Abstract
Previous studies suggested a role for adipokines in ageing and in several age-related diseases. The purpose of our study was to further elucidate adipokines involvement in neurodegeneration, investigating adiponectin, leptin and resistin in Alzheimer's disease (AD) and Frontotemporal Dementia (FTD). We enrolled for the study 70 subjects: 26 AD, 21 FTD, and 23 with other neurological (but not neurodegenerative) conditions (CTR, control group). According to a standardized protocol, we measured adipokines plasmatic levels, blood parameters of glucidic and lipidic metabolism, ESR, cerebrospinal fluid (CSF) markers of neurodegeneration (beta-amyloid, total-Tau, phosphorylated-Tau) and anthropometric parameters. In comparison with control group, we found lower resistin concentrations in patients with dementia, and in particular in AD (p < 0.001). In multivariate analysis, AD relative risk was reduced by resistin, when controlling for sex, age and anthropometric/metabolic parameters (RR = 0.71, P < 0.0001). Considering CSF biomarkers, we found a direct correlation between resistin and Aβ1-42 CSF concentration in patients (p < 0.001, r = 0.50). Lower resistin characterized AD patients in our study and AD, but not FTD, diagnosis risk was found to be inversely associated with resistin when controlling for confounders. We hypothesize that resistin-linked metabolic profile has to be reconsidered and further investigated in AD.
Keywords: Adipokines, Alzheimer's disease, Frontotemporal dementia, Adiponectin, Leptin, Resistin
Highlights
-
•
Adipose tissue has an endocrine function, releasing polypeptide hormones, the adipokines.
-
•
Impairment of adipokines circulating levels has been shown in neurodegenerative dementias.
-
•
We found lower resistin levels in Alzheimer's disease patients compared to control group.
-
•
Resistin plasmatic levels correlated with liquoral amyloid β1-42 concentrations in dementia patients.
-
•
Resistin could interact with amyloid β1-42 secretion and have a role in Alzheimer's disease pathogenesis.
Adipokines; Alzheimer's disease; Frontotemporal dementia; Adiponectin; Leptin; Resistin.
1. Introduction
Alzheimer's Disease (AD) and Frontotemporal Dementia (FTD) are frequent causes of cognitive decline, determining an enormous social burden in Western Societies [1]. Great scientific and economical efforts have been made in the last years towards the identification of more accurate and predictive biomarkers of neurodegeneration.
The pathogenic mechanisms underlying AD and FTD are still under investigation. Multiple, common processes are involved in both diseases, including protein misfolding, neuroinflammation, and impaired autophagy. In addition, in AD several studies showed a complex association between the disease and insulin resistance.
Adipose tissue has been historically considered only as a deposit of energy. Adipose tissue is now known to be an endocrine and secretory organ producing and releasing a variety of proinflammatory and anti-inflammatory factors, the so called adipocytokines or adipokines [2]. Although over 50 adipokines have been identified, the most well-known adipokines include adiponectin, leptin, resistin, visfatin, vaspin, and chemerin [3]. Adipokines play an important role in several physiological functions, including vascular tone, inflammation, weight, immune response, endothelial function, and insulin resistance [4].
Recent studies showed that adipokines could be involved in the pathogenesis of Alzheimer's disease and other types of dementia [5, 6]. Preliminary studies investigating the association between adipokines and AD found increased serum adiponectin levels [7, 8]. Contrariwise, further studies evaluating adiponectin concentrations have been inconclusive, showing no differences between patients with mild cognitive impairment (MCI), AD, or Vascular Dementia (VaD), and controls [9, 10, 11]. Some case–control studies showed reduced levels of circulating leptin in patients with AD [12, 13], but additional studies did not find any differences between AD and controls [9, 14]. Very recently, preliminary studies suggested an involvement of resistin in patients with AD, and in patients with FTD due to mutation of the progranulin gene [15].
Therefore, the aims of this study were to evaluate plasma concentrations of adiponectin, leptin, and resistin in patients with neurodegenerative disorders like AD and FTD, and to correlate the concentrations of these peptides with metabolic and insulin resistance parameters and CSF biomarkers of neurodegeneration.
2. Materials and methods
We examined a total of 70 subjects evaluated at the Department of Neuroscience and Mental Health of University Hospital “Città della Salute e della Scienza” of Torino.
Approval for this study was obtained from “Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino–A.O. Ordine Mauriziano–A.S.L. Città di Torino”.
