Abstract
Background and objective
Proton Pump Inhibitors (PPIs) reduce gastric acid production and they are indicated for myriad gastrointestinal conditions. Prolonged use of PPI has been linked to the risk of inflammatory bowel disease (IBD) though this fact is not well established. We aimed to conduct a systematic review and meta-analysis to estimate the risk of IBD occurrence with PPI use.
Methodology
The databases such as PubMed, Scopus, and Cochrane Library were accessed from inception to December 2020. Additionally, the bibliographic search and a random search in Google, Google Scholar, and ResearchGate were performed to find additional sources. The observational studies estimating the risk of IBD following the use of PPI, published in the English language were considered for this review. The methodological quality of included studies was assessed using the Modified Downs and Black checklist.
Results
Eight out of 2038 studies with 157,758 participants were included in this meta-analysis. A significantly higher risk of IBD (adjusted odds ratio [aOR] 2.43; 95% Confidence Interval [CI] 1.18–5.02; P=0.02; n=6) was observed in participants taking PPIs for any indication. Moreover, a significant association was observed between PPI exposure on the different types of IBD such as ulcerative colitis and Crohn’s disease together (aOR: 3.60; 95% CI: 1.10–11.74), collagenous colitis (OR: 4.73; 95% CI: 1.99–11.22) and lymphocytic Colitis (OR: 3.77; 95% CI: 2.91–4.87), but not with ulcerative colitis (P=0.47) and microscopic colitis (P=0.07) alone. Similarly, a significant association was observed among Europeans (aOR: 3.98; 95% CI: 2.36–6.71), but not with North American (aOR: 0.48; 95% CI: 0.01–26.71) studies. Overall the study quality was good.
Conclusion
The current evidence indicates that exposure to PPI is significantly associated with increased risk of IBD. Further, adequately powered studies from various parts of the world are needed for better quantification and generalizability of our findings.
PROSPERO Protocol Registration Number
CRD42020209674
Keywords: drug safety, gastrointestinal, inflammatory bowel disease, proton pump inhibitor, risk
Introduction
Inflammatory Bowel Diseases (IBDs) are a group of chronic conditions identified by the inflammation of the small and large intestine, where parts of the gastrointestinal tract (GIT) are attacked by the immune system [1]. This inflammatory condition has two major forms namely ulcerative colitis (UC) and Crohn’s disease (CD). A few investigators suggested that microscopic colitis (MC) may represent an attenuated form of IBD with transformation to classical IBD occurring in a subgroup of patients [2]. Several case reports and cohort studies suggested the possibility that IBD may either revert into MC or vice versa. MC could progress to IBD even considering MC as a milder or gentle form of IBD [3]. The clinical presentations of IBD include watery or bloody stools, with or without mucus, weight loss, vomiting, and abdominal pain. Prolonged inflammation of the gut may result in permanent damage of the intestine and ultimately, lower quality of life and increased healthcare costs [1].
In western countries, the incidence of IBD is approximately 0.9 to 11.6 per 100000/year for CD and 2 to 15 per 100000/year for UC [4]. In the United States, a population-based study showed the prevalence of MC to be 103.0 per 100,000 person-years [5]. Women have up to 30% higher risk of developing IBD than men. The exact etiology of IBD is unknown, however, these factors may play an important role including environmental factors such as bacterial and viral infections, nutritional and dietary habits, smoking and socioeconomic status, immunological factors like abnormal T cell responses, genetic susceptibility, immuno-genetic factors such as human leukocyte antigen and cytokine genes, and exposure to certain drugs. Drugs like non-steroidal anti-inflammatory drugs (NSAIDs), oral contraceptives, rituximab, and antibiotics have been linked to causing IBD due to their respective mechanisms of action [4]. More recently, the focus has been shifted to proton pump inhibitors (PPIs) being a causative agent for IBD, rather than therapeutic [6].
PPIs are acid-suppressing agents that block the gastric H+/ K+ ATPase proton pumps present on the intestinal epithelium, therefore inhibiting stomach gastric acid secretion. PPIs are widely used, prescribed, and are often the first-line agents for conditions like esophagitis, Zollinger-Ellison Syndrome, peptic ulcer disease [7], and in the management of IBD as well. Moreover, PPIs are among the most used over-the-counter medicine globally [8]. However, PPI may influence the local electrolyte balance and alter fluid acidification, which may cause immune and inflammatory reactions. Particularly, lansoprazole and omeprazole are known for contractile activity inhibition and induction of smooth muscle relaxation, having an effect on the actinomycin cytoskeleton, ultimately causing conformational and structural changes in the epithelial cells and their cytoskeleton as well as negatively impacting tight junction function [9]. Since PPIs alter gut microbiota, there may be an association between long-term PPI use and IBD risk [10]. PPIs have been associated with increased IBD flares as well [6]. Unfortunately, the limited evidence concerning the association between usage of PPIs to IBD risk is a major challenge in clinical settings. Thus we aimed to conduct a systematic literature review to identify and critically evaluate all the observational studies and to perform a meta-analysis to estimate the risk of IBD, including here MC (as a possible attenuated form of IBD) following the PPI exposure.
Methodology
Ethical consideration
This study did not require any ethical approval as this is a systematic review. Moreover, the protocol for this review is registered in the International prospective register of systematic reviews (PROSPERO) with a registration number CRD42020209674 [11]. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines [12] to report this systematic review, followed by a PICOS framework for the inclusion of relevant studies.
Criteria for considering studies for this review
Study design and participants
Observational studies such as cohort, cross-sectional, and case-control studies that assessed the risk of IBD in participants who had taken PPIs for any of their indications irrespective of their age and gender were considered for this review. Only the studies with full text available in the English language were selected. Reviews, descriptive studies, clinical trials, editorials, comments, conference abstracts and studies with insufficient information were excluded.
Exposure
We considered patients who had any exposure to PPI in the past 12 months, were currently using PPIs, or had been using them for a prolonged period for any of its indications, irrespective of age and gender. We considered all types of PPIs as per the author’s discretion.
Outcome considered
The outcome considered was the risk of IBD occurring after PPI use. We considered all types of IBD outcomes as the author’s discretion.
A detailed inclusion and exclusion criteria can be found in Supplementary file S1.
Search methods for identification of studies
Keywords and entry terms were collected from different databases and by reviewing similar papers published previously. A literature search was performed using a comprehensive search strategy in electronic databases such as MEDLINE/PubMed, Scopus, and Cochrane Library in December 2020 without any restriction. Bibliography of all the included studies and grey literature databases were searched for any additional research. Also, a random search in Google, Google Scholar, and Research Gate was performed to find any other relevant studies. Two authors were included in the preparation of search strategy and literature search process, and another reviewer cross-checked for the appropriateness of the use of entry terms and Boolean operators as well as missing any entry terms, and rectified if any. The keywords such as “proton pump inhibitor” “inflammatory bowel disease” “ulcerative colitis” “microscopic colitis” “Crohn’s disease” were used for the literature search. A detailed search strategy is provided in Supplementary file S2.
Study selection
The search results from different electronic databases were retrieved to a Microsoft Excel sheet. After duplicate removal, all the retrieved studies were subject to screening based on their title and abstract which was followed by a screening of its full-text against the predefined inclusion criteria. The reasons for the exclusion of studies were noted. Two independent reviewers were involved in the screening of studies and any disagreements were settled through mutual discussion or in consultation with a third reviewer.
Data extraction
Two authors independently analyzed the studies and the data were then extracted to a pre-framed data extraction grid in Microsoft Excel. Any discrepancies were settled through mutual discussion or consultation with a third reviewer. The last name of first author and year of publication were used to identify the studies. From each study, the following information was inspected and extracted (i) Author, Year, and Country (ii) Study Design (iii) Study Duration (iv) Number of Participants (v) Age, Gender % (vi) Number of IBD cases (vii) Outcomes (vii) Description of PPI use (viii) Definition of IBD (ix) Type of IBD (x) Number of IBD events (xi) Adjusted odds ratio (OR) along with 95% confidence interval (CI). We extracted the OR estimates that help to establish PPIs as a causal factor of IBD directly from the studies if it is available or it is calculated using the available information.
Quality assessment
The quality of evidence was assessed using Modified Downs and Black Checklist developed in 1998 [13]. It has 27 questions which have to be graded as “Yes”, “No” and “Unable to determine” as per the available information. Previously, studies have used a modified version by simplification of the power question and awarding only 1 point if a study had adequate power to recognize a clinically significant effect, where the probability value for the difference being due to chance is <5% if a study did not mention statistical power, it was deemed either “no” or “unable to determine” and given a score of 0. There are 5 sections which include the study quality (10 items), external validity (3 items), study bias (7 items), confounding and selection bias (6 items), and power (1 item). Each question if answered “yes” gets a score of 1, except for the 5th question which can get a score of 2 if answered “yes”. Thus the total score is out of 28. Each paper was assigned a grade of “excellent” (24–28 points), “good” (19–23 points), “fair” (14–18 points), or “poor” (<14 points) according to the score assigned.
