Abstract
Background and Objectives
Synaptic dysfunction and degeneration is a predominant feature of brain aging, and synaptic preservation buffers against Alzheimer disease (AD) protein-related brain atrophy. We tested whether CSF synaptic protein concentrations similarly moderate the effects of axonal injury, indexed by CSF neurofilament light [NfL]), on brain atrophy in clinically normal adults.
Methods
Clinically normal older adults enrolled in the observational Hillblom Aging Network study at the UCSF Memory and Aging Center completed baseline lumbar puncture and longitudinal brain MRI (mean scan [follow-up] = 2.6 [3.7 years]). CSF was assayed for synaptic proteins (synaptotagmin-1, synaptosomal-associated protein 25 [SNAP-25], neurogranin, growth-associated protein 43 [GAP-43]), axonal injury (NfL), and core AD biomarkers (ptau181/Aβ42 ratio; reflecting AD proteinopathy). Ten bilateral temporoparietal gray matter region of interest (ROIs) shown to be sensitive to clinical AD were summed to generate a composite temporoparietal ROI. Linear mixed-effects models tested statistical moderation of baseline synaptic proteins on baseline NfL-related temporoparietal trajectories, controlling for ptau181/Aβ42 ratios.
Results
Forty-six clinically normal older adults (mean age = 70 years; 43% female) were included. Synaptic proteins exhibited small to medium correlations with NfL (r range: 0.10–0.36). Higher baseline NfL, but not ptau181/Aβ42 ratios, predicted steeper temporoparietal atrophy (NfL × time: β = −0.08, p < 0.001; ptau181/Aβ42 × time: β = −0.02, p = 0.31). SNAP-25, neurogranin, and GAP-43 significantly moderated NfL-related atrophy trajectories (−0.07 ≤ β's ≥ −0.06, p's < 0.05) such that NfL was associated with temporoparietal atrophy at high (more abnormal) but not low (more normal) synaptic protein concentrations. At high NfL concentrations, atrophy trajectories were 1.5–4.5 times weaker when synaptic protein concentrations were low (β range: −0.21 to −0.07) than high (β range: −0.33 to −0.30).
Discussion
The association between baseline CSF NfL and longitudinal temporoparietal atrophy is accelerated by synaptic dysfunction and buffered by synaptic integrity. Beyond AD proteins, concurrent examination of in vivo axonal and synaptic biomarkers may improve detection of neural alterations that precede overt structural changes in AD-sensitive brain regions.
Neurodegeneration is commonly indexed using longitudinal brain atrophy on structural MRI, indicating a cumulative diminution of the neuropil.1 However, dynamic molecular alterations in neural integrity and functioning occur before observable atrophy on MRI.2 CSF markers that capture discrete components of neural structures, particularly axonal and synaptic proteins, have enhanced the characterization of these dysregulated neural pathways. Neurofilament light (NfL) is a well-studied marker of degeneration in large-caliber myelinated axons.3 More recent work highlights the important role of degeneration and dysfunction of the synapse through quantification of proteins reflecting presynaptic vesicular machinery (synaptosomal-associated protein-25 [SNAP-25] and synaptotagmin-1 [SYT-1]), postsynaptic calcium-mediated signaling pathway modulation (neurogranin), and axonal outgrowth regulation (growth-associated protein 43 [GAP-43]).4-7 As complementary markers of neural structure and function, joint modeling of NfL and synaptic proteins may facilitate our molecular understanding and detection of neural insults that precede overt structural brain changes in pathologies that target the axon and synapse.
CSF NfL and synaptic proteins are increasingly incorporated into brain aging and Alzheimer disease (AD) biomarker models to improve the prognostication of brain regions vulnerable to future atrophy.8,9 Independent of AD proteinopathy (CSF Aβ42 and ptau181), higher concentrations of both CSF NfL and synaptic proteins (reflecting greater axonal and synaptic damage, respectively) predict global and hippocampal volume loss in mild cognitive impairment (MCI)10,11 and steeper hippocampal atrophy, memory decline, and future conversion to MCI in cognitively unimpaired individuals.4,11-16
While synaptic dysfunction tracks with worsening progression of AD and other neurodegenerative diseases, mounting evidence highlights synaptic preservation as a core component of cognitive resilience, particularly against AD proteinopathy. In autopsy studies of individuals with pathologic AD, those who exhibited more normal synaptic protein levels were more likely to be cognitively unimpaired at death than those with synaptic failure despite similar burden of AD pathology.17,18 We recently reported that the deleterious effects of abnormal CSF AD proteins on the medial temporal lobe and total gray matter volumes were detected only in those with high (more abnormal) CSF synaptic protein levels, even in clinically normal adults.19 A longitudinal analysis similarly demonstrated that the association between low baseline CSF Aβ42 on temporal lobe atrophy and cortical thinning was moderated by baseline CSF neurogranin,20 a postsynaptic protein involved in hippocampal and neocortical plasticity.
