Abstract
Background and objective
Dengue is a vector-borne viral disease usually transmitted by Aedes mosquitoes. Around the world, the relationship between local vector density and frequency of dengue cases is being explored and needs further evidence. This study aimed to analyze the potential spatial relationships between the dengue vector (Aedes aegypti) and dengue cases in the megacity of Bangladesh during the 2019 dengue outbreak.
Methods
Vector density measures were used to estimate spatial associations with dengue case distribution. Location was determined for 364 dengue cases who were admitted to Dhaka Medical College Hospital over a period of 4 months. Data were collected using a semi-structured questionnaire, and prior consent was ensured before participation. The Moran global index, Getis-Ord Gi∗, ordinary least squares regression, geographically weighted regression and count data regression methods were used for spatial analysis.
Results
We found that dengue case distribution was not associated with immature Aedes aegypti mosquito (larvae) density across the city. The relationship between larval density measured by the Breteau Index (BI) and House Index (HI) with dengue cases was nonstationary and not statistically significant.
Conclusion
The location of dengue cases appears to be unrelated to vector distribution and vector density. These findings should prompt the search for other transmission risk factors.
Keywords: Tropical disease, Spatial analysis, Dengue, Hotspot, Megacity, Outbreak, Dhaka
1. Introduction
Dengue, a common mosquito-borne viral infectious disease, occurs in >100 countries around the world. Each year, up to 400 million people become infected with dengue, with approximately 100 million people manifesting clinical symptoms from infection and 22,000 ultimately dying from severe dengue [1]. Estimates of dengue cases reveal that the disease is more common in Asian countries, particularly the Philippines, Indonesia, and Thailand, with relatively negligible contributions from Bangladesh [2]. However, in the last two years, the sudden rise in the number of dengue cases in Bangladesh has surpassed all previous records, with evidence of 10,148 cases and 47 deaths in 2018 and a tenfold increase in the subsequent year (1,01,354 cases with 164 deaths by December 2019) [3]. Even amidst the coronavirus disease-2019 (COVID-19) pandemic, a total of 746 confirmed dengue cases were reported by November 2020 [4].
Identifying the presence of Aedes aegypti (vector of dengue virus) and achieving controlled eradication of this vector remain major challenges. High population densities, unplanned rapid urbanization and construction, and warm climates are responsible for the increased breeding of A. aegypti [3, 5, 6]. Additionally, insufficient and inadequate preparedness, lack of awareness of the general population, insufficiently resourced health care systems, and delayed and suboptimal vector-control programs further increased the Aedes population and the impact of dengue infection, resulting in deaths that could have been prevented [3, 7]. To date, no effective antiviral agents exist to treat dengue infection, and treatment therefore consists of supportive measures only [8]. Furthermore, no licensed vaccine against dengue infection is currently available, and dengue vaccine prototypes have not been as effective as anticipated [9, 10].
Aedes mosquito has adapted to human habitats and breed in relatively small containers that can hold water [11]. They can actively disperse over a very restricted range (28–199 m), and the majority of released mosquitos are usually recaptured in the same or adjacent houses from where they were released. The combination of small-scale breeding sites and a low level of mobility of the vector results in highly localized sites of disease transmission with dengue transmission around the sites where dengue vector density is high [11, 12, 13, 14]. Therefore, current efforts to control dengue transmission focus on the vector, using combinations of chemical and biological targeting of Aedes mosquito and management of breeding sites [8]. The World Health Organization (WHO) proposed several indices for Aedes aegypti surveillance [15, 16]. The Breteau Index (BI), House index (HI), and Container Index (CI) are used for larval surveys, and the Pupa Index (PI) is used for pupal surveys. For adult mosquito surveys, ovitraps are recommended. The BI and HI are commonly used for the determination of priority risk [16]. The rapid transition of the population in a megacity, high cost, lack of sufficient community health care workers and logistics operations were identified as barriers to the design of focused intervention in high BI or high HI areas [4, 17]. Furthermore, important gaps remain in knowledge regarding the relationship between the local level of Aedes aegypti infestation and the risk of dengue [18]. Studies that assessed the relationship between vector infestation and the rate of dengue incidence have frequently failed to demonstrate a clear association, and thus far, the results of such studies have been inconsistent [19, 20, 21]. Therefore, this study was designed to examine the extent of the spatial association of Aedes aegypti in hotspot areas of dengue-confirmed cases recorded in the 2019 outbreak in Bangladesh.
2. Subjects and methods
2.1. Research approach and design
We performed a cross-sectional study using data from dengue fever cases recorded during the 2019 outbreak in Bangladesh [22]. We included cases identified between May 1, 2020, and August 30, 2020. The criteria for dengue fever diagnosis followed from the national Guideline [23]. Among 747 cases, we included 364 confirmed cases from inside the geographic boundary of Dhaka city for final analysis.
