Abstract
Mycobacterium tuberculosis is associated with the activation of cytokine circuits both at sites of active tuberculosis in vivo and in cultures of mononuclear cells stimulated by M. tuberculosis or its components in vitro. Interactive stimulatory and/or inhibitory pathways are established between cytokines, which may result in potentiation or attenuation of the effects of each molecule on T-cell responses. Here we examined the interaction of transforming growth factor β1 (TGF-β1) and interleukin-10 (IL-10) in purified protein derivative (PPD)-stimulated human mononuclear cell cultures in vitro. TGF-β1 induced monocyte IL-10 (but not tumor necrosis factor alpha) production (by 70-fold, P < 0.02) and mRNA expression in the absence but not in the presence of PPD. Both exogenous recombinant (r) IL-10 and rTGF-β1 independently suppressed the production of PPD-induced gamma interferon (IFN-γ) in mononuclear cells from PPD skin test-positive individuals. Synergistic suppression of IFN-γ in cultures containing both rTGF-β1 and rIL-10 was only seen when the responder cell population were peripheral blood mononuclear cells (PBMC) and not monocyte-depleted mononuclear cells and when PBMC were pretreated with rTGF-β1 but not with rIL-10. Suppression of PPD-induced IFN-γ in PBMC containing both rTGF-β1 (1 ng/ml) and rIL-10 (100 pg/ml) was 1.5-fold higher (P < 0.05) than cultures containing TGF-β1 alone and 5.7-fold higher (P < 0.004) than cultures containing IL-10 alone. Also, neutralization of endogenous TGF-β1 and IL-10 together enhanced PPD-induced IFN-γ in PBMC in a synergistic manner. Thus, TGF-β1 and IL-10 together potentiate the downmodulatory effect on M. tuberculosis-induced T-cell production of IFN-γ, and TGF-β1 alone enhances IL-10 production. At sites of active M. tuberculosis infection, these interactions may be conducive to the suppression of mononuclear cell functions.
Cytokines play important roles in the regulation of host immune responses against intracellular pathogens, including Mycobacterium tuberculosis, by controlling the proliferation, differentiation, and effector functions of antigen-specific immune cells. Further, through autocrine and paracrine mechanisms, cytokines regulate the production and the biological effects of one another, thus augmenting or diminishing beneficiary or detrimental host responses towards infectious agents.
Active tuberculosis (TB) is associated with suppression of T-cell responses (12) and enhanced production and/or activity of immunosuppressive molecules such as transforming growth factor β1 (TGF-β1) and interleukin-10 (IL-10). TGF-β1 and IL-10 overlap with each other in many of their biological effects, including T-cell suppression, macrophage deactivation, modulation of proinflammatory cytokines, and interference with antigen-presenting-cell function (9, 23). In a study of TB patients from Karachi, Pakistan, neutralizing antibody to TGF-β1 normalized lymphocyte proliferation in response to purified protein derivative (PPD) and significantly increased PPD-induced production of gamma interferon (IFN-γ) in the peripheral blood mononuclear cells (PBMC) of patients with TB, but it had no effect on the PBMC of healthy PPD-reactive household contacts (HC) of patients (14). In addition, coculture with neutralizing antibody to IL-10 augmented T-cell proliferation to PPD in TB patients but not their HC. In contrast, stimulation of PBMC with PPD and the mycobacterial 30-kDa alpha antigen induced greater secretion of TGF-β, but not IL-10, in patients than HC. In recent studies of patients with active TB in Uganda, we have observed enhanced production of both IL-10 and TGF-β1 in PPD-activated PBMC culture supernatants from TB patients compared to healthy PPD skin test-reactive control subjects (12a). Further, coculture of PBMC from TB patients with neutralizing antibodies to either TGF-β1 or IL-10 significantly increased PPD-induced production of IFN-γ in TB patients. Whether TGF-β1 and IL-10 synergize with one another or function independently to enhance the suppression of the IFN-γ response in TB patients, however, is not known.
Recently, Maeda et al. showed that TGF-β enhanced production of IL-10 by peritoneal macrophages in both normal and tumor-bearing mice (15). However, an interaction between TGF-β and IL-10 in human mononuclear cell systems has not been investigated. M. tuberculosis and its constituents are strong inducers of a variety of cytokines by monocytes (25). In particular, TGF-β1 is induced by M. tuberculosis (13), its PPD (20), and its major cell wall lipoglycan, lipoarabinomannan (6). Further, TGF-β1 is present in human tuberculous granulomas (22).
