No | No | No |
| Gandolfi 2019 (79) | No | No | No | No | No | No | No | No | No | No | Yes | No |
| Gonzalez-Martinez 2020 (80) | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Garcia-Azorín 2018 (115) | No | No | No | No | No | No | Yes | No | No | No | No | No |
| Grazzi 2016 (117) | No | No | No | No | No | No | No | No | No | No | Yes | No |
| Grazzi 2015 (116) | No | No | No | No | No | No | Yes | No | No | No | Yes | No |
| Guerzoni 2017 (118) | No | No | No | No | Yes | No | No | No | No | No | No | Yes |
| Kennedy 2017 (119) | No | No | No | No | Yes | No | No | No | No | No | No | No |
| Lee 2016 (101) | Yes | No | No | No | No | No | No | No | No | No | No | No |
| Lin 2014 (102) | Yes | No | No | No | No | No | No | No | No | No | Yes | No |
| Navarrete Perez 2017 (120) | No | No | No | No | No | No | No | No | Yes | No | No | No |
| Negro 2016 (90) | Yes | Yes | No | Yes | Yes | No | No | No | No | No | No | Yes |
| Ornello 2020 (96) | Yes | No | No | No | No | Yes | No | Yes | No | No | Yes | No |
| Pedraza 2015 (121) | No | No | No | No | No | No | Yes | No | Yes | No | No | No |
| Quintas 2019 (122) | No | No | No | No | No | No | Yes | No | No | No | No | No |
| Ranoux 2017 (97) | No | No | No | Yes | No | Yes | No | No | Yes | No | No | No |
| Romoli 2017 (123) | No | No | No | No | No | Yes | Yes | No | No | No | Yes | No |
| Russo 2016 (91) | Yes | No | No | Yes | No | No | No | No | Yes | No | Yes | No |
| Santoro 2020 (98) | No | No | No | No | No | No | No | No | No | No | No | No |
| Sanz 2018 (92) | Yes | No | No | No | No | No | No | No | No | No | No | No |
| Sarchielli 2017 (85) | No | No | No | No | No | No | Yes | No | No | No | No | No |
| Stark 2019 (93) | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | No |
| Torres-Ferrus 2020 (94) | Yes | No | Yes | Yes | No | No | No | No | No | No | Yes | No |
| Taddei-Allen 2019 (78) | No | No | No | No | No | No | Yes | Yes | Yes | No | No | No |
| Vernieri 2019 (77) | Yes | No | No | Yes | No | No | No | No | No | No | Yes | No |
| Velasco-Juanes 2018 (123) | Yes | No | No | No | Yes* | No | No | No | No | No | No | No |
| Total | 16 | 03 | 02 | 06 | 06 | 02 | 14 | 04 | 08 | 02 | 17 | 04 |
CFB: change from baseline; HIT-6: Headache Impact Test-6; MSQ: Migraine-Specific Quality-of-Life Questionnaire; VAS: visual analog scale.
Bold font indicates outcome evaluated through meta-analysis. No studies reporting the endpoints of MSQ or migraine severity (VAS) were identified.
*Insufficient data (e.g., lack of standard deviation; standard error for CFB) to enable inclusion within meta-analysis.
Baseline clinical characteristics of patients within the included onabotulinumtoxinA real-world studies indicated heterogeneous patient populations (Supplementary Table 3). Mean (±standard deviation [SD]) age at study inclusion ranged from 39.3 (±10.2) (76) to 51.6 (±10.3) (77). Across the studies patients were, however, predominantly female (71% [78] to 92.5% [79]).
Duration of chronic migraine diagnosis ranged from a mean of 2.4 (±3.5) (80) to a mean of 25.7 (±12.3) years (81). The baseline number of headache days per month ranged from a mean of 18.9 (±5.5) (82) to a mean of 27.3 days (±4.7) (83) (Supplementary Table 3).
On patient-reported outcomes, mean HIT-6 scores at baseline ranged from a mean of 61.2 (SD not reported) (84) to a mean of 72.1 (±6.0) (85) (Supplementary Table 4). Mean baseline MSQ scores for role-function restrictive domain ranged from 36.2 (±17.8) (86) to 36.7 (SD not reported) (87). On the role-function preventive domain, mean scores ranged from 50.2 (±22.8) (REPOSE study) (86) to 51.4 (SD not reported) (87). On the emotional function domain, mean scores ranged from 38.0 (SD not reported) (87) to 42.4 (±25.6) (86) (Supplementary Table 4). Mean MIDAS scores at baseline ranged from 35.9 (±29.6) (88) to 117.13 (±94.8) (89) (Supplementary Table 4).
We also observed broadly comparable differences across studies for onabotulinumtoxinA dosage and the treatment protocol followed (Supplementary Table 5). Consequentially, the I2 statistics confirmed high overall study heterogeneity (Figures 2 –5) indicating that the random-effects models were the appropriate approach for interpretation of the meta-analysis results. However, we also reported the results of the fixed-effect models for completeness.
Figure 2.
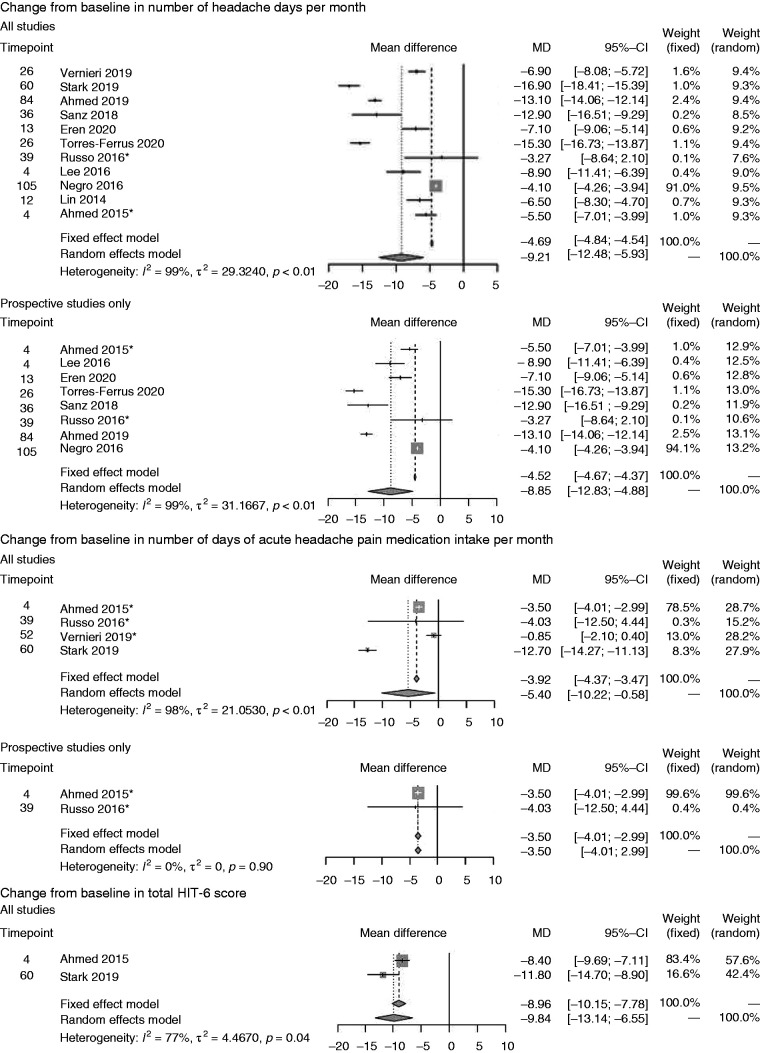
Primary analysis *Imputation of missing data applied. ¶ Comprises one prospective study only. CI: confidence interval; HIT: Headache Impact Test; MIDAS: Migraine Disability Assessment; MSQ: Migraine-Specific Quality of Life.
