Abstract
More than 2.1 million age-related fractures occur in the United States annually, resulting in an immense socioeconomic burden. Importantly, the age-related deterioration of bone structure is associated with impaired bone healing. Fracture healing is a dynamic process which can be divided into four stages. While the initial hematoma generates an inflammatory environment in which mesenchymal stem cells and macrophages orchestrate the framework for repair, angiogenesis and cartilage formation mark the second healing period. In the central region, endochondral ossification favors soft callus development while next to the fractured bony ends, intramembranous ossification directly forms woven bone. The third stage is characterized by removal and calcification of the endochondral cartilage. Finally, the chronic remodeling phase concludes the healing process.
Impaired fracture healing due to aging is related to detrimental changes at the cellular level. Macrophages, osteocytes, and chondrocytes express markers of senescence, leading to reduced self-renewal and proliferative capacity. A prolonged phase of “inflammaging” results in an extended remodeling phase, characterized by a senescent microenvironment and deteriorating healing capacity. Although there is evidence that in the setting of injury, at least in some tissues, senescent cells may play a beneficial role in facilitating tissue repair, recent data demonstrate that clearing senescent cells enhances fracture repair. In this review, we summarize the physiological as well as pathological processes during fracture healing in endocrine disease and aging in order to establish a broad understanding of the biomechanical as well as molecular mechanisms involved in bone repair.
Keywords: aging, bone, bone healing, fracture, fracture healing, osteoporosis, senescence
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
With the aging of the population and accumulation of comorbidities, impaired fracture healing is a considerable clinical problem
Four stages of fracture healing can be described, each of which may be impaired by aging and various endocrine diseases
Cellular senescence, which contributes to multiple comorbidities of aging including bone loss, may impair fracture healing
A better understanding of the cellular and molecular impairments of fracture healing with aging may lead to new therapeutic approaches to improve age-related deficits in fracture repair
In 2020, 53.5 million people were older than 65 years in the United States, representing 16% of the overall population (1). Owing to frailty and/or neuromuscular impairment as well as osteoporosis and comorbidities, the probability of falls and subsequent fractures in older individuals is high and the consequences are dire. In fact, the 1-year mortality after a hip fracture is estimated to be as high as 30% in people older than 65 (2). The tremendous socioeconomic burden of fractures requires an orthopedic awareness of the rise of patients with underlying preexisting medical conditions (3). Since osteoporosis and aging represent fundamental aspects in this growing patient cohort, an in-depth understanding of mechanisms underlying fracture repair is crucial for improved treatment approaches (4). Besides estrogen-deficiency, cellular senescence represents an independent pathogenetic path accompanying aging that may lead to osteoporosis and represents a promising future therapeutic approach (5).
Local Cellular and Molecular Mediators of Fracture Healing
Bone tissue is unique in that it can heal without a fibrous scar; however, the complex cellular and molecular interplay during the physiological and pathological bone-healing process is not fully understood (4). During the healing process, bones undergo endochondral ossification, intramembranous ossification, and appositional bone formation. Various key molecular players orchestrate the complex formation of new bone: The mechanical environment (ie, stability) of the fractured region, osteogenic and inflammatory cells, the vasculature providing mediators, and the scaffold underlying the newly formed bone are all important in the process of bone healing (6).
Importantly, the processes of primary (direct) and secondary (indirect) fracture healing are clearly distinct. The primary repair requires an anatomic reduction of the fracture site with high primary stability to facilitate osteoclastic remodeling of the fracture and creation of “cutting cones,” in humans forming new haversian channels (osteons). Direct intramembranous bone formation occurs with immediate cortical remodeling and without greater callus formation. The interfragmentary strain is reduced to a minimum and the fracture ends are anatomically restored (7). A primary “tunnel” is established by osteoclastic cells to clear the way for blood vessels, which are formed by endothelial cells and perivascular mesenchymal cells. These mesenchymal cells serve as osteoprogenitors and develop into osteoblastic cells, facilitated by the stable microenvironment (8).
The secondary (or indirect) fracture healing (via endochondral ossification) is characterized by micromotion between the fracture ends and subsequently the formation of a large callus, in which the de novo formation of embryonic bone is mimicked (9). Typically, this form of bone healing occurs after intramedullary nailing and external fixation of fractures. Indirect fracture healing comprises intramembranous (hard-callus) and endochondral (soft-callus) bone formation. In the former, mesenchymal precursor cells of the inner periosteal layer proceed with direct bone formation via differentiation into osteoblasts. Current data indicate that only newly formed osteoblasts contribute to bone repair, whereas mature osteoblasts appear not to be involved in this step (10, 11). This conclusion is based on genetic pulse-chase studies in C57BL/6 mice demonstrating a replicative population of stem cell/progenitors that dynamically replace osteolineage cells after injury. The mature osteoblasts present at the fracture site are almost completely (~ 90%) replaced after 7 days, and do not proliferate thereafter (10). No cartilaginous interim stage is built and the osteoprogenitor and undifferentiated mesenchymal cells directly form hard callus. In rabbit as well as murine endochondral bone formation, a newly built cartilage matrix layer, established by chondrocytes, is later converted into woven bone undergoing further remodeling into lamellar bone (8, 10, 12).
Chronological Progress of Bone Healing
Multiple attempts have been made to break down the complex temporal and spatial course of bone healing (13-16). Since an exhaustive picture lacks accessibility and may result in oversimplification, we tried to balance diverse paths for a comprehensive picture. For this purpose, we refer to a temporal arrangement in 4 sections, accompanied by a spatial framework. In general, 4 spatially divided regions can be distinguished: cortical bone, bone marrow, periosteum, and external (soft) tissue. However, these stages cannot be stringently distinguished and a simultaneous progression is more the rule than the exception. The main insights into the orchestrated process of bone formation were mostly obtained from mouse and rat fracture models. Regarding the time course, we refer predominantly to murine data.
First Healing Period: Fracture Hematoma and Inflammatory Response
Hematoma and Inflammation
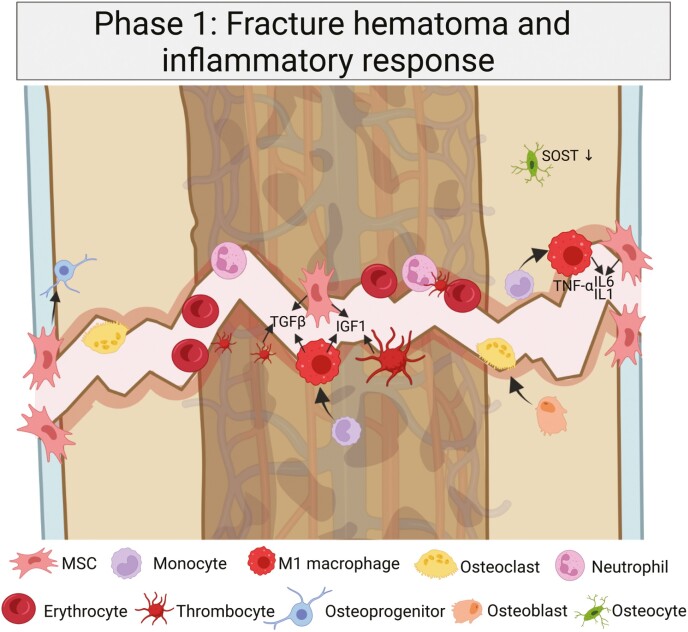
Regardless of the fracture type, the first bone-healing phase is invariably characterized by a robust early inflammatory response and hematoma formation around the fracture site (Fig. 1). Around this cleft, the bone marrow architecture is lost and a cellular reorganization occurs (17). The initial function of the cells present at the fracture site is to debride the fracture site and prime a signaling milieu in favor of the subsequent stages (4). Neutrophils migrate to the site of injury first and were shown to be replaced by monocytes in mice after 24 to 48 hours (18, 19). These monocytes differentiate into macrophages, secreting cytokines and growth factors (interleukin 1 [IL-1], IL-6, tumor necrosis factor α [TNFα], transforming growth factor β [TGFβ], inducible nitric oxide synthase [iNOS], fibroblast growth factor 2 [FGF2]). Platelets and erythrocytes, as well as granulocytes and lymphocytes, form an initial clot, which partially reduces bleeding and serves as a provisional fibrin matrix for the upcoming remodeling process. Periosteal cells were shown to proliferate and differentiate (mesenchymal stem cells, MSCs) into osteogenic and chondrogenic progenitors at the fracture site in mice, developing the subsequent reconstruction site (20). Transplantation experiments have demonstrated that inflammatory cells in the callus area originate from the bone marrow, while some osteochondral stem cells at the fracture site are derived from the local periosteum (21-23). The early hematoma displays an enrichment in MSCs secreting bone morphogenic proteins (BMPs) promoting the differentiation of progenitor cells to chondrogenic and osteogenic lineages (9). Next, an influx of immunoregulatory cells occurs, including granulocytes, lymphocytes, and macrophages, resulting in increased expression of cytokines and growth factors, as demonstrated in mice and humans (6, 24, 25). The overall role of IL-1, IL-6, and TNFα, which peak early in the healing period, is critical as this leads to the recruitment of inflammatory cells and MSCs.
Figure 1.
Initial steps after a traumatic fracture. Ruptured blood vessels lead to the formation of a hematoma by platelets and erythrocytes. Adherent mesenchymal stem cells (MSCs) are recruited and differentiate into osteoblastic and chondrogenic precursors. This results in a matrix of fibrinogen and a microenvironment that has a central hypoxic state, where angiogenesis is promoted and the key molecular players are insulin-like growth factor 1 (IGF-1), transforming growth factor β (TGFβ), and interleukin 1 (IL-1) and IL-6, which lead to the promotion of the next healing steps while maintaining an inflammatory state. A central cell in the first healing period is the classically activated macrophage (CAM = M1), which orchestrates the early inflammatory stage. Created with BioRender.com.
Macrophages act as coordinators of the healing process, and their MCP1/CCR2 profile is essential for MSC homing and migration (18, 26). Macrophages attract key cellular players in the initial healing phase since they secrete multiple inflammatory factors and cytokines (IL-1, IL-6, TNFα, iNOS, TGFβ, platelet-derived growth factor [PDGF], insulin-like growth factor [IGF], FGF2). Next to cytokine “homing,” the early coagulation leads to an “entrapment” of immunoregulatory cells, primarily granulocytes, monocytes/macrophages and leukocytes (B cells, T-helper [Th] more than T cytotoxic [Tcyt] cells) from blood and bone marrow. MSCs differentiate into an osteoblastic phenotype at the periosteum and surrounding soft tissue, which is promoted by the Wnt/β-catenin pathway, as demonstrated in Lrp5–/– knockout mice (27). On activation of this pathway, the number of osteoblasts is upregulated while the chondrocyte phenotype is decreased. T cells promote this osteoblastic differentiation as well. To limit the proinflammatory signaling, B cells express IL10, reducing the early inflammatory response.
Osteocytes and Osteoclasts
As one of the most abundant cell types, osteocytes decrease in the acute phase by apoptosis, mediated by caspase-3, and recruit osteoclasts to initiate bone resorption and release osteopontin (OPN) to promote the recruitment of multiple cell types, as shown in stress fractures in rat ulnae (28) and human femoral heads after fracture, where an age-related reduction in osteocyte viability was detected (13, 29). During the early healing phase, sclerostin (SOST) expression of osteocytes is downregulated directly after the fracture, demonstrated in fragility fracture patients with low-energy hip fracture (30). In the later stages, high SOST levels may facilitate endochondral ossification by preserving cartilage, since sclerostin preserves chondrocyte metabolism, especially in mechanical instability, as demonstrated in Sost-deficient mice (13, 31, 32). In the central hypoxic fracture area, the osteoclasts promote the debridement of necrotic areas and formation of new soft callus. The clearance of necrotic tissue and provisional matrix present after trauma is needed to further develop the provisional bone, which was demonstrated in rodents by Einhorn (17). As shown in CX3CR1CreERT2/Ai14 tdTomato reporter mice, circulating CX3CR1+ cells are recruited to the fractured bone by RANKL and macrophage colony-stimulating factor (M-CSF), lose CX3CR1 expression, and become osteoclasts to partially clear and embed the desharpened fragments from the forming callus area (33).
Matrix and Platelets
The structural backbone of newly built tissue is formed by platelets interacting with extracellular matrix collagen (type I [induced by IGF-1 and produced by osteoblasts] and type III) and fibronectin. Platelets secrete, among other factors, PDGF, vascular endothelial growth factor (VEGF), TGFβ, and IGF-1, resulting in both MSC differentiation and endothelial as well as fibroblastic cell proliferation. TGFβ (mainly TGFβ2 and 3) is initially produced by platelets and promotes the proliferation of MSCs as well as early osteoblastic and chondrocyte cells. In addition, TGFβ also induces the expression of matrix components (collagen I, fibronectin, OPN, osteonectin) and enhances bone formation, promotes angiogenesis, and recruits immunoregulatory cells, as revealed in rat models and human long bones (34, 35). Hence, chondrogenesis and endochondral bone formation are promoted. Thrombin and soluble fibrinogen form a biological matrix in the hematoma, regulating the lysis rate. A number of proteins (von Willebrand factor, tissue-type plasminogen activator, plasminogen activator inhibitor, and FGF2) regulate the early tissue formation. IL-1, IL-6, and TNFα are secreted by macrophages and other inflammatory cells, as well as mesenchymal cells in the periosteum, driving the initial phase of fracture healing and reducing the duration of cartilage formation (36). TNFα is indispensable for the initiation of an intramembranous stage on the periosteum, as shown in TNFα receptor (p55 and p75) knockout mice (37). A bone resident macrophage population referred to as “osteomacs” resides at the periosteum and endosteum and regulates osteoblast function, promoting fracture healing in mice (38). These CD68+ cells were also detected in human bone samples (39). The FGF level determines growth and differentiation of fibroblasts, osteoblasts, and chondrocytes, culminating in fibrin fibers sticking on early osteoblasts and subsequently leading to bone matrix accumulation (36).