Twenty-six patients with probable AD (male/female 10/16, mean age ±standard deviation 68.19 ± 8.46 years) and twenty-one patients with probable behavioral variant FTD were enrolled (male/female 10/11, mean age ± standard deviation 69.05 ± 7.37 years). Diagnosis of probable AD has been made according to NIA-AA (National Institute of Aging–Alzheimer Association) criteria [16]. Diagnosis of probable FTD has been made according to Rascovsky Criteria [17].
As a control group, CSF of 23 cognitive-spared patients (male/female 10/13, mean age ± standard deviation 63 ± 9.31 years) with other neurological (not neurodegenerative nor inflammatory) conditions was analyzed.
Comorbidities, information about neurodegenerative diseases in family members and onset symptoms were investigated, as well as neurological and neuropsychological examination.
All subjects involved in the study were of Caucasian origin. The demographic and clinical characteristics are summarized in Table 1.
Table 1.
Demographic data and clinical characteristics of patients with dementia and controls. P values <0.05 are in bold. P values in parentheses were obtained after FDR correction for multiple comparisons.
| AD | FTD | CONTROLS | p value patients vs controls | p value AD vs controls | p value FTD vs controls | p value AD vs FTD | |
|---|---|---|---|---|---|---|---|
| Subjects | 26 | 21 | 23 | _ | _ | _ | _ |
| Sex (F/M) | 16/10 | 11/10 | 13/10 | 1.00 | 0.78 | 1.00 | 0.57 |
| Age (Years) | 68.19 ± 8.45 | 69.05 ± 7.37 | 63.00 ± 9.31 | <0.01 (0.20) | 0.03 (0.30) | 0.02 (0.30) | 0.71 |
| BMI | 24.18 ± 3.64 | 25.44 ± 3.81 | 23.78 ± 2.69 | 0.27 | 0.66 | 0.10 | 0.27 |
Fasting plasmatic levels of total adiponectin, resistin, and leptin were analyzed using commercially available enzyme linked immunosorbent assay (ELISA) kits (Biovendor, Oxfordshire, UK). To quantify the adiponectin levels, the kit used had a limit of detection of 26 ng/ml, an interassay coefficient of variation (CV) of 6.7% and an intra-assay CV of 4.9%. The kit to measure leptin concentrations showed a limit of detection of 0.2 ng/ml, an interassay CV of 5.6% and an intra-assay CV of 5.9%, whereas the kit for resistin had a limit of detection of 0.012 ng/ml, an interassay CV of 7.6% and an intra-assay CV of 5.9%.
L/A ratio was calculated for each subject and the descriptive statistics was resumed in Table 2 for each study group.
Table 2.
Biochemical parameters of patients with dementia and controls. Values are expressed as mean ± standard deviation (SD). P values <0.05 are in bold. P values in parentheses were obtained after adjusting for sex, age, BMI, HOMA-IR, and other adipokines. NA: not available. P values in square brackets were obtained after FDR correction for multiple comparisons.
| AD | FTD | CONTROLS | p value patients vs controls | p value AD vs controls | p value FTD vs controls | p value AD vs FTD | |
|---|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 203.72 ± 45.42 | 188.89 ± 32.89 | 217.38 ± 37.55 | 0.22 | 0.42 | 0.03 [0.30] | 0.15 |
| HDL-cholesterol (mg/dL) | 65.45 ± 19.14 | 60.85 ± 14.33 | 63.54 ± 22.57 | 0.93 | 0.86 | 0.72 | 0.53 |
| Triglycerides (mg/dL) | 104.88 ± 47.80 | 98.11 ± 54.97 | 125.15 ± 100.24 | 0.55 | 0.78 | 0.40 | 0.49 |
| Fasting Insulin (microU/mL) | 7.05 ± 11.38 | 4.09 ± 3.35 | 4.09 ± 3.19 | 0.40 | 0.41 | 0.55 | 0.93 |
| Fasting Glucose (mg/dL) | 88.46 ± 11.58 | 92.05 ± 8.67 | 91.87 ± 11.53 | 0.72 | 0.38 | 0.71 | 0.14 |
| HOMA-IR | 1.77 ± 3.49 | 0.91 ± 0.88 | 0.97 ± 0.81 | 0.84 | 0.98 | 0.73 | 0.90 |
| ESR (mm/h) | 15.05 ± 17.21 | 8.94 ± 7.81 | 9.42 ± 8.24 | 0.67 | 0.41 | 0.85 | 0.22 |
| Adiponectin (μg/mL) | 14.34 ± 13.53 | 9.69 ± 7.01 | 10.19 ± 7.38 | 0.73 (0.33) | 0.44 (0.17) | 0.81 (0.86) | 0.34 (0.42) |
| Leptin (ng/mL) | 18.07 ± 17.52 | 16.12 ± 16.60 | 14.10 ± 10.96 | 0.36 (0.36) | 0.70 (0.19) | 0.91 (0.87) | 0.72 (0.26) |
| Leptin/Adiponectin ratio | 2.57 ± 3.31 | 2.64 ± 3.76 | 2.63 ± 4.19 | 0.68 (0.41) | 0.61 (0.28) | 0.87 (0.78) | 0.66 (0.35) |
| Resistin (ng/mL) | 10.21 ± 4.66 | 14.10 ± 8.40 | 13.58 ± 4.82 | 0.04 [0.34] (0.04) | <0.001 [0.03] (<0.01) | 0.38 (0.47) | 0.13 (0.31) |
