Abstract
Alzheimer’s disease (AD) is an incurable degenerative disease of the central nervous system and the most common type of dementia in the elderly. Despite years of extensive research efforts, our understanding of the etiology and pathogenesis of AD is still highly limited. Nevertheless, several hypotheses related to risk factors for AD have been proposed. Moreover, plant-derived dietary polyphenols were also shown to exert protective effects against neurodegenerative diseases such as AD. In this review, we summarize the regulatory effects of the most well-known plant-derived dietary polyphenols on several AD-related molecular mechanisms, such as amelioration of oxidative stress injury, inhibition of aberrant glial cell activation to alleviate neuroinflammation, inhibition of the generation and promotion of the clearance of toxic amyloid-β (Aβ) plaques, inhibition of cholinesterase enzyme activity, and increase in acetylcholine levels in the brain. We also discuss the issue of bioavailability and the potential for improvement in this regard. This review is expected to encourage further research on the role of natural dietary plant polyphenols in the treatment of AD.
Keywords: Alzheimer’s disease, polyphenols, antioxidant, anti-neuroinflammation, amyloid-β, bioavailability
1. Introduction
Alzheimer’s disease (AD), also known as senile dementia, is a neurodegenerative disease that occurs in the elderly worldwide and develops very slowly. AD is the most common type of dementia [1]. AD is characterized by a variety of pathological features, including cerebral cortex atrophy, decrease in brain weight [2], loss of locus coeruleus neuromelanin [3], accumulation of extracellular amyloid-β protein (Aβ), and the occurrence of neurofibrillary tangles (NFTs) due to abnormal tau protein hyperphosphorylation [4]. The pathogenesis of AD remains unclear to date. Several hypotheses have been developed in the past, such as the oxidative stress [5], cholinergic injury hypothesis [6], Aβ toxicity hypothesis, tau protein phosphorylation hypothesis [7], neuroinflammation hypothesis [8], and apolipoprotein E (ApoE) gene hypotheses [9]. The complexity of these hypotheses is considered to be the main reason for the repeated failure of anti-AD drugs. Thus, no clear conclusions have been drawn to date regarding the factors that determine the occurrence and development of AD. Therefore, the clinical treatment of AD remains a challenge for mankind.
Despite the fact that AD was first discovered more than a hundred years ago and that major pharmaceutical companies such as Merck and AstraZeneca have made heavy investments in developing drugs against AD, the failure rate remains very high. Only six drugs have been approved by the Food and Drug Administration (FDA) for AD treatment to date; however, many adverse drug reactions have also been reported for these drugs [10] (Table 1).
Table 1.
The mechanisms of action and adverse reactions of drugs approved by Food and Drug Administration (FDA) for Alzheimer’s disease (AD) treatment.
| Drugs | Mechanisms of Action | Main Limitations | Reference |
|---|---|---|---|
| Tacrine | Inhibition of acetylcholinesterase activity and increase in acetylcholine level | Oral administration leads to strong hepatotoxicity and gastrointestinal adverse reactions, which quickly leads to increase in transaminase activity | [11,12] |
| Donepezil | Inhibition of acetylcholinesterase activity and increase in acetylcholine level. Inhibition of aberrant glia cell activation to alleviate neuroinflammation | Low CNS selectivity, gastrointestinal toxicity (nausea, vomiting, anorexia, flatulence, loose stool, diarrhea, salivation, and abdominal colic) | [13,14,15] |
| Rivastigmine | Selective enhancement of Ach activity in the cerebral cortex and hippocampus. Improvements in cognitive function and deceleration of APP formation | Adverse reactions such as acute dystonia, nausea, vomiting, diarrhea, dizziness, and weight loss | [16,17,18] |
| Galantamine | Inhibition of acetylcholinesterase activity and increase in acetylcholine level. Regulation of nicotinic acid receptors outside the brain to increase Ach release | Severe cutaneous adverse drug reactions | [19] |
| Memantine | Antagonizing effect on NMDAR | Bradycardia | [20,21] |
| Aducanumab | Recognition of an epitope of Aβ, reduction of aggregated soluble and insoluble forms of Aβ. | ARIA, effusion, minor hemorrhage, and hemosiderosis | [22] |
CNS, central nervous system; Ach, acetylcholine; APP, amyloid precursor protein; NMDAR, N-methyl-D-aspartate-receptor; ARIA, amyloid-associated imaging abnormality.
Recently, the sodium oligomannate capsules (GV-971) for AD treatment were approved in China, which demonstrates the potential of natural products for the treatment of neurodegenerative diseases [23]. Therefore, the discovery and development of safe and effective anti-AD drugs from natural sources such as plants may provide a viable strategy [24]. Highly efficient anti-AD drugs with low toxicities may indeed be discovered through extensive studies on the effects of various natural products on AD-related molecular mechanisms [25].
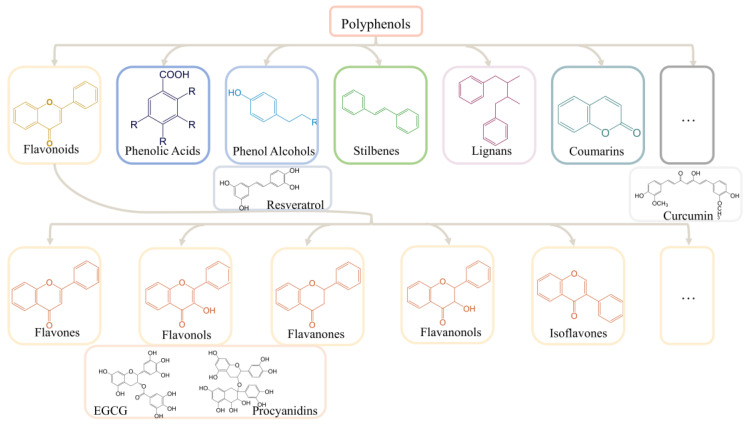
The focus of AD treatment strategies is also gradually shifting to alternative therapies. A diet-based lifestyle is the most effective that can be easily adopted by individuals among these alternative therapies [26,27]. Improving the dietary structure, such as choosing a Mediterranean or Asian diet rich in fruits and vegetables, is recognized globally as an effective means to prevent the development of AD [28,29,30]. Polyphenols are commonly found in vegetables, fruits, and cereals such as grapes, blueberries, and apples, in addition to tea and red wine [31,32,33,34,35]. Polyphenols have diverse and complex structures. Most polyphenols are found in glycosidic forms in plants [36]. Polyphenols are mainly classified into flavonoids and nonflavonoids based on the basic flavonoid structure, aglycone. Flavonoids include flavones, flavonols, flavanones, flavanonols, and isoflavones, whereas nonflavonoids are mainly classified into phenolic acids, phenolic alcohols, stilbenes, lignans, curcumins, and coumarins [37] (Figure 1). Polyphenols have been widely used for the treatment of age-related neurological disorders, including AD and cognitive disorders, due to their antioxidant and anti-inflammatory activities [38]. Many in vivo and in vitro experiments have confirmed the therapeutic effect of polyphenols on neurodegenerative diseases, including AD. These experiments also clarified several AD-related molecular mechanisms and led to the development of several hypotheses regarding the development of AD, such as oxidative stress, cholinergic, Aβ toxicity, tau protein, and neuroinflammation hypothesis [39,40,41,42].
Figure 1.
The classification of polyphenols. EGCG, ((−) -epigallocatechin-3-gallate).
However, at present, most of the experiments on the application of polyphenols in the treatment of AD are limited to in vitro experiments or animal experiments, and there are few clinical trials in vivo human studies, which may be related to the low bioavailability in the brain and the difficulty in penetrating the blood–brain barrier (BBB) [43]. Meanwhile, the clinical trials that have been carried out are often limited by the small sample size, the short study duration, and the individual differences of the subjects, and cannot obtain encouraging results like in vitro experiments [44]. These issues need to be further considered in future clinical experiment design involving in vivo human studies.
As of 25th September 2022, we have selected the relevant studies from PubMed, CNKI, and Google Scholar databases, using the following Medical Subject Headings (MeSH) keywords: “polyphenols”, “plant polyphenols”, “Alzheimer’s disease”, “resveratrol”, “curcumin”, “procyanidins”, “EGCG”, “bioavailability”, and so on. We identified the relevant papers and selected them following the inclusion criteria: full-text original articles, including both preclinical and clinical studies.
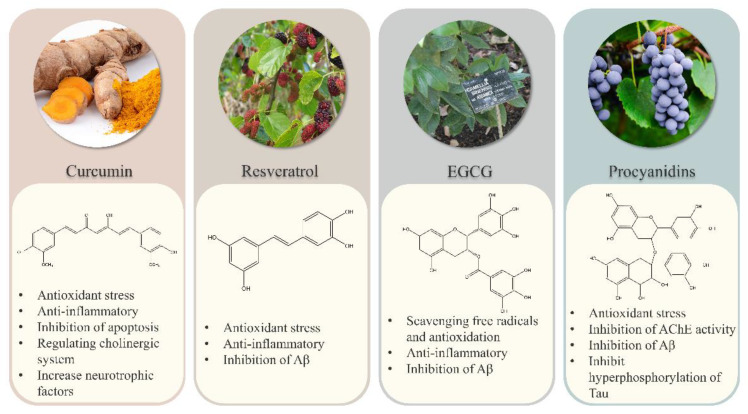
Here, we review the regulatory roles of the most widely reported dietary plant polyphenols, curcumin, resveratrol, proanthocyanidins, and EGCG, on widely recognized molecular mechanism hypotheses related to AD development. We also discuss the current application limitations of polyphenols and improved sample preparation methods in order to provide a reference for further functional research on plant polyphenols for the prevention and treatment of AD. We also expect that this review will provide a theoretical basis for the development of similar health products and drugs.
2. Polyphenols and Oxidative Stress
2.1. The Oxidative Stress in Alzheimer’s Disease
The brain has a greater lipid content and energy requirements than any other organ. The brain also has relatively weak endogenous antioxidant defenses and is thus vulnerable to an imbalance in redox homeostasis [45]. Therefore, free radicals may readily disrupt neuronal cell structure and function and lead to various neurodegenerative diseases, including AD and Parkinson’s disease (PD) [46]. Oxidative stress (OS) has previously been shown to be the primary cause of neuronal death and neuroinflammation in neurodegenerative diseases [47]. The aggregation of misfolded proteins, such as amyloid fibers and aberrantly phosphorylated tau protein, is a major pathological feature of AD that disrupts normal mitochondrial function and induces the production of large amounts of reactive oxygen species (ROS). This, in turn, leads to the oxidization of Aβ scavenger proteins and thus to further Aβ accumulation, as well as to hyperphosphorylation and Tau protein aggregation [48,49,50].
The antioxidant enzyme system is the first line of defenses against ROS in the human body. Activities of glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), and catalase (CAT) in the brains of AD patients have been reported to decrease, whereas malondialdehyde (MDA) activity is increased. This is accompanied by extensive protein and lipid peroxidation, which eventually leads to cell apoptosis [51]. SOD activity is particularly an important indicator of the degree of an oxidative stress injury in the nervous system. The regulation of SOD, GPX, GR, and CAT activities has thus become a focus of research for drug development against AD.
OS is also a significant feature of neuroinflammation and apoptosis. OS occurs in nerve cells; ROS produced by OS causes mitochondrial respiratory chain energy metabolism disorder, which leads to the irreversible opening of the mitochondrial permeability transition pore (MPTP) and a decrease in mitochondrial membrane potential in nerve cells. As a result, the release of cytochrome C (Cytc) from the mitochondria is increased, and a polymerized complex of Cytc and apoptosis protein activating factor-1 (Apaf-1) is formed. This complex activates the precursor of caspase-9, which in turn activates downstream caspase-3, and thereby starts the apoptotic response cascade, leading to the ultimate loss of synapses and neuronal apoptosis [52,53,54]. B-cell lymphoma-2 (Bcl-2) and mitogen-activated protein kinase (MAPK) family member proteins have also been reported to play key roles in AD development [55,56,57].
2.2. Polyphenols Alleviate AD Symptoms by Reducing Oxidative Stress
Curcumin (1,7-bis (4-Hydroxy-3-Methoxy phenol) -1,6-heptadiene-3,5-dione) is a lipophilic polyphenol obtained from the rhizome of the Asian ginger plant Curcuma longa [58]. Due to its antioxidant [59], anti-inflammatory [60], antibacterial [61], anti-mutagenic [62], and anti-cancer [63] properties, curcumin is widely used in many areas, such as food spices, cosmetics, food pigments, and colorants. Curcuma longa L.—the source plant of curcumin—has long been used as part of herbal medicine practices in Asian countries as well [64]. Curcumin can directly scavenge free radicals (ROS and reactive nitrogen species, RNS). In vivo and in vitro experiments have also confirmed the role of curcumin in cell protection via activation of nuclear factor erythroid 2-related factor 2 (Nrf2)/Heme Oxygenase-1 (HO-1) and Nrf2/Kelch-like ECH-associated protein 1 (Keap1) pathways [65,66,67]. The amount of (Nrf2) in the brains of AD patients has been shown to be significantly lower than that in healthy individuals [68]. This signaling pathway plays a key role in Aβ deposition and thus delays the onset and development of AD. Curcumin was also shown to reduce copper-induced oxidative stress and mitochondrial apoptosis in SH-SY5Y cells and thereby improve neurotoxicity [69]. Fermented curcuma, including curcumin as its main component, was found to exert neuroprotective effects on C6 and BV2 cells against OS induced by H2O2 [70] and on rats against high intraocular pressure. Curcumin can provide neuroprotection by reducing ROS species and inhibiting the apoptosis pathway [71]. Curcumin was in vivo found to improve the overall behavior by increasing the CAT level in the brain of depression model mice [72]. Finally, curcumin and mitochondria-targeted curcumin (MTC) were found to have neuroprotective effects on a mouse model of cerebellar oxidative damage [73].
Resveratrol (3,4 ‘, 5-trihydroxystilbene), also known as stilbene triphenol, is a polyphenol that is abundant in grapes, pomegranates, mulberry, blueberries, and Reynoutria japonica Houtt [74]. Resveratrol was first isolated from Veratrum in 1939. The beneficial effects of resveratrol on human health have been emphasized for the first time through the “French paradox” (low risk of cardiovascular disease among French, despite their high-fat and -cholesterol diet), which is related to large amounts of daily red wine consumption among the French population [75,76]. Resveratrol has two isomers: cis-resveratrol and trans-resveratrol. Trans-resveratrol is the main form, and its effectiveness is conferred by the lower steric hindrance of its side chain [77]. Resveratrol has anti-inflammatory [78], antioxidative [79], anti-infective [80], antibacterial [81], and anti-cancer effects [82]. The main mechanisms involved with respect to the effect of resveratrol in the context of AD mainly involve antioxidant stress, anti-inflammatory effects, and Aβ aggregation [83].
OS markers that indicate the progression of AD were shown to appear earlier than pathological changes, including Aβ plaques and neurofibrillary tangles [84]. The antioxidative stress effect of resveratrol is mainly determined by its structure: 4’-OH and conjugated double bonds facilitate the transfer of hydrogen atoms or electrons to free radicals [85,86]. Resveratrol was also found to facilitate the production of free radicals and reactive oxygen species in BV2 mice, malondialdehyde production induced by rotenone, and the downregulation of glutathione synthesis by enhancing the inhibition of signal transducer and activator of transcription 1 (STAT1) and Keap1, and finally upregulation of the expression of Nrf2 and solute carrier family 7 (cationic amino acid transporter, y+ system) member 11 (SLC7A11) proteins. Hence, it plays a role in antioxidative stress as well [87]. Administration of resveratrol to c57bl/6 mice exposed to MnCl2 not only resulted in an improvement of the cognitive function of mice but also increased ROS levels and inhibited MDA activity significantly. On the other hand, SOD and CAT activities, L-Glutathione (GSH) content, and the ratio of GSH/ glutathione (GSSG) in the hippocampus were significantly increased [88].
Procyanidins (PC) are polyphenols that consist of flavanol monomers and their polymers and are abundant in all kinds of fruits, vegetables, bark, and peel, and especially in grapes and grape seeds, which was demonstrated to be a much stronger antioxidant than vitamin C or E [89,90,91,92]. The favorable pharmacological activities of procyanidins in terms of free radical scavenging [93], and antioxidative [94], anti-inflammatory [95], hypoglycemic [96], hypolipidemic [97], anticancer, and antitumor [98] effects have been confirmed by numerous studies. Neuroprotective effects of PC with different structures have also been recently proven. PC have thus been used to treat and prevent neurodegenerative diseases, including AD [99]. A unique tricyclic structure confers PC to its antioxidant capacity [100]. PC were found to significantly improve the cognitive ability of elderly rats, reduce increased protein oxidation and lipid peroxidation levels, enhance their antioxidant defense abilities, and significantly restore Ach activities [101]. In a mouse OS injury model induced by extremely low-frequency electromagnetic fields (ELF-EMF), administration of lotus seed pod proanthocyanidins was found to significantly enhance the activities of SOD, CAT, GPx, GR, and GST, and also reduced MDA levels, and thereby exerted neuroprotective effects [102]. The phenolic profiles of hardy kiwifruits rich in procyanidin B2 were also found to allow the reversal of oxidative stress in SH-SY5Y and PC-12 cells induced by H2O2 by improving their ability to resist acetyl cholinesterase (AchE) and butyrylcholinesterase (BchE) action [103]. Grape seed proanthocyanidins were found to improve the cognitive decline of mice caused by isoflurane and prevent OS by interfering with SOD activity and the NR2B/CREB pathway [104].