Evidence synthesis and statistical analysis
A narrative synthesis was made employing all the extracted data and the results were presented in tabular form. Review Manager Software [14] was used for meta-analysis. Categorical results from individual studies were collected and presented as OR along with its 95% CI. Statistical heterogeneity was assessed using the I2 statistic and Cochrane P-value. For studies without a significant heterogeneity (I2 ≤50% or P≥0.10) the fixed-effects model was selected, whereas, for studies with substantial heterogeneity (I2 >50% or P≤0.10), the random-effects model was chosen.
Subgroup analysis involves splitting all the participant data into subgroup in order to make the comparison between them or to investigate the heterogeneity or to answer a specific question about the specific patient groups, type of intervention, or type of studies [15]. Subgroup analysis were performed based on the different types of IBD and the geographical distribution or location of the studies. The types such as CD, UC, and MC (LC and CC) were considered in the case of types of IBD; and geographical distribution was specified by the continent.
Sensitivity analysis and publication bias
To assess the stability and robustness of our results we performed a sensitivity analysis by excluding the study with the lowest weight in our main analysis. Publication bias was planned to be detected by visual inspection of funnel plots generated using RevMan and the statistically through Egger’s and Begg’s test. However, it could not be performed due to the fewer number of studies (less than ten) involved in the comparison analysis [15,16].
Results
Eligible studies and data summary
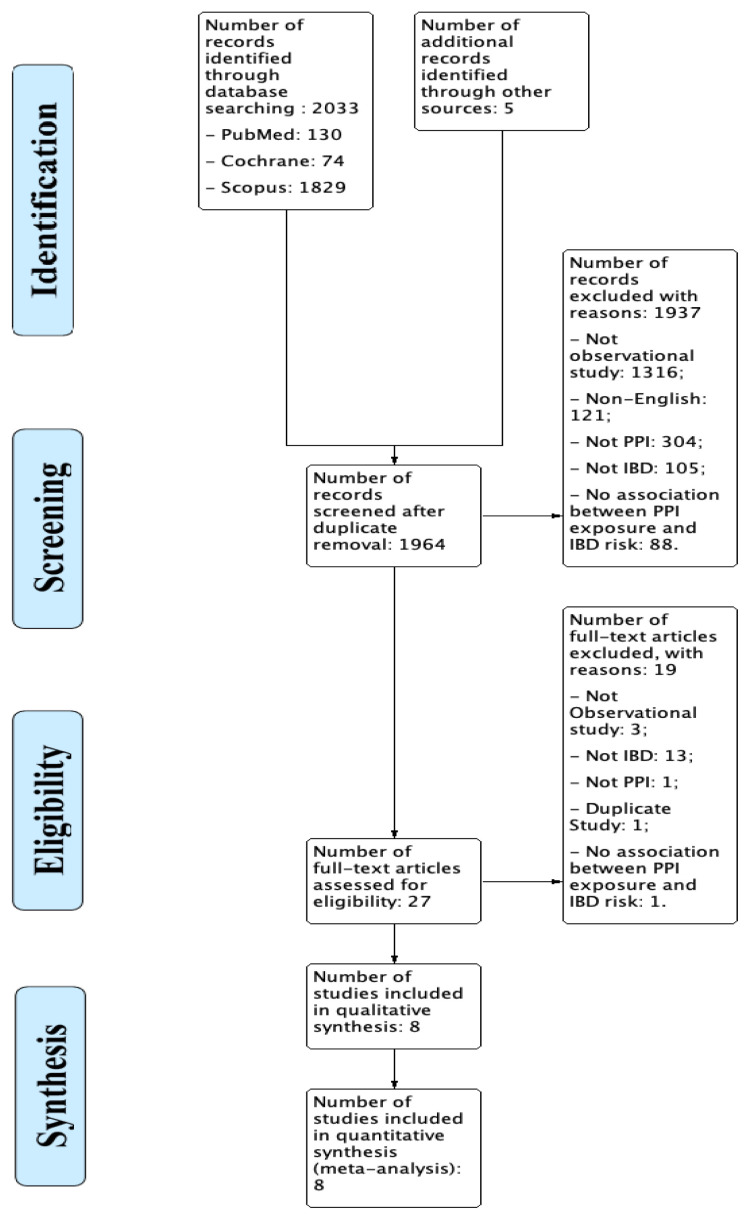
The literature search process identified 2033 records through database and 5 from an additional search of which 1964 studies were screened after duplicate removal. Using the given inclusion and exclusion criteria, these records were screened on their titles and abstracts, resulting in the inclusion of 27 articles which on further full-text assessment resulted in the exclusion of 19 articles and inclusion of 8 studies in this systematic review and meta-analysis. The process of the literature search is explained in figure 1.
Figure 1.
PRISMA Flow diagram for the study selection process.
Study summary and characteristics
A total of 8 relevant studies published between the years 2010 to 2020 were considered which includes 2 nested case controls, 5 case controls, and 1 cohort study. There were 157,758 participants totally from which 12435 IBD events occurred after exposure to PPIs irrespective of age and gender. The studies were performed in the United Kingdom [17], USA (n=2) [18,19], The Netherlands (n=3) [7,20,23], Spain [21] and Denmark [22]. One retrospective database cohort study by Esan et al. [17] assessed the sequelae of Campylobacter and Campylobacter and Non-typhoidal Salmonella infections followed from 2000–2015 and reported 27 UC and 10 CD cases among 15806 PPI users. Two nested case controls were conducted by Masclee et al. [20] and Schwartz et al. [19] which assessed the risk of IBD following the PPI exposure. Schwartz et al. [19] conducted the study between 1996 to 2016 reporting 285 cases and 1142 controls in the pediatric population. The total number of IBD cases that occurred were 3 UC and 3 CD cases. The study by Masclee et al. was over 13 years (1999–2012) which included 218 cases and 15045 community controls [20]. Finally, five case-control studies [9,18,21–23] included 120,597 participants were considered in which they reported a total number of 12,174 IBD events. A detailed description of study characteristics is illustrated in table I.
Table I.
Characteristics and summary of included studies.