In this study, we investigated the effect of TGF-β1 on the production of IL-10 and the interactions of these two cytokines on PPD-induced production of IFN-γ in vitro. We found that TGF-β1 specifically upregulated the production of IL-10 by human monocytes and that together TGF-β1 and IL-10 synergistically suppressed PPD-induced IFN-γ production in PBMC.
MATERIALS AND METHODS
Study subjects.
Twenty-two healthy volunteers were studied after informed consent was obtained. Ten subjects were PPD skin test positive, and twelve were PPD skin test negative. Among the participants in this study, 40% of the PPD skin test-reactive and 50% of the PPD skin test-nonreactive subjects were females. Subjects were drawn within 2 to 3 years of their PPD skin test examination.
Preparation of cells.
PBMC were prepared from whole heparinized blood by sedimentation over Ficoll-Paque (Pharmacia, Piscataway, N.J.) (4). To obtain monocytes (MN), PBMC were suspended at 107/ml in complete RPMI (BioWhittaker, Walkersville, Md.) containing penicillin (50 U/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), and HEPES (15 mM) and then incubated on plastic petri dishes (75 by 100 mm; Falcon Plastics, Oxnard, Calif.) that were precoated with 1 ml of pooled human serum (PHS) for 1 h at 37°C. To remove the nonadherent cells (NAC), the plates were washed three times with prewarmed RPMI. The adherent cell monolayers were then covered with cold phosphate-buffered saline (PBS) solution (BioWhittaker) and incubated at 4°C for 20 min. The adherent cells were gently scraped off the plates with a plastic scrapper and centrifuged at 250 × g for 10 min, and the resulting pellet was suspended at 106/ml in complete Iscove modified Dulbecco medium (IMDM) (BioWhittaker) (2 mM l-glutamine, 15 mM HEPES, penicillin [50 U/ml], streptomycin [50 mg/ml]) containing 1% PHS. Cells obtained in this manner were 90 to 94% peroxidase positive and 80 to 90% nonspecific esterase positive and contained <1% granulocytes as determined by Wright staining; these cells are referred to here as MN. The viability of MN as determined by exclusion of 0.2% trypan blue was >95% in all experiments. NAC were centrifuged at 250 × g for 10 min, and the resulting cell pellet was resuspended at 2 × 106 cells/ml in complete IMDM containing 1% PHS. NAC were shown to contain 1 to 2% MN by peroxidase staining.
Antigens, cytokines, and antibodies used.
PPD was purchased from the Statens Serum Institute (Copenhagen, Denmark) and was used at final concentrations of 5 and 20 μg/ml. Recombinant (r) TGF-β1, neutralizing chicken anti-TGF-β1 antibody, and chicken immunoglobulin Y were purchased from R&D Systems (Minneapolis, Minn.). rIL-10, neutralizing rat anti-human IL-10 antibody (clone JES3-9D7), and rat isotype control antibody were obtained from Pharmingen (San Diego, Calif.). The endotoxin content of all reagents as assessed by a chromogenic Limulus lysate assay (BioWhittaker) was <0.01 ng/mg of protein for PPD, the neutralizing antibodies, and isotype control antibodies and <15.0 ng/mg of protein for rTGF-β1 and rIL-10. To inactivate any residual lipopolysaccharide (LPS), polymyxin B (Sigma) was added to all cultures at 1 μg/ml. In preliminary experiments (n = 2), this concentration of polymyxin B reduced cytokine production by 30 ng of LPS per ml; tumor necrosis factor alpha (TNF-α) was reduced from 2.2 and 4.4 ng/ml to 0.7 and 0.9 ng/ml, respectively, and IL-10 was abrogated from 170 to 200 to 0 pg.
Generation of cytokine-containing supernatants.
PBMC (2 × 106/ml/well) in complete IMDM containing 1% PHS was incubated in 24-well tissue culture plates (Corning, Corning, N.Y.) with or without PPD. Culture supernatants were collected at 24 and 72 h of culture and stored frozen at −70°C until measurement of cytokine contents by enzyme-linked immunosorbent assay (ELISA). MN were seeded into 24-well tissue culture plates (106/ml/well) and preincubated for 24 h with or without rTGF-β1 (1 to 10 ng/ml) prior to the addition of PPD. Aliquots of the culture supernatant were collected at 24 and 72 h for assessment of cytokines by ELISA.
NAC diluted to 2 × 106 cells/ml in complete IMDM were incubated in 24-well tissue culture plates (2 × 106 cells/well) with or without rIL-10 (10 to 1,000 pg/ml) and TGF-β1 (0.1 to 10 ng/ml) in the presence or absence of PPD. Culture supernatants were collected at 72 h and stored at −70°C until assessed for cytokine content.
Immunoassay for cytokines.
