Abstract
Zinc is an essential trace element, and its deficiency causes taste dysfunction. Zinc accumulates in zinc transporter (ZnT)3-expressing presynaptic vesicles in hippocampal neurons and acts as a neurotransmitter in the central nervous system. However, the distribution of zinc and its role as a signal transmitter in taste buds remain unknown. Therefore, we examined the distribution of zinc and expression profiles of ZnT3 in taste cells and evaluated zinc release from isolated taste cells upon taste stimuli. Taste cells with a spindle or pyriform morphology were revealed by staining with the fluorescent zinc dye ZnAF-2DA and autometallography in the taste buds of rat circumvallate papillae. Znt3 mRNA levels were detected in isolated taste buds. ZnT3-immunoreactivity was found in phospholipase-β2-immunopositive type II taste cells and aromatic amino acid decarboxylase- immunopositive type III cells but not in nucleoside triphosphate diphosphohydrolase 2-immunopositive type I cells. Moreover, we examined zinc release from taste cells using human transient receptor potential A1- overexpressing HEK293 as zinc-sensor cells. These cells exhibited a clear response to isolated taste cells exposed to taste stimuli. However, pretreatment with magnesium-ethylenediaminetetraacetic acid, an extracellular zinc chelator - but not with zinc-ethylenediaminetetraacetic acid, used as a negative control - significantly decreased the response ratio of zinc-sensor cells. These findings suggest that taste cells release zinc to the intercellular area in response to taste stimuli and that zinc may affect signaling within taste buds.
Key words: zinc, zinc transporter, taste cell, taste bud, circumvallate papilla, lingual epithelium, taste signaling
Introduction
Zinc plays an important role in the sense of taste, and its deficiency causes taste disorders in humans. Zinc treatment has been reported as effective for some taste disorders.1-5 In rats, zinc deficiency is also reported to cause taste disorders by decreasing the number of taste buds.6-8 These results indicate that zinc is essential for the differentiation and proliferation of taste cells. However, the functional role of zinc in taste signaling remains unclear.
Zinc is abundant in the hippocampus of the central nervous system.9 It accumulates with glutamate in presynaptic vesicles of glutamatergic neurons owing to the action of ZnT3, a zinc transporter, and is released via exocytosis.10,11 Zinc is also an allosteric modulator of the P2X2 and N-methyl-D-aspartic acid (NMDA) receptors, which are ATP and glutamate receptors, respectively.12-16 Therefore, zinc is vital to the regulation of signal transduction in the central nervous system.
Taste buds are composed of taste cells, which are classified into types I–IV based on their morphology.17-19 Type I taste cells are glia-like supporting cells involved in the maintenance of taste buds.20-22 Type II taste cells have taste receptors for sweet, bitter, and umami tastants.23-25 ATP,24 5-HT,26 glutamate,27 epinephrine,28 GABA,29 acetylcholine,30 and other compounds act as signaling molecules in taste buds. Among these, ATP is a particularly vital signaling molecule released by type II taste cells in response to sweet, bitter, and umami stimuli to transmit information to type III taste cells or sensory nerve terminals.24,26,31-34 Type III taste cells have the sour receptor OTOP1 for sour tastants, which evoke 5- HT-release from type III taste cells in response to acid stimulation. 26,33,35-38 Interestingly, type III taste cells, but not type II cells, store 5-HT in vesicles located at synaptic sites and form typical chemical synapses with afferent nerve fibers.32,39-42 However, whether zinc directly plays a role in the taste signaling of taste buds or acts as a modulator of ATP signaling remains unclear. To address this knowledge gap, we aimed to clarify the presence and localization/expression profile of ZnT3 in the taste buds of rat circumvallate papillae and whether taste cells release zinc upon tastant stimulation.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats (total 43 rats, 6-week-old, 200–300 g body weight; Japan SLC, Hamamatsu, Japan) were randomly divided and housed in cages (3-4 rats per cage) with ad libitum access to food and water under a 12-h/12-h light/dark cycle in a controlled environment of approximately 55% relative humidity at 23°C. Rats were fed a normal diet (Oriental Yeast Co., Tokyo, Japan). The sample sizes were 3–5, the minimum number required to perform a significance test. None of the rats were excluded from the analysis. All experiments were performed strictly according to the ARRIVE guidelines as well as the Guidelines for Animal Experimentation of Kyoto Pharmaceutical University and Setsunan University.
The study protocol was authorized by the Experimental Animal Research Committee of Kyoto Pharmaceutical University (reference number: 16-12-005) and Setsunan University (reference number: K19-24).
Detachment of epithelial tissues in circumvallate papillae and isolation of taste buds
SD rats (3 rats per group) were transcardially perfused with saline under deep isoflurane anesthesia. Epithelial tissues containing circumvallate papillae were detached from the tongue by injecting an enzyme mixture comprising 2.5 mg/mL dispase II (Cat. No.: 4942078; Roche Applied Science, Tokyo, Japan), 1.0 mg/mL collagenase D (Cat. No.: 1088858; Roche Applied Science), and 1.0 mg/mL trypsin inhibitor (Cat. No.: T9128; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 20°C, and then the epithelial tissues were treated with RNAlater® solution (Sigma- Aldrich) at −20°C until reverse transcription (RT) within 30 min of collection, as reported previously.43 To isolate taste buds, detached epithelial tissues of circumvallate papillae were treated with the enzyme mixture at 37°C for 10 min, and the dispersed taste buds were collected using a glass capillary (World Precision Ins., Sarasota, FL, USA) under an inverted microscope (CKX41; Olympus, Tokyo, Japan).
