Abstract
Ischemic stroke (IS) is an acute cerebrovascular disease caused by sudden arterial occlusion, which is characterized by a high morbidity, mortality, and disability rate. It is one of the most important causes of nervous system morbidity and mortality in the world. In recent years, the search for new medicine for the treatment of IS has become an attractive research focus. Due to the extremely limited time window of traditional medicine treatment, some side effects may occur, and accompanied by the occurrence of adverse reactions, the frequency of exploration with natural medicine is significantly increased. Phosphatidylinositol-3-kinase/Protein kinase B (PI3K/Akt) signaling pathway is a classical pathway for cell metabolism, growth, apoptosis, and other physiological activities. There is considerable research on medicine that treats various diseases through this pathway. This review focuses on how natural medicines (including herbs and insects) regulate important pathophysiological processes such as inflammation, oxidative stress, apoptosis, and autophagy through the PI3K/Akt signaling pathway, and the role it plays in improving IS. We found that many kinds of herbal medicine and insect medicine can alleviate the damage caused by IS through the PI3K/Akt signaling pathway. Moreover, the prescription after their combination can also achieve certain results. Therefore, this review provides a new candidate category for medicine development in the treatment of IS.
Keywords: natural medicine, ischemic stroke, PI3K/Akt signaling pathway, treatment, new trend
1. Introduction
Ischemic stroke (IS) is an important cause of neurological morbidity, it can lead to high mortality and disability rates. Among all cerebrovascular diseases, IS has the highest incidence [1]. It accounts for more than 80% of all stroke types [2]. According to incomplete statistics, about 14 million people suffered from IS every year [3]. The main causes of IS include cardiogenic cerebral infarction, rupture of cerebrovascular atherosclerotic plaques, atherosclerotic plaques, and lacunar infarction caused by small vessel lesions [4]. With the aging of the population, the incidence of IS has increased dramatically. Many patients with basic diseases are more likely to suffer from IS, such as diabetes, hypertension, atrial fibrillation, and arterial disease. It was also found that hospitalized patients with Corona Virus Disease 2019 (COVID-19) had a risk of IS [5]. Therefore, the effective treatment of IS is of great significance to society as a whole. The treatment of IS includes surgical intervention and drug intervention. However, the cost and risk of surgical intervention is high. Therefore, drug intervention has become the most commonly used clinical measures, such as anti-platelet drugs and thrombolytic drugs [6]. However, the therapeutic time window of this kind of drug is extremely limited, with the risk of bleeding or even more serious consequences. This makes the search for new and effective natural medicines the key to the treatment of IS. Natural medicine, including herbal and animal medicine, is collectively referred to as traditional Chinese medicine in China [7]. It is widely used in the treatment of various diseases. Traditional Chinese Medicine (TCM) has been used effectively in the treatment of stroke for more than 2000 years in China. Over the years, this field has gleaned extensive clinical treatment experience [8]. Modern medicine has also found that natural medicine is effective in the treatment of IS, and it plays a significant preventive role in the early stage of IS [9]. In particular, natural medicine can treat IS through different signaling pathways, including Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT), Nuclear Factor kappa-B (NF-κB), Mitogen-Activated Protein Kinase (MAPK), Nuclear Factor erythroid 2-Related Factor 2 (Nrf2), and PI3K/Akt signaling pathways [10]. Among them, the PI3K/Akt signaling pathway is involved in many diseases and is presently being widely studied.
The PI3K/Akt signaling pathway is an indispensable signal mechanism in the biological process of mammals, which is involved in signal transduction. Such as cell development, differentiation, cell survival, protein synthesis, and metabolism [11]. PI3K is a dimer composed of regulatory subunit P85 and catalytic subunit p110, which can bind to growth Akt allosteric [8]. Akt, as the main molecule downstream of PI3K signaling pathway, includes three subtypes: Akt 1, Akt 2, and Akt 3. They are encoded by Akt α, Akt β, and Akt γ, respectively [12]. Activation of Akt can further regulate its downstream substrate and cause a series of cascade reactions, thus acting in apoptosis and cell cycle regulation. Abnormal activity of PI3K/Akt signaling pathway may cause a variety of diseases, such as diabetes, neurodegenerative diseases, stroke, and cancer [13]. It has been confirmed that the PI3K/Akt signaling pathway plays an important role in oxidative stress and inflammation in the course of IS [14]. In this paper, we reviewed the evidence for natural medicine treatment of IS through the PI3K/Akt signaling pathway in recent years. We also explained its mechanism to provide new treatment ideas for the clinical treatment of IS.
2. Correlation between PI3K/Akt Signaling Pathway and Ischemic Stroke
2.1. Structural Characteristics and Activation of PI3K and Akt
According to the structural and functional differences. PI3K can be divided into I, II, and III subtypes. The subtypes I and II participate in cellular signal transduction, while subtypes III participate in membrane transport. Indeed, type I PI3K is the most widely studied and most closely related to IS at present. It encompasses regulatory subunits (p85α, p85β, and p85γ) and catalytic subunits (p110α, p110β, p110δ, and p110γ), which can be directly activated by cell surface receptors [15]. IA PI3K is activated by receptor tyrosine kinase (RTK), G protein coupled receptor, and small G protein RAS. IB PI3K is activated only by G protein coupled receptors [16]. Subsequently, the p85 subunit binds to the tyrosine receptor on the cell membrane, further activating PI3K and phosphorylation. Then, the p110 catalytic subunit is activated to catalyze the conversion of phosphatidylinositol diphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3) [17]. PIP3 continues to phosphorylate and transmits its activation signal to Akt [18].
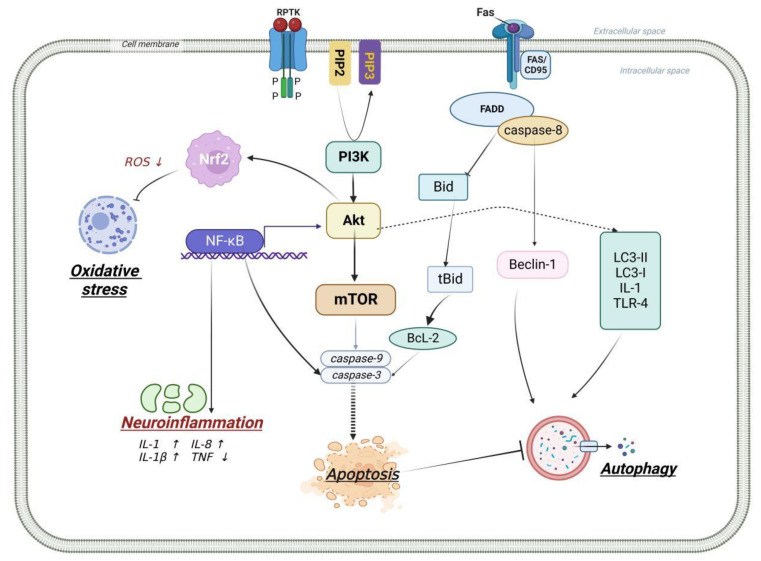
Akt is also known as protein kinase B (PKB). There are three subtypes: Akt1, Akt2, and Akt3 [19]. Different Akt subtypes are expressed in specific tissues. It indicates that Akt plays a key role in maintaining the physiological function of different tissues or organs [20]. Akt contains a special PH structural domain. If this domain has a defection, the activity of Akt also reduces or is even inactivated. After PI3K activation, the PH structural domain of Akt binds to the PI3K product PIP3 in order to promote the migration of Akt from intracellular to thin cell membrane and change the conformation. Then, the serine/threonine protein is phosphorylated, resulting in the complete activation of Akt [21]. Activated Akt further regulates its downstream substrate, causing a series of cascade reactions, and acts in apoptosis and cell cycle regulation [22] (Figure 1).
Figure 1.
Relationship between PI3K/Akt signaling pathway and apoptosis, inflammation, autophagy, and oxidative stress. After the activation of PI3K/AKT signaling pathway: (1) it can act on the downstream targets such as NF-κB, mammalian target of rapamycin (mTOR), and down-regulate the expression of cysteinyl aspartate specific proteinase-9 (Caspase-9), cysteinyl aspartate specific proteinase-3 (Caspase-3), and apoptosis-related protein B lymphocyte tumor-2 (Bcl-2) associated x protein (Bax), ultimate anti-apoptosis; (2) it up-regulates the expression of low complexity communications codec II/I (LC3-II/LC3-I), Beclin-1, interleukin-1 (IL-1), and toll-like receptor 4 (TLR4) to achieve the effect of anti-autophagy; (3) it inhibits the expression of pro-inflammatory factors induced by NF-κB to achieve the effect of anti-inflammation; (4) activated Akt further activates Nrf2, to achieve the effect of anti-oxidative stress.
2.2. Ischemic Stroke Activates PI3k/Akt Signaling Pathway, Link to the Relevant Cascade Reaction
2.2.1. The Pathological Mechanism of Ischemic Stroke
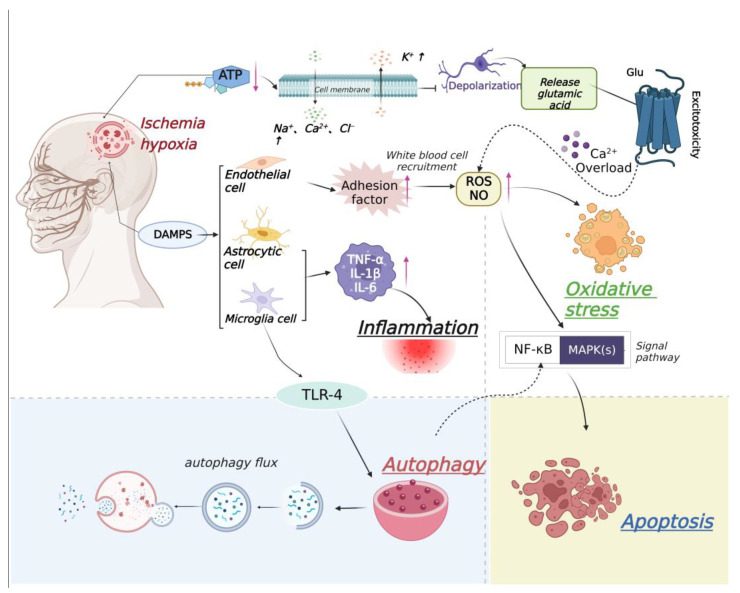
Ischemic stroke is a complex pathophysiological process. After cerebral ischemia, irreversible neuronal necrosis occurs in the central region due to the interruption of blood supply and energy depletion. For the ischemic penumbra, the disturbance of glucose energy metabolism leads to the decrease in Na+/K+-ATP enzyme activity and the imbalance of ion balance causes a large amount of Ca2+ influx, leading to the excessive release of glutamate and excitatory toxicity [23]. Glutamate further binds to the receptor and once again promotes the influx of Ca2+, leading to mitochondrial dysfunction and apoptosis. Meanwhile, after cerebral infarction, macrophages and microglia are activated to release vasoactive mediators and pro-inflammatory cytokines, which promote the infiltration of more leukocytes and cause neuroinflammation [24]. Inflammatory cells can also produce reactive oxygen species (ROS) and reactive nitrogen (RNS). This in turn reactivates inflammatory cells and a vicious cycle begins [25]. With the recovery of blood flow and oxygen supply after long-term infarction, ROS and RNS also increase oxidative stress. This aggravates the damage to the blood–brain barrier (BBB) and further leads to extravasation of plasma components, continued enhancement of inflammatory response, and obvious activation of autophagy-related signaling pathways [26] (Figure 2).
Figure 2.
Relationship between ischemic stroke and apoptosis, inflammation, autophagy, and oxidative stress. After cerebral ischemia, the activity of Na+/K+−ATP enzyme in ischemic penumbra decreases and the imbalance of ion homeostasis leads to cell membrane depolarization and Ca2+ influx, resulting in excessive release of glutamate and excitotoxicity. With the influx of a large amount of Ca2+, the adhesion factors of endothelial cells increase, which leads to white blood cell recruitment, increases the contents of ROS and nitric oxide (NO), and causes oxidative stress. Then, NF-κB and MAPK signaling pathways are activated, causing cell apoptosis. Microglial and astrocytic cells are also activated, which up-regulate tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), finally causing an inflammatory reaction. Meanwhile, microglia cell-mediated down-regulation of Toll-like receptor-4 (TLR-4) induce autophagy.
2.2.2. Neuroinflammation Caused by Ischemic Stroke with PI3K/Akt Signaling Pathway
Cerebral ischemia can disrupt the homeostasis between pro- and anti-inflammatory responses. Clinical studies have shown that inhibition of inflammatory response can reduce brain injury and improve neurological function. However, it has also been found that the inhibition of the inflammatory response may also worsen brain repair and long-term functional recovery after IS [27]. On the one hand, inflammatory reaction in cerebral ischemic area can cause excessive immune reaction and release inflammatory chemokine such as TNF-α and IL-6 into the blood, causing extensive damage to nerve cells [28]. On the other hand, a hypoxic environment promotes the activation of microglia and the release of superoxide, which can activate and recruit neutrophils. Eventually, the blood–brain barrier is destroyed [29]. Additionally, NF-κB is a key regulator in the process of inflammation, which regulates the active expression of pro-inflammatory genes [30]. Studies have shown that the activation of PI3K/Akt signaling pathway can inhibit the expression of pro-inflammatory factors induced by NF-κB, and transform M1 microglia into M2 microglia to reduce the inflammatory response [31,32]. Additionally, the activation of this pathway can also up-regulate the expression levels of interleukin-1RA (IL-1RA), interleukin-10 (IL-10), and interferon-β (IFN-β) and decrease the expression levels of proinflammatory cytokines, including IL-1, TNF-α, IL-6, interleukin-8 (IL-8), and chemokine (C-X-C motif) ligand 1 protein (CXCL1) [15].
Moreover, accumulating data indicate the role of PI3K/Akt signaling pathway in inflammation in IS. Many drugs have been found to inhibit inflammation of IS through the signaling PI3K/Akt signaling pathway. It has been confirmed that inhibition of chemokine CXCL8 may promote neuroglial activation and inhibit neuroinflammatory by regulating the PI3K/Akt/NF-κB signaling pathway in mice with IS [33]. Similarly, vinpocetine reduces inflammation induced by cerebral ischemia/reperfusion (I/R) injury in brain tissues through the PI3K/Akt signaling pathway [34]. Additionally, Bisperoxovanadium (BPV) protects nerves by up-regulating PI3K and Akt to inhibit the inflammatory response caused by cerebral I/R injury in rats with IS [35]. Fraxetin has a suppression effect on microglia-mediated neuroinflammation, and this effect is associated with the PI3K/Akt signaling pathway. Furthermore, the administration of fraxetin attenuated brain injury and behavioral deficits after IS [36]. In general, all these data suggest that alleviating brain inflammation through the PI3K/Akt signaling pathway is an effective way to improve the damage caused by IS.
2.2.3. Apoptosis Caused by Ischemic Stroke with PI3K/Akt Signaling Pathway
Apoptosis begins several hours after cerebral ischemia attack, which mainly appeared in ischemic penumbra. Bcl-2 family genes and cysteine protease (Caspase) family genes participate in ischemic neuronal apoptosis, which is a mitochondrial centered apoptosis process [37]. Under the stress of cerebral ischemia, the expression of Bcl2 decreases and the expression of Bax increases. It leads to caspase activation and an apoptosis-dependent cascade. Activated caspase (especially Caspase-1 and Caspase-3) can modify proteins and play a key role in the early stage of ischemia-mediated apoptosis [38]. Apoptosis has an inseparable relationship with the PI3K/Akt signaling pathway.
It has been found that there are a series of phosphorylation cascades in the PI3K/Akt signaling pathway, which can delay cell apoptosis [39], such as insulin-like growth factor 1 (IGF-1) up-regulating the yes-associated protein/transcription activator with PDZ binding motif (YAP/TAZ) by activating PI3K/Akt signal cascade, so as to reduce the apoptosis of nerve cells in IS [40]. Additionally, it has also been found that electroacupuncture (EA) preconditioning can inhibit the expression of pro-apoptotic genes and proteins in the middle cerebral artery (MCAO) model rats. The mechanism of reducing apoptosis can be achieved through the PI3K/Akt signaling pathway [41]. Moreover, resveratrol can significantly improve the neural function and attenuate neuronal apoptosis in IS rats through activating the PI3K/Akt signaling pathway [42]. Thus, it can be seen that the activation of the PI3K/Akt signaling pathway can alleviate the brain injury caused by IS by delaying apoptosis.
2.2.4. Oxidative Stress Caused by Ischemic Stroke with PI3K/Akt Signaling Pathway
ROS is a highly active substance formed by enzymatic or non-enzymatic reaction in mammalian cells, which is involved in many pathological processes during I/R [43]. Oxidative stress occurs when excessive production of ROS and/or ROS degradation is impaired, which is one of the important pathological mechanisms of IS [44]. The brain accounts for only 2% of the body weight, but oxygen consumption is close to 20%. This is why the brain produces more free radicals than other organs. Therefore, the exploration of oxidative stress reaction can better provide new ideas for the treatment of IS. After IS, the PI3K/Akt signaling pathway is activated. The downstream glycogen synthase kinase-3β (GSK-3β) is phosphorylated, resulting in the loss of the ability to activate NF-κB so as to reduce inflammation-mediated nerve cell injury [45]. At the same time, activated Akt further acts on Nrf2, a key regulator of oxidative stress. It prevents kelch-like epichlorohydrin-associated protein-1 (Keap1) from coupling with it and promotes the binding of the antioxidant response element (ARE) to it. Finally, it enhances the expression of antioxidant proteins [46].
