Abstract
This study aimed to investigate the association between changes in levels of trimethylamine N-oxide (TMAO) and its precursors and the prognosis of patients with acute myocardial infarction (AMI). Patients diagnosed with AMI were prospectively enrolled at Fuwai Hospital between March 2017 and January 2020. TMAO, betaine, choline, and L-carnitine were measured in 1203 patients at their initial admission and 509 patients at their follow-up of one month. Major adverse cardiovascular events (MACE), a composite of all-cause death, recurrence of MI, rehospitalization caused by HF, ischemic stroke, and any revascularization, were followed up. A decision tree by TMAO levels implicated that compared to those with low levels at admission, patients with high TMAO levels at both time points showed an increased risk of MACE (adjusted hazard ratio (HR) 1.59, 95% confidence interval (CI): 1.03–2.46; p = 0.034), while patients with high TMAO levels at admission and low levels at follow-up exhibited a similar MACE risk (adjusted HR 1.20, 95% CI: 0.69–2.06; p = 0.520). Patients with high choline levels at admission and follow-up showed an elevated MACE risk compared to those with low levels at both time points (HR 1.55, 95% CI: 1.03–2.34; p = 0.034). Repeated assessment of TMAO and choline levels helps to identify the dynamic risk of cardiovascular events.
Keywords: acute myocardial infarction, gut microbiome-driven metabolites, changes in trimethylamine N-oxide, changes in choline
1. Introduction
Increasing studies have suggested the associations between metabolites of intestinal microbiota and cardiovascular diseases [1,2]. Trimethylamine N-oxide (TMAO), a classic component of the metabolic pathway, has been extensively investigated and regarded as a biomarker for adverse cardiovascular events [3,4]. Studies have shown the association between TMAO levels and worse outcomes in cardiovascular diseases [4,5,6,7,8]. TMAO could also improve the identification of coronary culprit plaque rupture in acute myocardial infarction (AMI) [9]. Moreover, several studies have already focused on the impact of serial TMAO levels on cardiovascular diseases. Heianza et al. implied that long-term elevation in TMAO levels was associated with increased coronary heart disease (CHD) risk in healthy women, and repeated measurement of TMAO over ten years could identify high CHD risk [10]. Another study reported that elevated TMAO levels at enrolment and nine months after treatment were correlated with a higher risk of adverse events in patients with chronic heart failure (HF) [11]. However, little is known about changes in TMAO levels and prognosis in the population with AMI. The response of TMAO levels to pharmacological treatment in these patients is also unknown.
TMAO is generated from the oxidation of trimethylamine (TMA), which comes from betaine, choline, and L-carnitine [12]. A large body of studies has investigated the associations of betaine, choline, and L-carnitine with cardiometabolic risk and mortality among different populations. A study that included patients with stable coronary artery disease found that higher plasma choline and betaine levels were risk factors for major adverse cardiovascular events (MACE) in a concomitant increase in TMAO levels [13]. Moreover, the Jackson Heart Study results implied that high dietary betaine intake was correlated to the high CHD incidence, whereas higher dietary choline intake was not [14]. As for L-carnitine, Koeth et al. conducted a study that enrolled patients undergoing cardiac evaluation and reported that higher L-carnitine levels indicated an elevated risk of cardiovascular disease and incidence of MACE only with concurrently high TMAO levels [15]. However, a systematic review summarized that the results did not support a positive association between dietary choline or betaine and the incidence of cardiovascular diseases in healthy people [16]. Given the inconsistent results, the impact of betaine, choline, and L-carnitine should be further investigated.
Therefore, this study focused on patients with AMI and investigated the association of TMAO, betaine, choline, and L-carnitine levels with prognosis and further explored the association of changes in these levels during follow-up with prognosis.
2. Methods
2.1. Study Population
Between March 2017 and January 2020, we prospectively recruited patients admitted to the emergency department of Fuwai Hospital with a diagnosis of AMI. AMI is defined by the Fourth Universal Definition of Myocardial Infarction and guidelines, including elevated troponin I level and clinical evidence of ischemia such as sustained chest pain, new ST-segment changes, and new regional wall motion abnormality [17,18,19]. A flowchart of the patient selection process is presented in Supplemental Figure S1. Patients who were missing gut metabolite levels or follow-up records were excluded. Information on demographics, physical examinations, medical histories, laboratory results, echocardiography data, and medication at discharge was collected through the hospital information system. The Ethics Committee of Fuwai Hospital approved the current study, which conformed to the Declaration of Helsinki. All patients enrolled signed informed consent.
2.2. Outcomes and Follow-Up
The primary outcome of this study was MACE, including all-cause death, recurrence of MI, rehospitalization caused by HF, stroke, and any revascularization. HF was identified following guidelines and statements up to date, based on typical symptoms and signs, laboratory tests, echocardiogram, and X-ray findings [20,21]. Outpatient visits and telephone interviews were used to collect outcome data and conducted with patients at 1, 6, and 12 months after discharge and then annually. The Institutional Review Board of Fuwai Hospital approved the protocol for follow-up.
2.3. Sample Collection and Tests
Blood samples were taken from the radial or femoral artery before heparinization and percutaneous coronary intervention (PCI) at admission (V1) and the cubital vein approximately one month later when patients came to the clinic (V2). The samples were maintained with tubes containing ethylenediaminetetraacetic at 4 °C, centrifuged within 3 h, the supernatant was separated and stored at −80 °C until subsequent analysis. As described in previous studies, plasma levels of TMAO and its precursors were measured by stable isotope dilution high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry using an API 3200 triple quadrupole mass spectrometer (AB SCIEX, Framingham, MA, USA) with a d9-TMAO, d9-betaine, d9-choline, and d3-carnitine internal standard [22,23]. The other blood parameters were routinely measured in the hospital’s central laboratory.
2.4. Statistical Analyses
Baseline characteristics were displayed as mean ± standard deviation or median with interquartile range (IQR) for continuous variables and number (percentage) for categorical variables. Selecting the appropriate analysis method according to the number of groups and variable type compared the differences among groups. For patients who completed the second visit, the changes of continuous variables from V1 to V2 were compared by the Wilcoxon matched-pairs signed-rank test.
To investigate the association between TMAO and its precursors and cardiovascular risk in AMI, we grouped the patients based on plasma median and teritle levels of TMAO and its precursors, respectively. An analysis of the Kaplan–Meier curve and log-rank test was used to determine the event-free survival rates of the groups. Univariable and multivariable Cox proportional hazards regression analyses were used to compute the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). The variables with p < 0.1 in the univariable models were further analyzed in the multivariable Cox regression. Furthermore, the adjusted factors would add TMAO levels for the analysis of the precursors. Possible nonlinear relationships were accessed using restricted cubic spline (RCS) regression with TMAO as a continuous variable with four knots, which also adjusted for the confounders mentioned above.
To explore the relationship between changes in TMAO levels and subsequent MACE after V2, we classified patients with available data for both time points based on the respective median levels of TMAO at each time point. Then we created four groups, consisting of L/L group (low V1 and low V2), L/H group (low V1 and high V2), H/L group (high V1 and low V2), and H/H group (high V1 and high V2). Cox regression analysis examined the associations between each group and outcomes. The HRs of MACEs among groups were displayed in forest plots. A decision tree analysis was performed by the 𝜒2 automatic interaction detection to generate groups of different MACE risk and the association of resultant groups with MACE was confirmed by multivariable Cox regression and Kaplan–Meier survival curves. Furthermore, betaine, choline, and L-carnitine levels were analyzed similarly.
The SPSS software (version 26.0; IBM Corp., Armonk, NY, USA) and R (http://www.r-project.org/, (accessed on 1 March 2022) statistical packages were used to analyze the data. p < 0.05 was set out as statistically significant.
3. Results
3.1. Characteristics of Included Patients
Between March 2017 and January 2020, 1203 patients admitted to the emergency department of Fuwai Hospital and diagnosed with AMI were analyzed in this study. The baseline characteristics of the included patients are listed in Table 1. The median age of all patients was 61 (IQR 53–69) years, and 963 (80.0%) patients were male. The median plasma levels of TMAO, betaine, choline, and L-carnitine in all patients were 6.6 (IQR 4.0–11.6) µmol/L, 1.9 (IQR 1.5–2.4) µmol/L, 1.2 (IQR 1.0–1.5) µmol/L, and 50.6 (IQR 42.5–59.6) µmol/L, respectively. The patient characteristics grouped by the respective median and tertile levels of TMAO, betaine, choline, and L-carnitine are presented in Supplemental Tables S1–S4. Patients with the third tertile levels of TMAO and choline were older and presented with a higher rate of Killip II-IV, previous stroke, diabetes mellitus, and chronic kidney disease (CKD), and a higher frequency of MACE during follow-up. Moreover, the levels of betaine, choline and L-carnitine showed an increasing trend according to the tertile levels of TMAO.