Vascularization
Ruptured vessels from the periosteum, endosteum, and surrounding tissue further facilitate the early inflammatory response. As demonstrated in mice, a low oxygen tension at the site of the fracture results in a hypoxic niche in which the early stages of fracture healing occur (27, 40). In this environment, leukocytes and macrophages release VEGF and PDGF. Hypoxia-inducible factor (HIF) and VEGF are central to regulating angiogenesis and osteogenesis during the hypoxic stage. VEGF-dependent and angiopoietin-dependent pathways promote vascular growth in the developing callus.
Second Healing Period: Angiogenesis, Cartilage Formation/Soft Callus
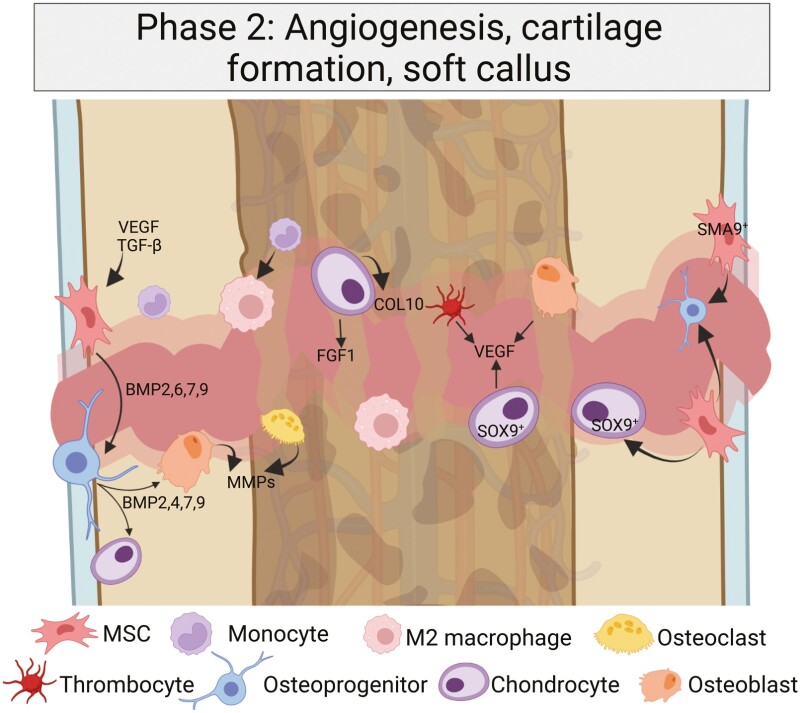
Since the remodeling process is neither strictly temporally nor spatially distinguishable, some distinct fracture areas will undergo the healing process faster while others are still in early healing stages. In the second healing period, which is best described as an anabolic phase, the new formation of vessels (angiogenesis) and formation of cartilage takes place in 2 distinct phases: endochondral ossification (soft-callus formation) and intramembranous ossification (hard-callus formation) (Fig. 2).
Figure 2.
Intramembranous ossification and endochondral ossification occur in parallel, but with different underlying mechanisms. Whereas periosteal mesenchymal stem cells (MSCs) adjacent to the fracture site form a hard callus via intramembranous ossification, the central area with a hypoxic core forms a soft callus via endochondral ossification. The macrophages are M2-polarized and alternatively activated (AAM), and the role of chondrocytes in the soft callus development becomes more prominent. Created with BioRender.com.
Endochondral Ossification (Central Parts, Soft Callus)
The endochondral ossification phase appears in the central parts of the fracture (as opposed to the peripheral intramembranous ossification), where avascular cartilaginous tissue is built to deliver a first “bridge” for mechanical microstability by chondrocytes (9, 41). This phase initiates the second stage of mesenchymal cell activation, a process in which undifferentiated MSCs differentiate into chondrocytes and osteoblasts from day 3 on. From days 3 to 7 onward, these cell types increase substantially in number, with a maximum on day 6 after the fracture; these MSCs (now SMA9+) expand and develop characteristics of osteochondroprogenitor cells, as shown in vivo in αSMACreERT2 mice (OPCs) (42). In these cells, the Runx2 gene acts as regulator of osteoblastogenesis, while downstream Sp7 (Osterix) suppresses Runx2, leading to a higher chondrogenic potential (43).
The overall cartilage building process in the soft callus starts with a collar of bone forming adjacent to the fractured bone site at the periosteum that stays connected to the soft callus (17). Undifferentiated MSCs proliferate here, giving rise to a subset of chondrocytes (Sox9+). These, together with fibroblasts, form a type II collagen/aggrecan structure from day 3 on. This constitutes the matrix for the avascular cartilaginous callus. The newly formed cartilage matrix mechanically bridges the fracture gap and provides stabilization; however, vertical (not transverse) cartilage is formed, as revealed in osteoclast-deficient mice (41, 44). The matrix is incrementally replaced by cartilage. After the chondrocytes mineralize the cartilaginous matrix, they become hypertrophic and produce collagen X, which appears to be dependent on TNFα in mice (37). GDF-5 (BMP14) as well as TGFβ2 and 3 reach their maximum on day 7, marking the maximum of cartilage formation (type II collagen) and underlining the importance of chondrogenesis. The woven bone also covers the external fibrocartilaginous callus surface and is thus the first step of mineralization of the fibrocartilage scaffold. The induction of IL-6 is essential for mineralization and early remodeling of the fracture callus, as shown in mice (6, 45, 46). This early remodeling requires the recruitment of osteoclasts, osteoblasts, and fibroblasts to the site of fracture.
In the inflammatory phase (phase 1), the formation of classically activated macrophages is promoted. In the later soft-callus phase (phase 2), the macrophages (now polarized into M2 and anti-inflammatory) are alternatively activated via IL-4 and IL-13 and participate in collagen deposition, as was demonstrated in mice (47, 48). These cells promote healing and downregulate the later phases of fracture healing (49). The high levels of IL-1, IL-6, and TNFα that drove the initial repair phase are continuously reduced during cartilage formation (before rising again in late bone remodeling).
Vascularization
The vascularization of soft-callus tissue is mainly driven by VEGF (primarily VEGF120 and VEGF164), FGF1, and TGFβ. VEGF levels of osteogenic cells are Runx2 dependent and driven by BMPs (from osteoblasts and osteoblast-like cells) (36). VEGF represents a downstream target of HIF1α and binds to the cartilage matrix during endochondral ossification. The final phase of endochondral ossification and remodeling is driven by bone matrix metalloproteinases, degrading cartilage and bone.
Intramembranous Ossification (Hard Callus)
The intramembranous ossification (formation of hard callus) recapitulates embryonic intramembranous ossification, where woven bone is formed (9). This bone formation route is mainly driven by osteoblasts and takes place on the mechanically more stable periosteal region, where MSCs differentiate into osteoblasts directly forming woven bone. Cytokines, including CXCL12 (under the control of HIF1α) from the periosteum lead to mobilization and adherence of these MSCs from the periosteum close to the fracture site and the bone marrow in the fracture area. These MSCs differentiate into osteoblastic cells and eventually begin to express an osteocyte phenotype, guided by E11 and Cx43 as shown in vivo for knockout mice, rats, and in vitro (13, 50-52).
Adjacent to the inner periosteal layer, the hard callus is formed out of type V and I collagen, mainly driven by BMP2, 4, and 7. However, to prevent excessive callus formation, the function of BMPs needs to be antagonized, which mainly happens by inhibitory molecules such as noggin (NOG) (53). During this phase, the inflammatory cytokines are highly released by MSCs (not by inflammatory cells) and their local descendants (osteoblasts and chondrocytes).
In summary, the chondrocytes produce cartilage spanning the fracture gap and connecting the bony ends to mechanically stabilize the fractured area. This soft callus serves as a scaffold for the endochondral bone formation, and a fibrocartilage tissue is formed in the central area. In contrast, osteoblasts form the cortical bone and osteoprogenitors form hard callus/woven bone on the fracture ends.
Third Healing Period: Cartilage Removal, Calcification/Hard Callus
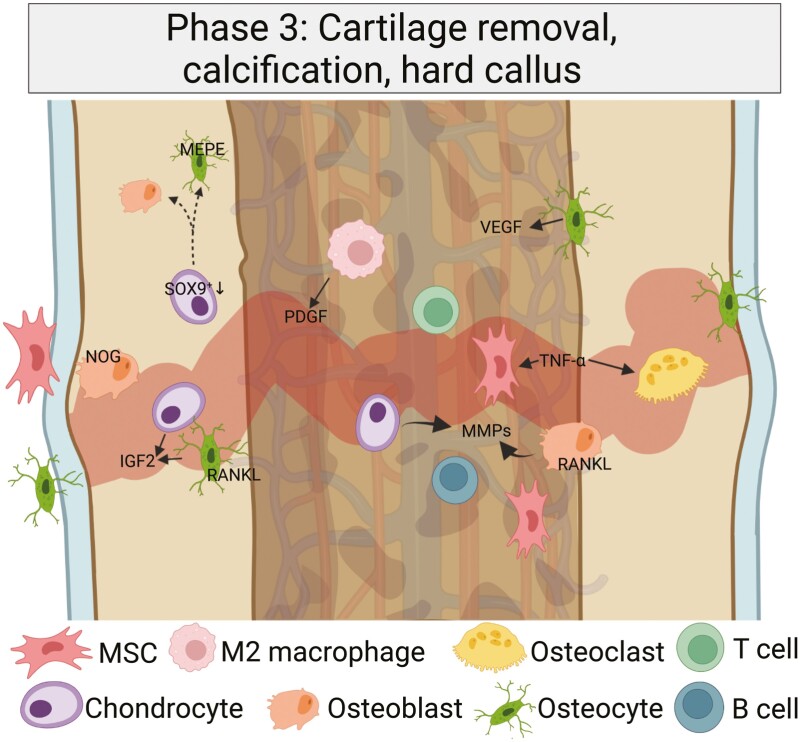
The third bone-healing stage is characterized by the gradual enlargement of cartilage and beginning calcification, and it marks the initiation of a more catabolic process although osteoblastic and osteoclastic processes alternate (Fig. 3). Intramembranous ossification and endochondral ossification are complementary processes required for the formation and remodeling of the callus area.
Figure 3.
Provisional cartilage is removed and the newly formed bone is calcified. In the third bone healing period, the central soft callus region undergoes a calcification process, while chondrocytes become hypertrophic and are partially removed. Osteocytes are more and more incorporated, while the previously built collagen II matrix is progressively degraded. Created with BioRender.com.
Intramembranous Ossification
During intramembranous ossification, the bone is directly formed without a cartilage scaffold. It takes place in mechanically more stable (peripheral) regions of the callus where it is restored to form a collagenous network (hard callus). The osteoprogenitor cells from the local periosteum contribute to the direct bone formation, while local endothelial cells transform into polymorphic cells with an osteoblastic phenotype (36).
Endochondral Ossification
After mechanical stability is reached, the previously formed cartilage becomes hypertrophic and mineralizes (36). The hypertrophic chondrocytes are therein “buried” and participate in the calcification of remaining extracellular matrix, and are subsequently removed by chondroclasts (54). Although controversial, there is evidence of a change in the morphology of hypertrophic chondrocytes, transitioning into an osteoblast/osteocyte phenotype with cellular canalicular extensions both from developmental and fracture models. Thus, using Col10a1int2-Cre;ROSAEYFP mice, Yang et al (55) first demonstrated the specificity of Col10a1int2 for hypertrophic chondrocytes, before Col10a1int2-Cre;ROSAEYFP mice were used to detect the coexpression of Col1a1, osteocalcin, and bone sialoprotein within YFP+ cells, strongly indicating an osteoblastic transition. The YFP+ cells accounted for 31% of all COL1A1+ osteoblasts in the metaphysis, and after 2 months, YFP+ cells were entrapped in the bone, morphologically representing osteocytes. In addition, using Col10a1-Cre;Osxflox/+ mice (which express enhanced green fluorescent protein only in Osx-expressing cells following Cre-mediated recombination), Zhou et al (56) demonstrated that Ocn+ and EGFP+ cells, representing mature osteoblasts on the bone surface, originated from hypertrophic chondrocytes. In addition, within fractured Agc1-CreERT2; 2.3-GFP;ROSA-tdTomato mouse tibiae, many Tom+ cells were positive for green fluorescent protein, indicating a transdifferentiation of chondrocytes to become osteoblasts. Similarly, Hu and colleagues (54) showed that within the transition zone, chondrocytes lose Sox9, Col2a1, and Col10a1 expression, and express Col1a1 and Runx2. Later, osteocalcin and OPN were detected in these cells as well. Performing lineage-tracing experiments (Ai9 to Col2CreERT and Agc1CreERT mice), the authors showed that chondrocyte-derived cells line the bone surface of newly formed bone, with the vascularization possibly triggering this transdifferentiation. Together, experimental evidence suggests that, at least in part, these hypertrophic chondrocytes become true osteoblasts (13, 47, 54). After vessel formation into formerly cartilaginous tissues has taken place, the entrapped hypertrophic chondrocytes lose their Sox9 expression, leading to enhanced Runx2 and β-catenin expression and subsequently expression of alkaline phosphatase, osterix, OPN, and osteocalcin. These transcriptomic changes were demonstrated to result in the calcification of the cartilage matrix. Upon mineralization, bone matrix is formed on top of the calcified matrix. The osteoblasts are marked by expression of the Wnt signaling components Dishevelled (Dsh) and β-catenin and gradually replace chondrocytes (57). Osteoblasts initially form woven bone (58), and M-CSF and RANKL recruit osteoclasts to resorb this bone, which is followed by the formation of new lamellar bone (41, 59).
