Abstract
Synthetic oligodeoxynucleotides (ODN) expressing unmethylated CpG motifs stimulate an innate immune response characterized by the production of polyreactive immunoglobulin M antibodies and immunomodulatory cytokines. This immune response has been shown to protect mice from challenge by Listeria monocytogenes and Francisella tularensis for up to 2 weeks. By repeatedly administering CpG ODN two to four times/month, we found that this protection could be maintained indefinitely. Protection was associated with a significant increase in the number of spleen cells that could be triggered by subsequent pathogen exposure to secrete gamma interferon and interleukin-6 in vivo (P < 0.01). ODN-treated animals remained healthy and developed neither macroscopic nor microscopic evidence of tissue damage or inflammation. Thus, repeated administration of CpG ODN may provide a safe means of conferring long-term protection against infectious pathogens.
Infectious bacteria are a major source of morbidity and mortality worldwide. The rapid induction of an innate immune response is one method by which the host limits the early spread of such pathogens (18, 20, 21). Innate immunity is triggered by the recognition of conserved determinants (such as lipopolysaccharide, mannans, or teichoic acid) expressed by infectious microorganisms (18). Recent studies indicate that bacterial DNA also stimulates an innate immune response (14, 17, 29). Specifically, hexameric motifs consisting of a central unmethylated CpG dinucleotide flanked by two 5′ purines and two 3′ pyrimidines (14, 24, 29) trigger the rapid production of polyreactive immunoglobulin M (IgM) antibodies and immunomodulatory cytokines in mice (5, 18, 20, 21). Due to a combination of CpG suppression and CpG methylation, unmethylated CpG hexamers are 20-fold more common in prokaryotic than eukaryotic genomes (reviewed in references 2, 8, and 23).
Synthetic oligodeoxynucleotides (ODN) that express CpG motifs mimic the immunostimulatory properties of bacterial DNA. CpG ODN induce lymphocytes and macrophages to secrete polyreactive antibodies and/or cytokines, including gamma interferon (IFN-γ), interleukin-6 (IL-6), IL-12, IL-18, and tumor necrosis factor alpha (1, 9, 14, 17). Based on the finding that immune recognition of unmethylated CpG motifs has been evolutionarily conserved in species ranging from fish to primates, we hypothesized that such recognition might confer a selective advantage on the host (17). Recent studies support this hypothesis, in that mice pretreated with CpG ODN resisted infection by pathogenic intracellular bacteria, such as Listeria monocytogenes and Francisella tularensis, as well as a variety of viral and parasitic agents (7, 10, 16, 30).
We and others found that the immune protection induced by CpG ODN persisted for approximately 2 weeks (7, 16). There are only a limited number of settings in which such short-term protection might be of therapeutic benefit. We therefore attempted to prolong protection by repeatedly administering ODN to normal mice. Results from this work shed light on the nature of the immune response induced by CpG ODN in vivo and on the safety profile of this novel form of immune modulation.
MATERIALS AND METHODS
Bacteria and growth conditions.
L. monocytogenes monocytogenes EGD (ATCC 15313) and F. tularensis LVS (ATCC 29684; American Type Culture Collection, Manassas, Va.) were grown in modified Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) as previously described (7). One-milliliter aliquots of bacteria were frozen in broth supplemented with 15% glycerol at −70°C and thawed for use. Viable bacteria were quantified by plating serial dilutions on Mueller-Hinton agar plates. All materials used in mouse inoculations, including bacteria, were diluted in phosphate-buffered saline (PBS) (BioWhittaker, Walkersville, Md.) containing <0.1 ng of endotoxin/ml.
Reagents.
