Abstract
Emerging studies indicate that the Hippo pathway, a highly conserved pathway that regulates organ size control, plays an important role in governing ovarian physiology, fertility, and pathology. Specific to the ovary, the spatiotemporal expression of the major components of the Hippo signaling cascade are observed throughout the reproductive lifespan. Observations from multiple species begin to elucidate the functional diversity and molecular mechanisms of Hippo signaling in the ovary in addition to the identification of interactions with other signaling pathways and responses to various external stimuli. Hippo pathway components play important roles in follicle growth and activation, as well as steroidogenesis, by regulating several key biological processes through mechanisms of cell proliferation, migration, differentiation, and cell fate determination. Given the importance of these processes, dysregulation of the Hippo pathway contributes to loss of follicular homeostasis and reproductive disorders such as polycystic ovary syndrome (PCOS), premature ovarian insufficiency, and ovarian cancers. This review highlights what is currently known about the Hippo pathway core components in ovarian physiology, including ovarian development, follicle development, and oocyte maturation, while identifying areas for future research to better understand Hippo signaling as a multifunctional pathway in reproductive health and biology.
Keywords: Hippo signaling, ovary, follicle, oocyte, YAP1, LATS1/2, ovarian cancer
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
The Hippo signaling pathway is an evolutionarily conserved pathway that regulates organ size via the control of cellular proliferation, differentiation, apoptosis, and cell fate
Regulation of the Hippo signaling cascade is cell-type and context dependent and is facilitated by various signals including the extracellular matrix, mechanical stress, G protein–coupled receptors, growth factor receptor tyrosine kinases, protein kinases and phosphatases, and cellular metabolism
The Hippo pathway and its effector YAP1 are critical for fine tuning ovarian physiology including cell fate determination, follicular activation, growth and differentiation, and steroidogenesis
In vivo mouse models and in vitro culture systems show that dysregulation of the Hippo pathway resulting in YAP1 over activation in granulosa cells contributes to loss of tissue homeostasis and reduced fertility
YAP1 is implicated in the ovarian PCOS phenotype and in metabolic disorders
Modulation of upstream Hippo pathway components leading to YAP1 activation leads to follicle activation and may improve assisted reproduction outcomes
Hyperactivation of YAP1 is implicated in the development and progression of ovarian cancer and resistance to chemotherapeutics
Introduction to Hippo Signaling
The ovary is a dynamic structure, undergoing extensive tissue remodeling with each reproductive cycle. This cyclic remodeling of the ovary includes the processes of folliculogenesis and atresia, ovulation, luteal formation and regression, and the accompanying vasculature and microenvironment changes, all of which are required for successful reproduction (1-3). Paracrine and endocrine-induced alterations in ovarian morphology begin during the development of the gonads and continues as the organ matures. The adult ovary undergoes multiple structural and developmental processes during the reproductive life span of women. Cellular proliferation and differentiation are regulated by a host of paracrine, autocrine, and endocrine factors. Errors in these processes either during fetal life or after menarche can result in ovarian dysgenesis, infertility, and pathology (4).
The follicle is the functional unit of the ovary. The most basic follicular structure is the primordial follicle, consisting of an oocyte surrounded by a few squamous granulosa cells and basal lamina (5). Intraovarian growth factors and secretion of pituitary gonadotropins influence the cyclic growth of ovarian follicles from gonadotropin-independent growth to gonadotropin-dependent growth (6, 7). The transition from primordial follicle to primary follicle occurs with a dramatic increase in cytoplasmic and nuclear volume in the oocyte along with changes in morphology and proliferation of the surrounding granulosa cells (5, 8). As the follicle progresses from a primary to a secondary stage follicle, the granulosa cells surrounding the oocyte continue to proliferate and increase in size (5). Other structural changes begin to occur during the transition to secondary follicle, such as the generation of the theca cell layer (5). The theca cells proliferate from a population of unspecialized mesenchymal cells in the ovarian stroma to form a layer adjacent to the basal lamina (9). Additionally, the follicle begins to vascularize, enabling the follicle to now secrete and respond to steroid hormones (5). In preantral follicles, theca cells respond to luteinizing hormone (LH), while follicle-stimulating hormone (FSH) binds to differentiated granulosa cells, resulting in the production and release of estradiol (10). The granulosa cells of the growing antral follicles compete for FSH, and a dominant follicle emerges, leaving the other follicles to undergo atresia. Estradiol secretion reaches its peak in mature follicles, triggering the LH surge that is required for ovulation (11). Postovulation, the remaining granulosa and theca cells of the ruptured follicle become luteinized and vascularized to form the corpus luteum, contributing to the increase in circulating progesterone and other hormones responsible for the establishment of pregnancy and early embryo development (12, 13).
The regulation of follicle development and subsequent luteal formation via endocrine signaling mechanisms have been well established (14-16). Research on intracellular signaling pathways in the ovary has identified several pathways that regulate follicle cell development, growth, and cell fate such as adenylyl cyclase/cAMP/protein kinase A (PKA) and phospholipase C/calcium/protein kinase C (PKC) (17-19), Notch (20), Hedgehog (21-23), and Wnt (24-26) signaling, members of the TGF-B superfamily (27-30), many receptor tyrosine kinases (RTKs) (31-35), and nuclear receptors (36). A new intracellular signaling pathway called the Hippo pathway (37, 38) has emerged having considerable influence on cellular differentiation and steroidogenesis in the ovary and will be the subject of this review.
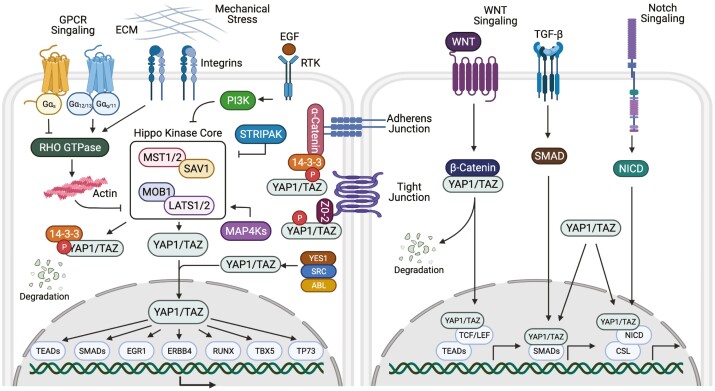
The Hippo signaling pathway, also referred to as Salvador/Warts/Hippo pathway was initially discovered as an integral regulator of imaginal disc size in Drosophila melanogaster (39) and later studies implicated the Hippo pathway in Drosophila ovarian development (40-44). The Hippo pathway also interacts with several of the pathways mentioned above to facilitate ovarian development and function as demonstrated by comprehensive studies in the Drosophila ovary (42, 44-49). This evolutionarily conserved pathway regulates organ size (50) via control of cell proliferation (51), apoptosis (52), and stem cell self-renewal (53), with dysfunction of the pathway contributing to a wide range of diseases (54), tissue overgrowth (51), and tumorigenesis (54). The foundation of this pathway consists of a protein kinase cascade (Fig. 1) of several negative regulators, inclusive of a complex formed by mammalian serine/threonine-protein kinase 4 and 3 (commonly called MST1/2) and Salvador family WW domain-containing protein 1 (SAV1). The MST/SAV1 complex phosphorylates and activates large tumor suppressors 1 and 2 (LATS1/2) and its regulatory protein Mps one binder 1 (MOB1). The activated LATS1/2 complex then phosphorylates yes-associated protein 1 (YAP1) and WW domain-containing transcription regulator 1 (WWTR1, also known as TAZ), the main effectors of this signaling pathway (55). Elevated phosphorylation of YAP1 and TAZ promotes interaction with 14-3-3 proteins, thus sequestering them to the cytoplasm or targeting the proteins for ubiquitin-mediated proteolytic degradation (56). Unphosphorylated YAP1 and TAZ translocate and accumulate in the nucleus when Hippo signaling is inactive, binding to TEA domain (TEAD) transcription factors, which promote the expression of genes involved in cell proliferation and survival (57).
Figure 1.
Diagrammatic representation of the Hippo signaling pathway and mechanism of action in mammalian cells. The Hippo signaling pathway has many upstream regulators, from left to right: G protein–coupled receptors (GPCRs), extracellular matrix (ECM) stiffness and mechanical stress, integrins, and growth factor receptor tyrosine kinases (RTK). When core Hippo components MST1/MST2 and LATS1/LATS2 and their respective regulatory proteins SAV1 and MOB1 are phosphorylated, the Hippo pathway is active, suppressing the transcriptional activity of YAP1/TAZ by phosphorylation and sequestering YAP1/TAZ to the cytoplasm where they bind to 14-3-3 proteins or undergo protein degradation. When the Hippo pathway is inactive, dephosphorylated YAP1/TAZ enter the nucleus and bind with TEA domain transcription factors, TEADs. YAP1 and TAZ can also interact with other nuclear transcription factors. Additionally, other signaling pathways (WNT, TGF-β, and Notch) may interact with YAP1 and TAZ in order to regulate target gene expression. Abbreviations: EGF, epidermal growth factor; G, guanine nucleotide binding protein; LATS1/2, large tumor suppressor 1 and 2; MAP4K, mitogen-activated protein kinase kinase kinase kinase; MOB1, Mps one binder 1; MST1/2, mammalian STE20-like; PI3K, phosphoinositide 3-kinase; SAV1, Salvador family WW domain-containing protein 1; STRIPAK, striatin-interacting phosphatase and kinase; TAZ, transcriptional coactivator with PTZ-binding motif also called WW domain-containing transcription regulator 1, WWTR1; TGF, transforming growth factor; YAP1, yes-associated protein 1, ZO-2, zona occludens-2.
Due to lack of direct DNA-binding activity, YAP1 and TAZ bind to a wide range of transcription factors including TEADs, TP73, ERBB4, EGR1, TBX5, SMADs, and RUNXs in order to regulate target gene expression (57). Regulation of the Hippo signaling cascade is cell-type and context dependent and is facilitated by various signals including cell polarity (58), mechanical stress (59), G protein–coupled receptors (60), microRNAs (61), and cellular metabolism (62). Hippo signaling can also be facilitated by contact-dependent or contact-independent mechanisms (58). One of the contact-dependent mechanisms involves sequestration of YAP1 at cellular junctions such as adherens junctions and tight junctions (56, 63, 64). Dependent on the response to cellular stimuli, the role of YAP1 and the respective binding transcription factor may provoke a range of responses, including proliferation or apoptosis, and differentiation or epithelial-mesenchymal transition.
The regulation of the Hippo kinase cassette (MST1/2, SAV, LATS1/2, MOB1) and effector molecules of the Hippo pathway, YAP1 and TAZ, have been reviewed recently (65, 66). The canonical Hippo pathway has a number of inputs that act directly or indirectly to modulate the activity of the Hippo kinase cascade (Fig. 1). The mitogen-activated protein kinase kinase kinase kinase (MAP4K) acts to coordinate MST1/2 and LATS1/2 activation serving to phosphorylate and inhibit YAP1 and TAZ. Conversely, MST1/2 and MAP4K are negatively regulated by a large protein complex called STRIPAK (striatin-interacting phosphatase and kinase) containing the protein phosphatase (PP2A), which dephosphorylates these protein kinases and inhibits their activity. STRIPAK complex interactions with the MST1/2 or MAP4K occur in response to conditions that inhibit the Hippo pathway (67).
Mechanical cues, such as extracellular matrix (ECM) stiffness and shear stress, are also potent regulators of YAP1/TAZ (68, 69). Active YAP1/TAZ enter the nucleus when cells are grown on a stiff ECM or are spread across a large surface. In contrast, YAP1/TAZ are inactive when cells are in a soft ECM or are compressed into a small area. Attachment of cells to the ECM activates Rho-GTPases, limiting LATS-dependent YAP1 phosphorylation. Disruption of F-actin blocks the effect of ECM attachment on YAP1 nuclear localization. ECM stiffness is important for cell proliferation and differentiation, and YAP1/TAZ activity plays a role in these cellular processes.
G protein–coupled receptors (GPCRs) also regulate the Hippo pathway. Serum, lysophosphatidic acid (LPA), and sphingosine-1-phosphate (S1P) activate YAP1 and TAZ via their Gα12/13 and Gαq/11 GPCRs that activate Rho GTPase, leading to increases in F-actin. In contrast, Gαs–coupled receptors via activation of protein kinase A (PKA) inhibit Rho GTPase and reduce actin stiffness resulting in repression of YAP1 and TAZ (70). Activated PKA can also directly phosphorylate LATS, leading to YAP1 phosphorylation (71).
The Hippo pathway can be inhibited by growth factors like insulin-like growth factor 1 (IGF1) and the epidermal growth factor (EGF) family of ligands (EGF, AREG) that activate receptor tyrosine kinases leading to the activation of phosphoinositide 3-kinase (PI3K) and AKT signaling. Activation of PI3K dismantles the Hippo kinase complex which inactivates LATS and consequently increases levels of YAP1 (72). Additionally, once activated AKT can directly phosphorylate MST1/2 preventing its activation and allowing YAP1 and TAZ to be active (73).
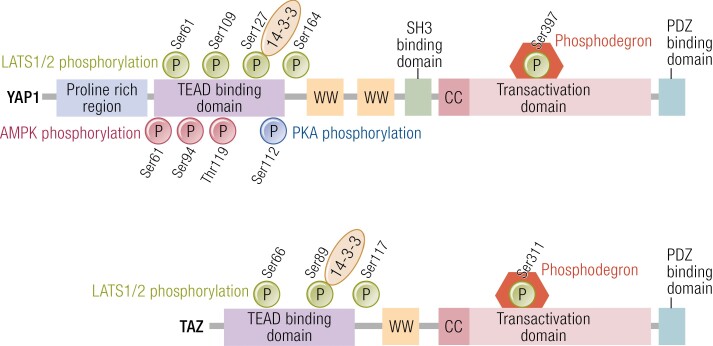
LATS1/2 kinases phosphorylate YAP1 at 5 serine motifs [Ser61, Ser109, Ser127, Ser164, Ser397 (also annotated as Ser381)] and TAZ at 4 serine motifs (Ser66, Ser89, Ser117, Ser311) (56, 74-76) (Fig. 2). Other protein kinases can also stimulate YAP1 and TAZ phosphorylation either directly or indirectly. Independent of Hippo activation, the energy sensor AMP-activated protein kinase (AMPK) can directly phosphorylate YAP1 at Ser61, Ser94, and Thr119 (77, 78). Phosphorylation at the Ser94 site disrupts the interaction between YAP1 and TEAD, thus limiting YAP1-mediated transcription (77, 78). AMPK can also affect YAP1 activity indirectly by phosphorylation of Angiomotin-like 1 (AMOTL1), which can promote LATS1/2 activity and sequester YAP1 to the cytoplasm (79). On the other hand, LATS1/2 kinase can be activated in AMPK knockout cells in response to energy stress (78), indicating that cellular energy stress can also activate the Hippo pathway via an AMPK-independent mechanism. Many other protein kinases such as PKA, cyclin-dependent kinase 1 (CDK1), Jun N-terminal kinases (JNK), and Src family tyrosine kinases have been shown to phosphorylate YAP1 and TAZ (70, 80), suggesting that YAP1/TAZ can be regulated by mechanisms independent of Hippo pathway kinases.
Figure 2.
Structural motifs and phosphorylation sites in YAP1/TAZ. YAP1 is phosphorylated by LATS1/2 at 5 serine residues (Ser61, Ser109, Ser127, Ser164, and Ser397) and TAZ at 4 serine residues (Ser66, Ser89, Ser117, and Ser311) (green circles). Phosphorylation of YAP1 Ser127 results in the restriction of YAP1 to the cytoplasm due to binding with 14-3-3 and phosphorylation at Ser397 activates a phosphodegron degradation motif leading to reduced levels of YAP1 and TAZ. Likewise, phosphorylation of TAZ Ser89 sequesters TAZ to the cytoplasm via binding with 14-3-3 and Ser 311 activates a phosphodegron degradation motif. Phosphorylation of YAP1 by AMP-activated protein kinase (AMPK) occurs at multiple sites shown by red circles. Phosphorylation at Ser 94 via AMPK disrupts the YAP-TEAD interactions, resulting in reduction of the transcription and YAP1 target genes. Protein kinase A (PKA) activates LATS and results in LATS mediated phosphorylation of YAP1 and TAZ. Phosphorylation of YAP1 at Ser 112 by PKA enables YAP1 to translocate to the nucleus (blue circle). Abbreviations: cc, coiled-coil domain; ww, WW domain.