Tea is one of the most popular beverages worldwide and an important component of the East Asian diet. Although from the current clinical research, it seems uncertain to draw the conclusion that the consumption of tea, especially green tea, is significantly related to the improvement of cognitive impairment in AD patients. However, scientists do not deny that tea and its effective ingredients have certain neuroprotective effects [105,106,107]. Especially in animal experiments and in vitro experiments, tea has shown a better effect on improving cognitive function [108,109]. EGCG is a natural and the main active ingredient in green tea. ECCG has antioxidative [110], anti-inflammatory [111], anti-viral [112], cardioprotective [113], and anticancer effects [114,115]. In recent years, EGCG has also attracted the attention of researchers due to its neuroprotective effects, especially for the prevention and treatment of neurodegenerative diseases such as AD [116,117]. In one study, a daily intake of 400 mg/kg green tea polyphenols rich in EGCG by rats with chronic cerebral hypoperfusion was found to significantly improve cognitive function, eliminate free oxygen radicals, enhance antioxidative capacity, and reduce the production of lipid peroxide and oxidative DNA damage [118]. When rats with Streptozocin (STZ) injection into the ventricles were treated with EGCG, the levels of ROS and GSH levels in the hippocampus were found to decrease, and memory function was found to improve [119]. AD pathology is also related to brain iron imbalance and its association with amyloid precursor protein (APP) plaque formation. To this end, EGCG was found to increase the level of transferrin receptor (TfR) protein and mRNA in SH-SY5Y cells in a dose-dependent manner, and thereby play a role in iron chelation and reduce the expression of Swedish mutant APP in Chinese hamster oocytes [120].
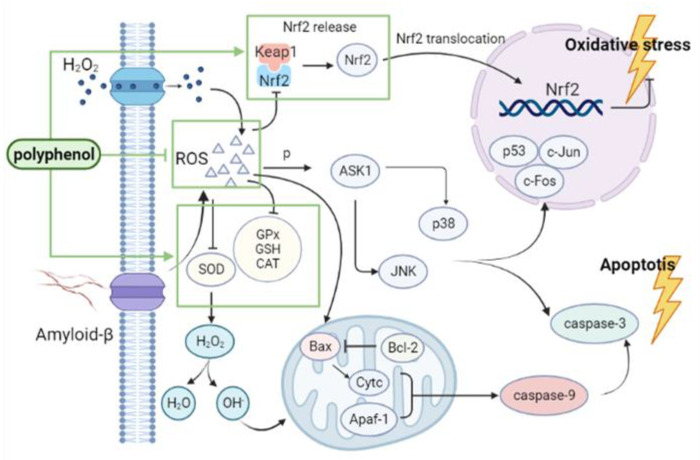
In general, the regulatory roles of polyphenols in the context of AD-related oxidative stress mechanisms are mainly based on the direct elimination of ROS; the regulation of SOD, CAT, and GSH levels; and the reduction of an oxidative stress injury in the brain through regulation of Nrf2/HO-1 and Nrf2/Keap1 pathways (Figure 2).
Figure 2.
Regulatory roles of polyphenols in the context of oxidative stress: polyphenols can directly clear ROS and inhibit Keap1 to upregulate Nrf2 expression; increase SOD, CAT, and GSH activities; inhibit oxidative stress response; and thus alleviate AD symptoms. Nrf2, nuclear factor erythroid 2-related factor 2; Keap1, Kelch-like ECH-associated protein 1; p53, tumor protein P53; ROS, reactive oxygen species; ASK1, DASH complex subunit ASK1; SOD, superoxide dismutase; GPx, glutathione peroxidase; GSH, L-glutathione; CAT, catalase; Bax, apoptosis regulator BAX; Bcl-2, apoptosis regulator Bcl-2; Cytc, Cytochrome C; Apaf-1, apoptotic peptidase activating factor 1.
3. Polyphenols and Neuroinflammatory Mechanisms
3.1. Neuroinflammation in Alzheimer’s Disease
Due to the prevention of immune cells and humoral factors from entering the brain by the blood-brain barrier (BBB) and the inability of brain cells to produce an innate immune response, the brain was previously believed to enjoy immune privilege [121]. However, an increasing number of epidemiological observations and autopsy results refuted this theory by demonstrating that brain tissue can flexibly regulate its innate paracrine inflammatory factors [122,123,124]. The repeated failure of drugs that directly affect Aβ in clinical trials led some scientists to believe that direct regulation of Aβ is an unrealistic therapeutic intervention and that attention should instead be directed toward neuroinflammation mechanisms in AD [125].
Microglia, astrocytes, and monocytes play a significant role in the occurrence and development of inflammatory reactions in the central nervous system (CNS) [8,126]. Microglia can be considered immune cells of the brain since, although they account for only about 10% of the brain tissue, they constitute the first line of defenses against diseases [127,128]. Upon their activation, microglia in their normal resting state quickly travel to the vicinity of Aβ plaques and differentiate into pro-inflammatory (M1 microglia) and anti-inflammatory (M2 microglia) phenotypes that secret pro-inflammatory or anti-inflammatory cytokines, respectively. Pro-inflammatory cytokines activate microglia and astrocytes, aggravate nerve damage, decrease Aβ clearance, and ultimately aggravate the development of AD [129,130]. Astrocytes are the most abundant cells in the brain and perform various biological functions, such as blood flow regulation, maintenance of the BBB, supply of energy metabolites to neurons, regulation of synaptic activity, control of the secretion of neurotrophic factors, and removal of dead cells. Similar to microglia, astrocytes can also differentiate into two different phenotypes: pro-inflammatory (A1 astrocytes) and anti-inflammatory (A2 astrocytes). Both types of astrocytes perform their functions through the secretion of cytokines and the formation of cross-talk between microglia and coordinate their responses to regulate neuroinflammation [131,132,133,134]. At the same time, monocytes can also join in the “talk”, actively participate in the immune response and inflammatory response in the brain, and secrete a large amount of monocyte chemotactic protein 1 (MCP-1) after astrocytes are stimulated by Aβ, triggering the inflammatory cascade reaction by combining it with C-C motif chemokine receptor 2 (CCR2), and gradually differentiate into macrophages in the process of recruitment into the brain, playing the Aβ clearance ability as good as microglia [135,136,137]. The significant decrease in the phagocytosis of monocytes to Aβ in AD patients leads to the deposition of Aβ, which also indicates that monocytes play an important role in the anti-inflammatory treatment of AD [138,139]. Therefore, the development of targeted drugs to inhibit neuroinflammatory processes, such as the inhibition of microglia and astrocyte activation, and the enhancement of the phagocytic ability of monocytes/macrophages to Aβ is of great significance for the treatment of neurodegenerative diseases such as AD.
3.2. Polyphenols Can Alleviate AD Symptoms by Inhibiting Glial Inflammatory Activation, Affecting Monocyte/Macrophage System, and Inhibiting Neuroinflammation
The inflammatory response is a key factor in the development of most neurodegenerative diseases, such as Parkinson’s syndrome, multiple sclerosis, and AD [140]. Curcumin was previously found to promote the polarization of BV2 cells to the anti-inflammatory M2 phenotype through the triggering receptor expressed on myeloid cells-2 (TREM2)/Toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) pathway in vitro, and thereby inhibit lipopolysaccharide (LPS)-induced neuroinflammation [141]. The expression of matrix metalloproteinase-9 (MMP-9) and interleukin 6 (IL-6) secreted by human astrocytes (U373-MG) was found to be downregulated by curcumin after LPS induction, confirming the potential of curcumin to regulate astrocyte-mediated inflammation in the central nervous system [142]. Curcumin was also injected intraperitoneally into c57bl/6 mice in a distal middle cerebral artery occlusion (dMCAO) model to improve infarct area and motor sensory function, promote the expression of M2 polarization markers in microglia, and inhibit the expression of M1 polarization markers [143]. Reactive astrocytes in the brains of gfap-il6 mice treated with Meriva curcumin (MC) were also found to be significantly reduced. Hence, plant-based curcumin may indeed improve the pathology of neuritis by reversing the harmful effects of chronic glial cell activation [144]. In addition, it has been verified that curcuminoids can significantly improve the phagocytosis of macrophages to Aβ in AD patients [145,146].
The anti-inflammatory properties of resveratrol may be attributed to the inhibition of the production of anti-inflammatory factors. Resveratrol was found to inhibit the polarization of LPS-stimulated microglia to the pro-inflammatory phenotype M1 in vitro and in vivo through PGC-1α and promote the polarization of anti-inflammatory phenotype M2 through CO activation of signal transducer and activator of transcription 6 (STAT6) and signal transducer and activator of transcription 3 (STAT3), and thereby play an anti-inflammatory role [147]. The production of classical pro-inflammatory factors tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and interleukin-18 (IL-18) released by primary astrocytes from the rat hippocampus and stimulated by LPS were also found to be inhibited by resveratrol. This anti-inflammatory effect is likely to be mediated through the regulation of NF-κB, HO-1, p38, and extracellular-signal-regulated kinases (ERK) pathways [148]. Resveratrol was also found to alleviate neuroinflammation in a rat astrocyte line (RA) and microglia (N9) caused by Aβ aggregation and plays an anti-inflammatory role by inhibiting NF-κB/p65 nuclear translocation [149]. In addition, resveratrol can reverse glutamate-induced upregulation of MCP-1 expression in rat hippocampus, reduce the release of inflammatory factor IL-1β, and alleviate neuroinflammation [150].
It was previously found that procyanidin dimers and trimers have significant anti-inflammatory activity [151,152,153,154]. Procyanidin A2 extracted from litchi seeds was found to significantly reduce the levels of TNF-α, IL-1β, and IL-6 released from the supernatant of BV2 neuroinflammatory model cells induced by Aβ1-42, and production levels of TNF-α, IL-1β, and inducible nitric oxide synthase (iNOS) by inhibiting the NF-B pathway to improve the inflammatory response [155]. Procyanidins were also found to inhibit LPS-induced p65 nuclear translocation and p38 MAPK phosphorylation in BV2 cells by regulating the p38 MAPK and NF-κB pathways. As a result, the inflammatory response is inhibited [156]. Proanthocyanidin treatment of mice with subcutaneous morphine-induced activation of spinal microglia attenuates chronic morphine tolerance by inhibiting the phosphorylation of NMDA-NR1, protein kinase C (PKC), and MAPKs in the spinal cord. Proanthocyanidin also inhibits the activation of microglia and NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome in vitro and in vivo [157]. In addition, both procyanidin dimer B1 and trimer C1 can inhibit the inflammatory response signaling in human monocytes [153].
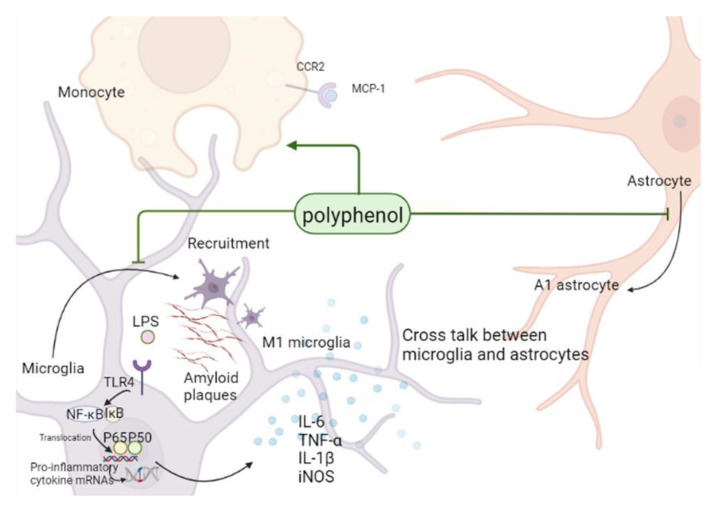
In conclusion, polyphenols inhibit the transformation of microglia and astrocytes to a pro-inflammatory phenotype and reactive astrocytes, respectively. Polyphenols further hinder the secretion of classical inflammatory factors such as TNF-α, IL-1β, and IL-6 by regulating the NF-κB pathway to improve inflammatory cross-talk in the brain and affecting monocyte/macrophage system to alleviate the symptoms of AD (Figure 3).
Figure 3.
Regulatory roles of polyphenols in the context of neuroinflammation: polyphenols inhibit the activation of astrocytes and transformation of microglia to pro-inflammatory phenotypes in the brain by inhibiting NF-κB pathway, reduce the secretion of inflammatory factors, improve the inflammatory environment in the brain and affect the phagocytosis of monocytes/ macrophages, to eventually alleviate AD symptoms. LPS, lipopolysaccharide; NF-κB, tumor necrosis factor-α; TLR4, Toll-like receptor 4; IL-6, interleukin-6; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; iNOS, inducible nitric oxide synthase.
4. Polyphenols and Amyloid Toxicity Mechanism
4.1. Amyloid Neurotoxicity in Alzheimer’s Disease
As the core of the amyloid hypothesis was first proposed in 1992, Aβ denotes short peptides composed of approximately 36–43 amino acids that are produced through hydrolysis of APP by β- and γ-secretory enzymes [158,159]. Amyloid plaque deposition in the brain as a result of the gradual aggregation and accumulation of Aβ is a major pathological feature of AD and has been recognized by scientists as the main cause of AD for a long time [160]. Aβ production is closely related to the single transmembrane protein APP, which is mainly located in the brain. Mutations in the APP gene locus and presenilin 1 and 2 (PSEN-1, -2) result in abnormal upregulation of beta-secretase 1 (BACE-1) and γ-site cutting enzyme activities. The resulting erroneous cleavage leads to the production and accumulation of excessive Aβ peptides. These peptides show toxic effects on axons and synapses; lead to neuronal degeneration, necrosis, and synaptic dysfunction; and eventually induce pathological changes [161,162]. However, an increasing number of researchers have started to question the relevance of this hypothesis to AD development. To this end, the connection between Aβ accumulation and AD development is not considered a simple causal relationship and may be explained by accelerated assembly of AD-like polymers, which in turn damages cholinergic neurons, and leads to excessive accumulation and activation of microglia to induce neuroinflammation. This, in turn, affects mitochondrial-dominated energy metabolism, leading to oxidative stress and abnormal phosphorylation of tau protein, creating a more complex feedback cascade that promotes the symptoms of AD [134,163,164,165,166,167].
4.2. Regulatory Role of Polyphenols in the Context of Amyloid Neurotoxicity
As mentioned above, the erroneous cleavage of β- and γ-secretase enzymes will eventually lead to large amounts of Aβ production, and the Aβ that cannot be cleared in time will deposit in the brain and eventually form toxic amyloid deposits, resulting in AD pathology. Therefore, in the current study, the relationship between polyphenols, Alzheimer’s disease, and amyloid toxicity hypothesis can be summarized as follows: polyphenols inhibit the production of Aβ by affecting the activity of secreted enzymes or inhibit the self-assembly of amyloid into fibrils by relying on their own aromatic rings with hydroxyl groups, and promote the depolymerization and clearance of Aβ [168,169]. In this way, the pathological phenomena of AD can be alleviated to a certain extent.
The effect of resveratrol on BACE1 and γ-secretase may occur via two distinct mechanisms. Nine resveratrol oligomers extracted from peony were found to inhibit BACE-1 expressed in baculovirus in a dose-dependent manner [170]. Resveratrol derivatives extracted from Vitis vinifera were also found to inhibit BACE-1 expression by baculovirus in a dose-dependent manner [171]. Resveratrol and its analogs were also found to decrease γ-secretase and increase α-secretase activities in Neuro2a neuroblastoma cells [172]. However, some studies have reported that resveratrol only facilitates the reduction of the Aβ content in cells and has, in fact, no effect on the α-, β-, or γ-secretase-mediated cleavage of APP or the stability of its C-terminal fragment. Resveratrol was also shown not to affect APP metabolism and Aβ production. The therapeutic potential of resveratrol for AD may therefore be based on its proteasome-dependent anti-amyloidosis activity [173]. The focus of research on the effect of resveratrol in AD thus shifted to Aβ clearance mechanisms such as activation of Aβ degradation enzymes [174], modulation of the plasminogen system [175], maintenance of the integrity of the BBB, and modulation of the expression of transporters and receptors to regulate the steady-state levels of Aβ [176].
Many clinical studies showed the central role of Aβ-polymerized fibrils or other self-assembly states in AD pathogenesis [177]. Procyanidins show their potential for AD treatment by affecting Aβ accumulation, which is manifested through the following mechanisms. First, the aggregation of Aβ is inhibited: several type-A procyanidins and procyanidins from apple were found to inhibit the aggregation of Aβ fibrils and show depolymerization activity as observed by experimental observations based on transmission electron microscopy (TEM) or thioflavin-T (Th-T) fluorescence staining [178,179]. This may be related to the fact that the catechol moiety is easily oxidized automatically and covalently bound to the nucleophilic amino acid residues of amyloid protein, rendering the structure of the β-fold unstable [180,181,182]. This may also be related to the presence of multiple hydroxyl groups in procyanidins themselves, which can be related to the strong interaction between amino acids that hinders the formation of β-fold forms [183]. Second, the particle size distribution of peptide aggregates is affected. Upon intervention by procyanidins, the particle size of Aβ decreases from the micron to the nanometer range. Third, the morphology of the peptide aggregates is modified. Following co-incubation with procyanidins, the fibrils of the Aβ peptide become smaller spherical particles. Finally, peptide depolymerization is promoted, and thereby mature fibrils are turned into shorter fibrils [184].
