Abstract
Data on the contribution of hepatitis B virus (HBV) infection and related comorbidities to liver-related mortality in Canada are limited. We assessed the concurrent impact of HBV infection, non-alcoholic fatty liver disease (NAFLD), and hepatitis C virus (HCV) coinfection on liver-related deaths in British Columbia (BC), Canada. We used data from the BC Hepatitis Testers Cohort (BC-HTC). We used Fine–Gray multivariable sub-distributional hazards models to assess the effect of HBV, NAFLD, and HCV coinfection on liver-related mortality, while adjusting for confounders and competing mortality risks. The liver-related mortality rate was higher among people with HBV infection than those without (2.57 per 1000 PYs (95%CI: 2.46, 2.69) vs. 0.62 per 1000 PYs (95%CI: 0.61, 0.64), respectively). Compared with the HBV negative groups, HBV infection was associated with increased liver-related mortality risk in almost all of the subgroups: HBV mono-infection (adjusted subdistribution hazards ratio (asHR) of 3.35, 95% CI 3.16, 3.55), NAFLD with HBV infection, (asHR 12.5, 95% CI 7.08, 22.07), and HBV/HCV coinfection (asHR 8.4, 95% CI 7.62, 9.26). HBV infection is associated with a higher risk of liver-related mortality, and has a greater relative impact on people with NAFLD and those with HCV coinfection. The diagnosis and treatment of viral and fatty liver disease are required to mitigate liver-related morbidity and mortality.
Keywords: hepatitis B virus, liver-related mortality, British Columbia, population-based cohort
1. Introduction
The World Health Organization (WHO) estimated that 296 million people were living with chronic hepatitis B virus (HBV) infection worldwide as of 2020 [1]. It was further reported that 820,000 people died of HBV-related causes in 2019 [1]. HBV-related cirrhosis and hepatocellular carcinoma are associated with a high morbidity and mortality [2]. Liver-related mortality in Canada has been increasing and is predicted to continue to rise due to hepatitis C virus (HCV) infection, as well as alcohol and non-alcohol-related liver diseases [3,4,5,6,7]. However, data on the contribution of HBV infection to liver-related mortality in Canada are scarce.
In 2015, approximately 285,000 Canadians were living with HBV [8]. Immigrants from East Asia (5–15%) had a higher prevalence than the Canadian-born population (0.24–0.5%) [9]. In British Columbia (BC), among the people diagnosed with HBV infection, 4.3% had acute HBV infection, while 95.7% had a chronic infection [10]. In addition, acute HBV infection was predominantly diagnosed among White people (78%), while 60% of chronic HBV infections were among people of an East Asian origin [10]. Populations with a higher prevalence of HBV infection include people from East Asia; gay, bisexual, and other men who have sex with men (gbMSM); and people who use injection drugs (PWID) [11,12,13]. Recent studies in BC showed that HBV infection is associated with a high morbidity and mortality [11].
Previous studies have reported that non-alcoholic fatty liver disease (NAFLD) and HBV/HCV coinfection are associated with a higher risk of liver cirrhosis and hepatocellular carcinoma [14,15]. The findings from a study conducted in the United States showed that in 2020, people with chronic HBV infection had a 13.3 fold increased risk of dying from liver-related mortality compared with people with no HBV infection [16]. People with chronic HCV infection are at higher risk of dying from liver-related death compared with people without HCV infection [17]. The treatment of HCV infection with direct-acting antiviral agents (DAAs) is associated with a reduction in liver-related mortality [6,18]. Several studies have shown that the risk of liver-related mortality is greater for individuals with coinfection of HBV and HCV than mono-infected patients [15,19,20]. The literature has shown a high risk of liver-related mortality among people with NAFLD compared with the general population [21]. However, to the best of our knowledge, no study has assessed the relationship between HBV infection, concurrent NAFLD and HCV co-infection, and liver-related mortality, each of which are independently associated with a higher mortality. In this longitudinal cohort study, we estimated the liver-related mortality rate and the concurrent impact of HBV infection, NAFLD, and HCV coinfection on liver-related deaths in BC using population-based data from the BC Hepatitis Testers Cohort (BC-HTC) [22].
2. Patients and Methods
2.1. Data Source, Design, and Study Population
This analysis is based on the BC-HTC, which includes more than 1.7 million individuals tested for HCV or HIV, or reported as a case of HCV, HIV, or HBV from 1990 to 31 December 2015. These data are integrated through a personal health number with various administrative healthcare datasets and registries including vital statistics, cancer registries, medical visits, hospital discharge data, emergency visits, and drug dispensations [22]. A more detailed description of BC-HTC has been reported previously [12,13,22].
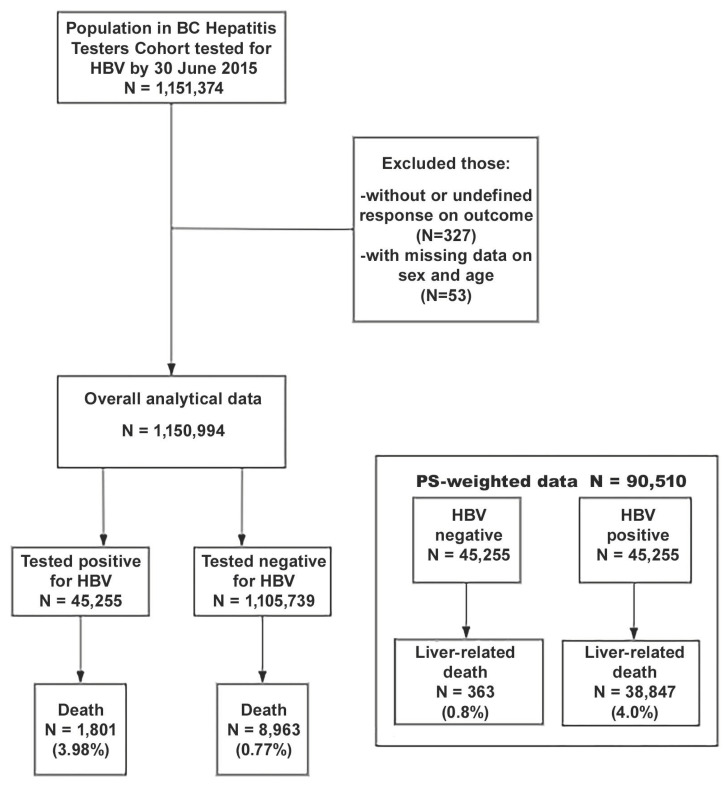
The current analysis included 1.1 million individuals who were ever tested for HBV as of 30 June 2015. We excluded people with missing data or an undefined response on outcome and those with unknown information regarding sex (Figure 1). This study was approved by the University of British Columbia Behavioral Research Ethics Board (H14-01649).
Figure 1.
Study population flowchart for liver-related mortality among a BC population cohort tested for HBV from 1990–2015. BC, British Columbia; HBV, hepatitis B virus; PS: propensity score.
