Abstract
The archipelagic country of Indonesia is inhabited by 300 ethnic groups, including the indigenous people of Tengger. Based on the reported list of medicinal plants used by the Tengger community, we have reviewed each of them for their phytochemical constituents and pharmacological activities. Out of a total of 41 medicinal plants used by the Tengerrese people, 33 species were studied for their phytochemical and pharmacological properties. More than 554 phytochemicals with diverse molecular structures belonging to different chemical classes including flavonoids, terpenoids, saponins and volatiles were identified from these studied 34 medicinal plants. Many of these medicinal plants and their compounds have been tested for various pharmacological activities including anti-inflammatory, antimicrobial, wound healing, headache, antimalarial and hypertension. Five popularly used medicinal plants by the healers were Garcinia mangostana, Apium graveolens, Cayratia clematidea, Drymocallis arguta and Elaeocarpus longifolius. Only A. graviolens were previously studied, with the outcomes supporting the pharmacological claims to treat hypertension. Few unexplored medicinal plants are Physalis lagascae, Piper amplum, Rosa tomentosa and Tagetes tenuifolia, and they present great potential for biodiscovery and drug lead identification.
Keywords: Tengger, phytochemistry, pharmacology, Cayratia clematidea, Drymocallis arguta, Elaeocarpus longifolius, Physalis lagascae, Piper amplum, Rosa tomentosa, Tagetes tenuifolia
1. Introduction
Since ancient human civilizations, mankind has used biotic resources including plants for clothing, cosmetics, food and medication. The World Health Organisation (WHO) estimated that more than 80 % of the world’s population rely on traditional medicines (TM) for their primary health needs [1]. Plants are the bulk ingredients used in these medicaments [1] with an estimated 50,000 plant species used worldwide, the majority of them contained within Asian medicines [2]. Most of the Asian medicinal plant knowledge is passed down uninterrupted from father to son using oral communication, or from master to apprentices using written scholarly traditions. The most popular scholarly medical traditions are Chinese traditional medicine [3], Indian Ayurvedic medicine [4] and Sowa Rigpa medicine (also practiced in Bhutan) [5]. The former oral traditions, which are predominantly practiced by remote tribes, are prone to disappearance or extinction [6,7].
In Indonesia there are more than 17,000 islands which are rich in biodiversity, especially the terrestrial plants. Indonesia has one of the highest numbers of higher plant species, with 22,500 species recorded so far. However, only a miniscule 4.4 % (1000 species) of these higher plant species are used as medicinal plants [8]. Since there are 300 ethnic groups/tribes in Indonesia, one would expect to find a rich medicinal plants diversity. One of these 300 thriving communities in Indonesia is the Tengger tribal community, residing in the Bromo mountain range (1600–2000 m above sea level, masl) of East Java, with the region known for its breathtaking views (Figure 1). The people still practice Hinduism from the old Majapahit Hindu Kingdom (1300–1500 A.D.) [9], which arrived in Indonesia in the first century through Indian traders, with Brahmin passengers as direct agents in transmitting Hinduism. For this reason, it is likely that Tenggerese ethnobotanical practices would resemble Indian Ayurvedic medicines. It is also expected that the mainstream Islamic traditional medical culture of Indonesia may have influenced the way Tenggerese medicines have evolved over centuries.
Figure 1.
Map of Indonesia. (A) Location of Tengger community within a Javanese island. (B) Bromo mountain range where Tenggerese community live.
There is no historical document to substantiate their influences, and there is a need for such studies in Indonesia. There are a few reports on the ethnobotanical studies of the Tenggerese community, including medicinal plant surveys in the Tenggeresse village of Wonokitri Village, Tosari subdistrict, Pasuruan Regency [10]. Another ethnobotanical survey was also previously reported in a different village, Ngadisari village, Sukapura district, Probolinggo Region [11]. Nevertheless, there is no comprehensive review on the phytochemical and pharmacological constituents of Tenggerese medicinal plants. In this review, we have collected the medicinal plants used by the Tenggerese community residing in the Wonokerso village, the oldest village in the Tenggerese community where the “Karo (blessing)” ceremony originated. The information regarding medicinal plants and their medicinal uses were collected through discussion and interviews with local physicians known as “dukuns”. The ethnopharmacological information was initiated in May 2015, and a list of dukuns medicinal plants was generated and are listed in Section 2 Table 1. Based on the list of plants in Table 1, we conducted a thorough literature search for each plant for their phytochemical and pharmacological activities. Figure 2 shows the schematic approach of this literature review. We have also consulted and compared the ethnobotanical information of our survey with the published information described previously from the surrounding villages. For example, Foeniculum vulgare and Acorus calamus, which were previously described as fever from the neighbouring villages, were also found in the current surveys described by dukuns [10,11,12].
Table 1.
Phytochemistry of Tenggerese medicinal plants collated from literature studies of similar species studied across the globe.
| Species | Family | Tenggeresse Ethnopharmacological Uses of Plants | Parts Used for Chemical Isolation | Countries (Chemical Studies Reported) | Isolated Compounds |
|---|---|---|---|---|---|
| Acorus calamus Linnaeus | Acoraceae | Fever | Leaves, rhizome, stem | India | β-Asarone, Camphene, Cymene, Calarene, α-Selinene, s-Cadinol, Isoshyobunone, β-Sesquiphellandrene, Preiso-calamendiol, Acorone [13]; (-)-4-Terpineol, Epieudesmin, Lysidine, (-)-Spathulenol, Borneol, Furyl ethyl ketone, Nonanoic acid, Bornyl acetate, Galgravin, Retusin, Butyl butanoate, Geranylacetate, Sakuranin, Acetic acid, Camphor, Isoelemicin, α-Ursolic acid, Acetophenone, Dehydroabietic acid, Isoeugenol Methylether, Apigenin, Dehydrodiisoeugenol, Linalool, Elemicin, Linolenic acid [14]; 2-Deca-4,7-dienol, Acoradin, Acoragermacrone, Acrenone, Aterpineol, β-Cadinene, Calacorene, Calamendiol, Galangin, Shyobunones, Sitosterol [15]; Calamusins A-I [16]. |
| Allium sativum Linnaeus | Alliaceae | Wound or cut | Rhizome | Iraq | E-Ajoene, Z-Ajoene, Alliin, Allicin, 2-Vinyl-4H-1,3-dithiin, Diallyl sulfide (DAS), Diallyl disulfide (DADS), Diallyl trisulfide (DATS), Allyl methyl sulfide (AMS) [17]. |
| Alyxia reinwardtii Blume | Apocynaceae | Fever, Rheumatism |
Stem | Thailand | Coumarin, 3-Hydroxycoumarin, 6-Hydroxycoumarin, 8-Hydroxycoumarin, Scopoletin, (+)-Pinoresinol, Zhebeiresinol and p-Hydroxybenzoic acid [18]. |
| Anredera cordifolia (Ten.) Steenis | Basellaceae | Itchiness, Wound |
Leaves | Brazil | Phytol, α-pinene, Larreagenin A, Vitexin, Isovitexin, Myricetin, Morin, Lupeol, β-Sitosterol, Ursolic acid [19]. |
| Apium graveolens Linnaeus | Apiaceae | Hypertension | Leaves | China | Apigenin, Luteolin, Chlorogenic acid [20]; Linalool, D-Limonene, 3-N-Butylphthalide (NBP) [21]. |
| Borreria laevis (Lam.) Griseb | Rubiaceae | Rheumatism | Aerial parts | Thailand | Borreline, Asperulosidic acid, 6-O-Acetylscandoside, 6α-Hydroxyadoxoside, Kaempferol 3-O-β-d-glucopyranoside, Kaempferol 3-O-rutinoside, Quercetin 3-O-β-d-galactopyranoside, Rutin [22,23]. |
| Brassica rapa Linnaeus | Brassicaceae | Fever, Hypertension, Nutrition |
Leaves, stem, flower buds, roots | Portugal | Kaempferol 3-O-sophoroside-7-O-glucoside, Kaempferol 3-O (feruloyl/caffeoyl)-sophoroside7-O-glucoside, Isorhamnetin 3,7-O-diglucoside, Isorhamnetin 3-O-glucoside [24]. |
| Capsicum pubescens Dun. | Solanaceae | Tonic after hard labour | Fruit | Mexico | Carotenoids (Violaxanthin, cis-Violaxanthin, Luteoxanthin, Antheraxanthin, Lutein, Zeaxanthin, β-Carotene), Ascorbic acid and Capsaicinoids (Capsaicin, Dihydrocapsaicin) [25]. |
| Cayratia clematidea (F. Müll.) Domin | Vitaceae | Stomach disorder | NA | NA | NA |
| Cinnamomum burmannii (Nees & T. Nees) Bl. | Laruaceae | Fever | China, Indonesia | Trans-Cinnamaldehyde, Coumarin, and Trans-Cinnamic acid [26]. Styrene, Benzaldehyde, Camphene, β-Pinene, Borneol, α-Terpineol, Procyanidin B1, Procyanidin B2, Procyanidin trimer, Catechin, Procyanidin dimer, Epicatechin, Coumarin, (E)-Cinnamic acid, (E)-Cinnamaldehyde, (Z)-Cinnamaldehyde, Cinnamyl alcohol, (E)-cinnamaldehyde, eugenol, and coumarin, procyanidin trimer, (E)-cinnamaldehyde, and (Z)-cinnamaldehyde [27]. catechin, epicatechin, procyanidin B2, quercitrin, 3,4-dihydroxybenzaldehyde, protocatechuic acid, and cinnamic acid [28]. (E)-Cinnamaldehyde, Cinnamyl alcohol, Coumarin, 3,4-Dihydrocoumarin, Kaempferol, Procyanidin dimer, Procyanidin trimer, Linalool [29] | |
| Cocos nucifera Linnaeus | Aracaceae | Foetus health | Fruit | India, Indonesia, Brazil, UK | 2-Furaldehyde diethyl acetal and Palmitic acid [30]; Jezonofol, Cirrhusin A, Cassigarol G, Maackin A, Treoguiacyl glycerol-8`-vanil ether acid, Erythroguiacyl glycerol-8′-vanillic acid ether, Apigenin-7-O-β-d-glucoside, Piceatannol, p-Hydroxybenzoic acid, Protocatechuic acid, and Vanillic acid [31]; Two phenol compounds-catechin and Chlorogenic acid [32]. |
| Cuminum cyminum Linnaeus | Apiaceae | Fever | Seed | USA, Iraq | Cuminaldehyde, α-Pinene, β-Pinene, γ-Cymene, γ-Terpinene, α-Terpinen-7-al and β-Terpinen-7-al [33]; Bergapten, Methoxsalen [34]; Luteolin, Apigenin-7-O-glucoside [35]. |
| Curcuma longa Linnaeus | Zingiberaceae | Fever, Headache, Wound |
Rhizome | Thailand, China, Belgium, Vietnam, Germany | Curcuminoids, Demethoxycurcumin, Bisdemethoxycurcumin [36]; Calebin-A [37]; α-Turmerone [38]; Epicatechins [39]; Cucurbitacin B, Curcumin [40]; Bisacurone B [41]; α-Curcumene, Zingiberene, Bisabolene, Sesquiphellandrene [42]; Turmeronol B, Turmeronol A, (E)-α-Atlantone [43]; Curlone [44]. |
| Datura metel Linnaeus | Solanaceae | Fever | Leaves, Flower | China | Daturafolisides, Daturametelin [45]; Dmetelisproside A, Citroside A, Staphylionoside D [46]; Baimantuoluolines, Baimantuoluoside [47]; Cyclosieversioside F, Astragaloside II, Ginsenoside Rg1, Astrojanoside A, Celerioside E [48]; Isofraxidin, Scopatone, Daturadiol (3),1,4-Benzenediol, Arenarine D, Vanillin, N-trans-Feruloyl-tyramine, Scopoletin, G-Sitosterol and Hyoscyamilactol [49]. |
