Abstract
Sleep and circadian rhythm disturbances are less-known risk factors for the development and suboptimal outcomes of diabetes. The goal of this narrative review is to highlight the importance of sleep and circadian rhythm disturbances in the development and outcomes of type 1 diabetes (T1D) and type 2 diabetes (T2D), assess current treatment options and the possible mediating mechanisms. We performed a literature search using PubMed and selected relevant English and Dutch papers. Disturbances of sleep and circadian rhythm are common in people with diabetes. They are associated with an increased risk of developing T2D as well as with suboptimal diabetes outcomes (including higher HbA1c levels and reduced quality of life) for T1D and T2D. Preliminary data suggest that treatment of sleep and circadian rhythm disturbances could improve diabetes outcomes in people with T1D and T2D. Finally, the association with medical parameters appears to be mediated by disturbance in hormones, and by suboptimal self-care including forgetting or postponing glucose monitoring or medication use as well as higher consumption of high fat/high sugary foods. Diabetes may also disturb sleep, for example through nocturnal hypoglycemia and nocturia. We concluded that sleep and circadian rhythm disturbances are closely linked with diabetes. More attention to sleep in regular diabetes care is warranted, while further research is needed on treatment of sleep and circadian rhythm disturbances in the prevention of diabetes and its suboptimal outcomes.
Keywords: sleep, sleep disorders, circadian rhythm, type 1 diabetes, type 2 diabetes
Introduction
Diabetes is a common metabolic non-communicable condition with an increasing prevalence. Almost 90% of the people with diabetes have type 2 diabetes (T2D) and the biggest part of the remaining 10% has type 1 diabetes (T1D).1 T1D and T2D have different pathophysiology; however, both types include altered glucose metabolism, which can lead to microvascular and macrovascular complications and premature mortality.2,3 Diabetes and its daily self-management may negatively affect a person’s quality of life and increase the risk of depression and other psychological problems.4,5
Apart from non-modifiable factors such as increasing age and genetic predisposition, the development of T2D is associated with obesity and lifestyle factors such as unhealthy eating, sedentary behavior and smoking.6 In both T1D and T2D, these lifestyle factors play a role in suboptimal diabetes outcomes, such as high HbA1c levels throughout the course of treatment.7,8 Lifestyle factors that are not often mentioned in this context, are sleep and circadian rhythm.
Sleep is defined as a state of inactivity with a reduced responsiveness to all external stimuli.9 It is a complex phenomenon, as it involves several dimensions (eg, disorders, deficiencies, descriptives) and levels of analysis (eg, self-report, objective measures such as polysomnography).10 Most sleep studies to date have focused on duration, quality and sleep timing (the placement of sleep in a day).11 The latter is important as sleep timing serves as a zeitgeber for the circadian rhythm. The circadian rhythms are the physical, mental, and behavioral changes that follow a 24-hour cycle. The endogenous rhythm responds primarily to light and dark. In this modern society with 24/7 light access, many face disturbances in sleep and circadian rhythm.12 This asynchrony between endogenous rhythms and behavioral sleep-wake schedules is termed circadian misalignment.13
The goal of this narrative review is to highlight the importance of sleep and circadian rhythm disturbances in the development and outcomes of T1D and T2D, current treatment options and the possible mediating mechanisms.
Materials and Methods
A literature search was performed in PubMed from inception until August 2022, using MeSH and tiab search terms indicating sleep: eg, “sleep”, “sleep duration”, “sleep disorder”, “sleep deprivation”, “circadian”, “social jeltag”, “insomnia”, “obstructive sleep apnea”, “restless legs syndrome”, “shift work”, “jetlag”; and diabetes mellitus: eg, “dm 1”, “insulin dependent”, “dm 2” and “non-insulin dependent”. Additionally, search terms for prevalence, treatment and health outcomes were used: eg, “morbidity”, “prevalence”, “chronobiology”, “sleep drug”, “sleep medication” and “health status”. All relevant English or Dutch language original and review studies were read by the authors and summarized. Observational (cross-sectional and longitudinal), experimental and interventional studies were included.
Results
Development of Diabetes
Sleep Duration
In the past decade, quite a large amount of evidence has been collected that shows an association between short sleep duration (often defined as <6h) and the development T2D. A recent meta-analysis of 21 experimental studies showed that sleep restriction reduced insulin sensitivity assessed by several methods, including glucose tolerance tests, homeostatic model assessment of insulin resistance and the hyperinsulinemic euglycemic clamp.14 In a second meta-analysis of observational studies, when compared to other lifestyle factors such as physical inactivity, short sleep duration was associated with a 1.48 higher odds (95% CI: 1.25, 1.76) of incident diabetes.15 An older meta-analysis of prospective observational studies with a follow-up duration of more than three years reported a relative risk (RR) of 1.28 (95% CI 1.03–1.60, p=0.024).16 A recent umbrella review focusing on all possible health outcomes of short sleep did not show a significant association of sleep duration with diabetes development, however there were only few meta-analyses with diabetes as an outcome included.17 The umbrella review did show limited evidence for a higher risk of obesity, an important T2D risk factor.17
In addition to short sleep, long sleep duration (often defined as >9h) has also been associated with the development of T2D. In the Anothaisintawee et al meta-analysis, when compared to other lifestyle factors such as physical inactivity, long sleep duration was associated with a 1.36 higher odds (1.12, 1.65) of incident diabetes.15 Cappuccio et al found a RR of 1.48 (95% CI 1.13–1.96, p=0.005).16 In the Gao et al umbrella review, long sleep was not associated with diabetes, but they did show a 5-times increased risk of stroke, dyslipidemia and cardiovascular mortality.17 Overall, there seems to be a U-shaped association between short and long sleep in the development of T2D, with the lowest risk at 7–8 hours per day.18
Sleep Quality
