Abstract
Context
The Kabi/Pfizer International Growth Database (KIGS) is a large, international database (1987-2012) of children treated with recombinant human growth hormone (rhGH) in real-world settings.
Objective
This work aimed to evaluate the safety and efficacy of rhGH from the full KIGS cohort.
Methods
Data were collected by investigators from children with growth disorders treated with rhGH (Genotropin [somatropin]; Pfizer). Safety was evaluated in all treated patients, and efficacy in those treated for 1 year or more. A subgroup included patients treated for 5 years or more (≥ 2 years prepubertal) who had reached near-adult height (NAH). Main outcomes included adverse events (AEs), serious AEs (SAEs), and height growth.
Results
The full KIGS cohort (N = 83 803 [58% male]) was treated for idiopathic GH deficiency (IGHD; 46.9%), organic GHD (10.0%), small for gestational age (SGA; 9.5%), Turner syndrome (TS; 9.2%), idiopathic short stature (ISS; 8.2%), and others (16.2%). Median rhGH treatment duration was 2.7 years and observation 3.1 years. SAEs occurred in 3.7% of patients and death in 0.4%. The most common SAEs were recurrence of craniopharyngioma (n = 151), neoplasm (n = 99), and cancer (n = 91); and scoliosis (n = 91). Median first-year delta height-SD score (SDS) (Prader) in prepubertal patients was 0.66 (IGHD), 0.55 (ISS), 0.58 (TS), and 0.71 (SGA). Median gains in NAH-SDS were 1.79 (IGHD), 1.37 (ISS), and 1.34 (SGA) for boys, and 2.07 (IGHD), 1.62 (ISS), 1.07 (TS), and 1.57 (SGA) for girls.
Conclusion
Data from KIGS, the largest and longest running international database of rhGH-treated children, show that rhGH is safe and increases short-term height gain and adult height across GHD and non-GHD conditions.
Keywords: children, short stature, growth hormone, KIGS, efficacy, safety
Effective growth-promoting treatment with pituitary-derived human growth hormone (hGH) was first reported more than 60 years ago (1). In 1985, Creutzfeldt-Jakob disease was linked to the use of pituitary-derived hGH leading to its discontinuation, which, shortly thereafter, was followed by the first full regulatory approval worldwide of recombinant hGH (rhGH) by the US Food and Drug Administration and by the European Medicines Agency in the same year (2). Initially approved only for pediatric GH deficiency (GHD), rhGH treatment currently is approved, depending on the country/region, for 8 indications in children (GHD, Prader-Willi syndrome [PWS], children born small for gestational age [SGA], Turner syndrome [TS], Noonan syndrome, idiopathic short stature [ISS], chronic renal failure [CRF], and short stature homeobox-containing gene deficiency [SHOX-D]); and 3 in adults (GHD, short bowel syndrome, and HIV wasting syndrome). Various registries/postmarketing surveillance databases were developed to follow rhGH-treated pediatric patients for long-term efficacy and safety in real-world clinical settings, and, in particular, to provide information regarding potential rare serious adverse consequences of rhGH therapy, including recurrent or new malignancies in children with preexisting malignancies, development of intracranial hypertension, unmasking of (type 2) diabetes mellitus, induction of stroke, and a possible association with increased overall mortality (3–7).
KIGS (Kabi/Pfizer International Growth Database) was an investigator driven and pharmaceutical company–supported surveillance study (8) first established as a survey in Sweden to determine the long-term safety and treatment outcomes of pediatric rhGH therapy (Genotropin [somatropin]; Pfizer). It started as a 5-year follow up of 500 patients from 3 countries in 1987, with enrollment quickly increasing to 5000 patients from 14 countries by 1989 (8), and continuing to evolve into a large outcomes research database of more than 80 000 rhGH-treated children with growth disorders from more than 50 countries by the time of data-lock June 30, 2012 (9). The database allows for analyses of relationships between clinical status, dosage schedule, treatment with rhGH before KIGS enrollment, and response to treatment. Prediction models have been developed based on KIGS results for individualized pediatric rhGH treatment to optimize management throughout the course of treatment and transition from pediatric to adult care. The large patient population enables evaluation of growth responses in children with rare growth disorders and comparisons of newly diagnosed patients with others who have the same established diagnosis. It has also contributed to our basic knowledge of growth, growth disorders, and rhGH treatment of pediatric patients through exploratory analyses of the collected study data.
The objective of the present report was to analyze and summarize the accumulated safety and efficacy data on rhGH-treated patients in KIGS until its closure, with follow-up in some cases for more than 18 years, and to compare and contrast our findings with those of other previously published, large rhGH registries/postmarketing surveillance databases (5, 10–14).
Materials and Methods
Study Design and Patients
Children with growth disorders who were treated with rhGH (Genotropin [somatropin]; Pfizer) according to approved indications or as prescribed by physicians in medical practice were enrolled. Patients who started with another rhGH brand, but switched to Genotropin, also were enrolled in KIGS. The treating physician diagnosed the specific growth disorder, which subsequently was categorized according to the KIGS Etiology Classification List (15) (Supplementary Table 1) (16). Treatment dosing and schedule were at the discretion of each physician. Safety and growth data were collected during patients’ routine clinical visits and entered using case report forms at the sole discretion of investigators and analyzed without further modification. Participation in any blinded clinical trial was not allowed while participating in KIGS. The study was carried out in accordance with the principles of the Declaration of Helsinki. Informed consents/assents were obtained from participants and their parents before study entry, according to the regulatory standards of the local institution or country at that time.
Study Outcomes
All adverse events (AEs; defined as any untoward medical occurrence in a patient) and serious AEs (SAEs; defined as any AE that is life-threatening, is an important medical event, or results in death, hospitalization/prolonged hospitalization, disability/permanent damage, or congenital anomaly/birth defect) were collected regardless of their relationship with rhGH. Coding of AEs and SAEs was initially based on the World Health Organization Adverse Reaction Terminology (WHO-ART) (17) and was later changed to the Medical Dictionary for Regulatory Activities (MedDRA) system organ classes (SOCs) and preferred terms (PTs) (MedDRA, version 14.1) after the launch of MedDRA (18). In MedDRA coding, “neoplasm” and “cancer” terms are both covered by the MedDRA SOC “Neoplasms benign, malignant, or unspecified.” “Neoplasm” terms are used to code for tumors that could be benign, malignant, or unspecified, whereas “cancer” is used for malignancy events, based on medical coders’ interpretation; coders are cautioned against assigning “tumor” events as “cancer” unless it is clear that malignancy is present. Causal relationship of AEs or SAEs with rhGH treatment was determined by investigators based on their opinions without central adjudication. Auxological data, including height, near-adult height (NAH), weight, and body mass index (BMI), were collected for the efficacy analysis.
Statistics
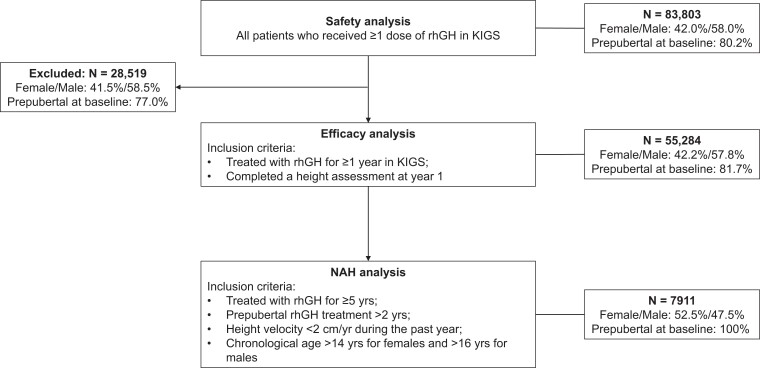
The safety cohort included all patients who received at least one dose of rhGH after KIGS enrollment (Fig. 1). Efficacy of rhGH treatment was analyzed in patients who had been treated for 1 year or more and completed a height assessment at year 1 (± 30 days) (see Fig. 1). The NAH subgroup comprises patients who received rhGH for 5 years or more (≥ 2 years of prepubertal treatment) and achieved NAH, which was defined by height velocity less than 2 cm/year during the last year and chronological age greater than 14 years for females and greater than 16 years for males (see Fig. 1). To evaluate the potential bias by the loss of patients from the safety to the efficacy cohort, a stepwise, multivariable logistic regression model was fitted for all the demographic variables, excluding variables that were not significant using the PROC LOGISTIC procedure in the SAS analytics software (SAS) (19). The effect on first-year height velocity of not including all rhGH-treated patients in the efficacy cohort was then analyzed with forward selection linear regression and the SAS REG procedure by using a model controlled for the significant dropout factors.
Figure 1.
Patient disposition and main analysis cohorts. Baseline prepubertal percentages were based on the number of patients with available puberty status data; n = 72 355; 23 246; 49 109; and 7911 for the safety, excluded, efficacy, and NAH sets, respectively. KIGS, Kabi/Pfizer International Growth Database; NAH, near adult height; rhGH, recombinant human growth hormone.
Continuous data are presented as medians (10th-90th percentiles) unless noted otherwise, and categorical data are presented as proportions of patients. Patients with missing values of a particular end point were excluded from the analysis for that end point. Frequencies of SAEs and death were compared among subgroups defined by patient characteristics. Growth outcomes were summarized for each diagnostic group. SD score (SDS) was calculated based on the Swiss references (Prader) according to chronological age- and sex-matched values for height (20) and according to Cole et al for BMI (21) except for the analysis comparing growth outcomes from different regions, which used the Japanese references (22) for outcomes from Japan. Mid-parental height (MPH) SDS was calculated according to Cole’s formula (MPH-SDS = [mother’s height-SDS + father’s height-SDS]/1.61) (23). Start of puberty was defined by Tanner breast stage II in girls and testicular volume 4 mL in boys (24, 25). Auxological data at different time points (rhGH start, year 1, puberty onset, and NAH) were compared in each diagnostic group among NAH patients. Efficacy also was compared by sex. All statistical analyses were conducted using SAS software (version 9.4) (SAS Institute Inc). A P value less than .05 was considered statistically significant. Analyses were exploratory and P values were not corrected for multiple comparisons.
Results
Demographics of Participants
The safety cohort included 83 803 children with growth disorders from 52 countries treated with rhGH. Of all patients, 62.2% were from Europe, 20.0% from the United States, 13.2% from Asia, and 4.7% from other parts of the world (Table 1). The United States, Germany, and Japan were the countries with the highest numbers of patients (16 737 [20.0%], 11 488 [13.7%], and 10 525 [12.6%], respectively). Additionally, Germany, the United States, France, and Japan were the countries with the longest follow-up in terms of patient-years (PY) (50 013; 46 254; 35 748; and 30 404 PY, respectively). Most patients were White (70%) and male (58%). In KIGS, children started rhGH treatment later in childhood (median age 10.7 years [range, 4.6-14.9]), and most (46.9%) were treated for idiopathic GHD (IGHD) (see Table 1). The initial dose of rhGH varied by diagnosis, with medians ranging from 0.17 mg/kg/wk (range, 0.08-0.26 mg/kg/wk) for craniopharyngioma to 0.33 mg/kg/wk (range, 0.15-0.39 mg/kg/wk) for CRF (Supplementary Table 2) (16). Duration of rhGH treatment in KIGS was 2.7 years (range, 0.3-7.2 years) (277 267 PY), and KIGS observation period was 3.1 years (range, 0.5-8.2 years) (322 576 PY) (see Table 1); neither duration differed by sex. In the safety cohort, 10 685 (12.8%) patients were treated for 6 months or less, 7143 (8.5%) for more than 6 months and 1 year or less, and 65 975 (78.7%) for more than 1 year.