2.2. Study area
The study was conducted in the Dhaka metropolitan city in Bangladesh. The city covers an area of 118 km2 (Latitude 23.71270 N, Longitude 90.41090 E) and is located on the eastern banks of the Buriganga River [24]. It is one of the most densely populated cities in the world, with nearly 23,000 people living per square kilometer as of 2020 [25]. This megacity is characterized by a hot, humid tropical climate, with a distinct monsoon season, an annual average temperature of 28 °C (82 °F), and monthly mean temperatures ranging between ∼28.7 °C (∼83.7 °F) in May and ∼29.0 °C (∼84.1 °F) in August [26].
Dhaka city is divided into two municipal zones: Dhaka North City Corporation (DNCC) and Dhaka South City Corporation (DSCC). These two-city corporations are again subdivided into ‘Ward’ (Ward is a level-4 administrative unit that contains at least one local community). A total of 129 wards (54 located in DNCC and 75 in DSCC), representing diverse socioeconomic and urban ecological settings, were purposefully chosen for the study. Before 2011, there was only one city corporation comprising 90 wards (‘old’ ward). As new administrative GIS maps are still unavailable, we used the old map for this analysis.
2.3. Dengue case and vector data
Dhaka Medical College Hospital (DMCH) is the busiest tertiary care hospital in Bangladesh and provides medical services to patients both within and outside the metropolitan region of Dhaka. During the study period, patients who were admitted to the DMCH from outside Dhaka primarily originated from neighboring districts and hence were considered the representative site during the outbreak.
We included patients who were currently living within the metropolitan city only. The address of the patients was confirmed to the ward level, and their geographical residence was then converted to ‘old’ ward numbers to enable matching the location with available GIS maps. In parallel, we used premonsoon Breteau Index (BI) and House Index (HI) data as surrogates of immature Aedes mosquito distribution with permission from Communicable Disease Control (CDC), Bangladesh [27]. The HI is widely used to assess the distribution of Aedes mosquitoes in a locality. The BI builds a relationship between positive containers and the number of houses. The Container Index (CI) only provides information on the proportion of water holding containers that are positive for Aedes [16]. According to WHO guidelines [16] on vector surveillance, BI and HI are the commonly used indices for the determination of priority areas for control measures. Hence, the Container Index (CI) data were not used in this study. These entomological data were collected as a part of the Aedes aegypti vector survey and were converted to ‘old’ ward numbers for use.
2.4. Vector surveillance method
The premonsoon vector surveillance data were collected by the CDC in March 2019. Samples for Aedes larvae were collected from 998 households over a period of 10 days by a group of 30 members (10 supervisors and 20 collectors forming 10 teams, with each team having one supervisor and two collectors). Each household was visited once. Ten households were selected in a judgment sampling method using GPS coordinates from 100 sites located in 98 wards of DNCC and DSCC. One site was selected from each ward, and two extra sites were selected from two densely populated wards. Multistourized buildings, independent houses, construction sites and vacant plots was surveyed for potential breeding sources. Larvae were collected from different types of breeding sources, including plastic bucket, drum, bottle, mug, pots, flower tub & tray, water tank, clay pot, discarded tires, and tin pots, following the WHO guideline [16].
2.5. Ethics statement
The study protocol was approved by the Ethical Review Committee of Dhaka Medical College Hospital (Memo no: MEU-DMC/ECC/2019/251). Informed written consent was obtained from patients and parents/guardians of patients in case of children before proceeding with the interview and data collection process.
2.6. Statistical analysis
2.6.1. Software used during analysis
We used ArcGIS (Version 10.5) for spatial analysis, ordinary least squares regression (OLS) and geographically weighted regression (GWR) analysis. R (Version 4.0.1) was also used for count data regression and other descriptive analyses.
2.6.2. Spatial autocorrelation (Moran global index) and hotspot (Getis-Ord Gi∗) analysis
An initial Moran's global index (Moran's I) spatial autocorrelation statistic was run on dengue cases to determine the most appropriate distance to be considered for spatial relationships during hotspot analysis. We calculated the Z scores from Moran's I for a distance of 100 m to 2000 m in 100 m intervals and then plotted them on a graph. The distance corresponding to the first plateau of the curve (700 m) was retained as the desired distance for Getis-Ord Gi∗ (hot spot) statistics. During hotspot analysis, we used a fixed distance band method to conceptualize the spatial relationship between wards and applied 700 m as the desired distance. The remainder of the parameters were set to default.