All ODN were synthesized at the Center for Biologics Evaluation and Research, Food and Drug Administration (CBER/FDA) core facility. Immunostimulatory CpG ODN had the sequences GCTAGACGTTAGCGT and TCAACGTTGA. Control ODN had the same sequences, except the CpG motifs (underlined) were switched to GpC (GCTAGAGCTTAGGCT and TCAAGCTTGA). All ODN were tested for endotoxin content by chromogenic Limulus amoebocyte lysate assay (courtesy of Donald Hochstein, Division of Product Quality Control, CBER/FDA) and for protein contamination by the bicinchoninic acid protein assay kit (Pierce Chemicals). Both Limulus amoebocyte lysate activity and protein levels were undetectable.
Mice.
Specific-pathogen-free male BALB/c mice were obtained from Jackson Laboratories (Bar Harbor, Maine). All mice were housed in sterile microisolator cages in a barrier environment in the CBER/FDA specific-pathogen-free-animal facility. ODN treatment was initiated at 6 to 8 weeks of age. The mice were injected intraperitoneally (i.p.) with 50 μg of ODN and/or 103 50% lethal doses (LD50) of bacteria.
In some experiments, blood was obtained by retro-orbital puncture. This was used to prepare serum, which was stored at −20°C until use. The mice were sacrificed by cervical dislocation, and samples of spleen, lymph node, liver, kidney, adrenal gland, lung, heart, muscle, and intestine were removed. The tissues were either prepared for ELIspot analysis or fixed in 10% formalin for histologic analysis. Tissue sections were prepared and stained by American Histolabs (Rockville, Md.). Single-spleen-cell suspensions were prepared from fresh spleen in RPMI 1640 supplemented with 5% fetal calf serum.
Enzyme-linked immunosorbent assays (ELISAs).
Ninety-six-well Immulon I microtiter plates were coated with goat anti-mouse Ig (Southern Biotechnologies Associates, Birmingham, Ala.) in PBS (26). The plates were blocked with PBS-2% bovine serum albumin and overlaid with serially diluted serum. After a 2-h incubation, the plates were washed and treated with alkaline phosphatase-conjugated goat anti-mouse heavy-chain specific Ig (1:3,000; Southern Biotechnologies Associates). The plates were incubated at room temperature for 2 h, washed, and then developed with p-nitrophenylphosphate (Kierkegaard and Perry, Gaithersburg, Md.) in diethanolamine buffer (pH 9.8). The concentration of specific antibody was determined by comparison to a standard curve generated with a high-titer antiserum as previously described (13).
Cytokine-specific ELIspot assays.
Ninety-six-well Immulon 2 plates were coated with anti-IL-4 (MM450C; Endogen, Woburn, Mass.), anti-IL-6 (18071D; Pharmingen), or anti-IFN-γ (AMC-4834; Bio-source, Camarillo, Calif.) in PBS (pH 7.2) buffer for 4 h at room temperature as described previously (12). The plates were blocked with PBS–5% bovine serum albumin for 2 h and washed with PBS–0.025% Tween 20. A single suspension of lymphocytes prepared in sterile RPMI 1640 supplemented with 5% fetal calf serum was serially diluted in anti-cytokine-coated plates at a starting concentration of 106 cells/well. The plates were incubated at 37°C for 6 h in a 5% CO2-in-air incubator. The plates were washed and then treated with biotinylated anti-cytokine antibody followed by phosphatase-streptavidin (Pharmingen), as described previously (12). ELIspots were developed after the addition of BCIP (5-bromo-4-chloro-3-indolylphosphate) phosphatase (Sigma Diagnostics, St. Louis, Mo.) in agarose and visually quantitated. ELISA assays for quantitating serum cytokines were performed as described above, except serum rather than cells was added to plates coated with anti-cytokine antibody.
Statistical analysis.
All cytokine and Ig assays were conducted with a minimum of three to six independently studied mice/group. All challenge experiments were performed with a minimum of 5 to 10 mice/group. Statistical significance was evaluated by Student's t test.
RESULTS
Effect of long-term CpG ODN treatment on immunological reactivity.
