Abstract
By using a synthetic peptide approach, we mapped epitopes from the mycobacterial 65-kDa heat shock protein (HSP65) recognized by human T cells belonging to the Mycobacterium leprae memory repertoire. A panel of HSP65 reactive CD4+ T-cell lines and clones were established from healthy donors 8 years after immunization with heat-killed M. leprae and then tested for proliferative reactivity against overlapping peptides comprising both the M. leprae and Mycobacterium tuberculosis HSP65 sequences. The results showed that the antigen-specific T-cell lines and clones established responded to 12 mycobacterial HSP65 peptides, of which 9 peptides represented epitopes crossreactive between the M. tuberculosis and M. leprae HSP65 (amino acids [aa] 61 to 75, 141 to 155, 151 to 165, 331 to 345, 371 to 385, 411 to 425, 431 to 445, 441 to 455, and 501 to 515) and 3 peptides (aa 343 to 355, 417 to 429, and 522 to 534) represented M. leprae HSP65-specific epitopes. Major histocompatibility complex restriction analysis showed that presentation of 9 of the 12 peptides to T cells were restricted by one of the 2 HLA-DR molecules expressed from self HLA-DRB1 genes, whereas 3 peptides with sequences completely identical between the M. leprae and M. tuberculosis HSP65 were presented to T cells by multiple HLA-DR molecules: peptide (aa 61 to 75) was presented by HLA-DR1, -DR2, and -DR7, peptide (aa 141 to 155) was presented by HLA-DR2, -DR7, and -DR53, whereas both HLA-DR2 and -DR4 (Dw4 and Dw14) were able to present peptide (aa 501 to 515) to T cells. In addition, the T-cell lines responding to these peptides in proliferation assays showed cytotoxic activity against autologous monocytes/macrophages pulsed with the same HSP65 peptides. In conclusion, we demonstrated that promiscuous peptide epitopes from the mycobacterial HSP65 antigen can serve as targets for cytotoxic CD4+ T cells which belong to the human memory T-cell repertoire against M. leprae. The results suggest that such epitopes might be used in the peptide-based design of subunit vaccines against mycobacterial diseases.
The mycobacterial 65-kDa heat shock protein (HSP65) is among the antigens recognized by Mycobacterium leprae- and Mycobacterium tuberculosis-reactive human CD4+ T cells (10, 13, 15, 35, 42). Several lines of evidences suggest that the HSP65 antigen is relevant to subunit vaccine design against mycobacterial diseases: cellular immune responses to HSP65 induced by whole mycobacteria lead to activation of CD4+ Th1 cells with protective effector functions such as gamma interferon (IFN-γ) release and major histocompatibility complex (MHC) class II-restricted cytotoxic activity against antigen pulsed macrophages (6, 28, 38, 43, 46). HSP65 is presented to human CD4+ T cells in association with multiple HLA-DR molecules (28). Mycobacterial HSP65-reactive CD4+ T cells are present in the memory T-cell repertoire induced by M. leprae immunization in humans (32). Finally, immunization with the mycobacterial HSP65 antigen induced protection against M. leprae and M. tuberculosis in mouse models of infections (4, 54, 55). In addition, DNA vaccination of mice with the M. tuberculosis HSP65 antigen provided protection against challenge with M. tuberculosis (60).
These earlier studies suggest that the mycobacterial HSP65 represents a candidate antigen for subunit vaccine design. However, immunization with the complete HSP65 molecule may lead to adverse effects such as autoimmune responses and the induction of suppressor T cells (11, 37, 66, 67). An alternative strategy could be to identify and select HSP65 epitopes that are presented to CD4+ Th1 cells in association with multiple HLA class II molecules. Although, several epitopes of the mycobacterial HSP65 recognized by human T cells have previously been identified (2, 12, 14, 43, 47), their application to diagnosis or vaccine design is hampered by a stringent HLA-DR restriction requirement. These studies demonstrated that all investigated HSP65 epitopes could only be recognized by T cells in association with one of the two HLA-DR molecules expressed from the self-HLA-DRB1 genes (2, 12, 43, 47).
By using synthetic peptides covering both the M. tuberculosis and M. leprae HSP65 sequences, we have in this study identified three novel HSP65 epitopes, each presented to CD4+ T cells in association with multiple HLA-DR molecules. The finding that such promiscuous epitopes were targets for recognition by M. leprae-induced memory T cells in humans suggests that they are relevant to vaccine development.
MATERIALS AND METHODS
Antigens and peptides.
Armadillo-derived killed M. leprae preparations were kindly supplied by R. J. W. Rees from the World Health Organization (WHO)/Immunology of Leprosy (IMMLEP) Bank. The recombinant M. leprae and M. tuberculosis HSP65 were kindly provided by J. D. A. van Embden from the WHO/IMMLEP Bank. Two sets of peptides were used to identify the epitopes recognized by the mycobacterial HSP65-reactive T-cell lines. The first set comprised 50 peptides (P1 to P50) covering the amino acid (aa) sequence of the M. tuberculosis HSP65 (43). These peptides were 15-mers and overlapped with 5 aa. Another series of 20 peptides (P51 to P70; 13-mers), corresponding to the parts of the M. leprae HSP65 sequence that differed from the M. tuberculosis sequence by one or more amino acids, were synthesized by the Pepscan method as described previously (20).
Antigen-presenting cells (APC).
Heparinized venous blood was obtained from the Mycobacterium bovis BCG- and M. leprae-vaccinated subjects (5) and from healthy individuals of the staff of the National Hospital, Oslo, Norway. Peripheral blood mononuclear cells (PBMC) were separated by flotation of the blood on Lymphoprep gradients (Nycomed, Oslo, Norway) (23). Autologous and allogeneic irradiated (2,400 rads) PBMC from a panel of donors were used as APC in T-cell proliferation assays.
HLA typing of donors.
Donors for T-cell lines and APC were HLA typed serologically by the immunomagnetic method (64). Donors vaccinated with M. bovis BCG and M. leprae were, in addition, typed for Dw4 and Dw14 subtypes of DR4 by using alloreactive T-cell clones (52). All of the donors were HLA class II typed genomically by the hybridization of sequence-specific oligonucleotide probes to PCR-amplified DNA (51). Testing for the presence of the HLA-DRB4*0101 allele (encoding HLA-DR53) was done genomically for selected individuals and the vaccinated subjects.
HSP65-specific T-cell lines and clones.