| Aβ1-42 (pg/mL) | 402.38 ± 128.10 | 836.56 ± 305.7 | NA | NA | NA | NA | <0.0001 [0.006] |
| T-tau (pg/mL) | 223.98 ± 194.90 | 135.06 ± 104.15 | NA | NA | NA | NA | 0.21 |
| P-tau (pg/mL) | 64.93 ± 48.74 | 57.10 ± 54.90 | NA | NA | NA | NA | 0.36 |
Blood levels of fasting serum metabolite concentrations (glucose, insulin, total cholesterol, HDL-C, and triglycerides) and ERS were also measured using standard laboratory protocols (Table 2). Low-density lipoprotein-cholesterol (LDL-C) was estimated by Friedewald equation. The index of insulin sensitivity Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated as follows: HOMA-IR (Fasting serum insulin (mIU/L) × Fasting blood glucose (mmol/L))/22.5.
All patients underwent lumbar puncture with measurement of CSF levels of Aβ1-42, total tau, and phosphorylated tau, and these were evaluated by ELISA method with Innotest Kits (Fujirebio).
2.1. Statistical methods
D'Agostino-Pearson's test was used to assess normality of included variables. Quantitative variables were described as mean and standard deviation while categorical ones as count. Means were compared through Student's t-test or Mann-Whitney test depending on variables distribution. ANOVA or Kruskal-Wallis tests were used to compare at the same time variables derived from all three studied groups (CTR, AD and FTD). To correct p values obtained from multiple comparisons between groups, we used Benjamini and Hochberg method. We considered acceptable a false discovery rate (FDR) < 0.05. Quantitative variables were correlated through Pearson or Spearman correlation as opportune. Multivariate generalized linear models (GLMs) were performed to estimate the quantitative relationship between adipokines plasma concentration and other variables. Diagnosis (AD and FTD vs CTR) as outcome variable was studied through multinomial logistic regression considering adipokines levels as predictors and controlling for other variables. Preliminar check for multicollinearity was carried out before running multivariate models. All analyses were run with R software (www.r-project.org). The level of statistical significance was defined at p < 0.05.
3. Results
3.1. Clinical and biochemical characteristics of the study groups
No difference in sex, anthropometric and metabolic parameters and erythrocyte sedimentation rate (ERS) were found between cases and controls (Table 1 and Table 2). Control subjects were younger than patients (Table 1). As expected, Amyloid β1-42 (Aβ1-42) CSF concentrations were significantly lower in AD group in respect to FTD patients (Table 2).
Concerning gender differences, serum high-density lipoprotein cholesterol (HDL-C) concentrations were higher in females, as expected, together with total cholesterol levels, while there was no difference in mean body mass index (BMI, data not shown). In the overall population, we found higher leptin and adiponectin plasmatic concentrations in females when compared to males (p < 0.0001 and p = 0.02, respectively), concordantly with previous evidences [18].
3.2. Adipokines plasmatic concentrations in patients and controls
3.2.1. Adiponectin
Crude total adiponectin levels were not different among patients with AD and FTD in comparison with controls, also after adjusting for sex, age, BMI, HOMA-IR, and other adipokines (Table 2).
3.2.2. Leptin
No significant difference in leptin concentrations was found between overall cases and controls, also after adjusting for confounding variables (sex, age, BMI, HOMA-IR, and other adipokines). In the subgroup analysis, after correction for confounding variables (as above), leptin levels were found to be significantly increased in female patients with Alzheimer's disease in respect to female controls (p = 0.033, data not shown). No significant difference was found when AD vs FTD patients were compared (Table 2).
3.2.3. Leptin to adiponectin ratio (L/A ratio)
Leptin to adiponectin ratio was not significantly different between study groups, also after adjusting for sex, age, BMI, HOMA-IR and resistin (Table 2).
