Abstract
All endocrine glands are susceptible to neoplastic growth, yet the health consequences of these neoplasms differ between endocrine tissues. Pituitary neoplasms are highly prevalent and overwhelmingly benign, exhibiting a spectrum of diverse behaviors and impact on health. To understand the clinical biology of these common yet often innocuous neoplasms, we review pituitary physiology and adenoma epidemiology, pathophysiology, behavior, and clinical consequences. The anterior pituitary develops in response to a range of complex brain signals integrating with intrinsic ectodermal cell transcriptional events that together determine gland growth, cell type differentiation, and hormonal production, in turn maintaining optimal endocrine health. Pituitary adenomas occur in 10% of the population; however, the overwhelming majority remain harmless during life. Triggered by somatic or germline mutations, disease-causing adenomas manifest pathogenic mechanisms that disrupt intrapituitary signaling to promote benign cell proliferation associated with chromosomal instability. Cellular senescence acts as a mechanistic buffer protecting against malignant transformation, an extremely rare event. It is estimated that fewer than one-thousandth of all pituitary adenomas cause clinically significant disease. Adenomas variably and adversely affect morbidity and mortality depending on cell type, hormone secretory activity, and growth behavior. For most clinically apparent adenomas, multimodal therapy controlling hormone secretion and adenoma growth lead to improved quality of life and normalized mortality. The clinical biology of pituitary adenomas, and particularly their benign nature, stands in marked contrast to other tumors of the endocrine system, such as thyroid and neuroendocrine tumors.
Keywords: pituitary adenoma, acromegaly, prolactinoma, Cushing’s disease, aggressive pituitary tumor, hypothalamus
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
The anterior pituitary gland is organized during embryonic development into distinct structural and functional networks comprising cell-type specific lineages
Pituitary adenomas are commonly encountered, with most benign and remaining clinically inapparent
Disease-causing adenomas develop from somatic and germline mutations causing unregulated hormone secretion and growth characterized by chromosomal instability and cell senescence
Aggressive behavior is uncommon and malignant transformation a rare exception
Secretory adenomas cause clinical phenotypes (including acromegaly/gigantism, Cushing’s disease, and prolactinomas) determined by the type of excessive hormones secreted
Co-morbidities including mass effects are managed effectively by multimodal therapies
The anterior pituitary gland is composed of highly differentiated oral ectoderm-derived cells that express unique hormonal products largely determined by cell-specific transcription factor(s). Thus, lactotrophs express prolactin (PRL); somatotrophs express growth hormone (GH); corticotrophs express proopiomelanocortin (POMC), the precursor to adrenocorticotropic hormone (ACTH); gonadotrophs express follicle-stimulating hormone (FSH) and luteinizing hormone (LH); and thyrotrophs express thyrotrophin (thyroid-stimulating hormone; TSH). Pituitary adenomas, which are overwhelmingly benign, arise from one (or more) of these cell lineages, or from null cells expressing no discernible gene product (1-3).
Pituitary adenoma biology has long been a subject of fascination and intrigue because of the highly variable spectrum and diversity of behavior exhibited by these neoplasms, ranging from innocuity to malignancy, along with their widely varied impact on health. The rarity of significant endocrine disease arising from pituitary adenomas, despite their very high prevalence, has impeded a better understanding of their natural history. Furthermore, pituitary adenomas are not classified uniformly by pathologists, surgeons, endocrinologists, and radiologists, restraining improved understanding of their treatment and prognosis. Thus, considerable investigation has focused on mechanisms for pituitary adenoma formation, progression, behavior, and clinical consequences.
This comprehensive critical review elucidates the evidence underlying pituitary adenoma biology and natural history, focusing on cell biology, genetics, physiology, classification, and epidemiology, as well as the morbidity and mortality associated with clinical endocrine syndromes in patients harboring pituitary adenomas.
Human Anterior Pituitary Gland
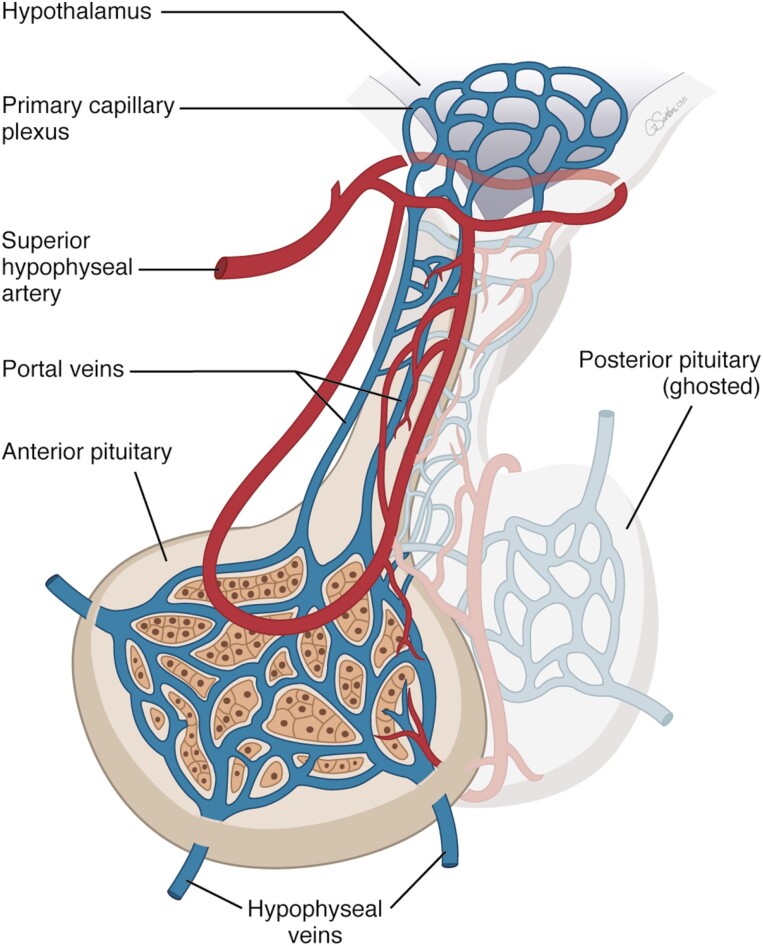
The pituitary comprises anatomically and functionally distinct anterior and posterior lobes. Hypothalamic neuropeptides traverse pituitary stalk portal vessels and signal to cognate pituitary cell surface receptors to induce or suppress systemic release of pituitary hormones, which elicit peripheral tissue endocrine and trophic effects.
Development
Several lines of evidence are consistent with the existence of pituitary stem cells, including identification of non-hormone-secreting, self-renewing primitive cells expressing SOX2 that exhibit differentiating capacity into hormone-secreting cell lineages (4-6) with subsequent persistent but slow postnatal proliferation (7, 8). Thus, mature hormone-secreting cells respond to physiological demands (9), enabling healthy developmental function.
Cell-specific terminal differentiation
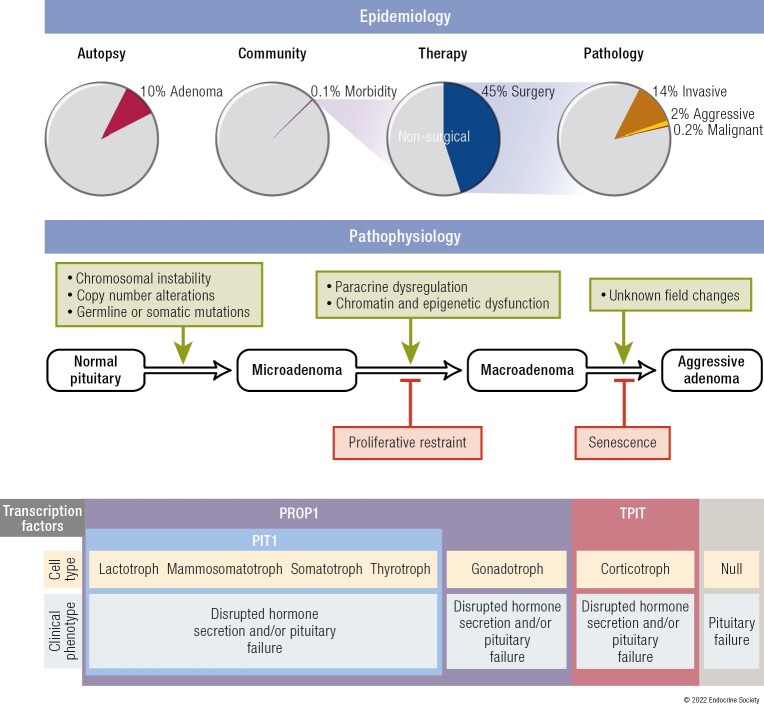
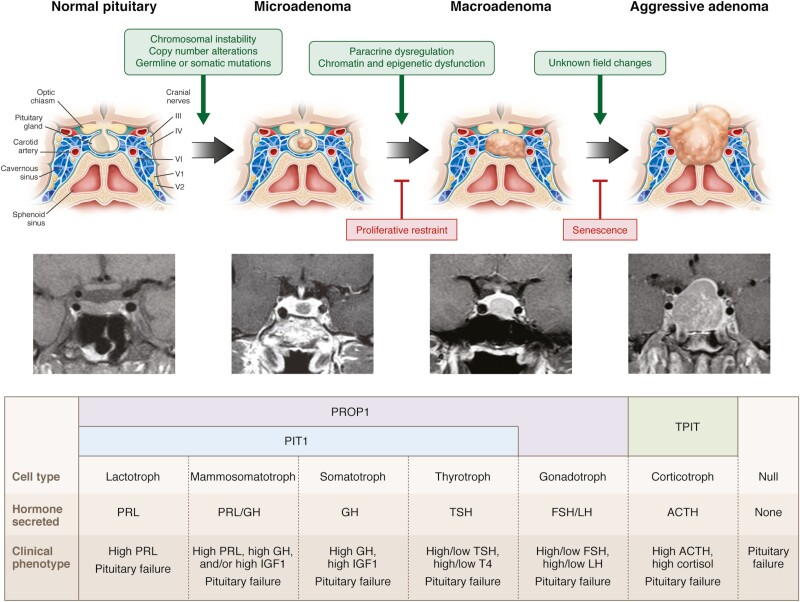
Embryonic cells of ectodermal origin derived from Rathke’s pouch follow temporally regulated and lineage-specific pathways to form distinctive terminally differentiated hormone-producing cells. Lineage differentiation is determined by expression of cell type-specific factors, and cell specification and proliferation are enabled by a finely balanced cascade of transcription and soluble factors (10-12) as reflected by PROP1 induction (13).
In turn, PROP1 induces expression of another transcription factor, PIT1 (also termed POU1F1), which determines lineage development of somatotrophs, lactotrophs, and thyrotrophs (14). Estrogen receptors abundantly expressed in PIT1-expressing cells favor PRL whereas thyrotroph embryonic factor (TEF) and GATA1 induce TSH expression. Gonadotroph development is driven by cell-specific expression of steroidogenic factor (SF1) and dosage-sensitive sex reversal, adrenal hypoplasia critical region (DAX1). Corticotrophs, expressing the ACTH precursor POMC, require T-box family member TBX19 (TPIT). Inactivating mutations of these factors may cause pituitary hormone deficit(s). Pioneer transcription factors that directly bind condensed chromatin also specify differentiation and may reflect cooperation between nuclear and nonnuclear determinants of pituitary cell hormone specificity (15).
Pituitary Cell Proliferation
Several lines of evidence point to a niche of adenoma progenitor cells as observed in the postnatal murine pituitary, where early stem cell–like progenitor cells may differentiate into hormone-synthesizing pituitary cells (16, 17). The role of progenitor cells in adenoma cytogenesis is exemplified by lineage-tracing of murine PAX7, a downstream nestin marker, also expressed in human corticotroph adenomas (18).
Although turnover of the mature pituitary cell is slow, the gland exhibits a plastic response to extrinsic stimuli (19). The pituitary gland enlarges during puberty, pregnancy, and in the setting of peripheral target gland failure. For example, longstanding thyroid failure results in thyrotroph hyperplasia (20), as low thyroxine levels drive thyrotrophin-releasing hormone (TRH) to enable thyrotroph proliferation. By contrast, lactotroph cell hyperplasia during pregnancy occurs mostly due to elevated estrogen levels, which directly stimulates the lactotrophs (21, 22).
Pituitary adenomas arise from hormone-secreting cell types with resultant clinical phenotypes determined by the cell of origin and specific overproduced hormone. Thus, lactotroph adenomas cause infertility and lactation, somatotroph adenomas lead to acromegaly/gigantism, corticotroph adenomas to hypercortisolism with Cushing’s disease, and thyrotroph adenomas to hyperthyroidism and goiter. Adenomas arising from gonadotroph cells are usually nonsecreting, and commonly present with hypogonadism (23, 24) (Fig. 1). Null cell adenomas may arise from a primitive precursor or from loss of lineage-specific tumorigenic factors.
Figure 1.
Pathogenesis of pituitary tumors. Pituitary adenomas arise from a differentiated hormone-expressing cell or from a null cell. Clinical phenotype is determined by the cell of origin and the presence or absence of autonomous, specific hormone hypersecretion.
Physiology of the Hypothalamic-Pituitary Axis
The pituitary gland responds to hypothalamic neuropeptides as well as hormonal signals from target organs. Hypothalamic control is mediated by adenohypophysiotrophic hormones secreted into the hypothalamic portal system and binding to anterior pituitary cell surface receptors (Fig. 2). These G protein coupled cell surface membrane receptors (GPCRs) expressed on pituitary cells are highly selective and specific for each of the hypothalamic hormones and elicit positive or negative signals to mediate specific pituitary hormone production. Hypothalamic neuropeptides expand committed progenitors during normal development and sustain proliferation of mature hormone-secreting cells.
Figure 2.
Hypothalamic-pituitary vascular and functional relationships.
Prader-Willi syndrome serves as a model of hypothalamic dysfunction and highlights the critical role of the hypothalamus in regulating pituitary function. It is a rare genetic neurodevelopmental disorder resulting from the loss of expression of maternally imprinted genes located in the paternal chromosomal region 15q11-13, characterized by cognitive disabilities, behavioral disorders, and hypothalamic dysfunction (25). Impaired pituitary development and function is increasingly recognized as the consequence of much of the phenotype of Prader-Willi syndrome. Pituitary hypoplasia occurs in 63% to 74% of patients, and GH deficiency, hypogonadism, hypothyroidism, ACTH deficiency, and premature adrenarche and/or precocious puberty are all observed.
Lactotroph Regulation
PRL is synthesized in randomly distributed acidophilic lactotrophs, which comprise about 20% of pituitary cells. PRL is weakly homologous to GH and is under tonic hypothalamic dopaminergic inhibition. Lactotrophs and somatotrophs are derived from a common progenitor (26) that may give rise to a tumor that secretes both PRL and GH. On histology, cytoplasmic PRL secretory granules may be densely packed or appear as clusters. Estrogen causes lactotroph cell hyperplasia, which occurs transiently during pregnancy. It is yet unclear whether estrogen pharmacotherapy causes prolactinoma formation or induces growth of preexisting adenomas. Prolactinomas are the most common type of pituitary adenoma, and incidence rates are considerably higher in women (discussed below). However, neither oral contraceptives, estrogen replacement, nor multiple pregnancies are linked to prolactinoma formation (27). Although prolactinomas have been reported after long-term high-dose estrogen therapy in transgender women, no increased risk has been reported in retrospective cohort studies (28, 29).
PRL secretion is under tonic inhibitory control by dopamine, produced by tubero-infundibular dopamine (TIDA) neurons in the dorsomedial arcuate nucleus of the hypothalamus. Dopamine reaches the lactotrophs via the hypothalamic-pituitary portal circulation and binds to lactotroph type 2 dopamine (D2) receptors to inhibit PRL secretion. PRL, in turn, participates in negative feedback to control its own release by increasing tyrosine hydroxylase activity, and thereby dopamine synthesis, in TIDA neurons. In PRL-deficient mice, dopamine is decreased in the median eminence, while mice lacking the D2 receptor develop hyperprolactinemia and lactotroph proliferation (30, 31). Dopamine D2 receptors signal through Gαi, and resultant inhibitory effects on adenylyl cyclase-mediated cellular transduction pathways suppress PRL secretion and lactotroph proliferation. These actions have been leveraged for development of dopamine receptor agonists such as bromocriptine and cabergoline for treatment of lactotroph tumors (32). While loss of Prlr leads to large pituitary tumors in mice, homozygous loss-of-function PRLR mutations in a human patient with hyperprolactinemia and inability to lactate was not associated with a pituitary tumor (33). Rather, physiologic, pharmacologic, or pathologic alterations in dopamine availability or action disrupt PRL regulation. Thus, PRL hypersecretion occurs with use of dopamine antagonists, as well as when the hypophyseal-portal system is disrupted by compression or stalk damage, regardless of mass etiology (34). As discussed below, PRL levels are elevated (~10-fold) during pregnancy, and PRL induces and maintains lactation, even while suppressing reproductive function.
Somatotroph Regulation
GH is the most abundant anterior pituitary hormone. Acidophilic somatotrophs constitute ~50% of pituitary cells, localized mainly in the lateral wings and containing prominent cytoplasmic secretory GH granules (23, 35). The pituitary GH gene (hGH-N) encodes a 22-kDa GH and a less abundant 20-kDa GH (36). Hypothalamic growth hormone–releasing hormone (GHRH) stimulates synthesis and secretion of GH (37) while somatostatin inhibits GH secretion (38). GH secretion is also stimulated by ghrelin, which is synthesized predominantly in the gastrointestinal tract. Somatostatin (SST) binds SST2 and SST5 receptor subtypes to preferentially signal to suppress GH (and TSH). Insulin-like growth factor 1 (IGF-1), the peripheral target hormone induced by GH, mediates many growth-promoting effects of GH and also feeds back negatively to suppress GH (39). Integrated effects of these complex neurogenic influences determine the final secretory pattern of GH production.
GHRH released by the hypothalamus interacts with its receptor, GHRHR, on the somatotroph cell membrane to increase activation of adenylyl cyclase through Gαs, leading to increased cyclic adenosine monophosphate (cAMP) production and activation of GH expression and cell proliferation. Overexpression of Ghrh in mice results in pituitary adenomas secreting excessive amounts of GH, and these effects are also seen with ectopic GHRH-secreting tumors in humans (40, 41). Induced cAMP pathway occurs with activating somatic mutations of Gαs-encoding GNAS, seen in up to 40% of somatotroph adenomas as well as in the McCune-Albright syndrome, and in the presence of increased protein kinase A activity, due either to loss of the inhibitory action of the regulatory subunit PRKAR1A or to increased PRKACB catalytic subunit activity as seen in Carney complex (42). GPR101, an orphan GPCR that couples to Gs and Gq/11, leads to increased cAMP and stimulation of GH secretion. Accordingly, germline or somatic GPR101 microduplication on chromosome Xq26.3 results in X-linked acrogigantism (X-LAG), a rare condition associated with somatotroph adenoma development and early-onset gigantism (43). These and other familial and inherited disorders are discussed below.
Ghrelin is a 28-amino acid peptide that binds the GH secretagogue receptor (44) to stimulate pituitary GH release, an action potentiated with GHRH, which acts as an allosteric co-agonist for the ghrelin receptor. Hypothalamic ghrelin exerts a range of central actions on appetite and metabolism (45-47), but a role in pituitary tumorigenesis has not been defined.
Somatostatin acts on pituitary SST2 and SST5 receptors to signal predominantly via inhibitory Gαi pathways, leading to inhibition of adenylyl cyclase as well as effects on potassium and calcium ion channels, culminating in reduced GH secretion and decreased somatotroph proliferation. These properties have been applied for therapeutic intervention with development of somatostatin receptor ligands (SRLs) (48).
Corticotroph Regulation
Basophilic ACTH-secreting corticotroph cells constitute ~20% of pituitary cells. They are located mainly in the central median wedge and contain abundant cytoplasmic neurosecretory granules, often with perinuclear vacuoles. They express POMC, which gives rise to ACTH as well as other products, including β-lipotrophin, endorphins, and enkephalins. Pituitary POMC gene transcription is primarily under positive regulation by corticotrophin-releasing hormone (CRH) and negative regulation by glucocorticoids. Vasopressin, cytokines, catecholamines, and vasoactive intestinal polypeptide activate pituitary corticotroph POMC gene expression while somatostatin and atrial natriuretic peptide inhibit its expression (49, 50). POMC gene expression is regulated differently in extrapituitary tissues than in the pituitary (51).
The CRH type 1 receptor is predominantly expressed on the corticotroph, and receptor activation increases cAMP, protein kinase A, and CREB induction to the promoter, leading to POMC transcription. Vasopressin is co-secreted with CRH and potentiates CRH action, as do β-adrenergic catecholamines, to enhance POMC transcription and ACTH production. Normal pituitary corticotrophs also express somatostatin SST2 and SST5 receptors, and somatostatin inhibits ACTH secretion, albeit in a glucocorticoid-sensitive manner (52). Dopamine receptors have not been characterized in normal human corticotrophs, although they are highly expressed in a subset of human corticotroph adenomas (53).
The hypothalamic-corticotroph-adrenal axis maintains overall cell homeostasis and transduces neuroendocrine stress responses by integrating peripheral and central signals, resulting in appropriate adrenal steroidogenesis. Responses to stressors, including pain, infection, inflammation, hemorrhage, hypovolemia, trauma, psychological stress, hypoglycemia, and critical illness, are mediated mostly by CRH, but also involve vasovagal, catecholamine, and cytokine activation (50, 54).
Gonadotroph Regulation
Basophilic gonadotrophs, comprising up to 10% of pituitary cells, are mainly located centrally and laterally and express FSH and/or LH-β-subunits within the cell. The secreted glycoprotein hormones FSH and LH comprise a common α-subunit as well as a unique β-subunit that confers hormone specificity (55). Hypothalamic gonadotrophin-releasing hormone (GnRH) regulates both pulsatile LH and FSH secretion, and determines reproductive cycles. Kisspeptin and activins also induce LH/FSH, while inhibins suppress their secretion (56), and FSH and LH regulate germ cell development and maturation and sex steroid synthesis. Primary gonadal failure is associated with gonadotroph hyperplasia, reflecting loss of feedback suppression by sex steroids.
Hypothalamic GnRH neurons are pivotal integrators of central and peripheral signals in regulating the pituitary-gonadal axis. Neurotransmitters that directly or indirectly modulate GnRH secretion include norepinephrine, dopamine, serotonin, γ-aminobutyric acid (GABA), glutamate, opiates, neuropeptide Y (NPY), and galanin. Glutamate and norepinephrine generally provide stimulatory drive, whereas GABA and opioid peptides are inhibitory. Kisspeptins, encoded by the KISS1 gene, and their cognate receptor, KISS1R, are key GnRH secretagogues (57-59). Neurokinin B, a member of the substance P–related tachykinin family, is co-expressed with kisspeptin in the hypothalamus and appears to act through control of kisspeptin secretion to modulate GnRH release. Indeed, hyperprolactinemia suppression of gonadotrophins is mediated at the level of kisspeptin neurons (60, 61). Substance P also modulates GnRH secretion. Leptin, a product of peripheral adipose tissue, is a positive regulator of the hypothalamic-pituitary-gonadal axis. This adipokine enables a pivotal link between body fat and reproduction, signaling energy availability centrally. Hypothalamic GnRH secretion is pulsatile, resulting in episodic gonadotroph stimulation. Thus, in patients with GnRH deficiency, restoration of gonadotrophin secretion can be achieved after exogenous pulsatile GnRH treatment, whereas continuous GnRH exposure suppresses gonadotrophin secretion. Although GnRH is trophic to gonadotrophins, there is no clear evidence for a role of GnRH in the pathogenesis of gonadotroph adenomas.
Thyrotroph Regulation
Basophilic thyrotrophs constitute approximately 5% of the pituitary cell population, located mainly in the antero-medial portion of the gland. Hypothalamic TRH induces TSH production, visible as granular deposits. TRH also induces PRL secretion, likely explaining the hyperprolactinemia typically observed with hypothyroidism. Thyroid hormones, dopamine, somatostatin, and glucocorticoids suppress TSH by overriding central TRH induction, while thyrotroph proliferation and TSH secretion are both unrestrained when negative feedback suppression by low thyroid hormone is removed (62).
Transcription of genes encoding the α and β TSH subunits is induced by TRH and suppressed by dopamine. Hypothalamic TRH neurons centrally regulate the hypothalamic-pituitary-thyroid axis setpoint by regulating pituitary TSH release. Hypothalamic TRH synthesis is, in turn, regulated primarily by thyroid hormones. Neuronal groups mediating other physiologic stimuli include adrenergic medullary input, which mediates stimulatory effects of cold exposure on the TRH neuron. TRH neurons also receive projections from 2 leptin-responsive neuronal populations that regulate energy homeostasis. POMC neurons, which promote weight loss, activate TRH neurons, while NPY/agouti-related protein (AGRP) neurons, which promote weight gain, inhibit TRH neurons. Fasting reduces TRH expression, which is mediated by suppression of POMC and stimulation of NPY/AGRP (63). Postnatal thyrotroph expansion is blocked in mice with disrupted Trh, illustrating the trophic effects of TRH on thyrotrophs.
