Abstract
It is now possible to estimate an individual’s brain age via brain scans and machine-learning models. This validated technique has opened up new avenues for addressing clinical questions in neurology, and, in this review, we summarize the many clinical applications of brain-age estimation in neuropsychiatry and general populations. We first provide an introduction to typical neuroimaging modalities, feature extraction methods, and machine-learning models that have been used to develop a brain-age estimation framework. We then focus on the significant findings of the brain-age estimation technique in the field of neuropsychiatry as well as the usefulness of the technique for addressing clinical questions in neuropsychiatry. These applications may contribute to more timely and targeted neuropsychiatric therapies. Last, we discuss the practical problems and challenges described in the literature and suggest some future research directions.
Keywords: brain age, neuropsychiatric disorder, neuroimaging, machine learning
1. Aging, Disease, and the Brain
The aging process in humans is associated with the progressive decline of various physiological and organ functions [1], and many diseases including cancer, cardiovascular disease, diabetes, and dementia are associated with aging [2]. It is not uncommon for elderly people to suffer from multiple diseases simultaneously. Since humans’ bodies change with age and as humans are living longer in several regions of the world, the aging process has become a key issue in public health, disease prevention, and treatment. Many discussions concerning the pathological meaning of aging in the context of epigenetic change, proteotoxic or oxidative stress, and telomere damage have thus been conducted [3].
The brain is also affected by aging [4]. In the early stage of life, the aging process is regarded as brain development in which the brain matures, and children usually experience an increase in their cognitive ability along with their physical growth. During late adulthood, the brain aging process has different effects, e.g., a decline of cognitive function, and advancing age is associated with neurodegeneration, particularly Alzheimer’s disease and other forms of dementia [5,6]. If the aging process of the brain could be measured precisely and accurately, the findings may have potential as biomarkers for neuropsychiatric disorders. In fact, frameworks to quantify the age of a human brain have been attempted for several decades [7]. Today, advances in medical imaging and analytical methods (especially machine learning) have allowed the calculation of an individual’s biological age from the extracted biological features [8]. The frameworks that are now used to estimate the age of an individual’s brain have the potential to provide useful, objective, and personalized biomarkers for neurological and psychiatric disorders.
2. Neuroimaging-Based Brain-Age Estimation
Telomere-related and epigenetics-related biomarkers have not shown sufficient predictive and deterministic value for estimating brain ages, and it has been suggested that phenotype-based estimation can generate a much closer indicator of brain age [4]. Neuroimaging is a widely available, less-invasive method to investigate the whole brain of humans, and with neuroimaging, the brain’s morphological and microstructural features can be obtained; these features are speculated to be suitable material for the estimation of the age of an individual brain. In fact, neuroimaging-based brain-age estimation has been increasingly applied to individuals with various neuropsychiatric disorders and general populations [8]. In this narrative review, we examined over 100 studies and introduce the recent findings and methodologies of this emerging technique. We conducted a search of the PubMed database in May 2022 using “brain-age estimation” and/or “brain-age prediction” as keywords, although we did not adopt rigorous systematic selection criteria of studies for this narrative review.
3. Theory and Methodology
3.1. Theory of Neuroimaging-Based Brain-Age Estimation
In 2010, Katja Franke and her peers developed a prediction model that was able to estimate a subject’s age based on brain imaging data and the use of a regression machine-learning model [9]. The output of a brain-age estimation framework has been called the “brain age-delta,” “brain predicted age difference (Brain-PAD),” “brain age gap estimation (BrainAGE),” and “brain age gap (BAG),” each of which is computed by deducting the estimated brain age from the subject’s chronological age. In this review, we refer to “brain age-delta” as the output of a brain-age estimation framework. The brain age-delta is known as a heritable biomarker for both monitoring cognitively healthy aging and identifying age-associated disorders [8]. There are three possibilities for a brain age-delta value: (i) a brain age-delta close to zero, representing normal brain aging, (ii) a positive brain age-delta (i.e., estimated brain age > chronological age), representing an older-appearing brain, and (iii) a negative brain age-delta (i.e., estimated brain age < chronological age), representing a younger-appearing brain.
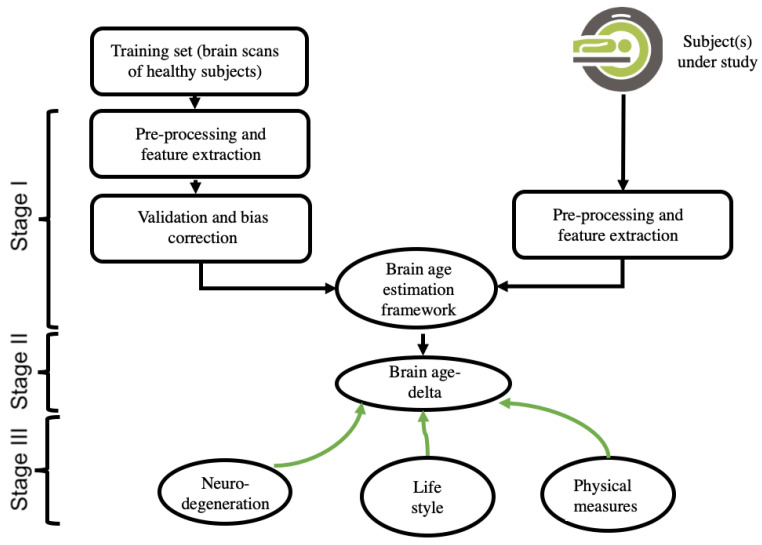
A brain-age estimation study is generally composed of three main stages: (i) creating a prediction model by using extracted brain features and a regression machine-learning model, validation, and bias correction; (ii) computing the brain age and brain age-delta for the subject under study; and (iii) interpreting the results, including the use of a within-group and/or a between-groups analysis. Figure 1 depicts the pipeline of a typical brain-age estimation study.
Figure 1.
This is a figure. Schemes follow the same formatting.
In the literature, the typical accuracy of the prediction of brain ages is from 2 years to 10 years in terms of mean absolute error (MAE) [10,11]. The prediction accuracy in a brain-age estimation framework depends on variables such as the type of input data, the feature extraction, and reduction strategies [12] and bias adjustment techniques [13], and machine-learning models [14]. In the following subsections, we provide a general overview of these variables.
3.2. Input Data and Feature-Extraction Methodologies of Neuroimaging
One of the key concerns among researchers attempting to develop a brain-age estimation framework is the selection of the input data. Each modality offers unique insights into the brain. For example, fluorodeoxyglucose-positron emission tomography (FDG-PET) scans provide information about the brain’s glucose metabolism, whereas magnetic resonance imaging (MRI) data provide information about the anatomy of the brain. Among the different brain, MRI modalities such as T1-weighted MRI images (T1w MRI), T2-weighted MRI images (T2w MRI), resting-state functional (f)MRI, and fluid-attenuated inversion recovery (FLAIR), the majority of brain-age estimation studies have used T1w MRI data. The main reason for using T1w MRI is because it is more readily available than other modalities [15]. Brain age frameworks generally require a large dataset for training a prediction model, and many public neuroimaging datasets such as ADNI (https://www.adni.loni.usc.edu, accessed on 31 October 2022), PPMI (https://www.ppmi-info.org, accessed on 31 October 2022), IXI (http://brain-development.org/ixi-dataset/, accessed on 31 October 2022), and OASIS (https://www.oasis-brains.org/, accessed on 31 October 2022) have provided a great number of T1W MRI scans for research studies.
Each brain imaging modality requires a specific feature extraction strategy. The feature extraction approaches for T1w MRI data can be classified into two categories: (i) voxel-wise methods (e.g., statistical parametric mapping [SPM], http://www.fil.ion.ucl.ac.uk/spm, accessed on 31 October 2022) [8,16,17], which use gray matter (GM) and/or white matter (WM) signal intensities as brain features; and (ii) region-wise methods (e.g., FreeSurfer, http://surfer.nmr.mgh.harvard.edu/, accessed on 31 October 2022) [18], which use the subcortical and cortical and measurements of volume, surface, and thickness values as brain features. Both voxel-wise and region-wise feature extraction approaches have been widely used in T1-w MRI-driven brain-age estimation studies [19,20,21].
A direct comparison of voxel-wise and region-wise metrics as well as their integration in the accuracy of brain age has been conducted [12]. For functional MRI-driven brain-age frameworks, the extracted features can be functional connectivity (FC) measures between brain regions or intrinsic connectivity networks and voxel-wise whole-brain FC measures (e.g., FSLNets, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets, accessed on 31 October 2022) [22,23]. In terms of the PET modality, the extracted features for estimating brain ages include measurements of brain metabolism (i.e., PET regional total glucose, aerobic glycolysis, oxygen) and cerebral blood flow [22,23]. White-matter microstructure measurements such as mean diffusivity, fractional anisotropy, axial diffusivity, and radial diffusivity have been employed as brain features for a diffusion tensor imaging (DTI)-based brain age framework [24].
3.3. Data Reduction, Validation, and Bias Adjustment Neuroimaging Methodologies
The ‘curse of dimensionality’ is one of the major concerns in developing a brain-age estimation framework, particularly when the number of brain features is far higher than the number of samples (e.g., in voxel-based feature extraction strategies). High-dimensional data can give rise to some substantial issues in a prediction model, such as overfitting and decreased computational efficiency. A data reduction technique that can decrease the high dimensionality of data and diminish redundant information is thus required. In the area of brain-age estimation, most studies have used the principal component analysis (PCA) strategy, which is an unsupervised learning technique [9,19]. The effect of the number of principal components on the accuracy of brain-age predictions has been investigated [9]. The number of principal components may influence the prediction accuracy in a brain age estimation framework. However, it can be adjusted to achieve maximum accuracy in the training set [9].
After a prediction model is developed, it is critical to validate the model’s prediction accuracy. Most studies in the field of brain-age estimation have used a K-fold cross-validation strategy (e.g., K = 5 or 10) to assess the prediction performance on a training set [12,14,16,21]. In the K-fold cross-validation technique, the data are randomly divided into K folds, and the learning process is repeated K times so that K-1 folds are used for training a prediction model, and the remaining fold is used as a test for each iteration. To assess the prediction accuracy, researchers generally use the coefficient of determination (R2) between the subjects’ chronological age and estimated age, the MAE, and root mean square error (RMSE) metrics.