TNF-α immunoreactivity was measured by using a pair of mouse monoclonal antibodies to TNF-α (Pharmingen) as the capture and detection antibodies. A standard curve was generated by using rTNF-α (Genzyme, Cambridge, Mass.) over a concentration range of 15.6 to 1,000 pg/ml. This assay is sensitive to 15.6 pg of TNF-α activity per ml. The IL-10 ELISA uses a pair of rat anti-human IL-10 antibodies as coating and capping antibodies (Pharmingen). A standard curve was generated by using rIL-10 (Pharmingen) over a concentration range of 15.6 to 1,000 pg/ml. This assay is sensitive to 15.6 pg of IL-10 activity per ml. IFN-γ immunoreactivity was assessed with a commercially available ELISA kit (Endogen, Boston, Mass.) with a sensitivity to 6.5 pg/ml.
Isolation and analysis of RNA.
Total RNA was extracted from MN or PBMC (5 × 106 to 10 × 106) by the guanidium-cesium method as described previously (19). For Northern blot analysis, 5 to 10 μg of RNA was electrophoretically separated through a 1% agarose–2.2 M formaldehyde gel containing ethidium bromide (0.5 mg/ml), transferred to nylon membranes, and cross-linked to the membranes by using UV light (Stratagene). Membranes were preincubated for at least 5 h in 1% fatty-acid-free bovine serum albumin (Sigma), 0.2 M sodium phosphate (pH 7.2), 3.75 M formamide, 0.001 M EDTA, and 7% sodium dodecyl sulfate (SDS) prior to hybridization with a buffer similar to the prehybridization solution containing a cDNA probe for human IL-10 (a 760-bp BglII-HindIII fragment from the IL-10 plasmid from the American Type Culture Collection, Rockville, Md.) or a riboprobe for human IFN-γ. Both probes were 32P labeled by random priming. Filters then were washed (1× SSC [0.15 M NaCl plus 0.015 M sodium citrate]–0.1% SDS at 55°C for 15 min) and exposed to Kodak XAR-5 film at −70°C with intensifying screens. To assess equal loading of RNA, each blot was stripped by treatment with 0.5× SSC–0.1% SDS and 50% formamide (65°C for 30 min) and then reprobed with a riboprobe for 18S rRNA (American Type Culture Collection).
Immunostaining for IL-10R.
PBMC were cultured in the absence or presence of rTGF-β1 (1 and 10 ng/ml) for 1 or 24 h. PBMC were then washed, resuspended in PBS, and divided into two tubes. One tube received biotin-conjugated rIL-10 (1 μl) (R&D Systems). After 60 min at 4°C, the PBMC were washed and received avidin-fluorescein isothiocyanate (FITC) (30 min at 4°C). The second (control) tube received avidin-FITC alone. PBMC were then analyzed by fluorescence-activated cell sorting (FACS); IL-10 staining of cells gated with characteristics of lymphocytes and MN were assessed separately.
Statistical analysis.
Data were analyzed by using the paired t test. A P value of <0.05 was considered significant.
RESULTS
Production of IL-10 and TNF-α by TGF-β1-pretreated MN.
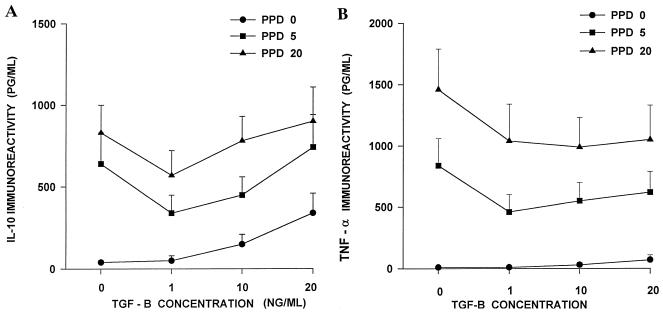
First, the effect of TGF-β1 on production of IL-10 and TNF-α by MN in the presence or absence of PPD of M. tuberculosis was examined. MN (106/ml) were preincubated for 24 h in complete IMDM alone or in medium containing rTCF-β1 (1, 10, and 20 ng/ml). Then, PPD (5 or 20 μg/ml) or medium was added to the cell cultures. An aliquot (50 μl) of culture supernatant was collected after 24 h for measurement of TNF-α, and the rest was collected at 72 h for IL-10 assessment. In preliminary work these time points were found to be optimal for the measurement of TNF-α and IL-10 by ELISA. In cultures without PPD, preincubation with rTGF-β1 induced IL-10 production in a dose-dependent manner (Fig. 1A). After preincubation with 20 ng of rTGF-β1 per ml, but not with 1 or 10 ng of rTGF-β1 per ml, significantly greater concentrations of IL-10 were present in MN cultures than in MN cultures without TGF-β1 (340 ± 120 versus 4 ± 1 pg/ml) (P < 0.05). In the presence of PPD, TGF-β1 showed a bimodal effect on IL-10 production (Fig. 1A). IL-10 concentrations were lower in cultures containing rTGF-β1 at 1 ng/ml plus PPD compared to cultures containing PPD alone. With higher amounts of rTGF-β1 (10 or 20 ng/ml), an increase in PPD-induced IL-10 concentration was seen. However, concentrations of IL-10 in PPD-activated MN cultures in the presence of rTGF-β1 (10 or 20 ng/ml) were not significantly different from those in the absence of TGF-β1. At all doses of TGF-β1, on the other hand, PPD-induced TNF-α levels were lower in wells with TGF-β1 than in the wells without it (Fig. 1B). Also, rTGF-β1 alone failed to induce the production of TNF-α in MN cultures. Therefore, the effect of TGF-β1 on production of IL-10 by MN is selective and does not include the upregulation of another monocyte cytokine, TNF-α.
