Abstract
Forsythia suspensa (Thunb.) has been widely used in traditional medicines in Asia. According to the 2020 edition of Chinese Pharmacopoeia, phillyrin is the main active ingredient in F. suspensa, which is effective in clearing heat, reducing swelling, and dispersing nodules. F. suspensa leaf is a non-toxic substance and it can be used to make a health tea. Here, we combine elicitors and transcriptomics to investigate the inducible biosynthesis of the phillyrin from the F. suspensa. After the fruits and leaves of F. suspensa were treated with different concentrations of methyl jasmonate (MeJA), the content of phillyrin in the fruits reached a peak at 200 µM MeJA for 12 h, but which was decreased in leaves. To analyze the differences in key enzyme genes involved in the phillyrin biosynthesis, we sequenced the transcriptome of F. suspensa leaves and fruits treated with 200 µM MeJA for 12 h. We hypothesized that nine genes related to coniferin synthesis including: F. suspensa UDP-glycosyltransferase (FsUGT); F. suspensa 4-coumarate coenzyme CoA ligase (Fs4CL); and F. suspensa Caffeoyl-CoA O-methyltransferase (FsCCoAOMT) etc. The qRT-PCR analysis of genes related to phillyrin biosynthesis was consistent with RNA-seq analysis. We also investigated the dynamic changes of genes in F. suspensa leaves and fruits at different time points after 200 µM MeJA treatment, which laid the foundation for further study of the molecular mechanisms regulating the biosynthesis of phillyrin.
Keywords: Forsythia suspensa, comparative transcriptome, MeJA treatment, phillyrin, phillyrin biosynthesis
1. Introduction
Forsythia suspensa (Thunb.) is utilized as a common traditional medicine in China, Japan, Korea, and many European countries. It is called ‘Lianqiao’ in China [1]. Based on the different harvest time, F. suspensa fruits can be classified into ‘Qingqiao’ and ‘Laoqiao’ forms. In folk medicine, the extract of the dried fruit has long been used to treat a variety of diseases, such as inflammation, pyrexia, gonorrhea, tonsillitis, and ulcers [2]. F. suspensa fruit is also the main active ingredient in many widely used classic Chinese patent medicine prescriptions, such as Shuanghuanglian injections [3] and Lianhua Qingwen granules [4]. Lianhua Qingwen is also recommended for the treatment of COVID-19 [5]. The dried ripe fruit of F. suspensa has also been prescribed for the treatment of diabetes in China [6]. In addition to the medicinal value of F. suspensa fruit, it has also been reported in recent years that the extract of F. suspensa leaves has antibacterial and other pharmacological effects [7]. In folk medicine, F. suspensa leaves are also used as tea [8]. Therefore, it is of great significance to study the secondary metabolites and their synthetic pathways in F. suspensa. In recent years, many active ingredients have been identified in F. suspensa, such as phenylethanoid glycosides, lignans, flavonoids, terpenes, and volatile oils [9]. Lignan and phenylethanol glycoside are the two main representative characteristic components in F. suspensa; more than 30 lignans and lignan glycosides, including (+)-forsythin, have been isolated from F. suspensa alone [10]. Phillyrin (also named forsythin), a kind of lignan substance, mitigates apoptosis and oxidative stress [11], has anti-viral and anti-inflammatory activity [12], and improves insulin resistance [13]. The biosynthesis of lignan (including phillyrin) consists of three stages: coniferyl alcohol, the precursor of lignan, is synthesized through the phenylpropanoid pathway; then, structurally diverse lignans are synthesized from coniferyl alcohol; and finally, lignans are modified by glycosylation to form lignan glycosides, which are accumulated and stored in plant cells and tissues [14].
The phenylpropane biosynthesis pathway shares some intermediates with the lignan biosynthesis pathways [15]. Based on previous study, phenylalanine ammonia-lyase enzyme (PAL), coumarate 3-hydroxylase (C3H), 4-coumarate CoA ligase (4CL), caffeoyl o-methyltransferase (COMT), caffeoyl-CoA o-methyltransferase (CCoAOMT), cinnamoyl-CoA reductase (CCR), cinnamyl alcohol dehydrogenase (CAD), dirigent protein (DIR), o-methyltransferase (OMT), and udp-glycosyltransferase (UGT), are key enzymes of the phillyrin-related secondary metabolism in plants. PAL is the enzyme that catalyzes the first step reaction of phenylpropanoid metabolism, connects the biological primary metabolism, and is the key and rate-limiting enzyme of phenylpropanoid metabolism [16]. C3H catalyzes the formation of caffeic acid from coumaric acid, which is methylated to form ferulic acid [17]. The enzyme 4CL activates cinnamic acid and its hydroxylated derivatives by forming the corresponding CoA thioesters, and it is one of the key enzymes in lignan biosynthesis [18]. Both COMT and CCoAOMT are substrate methylation enzymes, and lignan monomer synthesis requires a two-step methylation reaction at the 3′ and 5′ positions of the two-step methylation reaction; COMT and CCoAOMT are two methylation enzymes at different substrate levels that are associated with the specific synthesis of lignan monomers [19,20]. As an enzyme that catalyzes the first reaction of the lignan synthesis pathway, CCR is considered to be a potential control point for regulating the flow of carbon to lignans [21]. CAD catalyzes the formation of coniferyl dehyde to coniferyl alcohol, which is one of the key enzymes in lignan synthesis [14]. Although a coniferyl alcohol has yet to be identified, a dirigent protein (DIR) was shown to participate in the stereo-specific dimerization of E-coniferyl alcohol [22]. In the biological pathway for the formation of phillyrin from coniferyl alcohol, after the formation of (+)-epipinoresinol, phillygenin can be produced by oxygen methylation, possibly mediated by a specifc O-methylase [23]. Most lignans exist in plants in the form of glycosylation, which is catalyzed by UGT to generate lignan glycosides [24]. Methyl jasmonate (MeJA), a well-known exogenous inducing factor, participates in many plant processes, ranging from plant defense to growth and development [25]. As an abiotic inducer, MeJA can rapidly and selectively induce the expression of specific gene associated with specific biological process during plant secondary metabolism, thereby regulating the synthesis of secondary metabolites in plant cells [26,27]. MeJA-induced transcriptome changes have been analyzed in many different plant species including wheat and sweet basil [28,29]. In addition, genomic and transcriptomic information of F. suspensa is scarce in public databases. To identify the genes involved in the biosynthesis of phillyrin, the fruits and leaves of F. suspensa plants after 12 h of MeJA treatment were explored by transcriptional sequencing, based on the Illumina Novaseq 6000 sequencing platform, and the relevant genes in the phillyrin synthesis pathway were analyzed using Quantitative Real-Time PCR (qRT-PCR). This study provides a theoretical basis for further exploring the molecular regulation mechanism of secondary metabolites in F. suspensa and reveals the mechanism of the formation of phillyrin in F. suspensa.