Many brain-age estimation studies have reported age dependency on the prediction outputs, and this is considered a substantial issue in brain-age frameworks [13,21]. This bias, which could be a result of regression dilution bias, may adversely affect the predicted values and alter the interpretation of results. Several techniques have been proposed to diminish this bias (i.e., age dependency) [13,21,25]. For instance, Le and colleagues proposed using chronological age as a covariate in the statistical analyses and interpreting the results [26]. However, it should be highlighted that Le’s method is appropriate for group comparison only and not able to deliver bias-free brain age values at the individual level. A bias adjustment strategy is proposed in [21] (i.e., Cole’s method) that uses the intercept and slope of a linear regression model of estimated brain age against chronological derived from the training set. The bias-free Brain-age values in the test sets are then calculated by subtracting the intercept from the predicted brain age and dividing by the slope [21]. The most recent bias adjustment technique is suggested in [13] (i.e., Beheshti’s method) which computes offset values for test subjects on the basis of the intercept and slope of a linear regression model of brain age-delta against chronological age achieved from the training set. Then, the bias-free Brain-age values are computed by subtracting the offset values from the estimated brain-age values [13]. A direct comparison of these bias adjustment techniques has shown that Beheshti’s method greatly reduces the variance of the predicted ages, whereas Cole’s method increases it [13].
3.4. Machine-Learning Methodologies
One of the important steps in developing a brain-age estimation framework is choosing a regression machine-learning model. A regression model establishes a pattern between independent variables (here, brain features) and the corresponding dependent variable (a subject’s chronological age) based on the training dataset, and the model uses this pattern to predict the brain age based on unseen data (i.e., independent test datasets). The most widely used traditional regression algorithms include support vector regression (SVR) [19,23], relevance vector regression (RVR) [9], Gaussian process regression [21], an ensemble of gradient-boosted regression trees [25], and XGBoost [25]. It has been demonstrated that the type of regression algorithm used influences the prediction accuracy and the interpretation of outcomes in brain-age frameworks [14].
In addition to the traditional regression algorithms, deep learning models have become a prominent methodology in the area of brain-age estimation [11], as they can be used to develop more accurate prediction models. A major advantage of deep learning models is that they can be applied directly with 3D brain image data and incorporate feature extraction, data reduction, and prediction stages into a unified system. The main challenge of deep learning-based brain-age estimation frameworks is that this methodology requires a large dataset to train a model. In 2017, James Cole and his peers developed the first deep learning-based brain-age estimation framework, with a 3D convolutional neural network (CNN) that uses 3D gray matter and 3D white matter intensity maps as the input data [11]. Other deep learning architectures used in brain-age estimation frameworks include feed-forward neural networks, VGGNet [27], ResNet [28], U-Net [29], and an ensemble of CNN architectures [30].
4. Applications in Neuropsychiatry
4.1. Alzheimer’s Disease, Dementia, and Memory Impairment
One of the most active areas of brain-age research concerns Alzheimer’s disease (AD) and mild cognitive impairment (MCI) (Table 1) [17,23,31,32,33,34,35,36,37], because of their strong association with aging. Alzheimer’s disease is the most common cause of dementia, which is also a relevant issue in aging societies in many developed countries. The early detection of AD is important in terms of early cognitive intervention [38,39,40] as well as the future development of disease-modifying therapy [41], and neuroimaging plays key roles in ensuring accurate and early diagnoses, revealing the underlying pathophysiology, and monitoring the disease. An increased BAG in individuals with AD has been consistently reported, ranging from +2.88 to +9.29 years [17,20,37], and correlations between an increased BAG and cognitive dysfunction or white matter hyperintensity were also found [17,36]. Importantly, the predictability of progression from MCI to AD and the detectability of preclinical AD based on brain-age measures are also confirmed and would be clinically significant [31,34,37].
Table 1.
Neuroimaging-based brain age studies for dementia, cognitive impairment, and other neurological disorders.
| First Author [ref.] |
Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Alzheimer’s Disease and Cognitive Impairment | |||||
| Gaser [31] | 2013 | 133 pMCI, 62 sMCI | T1WI | RVR | BAG predicts conversion to AD, 10% greater risk of developing AD by each 1 additional yr of BAG |
| Lowe [32] | 2016 | 150 AD, 112 pMCI, 36 sMCI, 107HC | T1WI | RVR | Effect of APOEe4 on BrainAGE changing rates over time |
| Beheshti [17] | 2018 | 147 AD, 112 pMCI, 102 sMCI, 146 HCs | T1WI | SVR | BAG: +5.36 yr in AD, +3.15 yr in pMCI, +2.38 yr in sMCI. Correlation with cognitive function |
| Wang [33] | 2019 | 3688 people (middle age to elderly) | T1WI | CNN | BAG: related to incident dementia |
| Mohajer [35] | 2020 | 48 AD, 222 MCI, 60 HCs | T1WI | SVR | BAG was elevated in MCI and AD but was not associated with sleep-disordered breathing. |
| Ly [34] | 2020 | 74 AD, 283 MCI, 51 preclinical AD, 83 HCs | T1WI | GPR | BAG differentiated cognitively unimpaired Amyloid (+) from Amyloid (−). |
| Beheshti [23] | 2021 | 292 AD, 440 MCI, 548 HCs | FDG-PET | SVR | Younger BAG in females than in males in HCs group but not in MCI or AD groups |
| Habes [36] | 2021 | 1932 MCI/AD, 8284 HCs | T1WI | RBF-kernel | BAG associated with WMH as well as cognitive function |
| Parkinson’s disease | |||||
| Beheshti [20] | 2020 | 160 PD, 129 AD, 839 HCs | T1WI | SVR | GM-based BAG: +1.50 yr in PD, +9.29 yr in AD. WM-based BAG: +2.47 yr in PD, +8.85 yr in AD. WM-based BAG > GM-based BAG in PD |
| Eickhoff [42] | 2021 | 372 PD, 172 HCs | T1WI | SVR | BAG: +2.9 yr in PD. Associated with disease duration and cognitive and motor impairment. |
| Charisse [43] | 2022 | 83 PD-NC, 78 PD-MCI, 17 PD-D, 84 HCs | T1WI | SVR | RBA: +2.38 yr in PD-NC, +1.90 yr in PD-MCI, +3.52 yr in PD-D. Associated with attention deficits and working memory |
| Epilepsy | |||||
| Pardoe [44] | 2017 | 42 new FE, 94 refractory FE, 74 HCs | T1WI | GPR | BAG: +4.5yr in refractory FE, no significance in new FE |
| Hwang [45] | 2020 | 104 TLE, 151 HCs | T1WI, fMRI | SVR | T1-based BAG: +6.6 yr in TLE. fMRI-based BAG: +8.3 yr in TLE Association with clinical data |
| Sone [19] | 2021 | 318 epilepsy, 1,196 HCs | T1WI | SVR | BAG: >+4 yr in all types of epilepsies, +10.9 yr in TLE with psychosis |
| de Bézenac [46] | 2022 | 48 TLE, 37 HCs | T1WI | GPR | BAG: +7.97 yr in TLE, postsurgical reduction of BAG |
| Multiple sclerosis | |||||
| Cole [47] | 2020 | 1204 MS/CIS, 150 HCs | T1WI | GPR | BAG: +10.3 yr in MS, +13.3 yr in SPMS, predictive value for progression |
| Jacobs [48] | 2021 | 179 MS | T1WI | GPR | BAG: +6.54 yr in MS, associated with a physical disability |
| Traumatic brain injury | |||||
| Gan [49] | 2021 | 116 mTBI, 63 HCs | DTI | RVR | BAG: +2.59 yr in mTBI, associated with post-concussion complaints |
| Hellstrom [50] | 2021 | 123 mTBI | T1WI, DTI | XGBoost | No significant difference in BAG between APOEe4 carriers and non-carriers after mTBI |
| Pain | |||||
| Yu [51] | 2021 | 31 CLBP, 32 HCs | T1WI | GPR | Discrepancy in BAG between HCs and CLBP was greater in older individuals |
| Hung [52] | 2022 | 45 TN, 52 OA, 50 CLBP, 812 HCs | T1WI | GPR | BAG: +6.48 yr in TN, +9.80 yr in OA, no significance in BP. Female-driven elevation in BAG |
| Others | |||||
| Azor [53] | 2019 | 20 PWS, 40 HCs | T1WI | GPR | BAG: +7.24 yr in PWS, Not associated with IQ, hormonal or psychotropic medications, or abnormal behaviors |
| Cole [54] | 2017 | 162 HIV(+), 105 HIV(−) | T1WI | GPR | BAG: +2.15 yr in HIV(+), associated with cognitive performance |
AD: Alzheimer’s disease, BAG: brain age gap, CIS: clinically isolated syndrome, CLBP: chronic lower back pain, CNN: convolutional neural network, DTI: diffusion tensor imaging, FDG-PET: 18F-fluorodeoxyglucose PET, FE: focal epilepsy, fMRI: functional MRI, GPR: Gaussian process regression, HCs: healthy controls, ML: machine learning, MS: multiple sclerosis, mTBI: mild traumatic brain injury, NC: normal cognition, OA: osteoarthritis, PD: Parkinson disease, PD-D: PD with dementia, pMCI: progressive mild cognitive impairment, PWS: Prader-Willi syndrome, RBF: radial basis function, RVR: relevance vector regression, sMCI: stable mild cognitive impairment, SPMS: secondary progressive MS, SVR: support vector regression, T1WI: T1-weighted image, TLE: temporal lobe epilepsy, TN: trigeminal neuralgia.
4.2. Other Neurological Diseases
Parkinson’s disease (PD) is a common neurodegenerative movement disorder characterized by the degeneration of dopaminergic neurons in the substantia nigra [55]. Overall, it seems that an increase in brain age by 2–3 years occurs in PD [20,42,43], and such an increase is associated with cognitive or motor impairment (Table 1). A comparison study between PD and AD revealed a significant increase in the BAG in AD compared to PD.