Figure 3.

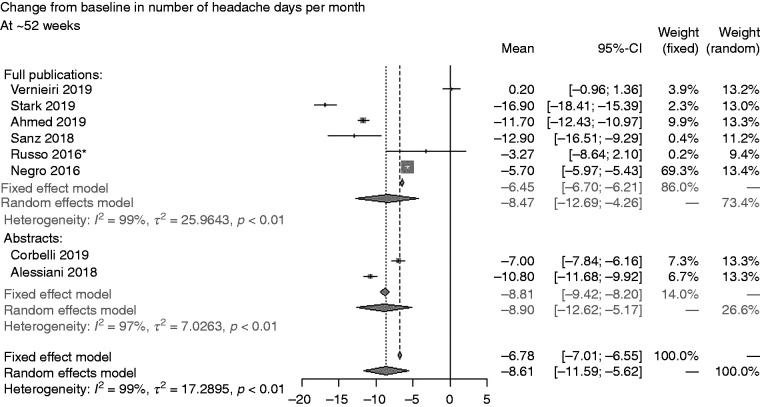
Sensitivity analysis 1: outcomes at study end *Imputation of missing data applied. ¶Comprises one prospective study only. CI: confidence interval; HIT-6: 6-item Headache Impact Test; MSQ: Migraine-Specific Quality-of-Life.
Figure 4.
Sensitivity analysis 2: broad publication criteria (i.e., inclusion of conference abstract/poster data).
Figure 5.
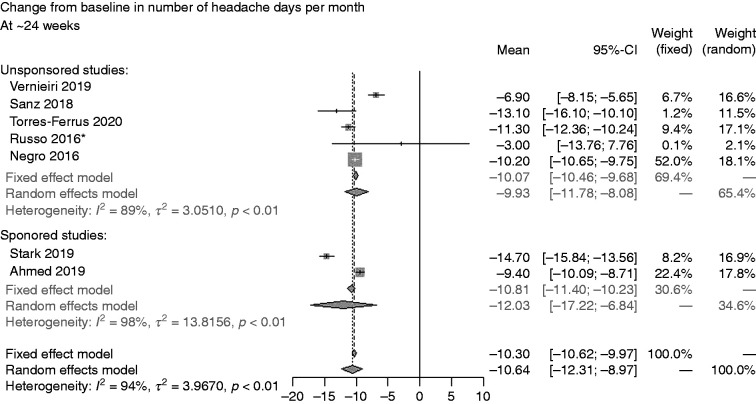
Sensitivity analysis 3: Unsponsored versus sponsored studies *Imputation of missing data applied. CI: confidence interval.
Primary analysis
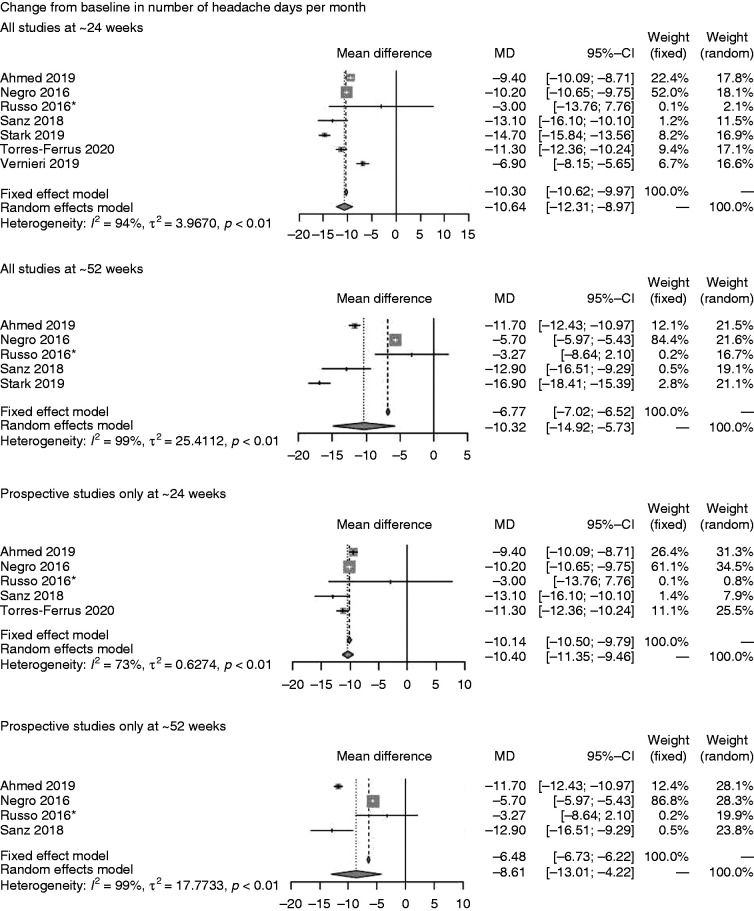
Seven studies assessed onabotulinumtoxinA on mean CFB in number of headache days per month at ∼24-weeks and five at ∼52-weeks (Figure 2A) (77,86,90–94). The random-effects models revealed mean treatment effects of onabotulinumtoxinA of –10.64 (95% confidence interval (CI; –12.31, –8.97)) at ∼24 weeks and –10.32 (CI: –14.92, –5.73) at ∼52 weeks. When considering prospective studies only, similar mean treatment effects were achieved at ∼24 weeks (–10.40 (CI: –11.35, –9.46)) but slightly lower at ∼52 weeks (–8.61 (CI: –13.01, –4.22)) on this endpoint.
