Abstract
Purpose
To determine the prevalence, characteristics, and causes of papillary and peripapillary hemorrhages (PPHs) in eyes with pathologic myopia (PM).
Methods
PM patients were retrospectively studied between 2011 and 2018. Fundus images were used to diagnose and classify the PPHs. Fundus fluorescein angiographic (FFA) and optical coherence tomographic (OCT) images were used to determine the status of the retinal vessels and tissue at and around the PPH sites. Visual field data determined by Goldmann perimetry and Humphrey visual field analyzer were also analyzed.
Results
A total of 2171 PM patients (3774 eyes) were examined. Eighty-eight patients (97 eyes) had PPHs (mean age 66.8 ± 11.9 years; mean axial length 30.79 ± 2.17 mm) for a prevalence of 4.05%. Thirty (30.9%) eyes recurred. Among the 90 eyes with a single-site PPH, the most common type and location were the conus type (49 eyes, 54.4%) and the temporal side (66 eyes, 73.3%), respectively. Regression analysis showed that patchy atrophy reduced the risk of recurrences than diffuse atrophy (P < 0.05), whereas a longer axial length and potential glaucoma increased the risk (both P < 0.05). FFA and OCT showed that PPHs developed in the area of straightened retinal arterioles (24 eyes), at or beside the peak of a ridge (10 eyes), in an area of compressed retinal tissue (two eyes).
Conclusions
PPHs are present in 4.05% of PM eyes, and they are most often located in the temporal peripapillary atrophic region of the retina. Axial elongation, mild myopic maculopathy, and potential glaucoma are risk factors for recurrences.
Keywords: disc hemorrhage, optic disc, myopia, pathologic myopia, peripapillary atrophy
It is known that a hemorrhage on or at the margin of the optic disc, classified as an optic disc hemorrhage (DH), is strongly associated with the development and progression of glaucoma.1–8 The prevalence of DHs in glaucomatous eyes has been reported to vary between 1.45% to 36.9% in different studies,9,10 and it has also been reported that DHs occur not only in glaucomatous eyes but also in normal eyes.9–12 The earlier studies on DHs were usually performed in glaucoma cohorts, and myopia was only considered as an influencing factor and eyes with high myopia were usually excluded from the DH studies. 3,8,13–17
Pathologic myopia (PM) is an important cause of blindness, especially in East Asia,18–20 and it is diagnosed by the presence of high myopia with a refractive error of < −6.0 diopters (D), an axial length >26.5 mm, and the presence of myopic maculopathy equal to or worse than diffuse atrophy or posterior staphylomas. 21,22 Earlier studies have shown that optic nerve (ON) damage was commonly seen in eyes with PM. 23–25 However, the relationship between DHs and ON damage has not been determined in eyes with PM.
We have observed that the DHs are located not only on the optic disc and its margins, but also within the peripapillary atrophic (PPA) areas surrounding the optic disc. Therefore we have renamed DHs as papillary and peripapillary hemorrhages (PPHs). Our search of PubMed using the terms: “disc hemorrhage” AND “high myopia”/“disc hemorrhage” AND “pathologic myopia” showed that there has not been a study that examined PPHs in eyes with PM, even though DHs are not rare in myopic eyes. Thus the purpose of this study was to determine the prevalence, clinical and morphological characteristics, and causes of PPHs in eyes with PM.
Methods
Data Collection
We reviewed the medical records of all patients diagnosed with PM who had been examined and followed up in the Advanced Clinical Center for Myopia at the Tokyo Medical and Dental University (TMDU) between January 2011 and December 2018. The procedures used in this study adhered to the tenets of the Declaration of Helsinki and were approved by the Ethics Committee of TMDU. All participants signed an informed consent form. The exclusion criteria were eyes with other diseases that could cause PPHs. Eyes with hemorrhages caused by choroidal neovascularization were also excluded.
Diagnosis and Classification
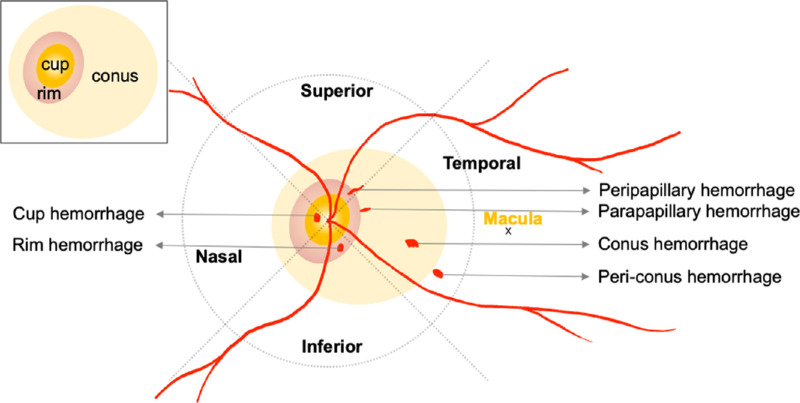
A PPH is a hemorrhage that develops on or around the optic disc in the area of PPA or in the periconus area close to the edge of the PPA. We divided the optic disc and its periphery into four quadrants (the temporal, superior, nasal and inferior), with the central retinal artery at the center and two lines separated by 90° forming the borders (Fig. 12). The PPHs were classified into six types (Figs. 1, 2): a cup hemorrhage was located on the optic disc cup, a rim hemorrhage was present on the neuroretinal rim, a peripapillary hemorrhage began within the optic disc and crossed the rim of the optic disc, a parapapillary hemorrhage was outside but adjacent to the edge of the optic disc, a conus hemorrhage was present in the area of a PPA, and a periconus hemorrhage was outside but close to the edge of the PPA.10
Figure 1.
Schematic drawing of posterior pole of an eye showing the six types of PPHs and their locations relative to the optic disc.
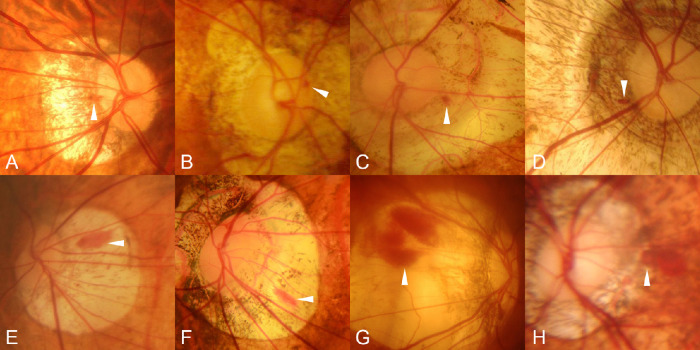
Figure 2.
