Abstract
Context
Diabetes is an age-related disease; however, the mechanism underlying senescent beta cell failure is still unknown.
Objective
The present study was designed to investigate whether and how the differentiated state was altered in senescent human beta cells by excluding the effects of impaired glucose tolerance.
Methods
We calculated the percentage of hormone-negative/chromogranin A–positive endocrine cells and evaluated the expressions of forkhead box O1 (FoxO1) and Urocortin 3 (UCN3) in islets from 31 nondiabetic individuals, divided into young (<40 years), middle-aged (40-60 years) and elderly (>60 years) groups. We also assessed adaptive unfolded protein response markers glucose-regulated protein 94 (GRP94), and spliced X-box binding protein 1 (XBP1s) in senescent beta cells and their possible contributions to maintaining beta cell identity and differentiation state.
Results
We found an almost 2-fold increase in the proportion of dedifferentiated cells in elderly and middle-aged groups compared with the young group (3.1 ± 1.0% and 3.0 ± 0.9% vs 1.7 ± 0.5%, P < .001). This was accompanied by inactivation of FoxO1 and loss of UCN3 expression in senescent human beta cells. In addition, we demonstrated that the expression levels of adaptive unfolded protein response (UPR) components GRP94 and XBP1s declined with age. In vitro data showed knockdown GRP94 in Min6-triggered cells to dedifferentiate and acquire progenitor features, while restored GRP94 levels in H2O2-induced senescent Min6 cells rescued beta cell identity.
Conclusion
Our finding highlights that the failure to establish proper adaptive UPR in senescent human beta cells shifts their differentiated states, possibly representing a crucial step in the pathogenesis of age-related beta cell failure.
Keywords: ageing, beta cell dedifferentiation, type 2 diabetes, unfolded protein response
Type 2 diabetes is an age-related disease that is accompanied by progressive beta cell failure to secrete adequate insulin to meet the increased insulin demand under metabolic stress (1–6). Previous studies have suggested that insulin resistance increases with age; however, insulin resistance alone does not account for age-related glucose intolerance (7, 8). Several human studies have consistently found an age-dependent decline of beta cell function, independent of insulin resistance (9–11). Age-related changes in beta cell proliferation capacity (12) and components of stimulus–secretion coupling (13, 14) have been studied as contributors to senescent beta cell dysfunction. Recently, Aguayo-Mazzucato et al sorted senescent (beta-gal+) beta cells from 7-8–month-old mice and found decreased beta cell identity (Insulin 1, MafA, Nkx6.1, and Pdx1) and increased disallowed genes (Ldha and Catalase) compared with nonsenescent (beta-gal−) beta cells (15). More importantly, a single-cell transcriptome analysis performed on human pancreas from 8 donors spanning 6 decades of life revealed that islet endocrine cells from older donors displayed increased levels of transcriptional noise and expressed more cell-atypic hormone mRNA (16). Such “fate drift” is emblematic of age-dependent transcriptional instability and endocrine cell reprogramming, which might contribute to beta cell failure during aging.
Beta cell dedifferentiation is considered as a novel mechanism of beta cell failure, in which beta cells lose their identity and differentiated state, revert to a progenitor-like stage, and even reprogram to another endocrine cell fate (17). Beta cell dedifferentiation was first reported in beta cell–specific forkhead box O1 (FoxO1)–deficient mice upon ageing and multiparous challenges (18), and was also observed in islets from other mouse models of obesity and type 2 diabetes (19–22). Direct evidence of human beta cell dedifferentiation in diabetes was provided by independent research groups, demonstrating a significant increase in hormone-negative/chromogranin A–positive endocrine cells in islets from individuals with type 2 diabetes (23, 24). In addition, our previous studies reported an increased rate of beta cell dedifferentiation not only in well-controlled type 2 diabetes (24) but also in diseases with increased susceptibility to diabetes, such as chronic pancreatitis (24) and pancreatic cancer (25). Interestingly, a recent study revealed that the gene profiles in senescent rodent beta cells were similar to those of the dedifferentiated beta cells. Many of these age-related changes were present both in glucose-intolerant C57BL/6J and in normoglycemic mice model (INKATTAC) (26). It is currently unknown whether and how ageing, a risk factor of type 2 diabetes, influences the differentiated state of human beta cells before blood glucose rises.
In the present study, we investigated the role of aging on beta cell dedifferentiation in nondiabetic subjects and explored the possible involvement of adaptive unfolded protein response (UPR) in age-related beta cell failure. Our data aim to provide clues for high susceptibility to type 2 diabetes in elderly subjects and candidates targeting age-related beta cell failure.
Materials and Methods
Patients and Human Pancreas
A total of 2065 subjects with partial pancreatectomy for various reasons performed in the Department of Surgery in Ruijin Hospital between January 2013 and August 2017 were recruited. By reviewing pathology diagnosis and CA19-9 levels, subjects who had been reported as having a malignant tumor were excluded. Cases were enrolled in the current study according to the following criteria: (1) pathological diagnosis of pancreatic serous cystadenoma or pancreatic mucinous cystadenoma; (2) nondiabetes defined as fasting blood glucose (FBG) ≤ 5.5 mmol/L according to American Diabetes Association guidelines (27); (3) nonobesity, defined as a body mass index (BMI) less than 30 kg/m2 according to American Association of Clinical Endocrinologist Medical Guidelines (28); (4) no history of diabetes and chronic pancreatitis; (5) adults with age >18 years old; and (6) with a complete dataset, including clinical characteristics, medical history, and laboratory tests.
A total of 31 nondiabetic patients were included in and divided into 3 different age groups, in other words, young group (<40 years), middle-aged group (40-60 years), and elderly group (>60 years) (Table 1 (29)). Paraffin sections of pancreatic tissues far from the margin of the pancreatectomy were obtained from the Department of Pathology in Ruijin Hospital for subsequent analysis. All of these tissues were reverified to ensure the final pathology diagnosis by a pathologist.
This study was approved by the Institutional Review Board of the Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine and was in accordance with the principles of the Declaration of Helsinki.
