Abstract
We examined the role of cytokines in the development of gamma interferon (IFN-γ)-secreting protective T cells following immunization with a culture filtrate subunit vaccine against Mycobacterium tuberculosis containing the adjuvant dimethyldioctadecylammonium bromide (DDA). Depletion of either interleukin-6 (IL-6) or IL-12 with specific neutralizing antibodies during vaccination reduced the priming of T cells for antigen-specific proliferation and IFN-γ secretion. Such reduction was also observed in IL-6 gene-disrupted mice as compared to wild-type animals. IL-6 was found to play a role in the initial differentiation of Th1 cells but not in their expansion. The defect found after IL-6 depletion or in IL-6-knockout mice was compensated by the inclusion of recombinant mouse IL-12 in the vaccine. The induction of protective immunity against an intravenous or an aerosol challenge with live, virulent M. tuberculosis was markedly reduced by neutralizing either IL-6 or IL-12 during immunization with the vaccine. Likewise, the effects of IL-6 neutralization were partially reversed by including IL-12 in the vaccine. Our data point to an important role of IL-6 and IL-12 in the generation of cell-mediated immunity to tuberculosis.
Tuberculosis still accounts for the deaths of around three million patients every year (13), and the emergence of multiple-drug-resistant microorganisms makes this disease a major health problem (14). The design of a tuberculosis vaccine that will perform better than Mycobacterium bovis BCG may aid in the solution of the tuberculosis epidemic. In that context, a subunit protein vaccine, composed of the secreted antigens of Mycobacterium tuberculosis, is a potential candidate (2, 19, 33, 34). These preparations will have to be administered together with an adjuvant that will prime T cells for a protective function as well as for secretion of gamma interferon (IFN-γ) upon challenge with their cognate antigen (10, 16). Many adjuvants have been tested in animal models, but few are accepted for medical use in human beings. Dimethyldioctadecylammonium bromide (DDA) is one of those adjuvants already used in human vaccines (46; for a review, see reference 18). It has already been demonstrated that this adjuvant will promote a type 1 T-helper-cell response, namely, when used in a tuberculosis subunit vaccine (24). Several studies have also shown that emulsifying short-term-culture filtrate (ST-CF) proteins from M. tuberculosis in DDA will lead to the development of an immune response that will give a considerable level of protection against a subsequent challenge with virulent tubercle bacilli (1, 24). However, the levels of protection are often below those conferred by BCG in such murine models. A possible way to improve the efficacy of such a vaccine would be to include cytokines that would boost the priming of the protective T cells. However, it is still unclear which cytokines intervene in the development of a T-cell response in an immunized organism.
Cytokines involved in the development of T cells in a type 1 pattern of response include interleukin-12 (IL-12) (50) and IL-18 (29, 32, 38, 48). On the other hand, IL-4 has the opposite effect by decreasing the expression of the beta 2 chain of the IL-12 receptor, thereby preventing the action of IL-12 on the T-helper-cell precursors (40, 47). The role of IL-6 is unclear since it has been shown that this cytokine is required for the induction of protective Th1 cells during experimental infections by Mycobacterium avium (5), M. tuberculosis (23), and Listeria monocytogenes (25–27), whereas others have shown that IL-6 is involved in the generation of Th2 responses (37). Additionally, it has been shown that IL-6 can act on the infected macrophages harboring mycobacteria and promote mycobacterial growth (12, 44) or antagonize the effects of bacteriostasis-inducing cytokines such as tumor necrosis factor alpha (7).
We therefore decided to investigate the roles of several cytokines involved in the response to a tuberculosis subunit vaccine that includes ST-CF from M. tuberculosis as the antigen and DDA as the adjuvant. Our data demonstrate that both IL-6 and IL-12 are required for an efficient priming of an IFN-γ response as well as for the generation of protective immunity against M. tuberculosis following such vaccination.
MATERIALS AND METHODS
Animals.
C57BL/6 female mice, aged 7 to 14 weeks, were purchased from the Gulbenkian Institute (Oeiras, Portugal). IL-6 gene-knockout (IL6-KO) mice and wild-type control mice derived from (C57BL/6 × 129)F2 interbreeding were a kind gift from Manfred Kopf (22) and were maintained at our animal facilities. IL6-KO mice with a C57BL/6 background were obtained in our laboratory by backcrossing the original strain into a C57BL/6 background for six generations and then screening the genomic DNA as described (22). C57BL/6 mice were used as controls in the experiments where these backcrossed IL6-KO mice were used.
Bacteria.
M. tuberculosis Erdman (batch 3) was grown at 37°C on Löwenstein-Jensen medium or suspended in modified Sauton medium enriched with 0.5% sodium pyruvate and 0.5% glucose (3).
Reagents.
Monoclonal antibodies specific for individual cytokines were purified from the ascitic fluid of nude mice injected intraperitoneally (i.p.) with the following hybridomas: MP5-20F3 secreting a rat immunoglobulin G1 (IgG1) specific for mouse IL-6 (DNAX, Palo Alto, Calif.); S4B6 secreting a rat IgG2a specific for mouse IL-2 (American Type Culture Collection, Manassas, Va.); 11B11 secreting a rat IgG1 specific for mouse IL-4 (DNAX); JES5-2A5 secreting a rat IgG1 specific for mouse IL-10 (DNAX); C15.1 and C15.6, two hybridomas secreting rat IgG1 specific for mouse IL-12 (The Wistar Institute, Philadelphia, Pa.); and GL113 secreting a rat IgG1 specific for β-galactosidase (DNAX). Ascites were delipidated with an organic solvent (1:4 mixture of 1-butanol and ethyl ether, respectively) and were sterile filtered before purification on a recombinant protein G agarose affinity column (Gibco BRL, Paisley, United Kingdom). Purified antibodies were dialyzed against phosphate-buffered saline (PBS) and were sterile filtered.