FIG. 1.
Effect of TGF-β1 on IL-10 and TNF-α production by MN. MN were cultured in the absence or presence of rTGF-β1 (1 to 20 ng/ml), without or with PPD (5 and 20 μg/ml). Culture supernatants were assessed for IL-10 at 72 h (A) and TNF-α at 24 h (B) by ELISA. Data represent the mean ± the standard error of the mean from 10 separate experiments.
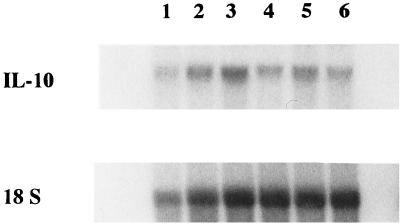
To assess whether increased TGF-β1-induced IL-10 production was also associated with increased expression of IL-10 mRNA, total RNA was extracted and analyzed by Northern blot analysis by using the IL-10 cDNA probe. Consistent with the cytokine ELISA data, TGF-β1 increased IL-10 mRNA expression modestly in the absence of PPD (Fig. 2). However, PPD-induced IL-10 expression was unaffected by TGF-β1.
FIG. 2.
Induction of IL-10 expression by TGF-β1. MN were cultured in the presence of medium alone (lanes 1 and 4) or with rTGF-β1 at 2 ng/ml (lanes 2 and 5) or 20 ng/ml (lanes 3 and 6). PPD (10 μg/ml) was added to lanes 4 to 6. Total RNA was assessed for the expression of IL-10 mRNA at 24 h by Northern analysis. A representative experiment (from three) is shown.
Effect of TGF-β1 and IL-10 on PPD-induced IFN-γ production.
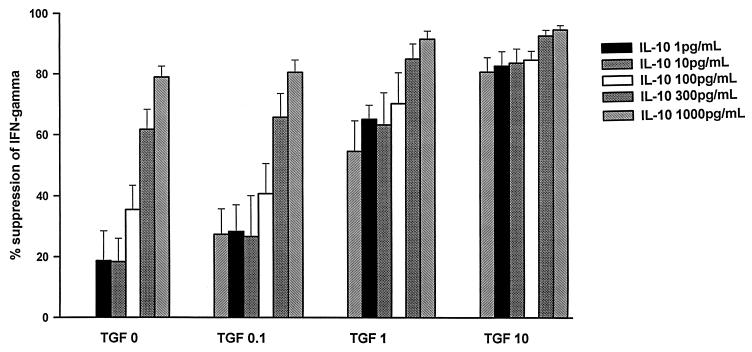
Next, we examined the interaction of IL-10 and TGF-β1 on PPD-induced T-cell production of IFN-γ. In preliminary experiments, PPD-induced IFN-γ production in PBMC and NAC was found to be optimal after 72 h of culture. NAC from PPD skin test-positive individuals were pretreated with IMDM alone or with IMDM containing rTGF-β1 (0.1, 1, or 10 ng/ml). After 24 h, PPD alone (5 μg/ml) or PPD together with rIL-10 (1 to 1,000 pg/ml) was added to the cultures. Supernatants were collected at 72 h after PPD stimulation for the assessment of IFN-γ immunoreactivity. The concentrations of cytokines used in this study bracket those found in M. tuberculosis antigen-stimulated PBMC cultures of TB patients (12a, 14). Suppression of IFN-γ in all culture supernatants was calculated in comparison to cultures of PPD-induced PBMC (or NAC). The IFN-γ immunoreactivity in cultures of NAC with PPD alone were assigned a 100% response and were not suppressed (0% suppression). In NAC cultures, a dose-dependent suppression of IFN-γ production was seen with either TGF-β1 or IL-10. IL-10 at 1 ng/ml showed an equivalent potency in suppression of IFN-γ compared to 10 ng of TGF-β1 per ml (Fig. 3). When the two cytokines were present together, an additive, but not synergistic, effect on suppression of PPD-induced IFN-γ production in NAC was observed. Further, even when NAC were precultured with rTGF-β1 or medium, washed, and then received PPD and IL-10, the suppressive effect of the two cytokines on IFN-γ production was additive but not synergistic (data not shown).