Immunostaining
Human pancreas samples were fixed and processed for immunohistochemistry according to the standard protocol, as previously described (30). Briefly, after being deparaffinized and rehydrated, antigen retrieval was performed by heating slides in antigen-unmasking solution (H-3300, Vector). All slides were incubated with primary antibodies and diluted in Dako Antibody Diluent (Dako; Burlington Canada) at 4°C overnight. The dilutions were as follows: guinea pig anti-insulin (1:10, Agilent Cat# IR002, RRID:AB_2800361), rabbit antiglucagon (1:200, Abcam Cat# ab10988, RRID:AB_297642), rabbit antisomatostatin (1:400, Millipore Cat# AB5494, RRID:AB_2255374), rabbit antipancreatic polypeptide (1:400, Millipore Cat# AB939, RRID:AB_92383), mouse antichromogranin A (1:100, Millipore Cat# MAB5268, RRID:AB_11213294), rabbit anti-UCN3 (1:200, Sigma-Aldrich Cat# HPA038281, RRID:AB_10672408), rabbit anti-FoxO1 (1:200, Cell Signaling Technology Cat# 2880, RRID:AB_2106495), rat anti-GRP94 (1:250, Thermo Fisher Scientific Cat# MA3-016, RRID:AB_2248666), rabbit anti-XBP1s (1:200, ABclonal Cat# A17007, RRID:AB_2772919), rabbit anti-ATF4 (1:100, Proteintech Cat# 10835-1-AP, RRID:AB_2058600), rabbit anti-ATF6 (1:200, Proteintech Cat# 24169-1-AP, RRID:AB_2876891), and rabbit anti-BIP (1:100, Proteintech Cat# 11587-1-AP, RRID:AB_2119855). Primary antibodies were detected using Alexa Fluor 488, 594, and 647 (1:500, Jackson Immuno Research Laboratories) as secondary antibodies. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI, Vector Laboratories; Burlingame, CA).
Senescence-associated beta-galactosidase (SA-beta-gal) staining of Min6 cells was performed using an SA-beta-gal staining kit (Beyotime Biotechnology, China, C0602) according to the manufacturer’s protocol.
Image Acquisition and Analysis
We captured immunofluorescence images using a Zeiss LSM 880 confocal microscope. We performed the quantifications blindly using ImageJ software (National Institutes of Health, Bethesda, MD, USA) to analyze individual cells in whole-slide images; at least 2 random sections and 818.1 ± 174.7 cells per donor were scored. We counted the Chromogranin A (CGA)-positive cells representing endocrine-derived cells first. CGA-positive and hormone-negative cells were identified as dedifferentiated cells, and insulin-positive and other pancreatic hormone–positive cells as multihormone cells. Fluorescent intensity was quantified blindly using ImageJ software (National Institutes of Health, Bethesda, MD, USA) with the EzColocalization plugin according to the protocol described by Stauffer et al (31).
Cell Culture
Min6 cells (Mouse insulinoma cells) were purchased from CAMS Cell Culture Center (Beijing, China), grown in Dulbecco’s modified Eagle’s medium (Gibco) containing 25.0 mmol/L glucose, 15% fetal bovine serum, 100 IU/mL penicillin, 100 µg/mL streptomycin, 10.2 mM L-glutamine, and 2.5 mM beta-mercaptoethanol at 37°C in a humidified 5% CO2 atmosphere. As for induction of senescence, Min6 cells were passaged and plated in 12-well cell culture plates for 24 hours. Cells were treated with 200 µM H2O2 for 48 hours and replaced by normal media for another 24 hours to generate senescence. Short hairpin RNA (shRNA) lentivirus targeting GRP94, XBP1, and control were constructed, packaged, purified, and titrated at GeneChem Co. Ltd. Min6 cells were infected with purified lentivirus at 50 multiplicity of infection for 48 hours. Overexpression constructs for mouse GRP94-Flag and Flag control plasmid transfection were conducted using Lipofectamine 3000 (Invitrogen) according to the manufacturer's manual.
Extraction of RNA and Quantitative Real-Time Polymerase Chain Reaction Analysis
Total cell RNA was extracted by Eastep® Super Total RNA Extraction Kit (Promega, LS1040) according to the manufacturer's protocols, and synthesis of cDNA was used with PrimerScript Master Mix (Takara). Duplicate samples for quantitative polymerase chain reaction were carried out using TB Green Premix Ex Taq (Takara) and performed with ICycler (ABI, USA). Primer sequences can be obtained elsewhere (Table 2 (29)).
Immunoblot Analysis
Min6 cells were lysed, quantified, and blotted as described before (30). Primary antibodies were listed as follows: rat anti-GRP94 (1:4000, Cell Signaling Technology Cat# 20292, RRID:AB_2722657); rabbit anti-XBP1s (1:1000, ABclonal Cat# A17007, RRID:AB_2772919). Horse radish peroxidase–conjugated alpha Tubulin (1:5000, Proteintech Cat# HRP-66031, RRID:AB_2687491) was used as an internal control to normalize band intensity.
Statistical Analysis
Data are presented as the mean ± SD or mean (minimum–maximum) unless otherwise noted. Statistical comparisons among the groups were performed using analysis of Fisher's exact tests for categorical variables, 1-way analysis of variance with Bonferroni's post hoc test or unpaired 2-tailed Student's t-test for continuous variables. Correlation coefficients were calculated by simple and multiple regression analyses. P < .05 was considered statistically significant. All statistical analyses were conducted using SPSS version 24.0 statistical software (IBM, Armonk, New York).
Results
Increase in Dedifferentiated Cells in Nondiabetic Senescent Human Islets
To explore whether beta cells underwent dedifferentiation during their progression to ageing, we collected pancreas samples from 31 nondiabetic individuals with age range 25-94 years, and divided them into young (<40 years, range 25-40 years), middle-aged (40-60 years, range 48-59 years), and elderly (>60 years, range 60–94 years) groups. The fasting blood glucose levels were comparable among the 3 groups (4.9 ± 0.5 vs 5.1 ± 0.3 vs 5.0 ± 0.6 mM, elderly vs middle-aged vs young, P > .05; Table 1) and were all within normal range according to American Diabetes Association guidelines (27). Subjects with history of diabetes, chronic pancreatitis, or pancreatic ductal adenocarcinoma, which had been reported to induce beta cell dedifferentiation (23–25), were excluded from the present study. All the subjects remained in the nonobesity range (28), and elderly subjects showed slightly increased BMI compared with young and middle-aged subjects (Table 1).
Table 1.