Table 1.
Demographic characteristics of the safety and efficacy cohorts
| Safety (N = 83 803) |
Efficacy (N = 55 284) |
Excluded (N = 28 519) |
|
|---|---|---|---|
| World region, n (%) | |||
| Europe | 52 135 (62.2) | 35 395 (64.0) | 16 740 (58.7) |
| USA | 16 737 (20.0) | 10 057 (18.2) | 6680 (23.4) |
| Asia | 11 023 (13.2) | 7668 (13.9) | 3355 (11.8) |
| Rest of the world | 3908 (4.7) | 2164 (3.9) | 1744 (6.1) |
| Race/ethnicity, n (%) | |||
| Black | 943 (1.1) | 540 (1.0) | 403 (1.4) |
| White | 59 022 (70.4) | 39 741 (71.9) | 19 281 (67.6) |
| Hispanic | 1989 (2.4) | 937 (1.7) | 1052 (3.7) |
| Asian | 12 082 (14.4) | 8470 (15.3) | 3612 (12.7) |
| Other | 2363 (2.8) | 1496 (2.7) | 867 (3.0) |
| Unknown | 7404 (8.8) | 4100 (7.4) | 3304 (11.6) |
| Sex, n (%) | |||
| Female | 35 183 (42.0) | 23 341 (42.2) | 11 842 (41.5) |
| Male | 48 620 (58.0) | 31 943 (57.8) | 16 677 (58.5) |
| Age at rhGH start, y | |||
| Mean ± SD | 10.2 ± 4.0 | 9.4 ± 3.8 | 10.7 ± 4.2 |
| Median (10th-90th percentile) | 10.7 (4.6 to 14.9) | 9.8 (4.2 to 14.1) | 11.2 (4.7 to 15.6) |
| Height SDS at rhGH start | n = 81 262 | n = 55 284 | n = 25 978 |
| Mean ± SD | −2.9 ± 1.3 | −3.0 ± 1.2 | −2.9 ± 1.4 |
| Median (10th-90th percentile) | −2.9 (−4.4 to −1.6) | −2.9 (−4.4 to −1.7) | −2.8 (−4.5 to −1.4) |
| Weight SDS at rhGH start | n = 81 262 | n = 55 284 | n = 25 978 |
| Mean ± SD | −1.9 ± 1.7 | −1.9 ± 1.7 | −1.8 ± 1.8 |
| Median (10th-90th percentile) | −1.9 (−3.7 to 0.2) | −1.9 (−3.7 to 0.1) | −1.8 (−3.7 to 0.3) |
| BMI SDS at rhGH start | n = 81 257 | n = 55 280 | n = 25 977 |
| Mean ± SD | −0.2 ± 2.2 | −0.2 ± 2.5 | −0.2 ± 1.5 |
| Median (10th-90th percentile) | −0.2 (−1.9 to 1.7) | −0.2 (−1.9 to 1.6) | −0.2 (−1.9 to 1.7) |
| Max GH peak, μg/L | n = 65 058 | n = 42 436 | n = 18 132 |
| Mean ± SD | 9. 5 ± 11.5 | 9.5 ± 11.6 | 9.2 ± 11.2 |
| Median (10th-90th percentile) | 7.4 (1.7 to 19.0) | 7.5 (1.8 to 19.0) | 7.2 (1.5 to 18.4) |
| Diagnosis of growth disorder, n (%) | |||
| Idiopathic GHD | 39 298 (46.9) | 25 810 (46.7) | 13 488 (47.3) |
| Isolated idiopathic GHD | 33 138 (39.5) | 21 263 (38.5) | 11 875 (41.6) |
| Combined idiopathic GHDa | 6160 (7.4) | 4547 (8.2) | 1613 (5.7) |
| Neurosecretory disfunction | 2187 (2.6) | 1537 (2.8) | 650 (2.3) |
| Congenital GHDb | 3323 (4.0) | 2189 (4.0) | 1134 (4.0) |
| Acquired GHD | |||
| Craniopharyngioma | 1381 (1.6) | 965 (1.7) | 416 (1.5) |
| Medulloblastoma | 998 (1.2) | 703 (1.3) | 295 (1.0) |
| Other cranial tumors | 1750 (2.1) | 1209 (2.2) | 541 (1.9) |
| Extracranial malignancy | 940 (1.1) | 680 (1.2) | 260 (0.9) |
| Non-GHD short stature disorders | |||
| Idiopathic short stature | 6867 (8.2) | 4336 (7.8) | 2531 (8.9) |
| Turner syndrome | 7714 (9.2) | 5580 (10.1) | 2134 (7.5) |
| Prader-Willi syndrome | 2338 (2.8) | 1501 (2.7) | 837 (2.9) |
| Other syndromesc | 2602 (3.1) | 1801 (3.3) | 801 (2.8) |
| Small for gestational age | 7936 (9.5) | 4892 (8.8) | 3044 (10.7) |
| Chronic renal failure | 2399 (2.9) | 1514 (2.7) | 885 (3.1) |
| Other causesd | 4070 (4.9) | 2567 (4.6) | 1503 (5.3) |
| Dose at rhGH start, mg/kg/wk | |||
| Mean ± SD | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.2 |
| Median (10th-90th percentile) | 0.2 (0.2 to 0.4) | 0.2 (0.2 to 0.4) | 0.2 (0.0 to 0.4) |
| Duration of rhGH treatment, y | |||
| Mean ± SD | 3.3 ± 2.8 | 4.2 ± 2.7 | 1.7 ± 2.2 |
| Median (10th-90th percentile) | 2.7 (0.3 to 7.2) | 3.5 (1.4 to 7.9) | 0.8 (0.0 to 4.7) |
| Duration of follow-up in KIGS, y | |||
| Mean ± SD | 3.9 ± 3.1 | 4.7 ± 3.0 | 2.2 ± 2.7 |
| Median (10th-90th percentile) | 3.1 (0.5 to 8.2) | 4.0 (1.6 to 9.0) | 1 (0.0 to 5.8) |
Abbreviations: BMI, body mass index; GH, growth hormone; GHD, growth hormone deficiency; KIGS, Pfizer International Growth Database; MPHD, multiple pituitary hormone deficiency; rhGH, recombinant human growth hormone; SDS, SD score; USA, United States of America.
Idiopathic GHD with additional pituitary hormone deficiencies.
Excluding GH receptor deficiency.
Other syndromes with 100 or more patients included Silver-Russell syndrome, Noonan syndrome, syndromes with/without chromosomal aberration, von Recklinghausen syndrome (neurofibromatosis), and gonadal dysgenesis.
Other causes with 100 or more patients included Kowarski-type bioinactive GH syndromes, other bioinactive GH syndromes, other functional GHD, head trauma, histiocytosis, other causes of acquired GHD, iatrogenic short stature due to medication, achondroplasia, hypochondroplasia, skeletal dysplasia, other chronic inflammatory disorders, precocious puberty, other endocrine disorders (not GHD).
Among all rhGH-treated patients, 55 284 met the criteria for the efficacy cohort (see Fig. 1); their characteristics are summarized in Table 1 and Supplementary Tables 3 and 4 (16). Of the 28 519 rhGH-treated patients excluded from the efficacy cohort (see Fig. 1), 17 828 had received less than 1 year of rhGH and 10 691 were missing height data at year 1. By linear regression modeling, age at rhGH start and maximum GH peak level were identified as the variables with a partial R-square equal to or greater than 5% (contribution to the total variability in height velocity explained). The effect of the missing data from the excluded patients on overall height velocity was calculated to be approximately −0.06 cm/year based on the differences between the safety and efficacy cohorts in median age and maximum GH peak level at rhGH start.
A total of 7911 patients from the efficacy cohort met the inclusion criteria for the NAH subgroup (see Fig. 1); their characteristics are presented in Supplementary Table 5 (16).
Safety
Adverse events
A total of 23 163 AEs was reported for 14.4% of patients; 3108 AEs were assessed by the investigators as potentially treatment related for 3.1% of patients (Table 2). The incidence rate of all-causality AE was 94.2 per 1000 PY. Drug discontinuation (temporary, permanent, or delayed) caused by AEs occurred in 1.6% of patients, and in 0.6% due to potential treatment-related AEs. The most common types of disorders (MedDRA SOC) for AEs were infections and infestations, which were reported in 3162 (3.8%) patients and more prevalent in children younger than 10 vs 10 years or older at rhGH start (5.8% vs 2.2%; P < .0001). AEs categorized in the SOC neoplasms as benign, malignant, and unspecified were reported in 842 (1%) patients. The most commonly reported AE by MedDRA PT was headache (all-causality in 1.2% and treatment related in 0.4%), followed by scoliosis (0.6% and 0.2%) (see Table 2), which was more prevalent in the PWS group (6.3%) than other groups. Multivariable analysis showed that the probability of reporting headache was significantly affected by sex (P = .0318), age at rhGH start, diagnosis, treatment duration, and initial or mean rhGH dose (P < .0001 for all except for sex); the probability was higher in females, younger patients, or patients with craniopharyngioma (Supplementary Table 5) (16). Craniopharyngioma AEs (mostly recurrent or worsening) were reported in 0.2% of all patients and considered related to rhGH treatment in 0.1% (see Table 2). In the craniopharyngioma diagnostic group, 11.9% of patients had a craniopharyngioma AE. Hemorrhagic stroke and bone tumor events were reported in a small number of all patients and most events were assessed as unrelated to treatment (see Table 2).
Table 2.