FIG. 3.
Effect of IL-10 and TGF-β on PPD-induced IFN-γ production by NAC. The NAC from PPD-reactive donors (n = 7) were precultured with rTGF-β1 (0.1 to 10 ng/ml) or medium for 24 h. Then, PPD (5 μg/ml) and rIL-10 (1 to 1,000 pg/ml) were added to the cultures. Culture supernatants were assessed for IFN-γ at 72 h by ELISA. The percent suppression of IFN-γ was calculated by assigning the IFN-γ in cultures that received PPD alone as the 100% response (0% suppression).
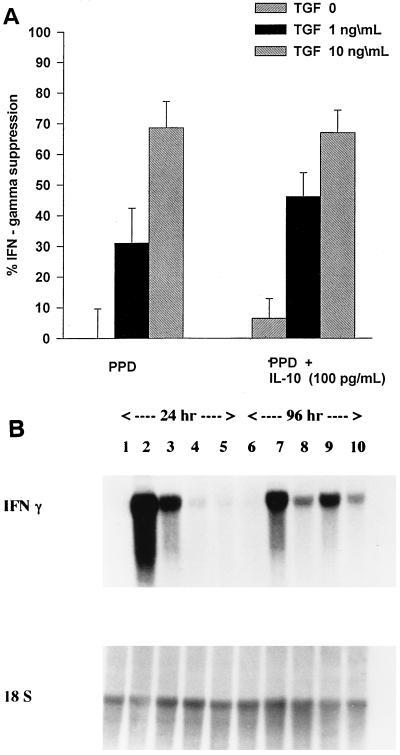
However, when PBMC were the responder population instead of NAC, a synergism of the two cytokines (TGF-β1 and IL-10) in the suppression of PPD-induced IFN-γ production was observed. In these experiments, PBMC were precultured with TGF-β1 (1 or 10 ng/ml) or medium for 24 h, and then PPD (5 μg/ml) with or without IL-10 (100 pg/ml) was added to cultures (Fig. 4A). Pretreatment with rTGF-β1 at 1 ng/ml, but not at 10 ng/ml, leads to a synergistic suppression of IFN-γ production when IL-10 (100 pg/ml) was added. Suppression of IFN-γ by rTGF-β1 (1 ng/ml) alone was at 30.9% (P < 0.05), and in the presence of both rTGF-β and rIL-10 (100 pg/ml) increased to 46% (P < 0.004). Suppression of IFN-γ by IL-10 (100 pg/ml) alone was ca. 8% (not significantly different than cultures with PPD alone). Pretreatment with IL-10 did not result in synergistic suppression of IFN-γ at any dose of TGF-β1 or IL-10 (data not shown).
FIG. 4.
Effect of IL-10 and TGF-β1 on PPD-induced IFN-γ production by PBMC. PBMC were precultured with rTGF-β1 (1 or 10 ng/ml) or medium for 24 h. Then, PPD (5 μg/ml) alone or together with rIL-10 (100 pg/ml) was added to the cultures. (A) Culture supernatants were assessed for IFN-γ immunoreactivity at 72 h, and the percentages of suppression of IFN-γ were calculated. (B) PBMC were cultured with medium alone (lanes 1 and 6), PPD (5 μg/ml) alone (lanes 2 and 7), PPD plus rTGF-β1 at 10 ng/ml (lanes 3 and 8), PPD plus rIL-10 at 100 pg/ml (lanes 4 and 9), and PPD plus both rTGF-β1 (10 ng/ml) and rIL-10 (100 pg/ml) (lanes 5 and 10). Total PBMC RNA was assessed for the expression of IFN-γ mRNA at 24 h (lanes 1 to 5) and at 96 h (lanes 6 to 10) by Northern analysis.