As with any other polyphenol, EGCG also inhibits the aggregation of Aβ fibrils in a dose-dependent manner [185]. Comparative evaluations of the capacities of several common polyphenols to inhibit Aβ aggregation showed that EGCG is a significantly stronger inhibitor of Aβ aggregation than other polyphenols, including curcumin and resveratrol [186]. The underlying mechanism may involve changes in the conformation of Aβ into an Aβ assembly without the “seeding” ability [187]. The aromatic ring of EGCG may also prevent the C-terminal of Aβ from obtaining a β-fold conformation. Thus, the oligomer structure is gradually stabilized and cannot form fibrils further [188]. Oral administration of EGCG (50 mg/kg) to APP/PS1 transgenic AD model mice for 4 months was found to significantly alleviate cognitive deficits in mice, improve dendritic integrity and synaptic protein expression levels in the brain, significantly reduce Aβ plaques in the hippocampus of mice, and alleviate the neuroinflammatory response by reducing microglial activation, inhibiting the secretion of pro-inflammatory cytokines IL-1β, and increasing the secretion of anti-inflammatory cytokines interleukin-10 (IL-10) and interleukin-13 (IL-13) [189]. EGCG was also found to increase α-secretase and reduce β- and γ-secretase activities by acting through the ERK/NF-κB pathway, thereby reducing the production of Aβ aggregates [190,191].
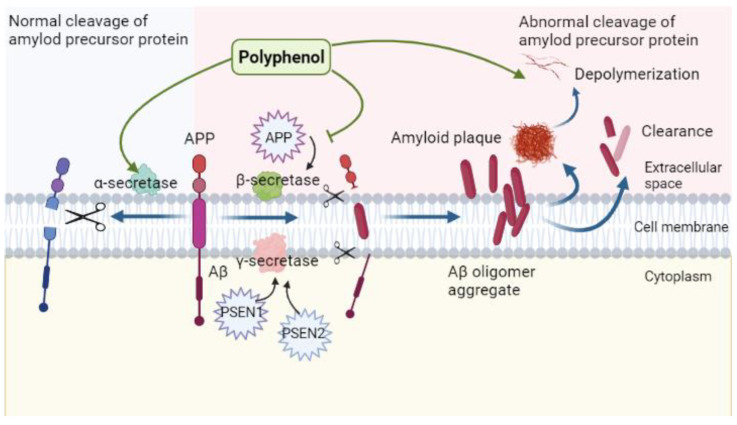
In conclusion, the inhibitory effect of polyphenols on Aβ aggregation is mainly based on the inhibition of Aβ production by affecting the activities of α-, β-, and γ-secretory enzymes, promoting the clearance, and depolymerization by affecting the conformation of Aβ fibrils, and reduction of neurotoxicity (Figure 4).
Figure 4.
Regulatory role of polyphenols in the context of amyloid neurotoxicity: polyphenols allow cleavage of Aβ correctly by promoting the activity of α-secretase, inhibit the activities of β-, and γ-secretases, prevent erroneous cleavage, promote Aβ plaque depolymerization and clearance, and reduces its neurotoxicity.
5. Polyphenols and Abnormal Tau Protein Phosphorylation
5.1. Abnormal Phosphorylation of Tau Protein in Alzheimer’s Disease
The tau protein hypothesis is mainly based on the abnormal deposition of neuronal tangles, a pathological phenomenon in AD [192]. The tau protein is soluble under normal conditions and regulates the stabilities of microproteins. However, its over-phosphorylation renders the protein insoluble, and the phosphorylated tau competes with binding to normal tau protein. This then leads to the depolymerization of microtubules and the formation of paired helices in neurons, thereby disrupting the normal physiological function of the tau protein [2,193,194]. As a marker of neuronal injury, the content of total tau protein in the cerebrospinal fluid of AD patients is higher than that in normal individuals. The proportion of abnormally phosphorylated tau protein is significantly increased as well [195]. Similar to the Aβ plaque deposition, abnormal phosphorylation of tau can also not be detected in the early stages of AD, yet previous findings indicate that the abnormal accumulation of tau is a better indicator of AD onset time [3]. Therefore, the current AD treatment methods based on tau protein are mainly divided into non-immunotherapeutic approaches based on stabilizing microtubules, inhibition of tau protein phosphorylation and aggregation, or inhibition of pathological tau diffusion, and active and passive immunotherapy approaches, including vaccination and monoclonal antibodies. Non-immunotherapeutic approaches are, in general, ineffective [196,197,198].
5.2. Regulatory Role of Polyphenols in the Context of Abnormal Tau Protein Phosphorylation
The role of curcumin in improving tau-based AD pathology has been preliminarily investigated [199]. Curcumin administration to human Tau (hTau) transgenic mice exhibiting the tau pathology was found to reduce the level of soluble tau dimers, reverse the interruption of the expression of molecular chaperones heat-shock protein 90 (HSP90), heat-shock protein 70 (HSP70), and heat-shock protein 72 (HSP72) in hTau expressing mice, and improve spatial learning and memory abilities [200]. Curcumin was also found to inhibit tau oligomerization by preventing β-folding, which is the initial step of tau aggregation, and decomposing the formed tau protein filaments in vitro [201]. Curcumin treatment of transgenic Caenorhabditis elegans with tau-induced neuronal dysfunction did not significantly affect tau aggregation, yet improved neuron functions and stabilized microtubules [202]. EGCG interacts with full-length tau protein via multiple residues to destabilize its structure and dissolve tau fibrils and oligomers while inhibiting the aggregation of full-length tau. Thus, the tau protein remains mostly in the unfolded monomer state [203,204]. EGCG was also found to enhance the clearance of abnormally phosphorylated tau in primary neurons by enhancing connexin expression in an in vitro model [205]. Oral administration of EGCG (50 mg/kg) to APPsw mice for 6 months significantly improved their cognitive ability and reduced the expression of hyperphosphorylated tau in the brain [206].
6. Influence of Polyphenol on Cholinergic Injury
6.1. Cholinergic Injury in Alzheimer’s Disease
The cholinergic injury hypothesis is a widely recognized mechanism of AD [207]. The significant loss in basal forebrain cholinergic neurons has attracted the attention of scientists as an early pathological feature of AD [208]. Previous findings revealed defects in the central cholinergic system responsible for regulating learning and memory in AD patients. One of the main reasons for this defect is the abnormal enhancement of AchE activity [209]. AchE inhibitors can therefore protect neurons by reversibly binding to the enzyme, thereby preventing acetylcholine hydrolysis, increasing the level of acetylcholine in the brain, and eventually enhancing cholinergic transmission. Indeed, drugs designed to inhibit AchE activity, such as donepezil, tacrine, and galantamine, have been widely used for AD treatment to date [210].
6.2. Regulatory Role of Polyphenols in the Context of Cholinergic Injury
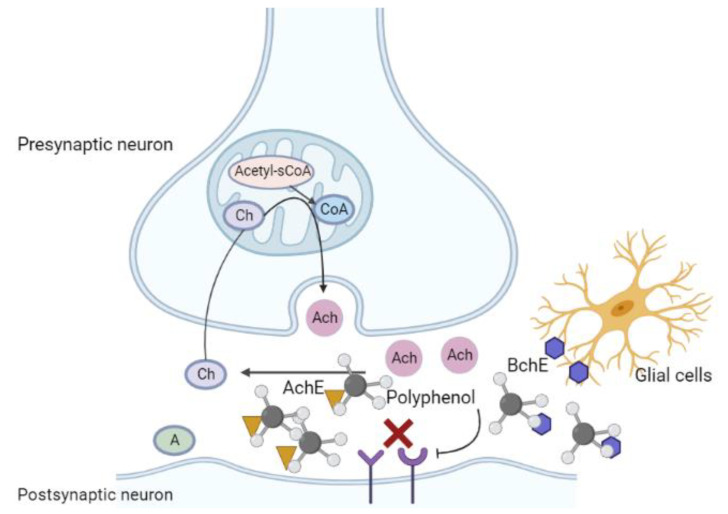
Polyphenols mainly inhibit the binding of cholinesterase to receptors, reduce its activity, and thus reduce the hydrolysis of Ach to restore cholinergic levels, improve cognitive ability, and alleviate AD symptoms (Figure 5). Curcumin has been reported to regulate the function of the cholinergic system and improve the levels of diminished nutritional factors in neurodegenerative diseases. Following the administration of curcumin to cadmium-treated (neurotoxic) rats, AchE activity and gene expression in the cerebral cortex were found to be significantly reduced, confirming the inhibitory effect of curcumin on AchE activity in the nervous system in vivo [211]. Curcumin can also inhibit AchE activity in the hippocampus and cortex of rats with dementia induced by intracerebroventricular (ICV) injection of STZ [212].
Figure 5.
Regulatory role of polyphenols in the context of cholinergic injury: polyphenols inhibit the binding of cholinesterase to receptors, reduce cholinesterase activity, reduce Ach hydrolysis, restore the cholinergic level in the brain, and alleviate AD symptoms. Ach, acetylcholine; AchE, acetyl cholinesterase; BchE, butyrylcholinesterase.
EGCG was also shown to have an inhibitory effect on AchE. A comparative evaluation of five natural flavane-3-alcohol (catechin) compounds isolated from green tea using the Ellman method: (-)—epicatechin (EC), catechin, (-)—epicatechin-3-gallate (ECG), (-)—epigallocatechin (EGC), and (-)—epigallocatechin-3-gallate (EGCG) revealed that only EGCG significantly inhibited AchE [213]. Oral administration of EGCG to rats with chronic brain dysfunction induced by an ICV injection of STZ reduced AchE activity in the hippocampus as well [119]. Other studies have further confirmed that the oral administration of EGCG (2 mg/kg/day, lasting for 30 days) increases the level of Ach in the cerebral cortex of aged rats [214]. Moreover, molecular docking simulations have also confirmed the interaction between green tea polyphenols through combined effects on AchE and BChE [215].
7. Polyphenols and ApoE in Alzheimer’s Disease
7.1. ApoE Gene in Alzheimer’s Disease
ApoE and its three isoforms, ApoE2, ApoE3, and ApoE4, play significant roles in plasma cholesterol metabolism, atherosclerosis, and hypercholesterolemia [216,217,218,219,220]. ApoE was also shown to be the main apolipoprotein lipid and cholesterol transporter in the central nervous system and is produced by nearly all nerve cell types, including astrocytes in the physiological state, microglia in the pathological state, and neurons following specific injuries [221,222,223,224]. AD is also considered by some a genetic disease caused by mutations in several genes, including ApoE. ApoE4—the ε4 allele of ApoE—has attracted attention as the strongest risk gene since it is most commonly found in patients with late-onset AD [224,225,226]. ApoE4 is associated with several pathological features of AD. For example, patients carrying this gene have more obvious speech memory and cognitive impairment, and Aβ plaque deposition and pathological tau protein phosphorylation occur earlier than in other AD patients [227,228]. ApoE4 not only affects Aβ-related mechanisms of AD [229,230,231,232,233] but also aggravates Tau-mediated neurodegeneration [234,235] and even promotes the expression of inflammatory cytokines. Hence, carriers of this gene develop a stronger systemic inflammatory response [236]. Although the exact mechanism is not yet clear, it can be argued that the ApoE gene hypothesis is closely related to other hypotheses of AD development.
7.2. Regulatory Role of Polyphenols in the Context of ApoE Gene
Our knowledge of polyphenols regulating ApoE gene function is mainly based on studies of atherosclerotic diseases. Only several studies have focused on the regulation of the ApoE gene by polyphenols in AD models [237]. Researchers have previously found that the secretion of inflammatory factors is reduced upon administration of the extract of Arabidopsis seedlings, which increases the yield of polyphenolsin mixed glial cell cultures extracted from the cerebral cortex of ApoE-targeted replacement (ApoE TR) mice or ApoE knockout (ApoE KO) mice. This suggests that polyphenols inhibit ApoE-derived neuroinflammation [238]. Other studies have confirmed that single polar phenolic compounds can improve the changes in cell redox state and cytotoxicity after induction and that kaempferol can regulate the pathogenic conformation of the ApoE4 form by providing ApoE-induced SK-N-SH cell blackcurrant extract (whose main components are resveratrol, quercetin, kaempferol, and epigallocatechin gallate) [239].
8. Polyphenols and Other Mechanisms in Alzheimer’s Disease Development
8.1. Polyphenols and Insulin Resistance
Most patients with AD develop many other complications as well, such as obesity, stroke, and type 2 diabetes mellitus (T2DM). These complications may coordinate or aggravate the development of AD [240]. A meta-analysis revealed that diabetic patients are more likely to convert from mild cognitive impairment (MCI) to AD compared to non-diabetic AD patients, which indicates that T2DM increases the vulnerability of patients to neurodegenerative diseases, including AD [241]. T2DM has been proven to be associated with AD through insulin resistance [242]. Insulin resistance refers to the decline in the efficiency of insulin in promoting glucose uptake and utilization and the compensatory secretion of excessive insulin by the body to produce hyperinsulinemia, which is the main cause and pathological phenomenon of T2DM [243]. Polyphenols were reported to effectively inhibit insulin resistance. For example, insulin resistance in APP/PS1 transgenic mice was found to decrease via upregulation of the PI3K/Akt signaling pathway upon curcumin treatment [244]. Resveratrol and EGCG were also found to enhance the activity of insulin-degrading enzyme (IDE) to decompose extracellular Aβ in neurons, and promote its clearance in hyperinsulinemia caused by insulin resistance [245,246].
8.2. Polyphenols and Mitochondrial Dysfunction
Mitochondrial dysfunction is one of the most important mechanisms in the early stages of AD, which is mainly caused by oxidative phosphorylation induced by insufficient glucose metabolism. Ultimately, dysfunctional mitochondria produce less ATP and more ROS. This, in turn, leads to pathological changes in AD, such as Aβ deposition, formation of NFTs, and synaptic degeneration [247]. The recombinant sirtuin (SIRT) family plays an important role in regulating mitochondrial proteins to maintain homeostasis during oxidative stress and apoptosis. Recombinant sirtuin 3 (SIRT3) has been proposed as a new target for AD treatment [248]. Previous studies have revealed that in the AD model presenilin (psev1-v97l) transgenic mice honokiol upregulates the expression of mitochondrial SIRT3, increases the amount of produced ATP, decreases the amount of produced ROS, improves the cognitive ability of mice, and thereby alleviates AD symptoms [249]. Other researchers found that urolithin A (UA), a type of ellagitannin found in pomegranate and walnuts, reduces mitochondrial membrane potential (MMP) and adenosine triphosphate (ATP) levels in a cellular model of early AD and increases the expression of genes involved in mitochondrial biogenesis and mitochondrial respiration (oxidative phosphorylation, OXPHOS). Urolithin A may thus also show therapeutic effects on AD [250].
8.3. Polyphenols and Faulty Autolysosome Acidification
The pathogenesis of AD has traditionally been accepted to originate from the formation of Aβ amyloid plaques. Here, amyloid plaques are first formed and then cause a series of toxic reactions to kill nerve cells. However, according to this concept, clearing plaques cannot remedy dead nerve cells according to this mechanism. This raises the question of whether Aβ plaques are the cause or effect. A recent study where a variety of AD mouse models were used disproved this traditional hypothesis and instead proposed that the occurrence of AD begins with the death of nerve cells caused by faulty autolysosome acidification of nerve cells, followed by the formation of extracellular amyloid plaques [251]. The hydrolases in lysosomes are only active in acidic environments, which are mainly maintained by the proton pump V-ATPase that pumps H+ ions from the cytoplasm into the lysosomes. Once lysosomal acidification is blocked, the pipeline of substrate removal stops, resulting in the formation and accumulation of many waste intermediates in lysosomes, including Aβ and APP. When the lysosome cannot bear rupture, the hydrolase is released into the cytoplasm to decompose the digestive cells and disintegrate them, eventually forming “extracellular” amyloid plaques [251,252]. Therefore, the main idea of treatment based on this mechanism is to restore the acidification function of the lysosomes. Previous experiments have shown that resveratrol activates adenosine 5‘-monophosphate (AMP)-activated protein kinase (AMPK) through pro-autophagic mechanisms and directly interacts with SIRT1 in vitro to induce autophagy by controlling SIRT1-mediated transcriptional regulation or mTOR-dependent signaling pathways. Resveratrol thus shows the potential to treat AD through these mechanisms [253,254,255,256]. Other studies have also revealed that EGCG resists incomplete autophagy induced by the hepatitis B virus (HBV) and inhibits its replication by enhancing lysosomal acidification [257]. Apple polyphenol extract (APE) has also been proven to restore lysosomal acidification by activating the SIRT1/AMPK signaling pathway, which may indicate another strategy for AD treatment [258].
8.4. Polyphenols and Disruption of the Intestinal Flora
The two-way communication system along the flora-gut-brain axis includes neural, immune, endocrine, and metabolic pathways, all of which have a profound impact on brain function and cognitive ability [259]. The relative abundance of bacterial species in patients with cognitive impairment was reported to change in previous studies. This change is manifested by an increase in the abundance of pro-inflammatory Escherichia coli/Shigella species and a decrease in the abundance of anti-inflammatory Escherichia coli [260]. On the other hand, the proportion of beneficial bacteria that synthesizes short-chain fatty acids (SCFA: acetate, propionate, butyrate) was found to decrease in the fecal flora of AD patients, whereas the abundance of pro-inflammatory bacteria was found to increase [261]. This suggests that the loss of intestinal homeostasis and inflammation caused by disruption of the intestinal flora may lead to the development of AD. Previous studies have revealed the effect of polyphenols on the richness, diversity, and composition of intestinal microbiota [262]. Curcumin was also found to improve the memory and cognitive abilities of APP/PS1 transgenic mice, increase intestinal bacterial diversity and reduce the abundance of Escherichia coli/Shigella [263]. In addition, a resveratrol-selenium-peptide nanocomposite was also found to improve cognitive impairment in AD model mice by reducing intestinal bacterial flora disruption due to oxidative stress and inflammation, such as Alistipes, Helicobacter pylori, Rikenella, Desulfovibrio, and Faecalibaculum [264]. The idea of treating AD by regulating intestinal flora has received increasing attention in the clinic, yet more research is still needed to confirm the proposed mechanisms.