Frequency of adverse events, serious adverse events, and treatment discontinuations (safety cohort N = 83 803)
| No. of patients, n (%) | All-causality | Treatment-related |
|---|---|---|
| No. of AEsa | 23 163 | 3108 |
| Patients with AEs | 12 055 (14.4) | 2638 (3.1) |
| Patients with dose reduction due to AEs | 180 (0.2) | 134 (0.2) |
| Patients with drug discontinuationb due to AEs | 1349 (1.6) | 504 (0.6) |
| AEs in ≥ 0.2% patients | ||
| Headache | 987 (1.2) | 328 (0.4) |
| Scoliosis | 514 (0.6) | 162 (0.2) |
| Upper respiratory tract infection | 474 (0.6) | 4 (0.0) |
| Arthralgia | 431 (0.5) | 129 (0.2) |
| Pyrexia | 425 (0.5) | 13 (0.0) |
| Ear infection | 408 (0.5) | 3 (0.0) |
| Influenza | 404 (0.5) | 4 (0.0) |
| Nasopharyngitis | 391 (0.5) | 5 (0.0) |
| Gastroenteritis | 273 (0.3) | 3 (0.0) |
| Tonsillitis | 244 (0.3) | 7 (0.0) |
| Abdominal pain | 228 (0.3) | 11 (0.0) |
| Varicella | 223 (0.3) | 1 (0.0) |
| Vomiting | 211 (0.3) | 22 (0.0) |
| Pain in extremity | 207 (0.2) | 67 (0.1) |
| Asthma | 207 (0.2) | 3 (0.0) |
| Elevated IGF-1c | 188 (0.2) | 146 (0.2) |
| Cough | 187 (0.2) | 6 (0.0) |
| Fatigue | 181 (0.2) | 30 (0.0) |
| Pneumonia | 181 (0.2) | 0 (0.0) |
| Craniopharyngioma | 170 (0.2) | 47 (0.1) |
| Otitis media | 170 (0.2) | 1 (0.0) |
| Bronchitis | 169 (0.2) | 2 (0.0) |
| Convulsion | 166 (0.2) | 16 (0.0) |
| Epilepsy | 152 (0.2) | 14 (0.0) |
| Back pain | 151 (0.2) | 24 (0.0) |
| Melanocytic nevus | 147 (0.2) | 62 (0.1) |
| Urinary tract infection | 143 (0.2) | 4 (0.0) |
| Diarrhea | 140 (0.2) | 3 (0.0) |
| Viral infection | 137 (0.2) | 2 (0.0) |
| Other AEs of interest | ||
| Neoplasm recurrence | 105 (0.1) | 23 (0.0) |
| Recurrent cancer | 91 (0.1) | 25 (0.0) |
| Benign intracranial hypertension | 54 (0.1) | 49 (0.1) |
| Epiphysiolysis | 80 (0.1) | 48 (0.1) |
| Cerebral hemorrhage | 7 (0.0) | 1 (0.0) |
| Hemorrhage intracranial | 3 (0.0) | 2 (0.0) |
| Intraventricular hemorrhage | 1 (0.0) | 0 |
| Subarachnoid hemorrhage | 1 (0.0) | 0 |
| Bone giant cell tumor | 1 (0.0) | 0 |
| Bone neoplasm | 3 (0.0) | 0 |
| Bone sarcoma | 4 (0.0) | 1 (0.0) |
| Ewing sarcoma | 1 (0.0) | 1 (0.0) |
| Ewing sarcoma recurrent | 1 (0.0) | 1 (0.0) |
| Osteochondroma | 10 (0.0) | 2 (0.0) |
| Osteoma | 2 (0.0) | 2 (0.0) |
| Enchondroma | 1 (0.0) | 0 |
| Enchondromatosis | 1 (0.0) | 1 (0.0) |
| Diabetes mellitus | 37 (0.0) | 19 (0.0) |
| Type 1 diabetes mellitus | 35 (0.0) | 15 (0.0) |
| Type 2 diabetes mellitus | 21 (0.0) | 12 (0.0) |
| Insulin-resistant diabetes | 2 (0.0) | 1 (0.0) |
| Diabetes mellitus malnutrition-related | 1 (0.0) | 0 |
| No. of SAEsa | 3981 | 657 |
| Patients with SAEs | 3108 (3.7) | 607 (0.7) |
| Patients with drug discontinuationb due to SAEs | 1030 (1.2) | 345 (0.4) |
| SAEs in ≥ 0.1% patients | ||
| Craniopharyngiomad | 151 (0.2) | 42 (0.1) |
| Neoplasm recurrence | 99 (0.1) | 23 (0.0) |
| Scoliosis | 91 (0.1) | 43 (0.1) |
| Recurrent cancer | 91 (0.1) | 26 (0.0) |
| Epiphysiolysis | 61 (0.1) | 38 (0.0) |
| Convulsion | 60 (0.1) | 6 (0.0) |
| Death | 59 (0.1) | 5 (0.0) |
| Vomiting | 47 (0.1) | 3 (0.0) |
| Pneumonia | 47 (0.1) | 0 (0.0) |
| Headache | 45 (0.1) | 11 (0.0) |
| Epilepsy | 43 (0.1) | 4 (0.0) |
| Appendicitis | 42 (0.1) | 1 (0.0) |
Abbreviations: AE, adverse event; IGF-1, insulin-like growth factor-1; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Multiple AEs/SAEs reported under the same MedDRA preferred term were counted once for the same patient.
Temporary, permanent, or delayed drug discontinuation.
Elevated IGF-1: outside the normal range defined by the laboratory used for the test.
These are craniopharyngioma recurrences rather than de novo cases.
Serious adverse events
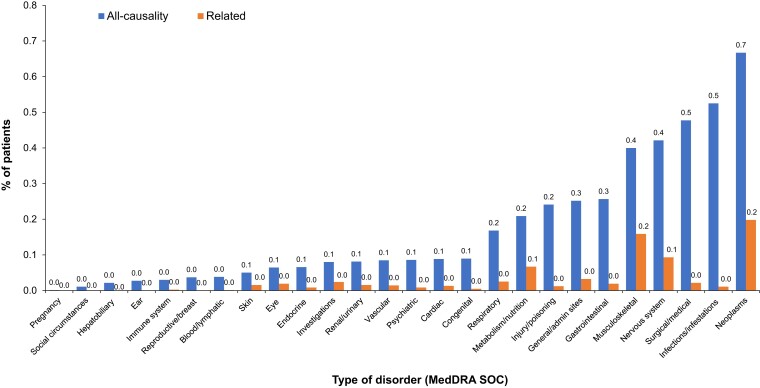
Overall, 3981 SAEs were reported for 3108 (3.7%) patients; 657 were considered treatment related in 607 patients (0.7%) (see Table 2). The most common type of disorders (MedDRA SOC) for SAEs was neoplasms (all-causality in 0.7% of patients and treatment related in 0.2%) (Fig. 2). SAEs led to discontinuation of rhGH in 1.2% of patients, and 0.4% due to related SAEs (see Table 2). The most common all-causality SAEs by MedDRA PT were craniopharyngioma (recurrent; 0.2%), neoplasm recurrence (benign, malignant, or unspecified; 0.1%), scoliosis (0.1%), and recurrent cancer (0.1%); and the most common treatment-related SAEs were craniopharyngioma (recurrent; 0.1%) and scoliosis (0.1%) (see Table 2).
Figure 2.
SAE frequency in the safety cohort by type of disorder (MedDRA SOC). Related SAEs are SAEs considered to be related to rhGH treatment based on investigators’ opinion without central adjudication. General/admin sites includes general disorders that affect several body systems or sites and administration site conditions. Investigations include adverse events based on results from clinical laboratory tests, radiologic tests, physical examinations, and physiologic tests; only MedDRA PTs representing investigation procedures and qualitative results are in this SOC. Neoplasms include benign, malignant, and unspecified neoplasms. admin, administration; MedDRA, Medical Dictionary for Regulatory Activities; PT, preferred term; rhGH, recombinant human growth hormone; SAE, serious adverse event; SOC, system organ class.
Effect of patient characteristics on serious adverse events
SAEs of all causality were more prevalent in patients who had a longer duration of treatment in KIGS (≥ 3 years) (Table 3). Analysis of SAEs by time interval (from rhGH start to the first event occurrence) among 5170 prepubertal patients and 666 pubertal patients over the first 5 years of treatment showed similar SAE frequencies between the 2 groups in each year (Supplementary Fig. 1) (16). SAEs generally occurred more frequently during the first year of treatment regardless of puberty status (see Supplementary Fig. 1) (16).
Table 3.
Total serious adverse events stratified by patient characteristics
| No.a | All-causality | Treatment-related | |
|---|---|---|---|
| Duration of rhGH treatment, y | |||
| < 3 y | 39 443 | 1039 (2.6) | 280 (0.7) |
| ≥ 3 y | 44 360 | 2069 (4.7) | 327 (0.7) |
| P | < .0001 | ≥ .999 | |
| Diagnosis | |||
| Idiopathic GHD | 39 298 | 743 (1.9) | 137 (0.3) |
| Neurosecretory dysfunction | 2187 | 41 (1.9) | 7 (0.3) |
| Congenital GHD | 3323 | 213 (6.4) | 21 (0.6) |
| Craniopharyngioma | 1381 | 268 (19.4) | 68 (4.9) |
| Medulloblastoma | 998 | 117 (11.7) | 20 (2.0) |
| Other cranial tumors | 1750 | 228 (13.0) | 56 (3.2) |
| Extracranial malignancy | 940 | 76 (8.1) | 19 (2.0) |
| Idiopathic short stature | 6867 | 73 (1.1) | 16 (0.2) |
| Turner syndrome | 7714 | 293 (3.8) | 68 (0.9) |
| Prader-Willi syndrome | 2338 | 175 (7.5) | 52 (2.2) |
| Other syndromes | 2602 | 163 (6.3) | 24 (0.9) |
| Small for gestational age | 7936 | 175 (2.2) | 19 (0.2) |
| Chronic renal failure | 2399 | 333 (13.9) | 61 (2.5) |
| Other causes | 4070 | 210 (5.2) | 39 (1.0) |
| P | < .0001 | < .0001 |
Abbreviations: GHD, growth hormone deficiency; rhGH, recombinant human growth hormone.
No. was used a denominator to calculated percentages in each row.
SAE frequency varied by diagnosis, highest among patients with craniopharyngioma (all-causality in 19.4% of patients and treatment related in 4.9%), followed by those with CRF (13.9% and 2.5%) and other cranial tumors (13.0% and 3.2%) (Table 3). No effect of rhGH dose on the frequency of SAEs was observed within each diagnostic group except for the IGHD group; the frequency of all-causality SAEs was significantly higher in IGHD patients with a higher (≥third quartile) vs lower (≤first quartile) mean rhGH dose during participation in KIGS (Supplementary Table 7 (16)). Overall, the frequency of all-causality and treatment-related SAEs were lower in the IGHD vs congenital GHD diagnostic groups (1.9% vs 6.4% for all-causality; 0.3% vs 0.6% for treatment related; see Table 3). In both groups, statistically significant differences were observed by baseline maximum GH peak level, with higher SAE frequency in the less than 5 µg/L subgroup; however, such a correlation was not found for treatment-related SAEs (Supplementary Table 8) (16). Common SAEs by type of disorders (MedDRA SOC) in each diagnostic group also varied (Supplementary Fig. 2) (16).
Supplementary Tables 9 and 10 (16) summarize SAEs (disorder types of interest) by patient characteristics. The difference by treatment duration was small, although it was statistically significant for all-causality cardiac, metabolism, musculoskeletal, and psychiatric disorders (Supplementary Table 9) (16). Among all diagnostic groups, all-causality neoplasms, metabolism disorders, and psychiatric disorders were more common in the craniopharyngioma group (12.7%, 0.9%, and 0.7%, respectively), cardiac disorders in the extracranial malignancy group (0.5%), musculoskeletal disorders in the PWS group (1.7%), and vascular disorders in the CRF group (1.0%) (see Supplementary Table 9 (16)). Frequencies of treatment-related SAEs within each diagnostic group were low for these disorders (see Supplementary Table 10) (16).
Mortality
In total, 307 (0.4%) patients died during the study (Table 4). Death occurred most frequently among patients treated for medulloblastoma (3.7%), other cranial tumors (2.5%), and extracranial malignancy (2.2%). No sex difference was observed. Deaths were reported for 0.5% of patients who had less than 2 years of rhGH exposure vs 0.3% of those treated for 2 years or more. Among all deaths, 31.9% attained age 10 to younger than 15 years, 28.3% 15 to younger than 20 years, and 18.2% 5 to younger than 10 years.
Table 4.