2.2. Outcome and Exposure
The outcome of this study is liver-related death. Assessment of liver-related deaths was based on the BC Vital Statistics Registry, including data up to 30 June 2020 [6]. Liver-related death was defined using the International Classification of Diseases, tenth (ICD-10) edition [23,24]. The underlying and contributory cause of death were both used for defining the causes of death. People with any of the following ICD-9 code: 571 (for the period 1990–2007) and ICD-10 codes (for the period 2008–2020): viral hepatitis (B15-19), sequelae of viral hepatitis (B942), liver cancer (C22), alcoholic liver disease (K70), and non-alcoholic liver disease (K71-77) were considered as liver-related deaths. Deaths from causes other than liver-related cause were considered as competing risks [25].
The exposure variable for this study was HBV test positivity, based on the BC viral hepatitis testing guidelines [26]. In BC HTC, an individual was confirmed as a case of HBV infection with a positive HBV DNA or HBVe antigen (HBeAg) test result, or those who had a record of receiving HBV treatment [10]. The HBV effect on mortality was extended to include the concurrent effect of HCV and NAFLD. NAFLD was defined by at least one physician billing or a hospitalization code related to another chronic non-alcoholic liver disease (IDC-9 code 571.8), other specified inflammatory liver diseases (ICD-10 code K-75.8), or fatty (change of) liver, not elsewhere classified (ICD-10 code K-71.0, tenth edition) [15,16,19]. Chronic HCV infections were diagnosed based on laboratory confirmation according to BC provincial guidelines for HCV testing from a positive HCV RNA test or based on treatment data [26].
2.3. Potential Risk Factors
Demographic variables, including age (categorized as <25, 25–34, 35–44, 45–54, 55–64, and 65 and above), sex, and ethnicity were identified as risk factors for HBV [27,28]. Ethnicity was determined with validated name recognition programs [29,30], and was classified into three categories (South Asian, East Asian, and Other (White, Black, Central Asian, Latin American, Pacific Islander, and West Asian individuals)). Socioeconomic status was also assessed using the Québec Index of Material and Social Deprivation, defined in five quintiles from Q1 to Q5 [31]. The material component consisted of indicators for education, employment, and income (persons without high school diplomas, ratio employment–population ratio, and average personal income). The social component comprised indicators related to marital status and family structure (persons living alone, persons separated, divorced or widowed, and single-parent families) [11]. We defined history of injection drug use (IDU), gbMSM, and problematic alcohol use at the time of the last HBV diagnosis or the last negative test (for negative individuals) using validated algorithms, and determined the occurrence of either one hospitalization or ambulatory care visit, or two physician visits pertaining to these risk factors [32,33]. Having other comorbidities such as diabetes, chronic kidney disease, hypertension, and hepatic complications liver cirrhosis were assessed using validated algorithms based on diagnostic codes and/or prescription drug records in administrative health datasets [33]. HIV infections were diagnosed based on laboratory confirmation according to BC provincial guidelines for HIV testing [26,34].
2.4. Statistical Analysis
The primary analysis for this study included the entire study population. We assessed the crude liver-related mortality rate by HBV infection status. Person-time started at the HBV positive test date for HBV positive individuals and the last HBV test date for negative individuals, and ended at death or end of follow-up (30 June 2020).
In this study, the distribution of characteristics in individuals who tested positive for HBV infection compared with those who tested negative was different. We compared the characteristics of those who tested negative and those who tested positive through standardized mean differences (SMD) [35]. We considered the SMD cut-off point at 0.2. To account for differences between individuals who tested positive and those who tested negative, we estimated the propensity scores (PS) for infection through a logistic regression model. The model included sex, ethnicity, material deprivation quintiles, HCV infection, HIV infection, diabetes, chronic kidney disease, injection drug use, alcohol use disorder, and non-alcoholic fatty liver disease. The criteria for covariate selection for the PS model was whether the covariates confounded the relationship between HBV and liver-related mortality or risk factors of liver-related mortality [36], in order to avoid an imbalance between variables. The inverse probability of the treatment weighting (IPTW) estimating average treatment effect (ATE) based on PS was conducted as the sensitivity analysis [37]. In IPTW, we matched each HBV positive individual with a negative individual.
Cumulative incidence curves and incidence rates per 1000 person-years (PY) of follow-up were calculated in the overall study population using the HBV infection status for each NAFLD group and HCV infection group. We created four-level variables that combined HBV infection and NAFLD status, as well as HBV infection and HCV status, to assess and compare the risk of liver-related mortality across the eight subgroups (Non-NAFLD, with NAFLD, Non-HCV, with HCV without HBV infection; Non-NAFLD, with NAFLD, Non-HCV, with HCV with HBV infection). A multivariable Fine–Gray proportional hazards model [38] was used to compute the adjusted sub-distributional hazard ratios (asHR) for liver-related mortality in each subgroup. Finally, we conducted a stratified analysis to compute asHR for the effect of HBV infection on liver-related mortality by NAFLD status and by HCV status. To assess the effect modification of HBV infection by NAFLD status and by HCV status, we included the interaction terms in the multivariable model and performed a likelihood ratio test based on the analysis of deviance [39]. In the sensitivity analysis using the IPTW method, the Fine–Gray models were fit in the overall dataset with the IPTW for ATE. The multivariable Fine–Gray model with IPTW also assessed the interaction term. The dataset was created in the Statistical Analysis System (SAS) [40] and was imported in R, version 4.2 [41], for the analyses.
3. Results
3.1. Study Participant’s Characteristics
Between 1990 and 2015, 1,151,241 people were tested for HBV in BC. After excluding people without data on sex, exposure, and outcome variables, our analysis was conducted on 1,150,994 individuals (Figure 1). Among these 1,150,994 individuals, 45,255 (4.09%) were HBV positive and 1801 (3.98%) individuals with HBV infection died from liver-related mortality. In total, 8963 (0.81%) individuals died from liver-related mortality among the 1,105,739 individuals without HBV infection. The median follow-up time for the study sample was 11.86 (interquartile range: 7.55–18.01) years. A higher proportion of those testing positive compared with negative were males (25,019 (55.3%) vs. 488,450 (44.2%)), born between 1945 and 1964 (21,107 (46.6%) vs. 331,100 (29.9%)), and of East Asian ethnicity (26,827 (59.3% vs. 148,383 (13.4%)). In the social deprivation categories, a higher proportion of those testing positive compared with negative was found in most deprived people (12,654 (28.0%) vs. 214,970 (19.4%)). Among the material deprivation categories, a higher proportion of those testing positive compared with negative was found in most privileged people (10,326 (22.8%) vs. 202,776 (18.3%)) (Table 1).
Table 1.