Although maintained synaptic integrity may attenuate adverse associations of Aβ42 and ptau181 with structural neuroimaging, how synaptic processes potentially buffer and/or contribute to early axonal changes and atrophy rates is unclear. In a cohort of cognitively unimpaired adults, we examined the relationship between baseline CSF NfL and longitudinal gray matter atrophy. We then evaluated the moderating role of synaptic integrity on this relationship, using a CSF panel of presynaptic and postsynaptic proteins. We hypothesized that NfL-related declines in gray matter volumes would be attenuated at lower levels of synaptic dysfunction, even when accounting for AD pathology (CSF ptau181/Aβ42 ratio).
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the UCSF Committee on Human Research. All participants provided written informed consent to study procedures.
Participants
Participants were 46 community-dwelling older adults enrolled in the observational Hillblom Longitudinal Aging Network study at the UCSF Memory and Aging Center. Participants underwent comprehensive neurologic and neuropsychologic evaluations, as well as a study partner interview to determine neurobehavioral status. All participants were reviewed and classified as clinically normal according to a consensus case conference with a board-certified neurologist and board-certified neuropsychologist and were deemed functionally intact based on the structured clinical interview with a study partner (Clinical Dementia Rating global score = 0). Participants were included in this analysis if they completed a baseline lumbar puncture and underwent neuroimaging visits, which occurred roughly 15–18 months apart. Study visits included in this analysis took place between October 2010 and January 2019. Of the 587 actively enrolled Hillblom Aging Network participants during this period, 68 completed a baseline lumbar puncture; 54 of those 68 had complete CSF biomarker data available for primary variables used in this analysis; and 46 of those 54 underwent neuroimaging visits. Given that the Hillblom Aging Network is an active longitudinal study with rolling enrollment, the number of neuroimaging visits per participant included in this analysis ranged from 1 (baseline only) to 6, with a mean number of 2.7 study visits per participant. Similarly, the duration between baseline lumbar puncture and final neuroimaging visit ranged from zero years (baseline only; n = 5) to 8.7 years, with a mean duration between baseline lumbar puncture and final neuroimaging visit of 3.7 years.
CSF Assays
Lumbar punctures were performed in the morning after a 12-hour fast, and CSF was collected, processed, and stored according to standard protocols.4 CSF Aβ42, Aβ40, and ptau181 were assayed using the Lumipulse platform, and the ptau181/Aβ42 ratio was selected to model AD-related proteinopathy in analyses.21 Assay methods for quantification of synaptic proteins (neurogranin, GAP-43, SNAP-25, and SYT-1) are described in detail elsewhere.19 CSF NfL was assayed using an in-house ELISA, also described elsewhere.22 For analysis, synaptic markers and ptau181/Aβ42 ratios were log10-transformed to improve distributions, and all CSF markers were standardized to Z-scores to facilitate interpretation of results.
Neuroimaging
Participants underwent longitudinal structural magnetic resonance imaging (MRI) at the UCSF Neuroscience Imaging Center using either a Siemens Trio Tim or Prisma Fit 3T scanner. Magnetization-prepared rapid gradient-echo sequences were used to obtain whole-brain T1-weighted images sagittally using the following parameters: repetition time = 2300 ms; inversion time = 900 ms; echo time = 2.98 ms; flip angle = 9°; and field of view = 240 × 256 mm with 1 × 1 mm in-plane resolution and 1-mm slice thickness. Parameters for both Trio and Prisma scanners had nearly identical parameters but slightly different echo times (Trio: 2.98 ms; Prisma: 2.9 ms).
Before processing, all T1-weighted images were visually inspected for quality control and those with excessive motion or image artifact were excluded. Magnetic field bias was corrected using the N3 algorithm.23 Tissue segmentation was performed using unified segmentation in SPM12.24 All segmentations were carefully inspected to ensure robustness of the process. Each participant's native space gray matter segmentation was normalized and modulated, by nonlinear and rigid-body transformations, to study-specific template space using DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra25). A Gaussian kernel of 4-mm full width half maximum was applied for smoothing of images. Transformations (linear and nonlinear) between DARTEL's space and International Consortium for Brain Mapping (ICBM) space were conducted to enable statistical comparisons.26 Finally, brain volumes of interest were quantified by translating a standard parcellation atlas27 into ICBM space and summing the gray matter within each region of interest (ROI).