Table 1.
The characteristics of included patients.
| Variables | Total Cohort (n = 1203) | Patients with Follow-Up Visit (n = 509) | ||
|---|---|---|---|---|
| V1 | V2 | p-Value | ||
| Male | 963 (80.0) | 423 (83.1) | ||
| Age (years) | 61.1 (53.0, 69.0) | 61.0 (53.4, 60.6) | ||
| BMI (kg/m2) | 25.8 (23.4, 27.8) | 25.7 (22.8, 27.8) | ||
| Killip (II- IV) | 153 (12.7) | 41 (8.1) | ||
| LVEF (%) | 55.0 (50.0, 60.0) | 56.0 (50.0, 60.0) | 60.0 (55.0, 63.0) | <0.001 |
| LVEF < 40% | 68 (5.7) | 24 (4.7) | 12 (2.4) | |
| MVD | 883 (73.4) | 365 (71.7) | ||
| PCI | 833 (69.2) | 359 (70.5) | ||
| Medical history | ||||
| Current Smoker | 875 (72.7) | 371 (72.9) | ||
| Hypertension | 786 (65.3) | 324 (63.7) | ||
| Hyperlipemia | 1117 (92.9) | 468 (91.9) | ||
| Diabetes Mellitus | 416 (34.6) | 177 (34.8) | ||
| Previous Stroke | 184 (15.3) | 79 (15.5) | ||
| CKD | 95 (7.9) | 25 (4.9) | ||
| PAD | 73 (6.1) | 35 (6.9) | ||
| Previous MI | 220 (18.3) | 98 (19.3) | ||
| Laboratory indexes | ||||
| TMAO (µmol/L) | 6.6 (4.0, 11.6) | 6.7 (4.0, 11.5) | 12.7 (8.1, 20.4) | <0.001 |
| Choline (µmol/L) | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.5) | 1.7 (1.4, 2.1) | <0.001 |
| Betaine (µmol/L) | 1.9 (1.5, 2.4) | 1.9 (1.5, 2.4) | 3.1 (2.4, 3.9) | <0.001 |
| L-carnitine1 (µmol/L) | 50.6 (42.5, 59.6) | 51.2 (43.5, 60.7) | 67.0 (51.5, 83.0) | <0.001 |
| eGFR (mL/min/1.732 m2 *) | 76.1 (64.1, 89.4) | 75.9 (65.8, 90.4) | 78.2 (65.3, 88.4) | 0.219 |
| ALT (IU/L) | 32.0 (21.0, 52.0) | 32.0 (20.0, 52.0) | ||
| AST (IU/L) | 90.0 (44.0, 191.0) | 87.0 (44.0, 187.0) | ||
| Baseline cTnI (ng/mL) | 1.0 (0.1, 5.5) | 1.1 (0.1, 6.2) | ||
| Peak cTnI (ng/mL) | 9.6 (2.1, 27.2) | 9.3 (2.0, 24.6) | ||
| Baseline NT-proBNP (ng/mL) | 301.8 (83.1, 1007.0) | 252.5 (70.4, 749.2) | ||
| Peak NT-proBNP (ng/mL) | 1186.0 (469.6, 2776.0) | 1056.0 (429.4, 2186.0) | ||
| hsCRP (mg/L) | 5.5 (1.8, 11.0) | 5.4 (1.8, 10.8) | 1.2 (0.5, 2.8) | <0.001 |
| LDL-C (mmol/L) | 2.6 (2.0, 3.2) | 2.6 (2.0, 3.2) | 1.8 (1.5, 2.3) | <0.001 |
| GRACE score | 108.0 (89.0, 127.0) | 107.0 (90.0, 125.0) | ||
| Medication at discharge | ||||
| Aspirin | 1142 (94.9) | 484 (95.1) | ||
| Ticagrelor | 565 (47.0) | 251 (49.3) | ||
| Clopidogrel | 607 (50.5) | 255 (50.1) | ||
| ACEI/ARB | 830 (69.0) | 356 (69.9) | ||
| Βeta Blocker | 1008 (83.8) | 443 (87.0) | ||
| Statins | 1139 (94.7) | 497 (97.6) | ||
| Adverse outcomes | ||||
| Death | 84 (7.0) | 24 (4.7) | ||
| reMI | 71 (5.9) | 27 (5.3) | ||
| reHF | 22 (1.8) | 12 (2.4) | ||
| Revascularization | 195 (16.2) | 71 (13.9) | ||
| Stroke | 41(3.4) | 18 (3.5) | ||
| MACE | 343 (28.5) | 125 (24.6) | ||
Continuous variables are presented as medians (25th−75th percentiles), and categorical variables are reported as counts (%). ACEIs/ARBs indicates angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; cTnI, cardiac troponin I; GRACE, the Global Registry of Acute Coronary Events; HF, heart failure; hsCRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular event; MI, myocardial infarction; MVD, multiple vessels disease; reHF, rehospitalization caused by heart failure; reMI, recurrent myocardial infarction; STMEI, ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro B-type natriuretic peptide; STMEI, ST-segment elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TMAO, trimethylamine-N-oxide. * Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease formula.
The follow-up levels of TMAO, betaine, choline, and L-carnitine were available in 509 (42.3%) patients. These patients showed an improvement in clinical variables (Table 1) after one month of treatment. The levels of low-density lipoprotein cholesterol (p < 0.001) and high-sensitivity C-reactive protein (p < 0.001) reduced significantly, while TMAO and its precursors all exhibited an increasing trend (p < 0.001). The Supplemental Table S5 summarizes characteristic differences between patients with and without TMAO measurements at V2. Patients without TMAO measurements at V2 presented with a higher prevalence of previous CKD (p = 0.001) and Killip II-IV (p < 0.001), as well as a higher incidence of MACE (p = 0.011) and all-cause death (p = 0.011).
3.2. Association between Plasma Levels of TMAO, Betaine, Choline, and L-Carnitine at Baseline and Adverse Outcomes
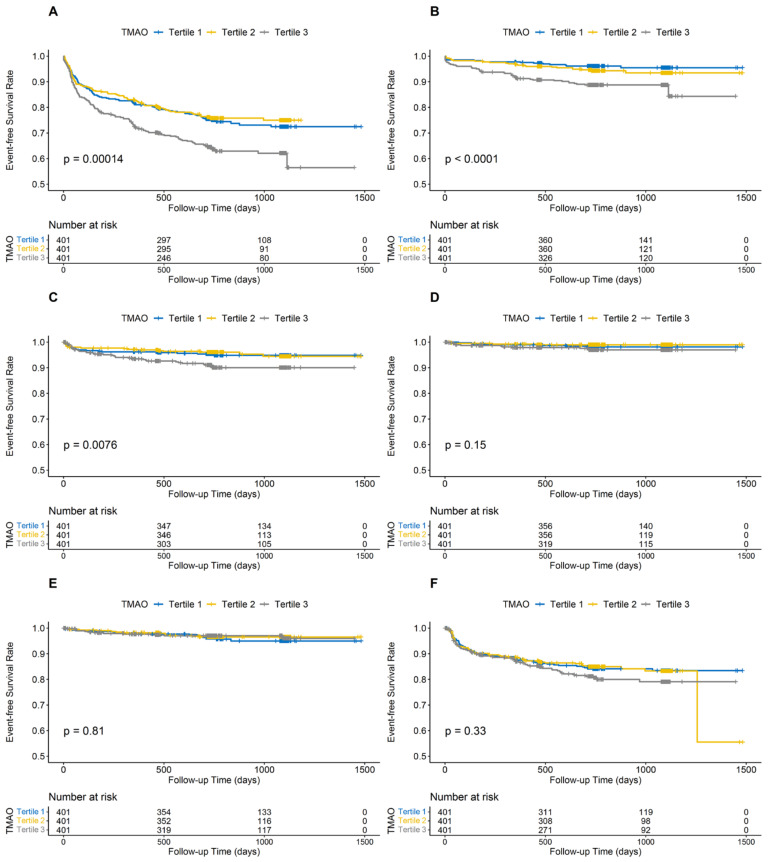
A total of 343 (28.5%) patients experienced MACE during a median time of 739 days follow-up. The Kaplan–Meier analysis of groups classified by the tertile TMAO levels in Figure 1 revealed that MACE risk in tertile 3 increased significantly (p < 0.001), as well as the risk for all-cause death (p < 0.001) and recurrent MI (p = 0.008). As shown in Table 2, compared with patients in tertile 1 (<4.76 µmol/L), those in tertile 3 had a higher MACE risk (>9.38 µmol/L, adjusted HR: 1.35, 95% CI: 1.02–1.78; p = 0.033), as well as risks of all-cause death and recurrent MI (HR: 2.06, 95% CI: 1.01–4.19; p = 0.047 and HR:1.95, 95% CI: 1.08–3.53; p = 0.027, respectively). As a continuous variable, the RCS regression analysis curved an S-shaped relationship between TMAO levels and HR for MACE and all-cause death (p for nonlinearity = 0.045 and 0.004, respectively) after adjusting for the confounding factors (Supplemental Figure S2).