Investigations of Hippo signaling in the ovary demonstrate that the pathway is an integral regulator of ovarian physiology, fertility, and pathology, although the precise role and concomitant mechanisms remain obscure. Emerging studies have demonstrated a role for Hippo pathway components in follicle activation and growth (81-88), infertility caused by reproductive disorders such as polycystic ovary syndrome (PCOS) (89, 90), premature ovarian insufficiency (82, 91), and ovarian cell neoplasia (92-95). To date, most studies done in the ovary have focused on the role of YAP1. In this review, we summarize the studies that have investigated the Hippo pathway and core components in ovarian physiology across different stages of ovarian follicle development and oocyte maturation with a focus on the mammalian Hippo pathway (Table 1), and we identify gaps in our understanding of this fascinating area of reproductive biology. Although some key molecular mechanisms that regulate Hippo signaling have been identified, many aspects of how these are modulated under physiological or stress conditions remain poorly explored.
Table 1.
Hippo pathway core components
| Mammalian gene | Binding domains |
|---|---|
| Mst1/2 | Ste20 Ser/Thr kinase |
| Sav1 | WW |
| Lats1/2 | NDR Ser/Thr kinase |
| Mob1 | Cys2-His2 zinc binding/Mob1/phocein |
| Yap/Taz (Wwtr1) | WW/PDZ and TEAD |
Methods
An extensive examination of the literature was performed to ensure a comprehensive review on Hippo signaling and ovarian function. The search was completed using the PubMed database (https://www.ncbi.nlm.nih.gov/pubmed) through April 2022. The search terms included Hippo pathway/signaling and general keywords (ovary, follicle, reproductive tract, ovarian cancer) to specific keywords (granulosa cell, oocyte, YAP/YAP1, LATS1/2, WWTR1, TAZ, SAV, MOB, polycystic ovarian syndrome, steroidogenesis) based on the authors’ knowledge on the topic. Further, the reference lists of identified articles were manually reviewed.
Hippo Signaling in the Ovary
Cellular Localization of Hippo Pathway Components
The dynamic functions of the Hippo pathway have led to studies investigating the capacity of the Hippo signaling cascade to regulate mammalian germ cell proliferation, follicle development, and luteal formation. Gene expression studies and immunostaining approaches demonstrate that MST1/2, LATS1/2, YAP1, and phospho-YAP1 are present in all follicle stages (primordial, primary, secondary, antral) within the oocyte, granulosa, and theca cells, as well as present in some atretic follicles and the corpus luteum (83, 84, 86, 96-100). Expression of the Hippo component YAP1 is observed in the granulosa and luteal cells of the mammalian ovary, with YAP1 localization to the nucleus of granulosa cells in follicles from the primary stage through the preovulatory stages (85). Postovulation, YAP1 is predominately localized to the cytoplasm in differentiated luteal cells (84, 85, 101).
Microarray analysis identified the expression of all Hippo signaling components in the bovine ovary. No differences were observed between mRNA expression levels of MST1, MST2, LATS2, MOB1A, and YAP1 in granulosa cells and theca cells of large antral follicles or the large and small steroidogenic luteal cells, their respective follicular counterparts (84). However, when cell types were analyzed in a group, MST2 transcripts were 4-fold more abundant than MST1 in the granulosa cells, theca cells, small luteal cells, and large luteal cells (84). Further, LATS1 abundance was ~5-fold greater than LATS2 in the bovine ovary, although LATS1 transcripts were reduced by around 40% in large luteal cells compared with granulosa cells (84). Notably SAV1 mRNA expression in bovine follicles was upregulated during the follicular to luteal transition. Similarly, TAZ mRNA transcripts were increased ~7.5 fold in bovine large luteal cells when compared with bovine granulosa cells, possibly contributing to the increase in luteal cell size as granulosa cells differentiate into luteal cells (84). These findings suggest that MST2 and LATS1 are the predominant upstream Hippo signaling components in the bovine ovary. Whether this relationship holds true for other species remains to be determined.
Reproductive aging in females is characterized by a considerable diminution in the number and quality of follicles and healthy oocytes, in addition to changes in the composition and architecture of the ovarian stroma (102). In mice, whole ovarian expression of MST1 declines with age and YAP1 levels increase before dropping off between 5 months and 16 months of age (96). Interestingly, ovarian expression of LATS2 initially decreases with age in the ovary, but a notable increase in expression occurs after 5 months, coinciding with the time that YAP1 levels decrease and the number of follicles decreases (96). Another study investigating the expression of Hippo pathway components during ovarian aging in mice determined that mRNA and protein expression of MST1 and LATS2, and protein abundance of phospho-YAP1 decreases with physiological ovarian aging (103). In contrast, YAP1 mRNA and protein expression gradually increases before falling after 10 months of age (103). In a model of pathologically induced ovarian aging, investigators observed that YAP1 and MST1 mRNA and protein levels are decreased, although an increase in LATS2 protein and mRNA is observed (103). Future studies are needed to understand the endocrine and paracrine controls that contribute to the dynamic changes in Hippo signaling components during ovarian development and aging. Furthermore, studies are needed to determine how YAP1/TAZ contributes to ovarian gene expression and function over the reproductive lifespan and how the changing ovarian stromal environment (such as increases in fibrotic matrix) contributes to mechanosensing and Hippo activation.
Regulation of Follicular Development
Current understanding of the role of the proximal Hippo pathway component MST1/2 and its adaptor protein SAV1 in the ovary is limited. Most of the experimental work has been focused on SAV1, the core binding partner of MST1 and MST2. In vitro experiments demonstrated that SAV1 alone is unable to phosphorylate the downstream protein kinase LATS1; however, in combination with MST1/2, SAV1 promotes phosphorylation of LATS1 (104). Studies using the avian follicle as a model system revealed that during avian ovarian follicle development, SAV1 mRNA transcript levels are highest in small follicles (< 1 mm) and decline as follicular development progresses to form the large preovulatory follicles (> 9 mm) (104). Similar to mammalian follicle selection, follicle-stimulating hormone receptor (FSHR) transcripts and protein levels are maintained and required within the granulosa cells of ovarian prehierarchical follicles in the hen (105). Overexpression of SAV1, which promotes Hippo signaling in hen granulosa cells, decreased cell proliferation and downregulated mRNA transcripts for FSHR, steroidogenic acute regulatory protein (STAR), and growth differentiation factor 9 (GDF9) (104). Genetic knockdown of SAV1 using small interfering RNA (siRNA)-attenuated Hippo activation, causes an increase in YAP1 activity and results in increased FSHR, STAR, and GDF9 mRNA expression and increased granulosa cell proliferation (104). These observations are of relevance to the mammalian ovary, as FSHR expression is critical for antral follicle development and GDF9 has been demonstrated to promote FSH-induced progesterone production and upregulation of STAR (106), an essential protein required for steroidogenesis. Taken together, these observations indicate that the upstream Hippo component SAV1/MST1/2 appears to play a suppressive role in ovarian granulosa cell proliferation, which may serve to prevent follicle selection during follicle development. It seems likely that SAV1 activation of Hippo signaling limits YAP1 activity, thus, reducing cell proliferation in the ovary. Further studies evaluating the physiological inputs to the MST/SAV complex will provide needed insight into the regulation of Hippo signaling and its control of ovarian function.
The LATS1/2 protein kinases are downstream of MST1/2 and are responsible for phosphorylating the Hippo effectors YAP1 and TAZ. Studies across several mammalian species have provided important clues about the LATS1/2 protein kinases in the ovary. Studies by Pisarska et al (107), identified LATS1 as a forkhead box L2 (FOXL2) interacting protein in the granulosa cells of small/medium follicles in the mouse ovary. This interaction expands the mechanistic role of the Hippo pathway, as FOXL2 is involved in virtually all stages of ovarian development and function (108), including cellular differentiation and estrogen and progesterone synthesis (107, 109). Furthermore, in vitro studies show that LATS1 can directly phosphorylate FOXL2, citing a potential role for LATS1 as a mechanism for controlling the transcriptional repressor activity of FOXL2 during follicle development (107). Although these early studies did not examine YAP1 or TAZ, they highlight the possible involvement of LATS protein kinases in noncanonical Hippo signaling (110).
Genetic manipulations of LATS protein kinases reveal that precise regulation of the Hippo pathway is required to maintain cellular identity. Studies in the female mouse show that global loss of the Lats1 allele does not impact the number of germ cells in mutant ovaries vs wild-type mouse ovaries at birth, although greater germ cell apoptosis was observed as the mouse ages, resulting in premature loss of follicles (111). In this same study, Lats1−/− newborn ovaries that were cultured in vitro exhibited abnormal follicle development, with a reduction in primordial and activated follicles and development of ovarian cysts (111). Similarly, the Lats1 null adult ovary displayed a reduction in the number of follicles, although they exhibit less granulosa cell apoptosis (112). Moreover, the adult ovaries lack corpora lutea, indicating a disruption in ovulation. The phenotype extends beyond the ovary with adult Lats1−/− female mice lacking mammary glands and developing ovarian stromal tumors (112). Overexpression of LATS2 in avian granulosa cells repressed granulosa cell proliferation and downregulated mRNA transcripts for FSHR, STAR, cytochrome P450 family 11 subfamilies A member 1 (CYP11A1), estrogen receptor isoform 1 (ESR1/ERα) and isoform 2 (ESR2/ERβ) (99). Inversely, genetic knockdown of LATS2 via siRNA resulted in enhanced granulosa cell proliferation and increased abundance of transcripts for STAR, CYP11A1, ESR1, and ESR2 (99). Interestingly, the overexpression of LATS2 increased transcripts of MST1, MST2, TEAD1, and TEAD3 while downregulating transcripts of YAP1 and SAV1. Conversely, the knockdown of LATS2 in avian granulosa cells decreased MST1, MST2, TEAD1, and TEAD3 mRNA transcript abundance while increasing YAP1 and SAV1 mRNA transcripts (99). Further, the loss of YAP1 in avian granulosa cells overexpressing LATS2 nullified the inhibitory effects of LATS2 on granulosa cell proliferation and steroidogenesis related gene expression, suggestive of a YAP1-LATS2 feedback loop (99). Other investigators have reported that LATS2 and YAP1 form a negative feedback loop to control YAP1 activity and prevent human ovarian surface epithelial cells from malignant transformation (113).
To examine the role of LATS1/2 more specifically in the ovary, investigators have developed mouse models with targeted deletion of both Lats1 and Lats2. Targeted deletion of both Lats1 and Lats2 caused a loss of granulosa cell identity and function, as well as a change in granulosa cell morphology (114). Using a mouse model of granulosa cell-specific deletion of Lats1 and Lats2 (Cyp19a1-Cre; Lats1fl/fl; Lats2fl/fl) investigators observed premature ovarian failure, demonstrated by an enlarged ovary (~10-fold compared with wild-type control), sterile phenotype, and multi-lineage transdifferentiation of granulosa cells into seminiferous tubule-like structures and bone, suggestive of epithelial-to-mesenchymal transition (114). In vitro experiments with granulosa cells isolated from Lats1fl/fl; Lats2fl/fl mice and infected with Ad-Cre to induce recombination resulted in significant loss of Lats1/2 transcripts and subsequent reductions in LATS1 protein and YAP1 phosphorylation. As expected, this resulted in an increase in total YAP1 protein expression in granulosa cells. The elevation of YAP1 resulted in decreased expression of mRNA transcripts for Wnt4, Fshr, Lhcgr, and Cyp19a1, genes previously identified as necessary for granulosa cell function and differentiation. The LATS1/2 deficient granulosa cells also underwent similar multi-lineage transdifferentiation as observed in vivo (114).
Granulosa cell-specific deletion of Lats1/2 under control of the anti-Müllerian hormone receptor type 2 (Amhr2) promoter (Lats1fl/fl; Lats2fl/fl; Amhr2-Cre) resulted in a reduction of follicle numbers in adult female mice, although corpora lutea were observed, indicative of functional ovulation (115). Further, histological evaluation revealed altered granulosa cell morphology and differentiation reminiscent of epithelial-to-mesenchymal transition (115). Interestingly, the ovarian surface epithelium contained cells that were suggestive of a neoplasm, but immunohistochemical analysis utilizing ovarian cancer markers did not indicate the presence of ovarian tumor cells, nor did the cells increase in number or become invasive as the animals aged (115). Collectively, these findings point to a prominent role for the Hippo pathway LATS protein kinases in maintaining ovarian homeostasis during ovarian follicle development. The differences observed between the 2 phenotypes could be accredited to the timing and activation of the promotor gene in vivo. The Amhr2-Cre would be active much earlier in ovarian development (embryonic day 12.5) than the Cyp19a1-Cre, which is active in granulosa cells of large preantral and antral follicles and luteal cells. Additionally, because LATS protein kinases may also target transcriptional regulators other than YAP1 and TAZ, additional approaches are needed to determine if the observed ovarian phenotypes were due solely to Hippo signaling or represent a combination of events in response to canonical and noncanonical Hippo signaling.
Most studies have focused on exploring the role of YAP1, the downstream effector of the Hippo signaling pathway. Active Hippo signaling results in phosphorylation of YAP1 and TAZ, resulting in sequestration of the transcriptional co-regulators in the cytoplasm. Studies in the KGN human granulosa tumor cell line revealed that forced expression of wild-type YAP1 or constitutively active YAP1S127A (this mutation prevents phosphorylation and maintains YAP1 activity) stimulates cell proliferation (85, 92). Similarly, ectopic expression of YAP1 drives cell cycle progression and proliferation of primary human granulosa cells and human granulosa cell lines in 3D spheroid culture systems (101). In contrast, genetic knockdown of YAP1 or pharmacological inhibition of YAP1/TEAD interactions in primary human granulosa cell cultures and KGN cells resulted in the suppression of cell proliferation and increased cell apoptosis (101). More evidence that suppression of the Hippo pathway is essential for granulosa cell proliferation was provided by studies employing siRNA targeting YAP1 and/or TAZ in primary cultures of bovine granulosa cells. Knockdown of either YAP1 or TAZ attenuated cell proliferation (84). Another study utilizing bovine granulosa cells determined that upon stimulation with bone morphogenic protein 2 (BMP2), YAP1 was dephosphorylated and translocated to the nucleus, resulting in increased mRNA abundance of the downstream YAP1/TEAD target and proliferation-related gene connective tissue growth factor (CTGF; also known as cellular communication network factor 2, CCN2) (116). Furthermore, disruption of the YAP1/TEAD complex with verteporfin treatment disrupted bovine granulosa cell proliferation (84, 116).
Genetic studies in mice also provide evidence for an important role for YAP1 in ovarian follicular development. Granulosa cell-specific deletion of Yap1 in mice (Foxl2-Cre crossbred with Yap1fl/fl) disrupted ovarian follicle development, resulting in reduced ovarian size with increased follicular atresia, and decreased litter size and offspring number (85). However, this group observed no impact on follicle development in conditional Yap1 knockout driven by the Cyp19a1-Cre promoter (85). A reason for the differences observed in these models could be explained by the very early depletion of Yap1 when follicles are forming and developing in the Foxl2-Cre mice compared to the Cyp19a1-Cre mice in which Yap1 is deleted in granulosa cells only after the formation of preantral follicles. The lack of a phenotype in the Cyp19a1-Cre mice could potentially be attributed to either a compensatory role for Taz, in which there would be little to no impact of the loss of Yap1 on follicle development; or changes in the expression of other transcriptional regulators during follicular development (36).
Given the Hippo pathway’s role in controlling tissue size and development, it is not a surprise to find that disruption of the Hippo pathway has been implicated in follicular activation, particularly in nonphysiological conditions. In mouse ovaries cultured in vitro, lentiviral knockdown of Yap1 suppressed follicle growth, resulting in more primordial follicles and fewer primary follicles, while overexpression of Yap1 results in follicle activation (83). Moreover, pharmacological inhibition of Yap1 via verteporfin disrupted follicle formation in the adult mouse ovary in vivo and the immature mouse ovary in vitro (85). Similarly, in vitro stimulation of the PI3K pathway, and subsequent treatment with a chemotherapeutic drug in cultured ovaries resulted in an increase of nonphosphorylated (active) YAP1 and follicle activation (87). On the contrary, the lentiviral overexpression of Yap1 in a chemotherapeutic-induced infertile mouse model increased the thickness of the ovarian surface epithelium, increased recruitment of follicles, and resulted in an increase in pup birth rate relative to respective controls. In this same study, the knockdown of Yap1 in a wild-type model resulted in no changes in the ovarian surface epithelium but resulted in decreased numbers of primordial follicles and lower birthrates relative to controls (117). These reports only begin to reveal the roles of each Hippo pathway component and how they are regulated during early follicle development and differentiation (Table 2).
Table 2.