For our primary outcome, we computed a composite ROI that summed bilateral gray matter volumes of the 10 atlas ROIs involving temporoparietal structures previously reported to be vulnerable to AD28: hippocampus, entorhinal cortex, parahippocampal gyrus, amygdala, fusiform gyrus, middle temporal, inferior temporal, temporal pole, precuneus, and inferior parietal. The total intracranial volume was also computed for each participant as the sum of total gray matter, white matter, and CSF volumes.
Statistical Analysis
We first examined Pearson correlations among Aβ42/40, ptau181, NfL, and synaptic proteins for descriptive purposes. Next, a series of linear mixed-effects models with random slopes and intercepts analyzed longitudinal temporoparietal volumetric changes by including years since baseline lumbar puncture visit (time) as a fixed and random effect, covarying for baseline age, sex, APOE status, intracranial volume, and scanner. To determine the relative contributions of baseline NfL and AD proteinopathy to temporoparietal trajectories, we tested interactions of NfL and ptau181/Aβ42 ratios with time (NfL × time, ptau181/Aβ42 × time), both separately and in a combined model. To address our primary aim of examining the moderating role of synaptic integrity in NfL-related temporoparietal volumetric change, separate models entered each synaptic protein as a moderator of the NfL × time interaction on temporoparietal trajectories (i.e., SNAP-25 × NfL × time, SYT-1 × NfL × time, neurogranin × NfL × time, GAP-43 × NfL × time), controlling for ptau181/Aβ42 ratios. The false discovery rate was set to 5% to account for multiple comparisons in these primary analyses examining synaptic protein moderation of NfL-related temporoparietal trajectories. To probe significant moderation effects of synaptic proteins, we calculated the effects of time on temporoparietal volumes when synaptic protein and NfL levels were 1 standard deviation above (z = 1) and below (z = −1) sample mean levels. To mitigate potential instability of model estimates because of our relatively small sample, we estimated 95% confidence intervals of standardized model coefficients using bootstrapping with 1,000 samples. All LME models were conducted using the lme4 package in R.
Data Availability
Anonymized, deidentified data from this report will be made available on request from any qualified investigator. UCSF Memory and Aging Center data requests can be sent to the corresponding author.
Results
Table 1 presents descriptive statistics for the study sample at baseline. Similar to our cross-sectional report,19 participants were on average aged 70 years (baseline age range = 53–86 years) with 17.3 years of education, 57% male, 39% APOE ε4+, and cognitively unimpaired (Mini-Mental State Examination [MMSE]; mean = 29/30). This sample was comparable with individuals in the larger Hillblom cohort who were not included in this analysis (n = 541) for age, education, and MMSE (all p's >0.05), but did have a higher proportion of men (57% vs 41%, p = 0.030) and APOE ε4+ (39% vs 22%, p = 0.001).
Table 1.
Baseline Characteristics (N = 46)
NfL Correlations With CSF Synaptic and AD Proteins
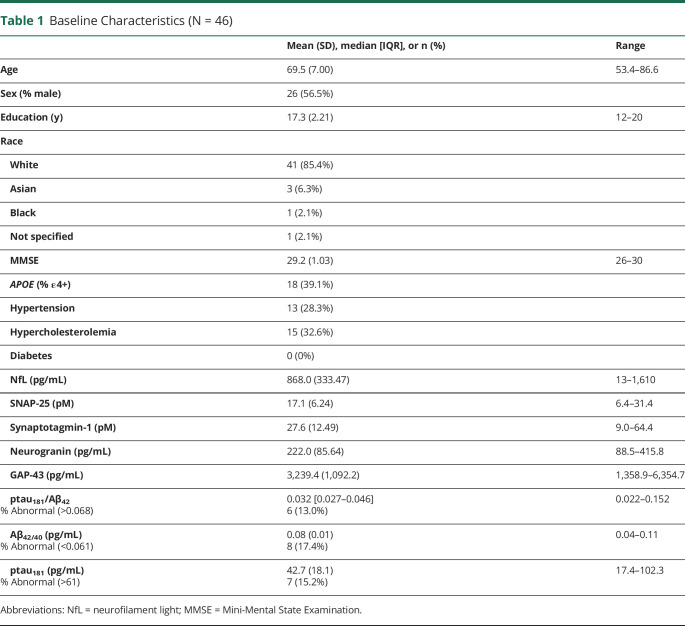
Figure 1 presents NfL correlations with asynaptic proteins. The associations between NfL and synaptic proteins ranged from small, positive, and statistically nonsignificant (SYT-1: r = 0.10, p = 0.507; Ng: r = 0.23, p = 0.120) to medium, positive, and statistically significant (SNAP-25: r = 0.32, p = 0.030; GAP-43: r = 0.36, p = 0.015). For AD proteins, NfL exhibited a medium, positive, and statistically significant association with ptau181/Aβ42 ratios (r = 0.35, p = 0.016). Our recent report extensively characterized the associations between AD and synaptic proteins in this cohort.19 Briefly, synaptic and AD proteins exhibited significant associations whereby the highest levels of AD proteinopathy (low Aβ42/40 and high ptau181) were observed at the highest levels of synaptic dysfunction.