| Author, Year, Country | Study Design | Study Duration | Number of Participants; Cases; Control | Age; Gender % | No. of IBD Events | Outcomes | Description of PPI Use | Confounders |
|---|---|---|---|---|---|---|---|---|
| Keszthelyi et al., 2010, The Netherlands [7] | Retrospective Case control study | 4 Years (November 2005–November 2009) | 450; Cases 95; Controls 355 | Mean Age 58 ± 1 years; 66% Female |
MC - 95 | The aOR = 4.5 (95% CI 2.0 – 9.5) | Patients having at least 1 PPI prescription 180 days prior to diagnosis. | Exposure to NSAIDS, BDZS, Diuretics and ACEI |
| Esan et al., 2020; United Kingdom [15] | Retrospective database cohort study | 15 years (2000–2015) | 20471; Cases 27 (UC) 10 (CD); Control 17564 | All age groups. Mean Age (SD) Control44.7 (21.2); 53.8% Males Cases 45.6 (17.2); 36.1% Males |
UC- 27; CD - 10 | The onset of UC with PPI use is aOR =1.5 (95% CI, 0.5–4.5) CD could not be assessed. | Length and dose of PPI exposure was not assessed; PPIs given 12 months before Campylobacter infection | Age, sex, smoking status, comorbidities, antibiotic use |
| Pascua et.al 2011, USA [16] | Case-control study | 5 years (2002–2007) | 544; Cases 26; Controls 518 (259 each for Random and diarrhoea) | Adults Median Age (Range) (years) Case 68.9 (18–92); 80% female Controls
|
LC – 15 CC - 11 |
The aOR is 0.06 (95% CI, 0.01 – 0.26) | PPI use within 12 months of the index date was considered | Race, BMI category and comorbidities (diabetes mellitus, hypertension, GERD, hyperlipidaemia) |
| Schwartz et al., 2019, USA [17] | Nested case -control | 20 Years (1996–2016) | 1427 Cases 285; Control 1142 | Age ≤21 years Mean Age 15.1 ± 2.6 years; 49.% Female |
UC – 3; CD – 3 | IBD with at least 1 receipt of PPI aOR= 3.6 (95% CI, 1.1–11.7) | 2 – 5 years before Index date with at least 1 prescription of PPI | Age, race, primary clinic Location, antibiotic Medication use, sex, and socio-economic status |
| Masclee et al., 2015, The Netherlands [18] | Nested case - control | 13 Years (1January 1999–March 2012) | 15263; Cases 218; Controls 15045 | Adults (≥18 years) Case 73.4% female Control 74.1% female |
MC - 218 | aOR = 7.3 (95% CI, 4.5–12.1) with PPI use and MC risk | Within 1 year before index date, assessing current use (<3 months) of PPI | Celiac disease, rheumatoid arthritis; hypothyroid disease; polyarthritis, diabetes mellitus Type 2 |
| Fernandes-Bañares et al., 2013, Spain [19] | Prospective case-control study | 3 years (March 2007 – May 2010) | 318; Cases 190; Controls 128 | Mean Age:,br>CC - 62.4 ± 1.4 years; 75% female LC 62.6 ± 1.9 years; 69% female Controls 62.4 ± 1.4; 74% female |
CC – 120 LC – 70 |
On calculation uOR for any PPI and LC risk was 2.63 (95% CI, 1.30 – 5.30) and for CC was 2.87 (95% CI 1.55 – 5.32) | Exposure was continuous or frequent (at least 3 days weekly) for 2 weeks or more | NR |
| Bonderup et al., 2018, Denmark [20] | Case-control study | 10 years (January 2004–December 2013) | 112033; Cases 10652; Controls 101381 | Adults Median Age (IQR) Cases CC– 68 (59–77); 76% Female LC – 66 (56–75); 65% female Controls CC - 68 (59–77); 76% Female LC - 66 (56–75); 64% female |
CC - 6250, LC - 4402 | CC risk with any PPI use, aOR= 6.98 (95% CI, 6.45 – 7.55). LC risk with any PPI aOR = 3.95 (95% CI 3.60 – 4.33) | Redemption of PPI prescription recorded in the Danish Prescription Registry prior to the index dates. | Use of NSAIDS, Antidepressants, antihypertensives, BDZS, past history of ischemic heart disease or hypothyroidism |
| Verhaegh et al., 2016, The Netherlands [21] | Case-control study | 21 Years (January 1992–December 2013) | 7252; Cases 1211; Controls 6041 | Adults Mean Age Cases 63.3; 73.2% Female Control 63.2; 73.2% Female |
MC - 1211 | Current PPI use showed an aOR of 3.37 (95% CI, 2.77 – 4.09) | Current users received their last dispensing 61–90 days before the index date | Autoimmune arthritis, Inflammatory Bowel Syndrome, NSAIDS and SSRI use |
ACEI: Angiotensin converting enzyme inhibitor; BDZS: Benzodiapines; BMI: Body mass index; CC: Collagenous Colitis; GERD: Gastro-Oesophageal Reflux Disease; LC: Lymphocytic Colitis; MC: Microscopic Colitis; NR: Not reported; NSAIDS: Non-steroidal anti-inflammatory drugs; OR: Odds Ratio; PPI: Proton pump inhibitor; SSRI: Selective Serotonin Reuptake Inhibitor.
Quality assessment of the evidence
The Modified Downs and Black checklist (1998) for the assessment of the methodological quality of included studies. Six of our studies were graded as “good” [9,18–20,22,23] quality evidence with a score ranging from 22–23 out of 28. One study was “excellent” [17] with a score of 24 and another study was deemed “fair” [21] with a score of 17. The included studies differed in their score due to the variation in methodology, sample size, duration of the study, confounding factor, study setting, and exposure to PPIs. None of the included studies mentioned the power of study or methods taken to increase their power. Some of the studies did not gather exact information about PPI consumption. A detailed quality assessment of the included studies and their score is provided as supplementary file S3.
Risk of IBD after PPI exposure
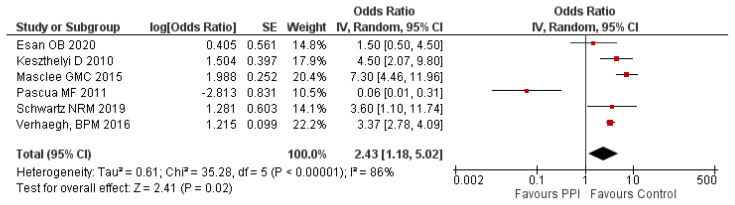
A study by Fernández-Bañares et al., [21] showed a significantly higher association between the use of lansoprazole and CC (aOR: 6.4; 95% CI: 1.3–32.1) and omeprazole in risk of LC (aOR: 2.7; 95% CI: 1.1–6.6). Moreover, a significantly higher risk of LC (OR: 2.63; 95% CI: 1.30–5.30) and CC (OR: 2.87; 95% CI: 1.55–5.32) was observed among those exposed to any PPI. Another nationwide Danish study by Bonderup [22] published in 2018 also reported a significantly higher LC risk (aOR: 3.95; 95% CI: 3.60–4.33) and CC risk (aOR: 6.98; 95% CI: 6.45–7.55) among those using any PPI. However, these studies were not included in this meta-analysis because they discussed the subtypes of MC. The meta-analysis of the 6 studies showed a significantly higher risk of IBD (aOR: 2.43; 95% CI: 1.18 – 5.02; P=0.02) with exposure to PPI compared to the control group. There was a significant level of heterogeneity (I2 = 86%) observed in the analysis. Hence, a random-effects model was used. A forest plot of meta-analysis on risk of IBD following the use of PPI is provided in figure 2.
Figure 2.
Meta-analysis on PPI use and risk of IBD.
Subgroup analysis
Types of IBD vs PPI exposure
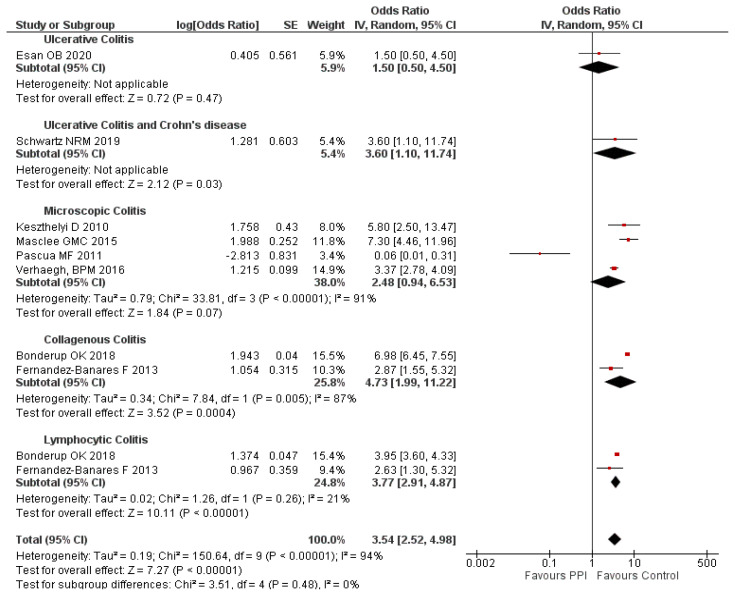
A subgroup analysis was done to study the effect of PPI use in different types of IBD. It is found that the use of PPI was significantly associated with a higher risk of UC and CD (aOR: 3.60; 95% CI: 1.10–11.74), MC (aOR: 2.48; 95% CI: 0.94–6.53), CC (OR: 4.73; 95% CI: 1.99–11.22) and LC (OR: 3.77; 95% CI: 2.91–4.87) compared to the control group. However, this association was not significant in patients with UC (aOR: 1.50; 95% CI: 0.5–4.5). The subgroup analysis doesn’t alter the heterogeneity except in LC type. This indicates that types of IBD may not be influencing the heterogeneity. The subgroup analysis between the type of IBD and PPI use is provided in figure 3.
Figure 3.
Subgroup analysis on PPI use and type of IBD.
Geographical location and IBD risk after PPI use
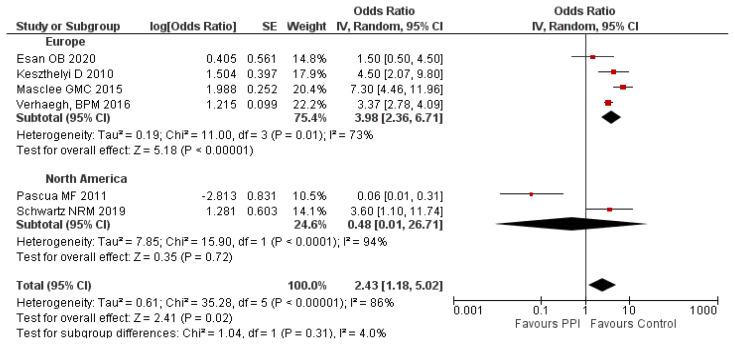
A subgroup analysis based on the geographical location of the study participants revealed a higher risk of IBD in the European population (aOR: 3.98; 95% CI: 2.36–6.71) whereas not in the North American population (OR = 0.48; 95% CI: 0.01–26.71) following the use of PPI compared to the control group. The subgroup analysis based on the geographical distribution doesn’t alter the level of heterogeneity, which indicates the non-influence of the geographical location in the heterogeneity of the overall analysis. The subgroup analysis based on the geographical location is given in figure 4.