Nonhormonal Cells
The pituitary contains a mixed population of nonhormonal supporting cells scattered throughout the gland as well as cells involved in autoimmune mechanisms (64, 65). These include folliculostellate cells (66), primitive undifferentiated null cells (67), and immune lymphocytes and macrophages (68), all of which may express intrapituitary cytokines that regulate pituitary function and contribute to tumorigenesis (50, 69).
Regulation of Pituitary Hormone Secretion
Central signals transduced by the pituitary to effect peripheral endocrine chemical messaging reflect a net consolidation of qualitative, temporal, and quantitative pathways. Pituitary hormone production requires integrated central control of hypothalamic neuropeptides, intrapituitary paracrine and autocrine signals, and target gland hormone feedback to generate uniquely timed and sized secretory hormone pulses to optimize peripheral hormone actions. In turn, target gland functions require timed pulses at each level, generating secretory profiles unique to each pituitary axis to effect peripheral tissue function in an axis-specific manner.
The chronobiology is unique for each axis. GH secretion is characterized by orderly secretory pulses that follow a distinct circadian pattern of predominant nocturnal release triggered by sleep onset, while ACTH exhibits a circadian profile of orderly episodic secretion peaking in the early morning followed by a fall to a later evening nadir. However, pituitary adenomas behave autonomously and do not respond appropriately to central or peripheral feedback signals. This disrupts the homeostatic transduction axis, leading to either endocrine hyperfunction or failure (19).
Pituitary Tumor Classification
Pituitary adenomas are classified by histology, genomics, surgical anatomy, and phenotypic behavior, each reflecting the multidisciplinary impact of their respective clinical biology.
Pathologic Classification
Cell lineage
Historically, histological classification of pituitary adenomas was based on pituitary hormone content as assessed by immunohistochemistry, as well as on the ultrastructural features of the cells. A change made in the fourth edition of the WHO Classification of Tumors of the Pituitary Gland in 2017 was the adaptation of a pituitary adenohypophyseal cell lineage as the main principle for classification (70-72). The 5th edition will include changes in classification of both neuroendocrine and non-neuroendocrine tumors (73), including a discussion of transitional terminology for pituitary neuroendocrine tumors (PitNET) (74, 75), with a goal of aligning disease coding across all neuroendocrine tumors (73, 75). The matter of whether pituitary neoplasms should be termed adenomas or neuroendocrine tumors has been the subject of an international workshop (76). For consistency and conceptual clarity, this review uses the term adenoma to designate neoplasms of pituitary cell origin unless otherwise stated.
Transcription factors are not only essential for cellular differentiation (77, 78) but also are meaningful for clinico-pathological practice due to their dependable expression in human pituitary tissues. PIT1 leads to differentiation of mammosomatotrophs, somatotrophs, lactotrophs, and thyrotrophs; TPIT drives the POMC lineage with differentiation of corticotrophs; and SF1 regulates gonadotroph cell differentiation (77-79). Accordingly, tumors are categorized into 4 large groups:
PIT1 lineage tumors encompass somatotroph, lactotroph, and thyrotroph adenomas and their several histological variants, as well as adenomas that may secrete/express 2 or more hormones, including mammosomatotroph and mixed somatotroph-lactotroph adenomas that secrete/express GH and PRL and rare plurihormonal adenomas that secrete/express GH, PRL, and TSH-β.
TPIT lineage tumors encompass corticotroph adenomas and its variants, including the common densely granulated corticotroph adenoma, the rare sparsely granulated corticotroph adenoma, and the Crooke’s cell adenoma, considered a high-risk tumor (discussed below).
SF1 lineage tumors encompass gonadotroph adenomas that may express the glycoprotein hormones FSH-β, LH-β, and α subunit in variable combinations, or may express only the SF1 transcription factor with minimal or no hormonal expression.
Adenomas without a distinct cell lineage differentiation include null cell adenomas and rare unclassified plurihormonal tumors with variable lineage combinations.
Details of the cell lineage family of tumors and variants are shown in Table 1. Adenomas in each of these categories may present clinically with evidence of hormone excess, that is, as hormone-secreting tumors, or as nonsecreting tumors. Immunohistochemistry directed toward GH, PRL, TSH-β, ACTH, FSH-β, LH-β, and, if possible, alpha-subunit of glycoproteins (αSU) is required for pathologic characterization. The application of the transcription factors PIT1, TPIT, and SF1 immunostaining complements characterization, particularly if a tumor is not classifiable by pituitary hormones alone. Immunohistochemical assessment of pituitary transcription factors is, however, critical in specific situations, including:
Table 1.
Pathologic classification of pituitary adenomas
| Lineage | Type | Morphological variants | Hormone and cytokeratin staining | Transcription factors |
|---|---|---|---|---|
| PIT1 | Lactotroph | Sparsely granulated | PRL | PIT1, ERα |
| Densely granulated | PRL | PIT1, ERα | ||
| Acidophilic stem cell | PRL, GH (focal and variable) | PIT1, ERα | ||
| Somatotroph | Densely granulated | GH ± αSU CK perinuclear staining |
PIT1 | |
| Sparsely granulated | GH CK highlights fibrous bodies |
PIT1 | ||
| Dual hormonal | Mammosomatotroph | GH + PRL (in same cells) ± αSU | PIT1, ERα | |
| Mixed somatotroph-lactotroph | GH + PRL (in different cells) ± αSU | PIT1, ERα | ||
| Thyrotroph | TSH-β, αSU | PIT1 | ||
| Plurihormonal | Immature PIT1 lineage | GH, PRL, TSH-β ± αSU (all focal) | PIT1 | |
| Mature PIT1 lineage | GH (predominant), PRL, TSH-β ± αSU | |||
| TPIT | Corticotroph | Densely granulated | ACTH | TPIT |
| Sparsely granulated | ACTH | TPIT | ||
| Crooke’s cell | ACTH CK forming ring-like appearance |
TPIT | ||
| SF1 | Gonadotroph | FSH-β, LH-β, αSU (various combinations) | SF1, GATA3, ERα | |
| No distinct lineage | Null cell | None or focal αSU | None | |
| Plurihormonal | Adenomas with unusual immunohistochemical combinations | Various combinations: ACTH/GH, ACTH/PRL | Unknown |
Abbreviations: αSU, alpha-subunit of glycoprotein hormones; ACTH, adrenocorticotropic hormone; CK, cytokeratin; ERα, estrogen receptor α; FSH, follicle-stimulating hormone; GH, growth hormone; LH, luteinizing hormone; PIT1, POU1F1a transcription factor; macroadenoma; PRL, prolactin; SF1, steroidogenic factor 1; TPIT, T-box family member TBX19; TSH, thyrotrophin (thyroid-stimulating hormone).
When an adenoma is not classifiable by hormone immunostains alone due to either focal/weak hormonal staining or staining for multiple hormones from different cell lineages;
When establishing the diagnosis of a null cell adenoma, now classified as a tumor immunonegative for pituitary hormones and transcription factors; and
When the presence of a pituitary transcription factor is inherent to a tumor definition, for example, plurihormonal PIT1-lineage adenomas.
Immunohistochemistry stains for other cofactors (estrogen receptor α [ERα], GATA3) and cellular components (cytokeratin) are helpful for subclassification of variants and subtypes (Table 1). With the combination of morphology and immunohistochemical markers, there is minimal necessity for ultrastructural analysis for adenoma classification (Fig. 3).
Figure 3.
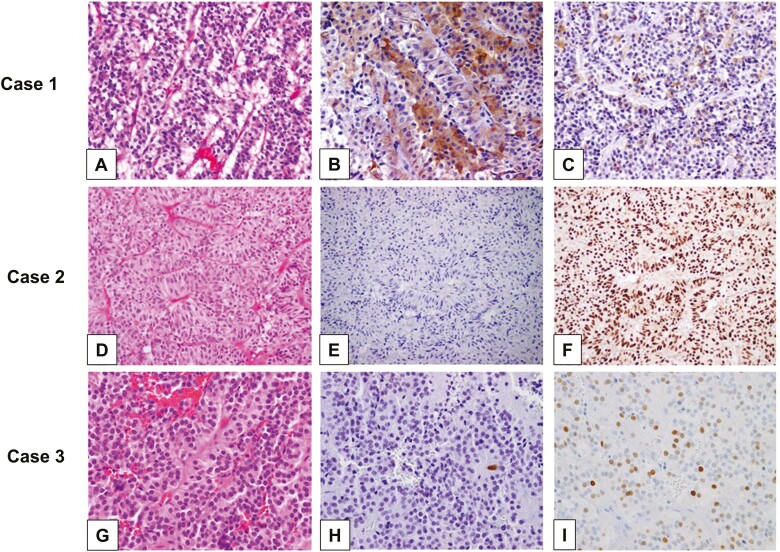
Representative pituitary adenomas classified by immunohistochemistry for pituitary hormones and transcription factors. (A-C, Case 1) (A) A gonadotroph adenoma showing typical chromophobic cells arranged in nests, with trabecular and sinusoidal arrangements. The majority of the gonadotroph adenomas express the gonadotrophins (B) FSH-β and (C) LH-β despite being clinically silent. (A: H&E; B: FSH-β immunohistochemistry [IHC]; C: LH-β IHC; A-C: 40× original magnification). (D-F, Case 2) (D) A gonadotroph adenoma showing typical histological appearance, but (E) completely devoid of gonadotrophin (FSH-β) expression and (F) expressing the gonadotroph-lineage transcription factor SF1. (D: H&E; E: FSH-β IHC; F: SF1 IHC; D-F: 40× original magnification). (G-I, Case 3) (G) A clinically nonsecreting adenoma with chromophobic appearance on H&E, showing (H) rare ACTH-positive cells and (I) multifocal positivity for TPIT, diagnosed as corticotroph adenoma (clinically silent). (G: H&E; H: ACTH IHC; I: TPIT IHC; G-I: 40× original magnification). Note that Case 2 and Case 3 most likely would be diagnosed as null cell adenomas if transcription factors were not considered.
The cell lineage classification is geared to align biological and clinical adenoma classifications more uniformly. For example, application of this classification has resulted in a shift in the reported prevalence of the so-called null cell adenomas due to their previous unclear pathologic classification. Once representing about 20% of all pituitary adenomas in large tumor registries (80) and almost a third of the hormone-negative nonfunctioning tumors (80, 81), null cell adenomas currently represent only 1% to 2% of all pituitary tumors (81-84). This raises the question whether these adenomas really exist or whether they reflect limitations of our diagnostic methodologies for further characterization of cell lineage (83, 85). Null cell adenomas classified by their lack of cell lineage differentiation by both pituitary hormone and transcription factor immunoexpression may have a more aggressive clinical behavior than other nonsecreting adenomas (86, 87).
Grading
The vast majority of pituitary adenomas are benign and slow growing, with a very low relapse rate over many years after surgical resection (88, 89). The fourth World Health Organization (WHO) classification grading scheme defined “pituitary adenoma” and “pituitary carcinoma,” with the latter comprising tumors with cerebrospinal and/or systemic metastasis (72). Importantly, it abandoned the “atypical adenoma” terminology, which had been introduced in the third edition, due to the lack of prognostic clinical value for this pathologic diagnosis, even while recognizing that these adenomas may be locally invasive, precluding clinical cure and demonstrating more aggressive clinical behavior (90-92). Morphologic features distinguishing indolent tumors from locally aggressive ones are still unidentified and, currently, no single prognostic parameter can determine the risk of growth or malignant progression (93-95). Evaluation of tumor proliferation (by mitotic count and/or Ki-67 labeling index) and of tumor invasion may be meaningful on an individual basis as both features correlate with more aggressive tumor behavior (96, 97). At this point, there is no significant evidence correlating genetic abnormalities driving invasive and/or metastatic pituitary tumors (98, 99).
Some histologic adenoma variants are recognized as having a more aggressive clinical behavior. These so-called high-risk lesions show proclivity for higher recurrence rates and resistance to standard therapies, and include sparsely granulated somatotroph adenomas, silent corticotroph adenomas, and Crooke’s cell adenomas, defined as corticotroph adenomas harboring larger percentage of cells with Crooke hyaline change characterized by cytoplasmic ring-like cytokeratin expression, as well as immature PIT1-lineage adenomas (100-105). In the upcoming fifth WHO classification, no new tumor grading system is introduced, although a terminology change of pituitary carcinoma to metastatic pituitary neuroendocrine tumor is recommended, in addition to the specific lineage characterization (eg, metastatic lactotroph pituitary neuroendocrine tumor) for tumors with discontinuous spread and distant metastasis (75).
Summary
Pathologic classification of pituitary adenomas is based on histological determination of cell lineage and associated transcription factors. Molecular analyses are not currently integrated into routine diagnosis as clinical correlates of genetic mechanisms underlying the pathogenesis of pituitary adenomas are as yet unclear (see below). Identification of potentially aggressive adenomas should be made on an individual basis by considering the adenoma subtype, proliferative potential, and tumor invasion assessment. Attention should be given to recognize “high-risk” tumor variants that have intrinsic substantial risk for recurrence and more adverse clinical behavior.
Genomic Classification
Pangenomic, high-throughput, large-scale omics analyses have been applied to study the transcriptome, miRNome, methylome, chromosomal, and sequence alterations in pituitary adenomas (106-108). Recent studies of large sample sets (ie, > 100) have enabled robust assessment of pituitary adenoma pangenomic profiles, improving understanding of the landscape of genetic and epigenetic alterations and forming the basis for a molecular classification of pituitary adenomas.
Large-scale transcriptome analysis has identified distinct pituitary adenoma groups based on gene expression profiles (99, 109). These groups generally correlate with the fourth WHO classification, but also offer specific insights relevant to clinical practice. For example, 2 corticotroph adenoma subtypes linked to hormonal secretory status have been identified, distinguishing between overt Cushing’s disease and silent corticotroph adenomas that exhibit a gene expression signature closer to that of gonadotroph adenomas. Transcriptome analysis also revealed that mixed lactotroph-somatotroph tumors share a gene expression profile with GH-secreting tumors rather than with pure lactotroph tumors. Gene expression signatures driving this molecular classification have been identified (99) and include increased expression of cell cycle genes in secretory corticotroph tumors vs overexpression of genes associated with oxidative phosphorylation in gonadotroph tumors and overexpression of MYC targets in lactotroph tumors. Furthermore, meta-analysis of microarray data from several studies showed overall dysregulation of differentially expressed genes related to metabolism in pituitary adenomas (109). Differences in gene expression profile between invasive and noninvasive pituitary adenomas have been suggested (110-112).
Pangenomic analysis of epigenetic changes also reveals specific molecular signatures for each group of pituitary adenomas, with the methylation pattern revealing a molecular classification (113-115). Methylation profiles differentiate somatotroph adenomas from gonadotroph and secretory corticotroph adenomas (99). Global hypomethylation is observed in somatotroph adenomas, mainly due to the CpG sites located in low CpG density regions (ie, the “open sea”). Of note, methylation level negatively correlates with cis-expression of key genes. For example, hypomethylation of the GH1 and SST5 gene promoter is associated with their overexpression in somatotroph adenomas; similarly, the POMC gene is hypomethylated in corticotroph adenomas (114).
The miRNome is a determinant of pituitary adenoma molecular classification, with at least 4 different molecular profiles of pituitary adenomas identifiable by miRNome analysis (99). Interestingly a specific cluster of 85 miRNA, known as MEG3, located on chromosome 14q32.2 and associated with somatotroph adenomas (116), is associated with GH secretion and a higher expression of PIT1 and DLK1. A main driving effect of this miRNA cluster in pituitary adenoma differentiation is supported by functional studies (116).
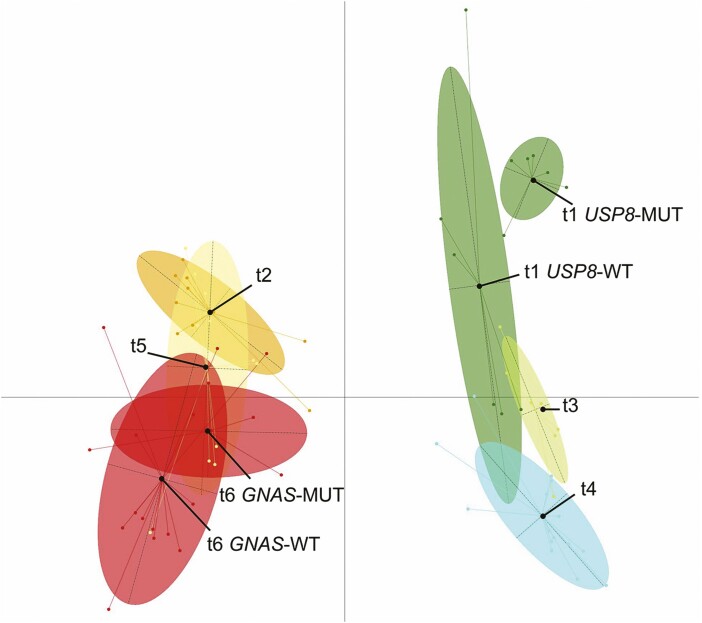
Integration of pangenomic genetic and epigenetic alterations in pituitary adenomas now provides a basis for an informed molecular classification to enable clinical investigation and histological analysis. This classification identifies PIT1 differentiation as the main driver (Fig. 4), while analysis of somatic mutations of GNAS and USP8 combined with transcriptome analysis identifies subgroups that correlate with specific genetic and epigenetic features and clinical/histological characteristics. Pangenomic classification also suggests that gonadotroph adenomas share genomic profiles with silent corticotroph and null cell adenomas (99).
Figure 4.
Pangenomic classification of pituitary adenomas. Multiple factor analysis of the transcriptome, miRNome, methylome, mutations, and chromosomal alterations in a series of 134 adenomas. t1: corticotroph adenomas with or without USP8 somatic mutation, t2: lactotroph adenomas, t3: silent corticotroph adenomas, t4: gonadotroph adenomas, t5: thyrotroph and plurihormonal adenomas, t6: somatotroph adenomas with or without GNAS somatic mutation. Reprinted with permission from Neou et al. (2020) (99).
Surgical Classification
Anatomic considerations
The pattern of pituitary adenoma growth is characterized either by expansion into or infiltration of surrounding parasellar tissues. Slow and expanding growth results in a mass with a well-circumscribed border that exerts increasing pressure on healthy nontumorous tissue and on the bony sella, displacing and compressing normal functional pituitary tissue and surrounding structures. By contrast, infiltrative growth results in penetration, incorporation, and destruction of adjacent tissues, resulting in a mass with poorly defined tumor margins. Initially, individual cells, tumor cell clusters, or tongues of adenoma tissue may infiltrate the dura, affecting bone, sphenoid sinus mucosa, cavernous sinuses, or other structures depending on growth direction. Up to 35% of adenoma types exhibit gross invasion, with macroadenomas showing higher rates (117, 118).
The direction of growth may be superior, inferior, anterior, posterior, or lateral to the sellar fossa, or a combination of patterns. Superior growth is the most common, as the diaphragm sella and its opening are a weak barrier to expansion. Tumors may compress and damage the optic nerves and chiasm; with a postfixed chiasm, the tumor may grow forward to the subfrontal area, whereas with a prefixed chiasm, growth is backward to the third ventricle and hypothalamus. Inferior growth produces sellar remodeling, enlargement, and bone resorption, leaving a free path for sphenoid sinus spread. Infiltrative tumors may directly penetrate the sphenoid bone and clivus, and, with further growth, may extend into the nasopharynx or nasal cavity. Anterior growth encroaches the planum sphenoidale, inferior surfaces of the frontal lobes, and ethmoid sinuses. Posterior growth produces expansion to the interpeduncular cistern and brainstem. Lateral growth may be either by expansion into or infiltration of the cavernous sinuses.
The behavior of pituitary adenomas is evaluated from changes in morphology and the degree of encroachment on regional anatomical structures such as the cavernous sinus. Although the pituitary gland appears to lack a capsule, there are reports the gland may be covered by a thin capsule, or that an adenoma capsule (or pseudocapsule) is simply compressed normal pituitary tissue (119-121). The medial wall of the cavernous sinus bordering the sella varies in structural thickness or defects. Thus, the tumor may invade, invaginate (122, 123), or extend to the cavernous sinus (124, 125).
Surgical classification and outcomes
The simplest way to characterize a pituitary adenoma is according to its size, using a 10-mm cutoff to define micro- vs macroadenoma. Tumors measuring > 40 mm are generally considered giant adenomas (126). No histological differences distinguish micro- from macroadenomas, nor are there morphological features that predict growth. By contrast, as tumor size is a predictor of more favorable surgical outcome, size-based classifications are clinically useful (127).
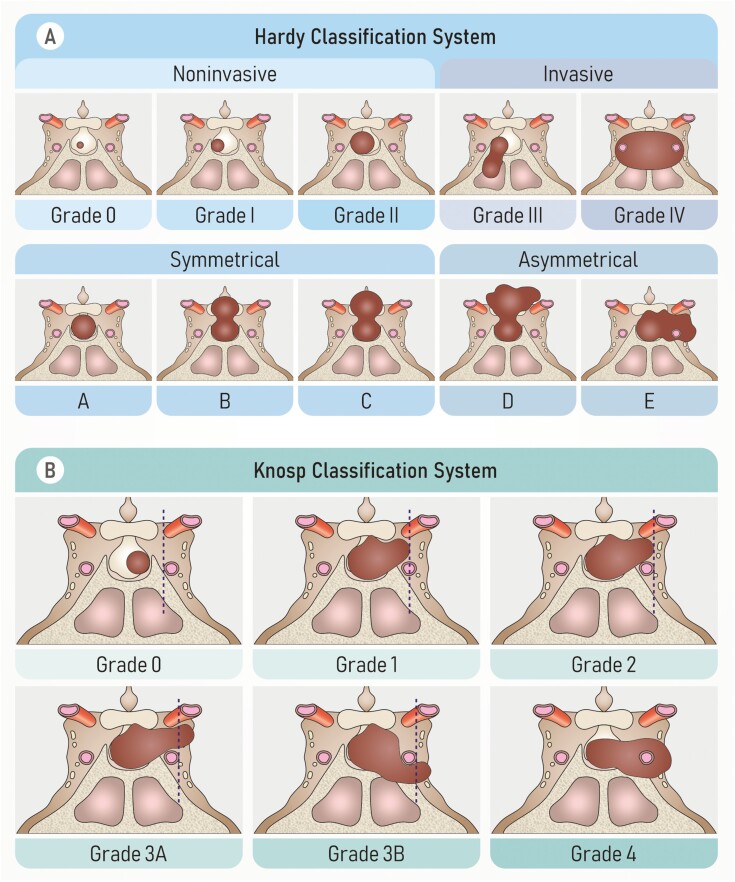
According to the Hardy classification (128), small, intrasellar, symmetrical pituitary tumors are noninvasive, whereas those causing bone destruction are invasive (Fig. 5A). As cavernous sinus involvement considerably limits surgical resection, and preoperative imaging aids in assessing its feasibility, Knosp (129, 130) classified adenomas based on the degree of cavernous sinus involvement, identifying the parasellar internal carotid artery on coronal magnetic resonance imaging (MRI) as a critical imaging landmark to gauge the presence of cavernous sinus invasion (Fig. 5B). This is a widely used classification due to its clarity and simplicity. The Knosp classification of adenomas correlates with surgical outcomes and biochemical remission. Indeed, resection of adenomas that invade the cavernous sinus has a low success rate (130).
Figure 5.
Classification systems used to characterize pituitary adenomas. (A) Hardy classification system. Sella turcica tumors can be noninvasive (grade 0, grade I, grade II), or invasive (grade III, grade IV). Suprasellar tumors can be symmetrical (grade A, grade B, grade C), or asymmetrical (grade D, grade E). (B) Knosp classification system. Grade 0, no cavernous sinus involvement; grades 1 and 2, the tumor invades the medial wall of the cavernous sinus, but does not go beyond a hypothetical line extending between the centers of the 2 segments of the internal carotid artery (grade 1) or it goes beyond such a line, but without passing a line tangent to the lateral margins of the artery itself (grade 2); grade 3A, the tumor extends laterally to the internal carotid artery into the superior cavernous sinus compartment; grade 3B, the tumor extends laterally to the internal carotid artery into the inferior cavernous sinus compartment; grade 4, total encasement of the intracavernous carotid artery. From Di Ieva A et al. (2014) (127).
With advanced understanding of pituitary adenoma pathogenesis and availability of novel medical therapies, anatomic classifications may diminish in practical value. New classifications will consider personalized biomarkers, response to therapy, and patient-centric determinants (105, 131).
Classification Based on Phenotypic Behavior
Although most pituitary adenomas are benign, an aggressive subgroup invade the sphenoid or cavernous sinus, present with multiple recurrences despite surgical or medical treatment, or, very rarely, develop distant metastases.
Invasive pituitary adenomas
Pituitary adenomas invading the sphenoid or cavernous sinus occur in up to 40% of surgical resections (96, 97, 132, 133). As noted above, complete surgical resection is not likely to be achieved when there is tumor invasion of the cavernous sinus, and the presence of residual tumor increases the likelihood of regrowth or of recurrence assessed by MRI from 10%–20% to 25%–50% in a 5-year study of nonsecreting adenomas (134). Nevertheless, despite its negative prognostic impact, invasion was not included in the fourth WHO classification, as intraoperative or histopathological evidence of tissue invasion was considered an imprecise and controversial biomarker (135). Rather, invasion, whether radiological or histological, is included with a cluster of other markers describing clinically aggressive adenomas (136).
