Abstract
Context
Hysterosalpingography (HSG) with oil-soluble contrast medium (OSCM) improves pregnancy rates. However, OSCM has high iodine content and long half-life, leading to potential iodine excess.
Objective
This work aimed to determine the pattern of iodine excess after OSCM HSG and the effect on thyroid function.
Methods
A prospective cohort study was conducted of 196 consecutive consenting eligible women without overt hypothyroidism or hyperthyroidism. All completed the study with compliance greater than 95%. Participants underwent OSCM HSG (Auckland, 2019-2021) with serial monitoring of thyrotropin (TSH), free thyroxine (FT4), and urine iodine concentration (UIC) for 24 weeks. The main outcome measure was the development of subclinical hypothyroidism (SCH), defined as a nonpregnant TSH greater than 4 mIU/L with normal FT4 (11-22 pmol/L) in those with normal baseline thyroid function.
Results
Iodine excess (UIC ≥ 300 μg/L) was almost universal (98%) with UIC peaking usually by 4 weeks. There was marked iodine excess, with 90% and 17% of participants having UIC greater than or equal to 1000 μg/L and greater than 10 000 μg/L, respectively. Iodine excess was prolonged with 67% having a UIC greater than or equal to 1000 μg/L for at least 3 months. SCH developed in 38%; the majority (96%) were mild (TSH 4-10 mIU/L) and most developed SCH by week 4 (75%). Three participants met the current treatment guidelines (TSH > 10 mIU/L). Thyroxine treatment of mild SCH tended to improve pregnancy success (P = .063). Hyperthyroidism (TSH < 0.3 mIU/L) occurred in 9 participants (5%).
Conclusion
OSCM HSG resulted in marked and prolonged iodine excess. SCH occurred frequently with late-onset hyperthyroidism occasionally. Regular thyroid function tests are required for 6 months following this procedure.
Keywords: iodine, hysterosalpingography, oil-soluble contrast, thyroid, pregnancy
Although oil-soluble contrast medium (OSCM) hysterosalpingography (HSG) was used for the assessment of tubal patency for many decades, it had largely been superseded by the use of water-soluble contrast medium (WSCM) or other methods (1, 2). Recently, OSCM HSG has been shown to improve pregnancy rates in women with unexplained infertility, with reported live birth rates between 23.9% to 39.7% (3–8). A recent large multicenter trial (H2Oil study) has confirmed the differential improvement in ongoing pregnancy rates with OSCM compared to WSCM (39.7% vs 29.1%) (4). While OSCM HSG is becoming a favored procedure in women with unexplained infertility, there is a need to establish its safety profile. The long history of OSCM use without major complications (9) provides a background for its safety and continued usage. However, concerns remain regarding the effect of iodine excess in women following OSCM exposure. The prototype OSCM used in HSGs, Lipiodol Ultra Fluide (Guerbet) has a high iodine content (480 000 μg/mL) and long half-life (50 days) (10). Ethiodol (Savage Laboratories), an OSCM previously used until 2010 in some countries, has an iodine content similar to Lipiodol (11). This is in contrast to the lower iodine content (250-350 mg/mL) (8, 12, 13) and shorter half-life (2-3 days) (10) of WSCM.
Each milliliter of retained contrast following an OSCM HSG would equate to an iodine release of more than 1100 µg/day (upper threshold of iodine intake without notable adverse events) (14) for more than 150 days, based on its iodine content and half-life. Thus, it is important to understand the levels and timing of iodine excess, and their potential effects on thyroid function.
While earlier studies have been published focusing on iodine excess and thyroid function, these were small (15) or retrospective (16, 17). Thus, the aim of this study was to define the safety profile of OSCM HSG more accurately in the context of iodine excess, particularly its effects on thyroid function.
Materials and Methods
This prospective, longitudinal cohort study of women undergoing OSCM HSG, the “Safety and Efficacy of Lipiodol in Fertility Investigations” (SELFI) study, was conducted in the Auckland region (New Zealand) in 2019 to 2021. The study was approved by the Northern B Health and Disability Ethics Committee (Ministry of Health; 19/NTB/52) and registered with the Australian New Zealand Clinical Trials Registry (ANZCTR: 12620000738921). This study is reported in line with the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines, and all participants provided written informed consent.
A total of 196 consecutive consenting women with infertility, primary (≥ 12 months of unsuccessfully attempting pregnancy with no live births [18]) or secondary (≥ 12 months of unsuccessfully attempting pregnancy with a previous live birth [18]), and planning to undergo OSCM HSG were enrolled. The details of sample size calculation and recruitment process are available in the published protocol (19). Inclusion and exclusion criteria are listed here, with the main inclusion criterion being referral for an OSCM HSG from a fertility specialist.
The inclusion criteria included the following:
1. Being of reproductive age (ie, 15-49 years); AND
2. Having primary or secondary infertility; AND
3. Referred for OSCM HSG in the Auckland region; AND
4a. Have probable tubal patency, based on the criterion of “low risk for preexisting tubal damage” as per Dreyer et al (4); OR
4b. If known to have damage to one or both fallopian tubes, having a referral for an OSCM HSG advised by the fertility specialist (for fertility enhancement from uterine bathing effect of Lipiodol); AND
5a. Have baseline normal thyroid function (ie, normal nonpregnant free thyroxine [FT4] and thyrotropin [TSH]); OR
5b. Have baseline subclinical hypothyroidism (defined as TSH > 4 mIU/L and normal FT4 levels), and not be on treatment with levothyroxine.
The exclusion criteria included the following:
1. Known contraindication for OSCM use (poppy seed or poppy seed oil allergy, or history of reactions to iodine); OR
2. Active thyroid disease, such as overt hypothyroidism, hyperthyroidism, or thyroid cancer, past history of thyroid cancer or thyroid surgeries; OR
3. Current treatment/recent treatment (within 3 months) with levothyroxine or antithyroid medications.
4. On medications known to affect thyroid function or iodine metabolism. These include lithium, amiodarone, tyrosine kinase inhibitors, interferons, immunomodulators, and checkpoint inhibitors.
