Abstract
Erythrocyte invasion by malaria parasites is mediated by specific molecular interactions. Sialic acid residues of glycophorin A are used as invasion receptors by Plasmodium falciparum. In vitro invasion studies have demonstrated that some cloned P. falciparum lines can use alternate receptors independent of sialic acid residues of glycophorin A. It is not known if invasion by alternate pathways occurs commonly in the field. In this study, we used in vitro growth assays and erythrocyte invasion assays to determine the invasion phenotypes of 15 P. falciparum field isolates. Of the 15 field isolates tested, 5 multiply in both neuraminidase and trypsin-treated erythrocytes, 3 multiply in neuraminidase-treated but not trypsin-treated erythrocytes, and 4 multiply in trypsin-treated but not neuraminidase-treated erythrocytes; 12 of the 15 field isolates tested use alternate invasion pathways that are not dependent on sialic acid residues of glycophorin A. Alternate invasion pathways are thus commonly used by P. falciparum field isolates. Typing based on two polymorphic markers, MSP-1 and MSP-2, and two microsatellite markers suggests that only 1 of the 15 field isolates tested contains multiple parasite genotypes. Individual P. falciparum lines can thus use multiple invasion pathways in the field. These observations have important implications for malaria vaccine development efforts based on EBA-175, the P. falciparum protein that binds sialic acid residues of glycophorin A during invasion. It may be necessary to target parasite ligands responsible for the alternate invasion pathways in addition to EBA-175 to effectively block erythrocyte invasion by P. falciparum.
Malaria parasites are obligate intracellular parasites that invade erythrocytes during the blood stage of their life cycle. The invasion of erythrocytes by malaria parasites is a multistep process that is mediated by specific molecular interactions between erythrocyte receptors and parasite ligands (2, 29). For example, the human malaria parasite Plasmodium vivax is absolutely dependent on the Duffy blood group antigen for the invasion of human erythrocytes (15). Duffy-negative erythrocytes lack the Duffy blood group antigen and are completely resistant to invasion by P. vivax. P. falciparum, on the other hand, does not require interaction with the Duffy blood group antigen for invasion and invades both Duffy-positive and Duffy-negative erythrocytes. Sialic acid residues of glycophorin A have been identified as invasion receptors for P. falciparum (3, 8, 16, 20). A 175-kDa P. falciparum protein, referred to as EBA-175 (for erythrocyte binding antigen-175 kDa), specifically binds sialic acid residues of glycophorin A to mediate erythrocyte invasion (4).
Unlike P. vivax, P. falciparum is not completely dependent on a single receptor for the invasion of human erythrocytes. Erythrocyte invasion studies with cloned P. falciparum lines revealed significant heterogeneity in the receptors used for invasion (9, 18, 21). P. falciparum clones such as HB3 and 7G8 invade neuraminidase-treated human erythrocytes, indicating that they can use sialic acid-independent invasion pathways (7). Other cloned parasite lines such as Dd2 and FCR3 are completely dependent on sialic acid residues for invasion but invade trypsin-treated erythrocytes that have lost glycophorin A. Dd2 and FCR3 can use sialic acid residues present on both glycophorin A as well as trypsin-resistant glycophorin B as receptors for invasion (7).
It is not known if the use of alternate invasion pathways observed with cloned P. falciparum lines actually occurs in the field. If it does, how commonly do P. falciparum field isolates use alternate invasion pathways that are independent of sialic acid residues of glycophorin A? The answer to this question has important implications for efforts to develop malaria vaccines based on EBA-175 that attempt to elicit antibodies to block binding of EBA-175 to erythrocytes and inhibit invasion. EBA-175 does not bind erythrocytes that lack either sialic acid residues or glycophorin A (4, 7) and therefore cannot mediate erythrocyte invasion by alternate pathways.
In this report, we have studied the invasion phenotypes of P. falciparum field isolates collected from different regions of India. In vitro parasite growth assays and erythrocyte invasion assays with normal, neuraminidase-treated, and trypsin-treated target erythrocytes were used to determine the invasion phenotypes of P. falciparum field isolates. These studies indicate that P. falciparum field isolates commonly use alternate invasion pathways that do not depend on sialic acid residues of glycophorin A. The parasite ligands responsible for the invasion of human erythrocytes by alternate pathways remain to be identified. It may be necessary to target such ligands in addition to EBA-175 in order to effectively block erythrocyte invasion by P. falciparum.