M. leprae HSP65-reactive T-cell lines were established from the PBMC of five healthy subjects 8 years after vaccination with killed M. leprae (28, 32). To establish the T-cell lines, 2 × 106 PBMC in 1 ml of complete medium (RPMI 1640 plus 10% AB serum and 1% penicillin-streptomycin) were cultured with M. leprae (5 × 107 bacilli/ml) in the wells of 24-well Costar plates (Costar, Cambridge, Mass.). The plates were incubated at 37°C in an atmosphere of 5% CO2 and 95% air. After 6 days of incubation, 100 U of recombinant interleukin-2 (Amersham, Amersham, United Kingdom) was added to the cultures twice a week for 4 weeks (17). To expand the mycobacterial HSP65-reactive T cells, the lines were restimulated with M. leprae HSP65 and autologous APC (15). Phenotypically, the T-cell lines were >95% CD4+ and <5% CD8+. M. leprae HSP65-specific T-cell clones were obtained from the M. leprae-induced T-cell lines by the limiting dilution technique (22, 26). Growing clones were expanded according to the protocols described previously (30). All of the T-cell clones established were CD4+ and CD8−.
T-cell proliferation assays.
Antigen-induced proliferation assays of T-cell lines and clones were performed as previously described (25, 39). In brief, 104 T cells were added to the wells of 96-well flat-bottom Costar plates together with 105 irradiated autologous or HLA-DR-typed allogeneic PBMC as APC. M. leprae (5 × 107 bacteria/ml), M. leprae HSP65 (10 μg/ml), and synthetic peptides (5 μg/ml) were added in triplicates. The total culture volume was adjusted to 200 μl. After 72 h of incubation, the cultures were pulsed with 0.045 MBq of [3H]thymidine (specific activity, 185 × 103 MBq/mM), and the radioactivity incorporated was determined by liquid scintillation counting (24). Median counts per minute (cpm) from triplicates were used to calculate stimulation index (SI), which is defined as cpm in cultures with amount of antigen per cpm in cultures without antigen. The proliferation of T cells in response to a given antigen was considered positive when the SI was >5 (19, 45). Such values in the tables are given in boldface.
Inhibition assays with monoclonal anti-HLA antibodies.
The inhibition of antigen-induced T-cell proliferation was studied as described previously (16, 20, 27, 40, 43, 44) with the monoclonal antibodies W6/32 (anti-HLA class I) and L243 (anti-HLA-DR), purchased from the American Type Culture Collection, as well as FN81 (anti-HLA-DQ), a gift from S. Funderud, Oslo, Norway. In brief, APC in the wells of 96-well flat-bottom plates were preincubated with the antibodies for 30 min at 37°C in an atmosphere of 5% CO2 and 95% air. After preincubation, antigen-induced proliferation of T-cell lines/clones was assessed as described above. The results were calculated in terms of percent inhibition, which is defined as follows: percent inhibition = (1 − [cpm in antigen-stimulated culture in the presence of antibody/cpm in antigen-stimulated cultures in absence of antibody]) × 100.
Cytotoxicity assays.
Cytotoxicity of T-cell lines against antigen-pulsed monocytes/macrophages was assessed by the neutral red release assay as previously described (20, 25, 30, 36, 40, 44). In brief, adherent monocytes/macrophages from 106 autologous irradiated PBMC in 24-well Costar plates were pulsed with the mycobacterial antigens. The T-cell clones were added to 105 cells/well. After 7 days of incubation at 37°C, the wells were washed to remove nonadherent T cells and the macrophages were allowed to take up neutral red for 30 min. The dye taken up by macrophages was released by adding 0.5 ml of 0.05 M acetic acid in 50% ethanol. The results are expressed as the percent cytotoxicity, which was calculated from spectrophotometric measurement of optical density at 540 nm (OD540) according to the following formula: percent cytotoxicity = ([OD540 control − OD540 experimental]/[OD540 control]) × 100, where the OD540 control = the OD540 of cultures with adherent cells plus T cells and the OD540 experimental = the OD540 of cultures with adherent cells plus T cells plus antigen.
RESULTS
Identification of peptides recognized by mycobacterial HSP65-reactive T-cell lines.
Five T-cell lines (TCL1, TCL2, TCL3, TCL4, and TCL5) responding to the M. leprae HSP65 in proliferation assays were established from PBMC of an equal number of donors 8 years after vaccination with heat-killed M. leprae. To map the T-cell epitopes recognized, all T-cell lines were tested for proliferative responses against 50 synthetic peptides (P1 to P50) covering the complete sequence of the M. tuberculosis HSP65 and 20 peptides (P51 to P70) covering the regions of the M. leprae HSP65 that contain one or more amino acid substitutions compared to the M. tuberculosis HSP65 sequence (30). The results showed that eight peptides corresponding to the M. tuberculosis HSP65 sequence (i.e., P7, aa 61 to 75; P13, aa 141 to 155; P14, aa 151 to 165; P29, aa 331 to 345; P34, aa 371 to 385; P38, aa 411 to 425; P40, aa 431 to 454; and P47, aa 501 to 515) stimulated one or more of the T-cell lines tested (Table 1). Among the stimulatory peptides, P29, P34, P38, and P40 were recognized by T-cell lines from single donors, and P7 and P47 stimulated T-cell lines from two donors, whereas the P14 and P13 were recognized by T-cell lines from three and four donors, respectively (Table 1). Among the 20 peptides corresponding to the M. leprae HSP65 sequence, two peptides (P61, aa 343 to 355; P62, aa 417 to 429) were stimulatory for T-cell lines from one donor each (Table 1).
TABLE 1.
Proliferation of the M. leprae HSP65-reactive T-cell lines in response to M. tuberculosis and M. leprae HSP65 peptidesa
| Peptide(s) (aa) | Proliferation (SI) of T-cell line:
|
||||
|---|---|---|---|---|---|
| TCL1 | TCL2 | TCL3 | TCL4 | TCL5 | |
| M. tuberculosis HSP65 | |||||
| P1–P6 | <5 | <5 | <5 | <5 | <5 |
| P7 (61–75) | <5 | <5 | <5 | 80 | 204 |
| P8–P12 | <5 | <5 | <5 | <5 | <5 |
| P13 (141–155) | <5 | 49 | 100 | 166 | 67 |
| P14 (151–165) | <5 | 166 | <5 | 106 | 49 |
| P15–P28 | <5 | <5 | <5 | <5 | <5 |
| P29 (331–345) | 15 | <5 | <5 | <5 | <5 |
| P30–P33 | <5 | <5 | <5 | <5 | <5 |
| P34 (371–385) | <5 | <5 | <5 | <5 | 19 |
| P35–P37 | <5 | <5 | <5 | <5 | <5 |
| P38 (411–425) | <5 | <5 | <5 | <5 | 46 |
| P39 | <5 | <5 | <5 | <5 | <5 |
| P40 (431–445) | <5 | <5 | 84 | <5 | <5 |
| P41–P46 | <5 | <5 | <5 | <5 | <5 |
| P47 (501–515) | <5 | 32 | <5 | 116 | <5 |
| P48–P50 | <5 | <5 | <5 | <5 | <5 |
| M. leprae HSP65 | |||||
| P51–P60 | <5 | <5 | <5 | <5 | <5 |
| P61 (343–355) | 220 | <5 | <5 | <5 | <5 |
| P62 (417–429) | <5 | <5 | <5 | 30 | <5 |
| P63–P70 | <5 | <5 | <5 | <5 | <5 |
cpm values in control wells lacking antigen or peptide ranged between 100 and 400. The values in boldface indicate positive responses.