Mortality stratified by patient characteristics
| No. of patients, n (%) | No.a | Mortality |
|---|---|---|
| Overall | 83 803 | 307 (0.4) |
| Likely related to rhGH treatmentb | 83 803 | 24 (0.0) |
| Diagnosis of growth disorder | ||
| Idiopathic GHD | 39 298 | 37 (0.1) |
| Neurosecretory dysfunction | 2187 | 4 (0.2) |
| Congenital GHD | 3323 | 24 (0.7) |
| Craniopharyngioma | 1381 | 24 (1.7) |
| Medulloblastoma | 998 | 37 (3.7) |
| Other cranial tumors | 1750 | 44 (2.5) |
| Extracranial malignancy | 940 | 21 (2.2) |
| Idiopathic short stature | 6867 | 2 (0.0) |
| Turner syndrome | 7714 | 14 (0.2) |
| Prader-Willi syndrome | 2338 | 12 (0.5) |
| Other syndromes | 2602 | 17 (0.7) |
| Small for gestational age | 7936 | 7 (0.1) |
| Chronic renal failure | 2399 | 37 (1.5) |
| Other causes | 4070 | 27 (0.7) |
| Sex | ||
| Female | 35 183 | 133 (0.4) |
| Male | 48 620 | 174 (0.4) |
| Duration of rhGH treatment, y | ||
| < 2 | 32 533 | 168 (0.5) |
| ≥ 2 | 51 270 | 139 (0.3) |
| Attained agec, y | ||
| < 5 | 33 (10.8) | |
| 5-<10 | 56 (18.2) | |
| 10-<15 | 98 (31.9) | |
| 15-<20 | 87 (28.3) | |
| ≥ 20 | 33 (10.8) |
Abbreviations: GHD, growth hormone deficiency; rhGH, recombinant human growth hormone.
No. was the denominator used to calculate percentages in each row unless noted otherwise.
According to investigators’ opinion.
Percentages were calculated with the number of total deaths (n = 307) as a denominator.
Cause of death was available for 280 patients, while causality could not be determined for 27 because of missing information. Most died of neoplasms (by MedDRA SOC; n = 84), primarily in patients with medulloblastoma (n = 27), other cranial tumors (n = 25), extracranial malignancy (n = 14), and craniopharyngioma (n = 9). The most common specified causes by MedDRA PT (in ≥ 5 patients) included neoplasm recurrence (benign, malignant, or unspecified; n = 19), recurrent cancer (n = 17), craniopharyngioma (n = 7), brain neoplasm (new or recurrent; n = 6), ill-defined disorder (n = 6), cerebral hemorrhage (n = 5), convulsion (n = 5), glioblastoma (n = 5), and pneumonia (n = 5). Among 24 deaths due to causes assessed as likely treatment related, 20 were tumor related, 1 was due to intracranial hemorrhage, and 3 had unspecified causes (Supplementary Table 11) (16). Among the 5 patients who died of cerebral hemorrhage, 3 were treated for GHD (craniopharyngioma, neurosecretory dysfunction, and congenital GHD), 1 for TS, and 1 for CRF.
Efficacy
Efficacy during the first year
Among patients of the efficacy cohort who remained prepubertal through the first year of rhGH exposure, median height-SDS at rhGH start was −3.72 (−5.64 to −2.45) to −2.02 (−3.84 to −0.21) (Table 5). Pubertal patients (pubertal at treatment start or entered puberty during year 1) had somewhat higher initial height-SDS vs prepubertal patients (see Table 5). After 1 year of treatment, height-SDS increased in all diagnostic groups. Median gain in height-SDS in prepubertal patients was the greatest with congenital GHD (1.01 [0.15 to 2.22]), while those with extracranial malignancy had the smallest gain (0.38 [−0.06 to 0.86]) (see Table 5). Pubertal patients had lower gains in height-SDS compared with prepubertal patients (see Table 5).
Table 5.
Growth outcomes at year 1 in the efficacy cohort
| Diagnosis | Prepubertala | Pubertala | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Initial Ht-SDS | Y 1 Ht-SDS | Delta Ht-SDS | n | Initial Ht-SDS | Y 1 Ht-SDS | Delta Ht-SDS | |
| IGHD | 13 882 | −3.15 (−4.65 to −2.11) |
−2.45 (−3.83 to −1.37) |
0.66 (0.22 to 1.40) |
8365 | −2.49 (−3.55 to −1.55) |
−2.00 (−3.12 to −0.99) |
0.48 (0.02 to 0.96) |
| NSD | 800 | −2.96 (−4.08 to −2.13) |
−2.30 (−3.38 to −1.47) |
0.63 (0.23 to 1.12) |
592 | −2.59 (−3.58 to −1.72) |
−2.14 (−3.23 to −1.09) |
0.41 (−0.04 to 0.89) |
| congGHD | 1740 | −3.40 (−5.53 to −1.56) |
−2.32 (−4.41 to −0.37) |
1.01 (0.15 to 2.22) |
340 | −2.65 (−4.34 to −1.01) |
−2.14 (−3.70v−0.43) |
0.49 (−0.06 to 1.14) |
| CP | 652 | −2.25 (−4.13 to −0.40) |
−1.51 (−3.44 to 0.34) |
0.75 (0.07 to 1.50) |
255 | −2.10 (−3.64 to −0.17) |
−1.43 (−2.99 to 0.34) |
0.52 (−0.12 to 1.18) |
| MB | 306 | −2.10 (−3.62 to −0.78) |
−1.58 (−3.24 to −0.29) |
0.51 (0.07 to 0.90) |
334 | −1.57 (−3.10 to −0.14) |
−1.27 (−2.80 to 0.18) |
0.32 (−0.14 to 0.84) |
| OCT | 512 | −2.23 (−4.00 to −0.51) |
−1.67 (−3.38 to 0.13) |
0.59 (0.01 to 1.25) |
585 | −1.45 (−3.26 to 0.35) |
−1.05 (−2.73 to 0.75) |
0.45 (−0.16 to 1.08) |
| ECM | 265 | −2.26 (−3.66 to −0.85) |
−1.96 (−3.39 to −0.37) |
0.38 (−0.06 to 0.86) |
358 | −1.52 (−3.01 to −0.26) |
−1.25 (−2.78 to 0.08) |
0.28 (−0.18 to 0.83) |
| ISS | 2125 | −3.20 (−4.35 to −2.30) |
−2.62 (−3.83 to −1.63) |
0.55 (0.18 to 1.00) |
1443 | −2.61 (−3.67 to −1.43) |
−2.19 (−3.36 to −0.99) |
0.39 (−0.10 to 0.85) |
| TS | 4067 | −3.27 (−4.52 to −2.10) |
−2.68 (−4.05 to −1.44) |
0.58 (0.05 to 1.08) |
1269 | −3.14 (−4.65 to −1.97) |
−2.72 (−4.23 to −1.48) |
0.44 (−0.08 to 0.93) |
| PWS | 1198 | −2.02 (−3.84 to −0.21) |
−1.02 (−2.84 to 0.73) |
0.94 (0.12 to 1.78) |
224 | −1.81 (−3.57 to 0.50) |
−1.40 (−3.35 to 0.84) |
0.30 (−0.29 to 1.14) |
| OS | 1290 | −3.72 (−5.64 to −2.45) |
−3.05 (−4.81 to −1.75) |
0.63 (0.16 to 1.30) |
388 | −2.93 (−4.34 to −1.19) |
−2.54 (−4.06 to −0.74) |
0.40 (−0.08 to 0.87) |
| SGA | 3487 | −3.25 (−4.48 to −2.30) |
−2.51 (−3.76 to −1.57) |
0.71 (0.30 to 1.23) |
997 | −2.68 (−3.73 to −1.76) |
−2.18 (−3.37 to −1.19) |
0.48 (−0.03 to 0.99) |
| CRF | 935 | −3.07 (−4.84 to −1.83) |
−2.40 (−4.20 to −1.07) |
0.63 (−0.06 to 1.41) |
408 | −2.56 (−4.62 to −1.34) |
−2.20 (−4.24 to −0.76) |
0.41 (−0.19 to 1.04) |
| Other | 1416 | −3.37 (−5.17 to −1.93) |
−2.79 (−4.59 to −1.28) |
0.56 (−0.01 to 1.28) |
874 | −2.51 (−3.98 to −0.63) |
−2.12 (−3.55 to −0.32) |
0.36 (−0.14 to 0.94) |
Data are presented as median (10th-90th percentile) unless noted otherwise.
Abbreviations: congGHD, congenital GHD; CP, craniopharyngioma; CRF, chronic renal failure; ECM, extracranial malignancy; GHD, growth hormone deficiency; Ht-SDS, height SD score; IGHD, idiopathic GHD; ISS, idiopathic short stature; MB, medulloblastoma; NSD, neurosecretory dysfunction; OCT, other cranial tumors; OS, other syndromes; PWS, Prader-Willi syndrome; SGA, small for gestational age; TS, Turner syndrome.
Patients who remained prepubertal during year 1.
Patients who already reached puberty at treatment start or went into puberty during year 1.
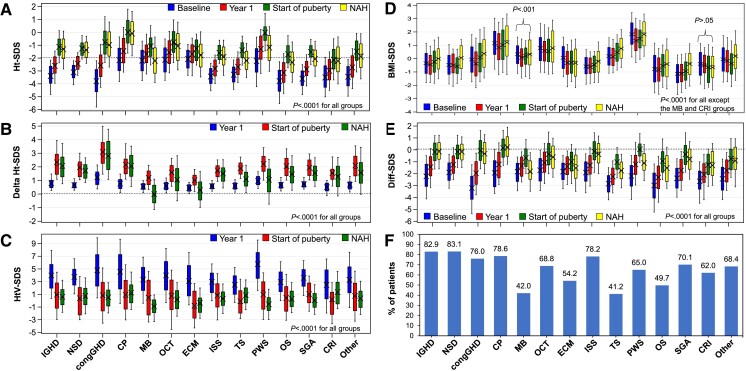
Efficacy to near-adult height
In the NAH subgroup, prepubertal height-SDS increased from baseline to year 1 and was higher at puberty onset (Fig. 3A). Median height-SDS at NAH was within normal range (> −2 SD) in patients with GHD diagnoses except for medulloblastoma, and in patients with ISS, PWS, and other causes (see Fig. 3A). Median total gain in NAH-SDS was greater than 0 in all diagnostic groups, except for patients with medulloblastoma (−0.13 [−1.14 to 1.10]). The greatest NAH-SDS gain was in patients with congenital GHD (2.77 [1.08-4.77]), followed by craniopharyngioma (1.98 [0.56-3.52]) and IGHD (1.89 [0.75-3.72]) (Fig. 3B). Longitudinal analysis showed larger height-SDS gains at puberty onset than year 1 for all diagnoses (see Fig. 3B). However, a decrease in delta height-SDS from puberty onset to NAH was observed in almost all diagnostic groups (see Fig. 3B). For patients with medulloblastoma or extracranial malignancy, median delta height-SDS at NAH was lower vs year 1 (see Fig. 3B). Height velocity-SDS was the highest at year 1, dropped to approximately 1 SD at puberty onset for most diagnoses, and remained similar at NAH (Fig. 3C). Changes in BMI-SDS over time were generally small (Fig. 3D). Median BMI-SDS was the highest in patients with PWS at all 4 time points evaluated, but remained less than +2.0 SD; it was lower at year 1 and puberty onset, but was similar at NAH, when compared with baseline (see Fig. 3D).
Figure 3.