3.2.4. Resistin
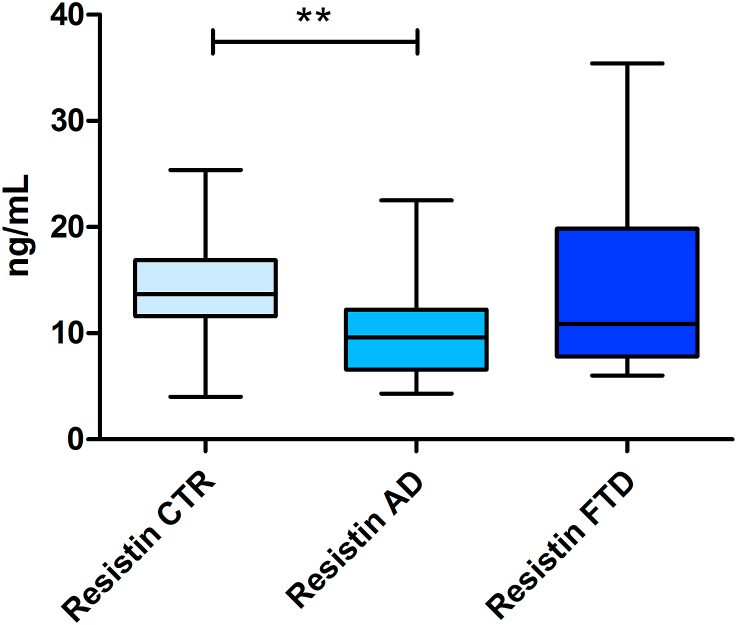
Resistin concentrations were found to be significantly different between study groups (p = 0.033). In particular, plasmatic values were lower in AD group than in control group (10.21 ± 4.66 ng/mL vs 13.58 ± 4.82 ng/mL, p < 0.01), also after adjusting for sex, age, BMI, HOMA-IR, and other adipokines (Table 2). No significant differences were found when comparing FTD patients to AD patients and controls (Figure 1).
Figure 1.
Resistin plasmatic concentrations in study groups. Box plot represents median, interquartile range and range (maximum–minimum) of resistin plasmatic concentration (ng/mL) of patients with AD, patients with FTD, and controls (CTR). ∗∗ (p < 0.01).
3.3. Correlations of adipokines with metabolic parameters in patients with dementia and controls
3.3.1. Adiponectin
In FTD patients, the adiponectin levels were negatively associated with fasting glucose (r = −0.54, p = 0.014). No further correlations between adiponectin levels and the other biochemical parameters were found.
3.3.2. Leptin
Considering all study groups, leptin directly correlated with HOMA-IR (p < 0.01, r = 0.31). As regards dementia patients, in AD we found a positive correlation between leptin and triglycerides (r = 0.518, p = 0.008). In FTD patients, a positive correlation between leptin and insulin (r = 0.506, p = 0.023), and between leptin and HOMA-IR (r = 0.524, p = 0.015) were found.
3.3.3. Leptin to adiponectin ratio
In the overall population, L/A ratio showed a direct correlation with triglycerides (p < 0.01, r = 0.34). As regards AD patients, L/A ratio directly correlated with BMI (p < 0.001, r = 0.62). Stronger correlations were found in FTD group with triglycerides, fasting insulin and HOMA-IR (p < 0.0001 and r > 0.8 in all three correlations). Moreover, L/A ratio correlated also with resistin in FTD group (p = 0.001, r = 0.65).
3.3.4. Resistin
As expected, resistin values correlated with indicators of overweight and obesity, i.e. body weight and BMI in all subjects included in our study (p < 0.01 and p = 0.01 respectively, r = 0.31 in both correlations). This relationship was even stronger in AD group (p < 0.001, r = 0.7 for both weight and BMI), while it was absent when considering FTD patients. Nonetheless, in this latter group, the resistin levels correlated with glucose (r = 0.522, p = 0.018), insulin (r = 0.522, p = 0.018), and HOMAIR (r = 0.563, p = 0.008).