Figure 1.
Kaplan—curve for cumulative event-free survival in groups stratified by TMAO tertile levels at enrollment. (A) major adverse cardiovascular event, (B) all-cause death, (C) myocardial infarction, (D) rehospitalization caused by heart failure; (E) stroke; (F) revascularization. TMAO, trimethylamine-N-oxide.
Table 2.
Association between TMAO levels at enrollment and all endpoints.
| Endpoint | Group | Event (n.%) | Crude HR (95%CI) | p-Value | Adjusted HR (95%CI) * | p-Value |
|---|---|---|---|---|---|---|
| MACE | ||||||
| ≤median | 149 (24.8) | [1] | [1] | |||
| >median | 194 (32.2) | 1.39 (1.12–1.72) | 0.003 | 1.27 (1.01–1.60) | 0.040 | |
| Tertile 1 | 102 (25.4) | [1] | [1] | |||
| Tertile 2 | 97 (24.2) | 0.95 (0.72–1.26) | 0.743 | 0.89 (0.67–1.19) | 0.429 | |
| Tertile 3 | 144 (35.9) | 1.54 (1.20–1.99) | 0.001 | 1.35 (1.02–1.78) | 0.033 | |
| Trend. test | 343 (28.5) | 1.26 (1.10–1.44) | 0.001 | 1.18 (1.02–1.36) | 0.025 | |
| All-cause death | ||||||
| ≤median | 31 (5.2) | [1] | [1] | |||
| >median | 53 (8.8) | 1.76 (1.13–2.74) | 0.013 | 1.22 (0.73–2.04) | 0.441 | |
| Tertile 1 | 16 (4.0) | [1] | [1] | |||
| Tertile 2 | 23 (5.7) | 1.45 (0.77–2.74) | 0.255 | 1.44 (0.68–3.02) | 0.341 | |
| Tertile 3 | 45 (11.2) | 2.97 (1.68–5.26) | < 0.001 | 2.06 (1.01–4.19) | 0.047 | |
| Trend. test | 84 (7.0) | 1.78 (1.34–2.36) | < 0.001 | 1.43 (1.02–2.01) | 0.036 | |
| reMI | ||||||
| ≤median | 24 (4.0) | [1] | [1] | |||
| >median | 47 (7.8) | 2.04 (1.25–3.33) | 0.005 | 2.01 (1.20–3.37) | 0.008 | |
| Tertile 1 | 19 (4.7) | [1] | [1] | |||
| Tertile 2 | 17 (4.2) | 0.90 (0.47–1.73) | 0.756 | 0.93 (0.48–1.81) | 0.836 | |
| Tertile 3 | 35 (8.7) | 1.96 (1.12–3.43) | 0.018 | 1.95 (1.08–3.53) | 0.027 | |
| Trend. test | 71 (5.9) | 1.46 (1.09–1.96) | 0.012 | 1.44 (1.06–1.97) | 0.021 | |
| reHF | ||||||
| ≤median | 8 (1.3) | [1] | [1] | |||
| >median | 14 (2.3) | 1.81 (0.76–4.31) | 0.182 | 1.02 (0.39–2.64) | 0.968 | |
| Tertile 1 | 7 (1.7) | [1] | [1] | |||
| Tertile 2 | 4 (1.0) | 0.57 (0.17–1.96) | 0.375 | 0.37 (0.09–1.45) | 0.152 | |
| Tertile 3 | 11 (2.7) | 1.67 (0.65–4.31) | 0.288 | 0.97 (0.34–2.80) | 0.961 | |
| Trend. test | 22 (1.8) | 1.36 (0.81–2.30) | 0.247 | 1.02 (0.57–1.82) | 0.940 | |
| Stroke | ||||||
| ≤median | 20 (3.3) | [1] | [1] | |||
| >median | 21 (3.5) | 1.09 (0.59–2.01) | 0.782 | 0.85 (0.44–1.66) | 0.638 | |
| Tertile 1 | 16 (4.0) | [1] | [1] | |||
| Tertile 2 | 13 (3.2) | 0.83 (0.40–1.72) | 0.607 | 0.77 (0.36–1.64) | 0.500 | |
| Tertile 3 | 12 (3.0) | 0.80 (0.38–1.70) | 0.566 | 0.52 (0.23–1.19) | 0.121 | |
| Trend. test | 41 (3.4) | 0.89 (0.61–1.30) | 0.556 | 0.72 (0.48–1.09) | 0.120 | |
| Revascularization | ||||||
| ≤median | 89 (14.8) | [1] | [1] | |||
| >median | 106 (17.6) | 1.25 (0.94–1.65) | 0.125 | 1.3 (0.97–1.75) | 0.083 | |
| Tertile 1 | 62 (15.5) | [1] | [1] | |||
| Tertile 2 | 61 (15.2) | 1.00 (0.70–1.42) | 0.978 | 0.96 (0.67–1.38) | 0.822 | |
| Tertile 3 | 72 (18.0) | 1.24 (0.89–1.75) | 0.209 | 1.25 (0.88–1.79) | 0.210 | |
| Trend. test | 195 (16.2) | 1.12 (0.94–1.33) | 0.205 | 1.12 (0.94–1.35) | 0.212 |
HR, hazard ratio; MACE, major adverse cardiovascular event; reHF, rehospitalization caused by heart failure; reMI, recurrent myocardial infarction; TMAO, trimethylamine-N-oxide. * Adjusted for the variables with p < 0.1 in the univariable models, including age, hypertension, diabetes, peripheral artery disease, chronic kidney disease, and previous history of stroke and MI, Killip II-IV, the Global Registry of Acute Coronary Events risk score, multiple vessels disease, percutaneous coronary intervention, and the peak value of cardiac troponin I and N-terminal pro B-type natriuretic peptide during hospitalization, as well as estimated glomerular filtration rate and left ventricular ejection fraction.
For patients grouped by choline tertile levels, the Kaplan–Meier analysis in Supplemental Figure S3 showed that there was no statistical difference in MACE risk, whereas patients in tertile 3 had higher risks of all-cause death (p < 0.001) and rehospitalization caused by HF (p = 0.024). Moreover, the Kaplan–Meier analysis of betaine tertile levels (Supplemental Figure S4) revealed that patients in tertile 3 had an increased risk of rehospitalization caused by HF (p = 0.026), while for L-carnitine (Supplemental Figure S5), there were no statistical differences in any endpoints. The details of Cox regression of relationships between all endpoints and choline, betaine, and L-carnitine levels are shown in Supplemental Tables S6–S8. After adjusting the confounders, the HR for rehospitalization caused by HF was higher in patients above the median choline levels (HR 2.90, 95% CI: 1.02–8.25; p = 0.045). Apart from this, the other HRs of all endpoints were not statistically different.