Live imaging of endogenous zinc in epithelial tissues containing taste buds
SD rats (3 rats per group) were perfused with saline under deep isoflurane anesthesia, and epithelial tissues, including taste buds, were removed from the tongue by treatment with 1.0 mg/mL collagenase D, 2.5 mg/mL dispase II, and 1.0 mg/mL trypsin inhibitor. As negative controls, rats received 0.7 g/kg diethyldithiocarbamate (DEDTC; Sigma-Aldrich) intraperitoneally 80 min before perfusion with saline. DEDTC was used as a chelating agent for Zn2+ and other group IIB metal ions.44 Subsequently, the detached epithelial tissues were treated with Tyrode’s solution (140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM D-glucose, 10 mM HEPES, and 10 mM sodium pyruvate) containing 100 mM ZnAF-2 DA (Enzo Life Sciences, Farmingdale, NY, USA) and 0.04% Pluronic F-127 for 90 min at 23°C, followed by washing in Tyrode’s solution. Fluorescence images were taken using an LSM510 META confocal laser microscope (Carl Zeiss, Jena, Germany).
Autometallography
Autometallography (AMG) was performed as described previously. 45,46 Rats (3 per group) were intraperitoneally administered 0.1% sodium selenium (20 mg/kg body weight). After 90 min, the rats were perfused with saline and 3% glutaraldehyde in 0.1 M phosphate buffer under deep anesthesia with isoflurane. The tongue tissues containing circumvallate papillae were post-fixed with 3% glutaraldehyde in a 0.1-M phosphate buffer for 2 h at 4 °C and immersed overnight in a 30% sucrose solution at 4°C.
Frozen sections (40-μm-thick) were prepared using a cryostat (CM1850; Leica, Wetzlar, Germany) and placed on glass slides (Matsunami Glass, Osaka, Japan). DEDTC was administered intraperitoneally 1 h before the administration of 0.1% sodium selenite as a negative control. Subsequently, tissue sections were incubated in the AMG developer for 3 h at 26°C. The AMG developer contained 30% gum arabic colloid, citrate buffer, 5.7% hydroquinone solution, and 0.73% silver lactate solution. After reaction with the AMG developer, 5% sodium thiosulfate was added for 10 min, and then the tissue sections were sealed with the EUKITT mounting medium (ORSAtec, Bobingen, Germany). Images were acquired using a Moticam1000 (Shimadzu, Kyoto, Japan).
RT-PCR analysis
cDNA was obtained from isolated taste buds using the CellAmp Whole Transcriptome Amplification Kit (Real Time) ver. 2 (Takara, Kusatsu, Japan) according to the manufacturer’s instructions. Total RNA from epithelial tissues containing taste buds was extracted and reverse-transcribed using the NucleoSpin RNA XS kit (Macherey-Nagel, Düren, Germany) and the PrimeScript RT reagent kit with gDNA Eraser (Takara) according to the manufacturer’s instructions. RT of total RNA from epithelial tissues was carried out at 37°C for 15 min and at 85°C for 5 s. RT-PCR was performed as nested PCR using rTaq DNA polymerase (Takara) for both the 1st and 2nd PCR. The primer sets and PCR conditions are listed in Table 1.
Immunohistochemical analysis
Rats (3 rats per group) were perfused transcardially with saline and 4% paraformaldehyde (Wako, Osaka, Japan) in 0.1 M phosphate buffer (pH 7.4) containing 0.2% picric acid (Wako) under 2% isoflurane deep anesthesia. Tongues containing circumvallate papillae were sectioned at 40 μm using a cryostat (CM1850; Leica). Free-floating sections were incubated with primary antibodies for 3 days at 4°C, followed by incubation for 1 day at 4°C with secondary antibodies, as shown in Table 2. Thereafter, the floating cross-sections were exposed to the guinea pig anti-ZnT3 antibody (1:100) and subsequently washed with PBS. Next, the sections were treated with the secondary antibody Alexa Fluor 488-labeled anti-guinea pig IgG (green, 1:1000). The nuclei were counterstained with Hoechst 33258 (blue, 10 μg/mL). A negative control test was performed by omitting the primary antibodies (data not shown). The anti-ZnT3 antibody for the adsorption test was adsorbed by the immunogen peptide (40 μg peptide per 3 μL antibody, 197-0P, Synaptic Systems, Germany) containing the same epitope sequence to generate the antibody. The cryosections were then treated with the adsorbed antibodies, followed by washing with PBS, and then Alexa Fluor 488-labeled anti-guinea pig IgG (green, 1:1000) was applied as the secondary antibody.
Table 1.