Emerging evidence has shown that the activation of the PI3K/Akt signaling pathway can effectively reduce oxidative stress induced by IS [47]. Studies have shown that basic fibroblast growth factor (bFGF) can inhibit endoplasmic reticulum stress induced by IS through activating PI3K/Akt and ERK1/2 pathways [48]. In addition, it was also found that that Aloperine (ALO) can improve cerebral I/R injury and alleviate oxidative stress via activating the PI3K/AKT signaling pathway in order to support the therapeutic potential of ALO against cerebral I/R injury in IS [49]. Similarly, the NX210 peptide can prevent oxidative stress and neuronal apoptosis in cerebral I/R via up-regulation of the Integrin-beta1/PI3K/Akt signaling pathway [50]. Moreover, total flavonoids of Chuju (TFCJ) may also reduce oxidative stress and apoptosis of IS by activating the PI3K/Akt/mTOR signal pathway [51]. In conclusion, the above evidence suggests that drugs can ameliorate oxidative stress after IS through the PI3K/Akt signaling pathway.
2.2.5. Autophagy Caused by Ischemic Stroke with PI3K/Akt Signaling Pathway
Autophagy is a catabolic pathway for cell self-protection, which achieves self renewal by decomposing damaged nerve cells [52]. In response to stress changes in the internal and external environment of the body, autophagy rapidly enters a bidirectional regulation state after IS [53]. Firstly, moderate autophagy can transform its degradation products and synthesize adenosine triphosphate (ATP) to provide energy for nerve cells. Secondly, excessive autophagy caused by increased ischemia will lead to the autophagic death of nerve cells [54].
Plenty of studies have shown that the PI3K/Akt signaling pathway is closely related to autophagy. One study showed that diosgenin injected intraperitoneally with 100mg/kg can improve LC3-II/LC3-I, Beclin-1 and IL-1 of I/R model rats by activating the PI3K/Akt signal pathway in order to reduce autophagy and play a neuroprotective role [55]. Additionally, mTOR is the downstream target of PI3K/Akt signaling pathway, can regulate a variety of intracellular signals and have an effect in autophagy [56]. A nested case–control study showed that the expression of mTOR protein in IS was significantly increased and the activation of the PI3K/Akt/mTOR signaling pathway autophagy signaling pathway was enhanced, which inhibited excessive autophagy of nerve cells [57]. It has been reported that the TCM prescription Tong-Qiao-Huo-Xue decoction can activate PI3K/Akt/mTOR signaling pathway to reduce brain microvascular endothelial cell (BMECs) autophagy after cerebral ischemia/reperfusion injury [58]. Similarly, Ibrutinib also can ameliorate autophagy and cerebral ischemia/reperfusion injury through the PI3K/Akt/mTOR signal pathway [59]. Moreover, inhibition of miR-124 can increase the PI3K/Akt/mTOR signaling pathway, thus inhibiting cell apoptosis and autophagy caused by IS [60]. Interestingly, we found that EA can also inhibit neuronal autophagy and apoptosis via the PI3K/Akt signaling pathway following IS, and it is a safe and effective therapy for ischemic stroke in both clinical and laboratory settings [41]. Therefore, the above evidence indicates that the improvement of IS may be achieved by activating the PI3K/Akt signaling pathway to reduce excessive autophagy.
Overall, IS is formed by the joint action of many pathological factors. Drugs activate the PI3K/Akt signaling pathway in the early stage of IS development, then crosstalk cell apoptosis, autophagy, oxidative stress, ischemia-induced inflammation, and other important mechanisms. These mechanisms trigger a series of cascade reactions, which initiate the protective effect on damaged nerve cells. However, it should be noted that these effects after IS do not exist alone, but overlap. In order to make better use of the PI3K/Akt signaling pathway, multi-target and multifaceted comprehensive interventions should be adopted in the treatment of IS.
3. Natural Medicine for the Treatment of Ischemic Stroke through PI3K/Akt Signaling Pathway
3.1. Herbal Medicine
After long-term research, it has been found that the following representative herbal medicine has significant therapeutic effects on ischemic stroke and the accumulated data confirm that the effects of these medicines are likely to be mediated by PI3K/Akt signaling pathway. In this section, we reviewed the effects of herbs and their active ingredients on IS and their potential mechanism on the PI3K/Akt signaling pathway (Table 1).
Table 1.
Effects of herbal medicine and their active ingredients on PI3K/Akt signaling pathway and their roles in ischemic stroke.
| Natural Medicine |
Plant Atlas |
Active Ingredients | Model | Regulation of PI3K/Akt Signaling Pathway |
Main Purposed Effects | Ref(s) |
|---|---|---|---|---|---|---|
|
Herbal
medicine |
||||||
| Chuanxiong |

|
Ligustrazine | PC12 Cells SD rats Human amniotic epithelial cells |
↑p-PI3K and p-Akt |
Anti-neuronal apoptosis Anti-inflammation Activating autophagy |
[61,62,63,64,65,66,67] |
|
Salvia
miltiorrhiza |

|
Tanshinone; Danshensu; Salvianolic Acid. |
SD rats Neural stem/precursor cells |
↑p-PI3K and p-Akt |
Inhibit apoptosis Anti-inflammation Anti-oxidative stress |
[68,69,70,71,72,73,74,75] |
|
Radix
Angelicae sinensis |

|
Angelica Polysaccharides; Ferulic Acid; Ligustilide. |
PC12 Cells SD rats CIRI rats |
↑p-PI3K and p-Akt |
Anti-oxidative stress Anti-neuronal apoptosis |
[76,77,78,79,80] |
|
Astragalus
membranaceus |

|
Astragalus Polysaccharides; Astragalus Saponins; Flavonoids. |
SD rats OGD/R HT22 cells |
↑p-PI3K and p-Akt |
Anti-neuronal apoptosis Anti-inflammation |
[81,82,83,84,85,86] |
| Safflower |

|
Safflower Yellow | SD rats | ↑p-PI3K, p-Akt, GSK3β | Anti-oxidative stress Anti-neuronal apoptosis Anti-inflammation |
[87,88,89,90,91,92,93] |
|
Ginkgo
biloba leaf |

|
Ginkgo Flavonoids; Ginkgolides. |
SD rats brain endothelial cell |
↑p-Akt, p-GSK3β, VEGF |
Anti-inflammation Anti-oxidative stress |
[94,95,96,97,98,99] |
|
Erigeron
breviscapus |

|
Flavonoids Scutellarin (Breviscapine) |
SD rats A375 cells |
↑p-Akt, eNOS | Anti-oxidative stress Anti-neuronal apoptosis |
[100,101,102,103] |
| Ginseng |

|
Ginsenoside | Mice SD rats |
↑p-Akt, p-mTOR, p-ERK |
Anti-oxidative stress Anti-neuronal apoptosis Anti-inflammation |
[104,105,106,107,108,109,110,111] |
|
Radix Paeoniae
Rubra |

|
Paeoniflorin | Mice SD rats |
↑p-PI3K and p-Akt |
Anti-inflammation Anti-neuronal apoptosis |
[112,113,114,115] |
|
Panax
notoginseng |

|
Panax Notoginseng; Saponins. |
Mice SD rats bEnd 3 cells H9c2 cells |
↑p-Akt/Akt, p-mTOR, Nrf2 |
Anti-neuronal apoptosis Anti-inflammation |
[116,117,118,119,120,121] |
|
Herbal
prescriptions |
||||||
| Buyang Huanwu Decoction |
Propyl Gallate; Formononetin; Hydroxysafflor Yellow A; Formononetin; Astragaloside IV; Inosine, Paeoniflorin; Paeonol; Ligustrazine; Ferulic Acid. |
HT22 cells mice |
↑p-PI3K, p-Akt, p-Bad |
Anti-neuronal apoptosis Anti-inflammation |
[122,123,124,125,126] | |
| Taohong Siwu decoction |
Hydroxysafflor Yellow A; Paeoniflorin; Paeonol; Ligustrazine; Ferulic Acid. |
SD rats | ↑p-Akt | Anti-inflammation Anti-neuronal apoptosis |
[127,128,129,130] | |
| Xiaoyao San | Saikoside; Ferulic Acid; Ligustilide; Atractylenolide; Paeoniflorin; Albiflorin; Liquiritin; Glycyrrhizic Acid; Pachymic Acid. |
PC12 cells | ↑p-PI3K and p-Akt |
Anti-neuronal apoptosis | [131,132] | |
| Danhong injection | Ferulic Acid; Cryptotanshinone; Quercetin; Anhydrosafflor Yellow B. |
SD rats | ↑p-PI3K, p-Akt, GSK3β |
Anti-inflammation Anti-neuronal apoptosis |
[133,134,135,136,137,138] | |
| Sanhua decoction | Flavonoids; Anthraquinones; Coumarins; Phenylpropanoid Glycosides; Alkaloids; Lignans. |
SD rats | ↑p-PI3K and p-Akt |
Anti-inflammation Anti-neuronal apoptosis |
[139,140,141] | |
| Xingnaojing injection |
Turmeric; Moschus; Borneolum Syntheticum; Fructus Gardeniae. |
SD rats | ↑p-Akt, eNOS | Anti-oxidative stress Anti-neuronal apoptosis |
[142,143,144,145] |
↑ signifies increase/activate
3.1.1. Chuanxiong
Chuanxiong (CX, Family: Umbelliferae) is the dried rhizome of Ligusticum chuanxiong Hort. CX has the effect of promoting blood circulation. It contains volatile oil, alkaloids, and other active ingredients. Among them, the most studied is the active component of alkaloid: ligustrazine [61]. It has been proven that ligustrazine can reduce the infarct area and water content of cerebral ischemia–reperfusion rats and improve the neurological function. It also can down-regulate the expression of Bax mRNA in hippocampal neurons and inhibit neuronal apoptosis [62]. Additionally, ligustrazine can control blood flow velocity, promote blood circulation, and play the role of anti-platelet aggregation and anti-thrombosis [63]. Additionally, the vitro experiments have proved that ligustrazine may have a significant neuroprotective effect on PC12 cells induced by glucose deprivation injury with inhibiting Bax/Bcl-2 and Caspase-3 apoptosis pathways [62]. It can be seen that ligustrazine may have a good effect on improving IS.
Various studies have reported that ligustrazine is related to the PI3K/Akt signaling pathway. It has been confirmed that ligustrazine may inhibit the proliferation and inflammation of rat pulmonary vascular smooth muscle cells (PASMCs) by activating PI3K/Akt signaling pathway, thus alleviating monocrotaline (MCT)-induced pulmonary hypertension in rats [64]. Additionally, ligustrazine can activate autophagy by regulating the PI3K/Akt/mTOR signaling pathway, so as to improve the neurocognitive impairment induced by endotoxin and reduce neuronal damage [65]. Ligustrazine can also play a neuroprotective effect on brain I/R injury in rats by activating the PI3K/Akt signaling pathway [66]. This evidence suggests that ligustrazine may improve IS-induced neuronal inflammation, autophagy, and apoptosis by regulating the PI3K/Akt signaling pathway.
Moreover, ligustrazine is also widely used in the clinic. Compared to conventional medicine treatment alone, ligustrazine plus conventional medicine treatment showed a significant difference in the reduction of stroke recurrence either at the end of 1-year follow-up or 3-years observation. The ligustrazine group showed a higher survival rate and significantly better effective rate than that of the control group at the end of a 1-year visit [67]. Thus, ligustrazine is a drug with high safety. Furthermore, ligustrazine comes from CX, which means that CX may be a potential drug for the treatment of IS. It can be confirmed by further study.
3.1.2. Salvia miltiorrhiza
Salvia miltiorrhiza (SM, Family: Labiatae) is the dried roots and rhizomes of Salvia miltiorrhiza Bunge. SM has the effect of promoting blood circulation, dispersing blood stasis, and relieving pain. The active ingredients include tanshinone, danshensu, and salvianolic acid. Studies have shown that SM possess good anti-inflammatory, antioxidant, and anti-apoptosis activities [68]. A large number of data show that there are three salvianolic acids have good therapeutic effect on IS. Salvianolic acid A (Sal A) can inhibit inflammation and apoptosis in mice through PI3K/Akt signaling pathway, and reduce nerve injury caused by IS [69]. Salvianolic acid B (Sal B) can induce the phosphorylation of Akt and mTOR and promote cell migration and increase angiogenesis in IS rats, thus reducing neuronal apoptosis and increasing the expression of stanniocalcin-1 (STC1) [70]. Additionally, Sal B also can maintain neural stem/progenitor cells self-renewal and promote proliferation through the PI3K/Akt signaling pathway, and improve cognitive impairment after IS in rats [71]. Based on the mechanism of network pharmacology and molecular docking, it has been revealed that salvianolic acid C (Sal C) may have a protective effect on the brain of IS rats through the PI3K/Akt signaling pathway [72]. Moreover, tanshinone is also associated with the PI3K/Akt signaling pathway and IS. It has been reported that tanshinone IIA (Tan IIA) may protect the neurons of primary cultured cortical neurons through PI3K/Akt signaling pathway [73]. Similarly, sodium Tan IIA sulfonate (STS) can prevent progressive and persistent brain injury and improve neurological dysfunction by activating the PI3K/Akt signaling pathway in rats with middle cerebral artery occlusion/reperfusion (MCAO/R) [74]. Additionally, in clinical treatment, the use of STS after stroke can improve the neurological function of patients with acute IS after recombinant tissue plasminogen activator (rt-PA) treatment by reducing BBB leakage and damage [75]. This evidence suggests that Salvia miltiorrhiza is not only safe in the treatment of IS, but also has a variety of compounds that can be studied. It is a potential drug for the treatment of IS.
3.1.3. Radix Angelicae sinensis
Radix Angelicae sinensis (RAs, Family: Umbelliferae) is the root of Angelica sinensis (Oliv.) Diels. RAs has a great effect of tonifying blood, regulating menstruation, and relieving pain. The main active ingredients are volatile oil, polysaccharides, and organic acids. Among them, angelica polysaccharides (ASP) has been proved to have the effect of anti-cerebral ischemia. It can increase the antioxidant activity of cortical neurons and the number of microvessels, thus improve the blood flow after cerebral ischemia [76]. Additionally, it has been reported that ASP can effectively improve neural function and neuronal cell apoptosis in CIRI rats by activating PI3K/Akt signaling pathway [77].
In addition, ferulic acid and ligustilide, the effective ingredients of angelica volatile oil have the effect of ameliorating IS. They have significant effects of anti-inflammation, antioxidation, angiogenesis, nerve regeneration, anti-platelet aggregation, anti-atherosclerosis, and protection of blood vessels. It is helpful to improve the neural function of IS [78]. According to prediction and docking simulation, it is shown that ferulic acid can synergistically affect IS through genes such as met-enkephalin (MEK) and NF-κB in PI3K, Akt cascade signals [79]. As for ligustilide, it has been found to attenuate hippocampal neuronal apoptosis induced by ischemia–reperfusion by activating PI3K/Akt signaling pathway [80]. The above evidence shows that the mechanism of Radix Angelicae sinensis in treating IS is by activating PI3K/Akt signal pathway.
3.1.4. Astragalus membranaceus
Astragalus membranaceus (AM, Family: Leguminosae) is the root of Astragalus memeranaceus (Fisch.) Bge. Var. mongholicus (Bge.) Hsiao. It has the functions of promoting diuresis, detumescence, blocking arthralgia, supporting toxin, and expelling pus. Additionally, the main active ingredients of AM are astragalus polysaccharides, saponins, and flavonoids. Studies have shown that AM has a wide range of effects on blood vessels. It includes reducing the inflammatory response after cerebral ischemia–reperfusion, scavenging oxygen free radicals, protecting vascular endothelial cells, reducing vascular permeability, increasing cerebral blood flow, and inhibiting apoptosis [81].
Astragaloside IV (AS-IV) is the main saponins of Astragalus membranaceus. AS-IV has been reported to promote the cell viability of HT22 after hypoxic glucose deprivation/reperfusion (OGD/R). It can also balance the expression of Bcl-2 and Bax in vitro and increase the expression of LC3II/LC3I. This indicate that AS-IV plays a neuroprotective role by down-regulating apoptosis by promoting the degree of autophagy and improve IS [82]. Moreover, in vivo and in vitro experiments have proved that AS-IV can down-regulate the expression of IL-17 protein, regulate cell apoptosis by regulating PI3K/Akt/GSK-3β signaling pathway, and reverse the promoting effect of IL-17 on the proliferation, neural regeneration, and cognitive dysfunction of neural stem cells (NSCs) after IS [83]. In addition, it was found that after administration of total flavonoids of AM in rats with cerebral ischemia, the expression of MDA, IL-1 β, TNF-α, Caspase-3, and Bax mRNA decreased significantly, while the activity of Bcl-2 mRNA, PI3K, and Akt protein increased significantly. It is suggested that the protective effect of total flavonoids of AM on cerebral ischemia–reperfusion injury is related to the anti-apoptotic effect caused by activating PI3K/Akt signaling pathway [84].