Aggressive pituitary adenomas
The term aggressive pituitary tumor has been used variably to describe invasive tumors, giant tumors, and refractory behavior, as there is currently a lack of an agreed definition for these adenomas. In light of these uncertainties, it is not possible to draw conclusions on their epidemiology or to identify predictive markers.
Definition.
The European Society of Endocrinology guidelines define an aggressive pituitary adenoma as a radiologically invasive tumor with an unusually rapid growth rate, or as a tumor presenting with clinically relevant growth despite optimal use of standard medical, surgical, and radiotherapeutic therapies (89). This is largely a clinical definition.
Although aggressive pituitary adenomas are usually macroadenomas at diagnosis, tumor size does not necessarily correlate with aggressive behavior, as exemplified by giant lactotroph tumors that can be quite responsive to medical treatment (137). Moreover, surgical success is not solely determined by tumor size (96, 130, 138).
The prevalence of aggressive pituitary adenomas has been estimated from surgical series. Based on reported percentages of invasive tumors and postoperative recurrences, approximately 2% of pituitary macroadenomas are aggressive (139), with the proportion influenced by tumor type, and higher for secretory tumors.
There is no consensus as to the definition of unusually rapid tumor growth, the hallmark of an aggressive tumor (140). As employed for other solid tumors, the longest diameter according to the RECIST 1.1 criteria may be adopted for objective evaluation pituitary neoplasms enabling rigorous assessment of tumor response to therapy (141), as this measure correlates with tumor volume (142). Thus, based on these criteria, significant tumor growth can be considered a 20% increase in diameter, and growth considered as unusually rapid when assessed over a standardized duration (143).
Predictive markers.
The major limitation in defining a pituitary adenoma as aggressive is the absence of predictive cell markers.
The fourth WHO classification recommends evaluation of tumor proliferation (ie, mitotic count and Ki-67 index) and tumor invasion as features of aggressive clinical behavior. However, cutoff values for these parameters are not specified (72). Although “high-risk” adenoma subtypes with poor prognosis have been identified, this histological classification does not grade clinical behavior (135). As an adjunct to the WHO classification, a 5-tiered grading system for clinical prognostication has been proposed, which combines indices of invasion and proliferation, specifically mitotic index > 2, Ki-67 ≥ 3%, and p53 immunopositivity (97). This grading system has been evaluated in at least 4 independent cohorts comprising 1992 patients (96, 144-146). Grade 2b (invasive and proliferative) tumors, which represented 5.4% to 8.8% of the surgical series, were associated with an increased likelihood of “aggressive” behavior, characterized by a high risk of recurrence or progression despite medical therapy (136).
Many studies have sought to identify molecular markers associated with tumor behavior. However, the confusion between invasiveness and aggressiveness, the low number of tumors analyzed, and the absence of prospective validation studies have largely precluded identification of biomarkers that distinguish aggressive or invasive pituitary adenomas from the very rare carcinomas. PTTG1 (147), which maintains chromosomal stability, appears to be a reliable marker of invasiveness. PTTG1 abundance is associated with tumorigenesis and invasion (22) and is highly expressed in tumors that recur (148, 149). Markers of cell cycle regulation, including cyclin D1, phosphorylated RB, CDKN1B, p21, and p16, are associated with recurrence or progression in some subtypes and in a few studies (149-152). A recent multi-omics study (99), however, did not identify a specific signature or pathway associated with prognosis. Chromosomal instability appears not to be associated with prognosis but rather with the functional properties of the tumor. Indeed, secreting tumors are more prone to exhibit copy number alterations (CNA) in comparison to nonsecreting null cell or gonadotroph tumors (99, 114, 153-156).
These reports suggest that additional mechanisms underlie tumor recurrence or resistance to medical treatment, including the contribution of soluble factors and the pituitary tumor microenvironment to aggressive behavior. For example, an association between high expression in the extracellular matrix of metalloproteinase 9 (MMP-9) and tumor invasion, angiogenesis, and proliferation has been demonstrated (157, 158). Cytokines and chemokines, tumor-associated fibroblasts, angiogenesis, and immune cells likely contribute to pituitary tumor pathogenesis and behavior, opening up new perspectives for identifying novel treatment targets for these tumors (64, 158-160).
Pituitary carcinomas
Definition.
In the absence of a specific pathological marker, the occurrence of cranio-spinal or systemic metastasis is required to classify a pituitary tumor as a carcinoma (72, 89). Despite this clear definition, differential diagnosis can be difficult in the presence of a well-differentiated neuroendocrine tumor of visceral origin with pituitary metastasis (161).
True pituitary carcinomas are exceedingly rare. Their prevalence, estimated from large pathology collections, ranges from 0.13% to 0.4% of all resected tumors (80, 97, 162). Interestingly, published cases describing pituitary carcinomas has increased dramatically following the first descriptions of successful treatment of these tumors with the oral alkylating agent temozolomide, leading to publication of case series or large cohort studies describing therapeutic results (163-165).
Natural history.
The majority of pituitary carcinomas originate from invasive macroadenomas, are resistant to medical treatment, and require multiple surgeries and radiation therapy to achieve tumor control (166, 167). From a review of the literature (168) and a survey of European Society of Endocrinology members (169), 112 cases of pituitary carcinomas were identified (65 men and 47 women), with a median age at diagnosis of 45 years (range, 9-75 years). Corticotroph and lactotroph tumors were the most frequent subtypes. The switch from a nonfunctioning to functioning tumor should alert the physician to the potential for an aggressive pituitary tumor and the risk of distant metastasis, especially with a switch from a silent corticotroph adenoma to overt Cushing’s disease.
De novo metastasis at the time of diagnosis is extremely rare. The median time from initial diagnosis to identification of metastases was 7 years, and the reported latency period was up to 31 years. Most metastases were intracranial and spinal, although metastases to liver, bone, lymph nodes, and lung were detected. Screening for metastasis is indicated when there is discordance between imaging results and biochemical findings, in the presence of nonpituitary site-specific symptoms in a patient with a known pituitary adenoma, or as pretherapeutic staging in the presence of an aggressive pituitary tumor. In these cases, in addition to whole-body computed tomography (CT) scan and brain and spinal MRI, functional imaging should be considered where available. Positron emission tomography (PET) using 68-Gallium DOTATOC may be helpful in identifying tumors/metastases, as well as for identifying candidates for peptide receptor radionuclide therapy (143).
Predictive markers.
The rarity of pituitary carcinomas is a major limitation for discovery of predictive markers. Most studies combine invasive and aggressive adenomas with pituitary carcinomas, enabling identification of markers associated with tumor progression but not specific to malignant tumors. As discussed above, upregulated cyclin D1, vascular endothelial growth factor, MMP-9, and p21Cip1 seem to be associated with disrupted tumor cell cycle progression, vasculogenesis, metastasis, and invasion, and may contribute to malignant transformation of pituitary adenomas to carcinomas (170).
ATRX and TP53 mutations may be more specifically associated with corticotroph carcinomas. In a study of 39 aggressive pituitary tumors and 9 carcinomas, investigators found that 5 corticotroph carcinomas and 1 GH-PRL carcinoma, as well as 3 aggressive corticotroph or lactotroph tumors, harbored somatic mutations in the ATRX gene, which is involved in heterochromatin remodeling and telomere maintenance (171). Loss of ATRX immunoexpression was confirmed in all tumors, suggesting that ATRX immunostaining may allow early identification of patients at risk of developing pituitary carcinomas. Interestingly, additional inactivating somatic mutations in tumor suppressor genes, specifically TP53, PTEN, RB1, and NF2, as well as CDKN2A/B deletion were identified in 8 of 9 ATRX-immunonegative tumors. Although TP53 is rarely mutated in pituitary adenomas, a higher-than-expected prevalence of TP53 mutations was observed in aggressive corticotroph tumors, and these were associated with chromosome instability (155).
The very limited number of patients with these tumors at any one center constrains identification and development of predictive markers, highlighting the need for multicenter international collaborations to inform risk stratification and optimization of therapeutic strategies.
Molecular Pathogenesis
More than 95% of all pituitary adenomas are sporadic (172). Whole genome sequencing studies have enabled major advances in elucidating their pathophysiology (173), yet the genetic background of a large proportion of pituitary tumors still remains unknown, largely because of technical challenges in studying surgically resected adenoma samples (174). The tissue pieces are often quite small, can contain intermingled normal pituitary tissue, vasculature, or mesenchymal cells, yield low-abundance mutations, and are genetically heterogeneous, all factors increasing background heterogeneity.
Pituitary tumors are monoclonal in composition (175, 176), with somatic, mosaic, and familial low penetrance variations being the potential causes of tumorigenesis (177). A recent genome-wide association study in a Han Chinese population of 771 pituitary adenomas and 2788 control subjects discovered 3 chromosomal susceptibility loci at 10p12.31, 10q21.1, and 13q12.13 with genome-wide significance (P < 5 × 10-8), suggesting that sporadic pituitary tumor formation also includes inherited genetic variations, although no specific gene mutations were found (178).
The mutational spectrum observed in pituitary tumors using whole genome or whole exome sequencing demonstrates lineage-dependent genetic diversity. The average number of somatic mutations in the coding region is low, with fewer than 10 per tumor sample (114, 154, 156, 179-181). Overall, commonly encountered oncogene mutations are not observed, and recurrent mutations are reported mostly in GNAS (for somatotroph adenomas) and USP8 (for corticotroph adenomas) and very rarely in NR3C1. Thus, molecular mechanisms include activating mutations in key pathways causing hormone hypersecretion and receptor mutations impairing hormone feedback mechanisms or activating intracellular pathways. Additional reported pro-proliferative and pro-secretory factors include chromosomal instability and DNA damage, senescence mechanisms, and molecular changes favoring both benign adenoma growth and hormonal secretory activity. Furthermore, nongenomic mechanisms may also contribute to adenoma pathogenesis and hormone hypersecretion, including soluble intrapituitary factors such as STAT3 activation (182) and Klotho (183, 184).
Activating Mutations
Targeted sequencing of candidate oncogenes and tumor suppressor genes has not yielded a high rate of oncogenic mutations. Thus, RAS mutations were reported in 3 rarely encountered metastatic pituitary carcinomas but not in the respective primary lesions (185); using droplet digital polymerase chain reaction (PCR), a mutant K-RAS was detected in a gonadotroph macroadenoma (186).
Recurrent GNAS mutations were identified in GH-secreting tumors (42) with a prevalence of around 40% (187). The mutations result in a substitution of highly conserved Arg201, or less frequently Gln227, with subsequent constitutive activation of the mutated Gsα subunit (187). Mechanistically, these mutations inhibit GTPase activity of the G protein alpha chain, increasing cAMP levels and turning Gsα into a constitutive active oncogene, termed Gsp. Its relevance was confirmed more recently in 2 whole genome and exome sequencing studies reporting a GNAS mutation rate of 25% and 31%, respectively (188, 189). Patients with somatotroph Gsp mutations are older at diagnosis and have smaller, less invasive tumors. Histopathologically, the tumors are densely granulated in comparison with nonmutated somatotrophinomas. Constitutive cAMP activation in somatotroph adenomas appears to mimic effects of excess GHRH signaling to induce both somatotroph proliferation with DNA damage, as well as GH secretion. Furthermore, somatotroph adenomas exhibit higher levels of PDE4D, further sustaining cAMP levels (156). Some studies reported a favorable response to SRLs, an observation disputed by others (187).
The pathogenesis of corticotroph adenomas was elucidated in 2015, when recurrent somatic heterozygous activating driver mutations were identified in the ubiquitin-specific protease USP8 gene (179, 190). The mutations appear to be specific to corticotroph tumors, with a prevalence ranging from 12% to 60% depending on study methodology and patient ethnicity (mean prevalence, 35.5%) (191, 192). USP8 mutations are primarily present in patients who are mostly female and of younger age, and who harbor microadenomas. Whole exome sequencing studies of USP wild-type tumors identified mutations in the deubiquitinase USP48, the glucocorticoid receptor NR3C1, the BRAF oncogene, and TP53 (193, 194). Moreover, USP8 mutant and USP8 wild-type corticotroph tumors cluster into 2 distinct groups with distinct transcriptomic profiles, thus offering a more robust molecular classification of these adenomas (99).
USP8 mutations affect the 14-3-3 protein binding site, a highly conserved area that protects USP8 from cleavage. Cleaved USP8 deubiquitinates EGFR, protecting it from lysosomal degradation. Recycled EGFR, in turn, leads to increased expression of POMC and ACTH release (195). USP8 mutations appear to have negligible effect on proliferation. Activating USP48 mutations were detected in 10% to 20% of wild-type USP8 tumors (193, 194). USP48 is also a deubiquitinase, and mutations occurred predominantly in female patients with smaller tumors. These mutations changed the structural conformation of USP48 and increased its catalytic activity toward the physiological substrates histone 2A and zinc finger protein Gli1, thereby enhancing POMC and ACTH secretion.
Activating somatic mutations in the p110-α catalytic subunit of PIK3CA have been identified in PRL- and ACTH-secreting and nonsecreting adenomas (196, 197). The mutations lead to constitutive activation of the AKT pathway and increased invasiveness (198).
Receptor Signaling Defects
Receptor signaling defects, a hallmark of endocrine disease pathophysiology, are present in patients with familial isolated pituitary adenomas (FIPA) harboring germline AIP mutations or in patients with X-LAG syndrome (discussed below). In sporadic pituitary tumors, whole genome sequencing identified a recurrent hotspot somatic mutation in splicing factor 3 subunit B1, SF3B1R625H, in about 20% of 227 prolactinomas. Mutant prolactinomas displayed higher PRL concentrations and shorter progression-free survival compared with wild-type tumors. The SF3B1R625H mutation caused aberrant splicing of estrogen-related receptor gamma and enhanced binding of PIT1, increasing PRL secretion and lactotroph proliferation (199). This mutation was not identified recently in another whole genome sequencing study of 16 lactotroph tumors (99).
Receptor signaling defects in the glucocorticoid receptor (GR) gene NR3C1 in corticotroph tumors may disrupt physiologic glucocorticoid feedback on tumor cells. A somatic frameshift mutation in NR3C1 with premature termination of the coding sequence was identified in tumor tissue of a patient with corticotroph tumor progression following bilateral adrenalectomy (Nelson’s syndrome) (200). Mutations in the coding region of NR3C1 are rare in Cushing’s disease: they were identified in 1 of 12 tumors studied by whole exome sequencing (190), but in none of 18 corticotroph tumors using Sanger sequencing (201), nor in 18 USP8 mutation-negative tumors using exome sequencing (194). Very recently, next-generation sequencing identified 3 NR3C1 mutations in 49 corticotroph adenomas (202). Clinical phenotypes were similar in patients harboring NR3C1 mutations and those with wild-type tumors. In vitro studies showed that the p.R469X mutant generated a truncated GR protein, and the p.D590G and p.Y693D GR mutants resulted in lower GR expression. The mutations reduced nuclear translocation of the GR following dexamethasone treatment in AtT-20 cells, increased cell proliferation, and attenuated suppression of POMC transcription.
Ectopic production of gastric inhibitory peptide receptor (GIPR) has been identified in subgroups of patients with acromegaly, with 184 of 496 (37%) patients with GH-secreting adenomas showing a paradoxical GH response to oral glucose tolerance testing. At diagnosis, these patients were older, had smaller tumors, higher basal GH normalized for tumor volume, and a lower rate of hyperprolactinemia, and they had a more favorable response to SRLs (203). In another study, GIPR expression was detected in 32% of samples, including all resected tissues from patients with paradoxical GH responses. GIPR-expressing somatotrophinomas did not show GNAS mutations (204, 205). GIPR expression was associated with a general hypermethylation phenotype, including in the GIPR gene, potentially driving ectopic expression (173, 204). It is interesting to speculate whether this represents a similar mechanism as described for GIPR expression in cortisol-producing primary bilateral macronodular adrenal hyperplasia (206).
Chromosomal Instability and DNA Damage
Studies on chromosomal alterations using CGC and exome and/or genome sequencing have reported either chromosomal losses or gains occurring most often on chromosomes 1, 2, 7, 8, 11, 18, 19, and 22 (154, 172, 207, 208). The alterations vary among adenoma types and range from extended chromosomal losses or gains to almost no change in adenomas with a “quiet” genome (99). Early studies demonstrated that adenoma PTTG overexpression leads to chromosomal instability and aneuploidy due to unfaithful centromere separation (22, 151, 209). The molecular basis of these earlier aneuploidy observations has been extended based on comparative genomic hybridization (CGH) analysis (205, 210, 211) and are shown to be more frequent in invasive adenomas (211). In a study of 42 pituitary macroadenomas, whole exome sequencing identified chromosome arm–level copy number alterations (CNA) across large fractions of the genome in 29% of samples. Chromosomal alterations are more frequent in secreting adenomas, especially in GH-secreting adenomas (154, 156, 180, 188, 212-214), and in atypical null cell adenomas (180). By contrast, alterations are less frequent and extensive in nonsecreting and gonadotroph adenomas (99, 154). In a prospective study of 159 resected adenomas, somatic CNA (SCNA) were overwhelmingly detected in secreting adenomas, with far fewer chromosomal abnormalities observed in nonsecreting adenomas. Using single-gene SCNA pathway analysis, cAMP pathways were identified in somatotroph adenomas, and both GH production and DNA damage were induced by a GHRH analogue of cAMP activation, thereby linking GH hypersecretion to SCNA and genome instability (156). The central role of constitutively elevated cAMP in eliciting DNA replicative stress, cell proliferation, and hormone hypersecretion may direct pituitary cells toward senescence rather than apoptosis (215) (see below). Taken together, an accumulating body of evidence suggests that sporadic pituitary adenomas have distinct copy number profiles that associate with hormonal and histologic subtypes and influence gene expression.
DNA methylation profiles show GH-secreting adenomas being dominated by hypomethylated sites (114, 181). Increased expression of GH and SST5 genes in GH-secreting adenomas and POMC gene in ACTH-secreting adenomas was associated with hypomethylation of the respective promoter regions (114). These findings were extended by CGH array analysis of 195 fresh-frozen pituitary adenomas showing CNA highest in lactotroph (median 38% of probes) compared to corticotroph (11%), somatotroph (5%), gonadotroph (0%), and immunonegative tumors (0%) (153).
Senescence Mechanisms
Cellular senescence characterized by largely irreversible cell cycle arrest constitutes an antiproliferative response, triggered by DNA damage, chromosomal instability and aneuploidy, loss of tumor suppressive signaling, or oncogene activation (216). Mechanisms underlying the invariably indolent growth pattern of pituitary adenomas has, thus, been explained by activation of cellular senescence (151). GH-secreting pituitary adenomas exhibit PTTG-provoked aneuploidy and DNA damage and abundantly express p21 as well as beta-galactosidase, a hallmark of senescence (151, 217). p21 induces both proliferative cell cycle arrest and senescence in somatotroph adenomas (151); in turn, induction of senescence stimulates GH expression and triggers the p53/p21 senescence pathway. p53 binds specific GH promoter motifs and enhances GH production in senescent pituitary adenoma cells, which further protects pituitary tumor cells from apoptosis (218).
Another pathway implicated in pituitary senescence is mediated by paracrine IL-6 signaling, leading to premature cell cycle arrest and evidence for pituitary tumor cell senescence (219). This cytokine also selectively induces PRL and ACTH production (220).
Summary
Overall, the available body of evidence suggests that recurrent cell-specific oncogenic mutations are uncommon. Unique activating mutations have been detected for USP8 in corticotrophinomas and GNAS in somatotrophinomas (179). Overall, the biology of pituitary adenomas is underpinned by somatic signaling driver pathways that induce chromosomal instability and senescence, accounting for both benign proliferative phenotypes as well as hormone hypersecretion (181).
Clinical Spectrum
Epidemiology
Epidemiological studies have provided valuable information on the clinical biology and clinical significance of pituitary adenomas (1, 221).
Overall prevalence
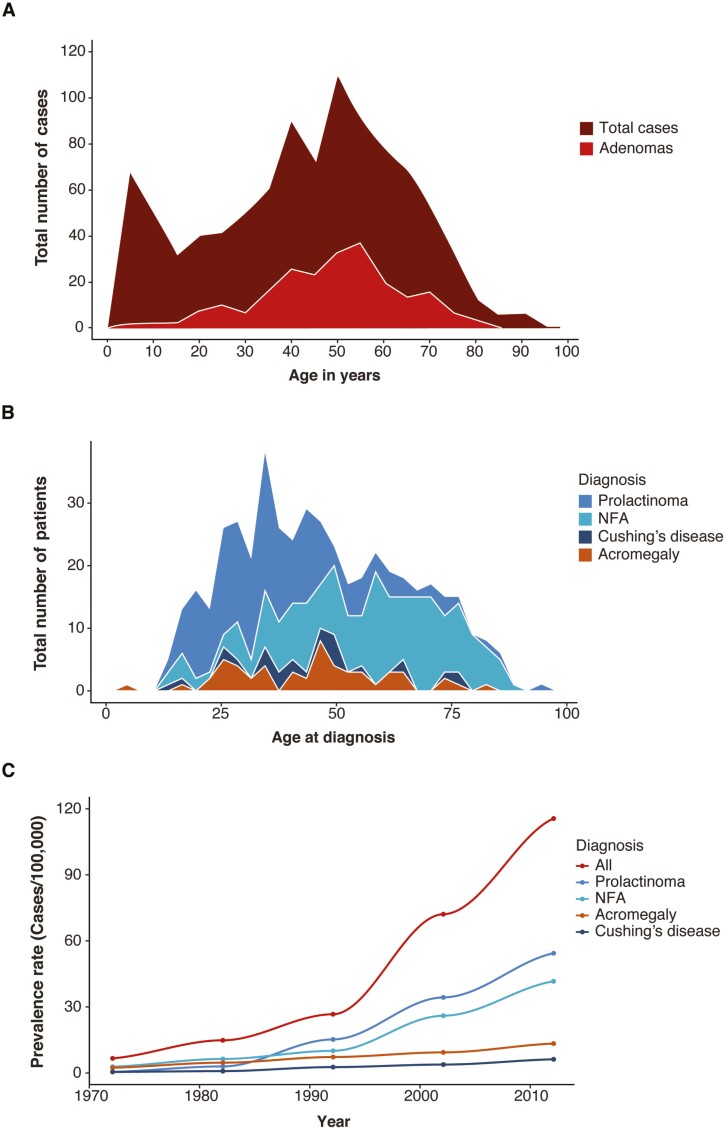
In 1924, Costello first reported that small adenomas were frequently present in pituitary glands of subjects deceased from causes unrelated to pituitary disease (222). Among 1000 unselected pituitary glands, 225 harbored adenomas and a few glands contained multiple distinct adenomas. In subjects aged from 2 years to 91 years, he observed equal gender distribution and peak frequency in the sixth decade (Fig. 6A). Studies from unselected autopsies have since confirmed a high prevalence of asymptomatic adenomas in the general population. In a comprehensive review of 16 studies encompassing more than 21 000 unselected autopsies, an average prevalence of 10% was reported, with immunological staining for PRL seen in up to 40% of these subclinical adenomas (224). Most lesions are < 3 mm in size; clinically inapparent macroadenomas are rare.
Figure 6.
Epidemiology of pituitary adenomas. (A) Number of pituitary adenomas and cases plotted at age of death in 1000 unselected autopsies reported in 1936 (222). (B) Number of patients with a prolactinoma, acromegaly, Cushing’s disease, or a nonfunctioning pituitary adenoma at age of diagnosis from a nationwide study in Iceland from 1955-2012 (223). NFA, nonfunctioning adenoma. (C) Increasing prevalence of clinically significant pituitary tumors during 1972–2012 showing a clear rise since around 1990, mainly explained by the increased prevalence of prolactinomas and nonfunctioning adenomas (223). NFA, nonfunctioning adenoma.
The subclinical prevalence of pituitary adenomas has also been estimated from imaging surveys. Studies employing CT scanning reported discrete pituitary lesions in about 10% of normal volunteers (225-227), while those using MRI observed pituitary abnormalities in 10% to 40% (228, 229) of subjects. The observed abnormalities were small, with none > 10 mm in diameter. However, this higher rate may also include nonpituitary pathological lesions that have a similar appearance.
Unlike prevalence derived from autopsies of people who died from causes unrelated to pituitary adenomas, the clinical significance of pituitary adenomas is derived from epidemiological studies of patients harboring known pituitary adenomas within a defined community (149, 223, 230-234). Based on registry studies and record reviews, the overall prevalence of pituitary adenomas is estimated to range from 680 to 1430 cases per million persons (Table 2). Thus, on a population level, the prevalence is nearly 1 case per 1000 persons, or approximately one-thousandth the number of subclinical adenomas reported from unselected autopsies (Table 3). The estimated incidence of clinically significant pituitary adenomas is 40 per million persons per year (149, 233, 234).
Table 2.