Primers and conditions for RT-PCR.
| Gene (Accession No.) | Primer pair | Primer sequences | Annealing | Cycle number | Product size | |
|---|---|---|---|---|---|---|
| temperature (°C) | (cycles) | (bp) | ||||
| Gapdh | F | 5 -TCATTGACCTGAACTACATGGTC-3 | 55 | 38 | 567 | |
| (NM _017008.4) | R | 5 -CGTTCAGCTCTGGGATGAC-3 | ||||
| Kcnq1 | 1st | F | 5 -CAGTGGATGCAGGGATGATG-3 | 52 | 25 | 511 |
| (NM_032073) | R | 5 -ACATCTCGCACATCGTAGGG-3 | ||||
| 2nd | F R | 5 -CTATGCTGCGGAGAATCCTGAC-3 5 -CGTGCTTGCTGGAATTTCTTC-3 | 40 | 456 | ||
| Ntpdase2 | 1st | F | 5 -GGGTGACTGCCAACTACCTG-3 | 55 | 15 | 911 |
| (NM_172030) | R | 5 -GACTGTGAAGAGCAGCAGGAG-3 | ||||
| 2nd | F R | 5 -CGACTCTATGGCCAGCATTACC-3 5 -GCAGGTGATCTCTGTGGCTTC-3 | 38 | 453 | ||
| Plcb2 | 1st | F | 5 -AAGGCATATCTGAGCCAAGG-3 | 52 | 25 | 535 |
| (NM_053478.1) | R | 5 -TTGCAAGGTGACAGGCACTG-3 | ||||
| 2nd | F R | 5 -GGACGATGAAACCTCAATAGCC-3 5 -GGCAGCTTCTACACGTTTGC-3 | 40 | 475 | ||
| Gnat3 | 1st | F | 5 -TAGTTCAGAGAGCAAGGAGTCAGC-3 | 55 | 15 | 520 |
| (NM_173139) | R | 5 -CCGGGAATGTAGAACGTCTTG-3 | ||||
| 2nd | F R | 5 -AAGAACTGGAGAAGAAGCTTCAGG-3 5 -AGGCTTGAATTCCTGGATCG-3 | 38 | 366 | ||
| Aadc | 1st | F | 5 -CACATCTGATCAGGCACATTCCTC-3 | 52 | 40 | 683 |
| (NM_012545) | R | 5 -TTAGCCGGAAGCAGACCAAC-3 | ||||
| 2nd | F R | 5 -AGCAATTCCTTCAGATGGCAAC-3 5 -TGAGACAGCTTCACGTGCTTTC-3 | 40 | 537 | ||
| Znt3 | 1st | F | 5 -TGCGTCGTGTGCTTCATCTTC-3 | 52 | 40 | 624 |
| (NM_001013243) | R | 5 -ATCTCGAAGTGTAGGAGCTGTGG-3 | ||||
| 2nd | F R | 5 -TGGCAGACATAGGCAGTATGATGG-3 5 -GCAGATAGAGAAGAGGAAGGTGCTG-3 | 40 | 500 | ||
Table 2.
Antibodies used for immunohistochemistry.
| Antigen | Primary antibody | Secondary Ab |
|---|---|---|
| ZnT3 | Guinea pig anti-ZnT3 Ab (1:100; 197 004, Synaptic Systems) | Goat anti-guinea pig IgG conjugated with Alexa Fluor® 488 (1:1000; Cat. No.: A11073, Thermo Fisher Scientific) |
| NTPDase2 | Sheep anti-NTPDase2 Ab (1:200; AF5797, R&D Systems) | Donkey anti-sheep IgG conjugated with Alexa Fluor® 594 (1:1000; Cat. No.: A11016, Thermo Fisher Scientific) |
| PLC-b2 | Rabbit anti-PLC-b2 Ab (1:1000; sc-206, Santa Cruz Biotechnology) | Goat anti-rabbit IgG conjugated with Alexa Fluor® 546 (1:1000; Cat. No.: A11010, Thermo Fisher Scientific) |
| AADC | Rabbit anti-AADC Ab (1:50; BML-AZ1030-0050, Enzo) | Goat anti-rabbit IgG conjugated with Alexa Fluor® 546 (1:1000; Cat. No.: A11010, Thermo Fisher Scientific) |
The sections were mounted on glass slides and sealed using a Prolong® antifade kit (Thermo Fisher Scientific, Waltham, MA, USA). Fluorescence micrographs were obtained using a confocal laser microscope (LSM510META).
Detection of zinc using zinc-sensor cells
We established HEK293T/human transient receptor potential A1 (hTRPA1) stable cells, which overexpressed hTRPA1, as zincsensor cells to validate zinc detection (Supplementary Figures 1 and 2). Briefly, the hTRPA1-pF5A vector was generated as follows: an hTRPA1-pF1K vector (pF1KB7348, Product ID FXC07217; Kazusa DNA Res. Inst., Kisarazu, Japan) was digested using SgfI and PmeI (New England Biolabs, Ipswich, MA, USA), and the digested fragment was ligated into the pF5A CMV-neo Flexi vector, which was digested using SgfI and PmeI according to the ligation high ver. 2 manual (TOYOBO, Osaka, Japan). HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% fetal bovine serum (Biowest, Miami, FL, USA). Transfection of the hTRPA1-pF5A vector into cells was performed using Lipofectamine 2000 (Thermo Fisher Scientific). To establish HEK293T/hTRPA1 stable cells, the transfectants were selected with 3 mg/mL geneticin (Thermo Fisher Scientific). The functionality of TRPA1 in stable cells was evaluated by the responses to AITC, a TRPA1 agonist, HC-030031, a TRPA1 antagonist, and Ca2+-free conditions. An increase in the intracellular Ca2+ level was determined using the Fura-2/AM ratio (ex. 340 nm/380 nm, em. 510 nm) with a microplate reader (Wallac 1420; PerkinElmer, Waltham, MA, USA).
