Abstract
The porcine epidemic diarrhea virus (PEDV) is a member of the coronavirus family, causing deadly watery diarrhea in newborn piglets. The global pandemic of PEDV, with significant morbidity and mortality, poses a huge threat to the swine industry. The currently developed vaccines and drugs are only effective against the classic GI strains that were prevalent before 2010, while there is no effective control against the GII variant strains that are currently a global pandemic. In this review, we summarize the latest progress in the biology of PEDV, including its transmission and origin, structure and function, evolution, and virus–host interaction, in an attempt to find the potential virulence factors influencing PEDV pathogenesis. We conclude with the mechanism by which PEDV components antagonize the immune responses of the virus, and the role of host factors in virus infection. Essentially, this review serves as a valuable reference for the development of attenuated virus vaccines and the potential of host factors as antiviral targets for the prevention and control of PEDV infection.
Keywords: PED, PEDV, swine, epidemiology, virulence, virus–host interaction
1. Epidemiology of PEDV
Porcine epidemic diarrhea (PED) is an infectious intestinal disease caused by the porcine epidemic diarrhea virus (PEDV) [1]. PEDV mainly affects piglets more severely than adult pigs, generally showing symptoms such as vomiting, liquid diarrhea, dehydration, anorexia, and weight loss [2]. The presence of PEDV was first reported on a farm in the United Kingdom in 1971 and the virus was first isolated in Belgium in 1978 [3]. In 1984, China confirmed the existence of PED antigen for the first time through the serum antigen test [1]. In early 2004, PEDV with low mortality did not receive much global attention. By 2010, high-mortality strains of PEDV emerged and spread globally, having an increasing impact on the global swine industry. In this regard, a comprehensive understanding of PEDV has become increasingly urgent. Here, we conduct a comprehensive review of the epidemiology of PEDV, including discussing the worldwide spread of PEDV variants, exploring the origins of PEDV variants around the world, and describing the structure and function of PEDV.
1.1. The Morbidity and Mortality of PEDV
Mortality was often associated with the age of recipient animals after PEDV infection [4]. In aged animals, although the morbidity may be close to 100%, the mortality rate is very low, between 1% and 3% [5]. However, the morbidity in piglets is more severe, with an average of 50% mortality in piglets within two weeks of birth [6,7]. In 2004, PEDV infection caused a regional outbreak with a low fatality rate. At this point, the morbidity in piglets was 46.4%, and the mortality rate was only 6.16% [1]. In the winter of 2010, a new variant of highly pathogenic GII PEDV appeared in southern China, which increased the morbidity and mortality of PEDV to more than 80–100% [8,9]. More than a million piglets, even vaccinated ones, died on pig farms in southern China, dealing a devastating blow to the swine industry [8,10]. In 2013, according to statistics, morbidity in the United States was nearly 100%, and piglet mortality was 90–95% [11,12]. As of 2014, the mortality rate of piglets infected with pathogenic strains in Germany was more than 70% [13]. From 2016 to 2018, the mortality rate of Mexican piglets was 80–95% [14]. In recent years, the most popular GII PEDV highly pathogenic variant has gradually spread around the world, and the threat to the global swine industry has become more and more serious.
1.2. Transmission of PEDV
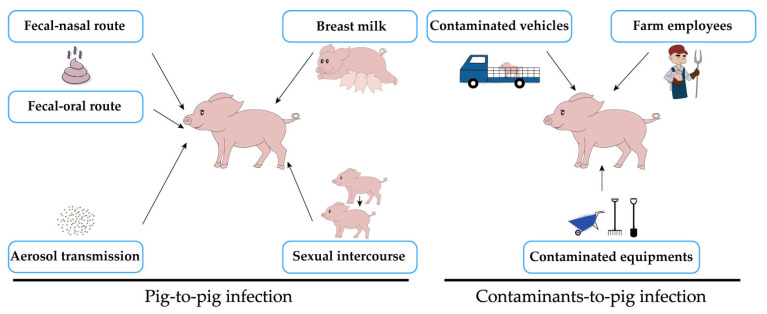
Transmission by direct and indirect contact is the main route of PEDV transmission between pigs. PEDV is transmitted between pigs at different ages and usually first infects finishing pigs, then the virus spreads to pregnant sows in the farrowing house, and subclinically infected sows then transmit PEDV to suckling piglets, eventually causing an epidemic of high mortality in piglets. Here, we summarize several routes of PEDV transmission between pigs (Figure 1).
Figure 1.
Several transmission routes of PEDV infection. Black arrows indicate transmission routes of PEDV.
PEDV spreads in several different ways during infection, and one of the most common is the fecal–oral route, that is, direct or indirect transmission through porcine feces, vomit, and other contaminants produced by the livestock process [7]. PEDV can be infected through the route of transmission from porcine feces to the nasal cavity, known as the fecal–nasal route [15]. In addition, PEDV can also be transmitted vertically to piglets through sow milk [8]. According to a study by Gallien et al. (2018) [5], the presence of PEDV can be detected in the semen of PEDV-infected boars, and prolonged virus shedding can be observed in the sperm-rich fraction, proving that PEDV can be sexually transmitted through boar semen [5]. A recent study showed that PEDV infected by nasal epithelium can be transferred to CD3+ T lymphocytes through synapses, and finally reaches the intestinal mucosa through blood circulation, resulting in intestinal infection [16], which is the mechanism of PEDV infection from the nasal cavity to the intestinal epithelium, and is also important evidence for aerosol transmission. In addition to pig–to–pig infection, the transmission of PEDV can also occur through contact with contaminated equipment [17], contaminated vehicles used for animal transport [18], or farm employees [16,17].
1.3. Genotyping, Distribution, and Origin of PEDV
PEDV continuously evolves in the process of adapting to environmental changes, and its frequent variants lead to the emergence of various virulent strains. At present, researchers have classified PEDV into the GI classical genogroup and the GII variant genogroup [19]. Genogroups GI and GII further evolved separately, and finally formed five subgroups (GIa, GIb, GIIa, GIIb, and GIIc). To further clarify the genotype characteristics of PEDV and the global prevalence of each genogroup, we discuss the distribution and origin of each genogroup of PEDV in Table 1.
Table 1.
Representative strains and distributions of each PEDV genotype.
| Genotype | Variation | Representative Strains | Distribution | Reference |
|---|---|---|---|---|
| GIa | Classical strains | CV777; DR13; LZC; CH-S; AVCT12; SM98; CHM2013 | Asia–China, Korea, Thailand; Europe–Belgium, Russia |
[19,20,21,22] |
| GIb | S-Indel vaccine strains relative to GIa | JS-2004-2; attenuated CV777; attenuated DR13; SD-M; SC1402; ZJ08; SQ2014; AH-M | Asia–China, Korea, Japan, Thailand | [1,19,23,24,25] |
| GIIa | Recombined non-S INDEL variant strains | AH2012; LZW; ZMDZY; GDZQ; FJZZ-9; GD-B; KNU-1305; Tottori2; MYG-1; Colorado; PC21A; PC22A; PC177; TC-PC177; MN; Kansas-166; Minnesota79; MEX/104/2013 | Asia–China, Korea, Japan; North America–USA, Mexico |
[6,19,22,23,26,27,28] |
| GIIb | Recombined non-S INDEL variant strains | AJ1102; LC; AH2012-12; YN1; CHGD-01; GD-1; GDS01; GD-A; ZJCZ4; HNPJ | Asia–China | [6,19,26,27,29] |
| GIIc | S-INDEL strains recombined between GIa and GIIa | ZL29; CH/HNQX-3/14; KNU-1406-1; OH851; Iowa106; Minnesota211; IL20697; GER/L00862/2014; 15V010; FR/001/2014 | Asia–China, Korea; North America–USA; Europe–Germany, Romania, Austria, Belgium, Italy, France, Hungary, Slovenija |
[1,6,19,22,25,29,30,31] |
Genogroup GI is divided into two subgroups, GIa and GIb. The GIa subgroup mainly consists of classic CV777, DR13, SM98, and other early strains, distributed in Europe and Asia [32]. The GIb strains mainly originate from Asian countries, especially China [33], which are generally early strains or cell-adapted attenuated vaccine strains caused by the insertion or deletion of the PEDV S gene. Compared with the GIa with a certain virulence, the GIb strains are less virulent, and many attenuated strains have been identified as candidates for attenuated vaccines, which we propose to term “S-Indel vaccine strains”. The most typical strains in the GIb subgroup include the early strain JS-2004-2 [22] and vaccine strains, such as the attenuated CV777 [34], attenuated DR13 [24], and SD-M [35]. In addition to the typical attenuated vaccine strains, there is also the CV777 vaccine strain-like AH-M classic strain, which has been circulating in China recently [36].
Since 2010, genogroup GII strains have been predominant globally, especially in China [26]. The GI and GII strains are not genetically closely related, and one of the most typical differences is that the GII strains have 11 different amino acid mutations (I116T, I356T, E365Q, T549S, G594S, N724S, A959V, S1044A, G1173D, S1232R, and R1298Q) [19]. Subgroup GIIa is an emerging non-S INDEL strain recombined within the S protein domain with higher virulence than genogroup GI, including those prevalent in Asia (AH2012 [37], LZW [38], ZMDZY [39], GDZQ [40], and Tottori2 [41]), and those prevalent in North America (Colorado [42], PC21A [43], and PC177 [44]), which occurs more frequently in Asia [33]. The GIIb subgroup is also a highly virulent non-S INDEL strain recombined within the S protein domain. All GIIb strains are currently circulating in China, including AJ1102 [45], LC [46], AH2012-12 [37], YN1 [47], and other typical strains [27,48,49,50]. However, no potential recombination occurred in the SH strain of GIIb [30], suggesting that amino acid substitutions in the S protein are also a pattern for the GIIb subgroup. The third important subtype of GII is GIIc, a kind of S-INDEL strain produced by recombination of the subgroups GIa and GIIa and the structural changes of the S gene [19,22]. These strains commonly have one amino acid insertion (aa 161–162) and two amino acid deletions (aa 59–62 and aa 140) in the N-terminal region of the S protein. Compared with other subgroups, GIIc also has three unique amino acid substitutions (L76, A/S92 and H/T113) and antigenic drift due to amino acid substitutions [19]. The less virulent GIIc strains, such as OH851, 15V010, CH/HNQX-3/14, and ZL29 [31,51,52,53], are mainly disseminated and prevalent in Europe, with a minority from the United States and Asia [4].
PEDV is mainly prevalent in Asia, North America, and Europe. Exploring the global prevalence and transmission of PEDV is thus relevant to understanding the origin of PEDV in various continents. The sporadic outbreaks of PEDV in 2005 occurred only in Asia, and they all belonged to the classic GIa strains [54]. In October 2010, a highly virulent GII variant which was different from the classic strain CV777 appeared in China for the first time [10]. Since then, China [8], South Korea [55], Japan [56], Vietnam [57], Thailand [58], and the Philippines [59] have suffered an outbreak of PEDV, successively. Among them, two pandemic strains, MG868 and BIG1024, emerged in South Korea, and these are likely to be the origin of the subsequent outbreak of PEDV in Asia [1]. In April 2013, when a highly virulent non-S INDEL GII strain was detected for the first time in the United States [60,61], PEDV appeared in the United States and rapidly spread throughout North America, including Canada [62] and Mexico [63]. Here, the three representative emerging strains, MN, IA1, and IA2, were closely related to the AH2012 strain in China [61]; however, the potential parental PEDV strain from which the North American outbreak originated has not been identified. In 2014, a newly discovered mild PEDV S-INDEL strain (GIIc) emerged in Germany [64] and began to circulate in many European countries such as the Netherlands [65], France [66], Belgium [53], Portugal [67] and Austria [68]. These mild variants were speculated to be homologous with the PEDV variant strain OH851, which was first detected in the United States earlier that same year [51].
1.4. Virion Structure and Function of PEDV
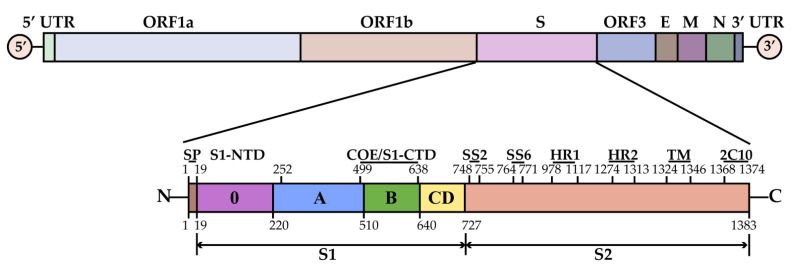
PEDV is a single-stranded positive-sense RNA virus with a diameter of 95–190 nm, a typical nested crown, and a genome size of approximately 28 kb, belonging to the genus alphacoronavirus [69,70]. PEDV consists of seven open reading frames (ORF1a, ORF1b, and ORF2–6), of which ORF1a (nt 297–12650) and ORF1b (nt 12605–20641) encode a total of sixteen nonstructural proteins, including nsp1~nsp16; ORF2 and ORF4–6 encode four structural proteins, including the spike (S) protein of 150–220 kDa, the envelope (E) protein of 7 kDa, the membrane (M) protein of 20–30 kDa, and the nucleocapsid (N) protein of 58 kDa; ORF3 encodes an accessory protein ORF3 [4,24,70,71] (Figure 2).
Figure 2.
Schematic diagram of the PEDV genome structure. Non-structural proteins, including nsp1–nsp16; structural proteins, including spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins; accessory proteins, ORF3. The spike protein consists of two subdomains, S1 and S2. The S1 subunit can be further subdivided into 6 domains, including signal peptide (SP, 1–18 aa, brown), domains 0 (19–219 aa, purple), domains A (220–509 aa, blue), domains B (510–639 aa, green), domains C and D (640–728 aa, yellow). The S1 subunit contains two receptor binding domains (RBDs): the N-terminal domain (S1-NTD, 19–252 aa) and the C-terminal domain (S1-CTD, 509–638 aa). The S2 subunit contains 3 domains, including two heptapeptide repeat domains (HR1 and HR2, 978–1117 aa and 1274–1313 aa) and a transmembrane domain (TM, 1324–1346 aa).
According to the molecular structure of the PEDV genome drawn, the ORF1a and ORF1b regions start from the 5′-end cap, and the polyproteins ppla and pp1ab translated from its replicase gene are post-translationally cleaved by internal proteases, resulting in 16 mature end-product nonstructural proteins nsp1–16 [4,70], mainly playing a role in virus infection; this can inhibit the immune responses of the host and create a favorable environment for virus infection and proliferation [71].
The S protein is located on the outer layer of the viral envelope, a type I glycoprotein, which is the most basic functional protein in PEDV [72]. Compared with the homology analysis of the corresponding regions of other coronaviruses, the S protein of PEDV is divided into two parts: S1 (19–726 aa) and S2 (727–1383 aa) [73]. The S1 subunit contains two RBDs, S1-NTD and S1-CTD [74], which mainly bind to receptors to facilitate viral attachment [75]. The S2 subunit contains three domains, two heptapeptide repeat domains (HR1 and HR2, 978–1117 aa and 1274–1313 aa) and a transmembrane domain (TM, 1324–1346 aa) [76], which mainly promote viral membrane fusion [77]. Four B cell epitopes have been characterized in the S protein, including the core neutralizing epitope (COE) (499–638 aa) on the S1 subunit, the epitopes SS2 (748–755 aa), SS6 (764–771 aa) and 2C10 (1368–1374 aa) on the S2 subunit [78], which become the main target for the development of vaccines or antibodies. The S protein plays an important role in viral pathogenicity, tissue tropism, infection, dissemination, and the trypsin-dependent proliferation of PEDV [73,79,80,81,82].
The accessory protein ORF3 is the characteristic protein of coronavirus and the only accessory protein in PEDV. ORF3 has a homotetrameric structure comprising four transmembrane regions, which are generally encoded by sequences located between the viral S and E genes [83]. The ORF3 protein of PEDV has a similar function to that of SARS coronavirus, and the intact ORF3 protein can function as an ion channel and regulate virus release during infection [84,85]. In addition, the ORF3 gene of PEDV has been shown to prolong the S phase of host cells by promoting the formation of vesicular structures, indicating its importance in the process of PEDV replication [86]. In general, ORF3 plays an important adjunctive role in PEDV infection, especially in the interaction between the virus and host cells.
The E protein is mainly localized in the endoplasmic reticulum, with a minor amount in the nucleus [87], whose molecular structure is divided into three regions, including a short amino-terminal hydrophilic region, an alpha-helical transmembrane domain of approximately 25 aa in length, and a long carboxyl-terminal region [88]. E protein functionally does not affect host cell proliferation and cell cycle, but it is implicated in the inflammatory response and persistence of PEDV infection [87]. Besides this, the E protein can also help evade host innate immunity by inhibiting RIG-I-mediated signaling [89]. Finally, the E protein tends to induce membrane curvature, and plays an important role in virion morphogenesis, assisting in the assembly of coronaviruses [90,91,92].
The M protein is an important membrane glycoprotein within the envelope, and the most abundant component in the viral envelope, which can interact with the E and S proteins encoded upstream of the viral structure, to complete the envelope assembly of the coronavirus [93]. The M protein can also interact with ORF3, and together with the N protein, participate in the assembly and budding of virus particles [94,95]. Additionally, the M protein suppresses the host immune responses by inducing neutralizing antibodies or inhibiting interferon-β (IFN-β) activity [96,97], while inducing cell-cycle arrest at S-phase through the cyclin A pathway during viral infection [98].
The N protein, called the nucleocapsid protein, is the main structural protein involved in the formation of the helical nucleocapsid, together with the viral genomic RNA [99]. It is currently known that in coronaviruses, the N protein is the only phosphorylated basic structural protein that is required for the efficient replication and transcription of viral RNA, and assists in the process of organizing the viral genome to help virion assembly [99,100,101]. During viral infection, PEDV N can regulate the cell cycle and suppress the immune responses by IFN regulatory factor 3 (IRF3) [102]. Meanwhile, PEDV N induces endoplasmic reticulum stress and inhibits cell-induced apoptosis through interaction with cellular phosphoproteins [103,104]. Surprisingly, the PEDV N protein may help to enhance the replication of viruses closely related to PEDV, such as porcine reproductive and respiratory syndrome virus (PRRSV), whereas it is inactive against unrelated viruses [105].
2. Factors Affecting Pathogenicity
Compared to GIIc strains with lower mortality rates in Europe, the prevalence of highly virulent GII strains with almost 100% mortality in Asia and the Americas caused greater losses to the swine industry. Moreover, due to the high heterogeneity of PEDV, vaccines developed for GI strains cannot effectively prevent and treat infections caused by highly virulent GII strains. Comparing the heterogeneity between variant strains and classical strains and analyzing the impact of these variants on virulence changes will help to parse out important factors affecting virus virulence and assess in a timely way the potential threat of emerging variants to the swine industry. Here, we describe the effects of PEDV viral proteins on virulence change and growth adaptation of strains from the perspective of gene heterogeneity. Finally, we try to find the key amino acids on the S protein that affect virus virulence and cell growth fitness, which have important reference values for the development of attenuated vaccines.