Identification of HLA molecules used in T-cell recognition of HSP65 and its synthetic peptides.
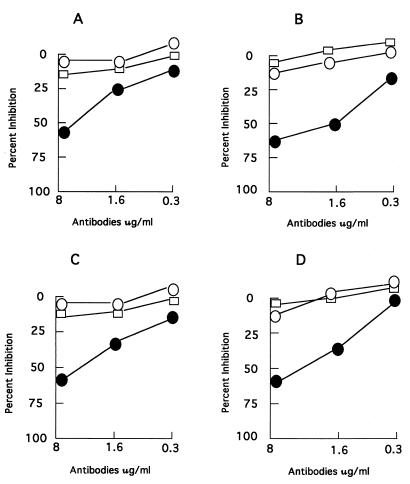
To determine the MHC-restriction of the T-cell lines, inhibition assays were performed with well-defined anti-HLA class I and class II antibodies by using M. leprae HSP65 as the antigen and irradiated autologous adherent cells as APC. The results showed that only anti-HLA-DR antibodies were able to inhibit the antigen-specific responses of all T-cell lines and clones in a dose-dependent manner (Fig. 1, representative results are given for two T-cell lines and two T-cell clones).
FIG. 1.
Inhibition of the proliferative response of T-cell lines and clones in the presence of anti-HLA class I and class II monoclonal antibodies. The T-cell lines and clones were established from the M. leprae-vaccinated donors as described in Materials and Methods. Two T-cell lines, one each from donor 2 (TCL2) (A) and donor 4 (TCL4) (B), and two T-cell clones, one each from donor 2 (C) and donor 4 (D) were stimulated with M. leprae HSP65 in the presence of defined monoclonal antibodies to HLA class I and class II molecules at the concentrations indicated. The percent inhibition (as defined in Materials and Methods) of the proliferative response is given for anti-HLA class I (□), anti-HLA-DQ (○), and anti-HLA-DR (●) antibodies.
To identify the HLA-DR molecules capable of presenting individual epitopes, each T-cell line was stimulated with the M. leprae HSP65 and the positive peptides in the presence of autologous and allogeneic HLA-DR-typed APC. The T-cell lines TCL1 (Table 2), TCL2 (Table 3), TCL4 (Table 5), and TCL5 (Table 6) responded to the M. leprae HSP65 in the presence of autologous and allogeneic APC expressing any one of the two HLA-DR molecules encoded by the self-HLA-DRB1 alleles. In contrast, the restriction pattern of TCL3 (Table 4) suggested that this T-cell line recognized the M. leprae HSP65 in association with HLA-DR53, which is encoded by the HLA-DRB4 gene.
TABLE 2.
Mapping of MHC restriction for proliferation of TCL1 in response to M. leprae HSP65 and peptide P61a
| APC donor | DR types | DW type(s) | Proliferation (SI) induced by:
|
|
|---|---|---|---|---|
| M. leprae HSP65 | Peptide P61 (aa 343–355) | |||
| 1 | 4,4 | 4,14 | 205 | 62 |
| 2 | 2,4 | 4 | 78 | 2.7 |
| 3 | 4,5 | 4 | 94 | 4.2 |
| 4 | 2,7 | 4.5 | 4.4 | |
| 5 | 1,2 | 1.5 | 0.7 | |
| 6 | 4,7 | 14 | 99 | 107 |
| 7 | 4,8 | 14 | ND | 82 |
| 8 | 2,9 | ND | 1.5 | |
| 9 | 2,5 | 0.9 | ND | |
| 10 | 3,3 | 0.5 | ND | |
| 11 | 2,3 | 0.5 | ND | |
DR restriction molecules were DR4 and DR4 (Dw14) for M. leprae HSP65 and P61, respectively. Autologous APC expressed HLA-DR4,4 (Dw4,14). The values given in boldface indicate positive responses. ND, not done.
TABLE 3.
Mapping of MHC restriction for proliferation of TCL2 in response to M. leprae HSP65 and peptides P13, P14, and P47a
| APC donor | DR types | Proliferation (SI) induced by:
|
|||
|---|---|---|---|---|---|
| M. leprae HSP65 | Peptide P13 (aa 141–155) | Peptide P14 (aa 151–165) | Peptide P47 (aa 501–515) | ||
| 1 | 4,4 | ND | 1.2 | 2.8 | 1.0 |
| 2 | 2,4 | 12 | 24 | 80 | 6.2 |
| 3 | 4,5 | 41 | 3.3 | 3.4 | 0.4 |
| 4 | 2,7 | 30 | 22 | 132 | 40 |
| 5 | 1,2 | 41 | 21 | 6.5 | ND |
| 6 | 4,7 | ND | 4.0 | 2.8 | 0.5 |
| 7 | 4,8 | 10 | ND | ND | ND |
| 9 | 2,5 | 124 | ND | ND | ND |
| 12 | 3,6 | 0.7 | ND | ND | ND |
DR restriction molecules were DR2,4, DR2, DR2, and DR2 for M. leprae HSP65 and P13, P14, and P47, respectively. Autologous APC expressed HLA-DR2,4. The values given in boldface indicate positive responses. ND, not done.
TABLE 5.
Mapping of MHC restriction for proliferation of TCL4 in response to M. leprae HSP65 and peptides P7, P13, P14, P47, and P62a
| APC donor | DR types | Proliferation (SI) induced by:
|
|||||
|---|---|---|---|---|---|---|---|
| M. leprae HSP65 | Peptide P7 (aa 61–75) | Peptide P13 (aa 141–155) | Peptide P14 (aa 151–165) | Peptide P47 (aa 501–515) | Peptide P62 (aa 417–429) | ||
| 1 | 4,4 | 3.3 | ND | ND | ND | ND | ND |
| 2 | 2,4 | 52 | 1.0 | 93 | 112 | 0.7 | 49 |
| 3 | 4,5 | 1.9 | ND | ND | ND | ND | ND |
| 4 | 2,7 | 129 | 63 | 180 | 85 | 30 | 21 |
| 5 | 1,2 | 195 | 1.4 | 72 | 91 | 1.7 | 18 |
| 14 | 7,7 | 60 | 58 | 137 | 2.0 | 0.7 | 0.8 |
| 16 | 3,8 | 1.7 | ND | ND | ND | ND | ND |
| 17 | 6,7 | 61 | 30 | 25 | 1.6 | 1.8 | 1.0 |
DR restriction molecules were DR2,7, DR7, DR2,7, DR2, and DR2 for M. leprae HSP65, P7, P13, P14, and P62, respectively. The DR restriction molecule for P47 could not be identified. Autologous APC expressed HLA-DR2,7. The values given in boldface indicate positive responses. ND, not done.
TABLE 6.