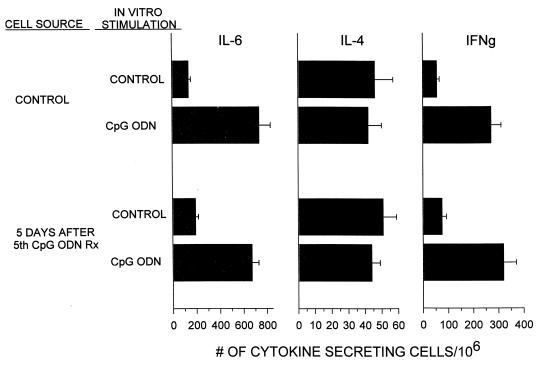
Previous studies showed that the innate immune response induced by a single injection of CpG ODN protected mice from infection by L. monocytogenes and F. tularensis for approximately 2 weeks (7, 16). In an effort to extend the duration of protection, BALB/c mice were repeatedly injected with 50 μg of CpG ODN. Initial experiments examined whether cells from long-term-treated animals remained responsive to this form of immune stimulation. Splenocytes from untreated animals and from mice injected with ODN for five successive weeks were incubated in vitro with CpG ODN. Cells from both control and ODN-treated mice responded by secreting IL-6 and IFN-γ in vitro, demonstrating that lymphocytes were not desensitized by repeated in vivo exposure to CpG motifs (Fig. 1).
FIG. 1.
BALB/c mice were injected i.p. for five consecutive weeks with 50 μg of CpG ODN. Spleen cell suspensions were prepared from naive mice or from mice 5 days after the last injection of ODN. The cells were then cultured in vitro for 8 h with or without 1 μg of CpG ODN/ml. The numbers of cells stimulated to secrete IL-6, IL-4, and IFN-γ were determined by ELIspot assay. The data represent the mean + standard deviation of three individually tested mice/group. All increases in IL-6 and IFN-γ production were significant (P < 0.01). Rx, treatment.
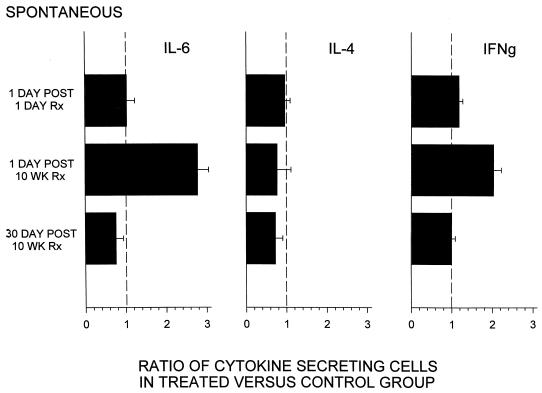
To monitor the effect of repeated ODN administration on immune activation in vivo, ongoing cytokine production was examined by ELIspot analysis of freshly isolated splenocytes. One day after a single injection of CpG ODN there was no detectable change in the number of spleen cells secreting IL-4, IL-6, or IFN-γ in vivo (Fig. 2). We also detected no change in numbers of cytokine-secreting cells or serum cytokine levels at any time point after a single ODN administration (data not shown). However, after 10 weekly injections, the number of IL-6- and IFN-γ-secreting spleen cells was increased >2-fold when compared to those in PBS-treated controls (P < 0.01 and P < 0.03, respectively). A modest decrease in the number of IL-4-secreting cells was also observed in long-term ODN-treated animals, although this did not reach statistical significance (Fig. 2). To determine whether these changes in immune activation persisted, the animals were studied 30 days after the cessation of therapy. As seen in Fig. 2, the number of cells secreting IFN-γ and IL-6 in animals that had been treated with CpG ODN for 4 months returned to baseline within a month of the discontinuation of therapy.
FIG. 2.