Prevalence of clinically significant pituitary adenomas
| Author | Year | Country | Population | Adenomas | Prevalence, 1 per N | Prevalence, N per million | Female, % | Macro,% |
|---|---|---|---|---|---|---|---|---|
| Daly (230) | 2006 | Belgium | 71,972 | 68 | 1064 | 940 | 68 | 43 |
| Fontana (231) | 2009 | Switzerland | 55,000 | 44 | 1241 | 800 | NR | 44 |
| Fernandez (232) | 2010 | England | 81,149 | 63 | 1289 | 780 | 67 | 41 |
| Raappana (233) | 2010 | Finland | 242,400 | 164 | 1471 | 680 | 71 | 46 |
| Gruppetta (149) | 2013 | Malta | 400,000 | 316 | 1321 | 790 | 70 | 43 |
| Tjornstrand (234) | 2014 | Sweden | 1,590,640 | 592 | 2686 | 370 | 52 | 66 |
| Agustsson (223) | 2015 | Iceland | 330,000 | 471 | 865 | 1430 | 60 | 55 |
| Average | 1420 | 830 | 65 | 48 |
Abbreviations: Macro, macroadenoma; NR, not reported.
Table 3.
Clinical epidemiology of pituitary adenomas
| Expected N per 1 million | |
|---|---|
| Subclinical (autopsies) | 100,000 |
| Clinically significant | 830 |
| Requiring surgery | 380 |
| Invasive | 53 |
| Carcinoma | 1 |
The overall prevalence of invasive adenomas in 6 studies evaluating 1705 patients was 14.2%, representing 6% of clinically significant pituitary adenomas (235). The prevalence of aggressive adenomas is estimated at 2% of surgically resected tumors (139) based on a consolidated definition that embraces invasion, histological markers of proliferation, and a clinical course of recurrence despite multimodal treatments. Malignant transformation is very rare. Studies of surgical specimens from the United States, Germany, France, and Canada totaling more than 12 000 tumors have identified a total of 30 carcinomas. This collectively yields an average prevalence of 0.25% among surgically resected adenomas (80, 97, 162, 236).
Prevalence rates for subclinical, clinically significant, and malignant pituitary adenomas is summarized in Table 3. Available data indicate that pituitary adenomas are common, exhibit a mostly benign natural history, and cause disease of variable severity in less than 0.1%, with a 1 in 100 000 risk of malignancy.
Morbidity and mortality
The burden of pituitary tumor-related morbidity can be gleaned from the distribution of adenoma types, the proportion requiring surgical treatment, and the fraction with aggressive behavior and malignant transformation, the latter of which add disproportionately to the burden of morbidity.
Clinically significant pituitary adenomas are more common in women than in men. About half of all tumors are macroadenomas and two-thirds are functional, secreting PRL, GH, and ACTH in order of descending frequency (Fig. 7A). A national database study in Iceland over 60 years from 1950 to 2012 observed that the prevalence rate increased after the 1990s, with contributions mainly from prolactinomas and nonsecreting adenomas (223) (Fig. 6B). Age-related prevalence differences in adenoma subtypes were also noted, with prolactinomas presenting at younger ages compared with nonsecreting adenomas (223) (Fig. 6C), while the age-related prevalence for acromegaly and Cushing’s disease was similar.
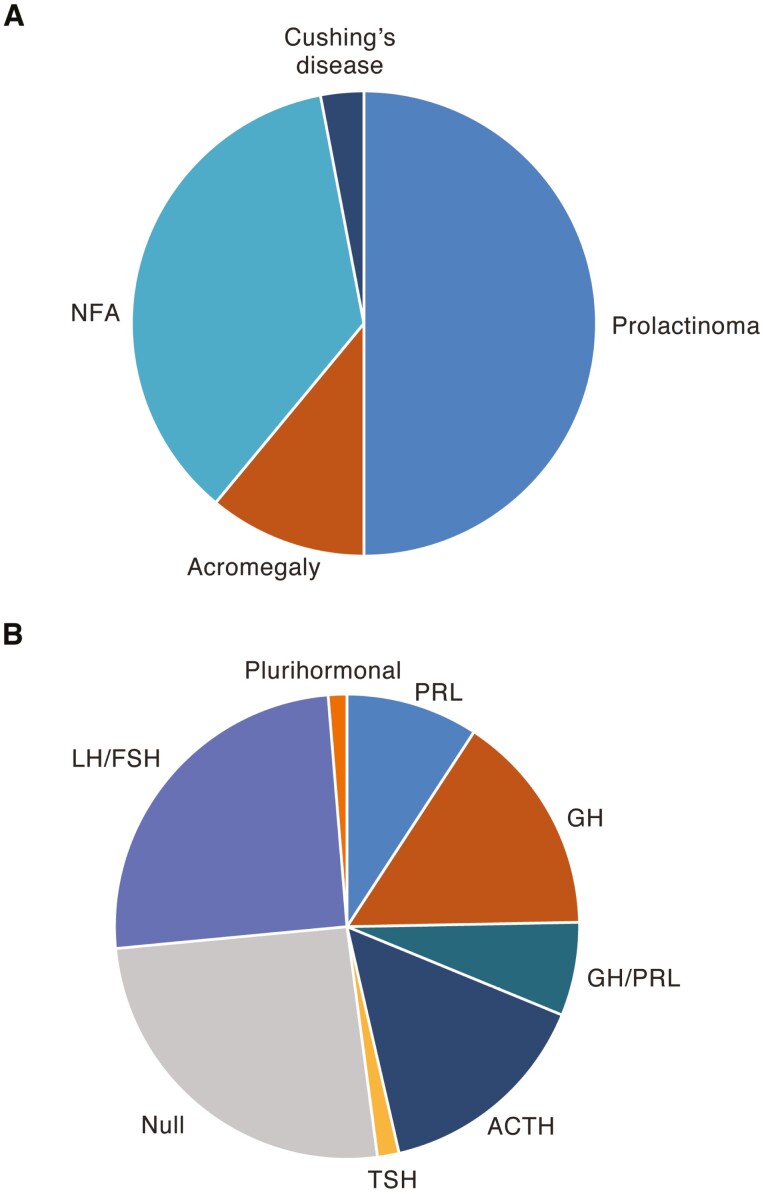
Figure 7.
Frequency of pituitary adenoma subtypes. (A) Frequency of PRL-, GH-, and ACTH-secreting and nonfunctioning adenomas in patients with clinically significant pituitary adenomas from 7 regions in Europe and the United Kingdom (149, 223, 230-234). NFA, nonfunctioning adenoma. (B) Distribution of surgically resected pituitary adenoma subtypes from the German Pituitary Tumor Registry classified by immunohistochemistry (80).
Acromegaly increases mortality about 2-fold, but this is reversed in both sexes by treatments that control GH hypersecretion (237-240). Cushing’s disease increases mortality up to 4-fold. Successful treatment reduces excess mortality, but rates are not usually restored to those seen in the general population (241-244).
Histological subtypes
In community and single-center surveys, about 45% of symptomatic tumors are surgically resected (230, 245, 246); this includes both nonsecreting and secreting adenomas, with the latter comprising a proportion treated primarily medically but remaining inadequately controlled. The distribution of histological types in more than 7000 specimens from the German Pituitary Tumor Registry (80) is shown in Fig. 7B. Classified based on immunohistochemistry staining, null and LH/FSH-staining adenomas together account for > 50% of cases; ACTH-positive adenomas comprise 15%, followed by GH, PRL, and mixed GH/PRL adenomas in descending frequency.
Clinical subtypes
Epidemiological studies assessing frequency of pituitary adenomas based on clinical diagnosis suggest that rates have increased in more recent years. However, the influence of factors unrelated to clinical biology of pituitary adenomas, including study methodology, improved diagnostic strategies, and physician awareness, remains to be determined (247).
Prolactinoma.
The estimated prevalence of prolactinomas is 444 to 540 per million (149, 223, 232), and the annual incidence is 16 to 26 per million, with much higher rates in women than in men (24-37 vs 7.6-9.0 per million, respectively) (223, 233, 234). The peak incidence in women occurs during the third and fourth decade of age; after menopause, the incidence rate is similar to that of men. A study from Korea based on a nationwide health insurance database reported an annual incidence of 23.5 per million, which is similar to previous studies, but they reported a considerably lower prevalence of 82.5 per million (248). Interestingly, 6% to 12% of prolactinomas were identified from evaluation of incidentally discovered pituitary adenomas (223, 233).
Acromegaly.
A review of treatment data between 1926 and 1996 estimated the annual incidence of acromegaly in New Zealand at approximately 3.3 per million (249). A nationwide Danish study of national health care registries and verified by patient records reported an annual incidence of 3.8 per million and a prevalence of 85 per million between 1991 and 2001, with a mean age at diagnosis of 48.7 years (250). A Finnish study reported an annual incidence of 3.4 per million and a median age at diagnosis of 40.5 years between 1992 to 2007 (233), and a regional study in Sweden reported a similar incidence of 3.5 per million between 2001 and 2011 (234). A nationwide Icelandic study based on patient registries and patient records estimated a higher annual incidence of 12 per million in 2012, and a prevalence of 136 per million (223), while a study in the United States using administrative claims data from 2008 to 2012 estimated the annual incidence was 11 cases per million and the prevalence was 78 per million (251). Taken together, these results suggest that the annual incidence of acromegaly has increased. However, it remains unclear whether this represents a true increasing incidence or whether it is due to methodological differences.
Cushing’s disease.
There are few studies on the incidence and prevalence of Cushing’s disease. In a nationwide study identifying Swedish patients through public health care registries, review of patient records to verify the diagnosis showed that 534 (41%) of 1317 cases of Cushing’s syndrome had confirmed Cushing’s disease, resulting in an incidence of Cushing’s disease of 1.6 cases per million per year. The incidence was somewhat higher between 2005 and 2013 as compared with rates between 1987 and 2004 (252). A higher annual incidence of 2.3 per million was reported from a recent Korean study based on health insurance database, but there was no patient record verification in this study (248). It is therefore likely that these studies relying on healthcare databases may have overestimated the incidence of Cushing’s disease. Indeed, 8 previous studies reported an annual incidence of between 1.2 and 2.4 per million, and nationwide studies from Denmark and New Zealand reported lower incidences of 1.2 and 1.3 per million per year, respectively (253, 254). Thus, there is uncertainty as to whether incidence has truly increased with time (233), or whether the apparent increased incidence of Cushing’s disease is the result of methodological differences.
Nonsecreting adenomas.
Based on national registry data, the incidence in Sweden of nonfunctioning pituitary adenomas increased from 6 to 11 per million between 1975 and 1991 (255). The estimated annual incidence was 20.3 per million at a later time period from 2002 to 2011, indicative of a rise in incidence (256). Similarly, in a regional Finnish study, the annual incidence was 10 per million between 1992 and 2007, with occurrences of incidentally discovered masses tripling over this time period (233).
Changes in incidence rates by sex and age are less clear (257). A Swedish regional report based on public health care registries and patient records reported an annual standardized incidence ratio of 18 per million between 2001 and 2011 that did not differ between men and women (234), while an Icelandic study showed the incidence clearly increased with time to 22 per million in women and 26 per million in men in 2012 (223). Overall, the incidence of nonfunctioning pituitary adenomas is low in childhood and peaks at age 60 to 65 years (233, 256). However, mean age at diagnosis is younger in women than in men (40-58 vs 58-62 years) despite approximate equal distribution between the sexes (149, 233, 256).
Of note, nearly all of these studies reported an increasing number of incidentally discovered pituitary adenomas. In the Icelandic and Finnish studies, nonsecreting adenomas were incidentally discovered in 30.5% and 51% of cases, respectively (223, 233). Thus, not surprisingly, the natural history of conservatively managed nonsecreting pituitary adenomas is not well studied (258). Nevertheless, a meta-analysis of incidentally discovered masses and of treated pituitary adenomas suggests that patients with macroadenomas are at higher risk for development of apoplexy, new pituitary hormone deficiencies, and visual field defects compared with patients with microadenomas (259).
Summary
Pituitary adenomas are common, with the overwhelming majority undetected and remaining harmless and indolent during the living years. Among the minority that are clinically significant, morbidity arises from hormonal hypersecretion or from growth and local invasion, with malignant transformation a very rare exception. The incidence of clinically relevant pituitary adenomas appears to be rising. However, results of epidemiological studies are affected by era and duration of study, availability of imaging and laboratory technology, and differences in methodological approaches, all of which influence detection and diagnosis.
Familial and Inherited Syndromes
Although most pituitary adenomas arise sporadically without a family history of pituitary or other tumors, about 5% have a familial form or a genetic tumor syndrome (260), many of which have identifiable molecular drivers. In general, adenomas arising from genetic mutations exhibit greater clinical aggressiveness, with earlier onset, accelerated tumoral growth and invasion, as well as relative treatment refractoriness (Table 4). Thus, although familial adenomas account for a small minority of cases, their management can be complex. Screening and familial genetic risk assessments are required in this population, but are not germane to management of the overwhelming majority of patients harboring sporadic pituitary adenomas (187).
Table 4.
Somatic and germline genetic causes of pituitary adenomas
| Gene | Phenotype | Pituitary features | |
|---|---|---|---|
| Germline | AIP | ~20% of FIPA | Younger age (<30 years) |
| ~13% of young sporadic macroadenomas ~23% of pediatric pituitary adenomas ~29% of gigantism |
Male predominance Large invasive adenomas |
||
| GPR101(mosaicism in sporadic males) | X-LAG syndrome 10% of gigantism |
Early onset (<36 months) Acromegaly features Increased appetite Hyperprolactinemia Hyperplasia |
|
| MEN1 | Up to 50% have pituitary adenoma | Mainly prolactinomas Plurihormonal and multiple adenomas Female predominance Larger and more invasive |
|
| CDKN1B | ~37% of MEN4 have pituitary adenoma | All subtypes | |
| PRKAR1A | Pigmentations, myxomas, hormone hypersecretion | Up to 12% have acromegaly | |
| PRKACB | Multiple adenomas with surrounding hyperplasia | ||
| SDHx, SSDHA, SDHB, SDHC, SDHD, SDHAF2 | ~30% have pheochromocytoma and/or paraganglioma and pituitary adenoma | Prolactinomas, somatotrophinomas, nonsecreting adenomas Extensive cytoplasmic vacuolization Mostly aggressive macroadenomas |
|
| MAX | 3PAs Aggressive pheochromocytoma |
Prolactinomas, somatotrophinomas | |
| NF1 | Neurofibromatosis type 1 | Acromegaly or gigantism | |
| DICER1 | Pleuropulmonary blastoma | Cushing’s disease with high mortality in early infancy | |
| CABLES1 | Corticotrophinomas | Invasive macroadenomas with high Ki-67 index | |
| Somatic | GNAS | 30-60% of somatotrophinomas | Smaller, densely granulated, older age |
| McCune-Albright syndrome (café-au lait macules, fibrous dysplasia, endocrine hyperactivity) | 10-15% acromegaly and/or gigantism Pituitary hyperplasia |
||
| PIKA3CA | All types | Mainly large adenomas | |
| USP8 | 30-60% of corticotrophinomas | Female predominance | |
| USP48 | ~20% of USP8 WT corticotrophinomas | No difference vs WT | |
| BRAF | ~16% of USP8 WT corticotrophinomas | Higher ACTH and cortisol |
Abbreviations: 3PAs, pituitary adenomas, paragangliomas, and pheochromocytomas; FIPA, familial isolated pituitary adenoma; WT, wild-type; X-LAG, X-linked acrogigantism. Adapted with permission from Vandeva et al. (2019) (187).
Multiple endocrine neoplasia type 1
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder caused by mutations in the tumor suppressor gene MEN1 (261). Pituitary adenomas occur in association with other endocrine tumors, typically of the parathyroids and pancreas, although other sites may be affected (262). Approximately 30% to 40% of these patients will have developed a clinically relevant pituitary adenoma during their lifetime (263); in 17% of adult patients and 30% of young patients, it is the first tumor diagnosed (263, 264). In general, MEN1-related pituitary adenomas are more likely to be larger and invasive and to express more than one pituitary hormone (263, 265, 266). In addition, cohort studies involving young patients with large or invasive macroadenomas report the occurrence of MEN1 mutations, as exemplified by studies of acrogigantism demonstrating that 1% also harbor MEN1 mutations (267, 268). Genetic testing guidelines recommend biochemical and imaging screening for pituitary adenomas in suspected carriers beginning from the age of 5 (262).
Multiple endocrine neoplasia type 4
Multiple endocrine neoplasia type 4 (MEN4) is caused by germline mutations of CDKN1B. It mimics MEN1 in affected patients with no other distinctive features (269, 270), yet is very rare. Fewer than 20 MEN4-related pituitary adenomas have been reported. Isolated cases of CDKN1B mutations in patients with Cushing’s disease have also been reported (271).
Carney complex
Carney complex is usually caused by germline mutations in PRKAR1A (272). The most frequent endocrine and nonendocrine features include skin lesions, myxomas, and tumors of the adrenal gland, testis, pituitary, and thyroid (273). Rare cases of PRKAR1A mutations in patients with Cushing’s disease have also been reported (274). Retrospective analyses suggest that approximately 10% of patients develop acromegaly (sometimes due to somatomammotroph hyperplasia), which occurs 10 to 20 years earlier than sporadic acromegaly (275-277). Up to three-quarters of these patients have abnormal GH/IGF-1 or PRL levels (275). Because PRKAR1A mutations do not play a role in sporadic acromegaly, testing for PRKAR1A mutations should be sought only from individuals with a family history of Carney complex or with syndromic presentation suggestive of the disorder (278).
McCune-Albright syndrome
First described in 1937, the McCune-Albright syndrome involves pituitary disease in the setting of multiple endocrine, bone, and cutaneous disorders (279, 280). Mosaicism for post zygotic activating GNAS mutations is the cause of the classical triad of café-au-lait skin macules, polyostotic fibrous dysplasia, and precocious puberty seen in these patients, with pituitary adenomas/hyperplasia a common finding (281). GH excess is an important feature, although adenomas commonly express both GH and PRL. GH excess affects nearly a third of patients (282) and exacerbates craniofacial fibrous dysplastic bony deformity (282-285), which may also cause optic or auditory nerve impingement (286, 287). GH excess may occur in early childhood (283), and this syndrome accounts for about 5% of pituitary gigantism (268). Treatment can be challenging: neurosurgical access to the sellar region is difficult and may be constrained by thick dysplastic skull base bone, and radiotherapy is often avoided due to the concern of inducing sarcomatous changes. Early and effective control of GH excess is a goal to avoid gigantism and other serious effects in these patients (288).
Familial isolated pituitary adenomas
Familial isolated pituitary adenoma (FIPA) is defined as kindreds with 2 or more pituitary adenomas in related members in the absence of syndromic conditions such as those discussed above. To date, hundreds of FIPA kindreds have been reported (289, 290) and it accounts for up to 2% of pituitary adenomas (291). FIPA can present homogeneously with the same clinical phenotype of pituitary adenoma in all affected members (eg, acromegaly), or heterogeneously with different subtypes in affected family members. Acromegaly is a prominent feature of FIPA, with approximately 35% of FIPA patients harboring somatotrophinomas (292). In general, pituitary adenomas in FIPA, including acromegaly, present 5 to 20 years earlier than in patients with sporadic adenomas, frequently with larger and more invasive neoplasms (291).
Not all genetic causes of FIPA are known. Among those identified are AIP mutations and chromosome Xq26.3 microduplications involving the GPR101 gene causing X-LAG syndrome, both of which are described below (43, 293). Together, these account for about 20% of FIPA cases, but rarely may also present sporadically in patients with no familial history of pituitary adenomas.
AIP mutations
Inactivating mutations or deletions of AIP exhibit autosomal dominant inheritance with incomplete penetrance (289). About 10% to 20% of AIP mutation carriers develop a clinically relevant pituitary adenoma (289, 290). While AIP-related pituitary adenomas can have any clinical phenotype, somatotrophinomas or mixed GH- and PRL-secreting adenomas comprise more than two-thirds of cases (289, 290, 294). Pituitary adenomas with AIP mutations exhibit aggressive features and early onset, with a median age of < 18 years (295). GH-secreting adenomas with AIP mutations are the main cause of pituitary gigantism, accounting for nearly 30% of cases (268). Pituitary adenomas due to AIP mutations are also typically larger and more invasive at diagnosis as compared to sporadic adenomas (295), and they are resistant to some medical therapies (295).
X-LAG syndrome
Sporadic and familial early childhood-onset pituitary gigantism may be caused by Xq26.3 microduplications (43, 296). Overexpression of GPR101 in somatotrophs leads to constitutive activation of multiple G proteins, including Gs and Gq/11, and stimulation of GH secretion through protein kinase A and protein kinase C (297). Low-expressing GPR101 ligands have been suggested, including endometrial GnRH1-5 and a leukocyte inflammatory modulator resolvin D5 (298-300). Given strong GPR101 somatotroph constitutive activity, it is unclear whether these putative ligands play a role in modulating pituitary function.
X-LAG syndrome is exceptionally rare, with only 40 patients described (301). Most occur sporadically due to constitutional duplications in females or somatic mosaicism in males. Three FIPA families have been identified, all with transmission from mother to son (260). Disease onset is typically in the first 12 months of life, and almost all patients are diagnosed by the age of 3 with a pituitary macroadenoma secreting high levels of GH and PRL (43, 296). The young age and large tumor size renders neurosurgical treatment difficult. X-LAG causes 10% of pituitary gigantism, but accounts for some of the tallest individuals reported (302, 303). Pituitary tumors in X-LAG grow relentlessly and are resistant to monotherapy (296, 304), often requiring multimodal therapies.
Other conditions
Pituitary adenomas, paragangliomas, and pheochromocytomas can rarely arise in the same patient due to shared pathogenic mechanisms (known as 3PA). Mutations of SDHx and MAX have been documented in these individuals (305-307). Different pituitary adenoma subtypes occur in the setting of 3PA, including acromegaly and prolactinomas.
Pregnancy
Increasing use of assisted reproductive techniques and advances in pituitary clinical and surgical treatments have resulted in more women with pituitary disorders achieving fertility and conceiving (308). Accordingly, pregnancy is now common in women harboring pituitary adenomas. However, during pregnancy, these adenomas may enlarge and pituitary volume increases by up to 136%, mostly due to estrogen-stimulated lactotroph hypertrophy and hyperplasia with a subsequent increased risk of sellar mass effect (309).
Pituitary adenomas, especially when associated with pituitary failure, may impair fertility and preconception, and affect endocrine outcomes throughout pregnancy (308, 310). Prior to pregnancy, hormone replacement therapy should be optimized in women with pituitary adenoma and hypopituitarism.
Evaluation of a newly discovered pituitary adenoma during pregnancy is challenging, as interpretation of biochemical testing can be difficult and imaging may be constrained due to radiation or gadolinium risks. Notably, the differential diagnosis in a pregnant woman with no known prior pituitary adenoma should include that of lymphocytic hypophysitis.
Most pregnant women with a pituitary adenoma should receive standard obstetrical care, with frequent maternal and fetal surveillance (308, 311). Those with macroadenomas and compressive symptoms would likely require cesarean section and should be managed by a multidisciplinary team.
Pituitary gland changes during pregnancy
Pituitary morphological adaptations result in altered pituitary hormonal secretion ensuring optimal fetal development. PRL secretion increases significantly throughout pregnancy due to hyperplasia, with the number of PRL-secreting lactotrophs usually increasing from up to 30% of cells to 60% to 70% by pregnancy end. Gonadotroph cells notably decrease, concordant with reduced gonadotrophin production. Although the number of corticotrophs and thyrotrophs remains stable, ACTH secretion increases moderately while TSH decreases slightly. Placental GH production rises continuously during pregnancy, suppressing pituitary GH secretion and somatotroph cell numbers by mid-second trimester (308). Notably, pituitary and placental GH share a 96% sequence homology; commercial assays do not distinguish between these 2 forms of GH during pregnancy. Furthermore, placental GH is not subjected to feedback regulation (308).
Lactotroph adenomas
Prolactinomas account for up to 75% of all pituitary adenomas in women (1). Dopamine agonist treatment is discontinued once pregnancy is confirmed, except in cases of large macroadenomas. Estrogen may induce prolactinoma cell proliferation, PTTG mRNA, and fibroblast and vascular endothelial growth factor expression (312), while rat antiestrogen treatment decreased both PRL levels and prolactinoma size (313).
Risk of overall microadenoma enlargement is ~5%, with 2% to 3% exhibiting significant growth, yet almost half of microadenomas > 5 mm may enlarge during pregnancy (314) (Table 5). Interestingly, pregnancy also may induce remission of some microprolactinomas, typically those overexpressing ERα (317). Likelihood of remission has been associated with smaller initial adenoma size and pituitary MRI normalization after pregnancy. Breastfeeding is unlikely to induce adenoma growth.
Table 5.