Mapping of MHC restriction for proliferation of TCL5 in response to M. leprae HSP65 and peptides P7, P13, and P14a
| APC donor | DR type | Proliferation (SI) induced by:
|
|||
|---|---|---|---|---|---|
| M. leprae HSP65 | Peptide P7 (aa 61–75) | Peptide P13 (aa 141–155) | Peptide P14 (aa 151–165) | ||
| 2 | 2,4 | 42 | 14 | 24 | 32 |
| 4 | 2,7 | 6.5 | 73 | 51 | 16 |
| 5 | 1,2 | 79 | 127 | 25 | 9.6 |
| 7 | 4,8 | 0.9 | ND | ND | ND |
| 9 | 2,5 | 16 | ND | ND | ND |
| 12 | 3,6 | 0.7 | ND | ND | ND |
| 16 | 1,7 | 52 | 130 | 0.8 | 0.5 |
| 17 | 6,7 | 0.7 | ND | ND | ND |
| 18 | 1,4 | 36 | 157 | 1.4 | 2.1 |
DR restriction molecules were DR1,2, DR1,2, DR2, and DR2 for M. leprae HSP65, P7, P13, and P14, respectively. Autologous APC expressed HLA-DR1,2. The values given in boldface indicate positive responses. ND, not done.
TABLE 4.
Mapping of MHC restriction for proliferation of TCL3 in response to M. leprae HSP65 and peptide P13a
| APC donor | DR types | DR53 typeb | Proliferation (SI) induced by:
|
|
|---|---|---|---|---|
| M. leprae HSP65 | Peptide P13 (aa 141–15) | |||
| 1 | 4,4 | +ve | 100 | ND |
| 2 | 2,4 | +ve | 40 | 20 |
| 3 | 4,5 | +ve | 63 | 26 |
| 5 | 1,2 | −ve | 0.4 | ND |
| 6 | 4,7 | +ve | 10 | 6.1 |
| 8 | 2,9 | +ve | 13 | 34 |
| 9 | 2,5 | −ve | 0.8 | 3.1 |
| 13 | 5,9 | +ve | 17 | 36 |
| 14 | 7,7 | +ve | 6.6 | 7.6 |
| 15 | 5,6 | −ve | 1.3 | 3.5 |
DR restriction molecules were DR53 and DR53 for M. leprae HSP65 and P13, respectively. Autologous APC expressed HLA-DR4,5 and DR53. The values given in boldface indicate positive responses. ND, not done.
The presence (+ve) or absence (−ve) of DR53 is indicated.
HLA-DR restriction analysis of T-cell lines with individual peptides showed that the peptides which stimulated T-cell lines from single donors were presented by one of the two HLA-DR molecules encoded by the self-HLA-DRB1 genes of the respective donors (data are shown for three representative peptides P61 [Table 2], P62 [Table 5], and P34 [Table 6]). Among the peptides recognized by more than one T-cell line, the peptide P14 was recognized in association with HLA-DR2 by all the three responding T-cell lines, i.e., TCL2 (Table 3), TCL4 (Table 5), and TCL5 (Table 6). Peptide P47, which was recognized by two T-cell lines, was restricted by HLA-DR2 for TCL2 (Table 3) and only by autologous APC for TCL4 (Table 5). Peptide P7 stimulating the T-cell lines from two donors was recognized in association with HLA-DR7 by TCL4 (Table 5) and with HLA-DR1 and HLA-DR2 by TCL5 (Table 6). Peptide P13, which stimulated T-cell lines from four donors, was recognized in association with HLA-DR2 by TCL2 (Table 3) and TCL5 (Table 6), with HLA-DR2 and HLA-DR7 by TCL4 (Table 5), and with HLA-DR53 by TCL3 (Table 4).
Cytotoxic activity of T-cell lines against monocytes/macrophages pulsed with HSP65 peptides.
The T-cell lines responding to peptides P7 and P13 in proliferation assays were also tested for cytotoxic activity against autologous monocytes/macrophages pulsed with these HSP peptides. In addition, a nonstimulatory peptide (P50) was used as a negative control antigen. The results revealed that the T-cell lines proliferating in response to a given peptide also showed cytotoxic activity against target cells pulsed with the same peptides. The T-cell lines TCL4 and TCL5 were able to lyse peptide P7 pulsed autologous monocytes/macrophages, whereas the T-cell lines TCL2, TCL4, and TCL5 lysed target T cells pulsed with peptide P13. None of the T-cell lines tested showed cytotoxic activity against autologous monocytes/macrophages pulsed with peptide P50 (Table 7).
TABLE 7.
Cytotoxic activity of T-cell lines against autologous monocytes/macrophages pulsed with peptides
| Peptide (aa) | % Cytotoxicity of T-cell line:
|
||
|---|---|---|---|
| TCL2 | TCL4 | TCL5 | |
| P7 (61–75) | 0 | 54 | 75 |
| P13 (141–155) | 99 | 91 | 85 |
| P50 (526–540) | 5 | −9 | 15 |
Identification of peptides and MHC molecules used in recognition of HSP65 peptides by T-cell clones.
In addition to T-cell lines, we also established M. leprae-reactive T-cell clones from the same five donors. Among the 140 M. leprae responding T-cell clones established, 29 and 18 T-cell clones responded to M. leprae and M. tuberculosis HSP65, respectively. When tested for proliferative responses against the M. leprae and M. tuberculosis HSP65 peptides, the peptides P13 (aa 141 to 155), P14 (aa 151 to 165), P29 (aa 331 to 345), P41 (aa 441 to 455), and P47 (aa 501 to 515) stimulated the M. leprae and M. tuberculosis HSP65 cross-reactive T-cell clones, whereas the peptides P61 (aa 343 to 355) and P69 (aa 522 to 534) stimulated the M. leprae HSP65-specific T-cell clones (Table 8).
TABLE 8.
Identification and HLA restriction of mycobacterial HSP65 peptides recognized by M. leprae and M. tuberculosis HSP65-reactive CD4+ T-cell clones
| Peptide (aa) | No. of T-cell clonesa responding | DR restrictionb |
|---|---|---|
| P13 (141–155) | 2 | DR7 |
| P14 (151–165) | 4 | DR2 |
| P29 (331–345) | 1 | DR4 |
| P41 (441–455) | 1 | DR4 |
| P47 (501–515) | 3 | DR4 |
| P61 (343–355) | 1 | DR4 (Dw14) |
| P69 (522–534) | 2 | DR4 |
The T-cell clones responding to peptides P13 (aa 141 to 155) and P14 (aa 151 to 165) were obtained from donors 4 and 2, respectively. All other T-cell clones were established from donor 1. All of the T-cell clones responding to the peptides P13 (aa 141 to 155), P14 (aa 151 to 165), P29 (aa 331 to 345), P41 (aa 441 to 455), and P47 (aa 501 to 515) proliferated in response to the M. leprae and M. tuberculosis HSP65, whereas the T-cell clones responding to the peptides P61 (aa 343 to 355) and P69 (aa 522 to 534) were M. leprae HSP65 specific (27).