In addition to sleep duration, there has been quite some evidence on the role of suboptimal sleep quality (often measured as difficulty falling asleep, frequent waking up during the night, low self-reported sleep quality) as well as sleep disorders (ie, insomnia, sleep apnea, restless leg syndrome) and the development of T2D. First, a recent meta-analysis including four studies that fragmented sleep or suppressed certain sleep stages showed that sleep fragmentation had no effect on markers of insulin sensitivity, while certain types of sleep stage (especially slow wave sleep) suppression did show reductions in insulin sensitivity, glucose tolerance and beta-cell function.14 Second, in an older systematic review by Cappuccio et al, they showed a relative risk of T2D incidence for difficulty in initiating sleep of 1.57 (1.25–1.97) and for difficulty in maintaining sleep, of 1.84 (1.39–2.43).16 In the Anothaisintawee et al meta-analysis, compared to other lifestyle factors, obstructive sleep apnea syndrome (OSA) (sleep-disordered breathing with repetitive upper airway collapse) was associated with a RR of 1.49 (95% CI: 1.27, 1.75) of incident diabetes, while difficulty maintaining sleep and difficulty initiating sleep were associated with a RR of 1.74 (95% CI: 1.30, 2.34) and 1.55 (95% CI: 1.21, 1.99).15 In the Gao et al umbrella review, suboptimal sleep quality was not significantly related to diabetes development, however there were only few meta-analyses included with diabetes as an outcome, reducing statistical power.17
Circadian Rhythm
In the past years, there has been more attention to not only sleep duration and quality, but also the role of sleep timing in metabolic functioning. In a recent meta-analysis of 5 experimental studies, which exposed participants to circadian misalignment, a negative association with insulin sensitivity was shown.14 Many observational studies have focused on the extreme form of circadian rhythm disturbance, which is shift work. A recent meta-analysis of 19 studies indicated that shift work was associated with an increased risk of T2D (relative risk = 1.10, 95% CI 1.05–1.14).19 In the Anothaisintawee et al meta-analysis, compared to other lifestyle factors, shift work was associated with a RR of 1.60 (95% CI: 1.20, 2.14) of incident diabetes.15
There are several studies, showing that a more subtle form of circadian disturbance, namely sleep variability (varying the time of going to bed and varying the time of getting up during the week, excluding shift work) is related to development of T2D. In a narrative review, Zuraikat et al 2020 showed that higher standard deviations (SD) across nights of sleep duration and onset or midpoint of sleep were associated with increased odds of having the precursor of T2D, namely the metabolic syndrome, as well as with higher Hba1c levels. Conversely, greater regularity of rest-activity patterns related to lower risk for T2D.20
Finally, there is a highly prevalent form of sleep variability and circadian disturbance, namely social jetlag. Social jetlag is defined as the discrepancy between work schedules, social obligations and biological need for sleep.21–23 Especially evening types, people with a late chronotype, have social jetlag.23,24 In our recent (unpublished) meta-analysis, we showed that in high-quality studies social jetlag was significantly associated with higher BMI as well as higher HbA1c levels, compared to no social jetlag. No statistically significant associations were observed for T2D development.25
Overall, limited to no evidence has been found on the role of (maternal) sleep (duration or quality) as well as circadian disturbance in the development of T1D. A prospective observational study found an association of sleep initiation and maintenance with adult-onset autoimmune diabetes (hazard ratio 95% CI 1.01–2.22), but there were only a limited number of people with “classical” T1D in this group.26 Another preliminary observational study suggests that maternal obesity, in the absence of maternal diabetes, is a risk factor for T1D in the offspring.27 Similarly, in a large observational study among adolescents, body mass index was associated with increased risk for incident T1D in early adulthood.28 Given the close link of obesity with sleep and circadian rhythm disturbances,29 these factors may indirectly contribute to T1D development. More research is, however, needed to confirm that hypothesis and enlighten the mechanisms.
Prevalence
Sleep and circadian rhythm disturbances are common in people with diabetes. The highest prevalence estimates even run over 90%, with problems varying from inadequate sleep, sleep deprivation, nocturia, apnea and leg symptoms.30 The present section focuses on prevalence comparisons with general sleep recommendations, people without diabetes and across diabetes types.
Sleep Duration
Many children <5 years with T1D did not meet the recommended sleeping time, which is approximately 10–13 hours per day, even if naps and overnight sleeping times were combined.31 Also, studies including older children between 5 and 12 years old demonstrated an average total sleeping time at the lower end of the recommended time in children with T1D.32 Similar patterns of short sleeping times were observed in adolescents or adults with T1D, when compared to peers without diabetes.33,34 Furthermore, a small meta-analysis showed that adults with T1D with optimal HbA1c levels (<53 mmol/mol (7%)) had a longer sleep duration (17.3 minutes), compared to those with a suboptimal HbA1c levels.35 Short sleep is not limited to T1D. Research demonstrated the prevalence of insufficient sleep (compared to age recommendations) ranged from 38% to 97% among children, adolescents and adults with T2D.36,37
Sleep Quality
Among parents of 2- to 12-year-old children with T1D, 67% reported their child met criteria for suboptimal sleep quality.38 In the meta-analysis by Reutrakul et al, adults with T1D reported more pronounced suboptimal sleep quality (MD 0.51; 95% CI = 0.33, 0.70), compared to those without T1D.35 This is in line with findings among people with T2D, as for example shown by Birhanu et al 2020, who found a prevalence of suboptimal sleep quality of 47.2% (95% CI: 42.5–52.1).39 In the Dutch Diabetes MILES study, we directly compared suboptimal sleep quality (PSQI-score >5) prevalence across diabetes types, and reported 31% suboptimal sleep in adults with T1D and 42% in adults with T2D.40 Finally, in a small study by Barone et al, adolescents with T1D had higher sleep variability, compared to their healthy peers.41
With regard to sleep problems, we have provided an elegant review on the topic last year.42 For example, we found the pooled prevalence of insomnia (symptoms) in people with T2D to be 39% (95% confidence interval, 34–44),43 while the prevalence of obstructive sleep apnea (OSA) in adults with T1D was 51.9% (95% CI = 31.2, 72.6).35 Overall, we showed lower sleep quality in people with diabetes, while the prevalence of sleep problems is higher than the general population.