Epilepsy is also a common neurological disorder, characterized by recurrent seizures associated with abnormal electrical activity in the brain. A brain with chronic epilepsy tends to present a BAG of +4 to +8 years (Table 1) [19,44,45,46], and comorbid psychosis may further increase the BAG by several additional years [19]. Interestingly, epilepsy surgery may reduce the abnormal BAG increase, regardless of postsurgical seizure freedom [46].
Multiple sclerosis (MS) is an autoimmune disease involving damage to the myelin sheaths of the brain and spinal cord [56]. The BAG in MS is relatively high at +6.5 to 10.3 years on average (Table 1), and it is particularly higher in secondary progressive MS (+13.3 years) [47,48]. An increased BAG is also suggested to predict MS progression.
A brain-age framework has also been applied to neurological and related disorders including traumatic brain injury [49,50], pain [51,52], Prader-Willi syndrome [53], and HIV infection [54] (Table 1).
4.3. Schizophrenia and Psychotic Disorders
Schizophrenia is a serious psychiatric disorder presenting symptoms that include psychosis, cognitive dysfunction, and negative symptoms. Increased brain age in schizophrenia and first-episode psychosis (FEP) has been reported (Table 2) [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], and consistent findings of a BAG increased by approx. 3–6 years in schizophrenia have been confirmed, with possible associations with cognitive dysfunction or polygenic risk. The BAG in individuals with FEP may be lower than that in schizophrenia, and, according to longitudinal studies, an acceleration of brain aging over time is suggested in this population. The BAG is also associated with schizotypal symptoms in relatives of patients with psychosis [72]. Early medication may reduce the BAG in psychosis [71].
Table 2.
Neuroimaging-based brain age studies for psychiatric disorders.
| First Author [ref.] |
Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Schizophrenia and Psychosis | |||||
| Koutsouleris [57] | 2014 | 141 SZ, 104 MDD, 57B PD, 89 ARMS, 127 HCs | T1WI | SVR | BAG: +5.5 yr in SZ, +4.0 yr in MDD, +3.1 yr in BPD, +1.7 yr in ARMS. |
| Schnack [58] | 2016 | 341 SZ, 386 HCs | T1WI | SVR | BAG: +3.36 yr in SZ, acceleration just after illness onset |
| Nenadic [59] | 2017 | 45 SZ, 22 BPAD, 70 HCs | T1WI | RVR | BAG: +2.56 yr in SZ, no significance in BPAD |
| Kolenic [60] | 2018 | 120 FEP, 114 HCs | T1WI | RVR | BAG: +2.64 yr in FES, associated with obesity |
| Hajek [62] | 2019 | 43 FES, 43 HCs, 96 offspring of BPAD (48 affected, 48 unaffected), 60 HCs | T1WI | RVR | BAG: +2.64 yr in FES, no significance in early BPAD |
| Chung [61] | 2019 | 476 CHR | N/A | N/A | BAG predicts conversion to psychosis in a univariate analysis but not in a multivariate analysis |
| Shahab [63] | 2019 | 81 SZ, 53 BPAD, 91 HCs | T1WI, DTI | RF | BAG: +7.8–8.2 yr in SZ, no significance in BPAD |
| Kuo [64] | 2020 | 26 SZ, 30 MDD, 19AD, 109 HCs | T1WI | LASSO, ICA | BAG: +5.69 yr in SCZ, +3.25 yr in AD, no significance in MDD. Association with large-scale structural covariance network |
| Tønnesen [65] | 2020 | 668 SZ, 185 BPAD, 990 HCs | DTI | XGBoost | Increased BAG in SZ (Cohen’s d = −0.29) and BPAD (Cohen’s d = 0.18) |
| Lee [66] | 2021 | 90 SZ, 200 HCs, 76 SZ, 87 HCs | T1WI | OLS, Ridge, LASSO, Elastic-Net, SVR, RVR | BAG: +3.8–5.2yr in SZ cohort 1, +4.5–11.7 yr in SZ cohort 2. Algorithm choice can be a cause of inter-study variability. |
| Lieslehto [67] | 2021 | 29 SZ, 61 HCs | T1WI | SVR | BAG: +1.3 yr at baseline, +7.7 yr at follow-up in SZ. It was suggested that BA captured treatment-related and global brain alterations. |
| McWhinney [68] | 2021 | 183FEP, 155 HCs | T1WI | RVR | BAG: +3.39 yr in FEP at baseline, longitudinal worsening was associated with clinical outcomes or higher baseline BMI |
| Teeuw [69] | 2021 | 193 SZ, 218 HCs | T1WI | SVR | BAG: correlation with polygenic risk, no correlation with epigenetic aging |
| Wang [70] | 2021 | 166 SZ, 107 HCs | DTI | RF | BAG: +5.903 in SZ >30 yrs old. Association with working memory and processing speed |
| Xi [71] | 2021 | 60 FES, 60 HCs | DTI | RVR | BAG: +4.932 yr in FES, +2.718. Decreased BAG after early medication |
| Demro [72] | 2022 | 163 psychosis, 103 relatives, 66 HCs | T1WI | SVR/RF | BAG increase in psychosis more than HCs or relatives. Associated with cognition or schizotypal symptoms in relatives |
| Mood disorders | |||||
| Bestteher [73] | 2019 | 38 MDD, 40 HCs | T1WI | RVR | BAG: no significant change in MDD |
| Van Gestel [74] | 2019 | 84 BPAD, 45 HCs | T1WI | RVR | BAG: +4.28 yr in BPAD without Li treatment, no significance in BPAD with Li treatment or HCs |
| de Nooij [75] | 2019 | 283AYA | T1WI | RVR | Reduction of BAG in young high-risk individuals who developed a mood disorder over 2-yr follow-up |
| Christman [76] | 2020 | 76 MDD (middle-age), 118 MDD (elderly), 130 HCs | T1WI | CNN | BAG: +3.69 yrs in geriatric MDD, no increase in mid-life MDD. Associated with cognitive and functional deficits in elderly |
| Ahmed [77] | 2021 | 95 late-life depression | T1WI | CNN | BAG: +4.36 yrs in late-life depression. Not associated with treatment response. |
| Ballester [78] | 2021 | 160 MDD, 111 HCs | T1WI | GPR | BAG: higher in older MDD than in younger MDD, associated with BMI in MDD, not associated with treatment response |
| Han [79] | 2021 | 2675 MDD, 4314 HCs | T1WI | Ridge regression | BAG: +1.08 yr in MDD with no specific association with clinical characteristics |
| Han [80] | 2021 | 220 MDD/Anxiety, 65 HCs | T1WI | Ridge regression | BAG: +2.78 yr in MDD, +2.91 yr in Anxiety. Association with somatic symptoms (+4.21 yr) and antidepressant use (−2.53 yr) |
| Dunlop [81] | 2021 | 109 MDD, 710 HCs | fMRI | SVR | BAG: +2.11 yr in MDD, associated with impulsivity and symptom severity |
| Others | |||||
| Liu [82] | 2022 | 90 OCD, 106 HCs | T1WI | GPR | BAP: +0.826 yr in OCD, associated with disease duration |
| Niu [83] | 2022 | 70 SP, 77 SAD, 70 MDD, 44 PTSD, 48 ODD, 81 ADHD | T1WI | Ridge regression | Multidimensional brain-age index is sensitive to distinct regional change patterns |
| Ryan [84] | 2022 | 1618 SMI, 11,849 HCs | DTI | RF, gradient boosting regression, LASSO | Additive effect of SMI and cardiometabolic disorders on brain aging, the greater effect of SMI than CMD |
| Comprehensive | |||||
| Kaufmann [85] | 2019 | 10,141 patients, 35,474 HCs | T1WI | XGBoost | BAG: d = +1.03 in dementia, +0.41 in MCI, +0.10 in MDD, +0.74 in MS, +0.29 in BPAD, +0.51 in SZ, +0.06 in ADHD, +0.07 in ASD |
| Bashyam [86] | 2020 | 353 AD, 833 MCI, 387 SZ, 12,689 HCs | T1WI | CNN | Successful discrimination for neuropsychiatric disorders |
| Kolbeinsson [87] | 2020 | 12,196 people who had not been stratified for health | T1WI | CNN | Identified risk factors, e.g., MS, diabetes, and beneficial factors, e.g., physical strength |
| Rokicki [88] | 2021 | 54 AD, 90 MCI, 56 SCI, 159 SZ, 135 BPAD, 750 HCs | T1WI, T2WI, ASL | RF | Highest accuracy by multimodal imaging model |
AD: Alzheimer’s disease, ARMS: at-risk mental state, ASL: arterial spin labeling, AYA: adolescence and young adult, BAG: brain age gap, BPAD: bipolar affective disorder, BPD: borderline personality disorder, CHR: clinical high-risk state for psychosis, CMD; cardiometabolic disease, CNN: convolutional neural network, DTI: diffusion tensor imaging, FEP: first episode psychosis, FES: first-episode schizophrenia, fMRI: functional MRI, GPR: Gaussian process regression, HCs: healthy controls, ICA: independent component analysis, MDD: major depressive disorder, ML: machine learning, OCD: obsessive-compulsive disorder, ODD: oppositional defiant disorder, OLS: ordinary least squares, PTSD: posttraumatic stress disorder, RF: random forest, RVR: relevance vector regression, SAD: social anxiety disorder, SMI: severe mental illness, SP: specific phobias, SVR: support vector regression, SZ: schizophrenia, T1WI: T1-weighted image, T2WI: T2-weighted image.
4.4. Mood Disorders
There have been several studies of brain age in mood disorders (Table 2), including bipolar affective disorder (BPAD) and major depressive disorder (MDD) [73,74,75,76,77,78,79,80,81]. Unlike schizophrenia, some studies reported no significant difference in the BAG in mood disorders [59,62,73], while others found an increase of approx. +2 to +4 years [76,77,79,80,81]. Overall, the aging abnormality in mood disorders would be mild to moderate. Two studies that focused on MDD in late life reported significantly increased brain age [76,77]. Interestingly, the BAG may be reduced by medications, such as lithium for BPAD or antidepressants for MDD [74,80].