2.1. Variation in Structural/Accessory/Non-Structural Proteins
The viral S protein, which is involved in receptor recognition and facilitates membrane fusion and anchoring, is a major factor in host tropism and pathogenesis of coronaviruses and may play important roles in virulence and host or tissue tropism [106,107,108]. To explore the effect of strain variation on virus virulence, in Table 2, we summarize the amino acid indels in the genome of several variant strains with the highly virulent parental strains as a reference.
Table 2.
Representative PEDV strains with protein variations that influence changes in viral virulence.
| Genotype | Strain | ORFs | Major Variation | Pathogenicity | Reference |
|---|---|---|---|---|---|
| GIIb | icPC22A-KDKE4A-SYA | Nsp16, S | Nsp16 (quadruple alanine substitutions[K45A-D129A-K169A-E202A]), S (Y1378A) | Attenuated | [109] |
| GII | FJzz1-F200 (FJzz1) | S1-NTD | 55I56G57E → 55K56Δ57Δ, 877–878SG →877–878RR | Attenuated | [110,111] |
| GII | Iowa106 (PC21A) | S1 | Deletion at nt 176–186 and nt 416–418, 6-nt insertion at nt 474–475 | Attenuated | [63,112] |
| GII | 5-17-V (KF452323) | S1-NTD | Deletion at aa 23–229 | Attenuated | [113] |
| GIIa | TC-PC177, icPC22A-S1Δ197 (PC21A, icPC22A) | S1-NTD | Deletion at aa 34–230 | Attenuated | [44,74] |
| GIIb | icPC22A-icΔ10aa (icPC22A) | S-CTs | ΔYxxΦEKVHVQ | Attenuated | [114] |
| GIIb | FL2013 (AJ1102) | S-CTs | Deletion at aa 1385–1391 | Attenuated | [1,115] |
| GIIb | KNU-141112-S DEL2/ORF3 (KNU-141112) | S, ORF3 | S N-DEL2 (a combination of S C-DEL5/ORF3 N-DEL70, and ORF3 C-DEL88) | Attenuated | [116] |
| GIa | CHM2013, SM98, AVCT12 (CV777) | S-CTs, ORF3, M | S (deletion at nt 4144–4165), ΔORF3 (deletion at nt 1–30), M (4–aa [MLVL]) insertion at nt 36–37) | Mild | [55,117,118] |
| GIIb | 17GXCZ-1ORF3d (17GXCZ-1ORF3c) | ORF3 | Deletion at nt 172–554 | Virulent | [119] |
| GIIa | HN2021 (HNCADC-2017) | ORF3 | Deletion at nt 207–373 | Virulent | [117] |
| GIb | attenuated DR13 (DR13) | ORF3, E | ORF3 (Deletion at nt 245–293), E (Deletion at nt 67–87) | Attenuated | [20,120,121] |
| GIIb | SH (LZW) | N | Deletion at aa 399–410 | Virulent | [30] |
S1 N-terminal domain: S1-NTD; S1 core neutralizing epitope: S1-COE; S C-terminals: S-CTs; 55I56G57E → 55K56Δ57Δ, deletion aa GE at residues 56 to 57.
The S1 subunit of the S protein is an important determinant of PEDV virulence, using reverse genetics [118]. Of these attenuated PEDV strains, most have a deletion in a stretch of amino acid sequences of the S gene, which clarifies the role of the S protein in virulence. The PEDV S protein contains two sorting motifs in the cytoplasmic tail: endocytosis signal-YxxΦ and ER retrieval signal (ERRS)-KVHVQ. Some studies have reported that deletion of the conserved motif of YxxΦEKVHVQ can prematurely truncate the S protein and reduce the pathogenicity in piglets [114], which clarifies the effect of the conserved motif of the S protein on virulence.
The S protein is not the only factor affecting viral virulence; ORF3 also plays a role in enhancing viral virulence. In contrast to the BJ2011C strain infected with a severe phenotype, the CHM2013-SBJ strain with a 70–aa deletion in ORF3 did not have any pathogenic properties during infection, underscoring the role of ORF3 in conferring virulence [122]. Similarly, the attenuated DR13 strain lacks 16 amino acids in the ORF3 gene, making it a candidate vaccine against PEDV infection [123]. However, not all ORF3 truncations are effective in reducing pathogenicity; the strains P55, 17GXCZ-1ORF3d and HN2021 with field naturally truncated ORF3 were all lethally virulent compared to field isolates with intact ORF3 [122,123,124]. Of course, the field strains CHM2013, SM98, and AVCT12 in which 10 amino acids were deleted continuously from the start codon resulting in a full-length truncation of ORF3, had mild phenotypes [55,117,118]. These could be explained by the fact that in field isolates, only the complete truncated full-length ORF3 strain may contribute to the reduction of pathogenicity. It has been shown that ORF3 enhances virus production through ion channel activity [120]. A mutation at residue 170 of ORF3 (Y170A) can significantly reduce its ionic activity [84], which may suggest that the Y170A mutation can be considered one of the targets for reducing virulence.
Studies have shown that the nonstructural protein nsp16, a 2′-O-methyltransferase (2′-O-MTase), also partially attenuates the pathogenicity of severe acute respiratory syndrome coronavirus (SARS-CoV) [121], and may be another important cofactor affecting virus virulence. Studies have confirmed that the attenuated KDKE4A-SYA mutant was constructed by inactivating 2′-O-MTase activity with quadruple alanine substitutions (K45A-D129A-K169A-E202A) and inactivating the endocytic signal of the S protein with mutation (Y1378A), which is a new candidate for live attenuated vaccines [109].
2.2. Sequence Heterogeneity of S, ORF3, E, M, and N Genes
The availability of attenuated vaccine strains is a major concern, due to the extensive genetic diversity of PEDV strains. To comprehensively study the genetic diversity of PEDV, here, we describe the contribution of each viral protein to the genetic diversity of PEDV from the perspective of viral genome conservation.
The study showed that in 28 Korean field samples, M (0–4%), ORF3 (0–3.9%), and E (0–5.3%) were more conservative than S (0.1–8.4%) and N (0.1–6.8%) [55]. Another report pointed out that compared with nsps, M, and N proteins, structural proteins S, and E, and accessory proteins ORF3 were subjected to higher mutational pressure and could generate mutations as high as 57.1% to 71.4%. The M and N proteins, on the other hand, have a higher degree of conservation and are not mutated [116].
In general, the structural protein S is recognized as the most genetically diverse protein with the highest mutation frequency, and the S gene is often used to construct a phylogenetic tree to study the evolutionary relationship between virus strains. Firstly, the state of S protein N-linked glycosylation may affect viral replication and cellular tropism of viral pathogens. The S1 N-terminal region is the region with the largest number of amino acid variations [81,125]. Variations in this region may lead to the generation or elimination of potential N-glycosylation sites [126]. It is not difficult to see that the N-terminal region of the S protein may be an important area in which to study the genetic correlation between virus strains. The glycosylation site at residue 725 of the S protein is also a common site of frequent loss [124], and its function is still unknown. Secondly, variation in the N-linked glycosylation site of the S protein may affect the antigenicity of PEDV [111]. The S protein epitope SS2 (748YSNIGVCK755) and epitope 2C10 (1368GPRLQPY1374), which can induce neutralizing antibodies against PEDV, were conserved in all the Chinese field strains [127]. However, the epitopes COE (aa 499–638) and SS6 (764LQDGQVKI771) show diversity in most field strains and are mutation-prone areas in the epitope regions [127]. Variations on the epitopes COE and SS6 of the S gene may be important references for the development of attenuated vaccines.
ORF3 and S genes have the most insertions and deletions of nucleotide fragments, and may be the two most heterogeneous structures. Much of the focus has been on ORF3 insertions or deletions, and the effects of mutations in ORF3 on virulence or other properties have rarely been examined. As a controversial accessory protein for virulence [122,128], the amino acid variation caused by its nucleotide mutation may be more related to the adaptability of the growth environment.
The E protein, like the S, is a cellular marker of PEDV growth adaptation and virulence attenuation [126]. However, genetic and phylogenetic analyses of the E gene have been rarely performed. Except for the attenuated DR13, the E gene of all Chinese PEDV strains has very high conservation [9]. So far, no relevant studies have verified the function of the E protein in virus virulence.
The M protein is involved in the viral envelope, and interacts with the virion core, playing an important role in the viral assembly process [129]. The M gene sequence analyses of 31 PEDV isolates obtained in Thailand and of six PEDV isolates obtained in China both showed that there were no insertions and deletions in the entire M gene, and the glycosylation sequence Asn-Phe-Thr (NFT) in the M gene was also highly conserved [21,130]. The relationship between M gene heterogeneity and virus pathogenicity has not yet been well examined.
The N protein is an important component of phylogenetic analysis [131,132,133], involved in packaging the viral genome to facilitate viral assembly [134]. The N gene is also highly conserved; none of the early Chinese isolates and the 15 Chinese field samples had insertions or deletions in the N gene, and the N-terminal part of the protein was more conserved than the C-terminal part [132,135]. PEDV strains with mutations in the N gene may evade the established host immune responses [136], enhancing the growth adaptability of the virus itself.
2.3. Potential Key Amino Acids
As a key protein that determines the virulence of PEDV, when the attenuated strain does not produce representative variants of deletion or insertion, the effect of S gene mutations on virus virulence and growth adaptability is particularly important, and cannot be ignored. On the one hand, mutations in the S gene may impair the recognition of porcine receptors and reduce the replication effect of PEDV in vivo [137]. On the other hand, mutation of the S gene may be a positive selection, leading to differences in its immunogenicity, which in turn diversifies the neutralization profiles of the virus [138]. To find functional amino acid sites that may be closely related to virus pathogenicity and growth fitness, in Table 3, we summarize the amino acid mutations accumulated in five cell-adapted attenuated strains and one field attenuated strain.
Table 3.
Amino acid changes on the S protein of PEDV attenuated strains.
| ORFs | PC22A-P120 [139] | CT-P120 [140] | PT-P96 [141] | YN144 [47] | FJzz1-F200 [110] | OH851 [51,64] | |
|---|---|---|---|---|---|---|---|
| S1 | domains 0 | 55–57IGE→K–, I166V | T144I | ∆144–145TG | 55–57IGE→K–, F128Y | S88A, N130D, N132K | |
| domains A | Q454K, D466G, ^477H | D265A | D405G, G428A | D265A, D378N, T491R | T361A | ||
| domains B/COE | F636R | F555S | |||||
| domains CD | S723R | A694D | |||||
| S2 | V881F, Q893K, A971V, G1009V, F1015L, E1379 stop | S888R, C1363G | S888R, S969A, I1022S, K1027R, L1253R, C1355F, C1359F | T780N, Q826H, I1011V, I1305L, C1355F | K774N, 888–889SG →RR, L901V, N1010D, I1340T, C1355F | G1162S, V1242L | |
1. The amino acid changes of the attenuated strains CT-P120, PT-P96, YN144, FJzz1-F200, PC22A-P120, and OH851 are based on the nucleotide sequences of CT-P10, PT-P5, YN15, PC22A-P3, FJzz1-F20, and GER/L00719/2014, respectively. 2. The residue positions on each domain were referenced to the S protein of the PC22A strain (GenBank: KM392224.1). 3. S1 subunit domains 0, residues 19 to 219; domains A, residues 220 to 509; domains B/COE, residues 510 to 639; domains CD, residues 640 to 728; S2 subunit, residues 729 to 1387 [142]. 4. ^477H, aa H was inserted at residue 477.
In these PEDV strains, several potentially important amino acid mutations have been elucidated in a review [137]. Amino acid mutations with different properties accumulate in the virus strains with an increasing number of passages, while the mutational spectrum is stable over time, which may increase the stability of the virus in the host. The accumulation of amino acid substitutions is accompanied by drastic changes in amino acid properties, especially mutations in the S1 subunit of the S protein, more than 93.3% of which result in transitions between hydrophilic and hydrophobic amino acids and changes in the charged properties of amino acids. However, the number of these transitions between hydrophilic and hydrophobic amino acids is essentially symmetrical in each strain, which may maintain the biochemical stability of viral proteins. As previously mentioned, the S1 subunit of the S protein is an important factor in determining PEDV virulence [118]. The PC220A-P120, PT-P96, YN144, FJzz1-F200, and OH851 strains were all mutated at domain 0 of the S1 subunit, which has the effect of binding sialic acid receptors on host cells to attach to virions [142]. Mutations in this domain may disrupt binding to sialic glycans and thereby reduce PEDV entry. The domains S A, S B, and S CD of the S1 subunit of the S protein have many B-cell epitopes that induce neutralizing antibodies [142,143], and mutations in these domains may affect the recognition of neutralizing antibodies. Especially in the CT-P120 and PT-P96 strains, the F636R and F636S mutations on the epitope COE are likely to reduce the reactivity of virus-neutralizing (VN) antibodies. The S2 subunit, which contains major immunodominant neutralizing epitopes [144], has a higher mutation frequency than the S1 subunit. More than 63% of mutations in the S protein occur in the S2 subunit. However, the mutations in the S2 subunit are milder. In YN144, PC22A-P120, and CT-P120 strains, 50–60% of the amino acid mutations did not change their biochemical properties, and the rest were changed in respect to charge properties. In PT-P96 and FJZZ1-F200 strains, there are still 85% of amino acid mutations with drastic changes in amino acid properties (charged, hydrophilic or hydrophobic), which may tolerate higher mutation pressure. Frequent mutations on the S2 subunit may be the main reason for the failure of neutralizing antibodies. Taken together, the attenuated virulence of these strains may be due to mutations in the S gene that impair the ability of receptor recognition and binding.
Studies have reported that trypsin can promote cell fusion and viral infection by cleaving the S protein [145]. Efficient viral infection of strains that result in enhanced cellular adaptability through serial passaging is generally more dependent on the presence of trypsin, and mutations in the S2 subunit can control the trypsin dependence of PEDV [146]. In these cell-adapted strains, mutations in the S2 subunit may be closely related to cleavage by trypsin [147]. It has been confirmed that the S888R (R885) mutation may promote the cellular adaptation of the virus by adding a new trypsin cleavage site [146]. Since trypsin cleaves only at arginine and lysine residues, we can speculate that the 888–889SG →RR, F1015L, K1027R, V1242L, and I1305L mutations in these strains might increase trypsin cleavage, to promote the cellular adaptation of PEDV. In addition, common mutations in these attenuated strains may also be a marker of cellular adaptation [146]. From Table 3, we can see that the variant at residue 144 of domain 0, the D265A substitution in domain S A, and the S888R and C1355F substitutions in the S2 subunit, all have higher mutation frequencies, which may be closely related to cell adaptability.
Unlike those cell-adapted strains, amino acid variations on the field strain OH851 were more associated with virulence. Piglets infected with the new variant OH851 with insertions and deletions in the S gene exhibited few or no clinical symptoms [51]. The field variant strain GER/L00719/2014 found in Germany and Portugal, although 99.5% similar to the American OH851 strain [13], had typical severe watery diarrhea symptoms and high mortality [64,67]. Sequence alignment revealed that changes in clinical pathogenicity may be due to the variations in key amino acids that affect virulence. Compared with cell-adapted strains, the variation in the field strain OH851 deserves more attention, because it is a likely target for the development of attenuated vaccines.
3. Virus-Host Interaction
PEDV prefers to infect and replicate in the swine intestinal tract, including the villous epithelial cells of the small intestine and jejunum, and the surface epithelial cells of the caecum and colon, of which the jejunum and ileum are the main sites of infection [43,148,149,150]. Infection with PEDV promotes fusion between cells, leading to continuous necrosis of cells, resulting in severe diarrhea and even death in piglets [7]. At the same time, PEDV is sensitive to the porcine liver, resulting in abnormal cholesterol metabolism genes, and disrupting the metabolic homeostasis of cholesterol [151,152].
A comprehensive understanding of the infection mechanism by which PEDV invades host cells and achieves replication has important implications for the prevention and control of viral infection. Firstly, PEDV relies on serine proteolysis and low pH to enter cells through endocytosis, which is the entry mechanism by which PEDV infects the host [153]. Secondly, PEDV mainly utilizes viral components to evade the host’s innate immune responses during host infection. It is well known that after the virus invades the host cells, interferon (IFN) plays an important role as a key signaling molecule to block virus infection [154], especially type I IFNs, which clear the virus by activating various immune cells and costimulatory molecules [155].
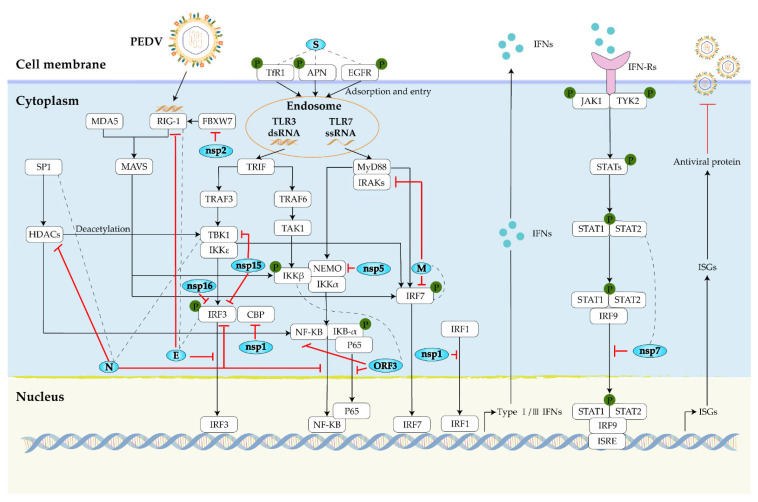
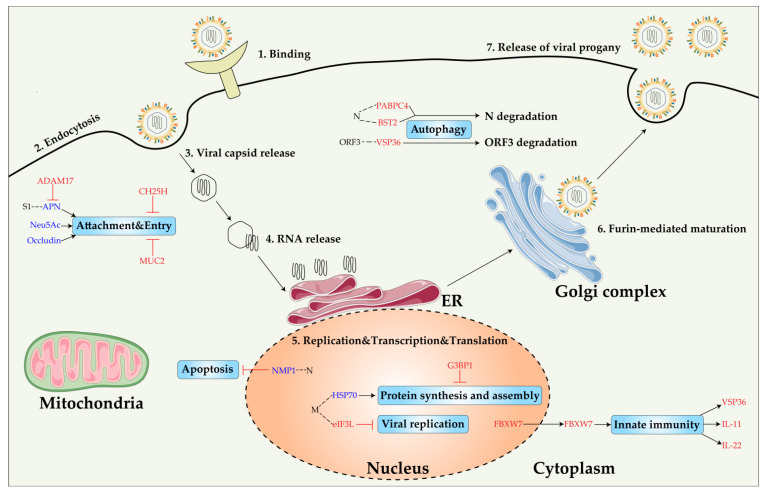
To better understand the biopathogenesis of PED, here, we map the network of PEDV-host interactions (Figure 3), and further elucidate the roles of PEDV components during viral infection. Moreover, host factors, especially functional receptors, may be potential antiviral targets for the effective control and elimination of PEDV. Here, we extend the map of pro/anti-viral host factors of PEDV and summarize the roles of the host factors during PEDV infection (Figure 4).