Figure 4.
Subgroup analysis on PPI use and risk of IBD based on geographical location.
Sensitivity analysis
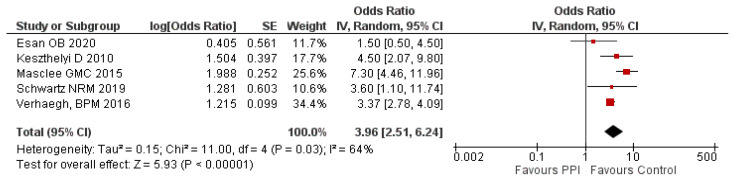
To check the robustness of our results, a sensitivity analysis was conducted by removing the study with the least weight (Pascua et. al; 10.5%), and the overall effect size was observed to be increased than before though there was no change in the significance of findings. There was a 3.96 fold (95% CI: 2.51–6.24; P=0.03) higher risk of IBD among the PPI users compared to the control group. The result of this analysis is given in figure 5.
Figure 5.
Sensitivity analysis on PPI use and risk of IBD.
Publication bias
Assessment of publication bias through a visual analysis of the funnel plot and Egger’s or Begg’s test was not performed as the comparison analysis comprises fewer than ten studies.
Discussion
PPIs were widely prescribed for various GIT disorders due to their acid-suppression mechanism [6]. However, there was conflicting evidence regarding the safety issues of PPIs and if they can induce IBD. Eight studies were included in this systematic review and the meta-analysis. This meta-analysis showed a significant association between PPI use and IBD risk. However, our analysis had high heterogeneity, hence caution should be taken while interpreting these findings. Moreover, we performed a subgroup analysis to explore the sources of heterogeneity. There is no clear understanding of the pathophysiology of PPI-induced IBD. However, there are certain hypotheses that may aid in its understanding. One theory is that the alteration of local electrolyte balance with fluid acidification due to colonic proton pump inhibition by PPIs may induce inflammatory reactions like IBD. Another theory states that omeprazole and lansoprazole may reduce contractile activity which can cause conformational changes in the epithelial cells cytoskeleton, and alter tight junction function [17].
PPI use can alter gut flora and cause dysbiosis, by promoting the movement of oral bacteria into lower regions of the GIT, causing pro-inflammatory microenvironment establishment [24] and an increased level of pro-inflammatory cells and mediators may trigger IBD [25]. Lansoprazole particularly may cause a marked inflammatory response and increased IBD risk due to its unique binding mechanism with cysteine-321 residue on proton pumps which may elicit their differential inhibition [26]. Pascua et al. [18] was the only study to show significantly lesser association between PPI use and IBD risk. The random controls group for a 12-month risk period was chosen to maintain homogeneity and the lack of common pathways argues against a causal relationship between the drug and IBD risk.
The subgroup analysis was done based on the type of IBD and the geographical location. In the overall risk analysis, two studies [21,22] were not included as they assessed LC and CC as individual entities and not IBD as a whole, however, they were included in the subgroup analysis. Fernandes-Bañares et al. [21] reported a higher risk of CC with lansoprazole and LC with omeprazole. Another study by Bonderup OK et al., [22] recorded a significantly higher CC risk and LC risk following the use of any PPI.
PPI use had the highest odds on development of CC and lowest with UC. A study by Bürgel et al., stated that the decreased Na+ and Cl− absorption and diminished epithelial resistance and tight junction proteins expression (like occludin and claudin-4) can cause diarrhea in CC [27]. From the sub-group analysis, it is observed that there was a non-significant higher risk of IBD in MC patients, which was not similar to the findings by Tong et al., where they reported a significantly higher risk of IBD in MC patients [28]. Another subgroup analysis was done to check the influence of geographical location and PPI use and development of IBD. North Americans were found to have no increased association on PPI use and IBD risk, while Europeans showed a higher risk of IBD. These differences between the regions may be due to differences in environmental factors, lifestyle, and genetic susceptibility or due to methodological differences [26].
Traditionally, IBD has been considered to be a condition of higher-income countries. In 2017, the USA was shown to have the highest age-standardized prevalence rate as compared to the rest of the world. Among European countries, the UK showed the highest age-standardized prevalence [27]. However, in our analysis of 2 studies from North America, the study conducted by Pascua et al. 2011 [18] was the only study which showed no increased association on PPI exposure.
The ORs retrieved were adjusted for various confounding factors. The most common covariates used for adjustment were - NSAID uses, history of autoimmune disease, age, sex, smoking status, and comorbidities. The quality assessment of our study was done using the Modified Downs and Black (1998) checklist. Six of the included studies were “good”, 1 was “excellent” and 1 was “fair”. This indicates that our evidence had good strength to draw a conclusion. Moreover, we performed a sensitivity analysis by excluding the study with the lowest weight (Pascua et al., 10.5%) and we did not observe a variation in the significance of the findings.
Additionally, our study has several strengths. To start with, this is the first study to discuss the use of PPI and the risk of different types of IBD among adults, though there are many systematic reviews available on various drugs induced adverse drug reactions [29–31]. Second, the quality assessment conducted showed that none of our studies were of “poor” quality, this increases the credibility of our findings. Third, the source population was large (157,758 participants) and collected using different validated electronically linked databases. Finally, the results of this study support our initial hypothesis that the risk of IBD can be significantly affected by PPI exposure. However, further cohort studies are required to strengthen these findings and to determine the chief pathophysiological mechanisms of PPI-induced IBD.
Our study has some limitations. Exclusion of non-English studies and restriction to the observational studies may lead to missing some important information. However, a comprehensive search strategy in numerous databases might have helped us to collate the maximum available pieces of evidence. The overall consumption of PPI use is difficult to determine due to its over-the-counter use in most countries. Since all of our studies had used an electronic linked database, the validity of IBD diagnosis cannot be confirmed. All of our included studies were conducted in the USA and Europe, thus reducing its global generalizability. Further, adequately powered studies are required across the world to strengthen our findings and to generalize the results to all populations.
Conclusion
Our study found a higher risk of IBD in patients who are exposed to PPI. The risk of collagenous colitis, lymphocytic colitis, and ulcerative colitis & Crohn’s disease was significantly associated with the use of PPI. Further observational studies are required across the world to generalize our findings to all other populations.
Supplementary Information
Supplementary S1.
Detailed inclusion and exclusion criteria.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study design |
|
|
| Patients |
|
|
| Exposure |
|
- |
| Comparator |
|
|
| Outcome |
|
|
| Language |
|
|
| Publication time-frame |
|
|
Supplementary File S2.
Search strategy in different databases.
A.
Search strategy in PubMed
| SEARCH NO. | QUERY | RESULTS |
|---|---|---|
| #1 | “Proton pump inhibitor” OR “Inhibitors, Proton Pump” OR “PPI” | 23,396 |
| #2 | “Omeprazole” OR “Prilosec” OR “Omeprazole Sodium” OR “Sodium, Omeprazole” OR “H 168-68” OR “H 168 68” OR “H 16868” OR “Omeprazole Magnesium” OR “Magnesium Omeprazole” | 12,306 |
| #3 | “lansoprazole” OR “lansoprazole sulfone” OR “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy) -2-pyridyl) methyl) sulfinyl) benzimidazole” OR “lansoprazole gastro” OR “AG 1749” OR “AG-1749” OR “AG1749” OR “agoston” OR “banalite” OR “lanson” OR “lansoprazole sodium” OR “sodium, lansoprazole” OR “lanzer monolithium” OR “spirin” OR “prevacid” OR “pro ulco” OR “promeco” OR “takepron” OR “ulnas” OR “soton” OR “gast” OR “frezal” | 13,481 |
| #4 | “Esomeprazole” OR “Esomeprazole Sodium” OR “Esomeprazole Strontium” OR “Strontium, Esomeprazole” OR “Esomeprazole Magnesium” OR “Nexium” OR “Esomeprazole Potassium” OR “Esomeprazole Strontium Anhydrous” | 1,706 |
| #5 | Pantoprazole OR “SK F 96022” OR “SKF-96022” OR “SK F-96022” OR “Protonix” OR “BY 1023” OR “BY-1023” OR “pantoprazole sodium” | 2,084 |
| #6 | “Dexlansoprazole” OR “Lansoprazole, R-Isomer” OR “R-Isomer Lansoprazole” OR “2-((R)-((3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole) OR R-Lansoprazole)” OR “R Lansoprazole” OR “Dexlansoprazole Sesquihydrate” OR “TAK 390MR” OR “TAK390MR” OR “TAK-390MR” OR “TAK-390” OR “TAK 390” OR “TAK390” OR “Dexilant” OR “T-168390” OR “T 168390” OR “T168390” | 142 |
| #7 | “ilaprazole” OR “IY 81149” OR “IY81149” OR “IY-81149” | 63 |
| #8 | “Rabeprazole” OR “2-((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methylsulfinyl)-1H-benzimidazole)” OR “Dexrabeprazole” OR “E 3810” OR “E3810” OR “Pariet” OR “Rabeprazole Sodium” OR “Sodium, Rabeprazole” OR “1H-Benzimidazole, 2-(((4-(3-methoxypropoxy)-3-methyl-2-pyridinyl)methyl)sulfinyl)-, Sodium Salt” OR “Aciphex” OR “LY-307640” OR “LY 307640” OR “LY307640” | 1,416 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #5 OR #6 OR #7 OR #8 | 45,724 |
| #10 | “Inflammatory Bowel Disease” OR “Bowel Diseases, Inflammatory” OR “Ulcerative Colitis” OR “Colitis Gravis” OR “Inflammatory Bowel Disease, Ulcerative Colitis Type” OR “Crohn’s Enteritis” OR “Regional Enteritis” OR “Crohn’s Disease” OR “Crohns Disease” OR “Inflammatory Bowel Disease” OR “Enteritis, Granulomatous” OR “Granulomatous Enteritis” OR “Enteritis” OR “Regional Ileocolitis” “Colitis, Granulomatous” OR “Granulomatous Colitis” OR “Ileitis, Terminal” OR “Terminal Ileitis” OR “Ileitis, Regional” OR “Regional Ileitides” OR “Regional Ileitis” | 57,169 |
| #11 | #9 AND #10 | 130 |
B.