Characteristic of the study subjects
| Subject profile | Young (n = 10) | Middle-aged (n = 10) | Elderly (n = 11) | P value |
|---|---|---|---|---|
| DM/CP/PDAC history | No | No | No | / |
| Age (years) | 33 (25-40) | 54 (48-59) | 72 (60–94) | <.001 |
| Female, n (%) | 8 (80.0%) | 8 (80.0%) | 4 (36.4%) | .057 |
| BMI (kg/m2) | 19.9 ± 2.8 | 22.5 ± 1.9 | 23.4 ± 2.9 | .011 |
| FBG (mmol/L) | 5.0 ± 0.6 | 5.1 ± 0.3 | 4.9 ± 0.5 | .689 |
| Smoking, no. (%) | 1 (10%) | 0 | 3 (27%) | .286 |
| % Dedifferentiation | 1.7 ± 0.5 | 3.0 ± 0.9 | 3.1 ± 1.0 | <.001 |
| % Multihormone | 2.5 ± 1.3 | 2.6 ± 1.1 | 2.9 ± 1.3 | .816 |
All continuous parameters are summarized by means ± SD or mean (minimum–maximum). P values were calculated from 1-way analysis of variance with Bonferroni's post hoc test for continuous variables, and Fisher's exact test for categorical variables.
Abbreviations: BMI, body mass index; CP, chronic pancreatitis; DM, diabetes mellitus; FBG, fasting blood glucose; PDAC, pancreatic ductal adenocarcinoma.
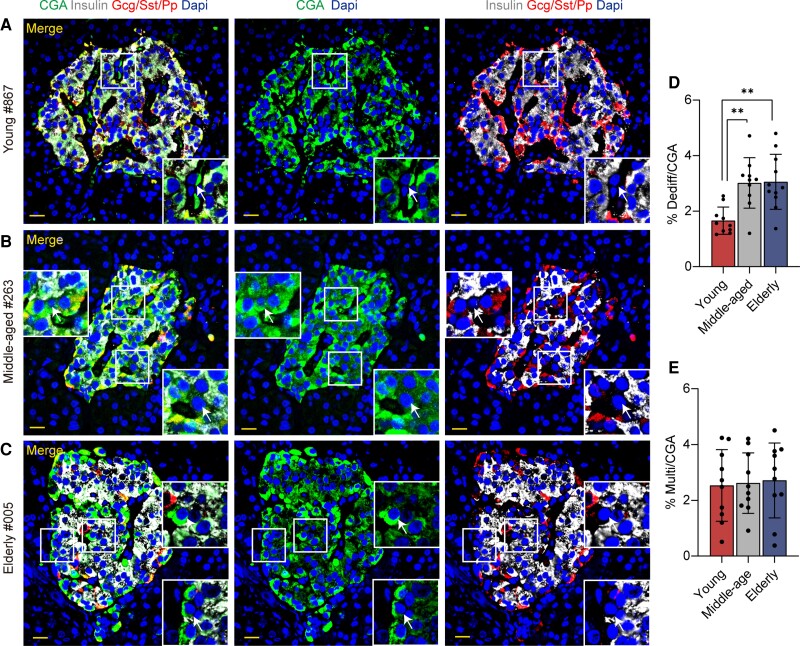
We first assessed the dedifferentiated cells and multihormone cells in pancreatic islets of all the subjects. As previously described (24, 25), the dedifferentiated cells were referred to as CGA-positive/hormone-negative cells (white arrows in Fig. 1A-1C), whereas multihormone cells were defined as coexpressing insulin and Gcg/Sst/Pp (yellow arrows in Fig. 1A (29)). Consistent with our previous reports (24, 25), regardless of age, the mean ratio of dedifferentiated cells was 2.6% (ranging from 1.2% to 4.8%) in human islets from 31 nondiabetic subjects. Interestingly, an almost 2-fold increase in the proportion of dedifferentiated cells was detected in middle-aged and elderly groups compared with the young group (3.1 ± 1.0% vs 3.0 ± 0.9% vs 1.7 ± 0.5%, elderly vs middle-aged vs young, P < .001; Fig. 1D and Table 1). We further calculated the ratio of dedifferentiated cells in different portions of the pancreas, namely, the head, body, and tail of the pancreas: there is a tendency for dedifferentiated cells to increase in the middle-aged and elderly groups compared with the young group, but this did not achieve statistical significance due to limited sample size (Fig. 1A and 1C (29)). We did not detect significant differences in the ratio of multihormone cells among the 3 groups (Fig. 1E). These data indicate that the ratio of dedifferentiated cells increased during aging.
Figure 1.
Representative immunofluorescence images of dedifferentiated cells from nondiabetic islets. (A-C) Immunofluorescence on pancreatic sections from young (A, case 867), middle-aged (Bb, case 263) and elderly (C, case 005) groups using insulin (white), cocktail of Gcg/Sst/Pp (red), CGA (green), and Dapi (blue) are shown. Boxes show higher magnification of the indicated region in the islets with white arrows demonstrating the dedifferentiated cells. (D-E) Quantitative analysis of dedifferentiated cells/CGA-positive cells and multihormone cells/CGA-positive cells. Scale bars, 20 μm. All data are expressed as mean ± SD. n = 10 in young group, n = 10 in middle-aged group, n = 11 in elderly group. **P < .01.
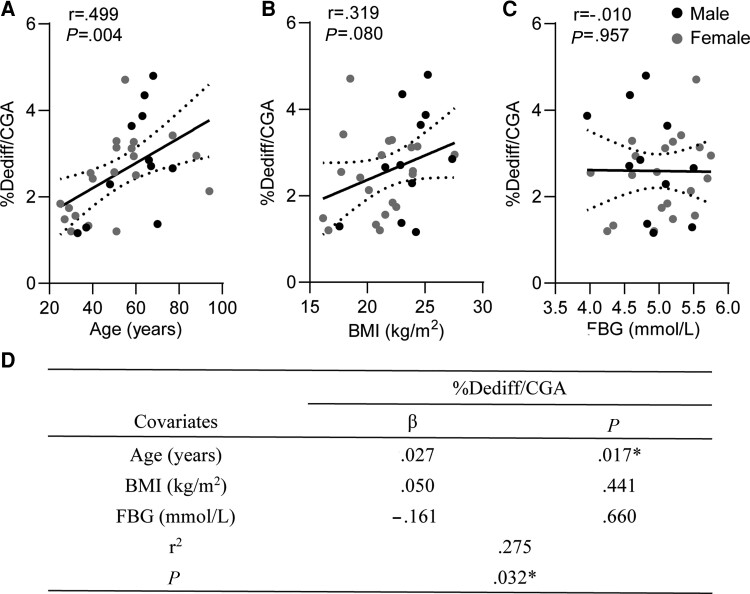
We then performed a univariate correlation analysis between the percentage of dedifferentiated cells in all subjects and their clinical parameters. We found the percentage of dedifferentiated cells exhibited a significantly positive correlation with age (r = 0.499, P = .004; Fig. 2A), but not with BMI (r = 0.319, P = .080; Fig. 2B) or FBG (r = −0.010, P = .957; Fig. 2C). Furthermore, according to multiple regression analyses, age (β = .027, P = .017; Fig. 2D), but not BMI and FBG, was independently and positively associated with the percentage of dedifferentiated cells.