3.3. Association between Changes in Levels of TMAO, Betaine, Choline, and L-Carnitine and Adverse Outcomes
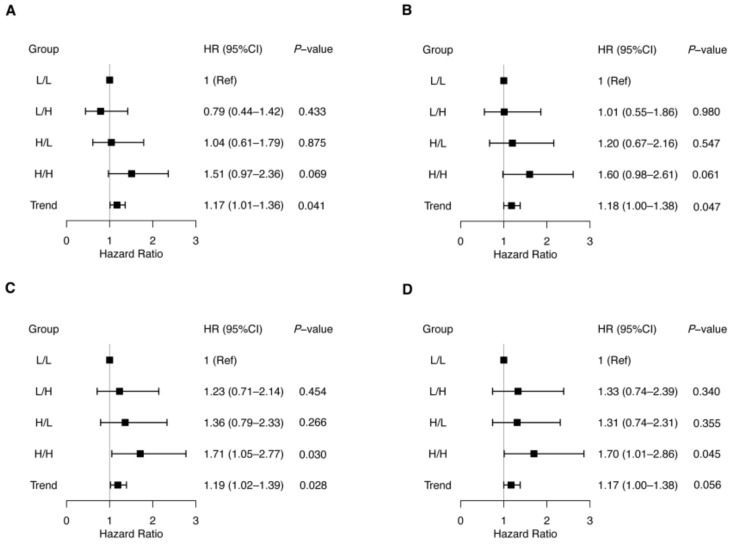
To investigate the associations between serial levels of TMAO following treatment and subsequent adverse events, we divided the patients with available TMAO levels of V1 and V2 into four groups according to the median levels of each visit (V1: 6.7 µmol/L; V2: 12.7 µmol/L). These groups were comprised of L/L group (n = 155), L/H group (n = 99), H/L group (n = 99), and H/H group (n = 156). The forest plot in Figure 2A showed that compared to the L/L group, no statistical differences were observed in each group (L/H group: HR 0.79, 95% CI: 0.44–1.42, p = 0.433; H/L group: HR 1.04, 95% CI: 0.61–1.79, p = 0.875; H/H group: HR 1.51, 95% CI: 0.97–2.36, p = 0.069), while the trend showed significantly increasing (adjusted HR 1.18, 95% CI: 1.00–1.38, p = 0.047). Moreover, there was no statistical significance in TMAO levels and individual endpoints (Table 3).
Figure 2.
Forest plot of hazard ratios for major adverse cardiovascular events in groups according to trimethylamine N-oxide (TMAO) (A,B) and choline (C,D) levels at enrollment (V1) and follow-up visit (V2). Patients with available TMAO and choline levels of V1 and V2 were divided into four groups according to the median levels of each visit (TMAO: 6.7 µmol/L and 12.7 µmol/L, choline: 1.2 µmol/L and 1.7 µmol/L for V1 and V2, respectively). L/L, low V1 and low V2; L/H, low V1 and high V2; H/L, high V1 and low V2; H/H, high V1 and high V2. Cox proportional hazards regression was used to compare the risk of major adverse cardiovascular events among the four groups of patients using L/L as the reference on each occasion [(A,C) unadjusted, (B) adjusted with age, hypertension, diabetes, peripheral artery disease, chronic kidney disease, and previous history of stroke and MI, Killip II-IV, the Global Registry of Acute Coronary Events risk score, multiple vessels disease, percutaneous coronary intervention, and the peak value of cardiac troponin I and N-terminal pro-B-type natriuretic peptide during hospitalization, as well as estimated glomerular filtration rate and left ventricular ejection fraction at V2; (D) adjusted with these factors and TMAO levels at V2].
Table 3.
Association between TMAO levels of V1 and V2 and all endpoints.
| Endpoint | Group | Event (n.%) | Crude HR (95%CI) | p-Value | Adjusted HR (95%CI) * | p-Value |
|---|---|---|---|---|---|---|
| MACE | ||||||
| L/L | 33 (21.3) | [1] | [1] | |||
| L/H | 17 (17.2) | 0.79 (0.44–1.42) | 0.433 | 1.01 (0.55–1.86) | 0.980 | |
| H/L | 22 (22.2) | 1.04 (0.61–1.79) | 0.875 | 1.20 (0.67–2.16) | 0.547 | |
| H/H | 47 (30.1) | 1.51 (0.97–2.36) | 0.069 | 1.60 (0.98–2.61) | 0.061 | |
| Trend. test | 119 (23.4) | 1.17 (1.01–1.36) | 0.041 | 1.18 (1.00–1.38) | 0.047 | |
| Group 1 | 50 (19.7) | [1] | [1] | |||
| Group 2 | 22 (22.2) | 1.14 (0.69–1.88) | 0.614 | 1.20 (0.69–2.06) | 0.520 | |
| Group 3 | 47 (30.1) | 1.65 (1.11–2.45) | 0.014 | 1.59 (1.03–2.46) | 0.034 | |
| Trend. test | 119 (23.4) | 1.28 (1.05–1.57) | 0.015 | 1.26 (1.02–1.57) | 0.036 | |
| All-cause death | ||||||
| L/L | 5 (3.2) | [1] | [1] | |||
| L/H | 4 (4.0) | 1.26 (0.34–4.68) | 0.734 | 1.95 (0.40–9.56) | 0.410 | |
| H/L | 4 (4.0) | 1.28 (0.34–4.78) | 0.709 | 3.14 (0.62–15.94) | 0.168 | |
| H/H | 11 (7.1) | 2.28 (0.79–6.57) | 0.126 | 2.55 (0.62–10.49) | 0.196 | |
| Trend. test | 24 (4.7) | 1.32 (0.93–1.85) | 0.117 | 1.30 (0.87–1.94) | 0.196 | |
| Group 1 | 9 (3.5) | [1] | [1] | |||
| Group 2 | 4 (4.0) | 1.17 (0.36–3.79) | 0.797 | 1.19 (0.34–4.15) | 0.786 | |
| Group 3 | 11 (7.1) | 2.07 (0.86–5.01) | 0.104 | 1.66 (0.60–4.59) | 0.330 | |
| Trend. test | 24 (4.7) | 1.45 (0.92–2.26) | 0.107 | 1.29 (0.77–2.15) | 0.327 | |
| reMI | ||||||
| L/L | 5 (3.2) | [1] | [1] | |||
| L/H | 2 (2.0) | 0.63 (0.12–3.25) | 0.582 | 0.78 (0.15–4.20) | 0.774 | |
| H/L | 8 (8.1) | 2.62 (0.86–8.01) | 0.091 | 3.30 (1.00–10.84) | 0.050 | |
| H/H | 8 (5.1) | 1.65 (0.54–5.06) | 0.377 | 1.89 (0.58–6.16) | 0.290 | |
| Trend. test | 23 (4.5) | 1.27 (0.90–1.79) | 0.178 | 1.33 (0.93–1.91) | 0.116 | |
| Group 1 | 7 (2.8) | [1] | [1] | |||
| Group 2 | 8 (8.1) | 3.06 (1.11–8.43) | 0.031 | 3.33 (1.18–9.42) | 0.023 | |
| Group 3 | 8 (5.1) | 1.93 (0.70–5.33) | 0.204 | 2.55 (0.90–7.22) | 0.078 | |
| Trend. test | 23 (4.5) | 1.37 (0.87–2.17) | 0.174 | 1.59 (0.99–2.55) | 0.057 | |
| reHF | ||||||
| L/L | 3 (1.9) | [1] | [1] | |||
| L/H | 2 (2.0) | 1.05 (0.18–6.29) | 0.957 | 3.39 (0.19–59.02) | 0.403 | |
| H/L | 2 (2.0) | 1.06 (0.18–6.34) | 0.950 | 0.23 (0.01–6.30) | 0.384 | |
| H/H | 4 (2.6) | 1.36 (0.30–6.07) | 0.689 | 0.19 (0.02–1.94) | 0.160 | |
| Trend. test | 11 (2.2) | 1.10 (0.68–1.80) | 0.692 | 0.54 (0.26–1.09) | 0.087 | |
| Group 1 | 5 (2.0) | [1] | [1] | |||
| Group 2 | 2 (2.0) | 1.04 (0.20–5.35) | 0.964 | 0.52 (0.06–4.44) | 0.551 | |
| Group 3 | 4 (2.6) | 1.33 (0.36–4.96) | 0.670 | 0.94 (0.16–5.49) | 0.943 | |
| Trend. test | 11 (2.2) | 1.15 (0.59–2.23) | 0.677 | 0.93 (0.38–2.31) | 0.883 | |
| Stroke | ||||||
| L/L | 5 (3.2) | [1] | [1] | |||
| L/H | 2 (2.0) | 0.62 (0.12–3.20) | 0.570 | 0.54 (0.1–3.00) | 0.484 | |
| H/L | 4 (4.0) | 1.28 (0.34–4.77) | 0.713 | 1.12 (0.26–4.75) | 0.880 | |
| H/H | 7 (4.5) | 1.45 (0.46–4.56) | 0.528 | 0.93 (0.23–3.74) | 0.914 | |
| Trend. test | 18 (3.5) | 1.18 (0.80–1.74) | 0.402 | 1.02 (0.64–1.62) | 0.946 | |
| Group 1 | 7 (2.8) | [1] | [1] | |||
| Group 2 | 4 (4.0) | 1.5 (0.44–5.13) | 0.516 | 1.34 (0.38–4.75) | 0.649 | |
| Group 3 | 7 (4.5) | 1.7 (0.60–4.85) | 0.321 | 1.13 (0.35–3.71) | 0.836 | |
| Trend. test | 18 (3.5) | 1.3 (0.78–2.18) | 0.314 | 1.07 (0.60–1.91) | 0.818 | |
| Revascularization | ||||||
| L/L | 21 (13.5) | [1] | [1] | |||
| L/H | 8 (8.1) | 0.58 (0.26–1.31) | 0.190 | 0.71 (0.31–1.64) | 0.429 | |
| H/L | 11 (11.1) | 0.80 (0.39–1.66) | 0.551 | 0.91 (0.41–2.01) | 0.823 | |
| H/H | 29 (18.6) | 1.42 (0.81–2.49) | 0.221 | 1.90 (1.05–3.45) | 0.035 | |
| Trend. test | 69 (13.6) | 1.16 (0.95–1.41) | 0.152 | 1.26 (1.03–1.55) | 0.026 | |