ST-CF was produced at the Statens Seruminstitut as described previously (3). Briefly, M. tuberculosis (4 × 106 CFU/ml) was grown in modified Sauton medium without Tween 80 on an orbital shaker for 7 days. The culture supernatants were sterile filtered and concentrated on an Amicon YM3 membrane (Amicon, Danvers, Mass.).
Recombinant mouse IL-12 was obtained from the Genetics Institute (Cambridge, Mass.), and recombinant human IL-6 was obtained from Ares-Serono (Geneva, Switzerland). Tissue culture reagents were from Gibco, and bacterial culture media were from Difco (Detroit, Mich.).
Experimental vaccine.
The experimental vaccine consisted of a mixture of ST-CF and DDA (Eastman Kodak Inc., Rochester, N.Y.). DDA was dissolved in bidistilled water, warmed in a water bath at 80°C for 10 min, cooled at room temperature, and mixed with an equal volume of ST-CF, so as to inject each animal with 250 μg of DDA and 50 μg of ST-CF in a total volume of 200 μl. Whenever recombinant IL-12 was used in the vaccine, it was added directly to the mixture of ST-CF and DDA in a dose of 0.5 μg per animal. Similarly, in some experiments, different doses of recombinant human IL-6 were given with the emulsion of antigen and DDA.
Immunizations.
Mice were injected subcutaneously (s.c.) at the dorsal base of the tail, three times at weekly intervals. Each 200-μl dose of the vaccine was divided in two and then injected in two separate sites. Monoclonal antibodies specific for different cytokines or an isotype-matched control antibody with irrelevant specificity was administered i.p. on the day of the first and third immunizations, 2 to 3 h before the vaccine, in a dose of 2 mg per animal. In two experiments, only two administrations of the vaccine were done, 2 weeks apart from each other. In some experiments, recombinant cytokines were mixed with the vaccine given as described above.
Lymphocyte cultures.
Lymphocytes were obtained by preparing single-cell suspensions either from lymph nodes (inguinal and iliac) or from spleens by dispersion of the tissue through a sterilized stainless steel mesh as described previously (4). Erythrocytes were lysed with a solution containing 155 mM ammonium chloride and 10 mM potassium bicarbonate buffer (3 ml of solution per spleen), and cells were thoroughly washed. Isolated cells were cultured in microtiter wells, each containing 2 × 105 cells in a volume of 200 μl of RPMI 1640 medium supplemented with 5 × 10−5 M 2-mercaptoethanol, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 2 mM 2-glutamine, and 10% (vol/vol) fetal calf serum. ST-CF was used to stimulate cells at a concentration of 4 μg/ml. Cell proliferation was investigated by pulsing cultures after 48 h of incubation (0.5 μCi of [3H]thymidine per well). After 18 to 20 h of incubation, plates were harvested and processed for liquid scintillation counting. All tests were carried out in triplicate. Supernatants from the cultures were also tested for the determination of cytokines by harvesting parallel cultures after 48 h of incubation, except where indicated otherwise. For the enzyme-linked immunospot (ELISPOT) assay, cells were cultured in 24-well plates, each well containing 4 × 106 cells in a volume of 1 ml of RPMI 1640 medium supplemented with 5 × 10−5 M 2-mercaptoethanol, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 2 mM 2-glutamine, and 5% (vol/vol) fetal calf serum.
Cytokine enzyme-linked immunosorbent assay.
The cytokine content in supernatants was determined by enzyme-linked immunosorbent assay by using the antibody pairs specific for IFN-γ secreted by hybridoma cell line R4-6A2 (American Type Culture Collection) as coating antibody and by AN18 (DNAX) as detecting antibody. The standards were made of recombinant IFN-γ from Genzyme (Cambridge, Mass.). The sensitivity of the assay was such that it could detect 80 pg of the cytokine per ml.
ELISPOT technique.
The ELISPOT assay was performed as described by Muller et al. (31) with the minor modifications introduced by Brandt et al. (9). Briefly, microtiter plates were coated with 2.5 μg of monoclonal rat anti-mouse IFN-γ (R4-6A2 cell line) per ml and were incubated overnight at 4°C. Plates were emptied and blocked for 2 h, followed by washing with PBS containing 0.05% Tween 20. Analyses were conducted on cells pooled from three mice. Cells were stimulated as described above with 4 μg of ST-CF per ml of modified RPMI 1640 medium for 18 to 22 h and were subsequently cultured for 6.5 h directly on the ELISPOT plates. For each group of cultured cells, six serial twofold dilutions were prepared with a starting concentration of 4 × 105 cells (every sample was run in duplicate). Cells were removed by washing the plates, and the site of cytokine secretion was detected by biotin-labeled rat anti-mouse IFN-γ monoclonal antibody (AN-18 cell line) and phosphatase-conjugated streptavidin. The enzyme reaction was developed with 0.9 mg of 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Sigma Chemical Co., St. Louis, Mo.) per ml of substrate buffer (0.74 mM MgCl2, 0.1% NaN3, 0.01% Triton X-405, and 9.6% 2-amino-2-methyl-1-propanol, pH 10.25) containing 0.6% agarose. Blue spots were counted microscopically. The relationship between the number of spots developed per well and the number of input cells per well was determined. Data are presented as the number of spots per 4 × 105 spleen cells.
Infection and bacterial enumeration in organs.
Groups of five mice were immunized s.c. with 5 × 104 CFU of BCG (Danish strain 1331). Other groups of five mice were immunized with three weekly doses of ST-CF (50 μg) in DDA (250 μg) with or without recombinant IL-12 (0.5 μg with each immunization, mixed in the vaccine). These latter groups received i.p. injections of either a control antibody with irrelevant specificity or anti-IL-6 or anti-IL-12 monoclonal antibodies with the first and third immunizations and 2 weeks after the last immunization (2 mg of antibody per dose). Mice were infected intravenously (i.v.) by injection with 0.1 ml of a solution containing 5 × 105 CFU of M. tuberculosis Erdman batch 3 per ml via the lateral vein of the tail 6 weeks after the last immunization, and the mice were sacrificed 2 weeks after infection. In some experiments, mice were subjected to an aerosol challenge with M. tuberculosis Erdman batch 3, leading to a pulmonary seeding which induced 15 to 20 lung granulomas 6 weeks after the last immunization, and these mice were sacrificed 6 weeks after infection. Animals were killed by cervical dislocation, and the organs were removed for bacterial enumeration. The organs were homogenized in PBS, and serial threefold dilutions were plated on Middlebrook 7H10 agar plates. After 4 weeks of incubation at 37°C, the numbers of bacteria were determined. The resulting values are presented as means of log10 CFU per organ ± 1 standard deviation or as log10 units of resistance, corresponding to the difference between the log10 CFU in control (nonimmune) mice and the log10 CFU in the immunized groups.
