Abstract
Two unique isolates of Borrelia burgdorferi, differing in plasmid content and outer surface protein C expression, were cultured on sequential captures of a single free-living Peromyscus leucopus mouse and were examined for differences in transmissibility. Both isolates were transmissible from inoculated C.B-17 mice to larval Ixodes scapularis ticks and, subsequently, from infected nymphal ticks to C3H/HeJ mice. Plasmid and protein analyses suggested that the original isolates were a mixed population of B. burgdorferi, and cloning by limiting dilution resulted in the identification of two clonal groups. In addition to being heterogeneous in plasmid and genomic macrorestriction analyses, the clones varied with respect to the electrophoretic mobilities and antigenicity of their OspC proteins, as shown by their reactivity to a panel of monoclonal antibodies. Plasmid analysis of sequential isolates from C3H mice experimentally infected with the primary isolate or various mixtures of its subclones showed an apparently random fluctuation in clonal dominance in the majority of mice. Surprisingly, mice infected with each subclone were permissive to superinfection with the heterologous subclone, despite the presence of anti-B. burgdorferi antibodies at the time of the secondary challenge. These results show conclusively that mice captured at Lyme disease enzootic sites may be infected by mixed populations of genetically and antigenically distinct B. burgdorferi clones and that these infections can be acquired by coinfection or by sequential infection. The lack of cross-immunization between clones existing within a naturally occurring population may play a role in the maintenance of the genetic heterogeneity of B. burgdorferi in nature.
The maintenance of Borrelia burgdorferi, the causative agent of Lyme disease (6, 9), in Ixodes scapularis, the principal vector of the disease to humans in the northeastern and upper midwestern regions of the United States (33, 44), is dependent on a zoonotic cycle involving tick vectors and vertebrate hosts. This is due to the near absence of transovarial transmission of the spirochete in I. scapularis ticks (36). In this zoonotic cycle, vertebrate hosts play a dual role in providing a blood meal for each stage of the tick and, for certain vertebrate hosts (11, 22, 27, 42), in serving as a reservoir of B. burgdorferi for transmission to feeding I. scapularis ticks. Among the potential reservoir-competent hosts, the white-footed mouse, Peromyscus leucopus, is the principal host in the northeastern and upper midwestern regions of the United States (27). Mice mount a specific immune response to B. burgdorferi (16, 24, 25), yet field (1, 16) and laboratory studies (41, 47) have established that mice develop chronic infection with B. burgdorferi. Paradoxically, however, mice cured of infection by antibiotic treatment are immune to rechallenge by the same organism for months to a year (5, 35).
Longitudinal mark and recapture studies of naturally infected reservoir mice are useful for studying the natural history of B. burgdorferi in its reservoir host. As part of a previous longitudinal study of a population of mice at an enzootic site in Maryland, we found the majority of B. burgdorferi isolates first cultured from sequentially captured mice to have relatively homogeneous plasmid and protein profiles (17). However, in some cases, changes in expression of OspC, most often accompanied by changes in plasmid content, were observed. Previous studies in our laboratory have shown that isolates of B. burgdorferi recovered after long-term infection may occasionally lose plasmids, but otherwise they appear to be relatively stable at the level of protein expression, genomic macrorestriction analysis, and the ospA (34) and ospC (45) sequences. An additional explanation for the observed alterations in the field isolates would be coinfection or superinfection by distinct B. burgdorferi clones; fluctuation in population dominance over time could then account for apparent genotypic and phenotypic differences.
The purpose of this study was (i) to determine the basis for the genotypic and phenotypic changes observed in sequential isolates of B. burgdorferi cultured from several mice in our previous study and (ii) to determine if those changes had an effect on the ability of the spirochetes to be acquired and transmitted by I. scapularis ticks and to establish infection by various routes. In this report we demonstrate that a representative isolate from one mouse in our previous study was composed of genotypically and phenotypically heterogeneous subpopulations of B. burgdorferi which were both capable of experimental transmission. We observed a random fluctuation in the composition of the population of spirochetes in mice experimentally infected with defined mixtures of each subpopulation. Furthermore, challenge of mice experimentally infected by one population member with the heterogeneous population member showed both members to be capable of superinfection, despite the development of specific immune responses against B. burgdorferi.
MATERIALS AND METHODS
B. burgdorferi isolates.
Primary isolates of B. burgdorferi from wild-caught mouse 225 (17) and experimentally infected mice were maintained in BSK II medium (BSK II) (2) containing 6% rabbit serum and 10 μg of rifampin, 4 μg of amphotericin B, and 1,000 μg of phosphomycin per ml at 34°C as previously described (17). Subsequent passages of the spirochete were grown in BSK II medium to which no antibiotics were added.
Experimental tick transmission of B. burgdorferi.
B. burgdorferi isolates 225a (passage 3 [p3]) and 225c (p4) were grown to early log phase and enumerated twice in a Petroff-Hausser counting chamber by using dark-field microscopy. For each isolate of B. burgdorferi, a 3-week-old C.B-17 mouse and a C.B-17 scid/scid (SCID) mouse (obtained from a breeding colony at Johns Hopkins University) were inoculated intradermally with 105 spirochetes at the base of the tail. One month postinoculation, 20 I. scapularis larval ticks (first-generation larvae from female ticks provided by J. Oliver, Georgia Southern University, Statesboro) were placed on the back of each infected mouse and allowed to feed to repletion. Collected replete larvae were placed in vials containing a moist plaster of paris base and held at 16°C in a photoperiod of 16 h of light and 8 h of dark.
Three months after the larval tick feeding, four molted nymphal ticks from each cohort of exposed ticks were placed on two 5-week-old C3H.HeJ mice (The Jackson Laboratories, Bar Harbor, Maine) and allowed to feed to repletion. One month after the tick feeding, the C3H mice were euthanized by inhalation of CO2, and kidney, spleen, bladder, and ear tissues were cultured as previously described (18).
Plasmid, protein, and genomic macrorestriction analyses.