Figure 1. CSF NfL Correlations With CSF Synaptic Proteins.
Abbreviations: NfL = neurofilament light.
Baseline NfL and AD Proteinopathy Effects on Gray Matter Trajectories
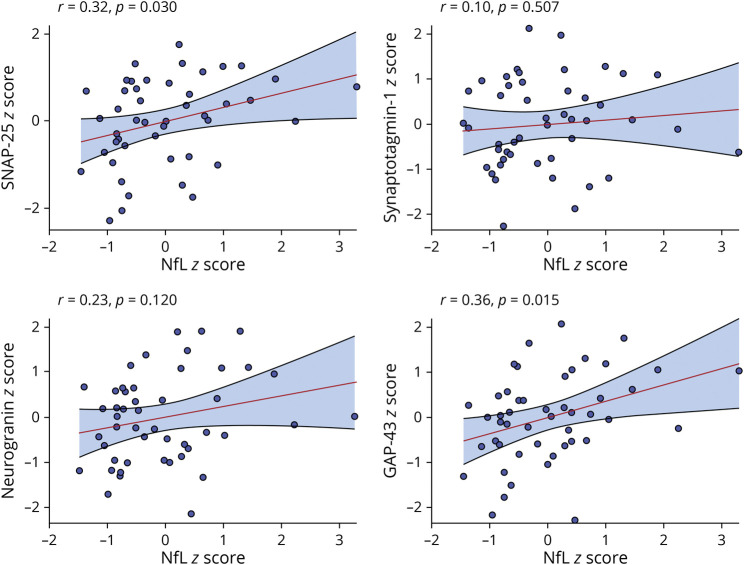
Time was significantly associated with declining temporoparietal volumes (β [95% CI] = −0.20 [−0.25 to −0.15]); p < 0.001). Figure 2 displays the effects of baseline NfL and ptau181/Aβ42 ratios on temporoparietal trajectories. The negative slope of time on temporoparietal volumes was significantly moderated by both baseline NfL (p < 0.001) and baseline ptau181/Aβ42 ratios (p = 0.028) in separate models; however, only NfL remained a significant moderator of temporoparietal trajectories when both terms were entered in a combined model (NfL × time: β [95% CI] = −0.08 [−0.12 to −0.04]; p < 0.001; ptau181/Aβ42 × time: β [95% CI] = −0.02 [−0.06 to 0.01]; p = 0.252). Specifically, the negative slope of time on temporoparietal volumes was steeper at higher (+1SD) levels of NfL (time: β [95% CI] = −0.26 [−0.31 to −0.21]) compared with lower (-1SD) levels of NfL (time: β [95% CI] = −0.11 [−0.17 to −0.05]). By contrast, the slope of time on temporoparietal volumes did not differ when the ptau181/Aβ42 ratio was high (time: β [95% CI] = −0.20 [−0.27 to −0.15]) vs low (time: β [95% CI] = −0.16 [−0.22 to −0.11]).
Figure 2. CSF NfL and ptau181/Aβ42 Effects on Gray Matter (Temporoparietal) Trajectories.
All interactions were modeled continuously—biomarker levels split into ± 1 standard deviation for illustration purposes. Abbreviations: NfL = neurofilament light.