2. Materials and Methods
2.1. Plant Materials and Treatment
F. suspensa used in this study was grown in 2021 at the F. suspensa medicine source base in Yiyang, Luoyang, China. In this study, fruit, and leaf tissues of F. suspensa plants, were treated with 50, 200, and 400 µM of MeJA. Preparation of MeJA (Sigma-Aldrich, 95%, Sigma, St. Louis, MO, USA) stock solution: 218 µL MeJA stock solution was dissolved in 2 mL absolute ethanol, then diluted with ddH2O to make the 10 mM MeJA solution. The high (400 µM), medium (200 µM), and low (50 µM) concentrations of MeJA treatment solution were obtained by diluting the 10 mM MeJA solution, respectively. During the experiment, final concentrations of MeJA solutions (50, 200, and 400 µM) were sprayed on the fruit and leaf surfaces of F. suspensa plants until the solution covered the leaf and fruit surfaces just dripping down. Control plants were sprayed with an equal volume of ethanol in ddH2O. For each treatment, each replicate was considered and three plants were collected. Leaves and fruits from treatments 0 h (as control), 7 h, 12 h, 24 h, 48 h were collected, frozen in liquid nitrogen, and stored at −80 °C. The F. suspensa fruit treated with 50 µM MeJA, 200 µM MeJA, and 400 µM MeJA were represented as M1F, M2F and M3F, respectively. The F. suspensa leaf treated with 50 µM MeJA, 200 µM MeJA, and 400 µM MeJA, were represented as M1L, M2L, and M3L, respectively.
2.2. Determination of Phillyrin Content in F. suspensa Fruits and Leaves
High performance liquid chromatography (HPLC, Agilent 1260 Infinity, Agilent Technologies Co. Ltd., Palo Alto, CA, USA) was used to determine the content of phillyrin in F. suspensa fruit and leaf. The MeJA-treated F. suspensa fruits and leaves were lyophilized and homogenized in a sterilized mortar. The extract was sonicated with 15 mL of methanol (70% v/v) for 30 min for a total of 0.5 g. The extract was cooled to room temperature and centrifuged at 4000 rpm for 10 min. The filtrate was then filtered through a 0.22 µm organic microporous membrane and stored under seal.
HPLC conditions: the mobile phase consisted of acetonitrile (A)-water (B) with gradient elution (0–40 min, 25% A) was at flow rate 1.0 mL min−1, column temperature 30 °C, the column was manufactured by Thermo Fisher Scientific (Waltham, MA, USA) Co., Ltd., with a diameter of 4.6 mm, a length of 250 mm, and a particle size of 5 μm. Detection wavelength 277 nm. The injection volume of the HPLC method was 10 µL. For the quantification of phillyrin, standards were used. The results were described in grams per kilogram of fresh weight. All experiments were repeated in triplicate.
2.3. RNA Extraction, cDNA Library Construction and Illumina Sequencing
We analyzed the content of phillyrin at different time periods after different concentrations of MeJA treatment by HPLC, and selected F. suspensa fruits (M2F)/leaves (M2L) treated with 200 µM MeJA for 12 h and F. suspensa fruits (CKF)/leaves (CKL) treated with control solution for 0 h for transcriptome sequencing including 12 samples with three replicates for each material. Total RNA was extracted from the tissue using TRIzol® Reagent (Invitrogen, Carlsbad, CA, USA) (Plant RNA Purification Reagent for plant tissue) according the manufacturer’s instructions (Invitrogen) and genomic DNA was removed using DNase I (TaKara, Japan). RNA degradation and contamination was monitored on 1% agarose gels. Then, the integrity and purity of the total RNA quality was determined by 2100 Bioanalyser (Agilent Technologies Co. Ltd., Palo Alto, CA, USA) and quantified using the ND-2000 (NanoDrop Technologies, Wilmington, DE, USA). Only high-quality RNA sample (OD260/280 = 1.8~2.2, OD260/230 = 1.8~2.2, RIN = 8.8~10.0, 28S:18S = 1.4~1.8) was used to construct sequencing library. RNA purification, reverse transcription, library construction and sequencing were performed at Shanghai Majorbio Bio-pharm Biotechnology Co., Ltd. (Shanghai, China) according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). Paired-end RNA-seq sequencing library was sequenced with the Illumina NovaSeq 6000 sequencer (Illumina, San Diego, CA, USA) (2 × 150 bp read length). Each sample contained three biological replicates.
2.4. Transcriptome Assembly and Annotation
The high-quality clean reads were obtained after removing reads containing adapters and low-quality reads. Transcriptome de novo assembly was performed using the Trinity program [30]. Functional annotation of the unigenes was carried out using BLASTx program with an E-value threshold of 1 × 10−5 against the NCBI non-redundant protein (Nr) datbase [31], Swiss-Prot protein database, COG database [32], and Kyoto Encyclopedia of Genes and Genomes (KEGG) database [33]. GO annotation was analyzed by Blast2GO software (Version 2.2.31+) [34]. Transcription factors (TFs) were identified using PlantTFDB database with default parameters [35].
2.5. Differentially Expressed Genes (DEGs) Analysis
The high-quality RNA reads were counted against bowtie’s paired results [36] by RSEM (http://deweylab.biostat.wisc.edu/rsem/). Differential expression analyses among the two treatments (CKL-vs.-M2L and CKF-vs.-M2F with three biological replicates per treatment) were conducted using edgeR software (Version 3.24.3) [37]. Genes with a fold change ≥ 2 and a false discovery rate (FDR) < 0.05 in a comparison were defined as significant DEGs. DEGs were then subjected to enrichment analysis of GO functions and KEGG pathways. Heatmaps of DEGs were analyzed using HemI [38].
2.6. Quantitative Real-Time PCR (qRT-PCR)
The RNA samples (1 μg) with A260/A230 ratios between 1.8 and 2.2 were used to synthesize the first strand of cDNA with the First Strand cDNA Synthesis Kit (CISTRO, Guangzhou, China) in a 20 μL reaction mixture according to the manufacturer’s protocol. The cDNA samples were diluted 10-fold with distilled water prior to the qRT-PCR analysis. The qRT-PCR analysis was performed using ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and Roche Light Cycle96 Real-Time PCR system with the following reaction conditions: initial denaturation at 95 °C for 30 s, followed by a two-step program of 95 °C for 10 s and 60 °C for 30 s for 40 cycles, with a melting curve analysis at 95 °C for 15 s, then from 60 °C to 95 °C at a rate of 0.2 °C/s. Candidate genes related to phillyrin were identified from F. suspensa transcriptome data, the longest CDS sequence was selected, and the specific primers for the target genes were designed using Oligo 7.0, and are listed in Table S1. The UKN1 + SDH + G6PD genes were used as reference gene group and the relative expression levels were determined according the 2−ΔΔCt method [39,40]. The specificity of the primer pair was verified by the presence of a single peak in the melt curve analysis during the qRT-PCR process (Figure S1). All biological replicates were performed in triplicate.