Plasmid profile analysis was performed on each isolate of B. burgdorferi as described by Barbour (3). Plasmid DNA was transferred from low-percentage agarose gels to nylon membrane by the technique of Southern (43) and probed with pBHB63 specific for the 16-kb linear plasmid (15) labeled by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, N.J.). Protein profile analysis by polyacrylamide gel electrophoresis PAGE (21) and immunoblotting of spirochetal proteins were performed on spirochetes which were harvested from log-phase cultures and washed twice with cold, sterile phosphate-buffered saline (PBS) (pH 7.0), as previously described (17). The total protein concentration was determined by the Bradford method (8), and 40 μg of each protein was loaded into each lane in a 12-by-14-cm gel. Electrophoretically separated proteins of B. burgdorferi were transferred to nitrocellulose membrane and were reacted with monoclonal antibodies (MoAbs) 6C4, 1F8, 10C5, 2E3, 12E5, and 2B8 of the OspC-specific L22 series (provided by B. Wilske, University of Munich, Munich, Germany). Genomic macrorestriction analysis was performed on MluI-digested B. burgdorferi isolates as previously described (28). Briefly, after harvest from BSK medium, spirochetal DNA cast in agarose plugs was digested overnight at 37°C by incubation in restriction buffer containing 30 U of MluI. The digested DNA was loaded in a 1.2% agarose gel (Fastlane; FMC Bioproducts, Rockland, Maine) for electrophoresis (Chef DRII; Bio-Rad, Hercules, Calif.). For protein, plasmid, and genomic macrorestriction analyses, spirochetes were harvested from log-phase cultures by centrifugation at 2,000 × g.
Cloning by limiting dilution.
B. burgdorferi isolates 225a (p3) and 225c (p4) were cloned twice by limiting dilution in 96-well flat-bottomed tissue culture plates incubated at 34°C in an atmosphere containing 5% CO2. In order to avoid aggregated clumps of spirochetes, the isolates were cloned early during growth in culture. The spirochetes were quantitated twice, and dilutions containing 300 to 0.06 spirochetes were cultured in 100 μl of BSK II medium per well. Two weeks later, the spirochetes in wells of the last positive dilution were transferred into 1.5 ml of medium and were incubated for 14 additional days. At that time the spirochetes were transferred into 12.5 ml of medium, incubated for a time sufficient to reach log phase, and were divided into aliquots for cryopreservation, plasmid profile, and protein analyses. The cloning process was repeated on a frozen aliquot of selected primary clones using the same protocol.
OspC amplification and sequence analysis.
A 632-bp fragment ospC was amplified from subclones 2E7′ and 3B6′ of isolate 225c by using primers PC-1s (5′-AATGAAAAAGAATACATTAAGTGCA-3′) and PC-2a (5′-TTAAGGTTTTTTTGGACTTTCTGC-3′), corresponding to ospC nucleotide positions 305 and 938 of isolate B31 (GenBank BBU01894). Amplifications were performed in a 100-μl reaction mixture containing 1× PCR buffer (Boehringer Mannheim Corp., Indianapolis, Ind.), 10% (vol/vol) glycerol, 1.5 mM MgCl2, 0.2 mM (each) deoxynucleoside triphosphate, 2.5 U of Taq DNA polymerase (Boehringer Mannheim), and 100 pmol each of primers PC-1 and PC-2. Amplification conditions were an initial cycle of 4 min at 94°C, for denaturation, 40 cycles consisting of a 1-min denaturation at 94°C, a 1-min annealing at 45°C, and a 2-min extension at 72°C, followed by a final cycle of a 4-min extension at 72°C. For PCR, spirochetes were grown in culture, harvested by centrifugation, and washed 1× in PBS. Spirochetes were resuspended in distilled water (dH2O) and heated to 100°C for 10 min before 10 μl was added to each reaction. The PCR master mix was prepared, and template DNA was added in a room in which PCR product was not analyzed. A negative control reaction (dH2O) was included in each group of PCR reactions.
Amplification products produced by primers PC-1s and PC-2a were purified (Wizard PCR Prep; Promega, Madison, Wis.), and the sense and antisense sequences were determined by using the amplification primers and internal primers OspC 605s (5′-GAGCTTAGCAATAT-3′) and OspC 607a (5′-CTCCCGCTAACAAT-3′) (the nucleotide position refers to GenBank BBU01894) in an automated DNA sequencing instrument (ABI 373; Perkin-Elmer Applied Biosystems, Foster City, Calif.). Consensus ospC sequences were created by assembling contiguous sequences with the Gel Assemble program of the Wisconsin Package (13), and these were aligned and translated by using the PileUp and Translate functions, respectively, of the Wisconsin Package (13). The ospC nucleotide sequence for subpopulation members represented by subclones 2E7′ and 3B6′ have been submitted to GenBank under accession numbers AF074465 and AF074464, respectively.
Population dynamics in experimentally infected mice.
Six groups of 10 3- to 4-week-old C3H.RKK mice, obtained from a breeding colony at the Mayo Foundation, were inoculated intradermally (i.d.) with 2 × 103 spirochetes of B. burgdorferi isolate 225c and 50:50, 90:10, and 10:90 mixtures of subclones 2E7′ or 3B6′ totalling 2 × 103 organisms. Mice were also inoculated with 2 × 103 of subclones 2E7′ or 3B6′. A 2-mm-diameter ear biopsy sample was obtained from all mice at 3, 5, 11, 19, 27, 41, and 52 weeks postinoculation for culturing as previously described. Plasmid profile analysis was performed on sequential isolates for individual mice representing each inoculation group. To determine the sampling variability of culture and plasmid profile analysis in ear biopsy samples obtained from mice infected with a mixture of subclones 2E7′ or 3B6′, we inoculated three mice with a mixture of 104 spirochetes of each subclone and then cultured three replicate samples from the same ear of each mouse at 2 and 4 weeks postinoculation for plasmid analysis. Additionally, we evaluated the sensitivity of plasmid analysis in the detection of a mixed population of spirochetes cultured from an infected animal by using mixtures of each subclone totalling 5 × 108 spirochetes. These mixtures contained subclones 2E7′ and 3B6′, respectively, in the following ratios: 50:50, 75:25, 90:10, 95:5, 99:1, 25:75, 10:90, 5:95, and 1:99. The prepared mixtures were analyzed directly for plasmid content as previously described.
Superinfection studies.