4.5. Other Psychiatric Disorders
The brain age in other psychiatric disorders such as obsessive-compulsive disorder (OCD) and specific phobias has been investigated [82,83] (Table 2), and a relatively large study reported contributions of both severe mental illness and cardiometabolic disorders to an increased BAG [84].
4.6. Comprehensive Studies
Brain-age findings across various neuropsychiatric disorders have been obtained in comprehensive studies [85,86,87,88] (Table 2). Overall, these studies successfully identified neuropsychiatric disorders and risk factors by using brain age, and it was indicated that a multimodal imaging model may have high accuracy [88]. In particular, an investigation of a large sample (>10,000 patients and 35,000 healthy controls) revealed the effect sizes of a BAG in various conditions, which should be regarded as a reliable standard of BAG scores so far [85]. According to this study, the strongest aging of the brain is seen in dementia, followed by MS, schizophrenia, and MCI.
5. Applications to General Populations
Targeting a general population or individuals without neuropsychiatric diagnoses is another important topic in neuroimaging-based brain-age framework research, as it may clarify how to keep our brains healthy and avoid the risks of accelerated aging [15,16,21,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129]. The most consistent significant risk factor could be diabetes (Table 3). In fact, diabetes has been consistently reported to adversely affect the aging of the brain. Alcohol consumption and smoking were also associated with an increased BAG in some studies [15,16,121,122,123,128]. Other factors that were suggested to be associated with an increased BAG include mortality, allostatic load, lung function, exposure to famine in early gestation, recidivism, chronic pain, cardiovascular risk, chemotherapy for cancer, lead exposure in childhood, hypertension, premature birth, male sex, worry and rumination, neighborhood disadvantage, sleep apnea, obesity, and physical strength (Table 3).
Table 3.
Neuroimaging-based brain age studies for general populations or those without neuropsychiatric diagnoses.
| First Author [ref.] |
Year | Cohort | Imaging Modality | ML Algorithm | Main Findings |
|---|---|---|---|---|---|
| Franke [89] | 2013 | 185 people | T1WI | RVR | BAG: +4.6 yr in T2DM, Acceleration by +0.2 yr per year |
| Franke [90] | 2014 | 228 elderly | T1WI | RVR | BAG associated with health markers with gender-specific pattern |
| Franke [91] | 2015 | 8 women | T1WI | RVR | BAG changes during the course of the menstrual cycle |
| Luders [92] | 2016 | 50 LTM, 50 HCs | T1WI | RVR | BAG: −7.5 yr in LTM |
| Cole [21] | 2018 | 669 people | T1WI | GPR | Higher BAG was associated with weaker grip strength, poorer lung function, slower walking speed, lower fluid intelligence, higher allostatic load, and increased mortality risk. |
| Franke [93] | 2018 | 118 elderly | T1WI | RVR | BAG: +4.3 yr in males whose mothers were exposed to famine in early gestation |
| Hatton [94] | 2018 | 359 men | T1WI | SVR | BAG associated with negative fateful life events in midlife |
| Kiehl [95] | 2018 | 1332 incarcerated males | T1WI | ICA | Brain age predicts recidivism, particularly when combined with other data. |
| Le [96] | 2018 | 20 healthy people | T1WI | SVR | BAG: −1.15 or −1.18 yr by taking ibuprofen |
| Luders [97] | 2018 | 14 healthy women after childbirth | T1WI | RVR | Brain age became younger in late postpartum by 5.4 yr. |
| Rogenmoser [98] | 2018 | 42 pro-musician, 45 amateurs, 38HCs | T1WI | RVR | BAG: −3.70 to −4.51 yr in musicians |
| Scheller [99] | 2018 | 34 elderly | T1WI | RVR | interaction of BAG and APOE variants, suggesting a compensation mechanism in the elderly |
| de Lange [100] | 2019 | 12,021 women | T1WI | XGBoost | BAG decrease with the number of previous childbirths |
| Cruz-Almeida [101] | 2019 | 47 elderly | T1WI | GPR | Increased BAG in elderly with chronic pain |
| Cole [15] | 2020 | 14,701 people | T1WI, FLAIR, T2*, DTI, fMRI | LASSO | BAS associated with stroke history, diabetes, smoking, alcohol, and cognitive measures |
| de Lange [102] | 2020 | 473 people | T1WI, DTI, fMRI | XGBoost | Associated with cardiovascular risk |
| de Lange [103] | 2020 | 19,787 women | T1WI | XGBoost | BAG decrease with the number of previous childbirths. Involvement of brain subcortical regions |
| Henneghan [104] | 2020 | 43 breast cancer with chemotherapy, 50 HCs | T1WI | SVR/RF | Trend-level increase on BAG after chemotherapy for breast cancer |
| Reuben [105] | 2020 | 564 people at 45 yr | T1WI | SVR/RF | BAG: +0.77 yr in those who had lead exposure in childhood |
| Seidel [106] | 2020 | 20 sepsis survivors with cognitive deficits, 44 HCs | T1WI | Kernel regression | BAG: +4.5 yr in sepsis survivor, associated with the severity of dyscognition |
| Anaturk [107] | 2021 | 537 elderly | T1WI, DTI, FLAIR | XGBoost | Relationship with cumulative lifestyle measures independent of cognitive age |
| Bittner [108] | 2021 | 622 elderly | T1WI | RVR | BAG: +5.04 months by combined lifestyle risk, +0.6 month by smoking, −0.55 month by physical activity |
| Cherbuin [109] | 2021 | 335 middle age, 351 elderly | T1WI | RVR | BAG: +51.1–65.7days by every additional 10-mmHg increase in BP |
| Dunas [110] | 2021 | 351 people | T1WI, DTI, fMRI | OLS, BRR, LASSO, ENET, SVR, RVR, GPR | BAG associated with current and past physical fitness and cognitive ability |
| Elliott [111] | 2021 | 869 middle-age | T1WI | SVR/RF | Associated with cognitive function, impaired brain health at age 3, and other signs of aging |
| Hedderich [112] | 2021 | 101 premature-born adults, 111 full-term controls | T1WI | RVR | BAG: +1.4 yr in premature-born adults, associated with low gestational age, low birth weight, and increased neonatal treatment intensity |
| Karim [113] | 2021 | 78 older adults | T1WI, T2WI, FLAIR | GPR | BAG associated with male sex, worry, and rumination |
| Rakesh [114] | 2021 | 166 adolescents | T1WI | SVR | increased BAG by neighborhood disadvantage, modulated by effortful control |
| Rosemann [115] | 2021 | 169 elderly | T1WI | GPR | No association with age-related hearing loss |
| Salih [116] | 2021 | 15,335 HCs | DTI | Bayesian ridge regression | Limbic tract-based BAG was most accurate and associated with daily life factors. Two SNPs were associated with BAG. |
| Sanders [117] | 2021 | 122 elderly | T1WI | XGBoost | BAG decrease in more physically active women but not men |
| Subramaniapillai [118] | 2021 | 1067 elderly | T1WI | Elastic net regression | Brain age was more associated with AD risk factors in women than in men. |
| Vidal-Pineiro [119] | 2021 | 6950 people | T1WI | LASSO, XGBoost | No association between cross-sectional brain age and longitudinal change. Association with congenital factors, suggesting a lifelong influence on brain structure from early life |
| Weihs [120] | 2021 | 690 people | T1WI | OLS | Brain age associated with AHI and ODI in PSG data |
| Angebrandt [121] | 2022 | 240 HCs, 231 HCs (middle age) | T1WI | SVR/RF | Dose-dependent relation between 90-day alcohol consumption and BAG |
| Beck [122] | 2022 | 790 healthy people | T1WI, DTI | XGBoost | T1-based BAG: associated with sBP, smoking, pulse, and CRP. DTI-based BAG: associated with phosphate, MCV |
| Bourassa [123] | 2022 | 910 people (midlife) | T1WI | SVR/RF | BAG in midlife is associated with smoking, obesity, and psychological problems during adolescence. |
| Giannakopoulos [124] | 2022 | 80 elderly | T1WI | RVR | BAG predicted a decrease in executive function over time. |
| Linli [125] | 2022 | 33,293 people | T1WI | XGBoost | BAG: +1.19 yr in active regular smokers, associated with the amount of smoking |
| Sone [16] | 2022 | 773 elderly | T1WI | SVR | BAG: associated with life satisfaction, alcohol use, and diabetes |
| Vaughan [126] | 2022 | 57 elderly | T1WI | GPR | BAG: associated with leg strength, moderating the relationship between strength and mobility |
| Wang [127] | 2022 | 165 elderly | T1WI | RVR | BAG: associated with female gender, higher education but not with APOE-e4 or family history of dementia |
| Whistel [128] | 2022 | 712 people | T1WI | SVR | Association of BAG in mid- to late-life with heavier smoking and alcohol consumption in early mid-life |
| Zheng [129] | 2022 | 1676 HCs | T1WI | RBF-kernel | BAG associated with worse cognitive outcomes over time |
BAG: brain age gap, CNN: convolutional neural network, DTI: diffusion tensor imaging, ENET: efficient neural network, fMRI: functional MRI, GPR: Gaussian process regression, HCs: healthy controls, ICA: independent component analysis, LTM: long-term meditation practitioner, ML: machine learning, OLS: ordinary least squares, RBF: radial basis function, RF: random forest, RVR: relevance vector regression, SVR: support vector regression, T1WI: T1-weighted image, T2DM: type 2 diabetes mellitus, T2WI: T2-weighted image.
In addition, several studies reported potentially protective factors associated with a reduced BAG: long-term meditation (−7.5 years), music composition (approx. −4 years), physical activity, taking ibuprofen, and life satisfaction (Table 3). Interestingly, it has been observed in more than one study that childbirth decreases the BAG in women, not only during the postpartum period but also in later life [100].
Thus, although the studies are diverse in terms of the methodologies used and the targeted factors, cumulative evidence will further expand our knowledge of how to improve the aging process of human brains. The strong and consistent risk for brain aging appears to be diabetes, followed by alcohol consumption. Other lifestyle-related risk factors, e.g., smoking or hypertension, may also be harmful but less consistent. Regarding beneficial effects, though the research focuses were diverse across studies, physical, mental, or creative activities may likely improve our brain age. It is unclear whether neuropsychiatric disorders, particularly dementia, could be prevented by improving brain aging. Further research is necessary to obtain real-world evidence regarding the utility of brain-age studies for this question.