Figure 2.
Correlation between the ratio of dedifferentiated cells and clinical parameters. (A-C) The Pearson correlations of the ratio of dedifferentiated cells with age (A), BMI (B), and FBG (C) in nondiabetic islets. (D) Multivariate association of the clinical variable with the ratio of dedifferentiated cells in nondiabetic islets. The r of Pearson correlation and P values are shown in each panel. *P < .05 was considered statistically significant.
FoxO1 Inactivation and UCN3 Reduction Were Observed in Nondiabetic Senescent Human Islets
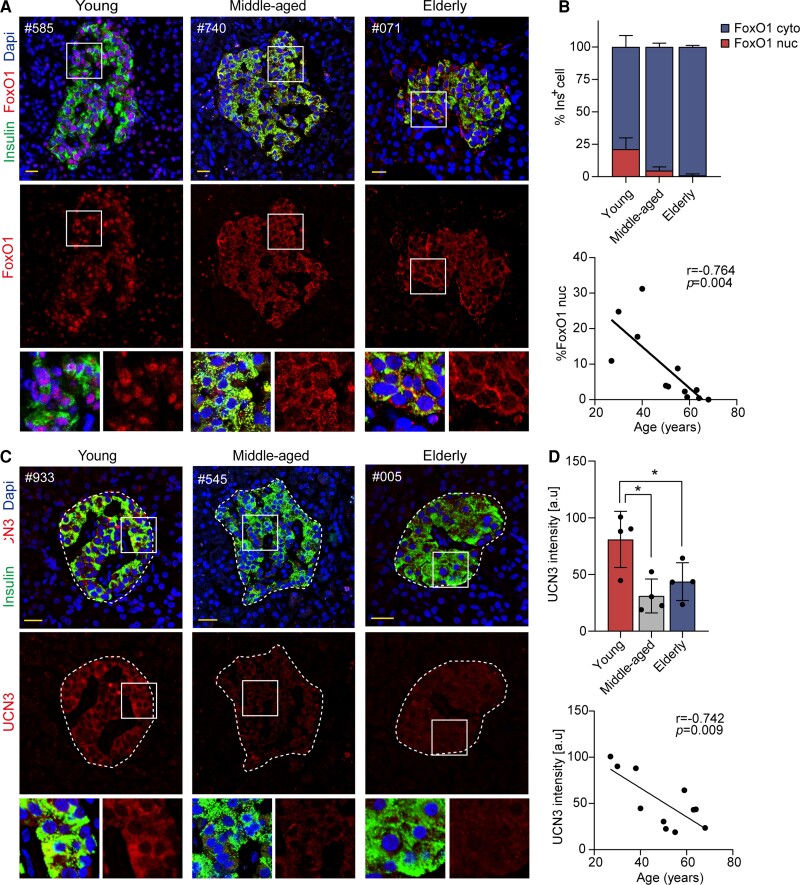
FoxO1 is an important transcription factor to enforce beta cell fate under physiologic stress including ageing (18, 32, 33). Translocation of FoxO1 from the nucleus to the cytoplasm decreases its activity (33). In the present study, we found at least 20% of insulin-positive cells had nuclear FoxO1 staining in young subjects, whereas FoxO1 expression was mostly detectable in the cytoplasm of beta cells from middle-aged and elderly subjects (Fig. 3A). We further calculated the number of beta cells with nuclear or cytoplasmic FoxO1 expression and found a robust reduction in fractions of beta cells with nuclear FoxO1 expression in middle-aged and elderly subjects compared with young ones (1.0 ± 1.2% vs 4.7 ± 2.8% vs 21.2 ± 8.8%, elderly vs middle-aged vs young, P < .001; Fig. 3B). Moreover, the percentage of beta cells with nuclear FoxO1 expression was negatively related with age (r = –0.764, P = .004; Fig. 3B).
Figure 3.
Expression of FoxO1 and UCN3 in nondiabetic elderly individuals. (A, C) Immunofluorescence images of pancreatic sections are shown with FoxO1 or UCN3 (red), insulin (green) and Dapi (blue) in young, middle-aged, and elderly groups. (B) Quantitative analysis of subcellular localization of FoxO1 in young, middle-aged, and elderly groups; correlation of quantified nuclear FoxO1 expression with age. (D) Quantification analysis of UCN3 intensity adjusted with islet area in young, middle-aged, and elderly groups; correlation of quantified UCN3 intensity with age. Scale bars, 20 μm. Data present means ± SD. The r of Pearson correlation and P values are shown in each panel. n = 4, *P < .05.
It is known that reduction of beta cell functional marker UCN3 (urocortin 3) is an early event during beta cell dedifferentiation in diabetes (34, 35). We performed immunofluorescent staining for UCN3 and insulin on the pancreas from the young, middle-aged, and elderly subjects, and found a remarkable decline in UCN3 expression in middle-aged and senescent human beta cells (43.7 ± 16.7 vs 31.1 ± 15.0% vs 80.9 ± 24.8, elderly vs middle-aged vs young, P < .05; Fig. 3C and 3D). Furthermore, quantified UCN3 intensity was negatively correlated with age (r = −0.742, P = .009; Fig. 3D).