One crucial molecule in the third healing period and endochondral ossification, binding to multiple BMP receptors, is NOG, which opposes BMP effects on osteoblastic differentiation and impairs osteoblastogenesis. NOG-overexpressing mice were more prone to fractures, osteopenia, and decreased bone formation, thus defining a role for NOG in the reduction of callus formation (60, 61).
The important function of TNFα includes the stimulation of osteoclastic function, which is essential for the resorption of mineralized cartilage in the later phases of the third healing stage. Synergistically with IL-1β, matrix mineralization by MSCs is also promoted by TNFα as shown in vitro (62, 63). As a consequence of these processes, the greatest amount of hard callus is reached on day 14 post fracture. At the same time, degradation of cartilage in endochondral ossification with matrix-metalloproteinases (especially MMP13 and MMP9) is initiated, degrading type II collagen to gelatin (36, 47). Matrix extracellular phosphoglycoprotein (MEPE), similar to DMP-1 in the middle-stage mineralization process, is present in the osteocytes in later stages of newly formed bone and is subsequently externalized in osteocyte lacunae (13, 64).
Fourth Healing Period: Chronic Bone Remodeling
The final remodeling phase in rodents begins approximately 3 weeks after fracture and can continue for months or years and should, therefore, be referred to as a chronic stage. In this phase, the woven bone is steadily replaced by superior lamellar bone while the overall callus size is reduced and the normal hematopoietic and trabecular structure restored (14, 17) (Fig. 4).
Figure 4.
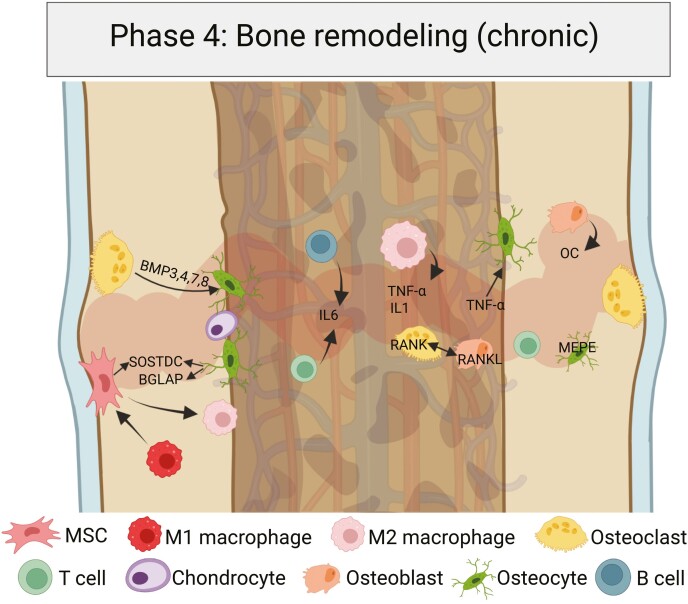
The fourth bone healing phase is characterized by bone remodeling. In a chronic process that can last several months, the preceding osteoblast/osteoclast proportions are gradually restored. Some osteocytes become apoptotic, some necrotic, and the hypertrophic callus region is reduced while favorable lamellar bone is built. Created with BioRender.com.
Molecular Pathways
In this stage, a resorptive cascade with remodeling of hard callus into lamellar bone with a central medullary area ends the process of bone healing. The major drivers are IL-1, IL-6, and TNFα (partially BMP-2) (36, 47, 65, 66). Certain BMPs, such as 3, 4, 7, and 8, usually peak in expression levels in the third week post fracture and drive cartilage resorption as well as osteoblastic recruitment (36, 67). Wnt is the crucial underlying pathway favoring osteogenesis and inhibiting chondrogenesis (13). In the late stages of fracture healing, sclerostin is upregulated and inhibits chondrogenic differentiation of subperiosteal MSCs via competitive binding of BMP receptors (13). TNFα promotes the apoptosis of osteocytes (6), while some chondrocytes become apoptotic or necrotic in the remaining soft callus. Multiple soluble factors are liberated in this process, including TGFβ, WNT10B, BMP6, SLIT3, MMP13, and MMP9, which are crucial for subsequent osteogenesis (47).
Cellular Compartment
In the cellular compartment, the remodeling process is orchestrated by osteoclasts, which are the key responsive elements although a morphological difference between chondroclasts and osteoclasts has not been formally demonstrated (6, 68). The hard callus is resorbed by osteoclasts, and lamellar bone is built by osteoblasts (47, 58).
In addition, MSC/macrophage crosstalk plays a crucial role for the regulation of bone healing (6, 69). M1-polarized macrophages (proinflammatory) promote mineralization of MSCs, in part by producing oncostatin M, while MSCs in turn promote the polarization shift from the M1 to M2 macrophage phenotype via TSG-6 and PGE2, decreasing proinflammatory cytokine secretion (6, 69).
Finally, the remaining osteoclasts become apoptotic via activation of their c-FMS receptor. In human bone, this promotes the restoration of the haversian system, a central vascular canal providing supply to a branching canalicular network, with the osteoblasts and osteocytes as central elements, and Mepe and osteocalcin as important regulators of that process (70-73). The ultimate stage of stability is usually not reached until several months after the injury. In fact, the complex remodeling process that reestablishes this haversian system takes months or years to complete (6, 74, 75).
Regulation of Fracture Healing by Systemic Factors
The bone healing process is influenced by systemic factors, endocrine diseases, and medications, of which the most important are discussed in detail next.
Parathyroid Hormone
Mechanistically, anabolic therapy with parathyroid hormone (PTH) was suggested to facilitate fracture healing via the Wnt signaling pathway (76). In preclinical murine femoral fracture models, daily administration of 1-34 PTH was found to facilitate fracture healing after 14 days. In contrast, a continuous infusion of PTH 1-34 (mimicking hyperparathyroidism) delayed fracture healing in very young mice, yet increased ultimate biomechanical properties on day 21, but not thereafter (77, 78). Treatment with 1-34 PTH improved angiogenesis and recruitment of MSCs to the callus area in mouse models, whereas endogenous PTH deficiency in PTH-knockout mice reduced RANKL expression in osteoblasts, thereby impairing osteoclast activity (77, 79).
While systematic prospective double-blind and controlled clinical trials for anabolic PTH therapy are not available, enhanced fracture healing in osteoporotic pelvic fractures after daily treatment with 100 μg PTH 1-84 was observed (although in a small study population, n = 21 treatment vs n = 44 controls), whereas the improvements after treatment of osteoporotic distal radius fractures with 20 μg 1-34 PTH (teriparatide) were minor, but early cortical bridging was detectable (80, 81). In lumbar fusion surgery, prospective studies have demonstrated improved union rates after daily subcutaneous administration of 20 μg PTH 1-34 as compared to oral bisphosphonate therapy in postmenopausal women (82-84). However, further randomized controlled trials (RCTs) in osteoporotic and stress fractures are ongoing (NCT02972424, NCT04196855).
Growth Hormone/Insulin-like Growth Factor 1
Increased growth hormone (GH) and IGF-1 levels are hallmarks of acromegaly. These patients have secondary osteoporosis with trabecular bone deficits and an increase in vertebral fracture rates, presumably from concomitant hormonal deficiencies (eg, hypogonadism) (85-87).
Evidence from human studies is limited and although a role in consolidation of delayed union is possible, the clinical effect of an impaired GH/IGF-1 axis on fracture healing has not been sufficiently characterized in human studies (88). Exposure of human MSCs to low doses of IGF-1 in vitro increased the early osteogenic differentiation of MSCs. This finding suggests that IGF-1 treatment could be a promising strategy for clinical trials in cases of impaired fracture healing; however, further in vivo evidence is lacking (89, 90). Nonetheless, clinical limitations are the expense and need for intravenous or subcutaneous administration (91).
Besides its role in osteogenic differentiation, IGF-1 has been implicated in acromegaly. Apart from a GH excess, the subsequent IGF-1 increase activates the Wnt/β-catenin pathway in acromegaly patients, leading to an increase of osteoblastic recruitment and, subsequently, enhanced callus formation (evidence from dystrophin knockout mice) (92). Moreover, IGF-1 is crucial for the M1/M2 macrophage transition in transwell experiments (93). Likewise, the important role of IGF1 becomes evident in Igf1r-knockout mice, in which a reduced callus size and lower bone volume after tibial fracture is accompanied by a mineralization defect and increased early osteoclast numbers—although the effects in an inducible Igf1r-knockout were inconsistent (94). In addition, local administration of a combination of IGF-1 and TGFβ resulted in an increased bone defect–healing capacity in old rats (95).
Mineral Metabolism
Ninety-nine percent of calcium present in the human body is deposited in bone, and it is known to play a crucial role in bone remodeling and mineralization (96). In rodent studies, a calcium deficit resulted in reduced callus mineralization and biomechanical parameters (97, 98), while calcium malabsorption could be counteracted by increased calcium release from the intact skeleton during fracture healing, as shown in mice (99). Supplementation of calcium in combination with vitamin D in osteoporotic C57BL/6 mice improved bone repair by reducing osteoclasts and increasing osteoblasts (100).
Although one of the most frequent ions in bone, the effect of magnesium on fracture healing has not been studied extensively. Interestingly, a link between systemic hypomagnesaemia and low BMD (and partly fracture risk) in men has been drawn in large epidemiological studies, while the effects on bone healing have not yet been experimentally defined (101, 102). In addition, according to a rat study, locally administered magnesium is beneficial in promoting fracture healing. In this study, a magnesium-containing nail facilitated femoral fracture repair by promoting calcitonin gene-related peptide–mediated osteogenic differentiation (103).
Vitamin D
Prospective clinical studies on the effect of vitamin D supplementation in healing bone are limited (104, 105). Most studies either pair vitamin D supplementation with calcium, or lack a control group. Case series report vitamin D deficiency to be associated with nonunion, but a causal connection has not been made (106). However, a large inception cohort study of 309 330 human fractures reported vitamin D deficiency to be associated with a slightly increased nonunion rate (odds ratio [OR], 1.14 [1.05-1.22]) (107). In a randomized, placebo-controlled study with 30 postmenopausal women suffering from proximal humerus fractures, supplementation with 800 IU vitamin D3 plus 1-g calcium improved BMD levels within the fracture after week 6 compared to the placebo group (108). In another RCT with 32 postmenopausal women with distal radius fractures, however, low-dose (700 IU) vitamin D3 treatment did not lead to statistically significant differences in peripheral quantitative computed tomography parameters at the fractured radius site 12 weeks post fracture (cortical and trabecular bone density, stiffness) vs a high-dose (1800 IU) vitamin D3 treatment group (109).
In a preclinical study, 24,25(OH)2D3 was injected subcutaneously (6.7 μg/kg daily) into wild-type C57BL/6 mice and improved stiffness on day 10, 18, and 21 in a tibial shaft fracture model, while a genetic lack of 24,25(OH)2D3 (Cyp24a1–/– mice) led to impaired endochondral callus formation, reduced callus volume, and stiffness (110, 111). In another femoral shaft fracture model, C57BL/6 J mice were fed a calcium/vitamin D–deficient diet for 8 weeks before being supplemented with calcium/vitamin D for 23 days. While the deficient diet itself increased osteoclast activity and reduced bone mass, supplementation after the fracture reduced bone resorption and improved bone repair, and did not lead to reduced BMD in the fracture callus compared to control diet, as was the case for permanent calcium/vitamin D deficiency (100).
In summary, the available data on vitamin D effects on human fracture healing remain inconclusive with a lack of large RCTs. There may be some beneficial effect of vitamin D and/or the combination with calcium, but this is mainly based on preclinical studies.
Impaired Fracture Repair in the Setting of Endocrine Diseases
Diabetes Mellitus
The Centers for Disease Control and Prevention reported that 10.5% of the US population (34.2 million) suffered from diabetes mellitus (DM) in 2020 (112). Importantly, diabetic patients are particularly prone to fractures and impaired bone healing (113, 114).
According to multiple meta-analyses, an increased fracture risk and susceptibility toward fracture malunion and reoperation in diabetic patients is not reflected by equally reduced BMD levels, at least in patients with type 2 DM (113, 115-119). Possible underlying reasons for the impaired bone healing in diabetic patients may be an increased concentration of TNFα at the fracture site, accompanied by a higher number of osteoclasts in the diabetic callus, as demonstrated in murine diabetic tibia fractures (120, 121). Further studies of diabetic bone healing in rodents revealed contradicting findings: Kayal et al (122) studied type 1 diabetic mice, which displayed increased chondrocyte apoptosis and osteoclastogenesis which accelerated cartilage loss and reduced endochondral bone formation; these deficits were reversed by insulin administration. By contrast, reduced osteoclastogenesis and osteoclast activity were detected in a type 2 diabetic rat model when glucose levels were high (123).