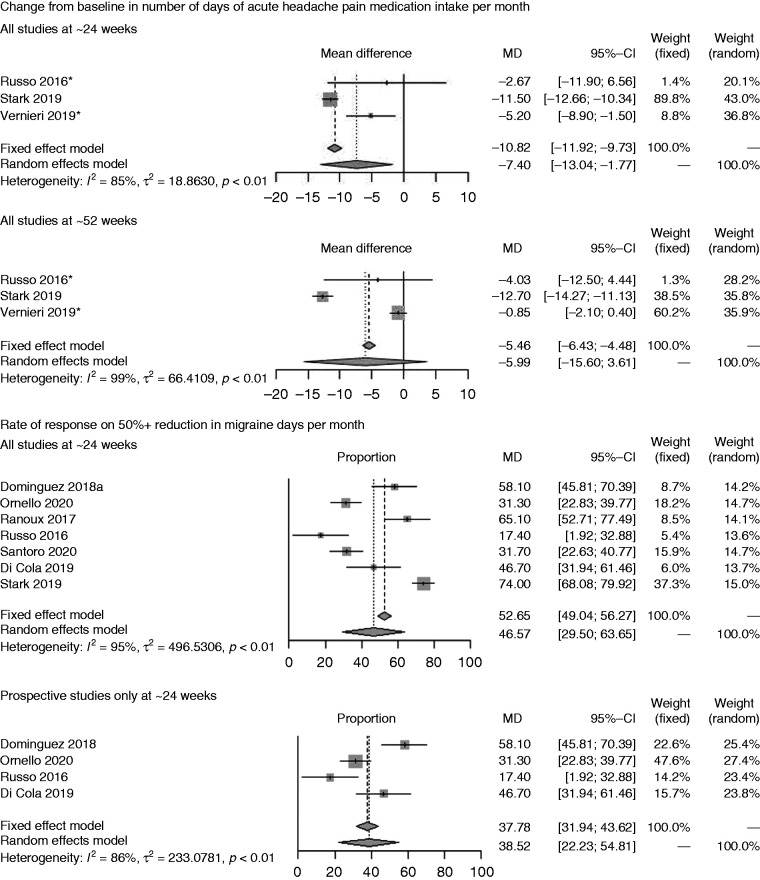
On CFB in number of days of acute headache pain medication intake per month (Figure 2B), onabotulinumtoxinA showed a mean of –7.40 (CI: –13.04, –1.77) on the endpoint at ∼24 weeks but the confidence intervals crossed zero at ∼52 weeks (77,91,93).
Seven studies reported the incidence of ≥50% reduction in migraine days per month (Figure 2C) at ∼24 weeks only and revealed an incidence of 46.57% (CI: 29.50%, 63.65%) for all studies and 38.52% (CI: 22.23%, 54.81%) for the group of prospective studies (88,91,93,95–98).
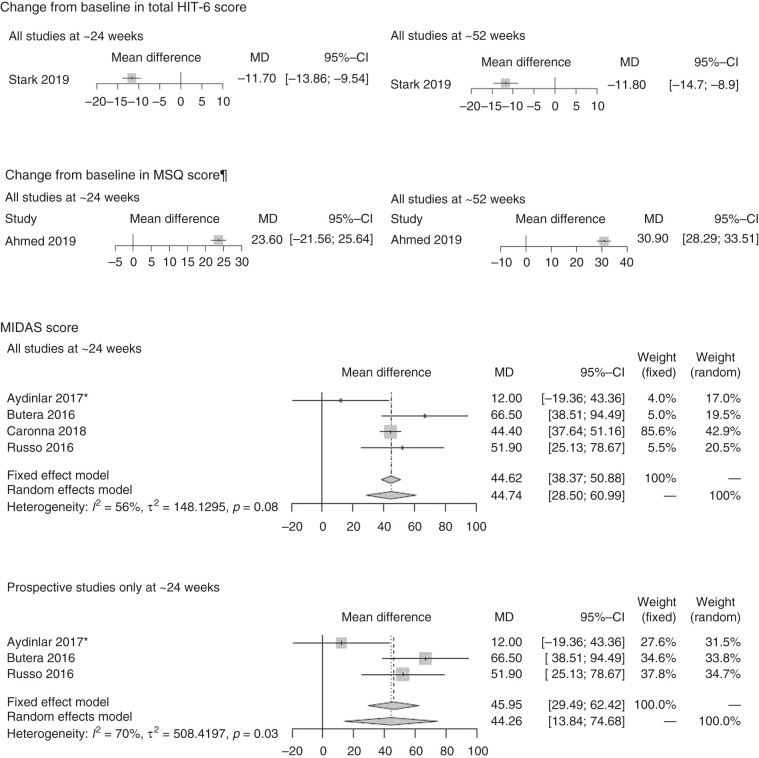
Data on mean CFB in total HIT-6 score (Figure 2D) were available in one study only (Stark 2019) (93) at ∼24 weeks (–11.70 [CI: –13.86, –9.54]) and ∼52 weeks (–11.80 [CI: 14.70, –8.90]). For mean CFB in MSQ score, we identified a single study only, which was also prospective (86) reporting the outcome at ∼24 weeks (23.60 [CI: 21.56, 25.64]) and ∼52 weeks (30.90 [CI: 28.29, 33.51]) (Figure 2E). On MIDAS score (Figure 2F), four studies (76,89,91,99) were available at ∼24 weeks only and showed onabotulinumtoxinA treatment effects based on a random-effects model of 44.74 (CI: 28.50, 60.99). Three prospective studies at ∼24 weeks showed similar treatment effects (44.26 [CI: 13.84, 74.68]) (76,89,91).
Sensitivity analysis
Sensitivity analysis 1, which assessed outcomes at study end, indicated that durability slightly waned over time for onabotulinumtoxinA on mean CFB in number of headache days per month (–9.21 [CI: –12.48, –5.93]) based on a random effects model considering all studies and compared with earlier assessments. However, the waning of effectiveness was less so considering prospective studies only (–8.85 [CI: –12.83, –4.88]) (Figure 3A) (79,83,86,90–94,100–102).
On number of days of acute headache pain medication intake per month, mean CFB was slightly lower at study end (–5.40 [CI: –10.22, –0.58]) (Figure 3B) (77,83,91,93) compared with earlier assessments. Only two studies provided data collected prospectively, Ahmed et al. (83) (4 weeks) and Russo et al. (91) (39 weeks) revealing less of a reduction on number of days of acute headache pain medication intake per month (−3.50 [CI: –4.01, –2.99]) for onabotulinumtoxinA (Figure 3B).
Data on mean CFB in total HIT-6 score (–9.84 [CI: –13.14, –6.55]) was only available for one study (83) at 4 weeks and 60 weeks (93) (Figure 3C), but a decrease over the long term was observed only over the 60-week study (93). The prospective study (4-week study) (83) assessed mean CFB in total HIT-6 score at the end of the study (Figure 3C) showing a mean CFB of –8.40 (CI: –9.69, –7.11). On CFB increase in MSQ score, the single prospective study (86) indicated a mean of 33.60 (CI: 30.03, 37.17) at 84 weeks (Figure 3D), an overall improvement compared with earlier assessments.
