Abstract
Background:
The different anthropometric indices have different predictive values of nonalcoholic fatty liver disease (NAFLD) in various populations. Since obesity is a common cause of NAFLD and diabetes, therefore, it is critical to correlate the various anthropometric indices as a risk factor in terms of NAFLD and diabetes in the Indian population. In view of reported association between obesity and NAFLD, the study was employed to analyze the relationship of various anthropometric indices (body mass index [BMI], a body shape index [ABSI], waist-height ratio [WHtR], etc.) with NAFLD and to comment, if possible, which among them has the highest predictive value in patients with type 2 diabetes.
Material and Methods:
Data of 220 diabetic patients (36–80 years) were analyzed. Anthropometric data were collected using standard methods. Routine biochemical investigations data were used. Ultrasonography was used to assess liver status for NAFLD.
Results:
Based on the results, Waist height ratio [WHtR] and BMI had better correlation with NAFLD than ABSI. The desirable WHtR cutoff value was 0.545 with 62% of sensitivity and 62% of specificity. The cut off for BMI and ABSI were 24.6 and 0.805, respectively, with 65% of sensitivity and 62% of specificity for BMI and 63% of sensitivity and 42% of specificity for ABSI.
Conclusion:
There is a strong association of BMI and ABSI with NAFLD in this study. Public health measures to limit overnutrition and management of obesity are essential to prevent NAFLD, and as its negative health effects on type 2 diabetes mellitus.
Keywords: Body mass index, body roundness index, nonalcoholic fatty liver disease, obesity, type 2 diabetes, waist circumference, waist height ratio
INTRODUCTION
Overweight and obesity are recognized as major public health issues in many countries worldwide. Obese and overweight people have an increased risk of chronic diseases such as type 2 diabetes, coronary heart disease, malignancies, and nonalcoholic fatty liver disease (NAFLD), according to decades of research.[1] Anthropometric measurements have varying prognostic capacities for diabetes, according to studies from various countries and ethnic groups worldwide.[2] NAFLD is a common disease that is highly prevalent in type 2 diabetes mellitus. NAFLD refers to excess accumulation of fat in the liver that develops from simple hepatic steatosis and progresses to liver cirrhosis.[3] The estimated prevalence of NAFLD is 6%–35% worldwide[3] with a lower rate among Asian countries which is about 5%–18%.[4] The occurrence of NAFLD varies depending on the population and is closely connected to the prevalence of metabolic disorders such as Type 2 diabetes mellitus (T2DM) and obesity.[5] The involvement of both NAFLD and T2DM raises the probability of developing diabetes complications, and the main factor to manage this is by maintaining good glycemic regulation and enhancing weight loss.[6] The rising prevalence of obesity in adolescents is associated with metabolic syndrome.[7] Studies have attempted to define anthropometric measures that can predict diabetes and prediabetes better.[8] As with metabolic syndrome, NAFLD is more correlated with the deposition of visceral fat.[9]
Precise and accurate visceral fat measurement may be critical for the identification and histological severity of patients with NAFLD risk.[10] Most commonly accepted and in use measure is body mass index (BMI), due to its time-tested and convenient approach[11] a lot of work has been done in estimating the accuracy of BMI in predicting diseases including NAFLD and type 2 DM.[12]
Many studies have proved that anthropometric parameters such as BMI and WC[13] are frequently associated with metabolic syndrome, insulin resistance, and histological findings (steatohepatitis and fibrosis) in patients with NAFLD[14] and may be helpful to evaluate risk and prognostic factors in these patients. However, with the advent of newer measure-based tools such as Waist height ratio (WHtR), a body shape index (ABSI), and body roundness index (BRI), and the possibility of improving the use of anthropometry screening tool for metabolic syndrome has arisen.[15] These tools incorporate some additional features such as pattern of fat deposition and not just the body weight, which may be attributed to bone weight as well.[16] These tools collectively help in diagnosing NAFLD as an indicator of adiposity and also provide an opportunity to target the treatment. Glycosylated hemoglobin (HbA1C), a marker of diabetes, serves as risk factor for NAFLD, and the study was done to find the association of HbA1C with NAFLD and its severity, compared with BMI, waist-to-hip ratio, and waist circumference (WC).[17] In varied populations, different obesity indices have different predictive values for NAFLD. Obesity is a common cause of NAFLD and diabetes, so it’s no surprise that NAFLD and diabetes are related. As a result, correlating the various anthropometric indices in terms of NAFLD and diabetes in the Indian population is important.