Synaptic Moderation of NfL-Related Gray Matter ROI Trajectories
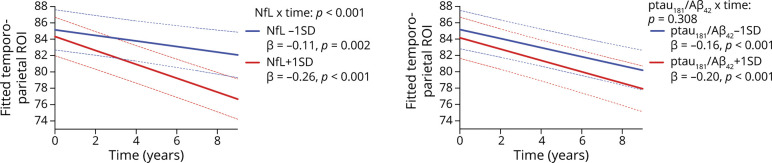
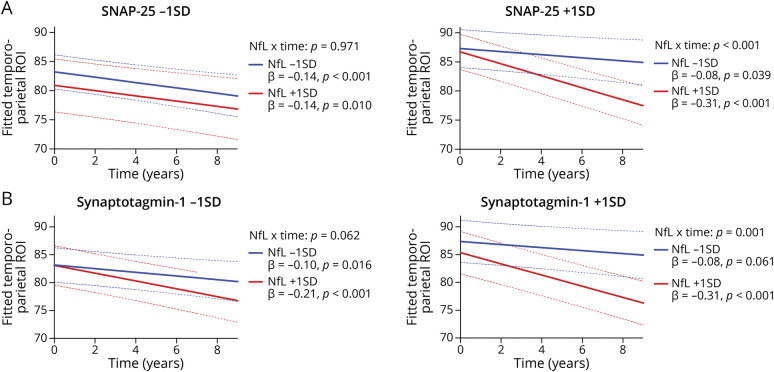
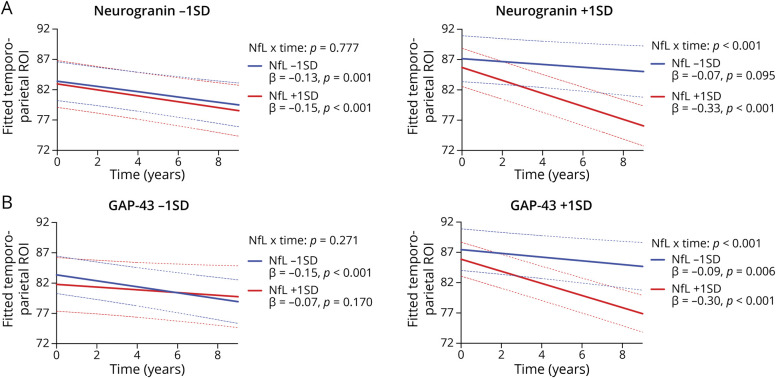
Controlling for ptau181/Aβ42 ratios, primary analyses entered synaptic proteins as moderators of NfL-related temporoparietal trajectories (Figures 3 and 4). The NfL × time interaction on temporoparietal volumes was significantly moderated by SNAP-25 (NfL × SNAP-25 × time: β [95% CI] = −0.06 [−0.10 to −0.02]; p = 0.011, FDR-adjusted p = 0.015), neurogranin (NfL × neurogranin × time: β [95% CI] = −0.06 [−0.10 to −0.02]; p = 0.010, FDR-adjusted p = 0.015), and GAP-43 (NfL × GAP-43 × time: β [95% CI] = −0.07 [−0.12 to −0.03]; p = 0.001, FDR-adjusted p = 0.004). Specifically, the deleterious effect of NfL on temporoparietal trajectories was observed at higher (more abnormal) synaptic protein levels, but not at lower (more normal) synaptic protein levels. The moderating effect of SYT-1 on the NfL × time interaction was not statistically significant (NfL × SYT-1 × time: β [95% CI] = −0.03 [−0.07 to −0.02]; p = 0.258), although a similar pattern was observed. As displayed in Figure 3, the magnitude of the slope of time on temporoparietal volumes was steepest when NfL and synaptic levels were high (β range: −0.33 to −0.30), whereas slopes were approximately 1.5–4.5 times weaker when NfL and/or synaptic levels were low (β range: −0.21 to −0.07).
Figure 3. CSF NfL Effect on Gray Matter (Temporoparietal) Trajectories Stratified by CSF SNAP-25 (A) and Synaptotagmin-1 (B).
All interactions were modeled continuously—biomarker levels split into ± 1 standard deviation for illustration purposes. Abbreviations: NfL = neurofilament light.
Figure 4. CSF NfL Effect on Gray Matter (Temporoparietal) Trajectories Stratified by CSF Neurogranin (A) and GAP-43 (B).
All interactions were modeled continuously—biomarker levels split into ± 1 standard deviation for illustration purposes. Abbreviations: NfL = neurofilament light; GAP-43 = growth-associated protein 43
Discussion
In our cohort of clinically normal older adults, higher baseline CSF NfL levels were associated with faster longitudinal atrophy in temporoparietal regions, but this relationship depended on the synaptic state. The combination of axonal injury (higher CSF NfL) and synaptic dysfunction (higher CSF SNAP-25, neurogranin, or GAP-43) predicted the steepest atrophy rates, whereas the association between baseline axonal injury and temporoparietal atrophy was markedly attenuated at low baseline levels of synaptic dysfunction. This pattern of synaptic moderation was statistically robust to AD proteinopathy (CSF ptau181/Aβ42 ratio) and was detected across presynaptic and postsynaptic proteins. Correlations between synaptic proteins and NfL were relatively modest, suggesting that synaptic dysfunction and axonal injury are dissociable yet complementary aspects of neurodegeneration. Axonal breakdown in the absence of synaptic dysfunction may not strongly relate to brain atrophy, or framed differently, maintenance of synaptic integrity may buffer neurodegenerative effects of axonal breakdown. Beyond traditional AD proteins, concurrent examination of axonal and synaptic markers informs the prediction of structural decline in AD-vulnerable brain regions in clinically normal adults.