Fundus photographs showing different types of PPHs. (A) Peripapillary hemorrhage in a 46-year-old woman with an axial length of 31.39 mm. (B) Rim hemorrhage in an 84-year-old man with an axial length of 29.02 mm (within two years). (C, D) Parapapillary hemorrhages in a 63-year-old man with an axial length of 32.89 mm and a 77-year-old woman with an axial length of 31.55 mm. (E–G) Conus hemorrhages in an 82-year-old man with an axial length of 32.40 mm (within 2 years), in a 57-year-old woman with an axial length of 31.48 mm (within two years), and in a 55-year-old woman with an axial length of 31.44 mm. (H) Periconus hemorrhage in a 25-year-old woman with an axial length of 35.54 mm (within three years).
General Examinations
Our patients were examined at three- to six-monthly intervals. The sex, age, axial length (within one year of the detection of the PPHs), intraocular pressure (IOP), refractive error (spherical equivalent), and best-corrected visual acuity (BCVA) were collected from the medical records. Color fundus photographs were examined to determine the characteristics of the PPHs.
The diagnosis of a recurrence was based on the findings of at least three types of consecutive images: hemorrhage, resolution, and re-hemorrhage. The absorption period referred to the interval between the first detection of a PPH and its complete absorption. Multiple times PPHs were defined as the detections of two or more occurrences of a PPH in an eye regardless of the location with a complete absorption in between. We further subdivided these eyes into recurrences at the same site and new hemorrhages at different sites.
Optical Coherence Tomography (OCT) and Fluorescein Fundus Angiography (FFA)
OCT examinations were performed with a spectral domain OCT device (Cirrus HD-OCT; Carl-Zeiss, Dublin, CA, USA) or a swept-source OCT device (DRI-Atlantis and DRI-Triton; Topcon Corp, Tokyo, Japan). The OCT scanning parameters were a scan length of 6 or 9 mm with 12 radial scans centered on the fovea or optic disc. An abrupt bending of the sclera in the OCT image was classified as a ridge when the vertical distance from the highest point of the sclera to a horizontal line starting at the edge of the optic disc was greater than 150 µm.25 FFAs were recorded with the Kowa VX-10i (Kowa Company, Ltd., Nagoya, Japan) device or an HRA Spectralis (Heidelberg Engineering, Heidelberg, Germany) device. All OCT and FFA results in the eight-year study period were used to determine the condition of the fundus at the site of the hemorrhage to obtain clues on the pathogenesis of the PPH.
Suspected ON Damage
The diagnosis of glaucoma in PM eyes is very difficult mainly because the optic disc is severely stretched and deformed, and the Humphrey field analyzer (HFA) findings are difficult to interpret because of coexisting large PPAs and the presence of myopic maculopathy lesions. Because HFA is not helpful, Xie et al.25 and Ohno-Matsui et al.23 used Goldmann perimetry instead of HFA, and they determined that ON damage was present when the visual field (VF) defects precisely matched the locations of the ON damage. Because of these difficulties, we have created two subgroups, which indicated that glaucoma may be present. The first group was the “possible glaucoma” group which included eyes whose IOP was >21 mm Hg and had VF defects that could not be explained by the myopic maculopathy lesions. The second group was the “possible ON damage” group which included eyes whose VF defects could not be explained by the myopic maculopathy lesions regardless of the IOP. All of the Goldmann perimetry findings or HFA findings in the absence of Goldmann perimetry findings were used to group eyes that had ever been treated by IOP-lowering topical medications during the eight-year period. Goldmann perimetry was performed with the refractive error fully corrected by disposable contact lenses. The results of the HFA C30-2 program (Zeiss Humphrey Systems, Dublin, CA, USA) with good reliability (fixation losses and false-negative and false-positive errors <33%)8 were supplementally used.
Statistical Analyses
All statistical analyses were performed with the SPSS 25.0 software (IBM Corp., released 2017; IBM SPSS Statistics for Windows, Version 25.0; IBM Corp., Armonk, NY, USA). P < 0.05 was taken to be statistically significant. The significance of the differences in the age was determined by Student's t tests, Mann-Whitney U tests or Welch's t tests. The axial length, IOP, and refractive error were determined by Student's t tests, Mann-Whitney U tests, one-way ANOVA, or Bonferroni tests. The statistical analyses of refractive error were calculated after excluding eyes with refractive surgery. BCVA and absorption period were determined by Mann-Whitney U tests or Kruskal-Wallis tests. The sex distribution, re-hemorrhaging status, and category of myopic maculopathy were determined by Pearson's χ2 tests or Fisher's exact tests. Risk factors for recurrences were determined by multivariate binary regression analyses.
Results
There were 3774 eyes of 2171 patients with PM in the registry of the Advanced Clinical Center for Myopia. All of the patients were examined between 2011 to 2018. In the end, 97 eyes of 88 patients that had a PPH and met the inclusion criteria were studied. The prevalence of PPH was 4.05% during the eight-year period. Of the 88 patients, nine patients had a PPH in both eyes, and seven patients had PPHs at multiple sites in one eye. Thirty-five eyes of 32 patients presented with one of the four types of PPHs: the cup, peripapillary, parapapillary, and rim type. The overall prevalence of the four types, which are referred to as disc-related PPHs, was 1.47%. On the other hand, 64 eyes of 61 patients had either the conus or periconus type, both of which were referred to as conus-related PPHs with an overall prevalence of 2.81%. Among these, three patients had different types of PPHs in the two eyes, and two eyes of two patients had PPHs at multiple sites. One had peripapillary and conus hemorrhages and the other had cup and conus hemorrhages.
Overall Characteristics of PPHs
A PPH was detected in 97 eyes of 88 patients (Table 1). The mean age of these patients was 66.8 ± 11.9 years, the mean axial length was 30.79 ± 2.17 mm, the mean refractive error was −15.02 ± 5.46 D, and the mean IOP was 15.03 ± 3.36 mm Hg. The median follow-up interval was 3.03 months, and the median follow-up period was 33.43 months. The median absorption period of the PPH was 182 days (range 28 to 1519 days). Thirty (30.9%) eyes developed a PPH multiple times, and 12 had a recurrence at the same site, 13 at a new site, and five had recurrences at the same site and also at a new site. Among these, the mean number of recurrences was 1.27 times (range 1 to 3).
Table 1.