With the background of various reports showing strong association between obesity and NAFLD, the study was employed to analyze the relationship of various anthropometric indices including BMI, ABSI, and WHtR with NAFLD and to comment if possible which among them has the highest predictive value in patients with T2DM. To better understand the association of anthropometric parameters with NAFLD, this prospective study was done in T2DM patients to predict the prevalence of NAFLD in Indian population.
METHODOLOGY
This observational prospective study was conducted in the Department of Medicine of a Medical College in South India, between July 2017 and July 2019 among patients attending the diabetes clinic with T2DM. Inclusion criteria: T2DM patients over 18 years of age and obtained written informed consent were included in the study. Exclusion criteria: patients with (a) males who drink more than 20 g of alcohol or females who drink 10 g per day. One drink was defined as having 10 g of ethanol and thus is equivalent to one can of beer, 4 ounces of wine, or 1.5 ounces (one-shot) of distilled spirits, (b) type 1 diabetes, (c) with steroid or statin intakes, (d) with any immunosuppressant drugs, and (e) chronic liver were excluded from the study at the time of screening. Required measurements were obtained for the anthropometric tools.
Height and weight were measured using standard scales available in the clinic, and the same scales were used for all patients to maintain uniformity. WC was measured using an inch tape. The tape was applied tightly to the skin surface so that the tape was taut but not tight. The measurement of WC was done midway between the inferior margin of ribs and the border of superior iliac crest.[18] All the patients underwent an ultrasound abdomen by a trained radiologist using a high-resolution B-mode ultrasonography system, Phillips IU22 Machine (3–5MHz transducer). The findings of the ultrasound were graded as per standard criteria [Table 1].
Table 1.
Ultrasound grades of fatty liver, in type 2 diabetes mellitus patient population
| Grades | USG finding | Count (%) |
|---|---|---|
| Normal | 85 (38.6) | |
| Grade 1: Fatty liver | Increased hepatic echogenicity with normal visualization of intrahepatic vessels and diaphragm | 95 (43.2) |
| Grade 2: Fatty liver | Moderately increase in echogenicity directing to impaired visualization of the diaphragm and intrahepatic vessels | 40 (18.2) |
| Fatty liver (Grade 1+2) | Marked increase in echogenicity with poor visualization of the posterior structures such as the diaphragm | 135 (61.3) |
USG: Ultrasound
The formula for calculating anthropometric tools: [19,20]
BMI = Weight (Kg)/(Height [m])²
ABSI = WC (cm)/(BMI [kg/m2]) 2/3 × (Height [m]) 1/2
WHtR = WC (cm)/Height (cm)
BRI = 364.2–365.5 (1 − [WC (m)/2 π]2/[0.5 × Height (m)]2) 1/2
Since cutoffs for anthropometric data depend on sex and race, predetermined grading systems for BMI for population were adapted to assess the present data.[21] For ABSI, grades were made based on the four quartiles of data. In addition, for each participating patient, routine laboratory investigations including blood sugar and glycated hemoglobin were also obtained.
The Statistical package for the social sciences (SPSS, version 24.0, [SPSS Inc., Chicago, Ill., USA]) software was used to analyze the data. Results are expressed as mean ± standard deviation or number and percentage as appropriate for qualitative and quantitative variables. T-test for parametric values and Kruskal–Wallis test for nonparametric values was used for comparison. For estimation of accuracy of a test and assessment of its optimum cutoff value, receiver operator curve (ROC) analysis was performed. A P < 0.05 was judged as statistical significance.
Ethical expert review
Approval sought from the regulatory and Institutional ethics committee (IEC no: 874/2017).