| Group 1 | 29 (11.4) | [1] | [1] | |||
| Group 2 | 11 (11.1) | 0.96 (0.48–1.92) | 0.911 | 1.12 (0.55–2.28) | 0.764 | |
| Group 3 | 29 (18.6) | 1.70 (1.02–2.85) | 0.042 | 2.21 (1.28–3.79) | 0.004 | |
| Trend. test | 69 (13.6) | 1.31 (1.00–1.70) | 0.048 | 1.48 (1.12–1.96) | 0.005 |
Patients were divided into four groups according to TMAO levels at the enrollment (V1) and follow-up visit (V2) relative to the median of each visit point (6.7 µmol/L and 12.7 µmol/L for V1 and V2, respectively). L/L, low V1 and low V2; L/H, low V1 and high V2; H/L, high V1 and low V2; H/H, high V1 and high V2. Group1: patients with TMAO levels below the median at V1; Group 2: patients with higher levels of TMAO at V1 and subsequently lower levels at V2; Group3: patients with both higher TMAO levels at V1 and V2. HR, hazard ratio; MACE, major adverse cardiovascular event; reHF, rehospitalization caused by heart failure; reMI, recurrent myocardial infarction; TMAO, trimethylamine-N-oxide. * Adjusted for the variables with p < 0.1 in the univariable models, including age, hypertension, diabetes, peripheral artery disease, chronic kidney disease, and previous history of stroke and MI, Killip II-IV, the Global Registry of Acute Coronary Events risk score, multiple vessels disease, percutaneous coronary intervention, and the peak value of cardiac troponin I and N-terminal pro B-type natriuretic peptide during hospitalization, as well as estimated glomerular filtration rate and left ventricular ejection fraction at V2.
We also grouped the 509 patients based on the median betaine, choline, and L-carnitine levels. As for choline, the median levels of V1 and V2 were 1.2 µmol/L and 1.7 µmol/L, respectively. These groups were comprised of L/L group (n = 147), L/H group (n = 107), H/L group (n = 107), and H/H group (n = 148). After adjusting for confounders and TMAO levels at V2, no statistical significance of MACE risk was seen in the L/H and H/L groups compared to the L/L group (HR 1.33, 95% CI: 0.74–2.39; p = 0.340 and HR 1.31, 95% CI: 0.74–2.31; p = 0.355, respectively; Figure 2D), while the H/H group showed an increased risk of MACE (adjusted HR 1.70, 95% CI: 1.01–2.86; p = 0.045; Figure 2D). However, there was no statistical significance in choline levels and individual endpoints (Supplemental Table S9). As for betaine and L-carnitine levels, no positive associations were obtained in composite and individual endpoints, and the details are shown in Supplemental Tables S10 and S11.
3.4. Decision Tree Analysis
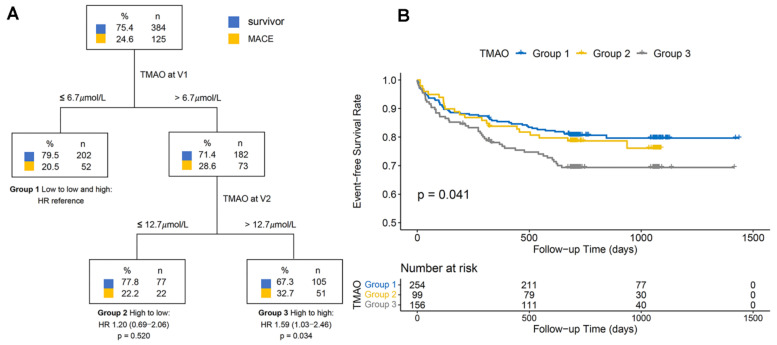
The decision tree analysis was performed to explore the TMAO levels at baseline and follow-up as biomarkers for risk stratification of MACE after V2 (Figure 3A). Classifying the patients according to the median TMAO levels at V1 and V2 created three risk groups and verified by the Kaplan–Meier analysis (Figure 3B) and multivariable Cox regression analysis (Table 3). The results revealed that patients who presented with high TMAO levels at V1 and low levels at V2 (Group 2, HR 1.20, 95% CI: 0.69–2.06; p = 0.520) had a similar risk for MACE to those with low TMAO levels at V1 (Group 1), whereas those who presented with high TMAO levels at V1 and V2 showed an increased MACE risk (Group 3, adjusted HR 1.59, 95% CI: 1.03–2.46; p = 0.034).
Figure 3.
Decision tree of risk stratification for major adverse cardiovascular events (MACE) using combined measurements at enrollment (V1) and follow-up visit (V2) for trimethylamine N-oxide (TMAO) (A). Kaplan–Meier curve for cumulative MACE-free survival in groups generated by decision tree (B). Decision tree using plasma TMAO level at V1 as the initial classifier, followed by plasma TMAO level at V2 enables effective selection of low- and high-risk groups of patients and increased cumulative event risk in Group 3 compared to Group 1. The number of events is shown below. Data are presented as adjusted hazard ratio (HR) and 95% confidence interval (CI). The adjusted factors included age, hypertension, diabetes, peripheral artery disease, chronic kidney disease, previous history of stroke and MI, Killip II-IV, the Global Registry of Acute Coronary Events risk score, multiple vessels disease, percutaneous coronary intervention, and the peak value of cardiac troponin I and N-terminal pro-B-type natriuretic peptide during hospitalization, as well as estimated glomerular filtration rate and left ventricular ejection fraction at V2.
However, the decision tree analysis could not be done based on the choline, betaine, and L-carnitine levels. In addition, we divided patients into three groups simply according to median choline levels at V1 and V2 without applying decision tree approach, namely Group 1 (patients with low choline levels at V1), Group 2 (high choline levels at V1 and subsequently low levels at V2) and Group 3 (high choline levels at V1 and V2). The Cox regression analysis (Supplemental Table S9) suggests that patients in Group 3 showed an increased risk of MACE (HR 1.55, 95% CI: 1.03–2.34; p = 0.034) compared to those in Group 1, whereas the difference diminished after adjusting for the confounders and TMAO levels of V2. Moreover, the Kaplan–Meier analysis (Supplemental Figure S6) shows no significant differences in MACE risk among groups (p = 0.100).
4. Discussion
This study set out to investigate the association of TMAO and its precursor (betaine, choline, and L-carnitine) levels with prognosis and the relationship between changes in these levels during follow-up and prognosis in a population with AMI. Firstly, the analysis in this study confirmed that TMAO levels at baseline were correlated to an increased risk of MACE, with an S-shaped relationship. Secondly, only patients with high TMAO levels at V1 and V2 showed an increase in MACE risk, while the risk of MACE in patients with high TMAO levels at V1 and low levels at V2 did not increase significantly. A similar condition was seen in choline levels. Changes in TMAO and choline levels during follow-up could indicate dynamic MACE risk.
