Abstract
Antimicrobial stewardship programs (ASPs) have made immense strides in optimizing antibiotic, antifungal, and antiviral use in clinical settings. However, although ASPs are required institutionally by regulatory agencies in the United States and Canada, they are not mandated for transplant centers or programs specifically. Despite the fact that solid organ transplant recipients in particular are at increased risk of infections from multidrug-resistant organisms, due to host and donor factors and immunosuppressive therapy, there currently are little rigorous data regarding stewardship practices in solid organ transplant populations, and thus, no transplant-specific requirements currently exist. Further complicating matters, transplant patients have a wide range of variability regarding their susceptibility to infection, as factors such as surgery of transplant, intensity of immunosuppression, and presence of drains or catheters in situ may modify the risk of infection. As such, it is not feasible to have a “one-size-fits-all” style of stewardship for this patient population. The objective of this white paper is to identify opportunities, risk factors, and ASP strategies that should be assessed with solid organ transplant recipients to optimize antimicrobial use, while producing an overall improvement in patient outcomes. We hope it may serve as a springboard for development of future guidance and identification of research opportunities.
Keywords: antibiotic prophylaxis, ethics and public policy, health services and outcomes research, infection and infectious agents, organ transplantation in general
1 |. ANTIMICROBIAL STEWARDSHIP: A NECESSITY IN SOT RECIPIENTS?
Antimicrobial stewardship programs (ASPs) have made immense strides in optimizing antibiotic, antifungal, and antiviral use in clinical settings. Their importance has been enhanced primarily by the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria announced initially in 2014.1 Following these landmark recommendations in the United States, The Joint Commission issued a recommendation that every hospital’s infection prevention team should include at a minimum—an infection diseases physician, a pharmacist, an infection preventionist, and a practitioner as members.2,3
In addition to the standards established by The Joint Commission for antimicrobial stewardship noted above, the Centers for Medicare and Medicaid Services (CMS) proposed a rule in 2016 that would require all acute care and critical access hospitals that participate in Medicare or Medicaid to implement an ASP.4 This rule was finalized in September 2019 and is intended to engage clinicians and hospital staff in fighting multidrug-resistant organisms (MDRO) that contribute innumerable complications and added costs to patients, especially those who are at higher risk for infections, such as transplant recipients.4,5 However, although ASPs are institutional requirements by regulatory agencies in the United States and Canada, they are not specifically mandated for transplant centers or programs. Furthermore, there are little rigorous data to support stewardship practices in solid organ transplant (SOT) populations, and thus, no transplant-specific requirements currently exist.
As noted in Table 1, SOT recipients are particularly at increased risk of MDRO infections, due to host and donor factors and immunosuppressive therapy. Additionally, a summary of MDROs with emerging new infections are detailed in Table 2, and the list is continually expanding as new reported resistance patterns emerge.
TABLE 1.
Organ-specific factors associated with multidrug-resistant organisms
| Prevalent resistant organism | Organ type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart | Kidney and pancreas | Liver | ||||||||||
| LVAD | Recipient | Recipient and donor | Recipient and donor | |||||||||
| MRSA | Nosocomial MDR GNR | VRE | Daptomycin-linezolid-resistant VRE | MDR GNR | Candida species | Ganciclovir- and letermovir-resistant CMV | MDR GNR | VRE | Candida species | Ganciclovir- and letermovir-resistant CMV | ||
| Source of infection | Blood | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes, occasionally | Yes | Yes | Yes |
| Urine | No | Yes | Yes, urinary stents | Yes | Yes, prior colonization and stents placed | Yes | No | Yes | Yes | Yes | No | |
| Pulmonary | No | No | No | No | No | No | Yes | No | No | No | Yes | |
| Abdominal | Drive line site | No | Yes | No | Yes | Yes | No | Yes, biliary drains, postoperative collections | Yes | Yes | No | |
| Comments | New study on role of phage therapy in treatment | Recovered from urine source often. Can be colonizer or complicate infection from urinary catheters, stents, or urinomas | Emerging pathogen | As with VRE | As with VRE | Donor mismatch at greatest risk | Frequently seen from SBP and antimicrobial prophylaxis. Bile tract heavily colonized with bacteria | Commonly seen in biliary tract | As above | Donor mismatch at greatest risk | ||
| Prevalent resistant organism | Organ type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Intestinal | ||||||||||||
| Cystic fibrosis recipient | Other recipient and donor | Recipient | |||||||||||
| MDR GNR+MDR-Achromobacter Stenotrophomonas spp. and Burkholderia spp. | MRSA | Azole-resistant Aspergillus spp. | Ganciclovir- and letermovir-resistant CMV | Drug-resistant influenza | MDR GNR | Ganciclovir- and letermovir-resistant CMV | Drug-resistant influenza | VRE | MDR GNR | MDR Candida spp. | Azole-resistant Aspergillus spp. | ||
| Source of infection | Blood | Yes | Yes | No | Yes | No | No | Yes | No | Yes | Yes | Yes | No |
| Urine | No | No | No | No | No | No | No | No | Yes | Yes | Yes | No | |
| Pulmonary | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | |
| Abdominal | No | No | No | No | No | No | No | No | Yes | Yes | Yes | No | |
| Comments | Frequent colonizers in sinuses and lungs of patients with cystic fibrosis | Emerging pathogen | Higher immunosuppression. Suboptimal antiviral prophylaxis dosing | Higher immunosuppression | |||||||||
Abbreviations: CMV, cytomegalovirus; GNR, gram-negative rod; LVAD, left ventricular assist device; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; SBP, spontaneous bacterial peritonitis; VRE, vancomycin-resistant enterococci.
TABLE 2.
Summary of emerging drug-resistant organisms in solid organ transplantation
| Emerging resistance | |
|---|---|
| Gram-positive bacteria | |
| Staphylococcus aureus | Clindamycin, trimethoprim-sulfamethoxazole, vancomycin, linezolid, and daptomycin |
| Enterococcus spp. | Daptomycin and linezolid |
| Streptococcus pneumoniae | Quinolone |
| Gram-negative bacteria | |
| E. coli | CRE and CPE |
| Klebsiella spp. | CRE and CPE |
| Haemophilus influenzae | Beta lactamase resistance |
| Enterobacter spp. | Pan drug resistance and CPE |
| Citrobacter/Serratia spp. | Pan drug resistance and CPE |
| Pseudomonas aeruginosa | Pan drug resistance and CPE |
| Stenotrophomonas maltophilia | Pan resistance |
| Burkholderia cepacia | Pan drug resistance |
| Achromobacter spp. | Carbapenems |
| Gram-negative anaerobes | |
| Bacteroides spp. | Metronidazole, piperacillin-tazobactam, and carbapenems |
| Fungi | |
| C. albicans | Echinocandins |
| C. glabrata | Echinocandins and amphotericin B |
| C. auris | Echinocandins |
| Aspergillus fumigatus | Voriconazole |
| Viruses | |
| Influenza | Oseltamivir and baloxavir marboxil |
| Cytomegalovirus | Ganciclovir and letermovir |
Abbreviations: Amp C, AmpC beta-lactamase enzymes; CPE, carbapenemase-producing Enterobacteriaceae; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended spectrum beta-lactamase; MRSA, methicillin-resistant Staphylococcus aureus.
The risk of infection depends on the type and timing of transplantation and can include donor-derived infections. The broad application of stewardship measures may not account for variables specific to the SOT population, such as timing since transplantation, depth and duration of immunosuppression, the type of organ transplanted, and donor-derived infections. Pretransplant patients, especially pre-lung transplant (i.e., those with cystic fibrosis) and pre-liver transplant, may already be colonized with MDROs from prolonged antimicrobial exposure for recurrent lung infections, spontaneous bacterial peritonitis, or recurrent bacterial cholangitis.
Additionally, a recent posttransplant patient with intense immunosuppression, catheters, and drains in situ will be at increased risk of infection—as compared with a patient who has had a transplant for more than 1 year. As such, a one-size-fits-all style of stewardship implementation is not feasible. To compound this issue, the lack of clinical data on specific ASP interventions and durations of effective therapy (Table 3) in these populations further necessitates the need of tailoring the ASP interventions among SOT populations.
TABLE 3.