HEK293T/hTRPA1 stable cells were used as zinc-sensor cells. Intracellular Ca2+ levels in Fluo-4/AM-treated HEK293T/hTRPA1 stable cells (Dojindo Lab., Kumamoto, Japan) were measured to evaluate zinc release from taste cells following taste stimulation, based on previous reports,47 demonstrating hTRPA1 activation by zinc. Fluo-4/AM was adopted to distinguish between zinc-sensor cells and isolated taste cells using a confocal laser microscope (LSM510META). In preliminary experiments, we confirmed the optimum concentrations of tastants that did not activate HEK293T/hTRPA1 stable cells. As taste stimuli, we used the recording medium (20 mM HEPES, 115 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 13.8 mM glucose) containing sweet (2 mM sucrose and 2 mM saccharin sodium), umami (2 mM monosodium glutamate), and bitter (2 μM quinine hydrochloride) tastants. For non-taste stimulus, we treated the cells with the recording medium without tastants. For the evaluation of zinc release from taste cells, HEK293T/hTRPA1 stable cells were cultured on four-well micro-inserts (ibidi GmbH, Gräfelfing, Germany) for 24 h, washed with the recording medium, and treated with 5 μM Fluo-4/AM for 1 h at 37°C. As a condition for the absence or presence of taste cells, taste mixture was injected to Fluo-4/AM-loaded HEK293T/hTRPA1 stable cells (Supplementary Figure 3 A,B, respectively). As a condition for the presence of 100 μM MgEDTA or 100 μM ZnEDTA, taste mixture was injected to taste cell-seeded Fluo-4/AM-loaded HEK293T/hTRPA1 stable cells (Supplementary Figure 3 C,D, respectively). As a condition for no taste stimulation, the recording medium was injected to taste cell-seeded Fluo-4/AM-loaded HEK293T/hTRPA1 stable cells (Supplementary Figure 3E). Cells were washed again with the recording medium and imaged under a confocal laser microscope (LSM510 META) at an excitation wavelength of 488 nm. One minute after the start of the measurement, the taste mixture was added, and the fluorescence intensity of Fluo-4 was measured every 2 s for 6 min. The fluorescence intensity of each cell type was calculated based on the obtained image data using the LSM 510 software (Carl Zeiss, Oberkochen, Germany). Furthermore, fluorescence intensity-time curves were constructed using the average fluorescence intensity before adding the taste mixture as 1.0.
Statistical analysis
Data are shown as the mean ± SD. Comparisons between two or more groups were performed using one-way ANOVA followed by the Dunnett’s multiple comparison test with the Prism software (version 8; GraphPad Inc., La Jolla, CA, USA). Differences with a p<0.05 were considered statistically significant.
Results
Detection of zinc-derived signals in taste cells of rat circumvallate papillae
We investigated the localization of endogenous zinc in the taste buds of the circumvallate papillae using ZnAF-2 DA, a zincspecific fluorescent probe. Fluorescent signals with a spindle or pyriform morphology were observed in the taste buds of the epithelial tissue of the circumvallate papillae (Figure 1 A,B). By contrast, the fluorescence signal derived from ZnAF-2 DA was clearly attenuated in the taste buds of the circumvallate papillary epithelium pretreated with DEDTC, a zinc and heavy metal ion chelator (Figure 1 C,D). AMG staining revealed the distribution of endogenous heavy metals in the circumvallate papillae epithelium. Partial staining was observed in cells with a spindle or pyriform morphology (Figure 2A). By contrast, AMG staining was not detected in the circumvallate papillary epithelial tissues pretreated with DEDTC (Figure 2B). These results indicate that zinc was located in the taste cells of taste buds.
Expression of zinc transporter ZnT3 in type II and III taste cells of rat circumvallate papillae
Isolated taste buds were collected from rat circumvallate papillae for semi-quantitative RT-PCR analysis. Isolated taste cells and circumvallate papillae were positive for taste cell markers, namely potassium voltage-gated channel, KQT-like subfamily, member 1 (Kcnq1),48 nucleoside triphosphate diphosphohydrolase 2 (Ntpdase2),20 phospholipase C beta-2 (Plcb2),49 guanine nucleotide-binding protein, alpha transducing 3 (Gnat3),34 and aromatic L-amino acid decarboxylase (Aadc),50 which were used as markers of types I, II, and III; type I; type II; type II; and type III, respectively. Znt3 mRNA was expressed in the isolated taste buds of rat circumvallate papillae (Figure 3).
ZnT3-immunoreactivity was detected in the taste buds of circumvallate papillae using immunohistochemistry; ZnT3- immunopositive cells showed a spindle or pyriform morphology (Figure 4). By contrast, the antigen pre-adsorbed group and negative controls without the anti-ZnT3 antibody did not exhibit fluorescence signals (Figure 4 B,C), demonstrating the specificity of the anti-ZnT3 antibody. These results indicate that ZnT3 is expressed in the taste buds of rat circumvallate papillae.
The localization of ZnT3 in taste buds was assessed using coimmunofluorescence staining with taste cell markers. ZnT3- immunoreactivity partly overlapped with the PLC-β2-signals detected in taste cells with spindle or pyriform morphology (arrowheads in Figure 5B and Supplementary Figure 4B). ZnT3- immunoreactivity was detected in a part of AADC-positive type III cells, with a spindle morphology (arrowheads in Figure 5C and Supplementary Figure 4C), whereas ZnT3-immunoreactivity was not detected in NTPDase 2 type I taste cell marker-positive taste cells (Figure 5A and Supplementary Figure 4A). As shown in Figure 5D, ZnT3-positive cells were observed in type I, II, and III taste cells (6.3±1.2%, 47.8±15.4%, and 42.2±15.6%, respectively). These results suggest that ZnT3 is expressed in type II and III taste cells rather than in type I taste cells.