General Characteristics of Patients With PPH
| Values | |
|---|---|
| Number of eyes (patients) | 97 (88) |
| Sex | |
| Female | 67 (69.1%) |
| Male | 30 (30.9%) |
| Age (y), mean (SD) | 66.8 (11.9) |
| Axial length (mm), mean (SD) | 30.79 (2.17) |
| IOP (mm Hg), mean (SD) | 15.03 (3.36) |
| Refractive error (D), mean (SD) | −15.02 (5.46) |
| Median BCVA [25%; 75%] | 0.70 [0.20; 1.00] |
| Median absorption period (d), [25%; 75%] | 182.00 [126.00; 329.00] |
| Re-hemorrhage status | |
| Single time hemorrhage | 67 (69.1%) |
| Multiple times hemorrhages* | 30 (30.9%) |
| Recurrence at the same site | 17 (17.5%) |
| New hemorrhage at a different site | 18 (18.6%) |
| Category of myopic maculopathy† | |
| Diffuse atrophy (C2) | 35 (36.1%) |
| Patchy atrophy (C3) | 42 (43.3%) |
| Macular atrophy (C4) | 20 (20.6%) |
| Possible ON damage (%) | 23 (23.7%) |
| Possible glaucoma (%) | 9 (9.3%) |
SD, standard deviation.
Five eyes recurred not only at the same site but also at a new site.
According to META-PM study classification.
Characteristics of PPH Eyes With “Possible ON Damage” and “Possible Glaucoma”
Twenty-three eyes (23.7%) were diagnosed as “possible ON damage,” and nine eyes (9.3%) were diagnosed as “possible glaucoma.” Comparison of the characteristics of the PPH eyes with and without “possible ON damage” (Table 2) showed that 23 eyes (23.7%) with “possible ON damage” had increased number of recurrences (12 [52.2%]; P = 0.012), whether at the same site (8 [34.8%]; P = 0.013) or at a different site (8 [34.8%]; P = 0.022). Other characteristics were not significantly different. In addition, comparisons between eyes with (9 [9.3%]) and without (88 [90.7%]) “possible glaucoma” showed no significant difference in all of the characteristics.
Table 2.
Comparison of Characteristics Between PPH Eyes With or Without “Possible ON Damage” and “Possible Glaucoma”
| Possible ON Damage | Possible Glaucoma | |||||
|---|---|---|---|---|---|---|
| Positive | Negative | P | Positive | Negative | P | |
| Number of eyes | 23 (23.7%) | 74 (76.3%) | — | 9 (9.3%) | 88 (90.7%) | — |
| Sex | 0.330 | 0.588 | ||||
| Female | 14 (60.9%) | 53 (71.6%) | 5 (55.56%) | 62 (70.45%) | ||
| Male | 9 (39.1%) | 21 (28.4%) | 4 (44.44%) | 26 (29.55%) | ||
| Age (y), mean (SD) | 66.1 (9.8) | 67.0 (12.5) | 0.497 | 66.11 (12.30) | 66.87 (11.88) | 0.856 |
| Axial length (mm), mean (SD) | 30.85 (1.99) | 30.77 (2.26) | 0.897 | 31.12 (2.63) | 30.75 (2.12) | 0.633 |
| IOP (mm Hg), mean (SD) | 15.11 (3.72) | 15.00 (3.26) | 0.893 | 17.11 (3.84) | 14.80 (3.26) | 0.064 |
| Refractive error (D), mean (SD) | −14.19 (4.67) | −15.20 (5.67) | 0.512 | −13.81 (5.04) | −15.09 (5.55) | 0.608 |
| Median BCVA, [25%; 75%] | 0.90 [0.55; 0.95] | 0.65 [0.20; 0.98] | 0.348 | 0.60 [0.10; 0.90] | 0.80 [0.20; 1.00] | 0.311 |
| Median absorption period (d), [25%; 75%] | 182.00 [140.00; 438.50] | 182.00 [126.00; 299.25] | 0.397 | 210.00 [182.00; 511.00] | 182.00 [126.00; 302.75] | 0.198 |
| Re-hemorrhage status, eyes | ||||||
| Multiple times hemorrhages† | 12 (52.2%) | 18 (24.3%) | 0.012* | 5 (55.56%) | 25 (28.41%) | 0.194 |
| Recurrence at the same site | 8 (34.8%) | 9 (12.2%) | 0.013* | 2 (22.22%) | 15 (17.05%) | 1.000 |
| New hemorrhage at a different site | 8 (34.8%) | 10 (13.5%) | 0.022* | 4 (44.44%) | 14 (15.91%) | 0.100 |
| Category of myopic maculopathy‡ | 0.405 | 0.175 | ||||
| Diffuse atrophy (C2) | 11 (47.8%) | 24 (32.4%) | 2 (22.22%) | 33 (37.50%) | ||
| Patchy atrophy (C3) | 8 (34.8%) | 34 (45.9%) | 3 (33.33%) | 39 (44.32%) | ||
| Macular atrophy (C4) | 4 (17.4%) | 16 (21.6%) | 4 (44.44%) | 16 (18.18%) | ||
SD, standard deviation.
Pearson's χ2 tests.
Some eyes with multiple times hemorrhages recurred not only at the same site but also at a new site, so the sum number of eyes of the next two-row might surpass this row.
According to META-PM study classification.
Risk Factors for Recurrences
Comparisons of eyes with single time PPHs to eyes with multiple times PPHs and the corresponding odds ratios (ORs) and 95% confidence intervals (CIs) are presented in Table 3. Controlling the confounding factors of sex, age, axial length, BCVA, category of myopic maculopathy and “possible ON damage” (regression 1 in Table 3), patchy atrophy reduced the risk of a recurrence than diffuse atrophy significantly (OR = 0.188; 95% CI, 0.036-0.796; P < 0.05). The increase in the axial length (OR = 1.741; 95% CI, 1.172-2.802; P < 0.05) and “possible ON damage” (OR = 3.920; 95% CI, 1.152-14.506; P < 0.05) enhanced the risk of a recurrence.
Table 3.