Four 3- to 4-week-old C3H.RKK mice were inoculated i.d. with 104 spirochetes of B. burgdorferi subclone 2E7′ or 3B6′. Infection in all mice was confirmed by culture 10 days after inoculation. One month later, the mice were anesthetized, bled from the periocular sinus, and challenged by i.d. inoculation with 104 spirochetes of the heterologous subclone. Serum was separated from each blood sample and stored at −20°C. At 3 and 6 weeks after challenge an ear biopsy sample was obtained from each mouse and cultured in BSK II medium. Cultures with spirochetal growth were subcultured into two tubes of BSK II medium for plasmid profile and genomic macrorestriction digest analyses. Antibodies specific for B. burgdorferi were detected in serum samples by immunoblot. Briefly, 100 μg of each subclone of B. burgdorferi was separated on a sodium dodecyl sulfate (SDS)–12.5% PAGE gel and transferred to nitrocellulose membrane, as previously described. Mouse sera were reacted to the transferred proteins at a dilution of 1:150 in Tris (50 mM) and sodium chloride (150 mM) containing 0.1% Tween 20 and 0.5% blocking solution (vol/vol) (BM Chemiluminescence; Roche Molecular Biochemicals, Indianapolis, Ind.) in a Miniblotter 25 apparatus (Immunetics, Inc., Cambridge, Mass.). Reactive antibodies were detected by horseradish peroxidase (POD)-labeled goat anti-mouse antibody (Roche) at a dilution of 1:4,000 according to the manufacturer's recommendations.
RESULTS
Sequential isolates are equally infectious.
B. burgdorferi isolates 225a and 225c were cultured sequentially 2.5 months apart from the same wild-caught P. leucopus as part of a longitudinal study on the maintenance of B. burgdorferi in a population of mice in Maryland (16). These primary isolates differed by the presence of plasmids migrating at 38, 36, and 16 kb in isolate 225a and the absence of those plasmids in isolate 225c. Isolate 225c contained a plasmid migrating at 17 kb, which was shown to be related to the 16-kb linear plasmid by hybridization studies (17). Additionally, relatively less OspC was expressed in isolate 225a than in 225c. To determine whether the genotypic and phenotypic differences detected in isolates 225a and 225c affected the ability of the spirochetes to be acquired and transmitted by vector ticks, between 8 and 12 replete larval ticks were fed on C.B-17 SCID mice which had been inoculated 1 month previously with 225a or 225c. The ear tissues removed from the inoculated mice at the completion of tick feeding were all culture positive for B. burgdorferi. The kidney, spleen, bladder, and ear tissues removed from the mice exposed to the newly molted nymphal ticks were uniformly culture positive for the spirochete, showing that the phenotypic differences between isolates 225a and 225c were not associated with obvious differences in infectivity or transmissibility.
Genotypic and phenotypic characteristics of the sequential isolates are unstable during secondary animal passage.
To determine whether the observed genetic and phenotypic differences between isolates 225a and 225c were due to either a loss or rearrangement of DNA or were due to clonal selection, we compared the plasmid profiles of isolates 225a and 225c and reisolated spirochetes from the inoculated and tick-infected mice. The plasmid profile of isolates 225a and 225c used to infect C.B-17 mice were the same as previously reported (17) (Fig. 1). However, the plasmid profiles of organisms obtained from various secondary infections varied dramatically. For example, the plasmid profile of the reisolate from mouse C.B-17 1, inoculated with 225a, was the same as that of the inoculum. However, the profile of spirochetes reisolated from mouse C.B-17 2, inoculated with 225c, unexpectedly matched that of 225a. In tick-exposed mice, the plasmid profile of mouse C3H 1, infected with ticks which fed upon the mouse inoculated with 225a, unexpectedly matched the profile of 225c. Finally, the plasmid profile of the reisolate from mouse C3H 2, was characteristic of both isolates 225a and 225c. Taken together, these observations suggested that clonal selection had occurred within the inoculated or tick-infected mice or within the ticks themselves.
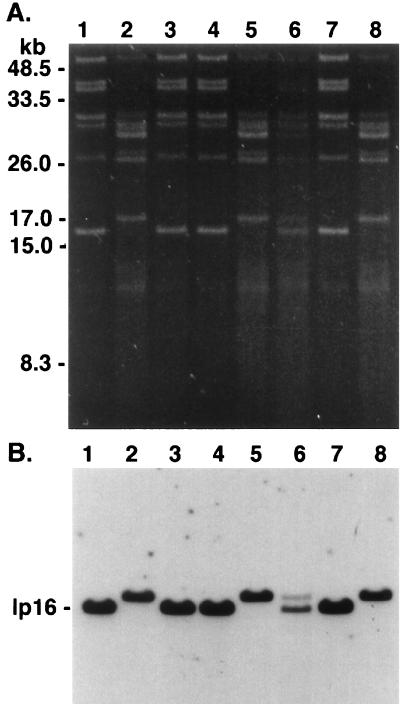
FIG. 1.
Plasmid profiles of B. burgdorferi isolates and subclones of isolate 225c. (A) Plasmid-enriched DNA was analyzed on a 0.2% agarose gel and stained with ethidium bromide. Lanes: 1 and 2, isolates 225a and 225c, respectively, which were cultured sequentially from a P. leucopus mouse; 3 and 4, isolates from mice C.B-17 1 and 2 inoculated 1 month previously with 225a and 225c and which served to infect larval I. scapularis ticks; 5 and 6, isolates from C3H 1 and 2 obtained 1 month after nymphal tick feeding; 7 and 8, subclones 2E7′ and 3B6′ obtained from 225c by limiting dilution. DNA molecular mass standards are indicated on the left. (B) Plasmid DNA was transferred to nylon membrane and was hybridized to pBHB63 (specific for the 16-kb linear plasmid) labeled by ECL. The gel photograph and hybridization were scanned into Adobe Photoshop 3.1, scaled to the column width, and labeled.
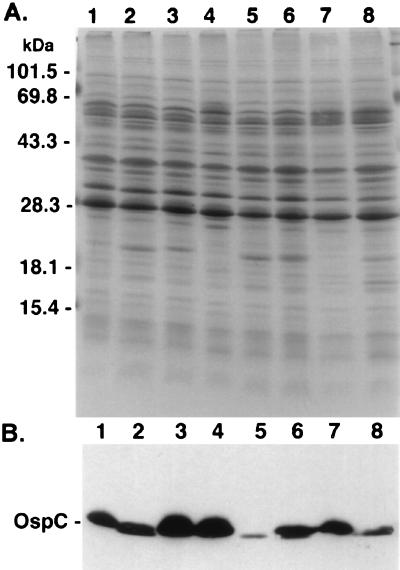
In agreement with our previous report (17), the protein profiles of isolates 225a and 225c were similar upon SDS-PAGE analysis, except that isolate 225c expressed more OspC than 225a (Fig. 2A). Based on the gel migration, the OspC protein of 225a and 225c showed apparent Mrs of 21 kDa; however, the OspC of 225c was slightly smaller. Both isolates reacted with OspC-specific antibody L32 1F8 (Fig. 2B). Consistent with the results of the plasmid analysis, both isolates from the C.B-17 mice expressed a slightly smaller OspC, based on its relative migration, while the isolate from C3H 1 expressed a slightly larger OspC. As anticipated from the plasmid analysis, the reisolate from mouse C3H 2 expressed two OspC proteins which reacted with antibody L32 1F8. These different OspC results corresponded to the presence of a 16- or a 17-kb linear plasmid or both. Expression of OspC was consistently less in those isolates containing a 16-kb plasmid, as previously described (17).