Growth outcomes in the NAH subgroup (data from girls and boys combined). A, Height-SDS. B, Changes in height-SDS vs start of rhGH treatment. C, Height velocity-SDS. D, BMI-SDS. E, Difference between height-SDS and MPH-SDS. F, Percentage of patients achieving an NAH within the range of MPH ± 1.5 SDS. BMI, body mass index; congGHD, congenital GHD; CP, craniopharyngioma; CRF, chronic renal failure; Diff-SDS, height SDS minus mid-parental SDS; ECM, extracranial malignancy; GHD, growth hormone deficiency; Ht-SDS, height-SDS; HtV, height velocity; IGHD, idiopathic GHD; ISS, idiopathic short stature; MB, medulloblastoma; NAH, near-adult height; NSD, neurosecretory dysfunction; OCT, other cranial tumors; OS, other syndromes; PWS, Prader-Willi syndrome; rhGH, recombinant human growth hormone; SDS, SD score; SGA, small for gestational age; TS, Turner syndrome. SDS was calculated based on Prader references (20) for height and the reference by Cole et al (21) for BMI.
Height-SDS minus MPH-SDS (Diff-SDS) also increased over time during follow-up until patients reached puberty, although its median was less than 0 for most diagnoses at any given time point (Fig. 3E). However, for patients with medulloblastoma, median Diff-SDS at NAH was lower than at baseline (−1.91 [−3.48 to −0.24] vs −1.66 [−3.45 to −0.60]) (see Fig. 3E). Patients with craniopharyngioma had the highest median Diff-SDS at NAH (0.15 [−1.24 to 1.91]) (see Fig. 3E). At NAH, 68.7% of all patients reached MPH ±1.5 SDS, 60.2% in girls and 77.9% in boys; the percentage was the highest in patients with neurosecretory dysfunction (83.1%), IGHD (82.9%), and craniopharyngioma (78.6%); and the lowest in those with medulloblastoma (42.0%) and TS (41.2%) (Fig. 3F).
Median age at the start of puberty (either induced or spontaneous) was the youngest both in boys (12.2 years [10.3-14.7 years]) and girls (10.8 years [9.4-13.5]) treated for medulloblastoma compared with other diagnoses (Tables 6 and 7). Girls with PWS also started puberty at a similarly young age (10.8 years [8.2-13.3]), although boys with PWS started puberty later (13.6 years; P = .0004 vs girls with PWS). Additionally, girls had more prepubertal gains in height-SDS than boys in most of the diagnostic groups (see Tables 6 and 7). Total gains in NAH-SDS were significantly higher in girls vs boys diagnosed with IGHD (P < .0001), neurosecretory dysfunction (P = .0079), congenital GHD (P < .0001), medulloblastoma (P = .0068), other cranial tumors (P = .0085), ISS (P = .0056), and other syndromes (P = .0006).
Table 6.
Growth outcomes in boys of the near-adult height subgroup
| Diagnosis | No.a | rhGH start | Puberty onseta | NAH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Ht-SDS | Age, y | Ht-SDS | Prepubertal gain | Age, y | Ht-SDS | Pubertal gaina | Total gain | ||
| IGHD | 2057 | 8.6 (4.5 to 12.1) |
−2.99 (−4.44 to −2.06) |
13.0 (11.4 to 15.2) |
−1.13 (−2.37 to 0.09) |
1.86 (0.98 to 3.65) |
17.7 (16.5 to 19.9) |
−1.16 (−2.67 to 0.13) |
0.16 (−0.76 to 0.97) |
1.79 (0.72 to 3.41) |
| NSD | 108 | 9.6 (5.4 to 11.7) |
−2.90 (−3.81 to −2.13) |
13.3 (11.7 to 14.5) |
−1.15 (−2.08 to −0.40) |
1.71 (0.96 to 2.83) |
17.3 (16.3 to 18.7) |
−1.30 (−2.36 to −0.43) |
0.11 (−0.46 to 0.51) |
1.52 (0.80 to 2.58) |
| congGHD | 227 | 6.1 (1.8 to 11.4) |
−3.43 (−4.87 to −2.20) |
13.1 (11.4 to 15.2) |
−0.73 (−2.23 to 1.29) |
2.61 (1.33 to 4.76) |
18.0 (16.5 to 20.0) |
−0.93 (−2.55 to 0.95) |
0.18 (−0.89 to 0.87) |
2.42 (0.95 to 4.18) |
| CP | 127 | 8.9 (4.7 to 12.4) |
−2.20 (−3.65 to −0.40) |
13.5 (12.2 to 16.6) |
−0.06 (−1.57 to 2.16) |
1.90 (1.14 to 3.24) |
18.7 (17.0 to 20.6) |
−0.07 (−1.85 to 1.40) |
−0.08 (−0.89 to 0.79) |
1.90 (0.56 to 3.25) |
| MB | 98 | 8.6 (6.1 to 11.6) |
−1.99 (−3.12 to −0.76) |
12.2 (10.3 to 14.7) |
−0.77 (−2.12 to 0.84) |
1.21 (0.38 to 1.91) |
17.6 (16.3 to 19.8) |
−2.14 (−3.85 to −0.36) |
−1.38 (−2.09 to −0.16) |
−0.23 (−1.19 to 0.98) |
| OCT | 125 | 9.7 (7.1 to 12.3) |
−2.02 (−3.36 to −0.51) |
13.0 (11.7 to 14.8) |
−0.73 (−1.99 to 1.04) |
1.35 (0.69v2.10) |
17.7 (16.4 to 20.3) |
−0.96 (−2.99 to 0.66) |
−0.31 (−1.01 to 0.51) |
0.82 (−0.51 to 2.29) |
| ECM | 87 | 10.4 (8.1 to 12.5) |
−1.95 (−3.28 to −1.01) |
13.4 (11.9 to 15.2) |
−1.20 (−2.47 to −0.29) |
1.04 (0.28 to 1.66) |
18.0 (16.6 to 20.2) |
−1.71 (−3.62 to 0.14) |
−0.77 (−2.00 to 0.20) |
0.34 (−1.05 to 1.65) |
| ISS | 230 | 9.6 (5.7 to 12.1) |
−3.06 (−4.22 to −2.28) |
12.8 (11.6 to 15.1) |
−1.52 (−2.47 to −0.70) |
1.45 (0.77 to 2.09) |
17.7 (16.5 to 19.9) |
−1.65 (−3.25 to −0.55) |
0.01 (−0.90 to 0.76) |
1.37 (0.44 to 2.25) |
| PWS | 71 | 8.6 (5.0 to 12.0) |
−2.08 (−3.38 to −0.37) |
13.6 (11.4 to 15.0) |
0.11 (−1.71 to 1.53) |
2.27 (1.02 to 3.42) |
18.0 (16.5 to 19.8) |
−0.87 (−2.40 to 0.56) |
−1.03 (−1.42 to −0.13) |
1.31 (−0.55 to 2.46) |
| OS | 183 | 8.8 (4.1 to 11.6) |
−3.41 (−4.86 to −2.21) |
13.1 (11.6 to 14.9) |
−1.73 (−2.76 to −0.21) |
1.52 (0.80 to 3.33) |
17.8 (16.4 to 19.6) |
−2.18 (−3.63 to −0.60) |
−0.29 (−1.27 to 0.37) |
1.31 (−0.01 to 2.66) |
| SGA | 149 | 8.8 (4.4 to 11.5) |
−3.20 (−4.70 to −2.28) |
12.7 (11.5 to 14.4) |
−1.45 (−2.43 to −0.61) |
1.58 (0.87 to 2.59) |
17.2 (16.3v19.1) |
−1.88 (−3.18 to −0.61) |
−0.14 (−0.91 to 0.91) |
1.34 (0.52 to 2.61) |
| CRF | 93 | 9.6 (5.1 to 12.4) |
−3.19 (−4.59 to −1.93) |
12.7 (11.3 to 15.6) |
−2.16 (−2.85 to −0.81) |
1.48 (0.92 to 1.89) |
17.9 (16.5v20.2) |
−2.01 (−4.01 to −0.35) |
0.20 (−1.67 to 0.98) |
1.30 (−0.18 to 2.46) |
| Other | 200 | 9.0 (4.8 to 12.0) |
−3.15 (−4.73 to −1.77) |
13.3 (11.4 to 15.8) |
−1.41 (−3.28 to −0.20) |
1.75 (0.56 to 2.93) |
17.9 (16.4 to 19.6) |
−1.48 (−3.51 to 0.13) |
−0.20 (−1.33 to 1.02) |
1.51 (0.02 to 3.17) |
Data are presented as median (10th, 90th percentile) unless noted otherwise.
Abbreviations: congGHD, congenital GHD; CP, craniopharyngioma; CRF, chronic renal failure; ECM, extracranial malignancy; GHD, growth hormone deficiency; Ht-SDS, height SD score; IGHD, idiopathic GHD; ISS, idiopathic short stature; MB, medulloblastoma; NAH, near-adult height; NSD, neurosecretory dysfunction; OCT, other cranial tumors; OS, other syndromes; PWS, Prader-Willi syndrome; rhGH, recombinant human growth hormone; SGA, small for gestational age.
For puberty onset and pubertal gain at NAH, N = 703 (IGHD), 23 (NSD), 97 (congGHD), 71 (CP), 29 (MB), 31 (OCT), 20 (ECM), 83 (ISS), 16 (PWS), 64 (OS), 54 (SGA), 23 (CRF), and 56 (Other).
Table 7.