Comparison of Characteristics of Eyes With Single-Time PPH to Eyes With Multiple-Times PPH
| Regression 1* | Regression 2† | |||||||
|---|---|---|---|---|---|---|---|---|
| Single-Time (67 Eyes) | Multiple-Times (30 Eyes) | B | OR (95%CI) | P | B | OR (95%CI) | P | |
| Sex | ||||||||
| Female | 44 (65.7%) | 23 (76.7%) | Ref. | Ref. | — | Ref. | — | |
| Male | 23 (34.3%) | 7 (23.3%) | −0.568 | 0.567 (0.135, 2.066) | 0.408 | −0.506 | 0.603 (0.143, 2.209) | 0.462 |
| Age (y), mean (SD) | 66.5 (12.7) | 67.4 (9.9) | 0.054 | 1.055 (0.990, 1.133) | 0.112 | 0.065 | 1.067 (0.999, 1.150) | 0.066 |
| Axial length (mm), mean (SD) | 30.55 (2.26) | 31.41 (1.83) | 0.554 | 1.741 (1.172, 2.802) | 0.012* | 0.622 | 1.863 (1.223, 3.112) | 0.009† |
| IOP (mm Hg), mean (SD) | 14.91 (3.56) | 15.32 (2.86) | — | — | — | — | ||
| Refractive error (D), mean (SD) | −14.85 (4.81) | −15.56 (7.57) | — | — | — | — | ||
| Median BCVA [25%; 75%] | 0.70 [0.25; 0.90] | 0.90 [0.12; 1.00] | 0.308 | 1.360 (0.361, 4.940) | 0.635 | 0.408 | 1.504 (0.350, 6.065) | 0.566 |
| Median absorption period (d), [25%; 75%] | 185.50 [126.00; 313.25] | 182.00 [126.00; 371.00] | — | — | — | — | ||
| Category of myopic maculopathy‡, eyes | 0.098 | 0.034 | ||||||
| Diffuse atrophy (C2) | 21 (31.3%) | 14 (46.7%) | Ref. | Ref. | — | Ref. | — | |
| Patchy atrophy (C3) | 33 (49.3%) | 9 (30.0%) | −1.669 | 0.188 (0.036, 0.796) | 0.031* | −2.158 | 0.116 (0.019, 0.526) | 0.009† |
| Macular atrophy (C4) | 13 (19.4%) | 7 (23.3%) | −2.040 | 0.130 (0.011, 1.125) | 0.080 | −3.279 | 0.038 (0.001, 0.500) | 0.027† |
| Possible ON damage | 11 (16.4%) | 12 (40.0%) | 1.366 | 3.920 (1.152, 14.506) | 0.032* | |||
| Possible glaucoma | 4 (6.0%) | 5 (16.7%) | 2.362 | 10.617 (1.578, 98.313) | 0.022† | |||
B, regression coefficient; Ref, reference; SD, standard deviation.
Multivariate binary regression analysis included sex, age, axial length, BCVA and category of myopic maculopathy, possible ON damage.
Multivariate binary regression analysis included sex, age, axial length, BCVA and category of myopic maculopathy, possible glaucoma.
According to META-PM study classification.
For the “possible glaucoma” eyes the regression analysis (regression 2 in Table 3) found that macular atrophy in addition to patchy atrophy also reduced the risk of recurrences significantly (OR = 0.038; 95% CI, 0.001-0.500; P < 0.05). Likewise, an increase in the axial length (OR = 1.863; 95% CI, 1.223-3.112; P < 0.05) and “possible ON damage” (OR = 10.617; 95% CI, 1.578-98.313; P < 0.05) enhanced the risk of a recurrence.
Characteristics of Each Type of Single-Site PPH
Among the 97 eyes, 90 eyes had a PPH at a single site (Table 4), and analyses showed that conus hemorrhage was the most common type (49 [54.4%]), followed by rim (12 [13.3%]), peripapillary (10 [11.1%]), parapapillary (9 [10%]), peri-conus (9 [10%]), and cup hemorrhage (1 [1.1%]). Conus and periconus hemorrhages were not reported in earlier studies of non-PM eyes and were thus considered to be characteristics of PM eyes.
Table 4.
Comparison of General Characteristics Between Eyes With Each Type of Single-Site PPH
| Cup | Peripapillary | Rim | Parapapillary | Conus | Periconus | P | |
|---|---|---|---|---|---|---|---|
| Number of eyes | 1 | 10 | 12 | 9 | 49 | 9 | |
| Male sex | 0 | 5 (50.0%) | 6 (50.0%) | 2 (22.2%) | 16 (32.7%) | 1 (11.1%) | 0.333 |
| Age (y), mean (SD) | 57.4 | 70.4 (12.7) | 64.6 (12.4) | 68.4 (7.8) | 68.1 (10.6) | 60.0 (20.3) | 0.657 |
| Axial length (mm), mean (SD) | 31.67 | 30.04 (2.14) | 32.32 (1.45) | 29.82 (2.25) | 30.85 (2.36) | 29.95 (1.61) | 0.070 |
| IOP (mm Hg), mean (SD) | 18.00 | 12.91 (4.99) | 15.47 (4.38) | 14.51 (2.54) | 15.42 (3.01) | 15.46 (1.85) | 0.323 |
| Refractive error (D), mean (SD) | −19.75 | −8.47 (5.13) | −19.17 (2.67)* | −16.81 (0.09) | −13.50 (4.70) | −20.81 (2.48)† | 0.003‡ |
| Median BCVA [25%; 75%] | 0.10 [NA; NA] | 0.90 [0.70; 1.05] | 0.75 [0.33; 0.98] | 0.60 [0.25; 0.95] | 0.70 [0.10; 1.00] | 0.60 [0.25; 0.95] | 0.651 |
| Median absorption period (d), [25%; 75%] | 119.00 [NA; NA] | 220.50 [106.75; 376.25] | 182.00 [78.75; 257.25] | 182.00 [126.00; 259.00] | 189.00 [126.00; 371.00] | 182.00 [129.50; 427.00] | 0.868 |
| Re-hemorrhage status, eyes (%) | |||||||
| Multiple times hemorrhages§ | 1 (100.0) | 3 (30.0) | 3 (25.0) | 3 (33.3) | 14 (28.6) | 3 (33.3) | 0.819 |
| Recurrence at the same site | 0 | 2 (20.0) | 2 (16.7) | 0 | 10 (20.4) | 2 (22.2) | 0.767 |
| New hemorrhage at a different site | 1 (100.0) | 2 (20.0) | 2 (16.7) | 3 (33.3) | 7 (14.3) | 1 (11.1) | 0.303 |
| Category of myopic maculopathy║ (%) | 0.028# | ||||||
| Diffuse atrophy (C2) | 1 (100.0) | 8 (80.0) | 3 (25.0) | 3 (33.3) | 11 (22.4) ** | 6 (66.7) | |
| Patchy atrophy (C3) | 0 | 2 (20.0) | 7 (58.3) | 4 (44.4) | 24 (49.0) | 2 (22.2) | |
| Macular atrophy (C4) | 0 | 0 | 2 (16.7) | 2 (22.2) | 14 (28.6) | 1 (11.1) | |
SD, standard deviation; NA, not available.
P = 0.033, compared with peripapillary, Bonferroni test.
P = 0.004, compared with peripapillary, Bonferroni test.
One-way ANOVA.
Some eyes with multiple times hemorrhages recurred not only at the same site but also at a new site, so the sum number of eyes of the next two-row might surpass this row.