FIG. 2.
Protein profiles of B. burgdorferi isolates and subclones of isolate 225c. (A) Whole-cell lysates (40 μg) were analyzed by 10 to 20% PAGE and stained with Coomassie blue. Loading of the lanes is identical to that described for Fig. 1. (B) Proteins from gel (prepared as in panel A) were transferred to nitrocellulose membrane, reacted with MAb L22 1F8 (specific for OspC), and detected by POD-labeled goat anti-mouse antibody. Migration of prestained molecular mass markers is indicated on the left side of panel A. The gel photograph and immunoblot were scanned into Adobe Photoshop 3.1, scaled to the column width, and labeled.
Isolates 225a and 225c are polyclonal mixtures of genotypically and phenotypically distinct clones.
The results of the transmission experiment suggested that the primary isolates 225a and 225c may have been comprised of subpopulations of B. burgdorferi with fluctuation of the predominant member over time. To test this hypothesis, we cloned isolate 225c twice by limiting dilution. Plasmid profiles of 18 primary clones that were obtained from 225c demonstrated that it was composed of a heterogeneous population of spirochetes comprising two distinct subpopulations. Based on the plasmid profiles, 13 of 18 primary clones (72%) corresponded closely to isolate 225c and the remaining 5 primary clones (28%) corresponded to 225a. Primary clones 2E7 and 3B6 were selected as representatives of the two subpopulations for a second round of cloning by limiting dilution; subclones were tested for identity with the parent primary clone by plasmid and protein analyses. Subclones cultured from primary clones 2E7 and 3B6 were found to be identical to the primary clones by plasmid profile (Fig. 1) and by protein analyses (Fig. 2), and the size and relative level of OspC expression in the secondary clones was also consistent with that observed in the primary clones. A representative of each subclone, designated 2E7′ and 3B6′, was selected for further analysis by genomic macrorestriction analysis, ospC sequence analysis, and antigenic reactivity with a panel of MAbs to OspC.
To determine whether isolate 225a, isolated from the wild-caught mouse 2.5 months prior to the isolation of isolate 225c, also was composed of a population of B. burgdorferi, we cloned isolate 225a by limiting dilution and performed plasmid profile analysis on the primary clones. The plasmid profile of 12 of 13 (92%) primary clones cultured from 225a matched that represented by 225c subclone 2E7′; the profile of the remaining clone of 225a matched the profile of 225c subclone 3B6′. This shows that the first isolate from mouse 225 was also comprised of both subpopulations and suggests that a shift may have occurred in the population structure from one consisting primarily of members represented by subclone 2E7′ (in isolate 225a) to one consisting primarily of members represented by 3B6′ (in isolate 225c).
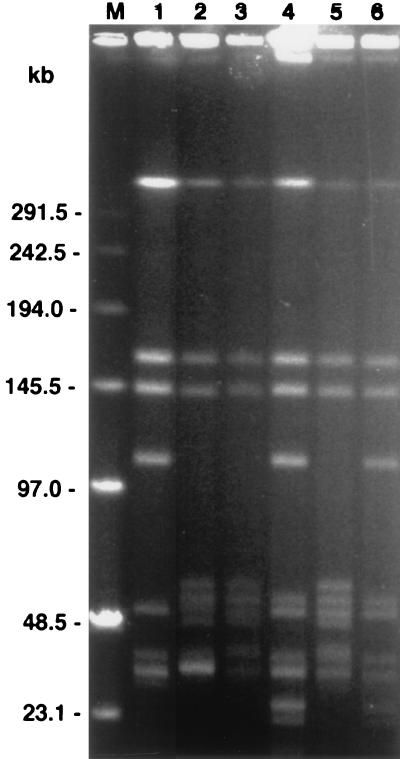
Genomic differences between the clones were also demonstrated; after MluI digestion, two different pulsed-field types (PFTs) were identified on pulsed-field gel electrophoresis (PFGE) of the secondary clones from isolate 225c (Fig. 3). The PFT of secondary clone 2E7′ was most similar to the PFT of B. burgdorferi isolate B31 (PFT B), as previously described (28). In contrast, the PFT of 3B6′ matched that of PFT A, indicating that, in addition to the heterogeneity of the plasmid content, isolate 225c was composed of distinct members exhibiting chromosomal polymorphism in addition to heterogeneity in plasmid content.
FIG. 3.
PFGE of MluI digests of B. burgdorferi isolates and subclones of isolate 225c. Lanes: 1, isolate WFMC4 (PFT A); 2, B31 (PFT B); 3 and 4, P. leucopus isolates 225a and 225c, respectively; 5 and 6, isolate 225c subclones 2E7′ and 3B6′, respectively; M, DNA pulse marker, 0.1 to 200 kb (Sigma). The gel photograph was scanned into Adobe Photoshop 3.1, scaled to the column width, and labeled.
Distinct population members harbor genetically and antigenically distinct OspCs because the primary clones of 225c expressed an OspC protein which varied slightly in size.
Based on the gel migration patterns, we amplified and sequenced ospC from subclones 2E7′ and 3B6′ in order to determine whether the ospC coding sequence was polymorphic. In the 603 bp compared, we found that the derived ospC sequence of subclone 2E7′ shared 85% identity on a DNA basis and 78% identity on an amino acid basis with the ospC sequence of subclone 3B6′ (Fig. 4). However, in the V2 variable region of OspC (amino acids 112 to 186 numbering from the N-terminal methionine [23]) subclones 2E7′ and 3B6′ shared only 65% identity on an amino acid basis and included an amino acid insertion or a deletion following amino acid 118 (Fig. 4). The peptide algorithm of the Wisconsin Package (13) predicted a protein molecular mass of 21.3 kDa for subclone 2E7′ and 21.5 kDa for 3B6′. As expected, the B. burgdorferi species-specific sequence (amino acids 23 to 34 numbering from the N-terminal methionine [23]) predicted for each subclone indicated that the spirochetes were B. burgdorferi sensu stricto.