Knockout/overexpression studies of Hippo pathway core components and ovarian phenotypes
| Study (first author, year) | Model system | Gene targeted | Overexpression or knockout | Findings |
|---|---|---|---|---|
| Fu et al., 2014 | KGN cell line | YAP1 | OE | Increase in granulosa cell proliferation and migration |
| KO | Inhibition of cell proliferation; changes in cell morphology | |||
| Ye et al., 2017 | Cyclophosphamide (CPA)-induced infertile mouse model | Yap1 | OE | Increased follicle number, litter size/birth rate, and ovarian surface epithelium thickness |
| KO | Decreased follicle number and pup birthrate | |||
| He et al., 2019 | Primary human ovarian surface epithelium cells | YAP1 | OE | Increase in cell proliferation; eventual cell senescence |
| KO | No changes in cell proliferation | |||
| TAZ | KO | No changes in cell proliferation | ||
| YAP1/TAZ | KO | Decrease in cell number | ||
| LATS2 | KO | Increase in cell proliferation; loss of cell senescence | ||
| Lv et al., 2019 | Foxl2-cre; Yap1fl/fl mouse model | Yap1 | KO | Decrease in ovarian follicle number/formation; decrease in ovarian size; increased number of atretic follicles; decreased litter size |
| Mouse model—in vitro ovary culture | Yap1 | Inhibition via VP | Disruption of follicle formation | |
| Mouse model—in vivo | Yap1 | Inhibition via VP | Reduced corpora lutea; increased granulosa cell apoptosis | |
| Plewes et al., 2019 | Primary bovine granulosa cells | YAP1 | KO | Reduced granulosa cell proliferation |
| TAZ | KO | Decreased granulosa cell proliferation | ||
| Hu et al., 2019 | Mouse model—in vitro ovary culture | Yap1 | OE | Decrease in primordial follicle number; increase in primary follicle number |
| KO | Increase in primordial follicle number; decrease in primary follicle number | |||
| Hu et al., 2019 | Tri-ortho-cresyl phosphate (TOCP) exposed mouse model | Yap1 | OE | Increase in follicle numbers; decreased atretic follicles; increased fertility |
| Lv et al., 2020 | Primary human granulosa cells from IVF patients | YAP1 | OE | Increased granulosa cell proliferation and growth; loss of granulosa cell identity |
| KO | Decreased granulosa cell proliferation and growth; increased cell apoptosis | |||
| KGN cell line | YAP1 | OE | Promotion of cell cycle progression; decrease in apoptotic cells; loss of granulosa cell identity | |
| KO | Reduced proliferation; increased apoptosis | |||
| HGrC1 cell line | YAP1 | OE | Increased granulosa cell proliferation and growth; decreased apoptosis; loss of granulosa cell identity | |
| KO | Suppressed granulosa cell proliferation | |||
| St John et al., 1999 | Lats1 -/- mouse model | Lats1 | KO | Reduced follicle number; disruption in ovulation |
| Sun et al., 2015 | Lats1 -/- mouse model | Lats1 | KO | Abnormal follicle development; development of ovarian cysts; increased germ cell apoptosis |
| Tsoi et al., 2019 | Lats1 fl/fl ; Lats2fl/fl; Cyp19a1-cre mouse model | Lats1/2 | KO | Enlargement of the ovary; infertility; loss of granulosa cell identity |
| Lats1 fl/fl ; Lats2fl/fl; Cyp19a1-cre mouse isolated granulosa cells | Lats1/2 | KO | Loss of granulosa cell identity and function | |
| St Jean et al., 2019 | Lats1 fl/fl ; Lats2fl/fl; Amhr2-cre mouse model | Lats1/2 | KO | Reduced follicle number; alterations in granulosa cell morphology and identity; thickening of the ovarian surface epithelium |
| Sun et al., 2021 | Primary hen granulosa cells | LATS2 | OE | Decrease in granulosa cell proliferation |
| KO | Increase in granulosa cell proliferation | |||
| Lyu et al., 2016 | Primary hen granulosa cells | SAV1 | OE | Decrease in granulosa cell proliferation |
| KO | Increase in granulosa cell proliferation |
Abbreviations: KO, knockout; OE, overexpression; VP, verteporfin.
It is clear that activation of the Hippo pathway results in the cessation of granulosa cell growth, while inhibition of the Hippo pathway results in the activation of YAP1 and proliferation of granulosa cells with subsequent follicle growth. Further, aberrant expression of YAP1 can contribute to lineage reprogramming in ovarian cells, potentially disrupting follicular development and overall ovarian homeostasis. Taken together, the evidence suggests that the Hippo pathway in ovarian somatic cells plays a prominent role in follicle development and function.
Hippo Components in Ovarian Germ Cells and Oocytes
Successful reproduction depends on proper oocyte development and maturation. Definitive temporal gene and protein expression and their respective cellular localization in germ cells is required for oocyte development. Single cell transcriptome analysis of oocytes collected from human in vitro fertilization (IVF) patients has identified YAP1 in mature oocytes (118). Immunohistochemical evaluation of the upstream Hippo signaling component MST1 in human ovarian cortical strips demonstrated a dynamic shift in MST1 expression in the oocyte during follicular development, with localization occurring in the oocyte cytoplasm in primordial and primary follicles and a gradual translocation to the oocyte nucleus in secondary and antral follicles (119-121). In mice, localization of YAP1 protein and mRNA varies within oocytes based on developmental stage. YAP1 is evenly distributed in the cytoplasm of murine oocytes at the germinal vesicle stage, with expression shifting to the nucleus after fertilization (121). Similar observations demonstrate that YAP1 expression is restricted to the cytoplasm in murine embryonic germ cells and throughout oocyte development, with phosphorylation at Ser112 regulated by protein kinase A (PKA) (119). Upon dephosphorylation of YAP1 at Ser112, YAP1 translocates to the nucleus of the growing oocyte, although it is unable to accumulate, suggesting that YAP1 is not intrinsically involved in murine oogenesis (119). Further support for the thesis that Yap1 is nonessential for oogenesis in mice is derived from experiments in which Yap1 was selectively deleted in oocytes by crossing Yap1-floxed mice with transgenic Gdf9-Cre mice (121). Oocyte-specific deletion of Yap1 had no impact on folliculogenesis, oocyte maturation, spindle organization, or fertilization (121). Importantly, upon fertilization, oocyte Yap1 is vital for embryonic development (122). Interestingly, expression of the coactivator Taz is minimal in the mouse oocyte (121). It appears therefore, that Hippo signaling in ovarian somatic cells rather than germ cells directs follicular growth and oocyte maturation.
Studies using murine cumulus oocyte complexes (COCs) reveal that the oocyte and other factors that control COC expansion have an important impact on the Hippo pathway (123). Removal of oocytes from COCs of immature mice resulted in increases in Sav1, Mob1b, and Lats1/2 transcripts in cumulus cells and the subsequent re-introduction of oocytes to the cumulus cells restored expression to the levels observed in intact COCs (123). To determine whether growth factors secreted from the oocyte, like GDF9 and BMP15, contributed to the regulation of Hippo signaling within the cumulus cell, COCs were treated with SB431542 to inhibit oocyte-initiated GDF9/BMP15-mediated SMAD2/3 signaling. Similar to the removal of oocytes, treatment with the inhibitor increased Sav1 and Lats2 mRNA, although no comparable increases in Lats1 or Mob1b were observed (123). Treatment of COCs with verteporfin to disrupt the YAP1/TEAD interaction hindered oocyte stimulation of granulosa cell proliferation in vitro and increased expression of transcripts that support progesterone secretion in the absence of ovulatory signaling (123). Together, this suggests that inhibition of YAP1/TEAD association can lead to premature differentiation of cumulus cells (123). These studies provide valuable clues about the role of the oocyte in controlling Hippo signaling and the role of Hippo signaling in the process of cellular differentiation.
In vitro stimulation of murine COCs with EGF which mimics the post-LH surge ovulatory increase of EGF-like growth factors, increased the abundance of many Hippo-related transcripts (Mob1b, Mst1/2, Lats1/2, and Taz), but not Yap1. In this study, levels of proteins for these components as determined by Western blot analysis displayed only modest changes or were greatly reduced in the intact COCs, especially LATS1 and YAP1 (123). In contrast, in vitro treatment of bovine granulosa cells with the YAP1/TEAD inhibitor verteporfin inhibited EGF stimulation of ERK1/2 and AKT phosphorylation (100), consistent with the findings in human granulosa cells, where YAP1 interacts with the EGFR singling pathway to promote cell proliferation (85). In vivo modeling of ovulation in the mouse with human chorionic gonadotropin treatment revealed increases in phosphorylation of YAP1 and TAZ after 24 hours and a significant reduction in total YAP1, but not TAZ protein (123). Understanding the role of the Hippo pathway during the ovulatory transition and response to gonadotropin control is pivotal for the development of new therapeutic approaches for improving fertility or contraception.
It is well accepted that human females, as well as females from other mammalian species, are born with a finite number of oocytes. However, a theory posits that mammalian ovarian stem cells exist as a potential source of germ cell renewal (124-127). Given the role of the Hippo pathway in stem cell renewal, some studies suggest an association between Hippo core components and ovarian stem cells (103, 117). Co-expression of the Hippo components Lats2, Mst1, and Yap1 is observed in vitro in isolated murine ovarian stem cells expressing the germ cell marker mouse vasa homologue (Mvh; also known as DEAD box protein 4, DDX4). The overexpression of Yap1 in these cells resulted in an increased abundance of specific markers for ovarian stem cells, Mvh and Oct4 (also known as POU Class 5 Homeobox1, POU5F1), suggesting that Yap1 promotes the differentiation of ovarian stem cells (117). Conversely, the knockdown of Yap1 via shRNA resulted in reduced abundance of Mvh and Oct4 and a decrease of multiplication efficiency in cells depleted of Yap1 (117), indicating that Yap1 expression is positively correlated with the proliferation and differentiation of murine ovarian stem cells.
Impacts of Hippo Signaling on Endocrine Function and Cellular Metabolism
Impacts on Endocrine Function
Hormone responsive estradiol biosynthesis is a hallmark of granulosa cells contributing to the timing and control of the reproductive cycle. In vitro studies utilizing siRNA-mediated knockdown of YAP1 in bovine granulosa cells revealed an 80% reduction in FSH-induced estradiol production relative to granulosa cells treated with nontargeting siRNA controls (84). Suppression of YAP1 activity via pharmacological inhibition or gene silencing also suppresses estradiol and progesterone production in KGN cells and primary human granulosa cells (85, 92). Paradoxically, other investigations using the human KGN cell line and primary human granulosa cells reported that overactivation of YAP1 also repressed production of estradiol and progesterone and eliminated accumulation of lipid droplets, presumptively as a result of the dedifferentiation of gonadotropin-responsive granulosa cells (85, 101). These divergent findings suggest that physiologic levels of YAP1 are required to balance normal proliferative and steroidogenic activities of granulosa cells. Forced overexpression of YAP1 results in cellular reprogramming and loss of granulosa identity. These observations are consistent with findings in murine granulosa cells showing that targeted deletion of Lats1/2 resulted in dedifferentiation or transdifferentiation of granulosa cells (114, 115). There is a great opportunity for future studies to identify the nuclear binding partners of YAP1 and TAZ and to determine how Yap1/Taz control transcriptional reprogramming during phases of follicular development and differentiation.
Studies performed on murine granulosa cells showed that treatment with testosterone and estradiol enhanced the expression and activity of Yap1 (97). As demonstrated by studies employing a luciferase reporter reflecting YAP1 activity, testosterone induces translocation of Yap1 from the cytoplasm to the nucleus within 30 minutes of treatment before being redistributed to the cytoplasm after 24 hours. Testosterone’s ability to stimulate transcription of the Yap1-activity reporter was prevented in cells depleted of YAP1 by treatment with siRNA targeting Yap1. This response was accompanied by a reduction in testosterone-stimulated proliferation of granulosa cells (97).
Studies utilizing genetic mouse models have also demonstrated that alteration in Hippo signaling adversely impacts gonadotropin levels and ovarian hormones. Global deletion of Lats1 in female mice results in decreased serum levels of LH and prolactin, although FSH levels remain unaffected (112). A recent study utilizing a mouse model of gonadotrope-specific deletion of Yap1 and Taz (GnRH receptor-IRES-Cre) revealed that Yap1 and Taz can regulate LH release, as demonstrated by increased levels of circulating LH after loss of Yap1 and Taz function (128). These mice also exhibited a hyperfertility phenotype characterized by higher ovulation rates and larger litter sizes (128). Further, in cultured pituitary cells from the Yap1 and Taz knockout increased both basal and GnRH-induced LH secretion, while inhibition of Yap1 via verteporfin also increased LH secretion in a similar manner in LβT2 gonadotrope-like cells (128).
Other studies show that ovarian specific deletion of Lats1 and Lats2 (Cyp19a1-Cre) results in increased ovarian weight, loss of granulosa cell identity, decreased progesterone levels (~6 fold lower than respective controls), and increased levels of FSH and LH, indicative of potential ovarian failure (114). Ovarian targeted Yap1 shRNA in mice decreased serum estradiol and FSH levels while lentiviral overexpression of Yap1 generates the opposite effect, increasing estradiol and FSH serum levels (117). Together, these findings implicate Hippo signaling and YAP1 in moderating the endocrine function of the ovary. The mechanism(s) behind how the Hippo pathway regulates steroidogenesis are subject to future investigation.
The Hippo and ERK signaling pathways may intersect in the control of ovarian function. The RAS-ERK1/2 signaling cascade is activated in the ovary upon the LH surge prior to ovulation (35). In various cell types, mitogen-activated protein kinases (MAPKs) may work in parallel with MST1/2 in the regulation of LATS1/2 (129). Using a superovulation model, in vivo human chorionic gonadotropin (hCG) injection into prepubertal mice increased the abundance of phosphorylated MST1/2 and phosphorylated YAP1 in granulosa cells of preovulatory follicles and in luteal cells after ovulation, thus decreasing nuclear YAP1. It is likely that ERK signaling mediated the response because in vivo deletion of Erk1/2 in granulosa cells (Erk1-/-; Erk2fl/fl; Cyp19a1-Cre) preserved YAP1 protein expression after hCG injection (97). Similar results were observed in vitro after forskolin/phorbol 12-myristate 13-acetate (PMA) treatment (mimicking the LH surge) and use of the ERK1/2 inhibitor U0126 (97). Treatment of murine granulosa cells with forskolin and PMA resulted in translocation of YAP1 from the nucleus to cytoplasm and the upregulation of genes associated with ovulation. Overexpression of Yap1 hindered their expression while the subsequent knockdown of Yap1 induced upregulation of the LH target genes (97). In a similar fashion, blocking YAP1 activity by direct injection of the YAP1/TEAD inhibitor verteporfin into the bovine follicle in vivo, obstructed ovulation in a dose-dependent manner (100). In bovine granulosa cells treated with verteporfin in vitro, downregulation of YAP1 target genes CTGF and CYR61 was observed, along with decreases in EGFR mRNA abundance (100). These important observations suggest that Hippo signaling plays a role in maintaining the state of cellular differentiation that allows for proper timing and execution of the ovulatory response to gonadotropins (Table 3). The ways in which these pathways interact to control the ovulatory process in large animal models, nonhuman primates, and humans await discovery.
Table 3.
Knockout/overexpression studies of Hippo pathway core components and impacts on endocrine function
| Study (first author, year) | Model system | Gene targeted | Overexpression/ knockout | Hormone measured | Findings |
|---|---|---|---|---|---|
| Fu et al., 2014 | KGN cell line | YAP1 | KO | E2 (conditioned media) | Reduces FSH-induced E2 production |
| Ye et al., 2017 | Lentiviral OE infertile mouse model | Yap1 | OE | E2, FSH (serum) | Increased FSH; no changes in E2 |
| Lentiviral KO wild-type mouse model | Yap1 | KO | E2, FSH (serum) | Decline in E2, FSH | |
| Lv et al., 2019 | KGN cell line 3D culture system | YAP1 | OE | E2, P4 (conditioned media) | Reduced E2, P4 |
| Plewes et al., 2019 | Primary bovine granulosa cells | YAP1 | KO | E2 (conditioned media) | Reduces FSH-induced E2 production |
| Lv et al., 2020 | KGN cell line | YAP1 | OE | E2, P4 (conditioned media) | Reduces FSH/FSK-induced E2, P4 production |
| Human granulosa cells from IVF patients | YAP1 | OE | E2, P4 (conditioned media) | Reduces FSH/FSK-induced E2, P4 production | |
| Lalonde-Larue et al., 2022 | Yap1 fl/fl ; Taz fl/fl ; Gnrhr GRIC mouse model | Yap1/Taz | KO | FSH, LH (serum) | Increases in LH; no changes in FSH |
| St. John et al., 1999 | Lats1 -/- mouse model | Lats1 | KO | LH, PRL, FSH (serum) | Deficiency in LH, PRL; no changes in FSH |
| Tsoi et al., 2019 | Lats1 fl/fl ; Lats2fl/fl; Cyp19a1-Cre mouse model | Lats1/2 | KO | E2, P4, LH, FSH (serum) | Decrease in P4; Increases in LH, FSH; E2 undetected (below assay threshold) |
Abbreviations: E2, estradiol; FSH, follicle-stimulating hormone; FSK, forskolin; KO, knockout; LH, luteinizing hormone; OE, overexpression; P4, progesterone; PRL, prolactin.