RESULTS
Totally, 220 Type 2 diabetic patients were enrolled for the study after inclusion criteria screening. The mean age of patients selected was 58.9 ± 11.3 years of 220, 138 (62.7%) were males and 82 (37.3%) were females. The average duration of known diabetes in these patients was 9.04 years (maximum of 30 years). The majority of the patients, i.e., 38% were diabetics for more than 10 years. Relevant demographic data of patients are represented in Table 2. Mean height estimated was 160.2 ± 10.4 cm and mean weight measured was 64.9 ± 11.7 kgs. Mean WC was 88.64 ± 9.8 cm and mean hip circumference was 100.1 ± 8.9 cm. Mean BMI estimated was 25.4 ± 4.3 (range: 14.1–39.6). Mean WC to height ratio was found to be 0.55 ± 0.06 as depicted in Table 3. Mean ABSI was calculated to be 0.817 ± 0.006 (range: 0.05–0.1) with interquartile range of 0.007 at 95% confidence interval. Mean BRI was 4.516 ± 1.398 (range: 1.29–11.13).
Table 2.
Demographic data of type 2 diabetes mellitus patients included in the study (n=220)
| Parameters | Findings (%) |
|---|---|
| Sex | |
| Male | 63 |
| Female | 37 |
| Age (years), mean±SD | 58.9±11.3 |
| Duration of T2DM (years), mean±SD | 9.04±1.89 |
| Hypertension | |
| Present | 55 |
| Treatment modalities | |
| Oral hypoglycemic agents | 69 |
| Insulin | 9 |
| Both | 14 |
| Treatment naive | 8 |
| Smoking | |
| Yes | 27 |
T2DM: Type 2 diabetes mellitus, SD: Standard deviation
Table 3.
Anthropometric and vital data of included patients (n=220)
| Parameters | Mean±SD |
|---|---|
| Age | 58.90±11.38 |
| Weight (kg) | 64.98±11.79 |
| Height (cm) | 160.24±10.45 |
| Waist circumference (cm) | 88.64±9.89 |
| Hip circumference (cm) | 100.19±8.98 |
| BMI | 25.36±4.34 |
| ABSI | 0.08±0.01 |
| WHR | 0.89±0.06 |
| Waist-height ratio | 0.55±0.06 |
| SBP | 128.09±17.71 |
| DBP | 80.09±9.65 |
| HbA1c | 8.51±2.07 |
SD: Standard deviation, WHR: Waist-to-hip ratio, BMI: Body mass index, ABSI: A body shape index, SBP: Systolic blood pressure, DBP: diastolic blood pressure, HbA1c: Glycosylated hemoglobin
Ultrasound abdomen revealed normal in 39% of patients, Grade I fatty liver in 43%, Grade II in 18%, and Grade III in none [Table 1]. All patients with Grade I or II fatty liver were considered as positive for NAFLD and analyzed as such as shown in Table 4.
Table 4.
Body mass index and a body shape index distribution in different ultrasound findings (normal and fatty liver)
| Anthropometric tool | Normal (n) | Fatty liver (n) | Total |
|---|---|---|---|
| BMI | n | n | |
| Normal | 32 | 33 | 65 |
| Preobese | 20 | 22 | 42 |
| Obese | 23 | 57 | 80 |
| Morbidly obese | 9 | 21 | 30 |
| Underweight | 1 | 2 | 3 |
| ABSI (quartile) | |||
| 1st | 31 | 30 | |
| 2nd | 23 | 30 | |
| 3rd | 18 | 37 | |
| 4th | 13 | 38 |
BMI: Body mass index, ABSI: A body shape index
ABSI grades made based on the cutoffs from the four quartiles Q1, Q2, Q3, and Q4 were 0.078, 0.082, 0.085, and 0.1, respectively. In the first quartile were 61 patients (27.7%), the second quartile had 53 patients (24.1%), the third quartile had 55 patients (25%), and finally, the fourth quartile included 51 cases (23.2%).