Circadian Rhythm
To our knowledge, there have been no reviews on the prevalence of circadian disturbances among people with diabetes. In studies among adults, the prevalence of social jetlag (≥1h) was 46% in T1D44 and 58% in those with T2D.45 However, given the age-dependence of social jetlag these numbers cannot be automatically generalized to the wider population. To illustrate, social jetlag appears highest in adolescents with T1D (mean, SD: 2.5, 1.2 hours)46 and young adults with T1D (mean, SD: 1.62, 0.87 hours),47 followed by adults with T1D (mean, SD: 53, 53 minutes;44 median, interquartile range: 49, 24–79 minutes48) and adults with T2D (median, range 15, 0–304 minutes49 and mean 35 minutes).50 One study directly compared sleep timing parameters between adults with and without T2D, finding a difference in self-reported social jetlag (median 43 vs 23 minutes in people with versus without T2D), suggesting there might be a difference in the prevalence.51
Diabetes Outcomes
The following section will summarize evidence related to the association of sleep and circadian rhythm disturbances with suboptimal diabetes outcomes (glycemic measures, diabetes complications and mortality) in people with diabetes.
Sleep Duration
In recent years, there has been quite an extensive line of research on the association between short sleep duration and suboptimal diabetes outcomes. For example, children with T1D who have insufficient sleep have significantly higher HbA1c levels, compared to children with T1D with sufficient sleep duration.52 A systematic review of Ji et al demonstrated shorter sleep to be associated with higher HbA1c levels in adolescents with T1D. Additionally, a review of Reutrakul et al found that adults with T1D who have short nights of sleep have a significantly higher HbA1c level (+0.24%), compared to adults with T1D sleeping >6h per night.35
These higher HbaA1c levels could be explained by decreased insulin sensitivity. An experimental study of Donga et al investigated the effect of a single night of sleep restriction (4 hours) on glucose tolerance and insulin sensitivity in people with T1D.53 The results showed that a single night of sleep restriction decreased insulin sensitivity with a reduction of insulin-stimulated glucose uptake by 14–21%. These findings are supported by other studies also in adults with T1D.11
For people with T2D, two meta-analyses containing >15 prospective studies showed a U-shaped association between sleep duration and HbA1c levels. Short sleep was associated with a 0.23% higher HbA1C levels and long sleep was associated with a 0.13% higher HbA1c levels.54,55 In addition to glycemic measures, the study of Meng et al demonstrated a higher risk of complications in adults with T2D and short sleep, including diabetic kidney disease and cardiovascular complications.56 Finally, a meta-analysis showed short sleep duration also to be associated with the occurrence of diabetic retinopathy (OR = 1.49, 95% CI 1.15–1.94).57
Sleep Quality
The review of Reutrakul et al suggested that participants with T1D reporting high sleep quality had lower HbA1c, compared to those with low sleep quality (MD = −0.19%; 95% CI = −0.30, −0.08).35 With regard to diabetes complications, a recent meta-analysis of 7 articles including more than 4500 people with diabetes found that low sleep quality related to a higher occurrence of diabetic retinopathy.57 With respect to sleep problems, our meta-analysis showed that in those with T2D having insomnia (symptoms), there are higher HbA1c levels (mean difference, 0.23% [0.1–0.4]) and higher fasting glucose levels (mean difference, 0.40 mmol/L [0.2–0.7]),43 compared to those without insomnia. In addition, among people with T1D with moderate-to-severe OSA there was a trend toward higher HbA1c levels (MD = 0.39%, 95% CI = −0.08, 0.87).35
Circadian Rhythm
Also, circadian disturbance has been related to suboptimal glycemic outcomes. First, compared with day work, shift work was associated with significantly higher HbA1c levels (shift workers had higher mean HbA1c levels than non-shift workers; B=0.67, p<0.05) in both those with T1D58 (ref) and (B = 0.059, p = 0.044) T2D.59 Second, higher sleep variability was significantly associated with suboptimal glycemic outcomes, including glucose levels and medication use (B = 0.100, p = 0.004) in people with T1D.60 Finally, also in our meta-analysis we showed that social jetlag was associated with suboptimal glycemic (ie, glucose) and metabolic outcomes (ie, hypertension) in people with diabetes (both types).25
Treatment
Treatment for sleeping problems may consist of pharmacological therapy or behavioral interventions. In a recent review assessing the effect of all types of sleep interventions on glucose metabolism,61 no meta-analysis could be conducted for sleep medication due to large heterogeneity of the studies. However, the authors did provide a narrative synthesis, suggesting mixed results. First, the studies on orexin receptor antagonists showed an increase in glucose and reduction in HOMA-IR.62,63 Second, the studies on benzodiazepines illustrated no change in insulin sensitivity, and a lowering effect on glucose.64,65 Third, several studies investigated melatonin, which showed no effect on glycemic parameters.61 However, Smirnova et al66 investigated lifestyle advice plus metformin and prolonged-release melatonin 2 mg, which showed a decrease in HOMA-IR as well as an improvement in sleep onset latency and nighttime awakenings. Overall, these studies suggest mixed results for sleep medication on glucose metabolism. Due to the highly addictive nature of the medication, the American Academy of Sleep Medicine prefers behavioral approaches, such as Cognitive Behavior Therapy (CBT) over medication for the treatment of sleeping problems.