MATERIALS AND METHODS
P. falciparum field isolates.
Cryopreserved P. falciparum field isolates were obtained from the Malaria Parasite Bank maintained by the Malaria Research Centre, Delhi, India. P. falciparum field isolates were collected from malaria patients from different regions of India and cryopreserved in liquid nitrogen by using standard procedures. Cloned P. falciparum lines Dd2 and 7G8 were provided by Tom Wellems, Laboratory of Parasitic Diseases, National Institutes of Health, Bethesda, Md. Parasites were thawed and cultured in candle jars according to the method of Trager and Jensen (28) for two to five cycles to a parasitemia of 2 to 5% before use in parasite growth or erythrocyte invasion assays. Parasite cultures were maintained in A+ erythrocytes at 5% hematocrit in RPMI 1640 medium (Gibco BRL Laboratories, Gaithersburg, Md.) supplemented with 10% AB+ human serum, gentamicin (10 μg/ml), 25 mM sodium bicarbonate, and 25 mM HEPES.
Enzymatic treatment of erythrocytes.
Normal A+ erythrocytes were collected in citrate-phosphate-dextrose solution, plasma was removed, and erythrocytes were washed three times in RPMI 1640. The cells were stored at 40 to 50% hematocrit in RPMI 1640 at 4°C for up to 2 weeks until used. Erythrocytes were treated with neuraminidase and trypsin as described earlier (9).
The efficiency of the neuraminidase and trypsin treatments was assayed by agglutination assays using monoclonal antibodies M2A1 and 12E.A1, directed against the M and N epitopes, respectively, of glycophorin A, using procedures described by the manufacturer (Gamma Biologicals, Houston, Tex.). Loss of agglutination confirmed complete removal of the M and N epitopes, which are sensitive to both neuraminidase and trypsin treatment.
In vitro parasite growth assay.
P. falciparum field isolates were tested for growth in normal, neuraminidase-treated, and trypsin-treated erythrocytes, using methods described earlier (6). Parasites were thawed and grown for two to five cycles to an asynchronous parasitemia of 2 to 5% in normal A+ erythrocytes. An inoculum from this culture was delivered to fresh 5-ml cultures containing either untreated, neuraminidase-treated, or trypsin-treated human erythrocytes to an initial parasitemia of 0.1 to 0.3%. Parasitemia was estimated at 24-h intervals for 7 days by scoring the number of infected erythrocytes per 1,000 erythrocytes on Giemsa-stained thin smears. To correct for growth in normal erythrocytes carried over during inoculation, an inoculum was also delivered in rhesus erythrocytes, which are known to be resistant to invasion by P. falciparum. The parasitemia in control, rhesus cultures can be attributed to invasion of human erythrocytes carried over with the inoculum. The corrected parasitemia in test erythrocytes was determined by subtracting the parasitemia in rhesus erythrocytes from the parasitemia in test cultures. Growth assays were set up in duplicate, and the average parasitemia ± range is reported.
In vitro erythrocyte invasion assay.
Erythrocyte invasion efficiencies of P. falciparum field isolates in normal and enzyme-treated erythrocytes were determined by methods described previously (7). Parasite cultures were synchronized by sorbitol treatment, and late-stage schizonts were enriched by flotation on 60% Percoll. Parasitized erythrocytes enriched to 60 to 90% parasitemia were washed twice with RPMI 1640, counted with a hemocytometer, and mixed with target erythrocytes in triplicate wells. Then 1 × 106 to 2 × 106 parasitized erythrocytes were mixed with 1 × 107 to 5 × 107 target erythrocytes in 0.5 ml of complete RPMI 1640 medium and incubated in a candle jar at 37°C for 16 to 20 h. Invasion efficiency was determined by counting the number of parasitized erythrocytes per 1,000 erythrocytes on Giemsa-stained thin smears. Rhesus erythrocytes, which are resistant to P. falciparum invasion, were used to correct for invasion into normal human erythrocytes carried over during inoculation. The invasion efficiency in test erythrocytes was determined by subtracting the parasitemia in rhesus cultures from the parasitemia in test cultures. Average invasion efficiencies ± standard deviation determined in two independent experiments are reported.
PCR-based typing of P. falciparum field isolates.