Decreased Adaptive UPR in Nondiabetic Senescent Human Beta Cells
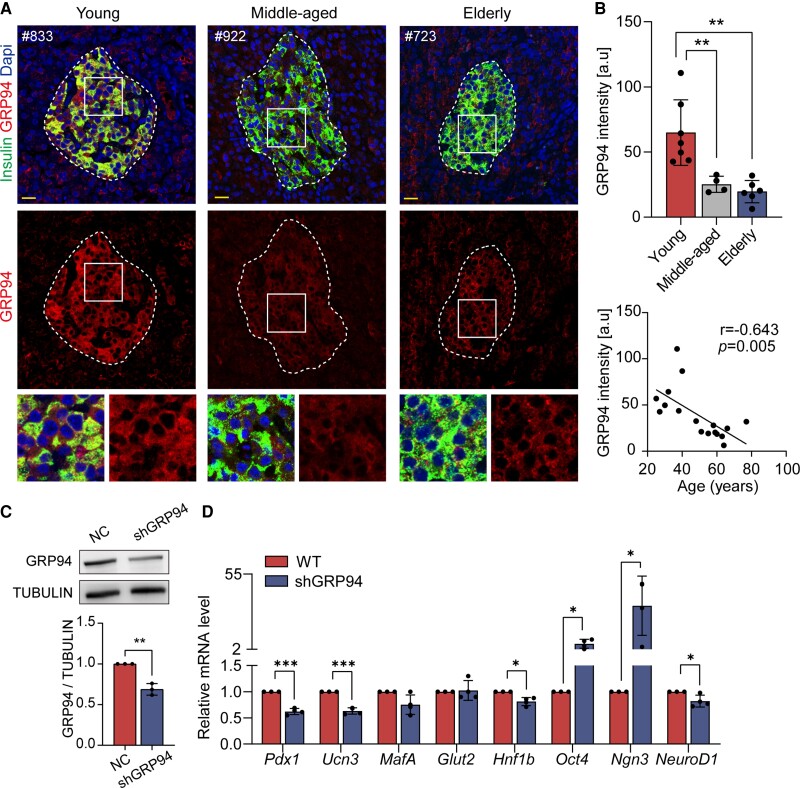
The accumulation of unfolded/misfolded proteins is a hallmark of ageing, and the potential of adaptive UPR to resolve endoplasmic reticulum (ER) stress has been implicated as a key mechanism of (de)compensatory beta cells (36, 37). In the present study, we found the expression level of GRP94 (glucose-regulated protein 94), a chaperone involved in adaptive UPR to execute protein quality control (38), was remarkably diminished in middle-aged and senescent human beta cells (19.9 ± 9.6 vs 25.2 ± 6.2 vs 57.3 ± 16.5, elderly vs middle-aged vs young, P < 0.01; Fig. 4A and 4B). Moreover, the quantified expression level of GRP94 was negatively associated with age (r = −0.643, P = .005; Fig. 4B). We then assessed another adaptive UPR marker XBP1s (spliced X-box binding protein 1), (39) and found XBP1s expression was also significantly decreased in senescent human beta cells (28.2 ± 15.8 vs 59.2 ± 32.0, elderly vs young, P < .05; Fig. 2A and 2E (29)). Quantified expression levels of XBP1s also exhibited a negative correlation with age (r = −0.654, P = .015; Fig. 2E (29)). However, we did not detect differences in expression levels of ATF4, ATF6, and BIP (Fig. 2B-D, F-H (29)). The above data indicate that failure to establish adaptive UPR might contribute to defects in senescent human beta cells.
Figure 4.
Attenuated GRP94 expression in nondiabetic elderly individuals influences beta cell identity. (A) Representative images of pancreatic section staining for GRP94 (red), insulin (green), and Dapi (blue) in young, middle-aged, and elderly groups. (B) Quantitative analysis of GRP94 intensity in young, middle-aged, and elderly groups; correlation of quantified GRP94 expression with age. (C) Immunoblot showed expression of GRP94 protein in ShGRP94 cells; band intensities normalized based on the corresponding TUBULIN intensity (n = 3). (D) Relative mRNA expression of transcription factors, genes involved in beta cell function, and progenitor cells in shGRP94 cells (n = 4). Scale bars, 20 μm. Data present means ± SD. The r of Pearson correlation and P values were shown in each panel. n = 7 in the young group, n = 4 in Middle-aged group, n = 6 in Elderly group. *P < .05, **P < .01, ***P < .001.
To examine the possible link between adaptive UPR and beta cell identity, we treated Min6 cells with GRP94 lentiviral shRNA for 48 hours. Successful knockdown of GRP94 (Fig. 4C) significantly decreased mRNA expression of pancreatic and duodenal homeobox1 (Pdx1), Ucn3, and Neuronal Differentiation 1 (NeuroD1) (Fig. 4D). Likewise, decreased expression of Pdx1 and V-maf musculoaponeurotic fibrosarcoma oncogene family protein A (MafA) were detected in shXBP1 Min6 cells (Fig. 3A and B (29)). Interestingly, genes highly expressed in progenitor cells, including octamer-binding transcription factor 4 (Oct4) and neurogenin 3 (Ngn3) were upregulated in shGRP94 and shXBP1 Min6 cells (Fig. 4D; Fig. 3B (29)). These results suggest that decrease in adaptive UPR (GRP94 or XBP1s) in senescent beta cells might trigger identity loss and acquisition of progenitor-like properties.
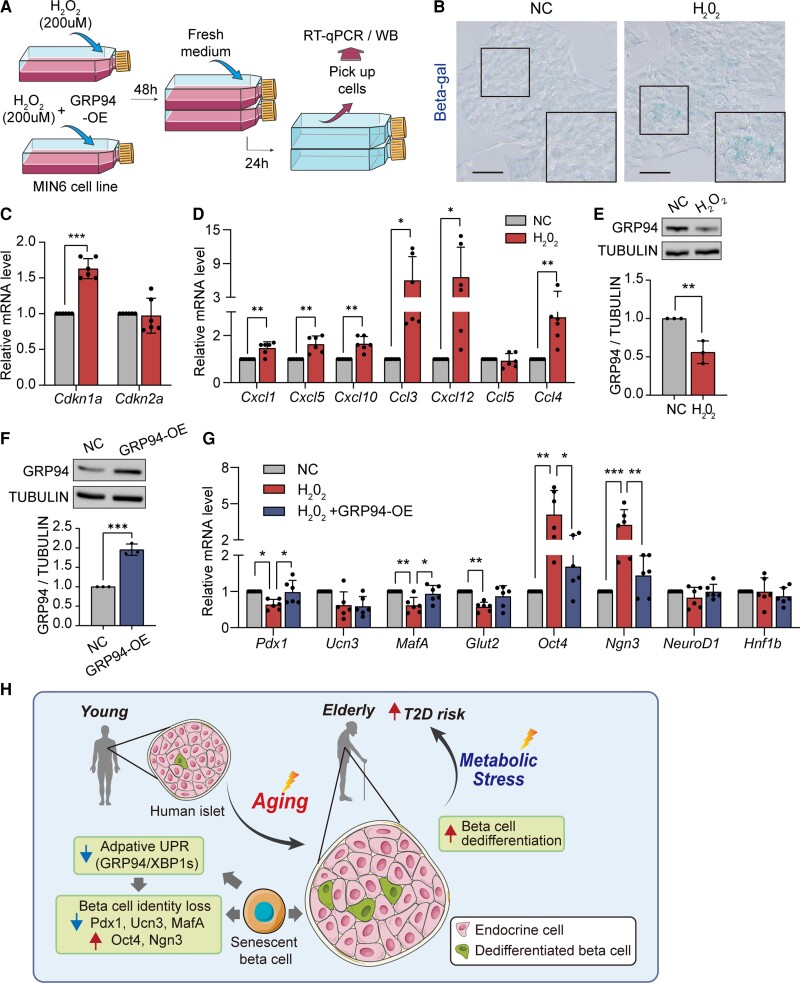
Adaptive UPR (GRP94) Mediates H2O2-induced Senescent Beta Cell Dedifferentiation
To investigate the correlation between adaptive UPR and beta cell identity in senescent murine beta cells, we treated Min6 cells with 200 µM H2O2 for 48 hours (Fig. 5A). Induction of senescence was evidenced by increased beta-gal activity (Fig. 5B), upregulation of Cdkn1a expression (Fig. 5C), and induction of senescence-associated secretory profile factors, namely, Cxcl1, Cxcl5, Cxcl10, Ccl3, Cxcl12, and Ccl4 (Fig. 5D). H2O2-induced senescent Min6 cells had comparable mRNA levels of Xbp1 (Fig. 3D (29)) but diminished GRP94 expression at both mRNA and protein levels (Fig. 5E; Fig. 3C (29)). H2O2-treated Min6 cells shifted their differentiated state, with decreased expression of key genes vital to beta cell identity, namely, Pdx1, MafA, and Glut2, and increased levels of progenitor markers, namely, Oct4 and Ngn3 (Fig. 5G).