2.7. Statistical Analyses
Data were analyzed with one-way ANOVA by using SPSS 17.0, and means were contrasted with Duncan’s multiple scope test at p < 0.05. All the experiments were carried out in triplicate.
3. Results
3.1. Effect of MeJA Induction on the Content of Phillyrin
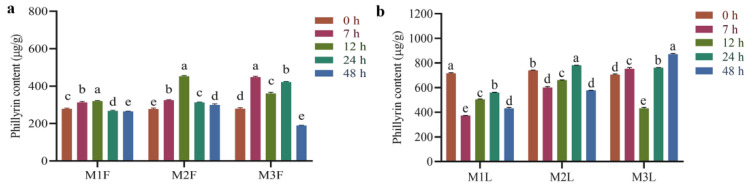
The effect of MeJA treatment on the content of phillyrin in the leaves and fruits of F. suspensa was shown in Figure 1. After 50 μM MeJA treatment, the phillyrin content in F. suspensa fruits was slightly increased compared with the control. Meanwhile, the content of phillyrin was gradually increased and then decreased after 200 μM MeJA treatment, peaking at 12 h with 1.63-fold compared with control. After 400 μM MeJA treatment, the content of phillyrin was obviously increased at 7 h and 24 h with 1.60 and 1.51-fold compared with control, respectively (Figure 1a). After 50 μM MeJA treatment of F. suspensa leaves, the content of phillyrin decreased compared to the control. After 200 μM MeJA treatment, the content of phillyrin decreased, then increased, and finally decreased with time. 400 μM MeJA treatment of F. suspensa leaves showed a decrease in phillyrin content at 12 h compared with the control (Figure 1b). This indicates that different parts of F. suspensa responded differently to the induction of different MeJA concentrations. The exogenous MeJA treatment of F. suspensa led to the induction of phillyrin in response to MeJA, but the induction effect varied among time and tissue parts. Due to F. suspensa leaves having been used to make health-care tea in folk tradition [8], we also treated them with 200 μM MeJA for 12 h to investigate the different mechanisms of phillyrin synthesis in F. suspensa leaves and fruits, under the same conditions of treatment.
Figure 1.
Phillyrin content of F. suspensa fruits and leaves treated with different MeJA. (a) Phillyrin content of F. suspensa fruits treated with different MeJA. (b) Phillyrin content of F. suspensa leaves treated with different MeJA. M1F: F. suspensa fruit treated with 50 µM MeJA; M2F: F. suspensa fruit treated with 200 µM MeJA; M3F: F. suspensa fruit treated with 400 µM MeJA. M1L: F. suspensa leaf treated with 50 µM MeJA; M2L: F. suspensa leaf treated with 200 µM MeJA; M3L: F. suspensa leaf treated with 400 µM MeJA. Data were analyzed by SPSS, followed by Duncan’s honestly significant difference test at p < 0.05. All Statistical analyses of data had three biological repeats. All data are displayed as means ± SD.
3.2. Illumina Sequencing and De Novo Assembly
To further understand the molecular mechanism underlying the response of phillyrin to MeJA, an RNA-seq analysis was performed. We established 12 cDNA libraries for transcriptome sequencing. Illumina Hiseq 6000 platform generated 98.21 Gb clean reads with an average Q30 value of 94.55% and an average GC content of 44%, Q20 > 98%, and Q30 > 94% (Table 1). The max length, min length, and N50 length, for the assembled unigenes were 15,856, 201, and 1851 bp, respectively. With regards to the length distribution of the unigenes, the total number of sequences of all unigene lengths of 200–500 bp in the transcriptome data of F. suspensa is much higher than the total number of sequences of other respective lengths (Figure S2).
Table 1.
Summary of sequencing and assembly for F. suspensa.
| Type | Unigene |
|---|---|
| Total number | 87,564 |
| Total base | 87,582,604 |
| Largest length (bp) | 15,856 |
| Smallest length (bp) | 201 |
| Average length (bp) | 1000.21 |
| GC percent (%) | 44 |
| N50 average length (bp) | 1851 |
| Q30 (%) | 94.55 |
| Q20 (%) | 98 |
All of the sequencing data were deposited in the National Center for Biotechnology Information (NCBI). The sequence read archive (SRA) accession number is SRP392325. The resulting fastq files were deposited on SRR21066404, SRR21066405, SRR21066406, SRR21066407, SRR21066412, SRR21066413 (leaf) and SRR21066408, SRR21066409, SRR21066410, SRR21066411, SRR21066414, SRR21066415 (fruit).
3.3. Functional Annotation of Unigenes
A total of 37,297, 25,361, 15,161, 30,017, 29,688, and 24,539 unigenes had significant hits (E-value ≤ 10−5) in the Nr (non-redundant protein) database, Swissprot, Kyoto Encyclopedia of Genes and Genomes (KEGG), the COG database and the Pfam database, respectively (Figure S3). A total of 60,093 CDS unigenes produced by the Illumina Hiseq 2500 platform, and most CDSs, were less than 1800 bp (Figure S4).
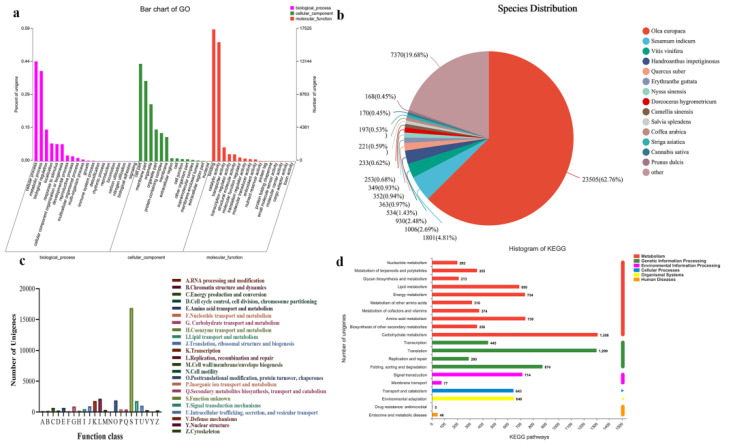
For GO annotation, 79,094 unigenes were allocated into three categories (“biological process”, “cellular component”, and “molecular function”). Among the biological processes, the unigenes were mainly involved in metabolic processes (8180, GO: 0008152), cellular processes (9058, GO: 0009987), and biological regulation (2432, GO: 0065007). Among the cellular components category, the unigenes were primarily associated with cellular fraction (7846, GO: 0044464), membrane fraction (7269, GO: 0044425), and organelles (4424, GO: 0043226). Among the molecular function categories, unigenes with catalytic activity (10,376, GO: 0003824), binding (12,606, GO: 0005488), and transporter protein activity (1323, GO: 0005215), accounted for a larger proportion (Figure 2a).