Zinc release from isolated taste cells by taste stimuli
TRPA1 is reportedly activated upon Zn2+ influx through TRPA1 and binding of Zn2+ to its intracellular domain,47 suggesting that TRPA1-expressing cells are able to detect extracellular zinc. Thus, TRPA1-expressing cells were validated as extracellular zincsensor cells (Supplementary Figures 1 and 2). First, in HEK293T/hTRPA1 stable cells, intracellular Ca2+ levels were increased upon treatment with AITC, a TRPA1 agonist. The increase was inhibited by pretreatment with HC-030031, a TRPA1 antagonist, or in Ca2+-free conditions (Supplementary Figure 1). Next, zinc treatment increased the intracellular Ca2+ levels in HEK293T/hTRPA1 stable cells compared with those in HEK296T cells (mock), whereas Ca2+-free conditions completely abolished the increase in intracellular Ca2+ levels (Supplementary Figure 2). These results indicate that HEK293T/hTRPA1 stable cells are permeable to zinc without affecting the channel functionality of TRPA1 and are useful zinc-sensor cells for evaluating extracellular zinc levels. We examined whether zinc was released from isolated taste cells by treating zinc-sensor cells with taste mixtures (Figure 6A). When isolated taste cells were treated with a taste mixture, the percentage of zinc-responding cells exhibiting greater than 2-fold increases in intracellular Ca2+ level compared with basal levels was 18.00±6.36% (mean ± SD, n=5; Figure 6 B,C; Supplementary Figure 3B). With 100 μM magnesium-ethylenediaminetetraacetic acid (MgEDTA), used as an extracellular zinc chelator, the percentage of zinc-responding cells decreased significantly (8.05±4.46%; mean ± SD, n=3; Figure 6 B,C; Supplementary Figure 3C), whereas 100 μM zinc-ethylenediaminetetraacetic acid (ZnEDTA), which has no zinc-chelating ability, did not affect the response (12.68±3.67%; mean ± SD, n=5; Figure 6 B,C; Supplementary Figure 3D). By contrast, the percentages of zincresponding cells under conditions of taste mixture treatment alone in the absence of taste cells and non-taste stimulus in the presence of taste cells were 0.11±0.19% and 3.26±3.61% (mean ± SD, n=3), respectively (Figure 6 B,C; Supplementary Figure 3 A,E). Thus, these results suggest that taste cells treated with tastants release zinc into the extracellular space.
Figure 1.
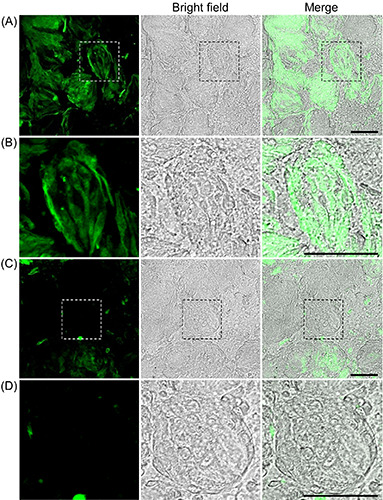
Fluorescence images of endogenous zinc in taste cells from the taste buds of rat circumvallate papillae. A) ZnAF-2 DA, a membrane- permeable zinc indicator, was loaded into taste bud-containing epithelial tissues at the circumvallate papillae. B) Higher magnification version of (A). C) Rats were pretreated with DEDTC, a zinc chelator, 80 min before perfusion with saline solution, and then taste bud-containing epithelial tissues were treated with ZnAF-2 DA. D) Higher-magnification version of (C). Data are presented as a representative image of at least three independent experiments. Scale bar: 50 mm.
Figure 2.
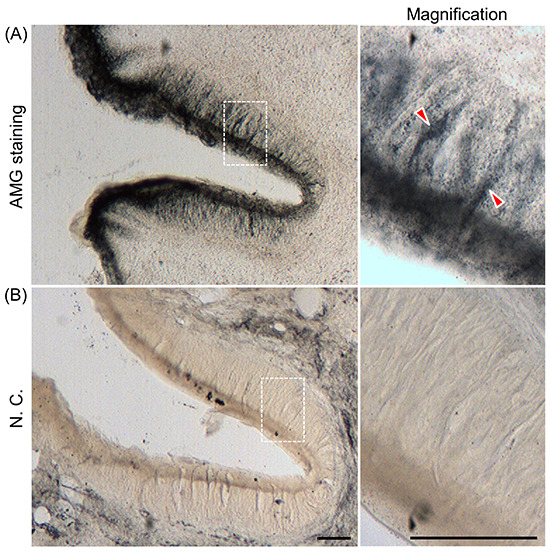
AMG staining in taste cells from the taste buds of rat circumvallate papillae. A) Distribution of AMG staining in circumvallate papillae is shown; the right panel shows a magnified version of the image in the dotted box; AMG staining was observed in taste cells (arrowheads). B) As a negative control (NC), rats were pretreated with DEDTC 1 h before injecting sodium selenite solution to chelate heavy metals; the right panel shows a magnified version of the image in the dotted box. Data are presented as a representative image of at least three independent experiments. Scale bar: 50 mm
Figure 3.