Baseline study participants’ characteristics at the first HBV testing by HBV infection status and the propensity score (PS)-weighting dataset from the British Columbia Hepatitis Testers Cohort 1990–2015.
| Overall Study Population | Inverse Probability of Treatment Weighted Dataset * | |||||
|---|---|---|---|---|---|---|
| Covariates | HBV Negative (n = 1,105,739) |
HBV Positive (n = 45,255) |
SMD | HBV Negative (n = 45,255) | HBV Positive (n = 45,255) |
SMD |
| Sex (%) | 0.224 | 0.025 | ||||
| Female | 657,289 (55.8) | 20,236 (44.7) | 25,071 (55.4) | 24483 (54.1) | ||
| Male | 488,450 (44.2) | 25,019 (55.3) | 20,183 (44.6) | 20,772 (45.9) | ||
| Age group, years (%) | 0.331 | 0.033 | ||||
| <25 | 257,063 (23.2) | 6021 (13.3) | 10,318 (22.8) | 9820 (21.7) | ||
| 25–34 | 301,363 (27.3) | 10,634 (23.5) | 12,264 (27.1) | 12,174 (26.9) | ||
| 35–44 | 215,920 (19.5) | 12,128 (26.8) | 8960 (19.8) | 9413 (20.8) | ||
| 45–54 | 151,346 (13.7) | 8804 (19.5) | 6290 (13.9) | 6336 (14.0) | ||
| 55–64 | 96,763 (8.8) | 4677 (10.3) | 3982 (8.8) | 3439 (9.0) | ||
| ≥65 | 83,284 (7.5) | 2991 (6.6) | 3394 (7.5) | 3439 (7.6) | ||
| Birth group (%) | 0.496 | 0.055 | ||||
| <1945 | 117,235 (10.6) | 5523 (12.2) | 4842 (10.7) | 5250 (11.6) | ||
| 1945–1964 | 331,100 (29.9) | 21,107 (46.6) | 13,848 (30.6) | 14,572 (32.2) | ||
| 1965–1974 | 217,614 (19.7) | 10,183 (22.5) | 8960 (19.8) | 8870 (19.6) | ||
| ≥1975 | 439,790 (39.8) | 8442 (18.7) | 17,604 (38.9) | 16,563 (36.6) | ||
| Ethnicity (%) | 1.189 | 0.031 | ||||
| Other * | 867,680 (78.5) | 16,933 (37.4) | 34,756 (76.8) | 34,213 (75.6) | ||
| East Asian | 148,383 (13.4) | 26,827 (59.3) | 6924 (15.3) | 7377 (16.3) | ||
| South Asian | 89,676 (8.1) | 1495 (3.3) | 3575 (7.9) | 3666 (8.1) | ||
| Social deprivation (%) | 0.276 | 0.060 | ||||
| Most privileged (Q1) | 243,399 (22.0) | 6975 (15.4) | 8372 (18.5) | 8553 (18.9) | ||
| Q2 | 212,109 (19.2) | 6879 (15.2) | 8101 (17.9) | 8508 (18.8) | ||
| Q3 | 208,340 (18.8) | 8055 (17.8) | 7965 (17.6) | 7648 (16.9) | ||
| Q4 | 220,325 (19.9) | 9970 (22.0) | 9006 (19.9) | 8870 (19.6) | ||
| Most deprived (Q5) | 214,970 (19.4) | 12,654 (28.0) | 11,540 (25.5) | 11,812 (26.1) | ||
| Unknown | 6596 (0.6) | 722 (1.6) | 272 (0.6) | 272 (0.6) | ||
| Material deprivation (%) | 0.183 | 0.024 | ||||
| Most privileged (Q1) | 202,776 (18.3) | 10,326 (22.8) | 9820 (21.7) | 8553 (18.9) | ||
| Q2 | 196,770 (17.8) | 9053 (20.0) | 8598 (19.0) | 8508 (18.8) | ||
| Q3 | 194,886 (17.6) | 7663 (16.9) | 8508 (18.8) | 8644 (19.1) | ||
| Q4 | 221,654 (20.0) | 7650 (16.9) | 9051 (20.0) | 9187 (20.3) | ||
| Most deprived (Q5) | 283,057 (25.6) | 9841 (21.7) | 8960 (19.8) | 9866 (21.8) | ||
| Unknown | 6596 (0.6) | 722 (1.6) | 272 (0.6) | 272 (0.6) | ||
| Hepatitis C virus (yes) (%) | 15,906 (1.4) | 4139 (9.1) | 0.349 | 2869 (26.9) | 1665 (29.9) | 0.066 |
| HIV† (yes) (%) | 3961 (0.4) | 1318 (2.9) | 0.202 | 226 (0.5) | 272 (0.6) | 0.015 |
| Diabetes (yes) (%) | 63,917 (5.8) | 2493 (5.5) | 0.012 | 1655 (15.5) | 887 (15.9) | 0.011 |
| Chronic Kidney Disease (yes) (%) | 5342 (0.5) | 466 (1.0) | 0.063 | 226 (0.5) | 272 (0.6) | 0.010 |
| Hypertension (%) | 132,663 (12.0) | 4403 (9.7) | 0.073 | 5431 (12.0) | 4707 (10.4) | 0.049 |
| Cirrhosis (yes) (%) | 3490 (0.3) | 659 (1.5) | 0.105 | 226 (0.5) | 226 (0.5) | 0.022 |
| Decompensated cirrhosis (yes) (%) | 3576 (0.3) | 450 (1.0) | 0.083 | 181 (0.4) | 181 (0.4) | <0.001 |
| Gay, bisexual and other men who have sex with men (%) | 65,394 (5.9) | 4,013 (8.9) | 0.232 | 2761 (6.1) | 3620 (8.0) | 0.082 |
| History of injection drug use (%) | 28,814 (2.6) | 2318 (5.1) | 0.131 | 1222 (2.7) | 1222 (2.7) | 0.004 |
| Alcohol use disorder (%) | 39,599 (3.6) | 1953 (4.3) | 0.038 | 1629 (3.6) | 1448 (3.2) | 0.022 |
| Non-alcoholic fatty liver disease (%) | 668 (0.1) | 52 (0.1) | 0.018 | 45 (0.1) | 45 (0.1) | 0.003 |
* The propensity scores (PS)-matched dataset used inverse probability weights (IPW) to estimate the average treatment effect (ATE). The PS were computed based on age at the first HBV testing, sex, birth group, ethnicity, social deprivation quintiles, HCV infection, HIV infection, diabetes, chronic kidney disease, history of injection drug use, alcohol use disorder, and non-alcoholic fatty liver disease. The IPW analysis includes 45,255 individuals with HBV infection and 45,255 individuals without HBV infection. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TB, tuberculosis; PS, propensity scores; IPW: inverse probability weighting; Q, quintile; SD, standard deviation; SMD, standardized mean difference. Other *: White, Black, Central Asian, Latin American, Pacific Islander, and West Asian individuals.
IPTW also reduced the differences in characteristics between individuals with HBV infection and those without it, as shown by the SMDs in Table 1.