5. Have used water-soluble contrast within 3 months before the OSCM HSG procedure; OR
6. Have used OSCM or any other oil-soluble contrast medium within 6 months before the procedure.
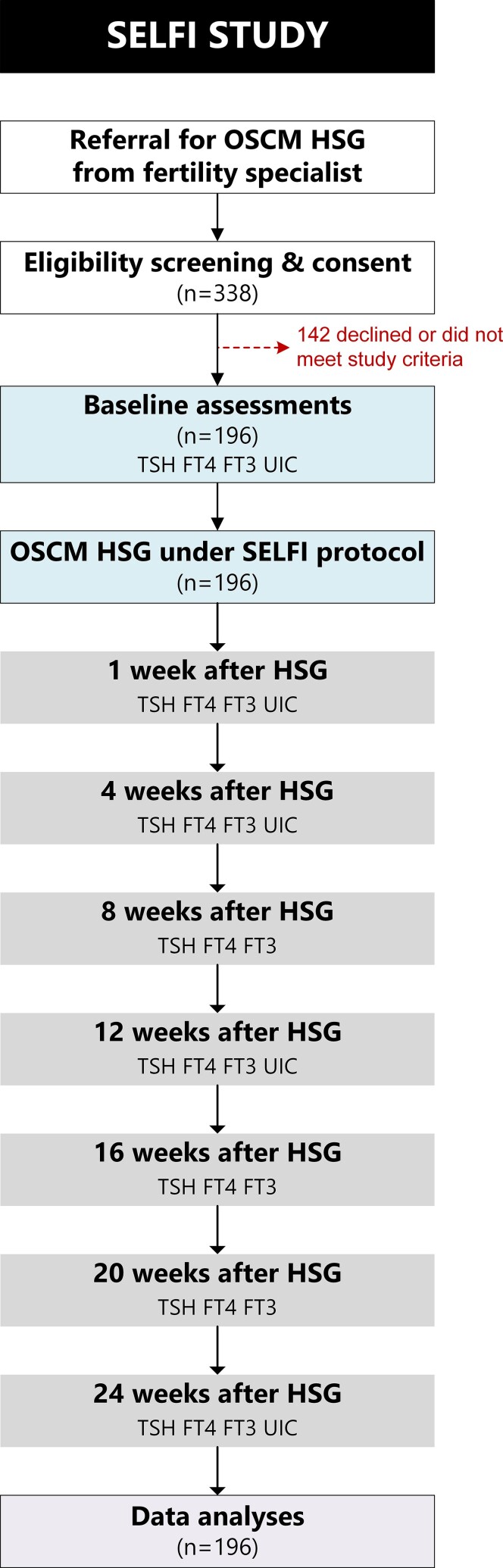
Participants underwent an OSCM HSG as per the SELFI protocol, including baseline and follow-up assessments (Fig. 1).
Figure 1.
Diagram showing the flow of participants throughout the SELFI Study and the timing of the various clinical investigations. FT3, free 3,5,3′-triiodothyronine; FT4, free thyroxine; HSG, hysterosalpingography; OSCM, oil-soluble contrast medium; SELFI, Safety and Efficacy of Lipiodol in Fertility Investigations; TSH, thyrotropin; UIC, urine iodine concentration.
The participants had baseline TSH, FT4, 3,5,3′-free triiodothyronine (FT3), thyroid peroxidase (TPO) antibody, and urine iodine concentration (UIC) measured before the HSG. UIC was obtained as a spot fasting first-morning urine sample.
All participants underwent an HSG with Lipiodol Ultra Fluide following a uniform SELFI protocol (19), which was performed in 2 private radiology clinics by 3 experienced radiologists (includes J.M.P., R.J.S.) and 1 infertility specialist (N.P.J.).
A delayed pelvic radiograph was obtained after 45 minutes, which was used for a semiqualitative assessment of OSCM peritoneum retention by one of the radiologists. Retention levels were graded as “none,” “minor,” “moderate,” or “extensive.” The clinicians undertaking the procedure and assessing the radiograph were blinded to the blood and urine investigation results. Participants had serial monitoring of TSH, FT4, and FT3 following OSCM HSG at weeks 4, 8, 12, 16, 20, and 24. UIC was measured at weeks 1, 4, 12, and 24. The follow-up tests were performed the exact day in most situations, with a margin of 2 days on either side of the organized follow-up date allowed in exceptional situations. Serum TSH, FT4, and FT3 were measured with an electrochemiluminescence immunoassay using Siemens ADVIA Centaur XP analyzer and the coefficient of variation for these assays was no more than 5% (20). Fasting first morning urine samples were used to measure UIC by inductively coupled plasma mass spectrometry (ICP-MS) using Agilent 7700 (coefficient of variation = 10.8%).
More detailed description of the HSG procedure, post-HSG radiograph, and blood and urine investigations are available in the published protocol (19). If abnormal thyroid function was detected (normal reference range: TSH 0.3-4.0 mIU/L and FT4 11-22 pmol/L), treatment was managed by the referring fertility clinician based on thyroid function tests, pregnancy status, and any plans for additional fertility treatment. Pregnancy was defined as a positive human chorionic gonadotropin. There was documentation of pregnancy and thyroid treatment throughout the study, as these would affect subsequent thyroid function test results. When analyzing TSH, FT4, and FT3 data, we excluded time points after pregnancy (to account for the associated normal decline in TSH) and after initiation of T4 replacement therapy, where relevant.
Abnormal thyroid function was defined as the following (19):
overt hypothyroidism—TSH greater than 4 mIU/L and low FT4 (< 11 pmol/L);
subclinical hypothyroidism (SCH)—TSH greater than 4 mIU/L and normal FT4 (11-22 pmol/L), with mild SCH having TSH 4 to 10 mIU/L;
overt hyperthyroidism—TSH less than 0.3 mIU/L and high FT4 (> 22 pmol/L); and
subclinical hyperthyroidism—TSH less than 0.3 mIU/L and normal FT4 (11-22 pmol/L)
Iodine status was defined based on UIC as per World Health Organization criteria (21): deficient (< 100 μg/L), sufficient (100-299 μg/L), and excessive (≥ 300 μg/L). These definitions by the World Health Organization are for population groups and not for individuals, with median UIC suggesting iodine status of the population (22). New Zealand soil is deficient in iodine, and fortification of iodized salt in bread is mandatory (23). However, mild iodine deficiency persists especially in women (24, 25) and recommended iodine supplementation is prescribed in women planning pregnancy (14). In our study, we allowed the women to continue the routine iodine supplements (150 μg iodine/tablet) or multivitamin supplements (220 μg iodine/tablet) prescribed by their respective fertility specialists. Iodine supplementation, if used, was started before this study. The recommended dosing would prevent iodine deficiency but not lead to iodine excess and the effect observed on the baseline samples. It would not have affected the interpretation of iodine excess from OSCM HSG.
Statistical Analysis
The instilled OSCM volume was compared between peritoneal retention groups using a Dwass, Steel, Critchlow–Fligner multiple comparison analysis based on pairwise 2-sample nonparametric Wilcoxon tests (26). The UIC at individual time points were then compared between the 3 groups with some level of peritoneal OSCM retention with the group without any observed retention, using general linear models adjusted for the baseline UIC concentrations. For these analyses, the pairwise adjusted mean differences are reported with respective Bonferroni-corrected 95% CIs, with α = .017 (ie, 0.05 ÷ 3) (27). The UIC time-weighted area under the curve (TwAUC) was also calculated for all participants with at least 3 out of 4 post-HSG UIC values measured, using the following formula:
where t0 and tn were the time in weeks of the participant's first and last UIC measurements, respectively. TwAUC data were also compared between the peritoneal retention groups, as described earlier.
TSH levels at individual time points after OSCM HSG were compared to baseline using paired t tests.
Pregnancy rates in women with SCH who were treated or not with T4 were compared with Fisher exact tests. The associations between TSH levels and UIC at baseline and the likelihood of developing SCH, hyperthyroidism, or pregnancy were assessed with generalized linear models using a modified Poisson procedure with robust error variances (28). Models also were adjusted for the participant's age group at baseline (< 35, 35-39.9, or ≥ 40 years) and the instilled OSCM volume, with the outcome likelihood reported as adjusted relative risks (aRR) and 95% CI.
If necessary, study parameters were log-transformed to approximate a normal distribution, with back-transformed least-squares means (adjusted means) and 95% CI reported. UIC TwAUC calculations and data analyses were performed using SAS v9.4 (SAS Institute). All statistical tests were 2-tailed.