Sensitivity analysis 2 based on all publications (full articles and abstracts) indicated CFB in number of headache days per month at ∼52 weeks of –8.61 (CI: –11.69, –5.62) (77,86,90–93,103,104); no data were available for ∼24 weeks (Figure 4). In sensitivity analysis 3, comparing data for this endpoint at ∼24 weeks indicated slightly less favourable results for unsponsored studies (–9.93 [CI: –11.78, –8.08]) versus sponsored studies (–12.03 [CI: –17.22, –6.84]) (Figure 5) (77,90–92,94).
Discussion
This meta-analysis indicates that outcomes for onabotulinumtoxinA in the real world measured at ∼24 weeks and ∼52 weeks are broadly comparable to the outcomes observed in the PREEMPT pivotal studies of onabotulinumtoxinA. For example, the n = 347 adults randomised to onabotulinumtoxinA every 12 weeks for two cycles in PREEMPT 2 experienced statistically significant and clinically meaningful improvements on the primary endpoint, mean CFB on number of headache days per month (–9.0 onabotulinumtoxinA vs –6.7 placebo, p < 0.001) (45). Our meta-analytic results showed a comparable magnitude of treatment effect for onabotulinumtoxinA on this measure at ∼24 weeks (–10.64 [CI: –12.31, –8.97]) and ∼52 weeks (–10.32 [CI: –14.92, –5.73]).
Moreover, whereas both PREEMPT 1 and 2 indicated statistically significant results for onabotulinumtoxinA versus placebo on selected secondary endpoints evaluated in our analysis (e.g., incidence of ≥50% reduction in migraine days per month, CFB in total HIT-6 scores, and CFB in MSQ scores) across all time points, our primary meta-analytic results pointed to similar levels of changes on outcomes for onabotulinumtoxinA in the real-world (67). Our analysis also showed similar CFB decrease in number of days of acute headache pain medication intake per month as in PREEMPT 2 (45).
In terms of real-world, long-term treatment effects compared with those observed in the COMPEL long-term, open-label clinical study of onabotulinumtoxinA, our findings were variable. For example, COMPEL observed a mean reduction in number of headache days per month (–10.7 [CI: –11.0, –10.0]) at 108 weeks for onabotulinumtoxinA (43). However real-world data suggested lower treatment effect on this outcome which had also slightly waned over time (–9.21 [CI: –12.48, –5.93]) from among the 11 studies that ranged from four weeks (84,93) to 105 weeks (90). Conversely, compared with the CFB for HIT-6 scores in COMPEL (–7.1 [CI: –7.8, –6.9]) at 108 weeks, two real-world studies (of four weeks [83] and 60 weeks [93] duration, respectively) together showed better improvement at study end (–9.84 [CI: –13.14, –6.55]). However, in considering these comparisons, it is notable that the COMPEL study evaluated the fixed-dose, fixed-site injection paradigm, which may not necessarily reflect real-world practice. Similarly, long-term mean CFB in HIT-6 score in our analysis was better than observed in the FORWARD multicenter, randomised, parallel-group, open-label prospective study of onabotulinumtoxinA 155 U every 12 weeks for three cycles. The comparator in this study was topiramate immediate-release 50–100 mg/day. Patients randomised to onabotulinumtoxinA exhibited significant (p < 0.001) mean (±SD) CFB in HIT-6 score of –5.6 (±7.2) (compared with –1.3 [±3.9] for topiramate) (42) to week 30 on the secondary endpoint of CFB in HIT-6 score. In terms of overall durability of treatment effect found across our studies, apart from a mean CFB increase in MSQ score in the single study by Ahmed et al. (86) at 84 weeks, the real-world data showed waning durability on other outcomes at end-of-study assessments.
A few important limitations to our meta-analysis must be considered for interpreting our findings. Firstly, there was high heterogeneity of results reported between studies (I2 ≥ 90% for most analysis) for many scenarios. High heterogeneity is expected in meta-analyses that include a large number of studies to enrich the sample (55). The meta-analyses presented in the current study are mostly based on single-arm trials with relatively narrow CIs, which influences the values of the statistical heterogeneity parameters. In particular, the narrower CIs of included studies, the higher I2. The fact that the CIs are narrow can be explained by potential overpowering, which is common in RWE. Indeed, given the same total sample size, the arm-wise sample size is larger for single-arm trials, hence the CIs are usually narrower (52–55). For example, a 99% I2 was estimated in the real-world meta-analysis of intravitreal aflibercept and intravitreal ranibizumab in age-related neovascular macular degeneration (105), and a 97% I2 in a meta-analysis on real-world statin use and risk of new-onset diabetes (106). Other comparable heterogeneities have also been reported elsewhere (105,106).
Nevertheless, we took the following steps to resolve the problem of high heterogeneity. First, we applied a random-effects model to account for the uncertainty of the pooled results (54). In this case, we did not expect a common ‘true effect', but rather a distribution of effects, as the population is heterogeneous across different observational studies (per age, disease severity, previous treatment, origin, ethnicity, socio-demographic data). Indeed, the CIs in the random-effects model reflected the range of results observed in all included studies. Hence, we presented the results across three different observation periods.
We also evaluated a series of scenarios and assessed potential sources of study heterogeneity by applying specific inclusion criteria to the meta-analysis, these included: retrospective versus prospective design, sponsorship status, and more technical sources allowing inclusion of results based on abstracts or studies for which data imputation procedures for missing data have been applied. We observed no substantial differences in the results following sensitivity analyses.
Many of the meta-analysis estimates (e.g., reduction in the number of days with migraine, at six or 12 months, or of moderate/severe headache days at 12 months, CFB in the total HIT-6 score at six months, CFB at six and 12 months of the MSQ score) were also based on only one or two studies (e.g., reduction in the number of moderate/severe headache days at six months, proportion of patients at six and 12 months with HIT-6 score of 60 or more). This is a result of the data identified, and some of the parameters were rarely investigated/reported in the selected studies (e.g., HIT-6 and MSQ scores); hence, this reduced the number of included studies and limited the ability to analyse the outcomes. Another limitation is the missing information to characterise the patients included in the meta-analysis, especially for some variables such as the number of previous prophylactic treatments received.
This meta-analysis has, nonetheless, systematically considered the totality of empirical evidence and provided insights regarding the agreement or disagreement of the results from different observational study designs (54). In addition, all studies were selected based on a NOS score of at least 4 out of a maximum of 9 (66). For example, for the primary endpoint (change from baseline in number of headache days per month) analysed in the primary analysis, only one of 11 studies rated 4, three rated 5, and seven out of 11 studies rated ≥6 or more on the NOS. Of the 56 full-text studies identified in the SLR, eight studies were of high quality, with a score ranging from 7–8 on NOS; 48 studies were of moderate quality with a score of 4–6 on NOS. The two studies deemed low quality (i.e., NOS score of 2–3) were excluded from the meta-analysis. Hence, given these study limitations and strengths, while no strong conclusions can be made on the findings, our results provide a broad indication of the treatment effects associated with onabotulinumtoxinA in the real world as observed in the RCTs.