In three experiments, the effect of TGF-β1 with or without IL-10 on PPD-induced expression of IFN-γ mRNA in PBMC was examined. For this purpose, PBMC were precultured with rTGF-β1 (10 ng/ml) or in medium alone for 2 h, and then PPD (5 μg/ml) with or without rIL-10 (100 pg/ml) was added to the cultures. Total RNA was extracted from PBMC after 24 h and after 4 days (96 h) of culture and was assessed for expression of IFN-γ mRNA by Northern analysis. Figure 4B is a representative blot. The suppressive effect of IL-10 alone was seen only in early (24-h) cultures, whereas that of TGF-β1 was sustained (24 h and 4 days). At both time points (24 h and 4 days), the IFN-γ message was lowest in cultures containing both TGF-β1 and IL-10.
Effect of neutralization of endogenous TGF-β1 and IL-10 on PPD-induced IFN-γ production.
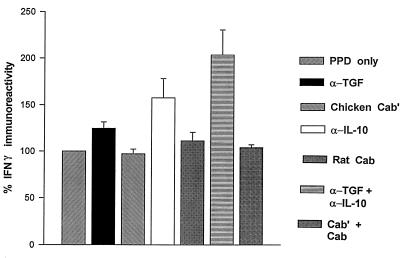
Next, we assessed the effects of neutralization of endogenous IL-10, TGF-β1, or both on PPD-induced IFN-γ production. In several experiments we found that the endogenous levels of TGF-β1 and IL-10 in PPD-induced PBMC cultures were 1 to 2 and 100 pg/ml, respectively (data not shown). PBMC from PPD-positive donors were cultured in complete IMDM with or without PPD (5 μg/ml) in the presence or absence of neutralizing antibodies to TGF-β1 (5 μg/ml), IL-10 (2 μg/ml), or both. In preliminary experiments, these amounts of neutralizing antibodies were found to abrogate the bioactivity of 5 ng of TGF-β1 per ml and the immunoreactivity of 300 pg of IL-10 per ml. Control experiments received the same amounts of respective isotype-matched control antibodies. Culture supernatants were collected at 72 h and assessed for IFN-γ immunoreactivity. Coculture with neutralizing antibody to TGF-β1 or IL-10 led to an increase in IFN-γ production above that in untreated PBMC cultures (Fig. 5); however, these improvements were not significantly different from PPD-induced IFN-γ production. When both antibodies were present, a synergistic effect on PPD-induced IFN-γ production was seen. PPD-induced IFN-γ levels doubled in cultures containing both neutralizing antibody to TGF-β1 and neutralizing antibody to IL-10 (P < 0.03).
FIG. 5.
Effect of endogenous IL-10 and TGF-β1 on PPD-induced IFN-γ production by PBMC. PBMC from nine separate donors were cultured with medium alone or with neutralizing antibody to TGF-β1 (5 μl), to IL-10 (2 μl), or to both cytokines. Control wells received isotype control antibodies for TGF-β1 (CAb), IL-10 (CAb′), or both (CAb plus CAb′). PPD (5 μg/ml) was added to all cultures. IFN-γ immunoreactivity was assessed in culture supernatants at 72 h. The percent increase in IFN-γ immunoreactivity compared to that of PPD alone (100%) is shown.
Effect of TGF-β on the expression of IL-10R in PBMC.
It was possible that the synergistic effect of TGF-β1 and IL-10 on the suppression of IFN-γ was secondary to upregulation of IL-10R expression on T cells or MN by TGF-β. Therefore, in some experiments (n = 4) we cultured PBMC in the absence or presence of rTGF-β1 (1 and 10 ng/ml) for 1 and 24 h and then performed immunostaining for IL-10R. For this purpose, biotin-conjugated rIL-10 (R&D Systems) and avidin-FITC were used. Control experiments received avidin-FITC alone. PBMC were then analyzed by FACS; IL-10 staining of cells gated with the characteristics of lymphocytes and MN were assessed separately. Table 1 shows the IL-10 staining results of PBMC at 24 h. IL-10 staining was observed on 77.9 ± 8.4% of the MN and was not affected by TGF-β1. Lymphocytes cultured with medium alone were 11.1 ± 2.6% (mean ± the standard deviation) IL-10R positive, and preincubation with TGF-β1 at 10 ng/ml increased the IL-10R reactivity to 17.1 ± 3.9%. However, these differences were not significant. No effect of TGF-β1 on IL-10R was observed at 1 h. Therefore, we conclude that TGF-β1 did not enhance the expression of IL-10R on mononuclear cells.
TABLE 1.