DR restriction molecules were identified by using the same panel of HLA-DR-typed autologous and allogeneic APC as described for the T-cell lines.
Inhibition assays with monoclonal anti-HLA antibodies showed that the HSP65 induced responses of all T-cell clones tested were inhibited by anti-HLA-DR antibodies (Fig. 1). Furthermore, testing for proliferative responses in the presence of autologous and allogeneic HLA-DR typed APC showed that each of the positive HSP65 peptides were recognized by the T-cell clones in association with only one of the two HLA-DR molecules encoded by the self-HLA-DRB1 alleles (Table 8).
DISCUSSION
The purpose of this study was to identify HSP65 epitopes that could serve as targets for M. leprae-induced memory T cells in humans. We have previously shown that intradermal immunization of humans with heat-killed M. leprae induces activation of CD4+ memory T cells of the IFN-γ-producing Th1 phenotype which exhibit cytotoxicity against antigen-pulsed macrophages (18, 21, 30, 32, 33, 43). All of these characteristics are associated with T-cell responses mediating protective immunity against mycobacterial infections (9, 48, 50, 61, 65). HSP65 epitopes recognized by CD4+ T cells from such donors may therefore be useful for the design of peptide vaccines against both tuberculosis and leprosy. In addition, species-specific epitopes may also replace the currently used diagnostic reagents such as tuberculin and lepromin, which both contain cross-reactive antigens (3, 56).
However, the application of individual T-cell epitopes to the development of vaccines, as well as diagnostic reagents, faces the problem of MHC restriction (16, 20, 27, 36, 40, 43, 47). A prerequisite for any antigen or epitope to induce cellular immune responses in an HLA heterogeneous human population is its presentation to CD4+ T cells in association with multiple HLA class II molecules. By using T-cell clones, we and others have previously demonstrated that only HLA-DR molecules expressed from the self-HLA-DRB1 genes of each donor tested could serve as restriction elements for peptide defined T-cell epitopes from the HSP65 antigen (29, 41, 47). The HLA-DRB1 locus is highly polymorphic and this, along with the fact that any defined DR molecule is only expressed in a minority of individuals at the population level (31, 51), suggests that such epitopes are not relevant to medical applications. To map a broader range of HSP65 T-cell epitopes relevant to prophylactic purposes, we have in this work established and screened both HSP65 responding T-cell lines and clones from M. leprae-immunized subjects for peptide reactivity in relation to MHC restriction.
The cumulative results obtained with T-cell lines and clones lead to the identification of 12 peptides from the mycobacterial HSP65 sequence which possess T-cell epitopes relevant to memory immune responses. A majority of the peptides represented epitopes cross-reactive between the M. tuberculosis and M. leprae HSP65 (aa 61 to 75, 141 to 155, 151 to 165, 331 to 345, 371 to 385, 411 to 425, 431 to 445, 441 to 455, and 501 to 515), and three peptides (aa 343 to 355, 417 to 429, and 522 to 534) represented M. leprae HSP65-specific epitopes. In addition, MHC restriction analysis showed that presentation of 9 of the 12 peptides to T cells were restricted by one of the HLA-DR molecules expressed from self-HLA-DRB1 genes, whereas 3 peptides were presented to T cells by multiple HLA-DR molecules.
Among the 12 HSP65 peptides identified in this study, four peptides (aa 411 to 425, 343 to 355, 417 to 429, and 522 to 534) were from the regions that differ between the M. leprae and M. tuberculosis HSP65 sequences by one or more amino acid (Table 9) (53). The peptide (aa 411 to 425) contains a single conservative substitution in the M. leprae HSP65 sequence (Table 9). However, this substitution did not affect T-cell recognition, since the M. leprae HSP65-induced T-cell line responded to the corresponding M. tuberculosis peptide (aa 411 to 425). In contrast, the other three peptides (aa 343 to 355, 417 to 429, and 522 to 534) were recognized as specific for the M. leprae HSP65 antigen. When compared to the M. tuberculosis HSP65 sequence, the M. leprae HSP65 peptides (aa 522 to 534, 417 to 429, and 343 to 355) showed 4-, 2-, and 1-aa substitutions, respectively (Table 9). Nonconservative substitutions of at least 1 aa were present in each peptide leading to M. leprae-specific T-cell recognition (Table 9). However, due to the fact that each peptide was recognized by T cells only in association with single serologically defined HLA-DR specificities, their potential as diagnostic reagents in HLA-DR heterogeneous populations will be limited.
TABLE 9.
Sequence alignment of the M. leprae HSP65 peptides recognized by T-cell clones which differ from the M. tuberculosis HSP65 sequence by one or more amino acidsa
| Peptide (aa) | Species | Sequence |
|---|---|---|
| P38 (411–425) | M. leprae | AGGGVTLLQAAPALD |
| M. tuberculosis | ------------T-- | |
| P61 (343–355) | M. leprae | RVAQIRTEIENSD |
| M. tuberculosis | ------Q------ | |
| P62 (417–429) | M. leprae | LLQAAPALDKLKL |
| M. tuberculosis | ------T--E--- | |
| P69 (522–534) | M. leprae | PEKTAAPASDPTG |
| M. tuberculosis | --- EK--V-G- |
Amino acid residues in the M. tuberculosis HSP65 sequence which are identical with those of M. leprae HSP65 sequences are represented by dashes. The sequence information is based on previously published data (53).
The M. leprae HSP65-specific epitope represented by peptide P62 (aa 417 to 429) has previously been reported to be recognized by HLA-DR2-restricted M. leprae-specific T-cell clones from a tuberculoid leprosy patient (2). In addition, it was shown that these T-cell clones cross-reacted with a homologous peptide derived from the third hypervariable region of the HLA-DR2 molecule sequence (2). Since HLA-DR2 is associated with tuberculoid leprosy in some populations, it was speculated whether T-cell responses against this peptide could be associated with immunopathological immune responses rather than protection (2). However, our present finding that the same peptide (aa 417 to 429) was recognized by M. leprae HSP65-specific T cells from a healthy donor in the context of HLA-DR2 suggests that such responses not necessarily are relevant to immunopathology.
Of the 12 HSP65 peptides recognized by the CD4+ T-cell lines and clones, 8 were completely identical in M. tuberculosis and M. leprae (53). Five of these peptides were presented to T cells in association with one of the two HLA-DR molecules expressed from the self-HLA-DRB1 genes, and three of them were presented in association with more than one HLA-DR molecule. Among the peptides presented to T-cell lines in the context of multiple HLA-DR molecules, peptide P7 (aa 61 to 75) was presented by HLA-DR1, -DR2, and -DR7, whereas peptide P13 (aa 141 to 155) was presented by HLA-DR2, -DR7, and -DR53. Peptide P7 (aa 61 to 75) was not recognized by the T-cell clones, and peptide P13 (aa 141 to 155) was recognized by T-cell clones from a single donor in association with HLA-DR7 (Table 8). The third peptide (aa 501 to 515) recognized in association with more than one HLA-DR molecule was presented to T cells in association with HLA-DR2 (Table 3) and HLA-DR4 (Table 8).