Impacts on Cellular Metabolism
Similar to events that activate Hippo signaling, environmental stimuli (e.g., energy stress, nutrients, oxygen) and upstream protein kinases (growth factor tyrosine kinases, Ca2+/calmodulin protein kinases, PKA) regulate cellular metabolism, which leads to a complex perspective on their regulation of YAP1 activity (130, 131). One such protein kinase is 5′-AMP-activated protein kinase (AMPK). AMPK activates catabolic processes such as autophagy, glucose transport, glycolysis, and glycogen synthesis or gluconeogenesis and is expressed in oocytes, ovarian follicle cells, and luteal cells (132-137). AMPK is activated in response to energy stress, i.e., reduced levels of ATP (elevated AMP/ATP ratio) (138). AMPK activation can also be triggered by upstream kinases, such as liver kinase B1 (LKB1) or in response to calcium flux via calcium/calmodulin dependent kinase kinase 2 (CAMKK2) (138, 139). Importantly, the activation of AMPK can be offset by gonadotropin activation of PKA signaling, which limits AMPK activity (133, 134). Studies suggest that AMPK serves to limit YAP1 activity. AMPK can directly phosphorylate YAP1, disrupting the interaction between YAP1 and the transcription factor TEAD, thus limiting YAP1-mediated transcription (77, 78). AMPK can also promote LATS1/2 activity and phosphorylation of YAP1, which reduces transcription activity by sequestering YAP1 in the cytoplasm (74). On the other hand, LATS1/2 kinase can be activated in response to energy stress in cells deficient in AMPK cells (78), indicating that cellular energy stress can also activate the Hippo pathway via an AMPK-independent mechanism.
Activation of AMPK inhibits gonadotropin-stimulated progesterone synthesis and induces autophagy in ovarian granulosa and luteal cells (132-134, 137, 140). Studies performed in the mouse ovary found that energy stress via glucose depletion increased AMPK activation and phosphorylation of MST1/2 (140). However, it did not affect phosphorylation of LATS1 and YAP1 (Ser127 or Ser94) when compared to ovaries treated with high levels of glucose (141). In this study, treatment with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), a known activator of AMPK, also elevated phosphorylation of LATS1, but paradoxically decreased phosphorylation of YAP1 at the site required for inactivation (Ser127) (141). Treatment with AICAR can also decrease progesterone production in granulosa and luteal cells (132-134, 137, 140). Thus, it is possible that dysregulation of AMPK activity can affect posttranslational modifications of YAP1 and ultimately change ovarian steroidogenesis. As FSH is a main driver of follicle development, Puri et al suggested that observed effects of FSH on YAP1 can be associated with cell differentiation rather than cell growth (142). A caveat with the studies mentioned above is that direct AMPK-YAP1 interactions were not established.
Another protein kinase responsible for regulating metabolic events in ovarian cells is mechanistic target of rapamycin (mTOR), which in contrast to AMPK, promotes anabolic processes, including ribosome biogenesis and protein, nucleotide, fatty acid, and lipid synthesis. In general, the activation of mTOR is dependent on nutrients and growth factors via (PI3K)/AKT and/or mitogen-activated protein kinase (MAPK)1/3 signaling pathways (143, 144). As described earlier for the Hippo signaling pathway, the mTOR signaling pathway is also important for follicular activation, growth and differentiation, and ovulation (145). The roles of Hippo and mTOR in organ size regulation are well established by their respective functions in controlling cell number and cell size (146). Several reports indicate that components of the Hippo pathway play an important role in the regulation of mTOR activity. For example, deletion of the upstream regulator of neurofibromin 2 (NF2), which promotes Hippo signaling, results in the activation of mTORC1 and stimulates growth of various cell types (147-149). Additionally, Yap1 regulates IGF1 (150) and the EGF ligand AREG, which activate mTOR and contribute to granulosa cell proliferation (85). Similar to depletion of Nf2, loss of Mst1 or Mst2 leads to mTORC1 activation (151). More recent studies indicate that mTORC2 can directly phosphorylate YAP1 at serine 436, positively regulating YAP1 activity, YAP1 dependent gene expression, cell growth, and motility in glioblastoma cells (149). Other lines of evidence suggest a reciprocal regulation of mTOR by YAP1 signaling. Nutrients such as amino acids (e.g., glutamine, arginine, leucine) are sufficient to activate mTOR (152). Experiments in systems other than the ovary indicate that activated, nuclear-localized YAP1 promotes the transcription of key metabolic enzymes. For instance, YAP1 stimulates the expression of glutamine synthetase, catalyzing the transformation of glutamate into glutamine, which then activates mTOR (153, 154). YAP1 can also upregulate expression of amino acid transporters that enhance mTOR activity (155). Thus, ample evidence suggests that both mTOR and Hippo signaling regulate ovarian growth, but the exact mechanisms operating during phases of follicular development remain to be solved experimentally.
The gonadotropins FSH and LH activate mTOR via the cAMP/PKA signaling pathway in ovarian granulosa and luteal cells, respectively (156, 157). Inhibition of mTOR activity impairs follicular function (158), but not luteal function or cellular viability (132), suggesting that mTOR activation is required for follicular maturation. Activation of AMPK, which activates Hippo, also inhibits mTOR and impairs the ability of FSH and LH to stimulate granulosa and luteal steroidogenesis (156, 159). How mTOR and AMPK pathways converge to regulate Hippo signaling in ovarian cells is complex and has not been carefully examined. Both FSH and LH can inhibit AMPK activity in their respective target cells (133, 134, 160) suggesting that the levels of YAP1 and TAZ phosphorylation likely fine tune the overall growth- and differentiation-promoting actions of gonadotropins. Studies are needed to fully characterize the YAP1 and TAZ regulated transcriptomes in response to hormones and under conditions of metabolic stress to identify genes and pathways critical for altering ovarian function and cellular fate.
Dysregulated energy metabolism represents a major contributing cause of female infertility (161). Lipids are an important source of mitochondrial energy production and cholesterol is a key precursor for steroid synthesis. Cellular lipid homeostasis is therefore essential for female fertility and regulated by a complex interplay between de novo synthesis, export, and storage (162). Multiple studies have demonstrated that excess cholesterol and fatty acids can negatively affect development competence of oocytes (163-173). It has been recently demonstrated that multiple members of the Hippo family influence lipid metabolism (174). Sterol regulatory-element binding proteins (SREBPs), key transcription factors that regulate the expression of genes involved in cholesterol synthesis, were found to be regulated by the Hippo components MST1 and LATS2 (175, 176). Overexpression of MST1, which would activate LATS2, led to the downregulation of SREBPs (176). Inhibition of Hippo signaling by in vitro knockdown of LATS2 caused activation of SREBP and cholesterol accumulation. Mice harboring a liver specific knockout of LATS2 displayed constitutive activation of SREBP, leading to aberrant lipid accumulation even when maintained under normal diet (175). Furthermore, the Hippo effector molecule, YAP1 has been shown to directly interact with SREB-1c and SREBP2 on the promoter’s fatty acid synthase (FASN) and 30-hydroxylmethyl glutaryl coenzyme A reductase (HMGCR) leading to stimulation of cholesterol synthesis (177). During the ovulatory process, LH stimulates expression of SREBP, resulting in de novo cholesterol synthesis (178). Lipid metabolism may also influence Hippo signaling by influencing the functional activity of YAP1 and TAZ. One such pathway is the mevalonate synthesis pathway, which converts acetyl-CoA into lipid precursors for generation of cholesterol and steroid hormones (179). Inhibition of the mevalonate pathway by statins, which inhibit HMGCR, leads to the downregulation of YAP1 activity, which was bought about by obstructing the activity of Rho GTPase through its effect on the F-actin cytoskeleton (180). In other studies, increased cholesterol levels in pathological conditions were shown to promote TAZ activity through a calcium-mediated signaling pathway (181). Generally, the YAP1/TAZ elevations in lipid synthesis were associated with increased cellular proliferation. Because cellular lipid homeostasis is essential for female fertility, an understanding of how Hippo signaling controls ovarian fatty acid and cholesterol metabolism during the ovarian reproductive cycle is critical to better understand follicular development and oocyte quality as well as the underpinnings of timely cholesterol production for cellular growth, differentiation, and steroidogenesis.
Reproductive Toxicity/Endocrine Disruptors
Endocrine-disrupting chemicals are exogenous substances and/or mixtures from natural or synthetic sources that disrupt the normal actions of endogenous hormones. Endocrine-disrupting chemicals can affect fertility via the alteration of ovarian steroidogenesis (182), mimicry of receptor signaling (183, 184), and impaired follicle and oocyte growth (185, 186). Zearalenone is an estrogenic mycotoxin present in contaminated grain products, including those used in animal feeds and breakfast cereals (187). The estrogenic effects of zearalenone on the female reproductive system are responsible for delayed pregnancy and fetal development (188, 189), disrupted germ cell meiotic progression and follicle assembly (190-193), and alterations in the ovarian proteome (194). Studies have shown that low doses of zearalenone can exert estrogen-like effects and carcinogenic properties, which can stimulate the proliferation of cells, while high doses can result in oxidative stress, DNA damage, and apoptosis (195).
In cultured mouse granulosa cells, exposure to zearalenone increased mRNA and protein expression of the Hippo pathway components Yap1, Taz, and Tead and resulted in an abnormal population of large, flattened granulosa cells (196). Bioinformatics analysis of RNA sequencing studies following similar treatment protocols revealed that zearalenone upregulated genes in granulosa cells associated with pathways linked to uncontrolled cell growth. Oral gavage of young female mice with zearalenone for 3 weeks also increased the expression of Yap1, Taz, Tead, and Smad3 in granulosa cells and disrupted ovarian follicle development (196). The impacts of chronic exposure to zearalenone on the ovary are unclear, although alterations in the cell cycle and Hippo signaling may potentially contribute to uncontrolled granulosa/ovarian cell growth.
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are widespread environmental contaminants found in several industrial and consumer products. These chemicals have been detected in nearly every person sampled in the US National Biomonitoring Program (197). Perfluorooctanoic acid (PFOA) is one of the primary PFAS compounds and is extensively manufactured. Observational studies in humans have shown PFOA to delay the age of menarche, disrupt menstrual cyclicity, and initiate early menopause via premature ovarian insufficiency (198-200). Similar studies in animals have revealed advanced puberty onset, inhibition of sex steroid hormones, and alterations in the composition of the follicular pool (201, 202). A recent study utilizing the immortalized human granulosa cell line HGrC1, a noncancerous human granulosa cell line that mimics granulosa cell behavior in earlier stages of development, revealed increased levels of YAP1 protein and its downstream target CTGF after PFOA exposure (203). The observations of increased YAP1 and CTGF protein expression was paired with increases in cell proliferation and migration (203). Upon inhibition of YAP1 with verteporfin, HGrC1 cell proliferation was repressed, further supporting a role for the Hippo pathway in PFOA-induced cell proliferation.
Another potential source of endocrine-disrupting chemicals includes exposure to plasticizers, chemical additives used in the manufacturing of products widely used in industry, agriculture, medical devices, and in food and beverage containers (204). Tri-ortho-cresyl phosphate (TOCP), a plasticizer commonly used in industry as a plastic softener and flame retardant, causes dose-dependent effects on murine ovarian follicle development, including decreased ovarian weight and follicle numbers relative to ovaries from mice that were exposed to vehicle alone (205). In mice exposed to TOCP, ovarian protein levels of the Hippo pathway components MST1 and LATS2 were increased and YAP1 expression was decreased (205), consistent with Hippo pathway activation. Posttranslational modifications of the Hippo pathway were observed in response to exposure to higher doses of TOCP. The phospho-MST1/MST1 ratio was decreased while in contrast the phospho-YAP1/YAP1 ratio was increased, suggestive of reduced survival of follicles due to activation of the Hippo pathway and subsequent loss of active YAP1-mediated growth-associated gene expression (205). Interestingly, ovarian overexpression of Yap1 in mice treated with TOCP provided a protective effect, evidenced by decreased numbers of atretic follicles and increased primordial, preantral, and antral follicle numbers, as well as increased fertility as measured by generation of live offspring (205). Chemical insults trigger stress responses and activation of Hippo signaling is essential for preserving tissue homeostasis and cell survival upon a chemical insult (Table 4). Taken together, these data further implicate the Hippo pathway as a key mediator of ovarian homeostasis in response to environmental toxicants. Much remains to be discovered about how YAP1 and TAZ interact with ovarian transcription factors to fine tune cell differentiation and cell fate after chemical exposure.
Table 4.
Chemical exposure impacts on Hippo pathway core components
| Study (first author, year) | Model system | Chemical exposure | Findings |
|---|---|---|---|
| Zhang et al., 2018 | Primary mouse granulosa cells | Zearalenone (30μM) | Altered granulosa cell morphology; increased transcripts of Yap1, Taz, Tead3; increased protein expression YAP1, TAZ |
| Mouse model—in vivo | Zearalenone (40-100 μg/kg) | Increased transcripts of Yap1, Taz; decrease in follicle number | |
| Hu et al., 2019 | Mouse model—in vivo | Tri-ortho-cresyl phosphate (TOCP) | Increased protein expression MST1, LATS2, decrease YAP1 protein; decline in follicle number; increase in atretic follicles; protective effect of YAP1 OE |
| Clark et al., 2022 | HGrC1 cell line | Perfluorooctanoic acid (PFOA) | Increased cell proliferation and migration; elevated YAP1 and CTGF protein expression |
Abbreviations: CTGF, connective tissue growth factor; LATS2, large tumor suppressor 2; MST1, mammalian serine/threonine-protein kinase 4; OE, overexpression; TAZ, WW domain-containing transcription regulator 1 (also known as WWTR1); TEAD, TEA domain transcription factor; YAP1, yes-associated protein 1.
Hippo Signaling in Ovarian Disorders
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a complex endocrine disorder affecting 5% to 15% of women of reproductive age (206). Clinically, women with PCOS have at least 2 of the 3 following characteristics: clinical and/or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology via ultrasound. PCOS is frequently associated with abdominal adiposity, insulin resistance, obesity, metabolic disorders, and cardiovascular risk factors. The etiology of this syndrome remains largely unknown, but mounting evidence suggests that PCOS might be a complex multigenic disorder with strong epigenetic and environmental influences. PCOS is associated with anovulatory or oligo-ovulatory infertility and increased risk of obstetric complications such as miscarriage, gestational diabetes, and preeclampsia (207); therefore, it is especially important to identify the molecular background of this disorder.
Several independent genomic studies have identified several loci significantly associated with PCOS (90, 208-210). A genome-wide association study (GWAS) identified 3 variants of YAP1 (rs11225138, rs11225161, rs11225166) that correlate with enhanced susceptibility for PCOS (90). Interestingly, genotype-phenotype correlation analysis showed that carriers of the rs11225161 and/or rs11225166 allele have impaired glucose tolerance, suggesting that women carrying these specific alleles of YAP1 may also have an increased risk of developing a metabolic disorder (90). Among the highly expressed genes in the ovaries of women with PCOS was the Hippo pathway component YAP1 (90, 210). Studies show that the YAP1 promoter is hypomethylated in PCOS patients compared to non-PCOS controls, which could contribute to the increased level of YAP1 mRNA and protein observed in PCOS patients (211). As described in previous sections of this review, YAP1 supports appropriate development and growth of ovarian follicles, while dysregulation in the Hippo pathway can lead to elevated expression of YAP1 and result in enlargement of the ovaries (114) and ovarian cysts (111), which are characteristics of PCOS (212).
Hormonal abnormalities in women with PCOS are associated with a higher concentrations of testosterone in blood serum, suggesting that an elevated androgenic environment contributes to the disorder (213). Hyperinsulinemia, a characteristic feature for women with PCOS, contributes to hyperandrogenism by stimulatory effects on androgen production by theca cells (214). In primary human granulosa cells, in vitro treatment with testosterone demonstrated a concentration-dependent inverse correlation with methylation status of the YAP1 promoter, with increases in testosterone concentration resulting in decreases in YAP1 promoter methylation (211). However, no changes in methylation of the YAP1 promoter were observed after treatment with either FSH or LH (211). The suggestion that ovarian androgens can elevate YAP1 expression in human granulosa cells is supported by studies in murine granulosa cells demonstrating that testosterone can enhance the expression and activity of YAP1 as discussed in a previous section of this review (97). Considering the hormonal imbalance in women with PCOS, it is likely that elevated concentrations of testosterone contribute to the elevated expression and activity of YAP1 in human ovarian cells, creating a favorable environment for PCOS development (Table 5). The elevated content of YAP1, observed in the ovaries of women with PCOS, is available for further posttranslational modifications that regulate its activity and consequently the expression of downstream genes.
Table 5.