Figure 3.
Schematic diagram of PEDV evading the host innate-immune signaling pathway. On the one hand, PEDV infection activates the host innate-immune signaling pathway. On the other hand, PEDV proteins exert inhibitory effects at different stages of the host innate immune response. Interaction of PEDV components with host proteins is indicated by dotted lines; downstream activation is indicated by black arrows; inhibition or degradation of target genes is indicated by red lines ending in dashes.
Figure 4.
Schematic illustration of PEDV infection in host cells and the role of pro/antiviral host factors in PEDV replication. There are four main stages in the PEDV life cycle: attachment, entry, replication, and release. Interaction of host proteins with PEDV proteins is indicated by dotted lines; proviral host factors are indicated in blue text; antiviral host factors are indicated in red text; downstream activation is indicated by black arrows; inhibition or degradation of target genes is indicated by red lines ending in dashes.
3.1. PEDV Proteins That Interact with Host Factors
Cell entry of the coronaviruses mainly depends on the interaction of the S protein with the receptor. The cleavage and activation of the S protein may depend on the hydrolysis of S protein by serine proteases at the S1/S2 junction and adjacent to the fusion peptide in the S2 region, during the virus entry process [153,156]. The S protein enhances TfR1 internalization by interacting with the extracellular domain of TfR1, facilitating viral entry [157]. The S1 subunit of the structural protein S is a key region for the recognition and binding of cellular receptors [158]. Both the C-terminal domain (S1-CTD) and N-terminal domain (S1-NTD) of the S1 subunit of coronaviruses can interact with receptors as RBDs [159]. In a variety of coronaviruses, S1-CTD recognizes and binds to the angiotensin converting enzyme 2 (ACE2), dipeptidyl peptidase 4 (DPP4), and aminopeptidase N (APN) to mediate the entry of SARS-CoV, human coronavirus NL63 (HCoV-NL63), Middle East respiratory syndrome coronavirus (MERS-CoV), transmissible gastroenteritis virus (TGEV) and porcine respiratory coronavirus (PRCV) [159,160,161,162,163,164,165,166,167,168]. Meanwhile, S1-NTD, as RBDs, interacts more with the carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1) or sugar receptors to promote the attachment and entry of viruses such as mouse hepatitis virus (MHV) and bovine coronavirus (BCoV) [169,170,171]. Similar to other coronaviruses, the RBDs of the receptor pAPN are located within the S1-CTD of PEDV, while the S1-NTD of PEDV is considered to be the RBDs of sialic acid glycans [72,172]. One study showed that the S protein inhibits IFN-mediated innate immune responses by binding to the epidermal growth factor receptor (EGFR) to increase PEDV infection [173]. As a transmembrane glycoprotein, EGFR may bind to S1-CTD, to promote virus entry, but the RBDs of the S protein that interacts with it remain to be verified.
The E and M proteins inhibit type I IFN immune responses by directly interacting with IRF3 and IFN regulatory factor 7 (IRF7), respectively [89,174]. In addition to evading innate immune responses, the E protein induces endoplasmic reticulum (ER) stress, upregulates IL-8 expression, and may play a role in inhibiting host-cell apoptosis [87]. It has been confirmed that the M protein interacts with five proteins (RIG-I, PPID, NHE-RF1, S100A11, CLDN4), among which the M protein may inhibit the proliferation of PEDV by interacting with S100A11 or PPID [175].
The structural protein N plays an important role in promoting viral replication and anti-innate immune responses. In TGEV, the N protein is not essential for RNA replication but is a key protein required for transcription [100]. While the role of PEDV N protein in RNA synthesis has not been elucidated, there has been a lot of indirect evidence for the role of N in PEDV replication. One piece of evidence is that the N protein colocalizes and interacts with nucleophospholipid (NPM1) in the nucleolus, promoting viral replication and inhibiting the apoptotic capacity of cells [104]. Similarly, the interaction of the N protein with p53 promotes viral replication mainly by inducing arrest in the S phase of the cell cycle [176]. Another piece of evidence is that the N protein interacts with heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1), an RNA-binding protein involved in pre-mRNA splicing and mRNA nucleo-cytoplasmic export in the nucleus [177], promoting PEDV replication [178]. In addition, the PEDV N protein has many mechanisms to evade the host immune responses, one of which is the most classical anti-IFN immune response: the N protein antagonizes the production of IFN-λ3 by blocking the nuclear translocation of NF-κB [179]; the N protein targets TANK-binding kinase 1 (TBK1) through direct interaction, which competitively inhibits the binding of TBK1 to IRF3, resulting in the inhibition of IRF3 activation and the subsequent production of type I IFN [102]. Another novel mechanism for evading host immune responses is the inhibition of HDAC1, an important regulator of innate immunity: the N protein in the nucleus interacts directly with Sp1, an important transcriptional regulator of histone deacetylase (HDAC) expression, indirectly inhibits the replication and transcription of multiple innate immune effectors regulated by HDAC1 [180,181].
ORF3 is mainly localized in the ER and triggers ER stress responses related to apoptosis or autophagy [182]. Accumulation of ORF3 is also found in the Golgi apparatus (Golgi), which is transported from the ER to the Golgi region via the exocytosis pathway [120]. We also found the presence of ORF3 on the surface of infected cells, and interacting with the S protein at the plasma membrane [183], which may be related to the ability to regulate viral replication, where the 170YLAI173 motif in the C-terminal part of ORF3 protein is crucial for the transport of ORF3 from the ER to the plasma membrane [120]. ORF3 inhibits NF-κB activation by inhibiting the phosphorylation and expression of nuclear factor P65 and interfering with the nuclear translocation of P65, thereby reducing the production of proinflammatory cytokines IL-6 and IL-8 [184]. Meanwhile, ORF3 interacts with IκB kinase β (IKBKB), induces IKBKB-mediated NF-κB promoter activity, and activates type I IFN induction, but inhibits Poly I: C mediated type I IFN production and induction [185]. These findings highlight the complex role of ORF3 in immune signaling and virus–host interactions.
The nonstructural proteins nsps encoded by ORF1a/b have distinct anti-host immune responses, possibly regulating viral replication by enhancing the translation of downstream ORFs [186]. Among them, nsp1 is the most potent suppressor of proinflammatory cytokines, interfering with IFN-induced immune processes [187,188]. Unlike the E protein interfering with IRF3 nuclear translocation, nsp1 does not interfere with IRF3 phosphorylation and nuclear translocation, but blocks the assembly of IRF3 and CREB-binding protein (CBP) enhanceosome by degrading CBP to suppress the innate immune responses of type I IFN [97]. Besides this, nsp1 also interferes with the nuclear translocation of interferon regulatory factor 1 (IRF1), and suppresses IRF1-mediated type III IFN immune responses [187]. The nsp2 acts through a novel mechanism of non-IFN-mediated host immune responses, which interacts with the innate antiviral factor FBXW7 and hinders the activation of the host innate antiviral response by targeting the ubiquitin-proteasome-mediated degradation of FBXW7 [189]. Interestingly, nsp4 is involved in the inflammatory response of the host, directing the expression of proinflammatory cytokines and chemokines, possibly inhibiting PEDV replication in vitro [190]. PEDV nsp5, encoding 3C-like protease, is another IFN antagonist as well as the N protein. Consistent with the foot-and-mouth disease virus (FMDV), hepatitis A virus (HAV), and PRRSV [191,192,193], PEDV nsp5 cleaves the NF-kappaB essential modulator (NEMO), disrupting type I IFN signaling [194]. The nsp6 induces autophagy through the PI3K/Akt/mTOR signaling pathway and has a similar function to its downstream nsp9 in promoting PEDV replication [195,196]. During PEDV infection, PEDV degrades the signal transducer and activator of transcription 1 (STAT1) proteins in a protease-dependent manner, to interfere with the type I IFN signaling pathway [197]. However, the functional protein of PEDV that degrades STAT1 remains unclear. There is only limited evidence that nsp7 is related to STAT1, and nsp7 can interact with the DNA-binding domains of STAT1/STAT2, blocking the nuclear translocation of STAT1, and further antagonizing IFN-α-induced JAK-STAT signaling [198]. In addition, nsp12, an RNA-dependent RNA polymerase, and nsp13, a helicase, participate in the replication process of PEDV by promoting the release of viral RNA [196,199]. The G-N-7 methyltransferase (G-N-7 MTase) activity of PEDV nsp14 plays an important role in regulating PEDV replication and type I and type III IFN immune responses [200]. Unlike the N protein sequestered binding of TBK1 and IRF3, nsp15 utilizes endoribonuclease (EndoU) activity to degrade the RNA of TBK1 and IRF3, to inhibit their mediated type I IFN response. The three residues (H226, H241, and K282) of nsp15 that affect the activity of EndoU may be the key amino acids for the inhibition of the antiviral activity [201]. The methyltransferase nsp16 is a more potent regulator of immune-related genes, which relies on KDKE tetrad to regulate the 2′-O-MTase activity, not only reducing IFN-β production but also inhibiting IRF3 phosphorylation, effectively modulating the host antiviral response to multiple viruses including PRRSV, vesicular stomatitis virus (VSV), and PEDV [202].
Collectively, each protein component of PEDV plays an important role in enhancing viral replication and assembly and evading the host’s innate immune responses. In the case of some nonstructural proteins, such as nsp1, nsp5, nsp6, and nsp13–16, although their roles in evading innate immune signaling have been elucidated, whether they interact with host chaperones remains to be investigated. The analysis of interacting proteins may be important for the elucidation of the molecular mechanisms that promote viral proliferation and evade host immune responses.
3.2. Proviral Host Factor
Host factors required for coronavirus replication are the main targets of antiviral drugs, especially pan-coronavirus host factors. Unraveling the host factors required for coronavirus to infect cells is critical to the development of antiviral drugs.
Each coronavirus has individual specific functional receptors. However, due to limitations such as frequent variants of PEDV and the lack of susceptible host cell lines, especially porcine-derived ones, the research on the infection mechanism of the PEDV virus and the mining of functional host factors have become difficult. difficult. In the process of infecting the host, the virus usually utilizes the components of the host cell to promote the entry, replication, and assembly of the virus, which we generally refer to as proviral host factors. Proviral-host factor inhibitors have also been a commonly used means of antiviral therapy. In Table 4, we summarize the proviral host factors of PEDV and their important functions during virus infection, and describe their mechanism of proviral effect in host cells.
Table 4.
An overview of host factors.
| Host Factors | Function | Role in VIRAL Infection | Reference | |
|---|---|---|---|---|
| Proviral factors | pAPN | Enzymatic cleavage of peptides; endocytosis; signal transduction | PEDV binds pAPN domain VII for entry into cells | [203,204] |
| Neu5Ac | As a cell surface glycoprotein; immune regulation and recognition; viral interactions | As a sugar coreceptor for PEDV entry cells | [73,205,206] | |
| Occludin | A tight junction protein; signal transduction; innate immune regulation | As a PEDV entry cofactor | [207,208] | |
| NPM1 | Ribosome assembly and chromatin remodeling; nuclear export; cell growth regulation | Interacts with N protein to promote PEDV growth | [104,209] | |
| HSP70 | Protein folding; participates in cellular processes; promotes viral replication. | Interacts with M protein to regulate PEDV replication, viral protein synthesis, and assembly | [210] | |
| EGFR | Regulates endocytic transport; regulates sorting after internalization and endocytosis | Inhibits I-IFN response through STAT3-mediated signaling | [211] | |
| Antiviral factors | BST2 | Regulates the transport of secreted cytokines; IFN-inducing markers | Binds and degrades the N protein of PEDV to inhibit PEDV replication | [212,205] |
| VPS36 | Regulation of protein sorting and MVB biogenesis | Promotes degradation of ORF3 by interacting with ORF3 | [206,213] | |
| CH25H | Regulates lipid metabolism, cholesterol homeostasis, inflammation, and immune responses | Inhibits entry of PEDV virions | [214,215] | |
| G3BP1 | Involves RNA recognition, host mRNA turnover and translation, SG formation | Induces antiviral SG formation and impairs PEDV replication | [216,217] | |
| FBXW7 | Regulates immune cells; tumor suppressor | Promotes host IFN-mediated antiviral response | [218] | |
| ADAM17 | Mediates cleavage and cleavage of cell surface proteins | Inhibits PEDV infection by regulating APN expression | [219] | |
| eIF3L | Regulates the physical stability of eIF3 assembly | Inhibits PEDV replication by interacting with M protein | [220,221] | |
| PABPC4 | An RNA processing protein that enhances translation and mRNA stability | Promotes degradation of N protein by interacting with N protein | [222] | |
| CD44 | Regulates signal transduction and tumor growth; cell adhesion | Activates NF-κB nuclear translocation and enhances protective cytokine release | [223,224] | |
| IL-11 | Regulates inflammation, apoptosis, epithelial regeneration and fertility | Anti-PEDV infection by activating the STAT3 signaling pathway | [225,226,227,228,229] | |
| IL-22 | Inhibits pro-inflammatory responses; protects the host gut barrier; maintains tissue integrity | Anti-PEDV infection by activating the STAT3 signaling pathway | [230,231,232] | |
| MUC2 | Regulates intestinal homeostasis | Regulates the replication of PEDV | [233,234] |
APN (CD13) is an ectoenzyme with membrane binding and signaling functions [204] and is a functional receptor for various coronaviruses such as human coronavirus 229E (HCoV-229E) and TGEV [168,235]. Porcine APN (pAPN) is a functional receptor of PEDV [203], and PEDV S1-NTD-CTD can effectively bind to domain VII of pAPN for entry into the host cells [72,236]. However, recent studies have found that APN is not essential for PEDV entry into cells [237], and the claim that pAPN acts as a functional receptor for PEDV is inaccurate. The increased susceptibility of pAPN transgenic mice to PEDV may be explained by pAPN promoting PEDV infection through its protease activity [238,239].
Sialic acid (SA), mainly the terminal component of glycoproteins and glycolipids, a monosaccharide derivative, exists on the cell surface and is a cofactor for viral attachment and entry into cells [240]. There are three forms of SA commonly found in mammals: 5-N-acetylneuraminic acid (Neu5Ac), 5-N-glycolylneuraminic acid (Neu5Gc) and 2-keto-deoxynonulosonic acid (Kdn) [241]. Sialylglycans are the functional receptors for influenza viruses such as avian and human infectious bronchitis coronavirus (IBV) and influenza A virus (IAV), but avian and human influenza viruses generally have different SA binding preferences [242,243]. SA facilitates the infection of multiple coronaviruses, among which MERS-CoV and TGEV preferentially bind Neu5Ac [244,245]. PEDV as betacoronaviruses (β-CoVs) also utilizes Neu5Ac as a sugar receptor. However, the binding ability of S protein to SA varies with PEDV strains [73].
Tight junction (TJ) proteins are multiprotein complexes formed by several different integral membrane proteins [246]. A variety of viruses use components of the TJ proteins as receptors to enter host cells through internalization pathways, including the coxsackie and adenovirus receptors (CAR) and the human occludin receptor [247,248,249]. The TJ protein occludin plays an important role in maintaining and repairing the intestinal mucosal barrier [208]. Recently, studies have shown that occludin is not effective at the attachment of PEDV, but occludin acts as a cofactor for PEDV entry into host cells, whose internalization is closely related to virus entry [250].
Nucleolar phosphoprotein nucleophosmin 1 (NPM1), which predominantly resides in the nucleolus, is a multifunctional protein that plays an important role in RNA transcription, ribosome assembly, DNA replication and repair, nuclear export, and cell growth regulation [209]. NPM1 promotes infection by a variety of viruses, including the human immunodeficiency virus type 1 (HIV-1) [251], Japanese encephalitis virus (JEV) [252], adenovirus [253], herpes simplex virus 1 (HSV-1) [254], Epstein–Barr Virus (EBV) [255], and Schmallenberg virus (SBV) [256], function at all stages of viral infection. As a proviral factor, NPM1 binds to the cap proteins of porcine circovirus types 2 and 3 (PCV2 and PCV3) to promote nucleolar localization and viral replication [257,258,259]. However, there is also evidence for NPM1 as an antiviral factor that limits the replication of the chikungunya virus (CHIKV) [260]. NPM1 binds to the N protein of PEDV and colocalizes in the nucleolus, ultimately promoting viral replication mainly by enhancing cell growth and survival [104]. The binding of NPM1 to other proteins of PEDV and its role in viral infection remains to be further explored.
Heat shock protein 70 (HSP70) is involved in the posttranslational folding of the protein, membrane protein localization, assembly and disassembly of the protein complex [261,262], and promotes viral replication during RNA virus infection [263]. HSP70 plays a proviral role by binding to the M protein during PEDV infection [210]. However, Hsp70 exerts antiviral effects by inhibiting the nuclear export of the influenza virus ribonucleoprotein complex (vRNP) and the replication process of VSV infection [264,265]. HSP70 has positive and negative dual regulatory roles on different virus types, showing the complexity of virus-chaperone protein interactions.
The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor that acts as a cofactor for viral entry into the host cells during hepatitis C virus (HCV), TGEV, and IAV infection [266,267,268,269]. EGFR signaling is a central pathway required for SARS-CoV-2 replication [270], and EGFR inhibition has emerged as a novel strategy to suppress endogenous antiviral defenses during host infection by respiratory viruses and PEDV [173,270]. However, at what stage EGFR plays a role in PEDV infection of host cells, has not been elucidated.
3.3. Antiviral Host Factor
In addition to host factors with proviral effects, some host factors resist the invasion of viruses by conferring protection on cells against virus infection, which are generally called antiviral host factors. Antiviral factors are positive regulators of the host’s innate immune system, and become potential targets for antiviral therapy. In Table 4, we summarize the antiviral host factors of PEDV and describe their antiviral effects in host cells.
Bone marrow stromal cell antigen 2 (BST2), also known as CD317, is an IFN-induced type II transmembrane glycoprotein [205]. BST2 inhibits the release of a large number of enveloped viruses, such as human HCoV-229E [271], human HCV [272], Dengue virus (DENV) [273], JEV [274], HIV [275,276], Ebola virus [277], Marburg virus (MARV) and Lassa virus (LASV) [278], HSV-1 [279], human cytomegalovirus (HCMV) [280] and other types of viruses. Studies have shown that BST2 combines with PEDV N protein to achieve protein degradation, thereby inhibiting the biological function of the N protein, while the role of BST2 in viral envelope release has not been elucidated.