| Daucus carota | Apiaceae | Eyesight | Roots, Stems, Flower | Italy, Korea | β-carotene, carotenoids [50]; β-Phellandrene, γ-Terpinene [51]; 6-methoxymellein [52]; Camphorene, Carotol, β-Bisabolene, Isoelemicin [53]. |
| Drymocallis arguta subsp. arguta | Rosaceae | Diarrhoea Anaemia |
NA | NA | NA |
| Elaeocarpus longifolius Bl. | Elaeocarpaceae | NA | NA | NA | |
| Erythrina variegata Linnaeus | Leguminoseae | Diarrhoea | Whole plant | China | Xanthoxyletin [54]; eryvarinols A and B [55]; Protocatechuic acid, Chlorogenic acid, and Caffeic acid [56]; Erythrinin B [57]. |
| Foeniculum vulgare | Apiaceae | Fever, Rheumatism |
Leaves, Stem | Serbia, Italy, Tunisia Turkey, Romania, China, India, Italy, Turkey, Algeria, Italy, Spain, Turkey, and Egypt |
Quercetin 3-glucuronide, Isoquercitrin, Rutin, Quercetin 3-arabinoside, Isorhammetin glycosides [58]; Dillapiol, Bergapten, Imperatorin, Psolaren [59]; Anethole, Limonene [60]; Gallic acid, Diosmin, Hesperidin, Kaempferol [61]; Carvacrol, Thymol, Anethol, p-Cymene and γ-Terpinene [62]; (E)-Anethole and p-Acetonylanisole [63]. α-Thujene, 1,8-Cineol, β-Ocimene, Linalool, Germacrene D, Anisketone, Apiol, n-Hexadecanoic acid, Cubebene, Benzene-1-methyl-4-(1-methylethyl)-p-cymene, 1,3,6-Octatriene, 3,7-dimethyl-, (E)-3-carene, 2-Heptene, 3-Methyl-butanal, β-Pinene, Camphene, Hexanal, α-Pinene, β-Phellandrene, α-Phellanrrene, β-Myrcene, 4-Carene, 2-Heptanohe, Limonene, 4-Methyl-bicyclo[3.1.0]hex-2-ene, Eucalyptol, α-Pinene, γ-Terpinene, 7-Dimethyl-1,3,7-octriene, 2,4-Dimethyl-benzenamine, 3-Carene, Cathine, 2-Heptanol, 2-Propyn-1-ol, 2,6-Dimethyl-2,4,6-octatriene, Fenchone, 1-Methyl-4-(1-methylethyl)-benzene, cis-Limonene oxide, trans-Limonene oxide, 6-Methylene-bicyclo[3.1.0]hexane, Sabinene hydrate, Fenchyl acetate, Camphor, Benzaldehyde, 1,3-Butanediol, Dicyclopropyl carbinol, Fenchol, 1-Octanol, 5-Methyl-2-heptanol, Tetradecyl-oxirane, Estragole, Trans-p-2,8-menthadien-1-ol, β-Terpinol, cis-p-2,8-Menthadien, 4-Methyl-1-(methylethyl)-3-cyclohexen, 2-Methyl-5-(1-methylethyl)-2-cyclohexen-1-one, Phenylmethyl-formic ester, 2,3-Cyclohexen-1-methanol, Epi-bicyclosesquiphellardrene, cis-p-Menth-2,8-dienol, 1,4-Dimethoxy-benzene, 1-Methoxy-4-(1-propenyl)-benzene, 1,2,4a,5,8,8a-Hexadehyde-naphthalene, 4-Methyl-bicyclo[3.1.1]hept-3-en-2-ol, trans-Anethole 73.20 73.27 66.71, Allantoic acid, 2-Methyl-5-(1-methylethyl)-phenol, Mannoheptulose, 2-Methyl-5-(1-methylethyl)-2-cyclohexen-1-ol, 1-Undecanol, Benzothiazole, E-Pinane, 2-Cyclohexen-1-ol, 2-Methyl-bezenemethanol, 4-Methoxy-benzaldehyde, 1,6-Hexanediol, 2-Methoxycyclohexanone, β-Elemenone, Mephenesin, 4φ-Methoxy-acetophenone, 2-Methyl-3-methylethyl-butanoic acid, Folic acid, 1-(Methoxyphenyl)-2-propanone, 1-Methyl-3-(1-methylethyl)-benzene, 4-Fluorohistamine, 1,2-Dimethoxy-4-(1-propenyl)-benzene, (E)-2-Hydroxy-4-cyano-stilbene, 1-(3-Methoxyphenyl)-1-propanone [12], eriodictyol-7-rutinoside, quercetin-3-rutinoside, and rosmarinic acid [64], quercetin-3-glucuronide, isoquercitrin, quercetin-3-arabinoside, kaempferol-3-glucuronide and kaempferol-3-arabinoside, and isorhamnetin glucoside [58], Quercetin-3-O-galactoside, kaempferol-3-O-rutinoside, and kaempferol-3-O-glucoside [65],Isorhamnetin 3-O-α-rhamnoside, quercetin, and kaempferol, quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, and quercetin 3-O-β-glucoside [66], quercetin, rutin, isoquercitrin [67], 3-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 5-Ocaffeoylquinic acid, 1,3-O-di-caffeoylquinic acid, 1,4-O-dicaffeoylquinic acid, and 1,5-O-di-caffeoylquinic acid [64], 3,4-dihydroxyphenethylalchohol-6-O-caffeoyl-β-Dglucopyranoside and 3′,8′-binaringenin [68]. |
| Garcinia mangostana Linnaeus | Clusiaceae | Stomach disorder | Fruit | India | α-Mangostin, β-Mangostin, γ-Mangostin, Garcinone-E, Methoxy-β-mangostin, Xanthone [69]; Mangostin, BR-Xanthone, Gartanin, 8-Desoxygartanin, Garcinone-D, Euxanthone, Xanthione [70]; Epichatechin, and Tannin [71]. |
| Jatropha gossypiifolia Linnaeus | Euphorbiaceae | Rheumatism | Whole plant, Stem, Leaves | India, Nigeria, Thailand | Gossypifan, Gossypilin, Gossypidien [72]; Gadain, Jatroiden [73]; Jatrodien [74]; Arylnaphthalene, Galic, Vanilic, Syringic, 2,5-Dihydroxy benzoic, Caffeic, Rosmarinic, and p-Coumaric [75]. |
| Kaempferia galanga Linnaeus | Zingiberaceae | Rheumatism | Rhizome | Thailand | (−)-Sandaracopimaradiene, Boesenberol, Sandaracopimaradien-1α,9α-diol, Kaempulchraol C, Kaempulchraol D [76]. |
| Malus prunifolia (Willd.) Borkh. | Rosaceae | Diarrhoea | Fruit | China | Citric acid, p-Coumaric acid, Hyperoside, Myricetin, Naringenin, Quercetin, Kaempferol, Gentiopicroside, Ursolic acid, and 8-Epiloganic acid [77]. |
| Manihot esculenta Crantz | Euphorbiaceae | Hypertension | Stem | Switzerland, China | Sporoge, Thecacorin, Longifoamide-B (Zeng Y, 2015); Yucalexin P-23, Yucalexin P-15, Protocatechuic acid, and Catalpinic acid [78]; Coniferaldehyde, Isovanillin, 6-Deoxyjacareubin, Scopoletin, Syringaldehyde, Pinoresinol, p-Coumaric acid, Ficusol, Balanophonin and Ethamivan [79]. |
| Musa paradisiaca Linnaeus | Musaceae | Diarrhoea Stomach disorder |
Fruit | Brazil, India | Cycloeucalenone, 31-Norcyclolaudenone, 24-Methylene-cicloartanol [80]; α-Thujene, γ-Terpinene, α- and β-Pinene, Sabinene, β-Myrcene, Limonene, α-Capaene, Caryophyllene and (Z,E)-α Farnesene, Aceteugenol, Palmitic acid, Stearic acid, Palmitin, and Stearin [81]. |
| Oryza sativa Linnaeus | Poaceae | Vitaliser | Seed, Roots | Japan, Korea | Momilactones A and B [82]; Momilactone D, Momilactone E, Momilactone A, Sandaracopimaradien-3-one, Oryzalexin A [83]; Oryzativol C [84]; Oryzativol A [85]; ferulic acid, γ-Oryzanol, and Phytic acid [86]; Vanillin, Methyl trans-ferulate, Trans-p-Coumaric acid Methyl ester, N-Benzoyltryptamine, and N-(Trans-cinnamoyl)tryptamine [87]. |
| Persea americana Mill. | Lauraceae | Hypertension | Seed | Brazil | Quercetin and Epicatechin [88]; Avocadene, Avocadyne, Avocadenol-A [89]; γ-Lactone Perseanolide [90]. |
| Physalis lagascae Roem. & Schult. | Solanaceae | Diarrhoea Stomach disorder |
NA | NA | NA |
| Piper amplum Kunth | Piperaceae | Rheumatism | NA | NA | NA |
| Piper betle Linnaeus | Piperaceae | Bleeding | Leaves | India, Myanmar, China | Estragole, Linalool, α-Copaene, Anethole, Caryophyllene, α-Terpinene, p-Cymene, 1,8-Cineole, β-Caryophyllene, α-Humulene, Allyl pyrocatechol, Allylcatechol, Methyl eugenol, Estragol (methyl chavicol), Chavibetol, Chavibetol acetate, Safrol, 4-Allyl-2-methoxy-phenolacetate, and 3-Allyl-6-methoxyphenol [91]; Pipeneolignan A, Piperneolignan B, Hydroxychavicol, p-Hydroxycinnamaldehyde, Diallylcatechol [92]; Pipercerebrosides A and B [93]; Piperolactam A [94]. |
| Rosa tomentosa Sm. | Rosaceae | Fever | NA | NA | NA |
| Rubus rosa L. H. Bailey | Rosaceae | Diarrhoea | Whole plant | USA | Elagic acid [95]. |
| Saccharum officinarum Linnaeus | Poaceae | Rheumatism | Stem | Brazil | Phenolic acid: p-Hydroxybenzoic, p-Hydroxycinnamic, Vanillic and Ferulic acid, Terpenoids: α-Tocopherol and β-Carotene, Flavonoid aglycone Tricin (5,7,4-trihydroxy-3,5-dimethoxyflavone) [96]. |
| Sechium edule | Cucurbitaceae | Fever (kindern) | Whole plant, Fruit | Mexico | Cinnamic acid, Linoleic, Palmitic, and Myristic acids [97]. |
| Sesbania grandiflora (L.)Pers. | Fabaceae | Fever | Leaves, Bark, Flowers | Indonesia | Gallic acid [98], 2-Arylbenzofuran [99]; Sesbagrandiflorains A and B [100]; Sesbagrandiflorain D and E, Spinosan A and Spinosan B [101]. |
| Solanum lycopersicum Linnaeus | Solanaceae | Nutrition | Fruit | USA, Japan, Korea, Chile | Monoterpenes, Glycoalkaloids, and Acyl sugars [102]; 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic acid (13-oxo-OTA), a linolenic acid derivative [103]; Steroidal saponins, Alkaloids, Cerebroside, Phenolic compounds, Sterols, and Nucleosides [104]; Guanosine [105]. |
| Solanum nigrum Linnaeus | Solanaceae | Hypertension, Tonic drink after hard labour |
Whole plant, Fruit, Seed | China, Korea | Lignanamides [106]; Solanine A, 7α-OH Khasianine, 7α-OH Solamargine and 7α-OH Solasonine [107]; Saponins, Solanigroside A and Solanigroside B [108]; Steroidal glycosides (β2-Solamargine, Solamargine, and Degalactotigonin), Saponin (degalactotigonin) [108]; Lunasin [109]. |
| Tagetes tenuifolia Cavanille | Asteraceae | Nasal bleeding | NA | NA | NA |
| Tamarindus indica Linnaeus | Fabaceae | Nausea | Fruit | India | 9,12-Octadecadienoic acid (Z,Z)-, Cis-vaccenic acid, n-Hexadecanoic acid, Beta-Sitosterol, and Octadecanoic acid [110]; Proanthcyanidins, (+)-Catechin, Procyanidin B2, (-)-Epicatechin, Procyanidin trimer, Procyanidin tetramer, Procyanidin pentamer, Procyanidin hexamer, Taxifolin, Apigenin, Eriodictyol, Luteolin and Naringenin [111]. |
| Zingiber officinale Roscoe | Zingiberaceae | Headache | Rhizome | Japan, Thailand | Myristicin, Plumbagin, Methyl piperate, 6-Shogaol, 6-Gingerol and Piperine [112]; Geranyl 6-O-α-l-arabinopyranosyl-β-d-glucopyranoside, Geranyl 6-O-β-d-apiofuranosyl-β-d-glucopyranoside, and Geranyl 6-O-β-d-xylopyranosyl-β-d-glucopyranoside [113]. |
NA: Not available.
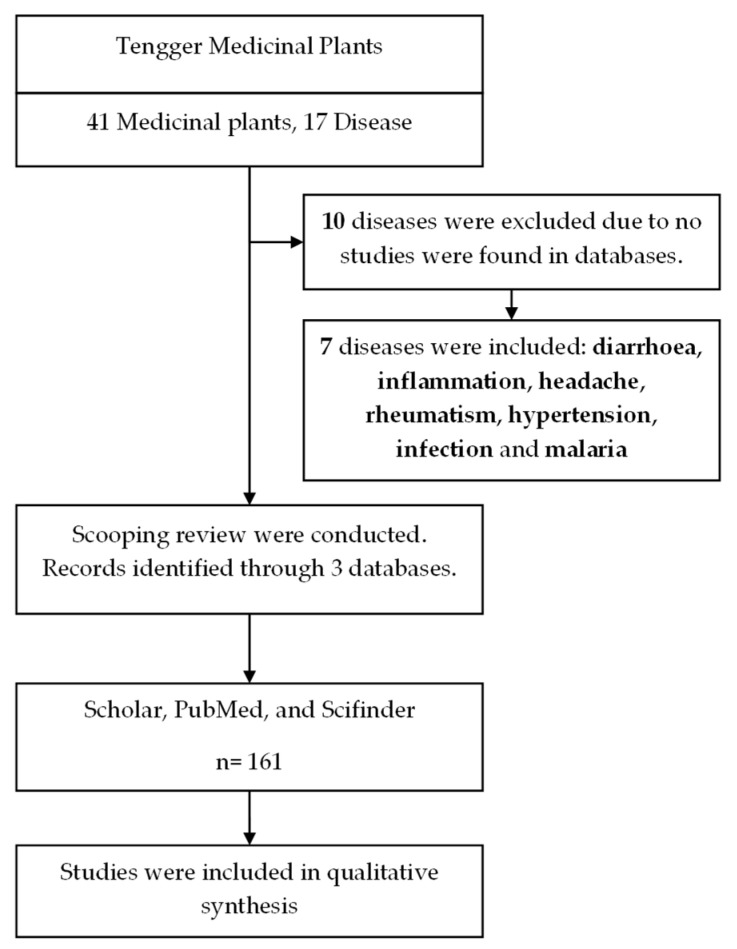
Figure 2.
Flow chart of our approach to scoping literature is presented here.
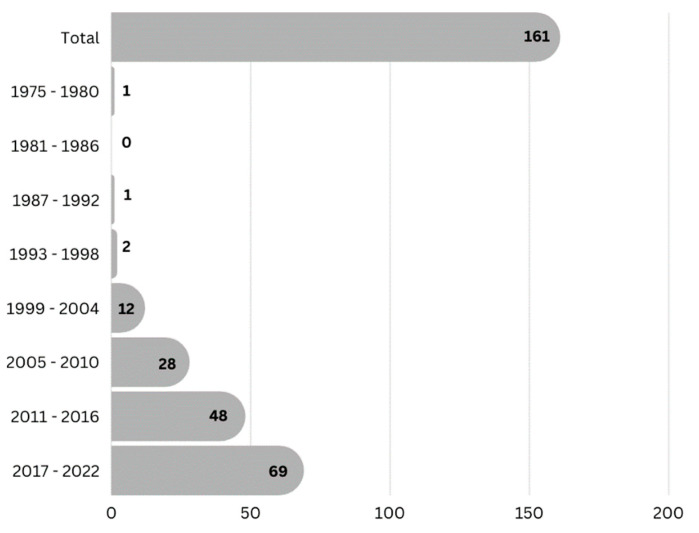
In order to retrieve the phytochemical information of medicinal plants from Google Scholar (https://scholar.google.com/, accessed on 9 August 2022), PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 9 August 2022) and Scifinder (https://scifinder.cas.org, accessed on 9 August 2022), we used keywords such as plant name, chemical constituent, phytochemical composition, and isolated compounds. Chemical names and molecular structures were authenticated using PubChem (https://pubchem.ncbi.nlm.nih.gov/, accessed on 11 August 2022) and Chemspider (http://www.chemspider.com/, accessed on 11 August 2022). To collect pharmacological information, data were searched using the same databases, with the bioactivity of each species collected based on Tenggerese traditional uses as part of the keywords. To maintain the quality of information, we included only Scopus and PubMed indexed articles. We retrieved the literature from 1975 to 2022, conducted meta-analysis and presented it in a bar graph, as shown in Figure 3.
Figure 3.
Retrieved articles related to phytochemistry and pharmacological studies of medicinal plants used by the Tenggeresse people. Papers were collected from a previous report (1975–2022) of the same plants studied across the globe for similar pharmacological claims (Google Scholar, PubMed, and SciFinder Scholar).