Statistical analysis.
The Student's t test using unpaired data and analysis of variance were used to compare data from the experiments.
RESULTS
In order to assess the requirements for the induction of IFN-γ-producing T cells, we initially immunized C57BL/6 mice with one, two, or three doses of the experimental vaccine consisting of 50 μg of ST-CF admixed with 0.25 mg of DDA given at weekly intervals. We chose such a narrow period for immunization in order to be able to modulate the response with neutralizing antibodies within the shortest period of time possible. All monoclonal antibodies used were previously tested in other models in our laboratory and were shown to be active in depleting the cytokines. Three weeks after the last administration, the animals were sacrificed, the spleens were collected, and spleen cell responses were analyzed in vitro after stimulation with ST-CF proteins. We saw that one immunization alone primed spleen cells for very low levels of IFN-γ secretion (441 ± 178 pg/ml) and for cell proliferation (3.2-fold increase over nonimmunized cells), but either two or three immunizations primed them for very high levels of antigen-specific IFN-γ production (16,107 ± 2,071 and 10,842 ± 1,650 pg/ml for two and three immunizations, respectively) and cell proliferation (4.9- and 4.2-fold increases in proliferation as compared to nonimmune cells for two and three immunizations, respectively), confirming previous findings (1, 24). We therefore chose to use two or three immunizations in the subsequent experiments.
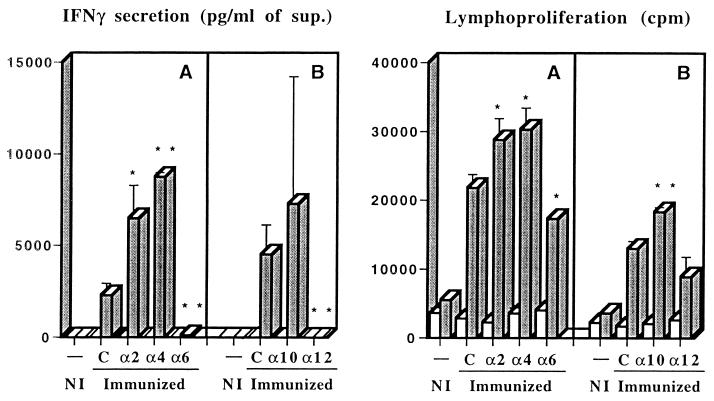
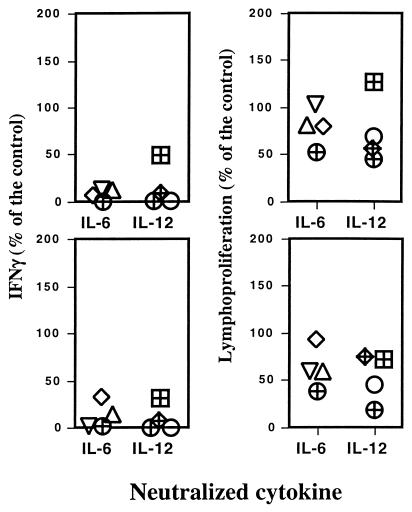
The requirement for endogenously produced cytokines in the development of an immune response characterized by priming for IFN-γ release was evaluated during the immunization procedure with the solution of ST-CF and DDA. Mice were vaccinated three times at weekly intervals, and cytokines were neutralized during the course of immunization by administering specific monoclonal antibodies. The effects of these antibodies were compared to those of a control monoclonal antibody recognizing an irrelevant antigen, β-galactosidase. Several independent experiments were performed, and they showed that neutralization of either IL-2, IL-4, or IL-10 increased the IFN-γ-dominated response to variable degrees (Fig. 1). On the other hand, an inhibition of the priming was observed as expected with the neutralization of IL-12 and, more interestingly, with the neutralization of IL-6 (Fig. 1). The effects of the antibody treatments on the proliferative responses were similar to the effects on the IFN-γ responses, although to a smaller degree. To confirm the observation that IL-6 and IL-12 were required for the priming of IFN-γ-secreting cells during immunization with the solution of ST-CF and DDA, we performed multiple independent experiments where each of these cytokines was neutralized as described above. The data for the different experiments are summarized in Fig. 2, which shows the response of the antibody-treated and immunized groups as compared to the immunized groups treated with the irrelevant antibody as percent response to the latter. Despite some variability, the neutralization of either IL-6 or IL-12 markedly and consistently reduced IFN-γ production, both with cells from the spleen (statistically significant reductions in four out of four experiments for IL-6 and in three out of four experiments for IL-12) or from the draining lymph nodes (statistically significant reductions in three out of four experiments for both IL-6 and IL-12). That decrease in IFN-γ was also accompanied by a decrease in the proliferation of cells from the lymph nodes (statistically significant reductions in three out of four experiments for IL-6 and in four out of four experiments for IL-12) that was not so evident with the splenocytes (statistically significant reductions in three out of four experiments for both IL-6 and IL-12). The decrease in the lymphoproliferative responses was less reproducible, most likely due to the requirement for a more complete depletion of the cytokines which may not have happened so consistently throughout all experiments.
FIG. 1.