Search strategy in Cochrane Library.
| SEARCH NO. | QUERY | RESULTS |
|---|---|---|
| #1 | “Omeprazole” OR “Prilosec” OR “Omeprazole Sodium” OR “Sodium, Omeprazole” OR “H 168-68” OR “H 168 68” OR “H 16868” OR “Omeprazole Magnesium” OR “Magnesium Omeprazole” | 4454 |
| #2 | “Proton pump inhibitor” OR “Inhibitors, Proton Pump” OR PPI | 3988 |
| #3 | “lansoprazole” OR “lansoprazole sulfone” OR “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy) -2-pyridyl) methyl) sulfinyl) benzimidazole” OR “lansoprazole gastro” OR “AG 1749” OR “AG-1749” OR “AG1749” OR “agoston” OR “banalite” OR “lanson” OR “lansoprazole sodium” OR “sodium, lansoprazole” OR “lanzer monolithium” OR “spirin” OR “prevacid” OR “pro ulco” OR “promeco” OR “takepron” OR “ulnas” | 2522 |
| #4 | “Esomeprazole” OR “Esomeprazole Sodium” OR “Esomeprazole Strontium” OR “Strontium, Esomeprazole” OR “Esomeprazole Magnesium” OR “Nexium” OR “Esomeprazole Potassium” | 1564 |
| #5 | “Pantoprazole” OR “SK F 96022” OR “SKF-96022” OR “SK F-96022” OR “Protonix” OR “BY 1023” OR “BY-1023” OR “pantoprazole sodium” | 1270 |
| #6 | “Dexlansoprazole” OR “Lansoprazole, R-Isomer” OR “R-Isomer Lansoprazole” OR “R Lansoprazole” OR “Dexlansoprazole Sesquihydrate” OR “TAK 390MR” OR “TAK390MR” OR “TAK- 390MR” OR “TAK-390” OR “TAK 390” OR “TAK390” OR “Dexilant” | 281 |
| #7 | “ilaprazole” OR “IY 81149” OR “IY81149” OR “IY-81149” | 57 |
| #8 | “Rabeprazole” OR “Dexrabeprazole” OR “E 3810” OR “E3810” OR “Pariet” OR “Rabeprazole Sodium” OR “Sodium, Rabeprazole” OR “Aciphex” OR “LY-307640” OR “LY 307640” OR “LY307640” | 1079 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 10871 |
| #10 |
“Inflammatory Bowel Disease” OR “Bowel Diseases, Inflammatory” OR “Ulcerative Colitis” OR “Colitis Gravis” OR “Inflammatory Bowel Disease, Ulcerative Colitis Type” OR “Crohn’s Enteritis” OR “Regional Enteritis” OR “Crohn’s Disease” OR “Crohns Disease” OR “Inflammatory Bowel Disease” OR “Enteritis, Granulomatous” OR “Granulomatous Enteritis” OR “Enteritis” OR “Regional Ileocolitis” “Colitis, Granulomatous” OR “Granulomatous Colitis” OR “Ileitis, Terminal” OR “Terminal Ileitis” OR “Ileitis, Regional” OR “Regional Ileitides” OR “Regional Ileitis” |
10118 |
| #11 | #9 AND #10 | 74 |
C.
Search strategy in Scopus.
| ID | Search Hits | RESULTS |
|---|---|---|
| #1 | ( TITLE-ABS-KEY ( “Proton pump inhibitor”) OR TITLE-ABS-KEY ( “Inhibitors, Proton Pump”) OR TITLE-ABS-KEY ( “PPI” ) ) | 58,331 |
| #2 | ( TITLE-ABS-KEY ( “Omeprazole”) OR TITLE-ABS-KEY ( “Prilosec”) OR TITLE-ABS-KEY ( “Omeprazole Sodium”) OR TITLE-ABS-KEY ( “Sodium, Omeprazole”) OR TITLE-ABS- KEY ( “H 168-68”) OR TITLE-ABS-KEY ( “H 168 68”) OR TITLE-ABS-KEY ( “H 16868”) OR TITLE-ABS-KEY ( “Omeprazole Magnesium”) OR TITLE-ABS-KEY ( “Magnesium Omeprazole” ) ) | 33,109 |
| #3 | (TITLE-ABS-KEY (“lansoprazole”) OR TITLE-ABS-KEY ( “lansoprazole sulfone”) OR TITLE-ABS- KEY ( “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy)-2-pyridyl) methyl) sulfinyl) benzimidazole”) OR TITLE-ABS-KEY ( “lansoprazole gastro”) OR TITLE-ABS-KEY ( AG 1749”) OR TITLE-ABS- KEY ( AG-1749”) OR TITLE-ABS-KEY ( AGl749”) OR TITLE-ABS-KEY ( “agoston”) OR TITLE- ABS-KEY ( “banalite”) OR TITLE-ABS-KEY ( “lanson”) OR TITLE-ABS-KEY ( “lansoprazole sodium”) OR TITLE-ABS-KEY ( “sodium, lansoprazole”) OR TITLE-ABS-KEY ( “lanzer monolithium”) OR TITLE-ABS-KEY ( “spirin”) OR TITLE-ABS-KEY ( “prevacid”) OR TITLE-ABS-KEY ( “pro ulco”) OR TITLE-ABS-KEY ( “promeco”) OR TITLE-ABS-KEY ( “takepron”) OR TITLE- ABS-KEY ( “ulnas”) OR TITLE-ABS-KEY ( “soton”) OR TITLE-ABS-KEY ( “gast”) OR TITLE-ABS- KEY ( “frezal”)) | 28,124 |
| #4 | TITLE-ABS-KEY ( “Esomeprazole”) OR TITLE-ABS-KEY ( “Esomeprazole Sodium”) OR TITLE-ABS-KEY ( “Esomeprazole Strontium”) OR TITLE-ABS-KEY ( “Strontium, Esomeprazole”) ORTITLE-ABS-KEY ( “Esomeprazole Magnesium”) OR TITLE-ABS-KEY ( “Nexium”) OR TITLE-ABS-KEY ( “Esomeprazole Potassium”) OR TITLE-ABS-KEY ( “Esomeprazole Strontium Anhydrous”)) | 7,271 |
| #5 | ( TITLE-ABS-KEY ( “Pantoprazole”) OR TITLE-ABS-KEY (“SKF 96022”) OR TITLE-ABS-KEY ( “SKF-96022”) OR TITLE-ABS-KEY ( “SK F-96022”) OR TITLE-ABS.KEY ( “Protonix”) OR TITLE-ABS-KEY ( “BY 1023”) OR TITLE-ABS-KEY ( “BY-1023”) OR TITLE-ABS-KEY ( “pantoprazole sodium”)) | 9246 |
| #6 | ( TITLE-ABS-KEY ( “Dexlansoprazole”) OR TITLE-ABS-KEY ( “Lansoprazole, R-lsomer”) OR TITLE-ABS-KEY ( “R-lsomer Lansoprazole”) OR TITLE-ABS-KEY ( “2-{(R)-{(3-Methyl-4-(2,2,2- trifluoroethoxy)-2-pyridinyl)methyl)sulfi nyl)-lH-benzimidazole) OR R-Lansoprazole)” ) OR TITLE-ABS-KEY ( “R lansoprazole”) OR TITLE-ABS-KEY ( “Dexlansoprazole Sesquihydrate”) OR TITLE-ABS-KEY ( “TAK 390MR”) OR TITLE-ABS-KEY ( “TAK390MR”) OR TITLE-ABS-KEY ( ‘TAK- 390MR:’) OR TITLE-ABS-KEY (‘TAK-390”) OR TITLE-ABS-KEY (‘TAK 390”) OR TITLE-ABSKEY (‘TAK390”) OR TITLE-ABS-KEY ( “Dexilant”) OR TITLE-ABS-KEY ( “T-168390”) OR TITLEABS-KEY ( “T 168390” ) OR TITLE-ABS-KEY ( “Tl68390” ) ) | 359 |
| #7 | ( TITLE-ABS-KEY ( “IY-81149”) OR TITLE-ABS-KEY ( “IY81149”) OR TITLE-ABS-KEY ( “IY 81149”) OR TITLE-ABS.KEY ( “ilaprazole”)) | 167 |
| #8 | ( TITLE-ABS-KEY ( “Rabeprazole”) OR TITLE-ABS-KEY ( “2-((4-(3-methoxypropoxy)-3-methylpyridin-2-ylmethylsulfinyl}-lH-benzimidazole)”) OR TITLE-ABS-KEY ( “Dexrabeprazole”) OR TITLE-ABS-KEY ( “E 3810”) OR TITLE-ABS-KEY ( “E3810”) OR TITLE-ABS-KEY ( “Pariet”) OR TITLE-ABS-KEY ( “Rabeprazole Sodium”) OR TITLE-ABS-KEY ( “Sodium, Rabeprazole”) OR TITLE-ABS-KEY ( “lH-Benzimidazole, 2-(((4-{3- methoxypropoxy}-3-methyl-2-pyridinyl)methyl)sulfinyl)-, Sodium Salt”) OR TITLE-ABSKEY (‘Aciphex”) OR TITLE-ABS.KEY ( “LY-307640”) OR TITLE-ABS-KEY ( “LY 307640”) OR TITLE-ABS-KEY ( “LY307640”)) | 4938 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 108601 |
| #10 | ( TITLE-ABS-KEY ( “Inflammatory Bowel Disease”) OR TITLE-ABS-KEY ( “Bowel Diseases, Inflammatory”) OR TITLE-ABS-KEY ( “Ulcerative Colitis”) OR TITLE-ABS-KEY ( “Colitis Gravis”) OR TITLE-ABS-KEY ( “Inflammatory Bowel Disease, Ulcerative Colitis Type”) OR TITLE-ABS-KEY ( “Crohn’s Enteritis”) OR TITLE-ABS-KEY ( “Regional Enteritis”) OR TITLE-ABSKEY ( “Crohn’s Disease”) OR TITLE-ABS-KEY ( “Crohns Disease”) OR TITLE-ABS-KEY ( “Inflammatory Bowel Disease”) OR TITLE-ABS-KEY ( “Enteritis, Granulomatous”) OR TITLE-ABS-KEY ( “Granulomatous Enteritis”) OR TITLE-ABS-KEY (“Enteritis”) OR TITLE-ABSKEY ( “Regional lleocolitis”) OR TITLE-ABS-KEY ( “Colitis, Granulomatous”) OR TITLE-ABSKEY ( “Granulomatous Colitis”) OR TITLE-ABS-KEY ( “Ileitis, Terminal”) OR TITLE-ABS-KEY ( “Terminal Ileitis”) OR TITLE-ABS-KEY ( “Ileitis, Regional”) OR TITLE-ABS-KEY ( “Regional Ileitides”) OR TITLE-ABS-KEY ( “Regional Ileitis”)) | 177,263 |
| #11 | #9 AND #10 | 1829 |
Supplementary file S3.
Detailed methodological assessment of included studies.
| Question No. | Bonderup et al. | Esan et al. | Fernández-Bañares et al. | Keszthelyi et al. | Masclee et al. | Pascua et al. | Schwartz et al. | Verhaegh et al |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 22 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 26 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 27 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (Out of 28) | 22 | 24 | 17 | 23 | 23 | 23 | 22 | 22 |