The Kruskal–Wallis test showed no significant difference in the distribution of fatty liver grades across age, sex, duration of diabetes, treatment modality, smoking status, diet, lipid profile, glycemic control, and liver function tests, except serum aspartate transaminase (AST) (P = 0.03). On comparing grades of fatty liver on ultrasound with anthropometric data, significant association was noted with BMI (P = 0.016), ABSI (P = 0.024), BRI (P = 0.010), and waist-height ratio (P < 0.01). Spearman correlation was applied to the parameters having significant association with ultrasound findings. Serum AST levels did not significantly correlate to fatty liver grades. However, Spearman’s test revealed significant correlation of grade of fatty liver with BMI, ABSI, BRI, and waist-height ratio. The highest coefficient of correlation was observed with waist-to-height ratio (rho = 0.271). The correlation coefficient for BRI was 0.237; BMI was 0.163, followed by ABSI with rho of 0.152.
ROC analysis was applied to all the anthropometric parameters to obtain the most appropriate cutoffs for predicting the presence of NAFLD in type 2 DM accurately [Figure 1]. Optimum results were noted with BMI and WHtR, giving cutoffs of 24.6 kg/m2> and 0.545, respectively.
Figure 1.
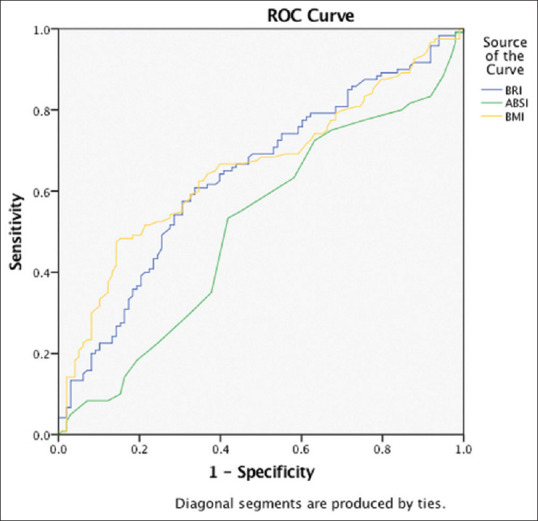
Receiver operator curve depicting accuracy of body mass index, a body shape index, and body roundness index in predicting nonalcoholic fatty liver disease
DISCUSSION
With advance technology of biomedical and the way to predict the NAFLD causes, the research has been progressed to evaluate the risk factors for NAFLD in type 2 diabetes. The present method of NAFLD evaluations varies with patients and disease criteria. The clinical burden of complications related to NAFLD is significant, as T2DM is considered to be an important risk factor in NAFLD development.[22] Since obesity is associated with NAFLD and T2DM, the prevention of excessive fat mass is important to avoid the progression of NAFLD. The use of basic anthropometric body composition indices such as BMI, WC, and WHtR has long been considered a realistic and useful method for evaluating adiposity. Various studies done on different populations such as Fan et al. in the Chinese population[23] and by Lahsaee et al. in the German population[24] to name a few recent ones have also shown BMI to be an independent risk factor for NAFLD. We established BMI of 24.6 as the cutoff value for NAFLD with a 65% sensitivity and 62% specificity.[25] BMI has its own shortcomings, and to overcome this WC, it is used as a surrogate measure. Krakauer et al. employed a new indicator, ABSI, to incorporate both BMI and WC.[25] Motamed et al.[15] proved from their large population-based study that when comparing various anthropometric measures, ABSI had poor association with NAFLD compared to BRI and WHtR. Our study confirms the same finding in the Indian population, and we concluded that even though BMI and ABSI had a strong correlation with NAFLD, BMI and WHtR had a superior predictive value than ABSI. We established a cutoff of 0.545 for WHtR predicting NAFLD, with a sensitivity of 62% and specificity of 62%. The cutoff is given by Motamed et al.[15] were 0.533 for men and 0.580 for women, which is again similar to the present study’s findings.