3.2. Liver-Related Mortality Rate among Participants
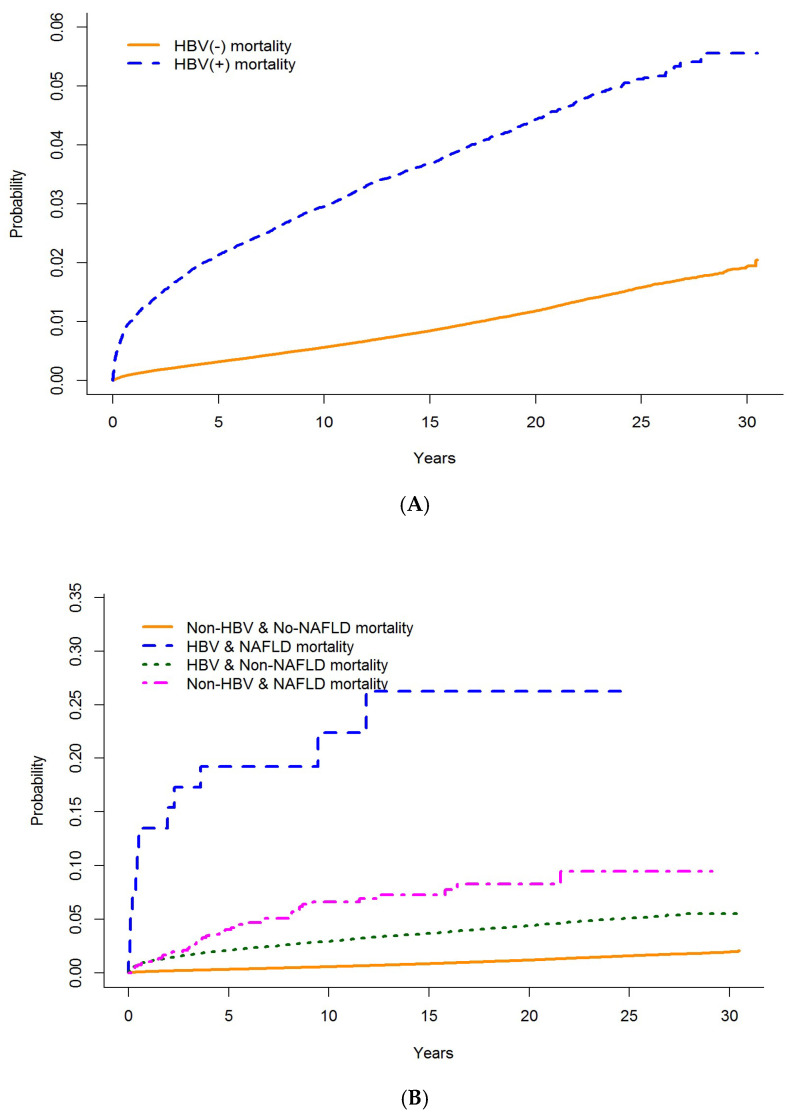
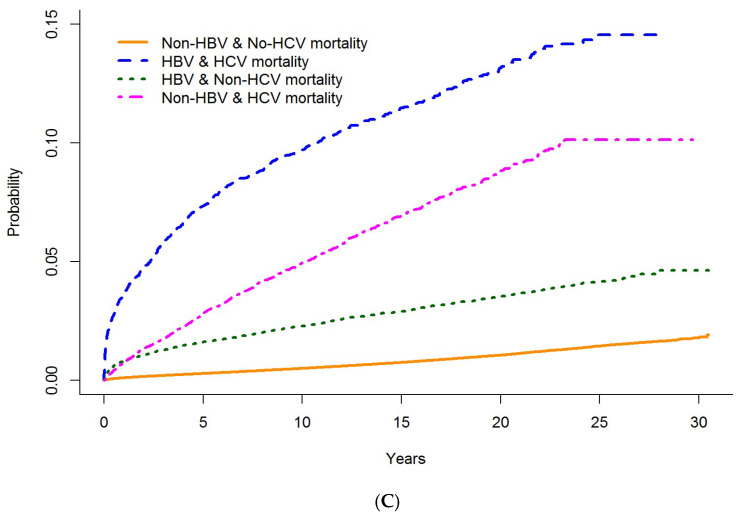
In the overall population, the highest mortality rate was found in people with HBV infection compared with those without (2.57 per 1000 PY (95% CI: 2.46, 2.69) vs. 0.62 per 1000 PY (95% CI: 0.61, 0.64), respectively). In people with NAFLD, among those without HBV infection, the incidence of liver-related mortality was 6.79/1000 PY (95% CI: 6.66, 6.94) and among people with infection, 27.97/1000 PY (95% CI: 15.88, 49.25). In people without NAFLD, among those without HBV infection, the incidence of liver-related mortality was 0.62/1000 PY (95% CI: 0.61, 0.63) and among people with HBV infection, 2.56/1000 PY (95% CI: 2.44, 2.68). In people with HCV infection, among those without HBV infection, the incidence of liver-related mortality was 5.48/1000 PY (95% CI: 5.17, 5.82) and among people with infection it was 10.39/1000 PY (95% CI: 9.53, 11.34). In people without HCV infection, among those without HBV infection, the incidence of liver-related mortality was 0.56/1000 PY (95% CI: 0.54, 0.57) and among people with infection, 1.99/1000 PY (95% CI: 1.88, 2.10). The Kaplan–Meier survival curves for liver-related mortality showed significantly higher cumulative mortality among people with HBV infection (vs. those without HBV infection) over time, with a much higher mortality among those with concurrent NAFLD or HCV coinfection (Figure 2).
Figure 2.
Cumulative mortality rate for people testing HBV positive vs. those testing negative in BC from 1990–2015. (A) Cumulative liver-related mortality in individuals with and without HBV infection. (B) Cumulative liver-related mortality in individuals with and without HBV infection and co-occurring NAFLD. (C) Cumulative liver-related mortality in individuals with and without HBV infection and HCV coinfection.
3.3. Effect of HBV Infection on Liver-Related Mortality
In the main analysis, after adjusting for the potential confounders and competing mortality risks, HBV infection was associated with a higher risk of liver-related mortality (asHR: 3.34, 95% CI: 3.15, 3.53). These findings from all of the population dataset were similar to those from the PS-weighted ATE (asHR:3.24, 95% CI: 2.99, 3.50) (Table 2). Other factors associated with an increased incidence of liver-related deaths include older age at HBV testing (asHR: 10.32, 95% CI: 8.20, 13.0 for 65 years old and above compared with individuals of 25 years and below), diabetes (asHR: 1.68, 95%CI: 1.59, 1.77), HCV infection (asHR: 3.68, 95% CI: 3.45, 3.93), NAFLD (asHR: 4.31, 95% CI: 3.32, 5.61), and alcohol use disorder (asHR: 3.58, 95% CI: 3.38, 3.80).
Table 2.