Summary of antimicrobial stewardship metrics reported in literature relevant to SOT patients7,8,13–16,19–21,24,46
| References | Population size, included SOT (Y/N) | Main interventions | Strategies | Program evaluation |
|---|---|---|---|---|
| Gouliouris et al, 201613 | N = 77 patients with candidemia, yes | Starting antifungal agent on same day as culture result Removal of central catheters within 4 days in nonneutropenic patients Repeat blood cultures Echo Eye exam Rationalization of therapy once susceptibility results become available |
Restriction Audit feedback Syndrome-specific intervention De-escalation |
1 month mortality Adherence to six elements of care bundle Markers of clinical severity at baseline (Charlson comorbidity index) |
| Antworth et al, 201314 | N = 78 patients with candidemia, unspecified | Appropriate antifungal therapy based on culture and susceptibility results Removal of IV catheters Repeat blood cultures at least every 48 h until negative Appropriate duration of antifungal therapy Eye exam |
Syndrome-specific intervention Education | Compliance with all bundle elements, LOS, and time to clearance of candidemia Clinical outcomes: recurrence of candidemia within 4 weeks, persistent candidemia >72 h Consumption outcome: appropriate duration of therapy |
| MacVane et al, 201646 | N = 85 patients with positive culture, unspecified | Identification of antimicrobial allergy or resistance markers or bug-drug mismatch Clarification of improper specimen/culture ordering Clinical liaison services to allow for correct therapy |
Audit feedback Syndrome-specific intervention De-escalation IV to PO Rapid diagnostics Education |
Appropriateness of antimicrobial changes Treatment of colonization and unnecessary microbiology workup Clarification of culture reporting Clarification of microbiology workup De-escalation of spectrum of activity Ensuring appropriate therapy for MDR organism Initiation of therapy, optimization of therapy, and reduced duration of therapy |
| Hamandi et al, 20147 | N = 531 patients SOT patients with infectious syndromes, yes | ID consultation within 48 h of admission vs. later than 48 h vs. none | None | 28-day in-hospital survival 30-day rehospitalization rates LOS Hospitalization costs |
| Lopez-Medrano et al, 201215 | N = 662 antifungal treatments, unspecified | Review of all new antifungal prescriptions and prescriptions susceptible to modification or discontinuation based on criteria of the investigator Substituting fluconazole for caspofungin or liposomal amphotericin B based on susceptibilities of Candida Stopping of antifungal therapy when colonization was considered IV to oral voriconazole when able Stopping empiric or prophylactic antifungal therapy when patient is at low risk |
Audit feedback Syndrome-specific intervention De-escalation IV to PO |
Antifungal expenses Quality of care: incidence of candidemia, % of persistent/relapsing candidemia, % fluconazole-resistant Candida, incidence of infection by filamentous fungi, and 12-month mortality |
| Shah et al, 201116 | N = 161 with candidemia, unspecified | Telephone call of positive culture to attending or ID physician Results of antifungal susceptibility testing in electronic health record |
Audit feedback Syndrome-specific intervention De-escalation |
Changes in antifungal therapy after susceptibility report, including de-escalation rates |
| Frenette et al, 201620 | N = 1424 hepatobiliary surgery or abdominal transplant procedures, yes | Annual feedback on SSI rates to each surgeon and recommendations to decrease SSI rates For transplant procedures, prolongation of antimicrobial therapy for positive Belzer fluid cultures Changes in antimicrobial prophylaxis protocol using preprinted orders Addition of chlorhexidine shower the night prior to procedure Change of sponges to chlorhexidine-impregnated wipes applied the night before and morning of surgery |
Audit feedback Syndrome-specific intervention Local guidelines Education |
Overall SSI rates Organ-space, deep incisional, and superficial incisional SSI rates Global conformity to the 2013 internal antimicrobial prophylaxis guidelines |
| Micallef et al, 201524 | N = 173 patients with antifungal treatment, yes | Diagnostic and therapeutic advice (stopping or change in antifungals, advising on side effects of drug interaction management, managing voriconazole serum assays, and de-escalation when warranted) Change to less costly agent or stopping therapy and returning unused stock when therapy was stopped or switched |
Audit feedback De-escalation Rapid diagnostics |
Drug switches Total cost of drugs |
| So et al, 201621 | N = 176 audits of SOT with infection, yes | Four audits performed over the study period to assess for concordance or discordance of antimicrobials with antimicrobial stewardship best guiding principles and public guidelines Transplant ID consultation |
Audit feedback Syndrome-specific intervention De-escalation Rapid diagnostics Education |
Concordance or discordance with antimicrobial stewardship best guiding principles and public guidelines |
| Rosa et al, 201619 | N = 1 889 cultures, yes | None | Antibiogram | Antimicrobial susceptibilities of most frequent gram-negative bacterial isolates from SOT recipients compared with institution’s antibiogram |
| So et al. 20198 | N = 318 patients with infectious syndromes, yes | Twice weekly academic detailing rounds with prescribers in SOT and SOT pharmacists (including recommendations on empiric antimicrobial selection, tailoring regimen based on investigation results, defining duration of treatment, referral to transplant ID consultation, modifying route of administration, dose, or frequency, and therapeutic drug monitoring) | Audit feedback Syndrome-specific intervention De-escalation IV to PO Local guidelines Rapid diagnostics Antibiogram Education |
Antimicrobial stewardship, concordant prescriptions before and after intervention via 4-point-prevalence surveys conducted in each period Antimicrobial stewardship, concordant prescriptions in patients consulted by transplant ID and frequency of each category of antimicrobial stewardship discordance Amount of antimicrobial consumption and antimicrobial cost per patient day Hospital-acquired C. difficile infections per 1000 patient day Hospital LOS Unplanned readmission within 30 days In-hospital 30-day mortality |
Abbreviations: ASP, antimicrobial stewardship program; ID, infectious disease; IV, intravenous; LOS, length of stay; PO, by mouth; SOT, solid organ transplant; SSI, surgical site infection.
The objective of this white paper is to identify opportunities, risk factors, and strategies that should be assessed with SOT recipients to optimize antimicrobial use (AU), while producing an overall improvement in patient outcomes. With bacterial, fungal, and viral pathogens developing ever-increasing resistance to available medications and challenges for future antimicrobial development, the timely development of such strategies is critical.
2 |. METHODS
The working group (WG) was composed of representatives from the Communities of Practice (CoP) of the American Society of Transplantation (AST), at the recommendations of the CoP chairs. The CoPs include Transplant Administration and Quality Management, Thoracic and Critical Care, Liver and Intestinal, Kidney and Pancreas, Pharmacy, and Infectious Diseases. We also invited representatives from the Centers for Disease Control and Prevention (CDC) and Society of Hospital Epidemiology of America (SHEA) to join the WG. After the introductory meeting, we created two subgroups, antimicrobial resistance (AMR) and antimicrobial stewardship (AMS). Each subgroup was jointly led by one of the cochairs (JH and MS) and a subgroup lead (GF and SMP). Each subgroup generated a list of topics pertaining to the current state of AMS and AMR in the transplant population. Upon consultation with a medical information specialist, a literature search strategy was devised to identify relevant publications to address the predefined topics. Initial drafts created by WG members were reviewed with the subgroup and then edited and summarized in a draft manuscript for readability. As the white paper was intended to serve as a springboard for future consensus guidance development and research opportunities, we did not assign evidence rating for the best practices recommendations based on current literature. Two patient reviewers recommended by the AST provided feedback on the manuscript. The draft was then reviewed by all members of the WG, the AST Infectious Diseases CoP Executive Committee and the AST Education Committee, the CDC, and SHEA. The WG Chair, cochairs, and subgroup leads made final revisions to the white paper based on reviewers’ comments.
3 |. CURRENT LANDSCAPE OF AMS INTERVENTIONS FOR SOT RECIPIENTS
As noted above, there are limited data regarding the implementation of ASP practices among SOT recipient populations. Although some studies have included SOT patients, the efficacy, safety, and optimal intervention strategies have not been widely evaluated. Additionally, process and outcome metrics have yet to be defined. Due to this lack of data, infectious disease and transplant societies make little to no mention of stewardship recommendations for SOT recipients in published guidelines. A 2016 survey of US transplant centers found that only 74% of institutional ASPs included coverage for adult SOT recipients.6 Involvement of transplant infectious diseases specialists in the care of transplant patients is associated with improved outcomes, as well as an increase in stewardship-concordant care.7,8 Preauthorization of formulary-restricted antimicrobials and prospective audit with feedback (PAF) are recommended by national guidelines and are core strategies for ASPs at transplant centers.6 Though limited data exist for either strategy, PAF has resulted in improved prescribing in a single-center report.8 The selection of optimal antimicrobial agents using transplant-specific antibiograms, allergy assessments, MDRO infection risk prediction, and rapid diagnostics are additional functions of ASPs that are currently not well studied in transplant populations. The small number of transplanted patients in some centers and the center’s expertise managing high risk/complex cases vs. lower risk may add to the complexity of measuring this information or setting benchmarks between transplant sites. Although some centers use syndrome-specific treatment duration guidance for SOT patients, the literature supporting this practice is currently limited. Optimization of antifungal medications through indication-specific guidance, diagnostic advice, and therapeutic drug monitoring has been successful at multiple centers and is recommended for immunocompromised patients in national guidelines.9 Improving the timing, route, and dosing of cytomegalovirus (CMV) specific antiviral agents is also a reported function of ASPs in SOT centers and has been associated with more appropriate prescribing and better CMV-specific outcomes.10 Finally, though rapid diagnostic tests are commonly used in SOT patient care, the in-house availability of such testing is varied and may be limited.6
4 |. QUALITY METRICS AND PERFORMANCE INDICATORS FOR AMS IN SOT RECIPIENTS
At their core, ASPs are quality improvement initiatives. To reflect and assess the impact of AMS on patient safety and optimization of care, we need valid and reliable measurements to determine gaps in current practices and opportunities for improvement.11 Metrics can also be used to guide future directions. Quality indicators are standardized, evidence-based measures to track clinical performance and outcomes.11 It is within the purview of ASPs to track and disseminate metrics such as antimicrobial consumption, appropriateness of prescribing, AMR patterns, and incidence of Clostridioides difficile infections.12 In this section, we aim to identify currently recommended AMS metrics already reported by hospital-based ASPs that are also applicable to the SOT population, as supported by literature.