Hippo pathway and PCOS
| Study (first author, year) | Model system | Method | Findings |
|---|---|---|---|
| Li et al, 2012 | Human GWAS study | SNP genotyping | YAP1 variants rs11225138, rs11225161, rs11225166 associated with PCOS susceptibility |
| Jiang et al., 2017 | Primary human granulosa cells from IVF patients | Bisulfite sequencing PCR | Hypomethylation of YAP1 promoter; increase in YAP1 mRNA and protein in women with PCOS; elevated levels of testosterone decreased YAP1 promoter methylation; no changes in YAP1 promoter methylation with FSH, LH |
Abbreviations: FSH, follicle-stimulating hormone; GWAS, genome-wide association study; IVF, in vitro fertilization; LH, luteinizing hormone; PCOS, polycystic ovary syndrome; PCR, polymerase chain reaction; SNP, single nucleotide polymorphism; YAP1, yes-associated protein 1.
As discussed in earlier sections of this review, the activity of YAP1 can be modulated by numerous upstream kinases, including AMPK via phosphorylation of YAP1 on Ser94, which disrupts the interaction of YAP1 with the transcription factor TEAD, thus repressing YAP1 target genes and restricting cell growth (77). Metformin, a commonly used therapeutic to treat women with PCOS with insulin resistance (215), is also an AMPK activator and has been shown to target YAP1 (216-218). Previous studies demonstrate that AMPK activation inhibits steroidogenesis in ovarian granulosa cells primarily by reducing the ability of gonadotropins to stimulate the transcription of genes required for steroidogenesis (140, 219). Because metformin activates AMPK in ovarian granulosa cells and impairs progesterone production (140), it is tempting to speculate that metformin via AMPK inhibits YAP1 interactions with transcriptional regulators in granulosa cells and directly or indirectly disrupts the steroidogenic machinery. Further, because the ovaries of PCOS patients appear fibrotic, it seems likely that the lower activity of AMPK observed in ovaries of women with PCOS (220, 221) could contribute to an interaction between YAP1 and TEAD and the transcription of genes encoding factors related to connective tissue growth and cellular communication network (CCN) growth factors. More studies should be undertaken to clarify the interactions between Hippo signaling and other signaling pathways to better understand their role in the etiology of PCOS.
Primary Ovarian Insufficiency
Ovarian Fragmentation
The Hippo signaling pathway is responsive to external stimuli, including a variety of ligands interacting with specific receptors and alterations in the physical environment and cell shape (222). In vitro activation therapy is an alternative for women who have previously experienced a poor response to gonadotrophin-mediated follicle stimulation. In this procedure, cortical strips from the ovary are surgically removed, fragmented, and transplanted back into the patient, or cultured in the presence of protein kinase B (Akt) stimulators prior to implantation (82, 223, 224). In this regard, fragmentation of the ovary has been shown to promote actin polymerization, resulting in disruption of Hippo signaling, reduced phosphorylation of YAP, and elevation of YAP1 abundance in the nucleus (82, 87, 98, 120, 225). The disruption of Hippo signaling leads to the transcription of YAP1-responsive genes including the expression of cellular communication network growth factors (CCN1, 2, 3, 5, and 6) and members of the inhibitor of apoptosis gene family (BIRC1 and 7) in follicular somatic cells, thus, promoting follicular growth (82, 225, 226). Upon fragmentation of the ovary, YAP1 expression was primarily located in the nucleus of the oocyte and granulosa cells in the cultured human ovary wedge sections, while YAP1 expression was observed in both the cytoplasm and nucleus of granulosa cells and oocytes in the intact cultured ovary controls (87). Further studies showed that treatment of murine ovaries or human granulosa cells with drugs that stimulate actin polymerization such as jasplakinolide and sphingosine-1-phosphate resulted in increased nuclear YAP1 localization and expression of the downstream growth factor Ccn2 and activation of follicle growth (225, 227). Likewise, Akt stimulation after ovarian fragmentation promoted greater secondary and antral follicle growth (82). In contrast, the Akt inhibitor MK2206 suppressed follicle growth, which was partially rescued upon overexpression of Yap1 (83). Similarly, in an ovarian cortex model that was microinjected with rhabdomyosarcoma cells, inhibition of YAP1/TEAD interaction via verteporfin and verteporfin plus a chemotherapeutic was able to effectively clear the tumor cells without any impacts on ovarian tissue or follicular integrity as measured by metabolic activity and Neutral red uptake (228). While this study was purely proof-of-principle, it would be interesting to see if these follicles could be activated and their subsequent viability after treatment with verteporfin and chemotherapy drugs. Together, procedures of mechanical stimulation and/or pharmaceutical administration in order to disrupt the Hippo pathway or YAP1/TEAD activity in order to control follicle growth have potential for treating infertility.
In ovarian samples derived from transgender men or oncology patients, in vitro ovarian fragmentation also led to activation of primordial follicles through inhibition of the Hippo pathway (120). The fragmentation and culture of human ovarian tissue reduced protein levels of phosphorylated YAP1 in the granulosa cells of primordial follicles and increased levels of total YAP1 upon follicle growth and activation (120). In parallel with the increase in nuclear YAP1 observed with follicle activation, the downstream YAP1 target CTGF was upregulated (120), lending further support for the role of the Hippo pathway in follicle activation. Interestingly, a notable difference in YAP1 was observed in the samples from oncology patients vs the transgender men, in that nuclear YAP1 expression in granulosa cells from the oncology patients was minimal, suggesting this could be possible due to the etiology of the oncological disease or hormone therapy prior to surgery (86, 120). These data reflect what has been reported in murine studies in which testosterone induces the translocation of YAP1 from the cytoplasm to the nucleus of granulosa cells (97), suggesting conserved regulators and mechanisms between species.
In contrast to the studies described above, another study reported that fragmentation of the human ovarian cortex did not induce changes in actin polymerization, as revealed by failure to observe changes in the ratios of globular and filamentous actin after tissue fragmentation (229). Further, levels of YAP1 and phosphorylated YAP1 proteins were unaltered, as were levels of transcripts for the YAP1 downstream targets CCN2, CCN3, and CCN5 (229). As a result, no apparent changes were observed in the number of growing follicles in fragmented cortex vs intact controls, though the overall number of total follicles was lower in the fragmented pieces relative to intact cortex (229). Further study is needed to clarify the molecular and physiological mechanisms of Hippo regulation and its role in follicle activation after fragmentation in human ovaries. Although some key molecular mechanisms that regulate Hippo signaling have been identified, many aspects of how these are modulated under physiological or stress conditions remain poorly explored.
Ovarian Cell Malignant Neoplasia
Ovarian cancer is the most lethal gynecological malignancy and the fifth leading cause of cancer death in women in the United States. American Cancer Society estimated that more than 21 500 women would be diagnosed with ovarian cancer, and around 14 000 women would die of this disease in 2020 (230). Globally, it is estimated that ovarian cancer claims the lives of more than 184 000 women annually (231). Ovarian cancer is sometimes referred to as a “silent killer” because most patients with ovarian cancer lack specific symptoms and are diagnosed in the advanced stages of the disease. Despite advances in surgical technique, postoperative care, and chemotherapy, ovarian cancer’s overall survival rate has only improved modestly in the past decades. This could be attributed to the fact that the risk factors and the etiology of ovarian cancer are not fully understood.
Ovarian cancer is not one disease. Ovarian cancers represent a spectrum of distinct types of cancers that may originate from organs (or tissues) other than ovaries. For example, accumulating evidence showed that high-grade serous ovarian cancer (HGSOC), the most common and lethal subtype of ovarian cancer, may originate from fallopian tube epithelium (232-234). In the ovary, ovarian cancer can arise from cells such as ovarian surface epithelial cells, granulosa cells, germ cells, theca cells, and specialized gonadal stromal cells. Accumulating evidence has now shown that the Hippo pathway plays a vital role in the development of ovarian cancer from ovarian cells.
Numerous studies demonstrate that YAP1 is an ovarian cancer oncogene (Table 6). YAP1 protein is localized in both the cytoplasm and nucleus in 98% of ovarian serous cystadenocarcinoma patients, while in normal ovarian tissues, only 25% of the samples display nuclear YAP1, and 50% of the samples display only cytoplasmic YAP1 expression (235). Consistently, a high level of nuclear YAP1 is associated with poor patient survival rate and is an independent prognostic marker for ovarian cancer outcome (93, 236-238). The involvement of the disrupted Hippo pathway in the development of ovarian cancer is further supported by multidimensional cancer genetic/genomic studies performed by the Cancer Genome Atlas Research Network (TCGA) (239). Data extracted from TCGA datasets (239) demonstrate that genes encoding the upstream tumor suppressors are frequently deleted, mutated, or downregulated, while genes encoding the downstream oncogenic proteins are frequently amplified or upregulated in the Hippo signaling cascade (95). TCGA data also clearly suggest high nuclear YAP1 is significantly related to a poor prognosis of ovarian serous cystadenocarcinoma (239). In addition to its potential role in ovarian cancer development, hyperactivation of YAP1 increases the resistance of ovarian cancer cells to drug-induced cell apoptosis, suggesting that the Hippo/YAP1 pathway may also be involved in ovarian cancer chemoresistance (238, 240, 241).
Table 6.
Knockout/overexpression studies of Hippo pathway core components and ovarian cancer phenotypes
| Study (first author, year) | Model system | Gene targeted | Overexpression/knockout | Findings |
|---|---|---|---|---|
| St John et al., 1999 | Lats1 -/- mouse model | LATS1 | KO | Development of ovarian stromal tumors |
| Xia et al., 2014 | A2780 cell line | YAP1 | OE | Increased cancer cell proliferation and migration; enhanced anchorage-independent growth; increased chemoresistance |
| KO | Decreased cancer cell proliferation and migration; increased chemosensitivity | |||
| OVCAR8 cell line | YAP1 | OE | Increased cancer cell proliferation and migration; enhanced anchorage-independent growth; chemoresistance | |
| KO | Decreased cancer cell proliferation and migration; increased chemosensitivity | |||
| He et al., 2015 | HOSE-T80 cell line | YAP1 | OE | Increased cancer cell proliferation; malignant transformation; enhanced anchorage-independent growth; induced tumorigenesis in vivo |
| KO | Suppressed cancer cell proliferation | |||
| TOV21G cell line | YAP1 | OE | Increased cancer cell proliferation; malignant transformation; enhanced anchorage-independent growth; induced tumorigenesis in vivo | |
| KO | Suppressed cancer cell proliferation | |||
| Hua et al., 2016 | FTSEC cell lines | YAP1 | OE | Increased cancer cell proliferation; malignant transformation; enhanced anchorage-independent growth; induced tumorigenesis in vivo |
| KO | Suppressed cancer cell proliferation | |||
| Lv et al., 2020 | HGrC1 cell line | YAP1 | OE | Induced tumorigenesis in vivo |
| HGL5 cell line | YAP1 | OE | Induced tumorigenesis in vivo |
Abbreviations: KO, knockout; LATS1, large tumor suppressor 1; OE, overexpression; YAP1, yes-associated protein 1.
Ovarian surface epithelial cells (OSE) or ovarian cortical inclusion cyst epithelial cells have long been considered as the cell of origin of epithelial ovarian cancer, including HGSOC (232). However, recent studies favor the concept that HGSOC may originate from the fallopian tube epithelial cells (FTE) (232, 242-246). Accumulating evidence suggests that OSE cells or epithelial cells from the inclusion cysts may be the cell of origin of the low-grade serous carcinoma (247). Interestingly, there is also evidence supporting the concept that low-grade serous carcinoma could progress to HGSOC (248-252). By introducing the same genetic abnormalities into both FTE and OSE cells, Zhang and colleagues (253) indicated that HGSOC might originate from either cell type. Therefore, the role of ovarian surface epithelial cells in the development of HGSOC cannot be excluded. Upon hyperactivation of YAP1 in immortalized FTE and OSE, the malignant transformation of both types of cells and the development of high-grade ovarian cancer was observed (93, 95). Consistent with these observations, early studies demonstrated that overexpression of YAP1 and phosphorylation-defective YAP1-5SA in immortalized OSE cells resulted in increased cell proliferation, resistance to cisplatin-induced apoptosis, faster cell migration, and anchorage-independent growth, whereas YAP1 knockdown resulted in increased sensitivity to cisplatin-induced apoptosis (237). In ovarian cancer cell lines, ectopic expression of hyperactivated YAP1 promotes cell proliferation and migration, while knockdown of YAP1 suppresses cancer cell growth in vivo and in vitro, suggesting that the Hippo/YAP1 signaling pathway also plays a role in ovarian cancer progression (93, 95).
The uncontrolled growth of granulosa cells also results in ovarian cancer. Ovarian cancers developed from ovarian granulosa cells are called granulosa cell tumors (GCT) and account for about 70% of malignant sex-cord stromal tumors (254). The mechanisms underlying GCT development, recurrence, and metastasis are poorly understood. Previous reports have found that YAP1 is highly expressed in human GCT tissues and overexpression of YAP1 protein significantly stimulates the proliferation and migration of the KGN GCT cell line, demonstrating that the Hippo pathway may be involved in the progression of GCT (92). Interestingly, whole transcriptome sequencing of human samples has demonstrated that a somatic mutation in FOXL2 (C402G; Cys134TrP) is present in almost all morphologically identified adult-type GCTs (255), suggesting that mutant FOXL2 is a potential driver in the pathogenesis of adult-type GCTs. Whether the Hippo pathway interacts with mutated FoxL2 to drive the development of GCT has not been studied.
Unexpectedly, recent studies demonstrated that hyperactivation of YAP1 in well-differentiated or luteinized granulosa cells could induce dedifferentiation and reprogramming of the cells, leading to the development of high-grade ovarian cancer with serous features (101). The hyperactivation of YAP1 stimulated expression of TERT and MYC, genes associated with stemness, suggesting that YAP1 dysregulation may result in the reprogramming of granulosa cells (101). This idea was supported by gene set enrichment analysis showing downregulation of genes associated with granulosa cell differentiation and epithelial-mesenchymal transition and upregulation of genes associated with cell stemness and pluripotency (101). Since granulosa cells are of mesenchymal lineage, these cells may be a candidate cell of origin of the mesenchymal subtype of HGSOC. More studies are required to confirm this speculation.
The Hippo pathway may also play a role in the rare cancers of the ovary. Malignant transformation of germ cells leads to the development of germ cell tumors. Although YAP1 is reported to be highly expressed in the germ cells (121), the role of YAP1 in the development of germ cell tumors has not been studied. Due to the nature of the germ cell tumor and a critical role of YAP1 in the maintenance of cell pluripotency, YAP1 may play a critical role in the germ cell development (256-260). The development of ovarian stromal cell tumors in the LATS1 knockout mice demonstrated that the Hippo pathway’s disruption might also contribute to the development of ovarian stromal tumors (112).
Mutation of the YAP1 oncogene is rare in ovarian cancer, suggesting that additional oncogenic perturbations may account for YAP1 activation and subsequent tumor progression. The exact mechanisms by which the Hippo pathway regulates ovarian cancer development is largely unknown. In primary human ovarian surface epithelial (hOSE) cells, the hyperactivation of YAP1 promotes cell proliferation until cellular senescence occurs after ~7 passages (113). Interestingly, siRNA depletion of either YAP1 or TAZ did not stop the proliferation of cultured hOSE cells, though double knockdown of YAP1 and TAZ effectively reduced cell proliferation (113). The suggested mechanism behind this observation is that sustained YAP1 activity resulted in a compensatory elevation in LATS2 expression, thus creating a negative feedback loop preventing the potential for malignant transformation (113). Hyperactivated YAP1 forms feedforward loops with receptors and signaling pathways in the epidermal growth factor family (ERBB) and fibroblast growth factor receptor family (FGFR) to drive the development of ovarian cancer cells from OSE and fallopian tube secretory epithelial cell (FTSEC) cell lines (93, 95, 261).
The GPCR ligand lysophosphatidic acid, mediated by RhoA-ROCK signaling, suppresses the Hippo pathway to drive the proliferation and migration of immortalized ovarian surface epithelial cells (261). Interestingly, protein kinase C iota (PRKCI) amplification, an early event in ovarian cancer development, has been shown to promote immune suppression by driving nuclear YAP1 accumulation, leading to the expression of proinflammatory cytokines, including tumor necrosis factor (TNF) (262). A more recent study demonstrates that arrestin Beta 1 (ARRB1) regulates YAP1 activity by interacting with Trio Rho guanine nucleotide exchange factor (TRIO), a member of the RhoGEF family that induces nuclear YAP1 accumulation and aberrant expression of TEAD genes in HGSOC. Interestingly, ARRB1 also acts as a nuclear anchor for mutated TP53 and YAP1, leading to the recruitment of diverse transcription factors, thus expanding the repertoire of ARRB1 induced downstream genes (263). Several studies also indicated that downregulation of myosin phosphatase targeting protein 1 (MYPT1) and overexpression of miR-30b or miR-149-5p suppressed the Hippo pathway to induce chemotherapeutic resistance (236, 241), possibly via regulating pluripotency genes influencing ovarian cancer stem cells (238, 240). Taken together, these studies demonstrate the dynamic ability of the Hippo pathway to control tissue homeostasis.