2.6.3. OLS and GWR regression analysis
OLS regression analysis was conducted using dengue cases as the dependent variable and BI and HI as independent variables. We conducted Moran's I statistics of the residuals for both models and found significant spatial autocorrelation. We then ran the GWR regression models keeping the same variable settings.
2.6.4. Count data regression
We used count data regression to find any significant association between the number of dengue patients per ward and Aedes aegypti larvae count expressed as BI and HI index. From an initial Poisson regression model, an overdispersion problem of dengue case data emerged; the Akaike information criterion (AIC) values were 843.675 and 846.767 for BI and HI explanatory variables, respectively. The residual deviances were 649.95 and 653.04 on 89 degrees of freedom, respectively, following a chi-square test with 1 degree of freedom. The dispersion parameters were 649.95/89 = 7.864 and 653.04/89 = 7.338, respectively. To account for the overdispersion problem, we tried to follow other count data regressions, such as negative binomial, zero-inflated Poisson, zero-inflated negative binomial, Hurdle Poisson and Hurdle negative binomial regression models. To select the optimal model, we used the AIC and Bayesian information criterion (BIC) values described in Supplementary Tables 1 and 2. The best fit was the Hurdle negative binomial (HNB) model for both BI and HI. Prior to incorporation of vector data in the models, we categorized them according to WHO guidelines [16]. A cutoff point of ≥20% was required to define high BI, and ≥5% was required to define high HI.
3. Results
3.1. Age, sex, and location of dengue cases
A total of 364 dengue patients residing within Dhaka city were admitted to the DMCH during the study period. The mean age was 26.3 ± 11.0 years [SD]. The majority of patients were aged between 21 and 30 years (42.2%) and were male (64.8%) (Supplementary Table 3).
Patients from other districts primarily resided in the neighboring districts of Dhaka (Figure 1).
Figure 1.
Spatial distribution of dengue patients admitted to Dhaka Medical College Hospital during the 2019 outbreak.
3.2. Spatial distribution of dengue cases and Aedes larvae in Dhaka city
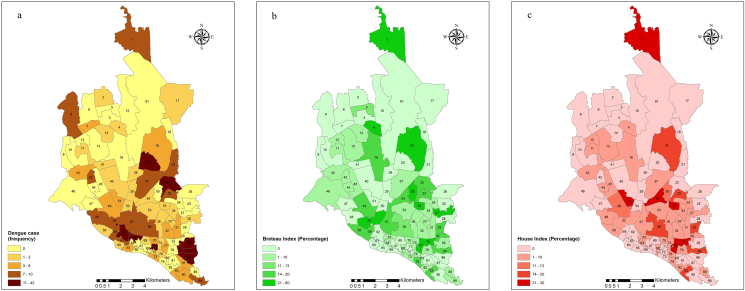
Within Dhaka, 85.6% of patients lived in the city, and 14.4% lived outside the city. Ward level (level-4 administrative areas of Bangladesh) data from those living within the city were used for spatial and hotspot analysis. Figure 2(a)–(c) shows the spatial distribution of dengue cases, the Breteau Index (BI) and the House Index (HI) across the different wards of the city. Dengue cases and Aedes larvae were clustered in the eastern and southern parts of the city. Dengue cases were more densely represented in wards 20, 22, 30, 62, 63, 84, 85, and 86 than in other wards. These wards were located in the Lalbag, Jatrabari, Mohakhali and Rampura police stations (level-3 administrative areas of the country). On the other hand, mosquito density as measured by BI and HI was much higher in wards 1, 4, 19, 27, 35, 52, 55, 75, and 77. These wards represented the Gulshan, Mirpur, Hazaribagh, Newmarket, Paltan, Kotowali and Wari police stations. Thus, there was an a priori mismatch between dengue case place of residence and clustered densities of mosquito larvae.
Figure 2.
Spatial distribution of dengue cases (a) and Aedes larvae calculated as BI (b) and HI (c) in different wards of Dhaka city; BI: Breteau Index; HI: House Index.