Results
Study Population
Table 1 describe the demographic characteristics of the study population (n = 196). Participants had a mean age of 36 years, were mostly of New Zealand European/European descent (60.2%), with a mean body mass index of 24.6. Seventy-five percent had primary infertility, mostly idiopathic or unexplained (66.3%); 71.9% of participants had no other fertility treatment (see Table 1). Median (interquartile range) UIC of the entire cohort was 152 μg/L (89-228 μg/L). At baseline, 4.1% of participants (8/196) had undiagnosed SCH; 29.2% were iodine deficient, 55.5% iodine sufficient, and 15.3% had iodine excess.
Table 1.
Demographic and clinical characteristics of the study population in the SELFI Study at baseline before hysterosalpingography
| Characteristic | Parameter | Level | n | Mean ± SD or % | Median (Q1-Q3) | Range |
|---|---|---|---|---|---|---|
| Demography | Age, y | 196 | 35.9 ± 4.4 | 36.2 (32.8-39.3) | 26.0-49.2 | |
| Ethnicity | NZ European/European | 118 | 60.2% | |||
| Indian | 37 | 18.9% | ||||
| Other Asian | 32 | 16.3% | ||||
| Mãori | 5 | 2.6% | ||||
| Pasifika | 4 | 2.0% | ||||
| Anthropometry | Height, cm | 152 | 164.0 ± 8.0 | 164 (160-170) | 144-185 | |
| Weight, kg | 158 | 66.5 ± 11.8 | 65.0 (59.0-72.0) | 59.0-130.0 | ||
| BMI | 152 | 24.6 ± 3.9 | 23.9 (21.9-27.2) | 18.5-43.2 | ||
| Clinical | TSH, mIU/L | 196 | 1.9 ± 1.0 | 1.8 (1.3-2.5) | 0.4-7.1 | |
| Infertility cause | Idiopathic | 130 | 66.3% | |||
| Endometriosis | 37 | 18.9% | ||||
| PCOS | 15 | 7.7% | ||||
| Other | 14 | 7.1% | ||||
| Infertility type | Primary | 147 | 75.0% | |||
| Secondary | 49 | 25.0% | ||||
| Fertility treatment | None | 141 | 71.9% | |||
| IVF | 31 | 15.8% | ||||
| IUI | 19 | 9.7% | ||||
| Unknown | 5 | 2.6% | ||||
| OSCM volume used, mL | 196 | 6.2 ± 2.6 | 6 (4-8) | 2.0-20.0 |
Abbreviations: BMI, body mass index; IUI, intrauterine insemination; IVF, in vitro fertilization; NZ, New Zealand; OSCM, oil-soluble contrast medium; PCOS, polycystic ovary syndrome; Q1, quartile 1; Q3, quartile 3; SELFI, Safety and Efficacy of Lipiodol in Fertility Investigations; TSH, thyrotropin.
No participants were lost to follow-up, as all 196 women assessed at baseline completed the study. Compliance with study investigations was very high, as each of the 4 key parameters was measured in more than 95% of participants at all time points after HSG or missed no more than 1 sample (UIC: 96%; TSH: 97%; FT4: 97%; FT3: 95%).
Iodine Levels Post Hysterosalpingography
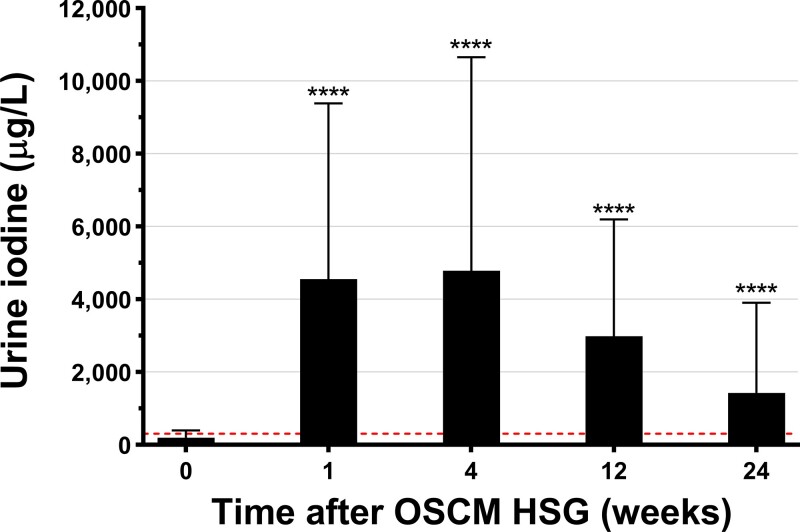
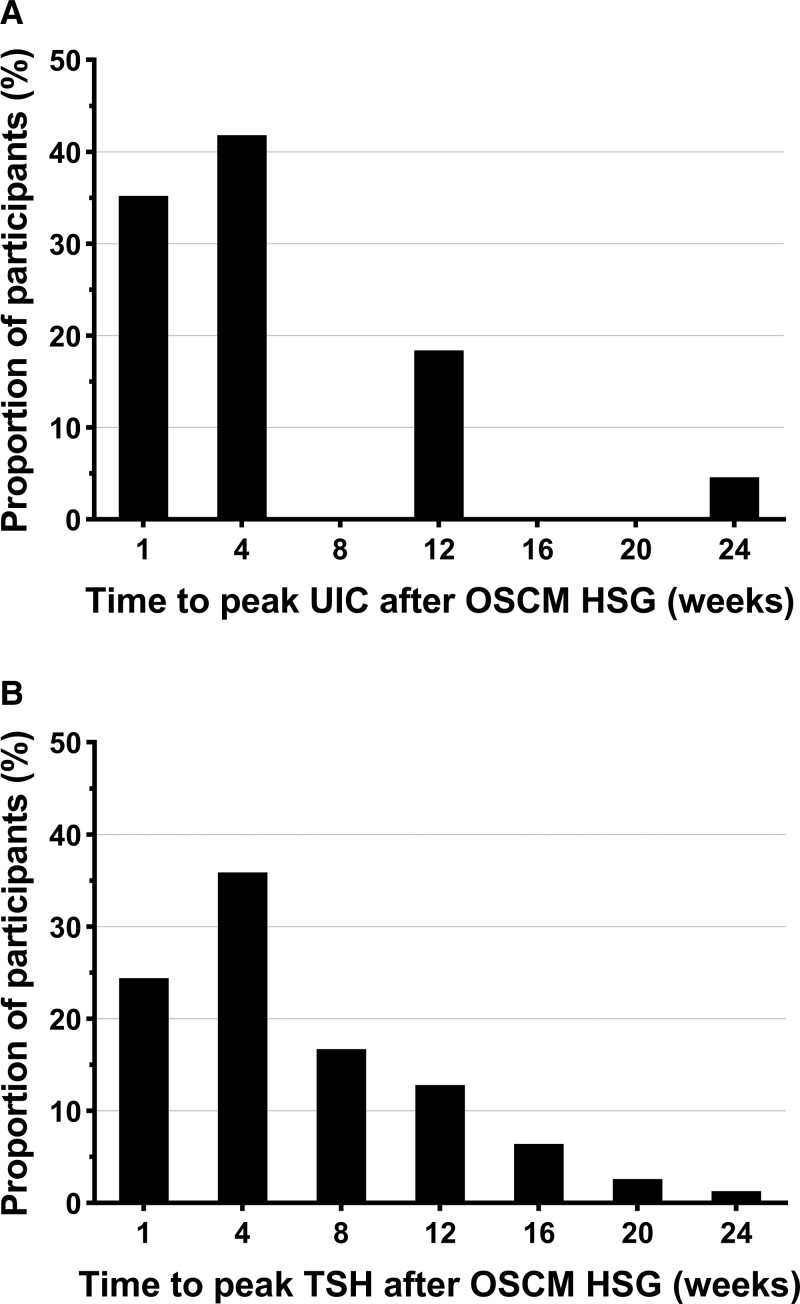
Iodine excess (UIC ≥ 300 µg/L) was almost a universal finding (98%) following the HSG. UIC peaked mostly at weeks 1 and 4 after the HSG, but even after 24 weeks the mean UIC was still 3-fold higher than the recommended normal range upper limit (Fig. 2). There was substantial variability in the magnitude and timing of peak iodine levels (Fig. 3A). A total of 90% and 17% of participants had at least one sample post HSG with UIC greater than or equal to 1000 μg/L and greater than 10 000 μg/L, respectively (Table 2). Notably, this iodine excess after HSG was persistent, as 67% had UIC measurements greater than or equal to 1000 μg/L for at least 3 months (see Table 2).