Impact of HBV infection on liver-related mortality between 1990–2015 in the British Columbia Hepatitis Testers Cohort, using multivariate * analysis Cox regression analysis.
| Overall Study Population | IPW-Dataset ATE ** | |||
|---|---|---|---|---|
| Covariates | asHR | 95%CI | asHR | 95%CI |
| HBV status | ||||
| Negative | Ref | Ref | ||
| Positive | 3.34 | (3.15, 3.53) | 3.24 | (2.99, 3.50) |
| Sex | ||||
| Female | Ref | |||
| Male | 1.52 | (0.21,10.91) | 1.99 | (1.75, 2.28) |
| Age, years | ||||
| <25 | Ref | |||
| 25–34 | 1.46 | (1.19, 1.79) | 1.34 | (0.68, 2.64) |
| 35–44 | 2.89 | (2.33, 3.57) | 2.3 | (1.18, 4.47) |
| 45–54 | 4.93 | (3.97,6.13) | 3.06 | (1.57, 5.97) |
| 55–64 | 7.34 | (5.87, 9.17) | 4.39 | (2.23, 8.66) |
| ≥65 | 10.32 | (8.20, 13.0) | 6.08 | (3.05, 12.09) |
| Birth group | ||||
| <1945 | Ref | |||
| 1945–1964 | 0.68 | (0.64, 0.73) | 0.49 | (0.41, 0.58) |
| 1965–1974 | 0.27 | (0.24, 0.31) | 0.23 | (0.16, 0.33) |
| ≥1975 | 0.12 | (0.10, 0.15) | 0.12 | (0.06, 0.24) |
| Ethnicity | ||||
| East Asian | Ref | |||
| South Asian | 1.24 | (1.11, 1.38) | 1.62 | (1.46, 1.78) |
| Other *** | 2.03 | (1.90, 2.16) | 0.93 | (0.70, 1.24) |
| Material deprivation | ||||
| Most privileged(Q1) | Ref | |||
| Q2 | 1.26 | (1.18, 1.35) | 1.13 | (0.92, 1.40) |
| Q3 | 1.26 | (1.18, 1.35) | 1.03 | (0.84, 1.27) |
| Q4 | 1.34 | (1.26, 1.43) | 1.21 | (0.99, 1.47) |
| Most deprived(Q5) | 1.49 | (1.40, 1.57) | 1.28 | (1.06, 1.54) |
| unknown | 1.46 | (1.20, 1.77) | 1.3 | (0.87, 1.95) |
| HCV status | ||||
| Negative | Ref | |||
| Positive | 3.68 | (3.45, 3.93) | 3.33 | (2.96, 3.76) |
| Diabetes | ||||
| No | Ref | |||
| Yes | 1.68 | (1.59, 1.77) | 1.86 | (1.58, 2.20) |
| Chronic Kidney Disease | ||||
| No | Ref | |||
| Yes | 1 | (0.84,1.18) | 0.99 | (0.67, 1.48) |
| Injection Drug Use | ||||
| No | Ref | |||
| Yes | 1.17 | (1.06,1.27) | 1.12 | (0.88, 1.41) |
| Alcohol use disorder | ||||
| No | Ref | |||
| Yes | 3.58 | (3.38, 3.80) | 2.56 | (2.13, 3.07) |
| Non-alcoholic fatty liver disease | ||||
| No | Ref | |||
| Yes | 4.31 | (3.32, 5.61) | 3.53 | (2.11, 5.91) |
* Multivariable models were adjusted with sex, age at first HBV testing date, birth cohort, ethnicity, material deprivation quintiles, HCV infection, diabetes, chronic kidney diseases, alcohol use disorder, injection drug use and other competing risks. asHR, sub-distributional adjusted hazard ratios; CI, confidence interval; HBV, hepatitis B virus. ** The PS-weighted dataset used inverse probability weights (IPW) estimating the average treatment effect (ATE). IPW were computed based on age at 1st HBV testing, sex, birth group, ethnicity, social deprivation quintiles, HCV infection, HIV infection, diabetes, chronic kidney disease, history of injection drug use, alcohol use disorder, and non-alcoholic fatty liver disease. Other ***: White, Black, Central Asian, Latin American, Pacific Islander, and West Asian individuals.
3.4. The Effect of NAFLD and HCV Infection in the HBV Liver-Related Mortality
In the multivariable model accounting for competing mortality, compared with those without NAFLD and without HBV infection, individuals without NAFLD but with HBV infection had a 3.35 times higher mortality risk (asHR 3.35, 95% CI: 3.16, 3.55). Similarly, individuals with NAFLD without HBV infection had a 4.49 times higher hazard of liver-related mortality (asHR 4.49, 95% CI: 3.35, 6.02), while individuals with both NAFLD and HBV infection had a 12.5 times higher hazard of liver-related mortality (asHR: 12.5, 95% CI: 7.08, 22.07). In the HBV-HCV model, compared with those without HCV infection and without HBV infection, individuals without HCV infection but with HBV infection had a 4.36 times higher risk of mortality (asHR: 4.36, 95% CI: 4.09, 4.64). Individuals with HCV infection without HBV infection had a 4.69 times higher risk of liver-related mortality (asHR: 4.69, 95% CI: 4.37, 5.03), while individuals with both HCV infection and HBV infection had a 8.4 times the risk of liver-related mortality (asHR: 8.4, 95% CI: 7.62, 9.26) compared with those without HCV infection and without HBV infection. The IPW ATE weighted analysis showed a similar asHR (Table 3).
Table 3.
Impact of HBV infection, NAFLD, and HCV on liver-related mortality between 1990–2015 in the British Columbia Hepatitis Testers Cohort, using the multivariable* Fine–Gray sub-distribution proportional hazards model analysis.
| Overall Study Population | IPW-Dataset ** ATE | |||
|---|---|---|---|---|
| Covariates | asHR | 95%CI | asHR | 95%CI |
| HBV and NAFL status | ||||
| HBV Negative without NAFLD | Ref | Ref | ||
| HBV Negative with NAFLD | 4.49 | (3.35, 6.02) | 4.52 | (3.33, 6.12) |
| HBV positive without NAFLD | 3.35 | (3.16, 3.55) | 3.32 | (3.13, 3.53) |
| HBV positive with NAFLD | 12.5 | (7.08, 22.07) | 12.08 | (5.87, 24.89) |
| HBV and HCV status | ||||
| HBV Negative without HCV | Ref | |||
| HBV Negative with HCV | 4.69 | (4.37, 5.03) | 4.82 | (4.48, 5.19) |
| HBV positive without HCV | 4.36 | (4.09, 4.64) | 4.3 | (4.04, 4.57) |
| HBV positive with HCV | 8.4 | (7.62, 9.26) | 8.66 | (7.77, 9.64) |
The impact of HBV infection and NAFLD was assessed using * Multivariable models and these were adjusted with sex, age at first HBV testing date, birth cohort, ethnicity, material deprivation quintiles, HCV infection, diabetes, chronic kidney diseases, alcohol use disorder, injection drug use, and other competing risks. ** The PS-weighted dataset used inverse probability weights (IPW) estimating the average treatment effect (ATE). IPW were computed based on age at the first HBV testing, sex, birth group, ethnicity, social deprivation quintiles, HCV infection, HIV infection, diabetes, chronic kidney disease, history of injection drug use, and alcohol use disorder. The impact of HBV infection and HCV coinfection was assessed using * Multivariable models and these were adjusted with sex, age at first HBV testing date, birth cohort, ethnicity, material deprivation quintiles, diabetes, chronic kidney diseases, alcohol use disorder, injection drug use, and other competing risks. ** The PS-weighted dataset used inverse probability weights (IPW) estimating the average treatment effect (ATE). IPW were computed based on age at 1st HBV testing, sex, birth group, ethnicity, social deprivation quintiles, HIV infection, diabetes, chronic kidney disease, history of injection drug use, alcohol use disorder, and non-alcoholic fatty liver disease. asHR, adjusted sub distributional hazard ratios; CI, confidence interval; HBV, hepatitis B virus, HCV, hepatitis C infection; NAFLD, non-alcoholic fatty liver diseases; IPW, inverse probability weighting; PS, propensity score.