In addition to identification of promiscuous epitopes, we also analyzed our data with respect to T-cell epitopes which may be recognized by several donors. An HLA-DR3-restricted dominant T-cell epitope of the mycobacterial HSP65 (aa 2 to 12) has previously been identified by using T-cell lines from several HLA-DR3-positive leprosy patients (63). In this study, two overlapping peptides (aa 141 to 155 and aa 151 to 165) with sequences identical between M. leprae and M. tuberculosis were recognized by T cells from all the three HLA-DR2-positive donors. Thus, these peptides could be considered as dominant epitopes for presentation to T cells in association with HLA-DR2 molecules. However, the HLA-DR2-restricted epitopes in P13 (aa 141 to 155) and P14 (aa 151 to 165) must represent two different sequences because these two peptides overlap by only 5 aa, and the minimum length of a peptide for binding to HLA-DR and presentation to T cells is 7 to 8 aa (20, 44, 62).
In contrast to the peptide P14 (aa 151 to 165), which was recognized only in the context of HLA-DR2, peptide P13 (aa 141 to 155) was also presented by HLA-DR7 and HLA-DR53 molecules. Different subsets of T cells probably existed in the polyclonal T-cell lines that recognized peptide P13 bound with HLA-DR2, -DR7, and -DR53. The binding of a given peptide to different HLA-DR molecules can generate unique epitopes recognized by specific T cells (8). This observation is supported from the experiments in which we raised T-cell clones from a T-cell line that responded to peptide P13 (aa 141 to 155) in association with HLA-DR2 and -DR7. The two M. leprae HSP65 responding T-cell clones established from this donor responded to peptide P13 only in the context of HLA-DR7 (Table 8). These results support the suggestion that different subsets of T cells recognized peptide P13 in the context of different HLA-DR molecules.
Our results show that at least three HSP65 peptides are recognized in association with multiple HLA-DR molecules. Of special interest is peptide P13 (aa 141 to 155), which was recognized in association with HLA-DR2 and -DR7, as well as with HLA-DR53. HLA-DR53 is the serologically defined product of the HLA-DRB4 gene and is coexpressed with the HLA-DRB1 gene products HLA-DR4, -DR7, and -DR9. T-cell epitopes presented by HLA-DR53 are of considerable importance with respect to prophylaxis due to its frequent expression (up to 80%) in populations where mycobacterial diseases are endemic (1). We have previously reported a peptide-defined T-cell epitope from a 24.1-kDa secreted lipoprotein common to M. leprae and M. tuberculosis that is also restricted by HLA-DR53 (20, 34, 45). Importantly, the CD4+ T-cell lines studied here also showed peptide-specific cytotoxic activity, a characteristic which is associated with the inhibition of mycobacterial growth within autologous macrophages (57).
On the basis of MHC restriction, cytotoxic activity, and representation among the targets of the M. leprae memory T-cell repertoire, we consider the promiscuous HSP65 T-cell epitopes identified here as relevant components to be included in experimental subunit vaccines. A major problem with peptide-based vaccines could be the poor immunogenicity in the absence of potent adjuvants. However, recent advances in DNA cloning and expression technologies have shown that peptides could be delivered in highly immunogenic form to the cellular immune system in the absence of classical adjuvants (7, 58, 59). Another potential limitation of using mycobacterial HSP65 or its peptides in a prophylactic vaccine against tuberculosis and leprosy could be the danger of inducing autoimmunity due to homology with the human HSP65 (11, 66, 67). Fortunately, none of the three promiscuous peptides of the mycobacterial HSP65 (aa 61 to 75, 141 to 155, and 501 to 515) belong to the regions of greatest homology between the mycobacterial and human HSP65 (49). In addition, these peptides have not been shown to possess epitopes recognized by T cells from patients with autoimmune diseases (49, 67).
ACKNOWLEDGMENTS
This study was supported by Kuwait University Research Administration Grant M1 114 and the Kuwait Foundation for Advancement of Sciences Project KFAS 97-0705. The recombinant antigens were provided through the WHO/IMMLEP Bank.
REFERENCES
- 1.Aizawa M. DRw53. In: Aizawa M, editor. HLA in Asia-Oceania. Sapporo, Japan: Hokkaido University Press; 1986. p. 175. [Google Scholar]
- 2.Anderson D C, van Schooten W C A, Barry M E, Janson A A M, Buchanon T M, de Vries R R P. A Mycobacterium leprae-specific human T-cell epitope cross-reactive with an HLA-DR2 peptide. Science. 1988;242:259–261. doi: 10.1126/science.2459778. [DOI] [PubMed] [Google Scholar]
- 3.Bloom B R. Learning from leprosy: a perspective on immunology and the Third World. J Immunol. 1986;137:i–x. [PubMed] [Google Scholar]
- 4.Gelber R H, Mehra V, Bloom B, Murray L P, Siu P, Tsang M, Brennan P J. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse foot pads. Infect Immun. 1994;62:4250–4255. doi: 10.1128/iai.62.10.4250-4255.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill H K, Mustafa A S, Godal T. Induction of delayed type hypersensitivity in human volunteers immunized with a candidate leprosy vaccine consisting of killed Mycobacterium leprae. Bull W H O. 1986;64:121–126. [PMC free article] [PubMed] [Google Scholar]
- 6.Haanen J B A G, Malefijt R D W, Res P C M, Kraakman E M, Ottenhoff T H M, deVries R R P, Spits H. Selection of a human T helper type 1-like cell subset by mycobacteria. J Exp Med. 1991;174:583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hetzel C, Janssen R, Ely S J, Kristensen N M, Bunting K, Cooper J B, Lamb J R, Young D B, Thole J E. An epitope delivery system for use with recombinant mycobacteria. Infect Immun. 1998;66:3643–3648. doi: 10.1128/iai.66.8.3643-3648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurcevic S, Travers P J, Hills A, Agrewala J N, Moreno C, Ivanyi J. Distinct conformations of a peptide bound to HLA-DR1 or DRB5*0101 suggested by molecular modelling. Int Immunol. 1998;8:1807–1814. doi: 10.1093/intimm/8.11.1807. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann S H E. The interplay between cytokines and T-cells in immunity to intracellular bacteria. In: Mustafa A S, Al-Attiyah R J, Nath I, Chugh T D, editors. T-cell subsets and cytokines interplay in infectious diseases. Basel, Switzerland: Karger AG; 1996. pp. 169–180. [Google Scholar]