Moreover, Astragalus polysaccharides (APS) is another important active ingredient in Astragalus membranaceus. Studies have shown that APS exerts anti-Parkinson via activating the PI3K/Akt/mTOR signaling pathway to increase cellular autophagy levels in vitro [85]. APS can also reduce the content of TNF-α by activating the PI3K/Akt signaling pathway [86]. This suggests that APS may improve autophagy and apoptosis of IS through PI3K/Akt signaling pathway.
In conclusion, AM has great development potential as a commonly used natural medicine. The study of AM to improve IS through the PI3K/Akt signaling pathway provides a new idea for the treatment of IS.
3.1.5. Safflower
Safflower (SF, Family: Compositae) is the flower of Carthamus tinctorius L. The main active ingredients of SF are alkaloids, flavonoids, and organic acids. These ingredients can effectively prevent and treat cardiovascular and cerebrovascular diseases such as atherosclerosis and thrombosis.
Safflower yellow (SY) is one of the effective flavonoids of SF, which is widely used for the treatment of acute ischemic stroke in China [87]. Studies have shown that SY can significantly reduce cerebral infarction area, increase cerebral vascular density, and reduce permeability. Finally, SF treatment leads to effective improvement of brain edema [87] and SY can also inhibit the formation of thrombus by inhibiting platelet activation and aggregation. Reduce brain injury caused by reperfusion by reducing blood viscosity, dilating blood vessels, and improving blood circulation [88]. Therefore, SY is widely concerned in the treatment of IS and emerging evidence has shown that SY may inhibit the recovery of cell proliferation by TNF-α through PI3K/Akt signaling pathway [89].
Hydroxysafflor yellow A (HSYA), a major active ingredient of the SF, has drawn more interest in recent year for its multiple pharmacological actions in the treatment of cerebrovascular and cardiovascular diseases [90]. It is reported that HSYA may inhibit foam cell formation and vascular endothelial cell dysfunction and inhibit the proliferation and migration of vascular smooth muscle cells through PI3K/Akt/mTOR signaling pathway. It can also inhibit platelet activation, thus reducing a series of injuries caused by IS [91]. Moreover, HSYA may play a protective role and inhibit apoptosis in focal cerebral ischemia model rats by activating PDGF-mediated PI3K/Akt signaling pathway [92]. Additionally, in clinical treatment, HSYA was safe and well-tolerated at all doses for treating IS patients with blood stasis syndrome (BSS). The medium (50 mg/d) or high dose (75 mg/d) might be the optimal dose for a phase III trial [93].
3.1.6. Ginkgo biloba leaf
Ginkgo biloba leaf (GBL, Family: Ginkgoaceae) are the dried leaves of Ginkgo biloba L. GBL is one of the oldest species in the world, known as “A living fossil full of treasure”. Ginkgo flavonoids and ginkgolides are the main active ingredients of GBL, which have certain therapeutic and preventive effects on ischemic cerebrovascular diseases.
Studies have shown that ginkgolides can reduce necrotic neurons and hippocampal lesions, reduce blood viscosity, dilate blood vessels, and increase cerebral blood flow [94]. Such as ginkgolide A (GA) has been shown to inhibit lipopolysaccharide (LPS)-induced inflammation in human coronary artery endothelial cells (HCAECs), and its anti-inflammatory activity may be related to the inhibition of PI3K/Akt signaling pathway [95]. Additionally, mechanism studies confirmed that ginkgolide B (GB) methane-sulfonate has angiogenic effect in vivo and in vitro through PI3K/Akt/ glycogen synthase kinase 3 (GSK3) signaling pathway. It is a potential anti-chronic IS drug [96]. Moreover, the combination of ginkgo flavonoids and ginkgolides can significantly reduce brain edema and antagonize platelet activating factor in order to reduce the volume of cerebral infarction and nerve injury [97]. Additionally, the combined application of the two can improve cerebral I/R injury through the PI3K/Akt/Nrf2 signaling pathway and multi-component intrabody process [98]. Furthermore, the existing clinical evidence shows that GBL preparations (GLP) have a good therapeutic effect on patients with IS and can improve their hemorheology indices. Moreover, GLP is shown to be relatively safe [99].
3.1.7. Erigeron breviscapus
Erigeron breviscapus (EB, Family: Compositae) is the whole grass of Erigeron breviscapus (Vant.) Hand.-Mazz. EB contains flavonoids, caffeic acid esters, aromatic acids, coumarins, pyranones, and other ingredients. Among them, flavonoids scutellarin (also known as breviscapine) and caffeic acid esters are the main active ingredients.
Scutellarin, the main bioactive flavonoid glycoside extracted form EB, has been reported to exert positive effects on anti-inflammatory reactions. It has been reported that scutellarin can regulate osteoarthritis through PI3K/Akt/mTOR signaling pathway in vitro [100]. Additionally, scutellarin can also inhibit epithelial-interstitial transformation and angiogenesis through PI3K/Akt/mTOR signaling pathway [101]. Meanwhile, scutellarin may play a therapeutic role in IS through PI3K/Akt/mTOR signaling pathway [102]. This evidence shows that scutellarin can improve the inflammatory response and neuronal apoptosis of IS through PI3K/Akt signaling pathway.
Emerging evidence shows that EB injection and scutellarin can inhibit the activation of MMP-9 and reduce the degradation of claudin-5 by inhibiting the synthesis of inducible nitric oxide synthase (iNOS). Finally, the structural integrity of BBB after ischemia was shown to be protected [103]. Moreover, the network pharmacological studies confirmed that EB may improve brain barrier damage caused by IS through PI3K/Akt signaling pathway [103]. Thus, it can be seen that EB has great potential in the treatment of IS. However, there is a lack of basic research on EB through PI3K/Akt signaling pathway in the treatment of IS. It is suggested that EB may become a new research breakthrough point.
3.1.8. Ginseng
Ginseng (Gs, Family: Araliaceae) is the root of Panax ginseng C.A. Meyer. The main active ingredient of Ginseng is ginsenoside (GS), which have always been referred to as “all-healing” and widely used for its extensively medicinal value. GS have diverse biological activity which might be related to inflammation, apoptosis, oxidative stress, and angiogenesis [104].
There are several types of GS that have been studied. It has been reported that ginsenoside-Rb1 (GS-Rb1) can protect the integrity of blood–brain barrier in IS by inhibiting free radicals derived from matrix metalloproteinase-9 and NADPH oxidase 4 (NOX4) induced by neuroinflammation [105]. GS-Rb1 can also inhibit autophagy of cardiomyocytes and reduce myocardial I/R injury, and the mechanism is mediated by the activation of the PI3K/Akt/mTOR signaling pathway [106]. In addition, ginsenoside-Rg1 (GS-Rg1) has been shown to promote cerebral angiogenesis through the PI3K/Akt/mTOR signaling pathway in recent years [107]. GS-Rg1 can also prevent cognitive impairment induced by isoflurane anesthesia in aged rats through antioxidant, anti-inflammatory, and anti-apoptotic effects mediated by the PI3K/Akt/GSK-3 signaling pathway [108]. Moreover, GS-Rd can reduce Tau phosphorylation after transient forebrain ischemia through the PI3K/Akt/GSK-3β signaling pathway. Moreover, the clinical studies showed that GS-Rd improves the main prognosis of acute IS and has an acceptable profile of adverse events [109]. Emerging evidence proves that GS-Rh1 exerts neuroprotective effects by activating the PI3K/Akt pathway in amyloid-beta induced SH-SY5Y cells [110]. GS can not only alleviate cerebral I/R injury in rats, but also activate PI3K/Akt and extracellular signal-regulated kinase 1/2 (ERK1/2) pathways and promote neurogenesis by increasing the expression of VEGF and brain-derived neurotrophic factor (BDNF) [111]. In conclusion, Gs can reduce the inflammatory reaction and apoptosis caused by IS in multi-angle by activating the PI3K/Akt signaling pathway. It has various active ingredients and is a high-quality natural medicine worthy of further study.
3.1.9. Radix Paeoniae Rubra
Radix Paeoniae Rubra (RPR, Family: Ranunculaceae) is the dried root of Paeonia lactifloral Pall, or Paeonia veitchii Lynch. The main active ingredient of RPR is glycosides, which have the effects of clearing heat and cooling blood, promoting blood circulation, and dispelling blood stasis. The role of paeoniflorin (PF) is particularly prominent. Studies have shown that PF is an active monomer that can promote vascular endothelial progenitor cells and angiogenesis in IS rat model [112]. PF is also the main anti-inflammatory component in RPR, which can inhibit the production of pro-inflammatory mediators such as TNF-α and IL-1β. It has been reported that PF improves functional recovery through repressing neuroinflammation and facilitating neurogenesis in rat IS model [113]. Additionally, the combination of PF and calycosin-7-glucoside can alleviate IS injury via the PI3K/Akt signaling pathway [114]. Clinical studies showed that the levels of apoptosis and inflammatory factors IL-1β decreased in PF treatment group. It is suggested that PF is a promising method for the treatment of brain I/R injury. However, additional preclinical studies are needed to more accurately evaluate the efficacy and safety of PF [115].
3.1.10. Panax notoginseng
Panax notoginseng (PN, Family: Araliaceae) is the dried root of Panax notoginseng (Burk.) F.H. PN has a variety of physiologically active ingredients, such as total saponins, volatile oil, amino acids, flavonoids, and polysaccharide.
Panax notoginseng saponins (PNS) is one of the ingredients with rich pharmacological effects. It can promote the mechanism of neural function recovery, and plays an important role in brain protection after cerebral infarction [116]. Additionally, it can also reduce leukocyte-mediated microvascular disturbance during the onset of IS [117]. According to the observed results, PNS may be an exogenous regulatory factor that activates the Nrf2 antioxidant signal through the PI3K/Akt signaling pathway to protect against OGD/R-induced BBB damage in vitro [118]. Moreover, PNS can also protect cardiomyocytes from apoptosis induced by ischemia in vivo and in vitro by activating PI3K/Akt signaling pathway [119]. Additionally, other studies have shown that PNS Rb1 can improve cognitive and sensorimotor disorders at least in part by regulating the Akt/mTOR/PTEN signaling pathway [120].
In clinical treatment, patients who received PNS combined with conventional treatments (CTs) showed significantly high improvements in neurological function among individuals with IS on the neurologic deficit score. Compared with CTs alone, PNS can significantly improve the overall response rate (ORR). In addition, the incidence of adverse reactions in PNS group was lower than that in control group and all of them were mild adverse.
In conclusion, the above evidence means that PNS may be effective and safe in treating IS on ameliorating the neurological deficit, improving activities of daily living function, and enhancing antiplatelet effects, and its mechanism is likely to be mediated by PI3K/Akt signaling pathway. However, more high-quality evidence is needed before it can be recommended for routine antiplatelet therapy in patients with IS [121].
3.2. Herbal Prescriptions
All the above natural medicines have special application in China, namely traditional Chinese medicine (TCM) prescription. TCM prescription combines effective natural medicines to treat related diseases, and each medicine can interact with each other to achieve the best therapeutic effect. TCM prescription has the characteristics of multi-target, multi-component, and multi-mechanism, which plays a synergistic effect through multiple mechanisms to produce a better effect in the treatment of cerebral ischemia. In this section, we reviewed the representative TCM prescriptions, thus revealing the relationship between it and the PI3K/Akt signaling pathway.
3.2.1. Buyang Huanwu Decoction
Buyang Huanwu Decoction (BHD) is composed of Astragalus membranaceus, Angelica sinensis, Radix Paeoniae Rubra, Chuanxiong, Safflower, and Peach kernel. BHD is commonly used in the clinical treatment of IS, coronary heart disease, and other vascular embolic diseases. The active ingredients in BHD play a potential pharmacological role in the treatment of IS by regulating the following multiple targets: (1) Propyl gallate, calycosin-7-O-beta-D-glucoside, paeonol, and ferulic acid can significantly inhibit apoptosis; (2) Propyl gallate and formononetin can significantly inhibit LPS-induced NO release; (3) HSYA and inosine can protect cells against the injuries caused by glutamate; and (4) Formononetin, AS-IV, astrISoflavan-7-O-beta-Dglucoside, inosine, PF, ononin, paeonol, propyl gallate, ligustrazine, and ferulic acid can significantly suppress the constriction of the thoracic aorta induced by KCl in rats [122]. In addition, the previous study confirmed that the ingredients of BHD may antagonize HT22 cell injury induced by cerebral I/R injury and oxygen-glucose deprivation in rats in vitro by reducing the inflammatory reaction and apoptosis, which is a novel IS protection strategy [123].
BHD is also closely related to the PI3K/Akt signaling pathway. It can promote vascular endothelial growth factor receptor-2 (VEGFR2) phosphorylation through the PI3K/Akt signaling pathway to restore neurological function and angiogenesis in mice with intracerebral hemorrhage, thus alleviating stroke [124]. Further studies have shown that BHD can significantly up-regulate p-PI3K, p-Akt, and phosphorylated Bcl-2-related death promoter (p-Bad), both in vivo and in vitro. It is suggested that BHD can achieve neuroprotection and promote nerve regeneration by activating PI3K/Akt/Bad signaling pathway, finally ameliorate IS [125].
In addition, BHD is a TCM prescription widely used in the rehabilitation of patients with IS in China. However, the methodological quality of the research it incorporates is relatively low [126]. The clinical efficacy and safety of BHD need to be well designed to ensure the clinical recommendation of BHD for the rehabilitation of patients with ischemic stroke.
3.2.2. Taohong Siwu Decoction
Taohong Siwu Decoction (THSWD) comes from “The Golden Mirror of Medicine”. It is composed of Peach kernel, Safflower, Radix rehmanniae, Radix Angelicae sinensis, Peony, and Chuangxiong. The main active ingredients of THSWD are HSYA, PF, paeonol, ligustrazine, and ferulic acid [127].
Studies have shown that THSWD can reduce the release of inflammatory factors and inhibit the activation of the complement signaling pathway, thus preventing IS [128]. Additionally, THSWD can also reduce the expression of the TNF-α pathway and reduce the necrosis of brain cells after cerebral ischemia, thus protecting the brain tissue of rats [129]. Moreover, we found that THSWD has a significant effect on the rat model of cerebral I/R injury. The mechanism may be to activate the PI3K/Akt signaling pathway to promote neovascularization after cerebral ischemia and restore neurological function in rats [130]. These results suggest that THSWD can reduce IS damage through the PI3K/Akt signaling pathway and may have a preventive effect. However, there is no clinical study on the treatment of IS with THSWD, which needs to be further confirmed.
3.2.3. Xiaoyao San
Xiaoyao San (XYS) comes from “Prescription of peaceful benevolent dispensary”. It is composed of Licorice, Radix Angelicae sinensis, Poria, Largehead atractylodes, White peony, and Radix bupleuri. The active ingredients of XYS contain saikoside, ferulic acid, ligustilide, atractylenolide, PF, albiflorin, liquiritin, glycyrrhizic acid, and pachymic acid [131]. XYS has the effect of soothing the liver and relieving depression, nourishing blood, and invigorating the spleen.
Emerging evidence demonstrates that XYS exerts anti-apoptotic and neuroprotective effects by activating the PI3K/Akt signaling pathway to inhibit Caspase-3 and Caspase-9 mediated apoptosis cascades, thus achieving the therapeutic effect of IS. XYS also has a good clinical effect in treating IS, with few adverse reactions [132].
3.2.4. Danhong Injection
Danhong injection (DHI) is composed of Danhong and Safflower. DHI has the effect of promoting blood circulation and removing blood stasis, dredging pulse, and relaxing collaterals. The active ingredients of DHI are ferulic acid, cryptotanshinone, quercetin, and anhydrosafflor yellow B [133].
Studies have shown that DHI can reduce cerebral edema and preserve ischemic penumbra after cerebral ischemia–reperfusion [134]. Additionally, DHI can also significantly down-regulate the phosphorylation levels of NF-κB and MAPK pathway proteins in ischemic brain tissue. It is suggested that DHI has an anti-neuroinflammatory effect and is helpful to improve the injury of central nervous system after IS [135]. It is worth noting that DHI is closely related to the PI3K/Akt signaling pathway. Studies have shown that DHI can significantly reduce the expression of pro-apoptotic factors Bad, Bax, and Bcl-2 through the PI3K/Akt signaling pathway, while the up-regulate the expression of anti-apoptotic factor Bcl-2 [136]. This evidence suggests that DHI may reduce the inflammatory response and apoptosis induced by IS through PI3K/Akt signaling pathway.
In addition, there are many clinical studies on the treatment of IS with DHI. Studies have shown that DHI injection combined with pivastatin can effectively inhibit inflammatory reaction and oxidative stress injury in patients with IS, and promote the recovery of neurological function [137]. Moreover, DHI has an important clinical improvement role in the NIHSS score, and no adverse events have been observed [138]. This evidence indicates that DHI is effective and safe in the treatment of IS.
3.2.5. Sanhua Decoction
Sanhua decoction (SHD) comes from “The Yellow Emperor’s Classic of Medicine”. It is composed of Rhubarb, Fructus aurantii immaturus, Magnolia officinalis, and Notopterygium. SHD mainly contains flavonoids, anthraquinones, coumarins, phenylpropanoid glycosides, alkaloids, and lignans [139].