Figure 5.
Overexpression of GRP94 partially prevents age-induced beta cell dedifferentiation in Min6 cells. (A) Flow chart of inducing senescence with/without overexpressing GRP94 in Min6 cells. Senescence was induced by treating with 200 µM H2O2 for 48 hours, followed by normal media for another 24 hours. (B-D) Beta-gal+ activity (B), relative mRNA expression of Cdkn1a (C), and senescence-associated secretory profile (SASP) factors (D) in H2O2-treated Min6 cells. (E) Protein levels of GRP94 in H2O2-treated Min6 cells; band intensities normalized on the basis of the corresponding TUBULIN intensity (n = 3). (F) Immunoblot shows expression of GRP94 protein in GRP94-OE cells; band intensities normalized on the basis of the corresponding TUBULIN intensity (n = 3). (G) Relative mRNA expression of transcription factors, genes involved in beta cell function, and progenitor cells in H2O2-treated Min6 cells with/without overexpressing GRP94. (H) Summary of the failure of establishing proper adaptive UPR in senescent beta cells triggering their dedifferentiation. n = 6. Data present means ± SD. *P < .05, **P < .01, ***P < .001.
To evaluate whether diminished GRP94 expression mediated H2O2-induced senescent beta cell dedifferentiation, we transfected senescent Min6 cells with a GRP94 flag plasmid to overexpress GRP94 during H2O2 treatment (Fig. 5F). Interestingly, restored GRP94 expression in senescent Min6 cells significantly reversed H2O2-induced downregulation of beta cell identity genes Pdx1 and MafA, and upregulation of progenitor genes Oct4 and Ngn3 (Fig. 5G). The above data provide evidence that impaired adaptive UPR during aging might be responsible for beta cell identity loss and acquisition of progenitor-like properties.
Discussion
The incidence and prevalence of type 2 diabetes increase with age (2, 4, 5). Age-related deterioration in glucose tolerance is attributed to reduced glucose-stimulated insulin secretion (11, 13, 14, 40) and/or decreased insulin sensitivity in peripheral tissues (9, 41). It is documented that beta cell identity loss is an important mechanism of type 2 diabetes. Recently, Aguayo-Mazzucato et al have reported that senescent beta cells in aging mice (7-8 months C57BL/6J) display a distinctive transcription profile characterized by downregulation of hallmark beta cell genes (including Insulin 1, Mafa, Nkx6.1, and Pdx1) and expression of disallowed genes, such as Ldha and catalase (15). It remains unclear whether the human beta cell differentiation state is also affected in elderly subjects before diabetes occurs, and therefore contributes to increased susceptibility to type 2 diabetes.
Consistent with our previous reports (24, 25), the mean ratio of dedifferentiated beta cells in nondiabetic human islets ranged from 1.2% to 4.8%. For the first time, we have provided evidence for age-related increase in dedifferentiated cells in nondiabetic humans. First, we found a 2-fold increase in the ratio of dedifferentiated cells in middle-aged (40-60 years) and elderly (>60 years) subjects compared with young adults (20-40 years). Second, age was independently and positively associated with the percentage of dedifferentiated cells in humans. Third, we observed subcellular changes of FoxO1 from the nucleus to the cytoplasm in senescent human beta cells, which is known to cause beta cell dedifferentiation (32, 42, 43). Our finding of age-dependent FoxO1 subcellular change reinforced a possible link between FoxO1 inactivation and beta cell dedifferentiation in the degeneration of stress response during aging (44). Fourth, a significant decrease in the expression of UCN3, which is closely related to insulin release (34, 45, 46), was detected in human beta cells from elderly subjects. The detection of early decrease in UCN3 expression in beta cells from nondiabetic elderly subjects provided evidence that senescent human beta cells are prone to identity loss and beta cell dysfunction. It has been proposed that mechanisms including glucotoxicity and its downstream pathways such as hypoxia (47, 48), oxidative stress (35, 49–51), inflammation (52), and ER stress (53–55) are involved in beta cell dedifferentiation. Loss of ER homeostasis and subsequent accumulation of unfolded and misfolded proteins have been proved to be a central molecular hallmark of aging and many degenerative diseases, including diabetes (36). ER homeostasis is balanced by adaptive UPR to resolve ER stress and maladaptive UPR leading to apoptosis (56, 57). It has been shown that adaptive UPR of beta cells was decreased in rodent diabetic models and human type 2 diabetes (58). Recently, several rodent studies have attributed the failure of beta cell adaptive UPR induction (ER chaperones and ER stress sensors) to the abnormalities of beta cell gene expression and diabetes progression (59–61). In the present study, we detected a robust reduction in adaptive UPR markers, namely, GRP94 and XBP1s, in beta cells from elderly subjects. GRP94 is an ER chaperone protein involved in UPR to execute protein quality control (38), loss of which leads to impaired islet development in mice (62). Meanwhile, another component of adaptive UPR, a transcriptionally active spliced form of XBP1 (XBP1s), is also required to maintain mature beta cell identity in mice (60, 63). Decreased expression of XBP1s was observed in islets of patients with type 2 diabetes (64). In the current study, we found knockdown of adaptive UPR components GRP94 or XBP1 in intact murine beta cells led to significant downregulation of key genes vital to beta cell identity (Pdx1, Ucn3, and MafA) and upregulation of progenitor markers (Oct4 and Ngn3). Furthermore, by using a chemically induced senescence cell model, we reproduced age-related human beta cell dedifferentiation in vitro, with decreased GRP94 expression, downregulation of beta cell identity genes (Pdx1, MafA, and Glut2), and upregulation of progenitor genes (Oct4 and Ngn3). Importantly, when GRP94 expression was restored in H2O2-treated Min6 cells, beta cell identity loss was at least partly rescued.