Prior studies have used BMI and WC as an anthropometric measure to predict NAFLD. Few studies conducted in European countries, Thailand, and China have demonstrated that BMI is associated in predicting NAFLD. Other studies which used BMI and WC along with other biochemical parameters have proved to have good association between NAFLD and T2DM. People with T2DM are at a higher risk of developing NAFLD, and obesity (BMI) further adds up to the risk.[26] It is thus known that BMI and WC have significant association with NAFLD in different ethnic groups. However, in comparison with other studies considering anthropometric measures, our study performed better using many physical measurement tools such as ABSI, BRI along with BMI, and WC and was found to be significant in the Indian population. Therefore, the models developed in this study help in identifying NAFLD in diabetes patients. The use of such anthropometric indices is highly encouraged because the accuracy of ultrasound diagnosis for NAFLD is limited as it depends on the level of fabricator and also the application of liver biopsy is invasive which may result in severe complications.[27]
The limitation in this study is the use of ultrasound to estimate NAFLD. Even though liver biopsy is considered the gold standard method, it’s not often used in NAFLD patients due to its high cost and bleeding risk. Since individual liver biopsies are neither indicated nor feasible in the given patient setting, ultrasound seemed more appropriate. Elastography was not performed as the patients were not found to have severe symptoms of NAFLD. This study is based on the Indian population and although in agreement with other studies, anthropometric measures are greatly influenced by ethnicity and cannot be extrapolated to other populations.
Based on easy to measure variables, this is a simple and noninvasive method of assessing NAFLD in diabetic patients. Anthropometric tools can be used in primary healthcare settings as well, where further investigation may be needed to confirm NAFLD in patients. In contrast to existing models, the simplicity and comprehensiveness of the nonlaboratory methods are not inferior, and further studies are needed to verify the feasibility of these models in clinical practice. It is hoped that these new models serve as noninvasive and cost-effective methods for detecting NAFLD in patients with T2DM.
CONCLUSION
WHtR, BMI, BRI, and ABSI (in that order) can be used as noninvasive markers for predicting the presence of NAFLD in type 2 diabetic patients, with appropriate limits for each measure. Further larger cohort studies on anthropometric tools and NAFLD in association with the Asian population would be helpful to better understand the mechanism involved the development of NAFLD in T2DM patients within normal BMI and this can be useful in targeting the treatment in the future. Since NAFLD is preventable with drugs and lifestyle changes, and affected individuals should be encouraged to adopt healthy lifestyles.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Banerjee S, Talukdar I, Banerjee A, Gupta A, Balaji A, Aduri R. Type II diabetes mellitus and obesity:Common links, existing therapeutics and future developments. J Biosci. 2019;44:150. [PubMed] [Google Scholar]
- 2.Ping Z, Pei X, Xia P, Chen Y, Guo R, Hu C, et al. Anthropometric indices as surrogates for estimating abdominal visceral and subcutaneous adipose tissue:A meta-analysis with 16,129 participants. Diabetes Res Clin Pract. 2018;143:310–9. doi: 10.1016/j.diabres.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–4. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 4.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis. 2016;20:205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–8. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benmohammed K, Valensi P, Benlatreche M, Nguyen MT, Benmohammed F, Pariès J, et al. Anthropometric markers for detection of the metabolic syndrome in adolescents. Diabetes Metab. 2015;41:138–44. doi: 10.1016/j.diabet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Hartwig S, Kluttig A, Tiller D, Fricke J, Müller G, Schipf S, et al. Anthropometric markers and their association with incident type 2 diabetes mellitus:Which marker is best for prediction?Pooled analysis of four German population-based cohort studies and comparison with a nationwide cohort study. BMJ Open. 2016;6:e009266. doi: 10.1136/bmjopen-2015-009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asghari S, Asghari-Jafarabadi M, Somi MH, Ghavami SM, Rafraf M. Comparison of calorie-restricted diet and resveratrol supplementation on anthropometric indices, metabolic parameters, and serum sirtuin-1 levels in patients with nonalcoholic fatty liver disease:A randomized controlled clinical trial. J Am Coll Nutr. 2018;37:223–33. doi: 10.1080/07315724.2017.1392264. [DOI] [PubMed] [Google Scholar]