5 |. AMS METRICS FOR SOT RECIPIENTS
AMS metrics pertaining to quality improvement can be categorized into outcome measures, process measures, and balancing measures.
As illustrated in Table 3, the literature reviewed in this section includes SOT patients in their study population, either as the target population or as part of a larger, immunocompromised host population.
5.1 |. Outcome measures
Outcome measures should be specific to the intervention(s) implemented. Therefore, if the interventions are syndrome-based, outcome measures should be reflective of that as well. For antifungal stewardship programs whose interventions target invasive fungal infections, outcome measures such as mortality, time to microbiologic clearance, incidence and recurrence of candidemia, and proportion of fluconazole-resistant isolates have been evaluated over the study periods.13–17 Detection and avoidance of adverse events, including toxicities, graft injury, and drug–drug interactions, are also valuable outcome measures to consider in reporting the impact of an ASP.18
For ASPs that have implemented interventions in collaboration with a microbiology laboratory, pertinent outcome measures that have been reported include SOT specific antibiograms comparing the susceptibilities of most common gram-negative isolates with those of the institution’s general antibiogram.19
A study evaluating collaborations of an AMS team with infection prevention and surgical teams reported a decrease in overall and specific surgical site infections in liver transplant recipients, as well as the proportion of inappropriate surgical antimicrobial prophylaxis as per local guidelines.20
One study assessed the appropriateness of AU in SOT recipients at baseline, in order to identify opportunities for AMS.21 The authors followed up with comparing appropriate use before vs. after an ASP was implemented within their multiorgan transplant program.8 Due to the absence of local guidelines specific to SOT recipients at the time, the authors used the CDC’s Core Elements of Antibiotic Stewardship22 as their adjudication framework.23 Hospital-acquired C. difficile infections, duration of hospitalization, unplanned readmission, and in-hospital mortality were additional outcome measures.8
5.2 |. Process measures
Process measures help us gauge whether applied interventions are heading in the intended direction and can be assessed for their congruence with outcome measures. They may also be more efficient to obtain than outcome measures—which often take longer to materialize and are more costly to gather. Process measures play important roles in accountability reporting to hospital administration as indicators of resource stewardship.12
Antimicrobial consumption and costs are common process measures in transplant-specific ASPs8,21 and in antifungal stewardship programs for immunocompromised populations.14–16,24 The National Healthcare Safety Network (NHSN) AU AMR modules25 were developed by CDC for general inpatient settings26 for participating hospitals. Although the modules were developed for a general patient population, uploaded data can be used for internal comparison, including unit-specific trends, and therefore can be applied to SOT units. Participating hospitals can also perform peer-to-peer comparison with external sites that have a comparable transplant population.
5.3 |. Balancing measures
Balancing measures are a necessity for systematic monitoring—to ensure that improvement in one aspect or area of care does not inadvertently or negatively impact another. Some process measures, such as length of stay and rehospitalization rates, are also balancing measures; and some outcome measures, such as surgical site infections, recurrence of infection, and mortality, are balancing measures as well.8,15,20 Some parameters such as surgical site infections are already collected for existing quality programs such as the National Surgical Quality Improvement Program27 and its transplant-specific quality improvement program TransQIP, jointly created by the American College of Surgeons and the American Society of Transplant Surgeons.28 Similar to outcome and process measures, balancing measures should be tailored to reflect possible consequences of AMS intervention(s) following implementation.
A relatively recent concept in ASP, with great potential to be utilized in SOT recipients, is Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR) analysis. DOOR analyses are constructed by assigning rankings to patients with better overall clinical outcomes and shorter durations of antibiotic use for similar overall clinical outcomes. RADAR is a novel methodology utilizing a superiority design and a two-step process that categorizes patients into an overall clinical outcome based on benefits and harms, then ranking patients with respect to DOOR.29
DOOR distributions allow comparison between different antibiotic use strategies. The proposed framework has potential in antimicrobial trials involving SOT recipients to account for the complex yet dynamic interplay between infection, rejection, impaired graft function, and adverse events and toxicities from antimicrobials and immunosuppressive medications.
However, a potential drawback in this methodology is its reliance on subjective assumptions based on the perspective of the person responsible for ranking outcomes within the DOOR–RADAR framework.30 Although DOOR–RADAR has potential to be used for global assessment of SOT patients’ experience with antimicrobial therapy, further research into its application is warranted.
An additional factor for consideration is improvement in the accessibility and transparency of transplant outcomes for SOT patients. Although currently these metrics are made available for review, they are often presented in ways that are difficult for nonhealthcare workers to interpret. Reporting mechanisms focused at conveying these outcomes in an accessible way for patients would be ideal. Organizations such as the United Network for Organ Sharing (UNOS) are in a prime position for relating antimicrobial and resistance-related outcomes directly to SOT patients, empowering them with information that may help them take charge of their healthcare decisions.
To summarize, measurements in quality improvement demonstrate baseline conditions and current performance, set goals for future directions, and monitor the effects of changes as they are made.31 ASPs are quality improvement initiatives; so for their interventions to be successful, metrics should ideally be determined concurrently with the design phase of the interventions. This will help to ensure that the metrics are feasible and attainable, tailored for the patient population, and specific to the interventions. As laid out in Table 3, the current literature on AMS interventions among SOT populations supports the application of these metrics, some of which are part of the wider institution-based AMS or SOT programs, which makes them efficient to collect, track, and disseminate to all key stakeholders.
6 |. BEST PRACTICE RECOMMENDATIONS FOR AMS IN SOT RECIPIENTS
Transplant-centered ASPs are in a unique position to impact AMR rates and improve patient outcomes in this vulnerable population. Multiple AMS strategies supported by the literature have been found to be successful.32 Although the AMS practices used were varied, implementation of some specific ASP-driven core elements22 that have been shown to be successful in affecting complex populations will likely impact transplant recipients as well.
6.1 |. Establish a multidisciplinary team
The creation of a multidisciplinary collaborative AMS team is one important approach. It is vital that representatives from each discipline are fully engaged in and supportive of the team’s stewardship efforts. Team members should include clinicians from both hospital medicine and those from the specific organ system (i.e., transplant pulmonary/cardiothoracic surgery vs. transplant hepatology/transplant surgery), a transplant infectious disease specialist, a pharmacist, and a representative from infection prevention, nursing, hospital epidemiology, micro-biologist, and quality assurance. Additionally, information technology staff are a critical part of the team, in order to help integrate stewardship protocols into the existing electronic medical record workflow.