It can be noted that the NOS is one of the most widely used scales for assessing quality and risk of bias in observational studies (66,107), as other tools, such as the ROBINS-I, are more commonly used to only assess the risk of bias of observational studies; therefore, each assesses different aspects in the design, execution, validity, and precision of a study (108).
While a cutoff threshold for the NOS distinguishing between ‘good’ and ‘poor’ quality studies is yet to be validated (66), we defined a score of 4 as the cutoff for our approach to selecting studies of adequate quality. We applied this for our primary analysis, including only the studies that satisfied this criterion. We further conducted a sensitivity analysis which included the studies with low NOS (i.e., <4 points) to assess the influence on the results of relaxing the inclusion criteria, including one study based on full text for the endpoint, Migraine Disability Assessment (MIDAS) score. We then included studies for which only the abstracts were available; for this analysis, the endpoints available for assessment included: CFB in number of headache days per month; CFB in number of days of acute headache pain medication intake per month; and ≥50% reduction in migraine days per month. It is notable that, in the results for second sensitivity analysis including abstract-only studies, we found that, not only were the differences unsubstantial, the small differences only further favoured onabotulinumtoxinA. Hence, by excluding these studies from the primary analysis, we have ensured that our main analysis was not only based on robust data, but also conservative. Finally, we also assessed the risk of publication bias by conducting a sensitivity analysis considering sponsored versus non-sponsored studies and found that the results were comparable.
Conclusions
This meta-analysis of onabotulinumtoxinA, in the context of clinical practice that reflects the heterogeneity of populations treated with onabotulinumtoxinA for preventive treatment of chronic migraine, broadly corroborates with the efficacy that has been observed in the pivotal RCTs and long-term, open-label studies of onabotulinumtoxinA. Ongoing research collecting real-world data on onabotulinumtoxinA and the newer treatments for chronic migraine will provide further insights into the effectiveness of chronic migraine preventive treatments in clinically generalisable patient populations.
Article highlights
A meta-analysis of onabotulinumtoxinA was conducted to provide context for the heterogeneity of populations prophylactically treated with onabotulinumtoxinA for chronic migraine in clinical practice.
In total, these data corroborate the efficacy that has been observed in the pivotal RCTs and long-term, open-label studies of onabotulinumtoxinA.
Supplemental Material
Supplemental material, sj-pdf-1-cep-10.1177_03331024221123058 for Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: A meta-analysis on 10 years of real-world data by Michel Lanteri-Minet, Anne Ducros, Clement Francois, Elzbieta Olewinska, Mateusz Nikodem and Laure Dupont-Benjamin in Cephalalgia
Acknowledgements
Medical writing assistance, under the direction of the authors, was provided by Gauri Saal, MA Economics (funded by AbbVie according to Good Publication Practice guidelines [http://www.ismpp.org/gpp3]) and Inès Bouajila, Health Economics & Outcomes Research Fellow from AbbVie.
The initial systematic literature review was performed by Bridge Medical, updated by Creativ-Ceutical, and funded by AbbVie.
Authors’ contributions: All authors read, contributed to, and approved all the drafts and the final manuscript:
CF was involved in the review of the systematic literature review (SLR) protocol, the meta-analysis plan, and interpretation of results.
MN was involved in the review of the SLR protocol, meta-analysis plan development, statistical analysis and interpretation of results.
EO was involved in the development of the SLR protocol, the extraction grid, and the reporting of the SLR.
LDB was involved in the review of the SLR protocol, the meta-analysis plan, and interpretation of results.
ML-M was involved in the review of the meta-analysis plan and interpretation of results.
AD was involved in the review of the meta-analysis plan and interpretation of results.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ML-M reports personal fees from Allergan; personal fees from Amgen; grants, personal fees from Eli Lilly; personal fees from Grunenthal; grants and personal fees from Lundbeck; grants and personal fees from Medtronic; grants, personal fees from Novartis; personal fees from Pfizer; personal fees from Reckitt Benckiser; personal fees from Sun Pharmaceutical; grants, personal fees from Teva Pharmaceuticals; personal fees from UPSA; personal fees from Zambon. AD reports personal fees from AbbVie/Allergan, Eli Lilly, Lundbeck, Novartis, and Teva. FC, MN, and EO work for Creativ-Ceutical, a consultancy company commissioned by AbbVie who provided the funding to perform the SLR update and the meta-analysis. LDB is an employee of AbbVie and holds stock options from GSK and AbbVie.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and publication of this article: The literature review and editorial assistance for the manuscript was funded by AbbVie. AbbVie participated in the review of the SLR protocol, the meta-analysis plan, interpretation of results; and in the writing, reviewing and approval of the final version. No honoraria or payments were made for authorship.
ORCID iD
Anne Ducros https://orcid.org/0000-0001-5560-5013
References
- 1.National Institute of Neurological Disorders. Migraine information page. https://www.ninds.nih.gov/Disorders/All-Disorders/Migraine-Information-Page (2012, accessed 11 May 2021).
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 3.The Migraine Trust. Chronic migraine, https://www.migrainetrust.org/about-migraine/types-of-migraine/chronic-migraine/ (2021, accessed 11 May 2021).
- 4.Steiner TJ, Stovener LJ, Jensen R H, et al. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 2020; 21: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Headache Society. Part I. The primary headaches. https://ichd-3.org/1-migraine/1-3-chronic-migraine/. (2019, accessed 12 September 2020).
- 6.Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010; 30: 793–803. [DOI] [PubMed] [Google Scholar]
- 7.Burch R, Hettie B. Longitudinal preventive medication use patterns in patients receiving onabotulinumtoxina treatment: A chart review study. Headache 2019; 59: 41–42. [Google Scholar]
- 8.Serrano D, Manack AN, Reed ML, et al. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health 2013; 16: 31–38. [DOI] [PubMed] [Google Scholar]
- 9.Agostoni EC, Barbanti P, Alabresi P, et al. Current and emerging evidence-based treatment options in chronic migraine: a narrative review. J Headache Pain 2019; 20: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford JH, Stauffer VL, McAllister P, et al. Functional impairment and disability among patients with migraine: evaluation of galcanezumab in a long-term, open-label study. Qual Life Res 2021; 30: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonafede M, Sapra S, Shah N, et al. Direct and indirect healthcare resource utilization and costs among migraine patients in the United States. Headache 2018; 58: 700–714. [DOI] [PubMed] [Google Scholar]
- 12.Raval AD, Shah A. National trends in direct health care expenditures among US adults with migraine: 2004 to 2013. J Pain 2017; 18: 96–107. [DOI] [PubMed] [Google Scholar]
- 13.Frampton JE, Silberstein SD. OnabotulinumtoxinA: a review in the prevention of chronic migraine. Drugs 2018; 78: 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence. Headaches in over 12s: diagnosis and management. CG150. https://www.nice.org.uk/guidance/cg150/chapter/Recommendations#management-2. (2021, accessed 16 May 2021). [PubMed]
- 15.Antonaci F, Dumitrache C, De Cillis I, et al. A review of current European treatment guidelines for migraine. J Headache Pain 2010; 11: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silberstein SD, Neto W, Schmitt J, et al. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol 2004; 61: 490–495. [DOI] [PubMed] [Google Scholar]
- 17.Brandes JL, Spaer JR, Diamond M, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004; 291: 965–973. [DOI] [PubMed] [Google Scholar]
- 18.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: A retrospective claims analysis. Cephalalgia 2017; 37: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AJMC. Emerging therapies and preventive treatments for migraine, https://ajmc.s3.amazonaws.com/_media/_pdf/AJMC_A787_Migrane_Whitepaper_posting.pdf (2017, accessed 5 February 2019).