9. Potential Regulation Effect of Polyphenols on Recently Identified Targets of AD
Mammalian STE20-like protein kinase (MST) is an important component of the Hippo pathway, in which MST1/2 regulates neuronal death and neuroinflammation [265,266]. A recent study revealed that Mst1 activity is increased with the accumulation of Aβ in the hippocampus of 5xfad mice. The overexpression of MST1/2 was also found to induce an AD-like phenotype in normal mice yet without a significant effect on Aβ levels. This was found to accelerate the decline in cognitive abilities of 2-month-old mice and caused damage to synaptic plasticity. It was also found to promote neuronal apoptosis by activating and phosphorylating p53. Knockout or chemical inactivation of Mst1 was found to be effective in alleviating AD symptoms [267]. Mst1 is therefore expected to become a potential target for the treatment of AD. Although there are currently only a few reports on the treatment of AD with polyphenols with this target, studies have already shown that tea polyphenols reverse the upregulation of MST1/2 in RAW264.7 cells after H2O2 application, inhibit oxidative stress through the MST/Nrf2 axis, and Keap1/Nrf2/HO-1 signaling pathways, and reduce the production of ROS, the release of NO, and the level of MDA in cells [268]. These findings highlight the therapeutic potential of polyphenols in alleviating AD symptoms by acting on this target.
Receptor-interacting protein kinase 1 (RIPK1), as a kind of serine/threonine protein kinase, a member of the RIP family, has been widely mentioned in research related to necrosis [269,270,271]. It was found that RIPK1 was highly expressed in AD patients, AD model mice, and microglia of the AD model [272]. In addition, RIPK1 inhibitors can reduce the levels of Aβ and p-tau in the cortex and hippocampus of APP/PS1 mice [273]. These data indicate that RIPK1 is expected to become a potential target for the treatment of AD. Although there are currently only a few reports aimed at this target to treat AD with polyphenols, some studies have shown that curcumin can reduce the levels of oxidative stress biomarkers and inflammatory cytokines and reduce the necrosis and apoptosis of chicken liver tissue by regulating TLR4/RIPK signal pathway [274]. In addition, some studies have also confirmed that resveratrol can inhibit myocardial cell necrosis and apoptosis by inhibiting TNF-a/receptor-interacting protein kinase 1 (RIP1)/RIP3/mixed-lineage kinase domain-like (MLKL) signaling pathway [275]. This suggests that the possible therapeutic ability of polyphenols on AD can be studied from this target in the future.
10. The Potential and Mechanisms of Action of Other Common Plant Polyphenols for AD Treatment
Polyphenols are widely found in various common plant species and show unlimited potential for screening of active ingredients for the prevention and treatment of AD. As mentioned above, polyphenols can alleviate AD symptoms through several mechanisms, including antioxidant stress, reduction of neuroinflammation, inhibition of Aβ production and aggregation, and inhibition of AchE activity. Curcumin, resveratrol, proanthocyanidins, and EGCG are extensively studied plant polyphenols in terms of their potential for the prevention and treatment of AD. However, the use of other plant polyphenols for AD treatment has also gradually attracted attention (Table 2).
Table 2.
Prevention and treatment potential of polyphenols on AD and its mechanism.
| Classification | Compounds | Main Sources | AD Model | Dose and Time | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Flavone | Apigenin | Apium graveolens, Petroselinum crispum, Citrus sinensis, Vitis vinifera, Allium sativum, Marchantia polymorpha | APP/PS1 mice | 40 mg/kg, 12 weeks | Antioxidant stress and inhibition of Aβ production | [276] |
| Aβ1–42 or LPS-induced coculture of cortical neurons and glial cells in neonatal Wistar rats | 1 μM, 24 h | Anti-inflammatory | [277] | |||
| Baicalin | Scutellaria baicalensis Georgi | APP/PS1 mice; Aβ1–42-induced BV2 and SH-SY5Y cells | 100 mg/kg, 33 days; 0, 10, 20, 40 µM, 24 h | Inhibiting NLRP3 inflammatory corpuscle activation by TLR4/NF-κB pathway | [278] | |
| Kaempferol | Allium tuberosum, Allium cepa, Vigna radiata, Cucurbita moschata, Solanum tuberosum, Solanum lycopersicum, Fragaria ananassa, Forsythia suspensa, Rosmarinus officinalis, Acacia farnesiana, Ginkgo, Mimosa pudica, Cinnamomum tamala | Female ovariectomized (OVX) Wistar rats induced by ICV of STZ | 10 mg/kg, 21 days | Antioxidative stress, anti-neuroinflammation | [279] | |
| Lipoprotein particles containing ApoE4-induced SK-N-SH cells | 20 μM, 24 h | Changing the conformation and function of ApoE4 | [239] | |||
| Luteolin | Arachis hypogaea, Brassica napus, Olea europaea, Fagopyrum esculentum, Theobroma, Capsicum annuum, Apium graveolens, Daucus carota, Cucumis sativus, Lactuca sativus, Punica granatum | 3 × Tg-AD mice | 20, 40 mg/kg, 3 weeks | inhibiting endoplasmic reticulum stress-dependent neuroinflammation | [280] | |
| Flavanol | Epicatechin | Tea-leaves, cocoa, grape seeds, Fagopyrum dibotrys, Amygdalus persica, Malus pumila | Methamphetamine (METH) induced HT22 cells | 10, 20 μM, 1 h | Antioxidant | [281] |
| Myricetin | Fragaria ananassa, Malus pumila, Spinacia oleracea, Aloe vera, Daucus carota, Fructus Mori, red wine | Primary rat cortical neurons induced by Aβ1–42 | 10μM, 48 h | Inhibiting the activity of BACE-1; inhibiting Aβ | [282] | |
| Quercetin | Citrus reticulata, Momordica charantia, Malus pumila, Allium cepa, Vitis vinifera, Vaccinium spp, Rubus idaeus | Aβ25–35-induced PC-12 cells | 10, 20, 40, 80 μM, 48 h | Antioxidant | [283] | |
| Okadaic acid (OA)-induced HT22 cells | 5, 10 μM, 12 h | Antioxidant, inhibiting tau hyperphosphorylation | [284] | |||
| APP/PS1 mice | 2 mg/kg, 7–8 months | inhibition of Aβ production | [285] | |||
| Phenolic acid | Caffeic acid | Solanum lycopersicum, Daucus carota, Fragaria ananassa, Semen Trigonellae, wheat wine, tea, coffee, apple juice | Mice induced by ICV of Aβ25–35 | 10, 50 mg/kg, 14 days | Antioxidant | [286] |
| Gallic acid | Vitis vinifera, Lilium brownii viridulum, Punica granatum, Rosa rugosa, Toxicodendron vernicifluum, Quercus palustris, Hamamelis mollis, Rhus chinensis, Terminalia chebula | APP/PS1 mice | 20 mg/kg, 6 months | Antioxidant stress, anti-inflammatory, affecting α-, β-, γ-secretase activity | [287] | |
| AlCl3-induced Wistar rats | 100 mg/kg, 60 days | Antioxidant | [288] | |||
| APP/PS1 mice | 30 mg/kg, 30 days | Inhibiting Aβ1–42 aggregation | [289] | |||
| Aβ1–42-induced primary microglia and BV2 cells | 5–50 μM, 24 h | Inhibiting NF-κB acetylation, antioxidant | [290] | |||
| Phenylpropanoid | Chlorogenic acid | coffee, Lycium chinense, Lonicera japonica, Eucommia ulmoides, Arctium lappa, Chrysanthemum indicum, Malus pumila, Prunus pseudocerasus, Camellia sinensis | APP/PS1 mice; Aβ25–35-induced SH-SY5Y cells | 40 mg/kg, 6 months; 3.125, 6.25, 12.5, 25, 50 µM, 24, 48 h | Inhibiting excessive autophagy and Aβ production by regulating mTOR/TFEB signaling pathway | [291] |
| Aβ25–35-induced primary rat hippocampal neurons | 12.5, 25, 50 μM, 2 h | Antioxidant | [292] | |||
| Ferulic acid | Triticum sativum, beer, coffee, berries, Avena sativa, Ananas comosus, Arachis hypogaea | APP/PS1 mice | 20 mg/kg, 30 days | Improving hippocampal capillary perfusion insufficiency | [293] | |
| ICR mice induced by ICV of Aβ1–42 | 14–19 mg/kg, 30 days | Antioxidant | [294] | |||
| Flavanonol | Dihydromyricetin | Vitis vinifera, Myrica rubra, Ampelopsis grossedentata, Ginkgo | SD rats induced by ICV of Aβ1–42 | 100, 200 mg/kg, 21 days | Anti-inflammatory by activating AMPK/SIRT1 signaling pathway | [295] |
| LPS-induced BV2 and primary microglias | 10, 30, 50 μM, 1 h | Anti-inflammatory by NF-κB signaling pathway | [296] | |||
| Isoflavone | Genistein | Glycine max | Wistar rats induced by bilateral ICV of Aβ1–42 | 10 mg/kg, 10 days | Attenuating synaptic toxicity, inhibiting tau hyperphosphorylation, inactivating ERK | [297] |
| Wistar rats induced by ICV of STZ | 150 mg/kg, 30, 90 days | Anti-inflammatory; Stimulating autophagy of Aβ40, 42 and p-tau | [298] | |||
| Aβ25–35-induced SH-SY5Y cells | 1, 10 nM, 24 h | Inhibiting Aβ induced apoptosis and Akt inactivation, inhibiting tau hyperphosphorylation | [299] | |||
| Flavonone | Hesperidin | Citrus reticulata Blanco | C57BL/6N induced by ICV of Aβ1–42; Aβ1–42-induced BV2 and HT22 cells | 50 mg/kg, 6 weeks; 50 μM, 24 h | Inhibiting oxidative stress by regulating Nrf2/HO-1; Inhibiting neuroinflammation by regulating TLR4/NF-κB; Inhibiting the expression of APP, BACE-1, Aβ | [300] |
| Ellagitannin | Punicalagin | Punica granatum | LPS-induced ICR; Primary rat cortical astrocytes and BV2 | 1.5 mg/kg, 4 weeks; 10, 20, 50 μM, 24 h | Anti-inflammatory | [301] |
| Isothiocyanate | Sulforaphane | Brassica oleracea, Nasturtium officinale, Brassica oleracea var. acephala DC, Brassica oleracea var. capitata, Brussels sprouts, Brassica rapa var. glabra Regel, Brassica juncea, Brassica oleracea var. botrytis Linnaeus | 5 × FAD mice | 5, 10 mg/kg, 2 months | Inhibiting Aβ, inhibiting phosphorylation tau | [302] |
| Benzotropolones | Theaflavin | Camellia sinensis | ICR mice induced by ICV of LPS; LPS, IFN-γ induced primary microglias | 10, 50 mg/kg, 3 days; 10, 30 μM, 12 h | Anti-inflammatory | [303] |
Previous studies have emphasized that in aging-related neurodegenerative diseases, including AD, plant polyphenols do not just affect a specific mechanism of the pathogenesis of these diseases, such as inhibition of Aβ production and aggregation, anti-inflammatory response, and antioxidant stress. The therapeutic effect on AD may also alleviate disease symptoms by regulating the links between multiple targets and mechanisms [304]. Some polyphenols were found to produce rapid benefits after a single administration, especially in terms of attention function and working memory, yet these polyphenols need to be consumed over a long period of time if adequate observations are to be made. Researchers also prefer to administer polyphenols in the early stage of the disease, based on the expectation that dietary intake of polyphenols is more suitable for the early stage of AD [43,305]. In this stage, polyphenols mainly play a therapeutic role in antioxidant stress, anti-inflammatory activity, inhibition of Aβ production and aggregation, and inhibition of AchE activity.
11. Limitations in the Application and Improvements in Preparation of Polyphenols
An increasing number of studies have revealed that a diet rich in polyphenols can prevent the onset of AD. Therefore, interest in easily obtained plant polyphenols with low toxicity has increased recently. Hence, the therapeutic potential of these compounds against AD has also been studied by more scientists over time. However, polyphenols also suffer from limitations that hinder their clinical use, such as low bioavailability and their unclear role in the BBB [306,307].
Although curcumin shows a certain degree of neuroprotective effect due to its strong hydrophobicity and low solubility, it has poor absorption and stability at physiological pH and suffers from rapid systemic elimination, poor bioavailability, and low brain bioavailability due to the BBB. The retention time of curcumin in the brain is very short [307,308,309]. To overcome this problem, different curcumin carriers, such as liposome curcumin, curcumin nanoparticles, and curcumin phospholipid complexes, have been developed previously [310]. Curcumin-loaded solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) were found to improve the stability of curcumin and increase its permeability through the BBB by a factor of 1.5 [311]. The melting crystallization method was also found to effectively improve the solubility of curcumin and wrap it into the cavity of lipid poly (lactic-co-glycolic acid) (PLGA) nanobubbles. The resulting curcumin-loaded lipid PLGA nanobubbles (Cur NBS) can pass through the BBB and deliver the drug to the brain core, which was found to significantly alleviate the symptoms in PD mice [312].
Similarly, the clinical use of resveratrol is also limited owing to its low bioavailability. Extensive metabolism in the gut and liver leads to an oral bioavailability of less than 1%, and dose escalation and repeated administration were found to be ineffective [313]. Since the success of the treatment of nervous system diseases largely depends on the bioavailability of drugs, novel strategies are needed for the use of resveratrol in a clinical context [314]. Currently, resveratrol delivery nanocarriers developed for the treatment of AD mainly include lipid core nano capsules (NC), polymer micelles, and SLN [315]. A comparison of resveratrol-loaded NC and free resveratrol in rats exposed to Aβ showed that resveratrol-loaded NC can play a neuroprotective role by increasing the concentration of resveratrol in brain tissue and reducing the harmful effects caused by Aβ, such as memory loss, learning difficulties, synaptophysin level reduction, and astrocyte and microglial activation [316]. Polymer micelles loaded with resveratrol can reduce the cytotoxicity of free resveratrol, protect PC12 cells from Aβ through antioxidant stress, and reduce the activity of Caspase-3 [317].
Although proanthocyanidins can promote health, only monomers and smaller oligomeric forms of proanthocyanidins can be absorbed. Proanthocyanidins with a degree of polymerization of more than four cannot be absorbed due to their large molecular sizes and the presence of the intestinal barrier [318]. High clearance and rapid metabolism of proanthocyanidins after oral administration were found to result in a significantly lower bioavailability (8–11%), as estimated from the blood area under the concentration–time curve (AUC) (0–24) values [319]. The absorption and metabolism of procyanidin B2 following oral administration in mice were also studied, and a total of 45 metabolites of procyanidin B2 were identified. The small intestine included the highest proportion of metabolites compared to other tissues. The liver was also found as the main binding reaction organ for procyanidin B2. Furthermore, procyanidins are among the few polyphenols that can penetrate the blood-brain barrier after oral absorption. The metabolites of procyanidin B2 in the mouse brain include hydration, sulfonation, and dihydrate products [320]. The optimization of the dosage form of procyanidins mostly involves the production of microcapsules, nano-emulsions, liposomes, and clathrate compounds in order to improve stability and bioavailability simultaneously [321].
Polyphenols have considerable application potential in the field of natural products as drugs and health products owing to their antioxidant, antibacterial, anti-inflammatory, and other favorable biological activities [322]. However, most polyphenols are insoluble and unstable, and their low absorption and bioavailability limit their clinical and industrial application [323]. With the development of improved nanomaterials for biomedical applications, polyphenol-loaded drug delivery systems that can improve the in vitro stability and bioavailability of polyphenols have been favored by researchers [324]. For example, polymer nanoparticles, lipid-based nanocarriers, gold–silver nanoparticles, silica nanoparticles of resveratrol, lipid nanoparticles, protein nanoparticles, and PLGA nanoparticles of EGCG have been developed in recent years [325].
12. Conclusions
In conclusion, recent scientific evidence shows that the development of AD is accompanied by pathological changes such as oxidative stress, Aβ deposition, abnormal phosphorylation of tau protein, neuroinflammation, and abnormal acetylcholine activity, in addition to changes in various other physiological mechanisms. Due to the complex molecular mechanisms related to AD, prevention and treatment can not only target a single protein or gene but also need to consider new strategies for multiple proteins and genes. Polyphenols, as secondary metabolites widely found in natural plants, especially curcumin, resveratrol, proanthocyanidins, EGCG, and other plant polyphenols, can alleviate the symptoms of AD patients through antioxidative, anti-inflammatory, Aβ inhibitory, and other neuroprotective effects when used as dietary supplements (Figure 6).
Figure 6.
Mechanisms of action of curcumin, resveratrol, proanthocyanidins, and EGCG in AD treatment: Curcumin, resveratrol, proanthocyanidins, and EGCG alleviate the pathological symptoms of AD mainly through antioxidant stress, anti-neuroinflammation, inhibition of Aβ production and inhibition of AchE.
Future research on polyphenols should focus on clinical applications, and especially the connection between in vitro and animal experiments. If the bioavailabilities of several powerful polyphenols need to be further improved, the preparation of nanoparticles, liposomes, and other new dosage forms that do not affect function while maintaining high bioavailability should be prioritized in clinical research. Moreover, it should also be recognized that in vitro AD experiments cannot completely simulate the long course of a disease that lasts for several years. Therefore, more long-term experiments and large-scale epidemiological and clinical studies are needed to determine whether polyphenols have sufficient beneficial effects on the slow development of AD. The combined use of several widely studied polyphenols is likely to have a greater effect. In addition, it is also necessary to evaluate the risks and safety of the use of polyphenols and investigate the possible adverse reactions to medication in clinical research. In general, the development of plant polyphenols with low toxicity and high availability as drugs has good potential to prevent and alleviate AD symptoms.