Figure 2.
Urine iodine concentration (UIC) at baseline and over 24 weeks (6 months) after oil-soluble contrast medium (OSCM) hysterosalpingography (HSG) in the SELFI Study. Data at each time point are means ± SD. ****P < .0001 for pairwise comparisons to baseline derived from paired t tests. Horizontal dashed line represents the upper UIC threshold for iodine excess (≥ 300 µg/L).
Figure 3.
Timing of peak for urine iodine concentrations (UIC) and thyrotropin (TSH) levels after oil-soluble contrast medium (OSCM) hysterosalpingography (HSG).
Table 2.
Proportion of participants in the SELFI Study with high levels of urine iodine concentrations recorded at baseline and after oil-soluble contrast medium (OSCM) hysterosalpingography (HSG) (n = 196)
| Assessment timing | Presence of urine iodine concentration above indicated threshold | ≥300 μg/L | ≥1000 μg/L | ≥10 000 μg/L | |
|---|---|---|---|---|---|
| Baseline | In baseline sample | Yes | 29 (14.8%) | 1 (0.5%) | nil |
| No | 154 (78.6%) | 182 (92.9%) | 183 (93.4%) | ||
| Unknown | 13 (6.6%) | 13 (6.6%) | 13 (6.6%) | ||
| After OSCM HSG | At least 1 sample at any time | Yes | 192 (98.0%) | 176 (89.8%) | 33 (16.8%) |
| No | 4 (2.0%) | 20 (10.2%) | 163 (83.2%) | ||
| ≥3 months | Yes | 166 (84.7%) | 132 (67.4%) | 1 (0.5%) | |
| No | 26 (13.3%) | 60 (2.0%) | 190 (96.9%) | ||
| Unknown | 4 (2.0%) | 4 (2.0%) | 5 (2.6%) | ||
| 6 months | Yes | 121 (61.7%) | 53 (27.0%) | nil | |
| No | 59 (30.1%) | 127 (64.8%) | 191 (97.5%) | ||
| Unknown | 16 (8.2%) | 16 (8.2%) | 5 (2.6%) |
Data are n (%).
Urine iodine concentrations were measured at four time points after OSCM HSG: weeks 1, 4, 12, and 24.
“Unknown” categories refer to participants with missing samples that prevented reliable calculation of a given rate.
Abbreviations: HSG, hysterosalpingography; OSCM, oil-soluble contrast medium; SELFI, Safety and Efficacy of Lipiodol in Fertility Investigations.
Peritoneal Oil-Soluble Contrast Medium Retention and Iodine Levels
Increased peritoneal OSCM retention (based on delayed radiograph of the pelvis) was associated with greater UIC levels (Supplementary Fig. S1 [29]; Supplementary Table S1 [30]). Iodine levels were highest at 4 weeks except in the group with no detected peritoneal retention (Supplementary Fig. S1 [29]). Compared to the “none” group, the greatest differences were observed at week 12, when UIC levels were 2.7, 6.7, and 12.5 times greater in the groups with “minor” (P = .001), “moderate” (P < .0001), and “extensive” (P < .0001) retention, respectively (Supplementary Fig. S1A [29]; Supplementary Table S1 [30]), with similar patterns observed for UIC TwAUC (Supplementary Fig. S1B [29]; Supplementary Table S1 [30]). Surprisingly, iodine levels also increased, albeit to a lesser level, in the “none” group, which defined those with bilateral fallopian tube occlusion (Supplementary Fig. S1 [29]; Supplementary Table S1 [30]), suggesting some OSCM retention without identifiable peritoneal spill.
Thyroid Hormone Levels Following Oil-Soluble Contrast Medium Hysterosalpingography
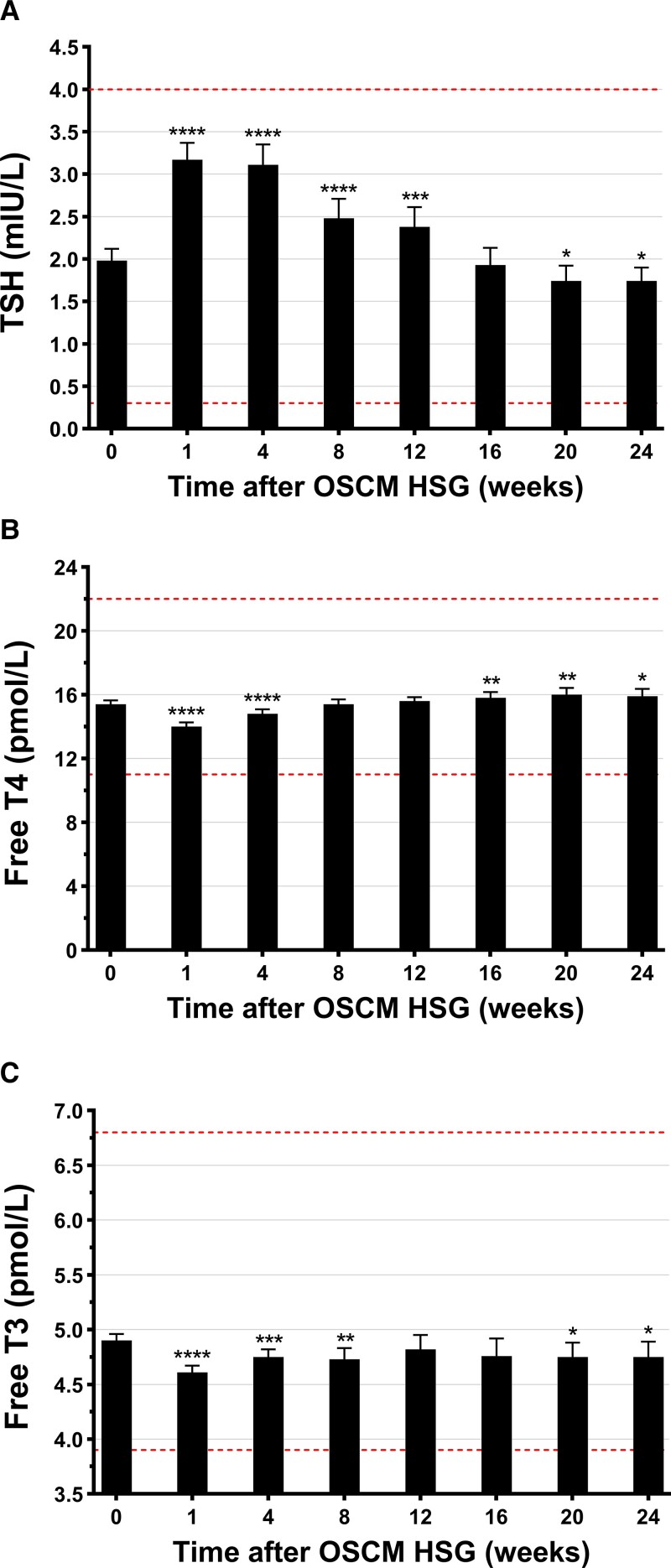
After HSG, TSH levels rose and remained elevated up to 12 weeks before decreasing to levels marginally lower than baseline (Fig. 4A). Timing to peak TSH varied between individuals, with the majority observed at week 4, although TSH peaked for some individuals as late as week 24 (Fig. 3B). Following HSG, mean TSH levels were 60% higher by week 1 (95% CI, 52%-68%; P < .0001) progressively decreasing thereafter but still 21% higher at week 12 (95% CI, 9%-32%; P < .001) (see Fig. 4A). TSH levels continued to decrease so that they were 12% lower at weeks 20 and 24 (95% CI, −21 to −3%; P = .011) (see Fig. 4A).