3.4. Correlations between adipokines and CSF neurodegeneration biomarkers
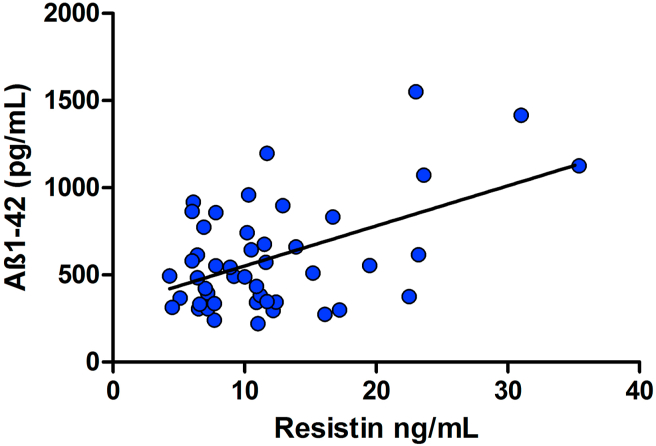
There was a significant correlation between resistin and Aβ1-42 in demented patients (p < 0.001, r = 0.50) (Figure 2). A similar result was found in the FTD group both concerning leptin and resistin in correlation with Aβ1-42 (p = 0.02 and r = 0.51; p < 0.01 and r = 0.57 respectively). L/A ratio showed a weak correlation with Aβ1-42 CSF concentrations in overall patients (p = 0.049, r = 0.29).
Figure 2.
Resistin values correlate with Aβ1-42 CSF concentrations in AD and FTD patients. Scatter plot represents the correlation between resistin plasmatic and Aβ1-42 CSF concentrations in patients (AD and FTD). The graphed line approximates the corresponding regression line (slope coefficient ±standard deviation: 23.0 ± 5.90).
3.5. Multivariate analysis
To gain further insights into adipokines role in this context, we performed a multivariate analysis primarily aimed to control for various potential confounders (adipokines-correlated factors such as gender, BMI and insulin resistance parameters as shown above).
Using this approach, we did not find any significant association between adipokines and diagnostic factors (neither AD nor FTD or both considered together) when controlling for sex, age, anthropometric/metabolic parameters (BMI, HDL-C, LDL-C, ESR, HOMA-IR), CSF markers (T-tau and Aβ1-42), and other adipokines. Other associations resulted significant as follows.
3.5.1. Adiponectin
Male gender was found to be an independent predictor of lower adiponectin values (p = 0.02), confirming gender difference shown by univariate approach. There was a significant inverse association with T-tau CSF levels (p = 0.01).
3.5.2. Leptin
We confirmed leptin values dependence from gender (negative association with male gender, p = 0.01), and positive association with HOMA-IR (p = 0.002).
3.5.3. Leptin to adiponectin ratio
L/A ratio was directly associated with age (p = 0.01), while inverse associations were found with HDL (p = 0.04) and ESR (p = 0.02).
3.5.4. Resistin
As regards resistin, while the diagnostic factor did not emerge as an independent explanatory variable, the only significant predictor (p = 0.01) was Aβ1-42 CSF concentration (0.012 estimated coefficient) controlling for all other above cited variables. Moreover, using multinomial logistic regression, adipokines levels were studied as predictors of dementia diagnosis (AD or FTD) compared to CTR as the reference group, controlling for sex, age and anthropometric/metabolic parameters (BMI, HDL-C, LDL-C, ESR, HOMA-IR). Using this approach, we estimated that the risk for AD was reduced by increased resistin (RR ratio 0.71, p < 0.0001), controlling for all other variables.
4. Discussion
Our study investigated the involvement of several adipokines in patients with Alzheimer's disease and Frontemporal dementia and provided some interesting findings. We found that plasmatic concentrations of resistin were significantly reduced in patients with Alzheimer's disease. A significant correlation between plasmatic resistin concentrations and CSF beta-amyloid was found in AD patients. Taken together, our data suggest a role for resistin in AD pathogenic mechanisms.
The results of our study are not in accord with the study of Kizilarslanoğlu et al, showing increased plasmatic resistin concentrations in AD [19]. A further study did not show any differences in resistin serum concentrations in AD patients in respect to controls but found increased resistin levels in dementia with vascular changes [20]. Several factors, like the different diagnostic criteria, the lack of controlling for known confounding variables such as BMI and insulin resistance parameters and the absence of CSF biomarkers, may explain the discrepancies between the two studies. Our data, on the contrary, confirm the relationship between CSF Aβ1-42 and resistin, suggesting that the concentrations of the two peptides covary both in the central nervous system and in the plasma. A previous work described a direct strong correlation between resistin and Aβ1-42 CSF values in a large Alzheimer's disease cohort [21]. Furthermore, we confirm relationship between different adipokines and the measures of insulin resistance.