Vacuolar Protein Sorting Associated Proteins 36 (VPS36) together with Vps22 and Vps25 form the ESCRT (Endosomal Sorting Complexes Required for Transport)-II complex [281], which plays a role in the multivesicular body (MVB) pathway of protein sorting and degradation, leading to the degradation of proteins in lysosomes [206]. VPS16 and VPS35 of the VPS family promote the infection of human coronavirus OC43 (HCoV-OC43), HCoV-229E, and SARS-CoV-2 coronaviruses [282,283]. However, VPS36 was found to interact with ORF3 during PEDV infection, inhibiting PEDV replication by promoting ORF3 degradation [213]. The VPS family may play different roles in the viral infection process.
Cholesterol-25-hydroxylase (CH25H) is a hydroxysterol belonging to the interferon-stimulated gene (ISG) family [284]. CH25H and its enzymatically catalyzed cholesterol product, 25-hydroxycholesterol (25HC), have diverse biological functions, including the regulation of cholesterol metabolism, the dual role of suppressing or increasing inflammation, and the regulation of B- and T-cell-mediated immune responses [214]. CH25H and 25HC also have broad antiviral activity, inhibiting the infection of VSV [285], rabies virus (RABV) [286], LASV [287], ZIKV [288], human rhinovirus (HRV) [289], porcine pseudorabies virus (PRV) [290], and PRRSV [291,292] through multiple mechanisms. Recent evidence suggests that CH25H and 25HC play an important role in hindering the entry of PEDV virions [215]. As an antiviral factor, CH25H may be an important target for broad-spectrum antiviral therapy.
Ras-GTPase-activating protein-binding protein 1 (G3BP1) is a multifunctional RNA-binding protein involved in various biological functions such as RNA recognition, mRNA turnover and translation, cell proliferation, apoptosis, and differentiation [293]. G3BP1 is also a key component in the assembly of SGs [294]. In response to stimuli, host cells form stress granules (SGs) to prevent viral protein synthesis and regulate viral replication [295]. SGs are more antiviral, and a variety of viruses including many RNA viruses [296,297,298,299,300,301], enteroviruses [302,303], coronaviruses [304], influenza viruses [305], and reoviruses [306,307], possibly inhibit the formation of SGs by cleaving G3BP1 or disrupting the eIF4GI-G3BP1 interaction. Similarly, G3BP1 has an antiviral role in PEDV infection, and PEDV promotes viral replication by inducing the degradation of G3BP1 [216,217]. however, CHIKV of the alphavirus genus has evolved a different mechanism to promote efficient replication by utilizing the SGs component G3BP1 [308]. Overall, G3BP1 functions as an antiviral factor in most viral infections.
The ubiquitin ligase FBXW7 is generally regarded as a tumor suppressor capable of targeting various oncogenic proteins for negative regulation [309]. Endogenous FBXW7 significantly inhibited human HCV replication [310], and a recent report demonstrated that FBXW7 is an innate antiviral factor. The nonstructural protein nsp2 mediates the degradation of FBXW7 by interacting with FBXW7, ultimately suppressing the host antiviral response [189]. Inhibiting the degradation of FBXW7 protein may be an effective strategy for antiviral therapy.
A disintegrin and metalloprotease 17 (ADAM17/CD156b), a member of the metalloprotease superfamily, is responsible for the cleavage of various cell surface proteins and may play a role after viral initial binding and release [219,311]. ADAM17 may have dual roles during viral infection. On the one hand, ADAM17 induces the replication of human papillomavirus (HPV) [311] and HIV-1 [312]. ADAM17 binds directly to E2 protein and plays a key role in CSFV entry [313], however regulation of ADAM17 activity does not affect SARS-CoV entry [314]. On the other hand, ADAM17 downregulates the expression of CD163, a functional receptor for PRRSV, and blocks viral entry [315]. ADAM17 also inhibits PEDV infection by regulating APN expression [316]. The mechanism of action of ADAM17 in viral infection requires more research evidence.
The L subunit of human eukaryotic translation initiation factor 3 (eIF3L) is one of the subunits of eukaryotic initiation factor (eIF3), which interacts with other eIF3 subunits and may play a role in increasing the physical stability of eIF3 [220]. The other subunit of eIF3, eIF3f, can specifically interfere with the 3′ end processing of mRNA, thereby inhibiting HIV-1 replication [317]. The eIF3L can interact with the M protein of PEDV and significantly inhibit virus replication, but how eIF3L inhibits virus infection remains to be further explored [221]. The eIF3L interacts with the NS5 protein of yellow fever virus (YFV) but has a weak role in inhibiting viral replication. During viral infection [318], more attention may be paid to the role of eIF3L in viral translation.
Poly(A) Binding Protein Cytoplasmic 4 (PABPC4) is a nuclear-cytoplasmic shuttle protein that interacts with the N protein of PEDV, SARS-CoV-2, MHV, IBV, and PDCoV multiple coronaviruses [222]. PABPC4 may inhibit coronavirus infection by degrading the N protein through the cargo receptor NDP52-mediated selective autophagy [319,320]. PABPC4 may be a broad anti-coronavirus host factor, which has important implications for the selection of antiviral targets.
CD44 is a transmembrane glycoprotein that plays an important role in cell adhesion, growth factor regulation, and signaling such as RTKs, Met, members of the TGFβ family, CXCL12 and its induced CXCR4 [321,322]. Furthermore, CD44 promotes tumorigenesis as a major cell surface receptor for hyaluronic acid (HA) [323]. The presence or absence of CD44 does not affect poliovirus replication [324], and its role in viral infection has also been overlooked. However, recent studies have shown that CD44 is an antiviral host factor that inhibits PEDV infection by activating NF-κB signaling [224]. Similarly, the Goujon C group identified CD44 as a novel anti-SARS-CoV-2 protein factor with potent activity, which has a certain impact on the infection of other coronaviruses such as HCoV-229E and MERS-CoV [325]. CD44 has broad anti-coronavirus activity, which may restrict viral entry by affecting viral internalization [325]. Similarly, chicken CD44 acts as a cellular receptor for the infectious bursal disease virus (IBDV), promoting IBDV binding and entry in B lymphocytes [326]. However, CD44 has less effect on influenza A virus IAV infection [325]. The complex role of CD44 in viral infection of different families and genera is highlighted.
Interleukin-11 (IL-11) is an inflammatory cytokine that plays an important role in inflammation suppression, anti-apoptosis, epithelial regeneration, and fertility [225,226,227,228]. There is evidence that IL-22 inhibits infection by a variety of viruses, including porcine enteric coronaviruses (PEDV, TGEV), porcine rotavirus (PoRV), human respiratory syncytial virus (RSV), and HIV-1 [229,327,328,329]. Both IL-11 and IL-22 promote the inhibition of PEDV by activating the STAT3 signaling pathway, and are two important antiviral factors during viral infection.
Mucin 2 (MUC2) is a protein secreted by goblet cells that constitutes the intestinal mucus layer that protects the intestinal epithelial cells [330,331]. MUC2 mainly has the function of maintaining the intestinal biological barrier. The expression of MUC2 mRNA was induced in PRRSV infection, which played a role in the maintenance of intestinal integrity [332]. Similarly, in PEDV infection experiments, the role of MUC2 in the antiviral was determined [234].
In addition to host genes involved in the viral infection process, MicroRNA-221-5p, a non-coding RNA involved in post-transcriptional regulation of genes, can also target the 3′UTR of the viral genome and activate the NF-κB signaling pathway to inhibit the replication of PEDV [333,334]. Noncoding RNA processing may become a new idea for antiviral therapy.
4. Conclusions and Perspectives
The high morbidity and high mortality that PEDV brings to piglets results in a rapid transmission of PEDV in pig populations, which has brought huge economic losses to the global swine industry. Considering the hazards caused by PEDV, preventive immunization is administered by vaccinating pregnant sows. However, due to the high heterogeneity of PEDV, there are currently no effective and safe vaccines to deal with the threat posed by PEDV. Exploring the genetic variation of PEDV and its virulence changes will help us to have a deeper understanding of PEDV and take more efficient diagnosis and treatment measures, accordingly. Similarly, given the problem that there are no efficient vaccines for PEDV treatment, exploring the pathogenesis of PEDV and mining the host factors that interact with PEDV will help us to find potential therapeutic targets, and then develop new prevention and control strategies. Mining host factors as the candidate targets for antiviral therapy, potentially addresses the challenges posed by PEDV genetic diversity to vaccine development. Based on the current state of research, future research should focus on finding the crucial functional receptor for PEDV or identifying key therapeutic targets, and finally developing alternative strategies to target the host proteins or regulators of immune responses, to control this pandemic.
Abbreviations
| BCoV | Bovine coronavirus |
| CHIKV | Chikungunya virus |
| DENV | Dengue virus |
| EBV | Epstein–Barr Virus |
| FMDV | Foot-and-mouth disease virus |
| HAV | Hepatitis A virus |
| HCMV | Human cytomegalovirus |
| HCoV-NL63 | Human coronavirus NL63 |
| HCoV-OC43 | Human coronavirus OC43 |
| HCoV-229E | Human coronavirus 229E |
| HCV | Hepatitis C virus |
| HIV-1 | Human immunodeficiency virus type 1 |
| HPV | Human papillomavirus |
| HRV | Human rhinovirus |
| HSV-1 | Herpes simplex virus 1 |
| IAV | Influenza A virus |
| IBDV | Infectious bursal disease virus |
| IBV | Human infectious bronchitis coronavirus |
| JEV | Japanese encephalitis virus |
| LASV | Lassa virus |
| MARV | Marburg virus |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MHV | Mouse hepatitis virus |
| PED | Porcine epidemic diarrhea |
| PEDV | Porcine epidemic diarrhea virus |
| PCV2 | Porcine circovirus types 2 |
| PCV3 | Porcine circovirus types 3 |
| PoRV | Porcine rotavirus |
| PRCV | Porcine respiratory coronavirus |
| PRV | Porcine pseudorabies virus |
| RBDs | Receptor binding domains |
| PRRSV | Porcine reproductive and respiratory syndrome virus |
| RABV | Rabies virus |
| RSV | Human respiratory syncytial virus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SBV | Schmallenberg virus |
| TGEV | Transmissible gastroenteritis virus |
| VSV | Vesicular stomatitis virus |
| YFV | Yellow fever virus |
Author Contributions
Writing—original draft preparation, Y.Z., Y.C. and X.W.; illustrations, J.Z., L.M., J.L. and L.Y.; writing—review and editing, H.Y., D.P. and H.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was funded by the Special Funds for Cultivation and Breeding of New Transgenic Organisms (No. 2016ZX08006003).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang D., Fang L., Xiao S. Porcine epidemic diarrhea in China. Virus Res. 2016;226:7–13. doi: 10.1016/j.virusres.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Have P., Moving V., Svansson V., Uttenthal A., Bloch B. Coronavirus infection in mink (Mustela vison). Serological evidence of infection with a coronavirus related to transmissible gastroenteritis virus and porcine epidemic diarrhea virus. Vet. Microbiol. 1992;31:1–10. doi: 10.1016/0378-1135(92)90135-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pensaert M.B., de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978;58:243–247. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015;12:193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallien S., Moro A., Lediguerher G., Catinot V., Paboeuf F., Bigault L., Berri M., Gauger P.C., Pozzi N., Authie E., et al. Evidence of porcine epidemic diarrhea virus (PEDV) shedding in semen from infected specific pathogen-free boars. Vet. Res. 2018;49:7. doi: 10.1186/s13567-018-0505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si F., Jiang L., Yu R., Wei W., Li Z. Study on the Characteristic Codon Usage Pattern in Porcine Epidemic Diarrhea Virus Genomes and Its Host Adaptation Phenotype. Front. Microbiol. 2021;12:738082. doi: 10.3389/fmicb.2021.738082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung K., Saif L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015;204:134–143. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun R.Q., Cai R.J., Chen Y.Q., Liang P.S., Chen D.K., Song C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012;18:161–163. doi: 10.3201/eid1801.111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X.M., Niu B.B., Yan H., Gao D.S., Yang X., Chen L., Chang H.T., Zhao J., Wang C.Q. Genetic properties of endemic Chinese porcine epidemic diarrhea virus strains isolated since 2010. Arch. Virol. 2013;158:2487–2494. doi: 10.1007/s00705-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W., Li H., Liu Y., Pan Y., Deng F., Song Y., Tang X., He Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg Infect. Dis. 2012;18:1350–1353. doi: 10.3201/eid1803.120002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cima G. PED virus reinfecting U.S. herds. Virus estimated to have killed 7 million-plus pigs. J. Am. Vet. Med. Assoc. 2014;245:166–167. [PubMed] [Google Scholar]
- 12.Stevenson G.W., Hoang H., Schwartz K.J., Burrough E.R., Sun D., Madson D., Cooper V.L., Pillatzki A., Gauger P., Schmitt B.J., et al. Emergence of Porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Invest. 2013;25:649–654. doi: 10.1177/1040638713501675. [DOI] [PubMed] [Google Scholar]
- 13.Hanke D., Jenckel M., Petrov A., Ritzmann M., Stadler J., Akimkin V., Blome S., Pohlmann A., Schirrmeier H., Beer M., et al. Comparison of porcine epidemic diarrhea viruses from Germany and the United States, 2014. Emerg. Infect. Dis. 2015;21:493–496. doi: 10.3201/eid2103.141165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reveles-Felix S., Carreon-Napoles R., Mendoza-Elvira S., Quintero-Ramirez V., Garcia-Sanchez J., Martinez-Bautista R., Saavedra-Montanez M., Mosqueda Gualito J.J., Sanchez-Betancourt J.I. Emerging strains of porcine epidemic diarrhoea virus (PEDv) in Mexico. Transbound Emerg. Dis. 2020;67:1035–1041. doi: 10.1111/tbed.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung K., Saif L.J., Wang Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045. doi: 10.1016/j.virusres.2020.198045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Wu Q., Huang L., Yuan C., Wang J., Yang Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018;9:3811. doi: 10.1038/s41467-018-06056-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman A.S., Krogwold R.A., Price T., Davis M., Moeller S.J. Investigating the introduction of porcine epidemic diarrhea virus into an Ohio swine operation. BMC Vet. Res. 2015;11:38. doi: 10.1186/s12917-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowe J., Gauger P., Harmon K., Zhang J., Connor J., Yeske P., Loula T., Levis I., Dufresne L., Main R. Role of transportation in spread of porcine epidemic diarrhea virus infection, United States. Emerg. Infect. Dis. 2014;20:872–874. doi: 10.3201/eid2005.131628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J., Fang L., Ye X., Chen J., Xu S., Zhu X., Miao Y., Wang D., Xiao S. Evolutionary and genotypic analyses of global porcine epidemic diarrhea virus strains. Transbound Emerg. Dis. 2019;66:111–118. doi: 10.1111/tbed.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S.J., Moon H.J., Luo Y., Kim H.K., Kim E.M., Yang J.S., Song D.S., Kang B.K., Lee C.S., Park B.K. Cloning and further sequence analysis of the ORF3 gene of wild- and attenuated-type porcine epidemic diarrhea viruses. Virus Genes. 2008;36:95–104. doi: 10.1007/s11262-007-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puranaveja S., Poolperm P., Lertwatcharasarakul P., Kesdaengsakonwut S., Boonsoongnern A., Urairong K., Kitikoon P., Choojai P., Kedkovid R., Teankum K., et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009;15:1112–1115. doi: 10.3201/eid1507.081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan B., Jiao D., Zhang R., Zhou J., Guo R., Yu Z., Shi D., Zhao Y., Gu J., Niu B., et al. Origin and epidemic status of porcine epidemic diarrhea virus variants in China. Transbound Emerg. Dis. 2020;67:1364–1370. doi: 10.1111/tbed.13444. [DOI] [PubMed] [Google Scholar]
- 23.Wang H., Zhang L., Shang Y., Tan R., Ji M., Yue X., Wang N., Liu J., Wang C., Li Y., et al. Emergence and evolution of highly pathogenic porcine epidemic diarrhea virus by natural recombination of a low pathogenic vaccine isolate and a highly pathogenic strain in the spike gene. Virus Evol. 2020;6:veaa049. doi: 10.1093/ve/veaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S.J., Song D.S., Ha G.W., Park B.K. Cloning and further sequence analysis of the spike gene of attenuated porcine epidemic diarrhea virus DR13. Virus Genes. 2007;35:55–64. doi: 10.1007/s11262-006-0036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Xia M., Ju D., Wu B., Ning C., Song N., Feng T., Chen F., Wang X., Wu Y., et al. Isolation, molecular characterization and an artificial infection model for a variant porcine epidemic diarrhea virus strain from Jiangsu Province, China. Arch. Virol. 2017;162:3611–3618. doi: 10.1007/s00705-017-3518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin C.M., Saif L.J., Marthaler D., Wang Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016;226:20–39. doi: 10.1016/j.virusres.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X., Zhang Q., Zhang L., Zhou P., Yang J., Fang Y., Dong Z., Zhao D., Li W., Feng J., et al. A newly isolated Chinese virulent genotype GIIb porcine epidemic diarrhea virus strain: Biological characteristics, pathogenicity and immune protective effects as an inactivated vaccine candidate. Virus Res. 2019;259:18–27. doi: 10.1016/j.virusres.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui J.T., Qiao H., Hou C.Y., Zheng H.H., Li X.S., Zheng L.L., Chen H.Y. Characteristics of the spike and ORF3 genes of porcine epidemic diarrhea virus in Henan and Shanxi provinces of China. Arch. Virol. 2020;165:2323–2333. doi: 10.1007/s00705-020-04744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Hayes J., Byrum B., Zhang Y. US variant porcine epidemic diarrhea virus: Histological lesions and genetic characterization. Virus Genes. 2016;52:578–581. doi: 10.1007/s11262-016-1334-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X.W., Wang M., Zhan J., Liu Q.Y., Fang L.L., Zhao C.Y., Jiang P., Li Y.F., Bai J. Pathogenicity and immunogenicity of a new strain of porcine epidemic diarrhea virus containing a novel deletion in the N gene. Vet. Microbiol. 2020;240:108511. doi: 10.1016/j.vetmic.2019.108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li R., Qiao S., Yang Y., Guo J., Xie S., Zhou E., Zhang G. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes. 2016;52:91–98. doi: 10.1007/s11262-015-1254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pensaert M.B., Martelli P. Porcine epidemic diarrhea: A retrospect from Europe and matters of debate. Virus Res. 2016;226:1–6. doi: 10.1016/j.virusres.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y., Chen Y., Han X., Yu Z., Wei Y., Zhang G. Porcine epidemic diarrhea virus in Asia: An alarming threat to the global pig industry. Infect. Genet. Evol. 2019;70:24–26. doi: 10.1016/j.meegid.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Piedimonte G., Tramacere M., Borghetti A.F. Cell-density control of amino acid transport in simian virus 40-transformed 3T3 cells. Biochem. Soc. Trans. 1979;7:1137–1139. doi: 10.1042/bst0071137. [DOI] [PubMed] [Google Scholar]