2. Phytochemistry of Tenggerese Medicinal Plants
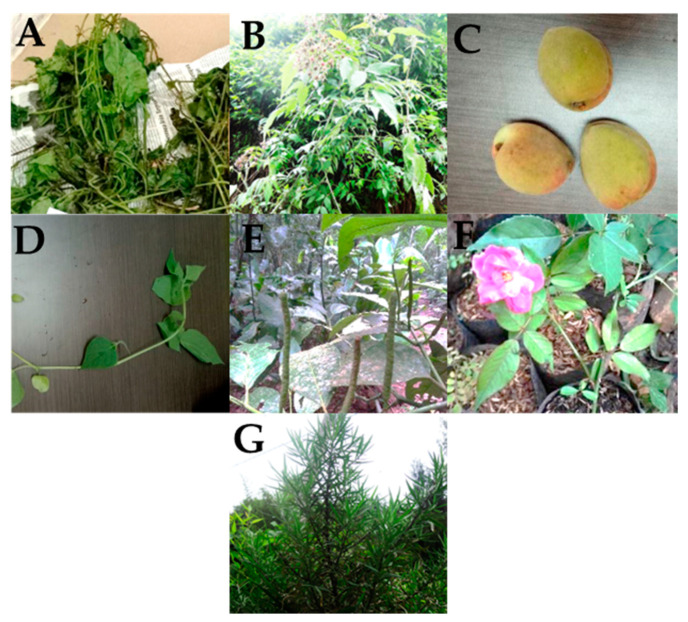
The analysis of the reported ethnobotanical studies of Tenggerese medicinal plants revealed 41 species of medicinal plants (Table 1). Of these, 33 were studied for their phytochemical composition, and seven species remain unstudied. More than 404 phytochemicals with diverse molecular structures were identified from the 33 medicinal plants studied (see Table 1). These phytochemicals belong to different chemical classes including flavonoids, terpenoids, alkaloids, saponins and volatiles. While a few plants were reported to contain two or three phytochemicals, other plants have been extensively studied, and as many as 30 phytochemicals were either detected or isolated from a single plant. For example, ellagic acid was the only phytochemical reported from Rubus rosa, and therefore further in-depth analysis of this plant is required. On the other hand, 48 phytochemicals have been identified from Acorus calamus (Table 1). Seven species that were not studied for their phytochemicals are Cayratia clemaidea, Drymocallis arguta, Elaeocarpus longifolius, Physalis lagascae, Piper amplum, Rosa tomentosa and Tagetes tenuifolia (Figure 4).
Figure 4.
Pictures of understudied medicinal plants used by the Indigenous people of Tengger. (A): Cayratia clemaidea; (B): Drymocallis arguta: (C): Elaeocarpus longifolius; (D): Physalis lagascae; (E): Piper amplum, (F): Rosa tomentosa; (G): Tagetes tenuifolia.
It is interesting to note that although the medicinal plants listed in Table 1 have been used for many generations by the people of Tengger in Indonesia, this review found that most of the phytochemical and pharmacological studies on these plants were reported from other countries, including North and South Americas, Europe, Middle East and East Asia, and South East Asian countries. There are only limited phytochemical and pharmacological studies reported on medicinal plants that grow in the Tengger region, or even Indonesia as a whole. The few medicinal plants that were extensively studied in Indonesia for their phytochemicals are C. burmanii, C. nucifera and S. grandiflora.
3. Biological Activities of Tenggerese Medicinal Plants
To provide a scientific basis to the traditionally claimed therapeutic indications of medicinal plants, it is critical to test the plants for their chemical and biological activities. This is often a challenging task for the traditional practitioners and researchers in Indonesia due to lack of expertise, technology and financial resources. However, since there are many overlapping medicinal plants between different cultures and countries, it is likely that some medicinal plants may have been studied previously. For example, a capsicum species which are used by Tengger people in alleviating post labour complication caused by inflammation [114] has been shown to possess antioxidant and anti-inflammatory activities. In these cases, in order to understand the scientific status of medicinal plants used by Tengger healers, a literature review on each of the plants listed in Table 1 was undertaken for their biological activities. Several medicinal plants have been studied for their biological activities including diarrhoea, wound healing, headache, rheumatism, hypertension, fever, and other disorders. We have discussed them separately.
3.1. Diarrhoea
Many medicinal plants were traditionally used for treating diarrhoea, e.g., the sap of Musa paradisiaca L. This plant was reported to possess anti-diarrheal activity in an animal model study [115]. The soluble plantain fibre of banana was also reported to prevent diarrhoea by blocking epithelial adhesion and M-cell translocation of intestinal pathogens [116]. A clinical study on children with acute watery diarrhoea who received green cooked banana supplement indicated a significant recovery of their health [117]. The dietary management of persistent diarrhea in hospitalized children showed that the green banana diet significantly shortened the duration of diarrhea by 18 h compared to the non-banana-supplemented group [118].
3.2. Wound Healing
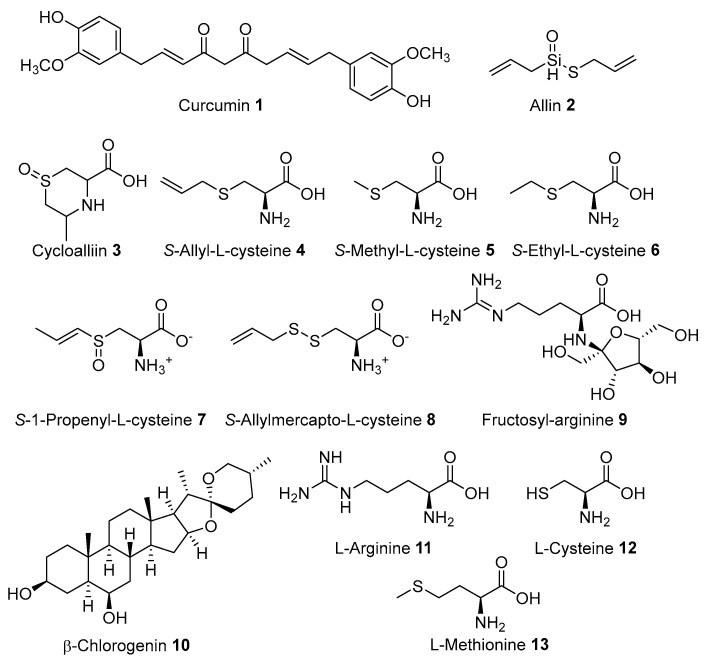
Of the many plants used by the Tenggerese healers for treating wounds, an in vivo preclinical experiment of leaf extract of Anredera cordifolia in skin burn recovery using albino rats showed a better healing process [119]. This might be related to the antioxidant, anti-inflammatory, and antibacterial properties of the plant. Similarly, a rhizome of Curcuma longa is prepared traditionally in wound healing by the Tenggeresse healers. Its rhizome is rich in curcumin 1, which has been reported for its wound healing, anti-inflammatory, anti-infectious, antibacterial and antioxidant activities [120]. In addition, curcumin 1 advances cutaneous wound healing through tissue remodelling, granulation, tissue formation, collagen deposition and epithelial regeneration, and increases fibroblast proliferation and vascular density [120]. The bulb of Allium sativum (garlic) poultice was applied in wound healing which is rich in allin 2, cycloalliin 3, S-allyl-l-cysteine 4, S-methyl-l-cysteine 5, S-ethylcysteine 6, S-1-proponyl-l-cysteine 7, S-allylmercapto-l-cysteine 8, fructosyl-arginine 9, and β-chlorogenin 10. It also consists of L-arginine 11, L-cysteine 12, and L-methionine 13 (Figure 5) [121]. These compounds were tested to have wound healing activity. Dermatologic application of garlic is correlated with its antioxidant components (S-allyl-l-cysteine 4 and S-allylmercapto-l-cysteine 8), which are organosulfur compounds. In addition, a randomized placebo-controlled double-blinded study on garlic powder revealed the powder increases capillary skin perfusions after 5 h administrations. The pre-clinical trial of aged garlic extract on chicken skin wounds indicated an increase in the re-epithelialization and profuse dose-dependent neovascularization [122].
Figure 5.
Wound healing compounds of medicinal plants used by Tenggerese (isolated from the same species found in other countries).
3.3. Headache
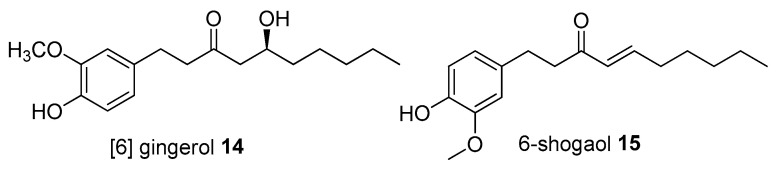
Zingiber officinale and Curcuma longa, which are frequently used in Tengger for the treatment of headache, possess several important pharmacological properties including analgesic and neuroprotective properties. A case report of a 42 year old patient with migraine/headache showed that they experienced a reduction in migraine attacks with much lower intensity after consuming ginger powder and using raw fresh ginger in their diet [123]. However, a double-blind placebo-controlled randomized clinical trial of Z. officinale revealed that the consumption of ginger did not have a substantial effect on migraine treatment. Nevertheless, the trial indicated significant activity in attenuating pain intensity [124]. This pain alleviating was associated with the modulatory effect of the trigeminal nociceptor in neurogenic inflammation, and also had neuroprotective effects by inhibition of the production of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α). 6-Gingerol 14 and 6-shogaol 15 (Figure 6) are the main chemical constituents of ginger [125]. Furthermore, a multimodal care for headache, which include C. longa as a management therapy, appeared to improve the patient’s symptoms. The tension score of the headache was 3 out of 10 (0 being no pain and 10 being highest pain) in the first week of treatment, with no migraine experienced after that [126]. Curcumin 1 could significantly reduce the neurochemical changes and nerve fibre degeneration [127]. In addition, curcumin 1 (isolated from C. longa) and capsaicin analog [6]-gingerol 14 (isolated from Z. officinale) were reported to possess significant analgesic activities [128,129]. These two plants are commonly used as cooking spices in many parts of the world.
Figure 6.
Analgesic compounds of medicinal plants used by Tenggerese (isolated from same species found in other countries).
3.4. Rheumatism and Anti-Inflammatory Agents
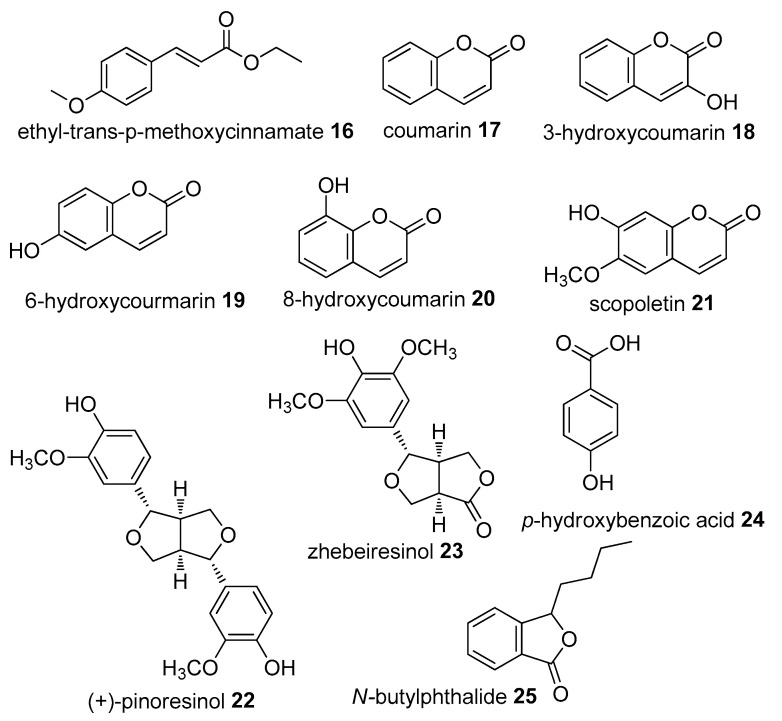
Seven medicinal plants prescribed by the Tenggerese healers to treat rheumatism are Alyxia reinwardtii, Borreria laevis, Foeniculum vulgare, Jatropha gossypiifolia, Kaempferia galanga, Saccharum officinarum, and Piper amplumi. Most are prepared as an ointment, juiced, or boiled to drink. Previous studies related to the pharmacological activities of F. vulgare, J. gossypiifolia, K. galanga, and S. officinarum supported the traditional claims of these plants as anti-rheumatoid. Locally known as “adas” in Tengger, the leaf of F. vulgare consists of monoterpene hydrocarbon and sesquiterpenes as the main components of their essential oils. The methanol extract of F. vulgare Mill. showed inhibitory effects against acute and subacute inflammatory diseases and possessed a central analgesic effect, validating its traditional use for arthritis [130]. An in vivo preclinical study of F. vulgare essential oils against the mouse ear edema model induced by TPA were reported to reduce the level of anti-inflammatory cytokines TNF-α, cyclooxygenase-2 (COX-2), IL-6, and p65 [131]. Additionally, a randomized double-blind trial of women with knee osteoarthritis showed that the extract capsule of F. vulgare significantly lowered the scores for pain, disability, total of WOMAC score and VAS variables [132]. In addition, the insignificant toxicity of F. vulgare infusion was reported based on an in vivo experiment using rats [133].
The plant K. galanga is used traditionally in rheumatism, and has been reported to possess significant anti-inflammatory activity in carrageenan-induced rats by limiting lipoxygenase (LOX), thereby suppressing the leukotriene B4 (LTB4) production [134]. Ethyl-trans-p-methoxycinnamate (EPMC) 16 is a dominant phytoconstituent in K. galanga. It showed significant anti-inflammatory activity with a minimum inhibitory concentration (MIC) of 100 mg/kg in a carrageenan-induced edema, and also showed non-selective inhibition activities of cyclooxygenases 1 and 2, with IC50 values of 1.12 μM and 0.83 μM, respectively [135]. EPMC rich extract suppress acute and chronic inflammation progression in animal models through neutrophil infiltration inhibition [136]. In another recent study, EPMC was also reported to have potential activity to inhibit granuloma tissue formation and suppress cytokine production including IL-1 and TNF-α. The significant analgesic effect of EPMC was also shown in a tail flick experiment of rodents [137].