6.2 |. Handshake stewardship
Given the complexity of SOT recipients, it is imperative for the team to have regular face-to-face interactions. Members of the ASP team rounding together in person (“handshake stewardship”) is an effective and sustainable ASP approach. Although it is time intensive, the structure of “handshake stewardship,” including multidisciplinary discussions and lack of antimicrobial restrictions, has been shown to be perceived as more efficient than chart review by each member of the team separately.33,34 The “handshake stewardship” strategy has been shown to be an effective way to enhance the visibility of the ASP in other patient populations. It has been associated with a reduction in AU and with enhancing the understanding of antimicrobial prescribing practices and clinical decision-making between each team member.35 “Handshake stewardship” may also contribute to a deeper understanding of the psychology of antimicrobial prescribing practices among the various transplant subspecialties. Additionally, a recent survey of the AST addressing perceptions and attitudes of transplant clinicians toward AMS revealed that involvement of the ASP team in bedside rounds was found to be the most favorable intervention.36
6.3 |. Prospective audit and formulary restriction
Associated processes that have been incorporated into the most successful ASPs are the use of PAF and formulary restrictions.32 A recent systemic review and meta-analysis demonstrated that these are critical components of an ASP.37 These interventions have been associated with a large reduction in targeted AU among patients who met stewardship review criteria.38 The impact of PAF is likely dependent on how frequently it is completed, how quickly it is implemented after antimicrobial prescriptions are initiated, and which antimicrobials are targeted. The impact is maximized if it occurs frequently and focuses not only on antimicrobials that are costly or broad spectrum but all antimicrobials used.39 Furthermore, PAF may be more effective than formulary restriction in transplant ASPs, as restriction was found to be the least favorable ASP intervention in a survey of transplant clinicians.8,36,40
6.4 |. Organ-specific guidelines
As described above, multidisciplinary and collaborative decisions regarding antimicrobial prescriptions are pivotal. However, additional systematic efforts to address dose optimization, intravenous to oral antimicrobial conversion, drug–drug interactions, and therapeutic drug monitoring are important components of transplant ASPs. These decisions have been deemed particularly beneficial for antiviral and antifungal therapeutics as well.9,41–43 Although evidence regarding optimal duration of therapy for common clinical syndromes in SOT is limited, generation of institution- and organ-specific guidelines for antimicrobial prophylaxis and empiric therapy may result in improvement in antimicrobial utilization. Such guidelines may be further informed by the development of transplant-specific antibiograms, which may show higher rates of resistant gram-negative infections in transplant units compared with institution-specific antibiograms.19 Furthermore, antimicrobial allergy labels have been associated with increased rates of AMR in a small cohort of liver transplant recipients.44 Although data regarding the impact of antimicrobial allergies in solid organ transplantation are currently limited, ASP-driven allergy de-labeling initiatives may optimize antimicrobial selection for individual patients and lead to a positive downstream effect on AMR in transplant recipients.
6.5 |. Active communication with microbiology laboratory
Collaboration between ASPs and the microbiology laboratory has been shown to reduce the time for communication of results.45,46 This is particularly germane to the transplant population, in which inadequate empiric antimicrobial therapy has been associated with mortality.47 This partnership is also crucial to improve and better inform diagnostic stewardship, or the appropriate application of laboratory testing in patient evaluation and management,48 in SOT. Multiplex polymerase chain reaction (PCR) panels, nucleic acid tests which rapidly detect genes conferring AMR, including CTX-M, KPC, NDM, OXA, VIM, IMP, vanA/B, and mecA/C,49–51 matrix-associated laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry, and other rapid diagnostic assays, may improve time to initiation of effective antimicrobials and aid de-escalation strategies. However, availability of rapid diagnostics varies,6 and their diagnostic and epidemiologic utility in solid organ transplantation warrants further study. Collaboration between ASPs and the microbiology laboratory may lead to improved understanding of new and evolving diagnostics and further inform their use in the setting of SOT.
SOT recipients are at significant risk for MDRO colonization and infection, and SOT candidates and recipients may drive institutional healthcare-associated infection rates. As such, collaboration between ASPs and hospital epidemiology is critical. In identifying trends in MDRO rates, ASPs work intricately with local infection prevention and control measures. In turn, hospital epidemiology aids in guiding surveillance procedures, interventions, and education targeting relevant pathogens.52 Partnership between ASPs, hospital epidemiology, and the quality assurance administrator also reinforce institutional and programmatic commitment to patient safety.
An additional practice that may improve the care of SOT recipients is the provision of patient and caregiver education on common antimicrobial treatment issues prior to transplant. By providing this information to patients and caregivers, SOT programs are able to arm patients with knowledge to advocate for best practices in the prescription of antimicrobial medications.
All of the interventions described above are variable in their demands on time and labor, and the degree to which they may be implemented at a specific center is dependent upon local resources. A major factor predicting the success of these strategies is the engagement of transplant teams and the support of transplant center administration. Moreover, although each AMS strategy is important independently, ASP success is likely related to the combined impact of these interventions. It will be crucial to develop standard metrics for each selected intervention so that each may be dynamically monitored in order to guide future center-specific AMS strategies. It will be vital to reach a consensus on which metrics will most optimally assess the impact of an ASP.
7 |. RESEARCH GAPS
AMS has been increasingly recognized as an essential quality improvement initiative in the management of infections by the infectious diseases community, accrediting agencies (e.g., The Joint Commission), and payers (e.g., CMS). ASPs are an excellent example of the continuum of improvement measures between SOT programs and hospital quality communication and collaboration. Unfortunately, implementing AMS strategies in immunocompromised hosts in general, and in SOT recipients in particular, has not been a priority. The majority of existing data is extrapolated from nonimmunocompromised hosts, and thus, their validity in the setting of SOT recipients remains unverified at best.
The first and foremost gap is the lack of a comprehensive understanding regarding the magnitude of AMR and antimicrobial consumption in SOT recipients. The studies that have been conducted in this highly specialized patient population are few and are primarily single-center studies. Robust multicenter epidemiological studies must be performed in order to assess current antimicrobial prescription practices and gauge the magnitude of AMR in SOT recipients. This objective can be initially achieved through the use of multicenter point prevalence studies, utilizing tools such as the “Antimicrobial Use and Resistance Modules” produced by NHSN or the National Antimicrobial Prescription Survey (NAPS). It is important for the transplant community to advocate for transplant quality assurance organizations to include SOT-specific data in NHSN. Accreditation of a transplant program by Medicare should include AU policies specific to SOT that is standardized across different centers through AMS.3,22,53–55
The two main cited barriers in the implementation of AMS protocols among SOT recipients were diagnostic uncertainty of infectious syndromes and the delay in the turnaround of diagnostic test results. Further complicating the issue are the lack of well-defined appropriateness measures, coupled with existing so-called “gold standards.” As evidenced from the review of literature delineated in Table 4, one commonly used parameter of appropriateness, the “duration of antimicrobial therapy in SOT recipients for common clinical syndromes,” is not well defined.
TABLE 4.
| Diagnosis | Duration evaluated | Number of patients | SOT patients | Result | Number RCT | |
|---|---|---|---|---|---|---|
| Short (days) | Long (days) | |||||
| Community-acquired pneumonia | 3 days57,59 5 days58,60–63 7 days64 |
7 days63 8 days59 10 days58,60–62,64 21 days57 |
8157 52858 12159 31260 18661 58162 46963 22864 42365 50166 |
457 058–62,65 N/A63,64,66 |
Similar outcome | 10 |
| Ventilator-associated pneumonia | 8 days67,68 | 15 days67,68 | 19767 22568 |
Excluded67,68 | Similar outcome | 2 |
| Pyelonephritis | 7 days69,70,72 5 days71,73,74 |
14 days69,70,72 10 days71,73,74 |
37869 24870 110971 5472 109373 10074 |
Excluded57,62,69,73,74 070 N/A71 |
Similar outcome | 6 |
| Intra-abdominal infection | Median: 4 days75 3 days76 |
Median: 8 days75 5 days76 |
51875 11176 |
075,76 | Similar outcome | 2 |
| Gram-negative bacteremia | 7 days77,78 | 14 days77,78 | 60477 50478 |
5177 Excluded78 |
Similar outcome | 2 |
| Cellulitis | 5 days79 6 days80,81 6 days82 |
10 days79–81 12 days82 |
16979 66780 66681 15182 |
N/A79,80,82 Excluded81 |
Similar outcome79–81 6-day course, more relapse by Day 9082 |
4 |
| Fever in the setting of neutropenia | Apyrexia and clinical recovery ×72 h | Absolute neutrophil count >500/mm3 | 15783 | 083 | Similar outcome | 1 |
Abbreviations: RCT, randomized controlled trials; SOT, solid organ transplant.
Although the AST has recently published guidelines for therapy of common clinical syndromes, they note that data to firmly support these strategies are currently lacking, as underscored by the low-strength ratings proposed for their recommendations. In the absence of randomized clinical trials, an excellent initial step would involve using the Delphi method to engage a panel of experts on appropriate use of antimicrobial prophylaxis and treatment of prevalent clinical syndromes, followed by robust cohort analyses with matching propensity scoring.
Although immediately postsurgery, SOT recipients will be followed as hospital inpatients by their transplant physicians; for the majority of an SOT recipient’s care over the lifetime of their transplanted organ, they may be seen by nontransplant physicians.
Due to the lack of formalized guidelines regarding the treatment of common infections (such as otitis, pharyngitis, and urinary tract infections) among SOT patients, the primary care team and other nontransplant physicians caring for SOT recipients may have a lower threshold to prescribe antimicrobials to this patient population due to their immunosuppressed status.
As such, in order to communicate transplant-specific antimicrobial data and best practices to the nontransplant physicians responsible for the majority of outpatient care for SOT recipients, it is essential that local treatment guidelines for common clinical syndromes are developed.