Our observation that baseline NfL was more strongly linked to temporoparietal atrophy than ptau181/Aβ42 ratios parallels a previous study reporting CSF NfL as a stronger predictor of hippocampal atrophy than CSF Aβ42 and ptau181 in a similar cohort solely comprising cognitively unimpaired older adults.12 Studies across the clinical AD spectrum show that NfL concentrations are associated with neurodegeneration in brain networks vulnerable to AD, including temporoparietal cortical thinning,29 declining cingulum microstructural integrity,20 and hippocampal atrophy with ventricular expansion.30 These studies also demonstrate that NfL predicts structural changes, irrespective of Aβ burden, supporting conclusions that NfL associations with neurodegeneration are likely nonspecific to AD pathology. However, a recent translational study reported Aβ-induced increases in CSF NfL corresponded to reduced parietotemporal cortex and hippocampal density in an early-stage AD animal model. The Aβ-specific effects of NfL in the same brain regions were replicated with neuroimaging data in humans.31 Thus, axonal injury as indexed by CSF NfL, although likely not AD-specific, prognosticates atrophy in brain regions sensitive to AD.
The “dying-back” hypothesis of neurodegeneration in AD posits that synaptic loss and axonal disconnection precede somatic cell death.32 Compatible with this notion, our data demonstrate synergistic effects of baseline synaptic proteins and NfL on longitudinal temporoparietal atrophy. Furthermore, the adverse impact of axonal injury on gray matter volumetric change was substantially buffered at lower (more normal) concentrations of CSF synaptic proteins, which encompassed proteins from presynaptic and postsynaptic compartments. To date, neurogranin is the most widely studied synaptic protein in human CSF AD biomarker studies. Lower CSF neurogranin has been shown to attenuate Aβ-related structural brain changes,20,29,30 likely because of its critical involvement in synaptic plasticity and regeneration through modulation of calmodulin in dendritic spines.33 GAP-43, although less studied than neurogranin, also facilitates long-term potentiation and synaptogenesis in medial temporal and neocortical structures.9,34,35 GAP-43 is also enriched at axon terminals and supports axonal regeneration,36 which may underlie its moderation of NfL-related atrophy and explain its significant correlation with NfL. SNAP-25, in coordination with other SNAP receptor (SNARE) complex proteins, governs presynaptic vesicular trafficking and also fast membrane transport to the axonal growth cone,37 a process that is disrupted during dying-back neurodegeneration.32,38 As a presynaptic vesicle cargo molecule, SYT-1 mediates neurotransmitter release in the hippocampus.39,40 In addition to supporting presynaptic homeostasis, some data suggest that SYT-1 facilitates axonal growth processes during neurodevelopment,41,42 although its role in axonal degeneration is unclear. Collectively, the observed synaptic-dependent associations between NfL and gray matter volumes are consistent with the known role of synaptic signaling in axonal regeneration and importantly add to the growing literature highlighting the synapse as a salient indicator of risk/resilience to neuropathology.19,43
In addition to synaptic-related effects on axonal function, NfL may also have a direct influence on synaptic function. Neurofilaments are predominantly involved in axonal scaffolding; however, they also support dendritic branching.44 Mouse knockout models show NfL-dependent modulation of synaptic neurotransmission and long-term potentiation in the hippocampus.45 Thus, the utility of joint elevations in CSF NfL and synaptic proteins in prognosticating gray matter atrophy could reflect multiple aspects of early synaptic dysfunction, in addition to axonal degradation, that ultimately progress toward gross atrophy. Future cellular work aimed at dissecting the differential roles of neurofilaments at synapses and axons, in the context of both AD and non-AD pathologies, may help clarify the mechanisms by which synaptic proteins mitigate NfL-related neurodegeneration.
Our findings should be interpreted in light of several limitations. Although our longitudinal study design and implementation of bootstrapped confidence intervals helps mitigate statistical power issues related to our relatively small study sample, we are still limited in our ability to estimate more complex model terms (e.g., 4-way interactions: AD proteins × NfL × synaptic proteins × time). Our longitudinal MRI data allowed modeling of atrophy trajectories, but lumbar punctures were only performed cross-sectionally. Repeated CSF collection would facilitate the temporal characterization of NfL and synaptic protein trajectories, alongside AD protein accumulation and structural MRI changes. Although temporoparietal volumes capture a classic neuroanatomic signature, particularly vulnerable to AD pathology, and CSF synaptic proteins are sensitive to AD, variance in these biomarkers in cognitively unimpaired adults may not exclusively signal AD-specific mechanisms, particularly when considering their dynamic interplay with NfL. Thus, our findings may reflect processes relevant to an AD neurobiological phenotype, but we cannot definitively rule out a role for non-AD pathologies contributing to observed volumetric changes. Although the higher proportion of men and APOE 4 carriers in our CSF subcohort, relative to the larger Hillblom cohort, may reflect increased motivation for these individuals to participate in lumbar puncture, our study data cannot definitively address this hypothesis. Furthermore, our study sample represents a relatively homogenous cohort, which necessitates replication across more demographically and socioeconomically diverse individuals who may possess different risk and resilience factors for brain health.