The herbal gel containing an aqueous extract of J. gossypiifolia was reported to have topical anti-inflammatory activity, either in acute or chronic models of inflammation. It also reduced the production of nitric oxide, leukocyte migration and inhibited edema formation. The flavonoids constituents may be hypothesized as the main active compounds in J. gossypiifolia [138]. In zymosan-induced arthritis mice, a mixture of fatty acids from S. officinarum wax oil (FAM) was reported to decrease the level of β-glucuronidase activity in the synovial fluid of treated mice. FAM also reduces bone erosion [139]. S. officinarum, K. galanga, and F. vulgare, which are used for treating rheumatism pain, have been reported to possess significant anti-inflammatory and analgesic properties when evaluated using a carrageenin-induced test, a hot plate and acetic acid-induced writhing tests [140]. Eight phenolic compounds that were isolated from A.reinwardtii Bl (coumarin 17, 3-hydroxycoumarin 18, 6-hydroxycoumarin 19, 8-hydroxycoumarin 20, scopoletin 21, (+)-pinoresinol 22, zhebeiresinol 23, and p-hydroxybenzoic acid 24) showed anti-inflammatory activities [18] (Figure 7). Brassica rapa [141] and A. reinwardtii [142] were reported to have anti-inflammatory, gastroprotective and antiulcer properties against indomethacin and aspirin-induced rats. In addition, Brassicaceae is composed of an anti-inflammatory agent producing veggie species such as B. oleracea, which significantly inhibits oxidative/nitrosative stress and lipoperoxidation, based on an ex vivo experiment [143].
Figure 7.
Compounds found in plants used by Tenggerese (isolated from same species found in other countries).
3.5. Hypertension
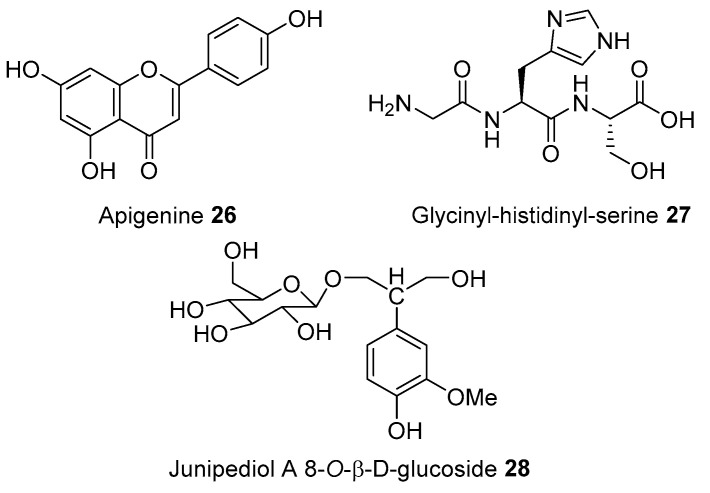
High blood pressure was habitually treated by Tenggerese using Apium graveolens, Brassica rapa, Manihot esculenta, Persera americana, and Solanum nigrum. The hexane, methanol, and aqueous ethanol extracts of A. graveolens seed was reported to reduce blood pressure in deoxycorticosterone acetate–induced hypertensive rats. Further studies revealed that N-butylphthalide 25 presented as the major constituent of the hexane extracts of A. graveolens, which might be responsible for lowering blood pressure activity. Apigenin 26 isolated from A. graveolens demonstrated anti-hypertensive effects in rats [144]. In addition, a randomized triple-blind, placebo-controlled, cross-over clinical trial of A. graveolens was reported to have beneficial effects in metabolic syndrome, including hypertension. Administration of A. graveolens extract could also alter the pharmacokinetic profile of oral anti-hypertensive drugs when given in combination, thereby enhancing their efficacy [145]. The oil of P. americana, commonly known as avocado, was also reported to decrease diastolic and systolic blood pressure by 21.2% and 15.5%, respectively. Besides its beneficial effect on hypertension, avocado oil was reported to suppress the reactive oxygen species (ROS) levels responsible for the pathogenesis of Angiotensin-II induced hypertension [146].
The aqueous extract of P. americana leaf showed a significant reduction in systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP), but had no beneficial effect on heart rate [147]. The crude extracts of other hypotensive medicinal plants such as B. rapa and M. esculenta inhibited angiotensin I-converting enzyme (ACE) [148]. The dual inhibition of ACE and renin are known to be more effective in lowering blood pressure. A protein-derived glycinyl-histidinyl-serine (GHS) 27 (identified from B. rapa) has been known to exhibit dual anti-hypertensive effects [149]. A. graveolens extract from which junipediol A 8-O-β-d-glucoside (1-β-d-glucosyloxy-2-(3-methoxy-4-hydroxyphenyl)propane-1,3-diol 28 was isolated also inhibited the angiotensin-converting enzyme (ACE) [150] (Figure 8).
Figure 8.
Antihypertensive compounds of plants used by Tenggerese (isolated from same species reported from other countries).
3.6. Antimicrobial Activities
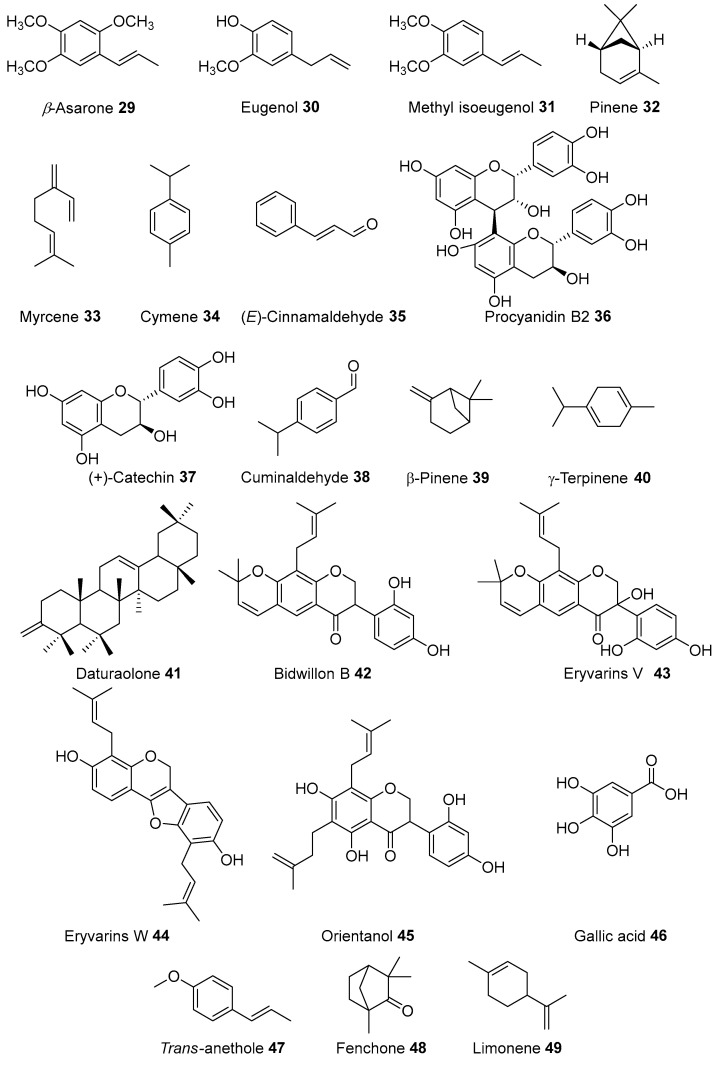
Some Tenggerese medicinal plants were reported to have potential antimicrobial properties against various strains. For example, Acorus calamus extract, used by Tengerrese traditional healers (dukuns) for treating fever, showed broad-spectrum bioactivities including inhibition of both gram negative and positive bacteria such as Helicobacter pylori [151], Propinibacterium acnes [152], Methycillin-resistant staphylococcus aureus (MRSA) [153], Enterobacter aaerogenes, Proteus mirabilis [154] multidrug-resistant enteric bacteria [155], and dengue virus (DENV) replication. In addition, A. calamus. was reported to contain antimicrobial compounds including β-asarone 29, eugenol 30, methyl isoeugenol 31, pinenes 32, myrcene 33, cymene 34 [156] and tatanan A [157]. (E)-Cinnamaldehyde 35, procyanidin B2 36 and (+)-catechin 37 isolated from Cinnamomum burmannii (Nees & T. Nees) Bl. showed antimicrobial activity [158]. Cuminaldehyde 38, β-pinene 39, and γ-terpinene 40 (Figure 9) isolated from Cuminum cyiminum seeds (locally known as ‘jinten’) showed antibacterial activities against Bacillus cereus, Staphylococcus aureus, and Escherichia coli. The oil from the seed of this plant increases membrane permeability leading to swelling, and the reduction of membrane function, thereby changing cell morphology and causing cell death [159]. Sechium edule was reported to possess antifungal activity against Candida spp and Aspergillus spp. [160]. D. metel showed antifungal activity against Aspergillus flavus, Microsporum canis, and Fusarium solani [161]. In addition, daturalone 41 isolated from Datura metel was reported to be effective against Klebsiella pneumoniae, Bacillus subtilis, Staphylococcus epidermis, and Staphylococcus aureus [161].
Figure 9.
Antimicrobial compounds of plants used by Tenggerese (isolated from same species grown in other countries).
C. burmanii extract, which contains E-cinnamaldehyde 35 and several polyphenols as a predominant volatile oil component, showed antimicrobial activities against B. cereus, Listeria monocytogenes, S. aureus Escherichia coli, and Salmonella anatum [158]. Crude polar extracts (n-butane and ethanol) from C. burmanii were reported to be effective against Listeria monocytogenes, Staphylococcus aureus, Escherichia coli O157:H7, and Salmonella anatum with the inhibition zone ranging from 7.28–24.32 mm in which n-butane extract indicated higher activity [27,29]. Curcuma longa, which contains curcumin, demonstrated a wide-spectrum of antimicrobial properties against Vibrio harveyi, Vibrio alginolyticus, Vibrio vulnificus, Vibrio parahaemolyticus, Vibrio cholerae, Bacillus subtilis, Bacillus cereus, Aeromonas hydrophila, Streptococcus agalactiae, Staphylococcus aureus, Staphylococcus intermedius, Staphylococcus epidermidis, and Edwardsiella tarda.
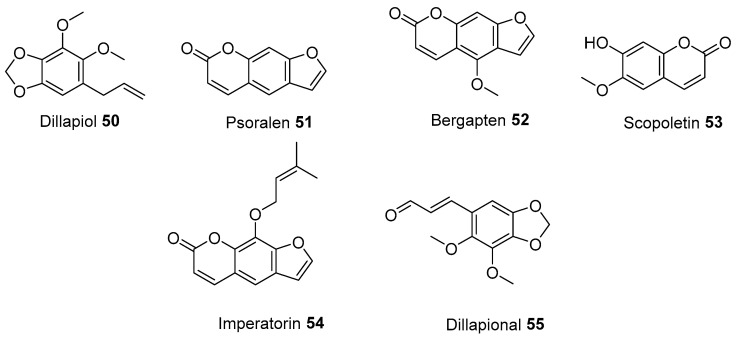
In addition, compounds isolated from these medicinal plants are known to exhibit various biological activities. For example, curcumin 1 elicited fungal inhibitory activities against four species of Rhizoctonia solani, Phytophthora infestans, Puccinia recondita, and Botrytis cinerea. The curcumin 1 also demonstrated antiviral activities against human immunodeficiency virus (HIV), hepatitis b virus (HBV), hepatitis c virus (HCV), and human papillomavirus (HPV) [162]. The isoflavonoid compounds such as bidwillon B 42, eryvarins V 43, and eryvarins W 44 [163], and orientanol E 45 [164], which were isolated from E. variegata, also demonstrated antibacterial activity against MRSA. Bidwillon B 42, in combination with mupirocin, was effective in eliminating MRSA infection of the nasal cavity and skin [165]. Gallic acid 46 and essential oils present in Sesbania grandiflora [98] and Foeniculum vulgare [166] were responsible for their antibacterial properties, respectively. The F. vulgare essential oil contains trans-anethole 47, fenchone 48, and limonene 49, which were reported to possess potent bioactivities against Mycobacterium tuberculosa, Shigella dysenteriae, Shigella flexneri, Vibrio cholerae, Staphylococcus aureus and Escherichia coli [166]. Essential oils in general are known for their antimicrobial properties and have great applications in making antimicrobial products, lotions, disinfectants and insect repellents (especially mosquitoes) [167]. Secondary metabolites such as dillapiole 50, psoralen 51, bergapten 52, scopoletin 53, imperatorin 54, and dillapional 55 from F. vulgare were reported to be responsible for antibacterial activity [12,59]. Indonesia is also gifted with a diverse array of lichens, which showed potent antibacterial properties–an area worth exploring for chemical and antimicrobial screening [168].
3.7. Antimalarial Activities
There are a number of medicinal plants used by Tenggerese healers for treating fever and malaria. The methanolic extract of the root of Sesbania species (used by dukuns for treating fever arising from malaria infection) was reported to have significant anti-plasmodial activity, with a minimum inhibition concentration value of 62.5 µg/mL [169]. The dichloromethane extract of Acorus calamus was reported to have antiplasmodial activity with an IC50 value of 5.07 µg/mL against the chloroquine-sensitive (CQS) strain of Plasmodium falciparum [170]. Further studies showed that curcumin 1 isolated from the roots of Curcuma longa inhibited P. falciparum growth with an IC50 of ~5 µM. Additionally, in mice infected with Plasmodium berghei, oral administration of curcumin 1 was reported to have significant activity in reducing the blood parasitemia by 80–90% [171]. A previous study reported that curcumin 1 was responsible for the inhibition of glycogen synthase Kinase-3β, which might be contributing to the antimalarial activity [172]. In addition, the moderate anti-malarial activities of Datura metel leaf methanol extract were reported with an IC50 value of 22 ± 0.6 µg/mL against P.falciparum [173]. The aqueous extract of Cuminum cyminum seeds was also reported to have plasmodial growth inhibition by 9% against P. falciparum strain FCR3 [174]. From Erythrina variegata, Warangalone 56 (8(3,3-dimethyl-allyl)-4′-hydroxy-2‴,2‴-dimethylpyran-[6,7,b] isoflavone) (Figure 10) had been isolated from stem bark, and possessed antimalarial activity with an IC50 value of 4.8 and 3.7 µg/mL against both the sensitive (3D7) and resistant (K1) strain of P. falciparum [175].
Figure 10.