Pituitary adenomas in pregnancy
| Symptomatic growth during pregnancy | Radiological growth during pregnancy | Peri-pregnancy change in adenoma size | |
|---|---|---|---|
|
PRL-secreting (311) 753 pregnancies 652 patients 13 studies |
All adenomas (n = 578): 9.0% (95% CI: 6.8-11.6) Microadenomas (n = 46): 15.2% (95% CI: 15.2; 6.3-28.9) Macroadenomas (n = 118): 30.5% (95% CI: 22.4-39.7) |
All adenomas (n = 376): 10.6% (95% CI: 7.7-14.2) Microadenomas (n = 104): 14.4% (95% CI: 8.3-22.7) Macroadenomas (n = 137): 13.9% (95% CI: 8.6-20.8) |
Population
n = 175 in 4 studies with available data before and after pregnancy No change (1 study) • 5.0 mm (95% CI: 1.6-14.4) before and 5.0 mm (95% CI: 0-12.0) after pregnancy Increased size (2 studies) • Range, 0.0-1.4 cm; mean 0.7 cm in macroadenomas • Median 4 mm (range, 2-8); 3.0 mm in macroadenomas and 0.5 mm in microadenomas Decreased size (1 study) • Median 1 mm (range, −19 to + 12); no change (range, −5 to + 6) in microadenomas and 6.5 mm reduction (range, −19 to + 12) in macroadenomas |
| PRL-secreting (315) | Macroadenomas with no prior surgery/radiation (n = 238): 21.0% Macroadenomas with prior surgery/radiation (n = 148): 4.7% |
N/A | N/A |
|
GH-secreting (311) 128 pregnancies 97 patients 4 studies |
All adenomas (n = 128): 7.0% (95% CI: 3.3-12.9) Microadenomas (n = 10): 30.0% (95% CI: 6.7-65.2) Macroadenomas (n = 97): 4.1% (95% CI: 1.1-10.2) |
All adenomas (n = 111): 5.4% (95% CI: 2.0-11.4) Microadenomas (n = 8): 25.0% (95% CI: 3.2-65.1) Macroadenomas (n = 82): 3.7% (95% CI: 0.8-10.3) |
N/A |
|
Clinically nonsecreting (311, 327) Observational study of > 2000 patients 71 confirmed pituitary macroadenomas |
Macroadenomas (n = 16): 37.5% (95% CI: 15.2-64.6) | Macroadenomas (n = 4): 25% (95% CI: 7.3-52.4) | N/A |
|
TSH-secreting (316) 3 cases |
Macroadenomas: 2 of 3 cases | Macroadenomas: 2 of 3 cases |
No change (1 case)
• Treated with continuous octreotide throughout pregnancy Increased size (2 cases) • Extensive enlargement during pregnancy despite continuation of bromocriptine • Increased at 6 months of pregnancy and octreotide withdrawal, reduction back toward baseline after octreotide reintroduction |
Prior to conception, a pituitary MRI obtained at baseline serves to guide subsequent management (318). Clinical examination each trimester for microadenomas and visual field exams for macroprolactinoma are warranted (311). Pituitary MRI without contrast should be undertaken in pregnant women with persistent headaches and/or vision changes and for those with macroadenoma. MRI without contrast (T1 and T2) has been suggested at 28 to 32 weeks, as T2-hypointense prolactinomas seem to be more prone to growth during pregnancy than hyperintense adenomas (312). Safety data on women with resistant prolactinomas requiring high cabergoline doses is lacking.
For large/invasive macroadenomas, continuation of dopamine agonist during pregnancy and frequent monitoring should be planned. In contrast to earlier recommendations, only routine endocrine follow-up has recently been recommended in pregnant women with small intrasellar microprolactinomas and normal prepartum pituitary function (311). In most patients with rapidly enlarging adenomas, dopamine agonist reinitiation controls tumor growth and surgery is rarely necessary (319). Induced delivery may be an option if pregnancy is sufficiently mature. Most women with prolactinomas can deliver vaginally, although preplanning for cesarean section should be considered if optic chiasm abutment is a risk. Rarely, postpartum imaging may reveal macroprolactinoma shrinkage or hemorrhage (309).
Somatotroph adenomas
Preconception assessment of disease activity, comorbidities, and fertility status in women with acromegaly is required. Pregnancy could, in rare cases, trigger growth of a GH-secreting adenoma, especially mixed lactotroph-somatotroph or mammosomatotroph phenotypes; withholding SRLs in patients with known acromegaly can also induce rebound adenoma growth (320).
In newly diagnosed acromegaly patients with mildly elevated IGF-1 levels or in those who underwent surgical tumor resection, IGF-1 levels will usually decrease during pregnancy as high estrogen levels increase GH hepatic resistance. Frequently, symptoms improve considerably during the first half of pregnancy. Symptomatic adenoma growth in pregnant women with acromegaly occurs in ~7% (95% CI, 3.3%-12.9%) (311). However, GH and IGF-1 levels do not correlate with tumor growth. Comorbidities associated with acromegaly should be closely monitored as risk for hypertension and diabetes are increased (321). Indeed, gestational diabetes in women with acromegaly is more common than in those without acromegaly (322).
Corticotroph adenomas
Corticotroph adenomas may express estrogen receptors, yet direct proliferative effect of estrogens has not been described. Although more than 25% of women of reproductive age with Cushing’s disease exhibited hypercortisolism within 1 year of childbirth (323), it is unclear whether this is due to pituitary corticotroph hyperactivity in the peripartum period or rather increased frequency of Cushing’s disease in young women (1). A large retrospective study found that both plasma ACTH and pituitary adenoma volume may increase during pregnancy in patients undergoing bilateral adrenalectomy, but pregnancy does not accelerate corticotroph tumor progression per se (324).
Treatment of endogenous Cushing’s syndrome during pregnancy has been reported in fewer than 200 cases (325). Maternal morbidity from hypertension, diabetes mellitus, heart failure, pre-eclampsia, and eclampsia is increased (326) as is the rate of preterm births (327). Prophylactic anticoagulation may be warranted in selected patients (311).
Nonsecreting adenomas
Pregnancy is very rare in patients with nonsecreting adenomas as these patients have impaired gonadotrophin activity and or/hyperprolactinemia (316). A large prospective, observational, population study of more than 2000 pregnant women reported 71 pituitary macroadenomas, of which 16 were nonsecreting; microadenomas were not analyzed in this study (328). Women with pituitary adenomas were 4 years older than the comparator group (P = 0.001) and none undergoing pre-pregnancy surgery and/or radiotherapy experienced symptomatic tumor expansion.
Women with nonsecreting adenomas are more likely to present with features consistent with tumor expansion such as visual symptoms (RR 4.59; 95% CI, 1.48-14.3). There is no evidence for adverse pregnancy outcomes in these patients, although cesarean section was more likely among those with nonsecreting adenomas vs control (RR 2.06) (328). By contrast, in the same study, women with a macroprolactinoma were not more likely to undergo cesarean deliveries compared with control (328). Given the almost invariably benign natural history of these adenomas, routine endocrine follow-up during pregnancy has not been recommended for smaller adenomas not impinging on critical structures (311). However, this approach should be individualized based on adenoma size, location, and previous history of tumor growth and/or treatment.
Pituitary apoplexy during pregnancy
Apoplexy and infarction risk factors during pregnancy include tumor growth with neurological and visual symptoms due to hemorrhage and infundibular and hypophyseal vessel compression. Cystic areas may reflect previous intratumoral hemorrhage and apoplexy predisposition. In a systematic review of 23 patients presenting with pituitary adenomas during pregnancy, including 12 with apoplexy (329), symptoms occurred mainly in the second trimester and included visual changes and headache. Apoplexy may occur rarely with microprolactinomas (330). Clinical suspicion requires urgent imaging, visual, and/endocrine evaluation. Treatment should be individualized; some patients with prolactinoma require observation and most require dopamine agonist reinitiation or surgery.
Summary
A normal pregnancy course is anticipated for most patients with a pituitary adenoma. Although most adenomas are small, and pregnancy-induced enlargement is negligible, some patients may exhibit symptomatic adenoma enlargement requiring urgent management. Surgical indications include vision loss and uncontrolled hypercortisolism in patients with corticotroph adenomas. Due to the rare but serious risk of maternal and fetal morbidity and mortality, specialized care is required. Vaginal delivery is preferred in women with a microadenoma. However, for a woman with a macroadenoma with visual impairment or severe headache, most would suggest cesarean section.
Long-term Comorbidities and Mortality Outcomes
Untreated macroadenomas enlarge by about 50% at 4 years (331). Central mass effects caused by pituitary macroadenomas may cause local compression with invasion, leading to cranial nerve defects and impaired pituitary function, particularly among those with nonsecreting adenomas (332, 333). Additional comorbidities may arise from inappropriate hormone replacement in patients with hypopituitarism, especially overtreatment with glucocorticoids (334) or thyroid hormones (335), leading to increased cardiovascular, metabolic, and bone comorbidities (336, 337) as well as mortality (338). Postsurgical regrowth of nonfunctioning adenomas and apoplexy may also occur (339). As most secreting adenomas express cell surface receptors for hypothalamic neuropeptides controlling hormone secretion and adenoma growth, selective ligands have been developed for therapy. Most PRL- and GH-secreting adenomas, and some ACTH- and TSH-secreting adenomas, respond well to medical therapy, which can provide sustained biochemical and tumor growth control (2, 340, 341). For example, although prolactinomas in middle-aged men are often large and highly invasive, they shrink remarkably after dopamine agonist therapy, exemplifying the benign nature of the adenomatous proliferation (Fig. 8).
Figure 8.
Cabergoline-induced shrinkage of a prolactinoma. Sequential MRIs, PRL levels, and visual fields over approximately 6 months of cabergoline treatment in a 27-year-old male with a 5 cm PRL-secreting tumor in the sphenoid with bilateral cavernous sinus extension and posterior extension to the clivus. Reprinted with permission from Dash et al. (2013) (342).
Prolactinoma
Prolactinoma is the most frequently encountered adenoma in clinical practice and are most frequently diagnosed in women of reproductive age presenting with menstrual irregularities and no other comorbidities (343). Due to the effectiveness of dopamine agonist therapy, few patients require other treatments to control hyperprolactinemia (319). However, diagnosis may be delayed, particularly in men who, unlike young women, often present with macroadenoma and mass-associated comorbidities rather than features of hyperprolactinemia (343). As higher doses of dopamine agonist therapy may be required for disease control, men may also be more at risk for adverse effects, including impulse disorders, which can impact treatment adherence (344).
Cardiometabolic comorbidities
The prevalence of metabolic syndrome is increased in patients with prolactinoma (345). Body mass index, fat mass (males only), and LDL cholesterol are increased, and HDL cholesterol is reduced compared with controls (346). The cause is not clear but may be due to hypogonadism induced by hyperprolactinemia as well as the action of PRL on key enzymes and transporters associated with glucose and lipid metabolism (343). Treatment with dopamine agonists may improve components of the metabolic syndrome in these patients (346).
Musculoskeletal comorbidities
Skeletal fragility occurs commonly, usually manifesting as reduced lumbar spine bone mineral density affecting trabecular more than cortical bone (347). There is a high prevalence (> 30%) of morphometric vertebral fractures and a 5-fold increased fracture rate in both women and men with prolactinomas compared with a control population (348, 349). Hypogonadism arising from hyperprolactinemia and PRL excess, per se, both contribute to bone loss. Low bone mineral density attributable to hypogonadism, longer disease duration, and elevated PRL levels is associated with fracture risk (347).
Mortality
Few studies have addressed mortality in prolactinomas. In a single study based on a National Health Insurance database from South Korea, there was no reported increase in the standardized mortality ratio of 136 patients followed over 10 years (350).
Acromegaly
Diagnostic delay contributes to long-term comorbidities and increases mortality in patients with acromegaly. This delay leads to increased risk of tumor growth, mass effects, hypopituitarism (351, 352), and poor disease control despite surgery, radiation therapy, and medical therapy (353) (Fig. 9).
Figure 9.
Clinical features of acromegaly.
Cardiometabolic and respiratory comorbidities
Cardiovascular morbidity has fallen in recent years because of earlier diagnosis, improved treatment (238), and increased awareness (354). Indeed, cardiovascular disease is no longer the leading cause of death in these patients (238, 239, 355). Hypertension, variably affecting 30% to 60% of patients, is a strong contributor to cardiovascular mortality (356). Increased sodium and water retention and plasma volume (357) may persist despite biochemical control (358, 359), and left ventricular hypertrophy, seen even in normotensive subjects, and more rarely, arrhythmia, often with prolonged QT interval, heart failure, and regurgitative valve disease, are all encountered (360). Ischemic heart disease prevalence is not markedly increased (361).
The cardiorespiratory response to exercise is reduced (362) and obstructive sleep apnea affects more than two-thirds of newly diagnosed patients, mainly due to pharyngeal soft tissue swelling (363). Sleep apnea is an independent cardiovascular risk factor (364) that may persist after acromegaly therapy (365).
Up to half of patients have impaired glucose tolerance and diabetes mellitus (366) due to GH-mediated insulin resistance and long-term decreased pancreatic insulin reserve (367). Diabetes mellitus increases cardiovascular mortality (368) and influences the choice of medical therapy, with pegvisomant generally preferred to the SRL pasireotide due to a greater risk for hyperglycemia with use of pasireotide (369).
Musculoskeletal comorbidities
Arthropathy in acromegaly results from cartilage hypertrophy and osteophyte growth that narrow joint spaces. Arthropathic pain, a symptom of progressive joint degeneration, is a major determinant of reduced quality of life, often leading to important loss of function over time (370). Patients often experience bilateral carpal tunnel syndrome due to median nerve enlargement (371). Morphometric vertebral fractures affect more than half of patients with active disease, who have a 3- to 8-fold higher prevalence than that of the general population (372). The risk of fracture is reduced with improved biochemical control (373) and is not affected by bone density and gonadal status (374).
Oncological comorbidities
Due to the pro-proliferative action of GH and IGF-1, there has been intense interest as to whether cancer rates are increased in acromegaly. The incidence of colorectal and renal cancer is increased, with a standardized incidence ratio of 1.5 and 4, respectively, reported in a recent Swedish registry study. However increased screening frequency may be a confounding factor because cancer-specific mortality rates were not increased (375). Excess mortality due to cancer may therefore be more closely linked to increased life expectancy in acromegaly rather than GH/IGF-1 excess per se (376).
Mortality
The mortality rate in acromegaly has decreased in comparison to previously reported rates (355). However, there is still an excess mortality compared to the general population, with recent reports of standardized mortality ratio ranging from 1.41 to 1.45 (250, 352, 377). Last recorded GH or IGF-1 level (378), diagnostic delay (352), and disease duration (377) are mortality determinants.
Cushing’s Disease
Patients with Cushing’s disease are often diagnosed when the pituitary adenoma is barely visible, and selective surgical removal of the microadenoma may be difficult (379). Furthermore, in patients with persistent disease after unsuccessful surgery, medical treatment is often not effective in achieving long-term biochemical control (380). Therefore, long-term comorbidities may occur, adversely impacting quality of life and survival (381) (Fig. 10).
Figure 10.
Clinical features of Cushing’s disease.
Cardiometabolic comorbidities
Because of an inherent hypercoagulable state, patients with Cushing’s disease have > 10-fold increased risk of thromboembolic events compared with the general population (382). Activated partial thromboplastin times are shortened, and high circulating levels of fibrinogen and factor VIII, as well as impaired fibrinolysis, all contribute to the increased risk (383).
Cardiovascular risk is also increased as most patients are overweight or obese, exhibiting increased visceral, subcutaneous, and total fat mass and decreased lean mass (384). These changes may also be mediated by glucocorticoid-induced central GH suppression (385). More than one-third of Cushing’s disease patients have diabetes mellitus (386) and more than half are dyslipidemic, with elevated triglycerides and LDL cholesterol and reduced HDL cholesterol (387). Patients are often hypertensive and have left ventricular hypertrophy, heart failure, and dilated cardiomyopathy (388).
Musculoskeletal comorbidities
Up to 50% of patients develop bone fractures, mostly vertebral. Fractures are often detected early in the course of the disease and may be seen at a subclinical stage using vertebral morphometry (389). Skeletal fragility due to long-term suppressed bone formation may be a direct result of cortisol excess or an indirect effect of suppressed GH/IGF-1 and hypothalamic-pituitary-gonadal axes as well as disrupted PTH pulsatility (390). Fractures may occur with normal bone mineral density as the quality of bone is deleteriously affected by cortisol excess (391) The protein catabolism of hypercortisolism causes myopathy, reducing physical capacity and function and quality of life. Myopathy may not recover after successful surgery in patients with low IGF-1 (392, 393).
Infections
Immune function is suppressed, increasing susceptibility to sepsis and opportunistic infections (394) reported in up to 50% as a result of immunosuppression and altered immune responses (395). Cushing’s disease may also be a predisposing factor for SARS-CoV-2 infection and severe COVID-19 (396).
Psychiatric disturbances
Psychiatric and neurocognitive symptoms, including depression and anxiety, mania, and psychosis, are common complications that reduce quality of life, and memory and attention may also be impaired. Depression and cognitive dysfunction may persist after treatment (397).
Mortality
The standardized mortality ratio is increased from 4- to 16-fold in patients with active/persistent Cushing’s disease compared with the general population, mainly from myocardial infarction and stroke (398). Recent epidemiological results show that death due to cardiovascular disease was increased > 4-fold and mortality rate was 5-fold higher among those with diabetes mellitus and 7-fold higher with persistent hypercortisolism (399). Risk did not revert to normal with disease remission (400).
Thyrotrophinoma
TSH-secreting adenomas are very rare, causing secondary hyperthyroidism, which is frequently misdiagnosed. Patients usually present with macroadenomas, and in one-third, co-manifestation of plurihormonal (often GH) secretion is observed (401). Response to medical therapy with SRLs is often favorable and can be used as a diagnostic test (401). However, not infrequently, despite surgery and SRL therapy, disease control remains suboptimal (402). Patients may experience cardiac complications such as atrial fibrillation (403), increasing the risk of stroke (350). In a retrospective case series, more than half of patients showed morphometrical vertebral fractures associated with age and serum free T4, but not with TSH levels (404). Patients with adenomas co-secreting TSH and ACTH have an increased a standardized mortality ratio of 1.9 compared with the general population (350).
Summation and Conclusion
The anterior pituitary gland develops from a range of complex brain signals integrating with intrinsic transcriptional events that together determine cell type differentiation and hormonal secretion, in turn regulating somatic growth, metabolism, nutrition, reproduction, and physical health. Although pituitary adenomas are common, the overwhelming majority remain indolent throughout life, with malignant transformation an extremely rare exception. Mechanisms underlying pathogenesis of these adenomas likely include disrupted intrapituitary signaling pathways promoting benign cell proliferation associated with chromosomal instability, with cellular senescence acting as a protective buffer against progression to malignancy.
Although representing less than one-thousandth of all pituitary adenomas, clinically relevant tumors variably and adversely affect morbidity and mortality depending on cell type, hormone secretory activity, and growth pattern. In most cases, multimodal therapy controlling hormone secretion and adenoma growth will lead to improved quality of life and a normalization of mortality rates to the same as that of the general population. The clinical biology of pituitary adenomas and particularly their benign nature stands in marked contrast to other tumors of the endocrine system such as thyroid (405) and neuroendocrine tumors (406).
Acknowledgments
The authors thank Shira Berman for assistance with manuscript preparation.
Glossary
Abbreviations
- αSU
alpha-subunit of glycoprotein hormones
- ACTH
adrenocorticotropic hormone
- cAMP
cyclic adenosine monophosphate
- CNA
copy number alteration
- CRH
corticotrophin-releasing hormone
- CT
computed tomography
- ERα
estrogen receptor α
- FIPA
familial isolated pituitary adenoma
- FSH
follicle-stimulating hormone
- GH
growth hormone
- GHRH
growth hormone–releasing hormone
- GIPR
gastric inhibitory peptide receptor
- GnRH
gonadotrophin-releasing hormone
- GPCR
G protein coupled receptor
- GR
glucocorticoid receptor
- IGF-1
insulin-like growth factor 1
- IHC
immunohistochemistry
- LH
luteinizing hormone
- MRI
magnetic resonance imaging
- NPY
neuropeptide Y
- PET
positron emission tomography
- PRL
prolactin
- POMC
proopiomelanocortin
- SCNA
somatic copy number alteration
- SF1
steroidogenic factor 1
- SRL
somatostatin receptor ligand
- SST
somatostatin
- TIDA
tubero-infundibular dopamine
- TPIT
T-box family member TBX19
- TRH
thyrotrophin-releasing hormone
- TSH
thyrotrophin (thyroid-stimulating hormone)
- WHO
World Health Organization
- X-LAG
X-linked acrogigantism
Contributor Information
Shlomo Melmed, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Ursula B Kaiser, Brigham & Women’s Hospital and Harvard Medical School, Boston, MA, USA.
M Beatriz Lopes, University of Virginia School of Medicine, Charlottesville, VA, USA.
Jerome Bertherat, Université de Paris, Assistance Publique–Hôpitaux de Paris, Paris, France.
Luis V Syro, Hospital Pablo Tobon Uribe and Clinica Medellin – Grupo Quirónsalud, Medellin, Colombia.
Gerald Raverot, Hospices Civils de Lyon and Lyon 1 University, Lyon, France.
Martin Reincke, University Hospital of LMU, Ludwig-Maximilians-Universität, Munich, Germany.
Gudmundur Johannsson, Sahlgrenska University Hospital & Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden.
Albert Beckers, University of Liège, Liège, Belgium.
Maria Fleseriu, Oregon Health & Science University, Portland, OR, USA.
Andrea Giustina, San Raffaele Vita-Salute University and IRCCS Hospital, Milan, Italy.
John A H Wass, University of Oxford, Oxford, UK.
Ken K Y Ho, The Garvan Institute of Medical Research and St. Vincents Hospital, Sydney, Australia.