4.1. Association between Levels of TMAO and Its Precursors and Prognosis
Substantial clinical studies have established that TMAO was strongly associated with poor outcomes in cardiovascular diseases [3,24]. Animal studies also have confirmed the causality between TMAO and atherosclerosis [25] and demonstrated that TMAO could accelerate atherosclerosis, thrombosis, cardiorenal fibrosis, and vascular inflammation through different pathways, resulting in the progression of CHD [26]. This study found that higher TMAO levels at baseline were independently correlated to an elevated MACE risk, as the same with the previous studies focused on acute coronary syndrome or stable coronary syndrome [7,27,28]. Thus, these findings implied that TMAO was a vital risk factor for cardiovascular events and measuring TMAO levels could help identify MACE risk in CHD patients.
Choline is critical in the process of lipid metabolism, cell membrane structure, and cholinergic neurotransmission [29]. As a dietary precursor of TMAO, choline is a risk factor for cardiovascular diseases and death. Betaine, a metabolite of choline, is also regarded as a potential risk factor for cardiovascular diseases. Wang et al. demonstrated that higher betaine (HR 1.33, 95% CI: 1.03–1.73; p < 0.05) and choline (HR 1.34, 95% CI: 1.03–1.74; p < 0.05) levels could each predict an elevated risk for cardiovascular events (including death, MI and stroke) after adjusted traditional cardiovascular risk factors in patients with stable coronary disease, while the addition of TMAO completely attenuated these association [13]. Yang et al. reported that plasma betaine levels were correlated to the disease severity among patients with pulmonary hypertension [30]. However, not all clinical studies of choline and betaine showed consistent results. The present study showed that high betaine and choline levels at baseline were not associated with an increased risk of MACE. Similarly, Trøseid et al. demonstrated that either choline or betaine was not associated with an increased risk of heart transplantation in patients with chronic HF [31]. As for another precursor of TMAO, L-carnitine plays a crucial role in lipid metabolism [32]. Koeth et al. implied a positive association of L-carnitine with poor prognosis among patients undergoing cardiac evaluation, while the association diminished after adjusting for TMAO levels [15]. However, the present study did not observe associations between L-carnitine levels at baseline and the risk of MACE. Regarding the inconsistent results of TMAO’s precursors and prognosis, a possible explanation may be that the study population and endpoints are different. Furthermore, the different approaches to measurement in studies may also influence the results. Although current studies would not draw a clear conclusion about this matter, these findings still remind us of the potential roles of choline, betaine, and L-carnitine in the risk management of cardiovascular diseases. More studies in this field are needed and may clarify the association between betaine, choline and L-carnitine and prognosis.
4.2. Association between Serial Levels of TMAO and Its Precursors and Prognosis
There were a few studies focused on serial levels of TMAO. Suzuki et al. conducted a study [11] of serial TMAO levels during a nine-month follow-up in patients with chronic HF. They demonstrated that patients with higher TMAO levels at both time points showed an elevated risk of all-cause death at 2 years (HR 2.10, 95% CI: 1.44–3.06; p < 0.001), whereas patients with higher TMAO levels at the first time and lower at the second time showed a similar risk of all-cause death at 2 years to those with lower TMAO levels at the first time. In addition, a study by Heianza et al. explored the association between ten-year changes in TMAO levels and CHD incidence in healthy women [10]. The results pointed out that long-term increases in TMAO levels could predict higher CHD risk (HR 1.79, 95% CI: 1.08–2.96; p = 0.023) compared to low TMAO levels at the first and second times [10]. Likewise, the present study found that TMAO levels at a follow-up of one month could help stratify the patients at high risk of MACE. The results indicated that patients with high TMAO levels at both time points had 1.59 times (95% CI: 1.03–2.46; p = 0.034) higher risk of MACE than those with low TMAO levels at V1, while the MACE risk of patients with high TMAO levels at V1 and low levels at V2 did not increase significantly compared to those with low TMAO levels at V1 (HR 1.20, 95% CI: 0.69–2.06; p = 0.520). However, the risk of MACE in the H/H group did not increase significantly compared to the L/L group; only the trend among the groups divided according to the respective median levels showed statistically significant (HR 1.17, 95% CI: 1.01–1.36; p for trend = 0.041). A possible explanation for this is that patients who did not complete the follow-up visit had a nearly two times higher mortality than those with follow-up data (8.6% vs. 4.7%, p = 0.011). In other words, the conditions of patients who completed the follow-up visit were relatively mild, implying a lower risk of MACE. Except for this, our earlier study observed that high TMAO levels were associated with more prevalence of culprit plaque rupture, which would result in high mortality [9]. This may partially explain the results of this study. In brief, these findings suggested that repeated assessment of TMAO levels during follow-up could predict changes in MACE risk.
Meanwhile, this study further explored whether the changes in levels of betaine, choline and L-carnitine were associated with prognosis. The results revealed that only those with higher choline levels at both time points were correlated to an increased risk of MACE compared to patients with lower levels at both time points (adjusted HR 1.70, 95% CI: 1.01–2.86; p = 0.045). Although these results were not very encouraging, this information could still remind us that repeated measurements of choline levels may be beneficial for long-term risk assessment in patients with AMI. Moreover, it is worth noting that this finding still needs to be verified in a large sample.
4.3. Metabolism and Function of TMAO and Its Precursors and Clinical Implications
TMA is generated in the gut from dietary betaine, choline, and other choline-containing compounds, and L-carnitine. These precursors are converted into TMA by various enzymes, such as betaine reductase, carnitine oxidoreductase, and choline TMA lyase [12]. Most TMA ingested or formed in the gut is rapidly absorbed into the portal circulation by passive diffusion and then oxidized to TMAO by hepatic flavin containing monooxygenases FMO3 and FMO1. Nearly 95% of TMA is oxidized and afterwards excreted in the urine in a 3:95 TMA: TMAO ratio within 24 h, only 4% is excreted in feces, and less than 1% is eliminated in the breath [33].
Numerous studies have investigated the mechanism of TMAO association with poor prognosis in cardiovascular diseases. Firstly, TMAO could suppress reverse cholesterol transport by modulating bile acid pool size and composition, accelerate foam cell formation by upregulating cluster of differentiation 36 and scavenger receptor A located on macrophages, and enhance uptake of oxidized low-density lipoprotein cholesterol [34,35]. Secondly, TMAO could activate inflammatory cascades via different pathways, such as mitogen-activated protein kinase signaling, nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 inflammasome, and nuclear factor-κB signaling pathway [36,37,38]. Furthermore, TMAO could enhance platelet reactivity through altering platelet calcium signaling and may diminish the anti-platelet effect [6,39]. These underlying mechanisms of TMAO might all contribute to the progression of cardiovascular diseases and imply that TMAO is a therapeutic target for cardiovascular diseases and deserves more attention and research. To date, some studies have found various methods to reduce TMAO levels. For example, ginkgolide B, an herbal component from Ginkgo biloba leaves, could inhibit the expression of flavin monooxygenase 3 (a hepatic enzyme in the transition from trimethylamine to TMAO) and then reduce TMAO levels [40]. Moreover, changing the intestinal microbiome’s composition also could reduce TMAO levels, including antibiotics, anti-diabetic treatment, and choline analogue [41,42,43]. However, the impact of reducing TMAO levels on the prognosis of cardiovascular disease remains unclear, and further studies are needed.
Except as a precursor of TMAO, choline is an essential nutrient for humans. Foods of animal origin, especially eggs and liver, are rich in choline; most foods we eat contain some amounts of choline or choline compounds. Choline has four primary metabolites: acetylcholine, betaine, phospholipids, and TMA [44]. Correspondingly, acetylcholine is the neurotransmitter and binds to receptors of the post-synaptic neuron in the central and peripheral nervous systems; phospholipids assure the structural integrity and signaling functions of cell membranes [44]. Betaine mainly comes from plant origins, such as spinach, beets and grains. It serves as an osmolyte and a methyl group donor and is involved in homocysteine methyltransferase reaction [29,45]. Moreover, phosphatidylcholine, synthesised from choline, is involved in very low-density lipoprotein production [44]. Studies also demonstrated that choline intake would lead to cholesterol and triglyceride transport from the liver to the vessels, causing cholesterol and triglyceride levels increased [46]. However, a study [47] reported that egg (a food rich in choline) intake could cause high-density lipoprotein cholesterol and choline concentrations to increase and no change in plasma low-density lipoprotein cholesterol or TMAO concentrations, which implied the complex association between choline and lipid metabolism. The specific interaction needs further investigation in the future.