Additional functions of ASPs that are currently not well studied in SOT populations include the selection of optimal antimicrobial agents using transplant-specific antibiograms, antimicrobial allergy assessments and de-labeling, MDRO infection risk prediction, and rapid diagnostics. However, assessment of antibiograms specific to transplant populations at the institutional or regional level may be helpful in this regard.19
Implementation of ASP in the SOT population may be more complicated than in some other patient populations. Emotional factors such clinician fears of the worst-case scenario may play a significant role in overprescription of antimicrobials. Behavioral components of interventions and reporting of behavioral economics principles in research are warranted. The necessity of incorporating a multidisciplinary team into the ASP is of paramount importance. A recent survey36 and a single-center study8 have highlighted the significance of involving a transplant infectious disease physician along with stakeholder engagement, as illustrated in Figure 1. A comparative evaluation of various implementation strategies including group audit and feedback, physician-specific audit and feedback, formulary restrictions, and handshake stewardship needs to be evaluated in future cluster randomized controlled trials to determine the optimal method of implementation. We believe that optimally, an ASP in SOT should be coled by a transplant infectious diseases physician and an immunocompromised-host infectious diseases pharmacist.
FIGURE 1.
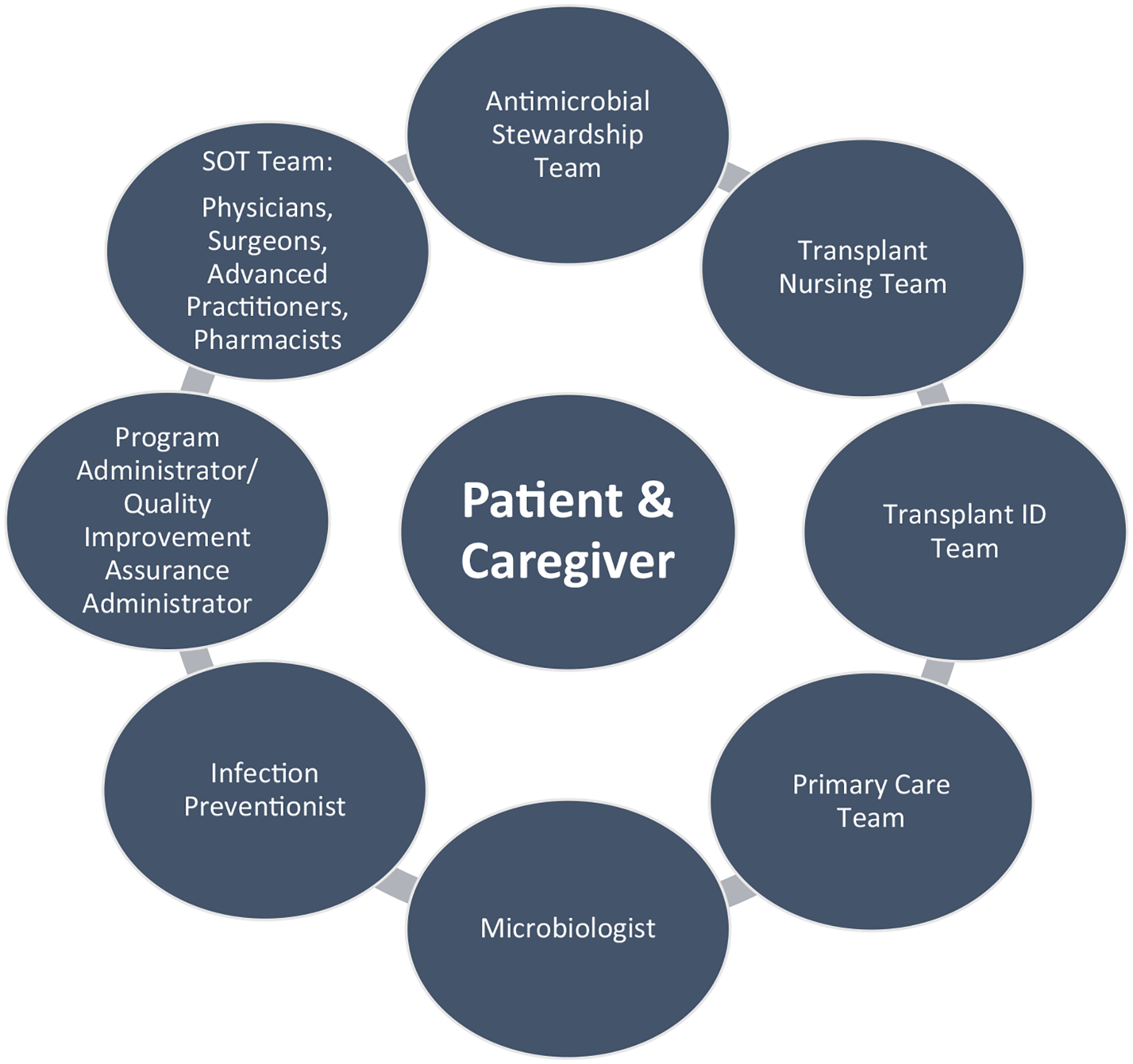
Key stakeholders in antimicrobial stewardship initiatives for solid organ transplant recipients. ID, infectious disease; SOT, solid organ transplant
AMS interventions are evaluated by several quality metrics and outcome measure assessments. In the literature, a few process, outcome, and balancing measures have been reported in various studies as elaborated in Section 3. However, there is a dearth of AMS studies that have been conducted in the SOT population specifically. Development of ASP process measures related to meaningful clinical outcomes in the SOT population is important. Some measures such as length of stay might not be as meaningful in SOT as in the general population, whereas other measures like drug interactions, allergies, potential toxicities, and development of antibiotic resistant pathogens may be more relevant in SOT. Global assessment measures such as DOOR–RADAR has a potential to be useful in SOT recipients. The relative utility of intervention specificity of these quality metrics and outcomes measures needs to be evaluated in large-scale studies. Use of multisite cluster randomization, crossover, time-series analysis, or stepped-wedge designs could potentially avoid institution-based biases.56
8 |. RECOMMENDATIONS
AMS recommendations for SOT patients are noted in Table 5.
TABLE 5.
Summary of key findings
| Section | Summary | References | Source of evidence | |
|---|---|---|---|---|
| Current landscape of antimicrobial stewardship interventions in SOT | A majority of ASPs in transplant centers include SOT recipients in their activities despite limited published data | [6] | Survey | |
| Transplant IDs specialists involvement in SOT recipient patient care is associated with improved outcomes and more stewardship-concordant care | [7,8] | Retrospective cohort | ||
| Prospective audit with feedback has successfully improved appropriate antimicrobial prescribing in SOT | [7] | Retrospective cohort | ||
| Antifungal and antiviral stewardship can improve appropriate prescribing and has been described in multiple centers | [10,13–17,24,84] | Retrospective cohort, guideline, and transfer | ||
| The in-house availability of rapid diagnostic and molecular tests frequently used in SOT patient care is varied and may be limited. Limited availability of rapid diagnostics hampers both the appropriate starting and stopping point of antimicrobial therapies | [6] | Survey | ||
| Quality metrics and performance indicators for antimicrobial stewardship in SOT patients | Outcome measures reflect the clinical effects of healthcare interventions on patient care | Appropriateness of antimicrobial prescribing based on predefined criteria or clinical practice guidelines | [9] | IDSA guidelines |
| Clinical outcome, such as cure of specific infectious syndromes | [9,85] | Expert opinion | ||
| Recurrence of infection | [9] | |||
| Mortality | [9] | |||
| Time to microbiologic clearance | [9] | |||
| Hospital-acquired Clostridioides difficile infection rate | [9] | |||
| Resistance patterns of specific organisms or institutional antibiograms | [19] | Retrospective review | ||
| Adverse events associated with antimicrobial use such as toxicity, graft injury, and drug-drug interaction | [9] | |||
| Process measures reflect the actions pertaining to healthcare delivery | Types of interventions made by antimicrobial stewardship teams, such as de-escalation or discontinuation of therapy | [9,22,24,84] | IDSA, retrospective cohort, Delphi panel, and expert opinion | |
| Proportion of antimicrobial stewardship recommendations accepted by clinical teams | [9] | |||
| Antimicrobial consumption measures include defined daily dose, days of therapy, or length of therapy. Antimicrobial use measures should be normalized to account for patient volume (i.e., days of therapy per 1000 patient day) | [9] | |||
| Antimicrobial cost (expenditure) | [9] | |||
| Hospital length of stay | [9] | |||
| Hospital admission requirement rate | [9] | |||
| Balancing measures reflect any unintended consequences resulting from a quality improvement initiative | Recurrence of infection | [8,22] | Retrospective and CDC expert opinion | |
| Surgical site infections mortality | [20] | |||
| Hospital length of stay | [22] | |||
| Rehospitalization rate | [22] | |||
Abbreviations: ASP, antimicrobial stewardship program; ID, infectious disease; SOT, solid organ transplant.
9 |. FUTURE DIRECTIONS
Although institutional ASPs have become more widespread and acceptance of their presence has become mainstream in the hospital environment, there are nevertheless further steps that we would like to see implemented over time.