3.3. Hotspot analysis of dengue cases and Aedes larvae in Dhaka city
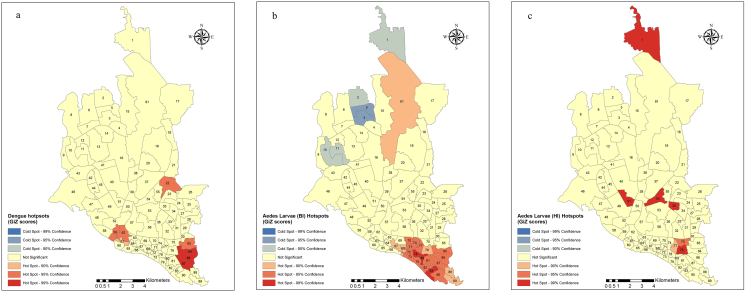
Hotspot analysis shows that wards 84, 86, and 87 located in Jatrabari were the most significant hotspots of dengue cases. This was followed by wards 59 and 62 located in Lalbag and ward 22 located in Rampura. There were no cold spots of dengue cases within Dhaka city (Figure 3a). Hotspot analysis of Aedes larval distribution in the city (Figure 3b and c) showed that according to BI, wards 78, 80, and 83 located in the Sutrapur and Shaympur police stations were the major hotspots of Aedes mosquito. These wards were surrounded by relatively less hot zones. In contrast, wards 2, 3, 5, 10, and 11 located in the Mirpur and Pallabi police stations were colder zones of the mosquito within the city. According to HI, wards 35, 51, 54, and 76 were the major hotspots covering Dhanmondi, Khilgaon, Ramna and Wari.
Figure 3.
Dengue hotspots (a) and mosquito hotspots as defined by BI (b) and HI (c) in Dhaka city; BI: Breteau Index; HI: House Index.
3.4. OLS regression models
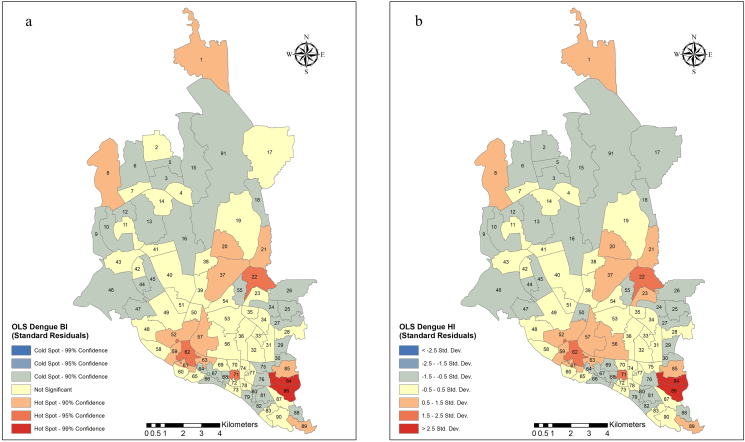
Ordinary least squares regression was conducted to evaluate the effect of Aedes distribution (as measured by BI and HI) on the number of dengue cases (Supplementary Table 4 and Figure 4(a) and (b)). We found that the dengue case distribution did not exhibit any significant correlation with BI (coefficient −0.019, p = 0.703, robust p = 0.614) or HI (coefficient −0.043, p = 0.584, robust p = 0.563). Additionally, both equations accounted for a very small proportion of the variance in dengue cases based on the Aedes vector density. High Jarque-Bera statistics (806.059 and 801.239 for models 1 and 2, respectively; df = 2 and p < 0.001 for both) indicated that the residuals of the regression lines were not normally distributed. Further analysis revealed that the residuals of both models were spatially autocorrelated (Model 1: Moran's I = 0.032, p = 0.029; Model 2: Moran's I = 0.034, p = 0.024).
Figure 4.
Spatial distribution of standardized residuals of an OLS to see the effect of BI (a) and HI (b) on the dengue distribution in Dhaka city. OLS: Ordinary least squares regression; BI: Breteau Index; HI: House Index.
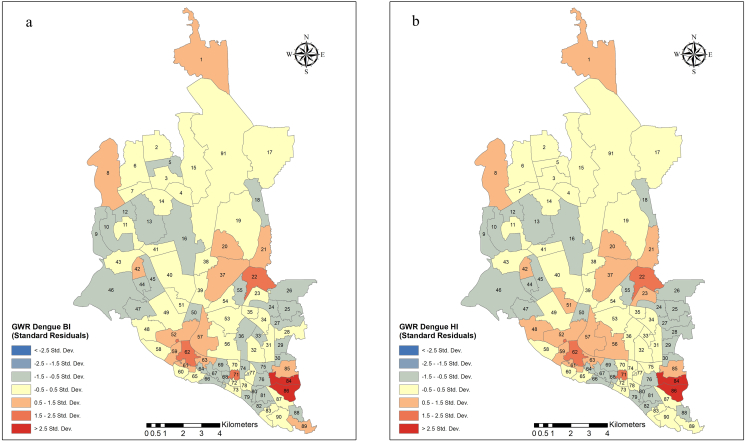
3.5. GWR models
We used GWR modeling to further explore the spatial diversities of dengue cases and BI or HI relationships. The summary results of the two models are shown in Supplementary Table 5, and the spatial distribution of the standard residuals is depicted in Figure 5(a,b). The dependent variable was the number of dengue cases per ward. The explanatory variables were BI for model 1 and HI for model 2. However, the GWR models did not perform better than the OLS models in explaining the distribution of dengue case densities (model 1 and model 2 explained 3% and 4% of the case variations, respectively).