Resistin is a small secreted protein playing a pivotal role in various metabolic, inflammatory, and autoimmune diseases, and it is mainly secreted by adipose tissue macrophages [22]. The resistin gene is located at 19p13.3, near the insulin receptor gene, and encodes a 12.5 kDa (108 amino acids) cysteine-rich polypeptide. The mechanisms by which resistin exerts its biological effects in humans are only partially understood. A link between the peptide and the adenylyl cyclase associated protein 1 (CAP1) as well as Toll-like receptor 4 (TLR-4) was suggested [23, 24]. Several intracellular signaling cascades are triggered by resistin: NF-kB signaling via activation of PI3K/AKT [25], the adenylate cyclase, cAMP, protein kinase A cascade, and the MAP kinase system [26]. Within the CNS, resistin production was observed in the hypothalamus, where resistin appears to modulate feeding behavior and energy intake [27], and inhibits the release of hypothalamic neuropeptides [28].
Different mechanisms may explain the involvement of resistin in AD pathogenesis. Inflammation is considered to play an important role in the disease, involving several pro-inflammatory and anti-inflammatory mediators [19, 29]. Previous findings has suggested that resistin may exert several pro-inflammatory properties, promoting the secretion of tumor necrosis factor (TNF)-α and interleukin (IL)-1β, -6, -8, and -12, and the generation of reactive oxygen species (ROS) [30]. An alternative explanation is related to insulin metabolism. Resistin counteracts the effects of insulin, decreasing glucose intake in adipocytes, muscle cells, and other tissues. Several studies have shown that insulin signaling is impaired in AD brains due to insulin resistance, ultimately resulting in the formation of neurofibrillary tangles (NFTs) [31]. Reduced resistin concentrations may further impair glucose metabolism in AD patients. Studies in experimental animals have shown that resistin clearly reduces glucose uptake, glycolytic rate, and ATP production in the hippocampus, a key region in AD pathogenesis [32]. In addition, resistin regulates hypothalamic functioning through insulin signaling modulation [24]. In particular, it modulates the phosphorylation (i.e. activation) of a key regulator of this pathway, AKT. At the same time, AKT hyperphosphorylation was found to be correlated with ex vivo brain amyloid burden and to a reduced cognitive function [33]. This finding seems to be coherent with the observation of a crosstalk between insulin CNS signaling and beta-secretase activity in beta-amyloid deposition [34]. Thus, resistin could exert an effect on central insulin resistance and collaterally on amyloid pathology by interfering with the same intracellular signaling pathway. Moreover, brain insulin sensitivity is linked to a reduced visceral fat accumulation and, in fact, to reduced resistin secretion. As a consequence, lower circulating resistin levels in AD patients could autogenerate a metabolic peripheral-central loop characterized by high central insulin sensitivity and low peripheral fat accumulation [35]. The central node of this loop is sustained by the same AKT hyperphosphorylation which could be linked to increased amyloid neuropathology. Finally, resistin can also lead to mitochondrial dysfunction. Physiologically, resistin stimulates mitochondrial metabolism, and a reduction in resistin signaling may alter mitochondrial transmembrane potentials, leading to irreversible mitochondrial damage.
In our study we found also that plasmatic leptin concentrations positively correlated with HOMA-IR, confirming a relationship with insulin resistance as previously described [36]. In particular, in our FTD patients, positive correlations between leptin and insulin, and between leptin and HOMA-IR were found, suggesting a condition of leptin resistance in this disease.
In conclusion, our study provides additional data concerning an involvement of resistin signaling in patients with Alzheimer's disease. Additional studies are needed in order to better elucidate the precise mechanisms related to resistin signaling impairment in the disease.
Declarations
Author contribution statement
Andrea Marcinnò: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Erica Gallo, Fausto Roveta, Alberto Grassini: Analyzed and interpreted the data; Wrote the paper.
Silvia Boschi: Performed the experiments; Analyzed and interpreted the data.
Innocenzo Rainero: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Elisa Rubino: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank the patients who participated in the study.