Research shows that SHD exerts neuroprotection through regulating the phosphorylated Tau level and promoting adult endogenous neurogenesis after cerebral I/R injury [140]. Additionally, SDH can also increase the levels of p-PI3K and p-Akt, and reduce the expression of tumor necrosis factor-α and IL-6 [141]. It is suggested that SDH may have a protective effect on brain injury caused by IS by regulating the PI3K/Akt signaling pathway and tumor necrosis factor. These conclusions provide a theoretical basis for the development of SHD as a new drug for the treatment of IS.
Clinically, SHD is also widely used to treat IS, especially in reducing blood viscosity and regulating circulatory disturbances, with almost no adverse reactions [141].
3.2.6. Xingnaojing Injection
Xingnaojing injection (XNJI) is derived from Angong Niuhuang Wan. It is composed of Turmeric, Moschus, Borneolum syntheticum, and Gardenia jasminoides. The main active ingredients of XNJI are muscone, camphor, curcumin A, curcumin B, curcumone, and curcumdione [142]. XNJI has the effect of cooling blood, activating blood circulation, opening orifices, and awakening the brain.
XNJI has been shown to prevent apoptosis of human microvascular endothelial cells through the PI3K/Akt/endothelial nitric oxide synthase (ENOS) pathway in a rat model of IS [143]. In addition, muscone is one of the active ingredients in XNJI. It has also been shown to significantly reduce neuronal apoptosis through the PI3K/Akt signaling pathway [144]. It indicates that XNJI may reduce oxidative stress and neuronal apoptosis induced by IS through PI3K/Akt signaling pathway.
Clinically, the reports showed that XNJI appears to be effective and safe for the emergency treatment of IS. The first 6 h after IS may be the best time to start taking medication [145]. However, more high quality randomized controlled trials are needed to determine the appropriate start-up time.
3.3. Animal Medicine
Most of the animal medicine used for the treatment of IS is insect medicine. Among them, earthworm, leech, and scorpion can inhibit thrombosis, lipid lowering, and platelet aggregation in varying degrees. Additionally, they can also improve the cerebral blood circulation and increase the blood oxygen supply in the cerebral ischemic area. Finally, they can reduce brain injury and promote the recovery of nerve cell function [146]. In this section, we reviewed the representative insect medicine and prescriptions related to PI3K/Akt signaling pathway in the therapeutics of IS (Table 2).
Table 2.
Effects of insect medicine and their active ingredients on PI3K/Akt signaling pathway and their main purposed effects.
| Natural Medicine | Insect Atlas | Active Ingredients | Model | Regulation of PI3K/Akt Signaling Pathway |
Main Purposed Effects | Ref(s) |
|---|---|---|---|---|---|---|
|
Insect
medicine |
||||||
| Earthworm |

|
Lumbrokinase | SD rats mice |
↑: p-PI3K and p-Akt |
Anti-neuronal apoptosis Anti-inflammation |
[147,148,149,150,151] |
| Leech |

|
Hirudin; Fibrinolysin. |
IPF rats SD rats mice |
↑: p-Akt | Anti-neuronal apoptosis Anti-inflammation |
[152,153,154,155,156] |
| Scorpion |

|
Adenosine; Dipeptides. |
SD rats K562 cell |
↑: p-Akt | Anti-neuronal apoptosis | [157,158,159,160] |
|
Insect
prescriptions |
||||||
| Tongxinluo capsule |
Ginsenoside; Hirudin; Denosine; Paeoniflorin. |
SD rats mice |
↑: p-Akt | Anti-neuronal apoptosis | [161,162,163,164,165] | |
| Naoxintong capsule |
Adenosine; Hirudin; Tanshinone; Danshensu; Salvianolic Acid. |
SD rats | ↑: p-Akt | Anti-neuronal apoptosis | [166,167,168,169] | |
| Shuxuetong injection |
Hirudin; Lumbrokinase. |
SD rats | ↑: p-Akt, VEGF | Anti-oxidative stress Anti-neuronal apoptosis |
[170,171,172,173] |
↑ signifies increase/activate.
3.3.1. Earthworm
Earthworm has the effects of clearing heat, calming shock, dredging collaterals, relieving asthma, and diuresis. It is mostly used for high fever, epileptic convulsions, limb paralysis, and hemiplegia. Modern studies have found that earthworms also have antithrombotic, immune, and anti-inflammatory effects. In addition, it is an important animal medicine in China’s current guidelines for the treatment of IS.
The main active ingredient of earthworm is lumbrokinase (LKs). It is an oral supplement to support and maintain healthy cardiovascular function and has a history of clinical treatment of cardiovascular disease for more than 10 years. Studies have shown that LKs treatment after ischemia can reduce myocardial I/R injury by activating SIRT1 signal pathway, thus reducing oxidative damage, inflammatory reaction and apoptosis [147]. Additionally, LKs can decrease the protein levels of caspase-8, caspase-9, and caspase-3 by enhancing the expression of survival-related proteins PI3K/Akt and Bcl-2 [148]. In addition, emerging evidence shows that earthworm extract can improve neurological dysfunction, histomorphology, and neuronal damage in mice with cerebral ischemic penumbra. It also can up-regulate the expression of PI3K and Bcl2 protein and increase the ratio of p-Akt/Akt [149]. It is suggested that earthworm may improve the neuronal damage in the penumbra of cerebral ischemia in IS mice, and its mechanism may be through the activation of the PI3K/Akt signaling pathway.
The report confirmed that LKs can also degrade fibrin and enhance the function of vascular endothelial cells during the clinical treatment of IS, thus significantly improving the hemorheology of patients with IS [150]. Moreover, LKs combined with aspirin has been shown to be more effective in reducing recurrence of IS than aspirin alone without increasing the incidence of massive bleeding and with higher safety [151]. The above results provide direct data for Chinese clinicians to choose the treatment plan of IS.
3.3.2. Leech
Leech, which has been recorded in the “Sheng Nong’s herbal classic”. It has high medicinal value and generally grows and breeds in inland fresh water. The main active ingredient of leech can be divided into two categories: one directly acts on the coagulation system such as hirudin, and the other is protease inhibitors such as fibrinolysin. Among them, hirudin has been widely used in stroke. Natural hirudin is the strongest specific thrombin inhibitor found so far. It has antithrombotic effect with low risk of bleeding, so it is widely used in clinic [152].
Hirudin protects BV-2 microglia against OGD/R and inhibits neuroinflammation mediated by Nod-like receptor protein 3 (NLRP3) inflammatory bodies. It is a promising early intervention drug for acute ischemic stroke [153]. Studies have shown that hirudin can reduce inflammatory cell infiltration and interstitial collagen accumulation through the PI3K/Akt signaling pathway [154]. Additionally, it has been reported that hirudin may protect the kidney by ameliorating renal autophagy impairment through modulating the PI3K/Akt pathway [155]. These results suggest that hirudin may attenuate IS-induced inflammation and autophagy through the PI3K/Akt signaling pathway.
Moreover, clinical treatment results of a study showed that hirudin combined with aspirin significantly reduced the safety risk of secondary prevention of non-valvular atrial fibrillation thrombotic stroke, indicating that hirudin has great potential in clinical treatment of IS [156].
3.3.3. Scorpion
At present, the global pharmacological research on scorpion is mainly focused on anti-thrombosis, anticoagulation, fibrinolysis, analgesia, anti-epilepsy, anti-tumor, and immune regulation. Low molecular compounds with anticoagulant activity have been isolated from scorpion venom, and their structures have been determined to be adenosine, dipeptide Leu Trp and Ile Trp [157].
Adenosine is a platelet agglutination inhibitor with anticoagulant properties [158]. Studies have shown that adenosine system plays a positive role in the treatment of IS and may open the way for the development of more targeted treatments and biomarkers for IS [159]. In addition, it has been confirmed that scorpion venom polypeptide extract (PESV) may inhibit the proliferation of K562 cells by regulating PI3K and p-Akt signal proteins, which has been involved in the development of prostate cancer [160]. Combined with the remarkable therapeutic effect of scorpion on stroke, its mechanism of the PI3K/Akt signaling pathway could be further studied.
3.4. Commonly Used Prescriptions of Traditional Chinese Medicine Containing Insects
3.4.1. Tongxinluo Capsule
Tongxinluo capsule (TXL) is composed of Ginseng, Leech, Scorpion, Ground Beetle, Centipede, Cicadae Periostracum, Radix Paeoniae Rubra, and Borneol. The main active ingredients are GS, hirudin, and PF. TXL has been widely used in China for the treatment of acute stroke and for neuroprotection.
Studies have shown that TXL can protect the blood-brain barrier after stroke by inhibiting lipoprotein receptor-related protein 1 (LRP-1) pathway [161]. After TXL treatment, the loss of neurons in prefrontal cortex was significantly reduced and the neurological function was significantly improved [162]. Additionally, TXL also alleviates cerebral microcirculatory disturbances against ischemic injury by modulating endothelial function and inhibiting leukocyte-endothelial cell interactions [163]. We also found that TXL can significantly up-regulate the expression of phosphoinositide-dependent kinase 1 (p-PDK1) and p-Akt by activating PI3K/Akt signaling pathway, which has a neuroprotective effect on brain I/R injury and neuronal apoptosis [164]. This suggests that TXL may improve IS-induced apoptosis and brain damage through PI3K/Akt signaling pathway.
TXL is superior to conventional treatment in improving clinical overall response rate and hemorheological indexes and is relatively safe. Due to the lack of existing research, more well-designed high-quality research is needed for further verification [165].
3.4.2. Naoxintong Capsule
Naoxintong capsule (NXT) is a clinical prescription for ischemic cerebrovascular disease, which is mainly composed of Scorpion, Leech, Salvia miltiorrhiza, Achyranthes bidentata, Frankincense, Myrrh, Suberect Spatholobus Stem, and Mulberry branch. The main active ingredients are adenosine, hirudin, tanshinone, danshensu, and salvianolic acid.
Emerging evidence has shown that NXT can effectively inhibit inflammation and oxidative stress, and reduce other pathological injuries caused by cerebral ischemia cascade reaction. Additionally, NXT can inhibit the expression of NF-κB and down-regulate the expression of inflammatory cytokines IL-1β, IL-6, and TNF-α. Thus, PI3K/Akt signaling pathway is activated, which plays anti-inflammatory, antioxidant, and cell protective roles [166,167]. This indicates that NXT may ameliorate IS-induced cellular inflammation, oxidative stress, and brain damage through the PI3K/Akt signaling pathway.
In clinical use, it has been found that NXT combined with aspirin may provide an alternative drug for the prevention of IS in elderly nonvalvular atrial fibrillation patients who are not tolerant to warfarin [168]. Additionally, a meta-analysis also confirmed the reliability of NXT in the efficacy and safety of IS [169].
3.4.3. Shuxuetong Injection
Shuxuetong (SXT) injection is a preparation of animal traditional Chinese medicine composed of hirudin in leech and lumbrokinase in earthworm. SXT has the effect of promoting blood circulation and removing blood stasis, and has been widely used in the treatment of IS.
An experimental study in vitro confirmed that SXT can inhibit the production of reactive oxygen species and mitochondrial superoxide by increasing the activity and expression of Bcl-2. It can also activate NF-κB and inhibit the expression of pro-inflammatory factors [170]. In addition, SXT can also restore and adjust the metabolic disorder of cerebral cortex and hippocampus in brain I/R rats [171]. Moreover, SXT has been proven to have the effect of promoting angiogenesis and improving cerebral perfusion [172]. Moreover, the exact target pathway of SXT in the treatment of IS has been confirmed by experimental studies. SXT can repair cerebral ischemic injury in rats by using the PI3K/Akt signaling pathway, MAPK signal pathway, HIF-1 signal pathway, and metabolic pathway-related proteins as targets [172]. Combined with the above evidence, it is suggested that SXT may alleviate IS-induced apoptosis and oxidative stress through the PI3K/Akt signaling pathway.
In clinical treatment, previous studies have shown that SXT is effective in the treatment of cerebral infarction with few adverse reactions. However, caution should be taken against the use with pregnant women and patients with hemorrhagic diseases [173].
3.5. Dosage of Natural Medicine in Animals and Humans
Most of the above natural medicines and their prescriptions can be used in animal experiments and clinics. Therefore, we also reviewed the commonly used medicine doses for the treatment of IS in this section. It can provide a dosage basis for the subsequent application of these natural drugs to IS.
Studies have shown that these drugs are administered in a variety of ways in rats or mice experiments, including oral administration, intravenous injection, intraperitoneal injection, and intramuscular injection. In addition, it has a wide range of administration ranging from 0.27 mg/kg to 100 mg/kg. It is suggested that great attention should be paid to the dose problem when using these drugs to treat IS. However, the clinical use of these drugs is incomplete. The current administration methods used to treat patients with IS are mainly oral and intravenous. It should be noted that the use of TCM prescriptions here is special in China’s clinical practice. For example, in the clinical use of BHD, the quality of each medicine in this prescription is adjusted on the basis of the original according to the patient’s disease. We have summarized the current animal and clinical doses in Table 3.
Table 3.
Doses of active ingredients and related prescriptions of natural medicine.
| Medicine | Dose in Animals | Dose in Human | Ref(s) |
|---|---|---|---|
| Active ingredients | |||
| Ligustrazine | 20 mg/kg/d in rat (i.p.) | 80–240 mg/d (i.v.) | [67,174] |
| Tanshinone IIA | 30 mg/kg/d in rat (i.v.) 10 mg/kg/d in mice (i.p.) |
60 mg/d (i.v.) | [75,175,176,177] |
| Ligustilide | 20–40 mg/kg/day in rat (p.o.) 5–20 mg/kg/d in mice (i.p.) |
/ | [178,179] |
| Hydroxysafflor Yellow A | 10–40 mg/kg/d in rat (i.v.) 2 mg/kg/d in mice (i.v.) |
25–70 mg/d (i.v.) | [93,180,181] |
| Ginsenoside Rd | 25 mg/kg/d in rat (i.p.) 10–50 mg/kg/d in mice (i.p.) |
10–20 mg/d (i.v.) | [182,183,184] |
| Ginsenoside-Rb1 | 25–100 mg/kg/d in rat (i.p.) | / | [185] |
| Ginsenoside-Rg1 | 20 mg/kg/d in rat (i.p.) 10–40 mg/kg/d in mice (i.p.) |
/ | [107,186] |
| Paeoniflorin | 40 mg/kg/d in rat (i.p.) | 3–9 g/d (i.v.) | [112,187] |
| Panax notoginseng Saponins | 25–100 mg/kg/d in rat (i.p.) 45 mg/kg/d in mice (i.p.) |
500 mg/d (i.v.) | [117,188,189] |
| Salvianolic Acid B | 10–20 mg/kg/d in rat (i.p.) | / | [70] |
| Breviscapus | 3–6 mL/kg in rat (i.v.) | / | [190] |
| Astragaloside IV | 2g/kg/d in rat (i.v.) 20 mg/kg in rat (i.p.) 200 mg/kg in mice (i.p.) |
/ | [82,83,191] |
| Lumbrokinase | / | 1,800,000 units/d (p.o.) | [151] |
| Hirudin | 10–40 mg/kg in mice (p.o.) | 2.25 g/d (p.o.) | [153,156] |
| Prescriptions | Formula/dose | ||
| Buyang Huanwu Decoction |
10–40 g/kg in rat (p.o.) 1.0 g/kg in mice (p.o), twice daily |
raw Astragalus 30 g, angelica 15 g, longan meat 15 g, antler gum 10 g, Salvia miltiorrhiza 10 g, frankincense 10 g, myrrh 10 g, and dried pine 5 g | [192,193,194,195] |
| Taohong Siwu decoction |
4.5–18 g/kg/d in rat (p.o.) | / | [196] |
| Danhong injection | 0.75–3 mL/kg in rat (i.v.), twice daily 3 mL/kg/d in mice (i.m.) |
20–40 mL/d (i.v.) | [134,138,197,198] |
| Sanhua decoction | 10 g/kg/d in rat (p.o.) | / | [140] |
| Xingnaojing injection |
0.75–3 mL/kg/d in rat (i.m.) 6 mg/kg/d in mice (i.m.) |
20 mL/12 h (i.v.) | [143,199] |
| Tongxinluo capsule |
100 mg/kg/d in rat (p.o.) 0.75–3.0 g/kg/d in mice (p.o.) |
1.56–3.12 g/d (p.o.) | [161,162,163] |
| Naoxintong capsule |
0.5 g/kg/d in rat (p.o.) | 2.4 g/d (p.o.) | [200,201,202] |
| Shuxuetong injection |
6 mL/kg/d in rat (i.v.) 0.27–1.08 mg/kg/d in rat (i.p.) |
6–10 mL/d (i.v.) | [171,172,203] |
/ Representative did not report.
4. Future Prospects
The pathological mechanism of ischemic stroke is complex, which involves the interaction of a variety of molecular signal transduction pathways. The PI3K/Akt signaling pathway is an important pathway in the pathogenesis of IS and plays an important role in anti-neuronal apoptosis, oxidative stress, inflammation, and autophagy.
Natural medicine, and its prescriptions, can improve and treat ischemic stroke by targeting the PI3K/Akt signaling pathway. Additionally, some progress has been made in its related research. This review systematically expounds the relationship between natural medicine and the PI3K/Akt signaling pathway and the pathogenesis of IS, which is convenient to understand the pathological process of IS as a whole and provide a theoretical basis for the treatment of IS with traditional natural medicine. Although in the current research, natural medicines and prescriptions have been shown to improve and treat IS by targeting the PI3K/Akt signaling pathway, there are still many mechanisms to be explored.