Figure 2.
Functional annotation of F. suspensa transcriptome. (a) Gene Ontology (GO) functional classification of assembled unigenes. (b) Homologous species distribution of F. suspensa annotated in the NR database. (c) Clusters of Orthologous Groups (COG) functional classifification of assembled unigenes. (d) Functional classifification and pathway assignment of assembled unigenes by Kyoto Encyclopedia of Genes and Genomes (KEGG).
Unigene sequences were searched against the NR database for annotation and revealed 23,505 unigenes (62.76%) matched to Olea europaea and 1801 unigenes (4.81%) matched to Sesamum indicum (Figure 2b).
To further evaluate the completeness of the transcriptome library and the validity of the annotation, the COG classification of unigenes was performed. Among the 23 COG categories, “L: Replication, recombination and repair” was the largest group, followed by “O: Posttranslational modification, protein turnover, chaperones and ion, protein turnover, chaperones” and “T: Signal transduction mechanisms”. The smallest group was “Y: Nuclear structure” with only two unigenes annotated to this category (Figure 2c).
The KEGG database was used to identify the biochemical pathways assigned to unigene sequences. In our results, a total of 10,325 unigenes were annotated to six metabolic pathways: metabolism, genetic information processing, environmental information processing, cellular processes, organismal systems, and human diseases. Metabolism had the largest number of pathways and human diseases had the smallest number of pathways. There were 356 unigenes matching to the biosynthesis of other secondary metabolism, and 224 unigenes of phenylpropanoid biosynthesis pathway (Figure 2d).
3.4. Analysis of Differentially Expressed Unigenes (DEGs)
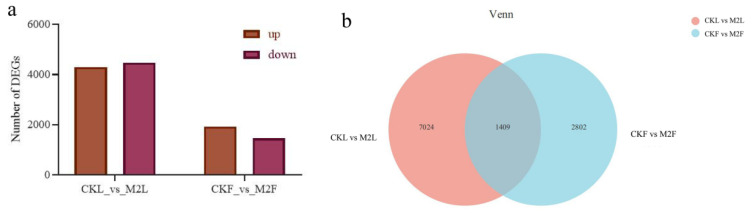
To obtain a comprehensive view of the gene expression profile associated with the response of phillyrin to MeJA, we used edgeR to identify the DEGs. A total of 4211 (2348 up-regulated and 1863 down-regulated) DEGs and 8433 (4134 up-regulated and 4299 down-regulated) DEGs were identified in fruits and leaves of F. suspensa treated 12 h with MeJA, respectively (Figure 3); this finding indicated that there were more upregulated than downregulated unigenes in F. suspensa fruits and more downregulated than upregulated unigenes in F. suspensa leaves under MeJA treatment. Furthermore, we used principal component analysis (PCA) to display the overall relationships between the transcriptome from the different samples (Figure S5), indicating the leaves and fruits of F. suspensa before and after MeJA treatment showed mutual aggregation within the group. Meanwhile, two different transcriptional variation tendencies were observed before and after the MeJA treatment of F. suspensa fruits, and F. suspensa leaves also showed the same trend.
Figure 3.
Statistics of DEGs in F. suspensa transcriptomes. (a) DEG numbers in comparisons of the CKF-vs.-M2F and CKL-vs.-M2L. (b) Venn diagram showing significantly DEGs at different tissue. CKL: F. suspensa leaf treated with control solution for 0 h; M2L: F. suspensa leaf treated with 200 µM MeJA; CKF: F. suspensa fruit treated with control solution for 0 h; M2F: F. suspensa fruit treated with 200 µM MeJA.
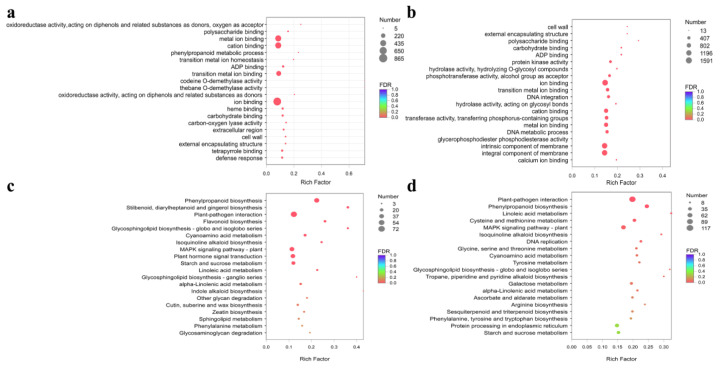
Functional enrichment analysis revealed that 3754 and 1822 DEGs were associated with GO terms in F. suspensa leaves and fruits, respectively (Figure 4). DEGs were mainly enriched in oxidoreductase activity (GO: 0016682), and phenylpropanoid metabolic process (GO: 0009698) (Figure 4a,b). The KEGG pathways analysis indicated DEGs were mainly involved in the metabolism of phenylpropanoid biosynthesis, plant-pathogen interaction, MAPK signaling pathway-plant, phenylalanine metabolism, and plant hormone signal transduction (Figure 4c,d).
Figure 4.
Enrichment analysis of differentially expressed genes in the GO and KEGG pathways. (a) GO pathway enrichment analysis in CKF-vs.-M2F. (b) GO pathway enrichment analysis in CKL-vs.-M2L. (c) KEGG pathway enrichment analysis in CKF-vs.-M2F. (d) KEGG pathway enrichment analysis in CKL-vs.-M2L. CKL: F. suspensa leaf treated with control solution for 0 h; M2L: F. suspensa leaf treated with 200 µM MeJA; CKF: F. suspensa fruit treated with control solution for 0 h; M2F: F. suspensa fruit treated with 200 µM MeJA.
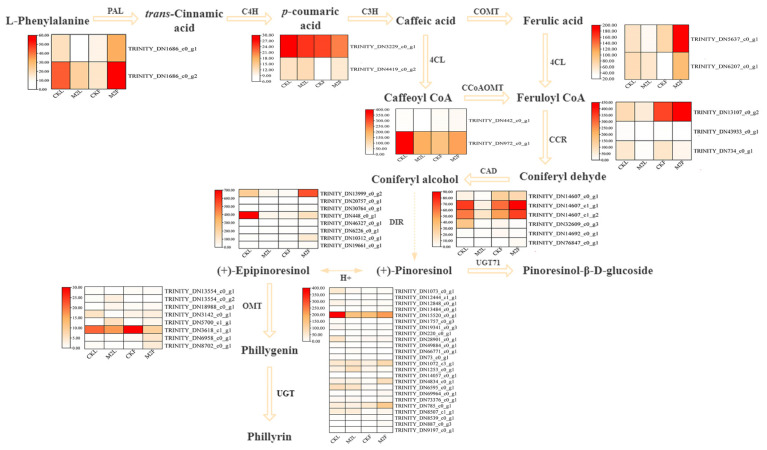
Previous study showed that phillyrin as a kind of lignan was tightly related to phenylpropanoid biosynthesis [41]. In the phillyrin biosynthesis pathway, three FsCCR, four FsCAD, six FsDIR, five FsOMT, one FsPAL, and thirteen FsUGT genes were significant different after MeJA treatment in F. suspensa leaves, respectively (Figure 3 and Figure 5). Meanwhile, two Fs4CL, two FsCAD, one FsCCR, four FsDIR, three FsOMT, two FsPAL, and twelve FsUGT were significant different in F. suspensa fruits. Furthermore, one FsUGT, three FsOMT, two FsDIR, one FsPAL, and one FsCCR were significant different both in F. suspensa leaves and fruits (Figure 3 and Figure 5). These results suggest that MeJA may induce the accumulation of phillyrin by regulating the expression of genes associated with phillyrin biosynthesis in F. suspensa.