- 10.Kaufmann S H E, Vath U, Thole J E R, van Embden J D A, Emmrich F. Enumeration of T-cells reactive with M. tuberculosis organisms and specific for the recombinant mycobacterial 64 kilodalton protein. Eur J Immunol. 1987;17:351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- 11.Lamb J R, Bal V, Mendez-Samperio P, Mehlert A, So A, Rothbord J, Jindal S, Young R A, Young D B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1:191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- 12.Lamb J R, Ivanyi J, Rees A D M, Rothbard J B, Howland K, Young R A, Young D B. Mapping of T-cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987;6:1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munk E M, Schoel B, Kaufmann S H E. T-cell responses of normal individuals towards recombinant protein antigens of Mycobacterium tuberculosis. Eur J Immunol. 1988;18:1835–1838. doi: 10.1002/eji.1830181128. [DOI] [PubMed] [Google Scholar]
- 14.Munk M E, Shinnik T M, Kaufmann S H E. Epitopes of the mycobacterial heat shock protein for human T-cells comprise different structures. Immunobiology. 1990;180:272–277. doi: 10.1016/S0171-2985(11)80334-7. [DOI] [PubMed] [Google Scholar]
- 15.Mustafa A S. Identification of T-cell activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bovis BCG and Mycobacterium w. Int J Lepr. 1988;50:265–273. [PubMed] [Google Scholar]
- 16.Mustafa A S. Isolation and characterization of the genes of pathogenic mycobacteria that express antigens for T-cell reactivity. Nutrition. 1995;11(Suppl. 5):653–656. [PubMed] [Google Scholar]
- 17.Mustafa A S. Restoration of proliferative response to M. leprae antigens in lepromatous T-cells against candidate antileprosy vaccines. Int J Lepr. 1996;64:257–267. [PubMed] [Google Scholar]
- 18.Mustafa A S. Recombinant mycobacterial antigens/epitopes recognized by human T-cells. In: Mustafa A S, Al-Attiyah R J, Nath I, Chugh T D, editors. T-cell subsets and cytokines interplay in infectious diseases. Basel, Switzerland: Karger AG; 1996. pp. 201–211. [Google Scholar]
- 19.Mustafa A S, Amoudy H A, Wiker H G, Abal A T, Ravn P, Oftung F, Andersen P. Comparison of antigen specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:535–543. doi: 10.1046/j.1365-3083.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 20.Mustafa A S, Deggerdal A, Lundin K E A, Meloen R M, Shinnick T M, Oftung F. An HLA-DRw53-restricted T-cell epitope from a novel Mycobacterium leprae protein antigen important to the human memory T-cell repertoire against M. leprae. Infect Immun. 1994;62:5595–5602. doi: 10.1128/iai.62.12.5595-5602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustafa A S, Deggerdal A, Oftung F. Identification of recombinant mycobacterial protein antigens as vaccine candidates. Immunology (Life Sci Adv) 1991;10:139–147. [Google Scholar]
- 22.Mustafa A S, Gill H K, Nerland A, Britton W J, Mehra V, Bloom B R, Young R A, Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986;319:63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- 23.Mustafa A S, Godal T. In vitro induction of human suppressor T-cells by mycobacterial antigens. BCG activated OKT4+ cells mediate suppression of antigen induced T-cell proliferation. Clin Exp Immunol. 1983;52:29–37. [PMC free article] [PubMed] [Google Scholar]
- 24.Mustafa A S, Godal T. BCG induced suppressor T-cells: optimal conditions for in vitro induction and mode of action. Clin Exp Immunol. 1985;62:474–481. [PMC free article] [PubMed] [Google Scholar]
- 25.Mustafa A S, Godal T. BCG induced CD4+ cytotoxic T-cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin Exp Immunol. 1987;69:255–262. [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa A S, Kvalheim G, Degre M, Godal T. Mycobacterium bovis BCG-induced human T-cell clones from BCG vaccinated healthy subjects: antigen specificity and lymphokine production. Infect Immun. 1986;53:491–497. doi: 10.1128/iai.53.3.491-497.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mustafa A S, Lundin K E A, Meloen R H, Shinnick T M, Coulson A F W, Oftung F. HLA-DR4 restricted T-cell epitopes from the mycobacterial 60,000 MW heat shock protein (hsp 60) do not map to the sequence homology regions with the human hsp 60. Immunology. 1996;87:421–427. doi: 10.1046/j.1365-2567.1996.448552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa A S, Lundin K E A, Oftung F. Human T-cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect Immun. 1993;61:5294–5301. doi: 10.1128/iai.61.12.5294-5301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustafa A S, Lundin K E A, Oftung F. Mapping of HLA-DR4 restricted T-cell epitopes from the mycobacterial HSP60 with synthetic peptides. In: Charron D, editor. Proceedings of the 12th International Histocompatibility Workshop and Conference. II. Paris, France: EDK; 1997. pp. 704–705. [Google Scholar]
- 30.Mustafa A S, Lundin K E A, Oftung F. Isolation of recombinant phage clones expressing mycobacterial T-cell antigens by screening a recombinant DNA library with human CD4+ Th1 clones. FEMS Immunol Med Microbiol. 1998;22:205–216. doi: 10.1111/j.1574-695X.1998.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 31.Mustafa A S, Nadeem S, Amoudy H A. Identification of HLA-DR molecules most frequently expressed by peripheral blood mononuclear cells of healthy subjects responding to mycobacterial antigens. Kuwait Med J. 1996;1996(Suppl.):323–327. [Google Scholar]
- 32.Mustafa A S, Oftung F. Long-lasting T-cell reactivity to Mycobacterium leprae antigens in human volunteers vaccinated with killed M. leprae. Vaccine. 1993;11:1108–1112. doi: 10.1016/0264-410x(93)90070-e. [DOI] [PubMed] [Google Scholar]
- 33.Mustafa A S, Oftung F. Identification of mycobacterial recombinant antigens recognized by human T-cells. Med Princ Pract. 1997;6:57–65. [Google Scholar]
- 34.Mustafa A S, Oftung F, Deggerdal A, Gill H K, Young R A, Godal T. Gene isolation using human T lymphocyte probes. Isolation of a gene that expresses an epitope recognized by T-cells specific for Mycobacterium bovis BCG and pathogenic mycobacteria. J Immunol. 1988;141:2729–2733. [PubMed] [Google Scholar]
- 35.Mustafa A S, Oftung F, Gill H K, Natvig I. Characteristics of human T-cell clones from BCG and killed M. leprae vaccinated subjects and tuberculosis patients. Lepr Rev. 1986;57(Suppl. 2):123–130. [PubMed] [Google Scholar]
- 36.Mustafa A S, Qvigstad E. HLA-DR restricted antigen induced proliferation and cytotoxicity mediated by CD4+ T-cell clones from subjects vaccinated with killed M. leprae. Int J Lepr. 1988;57:1–11. [PubMed] [Google Scholar]