FIG. 4.
Alignment of derived amino acid sequence of OspC from subclones 2E7′ and 3B6′ cloned from B. burgdorferi isolate 225c. Consensus sequence are indicated by capital letters, and nucleotide substitutions are indicated by dashes, with the appropriate substitution indicated by lowercase letters. The transcriptional start site is in boldface. Deletions are indicated by a period.
To determine whether the amino acid polymorphism detected between the ospC sequences of subclones 2E7′ and 3B6′ resulted in antigenic heterogeneity as well, immunoblotting of whole-cell lysate with a panel of MAbs produced against OspC was performed. On immunoblotting, subclone 2E7′ reacted with all 6 MAbs. However, subclone 3B6′ reacted with MAbs 6C4, 1F8, 10C5 (weakly), and 2B8 and not at all with MAbs 2E3 and 12E5 (data not shown). Isolate B31 reacted with all 6 MAbs and was used in this experiment as a positive control because the reactivity of B31 to this panel of MAbs was reported previously (19). This experiment demonstrated that antigenic heterogeneity between the subclones of isolate 225c most likely resulted from the amino acid variability between subclones.
Population dynamics in experimentally infected mice.
To evaluate the possibility that polyclonal infections would consistently shift in favor of a particular dominant member, we inoculated mice with the primary isolate 225c and defined mixtures of subclones 2E7′ and 3B6′ and then monitored the plasmid profiles of prospective isolates of B. burgdorferi. A pattern of selection for one population member as opposed to the heterologous member was not observed in spirochetes reisolated from mice of any inoculation group. The results for mice from each group which received the primary isolate 225c or defined mixtures of the subclones are represented for four of the sampling dates in Table 1. In the majority of mice inoculated with a population of spirochetes, the predominant population member apparently shifted randomly during the course of infection (mice 55, 17, 42, 43, 31, 38, and 52). Both population members were detectable in some mice by plasmid analysis at each sampling date (mice 60 and 32), whereas in others only one member was detectable throughout the sampling period (mice 5, 16, and 41). Even in inocula containing only 10% of one subclone, both subclones were still detectable after a year. In contrast, the plasmid profiles of mice inoculated with a single subclone remained unaltered and characteristic of that specific subpopulation member throughout the sampling period (data not shown).
TABLE 1.
Detection by plasmid profile analysis of predominant population members of B. burgdorferi isolate 225c in isolates from C3H mice inoculated with 225c and combinations of subclones 2E7′ and 3B6′
| Inoculuma | Mouse | Presence of 2E7′/3B6′ at (weeks postinfection)b:
|
|||
|---|---|---|---|---|---|
| 3 | 5 | 27 | 52 | ||
| 225c | 1 | +/+ | +/+ | +/+ | +/− |
| 2 | −/+ | −/+ | −/+ | −/+ | |
| 5 | −/+ | −/+ | −/+ | −/+ | |
| 55 | +/+ | +/+ | +/− | +/+ | |
| 56 | +/+ | +/− | +/− | +/− | |
| 57 | Neg | +/+ | +/− | +/− | |
| 60 | Neg | +/+ | +/+ | +/+ | |
| 2E7′-3B6′ (50:50) | 16 | −/+ | −/+ | −/+ | −/+ |
| 17 | −/+ | +/+ | +/− | −/+ | |
| 19 | Neg | −/+ | −/+ | −/+ | |
| 41 | +/− | +/− | +/− | +/− | |
| 42 | −/+ | −/+ | +/+ | −/+ | |
| 43 | +/− | −/+ | +/− | −/+ | |
| 2E7′-3B6′ (90:10) | 31 | −/+ | +/− | +/− | +/+ |
| 32 | +/+ | +/+ | +/+ | +/+ | |
| 2E7′-3B6′ (10:90) | 38 | +/+ | −/+ | −/+ | +/+ |
| 52 | +/+ | −/+ | −/+ | +/+ | |
Mice were infected with an intradermal inoculum of 2 × 103 spirochetes in BSK II medium.
Detection (+) of plasmid profile characteristic of subclone 2E7′ indicated to the left of the slash mark and of subclone 3B6′ to the right of slash mark.
Neg, culture negative.
In a separate experiment designed to test the sampling variability of plasmid analysis in cultures derived from replicate ear samples, we determined that the profiles of all three replicate ear cultures were in agreement at both 2 and 4 weeks postinoculation for each mouse (data not shown). This showed that the plasmid profile at a given time reflected the predominant population member and was probably not biased by sampling variability. In addition, we determined that plasmid analysis was able to detect a 5% minor member in an experimental mixture of subclones 2E7′ and 3B6′ totalling 5 × 108 spirochetes: the approximate yield from a cultured ear punch (data not shown).
Sequential infection by genetically and antigenically distinct B. burgdorferi subclones.
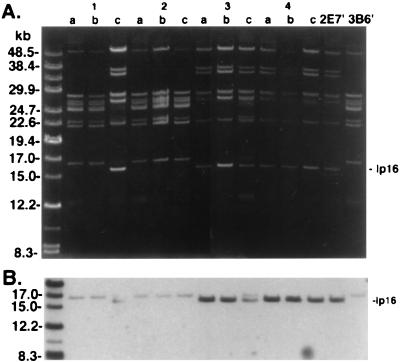
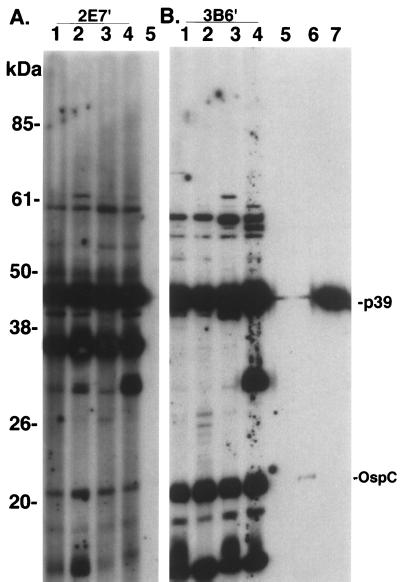
Superinfection was established in three of four mice inoculated with either subclone and challenged 1 month later by inoculation with the heterologous subclone (Fig. 5). In each animal in which superinfection was detected, the heterologous subclone was not detected by plasmid analysis until 2 months postchallenge. Superinfection occurred despite the development of an immune response to the initial inoculation, as shown by the presence of specific antibodies to B. burgdorferi upon immunoblot analysis (Fig. 6). All mice inoculated with subclones 2E7′ or 3B6′ developed antibodies to a number of antigens of B. burgdorferi, including OspC and p39. Immunoblot analysis also revealed a second notable difference between subclones 2E7′ and 3B6′. Prominent seroreactivity to a protein of approximately 28 kDa was detected in all mice infected with subclone 2E7′ when reacted to the homologous isolate (Fig. 6A). Reactivity to a protein migrating at approximately the same position was absent in sera from mice infected with subclone 3B6′ (Fig. 6B). The protein in question was probably OspD, which is encoded on the 38-kb linear plasmid of B. burgdorferi; subclone 2E7′ contained a 38-kb plasmid and 3B6′ did not. Superinfection was also established in one of two mice exposed to I. scapularis ticks infected with subclone 3B6′ and subsequently challenged 1 month later with ticks infected with subclone 2E7′ (data not shown). We were unsuccessful at establishing superinfection with 3B6′ in mice previously infected by tick bite with subclone 2E7′; however, uninfected control mice similarly exposed to ticks of the same cohort also remained uninfected with 3B6′, indicating that the challenge ticks were probably uninfected or carried a low level of infection.