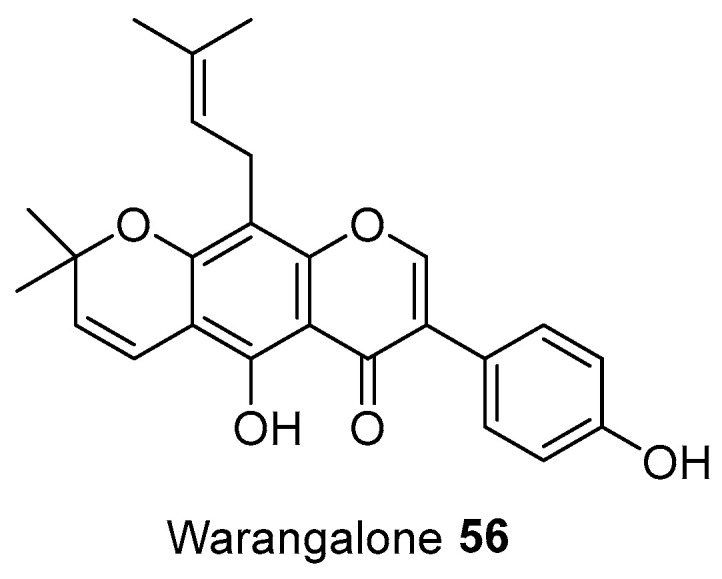
Antimalarial compound of plants used by Tenggeresse (isolated from same species grown in other countries).
4. Conclusions
This review evaluated 41 medicinal plants used by the indigenous people of the Tengger community, and revealed that 554 phytochemicals have been isolated from 33 plant species with flavonoids and terpenoids as the major chemical components. Most of the plants and their phytochemicals have been tested for various pharmacological activities including anti-inflammatory, antimicrobial, antimalarial, wound healing, headache, and hypertension. Although these medicinal plants grow plentifully in Indonesia, most of the studies were reported on the plants that grow in China, India and Thailand. Only three medicinal plants were phytochemically studied in Indonesia. The research cost and lack of modern laboratory equipment have limited Indonesian researchers in conducting extensive phytochemical and pharmacological studies. The few species that have not been evaluated scientifically presents great potential for biodiscovery. These medicinal plants are: Cayratia clemaidea, Drymocallis arguta, Elaeocarpus longifolius, Physalis lagascae, Piper amplum, Rosa tomentosa and Tagetes tenuifolia. The Cayratia clemaidea, Drymocallis arguta, Elaeocarpus longifolius and Physalis lagascae species has a potential application in treating diarrhea, as this is common among the Indonesian population, especially living in the rural areas where there is lack of food and water sanitation. In addition, Piper amplum, Rosa tomentosa and Tagetes tenuifolia are valuable for bioprospecting to discover new therapeutic agents to treat rheumatism, fever agents and nasal bleeding.
Acknowledgments
The authors thank the people of Tengger, the Faculty of Pharmacy for funding, Mohammad Hasan (Vice Chancellor, University of Jember), Sapanyana (Chief of Wonokerso Village) and Sutomo (Spiritual Leader of the People of Tengger). We thank Hildawati Ilham, Aries Syafitri, Syamsu Dhuha, Putri Sakinah, Virda Fitra Mandasari for technical support.
Author Contributions
Conceptualization, A.S.N., A.N.W.P., B.T., P.A.K. and P.W. methodology, A.S.N., A.N.W.P., B.T., P.A.K. and P.W.; software, A.S.N. and A.N.W.P.; validation, A.S.N., A.N.W.P., B.T., P.A.K. and P.W.; formal analysis, A.S.N., A.N.W.P., B.T. and P.W.; investigation, A.S.N., A.N.W.P., B.T., R.P.A., S.M., D.M.R., N.B.W. and P.W.; resources, A.S.N. and P.A.K.; data curation, A.S.N., A.N.W.P., B.T., R.P.A., S.M., D.M.R. and N.B.W.; writing—original draft preparation, A.S.N., A.N.W.P., B.T., R.P.A., S.M., D.M.R., N.B.W., P.A.K. and P.W.; writing—review and editing, A.S.N., D.M.R., N.B.W., P.A.K. and P.W.; visualization, A.S.N., D.M.R. and A.N.W.P.; supervision, A.S.N., P.A.K. and P.W.; project administration, A.S.N., B.T. and N.B.W.; funding acquisition, A.S.N., A.N.W.P. and B.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding. The APC was funded by MDPI as an invited paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wangchuk P., Tobgay T. Contributions of medicinal plants to the Gross National Happiness and Biodiscovery in Bhutan. J. Ethnobiol. Ethnomed. 2015;11:48. doi: 10.1186/s13002-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan H., Ma Q., Ye L., Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Rashid M.H., Kundu A., Mandal V., Wangchuk P., Mandal S.C. Preclinical and Clinical Trials of Indian Medicinal Plants in Disease Control. In: Sen S., Chakraborty R., editors. Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and Its Value. Springer; Singapore: 2020. pp. 119–142. [DOI] [Google Scholar]
- 5.Yeshi K., Gyal Y., Sabernig K., Phuntsho J., Tidwell T., Jamtsho T., Dhondup R., Tokar E., Wangchuk P. An integrated medicine of Bhutan: Sowa Rigpa concepts, botanical identification, and the recorded phytochemical and pharmacological properties of the eastern Himalayan medicinal plants. Eur. J. Integr. Med. 2019;29:100927. doi: 10.1016/j.eujim.2019.100927. [DOI] [Google Scholar]
- 6.Nugraha A.S., Keller P.a. Revealing indigenous Indonesian traditional medicine: Anti-infective agents. Nat. Prod. Commun. 2011;6:1953–1966. doi: 10.1177/1934578X1100601240. [DOI] [PubMed] [Google Scholar]
- 7.Roosita K., Kusharto C.M., Sekiyama M., Fachrurozi Y., Ohtsuka R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J. Ethnopharmacol. 2008;115:72–81. doi: 10.1016/j.jep.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Gewali M.B., Awale S. Aspects of Traditional Medicine in Nepal. Institute of Natural Medicine University of Toyama; Toyama, Japan: 2008. [Google Scholar]
- 9.Smith-Hefner N.J. A Social History of Language Change in Highland East Java. J. Asian Stud. 2011;48:257–271. doi: 10.2307/2057377. [DOI] [Google Scholar]
- 10.Azrianingsih R., Kusumahati A. Perception and appreciation of tenggerese of medicinal plants in Wonokitri Village, Tosari subdistrict, Pasuruan Regency. AIP Conf. Proc. 2018;2019:020016. doi: 10.1063/1.5061852. [DOI] [Google Scholar]
- 11.Jadid N., Kurniawan E., Himayani C.E.S., Andriyani, Prasetyowati I., Purwani K.I., Muslihatin W., Hidayati D., Tjahjaningrum I.T.D. An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS ONE. 2020;15:e0235886. doi: 10.1371/journal.pone.0235886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badgujar S.A.-O., Patel V.V., Bandivdekar A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014;2014:32. doi: 10.1155/2014/842674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paithankar V.V., Belsare S.L., Charde R.M., Vyas J.V. Acorus Calamus: An Overview. Int. J. Biomed. Res. 2011;2:518–529. doi: 10.7439/ijbr.v2i10.174. [DOI] [Google Scholar]
- 14.Balakumbahan R., Rajamani K., Kumanan K. Acorus calamus: An overview. J. Med. Plants Res. 2010;4:2740–2745. [Google Scholar]
- 15.Imam H., Riaz Z., Azhar M., Sofi G., Hussain A. Sweet flag (Acorus calamus Linn.): An incredible medicinal herb. Int. J. Green Pharm. 2013;7:288. doi: 10.4103/0973-8258.122053. [DOI] [Google Scholar]
- 16.Hao Z.-Y., Liang D., Luo H., Liu Y.-F., Ni G., Zhang Q.-J., Li L., Si Y.-K., Sun H., Chen R.-Y., et al. Bioactive Sesquiterpenoids from the Rhizomes of Acorus calamus. J. Nat. Prod. 2012;75:1083–1089. doi: 10.1021/np300095c. [DOI] [PubMed] [Google Scholar]
- 17.El-Saber Batiha G., Magdy Beshbishy A., GWasef L., Elewa Y.H., AAl-Sagan A., Abd El-Hack M.E., Taha A.E., MAbd-Elhakim Y., Prasad Devkota H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients. 2020;12:872. doi: 10.3390/nu12030872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rattanapan J., Sichaem J., Tip-Pyang S. Chemical constituents and antioxidant activity from the stems of Alyxia reinwardtii. Rec. Nat. Prod. 2012;6:288–291. [Google Scholar]
- 19.Souza L.F., de Barros I.B.I., Mancini E., Martino L.D., Scandolera E., Feo V.D. Chemical Composition and Biological Activities of the Essential Oil from Anredera cordifolia Grown in Brazil. Nat. Prod. Commun. 2014;9:1934578X1400900730. doi: 10.1177/1934578X1400900730. [DOI] [PubMed] [Google Scholar]
- 20.Liu G., Zhuang L., Song D., Lu C., Xu X. Isolation, purification, and identification of the main phenolic compounds from leaves of celery (Apium graveolens L. var. dulce Mill./Pers.) J. Sep. Sci. 2017;40:472–479. doi: 10.1002/jssc.201600995. [DOI] [PubMed] [Google Scholar]
- 21.Hedayati N., Bemani Naeini M., Mohammadinejad A., Mohajeri S.A. Beneficial effects of celery (Apium graveolens) on metabolic syndrome: A review of the existing evidences. Phytother. Res. 2019;33:3040–3053. doi: 10.1002/ptr.6492. [DOI] [PubMed] [Google Scholar]
- 22.Conserva L.M., Jesu Costa Ferreira J. Borreria and Spermacoce species (Rubiaceae): A review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacogn. Rev. 2012;6:46. doi: 10.4103/0973-7847.95866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noiarsa P., Yu Q., Matsunami K., Otsuka H., Ruchirawat S., Kanchanapoom T. (Z)-3-Hexenyl diglycosides from Spermacoce laevis Roxb. J. Nat. Med. 2007;61:406–409. doi: 10.1007/s11418-007-0151-x. [DOI] [Google Scholar]
- 24.Fernandes F., Valentão P., Sousa C., Pereira J.A., Seabra R.M., Andrade P.B. Chemical and antioxidative assessment of dietary turnip (Brassica rapa var. rapa L.). Food Chem. 2007;105:1003–1010. doi: 10.1016/j.foodchem.2007.04.063. [DOI] [Google Scholar]
- 25.Pérez-Vázquez M.A.K., Pacheco-Hernández Y., Lozoya-Gloria E., Mosso-González C., Ramírez-García S.A., Romero-Arenas O., Villa-Ruano N. Peppermint Essential Oil and Its Major Volatiles as Protective Agents against Soft Rot Caused by Fusarium sambucinum in Cera Pepper (Capsicum pubescens) Chem. Biodivers. 2022;19:e202100835. doi: 10.1002/cbdv.202100835. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad I., Arifianti A.E., Sakti A.S., Saputri F.C., Abdul M.i. Simultaneous natural deep eutectic solvent-based ultrasonic-assisted extraction of bioactive compounds of cinnamon bark and sappan wood as a dipeptidyl peptidase IV inhibitor. Molecules. 2020;25:3832. doi: 10.3390/molecules25173832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang Y., Li Y., Sun A., Liu X. Chemical compound identification and antibacterial activity evaluation of cinnamon extracts obtained by subcritical n-butane and ethanol extraction. Food Sci. Nutr. 2019;7:2186–2193. doi: 10.1002/fsn3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muhammad D.R.A., Tuenter E., Patria G.D., Foubert K., Pieters L., Dewettinck K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chem. 2021;340:127983. doi: 10.1016/j.foodchem.2020.127983. [DOI] [PubMed] [Google Scholar]
- 29.Rahayu D.U., Hakim R.A., Mawarni S.A., Satriani A.R. Indonesian Cinnamon (Cinnamomum burmannii): Extraction, Flavonoid Content, Antioxidant Activity, and Stability in the Presence of Ascorbic Acid. Cosmetics. 2022;9:57. doi: 10.3390/cosmetics9030057. [DOI] [Google Scholar]
- 30.Sethupathy S., Nithya C., Pandian S.K. 2-Furaldehyde diethyl acetal from tender coconut water (Cocos nucifera) attenuates biofilm formation and quorum sensing-mediated virulence of Chromobacterium violaceum and Pseudomonas aeruginosa. Biofouling. 2015;31:721–733. doi: 10.1080/08927014.2015.1102897. [DOI] [PubMed] [Google Scholar]
- 31.Elsbaey M., Ibrahim M.A.A., Bar F.A., Elgazar A.A. Chemical constituents from coconut waste and their in silico evaluation as potential antiviral agents against SARS-CoV-2. South Afr. J. Bot. 2021;141:278–289. doi: 10.1016/j.sajb.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lima E.B.C., de Sousa C.N.S., Vasconcelos G.S., Meneses L.N., Silva Pereira Y.F.E., Ximenes N.C., Santos Júnior M.A., Matos N.C.B., Brito R., Miron D., et al. Antidepressant, antioxidant and neurotrophic properties of the standardized extract of Cocos nucifera husk fiber in mice. J. Nat. Med. 2016;70:510–521. doi: 10.1007/s11418-016-0970-8. [DOI] [PubMed] [Google Scholar]
- 33.Zheljazkov V.D., Gawde A., Cantrell C.L., Astatkie T., Schlegel V. Distillation Time as Tool for Improved Antimalarial Activity and Differential Oil Composition of Cumin Seed Oil. PLoS ONE. 2015;10:e0144120. doi: 10.1371/journal.pone.0144120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldulaimi O. Screening of Fruits of Seven Plants Indicated for Medicinal Use in Iraq. Pharmacogn. Mag. 2017;13:S189–S195. doi: 10.4103/pm.pm_503_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neethu S., Veena S.K., Indulekha V.C., Eapen J., Radhakrishnan K.V. Phytoconstituents assessment and development of standardization protocol for ‘Nayopayam Kwatha’, a polyherbal Ayurvedic formulation. J. Ayurveda Integr. Med. 2021;12:489–499. doi: 10.1016/j.jaim.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wuthi-udomlert M., Grisanapan W., Luanratana O., Caichompoo W. Antifungal activity of Curcuma longa grown in Thailand. Southeast Asian J. Trop. Med. Public Health. 2000;31((Suppl. 1)):178–182. [PubMed] [Google Scholar]
- 37.Kim D.S., Kim J.Y. Total synthesis of Calebin-A, preparation of its analogues, and their neuronal cell protectivity against beta-amyloid insult. Bioorganic Med. Chem. Lett. 2001;11:2541–2543. doi: 10.1016/S0960-894X(01)00489-9. [DOI] [PubMed] [Google Scholar]
- 38.Aratanechemuge Y., Komiya T., Moteki H., Katsuzaki H., Imai K., Hibasami H. Selective induction of apoptosis by ar-turmerone isolated from turmeric (Curcuma longa L.) in two human leukemia cell lines, but not in human stomach cancer cell line. Int. J. Mol. Med. 2002;9:481–484. doi: 10.3892/ijmm.9.5.481. [DOI] [PubMed] [Google Scholar]