Previous studies demonstrate synaptic moderation of the negative effects of CSF AD proteins on brain health.19,20,30 Our results build on this finding and provide novel evidence that synaptic processes further moderate the relationship between CSF NfL and longitudinal brain atrophy in cognitively unimpaired adults. Taken together, our findings further implicate synaptic dysfunction as an accelerant of brain atrophy and synaptic integrity as a buffer against neurodegeneration in temporoparietal structures commonly targeted by AD. Future behavioral and/or pharmacologic interventions may consider inclusion of axonal and synaptic functioning markers that reflect relevant biological processes underlying neurodegenerative changes and possible treatment responsiveness.
Glossary
- AD
Alzheimer disease
- GAP-43
growth-associated protein 43
- NfL
neurofilament light
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- ROI
region of interest
- SNAP-25
synaptosomal-associated protein 25
- SYT-1
synaptotagmin-1
Appendix. Authors
Study Funding
This study was supported by NIH-NIA grants K23AG058752 (PI: K.B.C.), R01AG072475 (PI: K.B.C.), R01AG032289 (PI: JHK), R01AG048234 (PI: J.H.K.), F31AG064989 (PI: RS), and UCSF ADRC P30AG062422 (PI: BLM). Our work was also supported by the Larry L. Hillblom Network Grant (2014-A-004-NET; PI: J.H.K.) and the Alzheimer's Association (AARG-20-683875, PI: K.B.C.). H. Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018–02532); the European Research Council (#681712); Swedish State Support for Clinical Research (#ALFGBG-720931); the Alzheimer Drug Discovery Foundation (ADDF), United States (#201809–2016862); and the UK Dementia Research Institute at UCL. K. Blennow is supported by the Swedish Research Council (#2017-00915); the Swedish Alzheimer Foundation (#AF-930351, #AF-939721, and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006); the Swedish state under the agreement between the Swedish Government and the County Councils; the ALF agreement (#ALFGBG-715986 and #ALFGBG-965240); the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236); and the Alzheimer's Association 2021 Zenith Award (ZEN-21-848495).
Disclosures
H. Zetterberg has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). The remaining authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2(7):a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. [DOI] [PubMed] [Google Scholar]
- 4.Casaletto KB, Elahi FM, Bettcher BM, et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017;89(17):1782-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke MTM, Brinkmalm A, Foiani MS, et al. CSF synaptic protein concentrations are raised in those with atypical Alzheimer's disease but not frontotemporal dementia. Alzheimer's Res Ther. 2019;11(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milà-Alomà M, Brinkmalm A, Ashton NJ, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and pet: a cross-sectional study. Neurology. 97(21):e2065-e2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tible M, Å Sandelius, Höglund K, et al. Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology. 2020;95(8):e953. [DOI] [PubMed] [Google Scholar]
- 8.Park SA, Han SM, Kim CE. New fluid biomarkers tracking non-amyloid-β and non-tau pathology in Alzheimer's disease. Exp Mol Med. 2020;52(4):556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camporesi E, Nilsson J, Brinkmalm A, et al. Fluid biomarkers for synaptic dysfunction and loss. Biomarker Insights. 2020;15:1177271920950319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zetterberg H, Skillbäck T, Mattsson N, et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 2016;73(1):60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Portelius E, Zetterberg H, Skillbäck T, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain. 2015;138(pt 11):3373-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idland A-V, Sala-Llonch R, Borza T, et al. CSF neurofilament light levels predict hippocampal atrophy in cognitively healthy older adults. Neurobiol Aging. 2017;49(4):138-144. [DOI] [PubMed] [Google Scholar]
- 13.Kern S, Syrjanen JA, Blennow K, et al. Association of cerebrospinal fluid neurofilament light protein with risk of mild cognitive impairment among individuals without cognitive impairment. JAMA Neurol. 2019;76(2):187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merluzzi AP, Carlsson CM, Johnson SC, et al. Neurodegeneration, synaptic dysfunction, and gliosis are phenotypic of Alzheimer dementia. Neurology. 2018;91(5):e436-e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarawneh R, D'Angelo G, Crimmins D, et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 2016;73(5):561-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Therriault J, Kang MS, et al. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer's disease. Alzheimer's Res Ther. 2018;10(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boros BD, Greathouse KM, Gentry EG, et al. Dendritic spines provide cognitive resilience against Alzheimer's disease. Ann Neurol. 