Figure 5.
Spatial distribution of standardized residuals of a GWR to see the explanatory effect of BI (a) and HI (b) on dengue distribution in Dhaka city. GWR: Geographically weighted regression; BI: Breteau Index; HI: House Index.
3.6. Count data regression models
The AIC of the best-fitting HNB regression model for BI was 444.114 (Supplementary Table 6). The first part of the table contains the truncated negative binomial regression coefficient for the variable. A second part corresponds to the inflation model, which includes logit coefficients for predicting excess zeros. In the Dhaka city wards, high BI index areas (≥20%) had a 1.296 times higher case density than low BI index (<20%) areas with 95% CI (0.467–3.596). Counterintuitively, high BI index areas also had a 1.167 times higher chance of having ‘zero’ cases than areas with a low BI index (95% CI 0.532–2.560).
The HNB regression model was also the best fit for HI, as it had a low AIC value (441.953) (Supplementary Table 7). According to this model, in Dhaka city, wards with a high HI index (≥5%) had a 1.055 times higher number of dengue cases than wards with a low HI index (<5%) (95% CI 0.423–2.630). Again, contrary to expectations, wards with high HI were 3.229 times more likely to have ‘zero’ cases than wards with a low HI index.
4. Discussion
Geospatial analyses revealed that both dengue and immature mosquito (larval) density display some degree of spatial clustering in certain hotspot wards in the southern and eastern parts of Dhaka city. However, the construction of multiple previously validated regression models did not confirm such assumptions and was spatially nonstationary. None of the OLS, GWR or count data models revealed any significant associations between dengue case densities and immature mosquito densities in this study.
Spatiotemporal clustering of dengue cases in endemic zones occurs throughout the year [28, 29] and may become particularly prominent during outbreaks [29]. We examined data during the outbreak period and therefore, did not conduct temporal analysis. Evidence for clustering occurred in the southern and eastern parts of the city of Dhaka and might be due to the proximity of the affected person house to the hospital, which is located in the southern part of the city. Ali and colleagues evoked this possibility during the 2000 dengue outbreak in Dhaka city, when a similar spatial pattern was observed [30]. However, clustering of dengue cases as well as mosquitos is a common phenomenon found in dengue-endemic zones around the world [28, 29, 31, 32, 33].
Various vector, host and environmental factors might affect the regional distribution of dengue incidence. We explored the association of immature mosquito larval distribution in wards (measured by BI and HI) with dengue case distribution averaged according to ward-based regional units. GWR models pointed to a spatially nonstationary association between the variables, suggesting that other explanatory factors may be in play. Previously, Sulaiman et al. [34] and Lin and Wen [35] reported similar findings in Kuala Lumpur, Malaysia and Kaohsiung, Taiwan, respectively. One of these two studies applied linear associations and found no significant relationships between BI or HI and dengue cases [34]. Another study used GWR modeling and examined immature mosquito density as well as human case densities, and similar to current findings, it noted significant spatial nonstationary, indicating that higher dengue incidence in different city areas was independent of the concurrent presence of higher vector/host densities [35]. Conversely, Pham et al. noted a significant association of BI, HI and CI with dengue when adjusted for seasonality [36]. However, these investigators used a Poisson regression model instead of OLS. In Brazil, de Albuquerque et al. [21] also approached the problem on a finer geographic scale. They compared the distance between dengue cases or nondengue controls and ovitraps as well as egg density in two municipalities. These analyses led them to conclude on the presence of a positive spatial association between case and ovitrap positivity in one area, while no such association could be detected in the other area. Bowman et al. [37] systematically reviewed studies seeking the spatial relationship of dengue cases with vector indices published up to 2013. They reported that four out of thirteen studies found a statistically significant association between dengue and entomological indices.
A population density gradient study of the Dhaka metropolitan area by Khatun, Falgunee and Kutub [38] illustrated the density gradients of people across different thanas (level 3 administrative area) of Dhaka city. The maps generated in the study showed that the southern region of the city (also known as ‘old Dhaka’) is the most densely populated part of the city. In addition, the eastern region as well as some parts of the north and middle had foci of high population density. It is interesting to note that the spatial distribution of dengue cases in our study showed a nearly concordant pattern of clustering. Among places where dengue cases were more intensely present, the Lalbag and Jatrabari wards are located in the south and southeastern parts, respectively, while Mohakhali and Rampura are located in the middle and eastern parts of Dhaka, respectively. This similarity points toward a possible association of dengue incidence with population density, a pattern that was also apparent in the study from Taiwan [35]. Further studies examining the concordance between population density and dengue case density will be needed to confirm these preliminary observations.