Firstly, the current research on IS and the PI3K/Akt signaling pathway is relatively weak, and some studies have not verified the inhibition or activation of this pathway. Therefore, it is difficult to achieve results using its inhibitors or activators to verify forward and backward mechanisms. In addition, most of the experimental types are animal experiments and the research methods are simple. Clinical verification should be increased, and subsequent animal experiments and clinical observations can be combined to establish a unique database of IS. On this basis, new goals and ideas are put forward. Moreover, the target of PI3K/Akt signaling pathway in the treatment of IS is only supported by classical target proteins, which cannot judge the participation of this pathway in the process of IS as a whole. It needs to be further improved.
Overall, according to the promotion of the PI3K/Akt signaling pathway in the pathophysiological process of IS, it is suggested that the PI3K/Akt signaling pathway may be an effective way of targeted medicine therapy for IS and it can delay and hinder the occurrence and development of IS. Due to the multi-target therapeutic effect of natural medicine, the specific mechanism of action of related prescriptions and active components through the PI3K/Akt signaling pathway can be explored from the perspective of molecular biology. This can provide new drug development strategies for the improvement and treatment of IS.
Furthermore, transient ischemic attack (TIA) has also been the focus of clinical research in recent years, and it is closely related to IS. Stroke risk after TIA is highest in the first few days, but it is a benign and reversible cerebral ischemia syndrome. Therefore, early intervention of TIA can also reduce the probability of IS. We can also use TIA as a point to study the relationship between the PI3K/Akt signaling pathway and TIA and its possible mechanism, and discuss how natural medicine can treat TIA and IS through the PI3K/Akt signaling pathway from multiple perspectives and links.
Acknowledgments
The author thanks the College of Traditional Chinese Medicine of Xinjiang Medical University for its support.
Author Contributions
Writing—original draft preparation: X.L. and X.X.; writing—review and editing: W.L., L.Y. and X.H. Project guidance and financial support: W.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National key research and development program (No. 2017YFC1703901) and (No. 2017YFC1703902).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang W., Qiao O., Ji H., Zhang X., Han X., Zhang Y., Wang J., Li X., Gao W. Autophagy in vascular dementia and natural products with autophagy regulating activity. Pharmacol. Res. 2021;170:105756. doi: 10.1016/j.phrs.2021.105756. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 3.Farina M., Vieira L.E., Buttari B., Profumo E., Saso L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules. 2021;26:5001. doi: 10.3390/molecules26165001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y., Zhang X., Chen X., Wei Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review) Int. J. Mol. Med. 2022;49:15. doi: 10.3892/ijmm.2021.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oates C.P., Bienstock S.W., Miller M., Giustino G., Danilov T., Kukar N., Kocovic N., Sperling D., Singh R., Benhuri D., et al. Using Clinical and Echocardiographic Characteristics to Characterize the Risk of Ischemic Stroke in Patients with COVID-19. J. Stroke Cerebrovasc. Dis. 2022;31:106217. doi: 10.1016/j.jstrokecerebrovasdis.2021.106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu W.J., Yan F., Chao B.H., Cao L., Wang L. Treatment and 1-Year Prognosis of Ischemic Stroke in China in 2018: A Hospital-Based Study From Bigdata Observatory Platform for Stroke of China. Stroke. 2022;53:e415–e417. doi: 10.1161/STROKEAHA.121.038260. [DOI] [PubMed] [Google Scholar]
- 7.Trinh X.T., Long N.V., Van Anh L.T., Nga P.T., Giang N.N., Chien P.N., Nam S.Y., Heo C.Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int J. Mol. Sci. 2022;23:9573. doi: 10.3390/ijms23179573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu C., Zhang Q., Li Y., Li R., Feng J., Chen W., Ahmed W., Soufiany I., Huang S., Long J., et al. The PI3K/AKT Pathway-The Potential Key Mechanisms of Traditional Chinese Medicine for Stroke. Front. Med. (Lausanne) 2022;9:900809. doi: 10.3389/fmed.2022.900809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J.L., Zhao X.X., Zhang Y.Y., Wan H.T., He Y., Li X.H., Yu L., Jin W.F. Comparison of Traditional Chinese Medicine in the Long-Term Secondary Prevention for Patients with Ischemic Stroke: A Systematical Analysis. Front. Pharmacol. 2021;12:722975. doi: 10.3389/fphar.2021.722975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X.H., Yin F.T., Zhou X.H., Zhang A.H., Sun H., Yan G.L., Wang X.J. The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke. Molecules. 2022;27:3099. doi: 10.3390/molecules27103099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A., Mehan S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021;147:105067. doi: 10.1016/j.neuint.2021.105067. [DOI] [PubMed] [Google Scholar]
- 12.Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021;12:628690. doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roudsari N.M., Lashgari N.A., Momtaz S., Abaft S., Jamali F., Safaiepour P., Narimisa K., Jackson G., Bishayee A., Rezaei N., et al. Inhibitors of the PI3K/Akt/mTOR Pathway in Prostate Cancer Chemoprevention and Intervention. Pharmaceutics. 2021;13:1195. doi: 10.3390/pharmaceutics13081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q., Jin Z., Xu Z., Yang H., Li L., Li G., Li F., Gu S., Zong S., Zhou J., et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24:441–452. doi: 10.1007/s12192-019-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He X., Li Y., Deng B., Lin A., Zhang G., Ma M., Wang Y., Yang Y., Kang X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022;55:e13275. doi: 10.1111/cpr.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Xu P., Liu D., Wang R., Cui S., Zhang Q., Li Y., Yang W., Zhang D. TCM Regulates PI3K/Akt Signal Pathway to Intervene Atherosclerotic Cardiovascular Disease. Evid. Based Complement. Alternat. Med. 2021;2021:4854755. doi: 10.1155/2021/4854755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin T., Zhang Y., Botchway B.O.A., Zhang J., Fan R., Zhang Y., Liu X. Curcumin can improve Parkinson’s disease via activating BDNF/PI3k/Akt signaling pathways. Food Chem. Toxicol. 2022;164:113091. doi: 10.1016/j.fct.2022.113091. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M., Zhang X. The role of PI3K/AKT/FOXO signaling in psoriasis. Arch. Dermatol. Res. 2019;311:83–91. doi: 10.1007/s00403-018-1879-8. [DOI] [PubMed] [Google Scholar]
- 19.Szymonowicz K., Oeck S., Malewicz N.M., Jendrossek V. New Insights into Protein Kinase B/Akt Signaling: Role of Localized Akt Activation and Compartment-Specific Target Proteins for the Cellular Radiation Response. Cancers. 2018;10:78. doi: 10.3390/cancers10030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linton M.F., Moslehi J.J., Babaev V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019;20:2709. doi: 10.3390/ijms20112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M., Zhang J., Gong N. Role of the PI3K/Akt signaling pathway in liver ischemia reperfusion injury: A narrative review. Ann. Palliat. Med. 2022;11:806–817. doi: 10.21037/apm-21-3286. [DOI] [PubMed] [Google Scholar]
- 22.Revathidevi S., Munirajan A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang A.-J., Wang S., Wang P., Wang Y.R. Progress in Pathological Mechanism of Ischemic Stroke and Prevention and Treatment of Traditional Chinese Medicine. Chin. J. Exp. Tradit. Med. 2020;26:227–240. [Google Scholar]
- 24.Liu R., Song P., Gu X., Liang W., Sun W., Hua Q., Zhang Y., Qiu Z. Comprehensive Landscape of Immune Infiltration and Aberrant Pathway Activation in Ischemic Stroke. Front. Immunol. 2021;12:766724. doi: 10.3389/fimmu.2021.766724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu X.K., Zhang H.B., Shi S.S., Liang R.S., Wang C.H., Chen C.M., Yang W.Z. 5-LOX Inhibitor Zileuton Reduces Inflammatory Reaction and Ischemic Brain Damage Through the Activation of PI3K/Akt Signaling Pathway. Neurochem. Res. 2016;41:2779–2787. doi: 10.1007/s11064-016-1994-x. [DOI] [PubMed] [Google Scholar]
- 26.Su X.T., Wang L., Ma S.M., Cao Y., Yang N.N., Lin L.L., Fisher M., Yang J.W., Liu C.Z. Mechanisms of Acupuncture in the Regulation of Oxidative Stress in Treating Ischemic Stroke. Oxid. Med. Cell Longev. 2020;2020:7875396. doi: 10.1155/2020/7875396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayaraj R.L., Azimullah S., Beiram R., Jalal F.Y., Rosenberg G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonaventura A., Liberale L., Vecchie A., Casula M., Carbone F., Dallegri F., Montecucco F. Update on Inflammatory Biomarkers and Treatments in Ischemic Stroke. Int. J. Mol. Sci. 2016;17:1967. doi: 10.3390/ijms17121967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Y., Nie D., Wang L.J., Qiu H.C., Ma L., Dong M.X., Tu W.J., Zhao J. Microglial Polarization: Novel Therapeutic Strategy against Ischemic Stroke. Aging Dis. 2021;12:466–479. doi: 10.14336/AD.2020.0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.John Aaron Howell G.L.B.I. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther. Deliv. 2020;11:113–123. doi: 10.4155/tde-2019-0075. [DOI] [PubMed] [Google Scholar]
- 31.Joshi A.U., Orset C., Engelhardt B., Baumgart-Vogt E., Gerriets T., Vivien D., Kanse S.M. Deficiency of Factor VII activating protease alters the outcome of ischemic stroke in mice. Eur. J. Neurosci. 2015;41:965–975. doi: 10.1111/ejn.12830. [DOI] [PubMed] [Google Scholar]
- 32.Yang C., Hawkins K.E., Dore S., Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019;316:C135–C153. doi: 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv H., Li J., Che Y.Q. CXCL8 gene silencing promotes neuroglial cells activation while inhibiting neuroinflammation through the PI3K/Akt/NF-kappaB-signaling pathway in mice with ischemic stroke. J. Cell Physiol. 2019;234:7341–7355. doi: 10.1002/jcp.27493. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M., Hou S., Feng L., Shen P., Nan D., Zhang Y., Wang F., Ma D., Feng J. Vinpocetine Protects Against Cerebral Ischemia-Reperfusion Injury by Targeting Astrocytic Connexin43 via the PI3K/AKT Signaling Pathway. Front. Neurosci. 2020;14:223. doi: 10.3389/fnins.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao L.-L., Hao D.-L., Mao X.-W., Xu Y.-F., Huang T.-T., Wu B.-N., Wang L.-H. Neuroprotective effects of bisperoxovanadium on cerebral ischemia by inflammation inhibition. Neurosci. Lett. 2015;602:120–125. doi: 10.1016/j.neulet.2015.06.040. [DOI] [PubMed] [Google Scholar]
- 36.Deng S.-J., Ge J.-W., Xia S.-N., Zou X.-X., Bao X.-Y., Gu Y., Xu Y., Meng H.-L. Fraxetin alleviates microglia-mediated neuroinflammation after ischemic stroke. Ann. Transl. Med. 2022;10:439. doi: 10.21037/atm-21-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrabi S.S., Parvez S., Tabassum H. Ischemic stroke and mitochondria: Mechanisms and targets. Protoplasma. 2020;257:335–343. doi: 10.1007/s00709-019-01439-2. [DOI] [PubMed] [Google Scholar]
- 38.Tuo Q.Z., Zhang S.T., Lei P. Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Med. Res. Rev. 2022;42:259–305. doi: 10.1002/med.21817. [DOI] [PubMed] [Google Scholar]
- 39.Chen S., Peng J., Sherchan P., Ma Y., Xiang S., Yan F., Zhao H., Jiang Y., Wang N., Zhang J.H., et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 2020;17:168. doi: 10.1186/s12974-020-01853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong P., Zou Y., Zhang W., Tian Q., Han S., Xu Z., Chen Q., Wang X., Li M. The neuroprotective effects of Insulin-Like Growth Factor 1 via the Hippo/YAP signaling pathway are mediated by the PI3K/AKT cascade following cerebral ischemia/reperfusion injury. Brain Res. Bull. 2021;177:373–387. doi: 10.1016/j.brainresbull.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Wang M.M., Zhang M., Feng Y.S., Xing Y., Tan Z.X., Li W.B., Dong F., Zhang F. Electroacupuncture Inhibits Neuronal Autophagy and Apoptosis via the PI3K/AKT Pathway Following Ischemic Stroke. Front. Cell Neurosci. 2020;14:134. doi: 10.3389/fncel.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou Y., Wang K., Wan W., Cheng Y., Pu X., Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018;5:245–255. doi: 10.1016/j.gendis.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dringen R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000;62:649–671. doi: 10.1016/S0301-0082(99)00060-X. [DOI] [PubMed] [Google Scholar]
- 44.Ren J.X., Li C., Yan X.L., Qu Y., Yang Y., Guo Z.N. Crosstalk between Oxidative Stress and Ferroptosis/Oxytosis in Ischemic Stroke: Possible Targets and Molecular Mechanisms. Oxid. Med. Cell Longev. 2021;2021:6643382. doi: 10.1155/2021/6643382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon S.-H., Ma S.-X., Hwang J.-Y., Ko Y.-H., Seo J.-Y., Lee B.-R., Lee S.-Y., Jang C.-G. The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-kappa B Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells. Biomol. Ther. 2016;24:268–282. doi: 10.4062/biomolther.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J., Li Y., Lin S., Zhao W., Chen Y., Jin H. Collagen peptides from Acaudina molpadioides prevent CCl4-induced liver injury via Keap1/Nrf2-ARE, PI3K/AKT, and MAPKs pathways. J. Food Sci. 2022;87:2185–2196. doi: 10.1111/1750-3841.16142. [DOI] [PubMed] [Google Scholar]
- 47.Samakova A., Gazova A., Sabova N., Valaskova S., Jurikova M., Kyselovic J. The pi3k/Akt Pathway Is Associated With Angiogenesis, Oxidative Stress and Survival of Mesenchymal Stem Cells in Pathophysiologic Condition in Ischemia. Physiol. Res. 2019;68:S131–S138. doi: 10.33549/physiolres.934345. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z.G., Zhang H.Y., Xu X.L., Shi H.X., Yu X.C., Wang X.J., Yan Y.B., Fu X.B., Hu H.W., Li X.K., et al. bFGF inhibits ER stress induced by ischemic oxidative injury via activation of the PI3K/Akt and ERK1/2 pathways. Toxicol. Lett. 2012;212:137–146. doi: 10.1016/j.toxlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Li Z.M., Cao X., Xiao L.G., Zhou R.J. Aloperine protects against cerebral ischemia/reperfusion injury via activating the PI3K/AKT signaling pathway in rats. Exp. Ther. Med. 2021;22:1045. doi: 10.3892/etm.2021.10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang L., Zhu J., Yang L., Gan Y., Hu D., Zhao J., Zhao Y. SCO-spondin-derived peptide NX210 rescues neurons from cerebral ischemia/reperfusion injury through modulating the Integrin-beta1 mediated PI3K/Akt pathway. Int. Immunopharmacol. 2022;111:109079. doi: 10.1016/j.intimp.2022.109079. [DOI] [PubMed] [Google Scholar]
- 51.Wang C., Chen H., Jiang H.H., Mao B.B., Yu H. Total Flavonoids of Chuju Decrease Oxidative Stress and Cell Apoptosis in Ischemic Stroke Rats: Network and Experimental Analyses. Front. Neurosci. 2021;15:772401. doi: 10.3389/fnins.2021.772401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu K., Gao Y., Chu S., Chen N. Review of the effects and Mechanisms of microglial autophagy in ischemic stroke. Int. Immunopharmacol. 2022;108:108761. doi: 10.1016/j.intimp.2022.108761. [DOI] [PubMed] [Google Scholar]