BALB/c mice were injected i.p. with 50 μg of CpG ODN. Spleen cell suspensions were prepared 1 day after the first injection or 1 and 30 days after the 10th weekly injection of the animals. The level of immune activation in vivo was monitored by comparing the number of cells actively secreting cytokine in treated mice versus that in age-matched PBS-injected controls. The data represent the mean + standard deviation of five individually tested mice/group from two different experiments. The following changes were statistically significant: increased IL-6 (P < 0.01) and IFN-γ (P < 0.03) 1 day after the 10-week treatment (Rx).
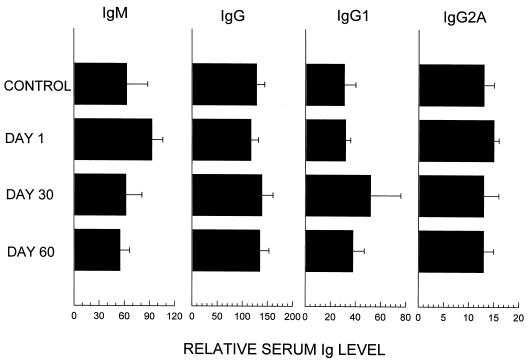
Serum Ig levels were examined in mice 1, 30, and 60 days after 4 months of weekly CpG ODN treatment. As seen in Fig. 3, a modest increase in serum IgM levels was observed in chronically treated mice. These returned to normal by 1 month after the cessation of treatment. No effect on total IgG, IgG1, or IgG2a levels was detected.
FIG. 3.
BALB/c mice were injected i.p. with 50 μg of CpG ODN every 1 to 2 weeks for 4 months. Sera from these animals were collected 1, 30, and 60 days after the last treatment and monitored by ELISA for anti-Ig levels. The data represent the mean + standard deviation of six individually tested mice/group from two different experiments.
Safety of long-term CpG ODN treatment.
Animals injected every week with CpG ODN remained physically vigorous: none became sick, lost weight, or died (Table 1). Mice were sacrificed 1 or 30 days after 4 months of treatment, and their organs (spleen, lymph nodes, muscle, intestine, heart, lung, adrenal gland, kidney, and liver) were fixed, stained, and examined histologically. There was no splenomegaly or hepatomegaly (Table 1). Coded samples from ODN-treated and control mice were analyzed by a pathologist (Allen Cheever). None of the organs showed macroscopic or microscopic evidence of damage or inflammation. The hematocrit and total white blood cell count in the peripheral blood of all animals at all time points were normal (data not shown).
TABLE 1.
Health assessment of mice chronically treated with CpG ODNa
| Parameter measured | Measurement 1 day after 10 weekly doses
|
|
|---|---|---|
| PBS control | CpG ODN treated | |
| Body wt (g) | 21.12 ± 0.38 | 21.18 ± 0.48 |
| Liver wt (g) | 0.97 ± 0.08 | 1.01 ± 0.05 |
| Spleen size (no. of cells [106]) | 85.60 ± 14.38 | 91.60 ± 10.16 |
BALB/c mice were injected i.p. with PBS or 50 μg of CpG ODN every week for over 2 months (10 injections). One day after the last injection, the animals were sacrificed. The total body and liver weights were recorded, as were the number of leukocytes in the spleen. The data represent the mean ± standard deviation of six individually tested mice/group from two different experiments.
Efficacy of long-term CpG ODN treatment.
Consistent with previous findings (7), animals treated only once with CpG ODN were fully protected from challenge by 103 LD50 of L. monocytogenes during the period from 3 to 14 days after injection (Table 2). Since it takes several days for optimal protection to develop, these mice were partially susceptible to infection during the first 2 days after treatment (Table 2).
TABLE 2.