Priming for antigen-specific IFN-γ and proliferative responses of spleen cells from mice immunized three times with a solution containing ST-CF and DDA as compared to an adjuvant alone (PBS and DDA) control (NI). Immunized animals received either nonimmune immunoglobulin (C) or monoclonal antibody specific for IL-2, IL-4, IL-6, IL-10, or IL-12 with the first and third immunizations. Immunizations were performed at weekly intervals, and the mice were sacrificed 3 weeks after the last immunization. Spleen cells were pooled from three mice and were cultured for 48 h in triplicate experiments. Shaded columns represent the response of spleen cells in the presence of 4 μg of ST-CF per ml, whereas the open columns correspond to unstimulated cultures. Data are presented as the means of the triplicate cultures ± 1 standard deviation. Statistically significant effects of the antibody treatments are labeled ∗ (for P < 0.05) and ∗∗ (for P < 0.01), according to Student's t test.
FIG. 2.
Effects of the neutralization of either IL-6 or IL-12 in the priming of T cells during the immunization of C57BL/6 mice with ST-CF plus DDA. Results are expressed as percentages of IFN-γ production at 48 h and the specific lymphocyte proliferations of spleen (upper panels) or lymph node (lower panels) cells from neutralizing-antibody-treated animals relative to the response of cells from control animals. Each symbol represents the results of one of eight independent experiments.
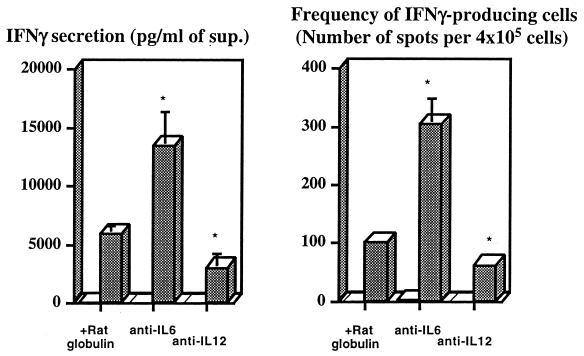
As the data on IL-12 were expected, we concentrated our studies on the more original observation that IL-6 is required for the priming of IFN-γ-secreting T cells. To test when IL-6 was required during in vivo immunization, we administered IL-6-neutralizing antibodies at different times during vaccination. As shown in Table 1 (experiment A), one dose of monoclonal antibody given with the first immunization reduced the priming of lymph node and splenic T cells for IFN-γ secretion, whereas the late administration of anti-IL-6 had the opposite effect in the lymph nodes, leading to a small reduction in the priming of spleen cells. These results suggested that IL-6 was not necessary for the responses of already primed cells. This was confirmed when we stimulated immune spleen cells in vitro and studied the effects of antibodies neutralizing this cytokine and compared these effects to those obtained by IL-12 neutralization. Neutralization of IL-12 had little effect, whereas the neutralization of IL-6 significantly enhanced IFN-γ production by immune splenocytes stimulated with the specific antigen (P < 0.05) as well as increased the frequency of IFN-γ-producing cells (P < 0.05) (Fig. 3). We also tested whether in vivo IL-10 neutralization offset the defect in IFN-γ priming observed in mice whose IL-6 had been neutralized during the immunization, since IL6-KO mice have been shown to be more susceptible to candidiasis and to have higher IL-10 responses underlying such susceptibility to infection (41). As shown in Table 1 (experiment B), that was not the case. While IL-10 neutralization alone led to a slight increase in the IFN-γ secretion of lymph node and spleen cells as compared to control animals, it did not affect the reduced IFN-γ response in animals treated with anti-IL-6 monoclonal antibodies during immunization (no statistically significant differences were found between those two groups). Similar data were obtained for the proliferative responses (data not shown).
TABLE 1.
IL-6 is required early in immunization and does not act through the inhibition of IL-10 secretion
| Antibody treatment | Administration of monoclonal antibody on day:
|
IFN-γ (pg/ml) obtained ind:
|
||||
|---|---|---|---|---|---|---|
| Experiment A
|
Experiment B
|
|||||
| 0 | 14 | Spleen | Lymph nodes | Spleen | Lymph nodes | |
| Control | − | − | 22,229 ± 2,607 | 5,480 ± 3,041 | 2,185 ± 744 | 1,133 ± 428 |
| Anti-IL-6 | + | + | 3,574 ± 226a | 2,132 ± 1,314e | 255 ± 95c | 154 ± 69c |
| Anti-IL-6 | − | + | 11,205 ± 449b | 11,435 ± 6,598e | ||
| Anti-IL-6 | + | − | 7,438 ± 2,534b | 1,119 ± 611e | ||
| Anti-IL-10 | + | + | 3,800 ± 1,517e | 3,220 ± 777c | ||
| Anti-IL-6 plus anti-IL-10 | + | + | 130 ± 79b | 100 ± 68c | ||
P < 0.001 (comparison between treated groups and controls by analysis of variance [for all P values]).
P < 0.01.
P < 0.05.
Only the results from antigen-stimulated cultures are shown since unstimulated cultures showed negligible levels of IFN-γ production. In experiment A, animals were injected s.c. (with ST-CF or PBS in DDA) twice with a 2-week interval, while in experiment B animals were immunized three times at weekly intervals. Cells from animals injected with PBS in DDA showed negligible production of IFN-γ, and they were not included here.
Not statistically significant.
FIG. 3.
Effects of the neutralization of either IL-6 or IL-12 during in vitro restimulation of immune spleen cells. Spleen cells from three C57BL/6 mice immunized three times with ST-CF in DDA at weekly intervals and sacrificed 3 weeks after the last immunization were pooled and cultured in triplicate in the absence (open columns) or presence (shaded columns) of 4 μg of ST-CF per ml. Either rat Ig or anti-IL-6 or anti-IL-12 antibodies (50 μg/ml) were added at the start of the cultures. The amounts of IFN-γ were measured 72 h later, and the numbers of IFN-γ-producing cells were determined by ELISPOT as described in Materials and Methods. Cells from nonimmune animals had undetectable IFN-γ responses, and data are not included here. Data are presented as the means of the triplicates ± 1 standard deviation. Statistically significant effects of the antibody treatments are labeled ∗ (for P < 0.05), according to the Student's t test.