Growth outcomes in girls of the near-adult height subgroup
| Diagnosis | No.a | rhGH start | Puberty onseta | NAH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Ht-SDS | Age, y | Ht-SDS | Prepubertal gain | Age, y | Ht-SDS | Pubertal gaina | Total gain | ||
| IGHD | 1186 | 7.6 (3.8 to 11.0) |
−3.62 (−5.41 to −2.46) |
11.9 (10.3 to 14.3) |
−1.13 (−2.35 to 0.19) |
2.45 (1.37 to 4.37) |
16.1 (14.5 to 18.7) |
−1.47 (−3.07 to 0.07) |
−0.18 (−1.05 to 0.70) |
2.07 (0.80 to 4.24) |
| NSD | 58 | 8.8 (5.0 to 11.1) |
−3.33 (−4.88 to −2.40) |
12.2 (10.9 to 14.5) |
−1.39 (−2.22 to 0.16) |
2.12 (1.30 to 3.74) |
15.7 (14.2 to 17.5) |
−1.40 (−2.73 to −0.24) |
−0.24 (−1.17 to 0.65) |
1.90 (0.91 to 2.96) |
| congGHD | 201 | 5.4 (1.3 to 9.3) |
−4.10 (−6.45 to −2.21) |
12.4 (10.0 to 14.5) |
−1.02 (−2.36 to 0.68) |
3.26 (2.16 to 5.86) |
16.8 (14.9 to 19.0) |
−1.13 (−2.81 to 0.61) |
−0.13 (−1.06 to 0.75) |
3.10 (1.32 to 5.16) |
| CP | 121 | 8.4 (4.6 to 11.3) |
−2.14 (−3.95 to −0.35) |
13.1 (11.1 to 15.9) |
0.03 (−1.69 to 1.58) |
1.90 (1.10 to 4.16) |
17.8 (15.5 to 20.6) |
−0.05 (−1.73 to 1.72) |
0.25 (−0.80 to 1.16) |
2.26 (0.59 to 4.08) |
| MB | 59 | 8.1 (4.9, 10.9) |
−2.52 (−4.38 to −0.63) |
10.8 (9.4 to 13.5) |
−1.81 (−2.92 to −0.73) |
1.21 (−0.21 to 2.59) |
16.6 (14.5 to 19.4) |
−2.20 (−4.14 to −0.37) |
−1.02 (−1.72 to −0.18) |
0.40 (−0.82 to 1.56) |
| OCT | 98 | 8.7 (5.6 to 11.6) |
−2.24 (−4.11 to −0.45) |
12.9 (8.9 to 16.4) |
−1.13 (−2.46 to 0.73) |
1.85 (0.54 to 3.05) |
17.1 (14.6 to 20.3) |
−0.97 (−2.89 to 0.87) |
−0.29 (−1.34 to 1.08) |
1.26 (−0.42 to 2.99) |
| ECM | 44 | 9.2 (6.7 to 11.0) |
−1.97 (−3.52 to −0.85) |
11.9 (10.3 to 13.5) |
−1.10 (−2.58, 0.15) |
1.00 (0.16 to 1.95) |
16.3 (14.9 to 19.9) |
−1.68 (−3.38 to −0.17) |
−1.09 (−1.66 to −0.48) |
0.28 (−0.76 to 1.76) |
| ISS | 129 | 8.1 (3.8 to 11.5) |
−3.72 (−4.79 to −2.80) |
11.8 (10.5 to 13.9) |
−1.74 (−2.37, −0.34) |
2.10 (1.30 to 2.61) |
16.2 (14.7 to 18.7) |
−2.17 (−3.67 to −0.62) |
−0.34 (−0.84 to 0.24) |
1.62 (0.56 to 2.85) |
| TS | 1631 | 8.0 (4.5 to 11.2) |
−3.28 (−4.43 to −2.14) |
13.2 (11.4 to 15.0) |
−1.38 (−2.55, −0.29) |
1.84 (1.13 to 2.72) |
16.7 (15.0 to 18.5) |
−2.18 (−3.51 to −0.85) |
−0.77 (−1.45 to −0.04) |
1.07 (0.10 to 2.12) |
| PWS | 82 | 7.4 (4.3 to 10.1) |
−2.48 (−4.39 to −0.82) |
10.8 (8.2 to 13.3) |
−0.24 (−1.44 to 0.72) |
2.30 (0.49 to 4.42) |
16.8 (14.5 to 19.1) |
−1.41 (−3.14 to 0.14) |
−0.86 (−2.02 to 0.18) |
1.35 (−0.75 to 2.60) |
| OS | 145 | 7.5 (4.0 to 10.9) |
−4.27 (−5.90 to −2.55) |
12.2 (10.3 to 15.0) |
−1.90 (−2.81 to −0.43) |
2.37 (1.38 to 3.52) |
16.1 (14.6 to 18.4) |
−2.46 (−3.92 to −0.96) |
−0.69 (−1.52 to 0.30) |
1.64 (0.44 to 3.04) |
| SGA | 185 | 7.6 (4.1 to 10.1) |
−3.72 (−4.94 to −2.83) |
11.1 (9.2 to 13.2) |
−1.57 (−2.60 to −0.52) |
2.16 (1.24 to 3.34) |
15.4 (14.3 to 17.0) |
−2.10 (−3.55 to −0.88) |
−0.36 (−1.30 to 0.33) |
1.57 (0.59 to 2.76) |
| CRF | 49 | 9.3 (5.2 to 13.3) |
−3.76 (−6.13 to −1.85) |
12.7 (11.3 to 16.7) |
−2.80 (−3.89 to −0.31) |
1.79 (−0.27 to 3.22) |
16.5 (15.1 to 19.7) |
−2.18 (−4.79 to −0.24) |
−0.28 (−1.45 to 1.47) |
1.59 (−0.14 to 3.30) |
| Other | 164 | 8.1 (4.5 to 10.9) |
−3.67 (−5.19 to −2.45) |
13.1 (11.2 to 15.0) |
−1.43 (−2.88 to −0.04) |
2.34 (1.34 to 4.02) |
16.3 (14.6 to 18.8) |
−1.96 (−3.94 to −0.20) |
−0.23 (−1.47 to 0.68) |
1.67 (−0.02 to 3.55) |
Data are presented as median (10th-90th percentile) unless noted otherwise.
Abbreviations: congGHD, congenital GHD; CP, craniopharyngioma; CRF, chronic renal failure; ECM, extracranial malignancy; GHD, growth hormone deficiency; Ht-SDS, height SD score; IGHD, idiopathic GHD; ISS, idiopathic short stature; MB, medulloblastoma; NAH, near-adult height; NSD, neurosecretory dysfunction; OCT, other cranial tumors; OS, other syndromes; PWS, Prader-Willi syndrome; rhGH, recombinant human growth hormone; SGA, small for gestational age; TS, Turner syndrome.
For puberty onset and pubertal gain at NAH, N = 434 (IGHD), 17 (NSD), 74 (congGHD), 57 (CP), 17 (MB), 33 (OCT), 13 (ECM), 47 (ISS), 777 (TS), 17 (PWS), 56 (OS), 57 (SGA), 15 (CRF), and 42 (Other).
Responses to rhGH were compared between patients in Europe, the United States, and Japan (which comprised > 90% of the full KIGS cohort). Among the 6 main indications for rhGH therapy, TS, PWS, CRF, and SGA were excluded from the analysis as fewer than 35 patients reached NAH in these groups from at least 1 of the 3 geographical areas; outcomes were compared for patients with IGHD and ISS (Supplementary Table 12) (16). Initial rhGH dose varied by area, being lowest in Japan and highest in the United States for both diagnoses. Japanese patients started treatment earlier than US and European patients. Median delta height-SDS was the smallest in Japan at year 1 regardless of puberty status and at puberty onset for both diagnostic groups. Patients with IGHD in the United States and Europe compared to those in Japan had higher NAH-SDS (approximately −1 SD vs −2 SD) and total gains in NAH-SDS (approximately 2 SD vs 1 SD). In patients with ISS, median NAH-SDS was the highest in US patients and similar between Japanese and European patients, while total gains in NAH-SDS were similar across the 3 areas. However, numbers of patients in the ISS group were low at NAH in Japan and the United States.
Discussion
Data on the full cohort of patients in KIGS showed that rhGH treatment was safe and well tolerated in pediatric patients with growth disorders as prescribed in real-world settings over a median observation period of 3 years. AEs/SAEs and deaths occurred at low frequencies, and the majority were assessed by investigators as not related to rhGH. rhGH treatment also was shown to be effective, as median height-SDS increased after 1 year of its use in all diagnostic groups both for prepubertal and pubertal patients. Total gains in height-SDS from treatment start to NAH were greater than 0 in most diagnostic groups.
Safety
The overall AE incidence rate of 94/1000 PY in the full KIGS cohort is lower than those in recent reports from the Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS; 175.3/1000 PY) (5) and the PATRO Children study (152.3/1000 PY) (13), which, similar to KIGS, collected all AEs regardless of causal relationship with rhGH treatment. However, given the nature of observational studies, the AE rate may be underestimated because of underreporting. Consistent with the findings in other surveillance studies (Supplementary Table 13 [16]) (5, 10, 26), SAE frequency varied largely by diagnosis, and was more prevalent in patients with organic GHD (congenital GHD, craniopharyngioma, medulloblastoma, other cranial tumors, and extracranial malignancy combined) and CRF, likely due to the preexisting risks with these diagnoses. A higher SAE frequency may be associated with a longer duration of rhGH exposure, although care should be taken when interpreting the statistical inferences, as small-magnitude differences could be statistically significant because of the large sample size.
The most prevalent AEs/SAEs in KIGS were generally common childhood illness or conditions associated with the underlying diagnoses. The most common AE (by MedDRA PT) was headache, which is a frequent complaint in children (27) and may be associated with intracranial hypertension. Younger patients or patients with craniopharyngioma had higher probability of reporting headache, in line with a previous KIGS report (28). The second most common AE was scoliosis, which is a common feature of patients with PWS or TS even when untreated and is not necessarily associated with rhGH treatment (3). As expected, we found that scoliosis was more commonly reported in patients with PWS than in patients with other indications. Other side effects that may be considered related to the use of rhGH, such as benign intracranial hypertension and epiphysiolysis, were infrequent in KIGS.
Previous reports of increased risks of mortality and hemorrhagic stroke after a mean follow-up of 17 years in French young adults with childhood rhGH treatment for GHD, ISS, or SGA from the Safety and Appropriateness of Growth hormone treatments in Europe (SAGhE) study raised safety concerns about pediatric rhGH therapy (29, 30). However, data from the entire data set of all 8 countries in SAGhE (7) and those from other rhGH registries of patients who underwent rhGH treatment during childhood did not support an association between rhGH use and premature death or stroke (5, 13, 14, 31–33). The increased mortality for the French SAGhE cohort compared with the general population was likely attributable to underlying etiologies (IGHD, ISS, SGA) rather than rhGH treatment. Our data in KIGS are reassuring, as intracranial hemorrhage events occurred at low frequencies in the full KIGS cohort and death in only 0.4% of patients during the study.
Our present analysis showed overall low frequencies of diabetes among 83 803 rhGH-treated children (35 type 1, 21 type 2, and 37 unspecified). Data from large observational studies, including a previous KIGS report on 23 333 patients, found no increased risk of type 1 diabetes during rhGH therapy (5, 10, 34); while the incidence of type 2 diabetes was higher than expected, it was likely driven by previous patient predisposition (10, 34). It is therefore important to monitor glucose homeostasis in patients with risk factors for developing type 2 diabetes when treating with rhGH (35).
Clinical evidence does not support an association of childhood rhGH therapy with neoplasia nor an increased risk of cancer recurrence in low-risk patients (36). A previous KIGS analysis assessing cancer incidence after a mean 3.6 years of rhGH exposure found no evidence of increased cancer risk (standardized incidence ratio [SIR] 1.26; 95% CI, 0.86-1.78) (37), which was corroborated by the results from both GeNeSIS (5) and the National Cooperative Growth Study (NCGS) (10). Although the risk of all-site cancer for patients with a history of childhood rhGH treatment was significantly increased in SAGhE (SIR 2.2; 95% CI, 1.9-2.6), that for patients with noncancer initial diagnoses was not (SIR 1.2; 95% CI, 1.0-1.6) (38). However, the same SAGhE report noted significantly increased risks for bone and bladder cancer in patients without prior cancer, suggesting potential effects of rhGH therapy on these cancer types (38). It should be noted, though, that the SAGhE study followed patients for an extended period of time into adulthood (∼ 15 years per patient), even after discontinuation of rhGH treatment (38), which allowed for detection of slow developing events during follow-up. In the present report, neoplasm AEs were reported in 1% of patients, comparable to the 1.6% reported in the PATRO Children study (13). Additionally, bone tumor events in KIGS were rare. Craniopharyngioma recurred in 11.9% of patients with that diagnosis, in agreement with the previously reported rate (11.7%) in KIGS after a median rhGH exposure of 2.8 years (39) and comparable to those observed in NCGS (8.7%) (10) and in GeNeSIS (13.7%) (5). Longer follow-up of KIGS patients and comparison with untreated patients would be necessary to better assess the incidence of cancer or tumor recurrence and its causal relationship with rhGH therapy.