Figure 5.
Putative pathway for phillyrin synthesis in F. suspensa. PAL, phenylalanine ammonia-lyase; C3H, Coumarate 3-hydroxylase; 4CL, 4-coumarate CoA ligase; CCoAOMT, caffeoyl-CoA O-methyltransferase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl alcohol dehydrogenase; DIR, dirgent protein; OMT, O-methyltransferase; UGT, UDP-glycosyltransferase. CKL: F. suspensa leaf treated with control solution for 0 h; M2L: F. suspensa leaf treated with 200 µM MeJA; CKF: F. suspensa fruit treated with control solution for 0 h; M2F: F. suspensa fruit treated with 200 µM MeJA.
3.5. Analysis of DEGs Related to Phillyrin Biosynthetic Pathway
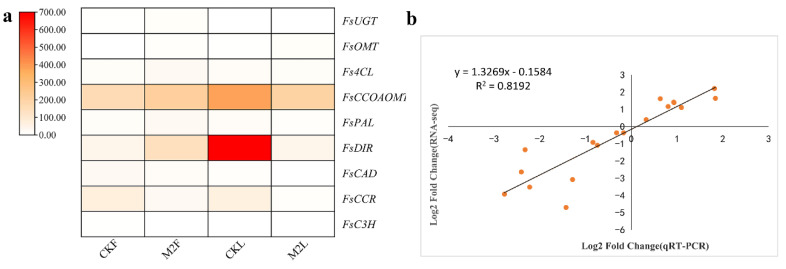
To verify the expression level of genes related to phillyrin biosynthesis exhibited by the identified DEGs in response to MeJA, the expression levels of nine genes (FsUGT, Fs4CL, FsCCoAOMT, FsOMT, FsPAL, FsDIR, FsCAD, FsCCR, and FsC3H) were detected by qRT-PCR. The expression profiles were generally consistent with the RNA-seq results (Figure 6a). Correlation analysis based on the qRT-PCR and transcriptome data also showed a significant correlation with a Spearman correlation coefficient of 0.819, which reflects the accuracy of transcriptome data (Figure 6b). To analyze the dynamic expression profiles of genes putatively related to phillyrin synthesis under MeJA treatment, genes expression level (FsUGT, Fs4CL, FsCCoAOMT, FsOMT, FsPAL, FsDIR, FsCAD, FsCCR, and FsC3H) in F. suspensa fruits and leaves after MeJA treatment in different time (0 h, 7 h, 12 h, and 24 h) were studied. The results revealed that MeJA activated or suppressed the expression of genes to variant degrees at different time points and in different tissues.
Figure 6.
Expression patterns of genes related to phillyrin biosynthesis. (a). Transcriptional profiles of DEGs related to phillyrin biosynthetic pathway. (b). Scatter plot and linear regression based on qRT-PCR and RNA-seq. The correlation coefficient was calculated using the Spearman correlation method. FsPAL, phenylalanine ammonia-lyase gene; FsC3H, Coumarate 3-hydroxylase gene; Fs4CL, 4-coumarate CoA ligase gene; FsCCoAOMT, caffeoyl-CoA O-methyltransferase gene; FsCCR, cinnamoyl-CoA reductase gene; FsCAD, cinnamyl alcohol dehydrogenase gene; FsDIR, dirgent protein gene; FsOMT, O-methyltransferase gene; FsUGT, UDP-glycosyltransferase gene. CKL: F. suspensa leaf treated with control solution for 0 h; M2L: F. suspensa leaf treated with 200 µM MeJA; CKF: F. suspensa fruit treated with control solution for 0 h; M2F: F. suspensa fruit treated with 200 µM MeJA.
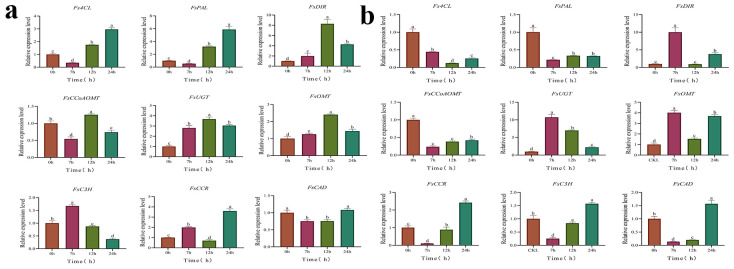
The expression of FsDIR, FsUGT, and FsOMT genes were up-regulated at all time points in 200 μM MeJA-treated F. suspensa fruit. In the presence of MeJA, the expression levels of Fs4CL, FsPAL, FsCCR, and FsCAD genes, were inhibited at the earlier time points and activated at the later time points. The expression level of FsCCoAOMT gene reached a maximum at 12 h, and then its expression decreased compared with the 0 h control. The expression levels of FsC3H gene reached a maximum at 7 h and were repressed thereafter compared with the 0 h control (Figure 7a). In F. suspensa leaves, Fs4CL, FsPAL, and FsCCoAOMT genes expression levels were down-regulated at all time points, and FsOMT and FsUGT genes expression were up-regulated within 24 h of MeJA treatment compared with the 0 h control. FsCCR, FsC3H, and FsCAD genes expression were repressed at early time points, afterwards, expression gradually increased (Figure 7b).
Figure 7.
Analysis of the expression profiles of nine genes related to phillyrin synthesis after 200 µM MeJA treatment for 0, 7, 12, and 24 h. (a). Relative expression levels of phillyrin biosynthesis genes in F. suspensa fruits are detected by qRT-PCR (0 h as 1). (b). Relative expression levels of phillyrin biosynthesis genes in F. suspensa leaves are detected by qRT-PCR (0 h as 1). The UKN1 + SDH + G6PD genes are used as the reference gene group to standardize the RNA samples for each reaction. All Statistical analyses of data had three biological repeats. Data are analyzed by SPSS, followed by Duncan’s honestly significant difference test at p < 0.05. All Statistical analyses of data had three biological repeats. FsPAL, phenylalanine ammonia-lyase gene; FsC3H, Coumarate 3-hydroxylase gene; Fs4CL, 4-coumarate CoA ligase gene; FsCCoAOMT, caffeoyl-CoA O-methyltransferase gene; FsCCR, cinnamoyl-CoA reductase gene; FsCAD, cinnamyl alcohol dehydrogenase gene; FsDIR, dirgent protein gene; FsOMT, O-methyltransferase gene; FsUGT, UDP-glycosyltransferase gene.