Currently, it is common to see a “one-size-fits-all” approach for stewardship across all hospitalized patients. However, SOT programs host a very specific patient population, which does not always conform to the ASP recommendations for other general hospital patients. Going forward, SOT programs should be encouraged to develop specific policies for ASP in their specific patient population. Ideally, this would be coled by a Transplant ID physician and ASP pharmacist with expertise in immunocompromised hosts.
In order to further legitimize the presence of specific ASPs for the SOT environment, we would like to see the inclusion of an ASP component within the CMS accreditation of SOT programs.
We would like to see the development and routine collection of metrics that track the duration of antimicrobial courses for common clinical syndromes and patient outcomes of ASP measures used in SOT programs. Through utilization of the AU Option, public health organizations such as the CDC ideally could highlight the importance of these metrics so that they are widely adopted.
Currently, allergy and drug interaction assessments are not included as a core safety component of ASP in SOT. Moving forward, we would like to ensure that allergy and drug interaction assessments are included as an essential step in ASPs.
Finally, as with the unsuitability of a “one-size-fits-all” approach for ASPs in SOT patients, consideration should be given for the development of an SOT-specific antibiogram as part of the ASP process, separate from a hospital’s antibiogram for the general patient population.
10 |. CONCLUSION
The field of AMS is evolving and gaining its due recognition in healthcare and society. It is of utmost importance that ASPs are integrated into care provided for SOT populations. As SOT patients are treated as outpatients for the majority of their care following surgery, it is essential that local guidelines are produced in order to guide the prescription of antimicrobials from their primary care team to this patient population for common clinical syndromes. Herein, we have provided a summary of the current landscape of AMS in SOT and highlighted the importance of ASP in this patient population. The summary of our key findings is highlighted in Table 6. The recognition of the uniqueness and complexities of ASPs in SOT needs to be recognized. There should be a concerted effort by the transplant community to develop appropriate implementation strategies and measurement tools and to conduct robust multicenter studies to better serve our SOT patients.
TABLE 6.
Recommendations for SOT in ASP
| No. | Recommendations |
|---|---|
| 1. | Multidisciplinary engagement is recommended for transplant-centered ASPs, including physicians from involved specialties, pharmacists, nurses, and others involved in the care of transplant recipients |
| 2. | Implementation of “Handshake stewardship” with ASP team involvement in daily inpatient rounds is recommended |
| 3. | Prospective audit and feedback is preferred over formulary restrictions in transplant-centered ASPs |
| 4. | Available institutional resources should provide a framework for the selection of ASP interventions (e.g., dose optimization, intravenous-to-oral antimicrobial conversion, therapeutic drug monitoring, development of antibiograms, allergy assessments and de-labelling, and creation of center- and organ-specific guidelines) |
| 5. | ASPs should collect SOT-specific outcomes data, follow metrics germane to selected interventions, and share this information with transplant teams. |
| 6. | Integration of the microbiology laboratory into ASP activities is necessary to improve antimicrobial utilization and inform diagnostic stewardship, including use of rapid diagnostic tests |
| 7. | Collaborations between ASPs and hospital epidemiology and infection prevention and control are necessary to inform local epidemiology and foster development of targeted surveillance procedures, interventions, and education |
Note: We intentionally did not include grades for these recommendations, as we believe that the current evidence is not strong enough in order to assign conclusive determination of the strength of these recommendations. These views represent the opinions of the American Society of Transplantation Infectious Disease Community of practice, Society of Healthcare Epidemiology of America (SHEA), and representatives of the Center for Disease Control and Prevention (CDC).
Abbreviations: ASP, antimicrobial stewardship program; SOT, solid organ transplant.
ACKNOWLEDGMENTS
We would like to give special thanks to Dr. Bruce Kaplan for his contributions to this work. Additionally, we would like to acknowledge our patient–reviewers from the Transplant Community Advisory of AST, Ms. Amy Silverstein and Ms. Molly McCarthy, for their feedback to improve this manuscript. We specifically acknowledge their comments on improving accessibility and transparency of stewardship-related outcomes in SOT patients, formalizing patient and caregiver antimicrobial education, and optimizing outpatient prescribing—where a majority of antimicrobials are used—by providing local treatment guidelines to nontransplant providers. We would also like to extend our sincerest thanks to Mr. Mark Richmond for their excellent editing and compilation of the manuscript.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Darshana M. Dadhania is on the advisory board for CareDx, Veloxis Pharmaceutical Inc., and AlloVir Inc., outside the submitted work. In addition, Dr. Dadhania has a patent #W02018187521A2 issued. Dr. Dilek Ince reports grants from Accelerate Diagnostics, Inc., Gilead Sciences Inc., and Leidos, Inc., outside the submitted work. Dr. Shahid Husain reports grant funding from Merck, Astellas, and Gilead Sciences Inc., outside the submitted work. In addition, Dr. Husain has received consultancy fees from Cidara outside the submitted work. All other authors have nothing to disclose.
Abbreviations:
- AMR
antimicrobial resistance
- AMS
antimicrobial stewardship
- ASP
antimicrobial stewardship programs
- AST
American Society of Transplantation
- AU
antimicrobial use
- CDC
Centers for Disease Control and Prevention
- CMS
Centers for Medicare and Medicaid Services
- CMV
cytomegalovirus
- CoP
Communities of Practice
- DOOR
Desirability of Outcome Ranking
- MALDI-TOF
matrix associated laser desorption/ionization-time of flight
- MDRO
multidrug-resistant organisms
- NAPS
National Antimicrobial Prescription Survey
- NHSN
National Healthcare Safety Network
- PAF
prospective audit and feedback service
- PCR
polymerase chain reaction
- RADAR
Response Adjusted for Duration of Antibiotic Risk
- SHEA
Society of Hospital Epidemiology of America
- SOT
solid organ transplant
- UNOS
United Network for Organ Sharing
- WG
working group
Footnotes
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. This manuscript is a work product of the American Society of Transplantation Infectious Disease Community of Practice and the Society of Health Care Epidemiology of America (SHEA).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.The President’s Council of Advisors on Science and Technology (PCAST). Report to the President on Combating Antibiotic Resistance. 2014. https://www.cdc.gov/drugresistance/pdf/report-to-the-resident-on-combating-antibiotic-resistance.pdf.
- 2.The Joint Commission. New Antimicrobial Stewardship Standard. R3 Report | Requirement, Rationale, Reference 2016. https://www.jointcommission.org/standards/r3-report/r3-report-issue-8-new-antimicrobial-stewardship-standard/.
- 3.The Joint Commission. Antimicrobial Stewardship in Ambulatory Health Care. R3 Report | Requirement, Rationale, Reference 2019; https://www.jointcommission.org/standards/r3-report/r3-report-issue-23-antimicrobial-stewardship-in-ambulatory-health-care/.
- 4.Centers for Medicare and Medicaid Services (CMS). Omnibus Burden Reduction (Conditions of Participation). Final Rule CMS-3346-F. 2019. https://www.cms.gov/newsroom/fact-sheets/omnibus-burden-reduction-conditions-participation-final-rule-cms-3346-f. Accessed July, 2020.
- 5.The Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats In The United States. 2019; 22. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
- 6.Seo SK, Lo K, Abbo LM. Current state of antimicrobial stewardship at solid organ and hematopoietic cell transplant centers in the US. Infect Control Hosp Epidemiol. 2016;37(10):1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamandi B, Husain S, Humar A, Papadimitropoulos EA. Impact of infectious disease consultation on the clinical and economic outcomes of solid organ transplant recipients admitted for infectious complications. Clin Infect Dis. 2014;59(8):1074–1082. [DOI] [PubMed] [Google Scholar]
- 8.So M, Morris AM, Nelson S, Bell CM, Husain S. Antimicrobial stewardship by academic detailing improves antimicrobial prescribing in solid organ transplant patients. Eur J Clin Microbiol Infect Dis. 2019;38(10):1915–1923. [DOI] [PubMed] [Google Scholar]
- 9.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorgenson MR, Descourouez JL, Schulz LT, et al. The development and implementation of stewardship initiatives to optimize the prevention and treatment of cytomegalovirus infection in solid-organ transplant recipients. Infect Control Hosp Epidemiol. 2020;41(9):1068–1074. [DOI] [PubMed] [Google Scholar]
- 11.Institute for Healthcare Improvement (IHI). Quality Improvement Essentials Toolkit. http://www.ihi.org/resources/Pages/Tools/Quality-Improvement-Essentials-Toolkit.aspx. Accessed July, 2020.