- 39.Saha A., Kuzuhara T., Echigo N., Suganuma M., Fujiki H. New role of (-)-epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev. Res. 2010;3:953–962. doi: 10.1158/1940-6207.CAPR-09-0247. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y., Zhang J., Zhou J., Huang Z., Hu H., Qiao M., Zhao X., Chen D. Synergistic effect of cucurbitacin B in combination with curcumin via enhancing apoptosis induction and reversing multidrug resistance in human hepatoma cells. Eur. J. Pharmacol. 2015;768:28–40. doi: 10.1016/j.ejphar.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Wang L., Zhu X., Wang D., Li X. Choleretic Activity of Turmeric and its Active Ingredients. J. Food Sci. 2016;81:H1800–H1806. doi: 10.1111/1750-3841.13348. [DOI] [PubMed] [Google Scholar]
- 42.Lv G.-P., Hu D.-J., Zhou Y.-Q., Zhang Q.-W., Zhao J., Li S.-P. Preparation and Application of Standardized Typical Volatile Components Fraction from Turmeric (Curcuma longa L.) by Supercritical Fluid Extraction and Step Molecular Distillation. Molecules. 2018;23:1831. doi: 10.3390/molecules23071831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akter J., Islam M.Z., Takara K., Hossain M.A., Sano A. Isolation and structural elucidation of antifungal compounds from Ryudai gold (Curcuma longa) against Fusarium solani sensu lato isolated from American manatee. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2019;219:87–94. doi: 10.1016/j.cbpc.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Le T., Beaufay C., Nghiem D., Pham T., Mingeot-Leclercq M.-P., Quetin-Leclercq J. Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules. 2019;24:1158. doi: 10.3390/molecules24061158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo R., Liu Y., Xu Z.-P., Xia Y.-G., Yang B.-Y., Kuang H.-X. Withanolides from the leaves of Datura metel L. Phytochemistry. 2018;155:136–146. doi: 10.1016/j.phytochem.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Guo R., Liu Y., Pan J., Guan W., Yang B.-Y., Kuang H.-X. A new sesquiterpenoid with cytotoxic and anti-inflammatory activity from the leaves of Datura metel L. Nat. Prod. Res. 2021;35:607–613. doi: 10.1080/14786419.2019.1590715. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y., Pan J., Sun Y.-P., Wang X., Liu Y., Yang B.-Y., Kuang H.-X. Immunosuppressive withanolides from the flower of Datura metel L. Fitoterapia. 2020;141:104468. doi: 10.1016/j.fitote.2019.104468. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y., Wu D.-D., Zhou Y.-Q., Wu J.-T., Qi Z.-T., Algradi A.M., Pan J., Guan W., Yang B.-Y., Kuang H.-X. A new ent-kaurane diterpenoid from the pericarps of Datura metel. J. Asian Nat. Prod. Res. 2021:1–7. doi: 10.1080/10286020.2021.1981874. [DOI] [PubMed] [Google Scholar]
- 49.Han X.-L., Wang H., Zhang Z.-H., Tan Y., Wang J.-H. Study on Chemical Constituents in Seeds of Datura metel from Xinjiang. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2015;38:1646–1648. [PubMed] [Google Scholar]
- 50.Miękus N., Iqbal A., Marszałek K., Puchalski C., Świergiel A. Green Chemistry Extractions of Carotenoids from Daucus carota L.-Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules. 2019;24:4339. doi: 10.3390/molecules24234339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Badalamenti N., Modica A., Ilardi V., Bruno M., Maresca V., Zanfardino A., Di Napoli M., Castagliuolo G., Varcamonti M., Basile A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021;1:1–6. doi: 10.1080/14786419.2021.2018588. [DOI] [PubMed] [Google Scholar]
- 52.Liu R., Choi H.S., Kim S.-L., Kim J.-H., Yun B.-S., Lee D.-S. 6-Methoxymellein Isolated from Carrot (Daucus carota L.) Targets Breast Cancer Stem Cells by Regulating NF-κB Signaling. Molecules. 2020;25:4374. doi: 10.3390/molecules25194374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaglio R., Barbera M., Aleo A., Lommatzsch I., La Mantia T., Settanni L. Inhibitory Activity and Chemical Characterization of Daucus carota subsp. maximus Essential Oils. Chem. Biodivers. 2017;14:e1600477. doi: 10.1002/cbdv.201600477. [DOI] [PubMed] [Google Scholar]
- 54.Rasul A., Khan M., Yu B., Ma T., Yang H. Xanthoxyletin, a coumarin induces S phase arrest and apoptosis in human gastric adenocarcinoma SGC-7901 cells. Asian Pac. J. Cancer Prev. APJCP. 2011;12:1219–1223. [PubMed] [Google Scholar]
- 55.Tanaka H., Hirata M., Etoh H., Watanabe N., Shimizu H., Ahmad M., Terada Y., Fukai T. Two diphenylpropan-1,2-diol syringates from the roots of Erythrina variegata. J. Nat. Prod. 2002;65:1933–1935. doi: 10.1021/np0201366. [DOI] [PubMed] [Google Scholar]
- 56.Liu Q., Yu J., Liao X., Zhang P., Chen X. One-step separation of antioxidant compounds from Erythrina variegata by high speed counter-current chromatography. J. Chromatogr. Sci. 2015;53:730–735. doi: 10.1093/chromsci/bmu115. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi M., Mahmud T., Yoshioka N., Shibuya H., Kitagawa I. Indonesian medicinal plants. XXI. Inhibitors of Na+/H+ exchanger from the bark of Erythrina variegata and the roots of Maclura cochinchinensis. Chem. Pharm. Bull. 1997;45:1615–1619. doi: 10.1248/cpb.45.1615. [DOI] [PubMed] [Google Scholar]
- 58.Kunzemann J., Herrmann K. [Isolation and identification of flavon(ol)-O-glycosides in caraway (Carum carvi L.), fennel (Foeniculum vulgare Mill.), anise (Pimpinella anisum L.), and coriander (Coriandrum sativum L.), and of flavon-C-glycosides in anise. I. Phenolics of spices (author’s transl)] Z. Fur Lebensm. Unters. Forsch. 1977;164:194–200. doi: 10.1007/BF01263030. [DOI] [PubMed] [Google Scholar]
- 59.Kwon Y.S., Choi W.G., Kim W.J., cKim W.K., Kim M.J., Kang W.H., Kim C.M. Antimicrobial constituents of foeniculum vulgare. Arch. Pharmacal. Res. 2002;25:154–157. doi: 10.1007/BF02976556. [DOI] [PubMed] [Google Scholar]
- 60.Senatore F., Oliviero F., Scandolera E., Taglialatela-Scafati O., Roscigno G., Zaccardelli M., De Falco E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia. 2013;90:214–219. doi: 10.1016/j.fitote.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Váradyová Z., Pisarčíková J., Babják M., Hodges A., Mravčáková D., Kišidayová S., Königová A., Vadlejch J., Várady M. Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus contortus. Exp. Parasitol. 2018;195:71–77. doi: 10.1016/j.exppara.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Štrbac F., Bosco A., Maurelli M.P., Ratajac R., Stojanović D., Simin N., Orčić D., Pušić I., Krnjajić S., Sotiraki S., et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep-In Vitro and In Vivo Studies. Vet. Sci. 2022;9:93. doi: 10.3390/vetsci9020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiboub W., Sassi A.B., Amina C.M.H., Souilem F., El Ayeb A., Djlassi B., Ascrizzi R., Flamini G., Harzallah-Skhiri F. Valorization of the Green Waste from Two Varieties of Fennel and Carrot Cultivated in Tunisia by Identification of the Phytochemical Profile and Evaluation of the Antimicrobial Activities of Their Essentials Oils. Chem. Biodivers. 2019;16:e1800546. doi: 10.1002/cbdv.201800546. [DOI] [PubMed] [Google Scholar]
- 64.Faudale M., Viladomat F., Bastida J., Poli F., Codina C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008;56:1912–1920. doi: 10.1021/jf073083c. [DOI] [PubMed] [Google Scholar]
- 65.Parejo I., Jauregui O., Sánchez-Rabaneda F., Viladomat F., Bastida J., Codina C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum vulgare) Using Liquid Chromatography−Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004;52:3679–3687. doi: 10.1021/jf030813h. [DOI] [PubMed] [Google Scholar]
- 66.Nassar M.I., Aboutabl E.S.A., Makled Y.A., El–Khrisy E.D.A., Osman A.F. Secondary metabolites and pharmacology of Foeniculum vulgare Mill. Subsp. Piperitum. Rev. Latinoam. Química. 2010;38:10. [Google Scholar]
- 67.Cherng J.-M., Chiang W., Chiang L.-C. Immunomodulatory activities of common vegetables and spices of Umbelliferae and its related coumarins and flavonoids. Food Chem. 2008;106:944–950. doi: 10.1016/j.foodchem.2007.07.005. [DOI] [Google Scholar]
- 68.Ghanem M.T.M., Radwan H.M.A., Mahdy E.S.M., Elkholy Y.M., Hassanein H.D., Shahat A.A. Phenolic Compounds from Foeniculum vulgare (Subsp. Piperitum) (Apiaceae) Herb and Evaluation of Hepatoprotective Antioxidant Activity. Pharmacogn. Res. 2012;4:5. doi: 10.4103/0974-8490.94735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dharmaratne H.R.W., Piyasena K.G.N.P., Tennakoon S.B. A geranylated biphenyl derivative from Garcinia malvgostana. Nat. Prod. Res. 2005;19:239–243. doi: 10.1080/14786410410001710582. [DOI] [PubMed] [Google Scholar]
- 70.Gopalakrishnan G., Banumathi B., Suresh G. Evaluation of the antifungal activity of natural xanthones from Garcinia mangostana and their synthetic derivatives. J. Nat. Prod. 1997;60:519–524. doi: 10.1021/np970165u. [DOI] [PubMed] [Google Scholar]
- 71.Ngawhirunpat T., Opanasopi P., Sukma M., Sittisombut C., Kat A., Adachi I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana) Pharm. Biol. 2010;48:55–62. doi: 10.3109/13880200903046138. [DOI] [PubMed] [Google Scholar]
- 72.Das B., Anjani G. ChemInform Abstract: Phytochemicals. Part 29. Gossypidien, a Lignan from Stems of Jatropha gossypifollia. Cheminform. 2010;30:1. doi: 10.1002/chin.199933258. [DOI] [Google Scholar]
- 73.Oduola T., Adeosun G., Oduola T., Avwioro G., Oyeniyi M. Mechanism of Action of Jatropha Gossypifolia Stem Latex As a Haemostatic Agent. Eur. J. Gen. Med. 2004;2:140–143. doi: 10.29333/ejgm/82329. [DOI] [Google Scholar]
- 74.Saishri R., Ravichandran N., Vadivel V., Brindha P. Pharmacognostic studies on leaf of Jatropha Gossypifolia L. Int. J. Pharm. Sci. Res. 2016;7:163–173. doi: 10.13040/IJPSR.0975-8232.7(1).163-73. [DOI] [Google Scholar]
- 75.Povichit N., Phrutivorapongkul A., Suttajit M., Chaiyasut C., Leelapornpisid P. Phenolic content and in vitro inhibitory effects on oxidation and protein glycation of some Thai medicinal plants. Pak. J. Pharm. Sci. 2010;23:403–408. [PubMed] [Google Scholar]
- 76.Tungcharoen P., Chatchai W., Tansakul P., Nakamura S., Matsuda H., Tewtrakul S. Anti-inflammatory effect of isopimarane diterpenoids from Kaempferia galanga. Phytother. Res. 2019;34:612–623. doi: 10.1002/ptr.6549. [DOI] [PubMed] [Google Scholar]
- 77.Wen C., Wang D., Li X., Huang T., Huang C., Hu K. Targeted isolation and identification of bioactive compounds lowering cholesterol in the crude extracts of crabapples using UPLC-DAD-MS-SPE/NMR based on pharmacology-guided PLS-DA. J. Pharm. Biomed. Anal. 2018;150:144–151. doi: 10.1016/j.jpba.2017.11.061. [DOI] [PubMed] [Google Scholar]
- 78.Li S.-S., Hu L.-F., Zhao Y.-X., Zuo W.-J., Zeng Y.-B., Li X.-N., Mei W.-L., Dai H.-F. A new diterpene from the stems of Manihot esculenta. J. Asian Nat. Prod. Res. 2011;13:961–964. doi: 10.1080/10286020.2011.600249. [DOI] [PubMed] [Google Scholar]
- 79.Yi B., Hu L., Mei W., Zhou K., Wang H., Luo Y., Wei X., Dai H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules. 2011;16:10157–10167. doi: 10.3390/molecules161210157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva A.A.S., Morais S.M., Falcão M.J.C., Vieira I.G.P., Ribeiro L.M., Viana S.M., Teixeira M.J., Barreto F.S., Carvalho C.A., Cardoso R.P.A., et al. Activity of cycloartane-type triterpenes and sterols isolated from Musa paradisiaca fruit peel against Leishmania infantum chagasi. Phytomedicine. 2014;21:1419–1423. doi: 10.1016/j.phymed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Fahim M., Ibrahim M., Zahiruddin S., Parveen R., Khan W., Ahmad S., Shrivastava B., Shrivastava A.K. TLC-bioautography identification and GC-MS analysis of antimicrobial and antioxidant active compounds in Musa × paradisiaca L. fruit pulp essential oil. Phytochem. Anal. PCA. 2019;30:332–345. doi: 10.1002/pca.2816. [DOI] [PubMed] [Google Scholar]
- 82.Quan N.V., Tran H.-D., Xuan T.D., Ahmad A., Dat T.D., Khanh T.D., Teschke R. Momilactones A and B Are α-Amylase and α-Glucosidase Inhibitors. Molecules. 2019;24:482. doi: 10.3390/molecules24030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho J.-G., Cha B.-J., Min Lee S., Shrestha S., Jeong R.-H., Sung Lee D., Kim Y.-C., Lee D.-G., Kang H.-C., Kim J., et al. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers. 2015;12:1356–1364. doi: 10.1002/cbdv.201400239. [DOI] [PubMed] [Google Scholar]
- 84.Lee T.K., Lee D., Yu J.S., Jo M.S., Baek S.C., Shin M.-S., Ko Y.-J., Kang K.S., Kim K.H. Biological Evaluation of a New Lignan from the Roots of Rice (Oryza sativa) Chem. Biodivers. 2018;15:e1800333. doi: 10.1002/cbdv.201800333. [DOI] [PubMed] [Google Scholar]
- 85.Kang H.R., Yun H.S., Lee T.K., Lee S., Kim S.-H., Moon E., Park K.-M., Kim K.H. Chemical Characterization of Novel Natural Products from the Roots of Asian Rice (Oryza sativa) that Control Adipocyte and Osteoblast Differentiation. J. Agric. Food Chem. 2018;66:2677–2684. doi: 10.1021/acs.jafc.7b05030. [DOI] [PubMed] [Google Scholar]
- 86.Manosroi A., Chutoprapat R., Abe M., Manosroi W., Manosroi J. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharm. Biol. 2012;50:208–224. doi: 10.3109/13880209.2011.596206. [DOI] [PubMed] [Google Scholar]