Impact of repeated CpG ODN administration on survivala
| Treatment | Duration of treatment (wk) | Day of challenge post-Rxc | % Survival |
|---|---|---|---|
| PBS | 1 | 1 | 0 |
| PBS | 1 | 7 | 0 |
| CpG ODN | 1 | 1 | 0 |
| CpG ODN | 1 | 3 | 100 |
| CpG ODN | 1 | 14 | 90 |
| CpG ODN | 1 | 21 | 0 |
| CpG ODN | 10 | 1 | 100 |
| CpG ODN | 10 | 14 | 100 |
| CpG ODN | 20 | 2 | 100 |
| CpG ODN | 20 | 13 | 100 |
| CpG ODN | 20 | 21 | 30 |
| CpG ODN | 20 | 28 | 0 |
| GpC ODNb | 20 | 1 | 0 |
| GpC ODNb | 20 | 14 | 0 |
BALB/c mice were injected i.p. every 2 weeks for up to 4 months with 50 μg of ODN. They were challenged with 103 LD50 of L. monocytogenes on the day shown after the last treatment. The data represent the percentage of mice surviving for >3 weeks. Each group shown consists of 5 to 15 animals. All cases of 90 to 100% survival are statistically significant (P < 0.05).
Note that these are control GpC ODN.
Rx, treatment.
To determine whether repeated administration of CpG ODN could extend the duration of protection, mice were treated every 2 weeks with 50 μg of ODN and then challenged with 103 LD50 of L. monocytogenes. Complete protection was observed both 1 and 14 days after five consecutive treatments administered over 2 months (Table 2). In contrast, mice injected with PBS or control ODN (in which the CpG dinucleotide was inverted to GpC) did not survive challenge. Extending this analysis, animals were treated with CpG ODN every 2 weeks for 4 months. As described above, these animals were completely protected from infection throughout the treatment period and for 2 weeks after the last dose of ODN. By 1 month after the end of therapy, they were fully susceptible to infection (Table 2).
Mice that survived L. monocytogenes challenge due to CpG ODN treatment were rechallenged with either L. monocytogenes or F. tularensis 6 weeks later. All mice infected with F. tularensis died, since the immunoprotective effects of the CpG ODN had waned. In contrast, all mice survived rechallenge with L. monocytogenes (Table 3). This confirms our earlier finding that animals exposed to a pathogen during the period of CpG ODN-induced protection develop long-lasting antigen-specific immunity (7).
TABLE 3.
Impact of repeated CpG ODN administration on survival after rechallengea
| Rx | Challenge | Rechallenge | No. surviving/no. challenged | % Survival |
|---|---|---|---|---|
| PBS | L. monocytogenes | 0/10 | 0 | |
| F. tularensis | 1/10 | 10 | ||
| CpG ODN | L. monocytogenes | 20/20 | 100 | |
| F. tularensis | 10/10 | 100 | ||
| CpG ODN | L. monocytogenes | L. monocytogenes | 10/10 | 100 |
| L. monocytogenes | F. tularensis | 0/10 | 0 |
BALB/c mice were injected i.p. with PBS or 50 μg of CpG ODN every 2 weeks for 2 months (five injections). One to 14 days after the last injection, the animals were challenged with 103 LD50 of L. monocytogenes or F. tularensis. The mice that survived L. monocytogenes challenge were then rechallenged 45 days later (after the cessation of CpG ODN therapy). The data show the number (and percentage) of mice surviving >3 weeks postchallenge. Rx, treatment.
Mechanism of action of CpG ODN.