4. Discussion
In this population-based Canadian cohort of more than a million individuals, we evaluated the effect of HBV infection and concurrent HCV co-infection and NAFLD on liver-related mortality risk. Individuals with HBV infection had a more than three times higher risk of dying from liver-related death compared with those without HBV infection. In people with both NAFLD and HBV infection, the risk of liver-related mortality was more than 12 times that of those without HBV infection and three times those with HBV or NFLD alone. Similarly, among people with both HBV and HCV coinfection, the risk of liver-related mortality was eight times compared with those without HBV or HCV and two times compared with HBV or HCV alone. These findings highlight that people with HBV infection who have concurrent NAFLD or HCV infection are at a much higher risk of liver-related mortality. Given the availability of highly effective treatments for HCV and HBV, there is a real opportunity to reduce this risk by early treatment for HBV or HCV among these individuals.
Previous studies have shown that the contribution of HBV mono-infection, NAFLD, and HCV alone to increased liver-related alone to mortality [42,43]. However, limited data are available on the concurrent HBV infection and NAFLD and HBV/HCV coinfection and liver-related mortality. Studies from Spain and Taiwan have reported a higher rate of liver-related mortality associated with HBV mono-infected [44,45]. An elevated risk of liver-related mortality among people with NAFLD has been reported from the United States of America (USA) [14]. The findings from the studies conducted in Canada, the Netherlands, and Israel among people with chronic HBV have reported that non-alcoholic steatohepatitis was associated with an increased risk of all-cause mortality [45,46], but their contribution to liver-related death was not assessed. A relatively small study from Spain reported a higher risk of death from liver disease with HCV infection among people with positive HBsAg. In summary, there are limited studies on the effect of HBV infection and NAFLD and HBV and HCV coinfection and liver-related mortality. Our findings suggest that for people with HBV infection, HCV coinfection and NAFLD substantially augment their risk of liver-related mortality.
Studies have reported that liver-related mortality rates among people with HBV mono-infection people vary between 0.1 per 100,000 PYs and 8.6 per 1000 PYs [47,48,49]. In a cohort of 39,109 individuals with HBV infection conducted in Australia from 1990 to 2002, the liver-related mortality among people with HBV infection was lower (1.17 per 1000 PYs) than our results [50]. This could be due to the differences in the periods of study; our study considered people from 1990 to 2020, and so the high mortality in our study could be related to an age difference in the population. In our study, the risk of death was high in older people. In another cohort study also conducted in Australia from 1993 to 2012, 4.8% of HBV positive patients died from liver-related mortality, with an increased rate of liver-related death in older individuals [48]. However, our results are far lower than those found in a study conducted in Italy, where the mortality rate among patients with HBV followed for 25 years was 15.7%; however, this study was conducted on only 70 HBV patients [2].
In addition to HCV coinfection and NALFD, other factors associated with a higher liver-related mortality among people with HBV infection included advanced age and alcohol use disorder. All of these are factors that were shown to contribute on their own to liver-related mortality through several studies in different areas. Other studies have shown the association of aging and alcohol disorder problems with liver-related complications, including death [51].
This study has several strengths. This is the largest population-based study to date to investigate the effect of HBV infection on mortality in BC. In addition, the study design allowed us to establish the temporality in the association of HBV and liver-related mortality. Even though this is an observational study, to avoid residual confounding and other biases, we used a robust approach, which allowed for a less biased assessment of the impact of HBV infection on liver-related mortality by balancing the baseline characteristics between individuals with and without HBV infection [52].
The findings of this study should be appraised in the context of the following limitations. First, in BC, HBV notifications are based on evidence of chronic infection. However, there is a probability of overestimating the impact of HBV on liver-related mortality as this study used data for all people who tested positive for HBV without the distinction of chronic or acute, so some individuals may have cleared HBV infection. Secondly, for people with multiple chronic diseases that are each potentially fatal, the decision about selecting the underlying cause of death can be subjective; however, where possible (i.e., liver-related mortality), a multiple cause definition was used to include important cause information that might have been otherwise overlooked. In addition, using administrative data has limitations because of a lack of sociodemographic and health risk information such as marital status, level of education, smoking, poor diet, and physical inactivity, which could be among the risk factors for liver-related mortality. As unmeasured confounders, these could introduce bias and underestimate the relationship between HBV and liver-related mortality [53]. Finally, the major limitation in this analysis relates to the need to account for treatment to assess the impact of non-cleared HBV. Future studies on liver-related mortality and all-cause mortality should consider impact of treatment. To account for residual confounding we used a double-robust approach of weighting based on PS, and by adjusting for covariates in the proportional hazards.
5. Conclusions
In this large population-based cohort of individuals tested for HBV in BC, we found that HBV infection is associated with a higher risk of liver-related mortality, with a greater relative impact in people with NAFLD and those with HBV/HCV co-infection. This indicates an urgent need for effective HBV and HCV treatment, as well as continued monitoring for liver-related diseases in order to reduce the risk of mortality. In addition, highly effective HBV vaccination prevents infection and hence reduces mortality, and thus must be part of the overall strategy to reduce the impact of HBV on health. The results from this study highlight the need for service integration to improve the overall wellbeing and survival of people with multiple co-occurring risk factors.
Acknowledgments
This study would not have happened without the contribution of the British Columbia population who provided the data used in this study, the British Columbia Centre for Disease Control, Providence Health Services Authority, British Columbia Ministry of Health, British Columbia Cancer Agency, and their respective program staff involved in data access, procurement, and data management for their assistance. We would like to thank them for their special contribution and support to this study.
Author Contributions
J.D.M. and N.Z.J. developed the study protocol. J.D.M., M.B., A.Y., S.W. and N.Z.J. developed the study design. S.W. and A.Y. created the dataset; J.D.M., S.W., H.A.V.G. and N.Z.J. analyzed, produced data tables and figures, and interpreted the data. J.D.M. wrote the paper with important contributions from D.J., P.A.A. and N.Z.J. All of the authors (J.D.M., D.J., M.B., P.A.A., G.C., A.Y., H.A.V.G., M.A., S.W., S.B., M.E.K., E.M.Y., A.R., M.K. and N.Z.J.) had full access to the data, participated in the data interpretation, and provided critical feedback on the manuscript. J.D.M. and N.Z.J. had the final authority of the manuscript submission, and all of the authors (J.D.M., D.J., M.B., P.A.A., G.C., A.Y., H.A.V.G., M.A., S.W., S.B., M.E.K., E.M.Y., A.R., M.K. and N.Z.J.) accepted responsibility for submission for publication. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the University of British Columbia Behavioral Research Ethics Board (H14-01649).
Informed Consent Statement
As this study used secondary data collected as part of routine healthcare encounters, informed consent was not required.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions”. Requests to access these datasets should be directed to naveed.janjua@bccdc.ca.