- 87.Cho J.-G., Huh J., Jeong R.-H., Cha B.-J., Shrestha S., Lee D.-G., Kang H.-C., Kim J.-Y., Baek N.-I. Inhibition effect of phenyl compounds from the Oryza sativa roots on melanin production in murine B16-F10 melanoma cells. Nat. Prod. Res. 2015;29:1052–1054. doi: 10.1080/14786419.2014.968155. [DOI] [PubMed] [Google Scholar]
- 88.Soldera-Silva A., Seyfried M., Campestrini L.H., Zawadzki-Baggio S.F., Minho A.P., Molento M.B., Maurer J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018;251:34–43. doi: 10.1016/j.vetpar.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 89.Louis M.R.L.M., Rani V.P., Krishnan P., Reegan A.D., Balakrishna K., Ignacimuthu S., Packiam S.M., Maheswaran R., Shirota O. Mosquito Larvicidal Activity of Compounds from Unripe Fruit Peel of Avocado (Persea americana Mill.) Appl. Biochem. Biotechnol. 2022;1:1–12. doi: 10.1007/s12010-022-03831-w. [DOI] [PubMed] [Google Scholar]
- 90.Reis I.M.A., Umehara E., Conceição R.S., de M. Oliveira L., Coelho Dos S.M., Jr., Costa-Silva T.A., Amaral M., Tempone A.G., Branco A., Lago J.H.G. γ-Lactones from Persea americana and Persea fulva—In Vitro and in Silico Evaluation of Trypanosoma cruzi Activity. Chem. Biodivers. 2021;18:e2100362. doi: 10.1002/cbdv.202100362. [DOI] [PubMed] [Google Scholar]
- 91.Nayaka N.M.D.M.W., Sasadara M.M.V., Sanjaya D.A., Yuda P.E.S.K., Dewi N.L.K.A.A., Cahyaningsih E., Hartati R. Piper betle (L): Recent Review of Antibacterial and Antifungal Properties, Safety Profiles, and Commercial Applications. Molecules. 2021;26:2321. doi: 10.3390/molecules26082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.San T.T., Wang Y.-H., Hu D.-B., Yang J., Zhang D.-D., Xia M.-Y., Yang X.-F., Yang Y.-P. A new sesquineolignan and four new neolignans isolated from the leaves of Piper betle, a traditional medicinal plant in Myanmar. Bioorganic Med. Chem. Lett. 2021;31:127682. doi: 10.1016/j.bmcl.2020.127682. [DOI] [PubMed] [Google Scholar]
- 93.Chen D.-Z., Xiong H.-B., Tian K., Guo J.-M., Huang X.-Z., Jiang Z.-Y. Two new sphingolipids from the leaves of Piper betle L. Molecules. 2013;18:11241–11249. doi: 10.3390/molecules180911241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amin S.A., Bhattacharya P., Basak S., Gayen S., Nandy A., Saha A. Pharmacoinformatics study of Piperolactam A from Piper betle root as new lead for non steroidal anti fertility drug development. Comput. Biol. Chem. 2017;67:213–224. doi: 10.1016/j.compbiolchem.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Miyasaki Y., Rabenstein J.D., Rhea J., Crouch M.-L., Mocek U.M., Kittell P.E., Morgan M.A., Nichols W.S., Van Benschoten M.M., Hardy W.D., et al. Isolation and characterization of antimicrobial compounds in plant extracts against multidrug-resistant Acinetobacter baumannii. PLoS ONE. 2013;8:e61594. doi: 10.1371/journal.pone.0061594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alves V.G., Souza A.G., Chiavelli L.U.R., Ruiz A.L.T.G., Carvalho J.E., Pomini A.M., Silva C.C. Phenolic compounds and anticancer activity of commercial sugarcane cultivated in Brazil. An. Acad. Bras. Cienc. 2016;88:1201–1209. doi: 10.1590/0001-3765201620150349. [DOI] [PubMed] [Google Scholar]
- 97.Trejo-Moreno C., Castro-Martínez G., Méndez-Martínez M., Jiménez-Ferrer J.E., Pedraza-Chaverri J., Arrellín G., Zamilpa A., Medina-Campos O.N., Lombardo-Earl G., Barrita-Cruz G.J., et al. Acetone fraction from Sechium edule (Jacq.) S.w. edible roots exhibits anti-endothelial dysfunction activity. J. Ethnopharmacol. 2018;220:75–86. doi: 10.1016/j.jep.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 98.Anantaworasakul P., Hamamoto H., Sekimizu K., Okonogi S. Biological activities and antibacterial biomarker of Sesbania grandiflora bark extract. Drug Discov. Ther. 2017;11:70–77. doi: 10.5582/ddt.2017.01013. [DOI] [PubMed] [Google Scholar]
- 99.Noviany N., Nurhidayat A., Hadi S., Suhartati T., Aziz M., Purwitasari N., Subasman I. Sesbagrandiflorain A and B: Isolation of two new 2-arylbenzofurans from the stem bark of Sesbania grandiflora. Nat. Prod. Res. 2018;32:2558–2564. doi: 10.1080/14786419.2018.1425858. [DOI] [PubMed] [Google Scholar]
- 100.Noviany N., Samadi A., Carpenter E.L., Abugrain M.E., Hadi S., Purwitasari N., Indra G., Indra A., Mahmud T. Structural revision of sesbagrandiflorains A and B, and synthesis and biological evaluation of 6-methoxy-2-arylbenzofuran derivatives. J. Nat. Med. 2020;75:66–75. doi: 10.1007/s11418-020-01445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tjahjandarie T.S., Tanjung M., Saputri R.D., Rahayu D.O., Gunawan Alfiah Nur I., Aldin M.F. Two new 2-arylbenzofurans from Sesbania grandiflora L. and their cytotoxicity towards cancer cell. Nat. Prod. Res. 2020;35:5637–5642. doi: 10.1080/14786419.2020.1821016. [DOI] [PubMed] [Google Scholar]
- 102.Kang J.-H., Shi F., Jones A.D., Marks M.D., Howe G.A. Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 2009;61:1053–1064. doi: 10.1093/jxb/erp370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takahashi H., Hara H., Goto T., Kamakari K., Wataru N., Mohri S., Suzuki H., Shibata D., Kawada T. 13-Oxo-9(Z),11(E),15(Z)-octadecatrienoic Acid Activates Peroxisome Proliferator-Activated Receptor γ in Adipocytes. Lipids. 2014;50:3–12. doi: 10.1007/s11745-014-3972-x. [DOI] [PubMed] [Google Scholar]
- 104.Seong S.H., Jung H.A., Choi J.S. Discovery of Flazin, an Alkaloid Isolated from Cherry Tomato Juice, As a Novel Non-Enzymatic Protein Glycation Inhibitor via in Vitro and in Silico Studies. J. Agric. Food Chem. 2021;69:3647–3657. doi: 10.1021/acs.jafc.0c07486. [DOI] [PubMed] [Google Scholar]
- 105.Fuentes E., Alarcón M., Astudillo L., Valenzuela C., Gutiérrez M., Palomo I. Protective mechanisms of guanosine from Solanum lycopersicum on agonist-induced platelet activation: Role of sCD40L. Molecules. 2013;18:8120–8135. doi: 10.3390/molecules18078120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li C.-X., Song X.-Y., Zhao W.-Y., Yao G.-D., Lin B., Huang X.-X., Li L.-Z., Song S.-J. Characterization of enantiomeric lignanamides from Solanum nigrum L. and their neuroprotective effects against MPP(+)-induced SH-SY5Y cells injury. Phytochemistry. 2019;161:163–171. doi: 10.1016/j.phytochem.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Gu X.-Y., Shen X.-F., Wang L., Wu Z.-W., Li F., Chen B., Zhang G.-L., Wang M.-K. Bioactive steroidal alkaloids from the fruits of Solanum nigrum. Phytochemistry. 2018;147:125–131. doi: 10.1016/j.phytochem.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X.-L., He X.-J., Zhou G.-X., Ye W.-C., Yao X.-S. Pregnane glycosides from Solanum nigrum. J. Asian Nat. Prod. Res. 2007;9:517–523. doi: 10.1080/10286020600782488. [DOI] [PubMed] [Google Scholar]
- 109.Jeong J.B., De Lumen B.O., Jeong H.J. Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Lett. 2010;293:58–64. doi: 10.1016/j.canlet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 110.Fagbemi K.O., Aina D.A., Olajuyigbe O.O. Soxhlet Extraction versus Hydrodistillation Using the Clevenger Apparatus: A Comparative Study on the Extraction of a Volatile Compound from Tamarindus indica Seeds. Sci. World J. 2021;2021:5961586. doi: 10.1155/2021/5961586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sudjaroen Y., Haubner R., Würtele G., Hull W.E., Erben G., Spiegelhalder B., Changbumrung S., Bartsch H., Owen R.W. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2005;43:1673–1682. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 112.Rattarom R., Sakpakdeejaroen I., Hansakul P., Itharat A. Cytotoxic activity against small cell lung cancer cell line and chromatographic fingerprinting of six isolated compounds from the ethanolic extract of Benjakul. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2014;97((Suppl. 8)):S70-5. [PubMed] [Google Scholar]
- 113.Sekiwa Y., Kobayashi A., Kubota K., Takenaka M. First isolation of geranyl disaccharides from ginger and their relations to aroma formation. Nat. Prod. Lett. 2001;15:267–274. doi: 10.1080/10575630108041291. [DOI] [PubMed] [Google Scholar]
- 114.Della Valle A., Dimmito M.P., Zengin G., Pieretti S., Mollica A., Locatelli M., Cichelli A., Novellino E., Ak G., Yerlikaya S., et al. Exploring the Nutraceutical Potential of Dried Pepper Capsicum annuum L. on Market from Altino in Abruzzo Region. Antioxidants. 2020;9:400. doi: 10.3390/antiox9050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yakubu M.T., Nurudeen Q.O., Salimon S.S., Yakubu M.O., Jimoh R.O., Nafiu M.O., Akanji M.A., Oladiji A.T., Williams F.E. Antidiarrhoeal Activity of Musa paradisiaca Sap in Wistar Rats. Evid.-Based Complement. Altern. Med. Ecam. 2015;4:1–9. doi: 10.1155/2015/683726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roberts C.L., Keita Å.V., Parsons B.N., Prorok-Hamon M., Knight P., Kennedy N., Söderholm J.D., Rhodes J.M., Campbell B.J. Soluble plantain fibre blocks epithelial adhesion and M-cell translocation of intestinal pathogens. Gut. 2011;60:A96. doi: 10.1136/gut.2011.239301.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gunasekaran D., Chandramohan A., Karthikeyan K., Balasubramaniam B., Jagadeesan P., Soundararajan P. Effect of Green Banana (Musa paradisiaca) on Recovery in Children With Acute Watery Diarrhea With No Dehydration: A Randomized Controlled Trial. Indian Pediatr. 2020;57:1114–1118. doi: 10.1007/s13312-020-2063-8. [DOI] [PubMed] [Google Scholar]
- 118.Alvarez-Acosta T., León C., Acosta-González S., Parra-Soto H., Cluet-Rodriguez I., Rossell M.R., Colina-Chourio J.A. Beneficial Role of Green Plantain [Musa paradisiaca] in the Management of Persistent Diarrhea: A Prospective Randomized Trial. J. Am. Coll. Nutr. 2009;28:169–176. doi: 10.1080/07315724.2009.10719768. [DOI] [PubMed] [Google Scholar]
- 119.Yuniarti W.M., Lukiswanto B.S. Effects of herbal ointment containing the leaf extracts of Madeira vine (Anredera cordifolia (Ten.) Steenis) for burn wound healing process on albino rats. Vet. World. 2017;10:808–813. doi: 10.14202/vetworld.2017.808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 121.Pazyar N., Feily A. Garlic in dermatology. Dermatol. Rep. 2011;3:e4. doi: 10.4081/dr.2011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ejaz S., Chekarova I., Cho J., Lee S., Ashraf S., Lim C. Effect of aged garlic extract on wound healing: A new frontier in wound management. Drug Chem. Toxicol. 2009;32:191–203. doi: 10.1080/01480540902862236. [DOI] [PubMed] [Google Scholar]
- 123.Mustafa T., Srivastava K.C. Ginger (Zingiber officinale) in migraine headache. J. Ethnopharmacol. 1990;29:267–273. doi: 10.1016/0378-8741(90)90037-T. [DOI] [PubMed] [Google Scholar]
- 124.Martins L.B., Rodrigues A.M.d.S., Monteze N.M., Tibaes J.R.B., Amaral M.H.A., Gomez R.S., Teixeira A.L., Ferreira A.V.M. Double-blind placebo-controlled randomized clinical trial of ginger (Zingiber officinale Rosc.) in the prophylactic treatment of migraine. Cephalalgia. 2019;40:88–95. doi: 10.1177/0333102419869319. [DOI] [PubMed] [Google Scholar]
- 125.Kim Y.J., Lim H.-S., Kim B.-Y., Seo C.-S., Jeong S.-J. Quantitative Analysis and Biological Efficacies regarding the Neuroprotective and Antineuroinflammatory Actions of the Herbal Formula Jodeungsan in HT22 Hippocampal Cells and BV-2 Microglia. Evid.-Based Complement. Altern. Med. 2017;2017:6360836. doi: 10.1155/2017/6360836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martin B.R. Multimodal Care for Headaches, Lumbopelvic Pain, and Dysmenorrhea in a Woman With Endometriosis: A Case Report. J. Chiropr. Med. 2021;20:148–157. doi: 10.1016/j.jcm.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Z., Huang P., Law S., Tian H., Leung W., Xu C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018;9:1374. doi: 10.3389/fphar.2018.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mittal N., Joshi R., Hota D., Chakrabarti A. Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytother. Res. 2009;23:507–512. doi: 10.1002/ptr.2662. [DOI] [PubMed] [Google Scholar]
- 129.Young H.-Y., Luo Y.-L., Cheng H.-Y., Hsieh W.-C., Liao J.-C., Peng W.-H. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharmacol. 2005;96:207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 130.Choi E.M., Hwang J.K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia. 2004;75:557–565. doi: 10.1016/j.fitote.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 131.Li C., Cai Q., Wu X., Tan Z., Yao L., Huang S., Zhang W., Hong Z., Chen Z., Zhang L.A.-O. Anti-inflammatory Study on the Constituents of Angelica sinensis (Oliv.) Diels, Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav., Angelica pubescence Maxim and Foeniculum vulgare Mill. J. Essent. Oils. 2022;71:1207–1219. doi: 10.5650/jos.ess22031. [DOI] [PubMed] [Google Scholar]