6. Strengths, Controversies, and Future Direction
As described above, a neuroimaging-based brain-age estimation can provide a reliable neuropsychiatric biomarker at the single-subject level. In addition, brain MRI—particularly T1-weighted structural MRI—is a widely available examination in most countries, which may support easier and wider clinical applications of brain-age analyses. Given that many studies have successfully used public databases to build a brain-age prediction model, the reproducibility and external validity of a brain-age model should be at an acceptable level. Thus, the strengths of brain age as a biomarker would be its use as a single-subject-level marker, widely available examination, and acceptable reproducibility. The processing of MRI scans, including normalization and machine-learning analysis, may require advanced techniques and could be a possible barrier for most facilities, but currently, there are several public tools, e.g., BARACUS (https://github.com/BIDS-Apps/baracus, accessed on 31 October 2022) and brainageR (https://github.com/james-cole/brainageR, accessed on 31 October 2022) which would help us apply a brain age model to the patients.
Controversies and limitations of the use of brain ages have also been suggested, particularly for the interpretation of study results. As reviewed herein, there is a level of overlapping of BAG scores across various disorders, which might limit the usefulness of the brain age for differential diagnoses in clinical analyses. It was also reported that individual variations in brain age were associated with early-life factors rather than longitudinal changes [119]. Moreover, the methodology is quite diverse in terms of imaging modalities, processing, and choice of machine-learning algorithm, and there is no established consensus about the optimal protocol for determining brain ages. Future studies should address these controversies and limitations.
In conclusion, neuroimaging-based brain-age estimation has been widely and increasingly researched for over 10 years, and many studies revealed its usefulness for neuropsychiatry. Considering the utility, availability, and reproducibility of neuroimaging-based brain-age estimations for single patients, brain age can be expected to become a useful personalized biomarker in neuropsychiatry.
Author Contributions
Both D.S. and I.B. contributed equally to this article including searching for literature and writing a manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by grants from the Japan Society for the Promotion of Science (KAKENHI) no. JP21K15720, the Japan Epilepsy Research Foundation (JERF TENKAN 22007), and the Uehara Memorial Foundation (all to D.S.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flatt T. A New Definition of Aging? Front. Genet. 2012;3:148. doi: 10.3389/fgene.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackburn E.H., Epel E.S., Lin J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–1198. doi: 10.1126/science.aab3389. [DOI] [PubMed] [Google Scholar]
- 3.Childs B.G., Durik M., Baker D.J., Van Deursen J.M. Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franke K., Bublak P., Hoyer D., Billiet T., Gaser C., Witte O., Schwab M. In vivo biomarkers of structural and functional brain development and aging in humans. Neurosci. Biobehav. Rev. 2020;117:142–164. doi: 10.1016/j.neubiorev.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 6.Azam S., Haque E., Balakrishnan R., Kim I.-S., Choi D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021;9:683459. doi: 10.3389/fcell.2021.683459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochla N.A.N., Fabian M.S., Parsons O.A. Brain-age quotients in recently detoxified alcoholic, recovered alcoholic and nonalcoholic women. J. Clin. Psychol. 1982;38:207–212. doi: 10.1002/1097-4679(198201)38:1<207::AID-JCLP2270380135>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 8.Franke K., Gaser C. Ten Years of BrainAGE as a Neuroimaging Biomarker of Brain Aging: What Insights Have We Gained? Front. Neurol. 2019;10:789. doi: 10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franke K., Ziegler G., Klöppel S., Gaser C., Alzheimer’s Disease Neuroimaging Initiative Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: Exploring the influence of various parameters. NeuroImage. 2010;50:883–892. doi: 10.1016/j.neuroimage.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Beheshti I., Gravel P., Potvin O., Dieumegarde L., Duchesne S. A novel patch-based procedure for estimating brain age across adulthood. NeuroImage. 2019;197:618–624. doi: 10.1016/j.neuroimage.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Cole J.H., Poudel R.P., Tsagkrasoulis D., Caan M.W., Steves C., Spector T.D., Montana G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage. 2017;163:115–124. doi: 10.1016/j.neuroimage.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 12.Beheshti I., Maikusa N., Matsuda H. The accuracy of T1-weighted voxel-wise and region-wise metrics for brain age estimation. Comput. Methods Programs Biomed. 2022;214:106585. doi: 10.1016/j.cmpb.2021.106585. [DOI] [PubMed] [Google Scholar]
- 13.Beheshti I., Nugent S., Potvin O., Duchesne S. Bias-adjustment in neuroimaging-based brain age frameworks: A robust scheme. NeuroImage Clin. 2019;24:102063. doi: 10.1016/j.nicl.2019.102063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beheshti I., Ganaie M.A., Paliwal V., Rastogi A., Razzak I., Tanveer M. Predicting Brain Age Using Machine Learning Algorithms: A Comprehensive Evaluation. IEEE J. Biomed. Health Inform. 2021;26:1432–1440. doi: 10.1109/JBHI.2021.3083187. [DOI] [PubMed] [Google Scholar]
- 15.Cole J.H. Multimodality neuroimaging brain-age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging. 2020;92:34–42. doi: 10.1016/j.neurobiolaging.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sone D., Beheshti I., Shinagawa S., Niimura H., Kobayashi N., Kida H., Shikimoto R., Noda Y., Nakajima S., Bun S., et al. Neuroimaging-derived brain age is associated with life satisfaction in cognitively unimpaired elderly: A community-based study. Transl. Psychiatry. 2022;12:25. doi: 10.1038/s41398-022-01793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beheshti I., Maikusa N., Matsuda H. The association between “Brain-Age Score”(BAS) and traditional neuropsychological screening tools in Alzheimer’s disease. Brain Behav. 2018;8:e01020. doi: 10.1002/brb3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valizadeh S., Hänggi J., Mérillat S., Jäncke L. Age prediction on the basis of brain anatomical measures. Hum. Brain Mapp. 2016;38:997–1008. doi: 10.1002/hbm.23434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sone D., Beheshti I., Maikusa N., Ota M., Kimura Y., Sato N., Koepp M., Matsuda H. Neuroimaging-based brain-age prediction in diverse forms of epilepsy: A signature of psychosis and beyond. Mol. Psychiatry. 2019;26:825–834. doi: 10.1038/s41380-019-0446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beheshti I., Mishra S., Sone D., Khanna P., Matsuda H. T1-weighted MRI-driven brain age estimation in Alzheimer’s disease and Parkinson’s disease. Aging Dis. 2020;11:618. doi: 10.14336/AD.2019.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole J., Ritchie S.J., Bastin M., Hernández M.C.V., Maniega S.M., Royle N., Corley J., Pattie A., Harris S.E., Zhang Q., et al. Brain age predicts mortality. Mol. Psychiatry. 2017;23:1385–1392. doi: 10.1038/mp.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal M.S., Blazey T.M., Su Y., Couture L.E., Durbin T.J., Bateman R.J., Benzinger T.L.-S., Morris J.C., Raichle M.E., Vlassenko A.G. Persistent metabolic youth in the aging female brain. Proc. Natl. Acad. Sci. USA. 2019;116:3251–3255. doi: 10.1073/pnas.1815917116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beheshti I., Nugent S., Potvin O., Duchesne S. Disappearing metabolic youthfulness in the cognitively impaired female brain. Neurobiol. Aging. 2021;101:224–229. doi: 10.1016/j.neurobiolaging.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Nemmi F., Levardon M., Péran P. Brain-age estimation accuracy is significantly increased using multishell free-water reconstruction. Hum. Brain Mapp. 2022;43:2365–2376. doi: 10.1002/hbm.25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler E.R., Chen A., Ramadan R., Le T.T., Ruparel K., Moore T.M., Satterthwaite T.D., Zhang F., Shou H., Gur R.C., et al. Pitfalls in brain age analyses. Hum. Brain Mapp. 2021;42:4092–4101. doi: 10.1002/hbm.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le T.T., Kuplicki R.T., McKinney B.A., Yeh H.-W., Thompson W.K., Paulus M.P., Tulsa 1000 Investigators. Aupperle R.L., Bodurka J., Cha Y.-H., et al. A Nonlinear Simulation Framework Supports Adjusting for Age When Analyzing BrainAGE. Front. Aging Neurosci. 2018;10:317. doi: 10.3389/fnagi.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng X., Lipton Z.C., Yang J., Small S.A., Provenzano F.A., Alzheimer’s Disease Neuroimaging Initiative. Australian Imaging Biomarkers and Lifestyle flagship study of ageing. Frontotemporal Lobar Degeneration Neuroimaging Initiative Estimating brain age based on a uniform healthy population with deep learning and structural magnetic resonance imaging. Neurobiol. Aging. 2020;91:15–25. doi: 10.1016/j.neurobiolaging.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ning K., Duffy B.A., Franklin M., Matloff W., Zhao L., Arzouni N., Sun F., Toga A.W. Improving brain age estimates with deep learning leads to identification of novel genetic factors associated with brain aging. Neurobiol. Aging. 2021;105:199–204. doi: 10.1016/j.neurobiolaging.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popescu S.G., Glocker B., Sharp D.J., Cole J.H. Local Brain-Age: A U-Net Model. Front. Aging Neurosci. 