L-carnitine, another precursor of TMAO, is found in animal products such as meat, fish, poultry, and milk. It could be transformed into betaine and γ-butyrobetaine [33]. First, L-carnitine plays a critical role in fat metabolism, transporting the activated long-chain fatty acids from the cytosol into the mitochondria and making them available for mitochondrial β-oxidation [48]. Second, carnitine may suppress the accumulation of lactic acid by reacting with acyl-coenzyme A to form acetyl-carnitine and coenzyme A, thereby enhancing high-intensity exercise performance [48]. Moreover, experimental data illustrated that carnitine administration could increase serum osteocalcin concentrations in animals, which suggested that carnitine might be helpful for the prevention and/or therapeutic treatment of osteoporosis and post-menopause syndrome [49]. Concerning the essential role of L-carnitine, enriching food types and maintaining normal L-carnitine levels is crucial.
5. Limitations
There were some limitations of this study. Firstly, we only assessed the levels of TMAO and its precursors before PCI and one month after admission. These two-time points did not perfectly reflect the changes in TMAO, betaine, choline, and L-carnitine levels. In addition, we did not collect the diet of these patients during follow-up. Secondly, we enrolled patients admitted to the emergency department with a relatively higher mortality risk. Attention should be paid to the characteristics of patients included in this study when interpreting and generalizing the results and conclusions. Thirdly, to some degree, the small number of patients who completed both visits resulted in fewer adverse events. Therefore, larger sample prospective studies on serial levels of TMAO, betaine, choline, and L-carnitine in different populations would help us establish greater accuracy on this matter.
6. Conclusions
Repeated assessment of TMAO and choline levels during follow-up could identify changes in MACE risk in patients with AMI. Serial increased levels of choline and TMAO at baseline and one-month follow-up each indicated an increased cardiovascular risk.
Abbreviations
| AMI | Acute Myocardial Infarction |
| CHD | Coronary Heart Disease |
| CIs | Confidence Intervals |
| CKD | Chronic Kidney Disease |
| HRs | Hazard Ratios |
| HF | Heart Failure |
| IQR | Interquartile Range |
| MACE | Major Adverse Cardiovascular Events |
| PCI | Percutaneous Coronary Intervention |
| RCS | Restricted Cubic Spline |
| TMAO | Trimethylamine N-oxide |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcdd9110380/s1. Figure S1: The flowchart of the patient selection process; Figure S2: Continuous hazard ratios across TMAO levels at enrollment for major adverse cardiovascular event (A), all-cause death (B), myocardial infarction (C), rehospitalization caused by heart failure (D), stroke (E), and revascularization (F); Figure S3: Kaplan-Meier curve for cumulative event-free survival in groups stratified by choline tertile levels at enrollment. A: major adverse cardiovascular event; B: all-cause death; C: myocardial infarction; D: rehospitalization caused by heart failure; E: stroke; F: revascularization; Figure S4: Kaplan-Meier curve for cumulative event-free survival in groups stratified by betaine tertile levels at enrollment. A: major adverse cardiovascular event; B: all-cause death; C: myocardial infarction; D: rehospitalization caused by heart failure; E: stroke; F: revascularization; Figure S5: Kaplan-Meier curve for cumulative event-free survival in groups stratified by L-carnitine tertile levels at enrollment. A: major adverse cardiovascular event; B: all-cause death; C: myocardial infarction; D: rehospitalization caused by heart failure; E: stroke; F: revascularization; Figure S6: Kaplan-Meier curve for cumulative MACE-free survival in groups stratified by choline levels at enrollment (V1) and follow-up visit (V2). Patients were divided into three groups according to the respective median levels of choline at V1 and V2, namely Group 1 (patients with low choline levels at V1), Group 2 (patients with high choline levels at V1 and subsequently low levels at V2) and Group 3 (patients with high choline levels at V1 and V2). MACE, major adverse cardiovascular event; Table S1: Patient characteristics according to the median and tertile levels of TMAO at enrollment; Table S2: Patient characteristics according to median and tertile levels of choline at enrollment; Table S3: Patient characteristics according to the median and tertile levels of choline at enrollment; Table S4: Patient characteristics according to the median and tertile levels of L-carnitine at enrollment; Table S5: Differences in characteristics at baseline between patients with and without TMAO measurements at follow-up visit; Table S6: Association between choline levels at enrollment and all endpoints; Table S7: Association between betaine levels at enrollment and all endpoints; Table S8: Association between L-carnitine levels at enrollment and all endpoints; Table S9: Association between choline levels of V1 and V2 and all endpoints; Table S10: Association between betaine levels of V1 and V2 and all endpoints; Table S11: Association between L-carnitine levels of V1 and V2 and all endpoints.
Author Contributions
N.L. and Y.W. contributed to data acquisition, data analysis, and wrote the manuscript. C.L., P.Z., L.S., H.Z., and H.Y. contributed to the study design and patient enrollment. N.L., Y.W., X.Z., R.C., J.Z., J.L., Y.C., and S.Y. contributed to the data acquisition. H.Z. and H.Y. reviewed and edited the intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Fuwai Hospital (protocol code is 2017-866 on 21 February 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
All authors declare no conflicts of interest.
Funding Statement
This study was supported by National Natural Science Foundation of China (No. 81970308), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016-I2M-1-009), Fund of “Sanming” Project of Medicine in Shenzhen (No. SZSM201911017), and Shenzhen Key Medical Discipline Construction Fund (No. SZXK001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomas M.S., Fernandez M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021;23:1–7. doi: 10.1007/s11883-021-00910-x. [DOI] [PubMed] [Google Scholar]
- 2.Li X.S., Obeid S., Klingenberg R., Gencer B., Mach F., Räber L., Windecker S., Rodondi N., Nanchen D., Muller O., et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017;38:814–824. doi: 10.1093/eurheartj/ehw582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guasti L., Galliazzo S., Molaro M., Visconti E., Pennella B., Gaudio G.V., Lupi A., Grandi A.M., Squizzato A. TMAO as a biomarker of cardiovascular events: A systematic review and meta-analysis. Intern. Emerg. Med. 2021;16:201–207. doi: 10.1007/s11739-020-02470-5. [DOI] [PubMed] [Google Scholar]
- 4.Schiattarella G.G., Sannino A., Toscano E., Giugliano G., Gargiulo G., Franzone A., Trimarco B., Esposito G., Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017;38:2948–2956. doi: 10.1093/eurheartj/ehx342. [DOI] [PubMed] [Google Scholar]
- 5.Wei H., Zhao M., Huang M., Li C., Gao J., Yu T., Zhang Q., Shen X., Ji L., Ni L., et al. FMO3-TMAO axis modulates the clinical outcome in chronic heart-failure patients with reduced ejection fraction: Evidence from an Asian population. Front. Med. 2022;2022 16:295–305. doi: 10.1007/s11684-021-0857-2. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W., Gregory J.C., Org E., Buffa J.A., Gupta N., Wang Z., Li L., Fu X., Wu Y., Mehrabian M., et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki T., Heaney L.M., Jones D.J.L., Ng L.L. Trimethylamine N-oxide and Risk Stratification after Acute Myocardial Infarction. Clin. Chem. 2017;63:420–428. doi: 10.1373/clinchem.2016.264853. [DOI] [PubMed] [Google Scholar]
- 8.Li N., Zhou J., Wang Y., Chen R., Li J., Zhao X., Zhou P., Liu C., Song L., Liao Z., et al. Association between trimethylamine N-oxide and prognosis of patients with acute myocardial infarction and heart failure. ESC Heart Fail. 2022 doi: 10.1002/ehf2.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Y., Sheng Z., Zhou P., Liu C., Zhao H., Song L., Li J., Zhou J., Chen Y., Wang L., et al. Plasma Trimethylamine N-Oxide as a Novel Biomarker for Plaque Rupture in Patients With ST-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2019;12:e007281. doi: 10.1161/CIRCINTERVENTIONS.118.007281. [DOI] [PubMed] [Google Scholar]