It should be noted that all subjects involved in this study had no history of diabetes with normal fasting glucose levels less than 5.5 mmol/L, thus it is difficult to investigate whether the proportion of beta cell dedifferentiation will lead to changes in fasting glucose levels. We should also notice that the proportion of dedifferentiated beta cells is relatively low, even in elderly subjects (∼3%). Thus, the clinical significance of age-related beta cell dedifferentiation on the development of diabetes remains unclear. As Saisho et al previously reported that beta cell mass and apoptosis were unchanged in nondiabetic elderly population, whereas the mean individual beta cell cross-sectional area and beta cell nuclear diameter were both increased with age (65), indicating beta cell (de)compensation instead of beta cell loss is the main phenotypic change during the process of ageing. This finding actually supports our in vivo and in vitro data that UPR defects and beta cell dedifferentiation appear to be one of the underlying mechanisms of age-related beta cell failure.
To our knowledge, this is the first study showing that age-related defects in adaptive UPR might contribute to beta cell identity loss and acquisition of progenitor-like properties. Our findings highlight that the failure to establish proper adaptive UPR (GRP94/XBP1s) in senescent human beta cells might shift their differentiated state by reducing identity-related genes and reverting to a progenitor-like state, thus supporting predisposition of beta cell senescence in diabetes risk under the metabolic stress (Fig. 5H). Understanding the phenotypic change in senescent human beta cells would help us to search for potential targets for age-related beta cell failure.
Acknowledgments
We thank Ying Huang and all members of the Core Facility of Basic Medical Sciences of SJTU for their technical support. Figure 5H was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License. (https://creativecommons.org/licenses/by/3.0/)
Abbreviations
- BMI
body mass index
- FBG
fasting blood glucose
- FoxO1
forkhead box O1
- UPR
unfolded protein response
- UCN3
Urocortin 3
Contributor Information
Jiaxi Song, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Qicheng Ni, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Jiajun Sun, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Jing Xie, Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Jianmin Liu, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Guang Ning, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Weiqing Wang, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Qidi Wang, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China; Sino-French Research Center for Life Sciences and Genomics, Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Financial Support
This work was supported by the National Natural Sciences Foundation of China (81870527, 82070795, 82100835, 91857205, 81730023) and the Shanghai Sailing Program (21YF1426900).
Author Contributions
J.X.S. designed and performed the experiments, analyzed the data, and wrote the manuscript. Q.C.N. contributed to conception, interpretation of data, and revised the manuscript. J.J.S. collected human samples. J.X. collected human samples and ensured the final pathology diagnosis of all tissues. G.N. and J.M.L. contributed to the discussion. W.Q.W. supervised the project and contributed to writing the manuscript. Q.D.W. conceived the project, and wrote and revised the manuscript. All authors critically reviewed and approved the final version of the manuscript. W.Q.W and Q.D.W. take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors have nothing to disclose.
Data Availability
All data generated or analyzed during this study are included in the article. Further information about the data are available from the corresponding author upon request.
References
- 1. Kennedy BK, Berger SL, Brunet A, et al. . Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. [DOI] [PubMed] [Google Scholar]
- 3. Wang L, Gao P, Zhang M, et al. . Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu G, Liu B, Sun Y, et al. . Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colhoun HM, McKnight J. Diabetes in Scotland: a rising tide. Lancet Diabetes Endocrinol. 2020;8(5):375–376. [DOI] [PubMed] [Google Scholar]
- 6. Bloomgarden Z, Ning G. Diabetes and aging. J Diabetes. 2013;5(4):369–371. [DOI] [PubMed] [Google Scholar]
- 7. Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E. Independent influence of age on basal insulin secretion in nondiabetic humans. European group for the study of insulin resistance. J Clin Endocrinol Metab. 1999;84(3):863–868. [DOI] [PubMed] [Google Scholar]
- 8. Gunasekaran U, Gannon M. Type 2 diabetes and the aging pancreatic beta cell. Aging (Albany NY). 2011;3(6):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Basu R, Breda E, Oberg AL, et al. . Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. [DOI] [PubMed] [Google Scholar]
- 10. Xiao J, Weng J, Ji L, et al. . Worse pancreatic beta-cell function and better insulin sensitivity in older Chinese without diabetes. J Gerontol A Biol Sci Med Sci. 2014;69(4):463–470. [DOI] [PubMed] [Google Scholar]
- 11. Geloneze B, de Oliveira MdS, Vasques AC, Novaes FS, Pareja JC, Tambascia MA. Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Metabolism. 2014;63(7):922–929. [DOI] [PubMed] [Google Scholar]
- 12. Kushner JA. The role of aging upon beta cell turnover. J Clin Invest. 2013;123(3):990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gregg T, Poudel C, Schmidt BA, et al. . Pancreatic beta-cells from mice offset age-associated mitochondrial deficiency with reduced KATP channel activity. Diabetes. 2016;65(9):2700–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Westacott MJ, Farnsworth NL, St Clair JR, et al. . Age-dependent decline in the coordinated [Ca(2+)] and insulin secretory dynamics in human pancreatic islets. Diabetes. 2017;66(9):2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguayo-Mazzucato C, Andle J, Lee TB Jr, et al. . Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30(1):129–142.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enge M, Arda HE, Mignardi M, et al. . Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171(2):321–330.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swisa A, Glaser B, Dor Y. Metabolic stress and compromised identity of pancreatic beta cells. Front Genet. 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puri S, Akiyama H, Hebrok M. VHL-mediated disruption of Sox9 activity compromises beta-cell identity and results in diabetes mellitus. Genes Dev. 2013;27(23):2563–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4(6):1262–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19(5):872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin HV, Ren H, Samuel VT, et al. . Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60(3):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cinti F, Bouchi R, Kim-Muller JY, et al. . Evidence of beta-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab. 2016;101(3):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun J, Ni Q, Xie J, et al. . β-Cell dedifferentiation in patients with T2D with adequate glucose control and nondiabetic chronic pancreatitis. J Clin Endocrinol Metab. 2019;104(1):83–94. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Ni Q, Sun J, et al. . Paraneoplastic beta cell dedifferentiation in nondiabetic patients with pancreatic cancer. J Clin Endocrinol Metab. 2020;105(4):dgz224. [DOI] [PubMed] [Google Scholar]