2017;82(4):602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold SE, Louneva N, Cao K, et al. Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer's disease. Neurobiol Aging. 2013;34(1):157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casaletto KB, Zetterberg H, Blennow K, et al. Tripartite relationship among synaptic, amyloid, and tau proteins. Neurology. 2021;97(3):e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielke MM, Przybelski SA, Lesnick TG, et al. Comparison of CSF neurofilament light chain, neurogranin, and tau to MRI markers. Alzheimer's Demen. 2021;17(5):801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell MR, Ashrafzadeh-Kian S, Petersen RC, et al. P-tau/Aβ42 and Aβ42/40 ratios in CSF are equally predictive of amyloid PET status. Alzheimer's & Dementia: Diagnosis, Assess Dis Monit. 2021;13(1):e12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaetani L, Höglund K, Parnetti L, et al. A new enzyme-linked immunosorbent assay for neurofilament light in cerebrospinal fluid: analytical validation and clinical evaluation. Alzheimer's Res Ther. 2018;10(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87-97. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839-851. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95-113. [DOI] [PubMed] [Google Scholar]
- 26.Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. Neuroimage. 1995;2(2):89-101. [DOI] [PubMed] [Google Scholar]
- 27.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer's disease severity. Neuroimage Clin. 2016;11:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira JB, Westman E, Hansson O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer's disease. Neurobiol Aging. 2017;58:14-29. [DOI] [PubMed] [Google Scholar]
- 30.Mattsson N, Insel PS, Palmqvist S, et al. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer's disease. EMBO Mol Med. 2016;8(10):1184-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang MS, Aliaga AA, Shin M, et al. Amyloid-beta modulates the association between neurofilament light chain and brain atrophy in Alzheimer's disease. Mol Psychiatry. 26(10):5989-6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA. Axonal degeneration in Alzheimer's disease: when signaling abnormalities meet the axonal transport system. Exp Neurol. 2013;246:44-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerendasy DD, Gregor Sutcliffe J. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Mol Neurobiol. 1997;15(2):131-163. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosciences. 1997;20(2):84-91. [DOI] [PubMed] [Google Scholar]
- 35.Dhiman K, Villemagne VLL, Eratne D, et al. Elevated levels of synaptic protein GAP-43 associate with brain tauopathy, atrophy and cognition in Alzheimer's disease. Alzheimer's Demen. 2020;16:e044098. [Google Scholar]
- 36.Chung D, Shum A, Caraveo G. GAP-43 and BASP1 in axon regeneration: implications for the treatment of neurodegenerative diseases. Front Cel Develop Biol. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ulloa F, Cotrufo T, Ricolo D, Soriano E, Araújo SJ. SNARE complex in axonal guidance and neuroregeneration. Neural Regen Res. 2018;13(3):386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muresan V, Muresan Z. Is abnormal axonal transport a cause, a contributing factor or a consequence of the neuronal pathology in Alzheimer's disease? Future Neurol. 2009;4(6):761-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Öhrfelt A, Brinkmalm A, Dumurgier J, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer's disease. Alzheimer's Res Ther. 2016;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guedes-Dias P, Holzbaur ELF. Axonal transport: driving synaptic function. Science (New York, NY). 2019;366(6462):eaaw9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greif KF, Asabere N, Lutz GJ, Gallo G. Synaptotagmin-1 promotes the formation of axonal filopodia and branches along the developing axons of forebrain neurons. Develop Neurobiol. 2013;73(1):27-44. [DOI] [PubMed] [Google Scholar]
- 42.Inoue Y, Kamikubo Y, Ezure H, et al. Presynaptic protein Synaptotagmin1 regulates the neuronal polarity and axon differentiation in cultured hippocampal neurons. BMC Neurosci. 2015;16:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latimer CS, Burke BT, Liachko NF, et al. Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathologica Commun. 2019;7(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan A, Sershen H, Veeranna, et al. Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry. 2015;20(8):986-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan A, Veeranna, Sershen H, et al. Neurofilament light interaction with GluN1 modulates neurotransmission and schizophrenia-associated behaviors. Transl Psychiatry. 2018;8(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized, deidentified data from this report will be made available on request from any qualified investigator. UCSF Memory and Aging Center data requests can be sent to the corresponding author.