Efficacy
Response to first-year rhGH treatment is considered an important indicator of future gains in height (40, 41). The efficacy of rhGH was shown in our analysis by positive prepubertal gains in height-SDS after 1 year of treatment in all diagnostic groups. These results are consistent with previous KIGS analyses of patients treated for GHD, TS, SGA, and PWS (42, 43), and are also in line with findings from postmarketing studies of other pediatric rhGH therapies (12, 13, 26, 44). Patients with extracranial malignancy had the smallest height-SDS gain at year 1, which could be at least partially related to the later initiation of rhGH vs other groups. Multiple regression analyses have revealed an inverse association between first-year growth response and age at rhGH start in children with GHD, ISS, and SGA, and girls with TS (45–47). Additionally, prior irradiation and body site of irradiation treatment could also affect growth responses to rhGH (48).
At NAH, patients in most diagnostic groups attained heights within the normal range (median height-SDS > –2), and the majority achieved MPH±1.5 SDS. Height-SDS gains were observed at puberty onset and at NAH both for patients with GHD and those with normal GH secretion, although patients with medulloblastoma had an overall loss in height-SDS at NAH vs rhGH start, despite age of treatment onset and rhGH dosage being comparable to patients with IGHD. Poor height response was also noted in a previous KIGS report on 113 patients with medulloblastoma who were rhGH treated until NAH (49). Relatively early puberty, prior craniospinal irradiation or chemotherapy, and/or cautious rhGH dosing could all affect the height response in patients with medulloblastoma. However, it is important to note that growth performance appears to be worse without rhGH treatment in this group of patients (50).
Some differences in growth response by patient characteristics were found. In general, girls appeared to have more prepubertal or total gains in height-SDS than boys. Timing of puberty initiation was not accelerated by rhGH treatment, but was slightly delayed compared to the estimated age of 9 to 11 years for girls and 10 to 12 years for boys in the general population (51, 52). We also found a geographical area–specific difference in growth outcomes; the smaller gains in NAH-SDS in Japanese patients vs US and European patients may be caused by an ethnic difference and/or by the lower rhGH doses given to Japanese patients.
Limitations and Strengths
Our study has limitations that are inherent to observational studies. Safety data reporting was at the discretion of investigators, and biases cannot be excluded because of AE underreporting and the lack of additional appraisal of causal relationships between AEs and rhGH treatment. Challenges in AE coding with MedDRA (eg, an AE being coded as different PTs, misinterpretation or bias by the medical coder) may also lead to inconsistency in AE reporting and potential underestimations of certain AEs (53, 54). Additionally, changes in GH assays over the time period, different stimulation tests and cutoffs used, and nonstandardized categorization may have led to inconsistencies in diagnoses; and some confounders (eg, adherence, socioeconomic factors, concomitant use of medications, heterogeneity of data by country) were not considered in our analysis. Approximately one-third of KIGS patients in the safety cohort were excluded from the efficacy analysis, which could also cause patient selection bias, although its effect on efficacy outcomes was estimated to be limited. Additionally, a single set of standards (Swiss references) was used in the efficacy analysis and the responses of patients of certain ethnicities/countries could be underestimated. In KIGS, patients were followed during pediatric rhGH treatment only, while ongoing, longer-term surveillance of these patients with posttreatment data is important to reveal longer-term sequelae such as cancer and cardiometabolic conditions as individuals attain ages at which these events occur more commonly.
Nevertheless, data from KIGS reflect real-life clinical practice situations that are complementary to clinical trials of rhGH therapy. In KIGS, a total of 83 803 patients were recruited globally and received rhGH, representing the largest cohort of rhGH-treated children studied to date. This large patient population and a long total observation of 322 576 PY makes it possible to collect rare events that cannot be observed in clinical trials. Patients were treated for a wide range of growth disorders and had characteristics representative of children with impaired growth commonly seen in clinical practice. Furthermore, additional classification of patients with GHD to different diagnostic groups in the present analysis allows for a more detailed review and comparison of the safety and efficacy by underlying disease.
Conclusions
Data compiled in KIGS, the largest and longest running global database of rhGH-treated children, complement results from clinical trials and confirm the safety and efficacy of pediatric rhGH therapy as used in clinical practice. The results for the full KIGS cohort of 83 803 patients with a follow-up of 322 576 PY reinforce that pediatric rhGH therapy effectively increases short-term height gain and adult height for patients with GHD and non-GHD conditions, with no new SAEs recognized.
Acknowledgments
The authors would like to acknowledge the medical writing assistance of Hui Zhang, PhD, and Kathleen Ohleth, PhD, CMPP, of Precise Publications, LLC, which was supported by Pfizer. We are grateful to all the participating patients and investigators for providing the data.
Abbreviations
- AE
adverse event
- BMI
body mass index
- CRF
chronic renal failure
- Diff-SDS
height-SDS minus MPH-SDS
- GeNeSIS
Genetics and Neuroendocrinology of Short Stature International Study
- GH
growth hormone
- GHD
growth hormone deficiency
- hGH
human growth hormone
- IGHD
idiopathic GHD
- KIGS
Kabi/Pfizer International Growth Database
- ISS
idiopathic short stature
- MedDRA
Medical Dictionary for Regulatory Activities
- MPH
mid-parental height
- NAH
near-adult height
- NCGS
National Cooperative Growth Study
- PT
preferred term
- PWS
Prader-Willi syndrome
- PY
patient-years
- rhGH
recombinant human growth hormone
- SAE
serious adverse event
- SAGhE
Safety and Appropriateness of Growth hormone treatments in Europe
- SGA
small for gestational age
- SIR
standardized incidence ratio
- SOC
system organ class
- TS
Turner syndrome
Contributor Information
Mohamad Maghnie, Department of Pediatrics, IRCCS Giannina Gaslini, Genova 16124, Italy; Department of Neuroscience, Rehabilitation, Ophthalmology, Genetics, Maternal and Child Health - DINOGMI, University of Genova, Genova 16124, Italy.
Michael B Ranke, Department of Pediatric Endocrinology, University Children´s Hospital, Tübingen 72076, Germany.
Mitchell E Geffner, The Saban Research Institute, Children's Hospital Los Angeles, Los Angeles, California 90027, USA.
Elpis Vlachopapadopoulou, Department of Endocrinology, Growth and Development, Aglaia Kyriakou Children's Hospital, Athens 11527, Greece.
Lourdes Ibáñez, Endocrinology, Pediatric Research Institute Sant Joan de Déu, Barcelona 08950, Spain; Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), Instituto de Salud Carlos III, Madrid 28029, Spain.
Martin Carlsson, Rare Disease, Biopharmaceuticals, Pfizer, New York, NY 10017, USA.
Wayne Cutfield, Liggins Institute, University of Auckland, Auckland 1142, New Zealand.
Raoul Rooman, PendoCon, Putte 2580, Belgium.
Roy Gomez, European Medical Affairs, Pfizer, Brussels 1070, Belgium.
Michael P Wajnrajch, Rare Disease, Biopharmaceuticals, Pfizer, New York, NY 10017, USA; Department of Pediatrics, New York University Langone Medical Center, New York, NY 10016, USA.
Agnès Linglart, Department of Pediatric Endocrinology and Diabetology for Children, AP-HP, Bicêtre Paris Saclay, Le Kremlin Bicêtre 94270, France; APHP, Reference Center for Rare Disorders of the Calcium and Phosphate Metabolism, Filière OSCAR and Plateforme d’Expertise Maladies Rares Paris-Sud, Bicêtre Paris Saclay Hospital, Le Kremlin Bicêtre 94270, France.
Renata Stawerska, Department of Endocrinology and Metabolic Diseases, Polish Mother’s Memorial Hospital-Research Institute, Lodz 93-338, Poland; Department of Pediatric Endocrinology, Medical University of Lodz, Lodz 93-338, Poland.
Peter E Clayton, Developmental Biology and Medicine, Faculty of Biology Medicine and Health, Manchester NIHR Academic Health Science Centre, University of Manchester, Manchester M13 9PL, UK.
Feyza Darendeliler, İstanbul University, Istanbul Faculty of Medicine, Pediatric Endocrinology Unit, İstanbul 34452, Turkey.
Anita C S Hokken-Koelega, Pediatrics, Subdivision of Endocrinology, Erasmus University Medical Center, Rotterdam 3015 GD, the Netherlands.
Reiko Horikawa, Division of Endocrinology and Metabolism, National Center for Child Health and Development, Tokyo 157-8535, Japan.
Toshiaki Tanaka, Tanaka Growth Clinic, Tokyo 158-0097, Japan.
Helmuth-Günther Dörr, Division of Pediatric Endocrinology, Department of Pediatrics and Adolescent Medicine, Friedrich-Alexander University of Erlangen-Nürnberg, Erlangen 91054, Germany.
Kerstin Albertsson-Wikland, Department of Physiology/Endocrinology, Institute of Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Gothenburg 405 30, Sweden.
Michel Polak, Université de Paris Cité; Hôpital Universitaire Necker Enfants Malades, Paris 75015, France.
Adda Grimberg, Division of Pediatric Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania 19104, USA.
Financial Support
The KIGS patient database was created and supported by Pfizer, Inc. Medical writing support was provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, CMPP, at Precise Publications, LLC and was funded by Pfizer.