According to META-PM study classification
χ2 test
P = 0.002, compared with peripapillary in C2, the Fisher's exact test.
Statistical examinations of the different types of single-site PPH (Table 4) showed that there was no significant difference in the sex distribution, age, axial length, BCVA, absorption period, and re-hemorrhage status. The eyes with a rim or periconus hemorrhage were significantly more myopic than the eyes with a peripapillary hemorrhage (−19.17 ± 2.67 D vs. −8.47 ± 5.13 D, P = 0.033; −20.81 ± 2.48 D vs. −8.47 ± 5.13 D, P = 0.004). Different types of PPHs had different dominant categories of background myopic maculopathy (P < 0.05). All of the characteristics were not significantly different between the conus-related PPHs and disc-related PPHs.
The location of each type of single-site hemorrhage is shown in Table 5. Among the 90 eyes, a PPH was most commonly seen on the temporal side (66 [73.3%]), followed by the inferior (10 [11.1%]), the superior (8 [8.9%]), and the nasal side (6 [6.7%]) of the optic disc. The conus type (42 [85.7%]) of PPH was dominant on the temporal side, followed by the peripapillary (7 [70.0%]), periconus (6 [66.7%]), parapapillary (6 [66.7%]), and the rim (5 [41.7%]) type. The different types had different distributions (P = 0.006).
Table 5.
Location of Each Type of Single-Site Papillary and Peripapillary Hemorrhage
| Cup | Peripapillary | Rim | Parapapillary | Conus | Periconus | Total | P | |
|---|---|---|---|---|---|---|---|---|
| Number of eyes | 1 | 10 | 12 | 9 | 49 | 9 | 90 | |
| Location (%) | 0.006* | |||||||
| Inferior | 0 | 0 | 2 (16.7) | 1 (11.1) | 4 (8.2) | 3 (33.3) | 10 (11.1) | |
| Nasal | 1 (100.0) | 1 (10.0) | 2 (16.7) | 1 (11.1) | 1 (2.0)† | 0‡ | 6 (6.7) | |
| Superior | 0 | 2 (20.0) | 3 (25.0) | 1 (11.1) | 2 (4.1) | 0 | 8 (8.9) | |
| Temporal | 0 | 7 (70.0) | 5 (41.7) | 6 (66.7) | 42 (85.7)§ | 6 (66.7) | 66 (73.3) |
Fisher's exact test.
Compared with cup, Bonferroni test.
Compared with cup, Bonferroni test.
Compared with rim, Bonferroni test.
FFA and OCT Findings of PPHs and Surrounding Tissue
Among the 97 eyes, 63 eyes had undergone a FFA examination, and 21 eyes were tested on the day when the hemorrhage was first detected. In 24 of the 63 (38.1%) eyes, the PPH was found in the area of straightened retinal arterioles running from the optic disc. The retinal veins in some eyes had an abnormal bending to <60° at the artery and vein (A-V) crossing site (Fig. 3). Capillary telangiectasia was found in 14 eyes and retinal microaneurysms in 13 of these 24 eyes (58.3%, 54.2%, respectively; Figs. 3G, 3H). Of these, 12 (50.0%) eyes had all three types of FFA abnormalities. Three (4.8%) of 63 eyes showed hyperfluorescence due to dye leakage at the hemorrhagic site (Figs. 4, 5). In two eyes, telangiectasic capillaries were seen running along the retinal arterioles in the dye leakage area in the early phase of FFA (Fig. 4B). OCT examination showed that two of these three eyes had a compression of the retinal tissue at the hemorrhagic site (Figs. 4, 5). In 1 eye, the retina around the compressed area was thin (Figs. 4D–G).
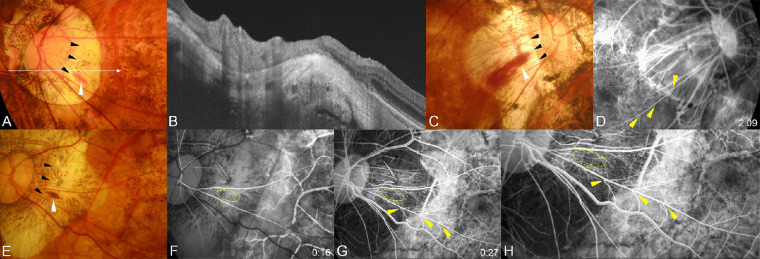
Figure 3.
Hemorrhages present at the peak of a scleral ridge and a straightened segment of retinal arterioles running away from the optic disc and telangiectasia of the surrounding capillaries. (A) Fundus photograph of the left eye of a 55-year-old woman with an axial length of 31.42 mm showing a typical flame-shaped conus hemorrhage (white arrowhead). The peak of the ridge can be seen (black arrowheads). The long arrow indicates the OCT scan line for the image shown in B. (B) OCT image showing a prominent scleral ridge at the hemorrhagic site. (C) Fundus photograph of the right eye of a 68-year-old woman with an axial length of 31.19 mm showing a typical flame-shaped conus hemorrhage (white arrowhead). The peak of the ridge can be seen at one end of the hemorrhage (black arrowheads). (D) Fluorescein angiogram taken one year before the hemorrhage at two minutes after the dye injection showing a marked bending of the retinal venules on the straightened artery (arrowheads). (E) Fundus photograph of the left eye of a 71-year-old woman with an axial length of 31.78 mm showing a typical flame-shaped conus hemorrhage (white arrowhead). The peak of the ridge can be seen (black arrowheads). (F, G) Fluorescein angiograms taken one year before the hemorrhage at 16 seconds after the dye injection (F) and at 27 seconds after dye injection (G). The hemorrhage site is circled in yellow. A marked bending of the retinal venules can be seen especially at the A/V crossing sites of the straightened artery (arrowheads). The closer the bending site is to the optic disc, the sharper is the bending angle. Many retinal microaneurysms and retinal capillary telangiectasias can be seen in the temporal part of the conus. (H) Magnified image shows capillary telangiectasia and microaneurysms.