- 37.Mutis T, Cornelisse Y E, Datema G, van den Elsen P J, Ottenhoff T H M, de Vries R R P. Definition of a human suppressor T-cell epitope. Proc Natl Acad Sci USA. 1994;91:9456–9460. doi: 10.1073/pnas.91.20.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutis T, Cornelisse Y E, Ottenhoff T H M. Mycobacteria induce CD4+ T-cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–2195. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 39.Oftung F, Borka E, Mustafa A S. Mycobacterium tuberculosis reactive T-cell clones from naturally converted PPD-positive healthy subjects: recognition of the M. tuberculosis 16-kDa antigen. FEMS Immunol Med Microbiol. 1998;20:319–325. doi: 10.1111/j.1574-695X.1998.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 40.Oftung F, Geluk A, Lundin K E A, Meloen R H, Thole J E R, Mustafa A S, Ottenhoff T H M. Mapping of multiple HLA-class II epitopes of the mycobacterial 70-kilodalton heat shock protein. Infect Immun. 1994;62:5411–5418. doi: 10.1128/iai.62.12.5411-5418.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oftung F, Lundin K E A, Geluk A, Ottenhoff T H M, Shinnick T M, Meloen R, Mustafa A S. Primary structure and MHC restriction of peptide defined T-cell epitopes from recombinantly expressed mycobacterial protein antigens. Med Princ Pract. 1997;6:66–73. [Google Scholar]
- 42.Oftung F, Mustafa A S, Husson R, Young R A, Godal T. Human T-cell clones recognize two abundant Mycobacterium tuberculosis protein antigen expressed in Escherichia coli. J Immunol. 1987;138:927–931. [PubMed] [Google Scholar]
- 43.Oftung F, Mustafa A S, Shinnick T M, Houghton R A, Kvalheim G, Degre M, Lundin K E A, Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T-cells. J Immunol. 1988;141:2749–2754. [PubMed] [Google Scholar]
- 44.Oftung F, Shinnick T M, Mustafa A S, Lundin K E A, Godal T, Nerland A H. Heterogeneity among human T-cell clones recognizing an HLA-DR4,Dw4-restricted epitope from the 18 kDa antigen of Mycobacterium leprae defined by synthetic peptides. J Immunol. 1990;144:1478–1483. [PubMed] [Google Scholar]
- 45.Oftung F, Wiker H G, Deggerdal A, Mustafa A S. A novel mycobacterial antigen relevant to cellular immunity belongs to a family of secreted lipoproteins. Scand J Immunol. 1997;46:445–451. doi: 10.1046/j.1365-3083.1997.d01-150.x. [DOI] [PubMed] [Google Scholar]
- 46.Ottenhoff T H M, Ab B K, van Embden J D A, Thole J E R, Kiessling R. The recombinant heat shock protein of Mycobacterium bovis bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988;168:1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ottenhoff T H M, Haanen J B A G, Geluk A, Mutis T, Ab B K, Thole J E R, van Schooten W C A, van den Elsen P J, de Vries R R P. Regulation of mycobacterial heat-shock protein reactive T-cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991;121:171–191. doi: 10.1111/j.1600-065x.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 48.Ottenhoff T H M, Kumararatne D, Casanova J L. Novel immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–494. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 49.Quayle A J, Wilson K B, Li S G, Kjeldsen-Kragh J, Oftung F, Shinnick T, Sioud M, Forre O, Capra J D, Natvig J B. Peptide recognition, T cell receptor usage and HLA restriction elements of human heat-shock protein (hsp) 60 and mycobacterial 65-kDa hsp-reactive T cell clones from rheumatoid synovial fluid. Eur J Immunol. 1992;22:1315–1322. doi: 10.1002/eji.1830220529. [DOI] [PubMed] [Google Scholar]
- 50.Ravn P, Boesen H, Pedersen B K, Andersen P. Human T-cell responses induced by vaccination with Mycobacterium bovis Bacillus Calmette-Guerin. J Immunol. 1997;158:1949–1955. [PubMed] [Google Scholar]
- 51.Ronningen K S, Spurkland A, Markussen G, Iwe T, Vartdal F, Thorsby E. Distribution of HLA class II alleles among Norwegian Caucasians. Hum Immunol. 1990;29:275–281. doi: 10.1016/0198-8859(90)90041-m. [DOI] [PubMed] [Google Scholar]
- 52.Sharrock C, Lundin K E A, Zeir K, Zeevi A, Thomson M, Eirmann T, Hansen J A, Mickelson E, Flomenberg N. T-cell recognition of HLA class II molecules DR4 and DQw3. In: Dupont B, editor. Immunobiology of HLA. Vol. 1. Heidelberg, Germany: Springer-Verlag, KG; 1988. pp. 509–513. [Google Scholar]
- 53.Shinnick T M, Sweetser D, Thole J, van Embden J, Young R A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987;55:1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva C L, Lowrie D B. A single mycobacterial protein (HSP 65) expressed by a transgenic antigen-presenting cell vaccinates mice against tuberculosis. Immunology. 1994;82:244–248. [PMC free article] [PubMed] [Google Scholar]
- 55.Silva C L, Silva M F, Pietro R C L R, Lowrie D B. Protection against tuberculosis by passive transfer with T-cell clones recognizing mycobacterial heat-shock protein 65. Immunology. 1994;83:341–346. [PMC free article] [PubMed] [Google Scholar]
- 56.Snider D E. The tuberculin skin test. Am Rev Respir Dis. 1982;125:108–118. doi: 10.1164/arrd.1982.125.3P2.108. [DOI] [PubMed] [Google Scholar]
- 57.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 58.Suzue K, Young R A. Adjuvant-free HSP70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–879. [PubMed] [Google Scholar]
- 59.Suzue K, Zhou X, Eisen H N, Young R A. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci USA. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tascon R E, Colston M J, Ragno S, Stavropoulos E, Gregoy D, Lowrie D B. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 61.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van der Zee R, van Eden W, Meloen R H, Noordzij A, van Embden J D A. Efficient mapping and characterization of a T-cell epitope by simultaneous synthesis of multiple peptides. Eur J Immunol. 1989;19:43–47. doi: 10.1002/eji.1830190108. [DOI] [PubMed] [Google Scholar]
- 63.Van Schooten W C A, Elferink D G, Van Embden J, Anderson D C, de Vries R R P. DR-3 restricted T-cells from different HLA-DR3 positive individuals recognize the same peptide (amino acids 2–12) of the mycobacterial 65-kDa heat shock protein. Eur J Immunol. 1989;19:2075–2079. doi: 10.1002/eji.1830191116. [DOI] [PubMed] [Google Scholar]
- 64.Vartdal F, Gaudernack G, Funderud S, Bratlie A, Lea T, Ugelstad J, Thorsby E. HLA class I and II typing using cells positively selected from blood by immunomagnetic isolation—a fast and reliable technique. Tissue Antigens. 1986;28:301–312. doi: 10.1111/j.1399-0039.1986.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 65.Xing Z, Wang J, Croitoru K, Wakeham J. Protection by CD4 or CD8 T cells against pulmonary Mycobacterium bovis bacillus Calmette-Guerin infection. Infect Immun. 1998;66:5537–5542. doi: 10.1128/iai.66.11.5537-5542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young R A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- 67.Zugel U, Kaufmann S H E. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12:19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]