FIG. 5.
Plasmid profiles B. burgdorferi isolates from superinfection experiment. (A) Plasmid-enriched DNA was analyzed on a 0.2% agarose gel and stained with ethidium bromide. Mice 1 and 2 were inoculated with 104 3B6′ spirochetes and challenged 4 weeks later by inoculation of the same number of subclone 2E7′ spirochetes. Sequential isolates of B. burgdorferi are shown for each animal as denoted by a to c, with culture a obtained 1 month postinfection and cultures b and c obtained 1 and 2 months, respectively, after challenge. Mouse 1 was superinfected as shown by the appearance of plasmids at 36 and 38 kDa and by the appearance of a 16-kb linear plasmid which migrated just below the faintly appearing 17-kb plasmid. Similarly, mice 3 and 4 were inoculated with subclone 2E7′ and subsequently challenged with subclone 3B6′. Mouse 3 was superinfected as shown by the faintly appearing 17-kb linear plasmid, which migrated just above the 16-kb plasmid. Superinfection was not detected in mouse 2 or 4. Lanes 2E7′ and 3B6′, plasmid DNA from subclones used to infect mice. DNA molecular mass standards indicated on the left. (B) Plasmid DNA was transferred to nylon membrane and was hybridized to pBHB63 (specific for the 16-kb linear plasmid) labeled by ECL. The gel photograph and hybridization were scanned into Adobe Photoshop 3.1, scaled to the column width, and labeled.
FIG. 6.
Immunoblot analysis of sera from mice in the superinfection experiment. Serum (diluted 1:150) from mice infected with either subclone 2E7′ (lanes 1 to 4 in panel A) or 3B6′ (lanes 1 to 4 in panel B) were reacted against the homologous B. burgdorferi subclone antigens (2E7′ in panel A and 3B6′ in panel B). Serum from an uninoculated mouse is included in lane 5 of each panel. Lanes 6 and 7, MAbs to B. burgdorferi-specific antigens ospC and p39, respectively. All sera were detected with POD-labeled secondary antibody (BM Chemiluminescence; Roche). Seroreactivity at approximately 30 kDa in panel A probably corresponds to OspD expression in 2E7′, which is encoded on the 38-kb plasmid (subclone 3B6′ lacks plasmids at 36 and 38 kb). Prestained molecular mass markers (Life Technologies) are indicated on the left. The immunoblots were scanned into Adobe Photoshop 3.1, scaled to the column width, and labeled.
DISCUSSION
The present study begins to address the ecological significance of observations made during a 2-year study of wild-caught mice naturally infected with B. burgdorferi (16). Previously, we reported on a number of genotypic and phenotypic changes identified in sequential isolates of B. burgdorferi obtained from mice in that study (17). Using B. burgdorferi sequentially isolated from one representative mouse from the previous study, we have determined that the isolates were composed of a mixed population with subpopulation members which were genetically and antigenically distinct. We hypothesized that other mice in the same cohort were likely similarly infected with a population of spirochetes and that the genotypic and phenotypic changes which we observed in sequential isolates from the same mouse were probably due to changes in the relative proportion of subpopulation members. This hypothesis was supported by similar findings when laboratory mice were inoculated with isolate 225c or with defined mixtures of its cloned subpopulation members. We also found that mice developed a specific immune response to one subpopulation member but were nevertheless permissive to challenge by another subpopulation member. These findings suggest that genotypic and phenotypic heterogeneity, shifting clonal dominance in B. burgdorferi coinfections, and superinfection with B. burgdorferi may play a role in the maintenance of the organism in reservoir mammals and possibly also in reinfection, superinfection, or coinfection in human patients.
Genotypic and phenotypic heterogeneity in Borrelia isolates can be the result of genetic or antigenic variation of a clonal population, as described for the VMP protein of B. hermsii (4) and more recently for the vlsE and OspC proteins of B. burgdorferi, respectively (39, 48). However, in our case, the heterogeneity was the result of a mixed infection with B. burgdorferi. Previously, a mixed infection of Apodemus speciosus mice with B. afzelii and B. burgdorferi group IV, which were differentiated by rRNA ribotyping, was reported (30). More recently, mixed infections of B. afzelii and B. garinii have been reported in rodents captured in Russia (37). In North America, infection of reservoir hosts with a population of B. burgdorferi sensu stricto has been postulated by Guttman et al. (14) and Wang et al. (46) based on the molecular detection of two or more ospA or ospC variants by single-stranded conformation analysis analysis of PCR products, respectively, in adult I. scapularis ticks. Infection of chipmunks (Tamias striatus) with B. burgdorferi encoding more than one ospA type has been suggested indirectly by SSCP analysis of nymphal ticks collected as replete larvae from captured animals (39). However, the demonstration of different SSCP types in a PCR reaction derived from a sample is not conclusive proof of mixed infections, since one clone could conceivably harbor many variant plasmids. Our study is the first to demonstrate by limiting dilution and subsequent genetic and phenotypic analyses a heterogeneous population of B. burgdorferi sensu stricto within reservoir mice.