CBT-based behavioral strategies consist of cognitive techniques (eg, reducing catastrophizing cognitions about sleep), sleep hygiene, stimulus control, relaxation and mindfulness exercises, and sleep restriction therapy. Multiple studies demonstrated positive effects of CBT-based sleeping interventions on sleep quality,61 glucose and HbA1c levels (although not always statistically significant)61 as well as diabetes self-care management.67 Approaches may be tailored to specific sample needs. For example, while the “Sleep Coach” intervention for adolescents with T1D includes education on sleep habits, coping techniques and relaxation/mindfulness,68 the adapted version for children aged 5–9 years and their parents (“Sleep coach Jr”) also addresses sleeping difficulties such as bedtime resistance and nighttime waking with developmentally appropriate strategies including a “bedtime pass”.69 Another possible behavioral intervention less used in regular care but investigated in research is sleep extension. The aim of sleep extension is to prolong the time in bed with 1–1.5 hours, using behavioral strategies. A recent meta-analysis from 42 studies in the general population, including people with diabetes, showed that interventions resulted in a significantly higher sleep duration, 0.80h (95% CI 0.28 to 1.31). Subgroup analyses revealed that studies directly intervening on sleep duration (ie, specifying the sleep schedule) had larger effects compared to indirect methods (coaching, educational approaches).70 In a preliminary randomized trial among young people with T1D, intervention participants did increase sleep duration as compared to the control group (41 vs 6 minutes), however less than half were likely to continue with the changes in their sleep schedule.71 A second review on the metabolic effects of sleep extension, also in the general population (including T1D and T2D), demonstrated a lower HOMA-IR and improved beta cell function after sleep extension.61
Additionally, there are treatments for specific sleep problems, such as Continuous Positive Airway Pressure (CPAP) or mandibular devices for sleep apnea, which all have been related to improved glycemic outcomes when used in people with diabetes.42 But also pramipexole treatment for restless leg syndrome showed a decrease in related complaints as well as a change in HbA1c levels of −3.2 mmol/mol (95% CI −4.4, −2.2).72 On a more general level, there is the option of weight loss therapy, which in addition to improving insulin sensitivity, also may improve sleep and reduce severity of sleep problems such as OSA.42
With respect to circadian disturbances, the general consensus from the National Institute of Health is to avoid shift work in order to prevent its negative effects on health.73 The field of interventions to restore circadian disturbance and thereby improve glycemic outcomes is limited. There are several options, including education and behavioral therapy, but none has been tested with regard to their endocrine or metabolic effects.74 There is one interesting environmental example related to circadian disturbance that has been tested. Bright light therapy (BLT), which is known for its activating and synchronizing effects, was shown to reduce depressive symptoms as well as improve insulin sensitivity in people with T2D and depression.75 However, much more work is needed to assess if and how treating circadian disturbances can prevent diabetes development and improve diabetes outcomes. Finally, technological developments in glucose management such as algorithm-driven partially automated insulin delivery are showing potential for improving not only glycemic outcomes but also sleep.76
Mechanisms
Evidence suggests that there is a bidirectional relationship of sleep and circadian rhythm disturbances with diabetes.77,78 On the one hand, disturbances in sleep and circadian rhythm may contribute to disturbed glycemic outcomes via direct biological mechanisms (including decreased brain glucose utilization and overactivation of the HPA-axis, disturbed satiety hormones such as leptin and ghrelin)78 and indirectly via suboptimal self-care.79 Suboptimal sleep may negatively affect cognitive processes central to self-care, including accuracy, attention, decision-making, planning and problem solving.80 In a study among adults with T1D, 12% and 33% reported that sleep disruptions affected bolus calculations and decision-making in diabetes care.81 Among young people with T1D suboptimal sleep quality was associated with glucose monitoring difficulties, eg, distractibility and procrastination82 as well as forgetfulness or “laziness” about diabetes self-care.83 Suboptimal sleep may also lead to more unhealthy food choices, including more high fat/high sugar products, and to more sedentary behavior.84 Conversely, increases in sleep of as little as 15–20 minutes have been associated with one additional insulin administration and fingerprick.85 Suboptimal sleep may also hinder self-care through its negative impact on quality of life, across domains such as physical and mental health, family and school/work.81,86 In both T1D and T2D, suboptimal sleep quality relates to higher daytime sleepiness, fatigue, diabetes-specific distress and symptoms of depression and anxiety.40 Not surprisingly, this may create a vicious cycle between emotional distress, reduced self-care, increased glucose levels, and suboptimal sleep.77
On the other hand, diabetes may contribute to the development of disturbed sleep and circadian rhythm directly (nocturnal hypo- and hyperglycemia, nocturia) and indirectly through its management (eg, bodily-worn technological devices; alarms; difficulty falling asleep after correcting glucose excursions), complications (eg, neuropathic pain) and co-morbidities (eg, obesity).11 A special mention should be made for depression, as there is large overlap between the constructs of sleep problems and depression. For example, part of the associations of sleep disturbance with diabetes development and suboptimal outcomes can be explained by depression, illustrated by a reduction in the strength of these associations when adjusting for depression status.15 This is especially true for the associations regarding long sleep.
Discussion
This review illustrates the close link of sleep and circadian rhythm disturbances with diabetes. Suboptimal sleep is associated with an increased risk of developing T2D as well as with suboptimal diabetes outcomes for people with T1D and T2D as depicted in summary Figure 1. Preliminary data suggest that treatment of sleep and circadian rhythm disturbances in people with T1D and T2D could improve not only sleep but also diabetes outcomes. Finally, this association appears to be mediated by disturbances in hormones and changes in self-care behavior. In turn, diabetes and its management may also lead to disturbed sleep.
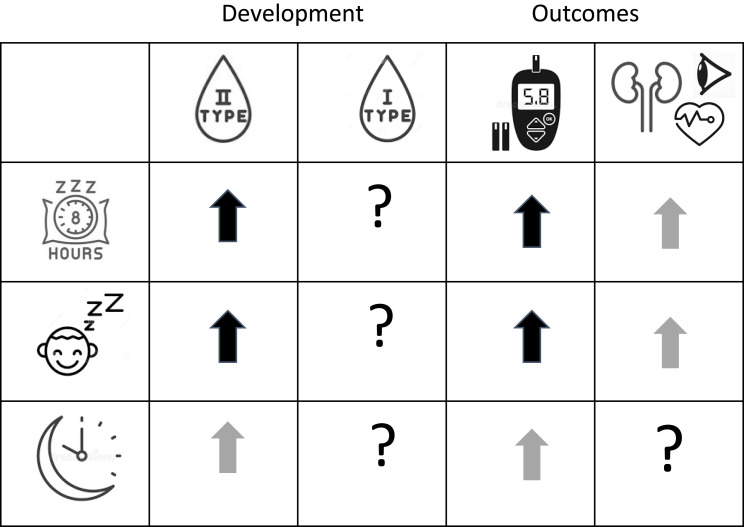
Figure 1.