In human studies, Mangialardi et al (124) demonstrated dysfunction of CD146+ pericytic cells in patients with type 2 DM, possibly reducing the cellular and vascular supply at the fracture site. Along with the increased tendency of diabetic MSCs to differentiate into adipocytes instead of osteoblasts, the reason for an increased fracture rate in diabetic patients may be both quantitative and qualitative (125).
Interestingly, local administration of insulin using an intramedullary delivery system at the fracture site was shown to contribute to fracture healing both in nondiabetic rats and rats with type 1 DM (126, 127). In female type 1 diabetic mice, delayed fracture healing was reversed by systemic insulin (and vitamin D3) treatment, possibly due to increased IGF-1 production in the fracture callus (128).
Glucocorticoid Excess
Glucocorticoids (GCs) not only reduce overall bone mass (secondary osteoporosis), but also affect the inflammatory bone-healing process. The induction of inflammatory mediators in the initial healing cascade is markedly reduced with GC treatment. GCs favor differentiation of specific anti-inflammatory phenotypes of monocytes and macrophages (especially M2c) via activation of the adenosine receptor A3 and inhibit the production of vasodilators such as VEGF, as demonstrated by in vitro experiments (129-132). Prolonged GC treatment, similar to GC-induced osteoporosis, delays chondrocyte hypertrophy, attenuating endochondral bone healing via reduced production of the chondrogenic differentiator TGFβ in murine experiments (133). Interestingly, the early healing period (intramembranous ossification) appeared to be less altered after GC treatment compared to the late (endochondral ossification) healing period in this model (133). Correspondingly, the healing of murine shaft fractures was delayed after treatment with dexamethasone, whereas the metaphyseal healing of femoral fractures was enhanced, confirming an inhibitory effect of GC mainly in endochondral ossification (134). A similar effect on mainly endochondral ossification was demonstrated after 3 months of prednisone treatment, delaying endochondral ossification in mice (135)
Using a global GC receptor knockout model (GRgtROSACreERT), Rapp et al (131, 135-137) demonstrated impaired fracture healing via an increase in the early inflammatory response and reduced late endochondral ossification, possibly due to negative regulation of osteoclast recruitment and osteoblast accumulation. Interestingly, an impaired GC receptor dimerization ability protected from systemic trauma-induced compromised fracture healing (138). GC treatment impaired osteogenic differentiation via cyclooxygenase-2 (COX-2) and RUNX2 inhibition in a mandible bone defect rat model (139). Although these animal studies provide insights into possible effects of GCs on fracture healing, there is a paucity of clinical studies evaluating bone healing after GC treatment (11).
Aging
Aging is associated with multiple disorders, including DM, osteoporosis, atherosclerosis, cancer, Alzheimer, and Parkinson disease. It has been challenging to separate the aging process from comorbidities such as osteoporosis and their effect on fracture healing (140). Although a slower and less reliable fracture healing process in older individuals appears likely, there is a considerable lack of prospective clinical trial data to verify this (4, 141). Summarized below is evidence from studies examining fracture nonunions that provides some, albeit inconsistent, evidence for age as a risk factor for fracture nonunion.
Zura et al (142) found in a prospective cohort study using 2.5 million Medicare patients, 56 492 fractures, and 1440 nonunions that increasing age is linked to a decreased risk of fracture nonunion. Based on their findings, the authors suggested that increased survival may reduce the impediments to healing. The bones most prone to nonunions (highest OR) were the scaphoid, femur, humerus and tibia + fibula, followed by the clavicle. As acknowledged by the authors, however, a weakness of this study is a potential lack of observing nonunions in older individuals due to inconsistent follow-up. A likewise higher risk for younger patients for nonunion was reported by Mills et al (143), who prospectively analyzed 4715 nonunions over 5 years. The highest nonunion rates appeared in the 30- to 44-year age group, and the most susceptible bones were the tibia + fibula, followed by the clavicle and humerus. A reason for a reduced nonunion rate in femoral fractures in older individuals may be the different treatment of choice (patients > 65 years are more likely to receive arthroplasty, whereas younger patients are internally fixated to a greater extent). A weakness of this study is that just symptomatic nonunions were treated, and operatively treated nonunions were included. In addition, the data were derived solely from the NHS Scotland database (excluding patients within the private sector) (143). To overcome site-specific differences, a retrospective review analyzed 1003 reamed intramedullary nailed tibial fractures in a Scottish trauma center over 22 years, and found that age significantly influenced fracture nonunion, with middle-aged patients (30-49 years) having the highest risk of developing this complication. Selection bias and the retrospective design as well as a biased cohort selection limit the applicability of that study (ie, patients aged 70-79 had a comparable nonunion rate to patients in their 30s and 40s, around 15%) (144). Overall, these studies do not support a higher rate of nonunions in the older population. However, the study by Zura et al (142) reports distinct comorbidities leading to higher nonunion rates, including smoking, alcoholism, obesity, cardiovascular disease, osteoarthritis, osteoporosis, and type 2 DM, introducing a possible confounding effect of a chronic inflammatory state. In this context, aging might be a modifying factor, and although female sex was not predictive of nonunions in this study, the nonunion number in the Mills study peaked in women aged 65 to 74 years. It may subsequently be advisable to evaluate these postmenopausal women separately to detect the influence of menopause on fracture healing ability (142, 143).
The tibia, followed by the clavicle and the humerus, are the primary bones prone to nonunion (145). In fact, perhaps the largest prospective study assessing nonunions in clavicular fractures highlighted age consistently with the fracture displacement as risk factors for nonunion (146). In osteoporotic vertebral fractures, Inose et al (147) did not verify age as a risk factor for nonunion in a unique prospective multicenter study. In retrospective studies on clavicular fractures, age remained a significant factor (OR, 1.07), whereas in tibial diaphyseal fractures and scaphoid fractures, it was not (148, 149). Intriguingly, in a meta-analysis of 41 429 tibial fractures, age older than 60 years was demonstrated to be predictive for fracture nonunion (150). The importance of age in the appearance of nonunion, however, has been questioned because in retrospective studies of nonunions, age could not be confirmed as significantly influencing the time to union in multivariate analyses (151). Thus, overall, age cannot consistently be identified as a risk factor for nonunion in generally every bone. However, older age likely raises the risk of a pseudarthrosis in certain patients and sites (ie, clavicle, tibia). Because comorbidities such as smoking and DM have a detrimental effect on bone healing as shown at the molecular level (113, 119, 152, 153), studies on the effect of aging alone require careful design (154).
Age-related Changes in Fracture Repair
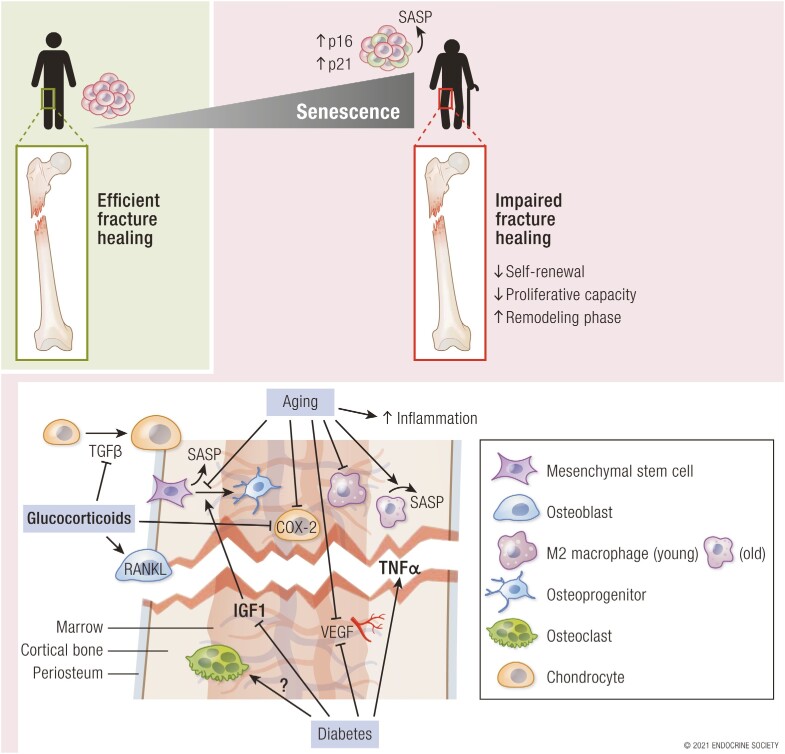
The single largest risk factor for various chronic diseases responsible for the majority of morbidity and mortality is aging (155). Based on the “geroscience hypothesis,” the treatment of underlying aging mechanisms may lead to new therapeutic approaches for our aging population (156, 157). Since the identification of senescent cells in the bone microenvironment, numerous studies have identified changes at the cellular and molecular level in the bone microenvironment. Drivers of these aging-related effects are accumulating senescent cells, which are characterized by high p16Ink4a and p21Cip1 levels, causing detrimental effects via secretion of a senescence-associated secretory phenotype (SASP). However, the effects of these senescent cells and their secretome on the dynamic bone-healing process are largely unexplored (5, 158-160).
Generally, the overall molecular program of fracture healing appears to be unimpaired in aged mice, while the callus volume, mineral content, as well as rigidity and breaking load are reduced in older mice and humans (154, 161). Interestingly, it was demonstrated that a “rejuvenation” of the bone marrow affected bone healing: The experimental transplantation of bone marrow from young (Rosa26) into old (irradiated C57BL/6J) mice resulted in larger calluses and more bone mass in the early healing period and rapid callus remodeling at later stages of healing in older mice, indicating that aging of bone marrow cells is affecting the later stages of bone healing as well (114-116). The particular cellular components of fracture healing are each differently affected by aging.
Vascular Supply
A reduced vascular capacity and angiogenesis delay fracture healing in older individuals that is not necessarily associated with DM (4, 47). Vascular supply/perfusion is decreased in the aged organism, subsequently impairing the number of total cells directed to the fracture area (162). One proposed mechanism is reduced endothelium-dependent vasodilatation, as described in femoral fractures in old rats through impairments in NOS signaling (162). Another possible underlying mechanism might be reduced VEGF release: Because the early inflammatory phase and chondrogenesis are delayed in older animals due to a prolonged increase in TNFα expression (mainly by MSCs and macrophages), the overall VEGF release is reduced, which is also a major contributor to delayed bone healing in diabetic mice (163). However, it has also been demonstrated that low levels of TNFα promote the osteogenesis of muscle-derived stem cells near the fracture site in the murine model, expediting fracture repair (65). Interestingly, TNF blockade via subcutaneous sTNFR1 administration accelerated fracture healing in the aged mouse model and appeared to be mediated by p21 (Cdkn1a) (164). Overall, the precise role of TNFα in aging-related fracture healing is not fully understood.
Macrophages
The importance of macrophages during bone healing is underlined by the fact that their deletion in the inducible Mafia (macrophage Fas-induced apoptosis) transgenic mouse model in murine femoral fractures resulted in a completely abolished callus formation (69, 165). As the macrophages, which are polarized toward an M1 state throughout the early healing period, contribute substantially to the fracture healing process, the effect of their age-related impairment is crucial. Aged macrophages are less responsive to granulocyte macrophage colony-stimulating factor (GM-CSF) stimulation, resulting in decreased proliferation, a chronic inflammatory state, and impaired bone healing. The GM-CSF–dependent stimulation decreases with age while the polarization toward an M2 phenotype was reported to be favored, possibly due to increased levels of S-endoglin (166, 167). The alternatively activated M2 macrophages show increased survival and reduced secretion of proinflammatory factors with age, and appear more often in the late healing stages (18, 69). However, Mahbub et al (168) demonstrated in vitro that an overall impaired polarization in elderly macrophages might dysregulate the host response. Either way, the role of these aged macrophages on fracture healing in older individuals appears to be more detrimental than helpful: In elderly mice, the blockade of macrophage recruitment to the fracture site subsequentially enhanced fracture healing (169). Interestingly, in ovariectomized mice, a reduction of both M1 and M2 macrophages in early healing stages might contribute to impaired fracture healing (170).
The pivotal effect of macrophages for the aged vs young healing process has been demonstrated for F4/80+ cells in heterochronic parabiosis studies by Vi et al (171): Macrophages that were isolated from old mice (age 20 months) and transplanted into young (age 4 months) animals led to reduced bone volume fraction and calcified callus volume after 4 weeks of tibia healing. By contrast, macrophages isolated from young mice and transplanted into old mice were able to rejuvenate the otherwise impaired healing capacity (171).
The close connections of macrophages and MSCs are likewise crucial for the healing process. Macrophages are known to recruit MSCs via CCL2 and CXCL8 (69), and a reciprocal immunomodulatory function via reduction of proinflammatory cytokines and induction of IL-10 in macrophages by MSCs has been demonstrated in vitro (69, 172). Additionally, MSCs modulate macrophage chemotaxis, and shift the monocyte polarization toward M2 macrophages in vitro (173). The exact nature of the macrophage/MSC interaction is still not fully understood, however, and direct cell-cell contact as well as soluble mediators are likely involved (69).