- 10.Heianza Y., Ma W., DiDonato J.A., Sun Q., Rimm E.B., Hu F.B., Rexrode K.M., Manson J.E., Qi L. Long-Term Changes in Gut Microbial Metabolite Trimethylamine N-Oxide and Coronary Heart Disease Risk. J. Am. Coll. Cardiol. 2020;75:763–772. doi: 10.1016/j.jacc.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T., Yazaki Y., Voors A.A., Jones D.J.L., Chan D.C.S., Anker S.D., Cleland J.G., Dickstein K., Filippatos G., Hillege H.L., et al. Association with outcomes and response to treatment of trimethylamine N-oxide in heart failure: Results from BIOSTAT-CHF. Eur. J. Heart Fail. 2019;21:877–886. doi: 10.1002/ejhf.1338. [DOI] [PubMed] [Google Scholar]
- 12.Janeiro M.H., Ramírez M.J., Milagro F.I., Martínez J.A., Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10:E1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z., Tang W.H.W., Buffa J.A., Fu X., Britt E.B., Koeth R.A., Levison B.S., Fan Y., Wu Y., Hazen S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014;35:904–910. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millard H.R., Musani S.K., Dibaba D.T., Talegawkar S.A., Taylor H.A., Tucker K.L., Bidulescu A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: The Jackson Heart Study. Eur. J. Nutr. 2018;57:51–60. doi: 10.1007/s00394-016-1296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer K.A., Shea J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2017;9:711. doi: 10.3390/nu9070711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 18.Collet J.P., Thiele H., Barbato E., Barthelemy O., Bauersachs J., Bhatt D.L., Dendale P., Dorobantu M., Edvardsen T., Folliguet T., et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 19.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 20.Bozkurt B., Coats A., Tsutsui H., Abdelhamid M., Adamopoulos S., Albert N., Anker S.D., Atherton J., Butler J., Drazenr M.H., et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021;27:387–413. doi: 10.1016/j.cardfail.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 21.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G., Coats A.J., Falk V., Gonzalez-Juanatey J.R., Harjola V.P., Jankowska E.A., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 22.Yu W., Xu C., Li G., Hong W., Zhou Z., Xiao C., Zhao Y., Cai Y., Huang M., Jin J. Simultaneous determination of trimethylamine N-oxide, choline, betaine by UPLC-MS/MS in human plasma: An application in acute stroke patients. J. Pharm. Biomed. Anal. 2018;152:179–187. doi: 10.1016/j.jpba.2018.01.049. [DOI] [PubMed] [Google Scholar]
- 23.Wang G., Zhao D., Chen H., Ding D., Kou L., Sun L., Hao C., Li X., Jia K., Kan Q., et al. Development and validation of a UPLC-MS/MS assay for the determination of gemcitabine and its L-carnitine ester derivative in rat plasma and its application in oral pharmacokinetics. Asian J. Pharm. Sci. 2017;12:478–485. doi: 10.1016/j.ajps.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farhangi M.A., Vajdi M. Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: Results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2020;60:2801–2823. doi: 10.1080/10408398.2020.1770199. [DOI] [PubMed] [Google Scholar]
- 25.Gregory J.C., Buffa J.A., Org E., Wang Z., Levison B.S., Zhu W., Wagner M.A., Bennett B.J., Li L., DiDonato J.A., et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 2015;290:5647–5660. doi: 10.1074/jbc.M114.618249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Witkowski M., Weeks T.L., Hazen S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lever M., George P.M., Slow S., Bellamy D., Young J.M., Ho M., McEntyre C.J., Elmslie J.L., Atkinson W., Molyneux S.L., et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS ONE. 2014;9:e114969. doi: 10.1371/journal.pone.0114969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ringel C., Dittrich J., Gaudl A., Schellong P., Beuchel C.F., Baber R., Beutner F., Teren A., Engel C., Wirkner K., et al. Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome. Atherosclerosis. 2021;335:62–67. doi: 10.1016/j.atherosclerosis.2021.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Zeisel S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y., Xu J., Zhou J., Xue J., Gao J., Li X., Sun B., Yang B., Liu Z., Zhao Z., et al. High Betaine and Dynamic Increase of Betaine Levels Are Both Associated With Poor Prognosis of Patients With Pulmonary Hypertension. Front. Cardiovasc. Med. 2022;9:852009. doi: 10.3389/fcvm.2022.852009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Troseid M., Ueland T., Hov J.R., Svardal A., Gregersen I., Dahl C.P., Aakhus S., Gude E., Bjorndal B., Halvorsen B., et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 32.Flanagan J.L., Simmons P.A., Vehige J., Willcox M.D., Garrett Q. Role of carnitine in disease. Nutr. Metab. 2010;7:30. doi: 10.1186/1743-7075-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisel S.H., Warrier M. Trimethylamine N-Oxide, the Microbiome, and Heart and Kidney Disease. Annu. Rev. Nutr. 2017;37:157–181. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- 34.Canyelles M., Tondo M., Cedo L., Farras M., Escola-Gil J.C., Blanco-Vaca F. Trimethylamine N-Oxide: A Link among Diet, Gut Microbiota, Gene Regulation of Liver and Intestine Cholesterol Homeostasis and HDL Function. Int. J. Mol. Sci. 2018;19:3228. doi: 10.3390/ijms19103228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vourakis M., Mayer G., Rousseau G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021;22:8074. doi: 10.3390/ijms22158074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L., Lusis A.J., Shih D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB. J. Am. Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S., Henderson A., Petriello M.C., Romano K.A., Gearing M., Miao J., Schell M., Sandoval-Espinola W.J., Tao J., Sha B., et al. Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell. Metab. 2019;30:1141–1151.e5. doi: 10.1016/j.cmet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Chen M.L., Zhu X.H., Ran L., Lang H.D., Yi L., Mi M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017;6:e006347. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W., Wang Z., Tang W.H.W., Hazen S.L. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation. 2017;135:1671–1673. doi: 10.1161/CIRCULATIONAHA.116.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv Z., Shan X., Tu Q., Wang J., Chen J., Yang Y. Ginkgolide B treatment regulated intestinal flora to improve high-fat diet induced atherosclerosis in ApoE(-/-) mice. Biomed. Pharm. 2021;134:111100. doi: 10.1016/j.biopha.2020.111100. [DOI] [PubMed] [Google Scholar]
- 41.Tang W.H., Wang Z., Levison B.S., Koeth R.A., Britt E.B., Fu X., Wu Y., Hazen S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xourgia E., Papazafiropoulou A., Papanas N., Melidonis A. Anti-diabetic treatment leads to changes in gut microbiome. Front. Biosci. Landmark Ed. 2019;24:688–699. doi: 10.2741/4743. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z., Roberts A.B., Buffa J.A., Levison B.S., Zhu W., Org E., Gu X., Huang Y., Zamanian-Daryoush M., Culley M.K., et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiedeman A.M., Barr S.I., Green T.J., Xu Z., Innis S.M., Kitts D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients. 2018;10:1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueland P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011;34:3–15. doi: 10.1007/s10545-010-9088-4. [DOI] [PubMed] [Google Scholar]
- 46.Rajaie S., Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: Review of epidemiological evidence. ARYA Atheroscler. 2011;7:78–86. [PMC free article] [PubMed] [Google Scholar]
- 47.DiMarco D.M., Missimer A., Murillo A.G., Lemos B.S., Malysheva O.V., Caudill M.A., Blesso C.N., Fernandez M.L. Intake of up to 3 Eggs/Day Increases HDL Cholesterol and Plasma Choline While Plasma Trimethylamine-N-oxide is Unchanged in a Healthy Population. Lipids. 2017;52:255–263. doi: 10.1007/s11745-017-4230-9. [DOI] [PubMed] [Google Scholar]
- 48.Pekala J., Patkowska-Sokola B., Bodkowski R., Amroz D., Nowakowski P., Lochynski S., Librowski T. L-carnitine--metabolic functions and meaning in humans life. Curr. Drug. Metab. 2011;12:667–678. doi: 10.2174/138920011796504536. [DOI] [PubMed] [Google Scholar]
- 49.Hooshmand S., Balakrishnan A., Clark R.M., Owen K.Q., Koo S.I., Arjmandi B.H. Dietary l-carnitine supplementation improves bone mineral density by suppressing bone turnover in aged ovariectomized rats. Phytomedicine. 2008;15:595–601. doi: 10.1016/j.phymed.2008.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.