- 53.Wang J.-Y., Liu P., Harris P.D.R., Sagi A., Field R.E., Sochart D.H., Tucker K., Asopa V. Severe Global Cerebral IschemiaYInduced Programmed Necrosis of Hippocampal CA1 Neurons in Rat Is Prevented by 3-Methyladenine: A Widely Used Inhibitor of Autophagy. J. Neuropathol. Exp. Neurol. 2011;70:314–322. doi: 10.1097/NEN.0b013e31821352bd. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Fang Y., Huang Q., Xu P., Lenahan C., Lu J., Zheng J., Dong X., Shao A., Zhang J. An updated review of autophagy in ischemic stroke: From mechanisms to therapies. Exp. Neurol. 2021;340:113684. doi: 10.1016/j.expneurol.2021.113684. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y., Tian Z., Wan H., Liu W., Kong F., Ma G. Deltonin Ameliorates Cerebral Ischemia/Reperfusion Injury in Correlation with Modulation of Autophagy and Inflammation. Neuropsychiatr. Dis. Treat. 2020;16:871–879. doi: 10.2147/NDT.S227988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang B., Li Y., Ma Y., Zhang X., Yang L., Shen X., Zhang J., Jing L. Selenium attenuates ischemia/reperfusion injury-induced damage to the blood-brain barrier in hyperglycemia through PI3K/AKT/mTOR pathway-mediated autophagy inhibition. Int. J. Mol. Med. 2021;48:1–11. doi: 10.3892/ijmm.2021.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen X., Huang Y., Xie Y., Wang J., Ma Y., Wang G., Gao Y., Lan R. Nested case-control study on the effect of PI3K autophagy signaling pathway on the recurrence of ischemic stroke. China J. Tradit. Chin. Med. Pharm. 2021;36:3713–3716. [Google Scholar]
- 58.Hua Y., Zhai Y., Wang G., Wang N., Wu Q., Huang Q., Seto S., Wang Y. Tong-Qiao-Huo-Xue decoction activates PI3K/Akt/mTOR pathway to reduce BMECs autophagy after cerebral ischemia/reperfusion injury. J. Ethnopharmacol. 2022;298:115585. doi: 10.1016/j.jep.2022.115585. [DOI] [PubMed] [Google Scholar]
- 59.Jin L., Mo Y., Yue E.L., Liu Y., Liu K.Y. Ibrutinib ameliorates cerebral ischemia/reperfusion injury through autophagy activation and PI3K/Akt/mTOR signaling pathway in diabetic mice. Bioengineered. 2021;12:7432–7445. doi: 10.1080/21655979.2021.1974810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao W., Yan Y., Bao T.H., Jia W.J., Yang F., Wang Y., Zhu Y.H., Yin M., Han J.H. Ischemic postconditioning exerts neuroprotective effect through negatively regulating PI3K/Akt2 signaling pathway by microRNA-124. Biomed. Pharmacother. 2020;126:109786. doi: 10.1016/j.biopha.2019.109786. [DOI] [PubMed] [Google Scholar]
- 61.Liu J.-L., Zheng S.-L., Fan Q.-J., Yuan J.-C., Yang S.-M., Kong F.-L. A Fast HPLC Quantitative Determination of Ligustrazine in Rhizome of Ligusticum chuanxiong. Asian J. Chem. 2014;26:5026–5028. doi: 10.14233/ajchem.2014.16303. [DOI] [Google Scholar]
- 62.Zhao T., Fu Y., Sun H., Liu X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life. 2018;70:60–70. doi: 10.1002/iub.1704. [DOI] [PubMed] [Google Scholar]
- 63.Zhang L., Du J.R., Wang J., Yu D.K., Chen Y.S., He Y., Wang C.Y. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi. 2009;129:855–859. doi: 10.1248/yakushi.129.855. [DOI] [PubMed] [Google Scholar]
- 64.Huang H., Kong L., Luan S., Qi C., Wu F. Ligustrazine Suppresses Platelet-Derived Growth Factor-BB-Induced Pulmonary Artery Smooth Muscle Cell Proliferation and Inflammation by Regulating the PI3K/AKT Signaling Pathway. Am. J. Chin. Med. 2021;49:437–459. doi: 10.1142/S0192415X21500208. [DOI] [PubMed] [Google Scholar]
- 65.Li G., Liu S., Wang H., Pan R., Tang H., Yan X., Wang Y., Fu Y., Jing F., Dong J. Ligustrazine ameliorates lipopolysaccharide-induced neurocognitive impairment by activating autophagy via the PI3K/AKT/mTOR pathway. Int. J. Mol. Med. 2020;45:1711–1720. doi: 10.3892/ijmm.2020.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Y., Du J., Cui F., Chen L., Li K. The protective effect of ligustrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Hum. Exp. Toxicol. 2019;38:1168–1177. doi: 10.1177/0960327119851260. [DOI] [PubMed] [Google Scholar]
- 67.Ni X.J., Liu S.N., Guo X.F. Medium- and long-term efficacy of ligustrazine plus conventional medication on ischemic stroke: A systematic review and meta-analysis. J. Tradit. Chin. Med. 2013;33:715–720. doi: 10.1016/S0254-6272(14)60002-9. [DOI] [PubMed] [Google Scholar]
- 68.Su C.-Y., Ming Q.-L., Rahman K., Han T., Qin L.-P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015;13:163–182. doi: 10.1016/S1875-5364(15)30002-9. [DOI] [PubMed] [Google Scholar]
- 69.Chien M.Y., Chuang C.H., Chern C.M., Liou K.T., Liu D.Z., Hou Y.C., Shen Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free. Radic. Biol. Med. 2016;99:508–519. doi: 10.1016/j.freeradbiomed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Bi S.J., Dong X.Y., Wang Z.Y., Fu S.J., Li C.L., Wang Z.Y., Xie F., Chen X.Y., Xu H., Cai X.J., et al. Salvianolic acid B alleviates neurological injury by upregulating stanniocalcin 1 expression. Ann. Transl. Med. 2022;10:739. doi: 10.21037/atm-21-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhuang P., Zhang Y., Cui G., Bian Y., Zhang M., Zhang J., Liu Y., Yang X., Isaiah A.O., Lin Y., et al. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS ONE. 2012;7:e35636. doi: 10.1371/journal.pone.0035636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y.T., He Y., Wei X.Y., Wan H.T., Ding Z.S., Yang J.H., Zhou H.F. Network Pharmacology and Molecular Docking-Based Mechanism Study to Reveal the Protective Effect of Salvianolic Acid C in a Rat Model of Ischemic Stroke. Front. Pharmacol. 2022;12:799448. doi: 10.3389/fphar.2021.799448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang Q., Han R., Xiao H., Shen J., Luo Q., Li J. Neuroprotective effects of tanshinone IIA and/or tetramethylpyrazine in cerebral ischemic injury in vivo and in vitro. Brain Res. 2012;1488:81–91. doi: 10.1016/j.brainres.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z., Sun Y., Bian L., Zhang Y., Zhang Y., Wang C., Tian J., Lu T. The crosstalk signals of Sodium Tanshinone A Sulfonate in rats with cerebral ischemic stroke: Insights from proteomics. Biomed. Pharmacother. 2022;151:113059. doi: 10.1016/j.biopha.2022.113059. [DOI] [PubMed] [Google Scholar]
- 75.Ji B.Y., Zhou F., Han L.J., Yang J., Fan H.J., Li S.S., Li J.W., Zhang X., Wang X.Y., Chen X.Y., et al. Sodium Tanshinone IIA Sulfonate Enhances Effectiveness Rt-PA Treatment in Acute Ischemic Stroke Patients Associated with Ameliorating Blood-Brain Barrier Damage. Transl. Stroke Res. 2017;8:334–340. doi: 10.1007/s12975-017-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lei T., Li H., Fang Z., Lin J., Wang S., Xiao L., Yang F., Liu X., Zhang J., Huang Z., et al. Polysaccharides from Angelica sinensis alleviate neuronal cell injury caused by oxidative stress. Neural Regen. Res. 2014;9:260–267. doi: 10.4103/1673-5374.128218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu H.B., Chen J., Liu W.B., Li H., Yu Z.H., Zeng C. The Effect of Angelica sinensis Polysaccharide on Neuronal Apoptosis in Cerebral Ischemia-Reperfusion Injury via PI3K/AKT Pathway. Int. J. Polym. Sci. 2021:2021. doi: 10.1155/2021/7829341. [DOI] [Google Scholar]
- 78.Han Y., Chen Y., Zhang Q., Liu B.W., Yang L., Xu Y.H., Zhao Y.H. Overview of therapeutic potentiality of Angelica sinensis for ischemic stroke. Phytomedicine. 2021;90:153652. doi: 10.1016/j.phymed.2021.153652. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y.-P., Wang K.-X., Cai J.-Q., Li Y., Yu H.-L., Wu Q., Meng W., Wang H.-D., Yin C.-H., Wu J., et al. Detecting Key Functional Components Group and Speculating the Potential Mechanism of Xiao-Xu-Ming Decoction in Treating Stroke. Front. Cell Dev. Biol. 2022;10:7829341. doi: 10.3389/fcell.2022.753425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Q., Mao Z., Liu J., Huang J., Wang N. Ligustilide Attenuates Ischemia Reperfusion-Induced Hippocampal Neuronal Apoptosis via Activating the PI3K/Akt Pathway. Front. Pharmacol. 2020;11:979. doi: 10.3389/fphar.2020.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo W.Y., Liu C.H., Chen C.H., Hsieh C.L. Proteomics analysis of protein biomarkers in Astragalus membranaceus- and Astragaloside IV-treated brain tissues in ischemia-reperfusion injured rats. J. Tradit. Complement. Med. 2021;11:369–374. doi: 10.1016/j.jtcme.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Zhang Y., Jin X.F., Zhou X.H., Dong X.H., Yu W.T., Gao W.J. The Role of Astragaloside IV against Cerebral Ischemia/Reperfusion Injury: Suppression of Apoptosis via Promotion of P62-LC3-Autophagy. Molecules. 2019;24:1838. doi: 10.3390/molecules24091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun L., Han R., Guo F., Chen H., Wang W., Chen Z., Liu W., Sun X., Gao C. Antagonistic effects of IL-17 and Astragaloside IV on cortical neurogenesis and cognitive behavior after stroke in adult mice through Akt/GSK-3beta pathway. Cell Death Discov. 2020;6:74. doi: 10.1038/s41420-020-00298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma L.-L., Sun L.-M., Dong Y., Yi B.-H. Effects of Astragali Radix total flavonoids on oxidative stress inflammation and apoptosis of rats with cerebral ischemia-reperfusion injury. Chin. Tradit. Pat. Med. 2019;41:1811–1815. [Google Scholar]
- 85.Tan Y.Y., Yin L., Sun Z.F., Shao S.S., Chen W.B., Man X., Du Y.F., Chen Y. Astragalus polysaccharide exerts anti-Parkinson via activating the PI3K/AKT/mTOR pathway to increase cellular autophagy level in vitro. Int. J. Biol. Macromol. 2020;153:349–356. doi: 10.1016/j.ijbiomac.2020.02.282. [DOI] [PubMed] [Google Scholar]
- 86.Ke B., Ke X., Wan X.S., Yang Y.B., Huang Y.J., Qin J., Hu C.H., Shi L. Astragalus polysaccharides attenuates TNF-alpha-induced insulin resistance via suppression of miR-721 and activation of PPAR-gamma and PI3K/AKT in 3T3-L1 adipocytes. Am. J. Transl. Res. 2017;9:2195–2206. [PMC free article] [PubMed] [Google Scholar]
- 87.Fan S.Y., Lin N., Shan G.L., Zuo P.P., Cui L.Y. Safflower yellow for acute ischemic stroke: A systematic review of randomized controlled trials. Complement. Ther. Med. 2014;22:354–361. doi: 10.1016/j.ctim.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Li L.J., Li Y.M., Qiao B.Y., Jiang S., Li X., Du H.M., Han P.C., Shi J. The Value of Safflower Yellow Injection for the Treatment of Acute Cerebral Infarction: A Randomized Controlled Trial. Evid. Based Complement. Altern. Med. 2015;2015:478793. doi: 10.1155/2015/478793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang C., Gao Y., Zhang Z., Chi Q., Liu Y., Yang L., Xu K. Safflower yellow alleviates osteoarthritis and prevents inflammation by inhibiting PGE2 release and regulating NF-kappa B/SIRT1/AMPK signaling pathways. Phytomedicine. 2020;78:153305. doi: 10.1016/j.phymed.2020.153305. [DOI] [PubMed] [Google Scholar]
- 90.Yu L., Liu Z.L., He W.D., Chen H.F., Lai Z.L., Duan Y.H., Cao X.H., Tao J., Xu C., Zhang Q.J., et al. Hydroxysafflor Yellow A Confers Neuroprotection from Focal Cerebral Ischemia by Modulating the Crosstalk Between JAK2/STAT3 and SOCS3 Signaling Pathways. Cell. Mol. Neurobiol. 2020;40:1271–1281. doi: 10.1007/s10571-020-00812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue X., Deng Y., Wang J., Zhou M., Liao L., Wang C., Peng C., Li Y. Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine. 2021;91:153694. doi: 10.1016/j.phymed.2021.153694. [DOI] [PubMed] [Google Scholar]
- 92.Yan-ping T. Protection of Hydroxysafflor Yellow-A Combined with Paeoniflorin on CIRI Rats and Its influence on Expression of PI3K and AKT Protein. Anti. Infect. Pharm. 2018;15:746–750. [Google Scholar]
- 93.Hu M.Z., Zhou Z.Y., Zhou Z.Y., Lu H., Gao M., Liu L.M., Song H.Q., Lin A.J., Wu Q.M., Zhou H.F., et al. Effect and Safety of Hydroxysafflor Yellow A for Injection in Patients with Acute Ischemic Stroke of Blood Stasis Syndrome: A Phase II, Multicenter, Randomized, Double-Blind, Multiple-Dose, Active-Controlled Clinical Trial. Chin. J. Integr. Med. 2020;26:420–427. doi: 10.1007/s11655-020-3094-7. [DOI] [PubMed] [Google Scholar]
- 94.Feng Z., Sun Q., Chen W., Bai Y., Hu D., Xie X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019;25:57. doi: 10.1186/s10020-019-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhaocheng J., Jinfeng L., Luchang Y., Yequan S., Feng L., Kai W. Ginkgolide A inhibits lipopolysaccharide-induced inflammatory response in human coronary artery endothelial cells via downregulation of TLR4-NF-kappaB signaling through PI3K/Akt pathway. Pharmazie. 2016;71:588–591. doi: 10.1691/ph.2016.6576. [DOI] [PubMed] [Google Scholar]
- 96.Fei Y., Zhao B., Zhu J., Fang W., Li Y. XQ-1H promotes cerebral angiogenesis via activating PI3K/Akt/GSK3beta/beta-catenin/VEGF signal in mice exposed to cerebral ischemic injury. Life Sci. 2021;272:119234. doi: 10.1016/j.lfs.2021.119234. [DOI] [PubMed] [Google Scholar]
- 97.Han W., Fang W., Li Y., Xu J. Protective effects and mechanism of ginkgo flavonoids and ginkgolides on focal cerebral ischemia-reperfusion injury in rats. Lishizhen Med. Mater. Med. Res. 2019;30:89–91. [Google Scholar]
- 98.Guo Y., Mao M., Li Q., Yu X., Zhou L. Extracts of Ginkgo flavonoids and ginkgolides improve cerebral ischaemia-reperfusion injury through the PI3K/Akt/Nrf2 signalling pathway and multicomponent in vivo processes. Phytomedicine. 2022;99:154028. doi: 10.1016/j.phymed.2022.154028. [DOI] [PubMed] [Google Scholar]
- 99.Zhao S., Zheng H., Du Y.W., Zhang R.L., Chen P.L., Ren R., Wu S.X. The Clinical Efficacy of Ginkgo biloba Leaf Preparation on Ischemic Stroke: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2021;2021:4265219. doi: 10.1155/2021/4265219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ju S.H., Tan L.R., Liu P.W., Tan Y.L., Zhang Y.T., Li X.H., Wang M.J., He B.X. Scutellarin regulates osteoarthritis in vitro by inhibiting the PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 2021;23:83. doi: 10.3892/mmr.2020.11722. [DOI] [PubMed] [Google Scholar]
- 101.Li C.Y., Wang Q., Wang X.M., Li G.X., Shen S., Wei X.L. Scutellarin inhibits the invasive potential of malignant melanoma cells through the suppression epithelial-mesenchymal transition and angiogenesis via the PI3K/Akt/mTOR signaling pathway. Eur. J. Pharmacol. 2019;858:172463. doi: 10.1016/j.ejphar.2019.172463. [DOI] [PubMed] [Google Scholar]
- 102.Meng Z.-q., Wu J.-r., Zhu Y.-l., Zhou W., Fu C.-g., Liu X.-k., Liu S.-y., Ni M.-w., Guo S.-y. Revealing the Common Mechanisms of Scutellarin in Angina Pectoris and Ischemic Stroke Treatment via a Network Pharmacology Approach. Chin. J. Integr. Med. 2021;27:62–69. doi: 10.1007/s11655-020-2716-4. [DOI] [PubMed] [Google Scholar]
- 103.Liu G., Liang Y., Xu M., Sun M., Sun W., Zhou Y., Huang X., Song W., Liang Y., Wang Z. Protective mechanism of Erigeron breviscapus injection on blood-brain barrier injury induced by cerebral ischemia in rats. Sci. Rep. 2021;11:18451. doi: 10.1038/s41598-021-97908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang F., Yang M.Y., Le J.Q., Luo B.Y., Yin M.D., Li C., Jiang J.L., Fang Y.F., Shao J.W. Protective Effects and Therapeutics of Ginsenosides for Improving Endothelial Dysfunction: From Therapeutic Potentials, Pharmaceutical Developments to Clinical Trials. Am. J. Chin. Med. 2022;50:749–772. doi: 10.1142/S0192415X22500318. [DOI] [PubMed] [Google Scholar]