- 20.Ashina S. Identifying barriers to care-seeking, diagnosis, and preventive medication among those with migraine: Results of the OVERCOME study. Presented at the 62nd Annual Scientific Meeting of the American Headache Society. 2020; San Diego, California, USA.
- 21.Ford JH, Schroeder K, Nyhuis AW, et al. Cycling through migraine preventive treatments: Implications for all-cause total direct costs and disease-specific costs. J Manag Care Spec Pharm 2019; 25: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yelland E, Ghosh R, Murrary-Thomas T, et al. Treatment patterns and adherence in a UK primary care migraine population. Pharmacoepidemiol Drug Saf 2019; 28: 47. [Google Scholar]
- 23.Blumenfeld AM, Bloudeck LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache 2013; 53: 644–655. [DOI] [PubMed] [Google Scholar]
- 24.Ford JH, Jackson J, Milligan G, et al. A real-world analysis of migraine: a cross-sectional study of disease burden and treatment patterns. Headache 2017; 57: 1532–1544. [DOI] [PubMed] [Google Scholar]
- 25.Gibbs SN, Shah S, Deshpande CG, et al. United States patients' perspective of living with migraine: Country-specific results from the global “My Migraine Voice” survey. Headache 2020; 60: 1351–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozo-Rosich P, Lucas C, Watson D PB, et al . Characteristics of migraine patients visiting the European headache specialist centers: real-world evidence from the multinational BECOME study. J Headache Pain 2019; 20: 109.31822271 [Google Scholar]
- 27.US Food and Drug Administration. QULIPTA (atogepant), https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215206Orig1s000lbl.pdf. (2021, accessed 1 December 2021).
- 28.European Medicines Agency. Atogepant, https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002530-pip01-18. (2020, 1 December 2021 December).
- 29.US Food and Drug Administration. NURTEC (rimegepant), https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212728s006lbl.pdf. (2020, accessed 1 December 2021).
- 30.European Medicines Agency. Rimegepant, https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002812-pip02-20. (2021, accessed 1 December 2021).
- 31.US Food and Drug Administration. VYEPTI (eptinezumab-jjmr) injection, for intravenous use. Initial US Approval. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf (accessed 7 December 2021).
- 32.European Medicines Agency. Eptinezumab, https://www.ema.europa.eu/en/medicines/human/paediatric-investigation-plans/emea-002243-pip01-17-m01. (2021, accessed 1 December 2021).
- 33.US Food and Drug Administration. AIMOVIG (erenumab-aooe) injection, for subcutaneous use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf. (2018, accessed 16 May 2021).
- 34.European Medicines Agency. Erenumab, https://www.ema.europa.eu/en/medicines/human/EPAR/aimovig. (2021, accessed 1 December 2021).
- 35.European Medicines Agency. Ajovy (fremanezumab), http://www.ema.europa.eu/en/medicines/human/EPAR/ajovy. (2019, accessed 1 December 2021).
- 36.US Food and Drug Administration. AJOVY (fremanezumab), https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf. (2018, accessed 1 December 2021).
- 37.European Medicines Agency. Galcanezumab, https://www.ema.europa.eu/en/medicines/human/EPAR/emgality. (2021, accessed 1 December 2021).
- 38.US Food and Drug Administration. EMGALTY (galcanezumab), https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf. (2019, accessed 1 December 2021).
- 39.US Food and Drug Administration. BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103000s5302lbl.pdf. (2017, accessed 11 May 2021).
- 40.Electronic Medicines Compendium. Botox 200 Units Powder for solution for injection, https://www.medicines.org.uk/emc/product/436/smpc (2020, accessed 16 May 2021).
- 41.MHRA. M.H.p.R.A. BOTOX 50 Allergan Units Powder for solution for injection (Botulinum Toxin Type A), https://mhraproducts4853.blob.core.windows.net/docs/61c8e659aa05dd44228dd89360c8d8b77288ed18. (2021, accessed 1 December 2021).
- 42.Rothrock JF, Manack Adams A, Lipton RB, et al. FORWARD Study: Evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache 2019; 59: 1700–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blumenfeld AM, Stark RJ, Freeman MC, et al. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain 2018; 19: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hariton E, Locascio JJ. Randomised controlled trials – the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG 2018; 125: 1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diener H-C, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010; 30: 804–814. [DOI] [PubMed] [Google Scholar]
- 46.The Innovative Medicines Initiative (IMI). RWE Navigator, 2020. https://rwe-navigator.eu/policies-and-perspectives/. (accessed 6 June 2021).
- 47.London School of Economics and Political Science. Policy challenges around real world evidence adoption in Europe, https://www.lse.ac.uk/business-and-consultancy/consulting/assets/documents/rwe-in-europe-paper-v.pdf. (2018, accessed 6 June 2021).
- 48.Berger ML, Sox H, Willke RJ, et al. Good practices for real-world data studies of treatment and/or comparative effectiveness: Recommendations from the joint ISPOR-ISPE Special Task Force on real-world evidence in health care decision making. Pharmacoepidemiol Drug Saf 2017; 26: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viana M, Sances G, Ghiotto N, et al. Variability of the characteristics of a migraine attack within patients. Cephalalgia 2016; 36: 825–830. [DOI] [PubMed] [Google Scholar]
- 50.Becker WJ. The diagnosis and management of chronic migraine in primary care. Headache 2017; 57: 1471–1481. [DOI] [PubMed] [Google Scholar]
- 51.The American Headache Society. The American Headache Society Position Statement on Integrating New Migraine Treatments Into Clinical Practice. Headache 2019; 59: 1–18. [DOI] [PubMed] [Google Scholar]
- 52.Briere J-B, Bowrin K, Taieb V, et al. Meta-analyses using real-world data to generate clinical and epidemiological evidence: a systematic literature review of existing recommendations. Curr Med Res Opinion 2018; 34: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 53.Field AP. The problems in using fixed-effects models of meta-analysis on real-world data. Understanding Statistics 2003; 2: 105–124. [Google Scholar]
- 54.Tufanaru C, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evidence Imp 2015; 13: 196–207. [DOI] [PubMed] [Google Scholar]
- 55.Metelli S, Chaimani A. Challenges in meta-analyses with observational studies. Evidence Based Mental Health 2020; 23: 83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green JHS. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: JW Sons, 2008. [Google Scholar]
- 57.Stroup DF, Berline JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 58.Moher D Liberarti A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The Migraine Trust. Research. https://migrainetrust.org/what-we-do/our-commitment-to-research/ (2021, accessed 30 November 2021).