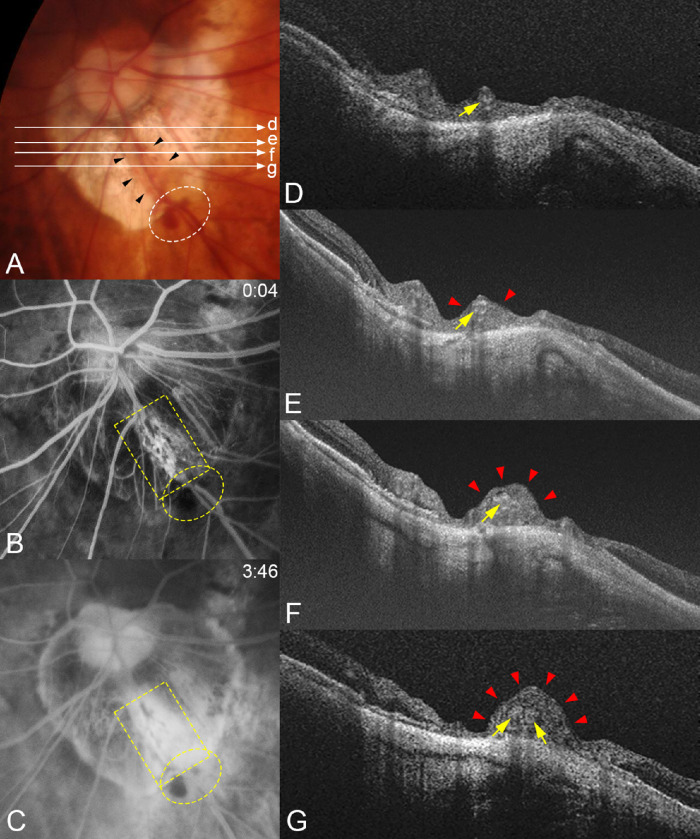
Figure 4.
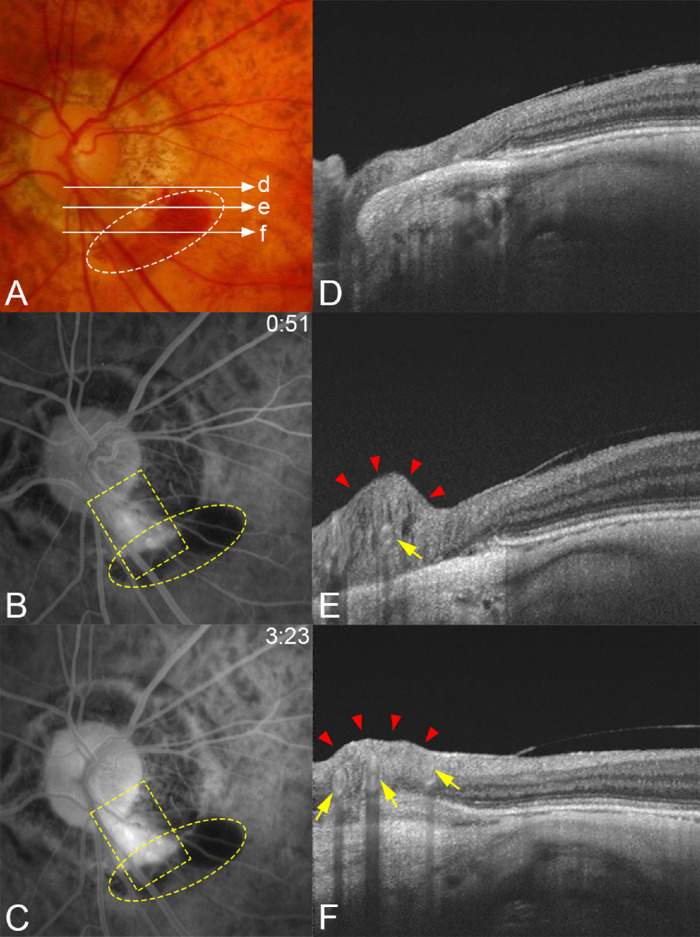
Compression of the retinal tissue and dye leakage from the compressed area in a patient with a periconus hemorrhage. (A) Fundus photograph of the left eye of a 74-year-old woman with an axial length of 29.80 mm showing periconus hemorrhage (white circle) with a blotchy shape. The fundus color is slightly orange in the area along the lower temporal retinal vein (arrowheads) crossing the conus area. Long arrows indicate OCT scan lines for images in D, E, F, and G. (B) Early phase of FFA at four seconds after the dye injection shows an area with many capillaries with microaneurysms along both sides of this lower temporal retinal vein (yellow square). Hemorrhages are present along the vessels (yellow circle). (C) At three minutes after the dye injection, a massive dye leakage is seen from the dilated capillaries in B. Periconus hemorrhages show blocked fluorescence (yellow circle). (D–G) OCT images corresponding to the scan lines shown in A. Images show that the retinal tissue is severely compressed (red arrowheads) especially at sites farther away from the optic disc along the vessel (arrows). The retina around the compressed area is thin, especially in G.
Figure 5.
Typical case showing a compression of the retinal tissue in a patient with a periconus hemorrhage. (A) Fundus photograph of the left eye of a 76-year-old woman with an axial length of 28.97 mm showing a blotchy shaped periconus hemorrhage (white circle). Long arrows indicate the OCT scan lines for images in D, E, and F. (B, C) Fluorescein angiograms. At 51 seconds after the dye injection showing leakage along the lower temporal retinal artery (yellow square) and hemorrhage developed adjacent to the margin of the myopic conus. Corresponding blocked fluorescence can be seen (yellow circle). (D, E) OCT images obtained two years after the hemorrhage of scan lines in A showing that the retinal tissue is severely compressed (red arrowheads) at the middle (E) in the area of conus from the optic disc along the retinal artery (arrow). (F) Three vessels (arrows) can be seen in the compressed retina (arrowheads).
Forty-one of the 97 (42.3%) eyes had a scleral ridge. Ten (24.4%) of these eyes had hemorrhages on or next to the peak of the ridge (Fig. 3). More specifically, five eyes had a hemorrhage at the peak of the ridge and the hemorrhages of the remaining eyes were located on the side of the peak of the ridge.
Discussion
Our results showed that the prevalence of PPH in eyes with PM was 4.05%. The definition of PPHs was somewhat broader in our study than that for DHs because PPHs included the conus and periconus types of PPHs that had not been reported and were thus specific to PM eyes. Our results showed that the prevalence of the conus-related PPHs (2.81%) was approximately twice as high as the disc-related PPHs (1.47%) in PM eyes. The prevalence of disc-related PPHs (1.47%) in the PM eyes was slightly higher than that of normal subjects (0%-1.08%) but on the very low end of the range of previously reported prevalence of DHs in glaucoma patients (1.45%-36.9%)9 In addition, a larger number of female patients with PM had PPHs, which we suggest was due to the larger female base with PM.26,27
Regression analyses (Table 3) showed that patchy atrophy reduced the risk of a repeat hemorrhaging while an elongation of the axial length increased the risk. These are new findings that show that in addition to the type and stage of glaucoma,10 the category of myopic maculopathy and axial length were risk factors for recurrences of PPHs in highly myopic eyes. These findings indicate that even though the choroid is thinner in patchy atrophy than in diffuse atrophy, the choroid forms new holes in Bruch's membrane in eyes with patchy atrophy,28,29 the mechanical stress caused by axial elongation may be released, and the risk of re-hemorrhaging is correspondingly reduced. This explanation needs to be confirmed in future studies.