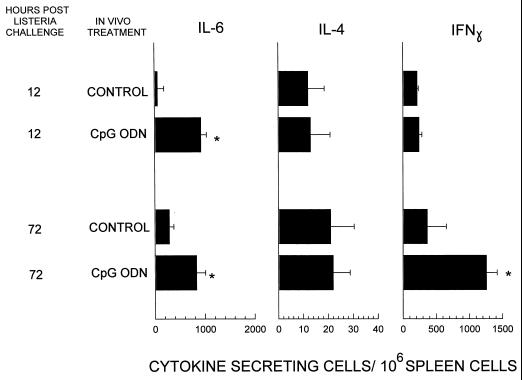
As shown above, a single 50-μg injection of CpG ODN protected BALB/c mice from infection but did not stimulate a detectable increase in the number of cells actively secreting Ig or cytokine in vivo. However, repeated administration of CpG ODN resulted in an increased number of spleen cells actively secreting IL-6 and IFN-γ in vivo. These findings led us to postulate that ODN might lower the activation threshold of the innate immune system subsequently exposed to a pathogen. To test this hypothesis, BALB/c mice were treated with CpG ODN or PBS and then challenged 3 days later with 103 LD50 of L. monocytogenes. As seen in Fig. 4, ODN-treated mice had significantly more cells actively secreting IL-6 (P < 0.01) within 12 h of listeria infection than did the negative controls. By 3 days after infection, there were significantly more cells secreting both IL-6 (P < 0.01) and IFN-γ (P < 0.01) in CpG ODN-pretreated mice.
FIG. 4.
BALB/c mice were injected i.p. with 50 μg of CpG ODN or PBS. The animals were challenged 3 days later with 103 LD50 of L. monocytogenes. The level of immune activation in vivo was monitored by ELIspot analysis 12 and 72 h after challenge. The data represent the mean + standard deviation of six individually tested mice/group from two different experiments. ∗, statistically significant increase in numbers of cytokine-producing cells in CpG ODN-treated versus control mice (P < 0.01).
DISCUSSION
The immune system has evolved two general mechanisms for combating infectious diseases. The first involves a rapid innate immune response characterized by the production of immunostimulatory cytokines and polyreactive antibodies. This early response helps limit a pathogen's spread prior to the development of antigen-specific sterilizing immunity (reviewed in references 18, 20, and 21). CpG motifs present in bacterial DNA induce such an innate immune response (2, 8, 14, 23, 24, 29). Recent reports demonstrate that CpG motifs improve host resistance to infection by a variety of bacterial, viral, and parasitic pathogens (7, 10, 16, 30). Protection is mediated by the release of IFN-γ, IL-12, and/or the activation of B and/or NK cells, depending upon the pathogen. CpG ODN also act as immune adjuvants, facilitating the development of pathogen-specific immunity (3, 4, 11, 15, 19, 22, 25).
There is considerable interest in the therapeutic potential of CpG ODN. ODN enter immunologically active cells within seconds, up-regulate mRNA within minutes, stimulate cytokine and IgM production within hours, and provide protection for approximately 2 weeks (7, 14, 17). In most situations where CpG ODN might be used therapeutically, such as preventing infection by pathogens for which vaccines are unavailable, long-term protection is required. Thus, developing a means to prolong the activity of CpG ODN would be of clear benefit. We examined whether repeatedly administering CpG ODN could continuously stimulate the innate immune system, thereby preventing protection from waning. As seen in Table 2, periodic injection of CpG ODN maintained host resistance to 103 LD50 of L. monocytogenes for the duration of therapy. Long-term protection against F. tularensis was also observed in animals treated repeatedly with CpG ODN (Table 3 and data not shown). Protection was unambiguously attributed to the activity of CpG motifs, since control ODN in which the CpG dinucleotide was inverted to GpC provided no protection.
Animals injected only once remained partially susceptible to infection for the 2 days immediately following ODN administration (Table 2 and reference 7). By treating mice every 2 weeks with ODN, immune activation was maintained at a level sufficient to provide continuous protection. Our mechanistic studies suggest that the CpG ODN had lowered the activation threshold of the innate immune system. This enabled the host to mount stronger and faster responses after infection, resulting in the control of an otherwise-lethal pathogen dose. The observation that ODN-pretreated mice had significantly more cells actively secreting IL-6 within 12 h of infection supports this conclusion. This increase cannot be accounted for by cell proliferation (even a single population doubling takes more than 12 h). Moreover, ODN treatment was not associated with an increase in spleen cell numbers (Table 1). Instead, CpG ODN enabled the innate immune system to mount a more vigorous immune response immediately after challenge. Different cellular elements may have contributed to this response over time, as shown by the subsequent rise in the number of IFN-γ-secreting cells (an effect also promoted by CpG ODN pretreatment). Ongoing studies are directed towards identifying precisely which cells contribute to each stage of this activation.