Summary of the literature to date on the association of sleep duration, sleep quality and sleep timing with the development as well as health outcomes (glycemic levels, complications) of type 1 and type 2 diabetes. ↑Increased risk or higher levels;?, no or insufficient data available. Bold black arrows, strong evidence based on large study sample or multiple studies; grey arrows, medium strength evidence; white arrows, evidence based on small sample or subgroup.
In general, improving sleep and circadian rhythm in people with diabetes could in turn improve glucose levels, thus providing an important aid in improving diabetes outcomes and ultimately improving quality of life.87 Unfortunately, studies on the effect of sleep and circadian rhythm interventions specifically in people with diabetes are limited, either based on small studies or absent. Most first-line treatments of sleep problems seem effective in people with diabetes, comparable to the general population, but with additional positive effects on glycemic measures and other health outcomes, as discussed above. Of high clinical relevance are people with diabetes who partake in shift work, requiring specific guidance in terms of meal preparation and insulin schedules in order to achieve optimal glycemic outcomes.
There are also some points of discussion, for example with regard to the ways to measure sleep and circadian rhythm in practice. The literature shows variation in outcome, depending on the way it is measured: objectively using electroencephalograms or accelerometers versus subjectively using a diary or questionnaires.88 For example, O’brien et al demonstrated this discrepancy: with actigraphy total sleeping time was approximately an hour less than self-reported sleeping time using diaries.89 However, despite this discrepancy, we feel the direction of the associations, namely disturbance of sleep and circadian rhythm to be associated with increased risk of diabetes development as well as suboptimal diabetes outcomes, to still be the same. Finally, there are still quite some research gaps that we identified throughout the review process. For example, limited knowledge is present on the prevalence of circadian disturbances in people with diabetes, and more research is also needed to illustrate the glycemic effects of treatment of sleep or circadian disturbances in people with diabetes. But also observations from clinical practice, such as the experience of more nightmares when having low blood sugar during the night,90 deserve further investigation.
With the advancement of measurement techniques, ecological momentary assessments (EMA) could aid the assessment of sleep, sleep disorders and circadian rhythm. EMA involves repeated sampling of participants´ behaviors and experiences in real-time and in the participants’ natural environments, often using smartphone applications.91 For example, one could ask regular questions on the status of a sleep problem and thus provide a more representative picture of the course of the condition by multiple assessments collected over a relatively short period. But also the use of activity trackers measuring sleep or even portable devices measuring sleep phases could provide enormous amounts of information on sleep and circadian rhythm in people with diabetes.92 Using EMA allows for better evaluation of within-person changes, which in chronic conditions such as diabetes could provide valuable information for the people living with them, health-care providers and researchers.93 A recent review of studies using EMA in people with diabetes to assess stress, anxiety, and depression showed that increases in those parameters predicted reductions in self-care behaviors93, suggesting that, despite limited research (only 10 studies included), EMA has potential clinical utility for diabetes care to measure sleep and circadian rhythm. However, we should make sure that the use of such technologies does not widen health inequalities since these technologies might not be widely available to all who may need them.
In Practice
Given their high prevalence and adverse consequences, sleep and circadian rhythm disturbances require more attention in the prevention and treatment of diabetes. Since 2017, the Standards of Medical Care of the American Diabetes Association include a recommendation to regularly assess sleep given its association with glycemic outcomes.94 These recommendations are yet to be widely implemented, while recent studies have further stressed the close link between suboptimal sleep and diabetes difficulties. This suggests that not only people with diabetes, but also their health professionals may benefit from more information on the reciprocal link between sleep and diabetes, including treatment options. By providing an overview of the present literature, this review contributes to increased awareness that sleep may be as central to physical and mental health as diet and physical activity.
Conclusions
We concluded that sleep and circadian rhythm disturbances are closely linked with diabetes. More attention to sleep in regular diabetes care is warranted, while further research is needed on treatment of sleep and circadian rhythm disturbances in the prevention of diabetes and its suboptimal outcomes.
Acknowledgments
We thank Fleur Pals for her contribution to the literature search.
Funding Statement
The authors did not receive funding for the writing of this manuscript.