Ideally, other meteorological, geographic, environmental and demographic factors should be included in the models. Additionally, an active search of dengue cases, an approach that is quite difficult to implement, might provide more accurate information on infection rates. Sharmin et al. modeled dengue data from 2000 to 2009 and found that the extent of cases recorded through passive reporting was only 2.8% (95% credible interval 2.7–2.8) in Dhaka city [39]. When they incorporated climate data, the authors noted a decrease in dengue transmission with a mean monthly temperature of 29 °C and when the average monthly rainfall was above 15 mm. Yue et al. explored the relationship of dengue incidence at the district level along with factors such as land type, land surface temperature, population density, and gross domestic product and found significant correlations in the OLS model [32].
We used ward-based dengue and immature mosquito density data instead of point data. Hence, our analysis was performed on a relatively coarse scale. This analysis assumed that the distribution of dengue within a delimited geographic boundary is likely homogenous. However, differences in the geographic surface of such areas might be problematic for the robust validity of such assumptions. Hence, we surmised that a count data regression model would be more appropriate to explore the association between dengue incidence and mosquito density. Among the various models explored herein, HNB for BI and HI performed better in terms of AIC values. Notwithstanding, the zero-hurdle models were necessary to account for the problem of zero inflation, as many cases were not reported. As previously discussed, Pham et al. [36] used Poisson regression analysis and found a significant association between dengue incidence and entomological indices. Sharmin et al. [40] developed a mixed effects model accounting for zero-inflation, spatial, and temporal random effects to explain the spatiotemporal variation in dengue cases in Bangladesh. They used dengue density measures at the district level and incorporated temperature and rainfall data but did not include vector indices in the equation. Our analysis revealed that HNB models also inferred spatial nonstationarity between dengue and mosquito densities.
4.1. Limitations
We noted several limitations pertaining to this study. First, we used passively reported dengue case data from a single tertiary care center, and the sample size was relatively small. Second, we were unable to collect ‘point’ data for cases. Third, we included only premonsoon Aedes larval density measured by BI and HI; therefore, potential temporal associations between dengue case incidence and vector density measures could not be explored. Fourth, an analysis of the clustering of dengue cases based on socioeconomic status and place of occupation was not possible due to a lack of relevant data. Last, incorporation of population density, population movement, climate, meteorological data and other factors affecting dengue distribution, such as the premise condition index, location index, breeding percentage and breeding preference ratio, was not possible due to the absence of available point data.
5. Recommendations
Further large-scale surveillance-based case‒control studies including population, vector, environmental, and geographic indices as explanatory variables should be conducted to explore various factors associated with dengue incidence in endemic regions such as Bangladesh. Additionally, point density data should be systematically obtained for both population and vector measures to test associations on a finer geographical scale that should then enable targeted interventions.
6. Conclusions
We found that dengue cases and immature mosquito indices show spatial clustering in certain parts of Dhaka city. A geospatial analysis of dengue density and immature mosquito indices at the ward level failed to reveal any significant relationship. Our analysis indicates spatial nonstationarity between dengue cases and mosquito densities during the 2019 outbreak. These findings should spark interest for further research in this area.
Declarations
Author contribution statement
Mohiuddin Sharif: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md. Abdullah Saeed Khan; Mohammad Jahid Hasan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tanzin Naher, Sujan Rudra, Jannatul Fardous: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
David Gozal: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Md Khalilur Rahman: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mohammad Robed Amin: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This project is a core research project of the Pi Research Consultancy Center, Bangladesh.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Centers for Disease Control and Prevention (CDC) About Dengue: what You Need to Know. https://www.cdc.gov/dengue/about/index.html [Internet]. 2021 [cited 2022 Jul 23]. Available from:
- 2.Wilder-Smith A., Ooi E.-E., Horstick O., Wills B. Dengue. Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- 3.Personal Communication. Ayesha akter, DGHS.