- 35.Zhao M., Sun Z., Zhang Y., Wang G., Wang H., Yang F., Tian F., Jiang S. Complete genome sequence of a Vero cell-adapted isolate of porcine epidemic diarrhea virus in eastern China. J. Virol. 2012;86:13858–13859. doi: 10.1128/JVI.02674-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo X., Hu H., Chen F., Li Z., Ye S., Cheng S., Zhang M., He Q. iTRAQ-based comparative proteomic analysis of Vero cells infected with virulent and CV777 vaccine strain-like strains of porcine epidemic diarrhea virus. J. Proteom. 2016;130:65–75. doi: 10.1016/j.jprot.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan B., Yu Z., Pang F., Xu X., Zhang B., Guo R., He K., Li B. Characterization of a pathogenic full-length cDNA clone of a virulent porcine epidemic diarrhea virus strain AH2012/12 in China. Virology. 2017;500:50–61. doi: 10.1016/j.virol.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P., Zhu J., Liu X., Guo J., Gu X., Ruan W. Isolation and recombinant analysis of variants of porcine epidemic diarrhea virus strains from Beijing, China. Virusdisease. 2019;30:294–301. doi: 10.1007/s13337-019-00513-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X.M., Niu B.B., Yan H., Gao D.S., Huo J.Y., Chen L., Chang H.T., Wang C.Q., Zhao J. Complete genome sequence of a variant porcine epidemic diarrhea virus strain isolated in central china. Genome Announc. 2013;1:e00243-12. doi: 10.1128/genomeA.00243-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song D., Chen Y., Peng Q., Huang D., Zhang T., Huang T., Zhang F., Zhou X., Tang Y. Full-Length Genome Sequence of a Variant Porcine Epidemic Diarrhea Virus Strain, CH/GDZQ/2014, Responsible for a Severe Outbreak of Diarrhea in Piglets in Guangdong, China, 2014. Genome Announc. 2014;2:e01239-14. doi: 10.1128/genomeA.01239-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami S., Miyazaki A., Takahashi O., Hashizume W., Hase Y., Ohashi S., Suzuki T. Complete Genome Sequence of the Porcine Epidemic Diarrhea Virus Variant Tottori2/JPN/2014. Genome Announc. 2015;3:e00877-15. doi: 10.1128/genomeA.00877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marthaler D., Jiang Y., Otterson T., Goyal S., Rossow K., Collins J. Complete Genome Sequence of Porcine Epidemic Diarrhea Virus Strain USA/Colorado/2013 from the United States. Genome Announc. 2013;1:e00555-13. doi: 10.1128/genomeA.00555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jung K., Wang Q., Scheuer K.A., Lu Z., Zhang Y., Saif L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014;20:662–665. doi: 10.3201/eid2004.131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou Y., Lin C.M., Yokoyama M., Yount B.L., Marthaler D., Douglas A.L., Ghimire S., Qin Y., Baric R.S., Saif L.J., et al. Deletion of a 197-Amino-Acid Region in the N-Terminal Domain of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Piglets. J. Virol. 2017;91:e00555-13. doi: 10.1128/JVI.00227-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bi J., Zeng S., Xiao S., Chen H., Fang L. Complete genome sequence of porcine epidemic diarrhea virus strain AJ1102 isolated from a suckling piglet with acute diarrhea in China. J. Virol. 2012;86:10910–10911. doi: 10.1128/JVI.01919-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen F., Pan Y., Zhang X., Tian X., Wang D., Zhou Q., Song Y., Bi Y. Complete genome sequence of a variant porcine epidemic diarrhea virus strain isolated in China. J. Virol. 2012;86:12448. doi: 10.1128/JVI.02228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F., Zhu Y., Wu M., Ku X., Ye S., Li Z., Guo X., He Q. Comparative Genomic Analysis of Classical and Variant Virulent Parental/Attenuated Strains of Porcine Epidemic Diarrhea Virus. Viruses. 2015;7:5525–5538. doi: 10.3390/v7102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan Y., Tian X., Li W., Zhou Q., Wang D., Bi Y., Chen F., Song Y. Isolation and characterization of a variant porcine epidemic diarrhea virus in China. Virol. J. 2012;9:195. doi: 10.1186/1743-422X-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei Z.Y., Lu W.H., Li Z.L., Mo J.Y., Zeng X.D., Zeng Z.L., Sun B.L., Chen F., Xie Q.M., Bee Y.Z., et al. Complete genome sequence of novel porcine epidemic diarrhea virus strain GD-1 in China. J. Virol. 2012;86:13824–13825. doi: 10.1128/JVI.02615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan H., Zhang J., Ye Y., Tong T., Xie K., Liao M. Complete genome sequence of a novel porcine epidemic diarrhea virus in south China. J. Virol. 2012;86:10248–10249. doi: 10.1128/JVI.01589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L., Byrum B., Zhang Y. New variant of porcine epidemic diarrhea virus, United States, 2014. Emerg. Infect. Dis. 2014;20:917–919. doi: 10.3201/eid2005.140195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg Infect. Dis. 2014;20:1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theuns S., Conceicao-Neto N., Christiaens I., Zeller M., Desmarets L.M., Roukaerts I.D., Acar D.D., Heylen E., Matthijnssens J., Nauwynck H.J. Complete genome sequence of a porcine epidemic diarrhea virus from a novel outbreak in belgium, january 2015. Genome Announc. 2015;3:e00506-15. doi: 10.1128/genomeA.00506-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun M., Ma J., Wang Y., Wang M., Song W., Zhang W., Lu C., Yao H. Genomic and epidemiological characteristics provide new insights into the phylogeographical and spatiotemporal spread of porcine epidemic diarrhea virus in Asia. J. Clin. Microbiol. 2015;53:1484–1492. doi: 10.1128/JCM.02898-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S.H., Lee J.M., Jung J., Kim I.J., Hyun B.H., Kim H.I., Park C.K., Oem J.K., Kim Y.H., Lee M.H., et al. Genetic characterization of porcine epidemic diarrhea virus in Korea from 1998 to 2013. Arch. Virol. 2015;160:1055–1064. doi: 10.1007/s00705-015-2353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masuda T., Murakami S., Takahashi O., Miyazaki A., Ohashi S., Yamasato H., Suzuki T. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch. Virol. 2015;160:2565–2568. doi: 10.1007/s00705-015-2522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vui D.T., Tung N., Inui K., Slater S., Nilubol D. Complete genome sequence of porcine epidemic diarrhea virus in Vietnam. Genome Announc. 2014;2:e00753-14. doi: 10.1128/genomeA.00753-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheun-Arom T., Temeeyasen G., Srijangwad A., Tripipat T., Sangmalee S., Vui D.T., Chuanasa T., Tantituvanont A., Nilubol D. Complete Genome Sequences of Two Genetically Distinct Variants of Porcine Epidemic Diarrhea Virus in the Eastern Region of Thailand. Genome Announc. 2015;3:e00634-15. doi: 10.1128/genomeA.00634-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paraguison-Alili R., Domingo C.Y. Phylogenetic tracking of current porcine epidemic diarrhea virus (PEDV) strains in the Philippines. Arch. Virol. 2016;161:2601–2604. doi: 10.1007/s00705-016-2938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diep N.V., Sueyoshi M., Izzati U., Fuke N., Teh A.P.P., Lan N.T., Yamaguchi R. Appearance of US-like porcine epidemic diarrhoea virus (PEDV) strains before US outbreaks and genetic heterogeneity of PEDVs collected in Northern Vietnam during 2012-2015. Transbound Emerg. Dis. 2018;65:e83–e93. doi: 10.1111/tbed.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y.W., Dickerman A.W., Pineyro P., Li L., Fang L., Kiehne R., Opriessnig T., Meng X.J. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the United States. mBio. 2013;4:e00737-13. doi: 10.1128/mBio.00737-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ojkic D., Hazlett M., Fairles J., Marom A., Slavic D., Maxie G., Alexandersen S., Pasick J., Alsop J., Burlatschenko S. The first case of porcine epidemic diarrhea in Canada. Can. Vet. J. 2015;56:149–152. [PMC free article] [PubMed] [Google Scholar]
- 63.Vlasova A.N., Marthaler D., Wang Q., Culhane M.R., Rossow K.D., Rovira A., Collins J., Saif L.J. Distinct characteristics and complex evolution of PEDV strains, North America, May 2013-February 2014. Emerg Infect. Dis. 2014;20:1620–1628. doi: 10.3201/eid2010.140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stadler J., Zoels S., Fux R., Hanke D., Pohlmann A., Blome S., Weissenbock H., Weissenbacher-Lang C., Ritzmann M., Ladinig A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015;11:142. doi: 10.1186/s12917-015-0454-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dortmans J., Li W., van der Wolf P.J., Buter G.J., Franssen P.J.M., van Schaik G., Houben M., Bosch B.J. Porcine epidemic diarrhea virus (PEDV) introduction into a naive Dutch pig population in 2014. Vet. Microbiol. 2018;221:13–18. doi: 10.1016/j.vetmic.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grasland B., Bigault L., Bernard C., Quenault H., Toulouse O., Fablet C., Rose N., Touzain F., Blanchard Y. Complete genome sequence of a porcine epidemic diarrhea s gene indel strain isolated in france in december 2014. Genome Announc. 2015;3:e00535-15. doi: 10.1128/genomeA.00535-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mesquita J.R., Hakze-van der Honing R., Almeida A., Lourenco M., van der Poel W.H., Nascimento M.S. Outbreak of Porcine Epidemic Diarrhea Virus in Portugal, 2015. Transbound Emerg. Dis. 2015;62:586–588. doi: 10.1111/tbed.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinrigl A., Fernandez S.R., Stoiber F., Pikalo J., Sattler T., Schmoll F. First detection, clinical presentation and phylogenetic characterization of Porcine epidemic diarrhea virus in Austria. BMC Vet. Res. 2015;11:310. doi: 10.1186/s12917-015-0624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woo P.C., Lau S.K., Lam C.S., Lau C.C., Tsang A.K., Lau J.H., Bai R., Teng J.L., Tsang C.C., Wang M., et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/jvi.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kocherhans R., Bridgen A., Ackermann M., Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137–144. doi: 10.1023/A:1011831902219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen Z., Yang Y., Yang S., Zhang G., Xiao S., Fu Z.F., Peng G. Structural and Biological Basis of Alphacoronavirus nsp1 Associated with Host Proliferation and Immune Evasion. Viruses. 2020;12:812. doi: 10.3390/v12080812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu C., Tang J., Ma Y., Liang X., Yang Y., Peng G., Qi Q., Jiang S., Li J., Du L., et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 2015;89:6121–6125. doi: 10.1128/JVI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W., van Kuppeveld F.J.M., He Q., Rottier P.J.M., Bosch B.J. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016;226:117–127. doi: 10.1016/j.virusres.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su Y., Hou Y., Wang Q. The enhanced replication of an S-intact PEDV during coinfection with an S1 NTD-del PEDV in piglets. Vet. Microbiol. 2019;228:202–212. doi: 10.1016/j.vetmic.2018.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reguera J., Santiago C., Mudgal G., Ordono D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8:e1002859. doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Z., Ma Z., Li Y., Gao S., Xiao S. Porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and vaccines. Microb. Pathog. 2020;149:104553. doi: 10.1016/j.micpath.2020.104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eckert D.M., Kim P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 78.Sun D., Feng L., Shi H., Chen J., Cui X., Chen H., Liu S., Tong Y., Wang Y., Tong G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet. Microbiol. 2008;131:73–81. doi: 10.1016/j.vetmic.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Diep N., Choijookhuu N., Fuke N., Myint O., Izzati U.Z., Suwanruengsri M., Hishikawa Y., Yamaguchi R. New tropisms of porcine epidemic diarrhoea virus (PEDV) in pigs naturally coinfected by variants bearing large deletions in the spike (S) protein and PEDVs possessing an intact S protein. Transbound Emerg. Dis. 2020;67:2589–2601. doi: 10.1111/tbed.13607. [DOI] [PubMed] [Google Scholar]
- 80.Liu C., Ma Y., Yang Y., Zheng Y., Shang J., Zhou Y., Jiang S., Du L., Li J., Li F. Cell Entry of Porcine Epidemic Diarrhea Coronavirus Is Activated by Lysosomal Proteases. J. Biol. Chem. 2016;291:24779–24786. doi: 10.1074/jbc.M116.740746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee D.K., Park C.K., Kim S.H., Lee C. Heterogeneity in spike protein genes of porcine epidemic diarrhea viruses isolated in Korea. Virus Res. 2010;149:175–182. doi: 10.1016/j.virusres.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wicht O., Li W., Willems L., Meuleman T.J., Wubbolts R.W., van Kuppeveld F.J., Rottier P.J., Bosch B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014;88:7952–7961. doi: 10.1128/JVI.00297-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jantraphakorn Y., Viriyakitkosol R., Jongkaewwattana A., Kaewborisuth C. Interaction Between PEDV and Its Hosts: A Closer Look at the ORF3 Accessory Protein. Front. Vet. Sci. 2021;8:744276. doi: 10.3389/fvets.2021.744276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang K., Lu W., Chen J., Xie S., Shi H., Hsu H., Yu W., Xu K., Bian C., Fischer W.B., et al. PEDV ORF3 encodes an ion channel protein and regulates virus production. FEBS Lett. 2012;586:384–391. doi: 10.1016/j.febslet.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu W., Zheng B.J., Xu K., Schwarz W., Du L., Wong C.K., Chen J., Duan S., Deubel V., Sun B. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. Proc. Natl. Acad. Sci. USA. 2006;103:12540–12545. doi: 10.1073/pnas.0605402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye S., Li Z., Chen F., Li W., Guo X., Hu H., He Q. Porcine epidemic diarrhea virus ORF3 gene prolongs S-phase, facilitates formation of vesicles and promotes the proliferation of attenuated PEDV. Virus Genes. 2015;51:385–392. doi: 10.1007/s11262-015-1257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu X., Zhang H., Zhang Q., Dong J., Liang Y., Huang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus E protein causes endoplasmic reticulum stress and up-regulates interleukin-8 expression. Virol. J. 2013;10:26. doi: 10.1186/1743-422X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torres J., Maheswari U., Parthasarathy K., Ng L., Liu D.X., Gong X. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 2007;16:2065–2071. doi: 10.1110/ps.062730007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng L., Wang X., Guo D., Cao J., Cheng L., Li X., Zou D., Zhang Y., Xu J., Wu X., et al. Porcine epidemic diarrhea virus E protein suppresses RIG-I signaling-mediated interferon-beta production. Vet. Microbiol. 2021;254:108994. doi: 10.1016/j.vetmic.2021.108994. [DOI] [PubMed] [Google Scholar]
- 90.Vennema H., Godeke G.J., Rossen J.W., Voorhout W.F., Horzinek M.C., Opstelten D.J., Rottier P.J. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 1996;15:2020–2028. doi: 10.1002/j.1460-2075.1996.tb00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fischer F., Stegen C.F., Masters P.S., Samsonoff W.A. Analysis of constructed E gene mutants of mouse hepatitis virus confirms a pivotal role for E protein in coronavirus assembly. J. Virol. 1998;72:7885–7894. doi: 10.1128/JVI.72.10.7885-7894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raamsman M.J., Locker J.K., de Hooge A., de Vries A.A., Griffiths G., Vennema H., Rottier P.J. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J. Virol. 2000;74:2333–2342. doi: 10.1128/JVI.74.5.2333-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen V.P., Hogue B.G. Protein interactions during coronavirus assembly. J. Virol. 1997;71:9278–9284. doi: 10.1128/jvi.71.12.9278-9284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Haan C.A., Kuo L., Masters P.S., Vennema H., Rottier P.J. Coronavirus particle assembly: Primary structure requirements of the membrane protein. J. Virol. 1998;72:6838–6850. doi: 10.1128/JVI.72.8.6838-6850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vennema H., de Groot R.J., Harbour D.A., Horzinek M.C., Spaan W.J. Primary structure of the membrane and nucleocapsid protein genes of feline infectious peritonitis virus and immunogenicity of recombinant vaccinia viruses in kittens. Virology. 1991;181:327–335. doi: 10.1016/0042-6822(91)90499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Woods R.D., Wesley R.D., Kapke P.A. Neutralization of porcine transmissible gastroenteritis virus by complement-dependent monoclonal antibodies. Am. J. Vet. Res. 1988;49:300–304. [PubMed] [Google Scholar]
- 97.Zhang Q., Shi K., Yoo D. Suppression of type I interferon production by porcine epidemic diarrhea virus and degradation of CREB-binding protein by nsp1. Virology. 2016;489:252–268. doi: 10.1016/j.virol.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu X.G., Zhang H.L., Zhang Q., Dong J., Huang Y., Tong D.W. Porcine epidemic diarrhea virus M protein blocks cell cycle progression at S-phase and its subcellular localization in the porcine intestinal epithelial cells. Acta. Virol. 2015;59:265–275. doi: 10.4149/av_2015_03_265. [DOI] [PubMed] [Google Scholar]