Data Sharing Statement
This review did not generate any new data.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
Dr Femke Rutters is an associate editor at Diabetologia and has received funding from the Dutch Diabetes Research Foundation related to sleep and diabetes. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.GBD Disease Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Zinman B, Cleary PA, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med. 2009;169(14):1307–1316. doi: 10.1001/archinternmed.2009.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am. 2013;42(3):529–544. doi: 10.1016/j.ecl.2013.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Smith KJ, Beland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74(2):89–99. doi: 10.1016/j.jpsychores.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11(11):1185–1200. doi: 10.7150/ijms.10001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang YB, Pan XF, Chen JX, et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63(1):21–33. doi: 10.1007/s00125-019-04985-9 [DOI] [PubMed] [Google Scholar]
- 8.Leroux C, Brazeau AS, Gingras V, Desjardins K, Strychar I, Rabasa-Lhoret R. Lifestyle and cardiometabolic risk in adults with type 1 diabetes: a review. Can J Diabetes. 2014;38(1):62–69. doi: 10.1016/j.jcjd.2013.08.268 [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie RP, Patel SR. The epidemiology of sleep and diabetes. Curr Diab Rep. 2018;18(10):82. doi: 10.1007/s11892-018-1055-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–U219. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nefs GM, Bazelmans E, Donga E, Tack CJ, de Galan BE. Sweet dreams or bitter nightmare: a narrative review of 25 years of research on the role of sleep in diabetes and the contributions of behavioural science. Diabet Med. 2020;37(3):418–426. doi: 10.1111/dme.14211 [DOI] [PubMed] [Google Scholar]
- 12.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75–89. doi: 10.1038/s41574-018-0122-1 [DOI] [PubMed] [Google Scholar]
- 13.Reutrakul S, Van Cauter E. Interactions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetes. Ann Ny Acad Sci. 2014;1311:151–173. doi: 10.1111/nyas.12355 [DOI] [PubMed] [Google Scholar]
- 14.Sondrup N, Termannsen AD, Eriksen JN, et al. Effects of sleep manipulation on markers of insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2022;62:101594. doi: 10.1016/j.smrv.2022.101594 [DOI] [PubMed] [Google Scholar]
- 15.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi: 10.2337/dc09-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao C, Guo J, Gong TT, et al. Sleep duration/quality with health outcomes: an umbrella review of meta-analyses of prospective studies. Front Med. 2021;8:813943. doi: 10.3389/fmed.2021.813943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan Z, Ma H, Xie M, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38(3):529–537. doi: 10.2337/dc14-2073 [DOI] [PubMed] [Google Scholar]
- 19.Gao YY, Gan T, Jiang LL, et al. Association between shift work and risk of type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of observational studies. Chronobiol Int. 2020;37(1):29–46. doi: 10.1080/07420528.2019.1683570 [DOI] [PubMed] [Google Scholar]
- 20.Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St-Onge MP. Sleep regularity and cardiometabolic health: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr Diabetes Rep. 2020;20(8). doi: 10.1007/s11892-020-01324-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–943. doi: 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 22.Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology-Basel. 2019;8(3). doi: 10.3390/biology8030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. doi: 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 24.Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29(9):1153–1175. doi: 10.3109/07420528.2012.719971 [DOI] [PubMed] [Google Scholar]
- 25.Bouman EJ, Beulens JWJ, Groeneveld L, et al. The association between social jetlag and (parameters of) metabolic syndrome and type 2 diabetes: a systematic review and meta-analysis. under review. J Sleep Res. 2022. doi: 10.1111/jsr.13770 [DOI] [PubMed] [Google Scholar]
- 26.Olsson L, Ahlbom A, Grill V, Midthjell K, Carlsson S. Sleep disturbances and low psychological well-being are associated with an increased risk of autoimmune diabetes in adults. Results from the Nord-Trondelag Health Study. Diabetes Res Clin Pract. 2012;98(2):302–311. doi: 10.1016/j.diabres.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 27.Lindell N, Carlsson A, Josefsson A, Samuelsson U. Maternal obesity as a risk factor for early childhood type 1 diabetes: a nationwide, prospective, population-based case-control study. Diabetologia. 2018;61(1):130–137. doi: 10.1007/s00125-017-4481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zucker I, Zloof Y, Bardugo A, et al. Obesity in late adolescence and incident type 1 diabetes in young adulthood. Diabetologia. 2022;65(9):1473–1482. doi: 10.1007/s00125-022-05722-5 [DOI] [PubMed] [Google Scholar]
- 29.Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3(5):383–388. doi: 10.1016/j.sleh.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plantinga L, Rao MN, Schillinger D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005–2008. Prev Chronic Dis. 2012;9:E76. [PMC free article] [PubMed] [Google Scholar]
- 31.Monaghan M, Herbert LJ, Cogen FR, Streisand R. Sleep behaviors and parent functioning in young children with type 1 diabetes. Child Health Care. 2012;41(3):246–259. doi: 10.1080/02739615.2012.685385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perfect MM. Sleep-related disorders in patients with type 1 diabetes mellitus: current insights. Nat Sci Sleep. 2020;12:101–123. doi: 10.2147/Nss.S152555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji XP, Wang YQ, Saylor J. Sleep and type 1 diabetes mellitus management among children, adolescents, and emerging young adults: a systematic review. J Pediatr Nurs. 2021;61:245–253. doi: 10.1016/j.pedn.2021.06.010 [DOI] [PubMed] [Google Scholar]
- 34.Denic-Roberts H, Costacou T, Orchard TJ. Subjective sleep disturbances and glycemic control in adults with long-standing type 1 diabetes: the Pittsburgh’s epidemiology of diabetes complications study. Diabetes Res Clin Pr. 2016;119:1–12. doi: 10.1016/j.diabres.2016.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reutrakul S, Thakkinstian A, Anothaisintawee T, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26–45. doi: 10.1016/j.sleep.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutil C, Chaput JP. Inadequate sleep as a contributor to type 2 diabetes in children and adolescents. Nutr Diabetes. 2017;7:e266–e266. doi: 10.1038/nutd.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeil J, Doucet E, Chaput JP. Inadequate sleep as a contributor to obesity and type 2 diabetes. Can J Diabetes. 2013;37(2):103–108. doi: 10.1016/j.jcjd.2013.02.060 [DOI] [PubMed] [Google Scholar]