Conflicts of Interest
The Canadian Network on Hepatitis C (CanHepC) Ph.D. fellowship supports J.D.M. and D.J. D.J. is also supported by the CIHR Frederick Banting and Charles Best Doctoral Award. M.E.K. is supported by the Michael Smith Foundation for Health Research Scholar award and holds research grants from the Natural Sciences and Engineering Research Council of Canada and BC SUPPORT Unit. Over the past 4 years, M.E.K. has received consulting fees from Biogen (unrelated to this project). S.B. received grant/research support from Gilead Sciences and Abbvie. E.Y. has received grant/research support from Paladin Laboratories, Pfizer Inc., Novodisc Inc., Genefit Inc., Intercept Inc., Madrigal Inc., Gilead Sciences, Merck Inc., Sonic Incytes. A.R. has received grant/research support from Abbvie, Assembly, Galmed, Gilead, Intercept, Janssen, Merck, Novartis, Novo-Nordisc, and Pfizer. M.K. has received grant/ research support from Roche, Merck, Siemens, Boehringer Ingelheim, and Hologic. N.Z.J. has participated in advisory boards and has spoken for AbbVie. S.W., M.B., P.A.A., S.B., G.C., A.Y., M.A., H.S., H.A.V.G. and Y.A. have no conflicts of interest to declare.
Funding Statement
The BC Centre for Disease Control and the Canadian Institutes of Health Research (CIHR) (Grant #HPC-178912, Grant #PJT-156066) supported this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Volume 53. World Health Organization; Geneva, Switzerland: 2021. [(accessed on 20 September 2021)]. pp. 1689–1699. Available online: https://www.who.int/publications/i/item/9789240027077. [Google Scholar]
- 2.Fattovich G., Olivari N., Pasino M., D′Onofrio M., Martone E., Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: Mortality after 25 years. Gut. 2007;57:84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Ventura-Cots M., Ballester-Ferré M.P., Ravi S., Bataller R. Public health policies and alcohol-related liver disease. JHEP Rep. 2019;1:403–413. doi: 10.1016/j.jhepr.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers R.P., Krajden M., Bilodeau M., Kaita K., Marotta P., Peltekian K., Ramji A., Estes C., Razavi H., Sherman M. Burden of Disease and Cost of Chronic Hepatitis C Virus Infection in Canada. Can. J. Gastroenterol. Hepatol. 2014;28:243–250. doi: 10.1155/2014/317623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janjua N.Z., Wong S., Abdia Y., Jeong D., Buller-Taylor T., Adu P.A., Samji H., Wilton J., Pearce M., Butt Z.A., et al. Impact of direct-acting antivirals for HCV on mortality in a large population-based cohort study. J. Hepatol. 2021;75:1049–1057. doi: 10.1016/j.jhep.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 7.Sherman M., Bilodeau M., Cooper C., Mackie D., Depew W., Villeneuve J.-P. Liver Disease in Canada: A Crisis in the Making. Canadian Liver Foundation; Toronto, ON, Canada: 2013. [Google Scholar]
- 8.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 9.Centre for Communicable Diseases and Infection Control . Brief Report: Hepatitis B Infection in Canada. Public Health Agency of Canada; Ottawa, ON, Canada: 2011. [(accessed on 19 November 2022)]. pp. 1–13. Available online: http://www.phac-aspc.gc.ca/id-mi/hepatitisBCan-hepatiteBCan-eng.php. [Google Scholar]
- 10.Binka M., Butt Z.A., Wong S., Chong M., A Buxton J., Chapinal N., Yu A., Alvarez M., Darvishian M., Wong J., et al. Differing profiles of people diagnosed with acute and chronic hepatitis B virus infection in British Columbia, Canada. World J. Gastroenterol. 2018;24:1216–1227. doi: 10.3748/wjg.v24.i11.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butt Z.A., Wong S., Rossi C., Binka M., Wong J., Yu A., Darvishian M., Alvarez M., Chapinal N., McKee G., et al. Concurrent Hepatitis C and B Virus and Human Immunodeficiency Virus Infections Are Associated With Higher Mortality Risk Illustrating the Impact of Syndemics on Health Outcomes. Open Forum Infect. Dis. 2020;7:ofaa347. doi: 10.1093/ofid/ofaa347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binka M., Butt Z.A., McKee G., Darvishian M., Cook D., Wong S., Yu A., Alvarez M., Samji H., Wong J., et al. Differences in risk factors for hepatitis B, hepatitis C, and human immunodeficiency virus infection by ethnicity: A large population-based cohort study in British Columbia, Canada. Int. J. Infect. Dis. 2021;106:246–253. doi: 10.1016/j.ijid.2021.03.061. [DOI] [PubMed] [Google Scholar]
- 13.McKee G., Butt Z.A., Wong S., Salway T., Gilbert M., Wong J., Alvarez M., Chapinal N., Darvishian M., Tyndall M.W., et al. Syndemic Characterization of HCV, HBV, and HIV Co-infections in a Large Population Based Cohort Study. eClinicalMedicine. 2018;4–5:99–108. doi: 10.1016/j.eclinm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong J.P., Pitts A., Younossi Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinou D., Deutsch M. The spectrum of HBV/HCV coinfection: Epidemiology, clinical characteristics, viralinteractions and management. Ann. Gastroenterol. 2015;28:221–228. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou K., Dodge J.L., Grab J., Poltavskiy E., Terrault N.A. Mortality in Adults with Chronic Hepatitis B Infection in the United States: A Population-Based Study. Aliment Pharmacol. Ther. 2020;52:382–389. doi: 10.1111/apt.15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piselli P., Serraino D., Fusco M., Girardi E., Pirozzi A., Toffolutti F., Cimaglia C., Taborelli M. Hepatitis C virus infection and risk of liver-related and non-liver-related deaths: A population-based cohort study in Naples, southern Italy. BMC Infect. Dis. 2021;21:667. doi: 10.1186/s12879-021-06336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallager S., Ladelund S., Christensen P.B., Kjær M.S., Roege B.T., Grønbæk K.E., Belard E., Barfod T.S., Madsen L.G., Gerstoft J., et al. Liver-related morbidity and mortality in patients with chronic hepatitis C and cirrhosis with and without sustained virologic response. Clin. Epidemiol. 2017;2017:501–516. doi: 10.2147/CLEP.S132072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brass V., Moradpour D. New insights into hepatitis B and C virus co-infection. J. Hepatol. 2009;51:423–425. doi: 10.1016/j.jhep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Thornton A.C., Jose S., Bhagani S., Chadwick D., Dunn D., Gilson R., Main J., Nelson M., Rodger A., Taylor C., et al. Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. AIDS. 2017;31:2525–2532. doi: 10.1097/QAD.0000000000001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haflidadottir S., Jonasson J.G., Norland H., Einarsdottir S.O., Kleiner D.E., Lund S.H., Björnsson E.S. Long term follow-up and liver-related death rate in patients with non-alcoholic and alcoholic related fatty liver disease. BMC Gastroenterol. 2014;14:166. doi: 10.1186/1471-230X-14-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janjua N.Z., Kuo M., Chong M., Yu A., Alvarez M., Cook D., Armour R., Aiken C., Li K., Rizi S.A.M., et al. Assessing Hepatitis C Burden and Treatment Effectiveness through the British Columbia Hepatitis Testers Cohort (BC-HTC): Design and Characteristics of Linked and Unlinked Participants. PLoS ONE. 2016;11:e0150176. doi: 10.1371/journal.pone.0150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Classification of Diseases: [9th] Ninth Revision Basic Tabulation List with Alphabetic Index. World Health Organization; Geneva, Switzerland: 1978. [(accessed on 18 October 2021)]. World Health Organization (WHO) Available online: https://apps.who.int/iris/handle/10665/39473. [Google Scholar]