- 61.American Headache Society. https://americanheadachesociety.org/. (accessed 30 November 2021).
- 62.European Headache Federation. https://www.ehf-headache.com/federation/publications/office-journal. (2021, accessed 30 November 2021).
- 63.American Academy of Neurology. https://www.aan.com/. (2021, accessed 30 November 2021).
- 64.International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. (2021, accessed 30 November 2021).
- 65.EU Clinical Trials Register. Clinical trials. https://www.clinicaltrialsregister.eu/. (2021, accessed 30 November 2021).
- 66.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (2015, accessed 6 July 2022).
- 67.Dodick DW, Turket CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010; 50: 921–936. [DOI] [PubMed] [Google Scholar]
- 68.Matharu M, Halker R, Pozo-Rosich P, et al. The impact of onabotulinumtoxinA on severe headache days: PREEMPT 56-week pooled analysis. J Headache Pain 2017; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003; 12: 963–974. [DOI] [PubMed] [Google Scholar]
- 70.Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1). Headache 2000; 40: 204–215. [DOI] [PubMed] [Google Scholar]
- 71.Stewart WF, Lipton RB, Downson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001; 56: S20–8. [DOI] [PubMed] [Google Scholar]
- 72.Diener H-C, Ashina M, Durand-Zaleski I, et al. Health technology assessment for the acute and preventive treatment of migraine: A position statement of the International Headache Society. Cephalalgia 2021; 41: 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 74.Murad MH, Montori VM, Ioannidis J PA, et al. Fixed-effects and random-effects models. In: Guyatt G, Rennie D, Meade MO, et al. (eds) Users’ Guide to the Medical Literature. A Manual for Evidence-Based Clinical Practice. 3rd ed. New York: McGraw-Hill, 2015. [Google Scholar]
- 75.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aydinlar EI, Dikmen PY, Kosak S, et al. OnabotulinumtoxinA effectiveness on chronic migraine, negative emotional states and sleep quality: a single-center prospective cohort study. J Headache Pain 2017; 18: 723–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vernieri F, Paolucci M, Altamura C, et al. Onabotulinumtoxin-A in chronic migraine: should timing and definition of non-responder status be revised? Suggestions from a real-life Italian multicenter experience. Headache 2019; 59: 1300–1309. [DOI] [PubMed] [Google Scholar]
- 78.Taddei-Allen P, et al. Comparing patient-reported outcomes in patients using CGRP antagonists or onabotulinumtoxinA for chronic migraine. J Managed Care Spec Pharm 2019; 25: S60. [Google Scholar]
- 79.Gandolfi M, Donisi V, Marchioretto F, et al. A prospective observational cohort study on pharmacological habitus, headache-related disability and psychological profile in patients with chronic migraine undergoing onabotulinumtoxinA prophylactic treatment. Toxins 2019; 11: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gonzalez‐Martinez A. Association between personality traits and onabotulinumtoxin A response in patients with chronic migraine. Headache 2020; 60: 153–161. [DOI] [PubMed] [Google Scholar]
- 81.d'Onofrio F, de Falco A, Costanzo A, et al. Impulse control disorders in chronic migraine with medication overuse after onabotulinumtoxinA: A single-center prospective cohort study. J Clin Neurosci 2020; 80: 152–155. [DOI] [PubMed] [Google Scholar]
- 82.Dikmen PY, Kosak S, Aydinlar EI, et al. A single-center retrospective study of onabotulinumtoxinA for treatment of 245 chronic migraine patients: survey results of a real-world experience. Acta Neurologica Belgica 2018; 118: 475–484. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed F, Zafar HW, Buture A, et al. Does analgesic overuse matter? Response to OnabotulinumtoxinA in patients with chronic migraine with or without medication overuse. Springerplus 2015; 4: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naprienko MV. The burden of migraine in real clinical practice: Clinical and economic aspects. Neurosci Behav Physiol 2020; 50: 20–26. [Google Scholar]
- 85.Sarchielli P, Romoli M, Corbelli et al. Stopping onabotulinum treatment after the first two cycles might not be justified: Results of a real-life monocentric prospective study in chronic migraine. Front Neurol 2017; 8: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmed F, Gaul C, Carcia-Monco JC, et al. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain 2019; 20: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boudreau G. Impact of onabotulinumtoxina on quality of life, health resource utilization, and work productivity in people with chronic migraine: Interim results from a prospective, observational study (PREDICT). Headache 2018; 58: 94. [Google Scholar]
- 88.Domínguez C, Pozo-Rosich P, Torres-Ferrus M, et al. OnabotulinumtoxinA in chronic migraine: predictors of response. A prospective multicentre descriptive study. Eur J Neurol 2018; 25: 411–416. [DOI] [PubMed] [Google Scholar]
- 89.Butera C, Colombo B, Bianchi F, et al. Refractory chronic migraine: is drug withdrawal necessary before starting a therapy with onabotulinum toxin type A? Neurol Sci 2016; 37: 1701–1706. [DOI] [PubMed] [Google Scholar]
- 90.Negro A, Curto M, Lionetto L, et al. A two years open-label prospective study of OnabotulinumtoxinA 195 U in medication overuse headache: A real-world experience. J Headache Pain 2016; 17: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Russo M, Manzoni GC, Taga A, et al. The use of onabotulinum toxin A (Botox®) in the treatment of chronic migraine at the Parma Headache Centre: a prospective observational study. Neurol Sci 2016; 37: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 92.Sanz AC. Experience with botulinum toxin in chronic migraine. Neurologia 2018; 33: 499–504. [DOI] [PubMed] [Google Scholar]
- 93.Stark C. Real-world effectiveness of onabotulinumtoxinA treatment for the prevention of headaches in adults with chronic migraine in Australia: A retrospective study. J Headache Pain 2019; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres-Ferrus M, Gallardo VJ, Alpuente A, et al. Influence of headache pain intensity and frequency on migraine-related disability in chronic migraine patients treated with OnabotulinumtoxinA. J Headache Pain 2020; 21: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Cola FS. et al., Response predictors in chronic migraine: Medication overuse and depressive symptoms negatively impact onabotulinumtoxin-A treatment. Front Neurol 2019; 10. [DOI] [PMC free article] [PubMed]