Acknowledgments
Figures were created with BioRender software, BioRender.com.
Author Contributions
Conceptualization, Y.W. and X.W.; validation, K.W., J.Y. and Q.Z.; writing—original draft preparation, Y.W.; writing—review and editing, K.W.; visualization, Q.Z.; supervision, J.Y.; funding acquisition, Y.W. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Postgraduate Research Innovation Project of Tianjin University of Traditional Chinese Medicine, grant YJSKC-20212004 (Y.W.) and the National Natural Science Foundation of China, grant 82204775 (Q.Z.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hodson R. Alzheimer’s disease. Nature. 2018;559:S1. doi: 10.1038/d41586-018-05717-6. [DOI] [PubMed] [Google Scholar]
- 2.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feulner T.M., Laws S.M., Friedrich P., Wagenpfeil S., Wurst S.H., Riehle C., Kuhn K.A., Krawczak M., Schreiber S., Nikolaus S., et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol. Psychiatry. 2010;15:756–766. doi: 10.1038/mp.2008.141. [DOI] [PubMed] [Google Scholar]
- 5.Tönnies E., Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017;57:1105–1121. doi: 10.3233/JAD-161088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannsen P. Long-term cholinesterase inhibitor treatment of Alzheimer’s disease. CNS Drugs. 2004;18:757–768. doi: 10.2165/00023210-200418120-00001. [DOI] [PubMed] [Google Scholar]
- 7.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020;15:40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calsolaro V., Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016;12:719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Serrano-Pozo A., Das S., Hyman B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20:68–80. doi: 10.1016/S1474-4422(20)30412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings J., Aisen P., Lemere C., Atri A., Sabbagh M., Salloway S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res. Ther. 2021;13:98. doi: 10.1186/s13195-021-00838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igartúa D.E., Martinez C.S., Del V.A.S., Prieto M.J. Combined Therapy for Alzheimer’s Disease: Tacrine and PAMAM Dendrimers Co-Administration Reduces the Side Effects of the Drug without Modifying its Activity. AAPS PharmSciTech. 2020;21:110. doi: 10.1208/s12249-020-01652-w. [DOI] [PubMed] [Google Scholar]
- 12.Arrieta J.L., Artalejo F.R. Methodology, results and quality of clinical trials of tacrine in the treatment of Alzheimer’s disease: A systematic review of the literature. Age Ageing. 1998;27:161–179. doi: 10.1093/ageing/27.2.161. [DOI] [PubMed] [Google Scholar]
- 13.Moss D.E., Perez R.G., Kobayashi H. Cholinesterase Inhibitor Therapy in Alzheimer’s Disease: The Limits and Tolerability of Irreversible CNS-Selective Acetylcholinesterase Inhibition in Primates. J. Alzheimers Dis. 2017;55:1285–1294. doi: 10.3233/JAD-160733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atri A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019;39:227–240. doi: 10.1055/s-0039-1678581. [DOI] [PubMed] [Google Scholar]
- 15.Imbimbo B.P. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs. 2001;15:375–390. doi: 10.2165/00023210-200115050-00004. [DOI] [PubMed] [Google Scholar]
- 16.Dhikav V., Anand K.S. Acute dystonic reaction with rivastigmine. Int. Psychogeriatr. 2013;25:1385–1386. doi: 10.1017/S104161021300029X. [DOI] [PubMed] [Google Scholar]
- 17.Hansen R.A., Gartlehner G., Webb A.P., Morgan L.C., Moore C.G., Jonas D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging. 2008;3:211–225. [PMC free article] [PubMed] [Google Scholar]
- 18.Pariente A., Sanctussy D.J., Miremont-Salamé G., Moore N., Haramburu F., Fourrier-Réglat A. Factors associated with serious adverse reactions to cholinesterase inhibitors: A study of spontaneous reporting. CNS Drugs. 2010;24:55–63. doi: 10.2165/11530300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-García M.P., Sánchez-Motillas J.M., Mateu-Puchades A., Díaz-Corpas T. Acute generalized exanthematous pustulosis induced by galantamine. Actas Dermosifiliogr. 2013;104:930–931. doi: 10.1016/j.ad.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Reisberg B., Doody R., Stöffler A., Schmitt F., Ferris S., Möbius H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 21.Howes L.G. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf. 2014;37:391–395. doi: 10.1007/s40264-014-0161-z. [DOI] [PubMed] [Google Scholar]
- 22.Salloway S., Chalkias S., Barkhof F., Burkett P., Barakos J., Purcell D., Suhy J., Forrestal F., Tian Y., Umans K., et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022;79:13–21. doi: 10.1001/jamaneurol.2021.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klose J., Griehl C., Roßner S., Schilling S. Natural Products from Plants and Algae for Treatment of Alzheimer’s Disease: A Review. Biomolecules. 2022;12:694. doi: 10.3390/biom12050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LI L., Wang X., Peng Y. Pharmacological research of natural products in the treatment of Alzheimer’s disease. Chin. Pharmacol. Bull. 2016;12:149–154, 155. [Google Scholar]
- 25.Sun Z., Ma P., Chen H., Zhu X., Fu Z. Research progress of anti-Alzheimer’s Disease drugs. Mod. Salt Chem. Ind. 2019;46:45–46. doi: 10.19465/j.cnki.2095-9710.2019.02.019. (In Chinese) [DOI] [Google Scholar]
- 26.Hu N., Yu J.T., Tan L., Wang Y.L., Sun L., Tan L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013;2013:524820. doi: 10.1155/2013/524820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jayatunga D.P.W., Hone E., Fernando W., Garg M.L., Verdile G., Martins R.N. Mitoprotective Effects of a Synergistic Nutraceutical Combination: Basis for a Prevention Strategy Against Alzheimer’s Disease. Front. Aging Neurosci. 2021;13:781468. doi: 10.3389/fnagi.2021.781468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukhari S.N.A. Dietary Polyphenols as Therapeutic Intervention for Alzheimer’s Disease: A Mechanistic Insight. Antioxidants. 2022;11:554. doi: 10.3390/antiox11030554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kępka A., Ochocińska A., Borzym-Kluczyk M., Chojnowska S., Skorupa E., Przychodzeń M., Waszkiewicz N. Healthy Food Pyramid as Well as Physical and Mental Activity in the Prevention of Alzheimer’s Disease. Nutrients. 2022;14:1534. doi: 10.3390/nu14081534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pusceddu M.M., Hernandez-Baixauli J., Puiggrós F., Arola L., Caimari A., Del Bas J.M., Baselga L. Mediterranean natural extracts improved cognitive behavior in zebrafish and healthy rats and ameliorated lps-induced cognitive impairment in a sex dependent manner. Behav. Brain Funct. 2022;18:5. doi: 10.1186/s12993-022-00190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watrelot A.A., Bouska L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and grapes. Food Chem. 2022;386:132703. doi: 10.1016/j.foodchem.2022.132703. [DOI] [PubMed] [Google Scholar]
- 32.Najjar R.S., Mu S., Feresin R.G. Blueberry Polyphenols Increase Nitric Oxide and Attenuate Angiotensin II-Induced Oxidative Stress and Inflammatory Signaling in Human Aortic Endothelial Cells. Antioxidants. 2022;11:616. doi: 10.3390/antiox11040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin Y., Li D., Liu F., Wang X., Cui Y., Li S., Li X. The Ameliorating Effects of Apple Polyphenol Extract on High-Fat-Diet-Induced Hepatic Steatosis Are SIRT1-Dependent: Evidence from Hepatic-Specific SIRT1 Heterozygous Mutant C57BL/6 Mice. J. Agric. Food Chem. 2022;70:5579–5594. doi: 10.1021/acs.jafc.2c01461. [DOI] [PubMed] [Google Scholar]
- 34.Lu J., Zhao S.X., Zhang M.Y., Ji P.Y., Chao S., Li L.J., Yin S., Zhao L., Zhao H., Sun Q.Y., et al. Tea polyphenols alleviate the adverse effects of diabetes on oocyte quality. Food Funct. 2022;13:5396–5405. doi: 10.1039/D1FO03770F. [DOI] [PubMed] [Google Scholar]
- 35.Tamargo A., Cueva C., Silva M., Molinero N., Miralles B., Bartolomé B., Moreno-Arribas M.V. Gastrointestinal co-digestion of wine polyphenols with glucose/whey proteins affects their bioaccessibility and impact on colonic microbiota. Food Res. Int. 2022;155:111010. doi: 10.1016/j.foodres.2022.111010. [DOI] [PubMed] [Google Scholar]
- 36.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L., Guo M.S., Zhang Y., Yu L., Wu J.M., Tang Y., Ai W., Zhu F.D., Law B.Y., Chen Q., et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell. Longev. 2022;2022:5288698. doi: 10.1155/2022/5288698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meshginfar N., Tavakoli H., Dornan K., Hosseinian F. Phenolic lipids as unique bioactive compounds: A comprehensive review on their multifunctional activity toward the prevention of Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021;61:1394–1403. doi: 10.1080/10408398.2020.1759024. [DOI] [PubMed] [Google Scholar]
- 39.Pluta R., Furmaga-Jabłońska W., Januszewski S., Czuczwar S.J. Post-Ischemic Brain Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Possible Therapeutic Role of Curcumin. Nutrients. 2022;14:248. doi: 10.3390/nu14020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ontario M.L., Siracusa R., Modafferi S., Scuto M., Sciuto S., Greco V., Bertuccio M.P., Trovato Salinaro A., Crea R., Calabrese E.J., et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and hidrox. Mech. Ageing Dev. 2022;203:111637. doi: 10.1016/j.mad.2022.111637. [DOI] [PubMed] [Google Scholar]
- 41.Dos Santos M.G., Schimith L.E., André-Miral C., Muccillo-Baisch A.L., Arbo B.D., Hort M.A. Neuroprotective Effects of Resveratrol in In vivo and In vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022;40:319–345. doi: 10.1007/s12640-021-00450-x. [DOI] [PubMed] [Google Scholar]
- 42.Ding J., Huang J., Yin D., Liu T., Ren Z., Hu S., Ye Y., Le C., Zhao N., Zhou H., et al. Trilobatin Alleviates Cognitive Deficits and Pathologies in an Alzheimer’s Disease Mouse Model. Oxid. Med. Cell. Longev. 2021;2021:3298400. doi: 10.1155/2021/3298400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W., Cui K., Li X., Zhao J., Zeng Z., Song R., Qi X., Xu W. Effect of Polyphenols on Cognitive Function: Evidence from Population-Based Studies and Clinical Trials. J. Nutr. Health Aging. 2021;25:1190–1204. doi: 10.1007/s12603-021-1685-4. [DOI] [PubMed] [Google Scholar]
- 44.Molino S., Dossena M., Buonocore D., Ferrari F., Venturini L., Ricevuti G., Verri M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016;161:69–77. doi: 10.1016/j.lfs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 45.Lee K.H., Cha M., Lee B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020;21:7152. doi: 10.3390/ijms21197152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang T., Sun Q., Chen S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Battaglini M., Marino A., Carmignani A., Tapeinos C., Cauda V., Ancona A., Garino N., Vighetto V., La Rosa G., Sinibaldi E., et al. Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation. ACS Appl. Mater. Interfaces. 2020;12:35782–35798. doi: 10.1021/acsami.0c05497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abramov A.Y., Berezhnov A.V., Fedotova E.I., Zinchenko V.P., Dolgacheva L.P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochem. Soc. Trans. 2017;45:1025–1033. doi: 10.1042/BST20170024. [DOI] [PubMed] [Google Scholar]
- 49.Owen J.B., Sultana R., Aluise C.D., Erickson M.A., Price T.O., Bu G., Banks W.A., Butterfield D.A. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: Implications for Aβ accumulation in AD brain. Free Radic. Biol. Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Casado A., Encarnación López-Fernández M., Concepción Casado M., de La Torre R. Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem. Res. 2008;33:450–458. doi: 10.1007/s11064-007-9453-3. [DOI] [PubMed] [Google Scholar]
- 52.Shen K., Wang Y., Zhang Y., Zhou H., Song Y., Cao Z., Kou J., Yu B. Cocktail of Four Active Components Derived from Sheng Mai San Inhibits Hydrogen Peroxide-Induced PC12 Cell Apoptosis Linked with the Caspase-3/ROCK1/MLC Pathway. Rejuvenation Res. 2015;18:517–527. doi: 10.1089/rej.2015.1697. [DOI] [PubMed] [Google Scholar]
- 53.Chen C., Mei Q., Wang L., Feng X., Tao X., Qiu C., Zhu J. TIGAR suppresses seizures induced by kainic acid through inhibiting oxidative stress and neuronal apoptosis. Biochem. Biophys. Res. Commun. 2019;515:436–441. doi: 10.1016/j.bbrc.2019.05.156. [DOI] [PubMed] [Google Scholar]
- 54.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang L., Pu Z., Li M., Wang K., Deng L., Chen W. Antioxidative and antiapoptosis: Neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci. 2020;243:117237. doi: 10.1016/j.lfs.2019.117237. [DOI] [PubMed] [Google Scholar]
- 56.Busche M.A., Hyman B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020;23:1183–1193. doi: 10.1038/s41593-020-0687-6. [DOI] [PubMed] [Google Scholar]
- 57.Aminzadeh A. Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: Roles of JNK, P38 MAPKs, and mitochondria pathway. Metab. Brain Dis. 2017;32:819–826. doi: 10.1007/s11011-017-9976-5. [DOI] [PubMed] [Google Scholar]
- 58.Agrawal D.K., Mishra P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010;30:818–860. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 59.Trujillo J., Chirino Y.I., Molina-Jijón E., Andérica-Romero A.C., Tapia E., Pedraza-Chaverrí J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013;1:448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin H., Guo Q., Li X., Tang T., Li C., Wang H., Sun Y., Feng Q., Ma C., Gao C., et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 61.Zheng D., Huang C., Huang H., Zhao Y., Khan M.R.U., Zhao H., Huang L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020;17:e2000171. doi: 10.1002/cbdv.202000171. [DOI] [PubMed] [Google Scholar]
- 62.Manjunatha J.R., Bettadaiah B.K., Negi P.S., Srinivas P. Synthesis of amino acid conjugates of tetrahydrocurcumin and evaluation of their antibacterial and anti-mutagenic properties. Food Chem. 2013;139:332–338. doi: 10.1016/j.foodchem.2013.01.081. [DOI] [PubMed] [Google Scholar]
- 63.Tomeh M.A., Hadianamrei R., Zhao X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019;20:1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akaberi M., Sahebkar A., Emami S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021;1291:15–39. doi: 10.1007/978-3-030-56153-6_2. [DOI] [PubMed] [Google Scholar]
- 65.Shahcheraghi S.H., Salemi F., Peirovi N., Ayatollahi J., Alam W., Khan H., Saso L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules. 2021;27:167. doi: 10.3390/molecules27010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bao W., Li K., Rong S., Yao P., Hao L., Ying C., Zhang X., Nussler A., Liu L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010;128:549–553. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 67.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000;28:1303–1312. doi: 10.1016/S0891-5849(00)00294-X. [DOI] [PubMed] [Google Scholar]
- 68.Kanninen K., Malm T.M., Jyrkkänen H.K., Goldsteins G., Keksa-Goldsteine V., Tanila H., Yamamoto M., Ylä-Herttuala S., Levonen A.L., Koistinaho J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol. Cell. Neurosci. 2008;39:302–313. doi: 10.1016/j.mcn.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Xiang B., Li D., Chen Y., Li M., Zhang Y., Sun T., Tang S. Curcumin Ameliorates Copper-Induced Neurotoxicity Through Inhibiting Oxidative Stress and Mitochondrial Apoptosis in SH-SY5Y Cells. Neurochem. Res. 2021;46:367–378. doi: 10.1007/s11064-020-03173-1. [DOI] [PubMed] [Google Scholar]
- 70.Eun C.S., Lim J.S., Lee J., Lee S.P., Yang S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 2017;17:367. doi: 10.1186/s12906-017-1880-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yue Y.K., Mo B., Zhao J., Yu Y.J., Liu L., Yue C.L., Liu W. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J. Ocul. Pharmacol. Ther. 2014;30:657–664. doi: 10.1089/jop.2014.0022. [DOI] [PubMed] [Google Scholar]
- 72.da Silva Marques J.G., Antunes F.T.T., da Silva Brum L.F., Pedron C., de Oliveira I.B., de Barros Falcão Ferraz A., Martins M.I.M., Dallegrave E., de Souza A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021;399:113002. doi: 10.1016/j.bbr.2020.113002. [DOI] [PubMed] [Google Scholar]
- 73.Hasan W., Kori R.K., Jain J., Yadav R.S., Jat D. Neuroprotective effects of mitochondria-targeted curcumin against rotenone-induced oxidative damage in cerebellum of mice. J. Biochem. Mol. Toxicol. 2020;34:e22416. doi: 10.1002/jbt.22416. [DOI] [PubMed] [Google Scholar]
- 74.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 75.Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. Nippon Kagaku Kaishi. 1939;60:1090–1100. doi: 10.1246/nikkashi1921.60.1090. [DOI] [Google Scholar]
- 76.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 77.Cardile V., Chillemi R., Lombardo L., Sciuto S., Spatafora C., Tringali C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z. Naturforsch. C J. Biosci. 2007;62:189–195. doi: 10.1515/znc-2007-3-406. [DOI] [PubMed] [Google Scholar]
- 78.Meng T., Xiao D., Muhammed A., Deng J., Chen L., He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021;26:229. doi: 10.3390/molecules26010229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia N., Daiber A., Förstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Filardo S., Di Pietro M., Mastromarino P., Sessa R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020;214:107613. doi: 10.1016/j.pharmthera.2020.107613. [DOI] [PubMed] [Google Scholar]