- 38.Jaser SS, Foster NC, Nelson BA, et al. Sleep in children with type 1 diabetes and their parents in the T1D Exchange. Sleep Med. 2017;39:108–115. doi: 10.1016/j.sleep.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birhanu TT, Salih MH, Abate HK. Sleep quality and associated factors among diabetes mellitus patients in a follow-up clinic at the University of Gondar comprehensive specialized hospital in Gondar, Northwest Ethiopia: a cross-sectional study. Diabet Metab Synd Ob. 2020;13:4859–4868. doi: 10.2147/Dmso.S285080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nefs G, Donga E, van Someren E, Bot M, Speight J, Pouwer F. Subjective sleep impairment in adults with type 1 or type 2 diabetes: results from Diabetes MILES-The Netherlands. Diabetes Res Clin Pr. 2015;109(3):466–475. doi: 10.1016/j.diabres.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 41.Barone MTU, Wey D, Schorr F, et al. Sleep and glycemic control in type 1 diabetes. Arch Endocrin Metab. 2015;59(1):71–78. doi: 10.1590/2359-3997000000013 [DOI] [PubMed] [Google Scholar]
- 42.Schipper SBJ, Van Veen MM, Elders PJM, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. 2021;64(11):2367–2377. doi: 10.1007/s00125-021-05541-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koopman ADM, Beulens JW, Dijkstra T, et al. Prevalence of Insomnia (Symptoms) in T2D and association with metabolic parameters and glycemic control: meta-analysis. J Clin Endocr Metab. 2020;105(3):614–643. doi: 10.1210/clinem/dgz065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rusu A, Ciobanu D, Bala C, Cerghizan A, Roman G. Social jetlag, sleep-related parameters, and glycemic control in adults with type 1 diabetes: results of a cross-sectional study. J Diabetes. 2019;11(5):394–401. doi: 10.1111/1753-0407.12867 [DOI] [PubMed] [Google Scholar]
- 45.Alabdulkarim A, Alayed O, Aloraini O, Almozini M, Aldawsari K, Bin Khathlan YZ. The association between social jetlag and glycemic control in diabetic patients at King Saud University Medical City. Cureus. 2020;12(7). doi: 10.7759/cureus.9215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Schnurbein J, Boettcher C, Brandt S, et al. Sleep and glycemic control in adolescents with type 1 diabetes. Pediatr Diabetes. 2018;19(1):143–149. doi: 10.1111/pedi.12538 [DOI] [PubMed] [Google Scholar]
- 47.Saylor J, Ji XP, Calamaro CJ, Davey A. Does sleep duration, napping, and social jetlag predict hemoglobin A1c among college students with type 1 diabetes mellitus?. Diabetes Res Clin Pr. 2019;148:102–109. doi: 10.1016/j.diabres.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larcher S, Gauchez AS, Lablanche S, Pepin JL, Benhamou PY, Borel AL. Impact of sleep behavior on glycemic control in type 1 diabetes: the role of social jetlag. Eur J Endocrinol. 2016;175(5):411–419. doi: 10.1530/Eje-16-0188 [DOI] [PubMed] [Google Scholar]
- 49.Kelly RM, Finn J, Healy U, et al. Greater social jetlag associates with higher HbA1c in adults with type 2 diabetes: a cross sectional study. Sleep Med. 2020;66:1–9. doi: 10.1016/j.sleep.2019.07.023 [DOI] [PubMed] [Google Scholar]
- 50.Zhu BQ, Kapella MC, Zhao XX, Fritschi C. Intra-individual variability in sleep is related to glycaemic control in adults with type 2 diabetes. J Adv Nurs. 2020;76(4):991–998. doi: 10.1111/jan.14290 [DOI] [PubMed] [Google Scholar]
- 51.Kelly RM, Healy U, Sreenan S, McDermott J, Coogan AN. An exploratory study of associations between sleep timing variability and cardiometabolic health in middle-aged adults with type 2 diabetes mellitus. Chronobiol Int. 2022;39(4):569–578. doi: 10.1080/07420528.2021.2005083 [DOI] [PubMed] [Google Scholar]
- 52.Farabi SS. Type 1 diabetes and sleep. Diabetes Spectr. 2016;29(1):10–13. doi: 10.2337/diaspect.29.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donga E, van Dijk M, van Dijk JG, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care. 2010;33(7):1573–1577. doi: 10.2337/dc09-2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91–101. doi: 10.1016/j.smrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Kim BK, Kim BS, An SY, et al. Sleep duration and glycemic control in patients with diabetes mellitus: Korea National Health And Nutrition Examination Survey 2007–2010. J Korean Med Sci. 2013;28(9):1334–1339. doi: 10.3346/jkms.2013.28.9.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng LL, Liu Y, Geng RN, Tang YZ, Li DQ. Association of diabetic vascular complications with poor sleep complaints. Diabetol Metab Syndr. 2016;8. doi: 10.1186/s13098-016-0195-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng ZZ, Wang CY, Li CH, et al. Meta-analysis of relationship of sleep quality and duration with risk of diabetic retinopathy. Front Endocrinol. 2022;13. doi: 10.3389/fendo.2022.922886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young J, Waclawski E, Young JA, Spencer J. Control of type 1 diabetes mellitus and shift work. Occup Med. 2013;63(1):70–72. doi: 10.1093/occmed/kqs176 [DOI] [PubMed] [Google Scholar]
- 59.Manodpitipong A, Saetung S, Nimitphong H, et al. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J Sleep Res. 2017;26(6):764–772. doi: 10.1111/jsr.12554 [DOI] [PubMed] [Google Scholar]
- 60.Chontong S, Saetung S, Reutrakul S. Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. J Sleep Res. 2016;25(4):438–444. doi: 10.1111/jsr.12393 [DOI] [PubMed] [Google Scholar]
- 61.Kothari V, Cardona Z, Chirakalwasan N, Anothaisintawee T, Reutrakul S. Sleep interventions and glucose metabolism: systematic review and meta-analysis. Sleep Med. 2021;78:24–35. doi: 10.1016/j.sleep.2020.11.035 [DOI] [PubMed] [Google Scholar]
- 62.Nakamura M, Nagamine T. Suvorexant as an orexin antagonist may regulate serum glucose levels in psychiatric patients with insomnia. Psychiat Clin Neuros. 2017;71(12):844. doi: 10.1111/pcn.12608 [DOI] [PubMed] [Google Scholar]
- 63.Nakamura M, Nagamine T. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov Clin Neurosci. 2017;14(3–4):30–37. [PMC free article] [PubMed] [Google Scholar]
- 64.Buxton OM, Pavlova MK, O’Connor SP, Wang W, Winkelman JW. Lack of change in glucose metabolism in eszopiclone-treated primary insomnia patients. Nat Sci Sleep. 2017;9:187–198. doi: 10.2147/Nss.S130505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu XY, Lin JH. Clinical evaluation of dexzopiclone in the treatment of type 2 diabetes mellitus accompanied by insomnia. Chin J New Drug. 2011;20(1):47–49. [Google Scholar]