- 99.McBride R., van Zyl M., Fielding B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6:2991–3018. doi: 10.3390/v6082991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zuniga S., Cruz J.L., Sola I., Mateos-Gomez P.A., Palacio L., Enjuanes L. Coronavirus nucleocapsid protein facilitates template switching and is required for efficient transcription. J. Virol. 2010;84:2169–2175. doi: 10.1128/JVI.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hurst K.R., Ye R., Goebel S.J., Jayaraman P., Masters P.S. An interaction between the nucleocapsid protein and a component of the replicase-transcriptase complex is crucial for the infectivity of coronavirus genomic RNA. J. Virol. 2010;84:10276–10288. doi: 10.1128/JVI.01287-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding Z., Fang L., Jing H., Zeng S., Wang D., Liu L., Zhang H., Luo R., Chen H., Xiao S. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J. Virol. 2014;88:8936–8945. doi: 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu X., Zhang H., Zhang Q., Huang Y., Dong J., Liang Y., Liu H.J., Tong D. Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 2013;164:212–221. doi: 10.1016/j.vetmic.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi D., Shi H., Sun D., Chen J., Zhang X., Wang X., Zhang J., Ji Z., Liu J., Cao L., et al. Nucleocapsid Interacts with NPM1 and Protects it from Proteolytic Cleavage, Enhancing Cell Survival, and is Involved in PEDV Growth. Sci. Rep. 2017;7:39700. doi: 10.1038/srep39700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liwnaree B., Narkpuk J., Sungsuwan S., Jongkaewwattana A., Jaru-Ampornpan P. Growth enhancement of porcine epidemic diarrhea virus (PEDV) in Vero E6 cells expressing PEDV nucleocapsid protein. PLoS ONE. 2019;14:e0212632. doi: 10.1371/journal.pone.0212632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hofmann H., Hattermann K., Marzi A., Gramberg T., Geier M., Krumbiegel M., Kuate S., Uberla K., Niedrig M., Pohlmann S. S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients. J. Virol. 2004;78:6134–6142. doi: 10.1128/JVI.78.12.6134-6142.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hulswit R.J., de Haan C.A., Bosch B.J. Coronavirus Spike Protein and Tropism Changes. Adv. Virus Res. 2016;96:29–57. doi: 10.1016/bs.aivir.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hou Y., Ke H., Kim J., Yoo D., Su Y., Boley P., Chepngeno J., Vlasova A.N., Saif L.J., Wang Q. Engineering a Live Attenuated Porcine Epidemic Diarrhea Virus Vaccine Candidate via Inactivation of the Viral 2′-O-Methyltransferase and the Endocytosis Signal of the Spike Protein. J. Virol. 2019;93:e00406-19. doi: 10.1128/JVI.00406-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen P., Zhao X., Zhou S., Zhou T., Tan X., Wu X., Tong W., Gao F., Yu L., Jiang Y., et al. A Virulent PEDV Strain FJzz1 with Genomic Mutations and Deletions at the High Passage Level Was Attenuated in Piglets via Serial Passage In Vitro. Virol. Sin. 2021;36:1052–1065. doi: 10.1007/s12250-021-00368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen P., Wang K., Hou Y., Li H., Li X., Yu L., Jiang Y., Gao F., Tong W., Yu H., et al. Genetic evolution analysis and pathogenicity assessment of porcine epidemic diarrhea virus strains circulating in part of China during 2011-2017. Infect. Genet. Evol. 2019;69:153–165. doi: 10.1016/j.meegid.2019.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin C.M., Annamalai T., Liu X., Gao X., Lu Z., El-Tholoth M., Hu H., Saif L.J., Wang Q. Experimental infection of a US spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original US PEDV infection. Vet. Res. 2015;46:134. doi: 10.1186/s13567-015-0278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tsai K.J., Deng M.C., Wang F.I., Tsai S.H., Chang C., Chang C.Y., Huang Y.L. Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced. Viruses. 2020;12:1378. doi: 10.3390/v12121378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hou Y., Meulia T., Gao X., Saif L.J., Wang Q. Deletion of both the Tyrosine-Based Endocytosis Signal and the Endoplasmic Reticulum Retrieval Signal in the Cytoplasmic Tail of Spike Protein Attenuates Porcine Epidemic Diarrhea Virus in Pigs. J. Virol. 2019;93:e01758-18. doi: 10.1128/JVI.01758-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X., Pan Y., Wang D., Tian X., Song Y., Cao Y. Identification and pathogenicity of a variant porcine epidemic diarrhea virus field strain with reduced virulence. Virol. J. 2015;12:88. doi: 10.1186/s12985-015-0314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee S., Son K.Y., Noh Y.H., Lee S.C., Choi H.W., Yoon I.J., Lee C. Genetic characteristics, pathogenicity, and immunogenicity associated with cell adaptation of a virulent genotype 2b porcine epidemic diarrhea virus. Vet. Microbiol. 2017;207:248–258. doi: 10.1016/j.vetmic.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lu Y., Huang W., Zhong L., Qin Y., Liu X., Yang C., Wang R., Su X., Du C., Mi X., et al. Comparative Characterization and Pathogenicity of a Novel Porcine Epidemic Diarrhea Virus (PEDV) with a Naturally Occurring Truncated ORF3 Gene Coinfected with PEDVs Possessing an Intact ORF3 Gene in Piglets. Viruses. 2021;13:1562. doi: 10.3390/v13081562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y.H., Li H.X., Chen X.M., Zhang L.H., Zhao Y.Y., Luo A.F., Yang Y.R., Zheng L.L., Chen H.Y. Genetic Characteristics and Pathogenicity of a Novel Porcine Epidemic Diarrhea Virus with a Naturally Occurring Truncated ORF3 Gene. Viruses. 2022;14:487. doi: 10.3390/v14030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki T., Terada Y., Enjuanes L., Ohashi S., Kamitani W. S1 Subunit of Spike Protein from a Current Highly Virulent Porcine Epidemic Diarrhea Virus Is an Important Determinant of Virulence in Piglets. Viruses. 2018;10:467. doi: 10.3390/v10090467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang D., Ge X., Chen D., Li J., Cai Y., Deng J., Zhou L., Guo X., Han J., Yang H. The S Gene Is Necessary but Not Sufficient for the Virulence of Porcine Epidemic Diarrhea Virus Novel Variant Strain BJ2011C. J. Virol. 2018;92:e00603-18. doi: 10.1128/JVI.00603-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song D.S., Yang J.S., Oh J.S., Han J.H., Park B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine. 2003;21:1833–1842. doi: 10.1016/S0264-410X(03)00027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Si F., Chen B., Hu X., Yu R., Dong S., Wang R., Li Z. Porcine Epidemic Diarrhea Virus ORF3 Protein Is Transported through the Exocytic Pathway. J. Virol. 2020;94:e00808-20. doi: 10.1128/JVI.00808-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Menachery V.D., Gralinski L.E., Mitchell H.D., Dinnon K.H., 3rd, Leist S.R., Yount B.L., Jr., McAnarney E.T., Graham R.L., Waters K.M., Baric R.S. Combination Attenuation Offers Strategy for Live Attenuated Coronavirus Vaccines. J. Virol. 2018;92:e00710-18. doi: 10.1128/JVI.00710-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Su M., Li C., Qi S., Yang D., Jiang N., Yin B., Guo D., Kong F., Yuan D., Feng L., et al. A molecular epidemiological investigation of PEDV in China: Characterization of co-infection and genetic diversity of S1-based genes. Transbound Emerg. Dis. 2020;67:1129–1140. doi: 10.1111/tbed.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li Y., Wang G., Wang J., Man K., Yang Q. Cell attenuated porcine epidemic diarrhea virus strain Zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine. 2017;35:7033–7041. doi: 10.1016/j.vaccine.2017.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen F., Yang J., Li A., Gong Z., Yang L., Cheng Q., Wang C., Zhao M., Yuan S., Chen Y., et al. Genetic characterization and phylogenetic analysis of porcine epidemic diarrhea virus in Guangdong, China, between 2018 and 2019. PLoS ONE. 2021;16:e0253622. doi: 10.1371/journal.pone.0253622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tian Y., Yang X., Li H., Ma B., Guan R., Yang J., Chen D., Han X., Zhou L., Song Z., et al. Molecular characterization of porcine epidemic diarrhea virus associated with outbreaks in southwest China during 2014–2018. Transbound Emerg. Dis. 2021;68:3482–3497. doi: 10.1111/tbed.13953. [DOI] [PubMed] [Google Scholar]
- 128.Chen X., Zeng L., Yang J., Yu F., Ge J., Guo Q., Gao X., Song T. Sequence heterogeneity of the ORF3 gene of porcine epidemic diarrhea viruses field samples in Fujian, China, 2010—2012. Viruses. 2013;5:2375–2383. doi: 10.3390/v5102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Escors D., Camafeita E., Ortego J., Laude H., Enjuanes L. Organization of two transmissible gastroenteritis coronavirus membrane protein topologies within the virion and core. J. Virol. 2001;75:12228–12240. doi: 10.1128/JVI.75.24.12228-12240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen J.F., Sun D.B., Wang C.B., Shi H.Y., Cui X.C., Liu S.W., Qiu H.J., Feng L. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes. 2008;36:355–364. doi: 10.1007/s11262-007-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J., Liu X., Lang H., Wang Z., Shi D., Shi H., Zhang X., Feng L. Genetic variation of nucleocapsid genes of porcine epidemic diarrhea virus field strains in China. Arch. Virol. 2013;158:1397–1401. doi: 10.1007/s00705-013-1608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z., Chen F., Yuan Y., Zeng X., Wei Z., Zhu L., Sun B., Xie Q., Cao Y., Xue C., et al. Sequence and phylogenetic analysis of nucleocapsid genes of porcine epidemic diarrhea virus (PEDV) strains in China. Arch. Virol. 2013;158:1267–1273. doi: 10.1007/s00705-012-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park J.Y., Pak S.I., Sung H.W., Kim J.H., Song C.S., Lee C.W., Kwon H.M. Variations in the nucleocapsid protein gene of infectious bronchitis viruses isolated in Korea. Virus Genes. 2005;31:153–162. doi: 10.1007/s11262-005-1788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li F., Zeng Y., Zhang R., Peng K., Jiang C., Xu Z., Zhu L. Genetic variations in S gene of porcine epidemic diarrhoea virus from 2018 in Sichuan Province, China. Vet. Med. Sci. 2020;6:910–918. doi: 10.1002/vms3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim S.J., Nguyen V.G., Huynh T.M., Park Y.H., Park B.K., Chung H.C. Molecular Characterization of Porcine Epidemic Diarrhea Virus and Its New Genetic Classification Based on the Nucleocapsid Gene. Viruses. 2020;12:790. doi: 10.3390/v12080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hou Y., Wang Q. Emerging Highly Virulent Porcine Epidemic Diarrhea Virus: Molecular Mechanisms of Attenuation and Rational Design of Live Attenuated Vaccines. Int. J. Mol. Sci. 2019;20:5478. doi: 10.3390/ijms20215478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu J., Shi H., Chen J., Zhang X., Ji Z., Yuan J., Shi D., Cao L., Zhu X., Dong H., et al. Neutralization of genotype 2 porcine epidemic diarrhea virus strains by a novel monoclonal antibody. Virology. 2017;507:257–262. doi: 10.1016/j.virol.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin C.M., Hou Y., Marthaler D.G., Gao X., Liu X., Zheng L., Saif L.J., Wang Q. Attenuation of an original US porcine epidemic diarrhea virus strain PC22A via serial cell culture passage. Vet. Microbiol. 2017;201:62–71. doi: 10.1016/j.vetmic.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu Y., Li W., Zhou Q., Li Q., Xu Z., Shen H., Chen F. Characterization and pathogenicity of Vero cell-attenuated porcine epidemic diarrhea virus CT strain. Virol. J. 2019;16:121. doi: 10.1186/s12985-019-1232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang Y.C., Kao C.F., Chang C.Y., Jeng C.R., Tsai P.S., Pang V.F., Chiou H.Y., Peng J.Y., Cheng I.C., Chang H.W. Evaluation and Comparison of the Pathogenicity and Host Immune Responses Induced by a G2b Taiwan Porcine Epidemic Diarrhea Virus (Strain Pintung 52) and Its Highly Cell-Culture Passaged Strain in Conventional 5-Week-Old Pigs. Viruses. 2017;9:121. doi: 10.3390/v9050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thavorasak T., Chulanetra M., Glab-Ampai K., Teeranitayatarn K., Songserm T., Yodsheewan R., Sae-Lim N., Lekcharoensuk P., Sookrung N., Chaicumpa W. Novel Neutralizing Epitope of PEDV S1 Protein Identified by IgM Monoclonal Antibody. Viruses. 2022;14:125. doi: 10.3390/v14010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li C., Li W., Lucio de Esesarte E., Guo H., van den Elzen P., Aarts E., van den Born E., Rottier P.J.M., Bosch B.J. Cell Attachment Domains of the Porcine Epidemic Diarrhea Virus Spike Protein Are Key Targets of Neutralizing Antibodies. J. Virol. 2017;91:e00273-17. doi: 10.1128/JVI.00273-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Okda F.A., Lawson S., Singrey A., Nelson J., Hain K.S., Joshi L.R., Christopher-Hennings J., Nelson E.A., Diel D.G. The S2 glycoprotein subunit of porcine epidemic diarrhea virus contains immunodominant neutralizing epitopes. Virology. 2017;509:185–194. doi: 10.1016/j.virol.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park J.E., Cruz D.J., Shin H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011;156:1749–1756. doi: 10.1007/s00705-011-1044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan Y., Sun L., Wang G., Shi Y., Dong W., Fu Y., Fu Z., Chen H., Peng G. The trypsin-enhanced infection of porcine epidemic diarrhea virus is determined by the S2 subunit of the spike glycoprotein. J. Virol. 2021;95:e02453-e20. doi: 10.1128/JVI.02453-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc. Natl. Acad. Sci. USA. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ducatelle R., Coussement W., Pensaert M.B., Debouck P., Hoorens J. In vivo morphogenesis of a new porcine enteric coronavirus, CV 777. Arch. Virol. 1981;68:35–44. doi: 10.1007/BF01315165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ducatelle R., Coussement W., Debouck P., Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. II. Electron microscopic study. Vet. Pathol. 1982;19:57–66. doi: 10.1177/030098588201900109. [DOI] [PubMed] [Google Scholar]
- 150.Debouck P., Pensaert M., Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV 777. Vet. Microbiol. 1981;6:157–165. doi: 10.1016/0378-1135(81)90007-9. [DOI] [Google Scholar]
- 151.Park J.E., Shin H.J. Porcine epidemic diarrhea virus infects and replicates in porcine alveolar macrophages. Virus Res. 2014;191:143–152. doi: 10.1016/j.virusres.2014.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu H.Y., Gu H., Qu H., Bao W., Li Y., Cai D. Aberrant Cholesterol Metabolic Genes Regulation in a Negative Feedback Loop Induced by an Alphacoronavirus. Front. Nutr. 2022;9:870680. doi: 10.3389/fnut.2022.870680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Park J.E., Cruz D.J., Shin H.J. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014;191:21–29. doi: 10.1016/j.virusres.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc. R Soc. Lond. B Biol. Sci. 1957;147:258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- 155.Raftery N., Stevenson N.J. Advances in anti-viral immune defence: Revealing the importance of the IFN JAK/STAT pathway. Cell Mol. Life Sci. 2017;74:2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamada Y., Liu D.X. Proteolytic activation of the spike protein at a novel RRRR/S motif is implicated in furin-dependent entry, syncytium formation, and infectivity of coronavirus infectious bronchitis virus in cultured cells. J. Virol. 2009;83:8744–8758. doi: 10.1128/JVI.00613-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang S., Cao Y., Yang Q. Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection. PLoS Pathog. 2020;16:e1008682. doi: 10.1371/journal.ppat.1008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xia S., Yan L., Xu W., Agrawal A.S., Algaissi A., Tseng C.K., Wang Q., Du L., Tan W., Wilson I.A., et al. A pan-coronavirus fusion inhibitor targeting the HR1 domain of human coronavirus spike. Sci. Adv. 2019;5:eaav4580. doi: 10.1126/sciadv.aav4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J. Virol. 2015;89:1954–1964. doi: 10.1128/JVI.02615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pohlmann S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA. 2005;102:7988–7993. doi: 10.1073/pnas.0409465102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312:1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Raj V.S., Mou H., Smits S.L., Dekkers D.H., Muller M.A., Dijkman R., Muth D., Demmers J.A., Zaki A., Fouchier R.A., et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mou H., Raj V.S., van Kuppeveld F.J., Rottier P.J., Haagmans B.L., Bosch B.J. The receptor binding domain of the new Middle East respiratory syndrome coronavirus maps to a 231-residue region in the spike protein that efficiently elicits neutralizing antibodies. J. Virol. 2013;87:9379–9383. doi: 10.1128/JVI.01277-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Godet M., Grosclaude J., Delmas B., Laude H. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 1994;68:8008–8016. doi: 10.1128/jvi.68.12.8008-8016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peng G., Sun D., Rajashankar K.R., Qian Z., Holmes K.V., Li F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA. 2011;108:10696–10701. doi: 10.1073/pnas.1104306108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wong A.H.M., Tomlinson A.C.A., Zhou D., Satkunarajah M., Chen K., Sharon C., Desforges M., Talbot P.J., Rini J.M. Receptor-binding loops in alphacoronavirus adaptation and evolution. Nat. Commun. 2017;8:1735. doi: 10.1038/s41467-017-01706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Delmas B., Gelfi J., L’Haridon R., Vogel L.K., Sjostrom H., Noren O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kubo H., Yamada Y.K., Taguchi F. Localization of neutralizing epitopes and the receptor-binding site within the amino-terminal 330 amino acids of the murine coronavirus spike protein. J. Virol. 1994;68:5403–5410. doi: 10.1128/jvi.68.9.5403-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peng G., Xu L., Lin Y.L., Chen L., Pasquarella J.R., Holmes K.V., Li F. Crystal structure of bovine coronavirus spike protein lectin domain. J. Biol. Chem. 2012;287:41931–41938. doi: 10.1074/jbc.M112.418210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Deng F., Ye G., Liu Q., Navid M.T., Zhong X., Li Y., Wan C., Xiao S., He Q., Fu Z.F., et al. Identification and Comparison of Receptor Binding Characteristics of the Spike Protein of Two Porcine Epidemic Diarrhea Virus Strains. Viruses. 2016;8:55. doi: 10.3390/v8030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang L., Xu J., Guo L., Guo T., Zhang L., Feng L., Chen H., Wang Y. Porcine Epidemic Diarrhea Virus-Induced Epidermal Growth Factor Receptor Activation Impairs the Antiviral Activity of Type I Interferon. J. Virol. 2018;92:e02095-17. doi: 10.1128/JVI.02095-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li S., Zhu Z., Yang F., Cao W., Yang J., Ma C., Zhao Z., Tian H., Liu X., Ma J., et al. Porcine Epidemic Diarrhea Virus Membrane Protein Interacted with IRF7 to Inhibit Type I IFN Production during Viral Infection. J. Immunol. 2021;206:2909–2923. doi: 10.4049/jimmunol.2001186. [DOI] [PubMed] [Google Scholar]