- 81.Vestergaard M., Ingmer H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents. 2019;53:716–723. doi: 10.1016/j.ijantimicag.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 82.Rauf A., Imran M., Butt M.S., Nadeem M., Peters D.G., Mubarak M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018;58:1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 83.Tellone E., Galtieri A., Russo A., Giardina B., Ficarra S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015;2015:392169. doi: 10.1155/2015/392169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castellani R.J., Rolston R.K., Smith M.A. Alzheimer disease. Dis. Mon. 2010;56:484–546. doi: 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stivala L.A., Savio M., Carafoli F., Perucca P., Bianchi L., Maga G., Forti L., Pagnoni U.M., Albini A., Prosperi E., et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 86.Sovak M. Grape Extract, Resveratrol, and Its Analogs: A Review. J. Med. Food. 2001;4:93–105. doi: 10.1089/109662001300341752. [DOI] [PubMed] [Google Scholar]
- 87.Li H., Shen Y., Xiao H., Sun W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol. Res. Pract. 2021;225:153576. doi: 10.1016/j.prp.2021.153576. [DOI] [PubMed] [Google Scholar]
- 88.Cong L., Lei M.Y., Liu Z.Q., Liu Z.F., Ma Z., Liu K., Li J., Deng Y., Liu W., Xu B. Resveratrol attenuates manganese-induced oxidative stress and neuroinflammation through SIRT1 signaling in mice. Food Chem. Toxicol. 2021;153:112283. doi: 10.1016/j.fct.2021.112283. [DOI] [PubMed] [Google Scholar]
- 89.Silva J.M.R.D., Rigaud J., Cheynier V., Cheminat A., Moutounet M. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. doi: 10.1016/S0031-9422(00)95213-0. [DOI] [Google Scholar]
- 90.Spranger I., Sun B., Mateus A.M., Freitas V., Ricardo-da-Silva J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008;108:519–532. doi: 10.1016/j.foodchem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Gebhardt S., Prior R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004;134:613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 92.Bagchi D., Garg A., Krohn R.L., Bagchi M., Tran M.X., Stohs S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 93.Mendoza-Wilson A.M., Castro-Arredondo S.I., Balandrán-Quintana R.R. Computational study of the structure-free radical scavenging relationship of procyanidins. Food Chem. 2014;161:155–161. doi: 10.1016/j.foodchem.2014.03.111. [DOI] [PubMed] [Google Scholar]
- 94.Truong V.L., Bak M.J., Jun M., Kong A.N., Ho C.T., Jeong W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014;62:8668–8678. doi: 10.1021/jf5027247. [DOI] [PubMed] [Google Scholar]
- 95.Martinez-Micaelo N., González-Abuín N., Ardèvol A., Pinent M., Blay M.T. Procyanidins and inflammation: Molecular targets and health implications. Biofactors. 2012;38:257–265. doi: 10.1002/biof.1019. [DOI] [PubMed] [Google Scholar]
- 96.Gonzalez-Abuin N., Pinent M., Casanova-Marti A., Arola L., Blay M., Ardevol A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015;22:39–50. doi: 10.2174/0929867321666140916115519. [DOI] [PubMed] [Google Scholar]
- 97.Li X., Chen Y., Li S., Chen M., Xiao J., Xie B., Sun Z. Oligomer Procyanidins from Lotus Seedpod Regulate Lipid Homeostasis Partially by Modifying Fat Emulsification and Digestion. J. Agric. Food Chem. 2019;67:4524–4534. doi: 10.1021/acs.jafc.9b01469. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y., Liu C., Niu Y., Xia J., Fan L., Wu Y., Gao W. Procyanidins mediates antineoplastic effects against non-small cell lung cancer via the JAK2/STAT3 pathway. Transl. Cancer Res. 2021;10:2023–2035. doi: 10.21037/tcr-20-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen J., Chen Y., Zheng Y., Zhao J., Yu H., Zhu J. Relationship between Neuroprotective Effects and Structure of Procyanidins. Molecules. 2022;27:2308. doi: 10.3390/molecules27072308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Katz D.L., Doughty K., Ali A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011;15:2779–2811. doi: 10.1089/ars.2010.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu J., Rong S., Xie B., Sun Z., Zhang L., Wu H., Yao P., Zhang X., Zhang Y., Liu L. Rejuvenation of antioxidant and cholinergic systems contributes to the effect of procyanidins extracted from the lotus seedpod ameliorating memory impairment in cognitively impaired aged rats. Eur. Neuropsychopharmacol. 2009;19:851–860. doi: 10.1016/j.euroneuro.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Luo X., Chen M., Duan Y., Duan W., Zhang H., He Y., Yin C., Sun G., Sun X. Chemoprotective action of lotus seedpod procyanidins on oxidative stress in mice induced by extremely low-frequency electromagnetic field exposure. Biomed. Pharmacother. 2016;82:640–648. doi: 10.1016/j.biopha.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 103.Jeong H.R., Kim K.J., Lee S.G., Cho H.S., Cho Y.S., Kim D.O. Phenolic Profiles of Hardy Kiwifruits and Their Neuroprotective Effects on PC-12 and SH-SY5Y Cells against Oxidative Stress. J. Microbiol. Biotechnol. 2020;30:912–919. doi: 10.4014/jmb.2001.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gong X., Xu L., Fang X., Zhao X., Du Y., Wu H., Qian Y., Ma Z., Xia T., Gu X. Protective effects of grape seed procyanidin on isoflurane-induced cognitive impairment in mice. Pharm. Biol. 2020;58:200–207. doi: 10.1080/13880209.2020.1730913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panza F., Solfrizzi V., Barulli M.R., Bonfiglio C., Guerra V., Osella A., Seripa D., Sabbà C., Pilotto A., Logroscino G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging. 2015;19:313–328. doi: 10.1007/s12603-014-0563-8. [DOI] [PubMed] [Google Scholar]
- 106.Ran L.S., Liu W.H., Fang Y.Y., Xu S.B., Li J., Luo X., Pan D.J., Wang M.H., Wang W. Alcohol, coffee and tea intake and the risk of cognitive deficits: A dose-response meta-analysis. Epidemiol. Psychiatr. Sci. 2021;30:e13. doi: 10.1017/S2045796020001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ide K., Matsuoka N., Yamada H., Furushima D., Kawakami K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules. 2018;23:2357. doi: 10.3390/molecules23092357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen T., Yang Y., Zhu S., Lu Y., Zhu L., Wang Y., Wang X. Inhibition of Aβ aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorg. Chem. 2020;105:104382. doi: 10.1016/j.bioorg.2020.104382. [DOI] [PubMed] [Google Scholar]
- 109.Amirpour M., Mirshekar M.A., Sedaghat G., Montazerifar F., Shourestani S., Arabmoazzen S., Naghizadeh M. The effects of green tea on cognitive impairments in the rat model of Alzheimer’s disease: Protection against inflammatory and oxidative damage. Nutr. Neurosci. 2022;25:2659–2667. doi: 10.1080/1028415X.2021.2003946. [DOI] [PubMed] [Google Scholar]
- 110.Liu B., Yan W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019;272:663–669. doi: 10.1016/j.foodchem.2018.08.086. [DOI] [PubMed] [Google Scholar]
- 111.Wang M., Zhong H., Zhang X., Huang X., Wang J., Li Z., Chen M., Xiao Z. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021;11:11014. doi: 10.1038/s41598-021-90398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Z., Zhang X., Bi K., He Y., Yan W., Yang C.S., Zhang J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021;114:11–24. doi: 10.1016/j.tifs.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eng Q.Y., Thanikachalam P.V., Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018;210:296–310. doi: 10.1016/j.jep.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 114.Yang L., Zhang W., Chopra S., Kaur D., Wang H., Li M., Chen P., Zhang W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets. 2020;21:1099–1104. doi: 10.2174/1389450121666200504080112. [DOI] [PubMed] [Google Scholar]
- 115.Luo K.W., Xia J., Cheng B.H., Gao H.C., Fu L.W., Luo X.L. Tea polyphenol EGCG inhibited colorectal-cancer-cell proliferation and migration via downregulation of STAT3. Gastroenterol. Rep. 2021;9:59–70. doi: 10.1093/gastro/goaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pervin M., Unno K., Ohishi T., Tanabe H., Miyoshi N., Nakamura Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules. 2018;23:1297. doi: 10.3390/molecules23061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Singh N.A., Mandal A.K., Khan Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG) Nutr. J. 2016;15:60. doi: 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu Y., Zhang J.J., Xiong L., Zhang L., Sun D., Liu H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J. Nutr. Biochem. 2010;21:741–748. doi: 10.1016/j.jnutbio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 119.Biasibetti R., Tramontina A.C., Costa A.P., Dutra M.F., Quincozes-Santos A., Nardin P., Bernardi C.L., Wartchow K.M., Lunardi P.S., Gonçalves C.A. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013;236:186–193. doi: 10.1016/j.bbr.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 120.Reznichenko L., Amit T., Zheng H., Avramovich-Tirosh Y., Youdim M.B., Weinreb O., Mandel S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: Implications for iron chelation in Alzheimer’s disease. J. Neurochem. 2006;97:527–536. doi: 10.1111/j.1471-4159.2006.03770.x. [DOI] [PubMed] [Google Scholar]
- 121.Hensley K. Neuroinflammation in Alzheimer’s disease: Mechanisms, pathologic consequences, and potential for therapeutic manipulation. J. Alzheimers Dis. 2010;21:1–14. doi: 10.3233/JAD-2010-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McGeer P.L., Schulzer M., McGeer E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/WNL.47.2.425. [DOI] [PubMed] [Google Scholar]
- 123.McGeer P.L., McGeer E.G. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol. Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 124.Frischer J.M., Bramow S., Dal-Bianco A., Lucchinetti C.F., Rauschka H., Schmidbauer M., Laursen H., Sorensen P.S., Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thawkar B.S., Kaur G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019;326:62–74. doi: 10.1016/j.jneuroim.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 126.Guo H., Zhao Z., Zhang R., Chen P., Zhang X., Cheng F., Gou X. Monocytes in the Peripheral Clearance of Amyloid-β and Alzheimer’s Disease. J. Alzheimers Dis. 2019;68:1391–1400. doi: 10.3233/JAD-181177. [DOI] [PubMed] [Google Scholar]
- 127.Lawson L.J., Perry V.H., Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 128.Wang D., Liu F., Zhu L., Lin P., Han F., Wang X., Tan X., Lin L., Xiong Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflammation. 2020;17:257. doi: 10.1186/s12974-020-01921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fu A.K., Hung K.W., Yuen M.Y., Zhou X., Mak D.S., Chan I.C., Cheung T.H., Zhang B., Fu W.Y., Liew F.Y., et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA. 2016;113:E2705–E2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang Y., Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 131.Kwon H.S., Koh S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liddelow S.A., Marsh S.E., Stevens B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020;41:820–835. doi: 10.1016/j.it.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Jha M.K., Jo M., Kim J.H., Suk K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 134.Kaur D., Sharma V., Deshmukh R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27:663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 135.Liu C., Cui G., Zhu M., Kang X., Guo H. Neuroinflammation in Alzheimer’s disease: Chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014;7:8342–8355. [PMC free article] [PubMed] [Google Scholar]
- 136.Zuroff L., Daley D., Black K.L., Koronyo-Hamaoui M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017;74:2167–2201. doi: 10.1007/s00018-017-2463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saresella M., Marventano I., Calabrese E., Piancone F., Rainone V., Gatti A., Alberoni M., Nemni R., Clerici M. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J. Alzheimers Dis. 2014;38:403–413. doi: 10.3233/JAD-131160. [DOI] [PubMed] [Google Scholar]
- 138.Gu B.J., Huang X., Ou A., Rembach A., Fowler C., Avula P.K., Horton A., Doecke J.D., Villemagne V.L., Macaulay S.L., et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol. 2016;132:377–389. doi: 10.1007/s00401-016-1596-3. [DOI] [PubMed] [Google Scholar]
- 139.Fiala M., Lin J., Ringman J., Kermani-Arab V., Tsao G., Patel A., Lossinsky A.S., Graves M.C., Gustavson A., Sayre J., et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005;7:221–232; discussion 255–262. doi: 10.3233/JAD-2005-7304. [DOI] [PubMed] [Google Scholar]
- 140.Yuan J., Amin P., Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019;20:19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang J., Zheng Y., Luo Y., Du Y., Zhang X., Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 142.Seyedzadeh M.H., Safari Z., Zare A., Gholizadeh Navashenaq J., Razavi S.A., Kardar G.A., Khorramizadeh M.R. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int. Immunopharmacol. 2014;22:230–235. doi: 10.1016/j.intimp.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 143.Liu Z., Ran Y., Huang S., Wen S., Zhang W., Liu X., Ji Z., Geng X., Ji X., Du H., et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017;9:233. doi: 10.3389/fnagi.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ullah F., Liang H., Niedermayer G., Münch G., Gyengesi E. Evaluation of Phytosomal Curcumin as an Anti-inflammatory Agent for Chronic Glial Activation in the GFAP-IL6 Mouse Model. Front. Neurosci. 2020;14:170. doi: 10.3389/fnins.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L., Fiala M., Cashman J., Sayre J., Espinosa A., Mahanian M., Zaghi J., Badmaev V., Graves M.C., Bernard G., et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2006;10:1–7. doi: 10.3233/JAD-2006-10101. [DOI] [PubMed] [Google Scholar]
- 146.Mohammadi A., Blesso C.N., Barreto G.E., Banach M., Majeed M., Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019;66:1–16. doi: 10.1016/j.jnutbio.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 147.Yang X., Xu S., Qian Y., Xiao Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 148.Bellaver B., Souza D.G., Bobermin L.D., Souza D.O., Gonçalves C.A., Quincozes-Santos A. Resveratrol Protects Hippocampal Astrocytes Against LPS-Induced Neurotoxicity Through HO-1, p38 and ERK Pathways. Neurochem. Res. 2015;40:1600–1608. doi: 10.1007/s11064-015-1636-8. [DOI] [PubMed] [Google Scholar]
- 149.Zhao H., Wang Q., Cheng X., Li X., Li N., Liu T., Li J., Yang Q., Dong R., Zhang Y., et al. Inhibitive Effect of Resveratrol on the Inflammation in Cultured Astrocytes and Microglia Induced by Aβ(1-42) Neuroscience. 2018;379:390–404. doi: 10.1016/j.neuroscience.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 150.Lee E.O., Park H.J., Kang J.L., Kim H.S., Chong Y.H. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J. Neurochem. 2010;112:1477–1487. doi: 10.1111/j.1471-4159.2009.06564.x. [DOI] [PubMed] [Google Scholar]
- 151.Han S., Gao H., Chen S., Wang Q., Li X., Du L.J., Li J., Luo Y.Y., Li J.X., Zhao L.C., et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019;9:15087. doi: 10.1038/s41598-019-51614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tian Y., Yang C., Yao Q., Qian L., Liu J., Xie X., Ma W., Nie X., Lai B., Xiao L., et al. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019;10:1895. doi: 10.3389/fimmu.2019.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Terra X., Palozza P., Fernandez-Larrea J., Ardevol A., Blade C., Pujadas G., Salvado J., Arola L., Blay M.T. Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free Radic. Res. 2011;45:611–619. doi: 10.3109/10715762.2011.564165. [DOI] [PubMed] [Google Scholar]
- 154.Byun E.B., Sung N.Y., Byun E.H., Song D.S., Kim J.K., Park J.H., Song B.S., Park S.H., Lee J.W., Byun M.W., et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2013;15:450–456. doi: 10.1016/j.intimp.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 155.Tang Y., Xiong R., Wu A.G., Yu C.L., Zhao Y., Qiu W.Q., Wang X.L., Teng J.F., Liu J., Chen H.X., et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. Int. J. Mol. Sci. 2018;19:2109. doi: 10.3390/ijms19072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan C., Wang C., Zhang L., Song L., Chen Y., Liu B., Liu W.T., Hu L., Pan Y. Procyanidins attenuate neuropathic pain by suppressing matrix metalloproteinase-9/2. J. Neuroinflamm. 2018;15:187. doi: 10.1186/s12974-018-1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cai Y., Kong H., Pan Y.B., Jiang L., Pan X.X., Hu L., Qian Y.N., Jiang C.Y., Liu W.T. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J. Neuroinflamm. 2016;13:53. doi: 10.1186/s12974-016-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thal D.R., Walter J., Saido T.C., Fändrich M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015;129:167–182. doi: 10.1007/s00401-014-1375-y. [DOI] [PubMed] [Google Scholar]
- 159.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 160.Rajmohan R., Reddy P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017;57:975–999. doi: 10.3233/JAD-160612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Patterson C., Feightner J.W., Garcia A., Hsiung G.Y., MacKnight C., Sadovnick A.D. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ. 2008;178:548–556. doi: 10.1503/cmaj.070796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sadleir K.R., Kandalepas P.C., Buggia-Prévot V., Nicholson D.A., Thinakaran G., Vassar R. Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer’s disease. Acta Neuropathol. 2016;132:235–256. doi: 10.1007/s00401-016-1558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomas K.R., Bangen K.J., Weigand A.J., Edmonds E.C., Wong C.G., Cooper S., Delano-Wood L., Bondi M.W. Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology. 2020;94:e397–e406. doi: 10.1212/WNL.0000000000008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Carvajal F.J., Inestrosa N.C. Interactions of AChE with Aβ Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011;4:19. doi: 10.3389/fnmol.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swerdlow R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimers Dis. 2018;62:1403–1416. doi: 10.3233/JAD-170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bloom G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 167.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 168.Zheng Q., Kebede M.T., Kemeh M.M., Islam S., Lee B., Bleck S.D., Wurfl L.A., Lazo N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules. 2019;24:2316. doi: 10.3390/molecules24122316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.So M., Kimura Y., Yamaguchi K., Sugiki T., Fujiwara T., Aguirre C., Ikenaka K., Mochizuki H., Kawata Y., Goto Y. Polyphenol-solubility alters amyloid fibril formation of α-synuclein. Protein Sci. 2021;30:1701–1713. doi: 10.1002/pro.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Choi C.W., Choi Y.H., Cha M.R., Kim Y.S., Yon G.H., Hong K.S., Park W.K., Kim Y.H., Ryu S.Y. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2011;77:374–376. doi: 10.1055/s-0030-1250370. [DOI] [PubMed] [Google Scholar]