4. Discussion
The content of phillyrin is an important index for evaluating the quality of F. suspensa. Phillyrin are effective in clearing heat and detoxifying, reducing swelling, and dispersing nodules [42]. It is important to further understand the phillyrin biosynthesis pathway and provide robust candidate genes for further functional investigations aimed at the improvement of phillyrin content and quality.
MeJA is a cyclopentanone derivative-like signaling substance commonly found in the plant kingdom that regulates plant growth and development, triggers cells to initiate protective mechanisms, and stimulates the expression of key enzymes of metabolic pathways [43]. Plant defense systems are initiated upon injury or by signals from exogenous plant hormones, such as MeJA. Moreover, lignans, at least in part, are believed to be involved in host defense systems [44,45,46]. In combination, the hormone is expected to enhance lignan biosynthesis [45,47]. We also found that MeJA could induce the accumulation of phillyrin in F. suspensa fruit (Figure 1a), but a certain concentration of MeJA could inhibit the synthesis of phillyrin in F. suspensa leaves (Figure 1b). The content of phillyrin in leaves without MeJA treatment was higher than that in fruits without MeJA treatment, which was consistent with the results of previous studies [48]. The efficacy of F. suspensa leaves recorded in traditional Chinese medicine classics is very similar to that of F. suspensa fruits, and the chemical composition is also similar [49]. According to the theory of traditional Chinese medicine, the effects of different parts of F. suspensa should be completely different, therefore, we performed RNA-seq detection on F. suspensa fruits and leaves to compare the molecular mechanism of phillyrin biosynthesis in F. suspensa fruits and leaves, laying a foundation for the comprehensive development and utilization of F. suspensa resources.
GO and KEGG results showed DEGs were mainly enriched in phenylpropanoid metabolic process in F. suspensa after MeJA treatment (Figure 4), the phenylpropanoid pathway, a common pathway of phillyrin biosynthesis pathway, can produce coniferyl alcohol, which are precursors of phillyrin [50], including Fs4CL, FsCCR, FsCAD, FsC3H, FsCCoAOMT, and FsPAL genes, which were significantly different in F. suspensa after MeJA treatment compared with control (Figure 3 and Figure 5). Research shows that during phillyrin biosynthesis, two coniferyl alcohol molecules are coupled to produce compounds of different configurations, and then converted to (+)-epipinoresinol. Furthermore, the (+)-epipinoresinol is converted to phillygenin, eventually forming phillyrin [23], including the FsDIR, FsOMT, and FsUGT gene, which were noticeably different in F. suspensa after MeJA treatment compared with control. When F. suspensa fruits were treated with different concentrations of MeJA, the content of phillyrin in the fruits increased, and most of the genes in its synthesis pathway showed an increasing trend; the expression of Fs4CL, FsCCR, FsCAD, FsC3H, FsCCoAOMT, and FsPAL in MeJA-treated F. suspensa leaves was suppressed for a certain period of time, while the expression of FsOMT and FsUGT increased, and phillyrin was also decreased (Figure 1 and Figure 7). Different parts of F. suspensa have different responses to MeJA induction, which has been reported in other plants [51]. This may indicate that genes, during the synthesis of the lignan precursor substance coniferyl alcohol, also have an important influence on the synthesis of the end product phillyrin; and that there are complex regulatory mechanisms involved in the transcription of genes to translation into proteins, from the processing and modification of proteins into active enzymes to secondary metabolites and accumulation.
The fruit of F. suspensa is the main part of the medicine; our study showed that MeJA treatment could effectively increase the content of phillyrin in F. suspensa fruits (Figure 1). The expression of FsOMT and FsUGT was up-regulated in both the fruits and leaves of F. suspensa after MeJA treatment. Previous study showed that Lycoris aurea LaOMT1 transcripts were significantly increased after MeJA treatment [52]; Bupleurum chinense DC. glycosyltransferase genes BcUGT1, BcUGT3, and BcUGT6 could be induced by MeJA with different degrees of up-regulation of expression [53]. The expression of FsCAD, FsCCR, and FsC3H did not correspond to the phillyrin content in F. suspensa fruit, and the expression of FsOMT and FsUGT did not correspond to the phillyrin content in F. suspensa leaves. The result was not a surprise because the formation of end products is collaborative with all genes in the synthesis pathway. The present study cannot adequately account for the role of these individual genes in phillyrin biosynthesis. Therefore, it is crucial to establish a transgenic system for F. suspensa in order to further confirm the function of genes in the phillyrin synthesis pathway. Recently, our team has been making a concerted effort to establish a system for transgenic F. suspensa with an emphasis on hairy roots and callus. In this transgenic system, a deeper understanding of the molecular mechanisms of phillyrin will be gained, and the biotechnological improvement of F. suspensa.
5. Conclusions
In this study, using de novo sequencing, a F. suspensa dataset containing 87,564 unigenes was constructed to molecular mechanism underlying the response of phillyrin to MeJA in F. suspensa leaves and fruits. A total of 4211 and 8433 DEGs were identified in the fruits and leaves of F. suspensa treated with MeJA for 12 h, respectively. GO and KEGG analyses indicated that DEGs were mainly enriched in phenylpropanoid metabolic process in F. suspensa, which was tightly associated with phillyrin biosynthesis. The qRT-PCR results showed that FsUGT, Fs4CL, FsCCoAOMT, FsOMT, FsPAL, and FsDIR were significantly up-regulated in F. suspensa fruits after MeJA treatment; the corresponding F. suspensa fruit also increased the content of phillyrin. While FsCAD Fs4CL, FsCCoAOMT, FsCCR, FsPAL, FsC3H, and FsDIR were significantly down-regulated in F. suspensa leaves, the content of phillyrin in F. suspensa leaves with the same treatment was reduced compared with that of the control. These qRT-PCR data were consistent with RNA-seq data. Taken together, the analysis of transcriptome results of F. suspensa leaves and fruits lay a foundation for the comprehensive development and utilization of F. suspensa resources. These results provide a robust theoretical basis for accelerating the study of the regulatory mechanism of MeJA-induced phillyrin biosynthesis in F. suspensa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo12111143/s1, Table S1: qRT-PCR primer information. Figure S1: Melt curve of representative qRT-PCR amplicons. Figure S2: The sequence length distribution of assembled unigenes. Figure S3: Gene function annotations in six databases (GO, KEGG, COG, NR, Swiss-Prot, Pfam) of F. suspensa. Figure S4: Length distribution of CDS in unigenes. Figure S5: Principal component analysis of expressed gene. CKL: F. suspensa leaf treated with control solution for 0 h; M2L: F. suspensa leaf treated with 200 µM MeJA; CKF: F. suspensa fruit treated with control solution for 0 h; M2F: F. suspensa fruit treated with 200 µM MeJA.