2021;13:761954. doi: 10.3389/fnagi.2021.761954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levakov G., Rosenthal G., Shelef I., Raviv T.R., Avidan G. From a deep learning model back to the brain—Identifying regional predictors and their relation to aging. Hum. Brain Mapp. 2020;41:3235–3252. doi: 10.1002/hbm.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaser C., Franke K., Klöppel S., Koutsouleris N., Sauer H., Alzheimer’s Disease Neuroimaging Initiative BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS ONE. 2013;8:e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Löwe L.C., Gaser C., Franke K., Alzheimer’s Disease Neuroimaging Initiative The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS ONE. 2016;11:e0157514. doi: 10.1371/journal.pone.0157514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Knol M.J., Tiulpin A., Dubost F., de Bruijne M., Vernooij M.W., Adams H.H.H., Ikram M.A., Niessen W.J., Roshchupkin G.V. Gray Matter Age Prediction as a Biomarker for Risk of Dementia. Proc. Natl. Acad. Sci. USA. 2019;116:21213–21218. doi: 10.1073/pnas.1902376116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ly M., Yu G.Z., Karim H.T., Muppidi N.R., Mizuno A., Klunk W.E., Aizenstein H.J. Improving brain age prediction models: Incorporation of amyloid status in Alzheimer’s disease. Neurobiol. Aging. 2019;87:44–48. doi: 10.1016/j.neurobiolaging.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohajer B., Abbasi N., Mohammadi E., Khazaie H., Osorio R.S., Rosenzweig I., Eickhoff C.R., Zarei M., Tahmasian M., Eickhoff S.B., et al. Gray matter volume and estimated brain age gap are not linked with sleep-disordered breathing. Hum. Brain Mapp. 2020;41:3034–3044. doi: 10.1002/hbm.24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Habes M., Pomponio R., Shou H., Doshi J., Mamourian E., Erus G., Nasrallah I., Launer L.J., Rashid T., Bilgel M., et al. The Brain Chart of Aging: Machine-learning analytics reveals links between brain aging, white matter disease, amyloid burden, and cognition in the iSTAGING consortium of 10,216 harmonized MR scans. Alzheimer’s Dement. 2020;17:89–102. doi: 10.1002/alz.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Millar P.R., Luckett P.H., Gordon B.A., Benzinger T.L., Schindler S.E., Fagan A.M., Cruchaga C., Bateman R.J., Allegri R., Jucker M., et al. Predicting brain age from functional connectivity in symptomatic and preclinical Alzheimer disease. NeuroImage. 2022;256:119228. doi: 10.1016/j.neuroimage.2022.119228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill N.T.M., Mowszowski L., Naismith S.L., Chadwick V.L., Valenzuela M., Lampit A. Computerized Cognitive Training in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am. J. Psychiatry. 2017;174:329–340. doi: 10.1176/appi.ajp.2016.16030360. [DOI] [PubMed] [Google Scholar]
- 39.Sherman D.S., Mauser J., Nuno M., Sherzai D. The Efficacy of Cognitive Intervention in Mild Cognitive Impairment (MCI): A Meta-Analysis of Outcomes on Neuropsychological Measures. Neuropsychol. Rev. 2017;27:440–484. doi: 10.1007/s11065-017-9363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nousia A., Siokas V., Aretouli E., Messinis L., Aloizou A.-M., Martzoukou M., Karala M., Koumpoulis C., Nasios G., Dardiotis E. Beneficial Effect of Multidomain Cognitive Training on the Neuropsychological Performance of Patients with Early-Stage Alzheimer’s Disease. Neural Plast. 2018;2018:2845176. doi: 10.1155/2018/2845176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Counts S.E., Ikonomovic M.D., Mercado N., Vega I.E., Mufson E.J. Biomarkers for the Early Detection and Progression of Alzheimer’s Disease. Neurotherapeutics. 2016;14:35–53. doi: 10.1007/s13311-016-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eickhoff C.R., Hoffstaedter F., Caspers J., Reetz K., Mathys C., Dogan I., Amunts K., Schnitzler A., Eickhoff S.B. Advanced brain ageing in Parkinson’s disease is related to disease duration and individual impairment. Brain Commun. 2021;3:fcab191. doi: 10.1093/braincomms/fcab191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charissé D., Erus G., Pomponio R., Gorges M., Schmidt N., Schneider C., Liepelt-Scarfone I., Riedel O., Reetz K., Schulz J.B., et al. Brain age and Alzheimer’s-like atrophy are domain-specific predictors of cognitive impairment in Parkinson’s disease. Neurobiol. Aging. 2021;109:31–42. doi: 10.1016/j.neurobiolaging.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Pardoe H.R., Cole J.H., Blackmon K., Thesen T., Kuzniecky R., Human Epilepsy Project Investigators Structural brain changes in medically refractory focal epilepsy resemble premature brain aging. Epilepsy Res. 2017;133:28–32. doi: 10.1016/j.eplepsyres.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Hwang G., Hermann B., Nair V.A., Conant L.L., Dabbs K., Mathis J., Cook C.J., Rivera-Bonet C.N., Mohanty R., Zhao G., et al. Brain aging in temporal lobe epilepsy: Chronological, structural, and functional. NeuroImage Clin. 2020;25:102183. doi: 10.1016/j.nicl.2020.102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Bézenac C.E., Adan G., Weber B., Keller S.S. Association of Epilepsy Surgery With Changes in Imaging-Defined Brain Age. Neurology. 2021;97:e554–e563. doi: 10.1212/WNL.0000000000012289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cole J.H., Raffel J., Friede T., Eshaghi A., Brownlee W.J., Chard D., De Stefano N., Enzinger C., Pirpamer L., Filippi M., et al. Longitudinal Assessment of Multiple Sclerosis with the Brain-Age Paradigm. Ann. Neurol. 2020;88:93–105. doi: 10.1002/ana.25746. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs S.A.H., Muraro P.A., Cencioni M.T., Knowles S., Cole J.H., Nicholas R. Worse Physical Disability Is Associated With the Expression of PD-1 on Inflammatory T-Cells in Multiple Sclerosis Patients With Older Appearing Brains. Front. Neurol. 2022;12:801097. doi: 10.3389/fneur.2021.801097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gan M.S., Shi W., Wang M.S., Sun M.Y., Yin B., Bai G., Jia X., Sun C., Niu M.X., Wang Z., et al. Accelerated Brain Aging in Mild Traumatic Brain Injury: Longitudinal Pattern Recognition with White Matter Integrity. J. Neurotrauma. 2021;38:2549–2559. doi: 10.1089/neu.2020.7551. [DOI] [PubMed] [Google Scholar]
- 50.Hellstrøm T., Andelic N., de Lange A.-M., Helseth E., Eiklid K., Westlye L. Apolipoprotein ɛ4 Status and Brain Structure 12 Months after Mild Traumatic Injury: Brain Age Prediction Using Brain Morphometry and Diffusion Tensor Imaging. J. Clin. Med. 2021;10:418. doi: 10.3390/jcm10030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu G.Z., Ly M., Karim H.T., Muppidi N., Aizenstein H.J., Ibinson J.W. Accelerated brain aging in chronic low back pain. Brain Res. 2021;1755:147263. doi: 10.1016/j.brainres.2020.147263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung P.S.-P., Zhang J.Y., Noorani A., Walker M.R., Huang M., Zhang J.W., Laperriere N., Rudzicz F., Hodaie M. Differential expression of a brain aging biomarker across discrete chronic pain disorders. Pain. 2022;163:1468–1478. doi: 10.1097/j.pain.0000000000002613. [DOI] [PubMed] [Google Scholar]
- 53.Azor A.M., Cole J.H., Holland A.J., Dumba M., Patel M.C., Sadlon A., Goldstone A.P., Manning K.E. Increased brain age in adults with Prader-Willi syndrome. NeuroImage Clin. 2019;21:101664. doi: 10.1016/j.nicl.2019.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cole J.H., Underwood J., Caan M.W., De Francesco D., Van Zoest R.A., Leech R., Wit F.W., Portegies P., Geurtsen G.J., Schmand B.A., et al. Increased brain-predicted aging in treated HIV disease. Neurology. 2017;88:1349–1357. doi: 10.1212/WNL.0000000000003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pringsheim T., Jette N., Frolkis A., Steeves T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 56.Oh J., Vidal-Jordana A., Montalban X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018;31:752–759. doi: 10.1097/WCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 57.Koutsouleris N., Davatzikos C., Borgwardt S., Gaser C., Bottlender R., Frodl T., Falkai P., Riecher-Rössler A., Möller H.-J., Reiser M., et al. Accelerated Brain Aging in Schizophrenia and Beyond: A Neuroanatomical Marker of Psychiatric Disorders. Schizophr. Bull. 2013;40:1140–1153. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schnack H.G., Van Haren N.E., Nieuwenhuis M., Pol H.H., Cahn W., Kahn R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry. 2016;173:607–616. doi: 10.1176/appi.ajp.2015.15070922. [DOI] [PubMed] [Google Scholar]
- 59.Nenadić I., Dietzek M., Langbein K., Sauer H., Gaser C. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res. Neuroimaging. 2017;266:86–89. doi: 10.1016/j.pscychresns.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Kolenic M., Franke K., Hlinka J., Matejka M., Capkova J., Pausova Z., Uher R., Alda M., Spaniel F., Hajek T. Obesity, dyslipidemia and brain age in first-episode psychosis. J. Psychiatr. Res. 2018;99:151–158. doi: 10.1016/j.jpsychires.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 61.Chung Y., Addington J., Bearden C.E., Cadenhead K., Cornblatt B., Mathalon D.H., McGlashan T., Perkins D., Seidman L.J., Tsuang M., et al. Adding a neuroanatomical biomarker to an individualized risk calculator for psychosis: A proof-of-concept study. Schizophr. Res. 2019;208:41–43. doi: 10.1016/j.schres.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 62.Hajek T., Franke K., Kolenic M., Capkova J., Matejka M., Propper L., Uher R., Stopkova P., Novak T., Paus T., et al. Brain Age in Early Stages of Bipolar Disorders or Schizophrenia. Schizophr. Bull. 2017;45:190–198. doi: 10.1093/schbul/sbx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shahab S., Mulsant B.H., Levesque M.L., Calarco N., Nazeri A., Wheeler A.L., Foussias G., Rajji T.K., Voineskos A.N. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2018;44:898–906. doi: 10.1038/s41386-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuo C.-Y., Lee P.-L., Hung S.-C., Liu L.-K., Lee W.-J., Chung C.-P., Yang A.C., Tsai S.-J., Wang P.-N., Chen L.-K., et al. Large-Scale Structural Covariance Networks Predict Age in Middle-to-Late Adulthood: A Novel Brain Aging Biomarker. Cereb. Cortex. 2020;30:5844–5862. doi: 10.1093/cercor/bhaa161. [DOI] [PubMed] [Google Scholar]