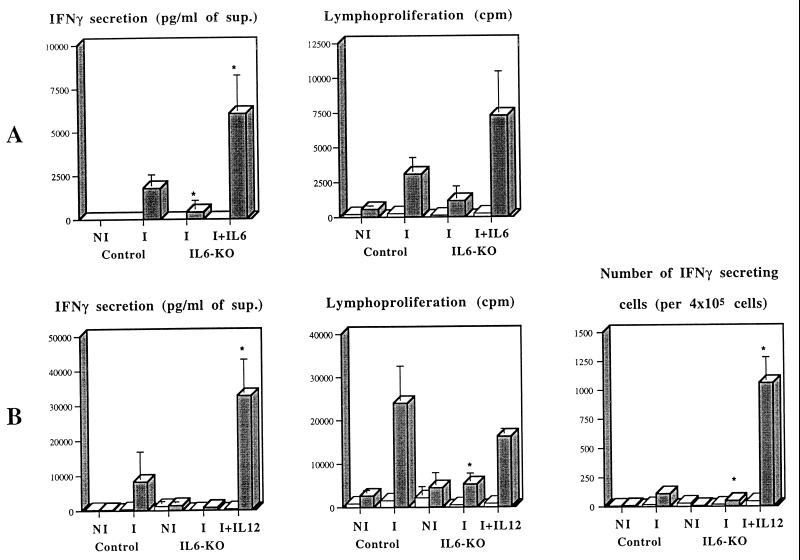
Since some antibodies against IL-6 have been demonstrated to enhance its half-life in vivo instead of blocking its activity (17), we decided to confirm that the results observed with the monoclonal antibody used in the previous experiments were indeed due to a neutralization of the activity of IL-6 and not due to its increased bioavailability. IL6-KO mice and control mice of similar genetic backgrounds (C57BL/6) were immunized two times with ST-CF in DDA at a 2-week interval, and then their spleens and iliac and inguinal lymph nodes were collected for stimulation in vitro 3 weeks after the second immunization. As shown in Fig. 4, splenocytes from IL6-KO mice were unable to produce significant amounts of IFN-γ, and their cell proliferation was decreased when compared to the control animals (P < 0.01 for both spleen and lymph node cells). Similar data were obtained with the lymph node cell preparations (not shown). In several different experiments, the number of cells isolated from the lymph nodes of IL6-KO mice were 10.6 to 75.4% lower than in wild-type animals, and such lower numbers were seen among all lymphocyte subpopulations (data not shown). We thus confirmed the requirement for the endogenous production of IL-6 in the priming of IFN-γ-secreting T cells after immunization with the mixture of ST-CF and DDA. In order to try to recover the defect observed in the IL6-KO mice, we performed immunizations in which different levels of recombinant IL-6 were included in the vaccine. In a first experiment, the inclusion of 0.5, 5, or 30 μg of recombinant human IL-6 in each one of the three doses of the vaccine failed to complement the genetic deficiency (data not shown). We therefore gave three 30-μg doses of IL-6, on the day of the first immunization and in the following two days (for a total dosage of 90 μg), with no further administration of IL-6 with the second dose of the vaccine. With this protocol, we recovered the defect in IFN-γ priming observed in the IL6-KO mice (Fig. 4A). The secretion of IFN-γ was significantly increased as compared to the immunized IL6-KO group (P < 0.05) as well as to the immunized wild-type controls (P < 0.05). Lymphoproliferation in IL6-KO cells was significantly increased by such treatment (P < 0.05).
FIG. 4.
Priming for antigen-specific IFN-γ and proliferative responses of spleen cells from IL6-KO mice or their controls (C57BL/6 mice) injected with a mixture of PBS and DDA (nonimmune [NI]) or with a mixture of ST-CF and DDA (immune [I]) and recovery of the defect in T-cell priming in IL6-KO mice by coadministration of either recombinant IL-6 or recombinant IL-12. (A) Wild-type (Control) and IL6-KO mice were immunized twice with the ST-CF–DDA vaccine at a 2-week interval. Recombinant human IL-6 (30 μg per dose) was given on days 0, 1, and 2 of the first vaccination by s.c. injection at the site of immunization. (B) Wild-type (Control) and IL6-KO mice were immunized three times with the ST-CF–DDA vaccine at weekly intervals. Recombinant mouse IL-12 (0.5 μg per dose) was given with each immunization. Spleen cells were collected from three immune mice or three nonimmune mice and were individually cultured for 96 h in triplicate. Shaded columns represent the response of spleen cells in the presence of 4 μg of ST-CF per ml, whereas the open columns correspond to unstimulated cultures. Data are presented as the means of the triplicates ± 1 standard deviation. Data were compared by analysis of variance, and differences to immune wild-type mice are labeled ∗ (P < 0.05).
An alternative to boost protective immunity after immunization with subunit vaccines involves the inclusion of IL-12 in the vaccine (6, 24, 45). We thus decided to test whether the defect in IL-6 could be compensated by IL-12. Preliminary experiments showed that either one or three administrations of IL-12 with the vaccine enhanced the IFN-γ and the lymphoproliferative responses in wild-type mice (data not shown). To analyze the effect of IL-12 administered with the vaccine in the IL6-KO mice, these animals were immunized three times with ST-CF in DDA plus IL-12 (one dose of the cytokine per immunization). The results (Fig. 4B) showed that the administration of IL-12 with ST-CF in DDA overcame the inability of IL6-KO mice to produce IFN-γ in response to this vaccine (P < 0.01) and that such cytokine secretion was even increased relative to control mice (P < 0.01 for spleen cells and P < 0.05 for lymph node cells). ELISPOT assays showed that the frequency of cells able to produce IFN-γ was also increased in the animals that received the ST-CF–DDA–IL-12 mixture (a 23-fold increase as compared to the immunized IL6-KO mice, corresponding to 10 times the frequency observed in immunized control mice), suggesting that IL-12 increased the number of IFN-γ-secreting cells in these mice rather than IFN-γ secretion of the cytokine on a per cell basis.