Concluding Remarks and Looking Forward
The Hippo pathway and downstream transcriptional effectors YAP1 and TAZ have emerged as an important signal transduction pathway in ovarian development and function, regulating several events such as cell fate determination, germ and follicle cell proliferation/migration, follicle activation/development, and apoptosis. The observations from the multi-species studies discussed herein have begun to elucidate the functional diversity and molecular mechanisms of Hippo signaling in the ovary, as well as identifying interactions with other signaling pathways and responses to various external stimuli, translating, and generating cellular responses that maintain ovarian tissue growth and homeostasis. It seems likely that transcriptional regulators activated by ovarian paracrine and endocrine factors interact with Yap1 and other Hippo pathway members to control follicle development, ovulation, and differentiation. Employment of approaches combining proteomics and transcriptomics will assist in identifying Hippo pathway components and their targets in specific ovarian compartments under physiological conditions. While several of the studies discussed in this review define distinct roles for Lats1, Lats2, and Yap1 in ovarian physiology, limited studies are available on our understanding of Mob1, Sav1, Mst1, Mst2, and Taz, and their impacts on pathway dynamics and regulation in the ovary remain to be elucidated. Further research is imperative in distinguishing the direct or indirect mechanisms of Hippo regulation in ovarian cells and how these mechanisms are directed during the various stages of follicle development. Moreover, disruption and/or dysregulation of the Hippo pathway has been implicated in the development of ovarian disorders, ovarian cancers, and impaired fertility, thus, providing another avenue for research into ovarian Hippo signaling. These investigations could possibly serve to identify cell-based molecular targets for prevention, diagnosis, and/or treatment of ovarian disorders. Further, understanding the role of Hippo signaling components during follicular maturation is necessary for improving applications in human reproductive technologies.
Despite the rapidly accumulating reports on the Hippo pathway’s contributions to ovarian function, some key questions remain unanswered, and along with new information, new questions are emerging. Listed here are some key questions for further study of this emerging signaling pathway.
(1) Where are Hippo pathway components localized in ovaries during the reproductive cycle? MST1 and MST2 are the core kinases of the mammalian Hippo tumor suppressor pathway; however, their localization and role during various stages of follicle development, ovulation, and luteal formation and regression are unknown. This may be key to understanding how external forces, hormones, and paracrine factors regulate upstream Hippo pathway components. Studies show that phosphorylated YAP1/TAZ are enriched in the cytoplasm and dephosphorylated YAP1/TAZ are present in the nucleus of oocytes and granulosa and luteal cells under various conditions. However, it remains to be determined where YAP1 and TAZ phosphorylation and dephosphorylation occur. A related question is how do non-LATS1/2 mediated YAP1 and TAZ phosphorylation sites (e.g., via PKA and AMPK) impact ovarian gene transcription?
(2) Does Hippo signaling regulate follicular and luteal size sensing? What Hippo signaling factors promote or limit follicular growth? Understanding these signals may improve in vivo and in vitro approaches to improve oocyte maturation and increase reproductive success. A related question is how are LATS1/2 regulated by actin remodeling and/or cellular stiffness? Ovarian stiffness and fibrosis contribute to reduced follicular activity, especially in the aging ovary, and approaches to alleviate fibrosis may improve fertility.
(3) How does the ovary integrate the many signaling mechanisms that impact Hippo signaling? In cancer, multiple studies have shown that there is an interactive regulation between Hippo signaling and metabolic signaling. Does the Hippo pathway act as a metabolic sensor in the ovary? Are endocrine or metabolic disturbances the cause or effect of altered ovarian morphology and infertility associated with PCOS?
(4) An equally intriguing question is how do gonadotropins fine tune Hippo signaling in vivo to achieve an exquisite balance of cellular proliferation and differentiation? Studies are needed to identify the cell-type specific responses occurring in follicular granulosa and theca cells, luteal steroidogenic and endothelial cells, as well as the ovarian stroma.
(5) How do YAP1 and TAZ direct ovarian gene expression? As a transcriptional co-regulator, YAP1 interacts with TEADs to regulate a transcription of genes that impact proliferation. However, evidence shows that YAP1 interacts with other transcription factors to provide a unique transcriptional landscape. Studies using state-of-the-art approaches are needed not only to identify YAP1/TAZ targets in ovarian cells, but also to determine how YAP1/TAZ intersect with other key transcription factors that are activated during distinct stages of the ovarian cycle. Equally important, what are the unique contributions of TAZ to ovarian function, as distinct from YAP1? Understanding how YAP1 and TAZ direct ovarian gene expression can provide new opportunities for strategies to improve fertility or contraception.
(6) How does Hippo signaling become deregulated in cancer? It is apparent that elevations of YAP1 contribute to disordered granulosa cellular identity and that that YAP activation is capable of cellular transformation leading to ovarian cancers. Although mutations in Hippo pathway genes are rare, the cell of origin for the various ovarian cancers may contribute other cancer-driving pathways that regulate the Hippo pathway.
Glossary
Abbreviations
- AMPK
adenosine monophosphate–activated protein kinase
- CCN1/2/3/5
cellular communication network factor 1/2/3 or 5
- COC
cumulus oocyte complex
- CTGF
connective tissue growth factor (also known as CCN2)
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FOXL2
forkhead box L2
- FSH
follicle-stimulating hormone
- FSHR
follicle-stimulating hormone receptor
- FTE
fallopian tube epithelial cells
- GCT
granulosa cell tumor
- GDF9
growth differentiation factor 9
- GPCR
G protein–coupled receptor
- HGSOC
high-grade serous ovarian cancer
- LATS1/2
large tumor suppressors 1 and 2
- LH
luteinizing hormone
- MAP4K
mitogen-activated protein kinase kinase kinase kinase
- MOB1
Mps one binder 1
- MST1/2
mammalian serine/threonine-protein kinase 4 and 3
- mTOR
mechanistic target of rapamycin
- OSE
ovarian surface epithelial cells
- PCOS
polycystic ovary syndrome
- PI3K
phosphoinositide 3-kinase
- PFAS
per/polyfluoroalkyl substances
- PFOA
perfluorooctanoic acid
- PKA
protein kinase A
- SAV1
Salvador family WW domain-containing protein 1
- siRNA
small interfering RNA
- SREBP
sterol regulatory-element binding protein
- STAR
steroidogenic acute regulatory protein
- TAZ
WW domain-containing transcription regulator 1 (also known as WWTR1)
- TEAD
TEA domain transcription factor
- TGF-B
transforming growth factor beta
- TOCP
tri-ortho-cresyl phosphate
- YAP1
yes-associated protein 1
Contributor Information
Kendra L Clark, Olson Center for Women’s Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE 68198, USA; Veterans Affairs Nebraska Western Iowa Health Care System, Omaha, NE 68105, USA.
Jitu W George, Olson Center for Women’s Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE 68198, USA; Veterans Affairs Nebraska Western Iowa Health Care System, Omaha, NE 68105, USA.
Emilia Przygrodzka, Olson Center for Women’s Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE 68198, USA; Veterans Affairs Nebraska Western Iowa Health Care System, Omaha, NE 68105, USA.
Michele R Plewes, Olson Center for Women’s Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE 68198, USA; Veterans Affairs Nebraska Western Iowa Health Care System, Omaha, NE 68105, USA.
Guohua Hua, Key Lab of Agricultural Animal Genetics, Breeding and Reproduction of Ministry of Education, College of Animal Science & Technology, Huazhong Agricultural University, Wuhan, Hubei 430070, China.
Cheng Wang, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA.
John S Davis, Olson Center for Women’s Health, Department of Obstetrics and Gynecology, University of Nebraska Medical Center, Omaha, NE 68198, USA; Veterans Affairs Nebraska Western Iowa Health Care System, Omaha, NE 68105, USA.
Financial Support
This work was supported by NIH F32HD106722 (K.L.C.), Olson Center for Women’s Health (J.W.G.), VA IK2BX004911 (M.R.P.), NIH R01CA197976 (C.W./J.S.D.), NIH R01CA201500 (C.W./J.S.D.), NIH P01AG029531 (J.S.D.), NIH R01HD092263 (J.S.D.), VA I01BX004272 (J.S.D.), and the Olson Center for Women’s Health (J.S.D.). J.S.D. is the recipient of a VA Senior Research Career Scientist Award (IK6BX005797).
Author Contributions
K.L.C, J.W.G, and J.S.D contributed to conception, study design, literature analysis, manuscript drafting, and revision; E.P., M.R.P., and G.H. contributed to literature analysis, manuscript drafting, and revision; C.W. contributed to manuscript drafting and editing.
Disclosures
All authors confirm that there is no conflict of interest.
References
- 1. Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58(1):44-50. [DOI] [PubMed] [Google Scholar]
- 2. Vanselow J, Christenson LK, Pate JL. Editorial: regulation of dynamic changes and remodeling events during the formation, rescue and regression of the corpus luteum. Front Endocrinol. 2020;11:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown HM, Russell DL. Blood and lymphatic vasculature in the ovary: development, function and disease. Hum Reprod Update. 2014;20(1):29-39. [DOI] [PubMed] [Google Scholar]
- 4. Ishizuka B. Current understanding of the etiology, symptomatology, and treatment options in premature ovarian insufficiency (POI). Front Endocrinol. 2021;12:626924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol. 1991;124:43-101. [DOI] [PubMed] [Google Scholar]
- 6. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev. 2015;36(1):1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gershon E, Dekel N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int J Mol Sci. 2020;21(12):4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gondos B. Granulosa cell-germ cell relationship in the developing rabbit ovary. J Embryol Exp Morphol. 1970;23(2):419-426. [PubMed] [Google Scholar]
- 9. Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37(7):1344-1349. [DOI] [PubMed] [Google Scholar]
- 10. Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Ziegler D, Fraisse T, de Candolle G, Vulliemoz N, Bellavia M, Colamaria S. Outlook: Roles of FSH and LH during the follicular phase: insight into natural cycle IVF. Reprod Biomed Online. 2007;15(5):507-513. [DOI] [PubMed] [Google Scholar]
- 12. Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28(1):117-149. [DOI] [PubMed] [Google Scholar]
- 13. Duncan W, Meiden R. The corpus luteum and women’s health. Life Cycle Corpus Luteum. 2017:249-275. [Google Scholar]
- 14. Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian Folliculogenesis. Results Probl Cell Differ. 2016;58:167-190. [DOI] [PubMed] [Google Scholar]
- 15. Robker RL, Hennebold JD, Russell DL. Coordination of ovulation and oocyte maturation: a good egg at the right time. Endocrinology. 2018;159(9):3209-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richards JS. From follicular development and ovulation to ovarian cancers: an unexpected journey. Vitam Horm. 2018;107:453-472. [DOI] [PubMed] [Google Scholar]
- 17. Conti M. Specificity of the cyclic adenosine 3’,5’-monophosphate signal in granulosa cell function. Biol Reprod. 2002;67(6):1653-1661. [DOI] [PubMed] [Google Scholar]
- 18. Liu KC, Ge W. Evidence for gating roles of protein kinase A and protein kinase C in estradiol-induced luteinizing hormone receptor (lhcgr) expression in zebrafish ovarian follicle cells. PLoS One. 2013;8(5):e62524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cottom J, Salvador LM, Maizels ET, et al. . Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278(9):7167-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie Q, Cheng Z, Chen X, Lobe CG, Liu J. The role of Notch signalling in ovarian angiogenesis. J Ovarian Res. 2017;10(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren Y, Cowan RG, Harman RM, Quirk SM. Dominant activation of the hedgehog signaling pathway in the ovary alters theca development and prevents ovulation. Mol Endocrinol. 2009;23(5):711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren Y, Cowan RG, Migone FF, Quirk SM. Overactivation of hedgehog signaling alters development of the ovarian vasculature in mice. Biol Reprod. 2012;86(6):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wijgerde M, Ooms M, Hoogerbrugge JW, Grootegoed JA. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology. 2005;146(8):3558-3566. [DOI] [PubMed] [Google Scholar]
- 24. Ricken A, Lochhead P, Kontogiannea M, Farookhi R. Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology. 2002;143(7):2741-2749. [DOI] [PubMed] [Google Scholar]
- 25. Hsieh M, Johnson MA, Greenberg NM, Richards JS. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology. 2002;143(3):898-908. [DOI] [PubMed] [Google Scholar]
- 26. Harwood BN, Cross SK, Radford EE, Haac BE, De Vries WN. Members of the WNT signaling pathways are widely expressed in mouse ovaries, oocytes, and cleavage stage embryos. Dev Dyn. 2008;237(4):1099-1111. [DOI] [PubMed] [Google Scholar]
- 27. Shimasaki S, Moore RK, Erickson GF, Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reproduction. 2003;61:323-337. [PubMed] [Google Scholar]
- 28. McNatty KP, Juengel JL, Reader KL, et al. . Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction. 2005;129(4):481-487. [DOI] [PubMed] [Google Scholar]
- 29. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191-206. [DOI] [PubMed] [Google Scholar]
- 30. Monsivais D, Matzuk MM, Pangas SA. The TGF-β Family in the Reproductive Tract. Cold Spring Harbor Perspect Biol. 2017;9(10):a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGee EA, Chun SY, Lai S, He Y, Hsueh AJ. Keratinocyte growth factor promotes the survival, growth, and differentiation of preantral ovarian follicles. Fertil Steril. 1999;71(4):732-738. [DOI] [PubMed] [Google Scholar]
- 32. Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68(5):1736-1741. [DOI] [PubMed] [Google Scholar]
- 33. Matos MH, Lima-Verde IB, Bruno JB, et al. . Follicle stimulating hormone and fibroblast growth factor-2 interact and promote goat primordial follicle development in vitro. Reprod Fertil Dev. 2007;(5):677-684. [DOI] [PubMed] [Google Scholar]
- 34. Chaves RN, Lima-Verde IB, Celestino JJ, et al. . Fibroblast growth factor-10 maintains the survival and promotes the growth of cultured goat preantral follicles. Domest Anim Endocrinol. 2010; 39(4):249-258. [DOI] [PubMed] [Google Scholar]
- 35. Fan HY, Liu Z, Shimada M, et al. . MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hughes CHK, Murphy BD. Nuclear receptors: Key regulators of somatic cell functions in the ovulatory process. Mol Aspects Med. 2021;78:100937. [DOI] [PubMed] [Google Scholar]
- 37. Ma S, Meng Z, Chen R, Guan KL. The Hippo pathway: biology and pathophysiology. Annu Rev Biochem. 2019;88:577-604. [DOI] [PubMed] [Google Scholar]
- 38. Dey A, Varelas X, Guan KL. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discovery. 2020;19(7):480-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114(4):445-456. [DOI] [PubMed] [Google Scholar]
- 40. Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol. 2007;17(21):1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 2007;17(21):1864-1870. [DOI] [PubMed] [Google Scholar]
- 42. Huang J, Kalderon D. Coupling of Hedgehog and Hippo pathways promotes stem cell maintenance by stimulating proliferation. J Cell Biol. 2014;205(3):325-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarikaya DP, Extavour CG. The Hippo pathway regulates homeostatic growth of stem cell niche precursors in the Drosophila ovary. PLoS Genet. 2015;11(2):e1004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsu TH, Yang CY, Yeh TH, Huang YC, Wang TW, Yu JY. The Hippo pathway acts downstream of the Hedgehog signaling to regulate follicle stem cell maintenance in the Drosophila ovary. Sci Rep. 2017;7(1):4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Borreguero-Munoz N, Fletcher GC, Aguilar-Aragon M, Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. The Hippo pathway integrates PI3K-Akt signals with mechanical and polarity cues to control tissue growth. PLoS Biol. 2019;17(10):e3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bray SJ. Notch signalling in context. Nat Rev Mol Cell Biol. 2016;17(11):722-735. [DOI] [PubMed] [Google Scholar]
- 47. Xu J, Gridley T. Notch signaling during Oogenesis in Drosophila melanogaster. Genet Res Int. 2012;2012:648207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu J, Poulton J, Huang YC, Deng WM. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS One. 2008;3(3):e1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen HJ, Wang CM, Wang TW, et al. . The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev Biol. 2011;357(2):370-379. [DOI] [PubMed] [Google Scholar]
- 50. Dong J, Feldmann G, Huang J, et al. . Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fallahi E, O’Driscoll NA, Matallanas D. The MST/Hippo pathway and cell death: a non-canonical affair. Genes. 2016;7(6):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fu V, Plouffe SW, Guan KL. The Hippo pathway in organ development, homeostasis, and regeneration. Curr Opin Cell Biol. 2017;49:99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21(4):212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bae SJ, Luo X. Activation mechanisms of the Hippo kinase signaling cascade. Biosci Rep. 2018(4);38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao B, Wei X, Li W, et al. . Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim MK, Jang JW, Bae SC. DNA binding partners of YAP/TAZ. BMB Rep. 2018;51(3):126-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boggiano JC, Fehon RG. Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev Cell. 2012;22(4):695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aragona M, Panciera T, Manfrin A, et al. . A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154(5):1047-1059. [DOI] [PubMed] [Google Scholar]