- 4.Hsan K., Hossain M.M., Sarwar M.S., Wilder-Smith A., Gozal D. Unprecedented rise in dengue outbreaks in Bangladesh. Lancet Infect. Dis. 2019;19(12):1287. doi: 10.1016/S1473-3099(19)30616-4. [DOI] [PubMed] [Google Scholar]
- 5.Sharmin S., Viennet E., Glass K., Harley D. The emergence of dengue in Bangladesh: epidemiology, challenges and future disease risk. Trans. R. Soc. Trop. Med. Hyg. 2015;109(10):619–627. doi: 10.1093/trstmh/trv067. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamun M.A., Misti J.M., Griffiths M.D., Gozal D. The dengue epidemic in Bangladesh: risk factors and actionable items. Lancet. 2019;394(10215):2149–2150. doi: 10.1016/S0140-6736(19)32524-3. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. 2009. https://apps.who.int/iris/handle/10665/44188 Available from: [PubMed]
- 9.Sabchareon A., Wallace D., Sirivichayakul C., Limkittikul K., Chanthavanich P., Suvannadabba S., et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomized, controlled phase 2b trial. Lancet. 2012;380(9853):1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 10.Halstead S.B. Dengue vaccine development: a 75% solution? Lancet. 2012;380(9853):1535–1536. doi: 10.1016/S0140-6736(12)61510-4. [DOI] [PubMed] [Google Scholar]
- 11.Haddawy P., Wettayakorn P., Nonthaleerak B., Su Yin M., Wiratsudakul A., Schöning J., et al. Large scale detailed mapping of dengue vector breeding sites using street view images. PLoS Neglected Trop. Dis. 2019;13(7):1–27. doi: 10.1371/journal.pntd.0007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington L.C., Scott T.W., Lerdthusnee K., Coleman R.C., Costero A., Clark G.G., et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 2005;72(2):209–220. [PubMed] [Google Scholar]
- 13.Hawley W.A. The biology of Aedes albopictus. J. Am. Mosq. Control Assoc. Suppl. 1988;1:1–39. [PubMed] [Google Scholar]
- 14.Honório N.A., Silva W. da C., Leite P.J., Gonçalves J.M., Lounibos L.P., Lourenço-de-Oliveira R. Dispersal of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in an urban endemic dengue area in the State of Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2003;98(2):191–198. doi: 10.1590/s0074-02762003000200005. [DOI] [PubMed] [Google Scholar]
- 15.Dengue control: vector surveillance. [cited 2020 Nov 28]. Available from: https://www.who.int/denguecontrol/monitoring/vector_surveillance/en/.
- 16.World Health Organization . second ed. World Health Organization; 2003. Regional Office for the Western Pacific. Guidelines for Dengue Surveillance and Mosquito Control.https://apps.who.int/iris/handle/10665/206987 Available from: [Google Scholar]
- 17.Dhar-Chowdhury P., Haque C.E., Driedger S.M. Dengue disease risk mental models in the city of Dhaka, Bangladesh: Juxtapositions and gaps between the public and experts. Risk Anal. 2016;36(5):874–891. doi: 10.1111/risa.12501. [DOI] [PubMed] [Google Scholar]
- 18.Focks, Dana A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors. World Health Organization; 2003. UNDP/World Bank/WHO special programme for research and training in tropical diseases.https://apps.who.int/iris/handle/10665/68575 Available from: [Google Scholar]
- 19.Getis A., Morrison A.C., Gray K., Scott T.W. Characteristics of the patial pattern of the dengue vector, Aedes aegypti, in Iquitos, Peru. Am. J. Trop. Med. Hyg. 2003;69(5):494–505. [PubMed] [Google Scholar]
- 20.Sanchez L., Vanlerberghe V., Alfonso L., Marquetti M.D.C., Guzman M.G., Bisset J., et al. Aedes aegypti larval indices and risk for dengue epidemics. Emerg. Infect. Dis. 2006;12(5):800–806. doi: 10.3201/eid1205.050866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Albuquerque B.C., Pinto R.C., Sadahiro M., Sampaio V.S., de Castro D.B., Terrazas W.C.M., et al. Relationship between local presence and density of Aedes aegypti eggs with dengue cases: a spatial analysis approach. Trop. Med. Int. Health. 2018;23(11):1269–1279. doi: 10.1111/tmi.13150. [DOI] [PubMed] [Google Scholar]
- 22.Hasan M.J., Tabassuma T., Sharifb M., Khana M.A.S., Bipashaa A.R., Basherc A., et al. Clinico-epidemiologic characteristics of the 2019 dengue outbreak in Bangladesh. Trans. R. Soc. Trop. Med. Hyg. 2020:1–8. doi: 10.1093/trstmh/traa126. [DOI] [PubMed] [Google Scholar]
- 23.Diseases Control Division (DGHS) fourth ed. 2018. National Guidelines for Clinical Management of Dengue Syndrome.https://old.dghs.gov.bd/index.php/en/publications/guideline (revised) Available from: [Google Scholar]