Acknowledgement
The authors would like to acknowledge the Department of Pharmacy Practice, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India, for all the support and infrastructure facilities for the best possible completion of this study.
References
- 1.Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113–120. doi: 10.2147/JIR.S65979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalili H, Burke KE, Roelstraete B, Sachs MC, Olén O, Ludvigsson JF. Microscopic Colitis and Risk of Inflammatory Bowel Disease in a Nationwide Cohort Study. Gastroenterology. 2020;158:1574–1583e2. doi: 10.1053/j.gastro.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco A, Fernández-Bañares F. Th1 Pathway: The Missing Link Between Inflammatory Bowel Disease and Microscopic Colitis? Dig Dis Sci. 2017;62:2609–2611. doi: 10.1007/s10620-017-4692-x. [DOI] [PubMed] [Google Scholar]
- 4.Xia B, Crusius J, Meuwissen S, Peña A. Inflammatory bowel disease: definition, epidemiology, etiologic aspects, and immunogenetic studies. World J Gastroenterol. 1998;4:446–458. doi: 10.3748/wjg.v4.i5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentile N, Yen EF. Prevalence, Pathogenesis, Diagnosis, and Management of Microscopic Colitis. Gut Liver. 2018;12:227–235. doi: 10.5009/gnl17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinink AR. Do Acid-Suppressing Medications in Inflammatory Bowel Disease Increase Risk for Flare? Digestion. 2017;95:186–187. doi: 10.1159/000464283. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Clarke JO. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2021. Proton Pump Inhibitors (PPI) [PubMed] [Google Scholar]
- 8.Venkataraman R, Rashid M, Shrestha H. Inappropriate Medication Use and Cost Comparison Analysis of Proton Pump Inhibitors: Evidence from an Indian Tertiary Care Facility. Curr Drug Saf. 2020;15:147–155. doi: 10.2174/1574886315666200311120151. [DOI] [PubMed] [Google Scholar]
- 9.Keszthelyi D, Jansen SV, Schouten GA, de Kort S, Scholtes B, Engels LG, et al. Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case–control study. Aliment Pharmacol Ther. 2010;32:1124–1128. doi: 10.1111/j.1365-2036.2010.04453.x. [DOI] [PubMed] [Google Scholar]
- 10.Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric Acid Suppression Is Associated with an Increased Risk of Adverse Outcomes in Inflammatory Bowel Disease. Digestion. 2017;95:188–193. doi: 10.1159/000455008. [DOI] [PubMed] [Google Scholar]
- 11.Rashid M, Thunga G, Chandran VP, Kantamneni R, Shastri SA, Suhita R, et al. Proton pump inhibitor use and risk of inflammatory bowel disease: A systematic review and meta-analysis. PROSPERO. 2020. p. CRD42020209674. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020209674. [DOI] [PMC free article] [PubMed]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Review Manager (RevMan). [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 15.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. Sep 23, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid M, Shamshavali K, Chhabra M. Efficacy and Safety of Nilutamide in Patients with Metastatic Prostate Cancer who Underwent Orchiectomy: A Systematic Review and Metaanalysis. Curr Clin Pharmacol. 2019;14:108–115. doi: 10.2174/1574884714666190112151202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esan OB, Perera R, McCarthy N, Violato M, Fanshawe TR. Incidence, risk factors, and health service burden of sequelae of campylobacter and non-typhoidal salmonella infections in England, 2000–2015: A retrospective cohort study using linked electronic health records. J Infect. 2020;81:221–230. doi: 10.1016/j.jinf.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 18.Pascua MF, Kedia P, Weiner MG, Holmes J, Ellenberg J, Lewis JD. Microscopic colitis and Medication Use. Clin Med Insights Gastroenterol. 2010;2010:11–19. doi: 10.4137/cgast.s4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz NRM, Hutfless S, Herrinton LJ, Amsden LB, Fevrier HB, Giefer M, et al. Proton Pump Inhibitors, H2 Blocker Use, and Risk of Inflammatory Bowel Disease in Children. J Pediatr Pharmacol Ther. 2019;24:489–496. doi: 10.5863/1551-6776-24.6.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol. 2015;110:749–759. doi: 10.1038/ajg.2015.119. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Bañares F, de Sousa MR, Salas A, Beltrán B, Piqueras M, Iglesias E, et al. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis. 2013;19:411–417. doi: 10.1002/ibd.23009. [DOI] [PubMed] [Google Scholar]
- 22.Bonderup OK, Nielsen GL, Dall M, Pottegård A, Hallas J. Significant association between the use of different proton pump inhibitors and microscopic colitis: a nationwide Danish case-control study. Aliment Pharmacol Ther. 2018;48:618–625. doi: 10.1111/apt.14916. [DOI] [PubMed] [Google Scholar]
- 23.Verhaegh BP, de Vries F, Masclee AA, Keshavarzian A, de Boer A, Souverein PC, et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43:1004–1013. doi: 10.1111/apt.13583. [DOI] [PubMed] [Google Scholar]
- 24.Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, et al. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J Gastroenterol. 2019;25:2706–2719. doi: 10.3748/wjg.v25.i22.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Putignani L, Del Chierico F, Vernocchi P, Cicala M, Cucchiara S, Dallapiccola B, et al. Gut Microbiota Dysbiosis as Risk and Premorbid Factors of IBD and IBS Along the Childhood-Adulthood Transition. Inflamm Bowel Dis. 2016;22:487–504. doi: 10.1097/MIB.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 26.Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433–443. doi: 10.1053/gast.2002.34784. [DOI] [PubMed] [Google Scholar]
- 27.Boland K, Nguyen GC. Microscopic Colitis: A Review of Collagenous and Lymphocytic Colitis. Gastroenterol Hepatol (N Y) 2017;13:671–677. [PMC free article] [PubMed] [Google Scholar]
- 28.Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:265–276. doi: 10.1038/ajg.2014.431. quiz 277. [DOI] [PubMed] [Google Scholar]
- 29.Kashyap A, Sreenivasan S, Rajan AK, Rashid M, Chhabra M. Ciprofloxacin-induced cutaneous adverse drug events: a systematic review of descriptive studies. J Basic Clin Physiol Pharmacol. 2021 Mar 16; doi: 10.1515/jbcpp-2020-0115. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Rashid M, Kashyap A, Undela K. Valproic acid and Stevens-Johnson syndrome: a systematic review of descriptive studies. Int J Dermatol. 2019;58:1014–1022. doi: 10.1111/ijd.14411. [DOI] [PubMed] [Google Scholar]
- 31.Rashid M, Rajan AK, Chhabra M, Kashyap A. Levetiracetam and cutaneous adverse reactions: A systematic review of descriptive studies. Seizure. 2020;75:101–109. doi: 10.1016/j.seizure.2020.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary S1.