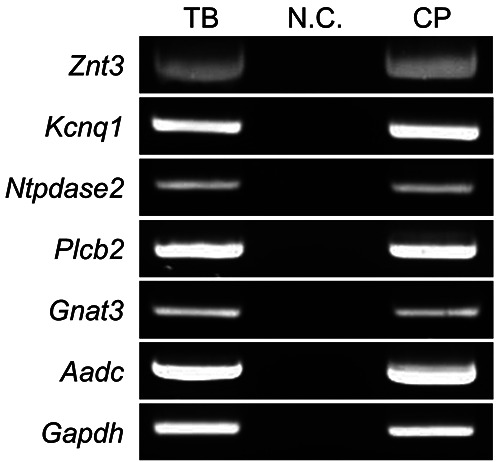
mRNA expression of Znt3 in isolated taste buds. Znt3 expression was examined using semi-quantitative RT-PCR. TB, NC, and CP indicate isolated taste buds, negative control (H2O; template in place of the cDNA), and taste bud-containing epithelial tissues (circumvallate papillae), respectively. Data are presented as a representative image of three independent experiments. Kcnq1, Ntpdase2, Plcb2, Gnat3, and Aadc were used as taste cell markers of types I, II, and III; type I; type II; type II; and type III, respectively.
Figure 4.
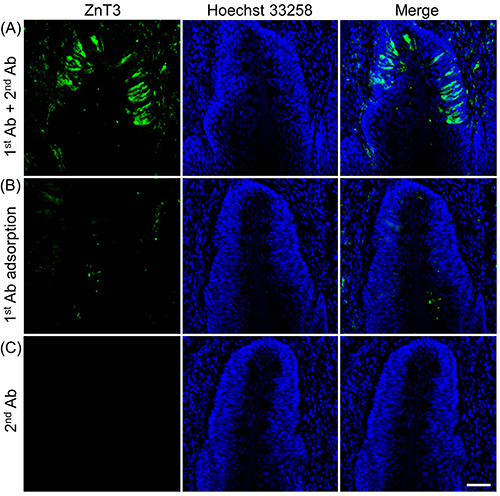
Immunohistochemical analysis of ZnT3 expression in the cross-sections of rat circumvallate papillae. A) Representative laser scanning microscopy images of immunohistochemistry with ZnT3 in the circumvallate papillae; the nuclei were counterstained with Hoechst 33258 (blue, 10 mg/mL). B) Immunostaining with an anti-ZnT3 antibody (1st Ab) adsorbed by the immunogen peptide containing the same epitope sequence to generate the antibody. C) The 2nd antibody was used alone as a negative control. Data are presented as a representative image of three independent experiments. Scale bar: 50 mm.
Figure 5.
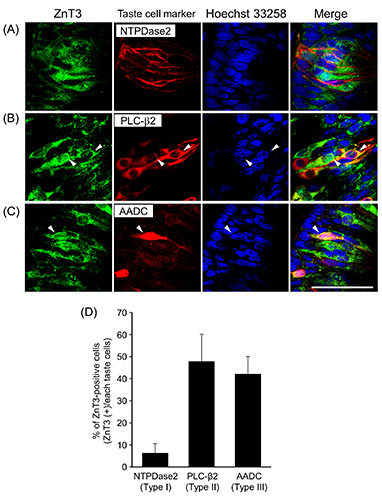
Immunohistochemical localization of ZnT3 and NTPDase2, PLC-b2, or AADC in cross-sections cut through the circumvallate papillae. Representative photomicrographs for double staining of ZnT3 (green) and the type I cell marker NTPDase2 (A; red), type II cell marker PLC-b2 (B; red), or type III cell marker AADC (C; red) are shown. Arrowheads show the colocalization of ZnT3 and taste cell markers in the cell bodies of taste cells. Data are presented as a typical image of three independent experiments. Scale bar: 50 mm. Immunopositive rates of ZnT3 in NTPDase2-, PLC-beta2-, and AADC-positive cells (D). Each value represents the mean ± SD (n=3).
Figure 6.
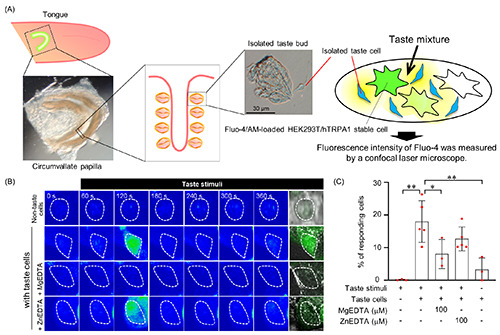
Zinc release from isolated taste cells upon taste stimulation. A) Schematic overview of the experiment to detect released zinc from isolated taste cells is shown. B) Representative images of Fluo-4/AM-loaded HEK293T/hTRPA1 stable (zinc-sensitive) cells are shown on the rainbow scale; the white dotted circle shows Fluo-4/AM-loaded HEK293T/hTRPA1 stable cells. C) Summary of the percentage of responding cells. Fluo-4/AM-loaded HEK293T/hTRPA1 stable cells were considered responders when [Ca2+]i increased more than 2-fold from the basal [Ca2+]i levels before taste stimuli. Each bar represents mean ± SD (n=3–5). *p<0.05; **p<0.01.
Discussion
In this study, we sought to elucidate the role of zinc in taste buds. Our key findings are as follows: i) zinc accumulates in the taste cells of rat circumvallate papillae; ii) ZnT3 is expressed in type II and type III taste cells; iii) treating isolated taste cells with tastants induces the activation of hTRPA1, expressed by HEK293T cells; however, this activation is blocked by pretreatment with an extracellular zinc chelator. Thus, we suggest that type II and type III taste cells release zinc into the extracellular space upon stimulation with tastants. In addition, zinc may function as a transmitter or modulator of taste signaling in taste cells.