References
- 1.Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C.P. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115(5):911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Blüher M., Mantzoros C.S. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64(1):131–145. doi: 10.1016/j.metabol.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.S., Chung J.H., Choi T.K., Suh S.Y., Oh B.H., Hong C.H. Peripheral cytokines and chemokines in Alzheimer’s disease. Dement. Geriatr. Cognit. Disord. 2009;28(4):281–287. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 6.Arnoldussen I., Gustafson D.R., Leijsen E., de Leeuw F.E., Kiliaan A.J. Adiposity is related to cerebrovascular and brain volumetry outcomes in the RUN DMC study. Neurology. 2019;93(9):e864–e878. doi: 10.1212/WNL.0000000000008002. [DOI] [PubMed] [Google Scholar]
- 7.Van Himbergen T.M., Beiser A.S., Ai M., Seshadri S., Otokozawa S., Au R., Thongtang N., Wolf P.A., Schaefer E.J. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch. Neurol. 2012;69(5):594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khemka V.K., Bagchi D., Bandyopadhyay K., Bir A., Chattopadhyay M., Biswas A., Basu D., Chakrabarti S. Altered serum levels of adipokines and insulin in probable Alzheimer's disease. J Alzheimers Dis. 2014;41(2):525–533. doi: 10.3233/JAD-140006. [DOI] [PubMed] [Google Scholar]
- 9.Warren M.W., Hynan L.S., Weiner M.F. Lipids and adipokines as risk factors for Alzheimer's disease. J Alzheimers Dis. 2012;29(1):151–157. doi: 10.3233/JAD-2012-111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dukic L., Simundic A.M., Martinic-Popovic I., Kackov S., Diamandis A., Begcevic I., Diamandis E.P. The role of human kallikrein 6, clusterin and adiponectin as potential blood biomarkers of dementia. Clin. Biochem. 2016;49(3):213–218. doi: 10.1016/j.clinbiochem.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Bigalke B., Schreitmüller B., Sopova K., Paul A., Stransky E., Gawaz M., Stellos K., Laske C. Adipocytokines and CD34+ progenitor cells in Alzheimer's disease. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0020286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieb W., Beiser A.S., Vasan R.S., Tan Z.S., Au R., Harris T.B., Roubenoff R., Auerbach S., DeCarli C., Wolf P.A., Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302(23):2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baranowska-Bik A., Bik W., Styczynska M., Chodakowska-Zebrowska M., Barcikowska M., Wolinska-Witort E., Kalisz M., Martynska L., Baranowska B. Plasma leptin levels and free leptin index in women with Alzheimer's disease. Neuropeptides. 2015;52:73–78. doi: 10.1016/j.npep.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Teunissen C.E., van der Flier W.M., Scheltens P., Duits A., Wijnstok N., Nijpels G., Dekker J.M., Blankenstein R.M., Heijboer A.C. Serum leptin is not altered nor related to cognitive decline in Alzheimer's disease. J. Alzheimers Dis. 2015;44(3):809–813. doi: 10.3233/JAD-141503. [DOI] [PubMed] [Google Scholar]
- 15.Zanardini R., Benussi L., Fostinelli S., Saraceno C., Ciani M., Borroni B., Padovani A., Binetti G., Ghidoni R. Serum C-peptide, visfatin, resistin, and ghrelin are altered in sporadic and GRN-associated frontotemporal lobar degeneration. J. Alzheimers Dis 2018. 2018;61(3):1053–1060. doi: 10.3233/JAD-170747. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., Mohs R.C., Morris J.C., Rossor M.N., Scheltens P., Carrillo M.C., Thies B., Sandra Weintraub S., Phelps C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G.P., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancuso P., Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 2019:10. doi: 10.3389/fendo.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kizilarslanoğlu M.C., Kara Ö., Yeşil Y., Kuyumcu M.E., Öztürk Z.A., Cankurtaran M., Rahatli S., Pakaştiçali N., Çinar E., Halil M.G., Sener B., Cankurtaran E.S., Arioğul S. Alzheimer disease, inflammation, and novel inflammatory marker: resistin. Turk. J. Med. Sci. 2015;45(5):1040–1046. [PubMed] [Google Scholar]