- 12.The Centers for Disease Control and Prevention (CDC). Core Elements of Hospital Antibiotic Stewardship Program. Antibiotic Prescribing and Use in Hospitals and Long-Term care. 2016. https://www.cdc.gov/antibiotic-use/core-elements/hospital.html. Accessed July, 2020. [Google Scholar]
- 13.Gouliouris T, Micallef C, Yang H, Aliyu SH, Kildonaviciute K, Enoch DA. Impact of a candidaemia care bundle on patient care at a large teaching hospital in England. J Infect. 2016;72(4):501–503. [DOI] [PubMed] [Google Scholar]
- 14.Antworth A, Collins CD, Kunapuli A, et al. Impact of an antimicrobial stewardship program comprehensive care bundle on management of candidemia. Pharmacotherapy. 2013;33(2):137–143. [DOI] [PubMed] [Google Scholar]
- 15.López-Medrano F, Juan RS, Lizasoain M, et al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital. Clin Microbiol Infect. 2013;19(1):56–61. [DOI] [PubMed] [Google Scholar]
- 16.Shah DN, Yau R, Weston J, et al. Evaluation of antifungal therapy in patients with candidaemia based on susceptibility testing results: implications for antimicrobial stewardship programmes. J Antimicrob Chemother. 2011;66(9):2146–2151. [DOI] [PubMed] [Google Scholar]
- 17.Johnson MD, Lewis RE, Dodds Ashley ES, et al. Core recommendations for antifungal stewardship: a statement of the Mycoses Study Group Education and Research Consortium. J Infect Dis. 2020;222(Suppl_3):S175–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Centers for Disease Control and Prevention (CDC). The Core Elements of Hospital Antibiotic Stewardship Programs. 2019:1–40. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf.
- 19.Rosa R, Simkins J, Camargo JF, Martinez O, Abbo LM. Solid organ transplant antibiograms: an opportunity for antimicrobial stewardship. Diagn Microbiol Infect Dis. 2016;86(4):460–463. [DOI] [PubMed] [Google Scholar]
- 20.Frenette C, Sperlea D, Leharova Y, Thirion DJG. Impact of an infection control and antimicrobial stewardship program on solid organ transplantation and hepatobiliary surgical site infections. Infect Control Hosp Epidemiol. 2016;37(12):1468–1474. [DOI] [PubMed] [Google Scholar]
- 21.So M, Yang DY, Bell C, Humar A, Morris A, Husain S. Solid organ transplant patients: are there opportunities for antimicrobial stewardship? Clin Transplant. 2016;30(6):659–668. [DOI] [PubMed] [Google Scholar]
- 22.The Centers for Disease Control and Prevention (CDC). Core Elements of Antibiotic Stewardship. 2019. https://www.cdc.gov/antibiotic-use/core-elements/index.html. Accessed November, 2020.
- 23.The Centers for Disease Control and Prevention (CDC). Antibiotic Stewardship Driver Diagram. https://www.cdc.gov/antibiotic-use/healthcare/pdfs/Antibiotic_Stewardship_Driver_Diagram.pdf. Accessed July, 2020.
- 24.Micallef C, Aliyu SH, Santos R, Brown NM, Rosembert D, Enoch DA. Introduction of an antifungal stewardship programme targeting high-cost antifungals at a tertiary hospital in Cambridge, England. J Antimicrob Chemother. 2015;70(6):1908–1911. [DOI] [PubMed] [Google Scholar]
- 25.The Centers for Disease Control and Prevention (CDC). National Healthcare Safety Network-Antimicrobial Use and Resistance Module. https://www.cdc.gov/nhsn/pdfs/pscmanual/11pscaurcurrent.pdf. Accessed July, 2020.
- 26.van Santen KL, Edwards JR, Webb AK, et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis. 2018;67(2):179–185. [DOI] [PubMed] [Google Scholar]
- 27.The American College of Surgeons (ACS). ACS National Surgical Quality Improvement Program. https://www.facs.org/quality-programs/acs-nsqip. Accessed July, 2020.
- 28.Parekh J, Ko C, Lappin J, Greenstein S, Hirose R. A transplant-specific quality initiative-introducing TransQIP: a joint effort of the ASTS and ACS. Am J Transplant. 2017;17(7):1719–1722. [DOI] [PubMed] [Google Scholar]
- 29.Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis. 2015;61(5):800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solomkin JS. A cool reception for desirability of outcome ranking (DOOR)/response adjusted for duration of antibiotic risk (RADAR) in intra-abdominal infections. Clin Infect Dis. 2017;65(9):1580–1581. [DOI] [PubMed] [Google Scholar]
- 31.Health Quality Ontario (HQO). Measurement for Quality Improvement. Quality Improvement Primers [Resource Guide]. 2013. http://www.hqontario.ca/Portals/0/documents/qi/qi-measurement-primer-en.pdf. Accessed July, 2020. [Google Scholar]
- 32.Morrill HJ, Caffrey AR, Gaitanis MM, LaPlante KL. Impact of a prospective audit and feedback antimicrobial stewardship program at a veterans affairs medical center: a six-point assessment. PLoS One. 2016;11(3):e0150795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacBrayne CE, Williams MC, Levek C, et al. Sustainability of handshake stewardship: extending a hand is effective years later. Clin Infect Dis. 2019;70(11):2325–2332. [DOI] [PubMed] [Google Scholar]
- 34.Baker DW, Hyun D, Neuhauser MM, Bhatt J, Srinivasan A. Leading practices in antimicrobial stewardship: conference summary. Jt Comm J Qual Patient Saf. 2019;45(7):517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurst AL, Child J, Pearce K, Palmer C, Todd JK, Parker SK. Handshake stewardship: a highly effective rounding-based antimicrobial optimization service. Pediatr Infect Dis J. 2016;35(10):1104–1110. [DOI] [PubMed] [Google Scholar]
- 36.So M, Hand J, Richmond M, Morris A, Husain S. Perceptions and attitude of transplant clinicians towards antimicrobial resistance and stewardship in transplant recipients: a survey of the American Society of Transplantation. Am J Transplant. 2019;19(Suppl 3):737.30091857 [Google Scholar]
- 37.Karanika S, Paudel S, Grigoras C, Kalbasi A, Mylonakis E. Systematic review and meta-analysis of clinical and economic outcomes from the implementation of hospital-based antimicrobial stewardship programs. Antimicrob Agents Chemother. 2016;60(8):4840–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palmay L, Elligsen M, Walker SAN, et al. Hospital-wide rollout of antimicrobial stewardship: a stepped-wedge randomized trial. Clin Infect Dis. 2014;59(6):867–874. [DOI] [PubMed] [Google Scholar]
- 39.Yeo C-L, Chan DS-G, Earnest A, et al. Prospective audit and feedback on antibiotic prescription in an adult hematology-oncology unit in Singapore. Eur J Clin Microbiol Infect Dis. 2012;31(4):583–590. [DOI] [PubMed] [Google Scholar]
- 40.Tamma PD, Avdic E, Keenan JF, et al. What is the more effective antibiotic stewardship intervention: preprescription authorization or postprescription review with feedback? Clin Infect Dis. 2017;64(5):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gagermeier JP, Rusinak JD, Lurain NS, et al. Subtherapeutic ganciclovir (GCV) levels and GCV-resistant cytomegalovirus in lung transplant recipients. Transpl Infect Dis. 2014;16(6):941–950. [DOI] [PubMed] [Google Scholar]
- 42.Padullés A, Colom H, Bestard O, et al. Contribution of population pharmacokinetics to dose optimization of ganciclovir-valganciclovir in solid-organ transplant patients. Antimicrob Agents Chemother. 2016;60(4):1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamdy RF, Zaoutis TE, Seo SK. Antifungal stewardship considerations for adults and pediatrics. Virulence. 2017;8(6):658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khumra S, Chan J, Urbancic K, et al. Antibiotic allergy labels in a liver transplant recipient study. Antimicrob Agents Chemother. 2017;61(5):e00078–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouza E, Muñoz P, Burillo A. Role of the clinical microbiology laboratory in antimicrobial stewardship. Med Clin North Am. 2018;102(5):883–898. [DOI] [PubMed] [Google Scholar]
- 46.MacVane SH, Hurst JM, Steed LL. The role of antimicrobial stewardship in the clinical microbiology laboratory: stepping up to the plate. Open Forum Infect Dis. 2016;3(4):ofw201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamandi B, Holbrook AM, Humar A, et al. Delay of adequate empiric antibiotic therapy is associated with increased mortality among solid-organ transplant patients. Am J Transplant. 2009;9(7):1657–1665. [DOI] [PubMed] [Google Scholar]
- 48.Patel R, Fang FC. Diagnostic stewardship: opportunity for a laboratory-infectious diseases partnership. Clin Infect Dis. 2018;67(5):799–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang CM, Chen XJ, Chih CC, et al. Rapid identification of blood-stream bacterial and fungal pathogens and their antibiotic resistance determinants from positively flagged blood cultures using the BioFire FilmArray blood culture identification panel. J Microbiol Immunol Infect. 2020;53(6):882–891. [DOI] [PubMed] [Google Scholar]
- 50.Rood IGH, Li Q. Review: molecular detection of extended spectrum-β-lactamase- and carbapenemase-producing Enterobacteriaceae in a clinical setting. Diagn Microbiol Infect Dis. 2017;89(3):245–250. [DOI] [PubMed] [Google Scholar]
- 51.Smith M, Diederen B, Scharringa J, Leversteijn-van Hall M, Fluit AC, Cohen SJ. Rapid and accurate detection of carbapenemase genes in Enterobacteriaceae with the Cepheid Xpert Carba-R assay. J Med Microbiol. 2016;65(9):951–953. [DOI] [PubMed] [Google Scholar]
- 52.Moody J, Cosgrove SE, Olmsted R, et al. Antimicrobial stewardship: a collaborative partnership between infection preventionists and healthcare epidemiologists. Infect Control Hosp Epidemiol. 2012;33(4):328–330. [DOI] [PubMed] [Google Scholar]
- 53.Abbo LM, Ariza-Heredia EJ. Antimicrobial stewardship in immunocompromised hosts. Infect Dis Clin North Am. 2014;28(2):263–279. [DOI] [PubMed] [Google Scholar]
- 54.Infectious Diseases Society of America (IDSA). Antimicrobial Stewardship Center of Excellence. 2020. https://www.idsociety.org/clinical-practice/antimicrobial-stewardship/. Accessed Feb, 2021.