- 96.Ornello R, Guerzoni S, Baraldi C, et al. Sustained response to onabotulinumtoxin A in patients with chronic migraine: real-life data. J Headache Pain 2020; 21: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ranoux D, Martiné G, Espagne-Dubreuilh G, Amilhaud-Bordier M, Caire F, Magy L, OnabotulinumtoxinA injections in chronic migraine, targeted to sites of pericranial myofascial pain: an observational, open label, real-life cohort study. J Headache Pain 2017; 18: 75. [DOI] [PMC free article] [PubMed]
- 98.Santoro A, Copetti M, Miscio AM, et al. Chronic migraine long-term regular treatment with onabotulinumtoxinA: a retrospective real-life observational study up to 4 years of therapy. Neurol Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caronna E. OnabotulinumtoxinA: an effective tool in the therapeutic arsenal for chronic migraine with medication overuse. Front Neurol 2018; 9: 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eren OE, Gaul C, Peikert A, et al. Triptan efficacy does not predict onabotulinumtoxinA efficacy but improves with onabotulinumtoxinA response in chronic migraine patients. Sci Rep 2020; 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee MJ, Lee C, Choi H, et al. Factors associated with favorable outcome in botulinum toxin A treatment for chronic migraine: A clinic-based prospective study. J Neurol Sci 2016; 363: 51–54. [DOI] [PubMed] [Google Scholar]
- 102.Lin K-H, Chen S-P, Fuh J-L, et al. Efficacy, safety, and predictors of response to botulinum toxin type A in refractory chronic migraine: A retrospective study. J Chin Med Assoc 2014; 77: 10–15. [DOI] [PubMed] [Google Scholar]
- 103.Alessiani M. Short-term psychodynamic psychotherapy versus onabotulinumtoxinA as preventive therapy in chronic migraine: A real world study. J Headache Pain 2018; 19: 42.29845369 [Google Scholar]
- 104.Corbelli I. Sustained efficacy and safety of onabotulinumtoxin Type A in chronic migraine patients: A multicentric prospective real-life study. Neurol Sci 2019; 40: S248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carrasco J, Pietshc G-A, Nicolas M-P, et al. Real-world effectiveness and real-world cost-effectiveness of intravitreal aflibercept and intravitreal ranibizumab in neovascular age-related macular degeneration: systematic review and meta-analysis of real-world studies. Adv Ther 2020; 37: 300–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Casula M. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2017; 27: 396–406. [DOI] [PubMed]
- 107.Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Meta-Anal 2017; 5: 80–84. [Google Scholar]
- 108.Argueta-Figueroa L. ROBINS-I or Newcastle Ottawa Scale (NOS) or AXIS: which has better validity and should there be a preference for either based on our field of work?, https://www.researchgate.net/post/ROBINS-I_or_Newcastle_Ottawa_Scale_NOS_or_AXIS_which_has_better_validity_and_should_there_be_a_preference_for_either_based_on_our_field_of_work/5ef23a4e177d9528634b6ce4/citation/download. (2020, accessed 5 July 2022).
- 109.Alpuente A, Gallardo VJ, Torres-Ferrus M, et al. Early efficacy and late gain in chronic and high-frequency episodic migraine with onabotulinumtoxinA. Eur J Neurol 2019; 26: 1464–1470. [DOI] [PubMed] [Google Scholar]
- 110.Andreou AP, Trimboli M, Al-Kaisy A, et al. Prospective real-world analysis of OnabotulinumtoxinA in chronic migraine post-National Institute for Health and Care Excellence UK technology appraisal. Eur J Neurol 2018; 25: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 111.Belvís R. Effectiveness of OnabotulinumtoxinA in chronic migraine. If early introduction: faster, cheaper and more satisfactory. J Headache Pain 2018; 18: P98–P98. [Google Scholar]
- 112.Boudreau G, Finkelstein I, Graboski C, et al. OnabotulinumtoxinA treatment improved health-related quality of life in adults with chronic migraine: results from a prospective, observational study (PREDICT) (714). Neurology 2020; 94: 714. [Google Scholar]
- 113.Boudreau G, Finkelstein I, Graboski C, et al . Onabotulinumtoxin a treatment improved health-related quality of life in adults with chronic migraine in the PREDICT Study: Results from study completers. Eur J Neurol 2020; 27: 154. [Google Scholar]
- 114.de Tommaso M, Brighina F, Delussi M. Effects of botulinum toxin A on allodynia in chronic migraine: an observational open-label two-year study. Eur Neurol 2019; 81: 37–46. [DOI] [PubMed] [Google Scholar]
- 115.Garcia-Azorin D. Habituation determined by algometry as a marker of response in chronic migraine. J Headache Pain 2018; 19. [Google Scholar]
- 116.Grazzi L, Usai S. Onabotulinum toxin A (Botox) for chronic migraine treatment: An Italian experience. Neurol Sci 2015; 36: 33–35. [DOI] [PubMed] [Google Scholar]
- 117.Grazzi L. Botulinum a toxin for treatment of chronic migraine with medication overuse. Cephalalgia 2016; 36: 21–22. [Google Scholar]
- 118.Guerzoni S, Pellesi L, Baraldi C, et al. Long-term treatment benefits and prolonged efficacy of OnabotulinumtoxinA in patients affected by chronic migraine and medication overuse headache over 3 years of therapy. Front Neurol 2017; 8: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kennedy G. Longer term outcomes for patients with chronic migraine treated with OnabotulinumtoxinA BOTOX and implications for a Headache Service: Real-life data for 120 patients treated at Sunderland Royal Hospital, UK. Cephalalgia 2017; 37: 333. [Google Scholar]
- 120.Navarrete Perez JJ. Wearing-off effect of onabotulinumtoxinA in chronic migraine: Evaluation in a series of 117 patients. Eur J Neurol 2017; 24: 547. [Google Scholar]
- 121.Pedraza MI. OnabotulinumtoxinA treatment for chronic migraine: experience in 52 patients treated with the PREEMPT paradigm. SpringerPlus 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quintas S, Garcia-Azorin D, Heredia P, et al. Wearing off response to onabotulinumtoxinA in chronic migraine: Analysis in a series of 193 patients. Pain Medicine 2019; 20: 1815–1821. [DOI] [PubMed] [Google Scholar]
- 123.Romoli M, Corbelli I, Bernetti L, et al. Stopping Onabotulinum treatment after the first 2 cycles might not be justified: Results of a real-life monocentric prospective study in chronic migraine. J Headache Pain 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Velasco-Juanes F. Clinical treatment of chronic and episodic migraine with onabotulinumtoxinA in a real-world setting. Drugs Therapy Persp 2018; 34: 335–343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cep-10.1177_03331024221123058 for Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: A meta-analysis on 10 years of real-world data by Michel Lanteri-Minet, Anne Ducros, Clement Francois, Elzbieta Olewinska, Mateusz Nikodem and Laure Dupont-Benjamin in Cephalalgia