Macrophages play a key role in eliminating senescent cells, and impairments in this process likely contribute to impaired tissue regeneration (174). Macrophages show critical regulatory activity in all stages of tissue repair by clearing senescent cells (175). Senescent cells display certain hallmarks associated with aging including increased expression of Cdkn2a (p16) and Cdkn1a (p21) (155, 156, 159). Interestingly, p16- and beta-galactosidase–positive macrophages are not necessarily senescent but may be cleared by senolytic compounds (176); the effect of this on tissue repair, including fracture healing, remains unclear.
Mesenchymal Stem Cells
The bone marrow progenitor cells from the periosteal region and bone marrow–derived stem cells, out of which osteogenic and chondrogenic progenitors are recruited, are reduced in quantity and quality, and a later initial periosteal reaction to the fracture can be detected in older mice (177).
However, the sole number of MSCs does not appear to be critical: While the total number of MSCs from older mice was demonstrated to be higher, not as many osteoprogenitor cells develop out of these as in younger mice (and are affected by sex) (178, 179). Additionally, their replicative lifespan has been demonstrated to be substantially shortened, oxidative damage is enhanced, and chondrogenic differentiation prolonged (16, 180, 181). As noted earlier, aged human MSCs have been described to overexpress TP53, CDKN2A (p16) and CDKN1A (p21), BAX, and MYC (18, 182, 183). Likewise, skeletal stem/progenitor cells (SSPCs) secrete SASP factors with aging, resulting in an activation of nuclear factor κB (NF-κB) in other SSPCs, inducing a state of senescence, leading to reduction of self-renewal and proliferation, and decreasing the total cell number. The osteogenic ability of these aged SSPCs, which express higher levels of Cdkn2a/p16 than their younger counterparts, has been shown to be reduced in vivo and in vitro, and this was attributed to an increase in NF-κB mediated inflammation (141).
Recently, the osteoimmunological effect on fracture healing has gained attention (184, 185). Owing to their low expression of MHC I and II molecules, MSCs do not induce an immunological response and can be used for transplantation experiments (186). Moreover, MSCs suppress T-cell proliferation and induce regulatory T cells to suppress the immune system, while T cells in general, particularly via interferon-γ (IFN-γ), reduce MSC-induced osteogenesis in murine allogeneic stem cell transplantation experiments (186, 187). These findings are corroborated by the detection of CD8+ effector memory T (TEMRA) cells in humans in peripheral blood and the fracture hematoma, which, by producing IFN-γ/TNFα, inhibited the osteogenic differentiation of MSCs (188).
In several meta-analyses, nonsteroidal anti-inflammatory drugs (NSAIDs) were reported to impair fracture healing (increase delayed union or nonunion in humans [189] and decrease biomechanical properties in animal models [190]). But it has likewise been shown that NSAIDS are able to inhibit the SASP-associated activation of NF-κB, thereby potentially enhancing bone regeneration (141, 191). However, there is still no final conclusion regarding whether the beneficial or detrimental effects of NSAIDs on bone healing are predominant because of the lack of appropriate prospective RCTs (192).
Lymphocytes
As noted earlier, the role of the inflammatory system in an aging organism has been elucidated and summed up with the term inflammaging (193). A link to the inflammatory mediator COX and lipoxygenases has been well described: The inflammatory response is more intense in aged female compared to young female mice, which delays bone healing, and can be reduced by genetic deletion of 5-lipoxygenase (194, 195) or pharmacologic inhibition via NSAIDs or derivates (191, 196). Regarding the cellular components of this inflammatory phase, the total number of T cells in general and regulatory T cells in particular is reduced in the old human organism (197). T-cell migration is controlled by the chemokine receptor CCR2. One underlying reason for the impaired immune function due to aging has been proposed as being a CCR2 (chemokine receptor for ligands including CCL2, CCL7, and CCL12) defect/deficiency, as demonstrated in mice (198). Apart from the osteochondral stem cells, some inflammatory cells originate in the bone marrow and their replacement in old mice via bone marrow transplantation from young mice results in larger calluses and earlier bone formation in the early phases as well as faster callus remodeling in the late stages (199). Proinflammatory cytokine expression from T cells as well as B cells in the callus is mediated by IL-10, inducing proper healing, while a missing early upregulation of IL-10 was associated with nonunion in human tibia fractures (200). Affirming the importance of IL-10 in fracture healing, B cells from patients with poor fracture healing had decreased levels of IL-10 compared to regularly healing patients.
Chondrocytes
Chondrocytes and their precursors change their size and function throughout the healing period and depending on age: In the early bone-healing process, chondrocytes do not express collagen type II in old mice but do so in young mice, indicating a faster adaptation to the acute fracture event (201). Correspondingly, chondrocyte maturation is present earlier (at day 5) in young mice but not in older and middle-aged mice (201). Older chondrocyte progenitors express significantly lower levels of COX-2, a critical marker in the early inflammatory phase regulating chondrogenesis, bone formation, and remodeling, as compared to young mice (202). This reduction of COX-2 expression leads to delayed remodeling in aged mice (202). In support of these data, pharmacological inhibition of COX-2 led to an impairment in endochondral ossification in rabbits (203). Accordingly, a downstream target of COX-2, EP4, has been targeted therapeutically to enhance bone healing (204).
Osteocytes
The osteocyte population translates mechanical signals to increase mineralization and accelerate metaphyseal bone healing in the osteoporotic mouse fracture model (31). Osteocytes, although long-lived and the most abundant cell population in bone, display increased rates of cell death during aging, leaving behind empty lacunae (31, 205). The preservation of osteocytes is on the other hand influenced by mechanical stimulation, steroids, and bisphosphonates as demonstrated in mice and partly in humans (206-209). Affecting the osteocyte-secreted antianabolic factor, sclerostin, sclerostin antibodies were approved for clinical therapy of severe osteoporosis in 2019, and have been demonstrated to improve bone healing in rat studies, but not in human tibia or femur bone healing (210-213). A targeted deletion of SOST leads to an earlier removal of cartilage, affecting especially the early remodeling phase (76, 214). This effect was consistent in delayed bone healing due to osteoporosis in rats (215, 216).
Similar to the aging process in MSCs, aging osteocytes show telomere dysfunction-associated foci, a senescence-associated distension of satellites, and secrete dysfunctional factors (SASP) linked to aging and chronic diseases (156, 158). One mechanism contributing to this osteocyte aging is a lack of cellular autophagy (217, 218).
Osteoclasts
More than the number of osteoclasts itself, the osteoblast/osteoclast balance is of crucial importance during the healing process to generate new bone and provide a proper reformation of woven and lamellar bone (15). Increased osteoclast activity has been proposed to be causal for delayed fracture healing due to higher concentrations of tartrate-resistant acid phosphatase (TRAP) and deoxy-pyridinoline in a senescence-accelerated osteoporotic mouse model (219). The molecular background of these mice indeed favors osteoclastogenesis: The increased IFN-γ expression in aged mice increases RANKL expression, driving osteoclastogenesis and bone loss (220, 221). In addition, a higher amount of TRAP-positive osteoclasts in the callus area was demonstrated in old mice (194).
Beneficial vs Adverse Effects of Cellular Senescence
While the initial descriptions of cellular senescence focused on its detrimental effects on aging, more recent work has shed light on its potential beneficial aspects, describing senescence as an evolutionary advantageous, yet double-edged process (222, 223).
A senescent cell does not divide further, which is why suppressing cancer development has been a potential explanation for cellular senescence (224). However, this nondividing cell causes a detrimental “bystander” effect on surrounding cells and whole tissues via secretion of reactive oxygen species and a miscellaneous cytokine cocktail, the SASP (225-228) (Fig. 5). Along with the senescent cells, the SASP contributes to inflammaging, which may also be protumorigenic (193, 229). The senescent phenotype is not limited to mitotic cells, and the elimination of these SASP-producing cells in mice has been demonstrated to be beneficial in ameliorating the effects of aging in multiple tissues, including the heart and bone (159, 230, 231). Overall, the removal of accumulated senescent cells has beneficial effects on aging phenotypes (223). It is therefore of importance to isolate, spatially restrict, or resolve an excessive accumulation of these senescent cells before they occupy cellular niches and lead to systemic disease and morbidity (232, 233). By mechanisms that are not fully understood, an imperfect clearance leads to the slow increase of the senescent cell burden with aging (234).
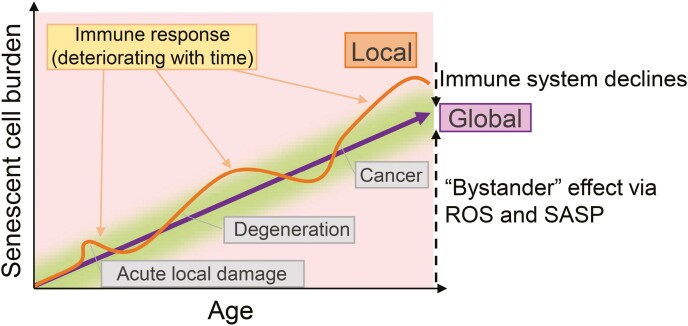
Figure 5.
Senescent cell burden in the aging organism. Acute tissue damage causes a local, rapid increase in senescent cell burden, briefly disturbing the healthy balance (green area), followed by a rapid immune response aimed at clearing those cells. However, senescent cell burden increases with age, because of the “bystander” effect and incomplete senescent cell clearance caused by a deteriorating immune system. Further disturbance of this delicate senescent cell balance occurs as a result of degenerative processes and cancer.
Importantly, a beneficial influence of senescent cells on murine skin and liver regeneration (235, 236), in the murine placenta (237) and in chicken as well as murine embryonic development (238) has been demonstrated (223). Moreover, a potentially important function of senescent cells in mice and amphibians has been demonstrated to lie in wound healing and tissue repair (223, 236, 239). In particular, the beneficial function of the SASP is mainly attributed to IL-6 in skeletal muscle, enabling cellular reprogramming, and PDGF-AA in wound healing, as demonstrated in mice (235, 240). In the injured skin, a transient induction of senescent cells is followed by rapid clearance at the early reparative stage. The recruitment of immune cells to the area of damage by the SASP subsequently leads to the removal of senescent cells after completed healing, pointing to a primary role of these cells in the damage-repair process (223, 241, 242). In a deteriorating immune system, this clearance potential, which is attributed largely to local macrophages, may be impaired (175, 235, 243, 244).
In terms of skeletal repair, we recently established the appearance of senescent cells within murine fracture healing in a time-dependent manner in young mice. In contrast to the aforementioned findings that describe senescent cells having a beneficial role in tissue repair (235, 236), using genetic (Cdkn2aLUC) and pharmacological (senolytic treatment with dasatinib + quercetin) models to clear senescent cells, we demonstrated that a reduction of senescent cells and the SASP within the callus improved its formation and biomechanical stability (245). Thus, the beneficial vs detrimental effects of senescent cells following injury may vary across tissues.
Summarizing these contradictory influences of senescent cells, the local immune clearance of these cells leads to a balance between beneficial and harmful effects, depending on the abundance and duration of the senescent cell burden. Indeed, some senescent cells are not replaced and yet play important local functional roles, whereas others accumulate and have a detrimental effect at a systemic level, driving organ dysfunction and aging (246, 247). These contrarian roles of senescent cells are depicted in Fig. 5 (223).
Conclusions
The cellular components of fracture healing that can be roughly subdivided into 4 temporal stages are all affected by the aging process. A number of age-related impairments in fracture healing have been identified, and further studies are needed to define the possible role of the accumulation of senescent cells with aging in modulating fracture healing. In addition, studies are needed to evaluate whether targeting senescent cells, or other impairments in fracture healing (eg, inflammaging), will enhance fracture healing in older individuals.
Glossary
Abbreviations
- BMD
bone mineral density
- BMP
bone morphogenic protein
- COX
cyclooxygenase
- DM
diabetes mellitus
- FGF2
fibroblast growth factor 2
- GC
glucocorticoid
- GH
growth hormone
- GM-CSF
granulocyte macrophage colony–stimulating factor
- HIF
hypoxia-inducible factor
- IFN-γ
interferon-γ
- IGF-1
insulin-like growth factor 1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- M-CSF
macrophage colony–stimulating factor
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor κB
- NOG
noggin
- NOS
nitric oxide synthase
- NSAIDS
nonsteroidal anti-inflammatory drugs
- OPN
osteopontin
- OR
odds ratio
- PDGF
platelet-derived growth factor
- PTH
parathyroid hormone
- RCT
randomized controlled trial
- SASP
senescence-associated secretory phenotype
- SOST
sclerostin
- SSPC
skeletal stem/progenitor cell
- TGFβ
transforming growth factor β
- TNFα
tumor necrosis factor α
- TRAP
tartrate-resistant acid phosphatase
- VEGF
vascular endothelial growth factor
Contributor Information
Dominik Saul, Kogod Center on Aging and Division of Endocrinology, Mayo Clinic, Rochester, Minnesota 55905, USA; Department of Trauma, Orthopedics and Reconstructive Surgery, Georg-August-University of Goettingen, 37073 Goettingen, Germany.
Sundeep Khosla, Kogod Center on Aging and Division of Endocrinology, Mayo Clinic, Rochester, Minnesota 55905, USA.
Financial Support
This work was supported by the National Institutes of Health (grant Nos. AG062413 and AG065868 to S.K.) and the German Research Foundation (No. 413501650; Deutsche Forschungsgemeinschaft DFG to D.S.).