In the hippocampus, ZnT3, expressed in presynaptic vesicles of glutamatergic neurons, accumulates zinc in presynaptic vesicles. Upon stimulation, as a neurotransmitter, zinc is released into the synaptic cleft through depolarization-induced exocytosis owing to an increase in intracellular Ca2+ levels.10,13 In the circumvallate papillae, we found that type III taste cells expressed ZnT3 (Figure 5). Because type III taste cells play crucial roles in synaptic transmission via exocytosis of synaptic vesicles,27,32 zinc may accumulate in vesicles of ZnT3-positive taste cells and be released via exocytosis as a signaling molecule. Additionally, zinc released from synaptic vesicles activates GPR39, which is expressed in the hippocampus and is an ionic zinc-sensing receptor.51 However, whether GPR39 receptors are expressed in gustatory nerve fibers remains to be determined.
Sour tastants induce 5-HT-release from type III taste cells,26,33 whereas sweet and bitter tastants indirectly cause 5-HT-release via actions of type III taste cells by ATP release from type II taste cells.52 In this study, the taste mixture containing sweet, bitter, and umami tastants evoked zinc release from taste cells, and activation of zinc-sensor cells was abolished by treatment with a zinc chelator (Figure 6). Thus, zinc and 5-HT may be released from type III taste cells via taste mixture-evoked ATP release from type II taste cells.
We also found that taste cells released zinc in response to taste stimuli (Figure 6). In taste buds, transient receptor potential melastatin 5 (TRPM5) is expressed in type II taste cells. It participates in regulating ATP release following taste stimuli, such as sweet, bitter, and umami tastants.31,53-58 The released ATP transmits signals by activating P2X2/P2X3 receptors on nerve terminals.24 Uchida et al. reported that extracellular zinc dose-dependently inhibits TRPM5 activation,59 suggesting that zinc released from taste cells upon taste stimuli might regulate taste cell depolarization by inhibiting the channel activity of TRPM5. By contrast, zinc is an allosteric modulator that enhances ATP responses via P2X2 receptors. 12,60,61 Therefore, it is considered that zinc released from taste cells upon taste stimulation may play a critical role in fine-tuning taste signaling by regulating ATP release and response in taste buds. More detailed investigations are warranted to decipher the relationship with other signaling molecules - not only with ATP - and clarify the roles of zinc in the regulation of taste signaling.
Overall, our findings indicate that ZnT3, expressed by type II and III taste cells, may mediate zinc accumulation in presynaptic vesicles, and taste stimuli-induced zinc release from taste cells may function as a transmitter or modulator for taste signaling. Further experiments using taste cells lacking ZnT3 would clarify the mechanism underlying fine-tuning of taste signaling through zinc released into the extracellular space of taste buds.
Acknowledgments
The authors thank Ms. Saki Matsumoto, a member of the Environmental Biochemistry laboratory (Kyoto Pharmaceutical University, Kyoto, Japan), and Ms. Monami Miyazaki, a member of the Integrative Pharmaceutical Sciences laboratory (Setsunan University, Osaka, Japan), for their help and support with the experiments associated with this research. The authors thank Editage (Cactus Communications, Tokyo, Japan), a professional English proofreading service, for editing the manuscript.
Funding Statement
Funding: This work was supported by JSPS KAKENHI Grant Number JP18K06673 and The Salt Science Research Foundation (1828).
References
- 1.Atkin-Thor E, Goddard BW, O’Nion J, Stephen RL, Kolff WJ. Hypogeusia and zinc depletion in chronic dialysis patients. Am J Clin Nutr 1978;31:1948-51. [DOI] [PubMed] [Google Scholar]
- 2.Heyneman CA. Zinc deficiency and taste disorders. Ann Pharmacother 1996;30:186-7. [DOI] [PubMed] [Google Scholar]
- 3.Mahajan SK, Prasad AS, Lambujon J, Abbasi AA, Briggs WA, McDonald FD. Improvement of uremic hypogeusia by zinc: a double-blind study. Am J Clin Nutr 1980;33:1517-21. [DOI] [PubMed] [Google Scholar]
- 4.Weismann K, Christensen E, Dreyer V. Zinc supplementation in alcoholic cirrhosis. A double-blind clinical trial. Acta Med Scand 1979;205:361-6. [DOI] [PubMed] [Google Scholar]
- 5.Hewlings S, Kalman D. A Review of zinc-l-carnosine and its positive effects on oral mucositis, taste disorders, and gastrointestinal disorders. Nutrients 2020;12:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou HC, Chien CL, Huang HL, Lu KS. Effects of zinc deficiency on the vallate papillae and taste buds in rats. J Formos Med Assoc 2001;100:326-35. [PubMed] [Google Scholar]
- 7.Hamano H, Yoshinaga K, Eta R, Emori Y, Kawasaki D, Iino Y, et al. Effect of polaprezinc on taste disorders in zinc-deficient rats. Biofactors 2006;28:185-93. [DOI] [PubMed] [Google Scholar]
- 8.Jakinovich W, Osborn DW. Zinc nutrition and salt preference in rats. Am J Physiol 1981;241:R233-9. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Clausell J, Danscher G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res 1985;337:91-8. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson CJ, Moncrieff DW. Zinc-containing neurons. Biol Signals 1994;3:127-39. [DOI] [PubMed] [Google Scholar]
- 11.Haug FM. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie 1967;8:355-68. [DOI] [PubMed] [Google Scholar]
- 12.Ma B, Ruan HZ, Burnstock G, Dunn PM. Differential expression of P2X receptors on neurons from different parasympathetic ganglia. Neuropharmacology 2005;48:766-77. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Sakurada N, Ando M, Kanno S, Oku N. Facilitation of zinc influx via AMPA/kainate receptor activation in the hippocampus. Neurochem In. 2009;55:376-82. [DOI] [PubMed] [Google Scholar]
- 14.Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK. Zinc effects on NMDA receptor gating kinetics. Biophys J 2011;100:1910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol 2005;566:747-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature 1987;328:640-3. [DOI] [PubMed] [Google Scholar]