The eyes in the “possible ON damage” or “possible glaucoma” groups had an increased risk of a recurrence. This provides an additional clue that can help diagnosing glaucoma in PM eyes (i.e., we need to consider the presence of glaucoma or ON damage if PPH recurs repeatedly in PM eyes). Prospective, longitudinal studies focusing on the PPHs and corresponding VF changes may also provide important clues for the pathogenesis and progression of ON damage in PM eyes.
The conus type of PPHs was the most common type (Table 4), and 85.7% of them were located on the temporal side of the optic disc. Previous studies reported that the parapapillary gamma zone developed and enlarged temporally from the disc border in highly myopic eyes and was usually largest in the temporal parapapillary region.30,31 These findings indicated that PPA progresses predominantly temporal to the optic disc and that the tissue of the PPA region is thinner22,32 and more fragile than the normal tissue of the fundus. This leads to a greater tendency for PPHs to develop on the temporal side of the optic disc, and they tend to be the conus type.
The earlier suggested pathogenesis of DHs in glaucomatous eyes9 might not be appropriate for PPHs in PM eyes especially for the conus-related PPHs. Our FFA results showed that 38.1% of the eyes with PPHs had straightened retinal arterioles that ran from the optic disc, and some of the venules at A-V crossings had an abnormal degree of bending (Fig. 3). In the eyes with abnormal bending of retinal venules at A-V crossings, capillary telangiectasia and retinal capillary microaneurysms were frequently seen. Hayashi et al.33 examined the FFA findings of 232 eyes with PM and showed the presence of capillary telangiectasia and capillary microaneurysms in the area with abnormal bending of the retinal veins at A-V crossing. These observations suggest that the sharp bending of the retinal venules at A-V crossings may slow the flow of blood from the retinal capillaries and the post-capillary venules toward the main trunk of the retinal venules as in myopia-induced retinal vein occlusion. It has been suggested that the vascular walls of such telangiectatic capillaries or microaneurysms tend to rupture, which may be one of the causes of PPHs. The stagnation of venous blood could be most prominent near the A-V crossing sites, which may explain why the PPHs tended to be found along the straightened sections of retinal arterioles (viz., at A-V crossing sites).
Interestingly, the retinal tissue in two eyes appeared to be compressed at the hemorrhagic site (Figs. 4, 5). No previous studies have reported similar findings. Although the pathogenesis of such compression was not determined, we suggest that the retinal arterioles become straightened as they run from the optic disc to the peripheral retina by the axial elongation. Shimada et al.34 examined vertical OCT sections between the macula and the ON in PM eyes and showed that retinal arterioles were not simply stretched but also protruded anteriorly (Figure 4 in their article). Such a straightening and anterior protrusion of retinal arterioles probably alters the morphology of the surrounding retinal tissue. The FFA findings (Fig. 4) showed that a group of capillaries and microaneurysms were compressed along the artery, which led to severe dye leakage from these capillaries. The OCT images also showed a compression of the retinal tissue along the retinal artery (Fig. 4). This is supported by how thick the retina was along the straightened arteriole and instead how thin the retina was on both sides of the compressed tissue (Figs. 4D–4G). Such abnormal compression may damage the walls of the retinal capillaries, which would then cause hemorrhaging in and around the compressed area. Based on our findings on the ridges (Fig. 3), we suggest that the mechanical damage produced by the ridges, such as a direct mechanical force applied to the upper retina or relative compression within the retina, which can damage the blood vessels, is one of the mechanisms for the formation of a PPH.
There are some limitations in our study. First, all our data were collected from patients examined in the Advanced Clinical Center for Myopia at TMDU, which might have led to a selection bias and might not be generalized. Second, the median follow-up interval was 3.03 months, so the PPH we examined may not be on the actual day of the onset of bleeding, and the absorption period we obtained (median, 182 days) may be longer than the actual absorption period. In addition, the PPH might have been missed during the interval. Third, this was a retrospective cohort study, and the impact of the absence of some clinical data and the value of testing not on the PPH day need to be considered. For example, disc-centered OCT examinations were not performed in most patients, leading to a lack of strong evidence for further exploration of the pathogenesis of disc-related PPHs. In addition, the lack of pre- and post-hemorrhagic VF testing makes it difficult to investigate the relationship between PPH and VF. Fourth, because most of the OCT results were obtained from 12 radial scans centered on the fovea, they rarely passed through the center of the PPH site, or if they did, the exact level of PPH might not be obtained due to the small area of the bleeding. Therefore future studies should be prospective with periodic serial FFA examinations or dense raster OCT scans centered on the disc to characterize and quantify the longitudinal changes. These tests should focus on the relationship between retinal vascular changes, PPHs, and VF defects. Fifth, eyes with glaucoma were not excluded from our study because of the difficulty in diagnosing glaucoma in PM eyes. So, a confounding effect of glaucoma is possible.
In conclusion, PPHs are not rare in eyes with PM with a prevalence of 4.05%. The PPHs were most often located in the temporal PPA region of the optic disc. Conus and periconus types of PPHs appear to be specific to PM with a prevalence of approximately twice that of disc-related PPHs. In addition, myopic axial elongation, mild myopic maculopathy, and the presence of glaucoma or ON damage were risk factors for the recurrence of a PPH. The pathogenesis of PPHs in PM eyes is probably different from that in glaucomatous eyes, and it may be mainly related to the mechanical tension generated by PM-associated lesions. These lesions can directly or indirectly damage the vessel walls. Such mechanical forces may play a role in pathologic myopic VF defects.
Acknowledgments
Supported by grants from the Japanese Society for Promotion of Science (number 19H03808). The funding organization had no role in the design or conduct of this research.