Disclosures
The authors have nothing to disclose.
References
- 1. United States Census Bureau. National population by characteristics: 2010-2019. Accessed August 11, 2021. https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html
- 2. Rose S, Maffulli N. Hip fractures. An epidemiological review. Bull Hosp Jt Dis. 1999;58(4):197-201. [PubMed] [Google Scholar]
- 3. Williams SA, Chastek B, Sundquist K, et al. . Economic burden of osteoporotic fractures in US managed care enrollees. Am J Manag Care. 2020;26(5):e142-e149. [DOI] [PubMed] [Google Scholar]
- 4. Clark D, Nakamura M, Miclau T, Marcucio R. Effects of aging on fracture healing. Curr Osteoporos Rep. 2017;15(6):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farr JN, Rowsey JL, Eckhardt BA, et al. . Independent roles of estrogen deficiency and cellular senescence in the pathogenesis of osteoporosis: evidence in young adult mice and older humans. J Bone Miner Res. 2019;34(8):1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loi F, Córdova LA, Pajarinen J, Lin T, Yao Z, Goodman SB. Inflammation, fracture and bone repair. Bone. 2016;86(8): 119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Augat P, Simon U, Liedert A, Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005;16(Suppl 2):S36-S43. [DOI] [PubMed] [Google Scholar]
- 8. Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci. 2015;28(1):57-71. [DOI] [PubMed] [Google Scholar]
- 9. Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl 3):S5-S7. [DOI] [PubMed] [Google Scholar]
- 10. Park D, Spencer JA, Koh BI, et al. . Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hachemi Y, Rapp AE, Picke AK, Weidinger G, Ignatius A, Tuckermann J. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J Mol Endocrinol. 2018;61(1):R75-R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao L, Wang X, Pomlok K, et al. . DDB1 promotes the proliferation and hypertrophy of chondrocytes during mouse skeleton development. Dev Biol. 2020;465(2):100-107. [DOI] [PubMed] [Google Scholar]
- 13. Choy MHV, Wong RMY, Chow SKH, et al. . How much do we know about the role of osteocytes in different phases of fracture healing? A systematic review. J Orthop Translat. 2020;21:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol. 2015;11(1):45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133-143. [DOI] [PubMed] [Google Scholar]
- 16. Ferretti C, Lucarini G, Andreoni C, et al. . Human periosteal derived stem cell potential: the impact of age. Stem Cell Rev Rep. 2015;11(3):487-500. [DOI] [PubMed] [Google Scholar]
- 17. Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;(355 Suppl):S7-S21. [DOI] [PubMed] [Google Scholar]
- 18. Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovtun A, Bergdolt S, Wiegner R, Radermacher P, Huber-Lang M, Ignatius A. The crucial role of neutrophil granulocytes in bone fracture healing. Eur Cell Mater. 2016;32:152-162. [DOI] [PubMed] [Google Scholar]
- 20. Duchamp de Lageneste O, Julien A, Abou-Khalil R, et al. . Periosteum contains skeletal stem cells with high bone regenerative potential controlled by periostin. Nat Commun. 2018;9:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873-884. [DOI] [PubMed] [Google Scholar]
- 22. Nakahara H, Bruder SP, Goldberg VM, Caplan AI. In vivo osteochondrogenic potential of cultured cells derived from the periosteum. Clin Orthop Relat Res. 1990;(259):223-232. [PubMed] [Google Scholar]
- 23. Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350(3):557-561. [DOI] [PubMed] [Google Scholar]
- 24. Nam D, Mau E, Wang Y, et al. . T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS One. 2012;7(6):e40044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun G, Wang Z, Ti Y, et al. . STAT3 promotes bone fracture healing by enhancing the FOXP3 expression and the suppressive function of regulatory T cells. APMIS. 2017;125(8):752-760. [DOI] [PubMed] [Google Scholar]
- 26. Ito H. Chemokines in mesenchymal stem cell therapy for bone repair: a novel concept of recruiting mesenchymal stem cells and the possible cell sources. Mod Rheumatol. 2011;21(2):113-121. [DOI] [PubMed] [Google Scholar]
- 27. Shiu HT, Leung PC, Ko CH. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J Tissue Eng Regen Med. 2018;12(4):e1911-e1925. [DOI] [PubMed] [Google Scholar]
- 28. Wu AC, Kidd LJ, Cowling NR, Kelly WL, Forwood MR. Osteocyte expression of caspase-3, COX-2, IL-6 and sclerostin are spatially and temporally associated following stress fracture initiation. Bonekey Rep. 2014;3:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dunstan CR, Evans RA, Hills E, Wong SY, Higgs RJ. Bone death in hip fracture in the elderly. Calcif Tissue Int. 1990;47(5):270-275. [DOI] [PubMed] [Google Scholar]
- 30. Caetano-Lopes J, Lopes A, Rodrigues A, et al. . Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing. PLoS One. 2011;6(2):e16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheung WH, Wong RMY, Choy VMH, Li MCM, Cheng KYK, Chow SKH. Enhancement of osteoporotic fracture healing by vibration treatment: the role of osteocytes. Injury. 2021;52Suppl 2:S97-S100. [DOI] [PubMed] [Google Scholar]
- 32. Bouaziz W, Funck-Brentano T, Lin H, et al. . Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Novak S, Roeder E, Kalinowski J, et al. . Osteoclasts derive predominantly from bone marrow-resident CX3CR1+ precursor cells in homeostasis, whereas circulating CX3CR1+ cells contribute to osteoclast development during fracture repair. J Immunol. 2020;204(4):868-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wildemann B, Schmidmaier G, Ordel S, Stange R, Haas NP, Raschke M. Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater. 2003;65(1):150-156. [DOI] [PubMed] [Google Scholar]
- 35. Sarahrudi K, Thomas A, Mousavi M, et al. . Elevated transforming growth factor-beta 1 (TGF-β1) levels in human fracture healing. Injury. 2011;42(8):833-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11-S25. [DOI] [PubMed] [Google Scholar]
- 37. Gerstenfeld LC, Cho TJ, Kon T, et al. . Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169(3):285-294. [DOI] [PubMed] [Google Scholar]
- 38. Alexander KA, Chang MK, Maylin ER, et al. . Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26(7):1517-1532. [DOI] [PubMed] [Google Scholar]
- 39. Chang MK, Raggatt LJ, Alexander KA, et al. . Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232-1244. [DOI] [PubMed] [Google Scholar]
- 40. Lu C, Rollins M, Hou H, et al. . Tibial fracture decreases oxygen levels at the site of injury. Iowa Orthop J. 2008;28:14-21. [PMC free article] [PubMed] [Google Scholar]
- 41. Little DG, Ramachandran M, Schindeler A. The anabolic and catabolic responses in bone repair. J Bone Joint Surg Br. 2007;89(4):425-433. [DOI] [PubMed] [Google Scholar]
- 42. Grcevic D, Pejda S, Matthews BG, et al. . In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30(2):187-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshida CA, Komori H, Maruyama Z, et al. . SP7 inhibits osteoblast differentiation at a late stage in mice. PLoS One. 2012;7(3):e32364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deckers MML, van Beek ER, van der Pluijm G, et al. . Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Miner Res. 2002;17(6):998-1007. [DOI] [PubMed] [Google Scholar]
- 45. Prystaz K, Kaiser K, Kovtun A, et al. . Distinct effects of IL-6 classic and trans-signaling in bone fracture healing. Am J Pathol. 2018;188(2):474-490. [DOI] [PubMed] [Google Scholar]
- 46. Wallace A, Cooney TE, Englund R, Lubahn JD. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res. 2011;29(9):1437-1442. [DOI] [PubMed] [Google Scholar]
- 47. Bahney CS, Zondervan RL, Allison P, et al. . Cellular biology of fracture healing. J Orthop Res. 2019;37(1):35-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schlundt C, El Khassawna T, Serra A, et al. . Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78-89. [DOI] [PubMed] [Google Scholar]
- 49. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loiselle AE, Paul EM, Lewis GS, Donahue HJ. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J Orthop Res. 2013;31(1):147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hadjiargyrou M, Rightmire EP, Ando T, Lombardo FT. The E11 osteoblastic lineage marker is differentially expressed during fracture healing. Bone. 2001;29(2):149-154. [DOI] [PubMed] [Google Scholar]
- 52. Staines KA, Prideaux M, Allen S, Buttle DJ, Pitsillides AA, Farquharson C. E11/podoplanin protein stabilization through inhibition of the proteasome promotes osteocyte differentiation in murine in vitro models. J Cell Physiol. 2016;231(6):1392-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoshimura Y, Nomura S, Kawasaki S, Tsutsumimoto T, Shimizu T, Takaoka K. Colocalization of noggin and bone morphogenetic protein-4 during fracture healing. J Bone Miner Res. 2001;16(5):876-884. [DOI] [PubMed] [Google Scholar]
- 54. Hu DP, Ferro F, Yang F, et al. . Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development. 2017;144(2):221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang G, Zhu L, Hou N, et al. . Osteogenic fate of hypertrophic chondrocytes. Cell Res. 2014;24(10):1266-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10(12):e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duan P, Bonewald LF. The role of the Wnt/β-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77(Pt A):23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ikebuchi Y, Aoki S, Honma M, et al. . Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 2018;561:195-200. [DOI] [PubMed] [Google Scholar]
- 60. Devlin RD, Du Z, Pereira RC, et al. . Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology. 2003;144(5):1972-1978. [DOI] [PubMed] [Google Scholar]
- 61. Aspenberg P, Jeppsson C, Economides AN. The bone morphogenetic proteins antagonist Noggin inhibits membranous ossification. J Bone Miner Res. 2001;16(3):497-500. [DOI] [PubMed] [Google Scholar]
- 62. Vallés G, Bensiamar F, Maestro-Paramio L, García-Rey E, Vilaboa N, Saldaña L. Influence of inflammatory conditions provided by macrophages on osteogenic ability of mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Osta B, Lavocat F, Eljaafari A, Miossec P. Effects of interleukin-17A on osteogenic differentiation of isolated human mesenchymal stem cells. Front Immunol. 2014;5:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lu C, Huang S, Miclau T, Helms JA, Colnot C. Mepe is expressed during skeletal development and regeneration. Histochem Cell Biol. 2004;121(6):493-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kon T, Cho TJ, Aizawa T, et al. . Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16(6):1004-1014. [DOI] [PubMed] [Google Scholar]
- 67. Lissenberg-Thunnissen SN, de Gorter DJJ, Sier CFM, Schipper IB. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop. 2011;35(9):1271-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pazzaglia UE, Reguzzoni M, Casati L, Sibilia V, Zarattini G, Raspanti M. New morphological evidence of the ‘fate’ of growth plate hypertrophic chondrocytes in the general context of endochondral ossification. J Anat. 2020;236(2):305-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pajarinen J, Lin T, Gibon E, et al. . Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yu B, Pacureanu A, Olivier C, Cloetens P, Peyrin F. Assessment of the human bone lacuno-canalicular network at the nanoscale and impact of spatial resolution. Sci Rep. 2020;10:4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Toyosawa S, Kanatani N, Shintani S, et al. . Expression of dentin matrix protein 1 (DMP1) during fracture healing. Bone. 2004;35(2):553-561. [DOI] [PubMed] [Google Scholar]
- 72. Lu C, Hansen E, Sapozhnikova A, Hu D, Miclau T, Marcucio RS. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26(10):1384-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ferron M, Wei J, Yoshizawa T, et al. . Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oliver RA, Yu Y, Yee G, Low AK, Diwan AD, Walsh WR. Poor histological healing of a femoral fracture following 12 months of oestrogen deficiency in rats. Osteoporos Int. 2013;24(10):2581-2589. [DOI] [PubMed] [Google Scholar]
- 75. Garcia P, Histing T, Holstein JH, et al. . Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur Cell Mater. 2013;26:1-12; discussion 12-14. [DOI] [PubMed] [Google Scholar]
- 76. Schupbach D, Comeau-Gauthier M, Harvey E, Merle G. Wnt modulation in bone healing. Bone. 2020;138:115491. [DOI] [PubMed] [Google Scholar]
- 77. Jiang X, Xu C, Shi H, Cheng Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS One. 2019;14(12):e0226163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yukata K, Kanchiku T, Egawa H, et al. . Continuous infusion of PTH1-34 delayed fracture healing in mice. Sci Rep. 2018;8:13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sun P, Wang M, Yin GY. Endogenous parathyroid hormone (PTH) signals through osteoblasts via RANKL during fracture healing to affect osteoclasts. Biochem Biophys Res Commun. 2020;525(4):850-856. [DOI] [PubMed] [Google Scholar]
- 80. Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93(17):1583-1587. [DOI] [PubMed] [Google Scholar]
- 81. Aspenberg P, Genant HK, Johansson T, et al. . Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res. 2010;25(2):404-414. [DOI] [PubMed] [Google Scholar]
- 82. Ohtori S, Inoue G, Orita S, et al. . Teriparatide accelerates lumbar posterolateral fusion in women with postmenopausal osteoporosis: prospective study. Spine (Phila Pa 1976). 2012;37(23):E1464-E1468. [DOI] [PubMed] [Google Scholar]
- 83. Seki S, Hirano N, Kawaguchi Y, et al. . Teriparatide versus low-dose bisphosphonates before and after surgery for adult spinal deformity in female Japanese patients with osteoporosis. Eur Spine J. 2017;26(8):2121-2127. [DOI] [PubMed] [Google Scholar]
- 84. Cho PG, Ji GY, Shin DA, Ha Y, Yoon DH, Kim KN. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J. 2017;26(3):691-697. [DOI] [PubMed] [Google Scholar]
- 85. Padova G, Borzì G, Incorvaia L, et al. . Prevalence of osteoporosis and vertebral fractures in acromegalic patients. Clin Cases Miner Bone Metab. 2011;8(3):37-43. [PMC free article] [PubMed] [Google Scholar]