- 10.Stefan N, Häring HU, Cusi K. Non-alcoholic fatty liver disease:Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–24. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 11.Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. 2018;360:k1274. doi: 10.1136/bmj.k1274. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Q, Laukkanen JA, Li Q, Li G. Body mass index is associated with type 2 diabetes mellitus in Chinese elderly. Clin Interv Aging. 2017;12:745–52. doi: 10.2147/CIA.S130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milić S, Lulić D, Štimac D. Non-alcoholic fatty liver disease and obesity:Biochemical, metabolic and clinical presentations. World J Gastroenterol. 2014;20:9330–7. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, et al. Body mass index and risk of nonalcoholic fatty liver disease:Two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101:945–52. doi: 10.1210/jc.2015-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motamed N, Rabiee B, Hemasi GR, Ajdarkosh H, Khonsari MR, Maadi M, et al. Body roundness index and waist-to-height ratio are strongly associated with non-alcoholic fatty liver disease:A population-based study. Hepat Mon. 2016;16:e39575. doi: 10.5812/hepatmon.39575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haraguchi N, Koyama T, Kuriyama N, Ozaki E, Matsui D, Watanabe I, et al. Assessment of anthropometric indices other than BMI to evaluate arterial stiffness. Hypertens Res. 2019;42:1599–605. doi: 10.1038/s41440-019-0264-0. [DOI] [PubMed] [Google Scholar]
- 17.Masroor M, Haque Z. HbA1C as a biomarker of non-alcoholic fatty liver disease:Comparison with anthropometric parameters. J Clin Transl Hepatol. 2021;9:15–21. doi: 10.14218/JCTH.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consultation WE. Waist circumference and waist-hip ratio. Report of a WHO Expert Consultation. Geneva, 2008. [Last accessed on 2017 Mar 17]. Available from: https://www.who.int .
- 19.Salamat MR, Shanei A, Salamat AH, Khoshhali M, Asgari M. Anthropometric predictive equations for estimating body composition. Adv Biomed Res. 2015;4:34. doi: 10.4103/2277-9175.150429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, et al. A body shape index and body roundness index:Two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health. 2015;15:794. doi: 10.1186/s12889-015-2150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaye L, Owiredu WK, Amidu N, Dapare PP, Adams Y. Comparative abilities of body mass index, waist circumference, abdominal volume index, body adiposity index, and conicity index as predictive screening tools for metabolic syndrome among apparently healthy ghanaian adults. J Obes. 2019;2019:8143179. doi: 10.1155/2019/8143179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorletti E, Byrne CD. Extrahepatic diseases and NAFLD:The triangular relationship between NAFLD, type 2-diabetes and dysbiosis. Dig Dis. 2016;34(Suppl 1):11–8. doi: 10.1159/000447276. [DOI] [PubMed] [Google Scholar]
- 23.Fan R, Wang J, Du J. Association between body mass index and fatty liver risk:A dose-response analysis. Sci Rep. 2018;8:15273. doi: 10.1038/s41598-018-33419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahsaee S, Ghazizade A, Yazdanpanah M, Enhesari A, Malekzadeh R. Assessment of NAFLD cases and its correlation to BMI and metabolic syndrome in healthy blood donors in Kerman. Gastroenterol Hepatol Bed Bench. 2012;5:183–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7:e39504. doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis H, Craig D, Barker R, Spiers G, Stow D, Anstee QM, et al. Metabolic risk factors and incident advanced liver disease in Non-Alcoholic Fatty Liver Disease (NAFLD):A systematic review and meta-analysis of population-based observational studies. PLoS Med. 2020;17:e1003100. doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue M, Yang X, Zou Y, Liu T, Su Y, Li C, et al. A non-invasive prediction model for non-alcoholic fatty liver disease in adults with type 2 diabetes based on the population of northern Urumqi, China. Diabetes Metab Syndr Obes. 2021;14:443–54. doi: 10.2147/DMSO.S271882. [DOI] [PMC free article] [PubMed] [Google Scholar]