CpG ODN also function as immune adjuvants. Several groups have shown that coadministering CpG ODN with protein- or DNA-based vaccines boosts the host's antibody and cytotoxic-T-lymphocyte response (3, 4, 11, 15, 19, 22, 25). In the context of their use as immunoprotective agents, we observed that animals treated with CpG ODN not only survived initial infection, they then developed an adaptive immune response resulting in long-term protection against subsequent challenge by the same organism (Table 3). At an immunologic level, we postulate that the presence of cells “primed” to rapidly release IL-6, IFN-γ, or other cytokines following contact with a pathogen in CpG ODN-treated mice may contribute to the induction of immunologic memory (Fig. 2).
As with all novel therapies, concern has been expressed that CpG ODN might have adverse effects on the host. Indeed, reports indicate that the toxicity of LPS (6) and d-galactosamine (27, 28) can be increased by the administration of CpG ODN. To examine the toxicity of CpG ODN in normal animals, we repeatedly injected BALB/c mice with 50 μg of CpG ODN, a dose that significantly exceeds the minimum amount of ODN required for protection (2 to 5 μg) (7). The chronic immune stimulation induced by this treatment could potentially promote an inflammatory, autoimmune, or other type of toxicity in the host, yet none of the nearly 200 mice injected multiple times with CpG ODN lost weight, became ill, or developed hepatomegaly or splenomegaly (prior to pathogen challenge). Stained tissue sections from ODN-treated and control mice showed no evidence of macroscopic or microscopic damage or inflammation. Blood smears from all animals were normal. In addition, none of the animals developed proteinuria or other manifestations of lupus-like disease. These studies do not indicate that therapeutic doses of CpG ODN are harmful under normal conditions.
In summary, our results demonstrate that the repeated administration of CpG ODN can significantly improve host resistance to infection for a prolonged period. In the present study, protection against L. monocytogenes and F. tularensis was maintained for >4 months. It seems likely that lifelong protection can be achieved by repeated ODN administration and that this protection will extend to the multiple microorganisms against which CpG ODN are effective (including viruses and parasites) (7, 10, 16, 30). Given the safety and efficacy of this dosing regimen, we predict that CpG ODN will be of considerable benefit in situations where effective vaccines are unavailable or where the risk of exposure to multiple pathogens is high.
ACKNOWLEDGMENTS
This review was supported in part by a grant from the National Vaccine Program and by Military Interdepartmental Purchase Request MM8926.
We thank Karen Elkins for providing seed stocks of the bacteria used in this study and Allen Cheever for his histologic analysis of tissue samples from control and ODN-treated mice.