- 86. Mazziotti G, Bianchi A, Porcelli T, et al. . Vertebral fractures in patients with acromegaly: a 3-year prospective study. J Clin Endocrinol Metab. 2013;98(8):3402-3410. [DOI] [PubMed] [Google Scholar]
- 87. Madeira M, Neto LV, de Paula Paranhos Neto F, et al. . Acromegaly has a negative influence on trabecular bone, but not on cortical bone, as assessed by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2013;98(4):1734-1741. [DOI] [PubMed] [Google Scholar]
- 88. Weiss S, Henle P, Bidlingmaier M, Moghaddam A, Kasten P, Zimmermann G. Systemic response of the GH/IGF-I axis in timely versus delayed fracture healing. Growth Horm IGF Res. 2008;18(3):205-212. [DOI] [PubMed] [Google Scholar]
- 89. Reible B, Schmidmaier G, Moghaddam A, Westhauser F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int J Mol Sci. 2018;19(6):1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Reible B, Schmidmaier G, Prokscha M, Moghaddam A, Westhauser F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in-vitro. Growth Factors. 2017;35(4-5):179-188. [DOI] [PubMed] [Google Scholar]
- 91. Kirby DJ, Buchalter DB, Anil U, Leucht P. DHEA in bone: the role in osteoporosis and fracture healing. Arch Osteoporos. 2020;15(1):84. [DOI] [PubMed] [Google Scholar]
- 92. Deng Z, Gao X, Sun X, Cui Y, Amra S, Huard J. Gender differences in tibial fractures healing in normal and muscular dystrophic mice. Am J Transl Res. 2020;12(6):2640-2651. [PMC free article] [PubMed] [Google Scholar]
- 93. Córdova LA, Loi F, Lin T, et al. . CCL2, CCL5, and IGF-1 participate in the immunomodulation of osteogenesis during M1/M2 transition in vitro. J Biomed Mater Res A. 2017;105(11):3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang T, Wang Y, Menendez A, et al. . Osteoblast-specific loss of IGF1R signaling results in impaired endochondral bone formation during fracture healing. J Bone Miner Res. 2015;30(9):1572-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Blumenfeld I, Srouji S, Lanir Y, Laufer D, Livne E. Enhancement of bone defect healing in old rats by TGF-beta and IGF-1. Exp Gerontol. 2002;37(4):553-565. [DOI] [PubMed] [Google Scholar]
- 96. Fischer V, Haffner-Luntzer M, Amling M, Ignatius A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur Cell Mater. 2018;35:365-385. [DOI] [PubMed] [Google Scholar]
- 97. Doepfner W. Consequences of calcium and-or phosphorus deficient diets on various parameters of callus formation and on growth rate in young rats. Br J Pharmacol. 1970;39(1):188P-189P. [PMC free article] [PubMed] [Google Scholar]
- 98. Namkung-Matthai H, Appleyard R, Jansen J, et al. . Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28(1):80-86. [DOI] [PubMed] [Google Scholar]
- 99. Haffner-Luntzer M, Heilmann A, Heidler V, et al. . Hypochlorhydria-induced calcium malabsorption does not affect fracture healing but increases post-traumatic bone loss in the intact skeleton. J Orthop Res. 2016;34(11):1914-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fischer V, Haffner-Luntzer M, Prystaz K, et al. . Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep. 2017;7(1):7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kunutsor SK, Whitehouse MR, Blom AW, Laukkanen JA. Low serum magnesium levels are associated with increased risk of fractures: a long-term prospective cohort study. Eur J Epidemiol. 2017;32(7):593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hayhoe RPG, Lentjes MAH, Luben RN, Khaw KT, Welch AA. Dietary magnesium and potassium intakes and circulating magnesium are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the EPIC-Norfolk cohort study. Am J Clin Nutr. 2015;102(2):376-384. [DOI] [PubMed] [Google Scholar]
- 103. Zhang Y, Xu J, Ruan YC, et al. . Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 2016;22(10):1160-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gorter EA, Hamdy NAT, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing: a systematic review of the literature. Bone. 2014;64:288-297. [DOI] [PubMed] [Google Scholar]
- 105. Chiavarini M, Naldini G, Fabiani R. The role of diet in osteoporotic fracture healing: a systematic review. Curr Osteoporos Rep. 2020;18(3):138-147. [DOI] [PubMed] [Google Scholar]
- 106. Brinker MR, O’Connor DP, Monla YT, Earthman TP. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21(8):557-570. [DOI] [PubMed] [Google Scholar]
- 107. Zura R, Xiong Z, Einhorn T, et al. . Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151(11):e162775. [DOI] [PubMed] [Google Scholar]
- 108. Doetsch AM, Faber J, Lynnerup N, Wätjen I, Bliddal H, Danneskiold-Samsøe B. The effect of calcium and vitamin D3 supplementation on the healing of the proximal humerus fracture: a randomized placebo-controlled study. Calcif Tissue Int. 2004;75(3):183-188. [DOI] [PubMed] [Google Scholar]
- 109. Heyer FL, de Jong JJ, Willems PC, et al. . The effect of bolus vitamin D3 supplementation on distal radius fracture healing: a randomized controlled trial using HR-pQCT. J Bone Miner Res. 2021;36(8):1492-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Martineau C, Kaufmann M, Arabian A, Jones G, St-Arnaud R. Preclinical safety and efficacy of 24R,25-dihydroxyvitamin D3 or lactosylceramide treatment to enhance fracture repair. J Orthop Translat. 2020;23:77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Martineau C, Naja RP, Husseini A, et al. . Optimal bone fracture repair requires 24R,25-dihydroxyvitamin D3 and its effector molecule FAM57B2. J Clin Invest. 2018;128(8):3546-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. Accessed January 17, 2021. https://www.cdc.gov/diabetes/data/statistics-report/index.html [Google Scholar]
- 113. Jiao H, Xiao E, Graves DT. Diabetes and its effect on bone and fracture healing. Curr Osteoporos Rep. 2015;13(5):327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ferrari SL, Abrahamsen B, Napoli N, et al. Bone and Diabetes Working Group of IOF . Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int. 2018;29(12):2585-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Murray CE, Coleman CM. Impact of diabetes mellitus on bone health. Int J Mol Sci. 2019;20(19):4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Moher D, Liberati A, Tetzlaff J, Altman DG;. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the Nurses’ Health Study. Diabetes Care. 2006;29(7):1573-1578. [DOI] [PubMed] [Google Scholar]
- 118. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427-444. [DOI] [PubMed] [Google Scholar]
- 119. Henderson S, Ibe I, Cahill S, Chung YH, Lee FY. Bone quality and fracture-healing in type-1 and type-2 diabetes mellitus. J Bone Joint Surg Am. 2019;101(15):1399-1410. [DOI] [PubMed] [Google Scholar]
- 120. Kayal RA, Tsatsas D, Bauer MA, et al. . Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res. 2007;22(4):560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Alblowi J, Kayal RA, Siqueira M, et al. . High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175(4):1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kayal RA, Alblowi J, McKenzie E, et al. . Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone. 2009;44(2):357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hu Z, Ma C, Liang Y, Zou S, Liu X. Osteoclasts in bone regeneration under type 2 diabetes mellitus. Acta Biomater. 2019;84:402-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mangialardi G, Ferland-McCollough D, Maselli D, et al. . Bone marrow pericyte dysfunction in individuals with type 2 diabetes. Diabetologia. 2019;62(7):1275-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vizoso FJ, Eiro N, Costa L, et al. . Mesenchymal stem cells in homeostasis and systemic diseases: hypothesis, evidences, and therapeutic opportunities. Int J Mol Sci. 2019;20(15):3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dedania J, Borzio R, Paglia D, et al. . Role of local insulin augmentation upon allograft incorporation in a rat femoral defect model. J Orthop Res. 2011;29(1):92-99. [DOI] [PubMed] [Google Scholar]
- 127. Gandhi A, Beam HA, O’Connor JP, Parsons JR, Lin SS. The effects of local insulin delivery on diabetic fracture healing. Bone. 2005;37(4):482-490. [DOI] [PubMed] [Google Scholar]
- 128. Cignachi NP, Ribeiro A, Machado GDB, et al. . Bone regeneration in a mouse model of type 1 diabetes: Influence of sex, vitamin D3, and insulin. Life Sci. 2020;263:118593. [DOI] [PubMed] [Google Scholar]
- 129. Ehrchen J, Steinmüller L, Barczyk K, et al. . Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109(3):1265-1274. [DOI] [PubMed] [Google Scholar]
- 130. Barczyk K, Ehrchen J, Tenbrock K, et al. . Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood. 2010;116(3):446-455. [DOI] [PubMed] [Google Scholar]
- 131. Ahmad M, Hachemi Y, Paxian K, Mengele F, Koenen M, Tuckermann J. A jack of all trades: impact of glucocorticoids on cellular cross-talk in osteoimmunology. Front Immunol. 2019;10:2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huang X, Li Y, Fu M, Xin HB. Polarizing macrophages in vitro. Methods Mol Biol. 2018;1784:119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang H, Shi X, Wang L, et al. . Intramembranous ossification and endochondral ossification are impaired differently between glucocorticoid-induced osteoporosis and estrogen deficiency-induced osteoporosis. Sci Rep. 2018;8(1):3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sandberg OH, Aspenberg P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Joint Res. 2015;4(10):170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Liu YZ, Akhter MP, Gao X, et al. . Glucocorticoid-induced delayed fracture healing and impaired bone biomechanical properties in mice. Clin Interv Aging. 2018;13:1465-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Azetsu Y, Chatani M, Dodo Y, et al. . Treatment with synthetic glucocorticoid impairs bone metabolism, as revealed by in vivo imaging of osteoblasts and osteoclasts in medaka fish. Biomed Pharmacother. 2019;118:109101. [DOI] [PubMed] [Google Scholar]
- 137. Rapp AE, Hachemi Y, Kemmler J, Koenen M, Tuckermann J, Ignatius A. Induced global deletion of glucocorticoid receptor impairs fracture healing. FASEB J. 2018;32(4):2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hachemi Y, Rapp AE, Lee S, et al. . Intact glucocorticoid receptor dimerization is deleterious in trauma-induced impaired fracture healing. Front Immunol. 2021;11:628287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li J, Wang X, Zhou C, et al. . Perioperative glucocorticosteroid treatment delays early healing of a mandible wound by inhibiting osteogenic differentiation. Injury. 2012;43(8):1284-1289. [DOI] [PubMed] [Google Scholar]
- 140. Nikolaou VS, Efstathopoulos N, Kontakis G, Kanakaris NK, Giannoudis PV. The influence of osteoporosis in femoral fracture healing time. Injury. 2009;40(6):663-668. [DOI] [PubMed] [Google Scholar]
- 141. Josephson AM, Bradaschia-Correa V, Lee S, et al. . Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019;116(14):6995-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zura R, Braid-Forbes MJ, Jeray K, et al. . Bone fracture nonunion rate decreases with increasing age: a prospective inception cohort study. Bone. 2017;95:26-32. [DOI] [PubMed] [Google Scholar]
- 143. Mills LA, Aitken SA, Simpson AHRW. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop. 2017;88(4):434-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dailey HL, Wu KA, Wu PS, McQueen MM, Court-Brown CM. Tibial fracture nonunion and time to healing after reamed intramedullary nailing: risk factors based on a single-center review of 1003 patients. J Orthop Trauma. 2018;32(7):e263-e269. [DOI] [PubMed] [Google Scholar]
- 145. Schmal H, Brix M, Bue M, et al. Danish Orthopaedic Trauma Society . Nonunion—consensus from the 4th annual meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020;5(1):46-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Robinson CM, Court-Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. J Bone Joint Surg Am. 2004;86(7):1359-1365. [DOI] [PubMed] [Google Scholar]
- 147. Inose H, Kato T, Ichimura S, et al. . Risk factors of nonunion after acute osteoporotic vertebral fractures: a prospective multicenter cohort study. Spine (Phila Pa 1976). 2020;45(13):895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Makaram NS, Leow JM, Clement ND, et al. . Risk factors associated with delayed and aseptic nonunion following tibial diaphyseal fractures managed with intramedullary nailing. Bone Jt Open. 2021;2(4):227-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Prabhakar P, Wessel L, Nguyen J, Stepan J, Carlson M, Fufa D. Factors associated with scaphoid nonunion following early open reduction and internal fixation. J Wrist Surg. 2020;9(2):141-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tian R, Zheng F, Zhao W, et al. . Prevalence and influencing factors of nonunion in patients with tibial fracture: systematic review and meta-analysis. J Orthop Surg Res. 2020;15:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Taormina DP, Shulman BS, Karia R, Spitzer AB, Konda SR, Egol KA. Older age does not affect healing time and functional outcomes after fracture nonunion surgery. Geriatr Orthop Surg Rehabil. 2014;5(3):116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hao Z, Li J, Li B, et al. . Smoking alters inflammation and skeletal stem and progenitor cell activity during fracture healing in mice. J Bone Miner Res. 2021;36(1):186-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Schmitz MA, Finnegan M, Natarajan R, Champine J. Effect of smoking on tibial shaft fracture healing. Clin Orthop Relat Res. 1999;( 365):184-200. [DOI] [PubMed] [Google Scholar]