- 65.Tønnesen S., Kaufmann T., de Lange A.-M.G., Richard G., Doan N.T., Alnæs D., van der Meer D., Rokicki J., Moberget T., Maximov I.I., et al. Brain Age Prediction Reveals Aberrant Brain White Matter in Schizophrenia and Bipolar Disorder: A Multisample Diffusion Tensor Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:1095–1103. doi: 10.1016/j.bpsc.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 66.Lee W.H., Antoniades M., Schnack H.G., Kahn R.S., Frangou S. Brain age prediction in schizophrenia: Does the choice of machine learning algorithm matter? Psychiatry Res. Neuroimaging. 2021;310:111270. doi: 10.1016/j.pscychresns.2021.111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieslehto J., Jääskeläinen E., Kiviniemi V., Haapea M., Jones P.B., Murray G.K., Veijola J., Dannlowski U., Grotegerd D., Meinert S., et al. The progression of disorder-specific brain pattern expression in schizophrenia over 9 years. npj Schizophr. 2021;7:32. doi: 10.1038/s41537-021-00157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McWhinney S., Kolenic M., Franke K., Fialova M., Knytl P., Matejka M., Spaniel F., Hajek T. Obesity as a Risk Factor for Accelerated Brain Ageing in First-Episode Psychosis—A Longitudinal Study. Schizophr. Bull. 2021;47:1772–1781. doi: 10.1093/schbul/sbab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teeuw J., Ori A.P., Brouwer R.M., de Zwarte S.M., Schnack H.G., Pol H.E.H., Ophoff R.A. Accelerated aging in the brain, epigenetic aging in blood, and polygenic risk for schizophrenia. Schizophr. Res. 2021;231:189–197. doi: 10.1016/j.schres.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Wang J., Kochunov P., Sampath H., Hatch K.S., Ryan M.C., Xue F., Neda J., Paul T., Hahn B., Gold J., et al. White matter brain aging in relationship to schizophrenia and its cognitive deficit. Schizophr. Res. 2021;230:9–16. doi: 10.1016/j.schres.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xi Y.-B., Wu X.-S., Cui L.-B., Bai L.-J., Gan S.-Q., Jia X.-Y., Li X., Xu Y.-Q., Kang X.-W., Guo F., et al. Neuroimaging-based brain-age prediction of first-episode schizophrenia and the alteration of brain age after early medication. Br. J. Psychiatry. 2021;220:339–346. doi: 10.1192/bjp.2021.169. [DOI] [PubMed] [Google Scholar]
- 72.Demro C., Shen C., Hendrickson T.J., Arend J.L., Disner S.G., Sponheim S.R. Advanced Brain-Age in Psychotic Psychopathology: Evidence for Transdiagnostic Neurodevelopmental Origins. Front. Aging Neurosci. 2022;14:872867. doi: 10.3389/fnagi.2022.872867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Besteher B., Gaser C., Nenadić I. Machine-learning based brain age estimation in major depression showing no evidence of accelerated aging. Psychiatry Res. Neuroimaging. 2019;290:1–4. doi: 10.1016/j.pscychresns.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Van Gestel H., Franke K., Petite J., Slaney C., Garnham J., Helmick C., Johnson K., Uher R., Alda M., Hajek T. Brain age in bipolar disorders: Effects of lithium treatment. Aust. N. Z. J. Psychiatry. 2019;53:1179–1188. doi: 10.1177/0004867419857814. [DOI] [PubMed] [Google Scholar]
- 75.De Nooij L., Harris M.A., Hawkins E.L., Clarke T.-K., Shen X., Chan S.W.Y., Ziermans T.B., McIntosh A.M., Whalley H.C. Longitudinal trajectories of brain age in young individuals at familial risk of mood disorder from the Scottish Bipolar Family Study. Wellcome Open Res. 2020;4:206. doi: 10.12688/wellcomeopenres.15617.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christman S., Bermudez C., Hao L., Landman B.A., Boyd B., Albert K., Woodward N., Shokouhi S., Vega J., Andrews P., et al. Accelerated brain aging predicts impaired cognitive performance and greater disability in geriatric but not midlife adult depression. Transl. Psychiatry. 2020;10:317. doi: 10.1038/s41398-020-01004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmed R., Ryan C., Christman S., Elson D., Bermudez C., Landman B.A., Szymkowicz S.M., Boyd B.D., Kang H., Taylor W.D. Structural MRI-Based Measures of Accelerated Brain Aging do not Moderate the Acute Antidepressant Response in Late-Life Depression. Am. J. Geriatr. Psychiatry. 2021;30:1015–1025. doi: 10.1016/j.jagp.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ballester P.L., Suh J.S., Nogovitsyn N., Hassel S., Strother S.C., Arnott S.R., Minuzzi L., Sassi R.B., Lam R.W., Milev R., et al. Accelerated brain aging in major depressive disorder and antidepressant treatment response: A CAN-BIND report. NeuroImage Clin. 2021;32:102864. doi: 10.1016/j.nicl.2021.102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han L.K.M., Dinga R., Hahn T., Ching C.R.K., Eyler L.T., Aftanas L., Aghajani M., Aleman A., Baune B.T., Berger K., et al. Brain aging in major depressive disorder: Results from the ENIGMA major depressive disorder working group. Mol. Psychiatry. 2020;26:5124–5139. doi: 10.1038/s41380-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han L.K.M., Schnack H.G., Brouwer R.M., Veltman D.J., van der Wee N.J.A., van Tol M.-J., Aghajani M., Penninx B.W.J.H. Contributing factors to advanced brain aging in depression and anxiety disorders. Transl. Psychiatry. 2021;11:402. doi: 10.1038/s41398-021-01524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dunlop K., Victoria L.W., Downar J., Gunning F.M., Liston C. Accelerated brain aging predicts impulsivity and symptom severity in depression. Neuropsychopharmacology. 2021;46:911–919. doi: 10.1038/s41386-021-00967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu L., Liu J., Yang L., Wen B., Zhang X., Cheng J., Han S., Zhang Y., Cheng J. Accelerated Brain Aging in Patients With Obsessive-Compulsive Disorder. Front. Psychiatry. 2022;13:852479. doi: 10.3389/fpsyt.2022.852479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niu X., Taylor A., Shinohara R.T., Kounios J., Zhang F. Multidimensional Brain-Age Prediction Reveals Altered Brain Developmental Trajectory in Psychiatric Disorders. Cereb. Cortex. 2022 doi: 10.1093/cercor/bhab530. [DOI] [PubMed] [Google Scholar]
- 84.Ryan M.C., Hong L.E., Hatch K.S., Gao S., Chen S., Haerian K., Wang J., Goldwaser E.L., Du X., Adhikari B.M., et al. The additive impact of cardio-metabolic disorders and psychiatric illnesses on accelerated brain aging. Hum. Brain Mapp. 2022;43:1997–2010. doi: 10.1002/hbm.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaufmann T., van der Meer D., Doan N.T., Schwarz E., Lund M.J., Agartz I., Alnæs D., Barch D.M., Baur-Streubel R., Bertolino A., et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019;22:1617–1623. doi: 10.1038/s41593-019-0471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bashyam V.M., Erus G., Doshi J., Habes M., Nasrallah I.M., Truelove-Hill M., Srinivasan D., Mamourian L., Pomponio R., Fan Y., et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain. 2020;143:2312–2324. doi: 10.1093/brain/awaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolbeinsson A., Filippi S., Panagakis Y., Matthews P.M., Elliott P., Dehghan A., Tzoulaki I. Accelerated MRI-predicted brain ageing and its associations with cardiometabolic and brain disorders. Sci. Rep. 2020;10:19940. doi: 10.1038/s41598-020-76518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rokicki J., Wolfers T., Nordhøy W., Tesli N., Quintana D.S., Alnæs D., Richard G., de Lange A.G., Lund M.J., Norbom L., et al. Multimodal imaging improves brain age prediction and reveals distinct abnormalities in patients with psychiatric and neurological disorders. Hum. Brain Mapp. 2020;42:1714–1726. doi: 10.1002/hbm.25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franke K., Gaser C., Manor B., Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front. Aging Neurosci. 2013;5:90. doi: 10.3389/fnagi.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Efranke K., Eristow M., Egaser C. Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly subjects. Front. Aging Neurosci. 2014;6:94. doi: 10.3389/fnagi.2014.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Franke K., Hagemann G., Schleussner E., Gaser C. Changes of individual BrainAGE during the course of the menstrual cycle. NeuroImage. 2015;115:1–6. doi: 10.1016/j.neuroimage.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 92.Luders E., Cherbuin N., Gaser C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage. 2016;134:508–513. doi: 10.1016/j.neuroimage.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Franke K., Gaser C., Roseboom T.J., Schwab M., de Rooij S.R. Premature brain aging in humans exposed to maternal nutrient restriction during early gestation. NeuroImage. 2018;173:460–471. doi: 10.1016/j.neuroimage.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 94.Hatton S.N., Franz C.E., Elman J., Panizzon M.S., Hagler D.J., Fennema-Notestine C., Eyler L.T., McEvoy L.K., Lyons M.J., Dale A.M., et al. Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol. Aging. 2018;67:1–9. doi: 10.1016/j.neurobiolaging.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiehl K.A., Anderson N.E., Aharoni E., Maurer J., Harenski K.A., Rao V., Claus E.D., Harenski C., Koenigs M., Decety J., et al. Age of gray matters: Neuroprediction of recidivism. NeuroImage Clin. 2018;19:813–823. doi: 10.1016/j.nicl.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le T.T., Kuplicki R., Yeh H.-W., Aupperle R.L., Khalsa S.S., Simmons W.K., Paulus M.P. Effect of Ibuprofen on BrainAGE: A Randomized, Placebo-Controlled, Dose-Response Exploratory Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2018;3:836–843. doi: 10.1016/j.bpsc.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luders E., Gingnell M., Poromaa I.S., Engman J., Kurth F., Gaser C. Potential Brain Age Reversal after Pregnancy: Younger Brains at 4–6 Weeks Postpartum. Neuroscience. 2018;386:309–314. doi: 10.1016/j.neuroscience.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogenmoser L., Kernbach J., Schlaug G., Gaser C. Keeping brains young with making music. Anat. Embryol. 2017;223:297–305. doi: 10.1007/s00429-017-1491-2. [DOI] [PubMed] [Google Scholar]