- 175.Dong S., Wang R., Yu R., Chen B., Si F., Xie C., Li Z. Identification of cellular proteins interacting with PEDV M protein through APEX2 labeling. J. Proteom. 2021;240:104191. doi: 10.1016/j.jprot.2021.104191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Su M., Shi D., Xing X., Qi S., Yang D., Zhang J., Han Y., Zhu Q., Sun H., Wang X., et al. Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication. J. Virol. 2021;95:e0018721. doi: 10.1128/JVI.00187-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.He Y., Smith R. Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell Mol. Life Sci. 2009;66:1239–1256. doi: 10.1007/s00018-008-8532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Z., Zeng W., Ye S., Lv J., Nie A., Zhang B., Sun Y., Han H., He Q. Cellular hnRNP A1 Interacts with Nucleocapsid Protein of Porcine Epidemic Diarrhea Virus and Impairs Viral Replication. Viruses. 2018;10:127. doi: 10.3390/v10030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shan Y., Liu Z.Q., Li G.W., Chen C., Luo H., Liu Y.J., Zhuo X.H., Shi X.F., Fang W.H., Li X.L. Nucleocapsid protein from porcine epidemic diarrhea virus isolates can antagonize interferon-lambda production by blocking the nuclear factor-kappaB nuclear translocation. J. Zhejiang Univ. Sci. B. 2018;19:570–580. doi: 10.1631/jzus.B1700283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu J., Mao J., Han X., Shi F., Gao Q., Wang T., Zhang Z., Shan Y., Fang W., Li X. Porcine Epidemic Diarrhea Virus Inhibits HDAC1 Expression To Facilitate Its Replication via Binding of Its Nucleocapsid Protein to Host Transcription Factor Sp1. J. Virol. 2021;95:e0085321. doi: 10.1128/JVI.00853-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Licciardi P.V., Karagiannis T.C. Regulation of immune responses by histone deacetylase inhibitors. ISRN Hematol. 2012;2012:690901. doi: 10.5402/2012/690901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zou D., Xu J., Duan X., Xu X., Li P., Cheng L., Zheng L., Li X., Zhang Y., Wang X., et al. Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 2019;235:209–219. doi: 10.1016/j.vetmic.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kaewborisuth C., He Q., Jongkaewwattana A. The Accessory Protein ORF3 Contributes to Porcine Epidemic Diarrhea Virus Replication by Direct Binding to the Spike Protein. Viruses. 2018;10:399. doi: 10.3390/v10080399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu Z., Cheng L., Xu J., Li P., Li X., Zou D., Zhang Y., Wang X., Wu X., Shen Y., et al. The accessory protein ORF3 of porcine epidemic diarrhea virus inhibits cellular interleukin-6 and interleukin-8 productions by blocking the nuclear factor-kappaB p65 activation. Vet. Microbiol. 2020;251:108892. doi: 10.1016/j.vetmic.2020.108892. [DOI] [PubMed] [Google Scholar]
- 185.Kaewborisuth C., Koonpaew S., Srisutthisamphan K., Viriyakitkosol R., Jaru-Ampornpan P., Jongkaewwattana A. PEDV ORF3 Independently Regulates IkappaB Kinase beta-Mediated NF-kappaB and IFN-beta Promoter Activities. Pathogens. 2020;9:376. doi: 10.3390/pathogens9050376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hofmann M.A., Senanayake S.D., Brian D.A. A translation-attenuating intraleader open reading frame is selected on coronavirus mRNAs during persistent infection. Proc. Natl. Acad. Sci. USA. 1993;90:11733–11737. doi: 10.1073/pnas.90.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang Q., Ke H., Blikslager A., Fujita T., Yoo D. Type III Interferon Restriction by Porcine Epidemic Diarrhea Virus and the Role of Viral Protein nsp1 in IRF1 Signaling. J. Virol. 2018;92:e01677-17. doi: 10.1128/JVI.01677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Q., Ma J., Yoo D. Inhibition of NF-kappaB activity by the porcine epidemic diarrhea virus nonstructural protein 1 for innate immune evasion. Virology. 2017;510:111–126. doi: 10.1016/j.virol.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li M., Wu Y., Chen J., Shi H., Ji Z., Zhang X., Shi D., Liu J., Tian J., Wang X., et al. Innate Immune Evasion of Porcine Epidemic Diarrhea Virus through Degradation of the FBXW7 Protein via the Ubiquitin-Proteasome Pathway. J. Virol. 2022;96:e0088921. doi: 10.1128/jvi.00889-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu L., Dong J., Wang Y., Zhang P., Liu Y., Zhang L., Liang P., Wang L., Song C. Porcine epidemic diarrhea virus nsp4 induces pro-inflammatory cytokine and chemokine expression inhibiting viral replication in vitro. Arch. Virol. 2019;164:1147–1157. doi: 10.1007/s00705-019-04176-2. [DOI] [PubMed] [Google Scholar]
- 191.Wang D., Fang L., Wei D., Zhang H., Luo R., Chen H., Li K., Xiao S. Hepatitis A virus 3C protease cleaves NEMO to impair induction of beta interferon. J. Virol. 2014;88:10252–10258. doi: 10.1128/JVI.00869-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang D., Fang L., Li K., Zhong H., Fan J., Ouyang C., Zhang H., Duan E., Luo R., Zhang Z., et al. Foot-and-mouth disease virus 3C protease cleaves NEMO to impair innate immune signaling. J. Virol. 2012;86:9311–9322. doi: 10.1128/JVI.00722-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Huang C., Zhang Q., Guo X.K., Yu Z.B., Xu A.T., Tang J., Feng W.H. Porcine reproductive and respiratory syndrome virus nonstructural protein 4 antagonizes beta interferon expression by targeting the NF-kappaB essential modulator. J. Virol. 2014;88:10934–10945. doi: 10.1128/JVI.01396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang D., Fang L., Shi Y., Zhang H., Gao L., Peng G., Chen H., Li K., Xiao S. Porcine Epidemic Diarrhea Virus 3C-Like Protease Regulates Its Interferon Antagonism by Cleaving NEMO. J. Virol. 2016;90:2090–2101. doi: 10.1128/JVI.02514-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lin H., Li B., Liu M., Zhou H., He K., Fan H. Nonstructural protein 6 of porcine epidemic diarrhea virus induces autophagy to promote viral replication via the PI3K/Akt/mTOR axis. Vet. Microbiol. 2020;244:108684. doi: 10.1016/j.vetmic.2020.108684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sutton G., Fry E., Carter L., Sainsbury S., Walter T., Nettleship J., Berrow N., Owens R., Gilbert R., Davidson A., et al. The nsp9 replicase protein of SARS-coronavirus, structure and functional insights. Structure. 2004;12:341–353. doi: 10.1016/j.str.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guo L., Luo X., Li R., Xu Y., Zhang J., Ge J., Bu Z., Feng L., Wang Y. Porcine Epidemic Diarrhea Virus Infection Inhibits Interferon Signaling by Targeted Degradation of STAT1. J. Virol. 2016;90:8281–8292. doi: 10.1128/JVI.01091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang J., Yuan S., Peng Q., Ding Z., Hao W., Peng G., Xiao S., Fang L. Porcine Epidemic Diarrhea Virus nsp7 Inhibits Interferon-Induced JAK-STAT Signaling through Sequestering the Interaction between KPNA1 and STAT1. J. Virol. 2022;96:e0040022. doi: 10.1128/jvi.00400-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ren J., Ding Z., Fang P., Xiao S., Fang L. ATPase and helicase activities of porcine epidemic diarrhea virus nsp13. Vet. Microbiol. 2021;257:109074. doi: 10.1016/j.vetmic.2021.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lu Y., Cai H., Lu M., Ma Y., Li A., Gao Y., Zhou J., Gu H., Li J., Gu J. Porcine Epidemic Diarrhea Virus Deficient in RNA Cap Guanine-N-7 Methylation Is Attenuated and Induces Higher Type I and III Interferon Responses. J. Virol. 2020;94:e00447-20. doi: 10.1128/JVI.00447-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wu Y., Zhang H., Shi Z., Chen J., Li M., Shi H., Shi D., Guo L., Feng L. Porcine Epidemic Diarrhea Virus nsp15 Antagonizes Interferon Signaling by RNA Degradation of TBK1 and IRF3. Viruses. 2020;12:599. doi: 10.3390/v12060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shi P., Su Y., Li R., Liang Z., Dong S., Huang J. PEDV nsp16 negatively regulates innate immunity to promote viral proliferation. Virus Res. 2019;265:57–66. doi: 10.1016/j.virusres.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li B.X., Ge J.W., Li Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007;365:166–172. doi: 10.1016/j.virol.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mina-Osorio P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Luo X., Guo L., Zhang J., Xu Y., Gu W., Feng L., Wang Y. Tight Junction Protein Occludin Is a Porcine Epidemic Diarrhea Virus Entry Factor. J. Virol. 2017;91:e00202-17. doi: 10.1128/JVI.00202-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sawada N. Tight junction-related human diseases. Pathol. Int. 2013;63:1–12. doi: 10.1111/pin.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yun J.P., Chew E.C., Liew C.T., Chan J.Y., Jin M.L., Ding M.X., Fai Y.H., Li H.K., Liang X.M., Wu Q.L. Nucleophosmin/B23 is a proliferate shuttle protein associated with nuclear matrix. J. Cell Biochem. 2003;90:1140–1148. doi: 10.1002/jcb.10706. [DOI] [PubMed] [Google Scholar]
- 208.Park J.Y., Ryu J., Park J.E., Hong E.J., Shin H.J. Heat shock protein 70 could enhance porcine epidemic diarrhoea virus replication by interacting with membrane proteins. Vet. Res. 2021;52:138. doi: 10.1186/s13567-021-01006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tomas A., Futter C.E., Eden E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kong N., Shan T., Wang H., Jiao Y., Zuo Y., Li L., Tong W., Yu L., Jiang Y., Zhou Y., et al. BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy. 2020;16:1737–1752. doi: 10.1080/15548627.2019.1707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kupzig S., Korolchuk V., Rollason R., Sugden A., Wilde A., Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 212.Schmidt O., Teis D. The ESCRT machinery. Curr. Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kaewborisuth C., Yingchutrakul Y., Roytrakul S., Jongkaewwattana A. Porcine Epidemic Diarrhea Virus (PEDV) ORF3 Interactome Reveals Inhibition of Virus Replication by Cellular VPS36 Protein. Viruses. 2019;11:382. doi: 10.3390/v11040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhao J., Chen J., Li M., Chen M., Sun C. Multifaceted Functions of CH25H and 25HC to Modulate the Lipid Metabolism, Immune Responses, and Broadly Antiviral Activities. Viruses. 2020;12:727. doi: 10.3390/v12070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang Y., Song Z., Wang M., Lan M., Zhang K., Jiang P., Li Y., Bai J., Wang X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet. Microbiol. 2019;231:129–138. doi: 10.1016/j.vetmic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sun L., Chen H., Ming X., Bo Z., Shin H.J., Jung Y.S., Qian Y. Porcine Epidemic Diarrhea Virus Infection Induces Caspase-8-Mediated G3BP1 Cleavage and Subverts Stress Granules To Promote Viral Replication. J. Virol. 2021;95:e02344-20. doi: 10.1128/JVI.02344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pandey K., Zhong S., Diel D.G., Hou Y., Wang Q., Nelson E., Wang X. GTPase-activating protein-binding protein 1 (G3BP1) plays an antiviral role against porcine epidemic diarrhea virus. Vet. Microbiol. 2019;236:108392. doi: 10.1016/j.vetmic.2019.108392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ramezani-Rad P., Leung C.R., Apgar J.R., Rickert R.C. E3 Ubiquitin Ligase Fbw7 Regulates the Survival of Mature B Cells. J. Immunol. 2020;204:1535–1542. doi: 10.4049/jimmunol.1901156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Y., Robertson J.D., Walcheck B. Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J. Biol. Chem. 2011;286:38980–38988. doi: 10.1074/jbc.M111.277087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Masutani M., Sonenberg N., Yokoyama S., Imataka H. Reconstitution reveals the functional core of mammalian eIF3. EMBO J. 2007;26:3373–3383. doi: 10.1038/sj.emboj.7601765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang R., Yu R., Chen B., Si F., Wang J., Xie C., Men C., Dong S., Li Z. Identification of host cell proteins that interact with the M protein of porcine epidemic diarrhea virus. Vet. Microbiol. 2020;246:108729. doi: 10.1016/j.vetmic.2020.108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kumar G.R., Shum L., Glaunsinger B.A. Importin alpha-mediated nuclear import of cytoplasmic poly(A) binding protein occurs as a direct consequence of cytoplasmic mRNA depletion. Mol. Cell Biol. 2011;31:3113–3125. doi: 10.1128/MCB.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 224.Li Y., Li J., Wang X., Wu Q., Yang Q. Role of intestinal extracellular matrix-related signaling in porcine epidemic diarrhea virus infection. Virulence. 2021;12:2352–2365. doi: 10.1080/21505594.2021.1972202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Garbers C., Scheller J. Interleukin-6 and interleukin-11: Same same but different. Biol. Chem. 2013;394:1145–1161. doi: 10.1515/hsz-2013-0166. [DOI] [PubMed] [Google Scholar]
- 226.Yang L., Wang R., Gao Y., Xu X., Fu K., Wang S., Li Y., Peng R. The protective role of interleukin-11 against neutron radiation injury in mouse intestines via MEK/ERK and PI3K/Akt dependent pathways. Dig. Dis. Sci. 2014;59:1406–1414. doi: 10.1007/s10620-013-3015-0. [DOI] [PubMed] [Google Scholar]
- 227.Uemura T., Nakayama T., Kusaba T., Yakata Y., Yamazumi K., Matsuu-Matsuyama M., Shichijo K., Sekine I. The protective effect of interleukin-11 on the cell death induced by X-ray irradiation in cultured intestinal epithelial cell. J. Radiat. Res. 2007;48:171–177. doi: 10.1269/jrr.06047. [DOI] [PubMed] [Google Scholar]
- 228.Agthe M., Garbers Y., Putoczki T., Garbers C. Interleukin-11 classic but not trans-signaling is essential for fertility in mice. Placenta. 2017;57:13–16. doi: 10.1016/j.placenta.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 229.Li Y., Wu Q., Jin Y., Yang Q. Antiviral activity of interleukin-11 as a response to porcine epidemic diarrhea virus infection. Vet. Res. 2019;50:111. doi: 10.1186/s13567-019-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zenewicz L.A., Yancopoulos G.D., Valenzuela D.M., Murphy A.J., Stevens S., Flavell R.A. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Basu R., O’Quinn D.B., Silberger D.J., Schoeb T.R., Fouser L., Ouyang W., Hatton R.D., Weaver C.T. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ouyang W., Rutz S., Crellin N.K., Valdez P.A., Hymowitz S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 233.Yamashita M.S.A., Melo E.O. Mucin 2 (MUC2) promoter characterization: An overview. Cell Tissue Res. 2018;374:455–463. doi: 10.1007/s00441-018-2916-9. [DOI] [PubMed] [Google Scholar]
- 234.Xiao Y., Zhou Y., Sun S., Wang H., Wu S., Bao W. Effect of Promoter Methylation on the Expression of Porcine MUC2 Gene and Resistance to PEDV Infection. Front. Vet. Sci. 2021;8:646408. doi: 10.3389/fvets.2021.646408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kamau A.N., Park J.E., Park E.S., Yu J.E., Rho J., Shin H.J. Porcine amino peptidase N domain VII has critical role in binding and entry of porcine epidemic diarrhea virus. Virus Res. 2017;227:150–157. doi: 10.1016/j.virusres.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Li W., Luo R., He Q., van Kuppeveld F.J.M., Rottier P.J.M., Bosch B.J. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017;235:6–13. doi: 10.1016/j.virusres.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Park J.E., Park E.S., Yu J.E., Rho J., Paudel S., Hyun B.H., Yang D.K., Shin H.J. Development of transgenic mouse model expressing porcine aminopeptidase N and its susceptibility to porcine epidemic diarrhea virus. Virus Res. 2015;197:108–115. doi: 10.1016/j.virusres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shirato K., Maejima M., Islam M.T., Miyazaki A., Kawase M., Matsuyama S., Taguchi F. Porcine aminopeptidase N is not a cellular receptor of porcine epidemic diarrhea virus, but promotes its infectivity via aminopeptidase activity. J. Gen. Virol. 2016;97:2528–2539. doi: 10.1099/jgv.0.000563. [DOI] [PubMed] [Google Scholar]
- 240.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burzynska P., Sobala L.F., Mikolajczyk K., Jodlowska M., Jaskiewicz E. Sialic Acids as Receptors for Pathogens. Biomolecules. 2021;11:831. doi: 10.3390/biom11060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wickramasinghe I.N., de Vries R.P., Grone A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.De Graaf M., Fouchier R.A. Role of receptor binding specificity in influenza A virus transmission and pathogenesis. EMBO J. 2014;33:823–841. doi: 10.1002/embj.201387442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Li W., Hulswit R.J.G., Widjaja I., Raj V.S., McBride R., Peng W., Widagdo W., Tortorici M.A., van Dieren B., Lang Y., et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA. 2017;114:E8508–E8517. doi: 10.1073/pnas.1712592114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Schultze B., Enjuanes L., Cavanagh D., Herrler G. N-acetylneuraminic acid plays a critical role for the haemagglutinating activity of avian infectious bronchitis virus and porcine transmissible gastroenteritis virus. Adv. Exp. Med. Biol. 1993;342:305–310. doi: 10.1007/978-1-4615-2996-5_47. [DOI] [PubMed] [Google Scholar]
- 246.Furuse M., Izumi Y., Oda Y., Higashi T., Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Torres-Flores J.M., Arias C.F. Tight Junctions Go Viral! Viruses. 2015;7:5145–5154. doi: 10.3390/v7092865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ploss A., Evans M.J., Gaysinskaya V.A., Panis M., You H., de Jong Y.P., Rice C.M. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Coyne C.B., Shen L., Turner J.R., Bergelson J.M. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zong Q.F., Huang Y.J., Wu L.S., Wu Z.C., Wu S.L., Bao W.B. Effects of porcine epidemic diarrhea virus infection on tight junction protein gene expression and morphology of the intestinal mucosa in pigs. Pol J. Vet. Sci. 2019;22:345–353. doi: 10.24425/pjvs.2019.129226. [DOI] [PubMed] [Google Scholar]
- 251.Fankhauser C., Izaurralde E., Adachi Y., Wingfield P., Laemmli U.K. Specific complex of human immunodeficiency virus type 1 rev and nucleolar B23 proteins: Dissociation by the Rev response element. Mol. Cell Biol. 1991;11:2567–2575. doi: 10.1128/mcb.11.5.2567-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tsuda Y., Mori Y., Abe T., Yamashita T., Okamoto T., Ichimura T., Moriishi K., Matsuura Y. Nucleolar protein B23 interacts with Japanese encephalitis virus core protein and participates in viral replication. Microbiol. Immunol. 2006;50:225–234. doi: 10.1111/j.1348-0421.2006.tb03789.x. [DOI] [PubMed] [Google Scholar]