- 154. Lopas LA, Belkin NS, Mutyaba PL, Gray CF, Hankenson KD, Ahn J. Fractures in geriatric mice show decreased callus expansion and bone volume. Clin Orthop Relat Res. 2014;472(11):3523-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Farr JN, Khosla S. Cellular senescence in bone. Bone. 2019;121:121-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhu Y, Tchkonia T, Pirtskhalava T, et al. . The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Farr JN, Fraser DG, Wang H, et al. . Identification of senescent cells in the bone microenvironment. J Bone Miner Res. 2016;31(11):1920-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Farr JN, Xu M, Weivoda MM, et al. . Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(11):1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Khosla S, Farr JN, Kirkland JL. Inhibiting cellular senescence: a new therapeutic paradigm for age-related osteoporosis. J Clin Endocrinol Metab. 2018;103(4):1282-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Meyer RA, Tsahakis PJ, Martin DF, Banks DM, Harrow ME, Kiebzak GM. Age and ovariectomy impair both the normalization of mechanical properties and the accretion of mineral by the fracture callus in rats. J Orthop Res. 2001;19(3):428-435. [DOI] [PubMed] [Google Scholar]
- 162. Prisby RD, Ramsey MW, Behnke BJ, et al. . Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res. 2007;22(8):1280-1288. [DOI] [PubMed] [Google Scholar]
- 163. Lim JC, Ko KI, Mattos M, et al. . TNFα contributes to diabetes impaired angiogenesis in fracture healing. Bone. 2017;99:26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wahl EC, Aronson J, Liu L, et al. . Restoration of regenerative osteoblastogenesis in aged mice: modulation of TNF. J Bone Miner Res. 2010;25(1):114-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Raggatt LJ, Wullschleger ME, Alexander KA, et al. . Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 2014;184(12):3192-3204. [DOI] [PubMed] [Google Scholar]
- 166. Sebastián C, Herrero C, Serra M, Lloberas J, Blasco MA, Celada A. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 2009;183(4):2356-2364. [DOI] [PubMed] [Google Scholar]
- 167. Aristorena M, Blanco FJ, de Las Casas-Engel M, et al. . Expression of endoglin isoforms in the myeloid lineage and their role during aging and macrophage polarization. J Cell Sci. 2014;127(Pt 12):2723-2735. [DOI] [PubMed] [Google Scholar]
- 168. Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32(1):18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Slade Shantz JA, Yu YY, Andres W, Miclau T III, Marcucio R. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma. 2014;28(Suppl 1):S10-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Chen L, Cheng S, Sun K, et al. . Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet Disord. 2021;22:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Vi L, Baht GS, Soderblom EJ, et al. . Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice. Nat Commun. 2018;9:5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Németh K, Leelahavanichkul A, Yuen PST, et al. . Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187-195. [DOI] [PubMed] [Google Scholar]
- 174. Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and ‘garb-aging.’ Trends Endocrinol Metab. 2017;28(3):199-212. [DOI] [PubMed] [Google Scholar]
- 175. Elder SS, Emmerson E. Senescent cells and macrophages: key players for regeneration? Open Biol. 2020;10(12):200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hall BM, Balan V, Gleiberman AS, et al. . Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY). 2016;8(7):1294-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56(2):123-129. [DOI] [PubMed] [Google Scholar]
- 178. Chen TL. Inhibition of growth and differentiation of osteoprogenitors in mouse bone marrow stromal cell cultures by increased donor age and glucocorticoid treatment. Bone. 2004;35(1):83-95. [DOI] [PubMed] [Google Scholar]
- 179. Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19(1):117-125. [DOI] [PubMed] [Google Scholar]
- 180. Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells. 2004;22(5):675-682. [DOI] [PubMed] [Google Scholar]
- 181. O’Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19(1):95-103. [DOI] [PubMed] [Google Scholar]
- 182. Shibata KR, Aoyama T, Shima Y, et al. . Expression of the p16INK4A gene is associated closely with senescence of human mesenchymal stem cells and is potentially silenced by DNA methylation during in vitro expansion. Stem Cells. 2007;25(9):2371-2382. [DOI] [PubMed] [Google Scholar]
- 183. Zhou S, Greenberger JS, Epperly MW, et al. . Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Ono T, Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteoporos Rep. 2017;15(4):367-375. [DOI] [PubMed] [Google Scholar]
- 185. Guder C, Gravius S, Burger C, Wirtz DC, Schildberg FA. Osteoimmunology: a current update of the interplay between bone and the immune system. Front Immunol. 2020;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kovach TK, Dighe AS, Lobo PI, Cui Q. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res. 2015;2015:752510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Dighe AS, Yang S, Madhu V, Balian G, Cui Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res. 2013;31(2):227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Reinke S, Geissler S, Taylor WR, et al. . Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5(177):177ra36. [DOI] [PubMed] [Google Scholar]
- 189. Wheatley BM, Nappo KE, Christensen DL, Holman AM, Brooks DI, Potter BK. Effect of NSAIDs on bone healing rates: a meta-analysis. J Am Acad Orthop Surg. 2019;27(7):e330-e336. [DOI] [PubMed] [Google Scholar]
- 190. Al-Waeli H, Reboucas AP, Mansour A, Morris M, Tamimi F, Nicolau B. Non-steroidal anti-inflammatory drugs and bone healing in animal models-a systematic review and meta-analysis. Syst Rev. 2021;10:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Saul D, Weber M, Zimmermann MH, et al. . Effect of the lipoxygenase inhibitor baicalein on bone tissue and bone healing in ovariectomized rats. Nutr Metab (Lond). 2019;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Marquez-Lara A, Hutchinson ID, Nuñez F Jr, Smith TL, Miller AN. Nonsteroidal anti-inflammatory drugs and bone-healing: a systematic review of research quality. JBJS Rev. 2016;4(3):e4. [DOI] [PubMed] [Google Scholar]
- 193. Franceschi C, Bonafè M, Valensin S, et al. . Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244-254. [DOI] [PubMed] [Google Scholar]
- 194. Biguetti CC, Rondina Couto MC, Rodrigues Silva AC, et al. . New surgical model for bone-muscle injury reveals age and gender-related healing patterns in the 5 lipoxygenase (5LO) knockout mouse. Front Endocrinol (Lausanne). 2020;11:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Saul D, Ninkovic M, Komrakova M, et al. . Effect of zileuton on osteoporotic bone and its healing, expression of bone, and brain genes in rats. J Appl Physiol. 2018;124(1):118-130. [DOI] [PubMed] [Google Scholar]
- 196. Cottrell JA, O’Connor JP. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J Bone Joint Surg Am. 2009;91(11):2653-2665. [DOI] [PubMed] [Google Scholar]
- 197. Cossarizza A, Ortolani C, Paganelli R, et al. . CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86(3):173-195. [DOI] [PubMed] [Google Scholar]
- 198. Melton DW, Roberts AC, Wang H, et al. . Absence of CCR2 results in an inflammaging environment in young mice with age-independent impairments in muscle regeneration. J Leukoc Biol. 2016;100(5):1011-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Xing Z, Lu C, Hu D, Miclau T III, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28(8):1000-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sun G, Wang Y, Ti Y, Wang J, Zhao J, Qian H. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin Exp Pharmacol Physiol. 2017;44(4):455-462. [DOI] [PubMed] [Google Scholar]
- 201. Lu C, Miclau T, Hu D, et al. . Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23(6):1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Naik AA, Xie C, Zuscik MJ, et al. . Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009;24(2):251-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Janssen MP, Caron MM, van Rietbergen B, et al. . Impairment of the chondrogenic phase of endochondral ossification in vivo by inhibition of cyclooxygenase-2. Eur Cell Mater. 2017;34:202-216. [DOI] [PubMed] [Google Scholar]
- 204. Li M, Thompson DD, Paralkar VM. Prostaglandin E2 receptors in bone formation. Int Orthop. 2007;31(6):767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe GH. Aging, osteocytes, and mechanotransduction. Curr Osteoporos Rep. 2017;15(5):401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Kousteni S, Chen JR, Bellido T, et al. . Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298(5594):843-846. [DOI] [PubMed] [Google Scholar]
- 208. Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104(10):1363-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128-3135. [DOI] [PubMed] [Google Scholar]
- 210. Kaplon H, Muralidharan M, Schneider Z, Reichert JM. Antibodies to watch in 2020. MAbs. 2020;12(1):1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Bhandari M, Schemitsch EH, Karachalios T, et al. . Romosozumab in skeletally mature adults with a fresh unilateral tibial diaphyseal fracture: a randomized phase-2 study. J Bone Joint Surg Am. 2020;102(16):1416-1426. [DOI] [PubMed] [Google Scholar]
- 212. Schemitsch EH, Miclau T, Karachalios T, et al. . A randomized, placebo-controlled study of romosozumab for the treatment of hip fractures. J Bone Joint Surg Am. 2020;102(8):693-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Suen PK, Zhu TY, Chow DHK, Le H, Zheng LZ, Qin L. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength of intact bones in adult male rats. Sci Rep. 2015;5:15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Morse A, Yu NYC, Peacock L, et al. . Endochondral fracture healing with external fixation in the Sost knockout mouse results in earlier fibrocartilage callus removal and increased bone volume fraction and strength. Bone. 2015;71:155-163. [DOI] [PubMed] [Google Scholar]
- 215. Liu Y, Rui Y, Cheng TY, et al. . Effects of sclerostin antibody on the healing of femoral fractures in ovariectomised rats. Calcif Tissue Int. 2016;98(3):263-274. [DOI] [PubMed] [Google Scholar]
- 216. McDonald MM, Morse A, Mikulec K, et al. . Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res. 2012;30(10):1541-1548. [DOI] [PubMed] [Google Scholar]
- 217. Piemontese M, Onal M, Xiong J, et al. . Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Sci Rep. 2016;6:24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Onal M, Piemontese M, Xiong J, et al. . Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem. 2013;288(24):17432-17440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Histing T, Kuntz S, Stenger D, et al. . Delayed fracture healing in aged senescence-accelerated P6 mice. J Invest Surg. 2013;26(1):30-35. [DOI] [PubMed] [Google Scholar]
- 220. Batoon L, Millard SM, Raggatt LJ, Pettit AR. Osteomacs and bone regeneration. Curr Osteoporos Rep. 2017;15(4):385-395. [DOI] [PubMed] [Google Scholar]
- 221. Cenci S, Toraldo G, Weitzmann MN, et al. . Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci U S A. 2003;100(18):10405-10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72-76. [DOI] [PubMed] [Google Scholar]
- 223. Kowald A, Passos JF, Kirkwood TBL. On the evolution of cellular senescence. Aging Cell. 2020;19(12):e13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Baker DJ, Wijshake T, Tchkonia T, et al. . Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Demaria M, O’Leary MN, Chang J, et al. . Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7(2):165-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Nelson G, Wordsworth J, Wang C, et al. . A senescent cell bystander effect: senescence-induced senescence. Aging Cell. 2012;11(2):345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Saul D, Kosinsky RL. Epigenetics of aging and aging-associated diseases. Int J Mol Sci . 2021;22(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Anderson R, Lagnado A, Maggiorani D, et al. . Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38(5):e100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Ogrodnik M, Zhu Y, Langhi LGP, et al. . Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29(5):1061-1077.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. López-Otín C, Kroemer G. Hallmarks of health. Cell. 2021;184(1):33-63. [DOI] [PubMed] [Google Scholar]
- 233. He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Karin O, Agrawal A, Porat Z, Krizhanovsky V, Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat Commun. 2019;10:5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Demaria M, Ohtani N, Youssef SA, et al. . An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Ritschka B, Storer M, Mas A, et al. . The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017;31(2):172-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Gal H, Lysenko M, Stroganov S, et al. . Molecular pathways of senescence regulate placental structure and function. EMBO J. 2019;38(18):e100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Gibaja A, Aburto MR, Pulido S, et al. . TGFβ2-induced senescence during early inner ear development. Sci Rep. 2019;9:5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Yun MH, Davaapil H, Brockes JP. Recurrent turnover of senescent cells during regeneration of a complex structure. Elife. 2015;4:e05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell. 2018;17(2):e12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Kale A, Sharma A, Stolzing A, Desprez PY, Campisi J. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing. 2020;17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev. 2018;43:17-25. [DOI] [PubMed] [Google Scholar]
- 243. Serrano M. Senescence helps regeneration. Dev Cell. 2014;31(6):671-672. [DOI] [PubMed] [Google Scholar]
- 244. Prata LGPL, Ovsyannikova IG, Tchkonia T, Kirkland JL. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin Immunol. 2018;40:101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Saul D, Monroe DG, Rowsey JL, et al. . Modulation of fracture healing by the transient accumulation of senescent cells. Elife. 2021;10:e69958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Grosse L, Wagner N, Emelyanov A, et al. . Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 2020;32(1):87-99.e6. [DOI] [PubMed] [Google Scholar]
- 247. Xu M, Pirtskhalava T, Farr JN, et al. . Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]