Disclosure: J. Xiong, None; R. Du, None; S. Xie, None; H. Lu, None; C. Chen, None; T. lgarashi-Yokoi, None; K. Uramoto, None; Y. Onishi, None; T. Yoshida, None; K. Kamoi, None; K. Ohno-Matsui, None
References
- 1. Budenz DL, Anderson DR, Feuer WJ, et al.. Detection and prognostic significance of optic disc hemorrhages during the Ocular Hypertension Treatment Study. Ophthalmology. 2006; 113: 2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung E, Demetriades AM, Christos PJ, Radcliffe NM. Structural glaucomatous progression before and after occurrence of an optic disc haemorrhage. Br J Ophthalmol. 2015; 99: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Moraes CG, Liebmann JM, Park SC, et al.. Optic disc progression and rates of visual field change in treated glaucoma. Acta Ophthalmol. 2013; 91: e86–91. [DOI] [PubMed] [Google Scholar]
- 4. De Moraes CG, Prata TS, Liebmann CA, Tello C, Ritch R, Liebmann JM. Spatially consistent, localized visual field loss before and after disc hemorrhage. Invest Ophthalmol Vis Sci. 2009; 50: 4727–4733. [DOI] [PubMed] [Google Scholar]
- 5. Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013; 120: 512–519. [DOI] [PubMed] [Google Scholar]
- 6. Ishida K, Yamamoto T, Sugiyama K, Kitazawa Y. Disk hemorrhage is a significantly negative prognostic factor in normal-tension glaucoma. Am J Ophthalmol. 2000; 129: 707–714. [DOI] [PubMed] [Google Scholar]
- 7. Sonnsjo B, Dokmo Y, Krakau T. Disc haemorrhages, precursors of open angle glaucoma. Prog Retin Eye Res. 2002; 21: 35–56. [DOI] [PubMed] [Google Scholar]
- 8. David RCC, Moghimi S, Do JL, et al.. Characteristics of central visual field progression in eyes with optic disc hemorrhage. Am J Ophthalmol. 2021; 231: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee EJ, Han JC, Kee C. A novel hypothesis for the pathogenesis of glaucomatous disc hemorrhage. Prog Retin Eye Res. 2017; 60: 20–43. [DOI] [PubMed] [Google Scholar]
- 10. Razeghinejad MR, Nowroozzadeh MH. Optic disk hemorrhage in health and disease. Surv Ophthalmol. 2017; 62: 784–802. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Xu L, Hu L, Wang Y, Yang H, Jonas JB. Frequency of optic disk hemorrhages in adult Chinese in rural and urban China: the Beijing Eye Study. Am J Ophthalmol. 2006; 142: 241–246. [DOI] [PubMed] [Google Scholar]
- 12. Tomidokoro A, Iwase A, Araie M, Yamamoto T, Kitazawa Y. Population-based prevalence of optic disc haemorrhages in elderly Japanese. Eye (Lond). 2009; 23: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 13. Ha A, Kim YK, Jeoung JW, Park KH. Impact of optic disc hemorrhage on subsequent glaucoma progression in mild-to-moderate myopia. PLoS One. 2017; 12: e0189706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim HS, Park KH, Jeoung JW, Park J. Comparison of myopic and nonmyopic disc hemorrhage in primary open-angle glaucoma. Jpn J Ophthalmol. 2013; 57: 166–171. [DOI] [PubMed] [Google Scholar]
- 15. Park HL, Lee J, Jung Y, Park CK. Optic disc hemorrhage and lamina cribrosa defects in glaucoma progression. Sci Rep. 2017; 7: 3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharpe GP, Danthurebandara VM, Vianna JR, et al.. Optic disc hemorrhages and laminar disinsertions in glaucoma. Ophthalmology. 2016; 123: 1949–1956. [DOI] [PubMed] [Google Scholar]
- 17. Kim YK, Park KH, Yoo BW, Kim HC. Topographic characteristics of optic disc hemorrhage in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2014; 55: 169–176. [DOI] [PubMed] [Google Scholar]
- 18. Wong YL, Saw SM. Epidemiology of pathologic myopia in Asia and worldwide. Asia Pac J Ophthalmol (Phila). 2016; 5: 394–402. [DOI] [PubMed] [Google Scholar]
- 19. Iwase A, Araie M, Tomidokoro A, et al.. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology. 2006; 113: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 20. Xu L, Wang Y, Li Y, et al.. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006; 113: 1134 e1131–1111. [DOI] [PubMed] [Google Scholar]
- 21. Ohno-Matsui K, Kawasaki R, Jonas JB, et al.. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol . 2015; 159: 877–883 e877. [DOI] [PubMed] [Google Scholar]
- 22. Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016; 52: 156–187. [DOI] [PubMed] [Google Scholar]
- 23. Ohno-Matsui K, Shimada N, Yasuzumi K, et al.. Long-term development of significant visual field defects in highly myopic eyes. Am J Ophthalmol. 2011; 152: 256–265 e251. [DOI] [PubMed] [Google Scholar]
- 24. Akagi T, Hangai M, Kimura Y, et al.. Peripapillary scleral deformation and retinal nerve fiber damage in high myopia assessed with swept-source optical coherence tomography. Am J Ophthalmol. 2013; 155: 927–936. [DOI] [PubMed] [Google Scholar]
- 25. Xie S, Kamoi K, Igarashi-Yokoi T, et al.. Structural abnormalities in the papillary and peripapillary areas and corresponding visual field defects in eyes with pathologic myopia. Invest Ophthalmol Vis Sci. 2022; 63: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du R, Xie S, Igarashi-Yokoi T, et al.. Continued increase of axial length and its risk factors in adults with high myopia. JAMA Ophthalmol. 2021; 139: 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mo Y, Wang MF, Zhou LL. Risk factor analysis of 167 patients with high myopia. Int J Ophthalmol. 2010; 3: 80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang Y, Du R, Nagaoka N, et al.. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology. 2019; 126: 1018–1032. [DOI] [PubMed] [Google Scholar]
- 29. Du R, Fang Y, Jonas JB, et al.. Clinical features of patchy chorioretinal atrophy in pathologic myopia. Retina. 2020; 40: 951–959. [DOI] [PubMed] [Google Scholar]
- 30. Jonas JB, Ohno-Matsui K, Panda-Jonas S. Myopia: anatomic changes and consequences for its etiology. Asia Pac J Ophthalmol (Phila). 2019; 8: 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonas JB, Fang Y, Weber P, Ohno-Matsui K. Parapapillary gamma and delta zones in high myopia. Retina. 2018; 38: 931–938. [DOI] [PubMed] [Google Scholar]
- 32. Jonas JB, Jonas SB, Jonas RA, Holbach L, Panda-Jonas S. Histology of the parapapillary region in high myopia. Am J Ophthalmol. 2011; 152: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 33. Hayashi W, Shimada N, Hayashi K, et al.. Retinal vessels and high myopia. Ophthalmology. 2011; 118:791–791 e792. [DOI] [PubMed] [Google Scholar]
- 34. Shimada N, Ohno-Matsui K, Nishimuta A, Tokoro T, Mochizuki M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology. 2007; 114: 2070–2076. [DOI] [PubMed] [Google Scholar]