To evaluate the roles of IL-6 and IL-12 in the induction of protective immunity by vaccination with ST-CF in DDA, the effects of the neutralization of these cytokines during immunization were tested as before by administering neutralizing monoclonal antibodies during immunization with three doses of the vaccine. These groups were compared to groups of animals immunized with ST-CF in DDA and given an irrelevant antibody as well as with a group immunized with BCG, the standard vaccine. Mice were then challenged with live, virulent M. tuberculosis, either i.v. or with an aerosol, 6 weeks after the last immunization. After i.v. challenge, bacterial enumeration was performed in the liver, where most of the inoculum is trapped, and in the lung, the preferential target for M. tuberculosis proliferation. The results are shown as the differences between the geometric means of CFU in the nonimmune mice and those in each of the immunized groups of mice (Table 2). In the liver, the vaccine offered less protection than BCG (P = 0.01), and its protective efficacy was reduced by neutralizing IL-6 (P < 0.05) and was ablated by neutralizing IL-12. In the lung, the protection afforded by vaccine was slightly superior to that afforded by BCG (P < 0.05), and again, neutralization of either IL-6 or IL-12 during immunization led to a reduction in its protective ability (P < 0.01 for both). In both organs, the inclusion of recombinant IL-12 increased the efficacy of the vaccine in control mice, but this increase was statistically significant only in the liver (P < 0.05). The addition of IL-12 to the vaccine compensated for the decrease induced by the anti-IL-6 treatment (P < 0.01 for both organs).
TABLE 2.
Protection afforded by BCG or the ST-CF–DDA mixture-based vaccine to an i.v. challenge or an aerosol of virulent M. tuberculosis Erdman
| Vaccine treatment | Results of i.v. challenge
|
Results of aerosol challenge
|
||||||
|---|---|---|---|---|---|---|---|---|
| Liver
|
Lung
|
Lung
|
Spleen
|
|||||
| Log10 of CFU | Log10 of resistance | Log10 of CFU | Log10 of resistance | Log10 of CFU | Log10 of resistance | Log10 of CFU | Log10 of resistance | |
| Untreated | 6.16 ± 0.07 | 5.54 ± 0.11 | 5.54 ± 0.09 | 4.64 ± 0.11 | ||||
| DDA | 6.02 ± 0.13 | 5.65 ± 0.16 | 5.68 ± 0.24 | 4.69 ± 0.14 | ||||
| BCG | 4.54 ± 0.11a | 1.62 | 4.36 ± 0.14a | 1.18 | 4.93 ± 0.26b | 0.61 | 3.99 ± 0.45b | 0.65 |
| Vaccined | ||||||||
| Control monoclonal antibody | 5.12 ± 0.31a | 0.90 | 4.15 ± 0.14a | 1.50 | 5.23 ± 0.16b | 0.45 | 4.11 ± 0.20a | 0.58 |
| Anti-IL-6 | 5.57 ± 0.12a | 0.45 | 4.74 ± 0.26a | 0.91 | 5.49 ± 0.18 | 0.19 | 4.66 ± 0.15 | 0.03 |
| Anti-IL-12 | 5.84 ± 0.49 | 0.18 | 5.08 ± 0.38c | 0.57 | 5.53 ± 0.31 | 0.15 | 4.56 ± 0.47 | 0.13 |
| Vaccine plus IL-12 | ||||||||
| Control monoclonal antibody | 4.77 ± 0.10a | 1.25 | 3.93 ± 0.29a | 1.72 | 4.90 ± 0.15a | 0.78 | 4.14 ± 0.18a | 0.55 |
| Anti-IL-6 | 4.98 ± 0.10a | 1.04 | 4.25 ± 0.15a | 1.40 | 5.35 ± 0.18c | 0.33 | 4.23 ± 0.18b | 0.46 |
P < 0.001.
P < 0.01.
P < 0.05.
Groups of five mice were immunized three times at weekly intervals with a mixture of ST-CF and DDA or with ST-CF and DDA plus 0.5 μg of IL-12. These mice received three doses of either an irrelevant antibody (control monoclonal antibody) or specific anti-IL-6 or anti-IL-12 monoclonal antibodies at 2-week intervals starting with the first immunization. A group of mice was immunized with BCG as a positive control for comparison of the efficacy of the vaccines. Mice were challenged as indicated in Materials and Methods. Results shown are the means of log10 CFU ± 1 standard deviation in the indicated organs as well as the log10 resistance calculated by subtracting mean log10 CFU of the immunized mice from mean log10 CFU of a nonimmunized group (untreated for the BCG vaccination and DDA alone for the ST-CF–DDA vaccine). The statistical significance (according to analysis of variance) of the protection afforded by each immunization as compared with the nonimmune group is presented as indicated above.
In mice that were similarly treated but were challenged with an aerosol, bacterial enumeration was performed in the target organ, in the lung, and in the spleen as a target for dissemination (Table 2). Protection induced by the vaccine was similar to that of BCG in both organs (no statistically significant differences were found). Neutralization of either IL-6 or IL-12 ablated vaccine-induced protection. The inclusion of recombinant IL-12 in the vaccine increased the efficacy of the vaccine in the lung (P < 0.05) and compensated for the lack of IL-6 in the spleen (P < 0.01), although not in the lung.