- 105.Chen W., Guo Y., Yang W., Zheng P., Zeng J., Tong W. Protective effect of ginsenoside Rb1 on integrity of blood-brain barrier following cerebral ischemia. Exp. Brain Res. 2015;233:2823–2831. doi: 10.1007/s00221-015-4352-3. [DOI] [PubMed] [Google Scholar]
- 106.Qin G.-W., Lu P., Peng L., Jiang W. Ginsenoside Rb1 Inhibits Cardiomyocyte Autophagy via PI3K/Akt/mTOR Signaling Pathway and Reduces Myocardial Ischemia/Reperfusion Injury. Am. J. Chin. Med. 2021;49:1913–1927. doi: 10.1142/S0192415X21500907. [DOI] [PubMed] [Google Scholar]
- 107.Chen J., Zhang X., Liu X., Zhang C., Shang W., Xue J., Chen R., Xing Y., Song D., Xu R. Ginsenoside Rg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur J. Pharm. 2019;856:172418. doi: 10.1016/j.ejphar.2019.172418. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Y., Zhang Z., Wang H., Cai N., Zhou S., Zhao Y., Chen X., Zheng S., Si Q., Zhang W. Neuroprotective effect of ginsenoside Rg1 prevents cognitive impairment induced by isoflurane anesthesia in aged rats via antioxidant, anti-inflammatory and anti-apoptotic effects mediated by the PI3K/AKT/GSK-3 pathway. Mol. Med. Rep. 2016;14:2778–2784. doi: 10.3892/mmr.2016.5556. [DOI] [PubMed] [Google Scholar]
- 109.Liu X., Wang L., Wen A., Yang J., Yan Y., Song Y., Liu X., Ren H., Wu Y., Li Z., et al. Ginsenoside-Rd improves outcome of acute ischaemic stroke—A randomized, double-blind, placebo-controlled, multicenter trial. Eur J. Neurol. 2012;19:855–863. doi: 10.1111/j.1468-1331.2011.03634.x. [DOI] [PubMed] [Google Scholar]
- 110.Park M., Kim S.-H., Lee H.-J. Ginsenoside Rh1 Exerts Neuroprotective Effects by Activating the PI3K/Akt Pathway in Amyloid-beta Induced SH-SY5Y Cells. Appl. Sci. 2021;11:5654. doi: 10.3390/app11125654. [DOI] [Google Scholar]
- 111.Liu X.Y., Zhou X.Y., Hou J.C., Zhu H., Wang Z., Liu J.X., Zheng Y.Q. Ginsenoside Rd promotes neurogenesis in rat brain after transient focal cerebral ischemia via activation of PI3K/Akt pathway. Acta Pharm. Sin. 2015;36:421–428. doi: 10.1038/aps.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu R.Y., Zheng Y., Han T., Lan J., He L.X., Shi J.Y. Angiogenic Actions of Paeoniflorin on Endothelial Progenitor Cells and in Ischemic Stroke Rat Model. Am. J. Chin. Med. 2021;49:863–881. doi: 10.1142/S0192415X21500415. [DOI] [PubMed] [Google Scholar]
- 113.Tang H., Wu L., Chen X., Li H., Huang B., Huang Z., Zheng Y., Zhu L., Geng W. Paeoniflorin improves functional recovery through repressing neuroinflammation and facilitating neurogenesis in rat stroke model. Peerj. 2021;9:e10921. doi: 10.7717/peerj.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang P.-C., Wang S.-X., Yan X.-L., He Y.-Y., Wang M.-C., Zheng H.-Z., Shi X.-G., Tan Y.-H., Wang L.-S. Combination of paeoniflorin and calycosin-7-glucoside alleviates ischaemic stroke injury via the PI3K/AKT signalling pathway. Pharm. Biol. 2022;60:1469–1477. doi: 10.1080/13880209.2022.2102656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang A.Z., Zhao W., Yan K.T., Huang P.P., Zhang H.W., Ma X.C. Preclinical Evidence of Paeoniflorin Effectiveness for the Management of Cerebral Ischemia/Reperfusion Injury: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022;13:1250. doi: 10.3389/fphar.2022.827770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu L., Zhu L., Zou Y., Liu W., Zhang X., Wei X., Hu B., Chen J. Panax notoginseng Saponins Promotes Stroke Recovery by Influencing Expression of Nogo-A, NgR and p75NGF, in Vitro and in Vivo. Biol. Pharm. Bull. 2014;37:560–568. doi: 10.1248/bpb.b13-00770. [DOI] [PubMed] [Google Scholar]
- 117.Wu T., Jia Z.H., Dong S.F., Han B., Zhang R., Liang Y.Y., Zhang S.F., Sun J.N. Panax notoginseng Saponins Ameliorate Leukocyte Adherence and Cerebrovascular Endothelial Barrier Breakdown upon Ischemia-Reperfusion in Mice. J. Vasc. Res. 2019;56:1–10. doi: 10.1159/000494935. [DOI] [PubMed] [Google Scholar]
- 118.Hu S., Wu Y., Zhao B., Hu H., Zhu B., Sun Z., Li P., Du S. Panax notoginseng Saponins Protect Cerebral Microvascular Endothelial Cells against Oxygen-Glucose Deprivation/Reperfusion-Induced Barrier Dysfunction via Activation of PI3K/Akt/Nrf2 Antioxidant Signaling Pathway. Molecules. 2018;23:2781. doi: 10.3390/molecules23112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen S., Liu J., Liu X., Fu Y., Zhang M., Lin Q., Zhu J., Mai L., Shan Z., Yu X., et al. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J. Ethnopharmacol. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 120.Yan Y.T., Li S.D., Li C., Xiong Y.X., Lu X.H., Zhou X.F., Yang L.Q., Pu L.J., Luo H.Y. Panax notoginsenoside saponins Rb1 regulates the expressions of Akt/ mTOR/PTEN signals in the hippocampus after focal cerebral ischemia in rats. Behav. Brain Res. 2018;345:83–92. doi: 10.1016/j.bbr.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 121.Wang L.D., Xu Z.M., Liang X., Qiu W.R., Liu S.J., Dai L.L., Wang Y.F., Guo C.Y., Qi X.H., Wang J., et al. Systematic Review and Meta-Analysis on Randomized Controlled Trials on Efficacy and Safety of Panax Notoginseng Saponins in Treatment of Acute Ischemic Stroke. Evid.-Based Complement. Altern. Med. 2021;2021:4694076. doi: 10.1155/2021/4694076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang W.-W., Xu F., Wang D., Ye J., Cai S.-Q. Buyang Huanwu Decoction ameliorates ischemic stroke by modulating multiple targets with multiple components: In vitro evidences. Chin. J. Nat. Med. 2018;16:194–202. doi: 10.1016/S1875-5364(18)30047-5. [DOI] [PubMed] [Google Scholar]
- 123.Jin Y.L., Dong L.Y., Wu C.Q., Qin J., Li S., Wang C.Y., Shao X., Huang D.K. Buyang Huanwu Decoction fraction protects against cerebral ischemia/reperfusion injury by attenuating the inflammatory response and cellular apoptosis. Neural Regen. Res. 2013;8:197–207. doi: 10.3969/j.issn.1673-5374.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui H.J., Yang A.L., Zhou H.J., Wang C., Luo J.K., Lin Y., Zong Y.X., Tang T. Buyang huanwu decoction promotes angiogenesis via vascular endothelial growth factor receptor-2 activation through the PI3K/Akt pathway in a mouse model of intracerebral hemorrhage. BMC Complement. Altern. Med. 2015;15:91. doi: 10.1186/s12906-015-0605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen X., Chen H., He Y., Fu S., Liu H., Wang Q., Shen J. Proteomics-Guided Study on Buyang Huanwu Decoction for Its Neuroprotective and Neurogenic Mechanisms for Transient Ischemic Stroke: Involvements of EGFR/PI3K/Akt/Bad/14-3-3 and Jak2/Stat3/Cyclin D1 Signaling Cascades. Mol. Neurobiol. 2020;57:4305–4321. doi: 10.1007/s12035-020-02016-y. [DOI] [PubMed] [Google Scholar]
- 126.Gao L., Xiao Z.R., Jia C.H., Wang W. Effect of Buyang Huanwu decoction for the rehabilitation of ischemic stroke patients: A meta-analysis of randomized controlled trials. Health Qual. Life Outcomes. 2021;19:79. doi: 10.1186/s12955-021-01728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang S.J., Jiang H.J., Liu Q.Q., Zhou Y.F., Cheng Y.F., Zhou T., Zhang J.M., He Y., Ren C.X., Pei J. A comparative study on the traditional versus modern yellow rice wine processing methods using Taohong Siwu Decoction for pharmaceutical production. J. Ethnopharmacol. 2022;290:115114. doi: 10.1016/j.jep.2022.115114. [DOI] [PubMed] [Google Scholar]
- 128.Pan L., Peng C., Wang L., Li L., Huang S., Fei C., Wang N., Chu F., Peng D., Duan X. Network pharmacology and experimental validation-based approach to understand the effect and mechanism of Taohong Siwu Decoction against ischemic stroke. J. Ethnopharmacol. 2022;294:115339. doi: 10.1016/j.jep.2022.115339. [DOI] [PubMed] [Google Scholar]
- 129.Wang N., Fei C., Chu F., Huang S., Pan L., Peng D., Duan X. Taohong Siwu Decoction Regulates Cell Necrosis and Neuroinflammation in the Rat Middle Cerebral Artery Occlusion Model. Front. Pharm. 2021;12:732358. doi: 10.3389/fphar.2021.732358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yuan-Hai Z. Effect of Taohongsiwu decoction on the angiogenesis and PI3K /AKT signal pathway in brain tissue of rats with cerebral ischemia reperfusion injury. J. Bengbu Med. Coll. 2017;42:36–56. [Google Scholar]
- 131.Lu J.D., Fu L.J., Qin G.Z., Shi P.L., Fu W.J. The regulatory effect of Xiaoyao San on glucocorticoid receptors under the condition of chronic stress. Cell. Mol. Biol. 2018;64:103–109. doi: 10.14715/cmb/2018.64.6.17. [DOI] [PubMed] [Google Scholar]
- 132.Xu Y., Chen W.Y., Chen Z.R., Huang M.Y., Yang F., Zhang Y. Mechanism of Action of Xiaoyao San in Treatment of Ischemic Stroke is Related to Anti-Apoptosis and Activation PI3K/Akt Pathway. Drug Des. Dev. Ther. 2021;15:753–767. doi: 10.2147/DDDT.S280217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Z.X., Wan H.F., Tong X., He Y., Yang J.H., Zhang L., Shao C.Y., Ding Z.S., Wan H.T., Li C. An integrative strategy for discovery of functional compound combination from Traditional Chinese Medicine: Danhong Injection as a model. Biomed. Pharmacother. 2021;138:111451. doi: 10.1016/j.biopha.2021.111451. [DOI] [PubMed] [Google Scholar]
- 134.Zeng M., Zhou H., He Y., Wang Z., Shao C., Yin J., Du H., Yang J., Wan H. Danhong injection alleviates cerebral ischemia/reperfusion injury by improving intracellular energy metabolism coupling in the ischemic penumbra. Biomed. Pharm. 2021;140:111771. doi: 10.1016/j.biopha.2021.111771. [DOI] [PubMed] [Google Scholar]
- 135.Du H., He Y., Pan Y., Zhao M., Li Z., Wang Y., Yang J., Wan H. Danhong Injection Attenuates Cerebral Ischemia-Reperfusion Injury in Rats Through the Suppression of the Neuroinflammation. Front. Pharm. 2021;12:561237. doi: 10.3389/fphar.2021.561237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feng C., Wan H., Zhang Y., Yu L., Shao C., He Y., Wan H., Jin W. Neuroprotective Effect of Danhong Injection on Cerebral Ischemia-Reperfusion Injury in Rats by Activation of the PI3K-Akt Pathway. Front. Pharm. 2020;11:298. doi: 10.3389/fphar.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu Y., Chen M.L., Xiao Q., Zhao Y.Y., Tian J. Observation of the clinical effects of Danhong injections combined with pitavastatin on blood lipid regulation in patients with ischemic strokes complicated with lipid abnormalities. Int. J. Clin. Exp. Med. 2019;12:3364–3375. [Google Scholar]
- 138.Du Y.W., Wang X.X., Zhong L.Q., Wu S.X. Comparative study of the effects of Danhong injection with different doses on ischemic stroke: A substudy of hospital-based Danhong injection registry. J. Tradit. Chin. Med. 2018;38:917–925. [PubMed] [Google Scholar]
- 139.Zhao Y.H., Wang M., Sun L., Jiang X., Zhao M., Zhao C.J. Rapid characterization of the chemical constituents of Sanhua decoction by UHPLC coupled with Fourier transform ion cyclotron resonance mass spectrometry. RSC Adv. 2020;10:26109–26119. doi: 10.1039/D0RA02264K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fu D.-L., Li J.-H., Shi Y.-H., Zhang X.-L., Lin Y., Zheng G.-Q. Sanhua Decoction, a Classic Herbal Prescription, Exerts Neuroprotection Through Regulating Phosphorylated Tau Level and Promoting Adult Endogenous Neurogenesis After Cerebral Ischemia/Reperfusion Injury. Front. Physiol. 2020;11:57. doi: 10.3389/fphys.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Zhang W., Zhang L., Wang W.J., Ma S.B., Wang M.M., Yao M.N., Li R.L., Li W.W., Zhao X., Hu D.M., et al. Network pharmacology and in vitro experimental verification to explore the mechanism of Sanhua decoction in the treatment of ischaemic stroke. Pharm. Biol. 2022;60:119–130. doi: 10.1080/13880209.2021.2019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Song Y.F., Chu Y., Ma X.H., Zheng H.R., Bai X.L., Zhou S.P., Yu B.Y. GC-MS/MS method for the determination and pharmacokinetic analysis of borneol and muscone in rat after the intravenous administration of Xingnaojing injection. J. Sep. Sci. 2017;40:4264–4271. doi: 10.1002/jssc.201700341. [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y.M., Qu X.Y., Zhai J.H., Tao L.N., Gao H., Song Y.Q., Zhang S.X. Xingnaojing Injection Protects against Cerebral Ischemia Reperfusion Injury via PI3K/Akt-Mediated eNOS Phosphorylation. Evid.-Based Complement. Altern. Med. 2018;2018:2361046. doi: 10.1155/2018/2361046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu Z., Li H., Ma W., Pan S. Network pharmacology to investigate the pharmacological mechanisms of muscone in Xingnaojing injections for the treatment of severe traumatic brain injury. Peerj. 2021;9:e11696. doi: 10.7717/peerj.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang L., Fan X., Chen Y., Liang X., Shen W., Zhang Y. Efficacy and Safety of Xingnaojing Injection for Emergency Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Pharm. 2022;13:839305. doi: 10.3389/fphar.2022.839305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dou W., Chen Y., Yang J., Liu T., Lu H., Yang P., Ye L. Clinical Research Progress in Insect Chinese Materia Medica in Treatment of Ischemic Stroke. Chin. J. Inf. TCM. 2019;26:142–144. [Google Scholar]
- 147.Wang Y.H., Li S.A., Huang C.H., Su H.H., Chen Y.H., Chang J.H.T., Huang S.S. Sirt1 Activation by Post-ischemic Treatment With Lumbrokinase Protects Against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2018;9:636. doi: 10.3389/fphar.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han C.K., Kuo W.W., Shen C.Y., Chen T.S., Pai P.Y., Tsai C.H., Lo F.Y., Ju D.T., Huang C.Y. Dilong Prevents the High-KCl Cardioplegic Solution Administration-Induced Apoptosis in H9c2 Cardiomyoblast Cells Mediated by MEK. Am. J. Chin. Med. 2014;42:1507–1519. doi: 10.1142/S0192415X14500943. [DOI] [PubMed] [Google Scholar]
- 149.Zhao L., Yuan Q., Zhang T., Jia Z., Yin M., Hu L. Exploration of the protective effects of Leech and Earthworm extracts on cerebral ischemic penumbra neurons in MCAO/R mice based on PI3K/AKT pathway. Tianjin J. Tradit. Chin. Med. 2022;39:1057–1063. [Google Scholar]
- 150.Ji H., Wang L., Bi H., Sun L., Cai B., Wang Y., Zhao J., Du Z. Mechanisms of lumbrokinase in protection of cerebral ischemia. Eur J. Pharm. 2008;590:281–289. doi: 10.1016/j.ejphar.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 151.Chen Y., Liu Y., Zhang J.J., Zhou K.H., Zhang X.C., Dai H.H., Yang B.L., Shang H.C. Efficacy and safety of lumbrokinase plus aspirin versus aspirin alone for acute ischemic stroke (LUCENT): Study protocol for a multicenter randomized controlled trial. Trials. 2022;23:1–12. doi: 10.1186/s13063-022-06200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Junren C., Xiaofang X., Huiqiong Z., Gangmin L., Yanpeng Y., Xiaoyu C., Yuqing G., Yanan L., Yue Z., Fu P., et al. Pharmacological Activities and Mechanisms of Hirudin and Its Derivatives—A Review. Front. Pharm. 2021;12:660757. doi: 10.3389/fphar.2021.660757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li W.Q., Qin Z.S., Chen S., Cheng D., Yang S.C., Choi Y.M.M., Chu B., Zhou W.H., Zhang Z.J. Hirudin alleviates acute ischemic stroke by inhibiting NLRP3 inflammasome-mediated neuroinflammation: In vivo and in vitro approaches. Int. Immunopharmacol. 2022;110:108967. doi: 10.1016/j.intimp.2022.108967. [DOI] [PubMed] [Google Scholar]