- 132.Alazadeh M., Azadbakht M., Niksolat F., Asgarirad H., Moosazadeh M., Ahmadi A., Yousefi S.S. Effect of sweet fennel seed extract capsule on knee pain in women with knee osteoarthritis. Complement. Ther. Clin. Pract. 2020;40:101219. doi: 10.1016/j.ctcp.2020.101219. [DOI] [PubMed] [Google Scholar]
- 133.Rifqiyati N., Wahyuni A. Fennel (Foeniculum vulgare) leaf infusion effect on mammary gland activity and kidney function of lactating rats. Nusant. Biosci. 2019;11:5. doi: 10.13057/nusbiosci/n110117. [DOI] [Google Scholar]
- 134.Dwita L.P., Hikmawanti N.P.E., Yeni, Supandi Extract, fractions, and ethyl-p-methoxycinnamate isolate from Kaempferia galanga Elicit anti-inflammatory activity by limiting leukotriene B4 (LTB4) production. J. Tradit. Complement. Med. 2021;11:563–569. doi: 10.1016/j.jtcme.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Umar M.I., Asmawi M.Z., Sadikun A., Atangwho I.J., Yam M.F., Altaf R., Ahmed A. Bioactivity-guided isolation of ethyl-p-methoxycinnamate, an anti-inflammatory constituent, from Kaempferia galanga L. extracts. Molecules. 2012;17:8720–8734. doi: 10.3390/molecules17078720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jagadish P.C., Latha K.P., Mudgal J., Nampurath G.K. Extraction, characterization and evaluation of Kaempferia galanga L. (Zingiberaceae) rhizome extracts against acute and chronic inflammation in rats. J. Ethnopharmacol. 2016;194:434–439. doi: 10.1016/j.jep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Muhammad Ihtisham U., Mohd Zaini A., Amirin S., Amin Malik Shah Abdul M., Fouad Saleih R.A.-S., Loiy Elsir Ahmed H., Rabia A., Mohamed B.K.A. Ethyl-p-methoxycinnamate isolated from kaempferia galanga inhibits inflammation by suppressing interleukin-1, tumor necrosis factor-α, and angiogenesis by blocking endothelial functions. Clinics. 2014;69:134–144. doi: 10.6061/clinics/2014(02)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xavier-Santos J.B., Félix-Silva J., Passos J.G.R., Gomes J.A.S., Fernandes J.M., Garcia V.B., de Araujo-Junior R.F., Zucolotto S.M., Silva-Junior A.A., Fernandes-Pedrosa M.F. Development of an effective and safe topical anti-inflammatory gel containing Jatropha gossypiifolia leaf extract: Results from a pre-clinical trial in mice. J. Ethnopharmacol. 2018;227:268–278. doi: 10.1016/j.jep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Ledon N., Casaco A., Remirez D., González A., Cruz J., Gonzalez R., Capote A., Tolón Z., Rojas E., Rodríguez V.J., et al. Effects of a mixture of fatty acids from sugar cane (Saccharum officinarum L.) wax oil in two models of inflammation: Zymosan-induced arthritis and mice tail test of psoriasis. Phytomedicine Int. J. Phytother. Phytopharm. 2007;14:690–695. doi: 10.1016/j.phymed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 140.Ledon N. Anti-inflammatory and analgesic effects of a mixture of fatty acids isolated and purified from sugar cane wax oil. Planta Med. 2003;2069:367–369. doi: 10.1055/s-2003-38880. [DOI] [PubMed] [Google Scholar]
- 141.Alotaibi B., Mokhtar F.A.-O., El-Masry T.A., Elekhnawy E., Mostafa S.A., Abdelkader D.H., Elharty M.E., Saleh A., Negm W.A.-O. Antimicrobial Activity of Brassica rapa L. Flowers Extract on Gastrointestinal Tract Infections and Antiulcer Potential Against Indomethacin-Induced Gastric Ulcer in Rats Supported by Metabolomics Profiling. J. Inflamm. Res. 2021;14:7411. doi: 10.2147/JIR.S345780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nugroho A.E., Wijayanti A.D., Mutmainah M., Susilowati R., Rahmawati N. Gastroprotective Effect of Combination of Hot Water Extracts of Licorice (Glycyrrhiza glabra), Pulasari Stem Bark (Alyxia reinwardtii), and Sembung Leaf (Blumea balsamifera) Against Aspirin-Induced Gastric Ulcer Model Rats. J. Evid. Based Complement. Altern. Med. 2016;21:NP77–NP84. doi: 10.1177/2156587216637469. [DOI] [PubMed] [Google Scholar]
- 143.Mollica A., Stefanucci A., Zengin G., Locatelli M., Macedonio G., Orlando G., Ferrante C., Menghini L., Recinella L., Leone S., et al. Polyphenolic composition, enzyme inhibitory effects ex-vivo and in-vivo studies on two Brassicaceae of north-central Italy. Biomed. Pharmacother. 2018;107:129–138. doi: 10.1016/j.biopha.2018.07.169. [DOI] [PubMed] [Google Scholar]
- 144.Chauhan N. Plants Used as Antihypertensive. Nat. Prod. Bioprospect. 2020;11:155–184. doi: 10.1007/s13659-020-00281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Siska S., Mun`im A., Bahtiar A., Suyatna F.D. Effect of Apium graveolens Extract Administration on the Pharmacokinetics of Captopril in the Plasma of Rats. Sci. Pharm. 2018;86:6. doi: 10.3390/scipharm86010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Márquez-Ramírez C., Paz J., Ortiz-Avila O., Raya-Farias A., González-Hernández J., Rodríguez-Orozco A., Salgado-Garciglia R., Saavedra-Molina A., Godínez-Hernández D., Cortés-Rojo C. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function and mitochondrial oxidative stress in hypertensive rats. Nutrition. 2018;54:60–67. doi: 10.1016/j.nut.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 147.Sokpe A., Mensah M., Koffuor G., Thomford K.P., Arthur R., Jibira Y., Baah M., Adedi B., Agbemenyah H. Hypotensive and Antihypertensive Properties and Safety for Use of Annona muricata and Persea americana and Their Combination Products. Evid. Based Complement. Altern. Med. 2020;6:1–13. doi: 10.1155/2020/8833828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rong H., Alashi A., Malomo S., Girgih A., Chao D., Ju X., Aluko R. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013;141:153–159. doi: 10.1016/j.foodchem.2013.02.087. [DOI] [PubMed] [Google Scholar]
- 149.He R., Malomo S.A., Girgih A.T., Ju X., Aluko R.E. Glycinyl-Histidinyl-Serine (GHS), a Novel Rapeseed Protein-Derived Peptide Has Blood Pressure-Lowering Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2013;61:8396–8402. doi: 10.1021/jf400865m. [DOI] [PubMed] [Google Scholar]
- 150.Simaratanamongkol A., Umehara K., Noguchi H., Panichayupakaranant P. Identification of a new angiotensin-converting enzyme (ACE) inhibitor from Thai edible plants. Food Chem. 2014;165:92–97. doi: 10.1016/j.foodchem.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 151.Prasad A., Devi A.T., Prasad M.N.N., Zameer F., Shruthi G., Shivamallu C. Phyto anti-biofilm elicitors as potential inhibitors ofHelicobacter pylori. 3 Biotech. 2019;9:53. doi: 10.1007/s13205-019-1582-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim W.-J., Hwang K.-H., Park D.-G., Kim T.-J., Kim D.-W., Choi D.-K., Moon W.-K., Lee K.-H. Major constituents and antimicrobial activity of Korean herb Acorus calamus. Nat. Prod. Res. 2011;25:1278–1281. doi: 10.1080/14786419.2010.513333. [DOI] [PubMed] [Google Scholar]
- 153.Aqil F., Ahmad I., Owais M. Evaluation of anti-methicillin-resistantStaphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol. J. 2006;1:1093–1102. doi: 10.1002/biot.200600130. [DOI] [PubMed] [Google Scholar]
- 154.Mickymaray S., Al Aboody M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina. 2019;55:289. doi: 10.3390/medicina55060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ahmad I., Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. Microbiol. Res. 2007;162:264–275. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 156.Sharma V., Singh I., Chaudhary P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014;28:1454–1466. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 157.Yao X., Ling Y., Guo S., He S., Wu W., Zhang Q., Zou M., Nandakumar K.S., Chen X., Liu S. Tatanan A from the Acorus calamus L. root inhibited dengue virus proliferation and infections. Phytomedicine. 2018;42:258–267. doi: 10.1016/j.phymed.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 158.Shan B., Cai Yz Fau-Brooks J.D., Brooks Jd Fau-Corke H., Corke H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007;55:5484–5490. doi: 10.1021/jf070424d. [DOI] [PubMed] [Google Scholar]
- 159.Wongkattiya N., Sanguansermsri P., Fraser I.H., Sanguansermsri D.A.-O. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019;16:1. doi: 10.1515/jcim-2018-0195. [DOI] [PubMed] [Google Scholar]
- 160.Ordoñez A.A., Ordoñez Rm Fau-Zampini I.C., Zampini Ic Fau-Isla M.I., Isla M.I. Design and quality control of a pharmaceutical formulation containing natural products with antibacterial, antifungal and antioxidant properties. Int. J. Pharm. 2009;378:51–58. doi: 10.1016/j.ijpharm.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 161.Bawazeer S., Rauf A. In Vitro Antibacterial and Antifungal Potential of Amyrin-Type Triterpenoid Isolated from Datura metel Linnaeus. BioMed. Res. Int. 2021;2021:1543574. doi: 10.1155/2021/1543574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zorofchian S., Kadir H., Hassandarvish P., Tajik H., Abu Bakar S., Zandi K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed. Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tanaka H., Atsumi I., Shirota O., Sekita S., Sakai E., Sato M., Murata J., Murata H., Darnaedi D., Chen I.-S. Three new constituents from the roots of Erythrina variegata and their antibacterial activity against methicillin-resistant Staphylococcus aureus. Chem. Biodivers. 2011;8:476–482. doi: 10.1002/cbdv.201000068. [DOI] [PubMed] [Google Scholar]
- 164.Tanaka H., Atsumi I., Hasegawa M., Hirata M., Sakai T., Sato M., Yamaguchi R., Tateishi Y., Tanaka T., Fukai T. Two New Isoflavanones from the Roots of Erythrina variegata. Nat. Prod. Commun. 2015;10:1934578X1501000330. doi: 10.1177/1934578X1501000330. [DOI] [PubMed] [Google Scholar]
- 165.Sato M., Tanaka H., Yamaguchi R., Kato K., Etoh H. Synergistic effects of mupirocin and an isoflavanone isolated from Erythrina variegata on growth and recovery of methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2004;24:241–246. doi: 10.1016/j.ijantimicag.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 166.Camacho-Corona M.D.R., Ramírez-Cabrera M.A., Santiago O.G., Garza-González E., Palacios I.d.P., Luna-Herrera J. Activity against drug resistant-tuberculosis strains of plants used in Mexican traditional medicine to treat tuberculosis and other respiratory diseases. Phytother. Res. PTR. 2008;22:82–85. doi: 10.1002/ptr.2269. [DOI] [PubMed] [Google Scholar]
- 167.Yeshi K., Wangchuk P. Chapter 11—Essential oils and their bioactive molecules in healthcare. In: Mandal S.C., Nayak A.K., Dhara A.K., editors. Herbal Biomolecules in Healthcare Applications. Academic Press; Cambridge, MA, USA: 2022. pp. 215–237. [DOI] [Google Scholar]
- 168.Nugraha A.S., Dayli I.R., Sukrisno Putri C.P.Z., Firli L.N., Widhi Pratama A.N., Triatmoko B., Untari L.F., Wongso H., Keller P.A., Wangchuk P. Isolation of Antibacterial Depside Constituents from Indonesian Folious Lichen, Candelaria fibrosa. J. Biol. Act. Prod. Nat. 2022;12:24–32. doi: 10.1080/22311866.2021.2021986. [DOI] [Google Scholar]
- 169.Maregesi S., Van Miert S., Pannecouque C., Feiz Haddad M.H., Hermans N., Wright C.W., Vlietinck A.J., Apers S., Pieters L. Screening of Tanzanian Medicinal Plants against Plasmodium falciparum and Human Immunodeficiency Virus. Planta Med. 2010;76:195–201. doi: 10.1055/s-0029-1186024. [DOI] [PubMed] [Google Scholar]
- 170.Nethengwe M. Antiplasmodial/Antipyretic activity of some Zulu medicinal plants. J. Med. Plants Res. 2012;6:1255–1262. doi: 10.5897/JMPR11.1319. [DOI] [Google Scholar]
- 171.Reddy R.C., Vatsala P.G., Keshamouni V.G., Padmanaban G., Rangarajan P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005;326:472–474. doi: 10.1016/j.bbrc.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 172.Ali A., Sudi S., Sidek H., Embi N., Basir R. The Antimalarial Effect of Curcumin Is Mediated by the Inhibition of Glycogen Synthase Kinase-3β. J. Med. Food. 2016;20:152–161. doi: 10.1089/jmf.2016.3813. [DOI] [PubMed] [Google Scholar]
- 173.Kamaraj C., Kaushik N.K., Rahuman A.A., Mohanakrishnan D., Bagavan A., Elango G., Zahir A.A., Santhoshkumar T., Marimuthu S., Jayaseelan C., et al. Antimalarial activities of medicinal plants traditionally used in the villages of Dharmapuri regions of South India. J. Ethnopharmacol. 2012;141:796–802. doi: 10.1016/j.jep.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 174.Sathiyamoorthy P., Lugasi-Evgi H., Schlesinger P., Kedar I., Gopas J., Pollack Y., Golan-Goldhirsh A. Screening for cytotoxic and antimalarial activities in desert plants of the negev and bedouin market plant products. Pharm. Biol. 1999;37:188–195. doi: 10.1076/phbi.37.3.188.6298. [DOI] [Google Scholar]
- 175.Herlina T., Supratman U., Soedjanaatmadja U., Subarnas A., Sutardjo S., Abdullah N., Hayashi H. Anti-malarial compound from the stem bark of Erythrina variegata. Indones. J. Chem. 2010;9:308–311. doi: 10.22146/ijc.21547. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.