DISCUSSION
IL-12 is well known to play a pivotal role in the priming of Th1-type immune responses, possibly aided by other cytokines, such as IL-18, IL-1, and tumor necrosis factor alpha. The precise role of IL-6 in T-cell differentiation is far from clarified. Deficient priming of IFN-γ responses in the absence of IL-6 was reported during infections by L. monocytogenes or M. tuberculosis (23, 25–27). However, others have shown that IL-6 can promote Th2 responses through the induction of IL-4 (37). Here we have shown that IL-6 was required for the priming of IFN-γ-secreting T cells during immunization with a subunit vaccine against tuberculosis previously shown to promote protective immunity to tuberculosis in a similar model (24). This was found using two different approaches: by depleting IL-6 during immunization using IL6-specific neutralizing monoclonal antibodies and by performing experiments in IL6-KO mice. This excluded the possibility of the antibodies chaperoning the cytokine instead of ablating its activity. IL-6 was required not only for the priming of IFN-γ-producing T cells but also for the induction of protective T cells, highlighting the role of IFN-γ as a fundamental molecule in protective immunity to tuberculosis. The deficiency in IL-6 led to reductions in both responses similar to the depletion of IL-12. We were able to recover the defect in IL6-KO mice by administering recombinant IL-6 early in vaccination and by adding recombinant IL-12 to the vaccine. In addition, IL-12 was effective in increasing the priming of T cells for IFN-γ secretion in normal mice and showed small but statistically significant effects in increasing the protective immunity granted by the vaccine. These results support the possibility of using IL-12 as an adjuvant in vaccines aimed at promoting cell-mediated immunity. This would be even more important if situations of deficient IL-6 production were found, either because of individual deficiencies or because of intrinsic properties of given adjuvants.
In this work, we found a high variability in the priming of cells for the secretion of IFN-γ. This effect has been consistently observed for this type of immunization in both of our two laboratories. Although the reason for such variability is not clear, some explanations can be envisaged. We know that the variability is not dependent on the batch of tuberculosis antigen, but the fact that the vaccine (i.e., ST-CF plus adjuvant) is prepared fresh for each inoculation could account for some variability in its immunizing activity between experiments. Alternatively, this immunization procedure might be very sensitive to environmental conditions (e.g., slight variations in the commensal flora of the animals).
Although the mechanism of the defect(s) in T-cell development in the absence of IL-6 is still unclear, several hypotheses are being tested. A simple explanation for our results could be that IL-6 is a cofactor for the development of the immune T cells that produce IFN-γ and mediate protection against the tuberculosis infection. The number of lymphocytes isolated from the lymph nodes of IL6-KO mice after immunization was consistently lower than the number isolated from immunized control animals. This could suggest that IL-6 was required for cell proliferation. A direct role of IL-6 in the proliferation or differentiation of T cells has been documented by several groups. IL-6 was shown to promote the proliferation of human T cells in response to CD2 ligation (15, 21, 28) or after mitogen stimulation (49) and to be involved in the generation of cytolytic T cells (30, 35, 36, 39). In our hands, and in keeping with previous observations by others (51), IL-6 reduced the responses of differentiated T cells as its neutralization in the cultures augmented IFN-γ production. However, it is still possible that IL-6 is mostly required for the initial proliferation and/or differentiation of T cells. In this study, only an early in vivo depletion of IL-6 led to the inhibition of priming for IFN-γ secretion. In contrast, late depletions had the opposite effect, mimicking our in vitro experiments. Also, only the early addition of IL-6 to the vaccine could lead to the recovery of the defect found in the IL6-KO mice. In contrast, a similar amount of IL-6 given with all immunizations was without effect. Our data are therefore consistent with an important role for IL-6 in the early expansion of immune T cells. Somehow, this requirement is overcome by IL-12 with regard to the IFN-γ responses but is not overcome in terms of the proliferative potential of the immune cells (Fig. 4). Consistent with these interpretations, Vink et al. (52) found that the effect of IL-6 on the in vitro proliferation of mitogen-stimulated murine T cells was critical for the initiation of the response but not for its maintenance. Also, Joseph et al. (20) have recently reported that IL-6 acted on purified murine T cells by promoting their proliferation and that such an effect was most important with naive rather than differentiated T cells. They also found that IL-6 promoted cytokine production in fully differentiated Th1 cells, whereas it decreased IFN-γ secretion in differentiating T cells, showing that IL-6 may have different functions on T cells according to their differentiation status, thereby clarifying the contradictory data previously reported in the literature. In our studies, the overall effects of the treatments on the proliferative responses were generally much less important than the effects on IFN-γ priming. This suggests that antigen-specific T cells that lack the ability to secrete IFN-γ may be induced in the absence of the two cytokines, IL-6 and IL-12.
Alternative mechanisms explaining the effects of IL-6 range from chemokine responses interfering with the recruitment of antigen-presenting cells and other immune cells (8, 42) to a role for neutrophils in T-cell priming (11, 41) or a role for the acute-phase reactants (22) or glucocorticoid metabolism (43) in the response to the vaccine.
In summary, we have found a major role for both IL-6 and IL-12 in the generation of protective immunity mediated by IFN-γ-secreting T cells following immunization with a subunit vaccine. Although the role of IL-12 seems to confirm its expected role as a major Th1 inducer, the mechanism involved in the action of IL-6 is not clear. Finally, the defect in IL-6-deficient mice could be overcome by IL-12, showing that this latter cytokine is an important candidate as an adjuvant in vaccines promoting cell-mediated immunity.
ACKNOWLEDGMENTS
This work was supported by grants from the Commission of the European Community's STD3 program (contract TS3*-CT/94-0313) and INCO/DC program (contract ERBIC18CT970254). I.S.L. receives a fellowship from the PRAXIS XXI program (Lisbon, Portugal).
We are grateful to M. Kopf for supplying breeders of IL6-KO mice, to the Genetics Institute for their gift of recombinant mouse IL-12, and to Ares-Serono for their gift of recombinant human IL-6.