Using a highly sensitive plasmid analysis assay capable of detecting as low as 5% of the total population of cultured spirochetes, we found that the predominant subpopulation member varied widely over time and that these variations apparently occurred randomly. We speculate that subpopulation fluctuations occur during the natural course of infection within the host, since similar plasmid profile fluctuations were observed in wild-caught and experimentally infected animals monitored over time. Definitive confirmation of population variations within the host might be achieved through a PCR-based detection method in which the relative level of subclone members is detected directly in tissue samples.
Although a recent study of B. burgdorferi genotypic and phenotypic patterns suggests that there may be factors within the host that activate preferentially the site-specific recombination of ospB and ospC, an alternate explanation, host selection of subpopulation members present in the challenge strain, was considered by the authors of that study (40). The results of our own study may be explained through the stable coexistence of B. burgdorferi subpopulations. This is supported by the fact that in the single passage of isolates 225a and 225c through a mammal-tick-mammal cycle; the minor subpopulation member was preserved in each case. The experimental infections in the study by Ryan et al. were conducted with presumably cloned B. burgdorferi and included two passages through mammals and ticks. Our studies suggest, however, that mixed populations of B. burgdorferi can be difficult to distinguish from clonal populations since minor population members can exist at a very low percentage of the overall total and yet reemerge during experimental infection.
Mixed infection of reservoir mammals with B. burgdorferi could result from cotransmission of mixed populations of spirochetes through coincident tick bites, as well as from sequential infection. In the transmission experiment, both subpopulation members were detected by plasmid analysis, thus demonstrating cotransmission. Superinfection was also observed experimentally, occurring in the majority of mice initially inoculated with one subclone of B. burgdorferi and challenged 1 month later by the heterologous subclone. Mice that developed a specific immune response to the initial infection, as demonstrated by immunoblot analysis, were still susceptible to infection by the second subpopulation member, despite development of specific (and presumably protective) OspC responses (38). Based on reactivity to a panel of MAbs to OspC, we were able to identify several differences in the antigenicity of OspC between subclones 2E7′ and 3B6′. Mice inoculated with a whole-cell bacterin (20) or recombinant OspC were susceptible to a challenge with strains heterologous in ospC. Furthermore, challenge of mice inoculated with recombinant OspC with the homologous strain of B. burgdorferi may be only partially successful at preventing infection (7, 12) and may be dependent on surface localization of the protein (7). In addition, the establishment of superinfection may have been potentiated by differential expression of OspD or other in vivo-expressed proteins between the subpopulation members.
The role of spirochetal genetic diversity in the maintenance of spirochetes at Lyme disease enzootic sites is unknown. The maintenance of spirochetal genetic diversity in reservoir mammals may be facilitated by the inability of a specific antibody response to one B. burgdorferi variant to protect an animal from infection with other heterogeneous variants, thus resulting in mixed infection. Stable coinfection with variants of the same parasite requires the absence of competition within the same host, such that the rate of transmission to other hosts or, in the case of B. burgdorferi, to vector ticks is unaffected by the presence of the other variants (29). While we have not quantitated the transmission rates of both B. burgdorferi subpopulation members, we have demonstrated that both are cotransmissible. Based on our limited experience with these two B. burgdorferi variants, it appears more likely that a stable coinfection is established in reservoir mice, as opposed to “clonal dominance” in which only the most virulent strain, the one that develops the greatest persistent parasite density in tissue, is maintained and transmitted (31). Recent studies in our laboratory of field isolates of B. burgdorferi from P. leucopus have shown that mixed infection with B. burgdorferi is more the rule than the exception (unpublished observations). Indeed, genetic diversity itself may arise from the lateral exchange of genetic material between spirochetal clones and species, as suggested by studies of ospC (10, 23) and ospD (26); this process is dependent on the infection of reservoir hosts or ticks with mixed spirochete populations.
ACKNOWLEDGMENTS
This work was supported in part by Public Health service grants AI45253, AI32403, and AI41497 and by cooperative agreement CCU510343 from the Centers for Disease Control and Prevention to D.H.P. and Public Health Service grant R55AI30042 to G.E.G.
We thank D. A. Mathiesen for technical assistance.
REFERENCES
- 1.Anderson J F, Johnson R C, Magnarelli L A. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J Clin Microbiol. 1987;25:1564–1566. doi: 10.1128/jcm.25.8.1564-1566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 3.Barbour A G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour A G, Tessier S L, Stoenner H G. Variable major proteins of Borrelia hermsii. J Exp Med. 1982;156:1312–1324. doi: 10.1084/jem.156.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W. Antigenic stability of Borrelia burgdorferi during chronic infections of immunocompetent mice. Infect Immun. 1993;61:4955–4961. doi: 10.1128/iai.61.12.4955-4961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 7.Bockenstedt L, Hodzic E, Feng S, Bourrel K, De Silva A, Montgomery R, Fikrig E, Radolf J, Barthold S. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun. 1997;65:4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Cleavinger, C., A. Seltman, and D. Persing. Several mechanisms of recombination contribute to ospC diversity in Borrelia burgdorferi. Submitted for publication.