PCR amplification of gene fragments encoding polymorphic regions of blood-stage parasite proteins, MSP-1 and MSP-2, and two microsatellite markers, TA62 and TA77, was used to type the P. falciparum field isolates and determine if they contain multiple clones. Genomic DNA was isolated from each P. falciparum isolate by the Chelex-boiling method described earlier (30). PCR typing of P. falciparum field isolates by using the two polymorphic markers MSP-1 and MSP-2 has been described elsewhere (12, 13, 25, 26).
(i) MSP-1.
Oligonucleotide primers based on conserved sequences flanking the polymorphic block 2 region of MSP-1 were used for PCR amplification (12, 13, 26). The PCR products were separated on 6% polyacrylamide gels and visualized by ethidium bromide staining. The PCR products were transferred to nylon membrane and typed by hybridization with 32P-labeled oligonucleotide probes based either on the major dimorphic forms, K1 and MAD20, which contain distinctive repeat sequences, or the third variant, R033, which lacks repeat sequences (12, 13, 26). Sequences of the PCR primers and oligonucleotide probes used as well as PCR amplification and hybridization conditions were identical to those described earlier (12, 13, 26).
(ii) MSP-2.
Oligonucleotide primers based on conserved sequences flanking the central polymorphic regions, blocks 2 and 3, of MSP-2 were used for PCR amplification (13, 25). The PCR products were separated on 6% polyacrylamide gels and visualized by ethidium bromide staining. The PCR products were transferred to nylon membrane and typed by hybridization using 32P-labeled oligonucleotide probes specific for the FC27 and CAMP-type MSP-2 sequences. The sequences of the PCR primers and oligonucleotide probes and the conditions for PCR and Southern hybridization were identical to those described by Kyes et al. (13).
(iii) TA62 and TA77.
The use of microsatellite polymorphisms to type P. falciparum strains has been described by Su and Wellems (27). Microsatellite markers comprise a variable number of simple sequence repeats that are widely distributed in the parasite genome. Two such microsatellite markers, TA62 and TA77, were PCR amplified by using oligonucleotide primers and PCR conditions described by Su and Wellems (27). One primer of each PCR primer pair was labeled with 32P to yield radiolabeled PCR products. The PCR products were separated on 6% polyacrylamide sequencing gels and detected by autoradiography.
RESULTS
Growth of P. falciparum field isolates in normal, neuraminidase-treated, and trypsin-treated human erythrocytes.
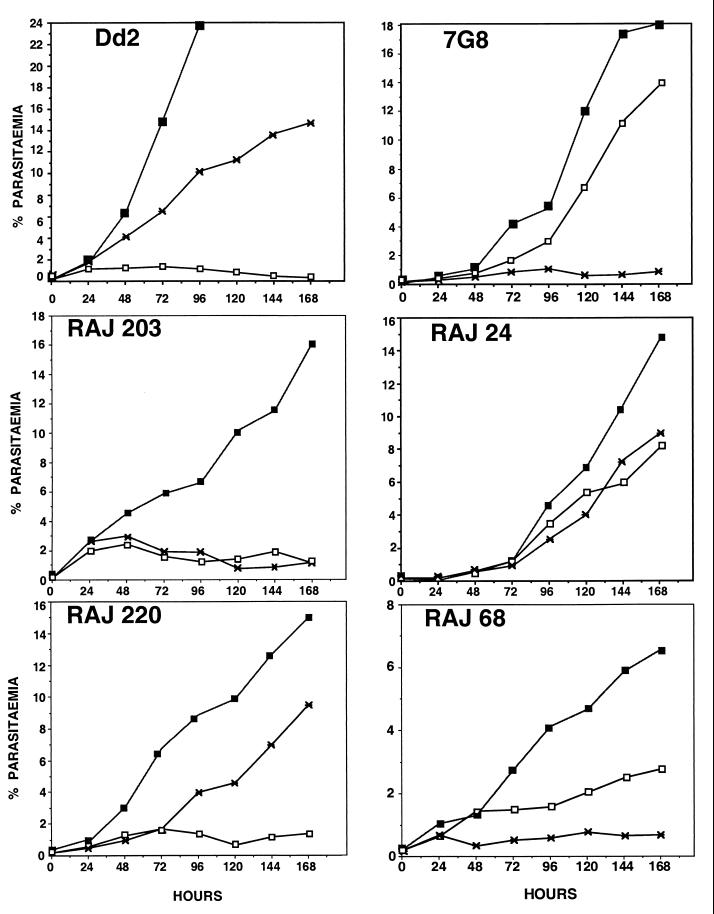