REFERENCES
- 1.Andersen P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–131. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen P, Askgaard D, Ljungqvist L, Bentzon M W, Heron I. T-cell proliferative response to antigens secreted from Mycobacterium tuberculosis. Infect Immun. 1991;59:1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appelberg R, Castro A G, Pedrosa J, Minóprio P. Role of interleukin 6 in the induction of protective T cells during mycobacterial infections in mice. Immunology. 1994;82:361–364. [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin S L, D'Souza C, Roberts A D, Kelly P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D M, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bermudez L E, Wu M, Petrofsky M, Young L S. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992;60:4245–4252. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas P, Delfanti F, Bernasconi S, Cota M, Polentarutti N, Mantovani A, Lazzarin A, Sozzani S, Poli G. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–265. [PubMed] [Google Scholar]
- 9.Brandt L, Oettinger T, Holm A, Andersen A B, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 10.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denis M, Gregg E O. Recombinant tumour necrosis factor-alpha decreases whereas recombinant interleukin-6 increases growth of a virulent strain of Mycobacterium avium in human macrophages. Immunology. 1990;71:139–141. [PMC free article] [PubMed] [Google Scholar]
- 13.Dolin P J, Raviglione M C, Kochi A. Global tuberculosis incidence and mortality during 1990–2000. Bull W H O. 1994;72:213–220. [PMC free article] [PubMed] [Google Scholar]
- 14.Dooley S W, Jarvis W R, Martone W J, Snider D E. Multidrug-resistant tuberculosis. Ann Intern Med. 1992;117:257–259. doi: 10.7326/0003-4819-117-3-257. [DOI] [PubMed] [Google Scholar]
- 15.Endler-Jobst B, Schraven B, Hutmacher B, Meuer S C. Human T cell responses to IL-1 and IL-6 are dependent on signals mediated through CD2. J Immunol. 1991;146:1736–1742. [PubMed] [Google Scholar]
- 16.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heremans H, Dillen C, Put W, van Damme J, Billiau A. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin associated with paradoxically increased IL-6 levels. Eur J Immunol. 1992;22:2395–2401. doi: 10.1002/eji.1830220932. [DOI] [PubMed] [Google Scholar]
- 18.Hilgers L A T, Snippe H. DDA as an immunological adjuvant. Res Immunol. 1992;143:494–503. doi: 10.1016/0923-2494(92)80060-x. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard R D, Flory C M, Collins F M. Immunization of mice with mycobacterial culture filtrate proteins. Clin Exp Immunol. 1992;87:94–98. doi: 10.1111/j.1365-2249.1992.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joseph S B, Miner K T, Croft M. Augmentation of naive, Th1 and Th2 effector CD4 responses by IL-6, IL-1 and TNF. Eur J Immunol. 1998;28:277–289. doi: 10.1002/(SICI)1521-4141(199801)28:01<277::AID-IMMU277>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Kasahara Y, Miyawaki T, Kato K, Kanegame H, Yachie A, Yokoi T, Taniguchi N. Role of interleukin 6 for differential responsiveness of naive and memory CD4+ T cells in CD2-mediated activation. J Exp Med. 1990;172:1419–1424. doi: 10.1084/jem.172.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 23.Ladel C H, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann S H E. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis sub-unit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Cheers C. The cellular source of interleukin-6 during Listeria infection. Infect Immun. 1993;61:2626–2631. doi: 10.1128/iai.61.6.2626-2631.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z, Simpson R J, Cheers C. Role of IL-6 in activation of T cells for acquired cellular resistance to Listeria monocytogenes. J Immunol. 1994;152:5375–5380. [PubMed] [Google Scholar]
- 27.Liu Z, Simpson R J, Cheers C. Role of interleukin-6 in T-cell activation during primary and secondary infection with Listeria monocytogenes. Infect Immun. 1995;63:2790–2792. doi: 10.1128/iai.63.7.2790-2792.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorré K, van Damme J, Verwilghen J, Baroja M L, Ceuppens J L. IL-6 is an accessory signal in the alternative CD2-mediated pathway of T cell activation. J Immunol. 1990;144:4681–4687. [PubMed] [Google Scholar]
- 29.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 30.Ming J E, Steinman R M, Granelli-Piperno A. IL-6 enhances the generation of cytolytic T lymphocytes in the allogeneic mixed leucocyte reaction. Clin Exp Immunol. 1992;89:148–153. doi: 10.1111/j.1365-2249.1992.tb06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller I, Kropf P, Louis J A, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect Immun. 1994;62:2575. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–90. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 33.Orme I M, Andersen P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 34.Pal P G, Horwitz M. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect Immun. 1992;60:4781–4792. doi: 10.1128/iai.60.11.4781-4792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quentmeier H, Klaucke J, Muhlradt P F, Drexler H G. Role of IL-6, IL-2, and IL-4 in the in vitro induction of cytolytic T cells. J Immunol. 1992;149:3316–3320. [PubMed] [Google Scholar]
- 36.Renauld J-C, Vink A, van Snick J. Accessory signals in murine cytolytic T cell responses. Dual requirement for IL-1 and IL-6. J Immunol. 1989;143:1894–1898. [PubMed] [Google Scholar]
- 37.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell R A. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 39.Rogers L A, Zlotnik A, Lee F, Shortman K. Lymphokine requirements for the development of specific cytotoxic T cells from single precursors. Eur J Immunol. 1991;21:1069–1072. doi: 10.1002/eji.1830210432. [DOI] [PubMed] [Google Scholar]
- 40.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky D H, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–325. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 43.Ruzek M C, Miller A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an interleukin (IL)-6-dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiratsuchi H, Johnson J L, Ellner J J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991;146:3165–3170. [PubMed] [Google Scholar]
- 45.Silva R A, Pais T F, Appelberg R. Evaluation of interleukin 12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 46.Stanfield J P, Gall D, Bracken P M. Single-dose antenatal tetanus immunization. Lancet. 1973;ii:215–219. doi: 10.1016/s0140-6736(73)90062-7. [DOI] [PubMed] [Google Scholar]
- 47.Szabo S J, Dighe A S, Gubler U, Murphy K M. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 49.Tosato G, Pike S E. Interferon-β2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988;141:1556–1562. [PubMed] [Google Scholar]
- 50.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 51.van Heyningen T K, Collins H L, Russell D G. IL-6 produced by macrophages infected with Mycobacterium species suppresses T cell responses. J Immunol. 1997;158:330–337. [PubMed] [Google Scholar]
- 52.Vink A, Uyttenhove C, Wauters P, van Snick J. Accessory factors involved in murine T cell activation. Distinct roles of interleukin-6, interleukin 1 and tumor necrosis factor. Eur J Immunol. 1990;20:1–6. doi: 10.1002/eji.1830200102. [DOI] [PubMed] [Google Scholar]