Funding
None
Disclosure Summary
The authors have nothing to disclose.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Melmed S. Pituitary-tumor endocrinopathies. N Engl J Med. 2020;382(10):937-950. [DOI] [PubMed] [Google Scholar]
- 2. Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA. 2017;317(5):516-524. [DOI] [PubMed] [Google Scholar]
- 3. Yavropoulou MP, Tsoli M, Barkas K, Kaltsas G, Grossman A. The natural history and treatment of non-functioning pituitary adenomas (non-functioning PitNETs). Endocr Relat Cancer. 2020;27(10):R375-R390. [DOI] [PubMed] [Google Scholar]
- 4. Caffarini M, Orciani M, Trementino L, Di Primio R, Arnaldi G. Pituitary adenomas, stem cells, and cancer stem cells: what’s new? J Endocrinol Invest. 2018;41(7):745-753. [DOI] [PubMed] [Google Scholar]
- 5. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30(7):790-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bilodeau S, Roussel-Gervais A, Drouin J. Distinct developmental roles of cell cycle inhibitors p57Kip2 and p27Kip1 distinguish pituitary progenitor cell cycle exit from cell cycle reentry of differentiated cells. Mol Cell Biol. 2009;29(7):1895-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87(3):933-963. [DOI] [PubMed] [Google Scholar]
- 8. Laporte E, Vennekens A, Vankelecom H. Pituitary remodeling throughout life: are resident stem cells involved? Front Endocrinol (Lausanne). 2020;11:604519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castinetti F, Davis SW, Brue T, Camper SA. Pituitary stem cell update and potential implications for treating hypopituitarism. Endocr Rev. 2011;32(4):453-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146(9):3985-3998. [DOI] [PubMed] [Google Scholar]
- 11. Chen J, Crabbe A, Van Duppen V, Vankelecom H. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol Endocrinol. 2006;20(12):3293-3307. [DOI] [PubMed] [Google Scholar]
- 12. Monahan P, Rybak S, Raetzman LT. The notch target gene HES1 regulates cell cycle inhibitor expression in the developing pituitary. Endocrinology. 2009;150(9):4386-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu X, Zhang J, Tollkuhn J, et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20(19):2739-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheung LYM, Camper SA. PROP1-dependent retinoic acid signaling regulates developmental pituitary morphogenesis and hormone expression. Endocrinology. 2020;161(2):bqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayran A, Sochodolsky K, Khetchoumian K, et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun. 2019;10(1):3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gleiberman AS, Michurina T, Encinas JM, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci USA. 2008;105(17):6332-6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA. 2008;105(8):2907-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosoyama T, Nishijo K, Garcia MM, et al. Postnatal Pax7 progenitor gives rise to pituitary adenomas. Genes Cancer. 2010;1(4):388-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112(11):1603-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alkhani AM, Cusimano M, Kovacs K, Bilbao JM, Horvath E, Singer W. Cytology of pituitary thyrotroph hyperplasia in protracted primary hypothyroidism. Pituitary. 1999;1(3-4):291-295. [DOI] [PubMed] [Google Scholar]
- 21. Ben-Jonathan N, Liu JW. Pituitary lactotrophs: endocrine, paracrine, juxtacrine, and autocrine interactions. Trends Endocrinol Metab. 1992;3(7):254-258. [DOI] [PubMed] [Google Scholar]
- 22. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999;5(11):1317-1321. [DOI] [PubMed] [Google Scholar]
- 23. Sanno N, Teramoto A, Osamura RY, et al. Pathology of pituitary tumors. Neurosurg Clin N Am. 2003;14(1):25-39, vi. [DOI] [PubMed] [Google Scholar]
- 24. Ezrin C, Kovacs K, Horvath E. A functional anatomy of the endocrine hypothalamus and hypophysis. Med Clin North Am. 1978;62(2):229-233. [DOI] [PubMed] [Google Scholar]
- 25. Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol. 2021;9(4):235-246. [DOI] [PubMed] [Google Scholar]
- 26. Montminy M. Cell differentiation. The road not taken. Nature. 1993;364(6434):190-191. [DOI] [PubMed] [Google Scholar]
- 27. Schlechte JA. Long-term management of prolactinomas. J Clin Endocrinol Metab. 2007;92(8):2861-2865. [DOI] [PubMed] [Google Scholar]
- 28. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. [DOI] [PubMed] [Google Scholar]
- 29. McFarlane T, Zajac JD, Cheung AS. Gender-affirming hormone therapy and the risk of sex hormone-dependent tumours in transgender individuals-a systematic review. Clin Endocrinol (Oxf). 2018;89(6):700-711. [DOI] [PubMed] [Google Scholar]
- 30. Steger RW, Chandrashekar V, Zhao W, Bartke A, Horseman ND. Neuroendocrine and reproductive functions in male mice with targeted disruption of the prolactin gene. Endocrinology. 1998;139(9):3691-3695. [DOI] [PubMed] [Google Scholar]
- 31. Horseman ND, Zhao W, Montecino-Rodriguez E, et al. Defective mammopoiesis, but normal hematopoiesis, in mice with a targeted disruption of the prolactin gene. EMBO J. 1997;16(23):6926-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Tang C, Wen G, et al. The mechanism and pathways of dopamine and dopamine agonists in prolactinomas. Front Endocrinol (Lausanne). 2018;9:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayashi T, Usui H, Tanaka H, Shozu M. Variant prolactin receptor in agalactia and hyperprolactinemia. N Engl J Med. 2018;379(23):2230-2236. [DOI] [PubMed] [Google Scholar]
- 34. Bernard V, Young J, Binart N. Prolactin - a pleiotropic factor in health and disease. Nat Rev Endocrinol. 2019;15(6):356-365. [DOI] [PubMed] [Google Scholar]
- 35. Melmed S, Braunstein GD, Horvath E, Ezrin C, Kovacs K. Pathophysiology of acromegaly. Endocr Rev. 1983;4(3): 271-290. [DOI] [PubMed] [Google Scholar]
- 36. Ribeiro de Oliveira Longo Schweizer J, Ribeiro-Oliveira A Jr, Bidlingmaier M. Growth hormone: isoforms, clinical aspects and assays interference. Clin Diabetes Endocrinol. 2018;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barinaga M, Bilezikjian LM, Vale WW, Rosenfeld MG, Evans RM. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature. 1985;314(6008):279-281. [DOI] [PubMed] [Google Scholar]
- 38. Olarescu NC, Gunawardane K, Hansen TK, Moller N, Jorgensen JOL. Normal physiology of growth hormone in adults. In: Feingold KR, Anawalt B, Boyce A, et al. eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. –2022. [Google Scholar]
- 39. Yamashita S, Melmed S. Insulinlike growth factor I regulation of growth hormone gene transcription in primary rat pituitary cells. J Clin Invest. 1987;79(2):449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lines KE, Stevenson M, Thakker RV. Animal models of pituitary neoplasia. Mol Cell Endocrinol. 2016;421:68-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thorner MO, Perryman RL, Cronin MJ, et al. Somatotroph hyperplasia. Successful treatment of acromegaly by removal of a pancreatic islet tumor secreting a growth hormone-releasing factor. J Clin Invest. 1982;70(5):965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340(6236):692-696. [DOI] [PubMed] [Google Scholar]
- 43. Trivellin G, Daly AF, Faucz FR, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656-660. [DOI] [PubMed] [Google Scholar]
- 45. Casanueva FF, Camina JP, Carreira MC, Pazos Y, Varga JL, Schally AV. Growth hormone-releasing hormone as an agonist of the ghrelin receptor GHS-R1a. Proc Natl Acad Sci USA. 2008;105(51):20452-20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tannenbaum GS, Epelbaum J, Bowers CY. Interrelationship between the novel peptide ghrelin and somatostatin/growth hormone-releasing hormone in regulation of pulsatile growth hormone secretion. Endocrinology. 2003;144(3):967-974. [DOI] [PubMed] [Google Scholar]
- 47. Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37(4):649-661. [DOI] [PubMed] [Google Scholar]
- 48. Gunther T, Tulipano G, Dournaud P, et al. International Union of Basic and Clinical Pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new nomenclature. Pharmacol Rev. 2018;70(4):763-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Drouin J. 60 YEARS OF POMC: transcriptional and epigenetic regulation of POMC gene expression. J Mol Endocrinol. 2016;56(4):T99-T112. [DOI] [PubMed] [Google Scholar]
- 50. Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J Clin Invest. 1999;104(9):1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann N Y Acad Sci. 2009;1163:17-30. [DOI] [PubMed] [Google Scholar]
- 52. Gatto F, Hofland LJ. The role of somatostatin and dopamine D2 receptors in endocrine tumors. Endocr Relat Cancer. 2011;18(6):R233-R251. [DOI] [PubMed] [Google Scholar]
- 53. Feelders RA, Newell-Price J, Pivonello R, Nieman LK, Hofland LJ, Lacroix A. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol. 2019;7(4):300-312. [DOI] [PubMed] [Google Scholar]
- 54. Cawley NX, Li Z, Loh YP. 60 YEARS OF POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56(4):T77-T97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol. 2018;60(3):R131-R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stamatiades GA, Kaiser UB. Gonadotropin regulation by pulsatile GnRH: signaling and gene expression. Mol Cell Endocrinol. 2018;463:131-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Abreu AP, Toro CA, Song YB, et al. MKRN3 inhibits the reproductive axis through actions in kisspeptin-expressing neurons. J Clin Invest. 2020;130(8):4486-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159(11):3723-3736. [DOI] [PubMed] [Google Scholar]
- 59. Navarro VM. Metabolic regulation of kisspeptin - the link between energy balance and reproduction. Nat Rev Endocrinol. 2020;16(8):407-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kaiser UB. Hyperprolactinemia and infertility: new insights. J Clin Invest. 2012;122(10):3467-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sonigo C, Bouilly J, Carre N, et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122(10):3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mariotti S, Beck-Peccoz P. Physiology of the hypothalamic-pituitary-thyroid axis. In: Feingold KR, Anawalt B, Boyce A, et al. eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000. –2022. [Google Scholar]
- 63. Fekete C, Lechan RM. Negative feedback regulation of hypophysiotropic thyrotropin-releasing hormone (TRH) synthesizing neurons: role of neuronal afferents and type 2 deiodinase. Front Neuroendocrinol. 2007;28(2-3):97-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marques P, Grossman AB, Korbonits M. The tumour microenvironment of pituitary neuroendocrine tumours. Front Neuroendocrinol. 2020;58:100852. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto M, Iguchi G, Bando H, et al. Autoimmune pituitary disease: new concepts with clinical implications. Endocr Rev. 2020;41(2):bnz003. [DOI] [PubMed] [Google Scholar]
- 66. Girod C, Trouillas J, Dubois MP. Immunocytochemical localization of S-100 protein in stellate cells (folliculo-stellate cells) of the anterior lobe of the normal human pituitary. Cell Tissue Res. 1985;241(3):505-511. [DOI] [PubMed] [Google Scholar]
- 67. Kovacs K, Horvath E, Ryan N, Ezrin C. Null cell adenoma of the human pituitary. Virchows Arch A Pathol Anat Histol. 1980;387(2):165-174. [DOI] [PubMed] [Google Scholar]
- 68. Principe M, Chanal M, Ilie MD, et al. Immune landscape of pituitary tumors reveals association between macrophages and gonadotroph tumor invasion. J Clin Endocrinol Metab. 2020;105(11):dgaa520. [DOI] [PubMed] [Google Scholar]
- 69. Arzt E, Pereda MP, Castro CP, Pagotto U, Renner U, Stalla GK. Pathophysiological role of the cytokine network in the anterior pituitary gland. Front Neuroendocrinol. 1999;20(1):71-95. [DOI] [PubMed] [Google Scholar]
- 70. Lopes MBS. World Health Ozganization 2017 classification of pituitary tumors. Endocrinol Metab Clin North Am. 2020;49(3):375-386. [DOI] [PubMed] [Google Scholar]
- 71. Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28(3):228-243. [DOI] [PubMed] [Google Scholar]
- 72. Osamura R, Lopes M, Grossman A, Kontogeorgos G, Trouillas J. Introduction. In: Lloyd R, Osamura R, Kloppel G, Rosai J, eds. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: IARC Press; 2017. [Google Scholar]
- 73. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lopes MBS, Gill AJ, Wesseling P, Brat DJ.. Tumours of the Sellar Region: Introduction. WHO Classification of Tumours of the Central Nervous System. 5th ed. Lyon: IARC Press; 2022. [Google Scholar]
- 75. Mete O, Osamura RY, Asa SL.. Pituitary Gland: Introduction. WHO Classification of Tumours of Endocrine Organs. 5th ed. Lyon: IARC Press; 2022. [Google Scholar]
- 76. Ho K, Fleseriu M, Kaiser U, et al. Pituitary neoplasm nomenclature workshop: does adenoma stand the test of time? J Endocr Soc. 2021;5(3):bvaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lloyd RV, Osamura RY. Transcription factors in normal and neoplastic pituitary tissues. Microsc Res Tech. 1997;39(2):168-181. [DOI] [PubMed] [Google Scholar]
- 78. Asa SL, Bamberger AM, Cao B, Wong M, Parker KL, Ezzat S. The transcription activator steroidogenic factor-1 is preferentially expressed in the human pituitary gonadotroph. J Clin Endocrinol Metab. 1996;81(6):2165-2170. [DOI] [PubMed] [Google Scholar]
- 79. Friend KE, Chiou YK, Laws ER Jr, Lopes MB, Shupnik MA. Pit-1 messenger ribonucleic acid is differentially expressed in human pituitary adenomas. J Clin Endocrinol Metab. 1993;77(5):1281-1286. [DOI] [PubMed] [Google Scholar]
- 80. Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007;156(2):203-216. [DOI] [PubMed] [Google Scholar]
- 81. Nishioka H, Inoshita N, Mete O, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol. 2015;26(4):349-355. [DOI] [PubMed] [Google Scholar]
- 82. Lenders NF, Wilkinson AC, Wong SJ, et al. Transcription factor immunohistochemistry in the diagnosis of pituitary tumours. Eur J Endocrinol. 2021;184(6):891-901. [DOI] [PubMed] [Google Scholar]
- 83. Manojlovic-Gacic E, Engstrom BE, Casar-Borota O. Histopathological classification of non-functioning pituitary neuroendocrine tumors. Pituitary. 2018;21(2):119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Silva-Ortega S, Garcia-Martinez A, Niveiro de Jaime M, et al. Proposal of a clinically relevant working classification of pituitary neuroendocrine tumors based on pituitary transcription factors. Hum Pathol. 2021;110:20-30. [DOI] [PubMed] [Google Scholar]
- 85. Kontogeorgos G, Thodou E. The gonadotroph origin of null cell adenomas. Hormones (Athens). 2016;15(2):243-247. [DOI] [PubMed] [Google Scholar]
- 86. Haddad AF, Young JS, Oh T, et al. Clinical characteristics and outcomes of null-cell versus silent gonadotroph adenomas in a series of 1166 pituitary adenomas from a single institution. Neurosurg Focus. 2020;48(6):E13. [DOI] [PubMed] [Google Scholar]
- 87. Almeida JP, Stephens CC, Eschbacher JM, et al. Clinical, pathologic, and imaging characteristics of pituitary null cell adenomas as defined according to the 2017 World Health Organization criteria: a case series from two pituitary centers. Pituitary. 2019;22(5):514-519. [DOI] [PubMed] [Google Scholar]
- 88. O’Sullivan EP, Woods C, Glynn N, et al. The natural history of surgically treated but radiotherapy-naive nonfunctioning pituitary adenomas. Clin Endocrinol (Oxf). 2009;71(5):709-714. [DOI] [PubMed] [Google Scholar]
- 89. Raverot G, Burman P, McCormack A, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1-G24. [DOI] [PubMed] [Google Scholar]
- 90. Ilie MD, Jouanneau E, Raverot G. Aggressive pituitary adenomas and carcinomas. Endocrinol Metab Clin North Am. 2020;49(3):505-515. [DOI] [PubMed] [Google Scholar]
- 91. Chatzellis E, Alexandraki KI, Androulakis II, Kaltsas G. Aggressive pituitary tumors. Neuroendocrinology. 2015;101(2):87-104. [DOI] [PubMed] [Google Scholar]
- 92. Raverot G, Castinetti F, Jouanneau E, et al. Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin Endocrinol (Oxf). 2012;76(6):769-775. [DOI] [PubMed] [Google Scholar]
- 93. Grimm F, Maurus R, Beschorner R, et al. Ki-67 labeling index and expression of p53 are non-predictive for invasiveness and tumor size in functional and nonfunctional pituitary adenomas. Acta Neurochir (Wien). 2019;161(6):1149-1156. [DOI] [PubMed] [Google Scholar]
- 94. Zaidi HA, Cote DJ, Dunn IF, Laws ER Jr. Predictors of aggressive clinical phenotype among immunohistochemically confirmed atypical adenomas. J Clin Neurosci. 2016;34:246-251. [DOI] [PubMed] [Google Scholar]
- 95. Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Ki-67 in pituitary neoplasms: a review--part I. Neurosurgery. 2009;65(3):429-4 37; discussion 437. [DOI] [PubMed] [Google Scholar]
- 96. Raverot G, Dantony E, Beauvy J, et al. Risk of recurrence in pituitary neuroendocrine tumors: a prospective study using a five-tiered classification. J Clin Endocrinol Metab. 2017;102(9):3368-3374. [DOI] [PubMed] [Google Scholar]
- 97. Trouillas J, Roy P, Sturm N, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(1):123-135. [DOI] [PubMed] [Google Scholar]
- 98. Hauser BM, Lau A, Gupta S, Bi WL, Dunn IF. The epigenomics of pituitary adenoma. Front Endocrinol (Lausanne). 2019;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Neou M, Villa C, Armignacco R, et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell. 2020;37(1):123-134.e5. [DOI] [PubMed] [Google Scholar]
- 100. Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol. 2013;168(4): 491-499. [DOI] [PubMed] [Google Scholar]
- 101. Bakhtiar Y, Hirano H, Arita K, et al. Relationship between cytokeratin staining patterns and clinico-pathological features in somatotropinomae. Eur J Endocrinol. 2010;163(4):531-539. [DOI] [PubMed] [Google Scholar]
- 102. Xu Z, Ellis S, Lee CC, et al. Silent corticotroph adenomas after stereotactic radiosurgery: a case-control study. Int J Radiat Oncol Biol Phys. 2014;90(4):903-910. [DOI] [PubMed] [Google Scholar]
- 103. Di Ieva A, Davidson JM, Syro LV, et al. Crooke’s cell tumors of the pituitary. Neurosurgery. 2015;76(5):616-622. [DOI] [PubMed] [Google Scholar]
- 104. Fountas A, Lavrentaki A, Subramanian A, Toulis KA, Nirantharakumar K, Karavitaki N. Recurrence in silent corticotroph adenomas after primary treatment: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2019;104(4):1039-1048. [DOI] [PubMed] [Google Scholar]
- 105. Cuevas-Ramos D, Carmichael JD, Cooper O, et al. structural and functional acromegaly classification. J Clin Endocrinol Metab. 2015;100(1):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. The Cancer Genome Atlas Research Network, Weinstein JN, Collisson EA, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45(10):1113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Assie G, Letouze E, Fassnacht M, et al. Integrated genomic characterization of adrenocortical carcinoma. Nat Genet. 2014;46(6):607-612. [DOI] [PubMed] [Google Scholar]
- 109. Hu J, Yin H, Li B, Yang H. Identification of transcriptional metabolic dysregulation in subtypes of pituitary adenoma by integrated bioinformatics analysis. Diabetes Metab Syndr Obes. 2019;12:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bao X, Wang G, Yu S, et al. Transcriptomic analysis identifies a tumor subtype mRNA classifier for invasive non-functioning pituitary neuroendocrine tumor diagnostics. Theranostics. 2021;11(1):132-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Galland F, Lacroix L, Saulnier P, et al. Differential gene expression profiles of invasive and non-invasive non-functioning pituitary adenomas based on microarray analysis. Endocr Relat Cancer. 2010;17(2):361-371. [DOI] [PubMed] [Google Scholar]
- 112. de Araujo LJ, Lerario AM, de Castro M, et al. Transcriptome analysis showed a differential signature between invasive and non-invasive corticotrophinomas. Front Endocrinol (Lausanne). 2017;8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mosella MS, Sabedot TS, Silva TC, et al. DNA methylation-based signatures classify sporadic pituitary tumors according to clinicopathological features. Neuro Oncol. 2021;23(8):1292-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salomon MP, Wang X, Marzese DM, et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, Cushing’s disease and endocrine-inactive subtypes. Clin Cancer Res. 2018;24(17):4126-4136. [DOI] [PubMed] [Google Scholar]
- 115. Garcia-Martinez A, Sottile J, Sanchez-Tejada L, et al. DNA methylation of tumor suppressor genes in pituitary neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104(4):1272-1282. [DOI] [PubMed] [Google Scholar]
- 116. Chen Y, Gao H, Liu Q, et al. Functional characterization of DLK1/MEG3 locus on chromosome 14q32.2 reveals the differentiation of pituitary neuroendocrine tumors. Aging (Albany NY). 2020;13(1):1422-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Scheithauer BW, Kovacs KT, Laws ER Jr, Randall RV. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986;65(6):733-744. [DOI] [PubMed] [Google Scholar]
- 118. Selman WR, Laws ER Jr, Scheithauer BW, Carpenter SM. The occurrence of dural invasion in pituitary adenomas. J Neurosurg. 1986;64(3):402-407. [DOI] [PubMed] [Google Scholar]
- 119. Campero A, Campero AA, Martins C, Yasuda A, Rhoton AL Jr. Surgical anatomy of the dural walls of the cavernous sinus. J Clin Neurosci. 2010;17(6):746-750. [DOI] [PubMed] [Google Scholar]
- 120. Chacko AG, Chacko G, Seshadri MS, Chandy MJ. The “capsule” of pituitary macroadenomas represents normal pituitary gland: a histopathological study. Br J Neurosurg. 2003;17(3):213-218. [DOI] [PubMed] [Google Scholar]
- 121. Serioli S, Doglietto F, Fiorindi A, et al. Pituitary adenomas and invasiveness from anatomo-surgical, radiological, and histological perspectives: a systematic literature review. Cancers (Basel). 2019;11(12):1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Goncalves MB, de Oliveira JG, Williams HA, Alvarenga RM, Landeiro JA. Cavernous sinus medial wall: dural or fibrous layer? Systematic review of the literature. Neurosurg Rev. 2012;35(2):147-1 53; discussion 153-144. [DOI] [PubMed] [Google Scholar]
- 123. Roux FX, Kalamarides M, Devaux B, et al. Intracavernous invagination of pituitary macro-adenomas. Ann Endocrinol (Paris). 1996;57(5):403-410. [PubMed] [Google Scholar]
- 124. Kawase T, van Loveren H, Keller JT, Tew JM. Meningeal architecture of the cavernous sinus: clinical and surgical implications. Neurosurgery. 1996;39(3):527-5 34; discussion 534-526. [DOI] [PubMed] [Google Scholar]
- 125. Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu JI. Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery. 2001;49(4):857-8 62; discussion 862-853. [DOI] [PubMed] [Google Scholar]
- 126. Shimon I. Giant prolactinomas. Neuroendocrinology. 2019;109(1):51-56. [DOI] [PubMed] [Google Scholar]
- 127. Di Ieva A, Rotondo F, Syro LV, Cusimano MD, Kovacs K. Aggressive pituitary adenomas--diagnosis and emerging treatments. Nat Rev Endocrinol. 2014;10(7):423-435. [DOI] [PubMed] [Google Scholar]
- 128. Hardy J. Transphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185-217. [DOI] [PubMed] [Google Scholar]
- 129. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993;33(4):610-61 7; discussion 617-618. [DOI] [PubMed] [Google Scholar]
- 130. Micko AS, Wohrer A, Wolfsberger S, Knosp E. Invasion of the cavernous sinus space in pituitary adenomas: endoscopic verification and its correlation with an MRI-based classification. J Neurosurg. 2015;122(4):803-811. [DOI] [PubMed] [Google Scholar]
- 131. Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229-2234. [DOI] [PubMed] [Google Scholar]
- 132. Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg. 2011;114(2):336-344. [DOI] [PubMed] [Google Scholar]
- 133. Meij BP, Lopes MB, Ellegala DB, Alden TD, Laws ER Jr. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96(2):195-208. [DOI] [PubMed] [Google Scholar]
- 134. Cortet-Rudelli C, Bonneville JF, Borson-Chazot F, et al. Post-surgical management of non-functioning pituitary adenoma. Ann Endocrinol (Paris). 2015;76(3):228-238. [DOI] [PubMed] [Google Scholar]
- 135. Lopes M. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521-535. [DOI] [PubMed] [Google Scholar]
- 136. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). 2020;12(2):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shimon I, Benbassat C, Hadani M. Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol. 2007;156(2):225-231. [DOI] [PubMed] [Google Scholar]
- 138. Graillon T, Castinetti F, Fuentes S, Gras R, Brue T, Dufour H. Transcranial approach in giant pituitary adenomas: results and outcome in a modern series. J Neurosurg Sci. 2020;64(1):25-36. [DOI] [PubMed] [Google Scholar]
- 139. Dekkers OM, Karavitaki N, Pereira AM. The epidemiology of aggressive pituitary tumors (and its challenges). Rev Endocr Metab Disord. 2020;21(2):209-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kasuki L, Raverot G. Definition and diagnosis of aggressive pituitary tumors. Rev Endocr Metab Disord. 2020;21(2):203-208. [DOI] [PubMed] [Google Scholar]
- 141. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 142. Imber BS, Lin AL, Zhang Z, et al. Comparison of radiographic approaches to assess treatment response in pituitary adenomas: is RECIST or RANO good enough? J Endocr Soc. 2019;3(9):1693-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Raverot G, Ilie MD, Lasolle H, et al. Aggressive pituitary tumours and pituitary carcinomas. Nat Rev Endocrinol. 2021;17(11):671-684. [DOI] [PubMed] [Google Scholar]