- 253.Samad M.A., Okuwaki M., Haruki H., Nagata K. Physical and functional interaction between a nucleolar protein nucleophosmin/B23 and adenovirus basic core proteins. FEBS Lett. 2007;581:3283–3288. doi: 10.1016/j.febslet.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 254.Lymberopoulos M.H., Bourget A., Ben Abdeljelil N., Pearson A. Involvement of the UL24 protein in herpes simplex virus 1-induced dispersal of B23 and in nuclear egress. Virology. 2011;412:341–348. doi: 10.1016/j.virol.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 255.Liu C.D., Chen Y.L., Min Y.L., Zhao B., Cheng C.P., Kang M.S., Chiu S.J., Kieff E., Peng C.W. The nuclear chaperone nucleophosmin escorts an Epstein-Barr Virus nuclear antigen to establish transcriptional cascades for latent infection in human B cells. PLoS Pathog. 2012;8:e1003084. doi: 10.1371/journal.ppat.1003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Passos-Castilho A.M., Marchand C., Archambault D. B23/nucleophosmin interacts with bovine immunodeficiency virus Rev protein and facilitates viral replication. Virology. 2018;515:158–164. doi: 10.1016/j.virol.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 257.Zhou J., Li J., Li H., Zhang Y., Dong W., Jin Y., Yan Y., Gu J., Zhou J. The serine-48 residue of nucleolar phosphoprotein nucleophosmin-1 plays critical role in subcellular localization and interaction with porcine circovirus type 3 capsid protein. Vet. Res. 2021;52:4. doi: 10.1186/s13567-020-00876-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Song J., Hou L., Wang D., Wei L., Zhu S., Wang J., Quan R., Jiang H., Shi R., Liu J. Nucleolar Phosphoprotein NPM1 Interacts With Porcine Circovirus Type 3 Cap Protein and Facilitates Viral Replication. Front. Microbiol. 2021;12:679341. doi: 10.3389/fmicb.2021.679341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zhou J., Dai Y., Lin C., Zhang Y., Feng Z., Dong W., Jin Y., Yan Y., Zhou J., Gu J. Nucleolar protein NPM1 is essential for circovirus replication by binding to viral capsid. Virulence. 2020;11:1379–1393. doi: 10.1080/21505594.2020.1832366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Abraham R., Singh S., Nair S.R., Hulyalkar N.V., Surendran A., Jaleel A., Sreekumar E. Nucleophosmin (NPM1)/B23 in the Proteome of Human Astrocytic Cells Restricts Chikungunya Virus Replication. J. Proteome Res. 2017;16:4144–4155. doi: 10.1021/acs.jproteome.7b00513. [DOI] [PubMed] [Google Scholar]
- 261.Hartl F.U., Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 262.Maggioni C., Braakman I. Synthesis and quality control of viral membrane proteins. Curr Top. Microbiol. Immunol. 2005;285:175–198. doi: 10.1007/3-540-26764-6_6. [DOI] [PubMed] [Google Scholar]
- 263.Nagy P.D., Wang R.Y., Pogany J., Hafren A., Makinen K. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology. 2011;411:374–382. doi: 10.1016/j.virol.2010.12.061. [DOI] [PubMed] [Google Scholar]
- 264.Hirayama E., Atagi H., Hiraki A., Kim J. Heat shock protein 70 is related to thermal inhibition of nuclear export of the influenza virus ribonucleoprotein complex. J. Virol. 2004;78:1263–1270. doi: 10.1128/JVI.78.3.1263-1270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.De Marco A., Santoro M.G. Antiviral effect of short hyperthermic treatment at specific stages of vesicular stomatitis virus replication cycle. Pt 8J. Gen. Virol. 1993;74:1685–1690. doi: 10.1099/0022-1317-74-8-1685. [DOI] [PubMed] [Google Scholar]
- 266.Lupberger J., Zeisel M.B., Xiao F., Thumann C., Fofana I., Zona L., Davis C., Mee C.J., Turek M., Gorke S., et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011;17:589–595. doi: 10.1038/nm.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Diao J., Pantua H., Ngu H., Komuves L., Diehl L., Schaefer G., Kapadia S.B. Hepatitis C virus induces epidermal growth factor receptor activation via CD81 binding for viral internalization and entry. J. Virol. 2012;86:10935–10949. doi: 10.1128/JVI.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hu W., Zhang S., Shen Y., Yang Q. Epidermal growth factor receptor is a co-factor for transmissible gastroenteritis virus entry. Virology. 2018;521:33–43. doi: 10.1016/j.virol.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Eierhoff T., Hrincius E.R., Rescher U., Ludwig S., Ehrhardt C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010;6:e1001099. doi: 10.1371/journal.ppat.1001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Klann K., Bojkova D., Tascher G., Ciesek S., Munch C., Cinatl J. Growth Factor Receptor Signaling Inhibition Prevents SARS-CoV-2 Replication. Mol. Cell. 2020;80:164–174.e164. doi: 10.1016/j.molcel.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wang S.M., Huang K.J., Wang C.T. BST2/CD317 counteracts human coronavirus 229E productive infection by tethering virions at the cell surface. Virology. 2014;449:287–296. doi: 10.1016/j.virol.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Pan X.B., Qu X.W., Jiang D., Zhao X.L., Han J.C., Wei L. BST2/Tetherin inhibits hepatitis C virus production in human hepatoma cells. Antiviral. Res. 2013;98:54–60. doi: 10.1016/j.antiviral.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 273.Pan X.B., Han J.C., Cong X., Wei L. BST2/tetherin inhibits dengue virus release from human hepatoma cells. PLoS ONE. 2012;7:e51033. doi: 10.1371/journal.pone.0051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Li M., Wang P., Zheng Z., Hu K., Zhang M., Guan X., Fu M., Zhang D., Wang W., Xiao G., et al. Japanese encephalitis virus counteracts BST2 restriction via its envelope protein E. Virology. 2017;510:67–75. doi: 10.1016/j.virol.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Le Tortorec A., Neil S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Neil S.J., Zang T., Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 277.Jouvenet N., Neil S.J., Zhadina M., Zang T., Kratovac Z., Lee Y., McNatt M., Hatziioannou T., Bieniasz P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sakuma T., Noda T., Urata S., Kawaoka Y., Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Blondeau C., Pelchen-Matthews A., Mlcochova P., Marsh M., Milne R.S., Towers G.J. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J. Virol. 2013;87:13124–13133. doi: 10.1128/JVI.02250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Viswanathan K., Smith M.S., Malouli D., Mansouri M., Nelson J.A., Fruh K. BST2/Tetherin enhances entry of human cytomegalovirus. PLoS Pathog. 2011;7:e1002332. doi: 10.1371/journal.ppat.1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Teo H., Perisic O., Gonzalez B., Williams R.L. ESCRT-II, an endosome-associated complex required for protein sorting: Crystal structure and interactions with ESCRT-III and membranes. Dev. Cell. 2004;7:559–569. doi: 10.1016/j.devcel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 282.Daniloski Z., Jordan T.X., Wessels H.H., Hoagland D.A., Kasela S., Legut M., Maniatis S., Mimitou E.P., Lu L., Geller E., et al. Identification of Required Host Factors for SARS-CoV-2 Infection in Human Cells. Cell. 2021;184:92–105.e116. doi: 10.1016/j.cell.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang R., Simoneau C.R., Kulsuptrakul J., Bouhaddou M., Travisano K.A., Hayashi J.M., Carlson-Stevermer J., Zengel J.R., Richards C.M., Fozouni P., et al. Genetic Screens Identify Host Factors for SARS-CoV-2 and Common Cold Coronaviruses. Cell. 2021;184:106–119.e114. doi: 10.1016/j.cell.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wilkins C., Gale M., Jr. Sterol-izing innate immunity. Immunity. 2013;38:3–5. doi: 10.1016/j.immuni.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 285.Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A., et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Yuan Y., Wang Z., Tian B., Zhou M., Fu Z.F., Zhao L. Cholesterol 25-hydroxylase suppresses rabies virus infection by inhibiting viral entry. Arch. Virol. 2019;164:2963–2974. doi: 10.1007/s00705-019-04415-6. [DOI] [PubMed] [Google Scholar]
- 287.Shrivastava-Ranjan P., Bergeron E., Chakrabarti A.K., Albarino C.G., Flint M., Nichol S.T., Spiropoulou C.F. 25-Hydroxycholesterol Inhibition of Lassa Virus Infection through Aberrant GP1 Glycosylation. mBio. 2016;7:e01808-16. doi: 10.1128/mBio.01808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Magoro T., Dandekar A., Jennelle L.T., Bajaj R., Lipkowitz G., Angelucci A.R., Bessong P.O., Hahn Y.S. IL-1beta/TNF-alpha/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J. Biol. Chem. 2019;294:14591–14602. doi: 10.1074/jbc.RA119.007555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Roulin P.S., Lotzerich M., Torta F., Tanner L.B., van Kuppeveld F.J., Wenk M.R., Greber U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 290.Wang J., Zeng L., Zhang L., Guo Z.Z., Lu S.F., Ming S.L., Li G.L., Wan B., Tian K.G., Yang G.Y., et al. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017;98:1467–1476. doi: 10.1099/jgv.0.000797. [DOI] [PubMed] [Google Scholar]
- 291.Ke W., Fang L., Jing H., Tao R., Wang T., Li Y., Long S., Wang D., Xiao S. Cholesterol 25-Hydroxylase Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication through Enzyme Activity-Dependent and -Independent Mechanisms. J. Virol. 2017;91:e00827-17. doi: 10.1128/JVI.00827-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Dong H., Zhou L., Ge X., Guo X., Han J., Yang H. Antiviral effect of 25-hydroxycholesterol against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2018;23:395–404. doi: 10.3851/IMP3232. [DOI] [PubMed] [Google Scholar]
- 293.Ge Y., Jin J., Li J., Ye M., Jin X. The roles of G3BP1 in human diseases (review) Gene. 2022;821:146294. doi: 10.1016/j.gene.2022.146294. [DOI] [PubMed] [Google Scholar]
- 294.Tourriere H., Chebli K., Zekri L., Courselaud B., Blanchard J.M., Bertrand E., Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 2003;160:823–831. doi: 10.1083/jcb.200212128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 295.McCormick C., Khaperskyy D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017;17:647–660. doi: 10.1038/nri.2017.63. [DOI] [PubMed] [Google Scholar]
- 296.Yang W., Ru Y., Ren J., Bai J., Wei J., Fu S., Liu X., Li D., Zheng H. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis. 2019;10:946. doi: 10.1038/s41419-019-2178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Wen W., Zhao Q., Yin M., Qin L., Hu J., Chen H., Li X., Qian P. Seneca Valley Virus 3C Protease Inhibits Stress Granule Formation by Disrupting eIF4GI-G3BP1 Interaction. Front. Immunol. 2020;11:577838. doi: 10.3389/fimmu.2020.577838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Humoud M.N., Doyle N., Royall E., Willcocks M.M., Sorgeloos F., van Kuppeveld F., Roberts L.O., Goodfellow I.G., Langereis M.A., Locker N. Feline Calicivirus Infection Disrupts Assembly of Cytoplasmic Stress Granules and Induces G3BP1 Cleavage. J. Virol. 2016;90:6489–6501. doi: 10.1128/JVI.00647-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Ng C.S., Jogi M., Yoo J.S., Onomoto K., Koike S., Iwasaki T., Yoneyama M., Kato H., Fujita T. Encephalomyocarditis virus disrupts stress granules, the critical platform for triggering antiviral innate immune responses. J. Virol. 2013;87:9511–9522. doi: 10.1128/JVI.03248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Visser L.J., Medina G.N., Rabouw H.H., de Groot R.J., Langereis M.A., de Los Santos T., van Kuppeveld F.J.M. Foot-and-Mouth Disease Virus Leader Protease Cleaves G3BP1 and G3BP2 and Inhibits Stress Granule Formation. J. Virol. 2019;93:e00922-18. doi: 10.1128/JVI.00922-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Panas M.D., Varjak M., Lulla A., Eng K.E., Merits A., Karlsson Hedestam G.B., McInerney G.M. Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection. Mol. Biol. Cell. 2012;23:4701–4712. doi: 10.1091/mbc.e12-08-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Yang X., Hu Z., Zhang Q., Fan S., Zhong Y., Guo D., Qin Y., Chen M. SG formation relies on eIF4GI-G3BP interaction which is targeted by picornavirus stress antagonists. Cell Discov. 2019;5:1. doi: 10.1038/s41421-018-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.White J.P., Cardenas A.M., Marissen W.E., Lloyd R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2:295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 304.Nabeel-Shah S., Lee H., Ahmed N., Burke G.L., Farhangmehr S., Ashraf K., Pu S., Braunschweig U., Zhong G., Wei H., et al. SARS-CoV-2 nucleocapsid protein binds host mRNAs and attenuates stress granules to impair host stress response. iScience. 2022;25:103562. doi: 10.1016/j.isci.2021.103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Khaperskyy D.A., Emara M.M., Johnston B.P., Anderson P., Hatchette T.F., McCormick C. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS Pathog. 2014;10:e1004217. doi: 10.1371/journal.ppat.1004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhao D., Li J., Wang Y., Li X., Gao L., Cao H., Zheng S.J. Critical role for G3BP1 in infectious bursal disease virus (IBDV)-induced stress granule formation and viral replication. Vet. Microbiol. 2020;248:108806. doi: 10.1016/j.vetmic.2020.108806. [DOI] [PubMed] [Google Scholar]
- 307.Choudhury P., Bussiere L.D., Miller C.L. Mammalian Orthoreovirus Factories Modulate Stress Granule Protein Localization by Interaction with G3BP1. J. Virol. 2017;91:e01298-17. doi: 10.1128/JVI.01298-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Scholte F.E., Tas A., Albulescu I.C., Zusinaite E., Merits A., Snijder E.J., van Hemert M.J. Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication. J. Virol. 2015;89:4457–4469. doi: 10.1128/JVI.03612-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wang Z., Inuzuka H., Fukushima H., Wan L., Gao D., Shaik S., Sarkar F.H., Wei W. Emerging roles of the FBW7 tumour suppressor in stem cell differentiation. EMBO Rep. 2011;13:36–43. doi: 10.1038/embor.2011.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chen J., Wu X., Chen S., Chen S., Xiang N., Chen Y., Guo D. Ubiquitin ligase Fbw7 restricts the replication of hepatitis C virus by targeting NS5B for ubiquitination and degradation. Biochem. Biophys. Res. Commun. 2016;470:697–703. doi: 10.1016/j.bbrc.2016.01.076. [DOI] [PubMed] [Google Scholar]
- 311.Mikulicic S., Finke J., Boukhallouk F., Wustenhagen E., Sons D., Homsi Y., Reiss K., Lang T., Florin L. ADAM17-dependent signaling is required for oncogenic human papillomavirus entry platform assembly. eLife. 2019;8:e44345. doi: 10.7554/eLife.44345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Arenaccio C., Chiozzini C., Columba-Cabezas S., Manfredi F., Affabris E., Baur A., Federico M. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism. J. Virol. 2014;88:11529–11539. doi: 10.1128/JVI.01712-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Yuan F., Li D., Li C., Zhang Y., Song H., Li S., Deng H., Gao G.F., Zheng A. ADAM17 is an essential attachment factor for classical swine fever virus. PLoS Pathog. 2021;17:e1009393. doi: 10.1371/journal.ppat.1009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Guo L., Niu J., Yu H., Gu W., Li R., Luo X., Huang M., Tian Z., Feng L., Wang Y. Modulation of CD163 expression by metalloprotease ADAM17 regulates porcine reproductive and respiratory syndrome virus entry. J. Virol. 2014;88:10448–10458. doi: 10.1128/JVI.01117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhang J., Guo L., Yang L., Xu J., Zhang L., Feng L., Chen H., Wang Y. Metalloprotease ADAM17 regulates porcine epidemic diarrhea virus infection by modifying aminopeptidase N. Virology. 2018;517:24–29. doi: 10.1016/j.virol.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Valente S.T., Gilmartin G.M., Mott C., Falkard B., Goff S.P. Inhibition of HIV-1 replication by eIF3f. Proc. Natl. Acad. Sci. USA. 2009;106:4071–4078. doi: 10.1073/pnas.0900557106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Morais A., Terzian A.C., Duarte D.V., Bronzoni R.V., Madrid M.C., Gavioli A.F., Gil L.H., Oliveira A.G., Zanelli C.F., Valentini S.R., et al. The eukaryotic translation initiation factor 3 subunit L protein interacts with Flavivirus NS5 and may modulate yellow fever virus replication. Virol. J. 2013;10:205. doi: 10.1186/1743-422X-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Holcomb D., Alexaki A., Hernandez N., Hunt R., Laurie K., Kames J., Hamasaki-Katagiri N., Komar A.A., DiCuccio M., Kimchi-Sarfaty C. Gene variants of coagulation related proteins that interact with SARS-CoV-2. PLoS Comput. Biol. 2021;17:e1008805. doi: 10.1371/journal.pcbi.1008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jiao Y., Kong N., Wang H., Sun D., Dong S., Chen X., Zheng H., Tong W., Yu H., Yu L., et al. PABPC4 Broadly Inhibits Coronavirus Replication by Degrading Nucleocapsid Protein through Selective Autophagy. Microbiol. Spectr. 2021;9:e0090821. doi: 10.1128/Spectrum.00908-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Orian-Rousseau V., Sleeman J. CD44 is a multidomain signaling platform that integrates extracellular matrix cues with growth factor and cytokine signals. Adv. Cancer Res. 2014;123:231–254. doi: 10.1016/B978-0-12-800092-2.00009-5. [DOI] [PubMed] [Google Scholar]
- 322.Ponta H., Sherman L., Herrlich P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 323.Heldin P., Basu K., Kozlova I., Porsch H. HAS2 and CD44 in breast tumorigenesis. Adv. Cancer Res. 2014;123:211–229. doi: 10.1016/B978-0-12-800092-2.00008-3. [DOI] [PubMed] [Google Scholar]
- 324.Bouchard M.J., Racaniello V.R. CD44 is not required for poliovirus replication. J. Virol. 1997;71:2793–2798. doi: 10.1128/jvi.71.4.2793-2798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Goujon C., Rebendenne A., Roy P., Bonaventure B., Valadao A.C., Desmarets L., Rouille Y., Tauziet M., Arnaud-Arnould M., Giovannini D., et al. Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal HCoVs. Res. Sq. 2021 doi: 10.21203/rs.3.rs-555275/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liu A., Pan Q., Wang S., Zhang Y., Li Y., Wang Y., Qi X., Gao L., Liu C., Zhang Y., et al. Identification of Chicken CD44 as a Novel B Lymphocyte Receptor for Infectious Bursal Disease Virus. J. Virol. 2022;96:e0011322. doi: 10.1128/jvi.00113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Favors S.E., Curd L.M., Gregg R.K. Use of the anti-inflammatory cytokine interleukin-11 to reverse HIV-1gp120 repression of a natural killer cell line. Cell Immunol. 2012;276:1–5. doi: 10.1016/j.cellimm.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 328.Xue M., Zhao J., Ying L., Fu F., Li L., Ma Y., Shi H., Zhang J., Feng L., Liu P. IL-22 suppresses the infection of porcine enteric coronaviruses and rotavirus by activating STAT3 signal pathway. Antiviral. Res. 2017;142:68–75. doi: 10.1016/j.antiviral.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Das S., St Croix C., Good M., Chen J., Zhao J., Hu S., Ross M., Myerburg M.M., Pilewski J.M., Williams J., et al. Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience. 2020;23:101256. doi: 10.1016/j.isci.2020.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Liu Y., Yu X., Zhao J., Zhang H., Zhai Q., Chen W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020;164:884–891. doi: 10.1016/j.ijbiomac.2020.07.191. [DOI] [PubMed] [Google Scholar]
- 331.McCauley H.A., Guasch G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 332.Zhao J., Wan S., Sun N., Sun P., Sun Y., Khan A., Guo J., Zheng X., Fan K., Yin W., et al. Damage to intestinal barrier integrity in piglets caused by porcine reproductive and respiratory syndrome virus infection. Vet. Res. 2021;52:93. doi: 10.1186/s13567-021-00965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zheng H., Xu L., Liu Y., Li C., Zhang L., Wang T., Zhao D., Xu X., Zhang Y. MicroRNA-221-5p Inhibits Porcine Epidemic Diarrhea Virus Replication by Targeting Genomic Viral RNA and Activating the NF-kappaB Pathway. Int. J. Mol. Sci. 2018;19:3381. doi: 10.3390/ijms19113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.