- 24.Khaleda S., Mowla Q.A., Murayama Y. Urban Development in Asia and Africa. The Urban Book Series. Springer; Singapore: 2017. Dhaka metropolitan area. [Google Scholar]
- 25.World Population Review Dhaka Population 2020 [Internet] 2020. https://worldpopulationreview.com/world-cities/dhaka-population [cited 2022 Jul 23]. Available from:
- 26.Weatherbase Dhaka, Bangladesh [Internet] 2022. https://www.weatherbase.com/weather/weather.php3?s=32914&cityname=Dhaka%2C+Dhaka%2C+Bangladesh [cited 2021 Mar 15]. Available from:
- 27.Mutsuddy P., Tahmina Jhora S., Shamsuzzaman A.K.M., Kaisar S.M.G., Khan M.N.A., Dhiman S. Dengue situation in Bangladesh: an epidemiological shift in terms of morbidity and mortality. Can. J. Infect Dis. Med. Microbiol. 2019;2019:2017–2022. doi: 10.1155/2019/3516284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majid N.A., Nazi N.M., Mohamed A.F. Distribution and spatial pattern analysis on dengue cases in Seremban District, Negeri Sembilan, Malaysia. Sustainability. 2019;11(13) [Google Scholar]
- 29.Lippi C.A., Stewart-Ibarra A.M., Romero M., Lowe R., Mahon R., Van Meerbeeck C.J., et al. Spatiotemporal tools for emerging and endemic eisease hotspots in small areas: an analysis of dengue and chikungunya in Barbados, 2013–2016. Am. J. Trop. Med. Hyg. 2020;103(1):149–156. doi: 10.4269/ajtmh.19-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M., Wagatsuma Y., Emch M., Breiman R.F. Use of a geographic information system for defining spatial risk for dengue transmission in Bangladesh: role for Aedes albopictus in an urban outbreak. Am. J. Trop. Med. Hyg. 2003;69(6):634–640. [PubMed] [Google Scholar]
- 31.Arboleda S., Jaramillo-O N., Peterson A.T. Spatial and temporal dynamics of Aedes aegypti larval sites in Bello, Colombia. J. Vector Ecol. 2012;37(1):37–48. doi: 10.1111/j.1948-7134.2012.00198.x. [DOI] [PubMed] [Google Scholar]
- 32.Yue Y., Sun J., Liu X., Ren D., Liu Q., Xiao X., et al. Spatial analysis of dengue fever and exploration of its environmental and socioeconomic risk factors using ordinary least squares: a case study in five districts of Guangzhou City, China, 2014. Int. J. Infect. Dis. 2018;75:39–48. doi: 10.1016/j.ijid.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Yang W., Fan J., Wang F., Jiang B., Liu Q. Spatial and temporal patterns of dengue in Guangdong Province of China. Asia Pac. J. Publ. Health. 2015;27(2):NP844–N853. doi: 10.1177/1010539513477681. [DOI] [PubMed] [Google Scholar]
- 34.Sulaiman S., Pawanchee Z.A., Arifin Z., Wahab A. Relationship between breteau and house indices and cases of dengue/dengue hemorrhagic fever in Kuala Lumpur, Malaysia. J. Am. Mosq. Control Assoc. 1996;12(3 PART 1):494–496. [PubMed] [Google Scholar]
- 35.Lin C.H., Wen T.H. Using geographically weighted regression (GWR) to explore spatial varying relationships of immature mosquitoes and human densities with the incidence of dengue. Int. J. Environ. Res. Public Health. 2011;8(7):2798–2815. doi: 10.3390/ijerph8072798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pham H.V., Doan H.T.M., Phan T.T.T., Tran Minh N.N. Ecological factors associated with dengue fever in a central highlands province, Vietnam. BMC Infect. Dis. 2011;11:1–6. doi: 10.1186/1471-2334-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bowman L.R., Runge-Ranzinger S., McCall P.J. Assessing the relationship between vector indices and dengue transmission: a systematic Review of the evidence. PLoS Neglected Trop. Dis. 2014;8(5) doi: 10.1371/journal.pntd.0002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatun H., Falgunee N., Kutub J.R. Analyzing urban population density gradient of Dhaka metropolitan area using geographic information systems (GIS) and census data. Geogr. Malaysian J. Soc. Sp. 2015;11(13):1–13. [Google Scholar]
- 39.Sharmin S., Glass K., Viennet E., Harley D. A Bayesian approach for estimating underreported dengue incidence with a focus on nonlinear associations between climate and dengue in Dhaka, Bangladesh. Stat. Methods Med. Res. 2018;27(4):991–1000. doi: 10.1177/0962280216649216. [DOI] [PubMed] [Google Scholar]
- 40.Sharmin S., Glass K., Viennet E., Harley D. Geostatistical mapping of the seasonal spread of underreported dengue cases in Bangladesh. PLoS Neglected Trop. Dis. 2018;12(11):1–13. doi: 10.1371/journal.pntd.0006947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.