Effect of TGF-β on expression of IL-10R on mononuclear cells at 24 h
| Expt | % IL-10 staining with:
|
|||||
|---|---|---|---|---|---|---|
| Medium alone
|
TGF-β1 (1 ng/ml)
|
TGF-β1 (10 ng/ml)
|
||||
| T cell | MN | T cell | MN | T cell | MN | |
| 1 | 18.1 | 90.1 | 21.3 | 90.5 | 26.2 | 88.5 |
| 2 | 9.2 | 70.4 | 12.3 | 78.8 | 15.9 | 78.1 |
| 3 | 5.8 | 75.5 | 5.2 | 68.3 | 7.2 | 76.5 |
| 4 | 11.3 | 75.9 | 18.5 | 87.6 | 19.2 | 90.0 |
DISCUSSION
In humans, both TGF-β1 and IL-10 are predominantly the products of activated monocytes/macrophages (2, 10). Importantly, the immunomodulatory effects of these two cytokines overlap to a great degree with each other; both TGF-β1 and IL-10 suppress T-cell functions (1, 7) and deactivate macrophages (3). Findings from this study suggest that these two cytokines are synergistic in their inhibition of PPD-induced production of IFN-γ in mononuclear cells in PPD-sensitized subjects. Further, a moderate effect of TGF-β1 on the enhancement of production of IL-10 by MN was shown. Thus, TGF-β1 both directly and indirectly (through induction of IL-10) may be suppressive of T-cell functions and the deactivation of macrophages in M. tuberculosis infection. However, the contribution of these interactions to the host immune response during TB and at sites of active M. tuberculosis infection is not presently clear.
IL-10 is tightly regulated at the transcriptional level (10, 16). It has been shown that TNF-α upregulates LPS-induced IL-10 expression in mononuclear phagocytes (26) and that IL-10 downregulates its own transcription (10). Further, it has been shown that TGF-β1 enhances the production of IL-10 by macrophages in normal and tumor-bearing mice (15). In the present study, we observed a specific enhancing effect of TGF-β1 on the production of IL-10 by human MN. IL-10 levels increased by ca. 70-fold (from a mean of 4 to 340 pg/ml) (P < 0.05) in MN cultures that contained significant amounts of rTGF-β1 (20 ng/ml). This effect was transcriptional, since TGF-β1 also modestly increased IL-10 mRNA in MN. However, in PPD-induced cultures, the effect of TGF-β1 on IL-10 production, like its other biologic effects (24), was bimodal; at lower concentrations TGF-β1 decreased, and at higher concentrations it increased IL-10 in MN cultures. However, these modulations of IL-10 by TGF-β1 in the presence of PPD were not significant. Since IL-10 downregulates its own transcription, it is possible that PPD-induced IL-10 masked any enhancing effect of TGF-β1 on IL-10 production in the MN cultures. Interestingly, human alveolar macrophages are limited in the production of TGF-β1 compared to blood MN, but not IL-10 (21). At sites of M. tuberculosis infection, where newly recruited MN constitute a sizable proportion of the mononuclear cells (18), local TGF-β1 (22) may upregulate the production of IL-10 by lung mononuclear phagocytes, thus enhancing immunosuppressive circuits.
Further, we showed a synergistic effect of TGF-β1 and IL-10 on M. tuberculosis-induced IFN-γ production in PBMC (Fig. 4) but not in nonadherent cells (Fig. 3). Previous studies have shown that IL-10 synergizes with both IL-4 and TGF-β1 in the inhibition of macrophage cytotoxicity (17). This synergistic inhibitory effect on IFN-γ in PBMC was shown both when exogenous cytokines (rIL-10 and rTGF-β1) were added and when endogenous cytokines (both IL-10 and TGF-β1) were neutralized. Whether the expression of this synergistic effect is mediated by a cell type or cytokine present in PBMC but not NAC (e.g., MN) needs to be investigated. Clearly, the small (1 to 2%) contamination of NAC with MN allows optimal induction of IFN-γ in cultures. In NAC cultures the suppression of PPD-induced IFN-γ by either IL-10 or TGF-β1 follow independent dose responses, suggesting separate modes of suppressing T-cell responses. However, the conditions for the expression of synergistic suppression of PPD-induced IFN-γ production is not present in cultures of NAC alone but is present in PBMC cultures. These data suggest that the synergistic effect of the deactivating cytokines (IL-10 and TGF-β1) on T-cell IFN-γ production appears to involve an early event involving antigen presentation by MN. In this regard, both TGF-β1 and IL-10 interfere with antigen presentation by MN through the downregulation of class II major histocompatibility complex molecules (5, 10). In addition, IL-10 interferes with the expression of the macrophage costimulatory functions (8, 11). Further, TGF-β1 interferes with both early and late activation events of T cells upon stimulation (1). Therefore, it is possible that the synergistic effect of TGF-β1 and IL-10 in downmodulation of PPD-induced IFN-γ in PBMC is mediated through a combined effect on the components of the antigen-presenting-cell function of mononuclear phagocytes leading to T-cell activation.