- 144. Lelotte J, Mourin A, Fomekong E, Michotte A, Raftopoulos C, Maiter D. Both invasiveness and proliferation criteria predict recurrence of non-functioning pituitary macroadenomas after surgery: a retrospective analysis of a monocentric cohort of 120 patients. Eur J Endocrinol. 2018;178(3):237-246. [DOI] [PubMed] [Google Scholar]
- 145. Asioli S, Righi A, Iommi M, et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: retrospective analysis on 566 patients from a tertiary care centre. Eur J Endocrinol. 2019;180(2):127-134. [DOI] [PubMed] [Google Scholar]
- 146. Guaraldi F, Zoli M, Righi A, et al. A practical algorithm to predict postsurgical recurrence and progression of pituitary neuroendocrine tumours (PitNET)s. Clin Endocrinol (Oxf). 2020;93(1):36-43. [DOI] [PubMed] [Google Scholar]
- 147. Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1997;11(4):433-441. [DOI] [PubMed] [Google Scholar]
- 148. Raverot G, Wierinckx A, Dantony E, et al. ; Hypopronos. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab. 2010;95(4):1708-1716. [DOI] [PubMed] [Google Scholar]
- 149. Gruppetta M, Mercieca C, Vassallo J. Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary. 2013;16(4):545-553. [DOI] [PubMed] [Google Scholar]
- 150. Park SH, Jang JH, Lee YM, Kim JS, Kim KH, Kim YZ. Function of cell-cycle regulators in predicting silent pituitary adenoma progression following surgical resection. Oncol Lett. 2017;14(6):7121-7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Chesnokova V, Zonis S, Kovacs K, et al. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci USA. 2008;105(45):17498-17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pei L, Melmed S, Scheithauer B, Kovacs K, Benedict WF, Prager D. Frequent loss of heterozygosity at the retinoblastoma susceptibility gene (RB) locus in aggressive pituitary tumors: evidence for a chromosome 13 tumor suppressor gene other than RB. Cancer Res. 1995;55(8):1613-1616. [PubMed] [Google Scholar]
- 153. Lasolle H, Elsensohn MH, Wierinckx A, et al. Chromosomal instability in the prediction of pituitary neuroendocrine tumors prognosis. Acta Neuropathol Commun. 2020;8(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bi WL, Horowitz P, Greenwald NF, et al. Landscape of genomic alterations in pituitary adenomas. Clin Cancer Res. 2017;23(7):1841-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Uzilov AV, Taik P, Cheesman KC, et al. USP8 and TP53 drivers are associated with CNV in a corticotroph adenoma cohort enriched for aggressive tumors. J Clin Endocrinol Metab. 2021;106(3):826-842. [DOI] [PubMed] [Google Scholar]
- 156. Ben-Shlomo A, Deng N, Ding E, et al. DNA damage and growth hormone hypersecretion in pituitary somatotroph adenomas. J Clin Invest. 2020;130(11):5738-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Turner HE, Nagy Z, Esiri MM, Harris AL, Wass JA. Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocrinol Metab. 2000;85(8):2931-2935. [DOI] [PubMed] [Google Scholar]
- 158. Ilie MD, Vasiljevic A, Raverot G, Bertolino P. The microenvironment of pituitary tumors-biological and therapeutic implications. Cancers (Basel). 2019;11(10):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yano H, Readhead C, Nakashima M, Ren SG, Melmed S. Pituitary-directed leukemia inhibitory factor transgene causes Cushing’s syndrome: neuro-immune-endocrine modulation of pituitary development. Mol Endocrinol. 1998;12(11): 1708-1720. [DOI] [PubMed] [Google Scholar]
- 160. Auernhammer CJ, Melmed S. The central role of SOCS-3 in integrating the neuro-immunoendocrine interface. J Clin Invest. 2001;108(12):1735-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ragni A, Nervo A, Papotti M, et al. Pituitary metastases from neuroendocrine neoplasms: case report and narrative review. Pituitary. 2021;24(5):828-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Alshaikh OM, Asa SL, Mete O, Ezzat S. An institutional experience of tumor progression to pituitary carcinoma in a 15-year cohort of 1055 consecutive pituitary neuroendocrine tumors. Endocr Pathol. 2019;30(2):118-127. [DOI] [PubMed] [Google Scholar]
- 163. Burman P, Lamb L, McCormack A. Temozolomide therapy for aggressive pituitary tumours - current understanding and future perspectives. Rev Endocr Metab Disord. 2020;21(2):263-276. [DOI] [PubMed] [Google Scholar]
- 164. Luo M, Tan Y, Chen W, et al. Clinical efficacy of temozolomide and its predictors in aggressive pituitary tumors and pituitary carcinomas: a systematic review and meta-analysis. Front Neurol. 2021;12:700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Syro LV, Rotondo F, Camargo M, Ortiz LD, Serna CA, Kovacs K. Temozolomide and pituitary tumors: current understanding, unresolved issues, and future directions. Front Endocrinol (Lausanne). 2018;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Kaltsas GA, Nomikos P, Kontogeorgos G, Buchfelder M, Grossman AB. Clinical review: diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab. 2005;90(5):3089-3099. [DOI] [PubMed] [Google Scholar]
- 167. Heaney AP. Clinical review: pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab. 2011;96(12):3649-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yoo F, Kuan EC, Heaney AP, Bergsneider M, Wang MB. Corticotrophic pituitary carcinoma with cervical metastases: case series and literature review. Pituitary. 2018;21(3):290-301. [DOI] [PubMed] [Google Scholar]
- 169. McCormack A, Dekkers OM, Petersenn S, et al. ; ESE survey collaborators. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265-276. [DOI] [PubMed] [Google Scholar]
- 170. Yang Z, Zhang T, Gao H. Genetic aspects of pituitary carcinoma: a systematic review. Medicine (Baltim). 2016;95(47):e5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Casar-Borota O, Boldt HB, Engstrom BE, et al. Corticotroph aggressive pituitary tumors and carcinomas frequently harbor ATRX mutations. J Clin Endocrinol Metab. 2021;106(4):1183-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Peculis R, Niedra H, Rovite V. Large scale molecular studies of pituitary neuroendocrine tumors: novel markers, mechanisms and translational perspectives. Cancers (Basel). 2021;13(6):1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Srirangam Nadhamuni V, Korbonits M. Novel insights into pituitary tumorigenesis: genetic and epigenetic mechanisms. Endocr Rev. 2020;41(6):821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Peculis R, Mandrika I, Petrovska R, et al. Pituispheres contain genetic variants characteristic to pituitary adenoma tumor tissue. Front Endocrinol (Lausanne). 2020;11:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Herman V, Fagin J, Gonsky R, Kovacs K, Melmed S. Clonal origin of pituitary adenomas. J Clin Endocrinol Metab. 1990;71(6):1427-1433. [DOI] [PubMed] [Google Scholar]
- 176. Jacoby LB, Hedley-Whyte ET, Pulaski K, Seizinger BR, Martuza RL. Clonal origin of pituitary adenomas. J Neurosurg. 1990;73(5):731-735. [DOI] [PubMed] [Google Scholar]
- 177. Shaid M, Korbonits M. Genetics of pituitary adenomas. Neurol India. 2017;65(3):577-587. [DOI] [PubMed] [Google Scholar]
- 178. Ye Z, Li Z, Wang Y, et al. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nat Genet. 2015;47(7):793-797. [DOI] [PubMed] [Google Scholar]
- 179. Reincke M, Sbiera S, Hayakawa A, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31-38. [DOI] [PubMed] [Google Scholar]
- 180. Song ZJ, Reitman ZJ, Ma ZY, et al. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016;26(11):1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Valimaki N, Schalin-Jantti C, Karppinen A, et al. Genetic and epigenetic characterization of growth hormone-secreting pituitary tumors. Mol Cancer Res. 2019;17(12):2432-2443. [DOI] [PubMed] [Google Scholar]
- 182. Zhou C, Tong Y, Wawrowsky K, Melmed S. PTTG acts as a STAT3 target gene for colorectal cancer cell growth and motility. Oncogene. 2014;33(7):851-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Schweizer J, Schilbach K, Haenelt M, et al. Soluble alpha klotho in acromegaly: comparison with traditional markers of disease activity. J Clin Endocrinol Metab. 2021;106(8):e2887-e2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zhou C, Jiao Y, Wang R, Ren SG, Wawrowsky K, Melmed S. STAT3 upregulation in pituitary somatotroph adenomas induces growth hormone hypersecretion. J Clin Invest. 2015;125(4):1692-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pei L, Melmed S, Scheithauer B, Kovacs K, Prager D. H-ras mutations in human pituitary carcinoma metastases. J Clin Endocrinol Metab. 1994;78(4):842-846. [DOI] [PubMed] [Google Scholar]
- 186. Aran V, Heringer M, da Mata PJ, et al. Identification of mutant K-RAS in pituitary macroadenoma. Pituitary. 2021;24(5):746-753. [DOI] [PubMed] [Google Scholar]
- 187. Vandeva S, Daly AF, Petrossians P, Zacharieva S, Beckers A. Somatic and germline mutations in the pathogenesis of pituitary adenomas. Eur J Endocrinol. 2019;181(6):R235-R254. [DOI] [PubMed] [Google Scholar]
- 188. Valimaki N, Demir H, Pitkanen E, et al. Whole-genome sequencing of growth hormone (GH)-secreting pituitary adenomas. J Clin Endocrinol Metab. 2015;100(10):3918-3927. [DOI] [PubMed] [Google Scholar]
- 189. Ronchi CL, Peverelli E, Herterich S, et al. Landscape of somatic mutations in sporadic GH-secreting pituitary adenomas. Eur J Endocrinol. 2016;174(3):363-372. [DOI] [PubMed] [Google Scholar]
- 190. Ma ZY, Song ZJ, Chen JH, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Treppiedi D, Barbieri AM, Di Muro G, et al. Genetic profiling of a cohort of Italian patients with ACTH-secreting pituitary tumors and characterization of a Novel USP8 gene variant. Cancers (Basel). 2021;13(16):4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wanichi IQ, de Paula Mariani BM, Frassetto FP, et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary. 2019;22(4):435-442. [DOI] [PubMed] [Google Scholar]
- 193. Sbiera S, Perez-Rivas LG, Taranets L, et al. Driver mutations in USP8 wild-type Cushing’s disease. Neuro Oncol. 2019;21(10):1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Chen J, Jian X, Deng S, et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat Commun. 2018;9(1):3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Fukuoka H, Cooper O, Ben-Shlomo A, et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J Clin Invest. 2011;121(12):4712-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lin Y, Jiang X, Shen Y, et al. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009;16(1):301-310. [DOI] [PubMed] [Google Scholar]
- 197. Murat CB, Braga PB, Fortes MA, Bronstein MD, Correa-Giannella ML, Giorgi RR. Mutation and genomic amplification of the PIK3CA proto-oncogene in pituitary adenomas. Braz J Med Biol Res. 2012;45(9):851-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Spada A, Mantovani G, Lania AG, et al. Pituitary tumors: genetic and molecular factors underlying pathogenesis and clinical behavior. Neuroendocrinology. 2022;112(1):15-33. [DOI] [PubMed] [Google Scholar]
- 199. Li C, Xie W, Rosenblum JS, et al. Somatic SF3B1 hotspot mutation in prolactinomas. Nat Commun. 2020;11(1):2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Karl M, Von Wichert G, Kempter E, et al. Nelson’s syndrome associated with a somatic frame shift mutation in the glucocorticoid receptor gene. J Clin Endocrinol Metab. 1996;81(1):124-129. [DOI] [PubMed] [Google Scholar]
- 201. Antonini SR, Latronico AC, Elias LL, et al. Glucocorticoid receptor gene polymorphisms in ACTH-secreting pituitary tumours. Clin Endocrinol (Oxf). 2002;57(5):657-662. [DOI] [PubMed] [Google Scholar]
- 202. Miao H, Liu Y, Lu L, et al. Effect of 3 NR3C1 mutations in the pathogenesis of pituitary ACTH adenoma. Endocrinology. 2021;162(11):bqab167. [DOI] [PubMed] [Google Scholar]
- 203. Occhi G, Losa M, Albiger N, et al. The glucose-dependent insulinotropic polypeptide receptor is overexpressed amongst GNAS1 mutation-negative somatotropinomas and drives growth hormone (GH)-promoter activity in GH3 cells. J Neuroendocrinol. 2011;23(7):641-649. [DOI] [PubMed] [Google Scholar]
- 204. Hage M, Chaligne R, Viengchareun S, et al. Hypermethylator phenotype and ectopic GIP receptor in GNAS mutation-negative somatotropinomas. J Clin Endocrinol Metab. 2019;104(5):1777-1787. [DOI] [PubMed] [Google Scholar]
- 205. Regazzo D, Losa M, Albiger NM, et al. The GIP/GIPR axis is functionally linked to GH-secretion increase in a significant proportion of gsp(-) somatotropinomas. Eur J Endocrinol. 2017;176(5):543-553. [DOI] [PubMed] [Google Scholar]
- 206. Lacroix A, Bolte E, Tremblay J, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion--a new cause of Cushing’s syndrome. N Engl J Med. 1992;327(14): 974-980. [DOI] [PubMed] [Google Scholar]
- 207. Thakker RV, Pook MA, Wooding C, Boscaro M, Scanarini M, Clayton RN. Association of somatotrophinomas with loss of alleles on chromosome 11 and with gsp mutations. J Clin Invest. 1993;91(6):2815-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Bates AS, Farrell WE, Bicknell EJ, et al. Allelic deletion in pituitary adenomas reflects aggressive biological activity and has potential value as a prognostic marker. J Clin Endocrinol Metab. 1997;82(3):818-824. [DOI] [PubMed] [Google Scholar]
- 209. Vlotides G, Eigler T, Melmed S. Pituitary tumor-transforming gene: physiology and implications for tumorigenesis. Endocr Rev. 2007;28(2):165-186. [DOI] [PubMed] [Google Scholar]
- 210. Pack SD, Qin LX, Pak E, et al. Common genetic changes in hereditary and sporadic pituitary adenomas detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2005;43(1):72-82. [DOI] [PubMed] [Google Scholar]
- 211. Trautmann K, Thakker RV, Ellison DW, et al. Chromosomal aberrations in sporadic pituitary tumors. Int J Cancer. 2001;91(6):809-814. [DOI] [PubMed] [Google Scholar]
- 212. Hage M, Viengchareun S, Brunet E, et al. Genomic alterations and complex subclonal architecture in sporadic GH-secreting pituitary adenomas. J Clin Endocrinol Metab. 2018;103(5):1929-1939. [DOI] [PubMed] [Google Scholar]
- 213. Falch CM, Sundaram AYM, Oystese KA, et al. Gene expression profiling of fast- and slow-growing non-functioning gonadotroph pituitary adenomas. Eur J Endocrinol. 2018;178(3):295-307. [DOI] [PubMed] [Google Scholar]
- 214. Newey PJ, Nesbit MA, Rimmer AJ, et al. Whole-exome sequencing studies of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2013;98(4):E796-E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Fagin JA, Petrini JH. Oncogene-induced DNA damage: cyclic AMP steps into the ring. J Clin Invest. 2020;130(11):5668-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7(5):257-266. [DOI] [PubMed] [Google Scholar]
- 217. Chesnokova V, Zonis S, Rubinek T, et al. Senescence mediates pituitary hypoplasia and restrains pituitary tumor growth. Cancer Res. 2007;67(21):10564-10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Chesnokova V, Zhou C, Ben-Shlomo A, et al. Growth hormone is a cellular senescence target in pituitary and nonpituitary cells. Proc Natl Acad Sci USA. 2013;110(35):E3331-E3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Sapochnik M, Haedo MR, Fuertes M, et al. Autocrine IL-6 mediates pituitary tumor senescence. Oncotarget. 2017;8(3):4690-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sapochnik M, Fuertes M, Arzt E. Programmed cell senescence: role of IL-6 in the pituitary. J Mol Endocrinol. 2017;58(4):R241-R253. [DOI] [PubMed] [Google Scholar]
- 221. Ho KKY, Fleseriu M, Wass J, et al. A tale of pituitary adenomas: to NET or not to NET: Pituitary Society position statement. Pituitary. 2019;22(6):569-573. [DOI] [PubMed] [Google Scholar]
- 222. Costello RT. Subclinical adenoma of the pituitary gland. Am J Pathol. 1936;12(2):205-216. 201. [PMC free article] [PubMed] [Google Scholar]
- 223. Agustsson TT, Baldvinsdottir T, Jonasson JG, et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur J Endocrinol. 2015;173(5):655-664. [DOI] [PubMed] [Google Scholar]
- 224. Molitch ME. Nonfunctioning pituitary tumors. Handb Clin Neurol. 2014;124:167-184. [DOI] [PubMed] [Google Scholar]
- 225. Chambers EF, Turski PA, LaMasters D, Newton TH. Regions of low density in the contrast-enhanced pituitary gland: normal and pathologic processes. Radiology. 1982;144(1):109-113. [DOI] [PubMed] [Google Scholar]
- 226. Wolpert SM, Molitch ME, Goldman JA, Wood JB. Size, shape, and appearance of the normal female pituitary gland. AJR Am J Roentgenol. 1984;143(2):377-381. [DOI] [PubMed] [Google Scholar]
- 227. Peyster RG, Adler LP, Viscarello RR, Hoover ED, Skarzynski J. CT of the normal pituitary gland. Neuroradiology. 1986;28(2):161-165. [DOI] [PubMed] [Google Scholar]
- 228. Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med. 1994;120(10):817-820. [DOI] [PubMed] [Google Scholar]
- 229. Chong BW, Kucharczyk W, Singer W, George S. Pituitary gland MR: a comparative study of healthy volunteers and patients with microadenomas. AJNR Am J Neuroradiol. 1994;15(4):675-679. [PMC free article] [PubMed] [Google Scholar]
- 230. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769-4775. [DOI] [PubMed] [Google Scholar]
- 231. Fontana E, Gaillard R. Epidemiology of pituitary adenoma: results of the first Swiss study. Rev Med Suisse. 2009;5(223):2172-2174. [PubMed] [Google Scholar]
- 232. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72(3):377-382. [DOI] [PubMed] [Google Scholar]
- 233. Raappana A, Koivukangas J, Ebeling T, Pirila T. Incidence of pituitary adenomas in Northern Finland in 1992-2007. J Clin Endocrinol Metab. 2010;95(9):4268-4275. [DOI] [PubMed] [Google Scholar]
- 234. Tjornstrand A, Gunnarsson K, Evert M, et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur J Endocrinol. 2014;171(4):519-526. [DOI] [PubMed] [Google Scholar]
- 235. Ho KKY, Fleseriu M, Wass J, et al. The tale in evolution: clarity, consistency and consultation, not contradiction and confusion. Pituitary. 2020;23(4):476-477. [DOI] [PubMed] [Google Scholar]
- 236. Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804-812. [DOI] [PubMed] [Google Scholar]
- 237. Arosio M, Reimondo G, Malchiodi E, et al. ; Italian Study Group of Acromegaly. Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur J Endocrinol. 2012;167(2):189-198. [DOI] [PubMed] [Google Scholar]
- 238. Maione L, Brue T, Beckers A, et al. ; French Acromegaly Registry Group. Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur J Endocrinol. 2017;176(5):645-655. [DOI] [PubMed] [Google Scholar]
- 239. Mercado M, Gonzalez B, Vargas G, et al. Successful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinic. J Clin Endocrinol Metab. 2014;99(12):4438-4446. [DOI] [PubMed] [Google Scholar]
- 240. Lenders NF, McCormack AI, Ho KKY. Management of Endocrine Disease: does gender matter in the management of acromegaly? Eur J Endocrinol. 2020;182(5):R67-R82. [DOI] [PubMed] [Google Scholar]
- 241. Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. J Clin Endocrinol Metab. 2011;96(3):632-642. [DOI] [PubMed] [Google Scholar]
- 242. van Haalen FM, Broersen LH, Jorgensen JO, Pereira AM, Dekkers OM. Management of endocrine disease: mortality remains increased in Cushing’s disease despite biochemical remission: a systematic review and meta-analysis. Eur J Endocrinol. 2015;172(4):R143-R149. [DOI] [PubMed] [Google Scholar]
- 243. Graversen D, Vestergaard P, Stochholm K, Gravholt CH, Jorgensen JO. Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur J Intern Med. 2012;23(3):278-282. [DOI] [PubMed] [Google Scholar]
- 244. Ragnarsson O, Olsson DS, Papakokkinou E, et al. Overall and disease-specific mortality in patients with Cushing disease: a Swedish nationwide study. J Clin Endocrinol Metab. 2019;104(6):2375-2384. [DOI] [PubMed] [Google Scholar]
- 245. Anagnostis P, Adamidou F, Polyzos SA, Efstathiadou Z, Panagiotou A, Kita M. Pituitary incidentalomas: a single-centre experience. Int J Clin Pract. 2011;65(2):172-177. [DOI] [PubMed] [Google Scholar]
- 246. Askitis D, Tsitlakidis D, Muller N, et al. Complete evaluation of pituitary tumours in a single tertiary care institution. Endocrine. 2018;60(2):255-262. [DOI] [PubMed] [Google Scholar]
- 247. Gittleman H, Ostrom QT, Farah PD, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004-2009. J Neurosurg. 2014;121(3):527-535. [DOI] [PubMed] [Google Scholar]
- 248. Park JS, Yun SJ, Lee JK, Park SY, Chin SO. Descriptive epidemiology and survival analysis of prolactinomas and Cushing’s disease in Korea. Endocrinol Metab (Seoul). 2021;36(3):688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Holdaway IM, Rajasoorya C. Epidemiology of acromegaly. Pituitary. 1999;2(1):29-41. [DOI] [PubMed] [Google Scholar]
- 250. Dal J, Feldt-Rasmussen U, Andersen M, et al. Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur J Endocrinol. 2016;175(3):181-190. [DOI] [PubMed] [Google Scholar]
- 251. Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and prevalence of acromegaly in a large US health plan database. Pituitary. 2016;19(3):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Ragnarsson O, Olsson DS, Chantzichristos D, et al. The incidence of Cushing’s disease: a nationwide Swedish study. Pituitary. 2019;22(2):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Bolland MJ, Holdaway IM, Berkeley JE, et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75(4):436-442. [DOI] [PubMed] [Google Scholar]
- 254. Lindholm J, Juul S, Jorgensen JO, et al. Incidence and late prognosis of cushing’s syndrome: a population-based study. J Clin Endocrinol Metab. 2001;86(1):117-123. [DOI] [PubMed] [Google Scholar]
- 255. Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, Jonsson B. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab. 2000;85(4):1420-1425. [DOI] [PubMed] [Google Scholar]
- 256. Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab. 2015;100(2):467-474. [DOI] [PubMed] [Google Scholar]
- 257. Ioachimescu AG, Handa T, Goswami N, Pappy AL 2nd, Veledar E, Oyesiku NM. Gender differences and temporal trends over two decades in acromegaly: a single center study in 112 patients. Endocrine. 2020;67(2):423-432. [DOI] [PubMed] [Google Scholar]
- 258. Esposito D, Olsson DS, Ragnarsson O, Buchfelder M, Skoglund T, Johannsson G. Non-functioning pituitary adenomas: indications for pituitary surgery and post-surgical management. Pituitary. 2019;22(4):422-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Fernandez-Balsells MM, Murad MH, Barwise A, et al. Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2011;96(4):905-912. [DOI] [PubMed] [Google Scholar]
- 260. Vasilev V, Daly AF, Zacharieva S, Beckers A. Clinical and molecular update on genetic causes of pituitary adenomas. Horm Metab Res. 2020;52(8):553-561. [DOI] [PubMed] [Google Scholar]
- 261. Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404-407. [DOI] [PubMed] [Google Scholar]
- 262. Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011. [DOI] [PubMed] [Google Scholar]
- 263. Verges B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87(2):457-465. [DOI] [PubMed] [Google Scholar]
- 264. Goudet P, Dalac A, Le Bras M, et al. MEN1 disease occurring before 21 years old: a 160-patient cohort study from the Groupe d’etude des Tumeurs Endocrines. J Clin Endocrinol Metab. 2015;100(4):1568-1577. [DOI] [PubMed] [Google Scholar]
- 265. Beckers A, Betea D, Valdes Socin H, Stevenaert A. The treatment of sporadic versus MEN1-related pituitary adenomas. J Intern Med. 2003;253(6):599-605. [DOI] [PubMed] [Google Scholar]
- 266. Trouillas J, Labat-Moleur F, Sturm N, et al. ; Groupe d'études des Tumeurs Endocrines. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol. 2008;32(4):534-543. [DOI] [PubMed] [Google Scholar]
- 267. Cuny T, Pertuit M, Sahnoun-Fathallah M, et al. Genetic analysis in young patients with sporadic pituitary macroadenomas: besides AIP don’t forget MEN1 genetic analysis. Eur J Endocrinol. 2013;168(4):533-541. [DOI] [PubMed] [Google Scholar]
- 268. Rostomyan L, Daly AF, Petrossians P, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22(5):745-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA. 2006;103(42):15558-15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Rostomyan L, Daly AF, Beckers A. Pituitary tumors associated with multiple endocrine neoplasia syndromes. In: Huhtaniemi I, Martini L, eds. Encyclopedia of Endocrine Diseases. Cambridge, MA: Academic Press; 2019:642-647. [Google Scholar]
- 271. Chasseloup F, Pankratz N, Lane J, et al. Germline CDKN1B loss-of-function variants cause pediatric Cushing’s disease with or without an MEN4 phenotype. J Clin Endocrinol Metab. 2020;105(6):1983-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26(1): 89-92. [DOI] [PubMed] [Google Scholar]
- 273. Kamilaris CDC, Faucz FR, Voutetakis A, Stratakis CA. Carney complex. Exp Clin Endocrinol Diabetes. 2019;127: 156-164. [DOI] [PubMed] [Google Scholar]
- 274. Kiefer FW, Winhofer Y, Iacovazzo D, et al. PRKAR1A mutation causing pituitary-dependent Cushing disease in a patient with Carney complex. Eur J Endocrinol. 2017;177(2):K7-K12. [DOI] [PubMed] [Google Scholar]
- 275. Espiard S, Bertherat J. Carney complex. Front Horm Res. 2013;41:50-62. [DOI] [PubMed] [Google Scholar]