- 171.Choi Y.H., Yoo M.Y., Choi C.W., Cha M.R., Yon G.H., Kwon D.Y., Kim Y.S., Park W.K., Ryu S.Y. A new specific BACE-1 inhibitor from the stembark extract of Vitis vinifera. Planta Med. 2009;75:537–540. doi: 10.1055/s-0029-1185311. [DOI] [PubMed] [Google Scholar]
- 172.Choi B., Kim S., Jang B.G., Kim M.J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. J. Funct. Foods. 2016;23:124–134. doi: 10.1016/j.jff.2016.02.024. [DOI] [Google Scholar]
- 173.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 174.Miners J.S., Barua N., Kehoe P.G., Gill S., Love S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2011;70:944–959. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- 175.Melchor J.P., Pawlak R., Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J. Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Santos L.M., Rodrigues D., Alemi M., Silva S.C., Ribeiro C.A., Cardoso I. Resveratrol administration increases Transthyretin protein levels ameliorating AD features- importance of transthyretin tetrameric stability. Mol. Med. 2016;22:597–607. doi: 10.2119/molmed.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qiang W., Yau W.M., Lu J.X., Collinge J., Tycko R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature. 2017;541:217–221. doi: 10.1038/nature20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tanaka T., Betkekar V.V., Ohmori K., Suzuki K., Shigemori H. Evaluation of Amyloid Polypeptide Aggregation Inhibition and Disaggregation Activity of A-Type Procyanidins. Pharmaceuticals. 2021;14:1118. doi: 10.3390/ph14111118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Toda T., Sunagawa T., Kanda T., Tagashira M., Shirasawa T., Shimizu T. Apple Procyanidins Suppress Amyloid β-Protein Aggregation. Biochem. Res. Int. 2011;2011:784698. doi: 10.1155/2011/784698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ishii T., Mori T., Tanaka T., Mizuno D., Yamaji R., Kumazawa S., Nakayama T., Akagawa M. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008;45:1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 181.Sato M., Murakami K., Uno M., Nakagawa Y., Katayama S., Akagi K., Masuda Y., Takegoshi K., Irie K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013;288:23212–23224. doi: 10.1074/jbc.M113.464222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bittner S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids. 2006;30:205–224. doi: 10.1007/s00726-005-0298-2. [DOI] [PubMed] [Google Scholar]
- 183.Shinde M.N., Barooah N., Bhasikuttan A.C., Mohanty J. Inhibition and disintegration of insulin amyloid fibrils: A facile supramolecular strategy with p-sulfonatocalixarenes. Chem. Commun. 2016;52:2992–2995. doi: 10.1039/C5CC10159J. [DOI] [PubMed] [Google Scholar]
- 184.Xu J., Zheng T., Huang X., Wang Y., Yin G., Du W. Procyanidine resists the fibril formation of human islet amyloid polypeptide. Int. J. Biol. Macromol. 2021;183:1067–1078. doi: 10.1016/j.ijbiomac.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 185.Park G., Xue C., Wang H., Guo Z. Distinguishing the Effect on the Rate and Yield of Aβ42 Aggregation by Green Tea Polyphenol EGCG. ACS Omega. 2020;5:21497–21505. doi: 10.1021/acsomega.0c02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chan S., Kantham S., Rao V.M., Palanivelu M.K., Pham H.L., Shaw P.N., McGeary R.P., Ross B.P. Metal chelation, radical scavenging and inhibition of Aβ₄₂ fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016;199:185–194. doi: 10.1016/j.foodchem.2015.11.118. [DOI] [PubMed] [Google Scholar]
- 187.Ahmed R., VanSchouwen B., Jafari N., Ni X., Ortega J., Melacini G. Molecular Mechanism for the (-)-Epigallocatechin Gallate-Induced Toxic to Nontoxic Remodeling of Aβ Oligomers. J. Am. Chem. Soc. 2017;139:13720–13734. doi: 10.1021/jacs.7b05012. [DOI] [PubMed] [Google Scholar]
- 188.Lopez del Amo J.M., Fink U., Dasari M., Grelle G., Wanker E.E., Bieschke J., Reif B. Structural properties of EGCG-induced, nontoxic Alzheimer’s disease Aβ oligomers. J. Mol. Biol. 2012;421:517–524. doi: 10.1016/j.jmb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 189.Bao J., Liu W., Zhou H.Y., Gui Y.R., Yang Y.H., Wu M.J., Xiao Y.F., Shang J.T., Long G.F., Shu X.J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020;40:18–27. doi: 10.1007/s11596-020-2142-z. [DOI] [PubMed] [Google Scholar]
- 190.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lee J.W., Lee Y.K., Ban J.O., Ha T.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 192.Naseri N.N., Wang H., Guo J., Sharma M., Luo W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019;705:183–194. doi: 10.1016/j.neulet.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 194.Wang J.Z., Xia Y.Y., Grundke-Iqbal I., Iqbal K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013;33((Suppl. 1)):S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- 195.Karikari T.K., Emeršič A., Vrillon A., Lantero-Rodriguez J., Ashton N.J., Kramberger M.G., Dumurgier J., Hourregue C., Čučnik S., Brinkmalm G., et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement. 2021;17:755–767. doi: 10.1002/alz.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoskin J.L., Sabbagh M.N., Al-Hasan Y., Decourt B. Tau immunotherapies for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2019;28:545–554. doi: 10.1080/13543784.2019.1619694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ruthirakuhan M., Herrmann N., Suridjan I., Abraham E.H., Farber I., Lanctôt K.L. Beyond immunotherapy: New approaches for disease modifying treatments for early Alzheimer’s disease. Expert Opin. Pharmacother. 2016;17:2417–2429. doi: 10.1080/14656566.2016.1258060. [DOI] [PubMed] [Google Scholar]
- 198.Anand R., Gill K.D., Mahdi A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology. 2014;76 Pt A:27–50. doi: 10.1016/j.neuropharm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 199.Sivanantharajah L., Mudher A. Curcumin as a Holistic Treatment for Tau Pathology. Front. Pharmacol. 2022;13:903119. doi: 10.3389/fphar.2022.903119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ma Q.L., Zuo X., Yang F., Ubeda O.J., Gant D.J., Alaverdyan M., Teng E., Hu S., Chen P.P., Maiti P., et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013;288:4056–4065. doi: 10.1074/jbc.M112.393751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rane J.S., Bhaumik P., Panda D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017;60:999–1014. doi: 10.3233/JAD-170351. [DOI] [PubMed] [Google Scholar]
- 202.Miyasaka T., Xie C., Yoshimura S., Shinzaki Y., Yoshina S., Kage-Nakadai E., Mitani S., Ihara Y. Curcumin improves tau-induced neuronal dysfunction of nematodes. Neurobiol. Aging. 2016;39:69–81. doi: 10.1016/j.neurobiolaging.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 203.Sonawane S.K., Chidambaram H., Boral D., Gorantla N.V., Balmik A.A., Dangi A., Ramasamy S., Marelli U.K., Chinnathambi S. EGCG impedes human Tau aggregation and interacts with Tau. Sci. Rep. 2020;10:12579. doi: 10.1038/s41598-020-69429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wobst H.J., Sharma A., Diamond M.I., Wanker E.E., Bieschke J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. doi: 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chesser A.S., Ganeshan V., Yang J., Johnson G.V. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016;19:21–31. doi: 10.1179/1476830515Y.0000000038. [DOI] [PubMed] [Google Scholar]
- 206.Rezai-Zadeh K., Arendash G.W., Hou H., Fernandez F., Jensen M., Runfeldt M., Shytle R.D., Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 207.Akıncıoğlu H., Gülçin İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini Rev. Med. Chem. 2020;20:703–715. doi: 10.2174/1389557520666200103100521. [DOI] [PubMed] [Google Scholar]
- 208.Shekari A., Fahnestock M. Retrograde axonal transport of BDNF and proNGF diminishes with age in basal forebrain cholinergic neurons. Neurobiol. Aging. 2019;84:131–140. doi: 10.1016/j.neurobiolaging.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 209.Singh M., Kaur M., Kukreja H., Chugh R., Silakari O., Singh D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013;70:165–188. doi: 10.1016/j.ejmech.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 210.Korabecny J., Spilovska K., Mezeiova E., Benek O., Juza R., Kaping D., Soukup O. A Systematic Review on Donepezil-based Derivatives as Potential Cholinesterase Inhibitors for Alzheimer’s Disease. Curr. Med. Chem. 2019;26:5625–5648. doi: 10.2174/0929867325666180517094023. [DOI] [PubMed] [Google Scholar]
- 211.Akinyemi A.J., Oboh G., Fadaka A.O., Olatunji B.P., Akomolafe S. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology. 2017;62:75–79. doi: 10.1016/j.neuro.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 212.Agrawal R., Mishra B., Tyagi E., Nath C., Shukla R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol. Res. 2010;61:247–252. doi: 10.1016/j.phrs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 213.Okello E.J., Mather J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients. 2020;12:1090. doi: 10.3390/nu12041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Srividhya R., Gayathri R., Kalaiselvi P. Impact of epigallo catechin-3-gallate on acetylcholine-acetylcholine esterase cycle in aged rat brain. Neurochem. Int. 2012;60:517–522. doi: 10.1016/j.neuint.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 215.Ali B., Jamal Q.M., Shams S., Al-Wabel N.A., Siddiqui M.U., Alzohairy M.A., Al Karaawi M.A., Kesari K.K., Mushtaq G., Kamal M.A. In Silico Analysis of Green Tea Polyphenols as Inhibitors of AChE and BChE Enzymes in Alzheimer’s Disease Treatment. CNS Neurol Disord. Drug Targets. 2016;15:624–628. doi: 10.2174/1871527315666160321110607. [DOI] [PubMed] [Google Scholar]
- 216.Utermann G. Isolation and partial characterization of an arginine-rich apolipoprotein from human plasma very-low-density lipoproteins: Apolipoprotein E. Hoppe Seylers Z. Physiol. Chem. 1975;356:1113–1121. doi: 10.1515/bchm2.1975.356.2.1113. [DOI] [PubMed] [Google Scholar]
- 217.Shore V.G., Shore B. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry. 1973;12:502–507. doi: 10.1021/bi00727a022. [DOI] [PubMed] [Google Scholar]
- 218.Wernette-Hammond M.E., Lauer S.J., Corsini A., Walker D., Taylor J.M., Rall S.C., Jr. Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J. Biol. Chem. 1989;264:9094–9101. doi: 10.1016/S0021-9258(18)81907-X. [DOI] [PubMed] [Google Scholar]
- 219.Wang T., Dong Y., Yao L., Lu F., Wen C., Wan Z., Fan L., Li Z., Bu T., Wei M., et al. Adoptive transfer of metabolically reprogrammed macrophages for atherosclerosis treatment in diabetic ApoE (-/-) mice. Bioact. Mater. 2022;16:82–94. doi: 10.1016/j.bioactmat.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Iwaki T., Arakawa T., Sandoval-Cooper M.J., Smith D.L., Donahue D., Ploplis V.A., Umemura K., Castellino F.J. Plasminogen Deficiency Significantly Reduces Vascular Wall Disease in a Murine Model of Type IIa Hypercholesterolemia. Biomedicines. 2021;9:1832. doi: 10.3390/biomedicines9121832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Y., Strickland M.R., Soranno A., Holtzman D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron. 2021;109:205–221. doi: 10.1016/j.neuron.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lindner K., Beckenbauer K., van Ek L.C., Titeca K., de Leeuw S.M., Awwad K., Hanke F., Korepanova A.V., Rybin V., van der Kam E.L., et al. Isoform- and cell-state-specific lipidation of ApoE in astrocytes. Cell Rep. 2022;38:110435. doi: 10.1016/j.celrep.2022.110435. [DOI] [PubMed] [Google Scholar]
- 223.Patel T., Carnwath T.P., Wang X., Allen M., Lincoln S.J., Lewis-Tuffin L.J., Quicksall Z.S., Lin S., Tutor-New F.Q., Ho C.C.G., et al. Transcriptional landscape of human microglia implicates age, sex, and APOE-related immunometabolic pathway perturbations. Aging Cell. 2022;21:e13606. doi: 10.1111/acel.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rapp A., Gmeiner B., Hüttinger M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie. 2006;88:473–483. doi: 10.1016/j.biochi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 225.Pagnon de la Vega M., Näslund C., Brundin R., Lannfelt L., Löwenmark M., Kilander L., Ingelsson M., Giedraitis V. Mutation analysis of disease causing genes in patients with early onset or familial forms of Alzheimer’s disease and frontotemporal dementia. BMC Genom. 2022;23:99. doi: 10.1186/s12864-022-08343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jin Y., Li F., Sonoustoun B., Kondru N.C., Martens Y.A., Qiao W., Heckman M.G., Ikezu T.C., Li Z., Burgess J.D., et al. APOE4 exacerbates α-synuclein seeding activity and contributes to neurotoxicity in Alzheimer’s disease with Lewy body pathology. Acta Neuropathol. 2022;143:641–662. doi: 10.1007/s00401-022-02421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Han S.H., Noh D.H., Jo E.J., Kam K.Y. Effects of Apolipoprotein E ɛ4 and Risk Factors on Domains of Cognition in Mild Cognitive Impairment and Dementia. J. Alzheimers Dis. 2022;87:1181–1188. doi: 10.3233/JAD-215075. [DOI] [PubMed] [Google Scholar]
- 228.Baek M.S., Cho H., Lee H.S., Lee J.H., Ryu Y.H., Lyoo C.H. Effect of APOE ε4 genotype on amyloid-β and tau accumulation in Alzheimer’s disease. Alzheimers Res. Ther. 2020;12:140. doi: 10.1186/s13195-020-00710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Shi D., Xie S., Li A., Wang Q., Guo H., Han Y., Xu H., Gan W.B., Zhang L., Guo T. APOE-ε4 modulates the association among plasma Aβ(42)/Aβ(40), vascular diseases, neurodegeneration and cognitive decline in non-demented elderly adults. Transl. Psychiatry. 2022;12:128. doi: 10.1038/s41398-022-01899-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Huang Y.A., Zhou B., Wernig M., Südhof T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell. 2017;168:427–441.e421. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dafnis I., Argyri L., Chroni A. Amyloid-peptide β 42 Enhances the Oligomerization and Neurotoxicity of apoE4: The C-terminal Residues Leu279, Lys282 and Gln284 Modulate the Structural and Functional Properties of apoE4. Neuroscience. 2018;394:144–155. doi: 10.1016/j.neuroscience.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 232.Huynh T.V., Davis A.A., Ulrich J.D., Holtzman D.M. Apolipoprotein E and Alzheimer’s disease: The influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 2017;58:824–836. doi: 10.1194/jlr.R075481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ma Q., Zhao Z., Sagare A.P., Wu Y., Wang M., Owens N.C., Verghese P.B., Herz J., Holtzman D.M., Zlokovic B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018;13:57. doi: 10.1186/s13024-018-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Shi Y., Holtzman D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018;18:759–772. doi: 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Farfel J.M., Yu L., De Jager P.L., Schneider J.A., Bennett D.A. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol. Aging. 2016;37:19–25. doi: 10.1016/j.neurobiolaging.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gale S.C., Gao L., Mikacenic C., Coyle S.M., Rafaels N., Murray Dudenkov T., Madenspacher J.H., Draper D.W., Ge W., Aloor J.J., et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J. Allergy Clin. Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cullen A.E., Centner A.M., Deitado R., Salazar J.F.A. The Impact of Dietary Supplementation of Whole Foods and Polyphenols on Atherosclerosis. Nutrients. 2020;12:2069. doi: 10.3390/nu12072069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ghura S., Tai L., Zhao M., Collins N., Che C.T., Warpeha K.M., LaDu M.J. Arabidopsis thaliana extracts optimized for polyphenols production as potential therapeutics for the APOE-modulated neuroinflammation characteristic of Alzheimer’s disease in vitro. Sci. Rep. 2016;6:29364. doi: 10.1038/srep29364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Mountaki C., Dafnis I., Panagopoulou E.A., Vasilakopoulou P.B., Karvelas M., Chiou A., Karathanos V.T., Chroni A. Mechanistic insight into the capacity of natural polar phenolic compounds to abolish Alzheimer’s disease-associated pathogenic effects of apoE4 forms. Free Radic. Biol. Med. 2021;171:284–301. doi: 10.1016/j.freeradbiomed.2021.05.022. [DOI] [PubMed] [Google Scholar]
- 240.Aubert L., Pichierri S., Hommet C., Camus V., Berrut G., de Decker L. Association between comorbidity burden and rapid cognitive decline in individuals with mild to moderate Alzheimer’s disease. J. Am. Geriatr. Soc. 2015;63:543–547. doi: 10.1111/jgs.13314. [DOI] [PubMed] [Google Scholar]
- 241.Cooper C., Sommerlad A., Lyketsos C.G., Livingston G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry. 2015;172:323–334. doi: 10.1176/appi.ajp.2014.14070878. [DOI] [PubMed] [Google Scholar]
- 242.Burillo J., Marqués P., Jiménez B., González-Blanco C., Benito M., Guillén C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells. 2021;10:1236. doi: 10.3390/cells10051236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Moller D.E., Flier J.S. Insulin resistance--mechanisms, syndromes, and implications. N. Engl. J. Med. 1991;325:938–948. doi: 10.1056/nejm199109263251307. [DOI] [PubMed] [Google Scholar]