- 26. Aguayo-Mazzucato C, van Haaren M, Mruk M, et al. . Β cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab. 2017;25(4):898–910.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes A . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S13–S28. [DOI] [PubMed] [Google Scholar]
- 28. Garvey WT, Mechanick JI, Brett EM, et al. . American Association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):1–203. [DOI] [PubMed] [Google Scholar]
- 29. Song J, Ni Q, Sun J, et al. Data from ageing impairs adaptive unfolded protein response and drives beta cell dedifferentiation in human. Figshare. 2022; Accessed August 2, 2022. 10.6084/m9.figshare.20335296.v4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li W, Zhang H, Nie A, et al. . mTORC1 pathway mediates beta cell compensatory proliferation in 60% partial-pancreatectomy mice. Endocrine. 2016;53(1):117–128. [DOI] [PubMed] [Google Scholar]
- 31. Stauffer W, Sheng H, Lim HN. Ezcolocalization: an ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci Rep. 2018;8(1):15764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitamura YI, Kitamura T, Kruse JP, et al. . Foxo1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2(3):153–163. [DOI] [PubMed] [Google Scholar]
- 33. Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9(10):615–623. [DOI] [PubMed] [Google Scholar]
- 34. Blum B, Roose AN, Barrandon O, et al. . Reversal of beta cell de-differentiation by a small molecule inhibitor of the TGFbeta pathway. Elife. 2014;3:e02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo S, Dai C, Guo M, et al. . Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123(8):3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frakes AE, Dillin A. The UPR(ER): sensor and coordinator of organismal homeostasis. Mol Cell. 2017;66(6):761–771. [DOI] [PubMed] [Google Scholar]
- 37. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eletto D, Dersh D, Argon Y. GRP94 In ER quality control and stress responses. Semin Cell Dev Biol. 2010;21(5):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ghosh R, Colon-Negron K, Papa FR. Endoplasmic reticulum stress, degeneration of pancreatic islet beta-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol Metab. 2019;27S:S60–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ihm SH, Matsumoto I, Sawada T, et al. . Effect of donor age on function of isolated human islets. Diabetes. 2006;55(5):1361–1368. [DOI] [PubMed] [Google Scholar]
- 41. Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284(1):E7–E12. [DOI] [PubMed] [Google Scholar]
- 42. Martinez SC, Cras-Meneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet beta-cell. Diabetes. 2006;55(6):1581–1591. [DOI] [PubMed] [Google Scholar]
- 43. Kawamori D, Kaneto H, Nakatani Y, et al. . The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281(2):1091–1098. [DOI] [PubMed] [Google Scholar]
- 44. Xiao X, Chen C, Guo P, et al. . Forkhead box protein 1 (FoxO1) inhibits accelerated beta cell aging in pancreas-specific SMAD7 mutant mice. J Biol Chem. 2017;292(8):3456–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Meulen T, Donaldson CJ, Caceres E, et al. . Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21(7):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30(3):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato Y, Inoue M, Yoshizawa T, Yamagata K. Moderate hypoxia induces beta-cell dysfunction with HIF-1-independent gene expression changes. PLoS One. 2014;9(12):e114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sato Y, Tsuyama T, Sato C, et al. . Hypoxia reduces HNF4alpha/MODY1 protein expression in pancreatic beta-cells by activating AMP-activated protein kinase. J Biol Chem. 2017;292(21):8716–8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem. 2005;280(12):11107–11113. [DOI] [PubMed] [Google Scholar]
- 50. Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic Biol Med. 2006;41(2):177–184. [DOI] [PubMed] [Google Scholar]
- 51. Kaneto H, Xu G, Fujii N, Kim S, Bonner-Weir S, Weir GC. Involvement of c-jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J Biol Chem. 2002;277(33):30010–30018. [DOI] [PubMed] [Google Scholar]
- 52. Nordmann TM, Dror E, Schulze F, et al. . The role of inflammation in beta-cell dedifferentiation. Sci Rep. 2017;7(1):6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Han D, Lerner AG, Vande Walle L, et al. . IRE1alpha Kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Szabat M, Kalynyak TB, Lim GE, et al. . Musashi expression in β-cells coordinates insulin expression, apoptosis and proliferation in response to endoplasmic reticulum stress in diabetes. Cell Death Dis. 2011;2(11):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lombardi A, Ulianich L, Treglia AS, et al. . Increased hexosamine biosynthetic pathway flux dedifferentiates INS-1E cells and murine islets by an extracellular signal-regulated kinase (ERK)1/2-mediated signal transmission pathway. Diabetologia. 2012;55(1):141–153. [DOI] [PubMed] [Google Scholar]
- 56. Eizirik DL, Pasquali L, Cnop M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349–362. [DOI] [PubMed] [Google Scholar]
- 57. Chan JY, Luzuriaga J, Maxwell EL, West PK, Bensellam M, Laybutt DR. The balance between adaptive and apoptotic unfolded protein responses regulates beta-cell death under ER stress conditions through XBP1, CHOP and JNK. Mol Cell Endocrinol. 2015;413(C):189–201. [DOI] [PubMed] [Google Scholar]
- 58. Herbert TP, Laybutt DR. A reevaluation of the role of the unfolded protein response in islet dysfunction: maladaptation or a failure to adapt? Diabetes. 2016;65(6):1472–1480. [DOI] [PubMed] [Google Scholar]
- 59. Title AC, Silva PN, Godbersen S, Hasenohrl L, Stoffel M. The miR-200-Zeb1 axis regulates key aspects of beta-cell function and survival in vivo. Mol Metab. 2021;53:101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee K, Chan JY, Liang C, et al. . XBP1 Maintains beta cell identity, represses beta-to-alpha cell transdifferentiation and protects against diabetic beta cell failure during metabolic stress in mice. Diabetologia. 2022;65(6):984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chan JY, Luzuriaga J, Bensellam M, Biden TJ, Laybutt DR. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes. 2013;62(5):1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim DS, Song L, Wang J, et al. . GRP94 Is an essential regulator of pancreatic beta-cell development, mass, and function in male mice. Endocrinology. 2018;159(2):1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108(21):8885–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Engin F, Nguyen T, Yermalovich A, Hotamisligil GS. Aberrant islet unfolded protein response in type 2 diabetes. Sci Rep. 2014;4:4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA. Butler PC. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013;36(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the article. Further information about the data are available from the corresponding author upon request.