- 55.Centers for Medicare and Medicaid Services (CMS). Medicare and Medicaid Programs; Regulatory Provisions To Promote Program Efficiency, Transparency, and Burden Reduction; Fire Safety Requirements for Certain Dialysis Facilities; Hospital and Critical Access Hospital (CAH) Changes To Promote Innovation, Flexibility, and Improvement in Patient Care. In: Centers for Medicare & Medicaid Services (CMS), ed. Vol 84 FR 51732. Federal Register, The Daily Journal of the United States Government. 2019:51732–51834 ( 51103 pages). https://www.federalregister.gov/agencies/centers-for-medicare-medicaid-services. Accessed July 09, 2021. [Google Scholar]
- 56.Morris AM, Calderwood MS, Fridkin SK, et al. Research needs in antibiotic stewardship. Infect Control Hosp Epidemiol. 2019;40(12):1334–1343. [DOI] [PubMed] [Google Scholar]
- 57.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505–511. [DOI] [PubMed] [Google Scholar]
- 58.Dunbar LM, Khashab MM, Kahn JB, Zadeikis N, Xiang JX, Tennenberg AM. Efficacy of 750-mg, 5-day levofloxacin in the treatment of community-acquired pneumonia caused by atypical pathogens. Curr Med Res Opin. 2004;20(4):555–563. [DOI] [PubMed] [Google Scholar]
- 59.Moussaoui RE, de Borgie CAJM, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332(7554):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257–1265. [DOI] [PubMed] [Google Scholar]
- 61.Leophonte P, Choutet P, Gaillat J, et al. Efficacy of a ten day course of ceftriaxone compared to a shortened five day course in the treatment of community-acquired pneumonia in hospitalized adults with risk factors. Med Mal Infect. 2002;32(7):369–381. [Google Scholar]
- 62.Tellier G, Chang JR, Asche CV, Lavin B, Stewart J, Sullivan SD. Comparison of hospitalization rates in patients with community-acquired pneumonia treated with telithromycin for 5 or 7 days or clarithromycin for 10 days. Curr Med Res Opin. 2004;20(5):739–747. [DOI] [PubMed] [Google Scholar]
- 63.File TM Jr, Mandell LA, Tillotson G, Kostov K, Georgiev O. Gemifloxacin once daily for 5 days versus 7 days for the treatment of community-acquired pneumonia: a randomized, multicentre, double-blind study. J Antimicrob Chemother. 2007;60(1):112–120. [DOI] [PubMed] [Google Scholar]
- 64.Léophonte P, File T, Feldman C. Gemifloxacin once daily for 7 days compared to amoxicillin/clavulanic acid thrice daily for 10 days for the treatment of community-acquired pneumonia of suspected pneumococcal origin. Respir Med. 2004;98(8):708–720. [DOI] [PubMed] [Google Scholar]
- 65.D’Ignazio J, Camere MA, Lewis DE, Jorgensen D, Breen JD. Novel, single-dose microsphere formulation of azithromycin versus 7-day levofloxacin therapy for treatment of mild to moderate community-acquired Pneumonia in adults. Antimicrob Agents Chemother. 2005;49(10):4035–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drehobl MA, De Salvo MC, Lewis DE, Breen JD. Single-dose azithromycin microspheres vs clarithromycin extended release for the treatment of mild-to-moderate community-acquired pneumonia in adults *. Chest. 2005;128(4):2230–2237. [DOI] [PubMed] [Google Scholar]
- 67.Chastre J, Wolff M, Fagon J-Y, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults. A randomized trial. JAMA. 2003;290(19):2588–2598. [DOI] [PubMed] [Google Scholar]
- 68.Capellier G, Mockly H, Charpentier C, et al. Early-onset ventilator-associated pneumonia in adults randomized clinical trial: comparison of 8 versus 15 days of antibiotic treatment. PLoS One. 2012;7(8):e41290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000;283(12):1583–1590. [DOI] [PubMed] [Google Scholar]
- 70.Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380(9840):484–490. [DOI] [PubMed] [Google Scholar]
- 71.Peterson J, Kaul S, Khashab M, Fisher AC, Kahn JB. A double-blind, randomized comparison of levofloxacin 750 mg once-daily for five days with ciprofloxacin 400/500 mg twice-daily for 10 days for the treatment of complicated urinary tract infections and acute pyelonephritis. Urology. 2008;71(1):17–22. [DOI] [PubMed] [Google Scholar]
- 72.Rudrabhatla P, Deepanjali S, Mandal J, Swaminathan RP, Kadhiravan T. Stopping the effective non-fluoroquinolone antibiotics at day 7 vs continuing until day 14 in adults with acute pyelonephritis requiring hospitalization: a randomized non-inferiority trial. PLoS One. 2018;13(5):e0197302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Klausner HA, Brown P, Peterson J, et al. A trial of levofloxacin 750 mg once daily for 5 days versus ciprofloxacin 400 mg and/or 500 mg twice daily for 10 days in the treatment of acute pyelonephritis. Curr Med Res Opin. 2007;23(11):2637–2645. [DOI] [PubMed] [Google Scholar]
- 74.Dinh A, Davido B, Etienne M, et al. Is 5 days of oral fluoroquinolone enough for acute uncomplicated pyelonephritis? The DTP randomized trial. Eur J Clin Microbiol Infect Dis. 2017;36(8):1443–1448. [DOI] [PubMed] [Google Scholar]
- 75.Sawyer RG, Claridge JA, Nathens AB, et al. Trial of short-course antimicrobial therapy for intraabdominal infection. New Engl J Med. 2015;372(21):1996–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basoli A, Chirletti P, Cirino E, et al. A prospective, double-blind, multicenter, randomized trial comparing ertapenem 3 vs >or=5 days in community-acquired intraabdominal infection. J Gastrointest Surg. 2008;12(3):592–600. [DOI] [PubMed] [Google Scholar]
- 77.Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis. 2019;69(7):1091–1098. [DOI] [PubMed] [Google Scholar]
- 78.von Dach E, Albrich WC, Brunel A-S, et al. Effect of C-reactive protein-guided antibiotic treatment duration, 7-day treatment, or 14-day treatment on 30-day clinical failure rate in patients with uncomplicated gram-negative bacteremia: a randomized clinical trial. JAMA. 2020;323(21):2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164(15):1669–1674. [DOI] [PubMed] [Google Scholar]
- 80.Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phos-phate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309(6):559–569. [DOI] [PubMed] [Google Scholar]
- 81.Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696–705. [DOI] [PubMed] [Google Scholar]
- 82.Cranendonk DR, Opmeer BC, van Agtmael MA, et al. Antibiotic treatment for 6 days versus 12 days in patients with severe cellulitis: a multicentre randomized, double-blind, placebo-controlled, non-inferiority trial. Clin Microbiol Infect. 2020;26(5):606–612. [DOI] [PubMed] [Google Scholar]
- 83.Aguilar-Guisado M, Espigado I, Martín-Peña A, et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): an open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017;4(12):e573–e583. [DOI] [PubMed] [Google Scholar]
- 84.Khanina A, Urbancic KF, Haeusler GM, et al. Establishing essential metrics for antifungal stewardship in hospitals: the results of an international Delphi survey. J Antimicrob Chemother. 2021;76(1):253–262. [DOI] [PubMed] [Google Scholar]
- 85.Clemente WT, Carratalà J. Why should quality metrics be used for infectious disease assessment, management and follow up in solid organ transplantation? Clin Microbiol Infect. 2021;27(1):12–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