- 244.Feng H.L., Dang H.Z., Fan H., Chen X.P., Rao Y.X., Ren Y., Yang J.D., Shi J., Wang P.W., Tian J.Z. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int. J. Immunopathol. Pharmacol. 2016;29:734–741. doi: 10.1177/0394632016659494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zheng Q., Kebede M.T., Lee B., Krasinski C.A., Islam S., Wurfl L.A., Kemeh M.M., Ivancic V.A., Jakobsche C.E., Spratt D.E., et al. Differential Effects of Polyphenols on Insulin Proteolysis by the Insulin-Degrading Enzyme. Antioxidants. 2021;10:1342. doi: 10.3390/antiox10091342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Krasinski C.A., Ivancic V.A., Zheng Q., Spratt D.E., Lazo N.D. Resveratrol Sustains Insulin-Degrading Enzyme Activity toward Aβ42. ACS Omega. 2018;3:13275–13282. doi: 10.1021/acsomega.8b01913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kapogiannis D., Mattson M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Govindarajulu M., Ramesh S., Neel L., Fabbrini M., Buabeid M., Fujihashi A., Dwyer D., Lynd T., Shah K., Mohanakumar K.P., et al. Nutraceutical based SIRT3 activators as therapeutic targets in Alzheimer’s disease. Neurochem. Int. 2021;144:104958. doi: 10.1016/j.neuint.2021.104958. [DOI] [PubMed] [Google Scholar]
- 249.Chen C.M., Liu S.H., Lin-Shiau S.Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin. Pharmacol. Toxicol. 2007;101:108–116. doi: 10.1111/j.1742-7843.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- 250.Esselun C., Theyssen E., Eckert G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021;22:8333. doi: 10.3390/ijms22158333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee J.H., Yang D.S., Goulbourne C.N., Im E., Stavrides P., Pensalfini A., Chan H., Bouchet-Marquis C., Bleiwas C., Berg M.J., et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat. Neurosci. 2022;25:688–701. doi: 10.1038/s41593-022-01084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Arotcarena M.L., Soria F.N., Cunha A., Doudnikoff E., Prévot G., Daniel J., Blanchard-Desce M., Barthélémy P., Bezard E., Crauste-Manciet S., et al. Acidic nanoparticles protect against α-synuclein-induced neurodegeneration through the restoration of lysosomal function. Aging Cell. 2022;21:e13584. doi: 10.1111/acel.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Armour S.M., Baur J.A., Hsieh S.N., Land-Bracha A., Thomas S.M., Sinclair D.A. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging. 2009;1:515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy. 2010;6:186–188. doi: 10.4161/auto.6.1.10817. [DOI] [PubMed] [Google Scholar]
- 255.Borra M.T., Smith B.C., Denu J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 256.Yessenkyzy A., Saliev T., Zhanaliyeva M., Masoud A.R., Umbayev B., Sergazy S., Krivykh E., Gulyayev A., Nurgozhin T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients. 2020;12:1344. doi: 10.3390/nu12051344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhong L., Hu J., Shu W., Gao B., Xiong S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015;6:e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li D., Cui Y., Wang X., Liu F., Li X. Apple polyphenol extract alleviates lipid accumulation in free-fatty-acid-exposed HepG2 cells via activating autophagy mediated by SIRT1/AMPK signaling. Phytother. Res. 2021;35:1416–1431. doi: 10.1002/ptr.6902. [DOI] [PubMed] [Google Scholar]
- 259.Moreno-Arribas M.V., Bartolomé B., Peñalvo J.L., Pérez-Matute P., Motilva M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients. 2020;12:3082. doi: 10.3390/nu12103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 261.Haran J.P., Bhattarai S.K., Foley S.E., Dutta P., Ward D.V., Bucci V., McCormick B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio. 2019;10:e00632-19. doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kumar Singh A., Cabral C., Kumar R., Ganguly R., Kumar Rana H., Gupta A., Rosaria Lauro M., Carbone C., Reis F., Pandey A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients. 2019;11:2216. doi: 10.3390/nu11092216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sun Z.Z., Li X.Y., Wang S., Shen L., Ji H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020;104:3507–3515. doi: 10.1007/s00253-020-10461-x. [DOI] [PubMed] [Google Scholar]
- 264.Li C., Wang N., Zheng G., Yang L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces. 2021;13:46406–46420. doi: 10.1021/acsami.1c14818. [DOI] [PubMed] [Google Scholar]
- 265.Fallahi E., O’Driscoll N.A., Matallanas D. The MST/Hippo Pathway and Cell Death: A Non-Canonical Affair. Genes. 2016;7:28. doi: 10.3390/genes7060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Khan M., Rutten B.P.F., Kim M.O. MST1 Regulates Neuronal Cell Death via JNK/Casp3 Signaling Pathway in HFD Mouse Brain and HT22 Cells. Int. J. Mol. Sci. 2019;20:2504. doi: 10.3390/ijms20102504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang H., Shang Y., Wang E., Xu X., Zhang Q., Qian C., Yang Z., Wu S., Zhang T. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog. Neurobiol. 2022;214:102280. doi: 10.1016/j.pneurobio.2022.102280. [DOI] [PubMed] [Google Scholar]
- 268.Li Q., Qiu Z., Wang Y., Guo C., Cai X., Zhang Y., Liu L., Xue H., Tang J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021;22:1473. doi: 10.3892/etm.2021.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Stanger B.Z., Leder P., Lee T.H., Kim E., Seed B. RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 270.Vanden Berghe T., Linkermann A., Jouan-Lanhouet S., Walczak H., Vandenabeele P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 271.Strilic B., Yang L., Albarrán-Juárez J., Wachsmuth L., Han K., Müller U.C., Pasparakis M., Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- 272.Ofengeim D., Mazzitelli S., Ito Y., DeWitt J.P., Mifflin L., Zou C., Das S., Adiconis X., Chen H., Zhu H., et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2017;114:e8788–e8797. doi: 10.1073/pnas.1714175114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang S.H., Lee D.K., Shin J., Lee S., Baek S., Kim J., Jung H., Hah J.M., Kim Y. Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Mol. Med. 2017;9:61–77. doi: 10.15252/emmm.201606566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li S., Liu R., Xia S., Wei G., Ishfaq M., Zhang Y., Zhang X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022;233:113319. doi: 10.1016/j.ecoenv.2022.113319. [DOI] [PubMed] [Google Scholar]
- 275.Hu Y., Pan H., Peng J., He J., Tang M., Yan S., Rong J., Li J., Zheng Z., Wang H., et al. Resveratrol inhibits necroptosis by mediating the TNF-α/RIP1/RIP3/MLKL pathway in myocardial hypoxia/reoxygenation injury. Acta Biochim. Biophys. Sin. 2021;53:430–437. doi: 10.1093/abbs/gmab012. [DOI] [PubMed] [Google Scholar]
- 276.Zhao L., Wang J.L., Liu R., Li X.X., Li J.F., Zhang L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules. 2013;18:9949–9965. doi: 10.3390/molecules18089949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Dourado N.S., Souza C.D.S., de Almeida M.M.A., Bispo da Silva A., Dos Santos B.L., Silva V.D.A., De Assis A.M., da Silva J.S., Souza D.O., Costa M.F.D., et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020;12:119. doi: 10.3389/fnagi.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Jin X., Liu M.Y., Zhang D.F., Zhong X., Du K., Qian P., Yao W.F., Gao H., Wei M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019;25:575–590. doi: 10.1111/cns.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kouhestani S., Jafari A., Babaei P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018;13:1827–1832. doi: 10.4103/1673-5374.238714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kou J.J., Shi J.Z., He Y.Y., Hao J.J., Zhang H.Y., Luo D.M., Song J.K., Yan Y., Xie X.M., Du G.H., et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022;43:840–849. doi: 10.1038/s41401-021-00702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kang Y., Lee J.H., Seo Y.H., Jang J.H., Jeong C.H., Lee S., Jeong G.S., Park B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019;27:145–151. doi: 10.4062/biomolther.2018.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Multifunction of myricetin on A beta: Neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. J. Neurosci. Res. 2008;86:368–377. doi: 10.1002/jnr.21476. [DOI] [PubMed] [Google Scholar]
- 283.Yu X., Li Y., Mu X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ (25-35) and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed. Res. Int. 2020;2020:8210578. doi: 10.1155/2020/8210578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Jiang W., Luo T., Li S., Zhou Y., Shen X.Y., He F., Xu J., Wang H.Q. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE. 2016;11:e0152371. doi: 10.1371/journal.pone.0152371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lu Y., Liu Q., Yu Q. Quercetin enrich diet during the early-middle not middle-late stage of alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018;10:1237–1246. [PMC free article] [PubMed] [Google Scholar]
- 286.Kim J.H., Wang Q., Choi J.M., Lee S., Cho E.J. Protective role of caffeic acid in an Aβ25-35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015;9:480–488. doi: 10.4162/nrp.2015.9.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Mori T., Koyama N., Yokoo T., Segawa T., Maeda M., Sawmiller D., Tan J., Town T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020;295:16251–16266. doi: 10.1074/jbc.RA119.012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ogunlade B., Adelakun S.A., Agie J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2022;45:651–662. doi: 10.1080/01480545.2020.1754849. [DOI] [PubMed] [Google Scholar]
- 289.Yu M., Chen X., Liu J., Ma Q., Zhuo Z., Chen H., Zhou L., Yang S., Zheng L., Ning C., et al. Gallic acid disruption of Aβ(1-42) aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019;124:67–80. doi: 10.1016/j.nbd.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 290.Kim M.J., Seong A.R., Yoo J.Y., Jin C.H., Lee Y.H., Kim Y.J., Lee J., Jun W.J., Yoon H.G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011;55:1798–1808. doi: 10.1002/mnfr.201100262. [DOI] [PubMed] [Google Scholar]
- 291.Gao L., Li X., Meng S., Ma T., Wan L., Xu S. Chlorogenic Acid Alleviates Aβ(25-35)-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des. Dev. Ther. 2020;14:1705–1716. doi: 10.2147/DDDT.S235969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shi M., Sun F., Wang Y., Kang J., Zhang S., Li H. CGA restrains the apoptosis of Aβ(25-35)-induced hippocampal neurons. Int. J. Neurosci. 2020;130:700–707. doi: 10.1080/00207454.2019.1702547. [DOI] [PubMed] [Google Scholar]
- 293.Wang N.Y., Li J.N., Liu W.L., Huang Q., Li W.X., Tan Y.H., Liu F., Song Z.H., Wang M.Y., Xie N., et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics. 2021;18:1064–1080. doi: 10.1007/s13311-021-01024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yan J.J., Cho J.Y., Kim H.S., Kim K.L., Jung J.S., Huh S.O., Suh H.W., Kim Y.H., Song D.K. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001;133:89–96. doi: 10.1038/sj.bjp.0704047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sun P., Yin J.B., Liu L.H., Guo J., Wang S.H., Qu C.H., Wang C.X. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci. Rep. 2019;39:BSR20180902. doi: 10.1042/BSR20180902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Weng L., Zhang H., Li X., Zhan H., Chen F., Han L., Xu Y., Cao X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int. Immunopharmacol. 2017;44:1–8. doi: 10.1016/j.intimp.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 297.Petry F.D.S., Hoppe J.B., Klein C.P., Dos Santos B.G., Hözer R.M., Bifi F., Matté C., Salbego C.G., Trindade V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021;87:108525. doi: 10.1016/j.jnutbio.2020.108525. [DOI] [PubMed] [Google Scholar]
- 298.Pierzynowska K., Podlacha M., Gaffke L., Majkutewicz I., Mantej J., Węgrzyn A., Osiadły M., Myślińska D., Węgrzyn G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology. 2019;148:332–346. doi: 10.1016/j.neuropharm.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 299.Petry F.D.S., Coelho B.P., Gaelzer M.M., Kreutz F., Guma F., Salbego C.G., Trindade V.M.T. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytother. Res. 2020;34:796–807. doi: 10.1002/ptr.6560. [DOI] [PubMed] [Google Scholar]
- 300.Ikram M., Muhammad T., Rehman S.U., Khan A., Jo M.G., Ali T., Kim M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019;56:6293–6309. doi: 10.1007/s12035-019-1512-7. [DOI] [PubMed] [Google Scholar]
- 301.Kim Y.E., Hwang C.J., Lee H.P., Kim C.S., Son D.J., Ham Y.W., Hellström M., Han S.B., Kim H.S., Park E.K., et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 302.Bahn G., Park J.S., Yun U.J., Lee Y.J., Choi Y., Park J.S., Baek S.H., Choi B.Y., Cho Y.S., Kim H.K., et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA. 2019;116:12516–12523. doi: 10.1073/pnas.1819541116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Ano Y., Ohya R., Kita M., Taniguchi Y., Kondo K. Theaflavins Improve Memory Impairment and Depression-Like Behavior by Regulating Microglial Activation. Molecules. 2019;24:467. doi: 10.3390/molecules24030467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Leri M., Scuto M., Ontario M.L., Calabrese V., Calabrese E.J., Bucciantini M., Stefani M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020;21:1250. doi: 10.3390/ijms21041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Macready A.L., Kennedy O.B., Ellis J.A., Williams C.M., Spencer J.P., Butler L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009;4:227–242. doi: 10.1007/s12263-009-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Li H., Wang Z. Comparison in antioxidant and antitumor activities of pine polyphenols and its seven biotransformation extracts by fungi. PeerJ. 2017;5:e3264. doi: 10.7717/peerj.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Askarizadeh A., Barreto G.E., Henney N.C., Majeed M., Sahebkar A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020;585:119476. doi: 10.1016/j.ijpharm.2020.119476. [DOI] [PubMed] [Google Scholar]
- 308.Tsai Y.M., Chien C.F., Lin L.C., Tsai T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011;416:331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 309.Hewlings S.J., Kalman D.S. Curcumin: A Review of Its Effects on Human Health. Foods. 2017;6:92. doi: 10.3390/foods6100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 311.Neves A.R., van der Putten L., Queiroz J.F., Pinheiro M., Reis S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021;331:108–117. doi: 10.1016/j.jbiotec.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 312.Yan Y., Chen Y., Liu Z., Cai F., Niu W., Song L., Liang H., Su Z., Yu B., Yan F. Brain Delivery of Curcumin Through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021;16:7433–7447. doi: 10.2147/IJN.S327737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Walle T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 314.Ghersi-Egea J.F., Mönkkönen K.S., Schmitt C., Honnorat J., Fèvre-Montange M., Strazielle N. Blood-brain interfaces and cerebral drug bioavailability. Rev. Neurol. 2009;165:1029–1038. doi: 10.1016/j.neurol.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 315.Andrade S., Ramalho M.J., Pereira M.D.C., Loureiro J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018;9:1261. doi: 10.3389/fphar.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Frozza R.L., Bernardi A., Hoppe J.B., Meneghetti A.B., Battastini A.M., Pohlmann A.R., Guterres S.S., Salbego C. Lipid-core nanocapsules improve the effects of resveratrol against Abeta-induced neuroinflammation. J. Biomed. Nanotechnol. 2013;9:2086–2104. doi: 10.1166/jbn.2013.1709. [DOI] [PubMed] [Google Scholar]
- 317.Lu X., Ji C., Xu H., Li X., Ding H., Ye M., Zhu Z., Ding D., Jiang X., Ding X., et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int. J. Pharm. 2009;375:89–96. doi: 10.1016/j.ijpharm.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 318.Rauf A., Imran M., Abu-Izneid T., Iahtisham Ul H., Patel S., Pan X., Naz S., Sanches Silva A., Saeed F., Rasul Suleria H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019;116:108999. doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 319.Stoupi S., Williamson G., Viton F., Barron D., King L.J., Brown J.E., Clifford M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C]procyanidin B2 in male rats. Drug Metab. Dispos. 2010;38:287–291. doi: 10.1124/dmd.109.030304. [DOI] [PubMed] [Google Scholar]
- 320.Xiao Y., Hu Z., Yin Z., Zhou Y., Liu T., Zhou X., Chang D. Profiling and Distribution of Metabolites of Procyanidin B2 in Mice by UPLC-DAD-ESI-IT-TOF-MS(n) Technique. Front. Pharmacol. 2017;8:231. doi: 10.3389/fphar.2017.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Wang Y., Zhang S., Deng H., Lin H. Research progress of methods to improve the stability of procyanidins. J. Guangdong Pharm. Univ. 2014;30:245–248. [Google Scholar]
- 322.Davatgaran-Taghipour Y., Masoomzadeh S., Farzaei M.H., Bahramsoltani R., Karimi-Soureh Z., Rahimi R., Abdollahi M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017;12:2689–2702. doi: 10.2147/IJN.S131973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zhang L., McClements D.J., Wei Z., Wang G., Liu X., Liu F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020;60:2083–2097. doi: 10.1080/10408398.2019.1630358. [DOI] [PubMed] [Google Scholar]
- 324.Pimentel-Moral S., Teixeira M.C., Fernandes A.R., Arráez-Román D., Martínez-Férez A., Segura-Carretero A., Souto E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018;260:85–94. doi: 10.1016/j.cis.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 325.Chen Z., Farag M.A., Zhong Z., Zhang C., Yang Y., Wang S., Wang Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021;176:113870. doi: 10.1016/j.addr.2021.113870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.