Figure 4.
Levels of A, thyrotropin (TSH); B, free thyroxine/tetraiodothyronine (T4); and C, free 3,5,3′-triiodothyronine (T3) at baseline and over the next 24 weeks (6 months) after oil-soluble contrast medium (OSCM) hysterosalpingography (HSG). Data at each time point are means ± 95% CI. *P < .05, **P < .01, ***P < .001, and ****P < .0001 from paired t tests for pairwise comparisons to baseline levels. Horizontal dashed lines represent the normal (reference) range for TSH, FT4 or FT3.
FT4 levels were consistently within the normal reference range after HSG. FT4 levels were 1.4 pmol/L lower than baseline at week 1 (95% CI, −1.6 to −1.2 pmol/L; P < .0001) and 0.6 pmol/L lower at week 4 (95% CI, −0.9 to −0.3 pmol/L; P < .0001) (see Fig. 4B); however, FT4 levels rose above baseline by week 16 (+0.4 pmol/L [95% CI, 0.1-0.8 pmol/L]; P = .005), remaining higher until week 24 (+0.5 pmol/L [95% CI, 0.1-0.9 pmol/L]; P = .027) (see Fig. 4B). After HSG, mean FT3 levels were 6% lower by week 1, persisting below baseline (but within the normal reference range) until week 24 (−3% [95% CI, −6% to −1%]; P = .027] (Fig. 4C).
Thyroid Dysfunction Following Soluble Contrast Medium Hysterosalpingography
The details of thyroid dysfunction following OSCM HSG in our participants are shown in Table 3. No women in the cohort developed overt hypothyroidism after HSG but SCH was common, occurring in 38%. Most cases of SCH developed not long after HSG, with 75% occurring by week 4% and 92% by week 12 (Supplementary Fig. S2A [31]). The peak TSH ranged between 4 and 10 mIU/L in 96% of those with SCH. Only 3 patients had TSH levels greater than 10 mIU/L (TSH range, 10.1-15.0 mIU/L), a recommended indication for T4 replacement treatment by the American Thyroid Association (ATA) guidelines (14). However, 60% of those with mild SCH also were treated with T4 by primary fertility specialists based on clinical discretion.
Table 3.
Incidence of thyroid dysfunction at baseline and within 6 months of oil-soluble contrast medium hysterosalpingography
| Thyroid dysfunction | Baseline | After HSGa |
|---|---|---|
| Subclinical hypothyroidism | 8/196 (4.1%) | 71/188 (37.8%)b |
| TSH 4-10 mIU/L | 8/8 | 68/71 |
| TSH > 10 mIU/L | Nil | 3/71 |
| Overt hypothyroidism | N/A | Nil |
| Subclinical hyperthyroidism | N/A | 4/196 (2.0%) |
| Overt hyperthyroidism | N/A | 5/196 (2.6%) |
Subclinical hypothyroidism: TSH > 4 mIU/L and FT4 ≥ 11 but ≤22 pmol/L at any assessment; overt hypothyroidism: TSH > 4 mIU/L and FT4 < 11 pmol/L at any assessment post HSG; subclinical hyperthyroidism: TSH < 0.3 mIU/L and FT4 ≥ 11 but ≤22 pmol/L at any assessment; and overt hyperthyroidism: TSH < 0.3 and FT4 > 22 pmol/L at any assessment.
Abbreviations: FT4, free thyroxine; HSG, hysterosalpingography; N/A, not applicable as subclinical and overt hypothyroidism were exclusion criteria for this study; TSH, thyrotropin.
Excluding the visits at or after a participant became pregnant, and visits at or after participants started on thyroxine treatment, where relevant.
Excluding those listed in footnote a above as well as participants with baseline subclinical hypothyroidism.
Overt hyperthyroidism (n = 5) and subclinical hyperthyroidism (n = 4) not attributable to other causes occurred in 5% of participants (see Table 3). None of these cases had positive thyroid antibodies (TPO and TSH receptor), indicating their hyperthyroidism was nonimmune. Most (8/9) developed hyperthyroidism later in the study period, from weeks 16 to 24 (Supplementary Fig. S2B [31]).
Factors Associated With the Development of Subclinical Hypothyroidism
There was an association between baseline UIC and the likelihood of SCH, and adjusted models showed that a UIC 10-fold lower at baseline was associated with a 2-fold higher risk of SCH after HSG (aRR = 2.05 [95% CI, 1.28-3.30]; P = .003). Following HSG, increasing UIC levels were associated with higher TSH levels throughout the study, with a repeated-measures analysis showing that a 10-fold increase in UIC was associated with a 0.6 mIU/L increase in TSH levels (95% CI, 0.4-0.8 mIU/L; P < .0001). Thus, those with lower UIC at baseline had a higher risk of developing SCH after an iodine load. In contrast, the higher UIC after the HSG was associated with higher TSH levels.
Unsurprisingly, TSH levels at baseline were higher in women who developed SCH than in those who did not (+0.6 mIU/L [95% CI, 0.4-0.8 mIU/L]; P < .0001). For every 1.0-mIU/L increase in TSH levels at baseline, the likelihood of SCH after HSG was nearly 2-fold higher (aRR = 1.92 [95% CI, 1.58-2.34]; P < .0001). Among the 79 women (40%) who had SCH sometime during the study (including at baseline), there was suggestion of higher pregnancy rates in those who were treated with T4 compared to those who were not (54% vs 36%; aRR 1.66 [95% CI, 0.97-2.84]; P = .063). Notably, when only women younger than 40 years at baseline were considered, the pregnancy rate after T4 treatment was higher than that of untreated women (63% vs 37%; P = .047), so that the likelihood of pregnancy with T4 treatment was 75% higher (aRR 1.75 (95% CI, 1.01-3.02); P = .046).