- 24.World Health Organization (WHO) International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) 5th ed. Volume 5 World Health Organization; Geneva, Switzerland: 2016. [Google Scholar]
- 25.Zhang Z. Survival analysis in the presence of competing risks. Ann. Transl. Med. 2017;5:47. doi: 10.21037/atm.2016.08.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.British Columbia Ministry of Health, British Columbia Medical Association . Hepatitis—Viral Hepatitis Testing. Government of British Columbia; Vancouver, BC, Canada: 2012. pp. 1–5. BC Guideline.ca. [Google Scholar]
- 27.Yuen M.F., Tanaka Y., Fong D.Y.T., Fung J., Wong D.K.H., Yuen J.C.H., But D.Y.K., Chan A.O.O., Wong B.C.Y., Mizokami M., et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J. Hepatol. 2009;50:80–88. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Pandyarajan V., Govalan R., Yang J.D. Risk Factors and Biomarkers for Chronic Hepatitis B Associated Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021;22:479. doi: 10.3390/ijms22020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lakha F., Gorman D.R., Mateos P. Name analysis to classify populations by ethnicity in public health: Validation of Onomap in Scotland. Public Health. 2011;125:688–696. doi: 10.1016/j.puhe.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Ryan R., Vernon S., Lawrence G., Wilson S. Use of name recognition software, census data and multiple imputation to predict missing data on ethnicity: Application to cancer registry records. BMC Med Informatics Decis. Mak. 2012;12:3. doi: 10.1186/1472-6947-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pampalon R., Hamel D., Gamache P., Philibert M.D., Raymond G. Simpson, A. An Area-based Material and Social Deprivation Index for Public Health in Québec and Canada. Can. J. Public Health. 2012;103((Suppl. S2)):17–22. doi: 10.1007/BF03403824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salway T., Butt Z.A., Wong S., Abdia Y., Balshaw R., Rich A.J., Ablona A., Wong J., Grennan T., Yu A. A Computable Phenotype Model for Classification of Men Who Have Sex With Men Within a Large Linked Database of Laboratory, Surveillance, and Administrative Healthcare Records. Front Digit. Health. 2020;2:547324. doi: 10.3389/fdgth.2020.547324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janjua N.Z., Islam N., Kuo M., Yu A., Wong S., Butt Z.A., Gilbert M., Buxton J., Chapinal N., Samji H., et al. Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int. J. Drug Policy. 2018;55:31–39. doi: 10.1016/j.drugpo.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 34.British Columbia Office of Provincial Health Officer . HIV Testing Guidelines for the Province of British Columbia. Office of the Provincial Health Officer; Victoria, BC, Canada: 2015. pp. 1–8. [Google Scholar]
- 35.Zhang Z., Kim H.J., Lonjon G., Zhu Y. Balance diagnostics after propensity score matching. Ann. Transl. Med. 2019;7:16. doi: 10.21037/atm.2018.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin P.C. A Tutorial and Case Study in Propensity Score Analysis: An Application to Estimating the Effect of In-Hospital Smoking Cessation Counseling on Mortality. Multivar. Behav. Res. 2011;46:119–151. doi: 10.1080/00273171.2011.540480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desai R.J., Franklin J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: A primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 38.Fine J.P., Gray R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 39.Zhang Z. Model building strategy for logistic regression: Purposeful selection. Ann. Transl. Med. 2016;4:4–10. doi: 10.21037/atm.2016.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedrow K. Natural Language Processing with SAS®: Special Collection. SAS Institute Inc.; Cary, NC, USA: 2018. [(accessed on 8 December 2021)]. 473p. Available online: https://github.com/PacktPublishing/Natural-Language-Processing-with-TensorFlow. [Google Scholar]
- 41.The R Development Core Team . R: A Language and Environment for Statistical Computing. Volume 2. Foundation for Statistical Computing; Vienna, Austria: 2017. [(accessed on 19 November 2022)]. Available online: https://www.r-project.org/ [Google Scholar]
- 42.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 43.Golabi P., Paik J.M., Eberly K., de Avila L., Alqahtani S.A., Younossi Z.M. Causes of death in patients with Non-alcoholic Fatty Liver Disease (NAFLD), alcoholic liver disease and chronic viral Hepatitis B and C. Ann. Hepatol. 2021;27:100556. doi: 10.1016/j.aohep.2021.100556. [DOI] [PubMed] [Google Scholar]
- 44.Aspinall E.J., Hutchinson S.J., Janjua N.Z., Grebely J., Yu A., Alavi M., Amin J., Goldberg D.J., Innes H., Law M., et al. Trends in mortality after diagnosis of hepatitis C virus infection: An international comparison and implications for monitoring the population impact of treatment. J. Hepatol. 2015;62:269–277. doi: 10.1016/j.jhep.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Choi H.S.J., Brouwer W.P., Zanjir W.M., de Man R.A., Feld J.J., Hansen B.E., Janssen H.L.A., Patel K. Nonalcoholic Steatohepatitis Is Associated With Liver-Related Outcomes and All-Cause Mortality in Chronic Hepatitis B. Hepatology. 2020;71:539–548. doi: 10.1002/hep.30857. [DOI] [PubMed] [Google Scholar]
- 46.Peleg N., Issachar A., Arbib O.S., Cohen-Naftaly M., Braun M., Leshno M., Barsheshet A., Shlomai A. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1:9–16. doi: 10.1016/j.jhepr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thio C.L., Seaberg E.C., Skolasky R., Jr., Phair J., Visscher B., Muñoz A., Thomas D.L. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/S0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 48.Alavi M., Grebely J., Hajarizadeh B., Amin J., Larney S., Law M.G., George J., Degenhardt L., Dore G.J. Mortality trends among people with hepatitis B and C: A population-based linkage study, 1993-2012. BMC Infect. Dis. 2018;18:215. doi: 10.1186/s12879-018-3110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asrani S.K., Larson J.J., Yawn B., Therneau T.M., Kim W.R. Underestimation of Liver-Related Mortality in the United States. Gastroenterology. 2013;145:375–382.e2. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amin J., Law M.G., Bartlett M., Kaldor J.M., Dore G.J. Causes of death after diagnosis of hepatitis B or hepatitis C infection: A large community-based linkage study. Lancet. 2006;368:938–945. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim H., Kisseleva T., Brenner D.A. Aging and liver disease. Curr. Opin. Gastroenterol. 2015;31:184–191. doi: 10.1097/MOG.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Austin P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pham A., Cummings M., Lindeman C., Drummond N., Williamson T. Recognizing misclassification bias in research and medical practice. Fam. Pract. 2019;36:804–807. doi: 10.1093/fampra/cmy130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions”. Requests to access these datasets should be directed to naveed.janjua@bccdc.ca.