- 60. Yu FX, Zhao B, Panupinthu N, et al. . Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150(4):780-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mitamura T, Watari H, Wang L, et al. . microRNA 31 functions as an endometrial cancer oncogene by suppressing Hippo tumor suppressor pathway. Mol Cancer. 2014;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. DeRan M, Yang J, Shen CH, et al. . Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9(2):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cox CM, Mandell EK, Stewart L, et al. . Endosomal regulation of contact inhibition through the AMOT:YAP pathway. Mol Biol Cell. 2015;26(14):2673-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karaman R, Halder G. Cell junctions in Hippo signaling. Cold Spring Harbor Perspect Biol. 2018;10(5):a028753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cho YS, Jiang J. Hippo-independent regulation of Yki/Yap/Taz: a non-canonical view. Front Cell Dev Biol. 2021;9:658481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kwon H, Kim J, Jho EH. Role of the Hippo pathway and mechanisms for controlling cellular localization of YAP/TAZ. FEBS J. 2021. [DOI] [PubMed] [Google Scholar]
- 67. Chen R, Xie R, Meng Z, Ma S, Guan KL. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat Cell Biol. 2019;21(12):1565-1577. [DOI] [PubMed] [Google Scholar]
- 68. Dupont S, Morsut L, Aragona M, et al. . Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179-183. [DOI] [PubMed] [Google Scholar]
- 69. Chang L, Azzolin L, Di Biagio D, et al. . The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature. 2018;563(7730):265-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim M, Kim M, Lee S, et al. . cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32(11):1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110(7):2569-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Straßburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367(2):187-196. [DOI] [PubMed] [Google Scholar]
- 74. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24(1):72-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283(9):5496-5509. [DOI] [PubMed] [Google Scholar]
- 76. Lei QY, Zhang H, Zhao B, et al. . TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mo JS, Meng Z, Kim YC, et al. . Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang W, Xiao ZD, Li X, et al. . AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17(4):490-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao B, Li L, Lu Q, et al. . Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614-1626. [DOI] [PubMed] [Google Scholar]
- 81. Grosbois J, Demeestere I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum Reprod. 2018;33(9):1705-1714. [DOI] [PubMed] [Google Scholar]
- 82. Kawamura K, Cheng Y, Suzuki N, et al. . Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110(43):17474-17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu LL, Su T, Luo RC, et al. . Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J Cell Physiol. 2019;234(2):1578-1587. [DOI] [PubMed] [Google Scholar]
- 84. Plewes MR, Hou X, Zhang P, et al. . Yes-associated protein (YAP1) is required for proliferation and function of bovine granulosa cells in vitro$. Biol Reprod. 2019;101(5):1001-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lv X, He C, Huang C, et al. . Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development. FASEB J. 2019;33(9):10049-10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. De Roo C, Lierman S, Tilleman K, et al. . Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod Biomed Online. 2017;34(6):557-566. [DOI] [PubMed] [Google Scholar]
- 87. Devos M, Grosbois J, Demeestere I. Interaction between PI3K/AKT and Hippo pathways during in vitro follicular activation and response to fragmentation and chemotherapy exposure using a mouse immature ovary model. Biol Reprod. 2020;102(3):717-729. [DOI] [PubMed] [Google Scholar]
- 88. Bernabé BP, Woodruff T, Broadbelt LJ, Shea LD. Ligands, receptors, and transcription factors that mediate inter-cellular and intra-cellular communication during ovarian follicle development. Reprod Sci. 2020;27(2):690-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McAllister JM, Legro RS, Modi BP, Strauss JF III. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li T, Zhao H, Zhao X, et al. . Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J Med Genet. 2012;49(4):254-257. [DOI] [PubMed] [Google Scholar]
- 91. Hsueh AJW, Kawamura K. Hippo signaling disruption and ovarian follicle activation in infertile patients. Fertil Steril. 2020;114(3):458-464. [DOI] [PubMed] [Google Scholar]
- 92. Fu D, Lv X, Hua G, et al. . YAP regulates cell proliferation, migration, and steroidogenesis in adult granulosa cell tumors. Endocr Relat Cancer. 2014;21(2):297-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. He C, Lv X, Hua G, et al. . YAP forms autocrine loops with the ERBB pathway to regulate ovarian cancer initiation and progression. Oncogene. 2015;34(50):6040-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. He C, Mao D, Hua G, et al. . The Hippo/YAP pathway interacts with EGFR signaling and HPV oncoproteins to regulate cervical cancer progression. EMBO Mol Med. 2015;7(11):1426-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hua G, Lv X, He C, et al. . YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene. 2016;35(17):2247-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xiang C, Li J, Hu L, et al. . Hippo signaling pathway reveals a spatio-temporal correlation with the size of primordial follicle pool in mice. Cell Physiol Biochem. 2015;35(3):957-968. [DOI] [PubMed] [Google Scholar]
- 97. Ji SY, Liu XM, Li BT, et al. . The polycystic ovary syndrome-associated gene Yap1 is regulated by gonadotropins and sex steroid hormones in hyperandrogenism-induced oligo-ovulation in mouse. Mol Hum Reprod. 2017;23(10):698-707. [DOI] [PubMed] [Google Scholar]
- 98. Masciangelo R, Hossay C, Chiti MC, et al. . Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J Assist Reprod Genet. 2020;37(1):101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun X, Niu X, Qin N, et al. . Novel insights into the regulation of LATS2 kinase in prehierarchical follicle development via the Hippo pathway in hen ovary. Poult Sci. 2021;100(12):101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dos Santos EC, Lalonde-Larue A, Antoniazzi AQ, et al. . YAP signaling in preovulatory granulosa cells is critical for the functioning of the EGF network during ovulation. Mol Cell Endocrinol. 2022;541:111524. [DOI] [PubMed] [Google Scholar]
- 101. Lv XH, Huang C, Hua G, et al. . Reprogramming of ovarian granulosa cells by YAP1 leads to development of high-grade cancer with mesenchymal lineage and serous features. Sci Bull. 2020;65(15):1281-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li CJ, Lin LT, Tsai HW, et al. . The molecular regulation in the pathophysiology in ovarian aging. Aging Dis. 2021;12(3):934-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li J, Zhou F, Zheng T, et al. . Ovarian germline stem cells (OGSCs) and the Hippo signaling pathway association with physiological and pathological ovarian aging in mice. Cell Physiol Biochem. 2015;36(5):1712-1724. [DOI] [PubMed] [Google Scholar]
- 104. Lyu Z, Qin N, Tyasi TL, et al. . The Hippo/MST pathway member SAV1 plays a suppressive role in development of the prehierarchical follicles in hen ovary. PLoS One. 2016;11(8):e0160896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Johnson AL. Ovarian follicle selection and granulosa cell differentiation. Poult Sci. 2015;94(4):781-785. [DOI] [PubMed] [Google Scholar]
- 106. Li J, Luo W, Huang T, Gong Y. Growth differentiation factor 9 promotes follicle-stimulating hormone-induced progesterone production in chicken follicular granulosa cells. Gen Comp Endocrinol. 2019;276:69-76. [DOI] [PubMed] [Google Scholar]
- 107. Pisarska MD, Kuo FT, Bentsi-Barnes IK, Khan S, Barlow GM. LATS1 phosphorylates forkhead L2 and regulates its transcriptional activity. Am J Physiol Endocrinol Metab. 2010;299(1):E101-E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pannetier M, Chassot AA, Chaboissier MC, Pailhoux E. Involvement of FOXL2 and RSPO1 in ovarian determination, development, and maintenance in mammals. Sex Dev. 2016;10(4):167-184. [DOI] [PubMed] [Google Scholar]
- 109. Kuo FT, Fan K, Bentsi-Barnes I, Barlow GM, Pisarska MD. Mouse forkhead L2 maintains repression of FSH-dependent genes in the granulosa cell. Reproduction 2012;144(4):485-494. [DOI] [PubMed] [Google Scholar]
- 110. Furth N, Aylon Y. The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell Death Differ. 2017;24(9):1488-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sun T, Pepling ME, Diaz FJ. Lats1 deletion causes increased germ cell apoptosis and follicular cysts in mouse ovaries. Biol Reprod. 2015;93(1):22. [DOI] [PubMed] [Google Scholar]
- 112. St John MA, Tao W, Fei X, et al. . Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet. 1999;21(2):182-186. [DOI] [PubMed] [Google Scholar]
- 113. He C, Lv X, Huang C, et al. . YAP1-LATS2 feedback loop dictates senescent or malignant cell fate to maintain tissue homeostasis. EMBO Rep. 2019;20(3):e44948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tsoi M, Morin M, Rico C, et al. . Lats1 and Lats2 are required for ovarian granulosa cell fate maintenance. FASEB J. 2019;33(10):10819-10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. St-Jean G, Tsoi M, Abedini A, et al. . Lats1 and Lats2 are required for the maintenance of multipotency in the Mullerian duct mesenchyme. Development 2019;146(20):DEV180430. [DOI] [PubMed] [Google Scholar]
- 116. Ito M, Yoshino O, Ono Y, et al. . Bone morphogenetic protein-2 enhances gonadotropin-independent follicular development via sphingosine kinase 1. Am J Reprod Immunol (New York, NY: 1989). 2020:e13374. [DOI] [PubMed] [Google Scholar]
- 117. Ye H, Li X, Zheng T, et al. . The Hippo Signaling pathway regulates ovarian function via the proliferation of ovarian germline stem cells. Cell Physiol Biochem. 2017;41(3):1051-1062. [DOI] [PubMed] [Google Scholar]
- 118. Yan L, Yang M, Guo H, et al. . Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol. 2013;20(9):1131-1139. [DOI] [PubMed] [Google Scholar]
- 119. Abbassi L, Malki S, Cockburn K, et al. . Multiple mechanisms cooperate to constitutively exclude the transcriptional Co-activator YAP from the nucleus during murine oogenesis. Biol Reprod. 2016;94(5):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. De Roo C, Lierman S, Tilleman K, De Sutter P. In-vitro fragmentation of ovarian tissue activates primordial follicles through the Hippo pathway. Hum Reprod Open. 2020;2020(4):hoaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yu C, Ji SY, Dang YJ, et al. . Oocyte-expressed yes-associated protein is a key activator of the early zygotic genome in mouse. Cell Res. 2016;26(3):275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sha QQ, Zheng W, Wu YW, et al. . Dynamics and clinical relevance of maternal mRNA clearance during the oocyte-to-embryo transition in humans. Nat Commun. 2020;11(1):4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sun T, Diaz FJ. Ovulatory signals alter granulosa cell behavior through YAP1 signaling. Reprod Biol Endocrinol. 2019;17(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hanna CB, Hennebold JD. Ovarian germline stem cells: an unlimited source of oocytes? Fertil Steril. 2014;101(1):20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Martin JJ, Woods DC, Tilly JL. Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. Cells. 2019;8(2):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Horan CJ, Williams SA. Oocyte stem cells: fact or fantasy? Reproduction. 2017;154(1):R23-r35. [DOI] [PubMed] [Google Scholar]
- 127. Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lalonde-Larue A, Boyer A, Dos Santos EC, Boerboom D, Bernard DJ, Zamberlam G. The Hippo pathway effectors YAP and TAZ regulate LH release by pituitary gonadotrope cells in mice. Endocrinology. 2022;163(1):bqab238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Meng Z, Moroishi T, Mottier-Pavie V, et al. . MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016;26(4):289-299. [DOI] [PubMed] [Google Scholar]
- 131. Koo JH, Guan KL. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018;28(2):196-206. [DOI] [PubMed] [Google Scholar]
- 132. Hou X, Arvisais EW, Davis JS. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology. 2010;151(6):2846-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Przygrodzka E, Hou X, Zhang P, Plewes MR, Franco R, Davis JS. PKA and AMPK signaling pathways differentially regulate luteal steroidogenesis. Endocrinology. 2021;162(4):bqab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Przygrodzka E, Monaco CF, Plewes MR, et al. . Protein Kinase A and 5’ AMP-activated protein kinase signaling pathways exert opposite effects on induction of autophagy in luteal cells. Front Cell Dev Biol. 2021;9:723563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Thomas RE, Armstrong DT, Gilchrist RB. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3’,5’-monophosophate levels. Biol Reprod. 2004;70(3):548-556. [DOI] [PubMed] [Google Scholar]
- 136. Tosca L, Chabrolle C, Uzbekova S, Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5’ monophosphate-activated protein kinase (AMPK). Biol Reprod. 2007;76(3):368-378. [DOI] [PubMed] [Google Scholar]
- 137. Tosca L, Crochet S, Ferré P, Foufelle F, Tesseraud S, Dupont J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J Endocrinol. 2006;190(1):85-97. [DOI] [PubMed] [Google Scholar]
- 138. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kim SJ, Tang T, Abbott M, Viscarra JA, Wang Y, Sul HS. AMPK phosphorylates Desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol Cell Biol. 2016;36(14):1961-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tosca L, Solnais P, Ferré P, Foufelle F, Dupont J. Metformin-induced stimulation of adenosine 5’ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol Reprod. 2006;75(3):342-351. [DOI] [PubMed] [Google Scholar]
- 141. Xu S, Wu X, Dong Y, et al. . Glucose activates the primordial follicle through the AMPK/mTOR signaling pathway. Clin Transl Med. 2020;10(3):e122. [Google Scholar]
- 142. Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein Kinase A: a master kinase of granulosa cell differentiation. Sci Rep. 2016;6:28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9-22. [DOI] [PubMed] [Google Scholar]
- 144. Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17(8):666-681. [DOI] [PubMed] [Google Scholar]
- 145. Papageorgiou K, Mastora E, Zikopoulos A, Grigoriou ME, Georgiou I, Michaelidis TM. Interplay between mTOR and Hippo Signaling in the ovary: clinical choice guidance between different gonadotropin preparations for better IVF. Front Endocrinol. 2021;12:702446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tumaneng K, Russell RC, Guan KL. Organ size control by Hippo and TOR pathways. Curr Biol. 2012;22(9):R368-R379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. James MF, Han S, Polizzano C, et al. . NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250-4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. López-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29(15):4235-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Holmes B, Benavides-Serrato A, Saunders JT, Kumar S, Nishimura RN, Gera J. mTORC2-mediated direct phosphorylation regulates YAP activity promoting glioblastoma growth and invasive characteristics. Neoplasia (New York, NY). 2021;23(9):951-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Xin M, Kim Y, Sutherland LB, et al. . Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signaling. 2011;4(196):ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhou D, Conrad C, Xia F, et al. . Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Guo Y, Cang X, Zhu L, et al. . PPP1CA/YAP/GS/Gln/mTORC1 pathway activates retinal Müller cells during diabetic retinopathy. Exp Eye Res. 2021;210:108703. [DOI] [PubMed] [Google Scholar]
- 154. Bertero T, Oldham WM, Cottrill KA, et al. . Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126(9):3313-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Park YY, Sohn BH, Johnson RL, et al. . Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2016;63(1):159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kayampilly PP, Menon KM. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148(8):3950-3957. [DOI] [PubMed] [Google Scholar]
- 157. Moravek MB, Shang M, Menon B, Menon K. HCG-mediated activation of mTORC1 signaling plays a crucial role in steroidogenesis in human granulosa lutein cells. Endocrine. 2016;54(1):217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yu J, Yaba A, Kasiman C, Thomson T, Johnson J. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PLoS One. 2011;6(7):e21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol. Endocrinol. 2010;24(9):1782-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5’-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology. 2009;150(2):929-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Della Torre S, Benedusi V, Fontana R, Maggi A. Energy metabolism and fertility: a balance preserved for female health. Nat Rev Endocrinol. 2014;10(1):13-23. [DOI] [PubMed] [Google Scholar]
- 162. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Leroy JL, Vanholder T, Mateusen B, et al. . Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130(4):485-495. [DOI] [PubMed] [Google Scholar]