In contrast to the baseline TSH levels, the presence of TPO antibodies was not associated with development of subsequent SCH (P = .71).
Discussion
This study highlights the thyroid function abnormalities that follow marked and prolonged iodine excess following an OSCM HSG. While increased iodine levels were universal, there was substantial variability in the magnitude and timing of the peak iodine excess, likely reflecting variable amounts of retained peritoneal OSCM. Moreover, OSCM produces a globular spill into the peritoneum (9, 32, 33) and a variable release of iodine from the globules of different sizes may have a role. Retained peritoneal OSCM was established as a reservoir of iodine/OSCM, suggesting that a delayed pelvic radiograph 45 minutes after an OSCM HSG may be a simple qualitative way of assessing iodine exposure and risk of developing thyroid dysfunction.
Mild but persistent SCH was the most common thyroid dysfunction in women following OSCM HSG, and this is consistent with findings from previous studies (15–17, 34). While the peak TSH levels were mostly between 4 and 10 mIU/L, this elevation persisted for weeks. While the mechanism of iodine excess causing SCH is unclear, animal studies point to an inhibition of pituitary deiodinase 2 (DiO2) (35). Because Dio2 catalyzes the conversion of T4 to T3, the reduced T3 leads to loss of negative feedback and increased TSH release from the pituitary and possibly explained the effect of chronic and severe iodine excess on thyroid hormone regulation by the hypothalamic-pituitary axis (35–37). The more commonly described Wolff-Chaikoff effect (38, 39), which likely was also present, does not explain the length of TSH elevation in these patients. Based on our findings, those with baseline iodine deficiency or SCH are at higher risk for developing SCH following an OSCM HSG and warrant closer monitoring post HSG.
Of some concern, hyperthyroidism occurred in almost 5% of our participants. This generally occurred later and is possibly explained by the Jod-Basedow phenomenon, where iodine load results in hypersecretion of thyroid hormones from 2 to 12 weeks after exposure (40). Extremely high iodine levels may have also caused an iodine-induced thyroiditis (41–43) that could have contributed not only to the SCH, but possibly to the later hyperthyroidism via destructive thyroiditis, similar to that observed with amiodarone-induced thyroiditis type 2 (44–46). None of the hyperthyroid participants had positive thyroid antibodies or resistant hyperthyroidism requiring steroids. We are aware of 2 other confirmed cases of hyperthyroidism among our participants that developed after the study period, suggesting that there should be a low threshold for later thyroid function testing if clinically indicated.
Among women who developed SCH, only 3 women had a TSH greater than 10 mIU/L and would have required T4 supplementation as per the ATA guidelines (14). However, there remains some controversy about the effect and management of mild SCH to optimize fertility and pregnancy outcomes. TSH levels in the 4 to 10 mIU/L range are a cause of concern given studies suggesting an effect on fertility (47, 48), increased pregnancy complications (49,50), and fetal neurocognition (51–56). In part, this reflects the varying TSH thresholds ranging from 2.5 to 10 mIU/L used to define SCH in studies looking at these outcomes (14). Moreover, ATA guidelines also suggest keeping TSH less than 2.5 mIU/L in women planning assisted reproduction (14). Thus, in our study, mild SCH was managed differently in each woman by the respective fertility specialists, based on their experience and the clinical scenario. Interestingly, in our subgroup analysis we found a trend toward higher pregnancy rates if women were started on T4 for mild SCH (compared to those untreated), suggesting a potential benefit in treating mild SCH in subfertile women. However, large randomized controlled trials focusing on the effect of mild SCH and its treatment in infertile women are required to confirm these observations. The inconsistencies in treatment threshold by clinicians also demand more studies and clarity in the guidelines for treating mild SCH in women trying to conceive.
The incidence of thyroid dysfunction is likely lower with WSCM HSG as indicated in a previous study (16); however, fertility rates are also lower as suggested by the H2Oil study. There is a concern that the H2Oil trial (4) used a much older WSCM that is very different in chemical and physical properties compared to modern WSCM (1) and therefore, more studies are warranted to define the efficacy and safety of WSCM used in current practice. The major strengths of this study were the large sample size (n = 196) and excellent follow-up rates. This is the largest prospective study undertaken so far to assess the iodine excess and subsequent thyroid dysfunction following an OSCM HSG. Moreover, it is the first study that confirms that the peritoneum is the repository of retained iodine and provides a simple tool to predict iodine excess and its consequences.
The main limitation of the study was that it relied on urine spot samples to measure systemic iodine load. While there are intraindividual variations in these measurements (eg, due to circadian variation, salt intake, and hydration status) (57, 58), spot samples remain the most practical assessment of iodine status. Although prescribed iodine supplements were continued, we did not assess the individual supplement's content and compliance with the supplements, which might have resulted in variations in baseline iodine levels. However, this dose was not of a magnitude to affect the interpretation of iodine excess from OSCM exposure. There was also a clinician-based variation in initiating T3 for SCH that occurred following OSCM HSG. To overcome this limitation and to avoid the effect of T4 treatment or the TSH-lowering effect of pregnancy, we removed from analyses the TSH, FT4, and FT3 values at the time points when the woman was either pregnant or on T4 (in cases where it was initiated post HSG by fertility specialists for SCH).
Conclusion
OSCM HSG caused almost universal marked and prolonged iodine excess with almost 40% of women developing SCH and 5% developing later-onset hyperthyroidism. These thyroid abnormalities could affect maternal health and fertility and may need treatment. Based on the aforementioned data, we recommend that monthly thyroid function tests be performed for a minimum of 6 months in women undergoing OSCM HSG. This study highlights the importance of regular thyroid function testing to identify and appropriately manage those with thyroid dysfunction and reinforces the need for the development of guidelines for appropriate thyroid function monitoring and treatment following an OSCM HSG.
Acknowledgments
We would like to acknowledge Guerbet for the research grant to the Liggins Institute. We would also like to thank Janene McMillan, Liggins Institute, University of Auckland, for the help with organizing clinical assessments; Alice Wang, Liggins Institute, University of Auckland, for assistance with the SELFI accounts; and Chris Varghese and Adora Husseini, University of Auckland, for research assistance. We would like to acknowledge the team at Auckland Radiology Group, Repromed fertility clinic, Fertility Plus, Fertility Associates, and Ascot Radiology, Auckland, for their support with recruitment.
Abbreviations
- aRR
adjusted relative risk
- ATA
American Thyroid Association
- FT3
free 3,5,3′-triiodothyronine
- FT4
free thyroxine
- HSG
hysterosalpingography
- OSCM
oil-soluble contrast medium
- SCH
subclinical hypothyroidism
- SELFI
Safety and Efficacy of Lipiodol in Fertility Investigations
- TPO
thyroid peroxidase
- TSH
thyrotropin
- TwAUC
time-weighted area under the curve
- UIC
urine iodine concentration
- WSCM
water-soluble contrast medium
Contributor Information
Divya M Mathews, Liggins Institute, University of Auckland, Auckland 1142, New Zealand.
Jane M Peart, Department of Radiology, Auckland Radiology Group, Auckland 1050, New Zealand.