- 11.Fish D, Daniels T J. The role of medium sized mammals as reservoirs of Borrelia burgdorferi in southern New York. J Wildl Dis. 1990;26:339–345. doi: 10.7589/0090-3558-26.3.339. [DOI] [PubMed] [Google Scholar]
- 12.Gilmore R, Kappel K, Dolan M, Burkot T, Johnson B. Outer surface protein C (OspC), but not p39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genetics Computer Group. Program manual for the Wisconsin Package, version 9. Wis: University of Wisconsin, Madison; 1994. [Google Scholar]
- 14.Guttman D, Wang P, Wang I, Bosler E, Luft B, Dykhuizen D. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J Clin Microbiol. 1996;34:652–656. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinnebusch J, Barbour A G. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992;174:5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmeister E, Ellis B, Glass G, Childs J. Longitudinal study of infection with Borrelia burgdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. Am J Trop Med Hyg. 1999;60:598–609. doi: 10.4269/ajtmh.1999.60.598. [DOI] [PubMed] [Google Scholar]
- 17.Hofmeister E K, Childs J E. Analysis of Borrelia burgdorferi sequentially isolated from Peromyscus leucopus captured at a Lyme disease enzootic site. J Infect Dis. 1995;172:462–469. doi: 10.1093/infdis/172.2.462. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeister E K, Markham R B, Childs J E, Arthur R R. Comparison of polymerase chain reaction and culture for detection of Borrelia burgdorferi in naturally infected Peromyscus leucopus and experimentally infected C.B-17 scid/scid mice. J Clin Microbiol. 1992;30:2625–2631. doi: 10.1128/jcm.30.10.2625-2631.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jauris-Heipke S, Liegl G, Preac-Mursic V, Rossler D, Schwab E, Soutschek E, Will G, Wilske B. Molecular analysis of genes encoding outer surface protein C (OspC) of Borrelia burgdorferi sensu lato: relationship to ospA genotype and evidence of lateral gene exchange of ospC. J Clin Microbiol. 1995;33:1860–1866. doi: 10.1128/jcm.33.7.1860-1866.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtti T, Munderloh U, Norton Hughes C, Engstrom S, Johnson R. Resistance to tick-borne spirochete challenge induced by Borrelia burgdorferi strains that differ in expression of outer surface proteins. Infect Immun. 1996;64:4148–4153. doi: 10.1128/iai.64.10.4148-4153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Levine J F, Wilson M L, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 23.Livey I, Gibbs C, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 24.Magnarelli L A, Anderson J F, Burgdorfer W, Chappell W A. Parasitism by Ixodes dammini (Acari; Ixodidae) and antibodies to spirochetes in mammals at Lyme disease foci in Connecticut, USA. J Med Entomol. 1984;21:52–57. doi: 10.1093/jmedent/21.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Magnarelli L A, Anderson J F, Kerwin K E, Fish D, McAninch J B. Serologic analyses of Peromyscus leucopus, a rodent reservoir for Borrelia burgdorferi, in northeastern United States. J Clin Microbiol. 1988;26:1138–1141. doi: 10.1128/jcm.26.6.1138-1141.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the OspD gene among Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mather T N, Wilson M L, Moore S I, Ribeiro J M C, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 28.Mathiesen D, Oliver J, Jr, Kolbert C, Tullson E, Johnson B, Campbell G, Mitchell P, Reed K, Telford III S, Anderson J, Lane R, Persing D. Genetic heterogeneity of Borrelia burgdorferi in the United States. J Infect Dis. 1997;175:98–107. doi: 10.1093/infdis/175.1.98. [DOI] [PubMed] [Google Scholar]
- 29.May R M, Nowak M A. Coinfection and the evolution of parasite virulence. Proc R Soc Lond B. 1995;261:209–215. doi: 10.1098/rspb.1995.0138. [DOI] [PubMed] [Google Scholar]
- 30.Nakao M, Miyamoto K. Mixed infection of different Borrelia species among Apodemus speciosus mice in Hokkaido, Japan. J Clin Microbiol. 1995;33:490–492. doi: 10.1128/jcm.33.2.490-492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak M, May R M. Superinfection and the evolution of parasite virulence. Proc R Soc Lond B. 1994;255:81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- 32.Norris S J, Carter C J, Howell J K, Barbour A G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface exposed, plasmid-encoded lipoprotein. Infect Immun. 1992;60:4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver J H, Jr, Owsley M R, Hutcheson H J, James A M, Chen C, Irby W S, Dotson E M, McLain D K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae) J Med Entomol. 1993;30:54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- 34.Persing D H, Mathiesen D, Podzorski D, Barthold S W. Genetic stability of Borrelia burgdorferi recovered from chronically infected immunocompetent mice. Infect Immun. 1994;62:3521–3527. doi: 10.1128/iai.62.8.3521-3527.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piesman J, Dolan M, Happ C, Luft B, Rooney S, Mather T, Golde W. Duration of immunity to reinfection with tick-transmitted Borrelia burgdorferi in naturally infected mice. Infect Immun. 1997;65:4043–4047. doi: 10.1128/iai.65.10.4043-4047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piesman J, Donahue J G, Mather T N, Spielman A. Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in field-collected larval Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1986;23:219. doi: 10.1093/jmedent/23.2.219. [DOI] [PubMed] [Google Scholar]
- 37.Postic D, Korenberg E, Gorelova N, Kovalevski Y, Bellenger E, Baranton G. Borrelia burgdorferi sensu lato in Russia and neighboring countries: high incidence of mixed isolates. Res Microbiol. 1997;148:691–702. doi: 10.1016/S0923-2508(99)80068-0. [DOI] [PubMed] [Google Scholar]
- 38.Probert W, Crawford M, Cadiz R, LeFebvre R. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis. 1997;175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 39.Qiu W, Bosler E, Campbell J, Ugine G, Wang I, Luft B, Dykhuizen D. A population genetic study of Borrelia burgdorferi sensu stricto from eastern Long Island, New York, suggested frequency-dependent selection, gene flow and host adaptation. Hereditas. 1997;127:203–216. doi: 10.1111/j.1601-5223.1997.00203.x. [DOI] [PubMed] [Google Scholar]
- 40.Ryan J, Levine J, Apperson C, Lubke L, Wirtz R, Spears P, Orndorff P. An experimental chain of infection reveals that distinct Borrelia burgdorferi populations are selected in arthropod and mammalian hosts. Mol Microbiol. 1998;30:365–379. doi: 10.1046/j.1365-2958.1998.01071.x. [DOI] [PubMed] [Google Scholar]
- 41.Schwan T G, Karstens R H, Schrumpf M E, Simpson W J. Changes in the antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete, during persistent infection in mice. Can J Microbiol. 1991;37:450–454. doi: 10.1139/m91-074. [DOI] [PubMed] [Google Scholar]
- 42.Smith R P, Jr, Rand P W, Lacombe E H, Telford III S R, Rich S M, Piesman J, Spielman A. Norway rats as reservoir hosts for Lyme disease spirochetes on Monhegan Island, Maine. J Infect Dis. 1993;168:678–691. doi: 10.1093/infdis/168.3.687. [DOI] [PubMed] [Google Scholar]
- 43.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 44.Steere A C, Malawista S E. Cases of Lyme disease in the United States: locations correlated with distribution of Ixodes dammini. Ann Intern Med. 1979;91:730–733. doi: 10.7326/0003-4819-91-5-730. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson B, Bockenstedt L K, Barthold S W. Expression and gene sequence of outer surface protein C of Borrelia burgdorferi reisolated from chronically infected mice. Infect Immun. 1994;62:3568–3571. doi: 10.1128/iai.62.8.3568-3571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang I, Dykhuizen D, Qiu W, Dunn J, Bosler E, Luft B. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright S D, Nielsen S W. Experimental infection of the white-footed mouse with Borrelia burgdorferi. Am J Vet Res. 1990;51:1980–1987. [PubMed] [Google Scholar]
- 48.Zhang J, Hardham J, Barbour A, Norris S. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]