- 66.Smirnova VO, Barykina IN, Salasyuk A, Khripaeva VY, Palashkin RV, Nedogoda SV. Slow release melatonine in metabolic syndrome sympthomatics correction. Russ J Cardiol. 2016;134(6):61–67. doi: 10.15829/1560-4071-2016-6-61-67 [DOI] [Google Scholar]
- 67.Alshehri MM, Alothman SA, Alenazi AM, et al. The effects of cognitive behavioral therapy for insomnia in people with type 2 diabetes mellitus, pilot RCT part II: diabetes health outcomes. Bmc Endocr Disord. 2020;20(1). doi: 10.1186/s12902-020-00612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jaser SS, Hamburger ER, Bergner EM, et al. Sleep coach intervention for teens with type 1 diabetes: randomized pilot study. Pediatr Diabetes. 2020;21(3):473–478. doi: 10.1111/pedi.12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaser SS, Bergner EM, Hamburger ER, et al. Pilot trial of a sleep-promoting intervention for children with type 1 diabetes. J Pediatr Psychol. 2021;46(3):304–313. doi: 10.1093/jpepsy/jsaa105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baron KG, Duffecy J, Reutrakul S, et al. Y Behavioral interventions to extend sleep duration: a systematic review and meta-analysis. Sleep Med Rev. 2021;60:101532. doi: 10.1016/j.smrv.2021.101532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perfect MM, Beebe D, Levine-Donnerstein D, Frye SS, Bluez GP, Quan SF. The development of a clinically relevant sleep modification protocol for youth with type 1 diabetes. Clin Pract Pediatr Psychol. 2016;4(2):227–240. doi: 10.1037/cpp0000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwatra V, Khan MA, Quadri SA, Cook TS. Differential diagnosis and treatment of restless legs syndrome: a literature review. Cureus. 2018;10(9). doi: 10.7759/cureus.3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Costa G. Shift work and health: current problems and preventive actions. Saf Health Work. 2010;1(2):112–123. doi: 10.5491/Shaw.2010.1.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez-Ortuno MM, Edinger JD. Internight sleep variability: its clinical significance and responsiveness to treatment in primary and comorbid insomnia. J Sleep Res. 2012;21(5):527–534. doi: 10.1111/j.1365-2869.2012.01010.x [DOI] [PubMed] [Google Scholar]
- 75.Brouwer A, van Raalte DH, Nguyen HT, et al. Effects of light therapy on mood and insulin sensitivity in patients with type 2 diabetes and depression: results from a randomized placebo-controlled trial. Diabetes Care. 2019;42(4):529–538. doi: 10.2337/dc18-1732 [DOI] [PubMed] [Google Scholar]
- 76.Nefs G. The psychological implications of automated insulin delivery systems in type 1 diabetes care. Front Clin Diabetes Healthc. 2022;3:846162. doi: 10.3389/fcdhc.2022.846162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monzon A, McDonough R, Meltzer LJ, Patton SR. Sleep and type 1 diabetes in children and adolescents: proposed theoretical model and clinical implications. Pediatr Diabetes. 2019;20(1):78–85. doi: 10.1111/pedi.12797 [DOI] [PubMed] [Google Scholar]
- 78.Barone MTU, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pr. 2011;91(2):129–137. doi: 10.1016/j.diabres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 79.Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39(1):74–82. doi: 10.1177/0145721712467683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Riegel B, Weaver TE. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nur. 2009;8(5):337–344. doi: 10.1016/j.ejcnurse.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barnard K, James J, Kerr D, Adolfsson P, Runion A, Serbedzija G. Impact of chronic sleep disturbance for people living with t1 diabetes. J Diabetes Sci Technol. 2016;10(3):762–767. doi: 10.1177/1932296815619181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turner SL, Queen TL, Butner J, Wiebe D, Berg CA. Variations in daily sleep quality and type 1 diabetes management in late adolescents. J Pediatr Psychol. 2016;41(6):661–669. doi: 10.1093/jpepsy/jsw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bergner EM, Williams R, Hamburger ER, et al. Sleep in teens with type 1 diabetes: perspectives from adolescents and their caregivers. Diabetes Educ. 2018;44(6):541–548. doi: 10.1177/0145721718799086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obesity. 2012;36(10):1346–1352. doi: 10.1038/ijo.2011.250 [DOI] [PubMed] [Google Scholar]
- 85.McDonough RJ, Clements MA, DeLurgio SA, Patton SR. Sleep duration and its impact on adherence in adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18(4):262–270. doi: 10.1111/pedi.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perfect MM, Patel PG, Scott RE, et al. Sleep, glucose, and daytime functioning in youth with type 1 diabetes. Sleep. 2012;35(1):81–88. doi: 10.5665/sleep.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pereira FH, Trevisan DD, Santos Lourenço D, da Silva JB, Lima MHM. Effect of educational strategies on the sleep quality of people with diabetes: randomized clinical trial. Aquichan. 2019;19(3):1–13. doi: 10.5294/aqui.2019.19.3.2 [DOI] [Google Scholar]
- 88.Kripke DF, Langer RD, Elliott JA, Klauber MR, Rex KM. Mortality related to actigraphic long and short sleep. Sleep Med. 2011;12(1):28–33. doi: 10.1016/j.sleep.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Brien E, Hart C, Wing RR. Discrepancies Between self-reported usual sleep duration and objective measures of total sleep time in treatment-seeking overweight and obese individuals. Behav Sleep Med. 2016;14(5):539–549. doi: 10.1080/15402002.2015.1048447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frier BM. How hypoglycaemia can affect the life of a person with diabetes. Diabetesm Metab Res. 2008;24(2):87–92. doi: 10.1002/dmrr.796 [DOI] [PubMed] [Google Scholar]
- 91.Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatr Neurosci. 2006;31(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- 92.Arnal PJ, Thorey V, Debellemaniere E, et al. The Dreem Headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep. 2020;43(11). doi: 10.1093/sleep/zsaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nam S, Griggs S, Ash GI, et al. Ecological momentary assessment for health behaviors and contextual factors in persons with diabetes: a systematic review. Diabetes Res Clin Pr. 2021;174:108745. doi: 10.1016/j.diabres.2021.108745 [DOI] [PubMed] [Google Scholar]
- 94.American Diabetes Association. Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(S1):S1–S135. doi: 10.2337/dc17-S001 [DOI] [PubMed] [Google Scholar]