Author Contributions
D.H. is the project administration and designed the experiments. X.L. and J.Z. performed the experiments and wrote the original draft. X.Z. prepared the samples for RNA extraction. H.L. used the software. H.X. and H.Z. conducted the RNA extraction and qRT-PCR analysis. X.Z., H.L. and H.S. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The sequencing data can be found in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under accession number PRJNA869193.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research was supported by the National Natural Science Foundation of China (U1404829), a key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302), Natural Science Foundation of Henan Province (202300410151). This research was also supported by a special fund for the construction of technical systems of the traditional Chinese medicine industry in Henan Province. Henan Province Chinese medicine industry science and technology commissioner service group.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xia E.Q., Ai X., Zang S., Guan T., Xu X., Li H. Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason. Sonochem. 2011;18:549–552. doi: 10.1016/j.ultsonch.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Li H., Chen F. Preparative isolation and purification of phillyrin from the medicinal plant Forsythia suspensa by high-speed counter-current chromatography. J. Chromatogr. A. 2005;1083:102–105. doi: 10.1016/j.chroma.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Zhou W., Di L., Shan J., Bi X., Chen L., Wang L. Intestinal absorption of forsythoside A in different compositions of Shuang–Huang–Lian. Fitoterapia. 2010;82:375–382. doi: 10.1016/j.fitote.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Hu K., Guan W., Bi Y., Zhang W., Li L., Zhang B., Liu Q., Song Y., Li X., Duan Z., et al. Efficacy and safety of Lianhua Qingwen capsules, a repurposed Chinese herb, in patients with Coronavirus disease 2019: A multicenter, prospective, randomized controlled trial [Phytomedicine 85 (2021) 153242] Phytomedicine. 2022;94:153800. doi: 10.1016/j.phymed.2021.153800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M., Gao Y., Yuan Y., Liu M., Gao Y., Yuan Y., Yang K., Shi S., Tian J., Zhang J. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: A systematic review and meta-analysis. Integr. Med. Res. 2021;10:100644. doi: 10.1016/j.imr.2020.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bu Y., Shi T., Meng M., Bu Y., Shi T., Meng M., Kong G., Tian Y., Chen Q., Yao X., et al. A novel screening model for the molecular drug for diabetes and obesity based on tyrosine phosphatase Shp2. Bioorg. Med. Chem. Lett. 2011;21:874–878. doi: 10.1016/j.bmcl.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 7.Xue H., Yuan W. Review on Pharmacological Research of Forsythia Suspensa Leaves. Lishizhen Med. Mater. Med. Res. 2009;20:1149–1150. [Google Scholar]
- 8.Yuan J., Qiu Z., Liu J.L., Liu H., Zhang S.L. Processing techniques of Forsythia Suspensa leaves green tea and analysis of nutrient active ingredients. J. Henan Univ. Sci. Technol. 2015;36:78–82. [Google Scholar]
- 9.Dong Z., Lu X., Tong X., Dong Y., Tang L., Liu M. Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics. Molecules. 2017;22:1466. doi: 10.3390/molecules22091466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satake H., Koyama T., Bahabadi S.E. Essences in metabolic engineering of lignan biosynthesis. Metabolites. 2015;5:270–290. doi: 10.3390/metabo5020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Y., You L., Ni B., Sai N., Wang W., Sun M., Xu R., Yao Y., Zhang Z., Qu C., et al. Phillyrin Mitigates Apoptosis and Oxidative Stress in Hydrogen Peroxide-Treated RPE Cells through Activation of the Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2020;2020:2684672. doi: 10.1155/2020/2684672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Q., Li R., Pan W., Huang W., Liu B., Xie Y., Wang Z., Li C., Jiang H., Huang J., et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-kappaB) signaling pathway. Phytomedicine. 2020;78:153296. doi: 10.1016/j.phymed.2020.153296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Saadeldeen F.S.A., Xu L., Zhao Y., Wei J., Wang D.H.M., Liu Z., Kang W. The Mechanism of Phillyrin from the Leaves of Forsythia suspensa for Improving Insulin Resistance. Biomed. Res. Int. 2019;2019:3176483. doi: 10.1155/2019/3176483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Yu S., Ma Y., Zhang T., Zhao G. Research progress in metabolic engineering and synthetic biology for natural lignan production. Chin. Tradit. Herb. Drugs. 2016;47:2556–2562. [Google Scholar]
- 15.Rahman M.M.A., Dewick P.M., Jackson D.E., Lucas John A. Biosynthesis of lignans in Forsythia intermedia. Phytochemistry. 1990;29:1841–1846. doi: 10.1016/0031-9422(90)85025-B. [DOI] [Google Scholar]
- 16.Fukasawa A.T., Kung S.D., Watson J.C. Phenylalanine ammonia-lyase gene structure, expression, and evolution in Nicotiana. Plant Mol. Biol. 1996;30:711–722. doi: 10.1007/BF00019006. [DOI] [PubMed] [Google Scholar]
- 17.Gertsch J., Tobler R.T., Brun R., Sticher O., Heilmann J. Antifungal, Antiprotozoal, Cytotoxic and Piscicidal Properties of Justicidin B and a New Arylnaphthalide Lignan from Phyllanthus piscatorum. Planta Med. 2003;69:420–424. doi: 10.1055/s-2003-39706. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A., Ellis B.E. 4-Coumarate:CoA ligase gene family in Rubus idaeus: cDNA structures, evolution, and expression. Plant Mol. Biol. 2003;31:327–340. doi: 10.1023/A:1022004923982. [DOI] [PubMed] [Google Scholar]
- 19.Anterola A., Lewis N. Trends in lignin modification: A comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry. 2002;61:221–294. doi: 10.1016/S0031-9422(02)00211-X. [DOI] [PubMed] [Google Scholar]
- 20.Guo D., Chen F., Inoue K., Blount J.W., Dixon R.A. Downregulation of caffeic acid 3-0-methyltransferase and caffeoyl CoA 3-0-methyltransferase in transgenic alfalfa: Impacts on lignin structure and implications for the biosynthesis of G and S lignin. Plant Cell Online. 2001;13:73. doi: 10.1105/tpc.13.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luderitz T., Grisebach H. Enzymic Synthesis of Lignin Precursors Comparison of Cinnamoyl-CoA Reductase and Cinnamyl Alcohol: NADP iDehydrogenase from Spruce (Picea abies L.) and Soybean (GZycine max L.) Eur. J. Biochem. 1981;119:115–124. doi: 10.1111/j.1432-1033.1981.tb05584.x. [DOI] [PubMed] [Google Scholar]