- 276. Espiard S, Vantyghem MC, Assie G, et al. Frequency and incidence of Carney complex manifestations: a prospective multicenter study with a three-year follow-up. J Clin Endocrinol Metab. 2020;105(3):e436-e446. [DOI] [PubMed] [Google Scholar]
- 277. Kurtkaya-Yapicier O, Scheithauer BW, Carney JA, et al. Pituitary adenoma in Carney complex: an immunohistochemical, ultrastructural, and immunoelectron microscopic study. Ultrastruct Pathol. 2002;26(6):345-353. [DOI] [PubMed] [Google Scholar]
- 278. Kaltsas GA, Kola B, Borboli N, et al. Sequence analysis of the PRKAR1A gene in sporadic somatotroph and other pituitary tumours. Clin Endocrinol (Oxf). 2002;57(4):443-448. [DOI] [PubMed] [Google Scholar]
- 279. Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N Engl J Med. 1937;216(17):727-746. [Google Scholar]
- 280. McCune DJ, Bruch H. Osteodystrophia fibrosa. Am J Dis Child. 1937; 54(4):806-848. [Google Scholar]
- 281. Collins MT, Singer FR, Eugster E. McCune-Albright syndrome and the extraskeletal manifestations of fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Salenave S, Boyce AM, Collins MT, Chanson P. Acromegaly and McCune-Albright syndrome. J Clin Endocrinol Metab. 2014;99(6):1955-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Javaid MK, Boyce A, Appelman-Dijkstra N, et al. Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: a consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis. 2019;14(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Dumitrescu CE, Collins MT. McCune-Albright syndrome. Orphanet J Rare Dis. 2008;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Lee JS, FitzGibbon EJ, Chen YR, et al. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J Rare Dis. 2012;7(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Boyce AM, Brewer C, DeKlotz TR, et al. Association of hearing loss and otologic outcomes with fibrous dysplasia. JAMA Otolaryngol Head Neck Surg. 2018;144(2):102-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Boyce AM, Glover M, Kelly MH, et al. Optic neuropathy in McCune-Albright syndrome: effects of early diagnosis and treatment of growth hormone excess. J Clin Endocrinol Metab. 2013;98(1):E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Vasilev V, Daly AF, Thiry A, et al. McCune-Albright syndrome: a detailed pathological and genetic analysis of disease effects in an adult patient. J Clin Endocrinol Metab. 2014;99(10):E2029-E2038. [DOI] [PubMed] [Google Scholar]
- 289. Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34(2):239-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Hernandez-Ramirez LC, Gabrovska P, Denes J, et al. Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J Clin Endocrinol Metab. 2015;100(9):E1242-E1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Daly AF, Jaffrain-Rea ML, Ciccarelli A, et al. Clinical characterization of familial isolated pituitary adenomas. J Clin Endocrinol Metab. 2006;91(9):3316-3323. [DOI] [PubMed] [Google Scholar]
- 292. Daly AF, Beckers A. Familial isolated pituitary adenomas (FIPA) and mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocrinol Metab Clin North Am. 2015;44(1):19-25. [DOI] [PubMed] [Google Scholar]
- 293. Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228-1230. [DOI] [PubMed] [Google Scholar]
- 294. Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92(5):1891-1896. [DOI] [PubMed] [Google Scholar]
- 295. Daly AF, Tichomirowa MA, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95(11):E373-E383. [DOI] [PubMed] [Google Scholar]
- 296. Beckers A, Lodish MB, Trivellin G, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22(3):353-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Abboud D, Daly AF, Dupuis N, et al. GPR101 drives growth hormone hypersecretion and gigantism in mice via constitutive activation of Gs and Gq/11. Nat Commun. 2020;11(1):4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Karlsson M, Zhang C, Mear L, et al. A single-cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31):eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Flak MB, Koenis DS, Sobrino A, et al. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J Clin Invest. 2020;130(1):359-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Cho-Clark M, Larco DO, Semsarzadeh NN, Vasta F, Mani SK, Wu TJ. GnRH-(1-5) transactivates EGFR in Ishikawa human endometrial cells via an orphan G protein-coupled receptor. Mol Endocrinol. 2014;28(1):80-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Trivellin G, Faucz FR, Daly AF, Beckers A, Stratakis CA. Hereditary endocrine tumours: current state-of-the-art and research opportunities: GPR101, an orphan GPCR with roles in growth and pituitary tumorigenesis. Endocr Relat Cancer. 2020;27(8):T87-T97. [DOI] [PubMed] [Google Scholar]
- 302. Beckers A, Fernandes D, Fina F, et al. Paleogenetic study of ancient DNA suggestive of X-linked acrogigantism. Endocr Relat Cancer. 2017;24(2):L17-L20. [DOI] [PubMed] [Google Scholar]
- 303. Beckers A, Petrossians P, Hanson J, Daly AF. The causes and consequences of pituitary gigantism. Nat Rev Endocrinol. 2018;14(12):705-720. [DOI] [PubMed] [Google Scholar]
- 304. Naves LA, Daly AF, Dias LA, et al. Aggressive tumor growth and clinical evolution in a patient with X-linked acro-gigantism syndrome. Endocrine. 2016;51(2):236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Daly AF, Castermans E, Oudijk L, et al. Pheochromocytomas and pituitary adenomas in three patients with MAX exon deletions. Endocr Relat Cancer. 2018;25(5):L37-L42. [DOI] [PubMed] [Google Scholar]
- 306. O’Toole SM, Denes J, Robledo M, Stratakis CA, Korbonits M. 15 YEARS OF PARAGANGLIOMA: the association of pituitary adenomas and phaeochromocytomas or paragangliomas. Endocr Relat Cancer. 2015;22(4):T105-T122. [DOI] [PubMed] [Google Scholar]
- 307. Xekouki P, Szarek E, Bullova P, et al. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab. 2015;100(5):E710-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Vila G, Fleseriu M. Fertility and pregnancy in women with hypopituitarism: a systematic literature review. J Clin Endocrinol Metab. 2020;105(3):e53-e65. [DOI] [PubMed] [Google Scholar]
- 309. Bronstein MD, Paraiba DB, Jallad RS. Management of pituitary tumors in pregnancy. Nat Rev Endocrinol. 2011;7(5):301-310. [DOI] [PubMed] [Google Scholar]
- 310. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888-3921. [DOI] [PubMed] [Google Scholar]
- 311. Luger A, Broersen LHA, Biermasz NR, et al. ESE Clinical Practice Guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur J Endocrinol. 2021;185(3):G1-G33. [DOI] [PubMed] [Google Scholar]
- 312. Kreutz J, Vroonen L, Cattin F, et al. Intensity of prolactinoma on T2-weighted magnetic resonance imaging: towards another gender difference. Neuroradiology. 2015;57(7):679-684. [DOI] [PubMed] [Google Scholar]
- 313. Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest. 2002;109(2):277-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Lebbe M, Hubinont C, Bernard P, Maiter D. Outcome of 100 pregnancies initiated under treatment with cabergoline in hyperprolactinaemic women. Clin Endocrinol (Oxf). 2010;73(2):236-242. [DOI] [PubMed] [Google Scholar]
- 315. Molitch ME. Endocrinology in pregnancy: management of the pregnant patient with a prolactinoma. Eur J Endocrinol. 2015;172(5):R205-R213. [DOI] [PubMed] [Google Scholar]
- 316. Huang W, Molitch ME. Pituitary tumors in pregnancy. Endocrinol Metab Clin North Am. 2019;48(3):569-581. [DOI] [PubMed] [Google Scholar]
- 317. Domingue ME, Devuyst F, Alexopoulou O, Corvilain B, Maiter D. Outcome of prolactinoma after pregnancy and lactation: a study on 73 patients. Clin Endocrinol (Oxf). 2014;80(5):642-648. [DOI] [PubMed] [Google Scholar]
- 318. Varlamov EV, Hinojosa-Amaya JM, Fleseriu M. Magnetic resonance imaging inthe management of prolactinomas; a review of the evidence. Pituitary. 2020;23(1):16-26. [DOI] [PubMed] [Google Scholar]
- 319. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265-273. [DOI] [PubMed] [Google Scholar]
- 320. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95(10):4680-4687. [DOI] [PubMed] [Google Scholar]
- 321. Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic complications of acromegaly and the impact of the current treatment landscape: an update. Endocr Rev. 2019;40(1):268-332. [DOI] [PubMed] [Google Scholar]
- 322. Vialon M, Grunenwald S, Mouly C, et al. Gestational diabetes and acromegaly: single-centre experience of 14 pregnancies. Clin Endocrinol (Oxf). 2019;91(6):805-809. [DOI] [PubMed] [Google Scholar]
- 323. Palejwala SK, Conger AR, Eisenberg AA, et al. Pregnancy-associated Cushing’s disease? An exploratory retrospective study. Pituitary. 2018;21(6):584-592. [DOI] [PubMed] [Google Scholar]
- 324. Jornayvaz FR, Assie G, Bienvenu-Perrard M, et al. Pregnancy does not accelerate corticotroph tumor progression in Nelson’s syndrome. J Clin Endocrinol Metab. 2011;96(4):E658-E662. [DOI] [PubMed] [Google Scholar]
- 325. Caimari F, Valassi E, Garbayo P, et al. Cushing’s syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine. 2017;55(2):555-563. [DOI] [PubMed] [Google Scholar]
- 326. Brue T, Amodru V, Castinetti F. Management of endocrine disease: management of Cushing’s syndrome during pregnancy: solved and unsolved questions. Eur J Endocrinol. 2018;178(6):R259-R266. [DOI] [PubMed] [Google Scholar]
- 327. Hochman C, Cristante J, Geslot A, et al. Pre-term birth in women exposed to Cushing’s disease: the Baby-Cush study. Eur J Endocrinol. 2021;184(3):469-476. [DOI] [PubMed] [Google Scholar]
- 328. Lambert K, Rees K, Seed PT, et al. Macroprolactinomas and nonfunctioning pituitary adenomas and pregnancy outcomes. Obstet Gynecol. 2017;129(1):185-194. [DOI] [PubMed] [Google Scholar]
- 329. Zoli M, Guaraldi F, Zoia C, et al. Management of sellar and parasellar tumors becoming symptomatic during pregnancy: a practical algorithm based on multi-center experience and systematic literature review. Pituitary. 2021;24(2):269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Kuhn E, Weinreich AA, Biermasz NR, Jorgensen JOL, Chanson P. Apoplexy of microprolactinomas during pregnancy: report of five cases and review of the literature. Eur J Endocrinol. 2021;185(1):99-108. [DOI] [PubMed] [Google Scholar]
- 331. Wass JA, Karavitaki N. Nonfunctioning pituitary adenomas: the Oxford experience. Nat Rev Endocrinol. 2009;5(9):519-522. [DOI] [PubMed] [Google Scholar]
- 332. Freda PU, Bruce JN, Khandji AG, et al. Presenting features in 269 patients with clinically nonfunctioning pituitary adenomas enrolled in a prospective study. J Endocr Soc. 2020;4(4):bvaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Pappy AL 2nd, Savinkina A, Bicknese C, Neill S, Oyesiku NM, Ioachimescu AG. Predictive modeling for pituitary adenomas: single center experience in 501 consecutive patients. Pituitary. 2019;22(5):520-531. [DOI] [PubMed] [Google Scholar]
- 334. Mazziotti G, Formenti AM, Frara S, et al. Management of endocrine disease: risk of overtreatment in patients with adrenal insufficiency: current and emerging aspects. Eur J Endocrinol. 2017;177(5):R231-R248. [DOI] [PubMed] [Google Scholar]
- 335. Mazziotti G, Mormando M, Cristiano A, et al. Association between l-thyroxine treatment, GH deficiency, and radiological vertebral fractures in patients with adult-onset hypopituitarism. Eur J Endocrinol. 2014;170(6):893-899. [DOI] [PubMed] [Google Scholar]
- 336. Gazzaruso C, Gola M, Karamouzis I, Giubbini R, Giustina A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH--an update. J Clin Endocrinol Metab. 2014;99(1):18-29. [DOI] [PubMed] [Google Scholar]
- 337. Mazziotti G, Doga M, Frara S, et al. Incidence of morphometric vertebral fractures in adult patients with growth hormone deficiency. Endocrine. 2016;52(1):103-110. [DOI] [PubMed] [Google Scholar]
- 338. Jasim S, Alahdab F, Ahmed AT, et al. Mortality in adults with hypopituitarism: a systematic review and meta-analysis. Endocrine. 2017;56(1):33-42. [DOI] [PubMed] [Google Scholar]
- 339. Pal A, Capatina C, Tenreiro AP, et al. Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol (Oxf). 2011;75(4):501-504. [DOI] [PubMed] [Google Scholar]
- 340. Melmed S. Pituitary medicine from discovery to patient-focused outcomes. J Clin Endocrinol Metab. 2016;101(3):769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Ho KK. The year in pituitary 2014. J Clin Endocrinol Metab. 2014;99(12):4449-4454. [DOI] [PubMed] [Google Scholar]
- 342. Dash S, Annamalai AK, Simpson HL, Sarkies N, Antoun NM, Mannion R. Acute shrinkage of a giant prolactinoma, masquerading as an erosive skull base tumour, with cabergoline. QJM. 2013;106(1):85. [DOI] [PubMed] [Google Scholar]
- 343. Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North Am. 2008;37(1):67-99, viii. [DOI] [PubMed] [Google Scholar]
- 344. Beccuti G, Guaraldi F, Natta G, et al. Increased prevalence of impulse control disorder symptoms in endocrine diseases treated with dopamine agonists: a cross-sectional study. J Endocrinol Invest. 2021;44(8):1699-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Andersen M, Glintborg D. Metabolic syndrome in hyperprolactinemia. Front Horm Res. 2018;49:29-47. [DOI] [PubMed] [Google Scholar]
- 346. Posawetz AS, Trummer C, Pandis M, et al. Adverse body composition and lipid parameters in patients with prolactinoma: a case-control study. BMC Endocr Disord. 2021;21(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. di Filippo L, Doga M, Resmini E, Giustina A. Hyperprolactinemia and bone. Pituitary. 2020;23(3):314-321. [DOI] [PubMed] [Google Scholar]
- 348. Mazziotti G, Porcelli T, Mormando M, et al. Vertebral fractures in males with prolactinoma. Endocrine. 2011;39(3):288-293. [DOI] [PubMed] [Google Scholar]
- 349. Mazziotti G, Mancini T, Mormando M, et al. High prevalence of radiological vertebral fractures in women with prolactin-secreting pituitary adenomas. Pituitary. 2011;14(4):299-306. [DOI] [PubMed] [Google Scholar]
- 350. Oh JS, Kim HJ, Hann HJ, et al. Incidence, mortality, and cardiovascular diseases in pituitary adenoma in Korea: a nationwide population-based study. Pituitary. 2021;24(1):38-47. [DOI] [PubMed] [Google Scholar]
- 351. Colao A, Grasso LFS, Giustina A, et al. Acromegaly. Nat Rev Dis Primers. 2019;5(1):20. [DOI] [PubMed] [Google Scholar]
- 352. Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Prolonged diagnostic delay in acromegaly is associated with increased morbidity and mortality. Eur J Endocrinol. 2020;182(6):523-531. [DOI] [PubMed] [Google Scholar]
- 353. Giustina A, Barkhoudarian G, Beckers A, et al. Multidisciplinary management of acromegaly: a consensus. Rev Endocr Metab Disord. 2020;21(4):667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Giustina A, Mancini T, Boscani PF, et al. ; Group CETAIS. Assessment of the awareness and management of cardiovascular complications of acromegaly in Italy. The COM.E.T.A. (COMorbidities Evaluation and Treatment in Acromegaly) Study. J Endocrinol Invest. 2008;31(8):731-738. [DOI] [PubMed] [Google Scholar]
- 355. Ritvonen E, Loyttyniemi E, Jaatinen P, et al. Mortality in acromegaly: a 20-year follow-up study. Endocr Relat Cancer. 2016;23(6):469-480. [DOI] [PubMed] [Google Scholar]
- 356. Vila G, Luger A, van der Lely AJ, et al. Hypertension in acromegaly in relationship to biochemical control and mortality: global ACROSTUDY outcomes. Front Endocrinol (Lausanne). 2020;11:577173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Kamenicky P, Viengchareun S, Blanchard A, et al. Epithelial sodium channel is a key mediator of growth hormone-induced sodium retention in acromegaly. Endocrinology. 2008;149(7): 3294-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Kamenicky P, Mazziotti G, Lombes M, Giustina A, Chanson P. Growth hormone, insulin-like growth factor-1, and the kidney: pathophysiological and clinical implications. Endocr Rev. 2014;35(2):234-281. [DOI] [PubMed] [Google Scholar]
- 359. Gonzalez B, Vargas G, de Los Monteros ALE, Mendoza V, Mercado M. Persistence of diabetes and hypertension after multimodal treatment of acromegaly. J Clin Endocrinol Metab. 2018;103(6):2369-2375. [DOI] [PubMed] [Google Scholar]
- 360. Kamenicky P, Maione L, Chanson P. Cardiovascular complications of acromegaly. Ann Endocrinol (Paris). 2021;82(3-4):206-209. [DOI] [PubMed] [Google Scholar]
- 361. Akutsu H, Kreutzer J, Wasmeier G, et al. Acromegaly per se does not increase the risk for coronary artery disease. Eur J Endocrinol. 2010;162(5):879-886. [DOI] [PubMed] [Google Scholar]
- 362. Giustina A, Boni E, Romanelli G, Grassi V, Giustina G. Cardiopulmonary performance during exercise in acromegaly, and the effects of acute suppression of growth hormone hypersecretion with octreotide. Am J Cardiol. 1995;75(15):1042-1047. [DOI] [PubMed] [Google Scholar]
- 363. Davi MV, Giustina A. Sleep apnea in acromegaly: a review on prevalence, pathogenetic aspects and treatment. Expert Rev Endocrinol Metab. 2012;7(1):55-62. [DOI] [PubMed] [Google Scholar]
- 364. Cao W, Wang X, Luo J, Huang R, Xiao Y. Impact of obstructive sleep apnea on cardiovascular risk in patients with acromegaly. Sleep Med. 2021;80:193-198. [DOI] [PubMed] [Google Scholar]
- 365. Davi MV, Dalle Carbonare L, Giustina A, et al. Sleep apnoea syndrome is highly prevalent in acromegaly and only partially reversible after biochemical control of the disease. Eur J Endocrinol. 2008;159(5):533-540. [DOI] [PubMed] [Google Scholar]
- 366. Berkmann S, Brun J, Schuetz P, Christ E, Mariani L, Mueller B. Prevalence and outcome of comorbidities associated with acromegaly. Acta Neurochir (Wien). 2021;163(11):3171-3180. [DOI] [PubMed] [Google Scholar]
- 367. Biagetti B, Aulinas A, Casteras A, Perez-Hoyos S, Simo R. HOMA-IR in acromegaly: a systematic review and meta-analysis. Pituitary. 2021;24(2):146-158. [DOI] [PubMed] [Google Scholar]
- 368. Frara S, Maffezzoni F, Mazziotti G, Giustina A. Current and emerging aspects of diabetes mellitus in acromegaly. Trends Endocrinol Metab. 2016;27(7):470-483. [DOI] [PubMed] [Google Scholar]
- 369. Chiloiro S, Giampietro A, Visconti F, et al. Glucose metabolism outcomes in acromegaly patients on treatment with pasireotide-LAR or pasireotide-LAR plus Pegvisomant. Endocrine. 2021;73(3):658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Claessen KM, Mazziotti G, Biermasz NR, Giustina A. Bone and joint disorders in acromegaly. Neuroendocrinology. 2016;103(1):86-95. [DOI] [PubMed] [Google Scholar]
- 371. Vouzouneraki K, Esposito D, Mukka S, et al. Carpal tunnel syndrome in acromegaly: a nationwide study. Eur J Endocrinol. 2021;184(2):209-216. [DOI] [PubMed] [Google Scholar]
- 372. Giustina A. Acromegaly and vertebral fractures: facts and questions. Trends Endocrinol Metab. 2020;31(4):274-275. [DOI] [PubMed] [Google Scholar]
- 373. Mazziotti G, Frara S, Giustina A. Pituitary diseases and bone. Endocr Rev. 2018;39(4):440-488. [DOI] [PubMed] [Google Scholar]
- 374. Chiloiro S, Giampietro A, Frara S, et al. Effects of pegvisomant and pasireotide LAR on vertebral fractures in acromegaly resistant to first-generation SRLs. J Clin Endocrinol Metab. 2020;105(3):dgz054. [DOI] [PubMed] [Google Scholar]
- 375. Esposito D, Ragnarsson O, Johannsson G, Olsson DS. Incidence of benign and malignant tumors in patients with acromegaly is increased: a nationwide population-based study. J Clin Endocrinol Metab. 2021;106(12):3487-3496. [DOI] [PubMed] [Google Scholar]
- 376. Ceccato F, Barbot M, Lizzul L, et al. Clinical presentation and management of acromegaly in elderly patients. Hormones (Athens). 2021;20(1):143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Wu JC, Huang WC, Chang HK, Ko CC, Lirng JF, Chen YC. Natural history of acromegaly: incidences, re-operations, cancers, and mortality rates in a national cohort. Neuroendocrinology. 2020;110(11-12):977-987. [DOI] [PubMed] [Google Scholar]
- 378. Thomas M, Berni E, Jenkins-Jones S, et al. Insulin-like growth factor-1, growth hormone and disease outcomes in acromegaly: a population study. Clin Endocrinol (Oxf). 2021;95(1):143-152. [DOI] [PubMed] [Google Scholar]
- 379. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Mancini T, Porcelli T, Giustina A. Treatment of Cushing disease: overview and recent findings. Ther Clin Risk Manag. 2010;6:505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Schernthaner-Reiter MH, Siess C, Micko A, et al. Acute and life-threatening complications in Cushing syndrome: prevalence, predictors, and mortality. J Clin Endocrinol Metab. 2021;106(5):e2035-e2046. [DOI] [PubMed] [Google Scholar]
- 382. Suarez MG, Stack M, Hinojosa-Amaya JM, et al. Hypercoagulability in Cushing Syndrome, prevalence of thrombotic events: a large, single-center, retrospective study. J Endocr Soc. 2020;4(2):bvz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. van der Pas R, de Bruin C, Leebeek FW, et al. The hypercoagulable state in Cushing’s disease is associated with increased levels of procoagulant factors and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab. 2012;97(4):1303-1310. [DOI] [PubMed] [Google Scholar]
- 384. Geer EB, Shen W, Strohmayer E, Post KD, Freda PU. Body composition and cardiovascular risk markers after remission of Cushing’s disease: a prospective study using whole-body MRI. J Clin Endocrinol Metab. 2012;97(5):1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. 2013;9(5):265-276. [DOI] [PubMed] [Google Scholar]
- 386. Mazziotti G, Formenti AM, Frara S, Maffezzoni F, Doga M, Giustina A. Diabetes in Cushing disease. Curr Diab Rep. 2017;17(5):32. [DOI] [PubMed] [Google Scholar]
- 387. Sun X, Feng M, Lu L, et al. Lipid abnormalities in patients with Cushing’s disease and its relationship with impaired glucose metabolism. Front Endocrinol (Lausanne). 2020;11:600323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Toja PM, Branzi G, Ciambellotti F, et al. Clinical relevance of cardiac structure and function abnormalities in patients with Cushing’s syndrome before and after cure. Clin Endocrinol (Oxf). 2012;76(3):332-338. [DOI] [PubMed] [Google Scholar]
- 389. Mancini T, Doga M, Mazziotti G, Giustina A. Cushing’s syndrome and bone. Pituitary. 2004;7(4):249-252. [DOI] [PubMed] [Google Scholar]
- 390. Canalis E, Bilezikian JP, Angeli A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone. 2004;34(4): 593-598. [DOI] [PubMed] [Google Scholar]
- 391. Frara S, Allora A, di Filippo L, et al. Osteopathy in mild adrenal Cushing’s syndrome and Cushing disease. Best Pract Res Clin Endocrinol Metab. 2021;35(2):101515. [DOI] [PubMed] [Google Scholar]
- 392. Vogel F, Braun LT, Rubinstein G, et al. Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J Clin Endocrinol Metab. 2020;105(12):e4490-e4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Vogel F, Braun L, Rubinstein G, et al. Patients with low IGF-I after curative surgery for Cushing’s syndrome have an adverse long-term outcome of hypercortisolism-induced myopathy. Eur J Endocrinol. 2021;184(6):813-821. [DOI] [PubMed] [Google Scholar]
- 394. Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The immune system in Cushing’s syndrome. Trends Endocrinol Metab. 2020;31(9):655-669. [DOI] [PubMed] [Google Scholar]
- 395. Vogel F, Reincke M. Endocrine risk factors for COVID-19: endogenous and exogenous glucocorticoid excess. Rev Endocr Metab Disord. 2022;23(2):233-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Frara S, Allora A, Castellino L, di Filippo L, Loli P, Giustina A. COVID-19 and the pituitary. Pituitary. 2021;24(3):465-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Piasecka M, Papakokkinou E, Valassi E, et al. Psychiatric and neurocognitive consequences of endogenous hypercortisolism. J Intern Med. 2020;288(2):168-182. [DOI] [PubMed] [Google Scholar]
- 398. Ntali G, Hakami O, Wattegama M, Ahmed S, Karavitaki N. Mortality of patients with Cushing’s disease. Exp Clin Endocrinol Diabetes. 2021;129(3):203-207. [DOI] [PubMed] [Google Scholar]
- 399. Roldan-Sarmiento P, Lam-Chung CE, Hinojosa-Amaya JM, et al. Diabetes, active disease, and afternoon serum cortisol levels predict Cushing’s disease mortality: a cohort study. J Clin Endocrinol Metab. 2021;106(1):e103-e111. [DOI] [PubMed] [Google Scholar]
- 400. Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol. 2016;4(7):569-576. [DOI] [PubMed] [Google Scholar]
- 401. De Herdt C, Philipse E, De Block C. Endocrine tumours: thyrotropin-secreting pituitary adenoma: a structured review of 535 adult cases. Eur J Endocrinol. 2021;185(2):R65-R74. [DOI] [PubMed] [Google Scholar]
- 402. Shimatsu A, Nakamura A, Takahashi Y, et al. Preoperative and long-term efficacy and safety of lanreotide autogel in patients with thyrotropin-secreting pituitary adenoma: a multicenter, single-arm, phase 3 study in Japan. Endocr J. 2021;68(7):791-805. [DOI] [PubMed] [Google Scholar]
- 403. Yoshiki K, Sasagawa Y, Shimojima M, et al. Thyrotropin-secreting pituitary adenomas induce left atrial enlargement with subclinical atrial fibrillation: an echocardiographic study. Pituitary. 2021;24(5):778-786. [DOI] [PubMed] [Google Scholar]
- 404. Frara S, Losa M, Doga M, et al. High prevalence of radiological vertebral fractures in patients with TSH-secreting pituitary adenoma. J Endocr Soc. 2018;2(9):1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Nabhan F, Ringel MD. Thyroid nodules and cancer management guidelines: comparisons and controversies. Endocr Relat Cancer. 2017;24(2):R13-R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.