REFERENCES
- 1.Ballas Z D, Rasmussen W L, Krieg A M. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol. 1996;157:1840–1847. [PubMed] [Google Scholar]
- 2.Bird A P. CpG-rich islands and the function of DNA methylation. Trends Genet. 1987;3:342–347. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 3.Branda R F, Moore A L, Lafayette A R, Mathews L, Hong R, Zon G, Brown T, McCormack J J. Amplification of antibody production by phosphorothioate oligodeoxynucleotides. J Lab Clin Med. 1996;128:329–338. doi: 10.1016/s0022-2143(96)90035-9. [DOI] [PubMed] [Google Scholar]
- 4.Brazolot Millan C L, Weeratna R, Krieg A M, Siegrist C A, Davis H L. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci USA. 1998;95:15553–15558. doi: 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation and protection of dendritic cells induced by double stranded RNA. J Exp Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowdery J S, Chace J H, Yi A, Krieg A M. Bacterial DNA induces NK cells to produce IFN gamma in vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 7.Elkins K L, Rhinehart-Jones T R, Stibitz S, Conover J S, Klinman D M. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2991–2998. [PubMed] [Google Scholar]
- 8.Gilkeson G S, Grudier J P, Karounos D G, Pisetsky D S. Induction of anti-double stranded DNA antibodies in normal mice by immunization with bacterial DNA. J Immunol. 1989;142:1482–1486. [PubMed] [Google Scholar]
- 9.Halpern M D, Kurlander R J, Pisetsky D S. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 10.Klinman D M. Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucleic Acid Drug Dev. 1998;8:181–184. doi: 10.1089/oli.1.1998.8.181. [DOI] [PubMed] [Google Scholar]
- 11.Klinman D M, Barnhart K M, Conover J. CpG motifs as immune adjuvants. Vaccine. 1998;17:19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 12.Klinman D M, Nutman T B. ELIspot assay to detect cytokine-secreting murine and human cells. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. Brooklyn, N.Y: Greene Publishing Associates; 1994. pp. 6.19.1–6.19.8. [Google Scholar]
- 13.Klinman D M, Steinberg A D. Proliferation of anti-DNA producing NZB B cells in a non-autoimmune environment. J Immunol. 1986;137:69–75. [PubMed] [Google Scholar]
- 14.Klinman D M, Yi A, Beaucage S L, Conover J, Krieg A M. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNγ. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovarik J, Bozzotti P, Love-Homan L, Pihlgren M, Davis H L, Lambert P, Krieg A M, Siegrist C A. CpG oligonucleotides can circumvent the TH2 polarization of neonatal responses to vaccines but fail to fully redirect TH2 responses established by neonatal priming. J Immunol. 1999;162:1611–1617. [PubMed] [Google Scholar]
- 16.Krieg A M, Homan L L, Yi A K, Harty J T. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–2434. [PubMed] [Google Scholar]
- 17.Krieg A M, Yi A, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–548. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 18.Marrack P, Kappler J. Submersion of the immune system by pathogens. Cell. 1994;76:323–332. doi: 10.1016/0092-8674(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 19.McCluskie M J, Davis H L. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–4466. [PubMed] [Google Scholar]
- 20.Medzhitov R, Janeway C A. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Janeway C A. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1998;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 22.Moldoveanu Z, Love-Homan L, Huang W Q, Krieg A M. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine. 1998;16:1216–1224. doi: 10.1016/s0264-410x(98)80122-9. [DOI] [PubMed] [Google Scholar]
- 23.Razin A, Friedman J. DNA methylation and its possible biological roles. Prog Nucleic Acid Res Mol Biol. 1981;25:33–52. doi: 10.1016/s0079-6603(08)60482-1. [DOI] [PubMed] [Google Scholar]
- 24.Roman M, Martin-Orozco E, Goodman J S, Nguyen M, Sato Y, Ronaghy A, Kornbluth R S, Richman D D, Carson D A, Raz E. Immunostimulatory DNA sequences function as T helper-1 promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 26.Sedgwick J D, Holt P G. A solid phase immunoenzymatic technique for the enumeration of specific antibody secreting cells. J Immunol Methods. 1983;57:301–309. doi: 10.1016/0022-1759(83)90091-1. [DOI] [PubMed] [Google Scholar]
- 27.Sparwasser T, Meithke T, Lipford G, Borschert K, Hicker H, Heeg K, Wagner H. Bacterial DNA causes septic shock. Nature. 1997;386:336–338. doi: 10.1038/386336a0. [DOI] [PubMed] [Google Scholar]
- 28.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-α-mediated shock. Eur J Immunol. 1997;27:1671–1679. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Yamamoto T, Katoaka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–4076. [PubMed] [Google Scholar]
- 30.Zimmermann S, Egeter O, Hausmann S, Lipford G B, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine Leishmaniasis. J Immunol. 1998;160:3627–3630. [PubMed] [Google Scholar]