Detailed inclusion and exclusion criteria.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Study design |
|
|
| Patients |
|
|
| Exposure |
|
- |
| Comparator |
|
|
| Outcome |
|
|
| Language |
|
|
| Publication time-frame |
|
|
Supplementary File S2.
Search strategy in different databases.
A.
Search strategy in PubMed
| SEARCH NO. | QUERY | RESULTS |
|---|---|---|
| #1 | “Proton pump inhibitor” OR “Inhibitors, Proton Pump” OR “PPI” | 23,396 |
| #2 | “Omeprazole” OR “Prilosec” OR “Omeprazole Sodium” OR “Sodium, Omeprazole” OR “H 168-68” OR “H 168 68” OR “H 16868” OR “Omeprazole Magnesium” OR “Magnesium Omeprazole” | 12,306 |
| #3 | “lansoprazole” OR “lansoprazole sulfone” OR “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy) -2-pyridyl) methyl) sulfinyl) benzimidazole” OR “lansoprazole gastro” OR “AG 1749” OR “AG-1749” OR “AG1749” OR “agoston” OR “banalite” OR “lanson” OR “lansoprazole sodium” OR “sodium, lansoprazole” OR “lanzer monolithium” OR “spirin” OR “prevacid” OR “pro ulco” OR “promeco” OR “takepron” OR “ulnas” OR “soton” OR “gast” OR “frezal” | 13,481 |
| #4 | “Esomeprazole” OR “Esomeprazole Sodium” OR “Esomeprazole Strontium” OR “Strontium, Esomeprazole” OR “Esomeprazole Magnesium” OR “Nexium” OR “Esomeprazole Potassium” OR “Esomeprazole Strontium Anhydrous” | 1,706 |
| #5 | Pantoprazole OR “SK F 96022” OR “SKF-96022” OR “SK F-96022” OR “Protonix” OR “BY 1023” OR “BY-1023” OR “pantoprazole sodium” | 2,084 |
| #6 | “Dexlansoprazole” OR “Lansoprazole, R-Isomer” OR “R-Isomer Lansoprazole” OR “2-((R)-((3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole) OR R-Lansoprazole)” OR “R Lansoprazole” OR “Dexlansoprazole Sesquihydrate” OR “TAK 390MR” OR “TAK390MR” OR “TAK-390MR” OR “TAK-390” OR “TAK 390” OR “TAK390” OR “Dexilant” OR “T-168390” OR “T 168390” OR “T168390” | 142 |
| #7 | “ilaprazole” OR “IY 81149” OR “IY81149” OR “IY-81149” | 63 |
| #8 | “Rabeprazole” OR “2-((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methylsulfinyl)-1H-benzimidazole)” OR “Dexrabeprazole” OR “E 3810” OR “E3810” OR “Pariet” OR “Rabeprazole Sodium” OR “Sodium, Rabeprazole” OR “1H-Benzimidazole, 2-(((4-(3-methoxypropoxy)-3-methyl-2-pyridinyl)methyl)sulfinyl)-, Sodium Salt” OR “Aciphex” OR “LY-307640” OR “LY 307640” OR “LY307640” | 1,416 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #5 OR #6 OR #7 OR #8 | 45,724 |
| #10 | “Inflammatory Bowel Disease” OR “Bowel Diseases, Inflammatory” OR “Ulcerative Colitis” OR “Colitis Gravis” OR “Inflammatory Bowel Disease, Ulcerative Colitis Type” OR “Crohn’s Enteritis” OR “Regional Enteritis” OR “Crohn’s Disease” OR “Crohns Disease” OR “Inflammatory Bowel Disease” OR “Enteritis, Granulomatous” OR “Granulomatous Enteritis” OR “Enteritis” OR “Regional Ileocolitis” “Colitis, Granulomatous” OR “Granulomatous Colitis” OR “Ileitis, Terminal” OR “Terminal Ileitis” OR “Ileitis, Regional” OR “Regional Ileitides” OR “Regional Ileitis” | 57,169 |
| #11 | #9 AND #10 | 130 |
B.
Search strategy in Cochrane Library.
| SEARCH NO. | QUERY | RESULTS |
|---|---|---|
| #1 | “Omeprazole” OR “Prilosec” OR “Omeprazole Sodium” OR “Sodium, Omeprazole” OR “H 168-68” OR “H 168 68” OR “H 16868” OR “Omeprazole Magnesium” OR “Magnesium Omeprazole” | 4454 |
| #2 | “Proton pump inhibitor” OR “Inhibitors, Proton Pump” OR PPI | 3988 |
| #3 | “lansoprazole” OR “lansoprazole sulfone” OR “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy) -2-pyridyl) methyl) sulfinyl) benzimidazole” OR “lansoprazole gastro” OR “AG 1749” OR “AG-1749” OR “AG1749” OR “agoston” OR “banalite” OR “lanson” OR “lansoprazole sodium” OR “sodium, lansoprazole” OR “lanzer monolithium” OR “spirin” OR “prevacid” OR “pro ulco” OR “promeco” OR “takepron” OR “ulnas” | 2522 |
| #4 | “Esomeprazole” OR “Esomeprazole Sodium” OR “Esomeprazole Strontium” OR “Strontium, Esomeprazole” OR “Esomeprazole Magnesium” OR “Nexium” OR “Esomeprazole Potassium” | 1564 |
| #5 | “Pantoprazole” OR “SK F 96022” OR “SKF-96022” OR “SK F-96022” OR “Protonix” OR “BY 1023” OR “BY-1023” OR “pantoprazole sodium” | 1270 |
| #6 | “Dexlansoprazole” OR “Lansoprazole, R-Isomer” OR “R-Isomer Lansoprazole” OR “R Lansoprazole” OR “Dexlansoprazole Sesquihydrate” OR “TAK 390MR” OR “TAK390MR” OR “TAK- 390MR” OR “TAK-390” OR “TAK 390” OR “TAK390” OR “Dexilant” | 281 |
| #7 | “ilaprazole” OR “IY 81149” OR “IY81149” OR “IY-81149” | 57 |
| #8 | “Rabeprazole” OR “Dexrabeprazole” OR “E 3810” OR “E3810” OR “Pariet” OR “Rabeprazole Sodium” OR “Sodium, Rabeprazole” OR “Aciphex” OR “LY-307640” OR “LY 307640” OR “LY307640” | 1079 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 10871 |
| #10 |
“Inflammatory Bowel Disease” OR “Bowel Diseases, Inflammatory” OR “Ulcerative Colitis” OR “Colitis Gravis” OR “Inflammatory Bowel Disease, Ulcerative Colitis Type” OR “Crohn’s Enteritis” OR “Regional Enteritis” OR “Crohn’s Disease” OR “Crohns Disease” OR “Inflammatory Bowel Disease” OR “Enteritis, Granulomatous” OR “Granulomatous Enteritis” OR “Enteritis” OR “Regional Ileocolitis” “Colitis, Granulomatous” OR “Granulomatous Colitis” OR “Ileitis, Terminal” OR “Terminal Ileitis” OR “Ileitis, Regional” OR “Regional Ileitides” OR “Regional Ileitis” |
10118 |
| #11 | #9 AND #10 | 74 |
C.