- 154.Li Y., Zhang L., Xiong W.J., Gao X., Xiong Y.Y., Sun W. A Molecular Mechanism Study to Reveal Hirudin’s Downregulation to PI3K/AKT Signaling Pathway through Decreasing PDGFR beta in Renal Fibrosis Treatment. Biomed Res. Int. 2022;2022:5481552. doi: 10.1155/2022/5481552. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 155.Yu H.X., Lin W., Yang K., Wei L.J., Chen J.L., Liu X.Y., Zhong K., Chen X., Pei M., Yang H.T. Transcriptome-Based Network Analysis Reveals Hirudin Potentiates Anti-Renal Fibrosis Efficacy in UUO Rats. Front. Pharmacol. 2021;12:741801. doi: 10.3389/fphar.2021.741801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song C.G., Bi L.J., Zhao J.J., Wang X., Li W., Yang F., Jiang W. The efficacy and safety of Hirudin plus Aspirin versus Warfarin in the secondary prevention of Cardioembolic Stroke due to Nonvalvular Atrial Fibrillation: A multicenter prospective cohort study. Int. J. Med. Sci. 2021;18:1167–1178. doi: 10.7150/ijms.52752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thien T.V., Anh H.N., Trang N.T.T., Van Trung P., Khoa N.C., Osipov A.V., Dubovskii P.V., Ivanov I.A., Arseniev A.S., Tsetlin V.I., et al. Low-molecular-weight compounds with anticoagulant activity from the scorpion Heterometrus laoticus venom. Dokl. Biochem. Biophys. 2017;476:316–319. doi: 10.1134/S1607672917050052. [DOI] [PubMed] [Google Scholar]
- 158.Tran T.V., Hoang A.N., Nguyen T.T.T., Van Phung T., Nguyen K.C., Osipov A.V., Ivanov I.A., Tsetlin V.I., Utkin Y.N. Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom. Toxins. 2017;9:343. doi: 10.3390/toxins9110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pasquini S., Vincenzi F., Casetta I., Laudisi M., Merighi S., Gessi S., Borea P.A., Varani K. Adenosinergic System Involvement in Ischemic Stroke Patients’ Lymphocytes. Cells. 2020;9:1072. doi: 10.3390/cells9051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.You X.J., Wu Y.R., Li Q.X., Sheng W., Zhou Q., Fu W. Astragalus-Scorpion Drug Pair Inhibits the Development of Prostate Cancer by Regulating GDPD4-2/PI3K/AKT/mTOR Pathway and Autophagy. Front. Pharmacol. 2022;13:895696. doi: 10.3389/fphar.2022.895696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang L.P., Hu L., Wei C., Zhang H., Liu S. Chinese medicine Tongxinluo capsule protects against blood-brain barrier disruption after ischemic stroke by inhibiting the low-density lipoprotein receptor-related protein 1 pathway in mice. J. Stroke Cerebrovasc. Dis. 2020;29:105071. doi: 10.1016/j.jstrokecerebrovasdis.2020.105071. [DOI] [PubMed] [Google Scholar]
- 162.Cheng X., Luo H.X., Zhou L.H., Wang L.X., Sun J.B., Huang Y., Luo E.L., Cai Y.F. Neuroprotective effect of the traditional Chinese herbal formula Tongxinluo: A PET imaging study in rats. Neural Regen. Res. 2014;9:1267–1274. doi: 10.4103/1673-5374.137573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu S., Wei C., Kang N., He Q.L., Liang J.Q., Wang H.T., Chang L.P., Chen D.H., Zhang Q.Y., Chang C.C., et al. Chinese medicine Tongxinluo capsule alleviates cerebral microcirculatory disturbances in ischemic stroke by modulating vascular endothelial function and inhibiting leukocyte-endothelial cell interactions in mice: A two-photon laser scanning microscopy study. Microcirculation. 2018;25:e12437. doi: 10.1111/micc.12437. [DOI] [PubMed] [Google Scholar]
- 164.Yu Z.H., Cai M., Xiang J., Zhang Z.N., Zhang J.S., Song X.L., Zhang W., Bao J., Li W.W., Cai D.F. PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J. Ethnopharmacol. 2016;181:8–19. doi: 10.1016/j.jep.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 165.Yang P., Liu P., Yang R.J. Systematic Review of Tongxinluo Capsule on the Therapeutic Effect and Hemorheology of Patients with Transient Ischemic Attack. Evid.-Based Complement. Altern. Med. 2021;2021:5541768. doi: 10.1155/2021/5541768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang X.R., Yin Z.Q., Cao P.C., Zheng S.H., Chen Y.L., Yu M.Y., Liao C.Z., Zhang Z.Y., Duan Y.J., Han J.H., et al. NaoXinTong Capsule ameliorates memory deficit in APP/PS1 mice by regulating inflammatory cytokines. Biomed. Pharmacother. 2021;133:110964. doi: 10.1016/j.biopha.2020.110964. [DOI] [PubMed] [Google Scholar]
- 167.Lv B., Deng L.N., Xie T., Wei X., Liu X., Tan W.X., Wang X.Y., Gao X.M. Evaluation of the anti-inflammatory and antioxidant pharmcodynamic compoents of naoxintong capsules as a basis of broad spectrum effects. Pharm. Biol. 2021;59:242–251. doi: 10.1080/13880209.2020.1870506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang H., Zhou X.K., Zheng L.F., Wu X.Y., Chen H. Comparison of Aspirin and Naoxintong Capsule (sic) with Adjusted-Dose Warfarin in Elderly Patients with High-Risk of Non-Valvular Atrial Fibrillation and Genetic Variants of Vitamin K Epoxide Reductase. Chin. J. Integr. Med. 2018;24:247–253. doi: 10.1007/s11655-015-2443-4. [DOI] [PubMed] [Google Scholar]
- 169.Li N., Yang F., Zhao Z.L., Jiang J., Lei Y. Efficacy and safety of Naoxintong capsule for acute ischemic stroke A protocol for systematic review and meta-analysis. Medicine. 2021;100:e27120. doi: 10.1097/MD.0000000000027120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sun Z.Y., Wang F.J., Guo H., Chen L., Chai L.J., Li R.L., Hu L.M., Wang H., Wang S.X. Shuxuetong injection protects cerebral microvascular endothelial cells against oxygen-glucose deprivation reperfusion. Neural Regen. Res. 2019;14:783–793. doi: 10.4103/1673-5374.249226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang T.Y., Jiao J.K., Shang J.F., Bi L., Wang H.H., Zhang C., Wu H.W., Cui Y.R., Wang P., Liu X. The Differences of Metabolites in Different Parts of the Brain Induced by Shuxuetong Injection against Cerebral Ischemia-Reperfusion and Its Corresponding Mechanism. Evid.-Based Complement. Altern. Med. 2022;2022:9465095. doi: 10.1155/2022/9465095. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 172.Liu X., Wang Q., Cui Y.R., Li X.Y., Yang H.J. In-depth transcriptomic and proteomic analyses of the hippocampus and cortex in a rat model after cerebral ischemic injury and repair by Shuxuetong (SXT) injection. J. Ethnopharmacol. 2020;249:112362. doi: 10.1016/j.jep.2019.112362. [DOI] [PubMed] [Google Scholar]
- 173.Fang H., Zhou H.L., Zhang J.C., Li Z.Y., Chen Z., Yuan R.R., Huang X.Q., Yang J.Y., Zhang J.Q., Wang S., et al. Effects of shuxuetong injection for cerebral infarction A protocol for systematic review and meta-analysis. Medicine. 2020;99:e21929. doi: 10.1097/MD.0000000000021929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tan F., Fu W.J., Cheng N.F., Meng D., Gu Y. Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion. Exp. Ther. Med. 2015;9:1757–1762. doi: 10.3892/etm.2015.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yuan Z.H., Gao Y.H., Pan C.H., Liu X., Wang L., Wang X.Y. Effect of Tanshinone IIA combined with Rehabilitation Training on Nerve Repair and Expression of Growth-associated Protein-43 of Peri-ischemic Cortex in Ischemic Stroke Rats. Lat. Am. J. Pharm. 2019;38:355–360. [Google Scholar]
- 176.Cai M., Guo Y.X., Wang S.Q., Wei H.D., Sun S.S., Zhao G.C., Dong H.L. Tanshinone IIA Elicits Neuroprotective Effect Through Activating the Nuclear Factor Erythroid 2-Related Factor-Dependent Antioxidant Response. Rejuvenation Res. 2017;20:286–297. doi: 10.1089/rej.2016.1912. [DOI] [PubMed] [Google Scholar]
- 177.Dai Z.K., Chen Y.F., Feng F., Luo Y. Tanshinone IIA pretreatment attenuates oxygen-glucose deprivation induced hippocampal neurons damage. Int. J. Clin. Exp. Med. 2017;10:10287–10296. [Google Scholar]
- 178.Ren C.H., Li N., Gao C., Zhang W., Yang Y., Li S.J., Ji X.M., Ding Y.C. Ligustilide provides neuroprotection by promoting angiogenesis after cerebral ischemia. Neurol. Res. 2020;42:683–692. doi: 10.1080/01616412.2020.1782122. [DOI] [PubMed] [Google Scholar]
- 179.Kuang X., Wang L.F., Yu L., Li Y.J., Wang Y.N., He Q., Chen C., Du J.R. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats: Involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic. Biol. Med. 2014;71:165–175. doi: 10.1016/j.freeradbiomed.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 180.Sun Y., Xu D.P., Qin Z., Wang P.Y., Hu B.H., Yu J.G., Zhao Y., Cai B., Chen Y.L., Lu M., et al. Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur. J. Pharmacol. 2018;818:604–609. doi: 10.1016/j.ejphar.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 181.Lv Y.N., Qian Y.S., Fu L.S., Chen X.Y., Zhong H.L., Wei X.H. Hydroxysafflor yellow A exerts neuroprotective effects in cerebral ischemia reperfusion-injured mice by suppressing the innate immune TLR4-inducing pathway. Eur. J. Pharmacol. 2015;769:324–332. doi: 10.1016/j.ejphar.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 182.Zhang G.Y., Xia F., Zhang Y.X., Zhang X., Cao Y.H., Wang L., Liu X.D., Zhao G., Shi M. Ginsenoside Rd Is Efficacious Against Acute Ischemic Stroke by Suppressing Microglial Proteasome-Mediated Inflammation. Mol. Neurobiol. 2016;53:2529–2540. doi: 10.1007/s12035-015-9261-8. [DOI] [PubMed] [Google Scholar]
- 183.Ye R.D., Yang Q.Z., Kong X.W., Han J.L., Zhang X., Zhang Y.X., Li P., Liu J.F., Shi M., Xiong L.Z., et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem. Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 184.Wang B.G., Zhu Q.S., Man X.X., Guo L., Hao L.M. Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury. Neural Regen. Res. 2014;9:1678–1687. doi: 10.4103/1673-5374.141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang S.Y., Li M.H., Guo Y., Li C., Wu L.O., Zhou X.F., Luo Y.H., An D., Li S.D., Luo H.Y., et al. Effects of Panax notoginseng ginsenoside Rb1 on abnormal hippocampal microenvironment in rats. J. Ethnopharmacol. 2017;202:138–146. doi: 10.1016/j.jep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 186.Chu S.F., Zhang Z., Zhou X., He W.B., Chen C., Luo P., Liu D.D., Ai Q.D., Gong H.F., Wang Z.Z., et al. Ginsenoside Rg1 protects against ischemic/reperfusion-induced neuronal injury through miR-144/Nrf2/ARE pathway. Acta Pharmakoi. Sin. 2019;40:13–25. doi: 10.1038/s41401-018-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li X.B., Shi F.G., Zhang R., Sun C.L., Gong C.T., Jian L.Y., Ding L. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin After Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. Clin. Ther. 2016;38:327–337. doi: 10.1016/j.clinthera.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 188.Feng L.D., Han F., Zhou L., Wu S.X., Du Y.W., Zhang D.D., Zhang C., Gao Y. Efficacy and Safety of Panax Notoginseng Saponins (Xueshuantong) in Patients With Acute Ischemic Stroke (EXPECT) Trial: Rationale and Design. Front. Pharmacol. 2021;12:648921. doi: 10.3389/fphar.2021.648921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xu Z.Y., Xu Y., Xie X.F., Tian Y., Sui J.H., Sun Y., Lin S., Gao X., Peng C., Fan Y.J. Anti-platelet aggregation of Panax notoginseng triol saponins by regulating GP1BA for ischemic stroke therapy. Chin. Med. 2021;16:12. doi: 10.1186/s13020-021-00424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang L., Tao Y.W., Luo L.L., Zhang Y., Wang X.B., Meng X.L. Dengzhan Xixin injection derived from a traditional Chinese herb Erigeron breviscapus ameliorates cerebral ischemia/reperfusion injury in rats via modulation of mitophagy and mitochondrial apoptosis. J. Ethnopharmacol. 2022;288:114988. doi: 10.1016/j.jep.2022.114988. [DOI] [PubMed] [Google Scholar]
- 191.Chen X., Wu H., Chen H.S., Wang Q., Xie X.J., Shen J.G. Astragaloside VI Promotes Neural Stem Cell Proliferation and Enhances Neurological Function Recovery in Transient Cerebral Ischemic Injuryvia Activating EGFR/MAPK Signaling Cascades. Mol. Neurobiol. 2019;56:3053–3067. doi: 10.1007/s12035-018-1294-3. [DOI] [PubMed] [Google Scholar]
- 192.Pan R.H., Cai J., Zhan L.C., Guo Y.H., Huang R.Y., Li X., Zhou M.C., Xu D.D., Zhan J., Chen H.X. Buyang Huanwu decoction facilitates neurorehabilitation through an improvement of synaptic plasticity in cerebral ischemic rats. BMC Complement. Altern. Med. 2017;17:173. doi: 10.1186/s12906-017-1680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sun H.L., Wu C.Y. Acupuncture combined with Buyang Huanwu decoction in treatment of patients with ischemic stroke. J. Int. Med. Res. 2019;47:1312–1318. doi: 10.1177/0300060518822923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Li H., Peng D., Zhang S.J., Zhang Y., Wang Q., Guan L. Buyang Huanwu Decoction promotes neurogenesis via sirtuin 1/autophagy pathway in a cerebral ischemia model. Mol. Med. Rep. 2021;24:791. doi: 10.3892/mmr.2021.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen H.J., Shen Y.C., Shiao Y.J., Liou K.T., Hsu W.H., Hsieh P.H., Lee C.Y., Chen Y.R., Lin Y.L. Multiplex Brain Proteomic Analysis Revealed the Molecular Therapeutic Effects of Buyang Huanwu Decoction on Cerebral Ischemic Stroke Mice. PLoS ONE. 2015;10:e0140823. doi: 10.1371/journal.pone.0140823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang M.M., Liu Z.Q., Hu S.S., Duan X.C., Zhang Y.Y., Peng C., Peng D.Y., Han L. Taohong Siwu Decoction Ameliorates Ischemic Stroke Injury Via Suppressing Pyroptosis. Front. Pharmacol. 2020;11:590453. doi: 10.3389/fphar.2020.590453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Orgah J.O., Ren J., Liu X.Y., Orgah E.A., Gao X.M., Zhu Y. Danhong injection facilitates recovery of post-stroke motion deficit via Parkin-enhanced mitochondrial function. Restor. Neurol. Neurosci. 2019;37:375–395. doi: 10.3233/RNN-180828. [DOI] [PubMed] [Google Scholar]
- 198.Xu J., Wang T.T., Guo F.F., Ji E.H., Zhang Y., Wu H.W., Tang S.H., Wei J.Y., Yang H.J. Systematical Identification of the Protective Effect of Danhong Injection and BuChang NaoXinTong Capsules on Transcription Factors in Cerebral Ischemia Mice Brain. Oxidative Med. Cell. Longev. 2020;2020:5879852. doi: 10.1155/2020/5879852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lai X.X., Cao K.G., Kong L.B., Liu Q., Gao Y., Xmas Study I. Xingnaojing for Moderate-to-severe Acute ischemic Stroke (XMAS): Study protocol for a randomized controlled trial. Trials. 2017;18:479. doi: 10.1186/s13063-017-2222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yu X.F., Zhu X.Y., Yuan C.X., Wu D.H., Zhao Y.W., Yang J.J., Wang C.D., Wu W.W., Liu X.Y., Liu Z.G., et al. Naoxintong Capsule for Secondary Prevention of Ischemic Stroke: A Multicenter, Randomized, and Placebo-Controlled Trial. Chin. J. Integr. Med. 2022:9. doi: 10.1007/s11655-022-3586-8. [DOI] [PubMed] [Google Scholar]
- 201.Wang H.Y., Zhou H.F., He Y., Yu L., Li C., Yang J.H., Wan H.T. Protective Effect of Naoxintong Capsule Combined with Guhong Injection on Rat Brain Microvascular Endothelial Cells during Cerebral Ischemia-Reperfusion Injury. Chin. J. Integr. Med. 2021;27:744–751. doi: 10.1007/s11655-020-3215-3. [DOI] [PubMed] [Google Scholar]
- 202.Wan J.Y., Wan H.F., Yang R.B., Wan H.T., Yang J.H., He Y., Zhou H.F. Protective effect of Danhong Injection combined Naoxintong Capsule on cerebral ischemia-reperfusion injury in rats with. J. Ethnopharmacol. 2018;211:348–357. doi: 10.1016/j.jep.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 203.Gu H.Q., Xie X.W., Jing J., Meng X., Lv W., Yu J.D., Lv X.P., Li H., Wang Y.L., Wang Y.J. Shuxuetong for Prevention of recurrence in Acute Cerebrovascular events with Embolism (SPACE) trial: Rationale and design. Stroke Vasc. Neurol. 2020;5:311–314. doi: 10.1136/svn-2019-000293. [DOI] [PMC free article] [PubMed] [Google Scholar]