- 17.Farbman AI. Fine structure of the taste bud. J Ultrastruct Res 1965;12:328-50. [DOI] [PubMed] [Google Scholar]
- 18.Takeda M, Hoshino T. Fine structure of taste buds in the rat. Arch Histol Jpn 1975;37:395-413. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Dzowo YK, Wilson CE, Russell RL, Kidd GJ, Salcedo E, et al. Three-dimensional reconstructions of mouse circumvallate taste buds using serial blockface scanning electron microscopy: I. Cell types and the apical region of the taste bud. J Comp Neurol 2020;528:756-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto- ATPase of type I cells in taste buds. J Comp Neurol 2006;497:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol 2009;517:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci 2000;12:3163-71. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 2010;190:285-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005;310:1495-9. [DOI] [PubMed] [Google Scholar]
- 25.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 2007;26:657-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci 2005;25:843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci 2010;30:14695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herness S, Zhao FL, Kaya N, Lu SG, Shen T, Sun XD. Adrenergic signalling between rat taste receptor cells. J Physiol 2002;543:601-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci 2011;31:5782-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol 2002;87:2643-9. [DOI] [PubMed] [Google Scholar]
- 31.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013;495:223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci 2006;26:3971-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 2009;29:13909-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol 2000;425:139-51. [DOI] [PubMed] [Google Scholar]
- 35.Liman ER, Kinnamon SC. Sour taste: receptors, cells and circuits. Curr Opin Physiol 2021;20:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, et al. An evolutionarily conserved gene family encodes protonselective ion channels. Science 2018;359:1047-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Jin H, Zhang W, Ding C, O’Keeffe S, Ye M, et al. Sour sensing from the tongue to the brain. Cell 2019;179:392-402.e15. [DOI] [PubMed] [Google Scholar]
- 38.Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC, Liman ER. Cellular and neural responses to sour stimuli require the proton channel otop1. Curr Biol 2019;29:3647-56.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinnamon JC, Taylor BJ, Delay RJ, Roper SD. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol 1985;235:48-60. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto S, Ueda H, Kagawa H. Immunocytochemistry on the localization of 5-hydroxytryptamine in monkey and rabbit taste buds. Acta Anat (Basel) 1987;128:80-3. [DOI] [PubMed] [Google Scholar]
- 41.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 2001;440:97-108. [DOI] [PubMed] [Google Scholar]
- 42.Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. J Comp Neurol 2007;505:302-13. [DOI] [PubMed] [Google Scholar]
- 43.Nishida K, Dohi Y, Yamanaka Y, Miyata A, Tsukamoto K, Yabu M, et al. Expression of adenosine A2b receptor in rat type II and III taste cells. Histochem Cell Biol 2014;141:499-506. [DOI] [PubMed] [Google Scholar]
- 44.Danscher G, Haug FM, Fredens K. Effect of diethyldithiocarbamate (DEDTC) on sulphide silver stained boutons. Reversible blocking of Timm’s sulphide silver stain for “heavy” metals in DEDTC treated rats (light microscopy). Exp Brain Res 1973;16:521-32. [DOI] [PubMed] [Google Scholar]
- 45.Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry 1981;71:1-16. [DOI] [PubMed] [Google Scholar]
- 46.Danscher G, Stoltenberg M. Zinc-specific autometallographic in vivo selenium methods: tracing of zinc-enriched (ZEN) terminals, ZEN pathways, and pools of zinc ions in a multitude of other ZEN cells. J Histochem Cytochem 2005;53:141-53. [DOI] [PubMed] [Google Scholar]
- 47.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 2009;5:183-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohmoto M, Matsumoto I, Misaka T, Abe K. Taste receptor cells express voltage-dependent potassium channels in a cell age-specific manner. Chem Senses 2006;31:739-46. [DOI] [PubMed] [Google Scholar]
- 49.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol 2004;468:311-21. [DOI] [PubMed] [Google Scholar]
- 50.Seta Y, Kataoka S, Toyono T, Toyoshima K. Immunohistochemical localization of aromatic L-amino acid decarboxylase in mouse taste buds and developing taste papillae. Histochem Cell Biol 2007;127:415-22. [DOI] [PubMed] [Google Scholar]
- 51.Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, et al. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci 2009;29:2890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 2007;104:6436-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang YA, Roper SD. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol 2010;588:2343-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, et al. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol 2010;104:896-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 2002;5:1169-76. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 2003;100:15160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 2003;112:293-301. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 2007;27:5777-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchida K, Tominaga M. Extracellular zinc ion regulates transient receptor potential melastatin 5 (TRPM5) channel activation through its interaction with a pore loop domain. J Biol Chem 2013;288:25950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wildman SS, King BF, Burnstock G. Zn2+ modulation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br J Pharmacol 1998;123:1214-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, et al. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J Neurophysiol 1999;81:2088-94. [DOI] [PubMed] [Google Scholar]