Robert G Sim, Department of Radiology, Auckland Radiology Group, Auckland 1050, New Zealand.
Neil P Johnson, Department of Obstetrics and Gynecology, Robinson Research Institute, University of Adelaide, Adelaide, South Australia 5006, Australia; Department of Reproductive Endocrinology and Fertility, Repromed Auckland and Auckland Gynecology Group, Auckland 1050, New Zealand.
Susannah O'Sullivan, Department of Endocrinology, Greenlane Clinical Centre, Auckland District Health Board, Auckland 1051, New Zealand.
José G B Derraik, Liggins Institute, University of Auckland, Auckland 1142, New Zealand; Department of Paediatrics: Child and Youth Health, Faculty of Medical and Health Sciences, University of Auckland, Auckland 1023, New Zealand.
Paul L Hofman, Liggins Institute, University of Auckland, Auckland 1142, New Zealand.
Financial Support
The study was funded by an institutional research grant to the Liggins Institute, University of Auckland (grant number: 5000278) by Guerbet Pharmaceuticals, the manufacturer of Lipiodol. Guerbet had no role in the study design, conduction of the study, data analysis or interpretation, decision to publish, manuscript preparation, or dissemination of results.
Disclosures
N.J. is involved in research with the University of Auckland and the University of Adelaide, which are funded by Guerbet; N.P.J. has undertaken paid consultancies for Guerbet; D.M.M. and P.L.H. are involved with University of Auckland research on Lipiodol safety through an unrestricted independent grant to the Liggins institute, Auckland by Guerbet; P.L.H. has received fees for speaking in 2 webinars sponsored by Guerbet; R.G.S. and J.M.P. have been paid for presenting and being an advisory board member by Guerbet; and R.G.S., J.M.P., and N.P.J. undertake Lipiodol HSGs as a part of their profession.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
Clinical Trial Information
Australian New Zealand Clinical Trials Registry registration number ANZCTR 12620000738921 (registered July 14, 2020).
References
- 1. Peart JM, Sim RG, Hofman PL. Therapeutic effects of hysterosalpingography contrast media in infertile women: what do we know about the H2O in the H2Oil trial and why does it matter? Hum Reprod. 2021;36(3):529–535. [DOI] [PubMed] [Google Scholar]
- 2. Baramki TA. Hysterosalpingography. Fertil Steril. 2005;83(6):1595–1606. [DOI] [PubMed] [Google Scholar]
- 3. Johnson NP, Farquhar CM, Hadden WE, Suckling J, Yu Y, Sadler L. The FLUSH trial—flushing with Lipiodol for unexplained (and endometriosis-related) subfertility by hysterosalpingography: a randomized trial. Hum Reprod. 2004;19(9):2043–2051. [DOI] [PubMed] [Google Scholar]
- 4. Dreyer K, van Rijswijk J, Mijatovic V, et al. Oil-based or water-based contrast for hysterosalpingography in infertile women. N Engl J Med. 2017;376(21):2043–2052. [DOI] [PubMed] [Google Scholar]
- 5. Alper MM, Garner PR, Spence JE, Quarrington AM. Pregnancy rates after hysterosalpingography with oil- and water-soluble contrast media. Obstet Gynecol. 1986;68(1):6–9. [PubMed] [Google Scholar]
- 6. de Boer AD, Vemer HM, Willemsen WN, Sanders FB. Oil or aqueous contrast media for hysterosalpingography: a prospective, randomized, clinical study. Eur J Obstet Gynecol Reprod Biol. 1988;28(1):65–68. [DOI] [PubMed] [Google Scholar]
- 7. Lindequist S, Rasmussen F, Larsen C. Use of iotrolan versus ethiodized poppy-seed oil in hysterosalpingography. Radiology. 1994;191(2):513–517. [DOI] [PubMed] [Google Scholar]
- 8. Rasmussen F, Lindequist S, Larsen C, Justesen P. Therapeutic effect of hysterosalpingography: oil- versus water-soluble contrast media—a randomized prospective study. Radiology. 1991;179(1):75–78. [DOI] [PubMed] [Google Scholar]
- 9. Peart JM, Sim R. Lipiodol hysterosalpingogram: a modified HSG technique to minimize risks associated with Lipiodol use. J Med Imaging Radiat Oncol. 2020;64(4):516–521. [DOI] [PubMed] [Google Scholar]
- 10. Miyamoto Y, Tsujimoto T, Iwai K, et al. Safety and pharmacokinetics of iotrolan in hysterosalpingography. Retention and irritability compared with Lipiodol. Invest Radiol. 1995;30(9):538–543. [DOI] [PubMed] [Google Scholar]
- 11. Kolbeck KJ. Lipiodol = ethiodol. J Vasc Interv Radiol 2011;22(3):419–420. [DOI] [PubMed] [Google Scholar]
- 12. Peart J. Higher reported rates of intravasation of oil-soluble contrast media—there may be a silver lining. Hum Reprod Open. 2020;2020(3):hoaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang F, Bai Y, Zhang Y, Faramand A. Oil-based versus water-based contrast for hysterosalpingography in infertile women: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2018;110(1):153–160.e3. [DOI] [PubMed] [Google Scholar]
- 14. Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 15. Kaneshige T, Arata N, Harada S, et al. Changes in serum iodine concentration, urinary iodine excretion and thyroid function after hysterosalpingography using an oil-soluble iodinated contrast medium (Lipiodol). J Clin Endocrinol Metab. 2015;100(3):E469–E472. [DOI] [PubMed] [Google Scholar]
- 16. So S, Yamaguchi W, Tajima H, et al. The effect of oil and water-soluble contrast medium in hysterosalpingography on thyroid function. Gynecol Endocrinol. 2017;33(9):682–685. [DOI] [PubMed] [Google Scholar]
- 17. Mekaru K, Kamiyama S, Masamoto H, Sakumoto K, Aoki Y. Thyroid function after hysterosalpingography using an oil-soluble iodinated contrast medium. Gynecol Endocrinol. 2008;24(9):498–501. [DOI] [PubMed] [Google Scholar]
- 18. Barnhart KT. Live birth is the correct outcome for clinical trials evaluating therapy for the infertile couple. Fertil Steril. 2014;101(5):1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathews DM, Peart JM, Sim RG, et al. The effect of acute and chronic iodine excess on thyroid profile and reproductive function of women using Lipiodol during hysterosalpingography and the potential impact on thyroid function of their offspring: the SELFI study protocol. Med Case Rep Study Protoc. 2021;2(8):e0148. [Google Scholar]
- 20. Barth JH, Spencer JD, Goodall SR, Luvai A. Reference intervals for thyroid hormones on Advia Centaur derived from three reference populations and a review of the literature. Ann Clin Biochem. 2016;53(Pt 3):385–389. [DOI] [PubMed] [Google Scholar]
- 21. WHO/UNICEF/ICCIDD . Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers. 3rd ed. World Health Organization; 2007. [Google Scholar]
- 22. Karakochuk CD, Michaux KD, Chai TL, et al. Median urinary iodine concentrations are indicative of adequate iodine status among women of reproductive age in Prey Veng, Cambodia. Nutrients. 2016;8(3):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pettigrew-Porter A, Skeaff S, Gray A, Thomson C, Croxson M. Are pregnant women in New Zealand iodine deficient? A cross-sectional survey. Aust N Z J Obstet Gynaecol. 2011;51(5):464–467. [DOI] [PubMed] [Google Scholar]