- 99.Scheller E., Schumacher L., Peter J., Lahr J., Wehrle J., Kaller C., Gaser C., Klöppel S. Brain Aging and APOE ε4 Interact to Reveal Potential Neuronal Compensation in Healthy Older Adults. Front. Aging Neurosci. 2018;10:74. doi: 10.3389/fnagi.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Lange A.-M.G., Kaufmann T., van der Meer D., Maglanoc L.A., Alnæs D., Moberget T., Douaud G., Andreassen O.A., Westlye L.T. Population-based neuroimaging reveals traces of childbirth in the maternal brain. Proc. Natl. Acad. Sci. USA. 2019;116:22341–22346. doi: 10.1073/pnas.1910666116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cruz-Almeida Y., Fillingim R., Riley J.L., III, Woods A.J., Porges E., Cohen R., Cole J. Chronic pain is associated with a brain aging biomarker in community-dwelling older adults. Pain. 2019;160:1119–1130. doi: 10.1097/j.pain.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Lange A.-M.G., Anatürk M., Suri S., Kaufmann T., Cole J.H., Griffanti L., Zsoldos E., Jensen D.E., Filippini N., Singh-Manoux A., et al. Multimodal brain-age prediction and cardiovascular risk: The Whitehall II MRI sub-study. NeuroImage. 2020;222:117292. doi: 10.1016/j.neuroimage.2020.117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Lange A.G., Barth C., Kaufmann T., Anatürk M., Suri S., Ebmeier K., Westlye L.T. The maternal brain: Region-specific patterns of brain aging are traceable decades after childbirth. Hum. Brain Mapp. 2020;41:4718–4729. doi: 10.1002/hbm.25152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Henneghan A., Rao V., Harrison R.A., Karuturi M., Blayney D.W., Palesh O., Kesler S.R. Cortical Brain Age from Pre-treatment to Post-chemotherapy in Patients with Breast Cancer. Neurotox. Res. 2020;37:788–799. doi: 10.1007/s12640-019-00158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reuben A., Elliott M.L., Abraham W.C., Broadbent J., Houts R.M., Ireland D., Knodt A.R., Poulton R., Ramrakha S., Hariri A.R., et al. Association of Childhood Lead Exposure With MRI Measurements of Structural Brain Integrity in Midlife. JAMA. 2020;324:1970–1979. doi: 10.1001/jama.2020.19998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seidel G., Gaser C., Götz T., Günther A., Hamzei F. Accelerated brain ageing in sepsis survivors with cognitive long-term impairment. Eur. J. Neurosci. 2020;52:4395–4402. doi: 10.1111/ejn.14850. [DOI] [PubMed] [Google Scholar]
- 107.Anatürk M., Kaufmann T., Cole J.H., Suri S., Griffanti L., Zsoldos E., Filippini N., Singh-Manoux A., Kivimäki M., Westlye L.T., et al. Prediction of brain age and cognitive age: Quantifying brain and cognitive maintenance in aging. Hum. Brain Mapp. 2020;42:1626–1640. doi: 10.1002/hbm.25316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bittner N., Jockwitz C., Franke K., Gaser C., Moebus S., Bayen U.J., Amunts K., Caspers S. When your brain looks older than expected: Combined lifestyle risk and BrainAGE. Anat. Embryol. 2021;226:621–645. doi: 10.1007/s00429-020-02184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cherbuin N., Walsh E.I., Shaw M., Luders E., Anstey K.J., Sachdev P.S., Abhayaratna W.P., Gaser C. Optimal Blood Pressure Keeps Our Brains Younger. Front. Aging Neurosci. 2021;13:694982. doi: 10.3389/fnagi.2021.694982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunås T., Wåhlin A., Nyberg L., Boraxbekk C.-J. Multimodal Image Analysis of Apparent Brain Age Identifies Physical Fitness as Predictor of Brain Maintenance. Cereb. Cortex. 2021;31:3393–3407. doi: 10.1093/cercor/bhab019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elliott M.L., Belsky D.W., Knodt A.R., Ireland D., Melzer T.R., Poulton R., Ramrakha S., Caspi A., Moffitt T.E., Hariri A.R. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol. Psychiatry. 2019;26:3829–3838. doi: 10.1038/s41380-019-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hedderich D.M., Menegaux A., Schmitz-Koep B., Nuttall R., Zimmermann J., Schneider S.C., Bäuml J.G., Daamen M., Boecker H., Wilke M., et al. Increased Brain Age Gap Estimate (BrainAGE) in Young Adults After Premature Birth. Front. Aging Neurosci. 2021;13:653365. doi: 10.3389/fnagi.2021.653365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karim H.T., Ly M., Yu G., Krafty R., Tudorascu D.L., Aizenstein H.J., Andreescu C. Aging faster: Worry and rumination in late life are associated with greater brain age. Neurobiol. Aging. 2021;101:13–21. doi: 10.1016/j.neurobiolaging.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rakesh D., Cropley V., Zalesky A., Vijayakumar N., Allen N.B., Whittle S. Neighborhood disadvantage and longitudinal brain-predicted-age trajectory during adolescence. Dev. Cogn. Neurosci. 2021;51:101002. doi: 10.1016/j.dcn.2021.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosemann S., Thiel C.M. No association between age-related hearing loss and brain age derived from structural neuroimaging data. Neuroimage Rep. 2021;1:100020. doi: 10.1016/j.ynirp.2021.100020. [DOI] [Google Scholar]
- 116.Salih A., Galazzo I.B., Raisi-Estabragh Z., Rauseo E., Gkontra P., Petersen S.E., Lekadir K., Altmann A., Radeva P., Menegaz G. Brain age estimation at tract group level and its association with daily life measures, cardiac risk factors and genetic variants. Sci. Rep. 2021;11:20563. doi: 10.1038/s41598-021-99153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanders A.-M., Richard G., Kolskår K., Ulrichsen K.M., Kaufmann T., Alnæs D., Beck D., Dørum E.S., de Lange A.-M.G., Nordvik J.E., et al. Linking objective measures of physical activity and capability with brain structure in healthy community dwelling older adults. NeuroImage Clin. 2021;31:102767. doi: 10.1016/j.nicl.2021.102767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subramaniapillai S., Rajagopal S., Snytte J., Otto A.R., Einstein G., Rajah M.N. Sex differences in brain aging among adults with family history of Alzheimer’s disease and APOE4 genetic risk. NeuroImage Clin. 2021;30:102620. doi: 10.1016/j.nicl.2021.102620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vidal-Pineiro D., Wang Y., Krogsrud S.K., Amlien I.K., Baaré W.F., Bartres-Faz D., Bertram L., Brandmaier A.M., Drevon C.A., Düzel S., et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. eLife. 2021;10:e69995. doi: 10.7554/eLife.69995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weihs A., Frenzel S., Wittfeld K., Obst A., Stubbe B., Habes M., Szentkirályi A., Berger K., Fietze I., Penzel T., et al. Associations between sleep apnea and advanced brain aging in a large-scale population study. Sleep. 2020;44:zsaa204. doi: 10.1093/sleep/zsaa204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Angebrandt A., Abulseoud O.A., Kisner M., Diazgranados N., Momenan R., Yang Y., Stein E.A., Ross T.J. Dose-dependent relationship between social drinking and brain aging. Neurobiol. Aging. 2021;111:71–81. doi: 10.1016/j.neurobiolaging.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beck D., Lange A.G., Pedersen M.L., Alnæs D., Maximov I.I., Voldsbekk I., Richard G., Sanders A., Ulrichsen K.M., Dørum E.S., et al. Cardiometabolic risk factors associated with brain age and accelerate brain ageing. Hum. Brain Mapp. 2021;43:700–720. doi: 10.1002/hbm.25680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bourassa K.J., Moffitt T.E., Ambler A., Hariri A.R., Harrington H., Houts R.M., Ireland D., Knodt A., Poulton R., Ramrakha S., et al. Association of Treatable Health Conditions During Adolescence With Accelerated Aging at Midlife. JAMA Pediatr. 2022;176:392. doi: 10.1001/jamapediatrics.2021.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giannakopoulos P., Montandon M.-L., Herrmann F.R., Hedderich D., Gaser C., Kellner E., Rodriguez C., Haller S. Alzheimer resemblance atrophy index, BrainAGE, and normal pressure hydrocephalus score in the prediction of subtle cognitive decline: Added value compared to existing MR imaging markers. Eur. Radiol. 2022 doi: 10.1007/s00330-022-08798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Linli Z., Feng J., Zhao W., Guo S. Associations between smoking and accelerated brain ageing. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 2021;113:110471. doi: 10.1016/j.pnpbp.2021.110471. [DOI] [PubMed] [Google Scholar]
- 126.Vaughan B.A., Simon J.E., Grooms D.R., Clark L.A., Wages N.P., Clark B.C. Brain-Predicted Age Difference Moderates the Association Between Muscle Strength and Mobility. Front. Aging Neurosci. 2022;14:808022. doi: 10.3389/fnagi.2022.808022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang M., Ren Q., Shi Y., Shu H., Liu D., Gu L., Xie C., Zhang Z., Wu T., Wang Z. The effect of Alzheimer’s disease risk factors on brain aging in normal Chineses: Cognitive aging and cognitive reserve. Neurosci. Lett. 2021;771:136398. doi: 10.1016/j.neulet.2021.136398. [DOI] [PubMed] [Google Scholar]
- 128.Whitsel N., Reynolds C.A., Buchholz E.J., Pahlen S., Pearce R.C., Hatton S.N., Elman J.A., Gillespie N.A., Gustavson D.E., Puckett O.K., et al. Long-term associations of cigarette smoking in early mid-life with predicted brain aging from mid- to late life. Addiction. 2021;117:1049–1059. doi: 10.1111/add.15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng Y., Habes M., Gonzales M., Pomponio R., Nasrallah I., Khan S., Vaughan D.E., Davatzikos C., Seshadri S., Launer L., et al. Mid-life epigenetic age, neuroimaging brain age, and cognitive function: Coronary artery risk development in young adults (CARDIA) study. Aging. 2022;14:1691–1712. doi: 10.18632/aging.203918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.