Search strategy in Scopus.
| ID | Search Hits | RESULTS |
|---|---|---|
| #1 | ( TITLE-ABS-KEY ( “Proton pump inhibitor”) OR TITLE-ABS-KEY ( “Inhibitors, Proton Pump”) OR TITLE-ABS-KEY ( “PPI” ) ) | 58,331 |
| #2 | ( TITLE-ABS-KEY ( “Omeprazole”) OR TITLE-ABS-KEY ( “Prilosec”) OR TITLE-ABS-KEY ( “Omeprazole Sodium”) OR TITLE-ABS-KEY ( “Sodium, Omeprazole”) OR TITLE-ABS- KEY ( “H 168-68”) OR TITLE-ABS-KEY ( “H 168 68”) OR TITLE-ABS-KEY ( “H 16868”) OR TITLE-ABS-KEY ( “Omeprazole Magnesium”) OR TITLE-ABS-KEY ( “Magnesium Omeprazole” ) ) | 33,109 |
| #3 | (TITLE-ABS-KEY (“lansoprazole”) OR TITLE-ABS-KEY ( “lansoprazole sulfone”) OR TITLE-ABS- KEY ( “2- (((3-Methyl-4- (2,2,2-trifluoroethoxy)-2-pyridyl) methyl) sulfinyl) benzimidazole”) OR TITLE-ABS-KEY ( “lansoprazole gastro”) OR TITLE-ABS-KEY ( AG 1749”) OR TITLE-ABS- KEY ( AG-1749”) OR TITLE-ABS-KEY ( AGl749”) OR TITLE-ABS-KEY ( “agoston”) OR TITLE- ABS-KEY ( “banalite”) OR TITLE-ABS-KEY ( “lanson”) OR TITLE-ABS-KEY ( “lansoprazole sodium”) OR TITLE-ABS-KEY ( “sodium, lansoprazole”) OR TITLE-ABS-KEY ( “lanzer monolithium”) OR TITLE-ABS-KEY ( “spirin”) OR TITLE-ABS-KEY ( “prevacid”) OR TITLE-ABS-KEY ( “pro ulco”) OR TITLE-ABS-KEY ( “promeco”) OR TITLE-ABS-KEY ( “takepron”) OR TITLE- ABS-KEY ( “ulnas”) OR TITLE-ABS-KEY ( “soton”) OR TITLE-ABS-KEY ( “gast”) OR TITLE-ABS- KEY ( “frezal”)) | 28,124 |
| #4 | TITLE-ABS-KEY ( “Esomeprazole”) OR TITLE-ABS-KEY ( “Esomeprazole Sodium”) OR TITLE-ABS-KEY ( “Esomeprazole Strontium”) OR TITLE-ABS-KEY ( “Strontium, Esomeprazole”) ORTITLE-ABS-KEY ( “Esomeprazole Magnesium”) OR TITLE-ABS-KEY ( “Nexium”) OR TITLE-ABS-KEY ( “Esomeprazole Potassium”) OR TITLE-ABS-KEY ( “Esomeprazole Strontium Anhydrous”)) | 7,271 |
| #5 | ( TITLE-ABS-KEY ( “Pantoprazole”) OR TITLE-ABS-KEY (“SKF 96022”) OR TITLE-ABS-KEY ( “SKF-96022”) OR TITLE-ABS-KEY ( “SK F-96022”) OR TITLE-ABS.KEY ( “Protonix”) OR TITLE-ABS-KEY ( “BY 1023”) OR TITLE-ABS-KEY ( “BY-1023”) OR TITLE-ABS-KEY ( “pantoprazole sodium”)) | 9246 |
| #6 | ( TITLE-ABS-KEY ( “Dexlansoprazole”) OR TITLE-ABS-KEY ( “Lansoprazole, R-lsomer”) OR TITLE-ABS-KEY ( “R-lsomer Lansoprazole”) OR TITLE-ABS-KEY ( “2-{(R)-{(3-Methyl-4-(2,2,2- trifluoroethoxy)-2-pyridinyl)methyl)sulfi nyl)-lH-benzimidazole) OR R-Lansoprazole)” ) OR TITLE-ABS-KEY ( “R lansoprazole”) OR TITLE-ABS-KEY ( “Dexlansoprazole Sesquihydrate”) OR TITLE-ABS-KEY ( “TAK 390MR”) OR TITLE-ABS-KEY ( “TAK390MR”) OR TITLE-ABS-KEY ( ‘TAK- 390MR:’) OR TITLE-ABS-KEY (‘TAK-390”) OR TITLE-ABS-KEY (‘TAK 390”) OR TITLE-ABSKEY (‘TAK390”) OR TITLE-ABS-KEY ( “Dexilant”) OR TITLE-ABS-KEY ( “T-168390”) OR TITLEABS-KEY ( “T 168390” ) OR TITLE-ABS-KEY ( “Tl68390” ) ) | 359 |
| #7 | ( TITLE-ABS-KEY ( “IY-81149”) OR TITLE-ABS-KEY ( “IY81149”) OR TITLE-ABS-KEY ( “IY 81149”) OR TITLE-ABS.KEY ( “ilaprazole”)) | 167 |
| #8 | ( TITLE-ABS-KEY ( “Rabeprazole”) OR TITLE-ABS-KEY ( “2-((4-(3-methoxypropoxy)-3-methylpyridin-2-ylmethylsulfinyl}-lH-benzimidazole)”) OR TITLE-ABS-KEY ( “Dexrabeprazole”) OR TITLE-ABS-KEY ( “E 3810”) OR TITLE-ABS-KEY ( “E3810”) OR TITLE-ABS-KEY ( “Pariet”) OR TITLE-ABS-KEY ( “Rabeprazole Sodium”) OR TITLE-ABS-KEY ( “Sodium, Rabeprazole”) OR TITLE-ABS-KEY ( “lH-Benzimidazole, 2-(((4-{3- methoxypropoxy}-3-methyl-2-pyridinyl)methyl)sulfinyl)-, Sodium Salt”) OR TITLE-ABSKEY (‘Aciphex”) OR TITLE-ABS.KEY ( “LY-307640”) OR TITLE-ABS-KEY ( “LY 307640”) OR TITLE-ABS-KEY ( “LY307640”)) | 4938 |
| #9 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 | 108601 |
| #10 | ( TITLE-ABS-KEY ( “Inflammatory Bowel Disease”) OR TITLE-ABS-KEY ( “Bowel Diseases, Inflammatory”) OR TITLE-ABS-KEY ( “Ulcerative Colitis”) OR TITLE-ABS-KEY ( “Colitis Gravis”) OR TITLE-ABS-KEY ( “Inflammatory Bowel Disease, Ulcerative Colitis Type”) OR TITLE-ABS-KEY ( “Crohn’s Enteritis”) OR TITLE-ABS-KEY ( “Regional Enteritis”) OR TITLE-ABSKEY ( “Crohn’s Disease”) OR TITLE-ABS-KEY ( “Crohns Disease”) OR TITLE-ABS-KEY ( “Inflammatory Bowel Disease”) OR TITLE-ABS-KEY ( “Enteritis, Granulomatous”) OR TITLE-ABS-KEY ( “Granulomatous Enteritis”) OR TITLE-ABS-KEY (“Enteritis”) OR TITLE-ABSKEY ( “Regional lleocolitis”) OR TITLE-ABS-KEY ( “Colitis, Granulomatous”) OR TITLE-ABSKEY ( “Granulomatous Colitis”) OR TITLE-ABS-KEY ( “Ileitis, Terminal”) OR TITLE-ABS-KEY ( “Terminal Ileitis”) OR TITLE-ABS-KEY ( “Ileitis, Regional”) OR TITLE-ABS-KEY ( “Regional Ileitides”) OR TITLE-ABS-KEY ( “Regional Ileitis”)) | 177,263 |
| #11 | #9 AND #10 | 1829 |
Supplementary file S3.
Detailed methodological assessment of included studies.
| Question No. | Bonderup et al. | Esan et al. | Fernández-Bañares et al. | Keszthelyi et al. | Masclee et al. | Pascua et al. | Schwartz et al. | Verhaegh et al |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 5 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 13 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 22 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 26 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| 27 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (Out of 28) | 22 | 24 | 17 | 23 | 23 | 23 | 22 | 22 |