However, it was also possible that the synergistic inhibition of IFN-γ by the deactivating cytokines was mediated through an enhancement of the IL-10 response. Therefore, we examined the effect of TGF-β1 on expression of IL-10R in PBMC in a limited number of experiments. TGF-β1 did not significantly upregulate the expression of IL-10R in MN or lymphocytes. Thus, the synergistic effect of IL-10 and TGF-β1 was not due to the upregulation of IL-10R. However, it is still possible that cell signalling pathways are enhanced in a synergistic manner in PBMC; this possibility needs to be investigated further.
In conclusion, the immunosuppressive activities of TGF-β1 and IL-10 may include both individual and combined components, depending on the concentrations and kinetics of expression of each of these two cytokines. At sites of active M. tuberculosis infection, this interaction may result in a predominant suppressive effect on mononuclear cell function.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (AI45244 and AI18471) and a grant from the U.S. Department of Veterans Affairs. C. Othieno was a recipient of a Fogarty grant award from the National Institutes of Health during 1995 and 1996.
REFERENCES
- 1.Ahuja S S, Paliogianni F, Yamada H, Balow J E, Boumpas D T. Effect of transforming growth factor-β on early and late activation events in human T cells. J Immunol. 1993;150:3109–3118. [PubMed] [Google Scholar]
- 2.Assoian R K, Fleurdelys B E, Stevenson H C, Miller P J, Madtes D K, Raine E W, Ross R, Sporn M B. Expression and secretion of type B transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020–6025. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor β and interleukin-10. J Biol Chem. 1992;267:23301–23308. [PubMed] [Google Scholar]
- 4.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig Suppl. 1968;97:9–29. [PubMed] [Google Scholar]
- 5.Czarniecki C W, Chiu H H, Wong G H W, McCabe S M, Palladino M A. Transforming growth factor-β1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988;140:4217–4224. [PubMed] [Google Scholar]
- 6.Dahl K E, Shiratsuchi H, Hamilton B, Ellner J J, Toossi Z. Selective induction of transforming growth factor β in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Prete G, De Carli M, Almerigogna F, Giudizi M G, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 8.de Waal-Malefyt R, Haanen J, Hergen S, Roncarolo M, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal-Malefyt R, Yssel H, Roncarolo M, Hergen S, de Vries J E. Interleukin-10. Curr Opin Immunol. 1992;4:314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 10.de Waal-Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Shevach E M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 12.Ellner J J. The human response in tuberculosis. J Infect Dis. 1997;176:1351–1359. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 12a.Hirsch, C. S. Unpublished data.
- 13.Hirsch C S, Yoneda T, Ellner J J, Averill L E, Toossi Z. Enhancement of intracellular growth of M. tuberculosis in human monocytes by transforming growth factor beta. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon-γ production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda H, Kusahara H, Ichimura Y, Ohtsuki M, Kurakata S, Shiraishi A. TGF-β enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- 16.Moore K W, O'Garra A, de Waal-Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 17.Oswald I P, Gazzinelli R T, Sher A, James S L. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 18.Shwander S K, Sada E, Torres M, Escobedo D, Sierra J G, Alt S, Rich E A. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis. 1996;173:1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 19.Toossi Z, Lapurga J P, Ondash R J, Sedor J R, Ellner J J. Expression of functional interleukin-2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J Clin Investig. 1990;85:1777–1784. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toossi Z, Young T, Averill L E, Hamilton B D, Shiratsuchi H, Ellner J J. Induction of transforming growth factor β1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–228. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toossi Z, Hirsch C S, Hamilton B D, Knuth C K, Friedlander M A, Rich E A. Decreased production of transforming growth factor β1 (TGF-β1) in human alveolar macrophages. J Immunol. 1996;156:3461–3468. [PubMed] [Google Scholar]
- 22.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of transforming growth factor-β (TGF-β) by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;54:465–473. [PubMed] [Google Scholar]
- 23.Wahl S M. Transforming growth factor beta: a cause and a cure. J Clin Immunol. 1992;2:61–71. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 24.Wahl S M, McCartney-Francism N, Allen J B, Dougherty E B, Dougherty S F. Macrophage production of TGF-β and regulation of TGF-β. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 25.Wallis R S, Ellner J J. Cytokines and tuberculosis. J Leukoc Biol. 1994;55:676–681. doi: 10.1002/jlb.55.5.676. [DOI] [PubMed] [Google Scholar]
- 26.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]