- 164. Trigatti B, Rayburn H, Viñals M, et al. . Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA. 1999;96(16):9322-9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest. 2001;108(11):1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fan HY, Sun QY. Involvement of mitogen-activated protein kinase cascade during oocyte maturation and fertilization in mammals. Biol Reprod. 2004;70(3):535-547. [DOI] [PubMed] [Google Scholar]
- 167. Yesilaltay A, Dokshin GA, Busso D, et al. . Excess cholesterol induces mouse egg activation and may cause female infertility. Proc Natl Acad Sci USA. 2014;111(46):E4972-E4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Quiroz A, Molina P, Santander N, Gallardo D, Rigotti A, Busso D. Ovarian cholesterol efflux: ATP-binding cassette transporters and follicular fluid HDL regulate cholesterol content in mouse oocytes†. Biol Reprod. 2020;102(2):348-361. [DOI] [PubMed] [Google Scholar]
- 169. McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev. 2011;24(1):59-67. [DOI] [PubMed] [Google Scholar]
- 170. Van Hoeck V, Rizos D, Gutierrez-Adan A, et al. . Interaction between differential gene expression profile and phenotype in bovine blastocysts originating from oocytes exposed to elevated non-esterified fatty acid concentrations. Reprod Fertil Dev. 2015;27(2):372-384. [DOI] [PubMed] [Google Scholar]
- 171. Valckx SD, Arias-Alvarez M, De Pauw I, et al. . Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. 2014;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Valckx SD, De Bie J, Michiels ED, et al. . The effect of human follicular fluid on bovine oocyte developmental competence and embryo quality. Reprod Biomed Online. 2015;30(2):203-207. [DOI] [PubMed] [Google Scholar]
- 173. Valckx SD, De Pauw I, De Neubourg D, et al. . BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod. 2012;27(12):3531-3539. [DOI] [PubMed] [Google Scholar]
- 174. Yamaguchi H, Taouk GM. A potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front Oncol. 2020;10:928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Aylon Y, Gershoni A, Rotkopf R, et al. . The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 2016;30(7):786-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Geng C, Zhang Y, Gao Y, et al. . Mst1 regulates hepatic lipid metabolism by inhibiting Sirt1 ubiquitination in mice. Biochem Biophys Res Commun. 2016;471(4):444-449. [DOI] [PubMed] [Google Scholar]
- 177. Shu Z, Gao Y, Zhang G, et al. . A functional interaction between Hippo-YAP signalling and SREBPs mediates hepatic steatosis in diabetic mice. J Cell Mol Med. 2019;23(5):3616-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Nakanishi T, Tanaka R, Tonai S, et al. . LH induces De Novo Cholesterol Biosynthesis via SREBP activation in granulosa cells during ovulation in female mice. Endocrinology. 2021;162(11):bqab166. [DOI] [PubMed] [Google Scholar]
- 179. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225-245. [DOI] [PubMed] [Google Scholar]
- 180. Sorrentino G, Ruggeri N, Specchia V, et al. . Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16(4):357-366. [DOI] [PubMed] [Google Scholar]
- 181. Wang X, Cai B, Yang X, et al. . Cholesterol stabilizes TAZ in hepatocytes to promote experimental non-alcoholic steatohepatitis. Cell Metab. 2020;31(5):969-986.e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94(1):3-21. [DOI] [PubMed] [Google Scholar]
- 183. Hale MD, McCoy JA, Doheny BM, Galligan TM, Guillette LJ Jr., Parrott BB. Embryonic estrogen exposure recapitulates persistent ovarian transcriptional programs in a model of environmental endocrine disruption. Biol Reprod. 2019;100(1):149-161. [DOI] [PubMed] [Google Scholar]
- 184. Knapczyk-Stwora K, Grzesiak M, Ciereszko RE, Czaja E, Koziorowski M, Slomczynska M. The impact of sex steroid agonists and antagonists on folliculogenesis in the neonatal porcine ovary via cell proliferation and apoptosis. Theriogenology. 2018;113:19-26. [DOI] [PubMed] [Google Scholar]
- 185. Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233(2):286-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol. 2006;191(3):549-558. [DOI] [PubMed] [Google Scholar]
- 187. Iqbal SZ, Rabbani T, Asi MR, Jinap S. Assessment of aflatoxins, ochratoxin A and zearalenone in breakfast cereals. Food Chem. 2014;157:257-262. [DOI] [PubMed] [Google Scholar]
- 188. Zhang Y, Jia Z, Yin S, et al. . Toxic effects of maternal zearalenone exposure on uterine capacity and fetal development in gestation rats. Reprod Sci. 2014;21(6):743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Zhu CC, Hou YJ, Han J, Cui XS, Kim NH, Sun SC. Zearalenone exposure affects epigenetic modifications of mouse eggs. Mutagenesis. 2014;29(6):489-495. [DOI] [PubMed] [Google Scholar]
- 190. Liu KH, Sun XF, Feng YZ, et al. . The impact of Zearalenone on the meiotic progression and primordial follicle assembly during early oogenesis. Toxicol Appl Pharmacol. 2017;329:9-17. [DOI] [PubMed] [Google Scholar]
- 191. Zhang GL, Sun XF, Feng YZ, et al. . Zearalenone exposure impairs ovarian primordial follicle formation via down-regulation of Lhx8 expression in vitro. Toxicol Appl Pharmacol. 2017;317:33-40. [DOI] [PubMed] [Google Scholar]
- 192. Hou YJ, Zhu CC, Xu YX, Cui XS, Kim NH, Sun SC. Zearalenone exposure affects mouse oocyte meiotic maturation and granulosa cell proliferation. Environ Toxicol. 2015;30(10):1226-1233. [DOI] [PubMed] [Google Scholar]
- 193. Zheng W, Wang B, Li X, et al. . zearalenone promotes cell proliferation or causes cell death? Toxins. 2018;10(5):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. González-Alvarez ME, McGuire BC, Keating AF. Obesity alters the ovarian proteomic response to zearalenone exposure†. Biol Reprod. 2021;105(1):278-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yang D, Jiang X, Sun J, et al. . Toxic effects of zearalenone on gametogenesis and embryonic development: a molecular point of review. Food Chem Toxicol. 2018;119:24-30. [DOI] [PubMed] [Google Scholar]
- 196. Zhang RQ, Sun XF, Wu RY, et al. . Zearalenone exposure elevated the expression of tumorigenesis genes in mouse ovarian granulosa cells. Toxicol Appl Pharmacol. 2018;356:191-203. [DOI] [PubMed] [Google Scholar]
- 197. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, National Report on Human Exposure to Environmental Chemicals. Updated March, 2022. https://www.cdc.gov/exposurereport/. [Google Scholar]
- 198. Lopez-Espinosa MJ, Fletcher T, Armstrong B, et al. . Association of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. 2011;45(19):8160-8166. [DOI] [PubMed] [Google Scholar]
- 199. Zhou W, Zhang L, Tong C, et al. . Plasma Perfluoroalkyl and Polyfluoroalkyl substances concentration and menstrual cycle characteristics in preconception women. Environ Health Perspect. 2017;125(6):067012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang S, Tan R, Pan R, et al. . Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in chinese women. J Clin Endocrinol Metab. 2018;103(7):2543-2551. [DOI] [PubMed] [Google Scholar]
- 201. Chen Y, Zhou L, Xu J, et al. . Maternal exposure to perfluorooctanoic acid inhibits luteal function via oxidative stress and apoptosis in pregnant mice. Reprod Toxicol. 2017;69:159-166. [DOI] [PubMed] [Google Scholar]
- 202. Du G, Hu J, Huang Z, et al. . Neonatal and juvenile exposure to perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS): Advance puberty onset and kisspeptin system disturbance in female rats. Ecotoxicol Environ Saf. 2019;167:412-421. [DOI] [PubMed] [Google Scholar]
- 203. Clark KL, George JW, Hua G, Davis JS. Perfluorooctanoic acid promotes proliferation of the human granulosa cell line HGrC1 and alters expression of cell cycle genes and Hippo pathway effector YAP1. Reprod Toxicol. 2022;110:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol. 2015;219:74-88. [DOI] [PubMed] [Google Scholar]
- 205. Hu L, Peng T, Huang J, et al. . Tri-ortho-cresyl phosphate (TOCP) induced ovarian failure in mice is related to the Hippo signaling pathway disruption. Reprod Toxicol. 2019;83:21-27. [DOI] [PubMed] [Google Scholar]
- 206. Chang S, Dunaif A. Diagnosis of polycystic ovary syndrome: which criteria to use and when? Endocrinol Metab Clin North Am. 2021;50(1):11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Rees DA, Jenkins-Jones S, Morgan CL. Contemporary reproductive outcomes for patients with polycystic ovary syndrome: a retrospective observational study. J Clin Endocrinol Metab. 2016;101(4):1664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Chen ZJ, Zhao H, He L, et al. . Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43(1):55-59. [DOI] [PubMed] [Google Scholar]
- 209. Cui L, Zhao H, Zhang B, et al. . Genotype-phenotype correlations of PCOS susceptibility SNPs identified by GWAS in a large cohort of Han Chinese women. Hum Reprod. 2013;28(2):538-544. [DOI] [PubMed] [Google Scholar]
- 210. Shi Y, Zhao H, Shi Y, et al. . Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44(9):1020-1025. [DOI] [PubMed] [Google Scholar]
- 211. Jiang LL, Xie JK, Cui JQ, et al. . Promoter methylation of yes-associated protein (YAP1) gene in polycystic ovary syndrome. Medicine. 2017;96(2):e5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Kawashima I, Kawamura K. Regulation of follicle growth through hormonal factors and mechanical cues mediated by Hippo signaling pathway. Syst Biol Reprod Med. 2018;64(1):3-11. [DOI] [PubMed] [Google Scholar]
- 213. Walters KA, Rodriguez Paris V, Aflatounian A, Handelsman DJ. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242(2):R23-R50. [DOI] [PubMed] [Google Scholar]
- 214. Bremer AA, Miller WL. The serine phosphorylation hypothesis of polycystic ovary syndrome: a unifying mechanism for hyperandrogenemia and insulin resistance. Fertil Steril. 2008;89(5):1039-1048. [DOI] [PubMed] [Google Scholar]
- 215. Shpakov AO. Improvement effect of metformin on female and male reproduction in endocrine pathologies and its mechanisms. Pharmaceuticals. 2021;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Tian Y, Tang B, Wang C, et al. . Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget. 2016;7(29):46230-46241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yuan X, Wei W, Bao Q, Chen H, Jin P, Jiang W. Metformin inhibits glioma cells stemness and epithelial-mesenchymal transition via regulating YAP activity. Biomed Pharmacother. 2018;102(1):263-270. [DOI] [PubMed] [Google Scholar]
- 218. Wu Y, Zheng Q, Li Y, et al. . Metformin targets a YAP1-TEAD4 complex via AMPKalpha to regulate CCNE1/2 in bladder cancer cells. J Exp Clin Cancer Res. 2019;38(23):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Abdou HS, Bergeron F, Tremblay JJ. A cell-autonomous molecular cascade initiated by AMP-activated protein kinase represses steroidogenesis. Mol Cell Biol. 2014;34(23):4257-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Carvajal R, Rosas C, Kohan K, et al. . Metformin augments the levels of molecules that regulate the expression of the insulin-dependent glucose transporter GLUT4 in the endometria of hyperinsulinemic PCOS patients. Hum Reprod. 2013;28(8):2235-2244. [DOI] [PubMed] [Google Scholar]
- 221. Oróstica L, García P, Vera C, García V, Romero C, Vega M. Effect of TNF-α on molecules related to the insulin action in endometrial cells exposed to hyperandrogenic and hyperinsulinic conditions characteristics of polycystic ovary syndrome. Reprod Sci. 2018;25(7):1000-1009. [DOI] [PubMed] [Google Scholar]
- 222. Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat Rev Mol Cell Biol. 2017;18(12):758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Suzuki N, Yoshioka N, Takae S, et al. . Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608-615. [DOI] [PubMed] [Google Scholar]
- 224. Zhai J, Yao G, Dong F, et al. . In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101(11):4405-4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Cheng Y, Feng Y, Jansson L, et al. . Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J. 2015;29(6):2423-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Díaz-García C, Herraiz S, Pamplona L, et al. . Follicular activation in women previously diagnosed with poor ovarian response: a randomized, controlled trial. Fertil Steril. 2022;117(4):747-755. [DOI] [PubMed] [Google Scholar]
- 227. Hu LL, Chang HM, Yi Y, et al. . CCN2 mediates S1P-induced upregulation of COX2 expression in human granulosa-lutein cells. Cells. 2019;8(11):1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Mulder CL, Eijkenboom LL, Beerendonk CCM, Braat DDM, Peek R. Enhancing the safety of ovarian cortex autotransplantation: cancer cells are purged completely from human ovarian tissue fragments by pharmacological inhibition of YAP/TAZ oncoproteins. Hum Reprod. 2019;34(3):506-518. [DOI] [PubMed] [Google Scholar]
- 229. Lunding SA, Andersen AN, Hardardottir L, et al. . Hippo signaling, actin polymerization, and follicle activation in fragmented human ovarian cortex. Mol Reprod Dev. 2020;87(6):711-719. [DOI] [PubMed] [Google Scholar]
- 230. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. [DOI] [PubMed] [Google Scholar]
- 231. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 232. Karnezis AN, Cho KR, Gilks CB, Pearce CL, Huntsman DG. The disparate origins of ovarian cancers: pathogenesis and prevention strategies. Nat Rev Cancer. 2017;17(1):65-74. [DOI] [PubMed] [Google Scholar]
- 233. Bowtell DD, Böhm S, Ahmed AA, et al. . Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kim O, Park EY, Klinkebiel DL, et al. . In vivo modeling of metastatic human high-grade serous ovarian cancer in mice. PLoS Genet. 2020;16(6):e1008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Steinhardt AA, Gayyed MF, Klein AP, et al. . Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39(11):1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Zhang X, George J, Deb S, et al. . The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30(25):2810-2822. [DOI] [PubMed] [Google Scholar]
- 237. Hall CA, Wang R, Miao J, et al. . Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70(21):8517-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Xia Y, Chang T, Wang Y, et al. . YAP promotes ovarian cancer cell tumorigenesis and is indicative of a poor prognosis for ovarian cancer patients. PLoS One. 2014;9(3):e91770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Xia Y, Zhang YL, Yu C, Chang T, Fan HY. YAP/TEAD co-activator regulated pluripotency and chemoresistance in ovarian cancer initiated cells. PLoS One. 2014;9(11):e109575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Muñoz-Galván S, Felipe-Abrio B, Verdugo-Sivianes EM, et al. . Downregulation of MYPT1 increases tumor resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness. Mol Cancer. 2020;19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Piek JM, van Diest PJ, Zweemer RP, et al. . Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451-456. [DOI] [PubMed] [Google Scholar]
- 243. Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26(32):5284-5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Dubeau L, Drapkin R. Coming into focus: the nonovarian origins of ovarian cancer. Ann Oncol. 2013; 24(Suppl 8):viii28-viii35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Perets R, Wyant GA, Muto KW, et al. . Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013; 24 Suppl 10:x16-21 [DOI] [PubMed] [Google Scholar]
- 247. Vang R, Shih Ie M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Shih Ie M, Chen L, Wang CC, et al. . Distinct DNA methylation profiles in ovarian serous neoplasms and their implications in ovarian carcinogenesis. Am J Obstet Gynecol. 2010;203(6):584.e581-584.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Dehari R, Kurman RJ, Logani S, Shih Ie M. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31(7):1007-1012. [DOI] [PubMed] [Google Scholar]
- 250. Boyd C, McCluggage WG. Low-grade ovarian serous neoplasms (low-grade serous carcinoma and serous borderline tumor) associated with high-grade serous carcinoma or undifferentiated carcinoma: report of a series of cases of an unusual phenomenon. Am J Surg Pathol. 2012;36(3):368-375. [DOI] [PubMed] [Google Scholar]
- 251. Nik NN, Vang R, Shih Ie M, Kurman RJ. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014;9:27-45. [DOI] [PubMed] [Google Scholar]
- 252. Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zhang S, Dolgalev I, Zhang T, Ran H, Levine DA, Neel BG. Both fallopian tube and ovarian surface epithelium are cells-of-origin for high-grade serous ovarian carcinoma. Nat Commun. 2019;10(1):5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Jamieson S, Fuller PJ. Molecular pathogenesis of granulosa cell tumors of the ovary. Endocr Rev. 2012;33(1):109-144. [DOI] [PubMed] [Google Scholar]
- 255. Shah SP, Köbel M, Senz J, et al. . Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med. 2009;360(26):2719-2729. [DOI] [PubMed] [Google Scholar]
- 256. Damjanov I. The road from teratocarcinoma to human embryonic stem cells. Stem Cell Rev. 2005;1(3):273-276. [DOI] [PubMed] [Google Scholar]
- 257. Lian I, Kim J, Okazawa H, et al. . The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24(11):1106-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22(7):339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Musah S, Wrighton PJ, Zaltsman Y, et al. . Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci USA. 2014;111(38):13805-13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15(6):642-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal. 2013;11(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Sarkar S, Bristow CA, Dey P, et al. . PRKCI promotes immune suppression in ovarian cancer. Genes Dev. 2017;31(11):1109-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Tocci P, Cianfrocca R, Di Castro V, et al. . beta-arrestin1/YAP/mutant p53 complexes orchestrate the endothelin A receptor signaling in high-grade serous ovarian cancer. Nat Commun. 2019;10(1):3196. [DOI] [PMC free article] [PubMed] [Google Scholar]