- 20.Bednarska-Makaruk M., Graban A., Wiśniewska A., Łojkowska W., Bochyńska A., Gugała-Iwaniuk M., Sławińska K., Ługowska A., Ryglewicz D., Wehr H. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology. 2017;18(4):561–580. doi: 10.1007/s10522-017-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung Y.Y., Toledo J.B., Nefedov A., Polikar R., Raghavan N., Xie S.X., Farnum M., Schultz T., Baek Y., Deerlin V.V., Hu W.T., Holtzman D.M., Fagan A.M., Perrin R.J., Grossman M., Soares H.D., Kling M.A., Mailman M., Arnold S.E., Narayan V.A., Lee V.M., Shaw L.M., Baker D., Wittenberg G.M., Trojanowski J.Q., Wang L.S. Identifying amyloid pathology-related cerebrospinal fluid biomarkers for Alzheimer's disease in a multicohort study. Alzheimers Dement (Amst) 2015;1(3):339–348. doi: 10.1016/j.dadm.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landecho M.F., Tuero C., Valentí V., Bilbao I., de la Higuera M., Frühbeck G. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. Nutrients. 2019;11(11):2664. doi: 10.3390/nu11112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S., Lee H.C., Kwon Y.W., Lee S.E., Cho Y., Kim J., Lee S., Kim J.Y., Lee J., Yang H.M., Mook-Jung I., Nam K.Y., Chung J., Lazar M.A., Kim H.S. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metabol. 2014;19(3):484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benomar Y., Gertler A., De Lacy P., Crépin D., Ould Hamouda H., Riffault L., Taouis M. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62(1):102–114. doi: 10.2337/db12-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Pacheco F., Martinez-Fuentes A.J., Tovar S., Pinilla L., Tena-Sempere M., Dieguez C., Castaño J.P., Malagon M.M. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148(1):401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez-Pacheco F., Vázquez-Martínez R., Martínez-Fuentes A.J., Pulido M.R., Gahete M.D., Vaudry H., Gracia-Navarro F., Diéguez C., Castaño J.P., Malagón M.M. Resistin regulates pituitary somatotrope cell function through the activation of multiple signaling pathways. Endocrinology. 2009;150(10):4643–4652. doi: 10.1210/en.2009-0116. [DOI] [PubMed] [Google Scholar]
- 27.Vázquez M.J., González C.R., Varela L., Lage R., Tovar S., Sangiao-Alvarellos S., Williams L.M., Vidal-Puig A., Nogueiras R., López M., Diéguez C. Central resistin regulates hypothalamic and peripheral lipid metabolism in a nutritional-dependent fashion. Endocrinology. 2008;149(9):4534–4543. doi: 10.1210/en.2007-1708. [DOI] [PubMed] [Google Scholar]
- 28.Brunetti L., Orlando G., Recinella L., Michelotto B., Ferrante C., Vacca M. Resistin, but not adiponectin, inhibits dopamine and norepinephrine release in the hypothalamus. Eur. J. Pharmacol. 2004;493(1-3):41–44. doi: 10.1016/j.ejphar.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Sharma M., Fitzpatrick A.L., Arnold A.M., Chi G., Lopez O.L., Jenny N.S., DeKosky S.T. Inflammatory biomarkers and cognitive decline: the ginkgo evaluation of memory study. J. Am. Geriatr. Soc. 2016;64(6):1171–1177. doi: 10.1111/jgs.14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silswal N., Singh A.K., Aruna B., Mukhopadhyay S., Ghosh S., Ehtesham N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem. Biophys. Res. Commun. 2005;334(4):1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 31.Rad S.K., Arya A., Karimian H., Madhavan P., Rizwan F., Koshy S., Prabhu G. Mechanism involved in insulin resistance via accumulation of β-amyloid and neurofibrillary tangles: link between type 2 diabetes and Alzheimer's disease. Drug Des. Dev. Ther. 2018;12:3999–4021. doi: 10.2147/DDDT.S173970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cisternas P., Martinez M., Ahima R.S., William Wong G., Inestrosa N.C. Modulation of glucose metabolism in hippocampal neurons by adiponectin and resistin. Mol. Neurobiol. 2019;56(4):3024–3037. doi: 10.1007/s12035-018-1271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arvanitakis Z., Wang H.Y., Capuano A.W., Khan A., Taïb B., Anokye-Danso F., Schneider J.A., Bennett D.A., Ahima R.S., Arnold S.E. Brain insulin signaling, alzheimer disease pathology, and cognitive function. Ann. Neurol. 2020;88(3):513–525. doi: 10.1002/ana.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jantrapirom S., Nimlamool W., Chattipakorn N., Chattipakorn S., Temviriyanukul P., Inthachat W., Govitrapong P., Potikanond S. Liraglutide suppresses tau hyperphosphorylation, amyloid beta accumulation through regulating neuronal insulin signaling and BACE-1 activity. Int. J. Mol. Sci. 2020;21(5):1725. doi: 10.3390/ijms21051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kullmann S., Valenta V., Wagner R., Tschritter O., Machann J., Häring H.U., Preissl H., Fritsche A., Heni M. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-15686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moonishaa T.M., Nanda S.K., Shamraj M., Sivaa R., Sivakumar P., Ravichandran K. Evaluation of leptin as a marker of insulin resistance in type 2 diabetes mellitus. Int. J. Appl. Basic Med. Res. 2017;7(3):176–180. doi: 10.4103/ijabmr.IJABMR_278_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.