Disclosures
M.M. has received research support from Merck Serono and Pfizer, and has consulted for Ascendis, BioMarin, Ferring, Merck Serono, Novo Nordisk, Pfizer, and Sandoz. M.B.R. has received speaker fees from Mediagnost, Merck, Pfizer, and Sandoz. M.E.G. has a research contract from Novo Nordisk; serves as a consultant for and/or on advisory boards for Adrenas, Eton Pharmaceuticals, Neurocrine Biosciences, Novo Nordisk, and Pfizer; is a member of a data safety monitoring board for Aeterna Zentaris and Ascendis; and receives royalties from McGraw-Hill and UpToDate. E.V. has received research support from Ascendis, OPKO, and Pfizer. W.C. has received research support from Pfizer. R.R. is a past member of the KIGS Strategic Advisory Board, and serves as a consultant for Pfizer. A.L. has received speaker fees from Alexion, Kyowa Kirin, Novo Nordisk, Pfizer, and Sandoz. A.C.S.H.-K. is a past member of the KIGS Strategic Advisory Board; recipient of investigator-initiated independent research grants from Novo Nordisk and Pfizer; and has received lecture fees from Merck-Serono, Novo Nordisk, and Pfizer. T.T. has consulted for JCR Pharmaceuticals. H.G.D. has received honoraria for lectures from Ferring, Ipsen, Novo Nordisk, and Pfizer. M.P. is on the advisory board for IPSEN Increlex Registry, Novo Nordisk, and Pfizer France; has received grants from Ipsen, Merck, Novo Nordisk, Pfizer, Sandoz, and Sanofi; and has French institutional public grants from ANR PHRC. A.G. has consulted for Pfizer and received an investigator-initiated independent research grant from Pfizer. R.S. has received independent research support from OPKO, Pfizer, and Sandoz. M.C., R.G., and M.P.W. are employees and stockholders/stock grant holders of Pfizer. L.I., P.E.C., F.D., R.H., and K.A.-W. have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Raben MS. Treatment of a pituitary dwarf with human growth hormone. J Clin Endocrinol Metab. 1958;18(8):901–903. [DOI] [PubMed] [Google Scholar]
- 2. Ranke MB, Wit JM. Growth hormone—past, present and future. Nat Rev Endocrinol. 2018;14(5):285–300. [DOI] [PubMed] [Google Scholar]
- 3. Allen DB, Backeljauw P, Bidlingmaier M, et al. GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol. 2016;174(2):P1–P9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Santen HM. Safety of GH after treatment for childhood cancer. Eur J Endocrinol. 2020;183(6):C15–C18. [DOI] [PubMed] [Google Scholar]
- 5. Child CJ, Zimmermann AG, Chrousos GP, et al. Safety outcomes during pediatric GH therapy: final results from the prospective GeNeSIS observational program. J Clin Endocrinol Metab. 2019;104(2):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stochholm K, Kiess W. Long-term safety of growth hormone—a combined registry analysis. Clin Endocrinol (Oxf). 2018;88(4):515–528. [DOI] [PubMed] [Google Scholar]
- 7. Savendahl L, Cooke R, Tidblad A, et al. Long-term mortality after childhood growth hormone treatment: the SAGhE cohort study. Lancet Diabetes Endocrinol. 2020;8(8):683–692. [DOI] [PubMed] [Google Scholar]
- 8. Wilton P. KIGS: structure and organization. In: Ranke MBPrice DA and Reiter OE, eds. Growth Hormone Therapy in Pediatrics—20 Years of KIGS. Karger; 2007:1–5. [Google Scholar]
- 9. Ranke MB, Lindberg A, Tanaka T, Camacho-Hübner C, Dunger DB, Geffner ME. Baseline characteristics and gender differences in prepubertal children treated with growth hormone in Europe, USA, and Japan: 25 years’ KIGS experience (1987-2012) and review. Horm Res Paediatr. 2017;87(1):30–41. [DOI] [PubMed] [Google Scholar]
- 10. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. [DOI] [PubMed] [Google Scholar]
- 11. Sävendahl L, Polak M, Backeljauw P, et al. Treatment of children with GH in the United States and Europe: long-term follow-up from NordiNet IOS and ANSWER Program. J Clin Endocrinol Metab. 2019;104(10):4730–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfäffle R, Land C, Schönau E, et al. Growth hormone treatment for short stature in the USA, Germany and France: 15 years of surveillance in the Genetics and Neuroendocrinology of Short-Stature International Study (GeNeSIS). Horm Res Paediatr. 2018;90(3):169–180. [DOI] [PubMed] [Google Scholar]
- 13. Pfäffle R, Bidlingmaier M, Kreitschmann-Andermahr I, et al. Safety and effectiveness of Omnitrope, a biosimilar recombinant human growth hormone: more than 10 years’ experience from the PATRO Children Study. Horm Res Paediatr. 2020;93(3):154–163. [DOI] [PubMed] [Google Scholar]
- 14. Sävendahl L, Polak M, Backeljauw P, et al. Long-term safety of growth hormone treatment in childhood: two large observational studies: NordiNet IOS and ANSWER. J Clin Endocrinol Metab. 2021;106(6):1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranke MB. The Kabi Pharmacia International Growth Study: aetiology classification list with comments. Acta Paediatr Scand Suppl. 1991;379:87–92. [DOI] [PubMed] [Google Scholar]
- 16. Maghnie M, Ranke MB, Geffner ME, et al. Supplemental data for “Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort.” 2022. Uploaded September 5, 2022. https://figshare.com/s/78ef3f75370d16db3bc6
- 17. International monitoring of adverse reactions to drugs, World Health Organization. Pharmaceuticals unit & WHO Collaborating Centre for International Drug Monitoring. Adverse reaction terminology, June 30, 1984. 1984 (DEM/NC/84.153. Unpublished). Uppsala: WHO Collaborating Centre for International Drug Monitoring.
- 18. Medical Dictionary for Regulatory Activities (MedDRA) . International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Accessed June 14, 2022. https://www.meddra.org/
- 19. SAS Institute Inc . SAS/STAT 9.1 User’s Guide. SAS Institute Inc. 2004. Accessed October 12, 2021. https://support.sas.com/documentation/onlinedoc/91pdf/sasdoc_91/stat_ug_7313.pdf
- 20. Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich Longitudinal Study of growth and development. Helv Paediatr Acta Suppl. 1989;52:1–125. [PubMed] [Google Scholar]
- 21. Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suwa S, Tachibana K, Maesaka H, Tanaka T, Yokoya S. Longitudinal standards for height and height velocity for Japanese children from birth to maturity. Clin Pediatric Endocrinol. 1992;1(1):5–13. [Google Scholar]
- 23. Ranke MB. Towards a consensus on the definition of idiopathic short stature. Horm Res. 1996;45(Suppl 2):64–66. [DOI] [PubMed] [Google Scholar]
- 24. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rhie YJ, Yoo JH, Choi JH, et al. Long-term safety and effectiveness of growth hormone therapy in Korean children with growth disorders: 5-year results of LG Growth Study. PLoS One. 2019;14(5):e0216927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sweeting H, West P. Health at age 11: reports from schoolchildren and their parents. Arch Dis Child. 1998;78(5):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darendeliler F, Karagiannis G, Wilton P. Headache, idiopathic intracranial hypertension and slipped capital femoral epiphysis during growth hormone treatment: a safety update from the KIGS database. Horm Res. 2007;68(Suppl 5):41–47. [DOI] [PubMed] [Google Scholar]
- 29. Carel JC, Ecosse E, Landier F, et al. Long-term mortality after recombinant growth hormone treatment for isolated growth hormone deficiency or childhood short stature: preliminary report of the French SAGhE study. J Clin Endocrinol Metab. 2012;97(2):416–425. [DOI] [PubMed] [Google Scholar]
- 30. Poidvin A, Touzé E, Ecosse E, et al. Growth hormone treatment for childhood short stature and risk of stroke in early adulthood. Neurology. 2014;83(9):780–786. [DOI] [PubMed] [Google Scholar]
- 31. Mo D, Hardin DS, Erfurth EM, Melmed S. Adult mortality or morbidity is not increased in childhood-onset growth hormone deficient patients who received pediatric GH treatment: an analysis of the Hypopituitary Control and Complications Study (HypoCCS). Pituitary. 2014;17(5):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albertsson-Wikland K, Mårtensson A, Sävendahl L, et al. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. 2016;101(5):2149–2159. [DOI] [PubMed] [Google Scholar]
- 33. Quigley CA, Child CJ, Zimmermann AG, Rosenfeld RG, Robison LL, Blum WF. Mortality in children receiving growth hormone treatment of growth disorders: data from the Genetics and Neuroendocrinology of Short Stature International Study. J Clin Endocrinol Metab. 2017;102(9):3195–3205. [DOI] [PubMed] [Google Scholar]
- 34. Cutfield WS, Wilton P, Bennmarker H, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355(9204):610–613. [DOI] [PubMed] [Google Scholar]
- 35. Grimberg A, DiVall SA, Polychronakos C, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. [DOI] [PubMed] [Google Scholar]
- 36. Raman S, Grimberg A, Waguespack SG, et al. Risk of neoplasia in pediatric patients receiving growth hormone therapy–a report from the Pediatric Endocrine Society Drug and Therapeutics Committee. J Clin Endocrinol Metab. 2015;100(6):2192–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilton P, Mattsson AF, Darendeliler F. Growth hormone treatment in children is not associated with an increase in the incidence of cancer: experience from KIGS (Pfizer International Growth Database). J Pediatr. 2010;157(2):265–270. [DOI] [PubMed] [Google Scholar]
- 38. Swerdlow AJ, Cooke R, Beckers D, et al. Cancer risks in patients treated with growth hormone in childhood: the SAGhE European Cohort Study. J Clin Endocrinol Metab. 2017;102(5):1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Darendeliler F, Karagiannis G, Wilton P, Ranke MB, Albertsson-Wikland K, Price DA; on Behalf of the KIGS International Board . Recurrence of brain tumours in patients treated with growth hormone: analysis of KIGS (Pfizer International Growth Database). Acta Paediatr. 2006;95(10):1284–1290. [DOI] [PubMed] [Google Scholar]
- 40. de Ridder MAJ, Stijnen T, Hokken-Koelega ACS. Prediction of adult height in growth-hormone-treated children with growth hormone deficiency. J Clin Endocrinol Metab. 2007;92(3):925–931. [DOI] [PubMed] [Google Scholar]
- 41. Kriström B, Dahlgren J, Niklasson A, Nierop AFM, Albertsson-Wikland K. The first-year growth response to growth hormone treatment predicts the long-term prepubertal growth response in children. BMC Med Inform Decis Mak. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ranke MB, Lindberg A. Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010;95(3):1229–1237. [DOI] [PubMed] [Google Scholar]
- 43. Bakker NE, Lindberg A, Heissler J, Wollmann HA, Camacho-Hübner C, Hokken-Koelega AC; KIGS Steering Committee . Growth hormone treatment in children with Prader-Willi syndrome: three years of longitudinal data in prepubertal children and adult height data from the KIGS database. J Clin Endocrinol Metab. 2017;102(5):1702–1711. [DOI] [PubMed] [Google Scholar]
- 44. Rohrer TR, Ceplis-Kastner S, Jorch N, et al. Ferring Arzneimittel GmbH . . Needle-free and needle-based growth hormone therapy in children: a pooled analysis of three long-term observational studies. Horm Res Paediatr. 2018;90(6):393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ranke MB, Lindberg A, Mullis PE, et al. Towards optimal treatment with growth hormone in short children and adolescents: evidence and theses. Horm Res Paediatr. 2013;79(2):51–67. [DOI] [PubMed] [Google Scholar]
- 46. Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008;93(2):352–357. [DOI] [PubMed] [Google Scholar]
- 47. Ranke MB, Lindberg A, Chatelain P, et al. Derivation and validation of a mathematical model for predicting the response to exogenous recombinant human growth hormone (GH) in prepubertal children with idiopathic GH deficiency. KIGS International Board. Kabi Pharmacia International Growth Study. J Clin Endocrinol Metab. 1999;84(4):1174–1183. [DOI] [PubMed] [Google Scholar]
- 48. Rose SR, Carlsson M, Grimberg A, et al. Response to GH treatment after radiation therapy depends on location of irradiation. J Clin Endocrinol Metab. 2020;105(10):e3730–e3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ranke MB, Price DA, Lindberg A, Wilton P, Darendeliler F, Reiter EO. Final height in children with medulloblastoma treated with growth hormone. Horm Res. 2005;64(1):28–34. [DOI] [PubMed] [Google Scholar]
- 50. Xu W, Janss A, Moshang T. Adult height and adult sitting height in childhood medulloblastoma survivors. J Clin Endocrinol Metab. 2003;88(10):4677–4681. [DOI] [PubMed] [Google Scholar]
- 51. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–693. [DOI] [PubMed] [Google Scholar]
- 52. Juul A, Teilmann G, Scheike T, et al. Pubertal development in Danish children: comparison of recent European and US data. Int J Androl. 2006;29(1):247–255. [DOI] [PubMed] [Google Scholar]
- 53. Schroll JB, Maund E, Gøtzsche PC. Challenges in coding adverse events in clinical trials: a systematic review. PLoS One. 2012;7(7):e41174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brown EG. Using MedDRA: implications for risk management. Drug Saf. 2004;27(8):591–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”