- 24. Ministry of Health, New Zealand. Biomedical Data Explorer 204/2015: New Zealand Health survey. Published February 13, 2020. Accessed May 12, 2022. https://www.health.govt.nz/publication/biomedical-results-2014-15-new-zealand-health-survey
- 25. Brough L, Jin Y, Shukri NH, Wharemate ZR, Weber JL, Coad J. Iodine intake and status during pregnancy and lactation before and after government initiatives to improve iodine status, in Palmerston North, New Zealand: a pilot study. Matern Child Nutr. 2015;11(4):646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Douglas CE, Michael FA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Methods. 1991;20(1):127–139. [Google Scholar]
- 27. Abdi H. Bonferroni and Šidák corrections for multiple comparisons. Encycl Meas Stat. 2007;3:103–107. [Google Scholar]
- 28. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 29. Mathews D, Hofman P, Johnson N, et al. Supplementary materials for “The SELFI Study: iodine excess and thyroid dysfunction in women undergoing oil-soluble contrast hysterosalpingography.” University of Auckland; 2022. Deposited September 9, 2022. 10.17608/k6.auckland.21077098.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathews D. Supplementary materials for “The SELFI Study: iodine excess and thyroid dysfunction in women undergoing oil-soluble contrast hysterosalpingography.” University of Auckland; 2022. Deposited August 11, 2022. 10.17608/k6.auckland.20469162.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathews D, Peart J, Sim R, et al. Supplementary materials for “The SELFI Study: iodine excess and thyroid dysfunction in women undergoing oil-soluble contrast hysterosalpingography.” University of Auckland; 2022. Deposited September 11, 2022. 10.17608/k6.auckland.21077122.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karasick S. Hysterosalpingography. Urol Radiol. 1991;13(1):67–73. [DOI] [PubMed] [Google Scholar]
- 33. Grigovich M, Kacharia VS, Bharwani N, Hemingway A, Mijatovic V, Rodgers SK. Evaluating fallopian tube patency: what the radiologist needs to know. Radiographics. 2021;41(6):1876–18961. [DOI] [PubMed] [Google Scholar]
- 34. Li R, Chen W, Liu Y, et al. The impact of preconceptional hysterosalpingography with oil-based contrast on maternal and neonatal iodine status. Reprod Sci. 2021;28(10):2887–2894. [DOI] [PubMed] [Google Scholar]
- 35. Li N, Jiang Y, Shan Z, Teng W. Prolonged high iodine intake is associated with inhibition of type 2 deiodinase activity in pituitary and elevation of serum thyrotropin levels. Br J Nutr. 2012;107(5):674–682. [DOI] [PubMed] [Google Scholar]
- 36. Sun Y, Du X, Shan Z, Teng W, Jiang Y. Effects of iodine excess on serum thyrotropin-releasing hormone levels and type 2 deiodinase in the hypothalamus of Wistar rats. Br J Nutr. 2022;127(11):1631–1638. [DOI] [PubMed] [Google Scholar]
- 37. Hussein AEAM, Abbas AM, El Wakil GA, Elsamanoudy AZ, El Aziz AA. Effect of chronic excess iodine intake on thyroid function and oxidative stress in hypothyroid rats. Can J Physiol Pharmacol. 2012;90(5):617–625. [DOI] [PubMed] [Google Scholar]
- 38. Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10(3):136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-induced hypothyroidism. Thyroid. 2001;11(5):501–510. [DOI] [PubMed] [Google Scholar]
- 40. Dunne P, Kaimal N, MacDonald J, Syed AA. Iodinated contrast-induced thyrotoxicosis. CMAJ. 2013;185(2):144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29(2-3):195–202. [DOI] [PubMed] [Google Scholar]
- 42. Rose NR, Bonita R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1(1-2):97–103. [DOI] [PubMed] [Google Scholar]
- 43. Ruwhof C, Drexhage HA. Iodine and thyroid autoimmune disease in animal models. Thyroid. 2001;11(5):427–436. [DOI] [PubMed] [Google Scholar]
- 44. Henry RK, Chaudhari M. In iodine-induced thyrotoxicosis, steroid therapy today could keep the surgical knife at bay. J Pediatr Endocrinol Metab. 2018;31(5):585–588. [DOI] [PubMed] [Google Scholar]
- 45. Takemoto K, Takada S. Thyroid storm associated with type 2 amiodarone-induced thyrotoxicosis due to long-term administration: a case report. Acute Med Surg. 2020;7(1):e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roti E, Uberti ED. Iodine excess and hyperthyroidism. Thyroid. 2001;11(5):493–500. [DOI] [PubMed] [Google Scholar]
- 47. Yoshioka W, Amino N, Ide A, et al. Thyroxine treatment may be useful for subclinical hypothyroidism in patients with female infertility. Endocr J. 2015;62(1):87–92. [DOI] [PubMed] [Google Scholar]
- 48. Verma I, Sood R, Juneja S, Kaur S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int J Appl Basic Med Res. 2012;2(1):17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneuer FJ, Nassar N, Tasevski V, Morris JM, Roberts CL. Association and predictive accuracy of high TSH serum levels in first trimester and adverse pregnancy outcomes. J Clin Endocrinol Metab. 2012;97(9):3115–3122. [DOI] [PubMed] [Google Scholar]
- 50. Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009;160(6):985–991. [DOI] [PubMed] [Google Scholar]
- 51. Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf). 2010;72(6):825–829. [DOI] [PubMed] [Google Scholar]
- 52. Liu Y, Chen H, Jing C, Li F. The association between maternal subclinical hypothyroidism and growth. Development, and childhood intelligence: a meta-analysis. J Clin Res Pediatr Endocrinol. 2018;10(2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy NC, Diviney MM, Donnelly JC, et al. The effect of maternal subclinical hypothyroidism on IQ in 7- to 8-year-old children: a case-control review. Aust N Z J Obstet Gynaecol. 2015;55(5):459–463. [DOI] [PubMed] [Google Scholar]
- 54. Fan X, Wu L. The impact of thyroid abnormalities during pregnancy on subsequent neuropsychological development of the offspring: a meta-analysis. J Matern Fetal Neonatal Med. 2016;29(24):3971–3976. [DOI] [PubMed] [Google Scholar]
- 55. Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs. 2012;72(1):17–33. [DOI] [PubMed] [Google Scholar]
- 56. van den Boogaard E, Vissenberg R, Land JA, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2011;17(5):605–619. [DOI] [PubMed] [Google Scholar]
- 57. Li C, Peng S, Zhang X, et al. The urine iodine to creatinine as an optimal Index of iodine during pregnancy in an iodine adequate area in China. J Clin Endocrinol Metab. 2016;101(3):1290–1298. [DOI] [PubMed] [Google Scholar]
- 58. Ji C, Lu T, Dary O, Legetic B, Campbell NR, Cappuccio FP;Sub-Group for Research and Surveillance of the PAHO–WHO Regional Expert Group for Cardiovascular Disease Prevention through Population-wide Dietary Salt Reduction . Systematic review of studies evaluating urinary iodine concentration as a predictor of 24-hour urinary iodine excretion for estimating population iodine intake. Rev Panam Salud Publica. 2015;38(1):73–81. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.