- 22.Davin L.B., Wang H.B., Crowell A.L., Davin L.B., Wang H.B., Crowell A.L., Bedgar D.L., Martin D.M., Sarkanen S., Lewis N.G. Stereoselective bimo-lecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science. 1997;275:362–367. doi: 10.1126/science.275.5298.362. [DOI] [PubMed] [Google Scholar]
- 23.Yuan W., Zhang S., He Z., He y., He S., Liu L., Sun X., Li Q. Comparative transcriptome analyses identify genes involved into the biosynthesis of forsythin and forsythoside A in Forsythia suspensa. Funct. Integr. Genom. 2022;22:731–741. doi: 10.1007/s10142-022-00887-z. [DOI] [PubMed] [Google Scholar]
- 24.Berim A., Ebel R., Schneider B., Petersen M. UDP-glucose:(6-methoxy)podophyllotoxin 7-O-glucosyltransferase from suspension cultures of Linum nodiflorum. Phytochemistry. 2008;69:374–381. doi: 10.1016/j.phytochem.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verpoorte R., Heijden R.V.D., Hoge J.H.C., Hoopen T. Plant cell biotechnology for the production of secondary metabolites. Pure Appl. Chem. 1994;66:2307–2310. doi: 10.1351/pac199466102307. [DOI] [Google Scholar]
- 27.Zhao J., Zhu W.H., Hu Q. Selection of fungal elicitors to increase indole alkaloid accumulation in Catharanthus roseus suspension cell culture. Enzym. Microb. Technol. 2001;28:666–672. doi: 10.1016/S0141-0229(01)00309-X. [DOI] [PubMed] [Google Scholar]
- 28.Diallo A.O., Agharbaoui Z., Badawi M.A., Ali-Benali M.A., Moheb A., Houde M., Sarhan F. Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat. J. Exp. Bot. 2014;65:2271–2286. doi: 10.1093/jxb/eru102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Misra R.C., Maiti P., Chanotiya C.S., Shanker K., Ghosh S. Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant Physiol. 2014;164:1028–1044. doi: 10.1104/pp.113.232884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruitt K.D., Tatusova T., Maglott D.R. NCBI Reference Sequence (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic. Acids Res. 2005;33:D501–D504. doi: 10.1093/nar/gki025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatusov R.L., Fedorova N.D., Jackson J.D., Jacobs A.R., Kiryutin B., Koonin E.V., Krylov D.M., Mazumder R., Mekhedov S.L., Nikolskaya A.N., et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M., Katayama T., Kawashima S., Okuda S., Tokimatsu T., et al. KEGG for linking genomes to life and the environment. Nucleic. Acids Res. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conesa A., Gotz S., Garcia-Gomez J.M., Terol J., Talón M., Robles M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 35.Jin J.P., Tian F., Yang D.C., Meng Y., Kong L., Luo J., Gao G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic. Acids Res. 2017;45:D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewey C.N., Bo L. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng W., Wang Y., Liu Z., Cheng H., Xue Y. HemI: A toolkit for illustrating heatmaps. PLoS ONE. 2014;9:e111988. doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Shen J., Wu Y., Jiang Z., Xu Y., Zheng T., Wang J., Cheng T., Zhang Q., Pan H. Selection and validation of appropriate reference genes for gene expression studies in Forsythia. Physiol. Mol. Biol. Plants. 2020;26:173–188. doi: 10.1007/s12298-019-00731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis N.G., Davin L.B., Sarkanen S. Lignin and Lignan Biosynthesis: Distinctions and Reconciliations. ACS Symp. 1998;697:1–27. [Google Scholar]
- 42.Zhou C., Lu M., Cheng J., Rohani E.R., Hamezah H.S., Han R., Tong X. Review on the Pharmacological Properties of Phillyrin. Molecules. 2022;27:3670. doi: 10.3390/molecules27123670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moharramnejad S., Azam A.T., Panahandeh J., Dehghanian Z., Ashraf M. Effect of Methyl Jasmonate and Salicylic Acid on In Vitro Growth, Stevioside Production, and Oxidative Defense System in Stevia rebaudiana. Sugar Tech. 2019;21:1031–1038. [Google Scholar]
- 44.Satake H., Ono E., Murata J. Recent advances in the metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. J. Agric. Food Chem. 2013;61:11721–11729. doi: 10.1021/jf4007104. [DOI] [PubMed] [Google Scholar]
- 45.Malik S., Biba O., Grúz J., Arroo R.R.J., Strnad M. Biotechnological approaches for producing aryltetralin lignans from Linum species. Phytochem. Rev. 2014;13:893–913. doi: 10.1007/s11101-014-9345-5. [DOI] [Google Scholar]
- 46.Zhao J., Davis L.C., Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Berim A., Spring O., Conrad J., Maitrejean M., Boland W., Petersen M. Enhancement of lignan biosynthesis in suspension cultures of Linum nodiflorum by coronalon, indanoyl-isoleucine and methyl jasmonate. Planta. 2005;222:769–776. doi: 10.1007/s00425-005-0019-9. [DOI] [PubMed] [Google Scholar]
- 48.Wang J., Fan S., Li A., Chen T., Sun B., Yang X. Analysis of the Forsythin and Forsythiaside A in different parts of Forsythia suspensa and its medicinal use discussion. Mod. Chin. Med. 2013;15:556–559. [Google Scholar]
- 49.Ye H., Wu Z., Li F.R. Problems and thoughts of folium Forsythia used for medical purpose. Chin. J. Clin. Ration. Drug Use. 2011;4:74–75. [Google Scholar]
- 50.Vanholme R., Demedts B., Morreel K., John R., Wout B. (2010) Lignin biosynthesis and structure. Plant Physiol. 2010;153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., Yang J., Deng K., He X., Zhan R., Tang L. Methyl Jasmonate affects metabolism and gene transcription of volatile terpenoids from Amomum villosum Lour. Mod. Tradit. Chin. Med. Mater. Med.-World Sci. Technol. 2014;16:1528–1536. [Google Scholar]
- 52.Sun B., Wang P., Wang R., Li Y., Xu S. Molecular Cloning and Characterization of a meta/para-O-Methyltransferase from Lycoris aurea. Int. J. Mol. Sci. 2018;19:1911. doi: 10.3390/ijms19071911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao Y., Xu J., Wei J., Sun J., Xu Y., Yang X., Zhang Y., Liu J., Sui C. Expression analysis of glycosyltransferase BcUGT1 from Bupleurum chinense DC. and its expression in E. coli and the target protein purification. Yao Xue Xue Bao = Acta Pharm. Sin. 2013;48:1345–1352. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The sequencing data can be found in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under accession number PRJNA869193.