In vitro parasite growth assays were performed with 15 P. falciparum field isolates. Two cloned P. falciparum lines, Dd2 and 7G8, were used as controls. Dd2 is known to invade trypsin-treated erythrocytes but not neuraminidase-treated erythrocytes (7). Conversely, 7G8 invades neuraminidase-treated erythrocytes but not trypsin-treated erythrocytes (7). The growth curves of Dd2 and 7G8 reflect their invasion phenotypes (Fig. 1). The growth curves of four P. falciparum field isolates are also shown in Fig. 1. The P. falciparum field isolate RAJ203 multiplies in normal human erythrocytes but not in neuraminidase-treated or trypsin-treated human erythrocytes. In contrast, the P. falciparum field isolate RAJ24 multiplies in both neuraminidase-treated and trypsin-treated human erythrocytes. The growth curve of the P. falciparum field isolate RAJ220 is similar to that of Dd2. Both RAJ220 and Dd2 multiply in trypsin-treated erythrocytes but not in neuraminidase-treated erythrocytes. In contrast, the P. falciparum field isolate RAJ68 has a growth curve similar to that of 7G8. Both RAJ68 and 7G8 multiply in neuraminidase-treated but not in trypsin-treated human erythrocytes.
FIG. 1.
Growth of P. falciparum field isolates in normal and enzyme-treated erythrocytes. Cloned P. falciparum lines (Dd2 and 7G8) and P. falciparum field isolates (RAJ203, RAJ24, RAJ220, and RAJ68) were inoculated in normal (closed squares), neuraminidase-treated (open squares), and trypsin-treated (asterisk) erythrocytes and cultured for 7 days. Parasitemia at 24-h intervals is shown.
The growth curves shown (Fig. 1) are representative of the growth curves of the 11 other P. falciparum field isolates that were tested. Table 1 shows the parasitemia of each P. falciparum field isolate in enzyme-treated erythrocytes as a percentage of the parasitemia in normal human erythrocytes on day 7 of the growth assay. If the day 7 parasitemia in enzyme-treated erythrocytes is greater than 20% of the day 7 parasitemia in normal erythrocytes, the parasite isolate is considered to be positive for growth in the enzyme-treated erythrocytes. By this criterion, only 3 of the 15 isolates tested do not multiply in either neuraminidase-treated or trypsin-treated human erythrocytes; in contrast, of the 15 isolates tested, 5 multiply in both neuraminidase-treated and trypsin-treated erythrocytes, 3 multiply in neuraminidase-treated but not in trypsin-treated erythrocytes, and 4 multiply in trypsin-treated but not in neuraminidase-treated erythrocytes. These data suggest that 12 of the 15 field isolates tested can use alternate invasion pathways that are independent of sialic acid residues of glycophorin A for invasion.
TABLE 1.
Growth of P. falciparum field isolates in normal and enzyme-treated human erythrocytesa
| Parasite isolate | Day 7 parasitemia (%; avg ± range) in normal) erythrocytes | Day 7 parasitemia (% of normal; avg ± range) in erythrocytes treated with:
|
|
|---|---|---|---|
| Neuraminidase | Trypsin | ||
| Dd2 | 23.8 ± 1.8b | 1.4 ± 0.7 (−) | 43.0 ± 1.4 (+) |
| 7G8 | 18.0 ± 1.4 | 74.0 ± 1.9 (+) | 3.8 ± 0.5 (−) |
| JDP8 | 12.2 ± 1.1 | 24.5 ± 1.0 (+) | 42.0 ± 2.9 (+) |
| RKL9 | 12.9 ± 0.4 | 59.8 ± 2.9 (+) | 47.7 ± 1.5 (+) |
| RKL12 | 13.1 ± 0.5 | 33.5 ± 4.3 (+) | 13.0 ± 0.2 (−) |
| RAJ24 | 14.8 ± 0.5 | 56.1 ± 4.6 (+) | 61.9 ± 1.7 (+) |
| RAJ68 | 6.6 ± 1.0 | 42.6 ± 3.2 (+) | 13.9 ± 3.8 (−) |
| RAJ86 | 9.4 ± 1.7 | 22.4 ± 3.3 (+) | 17.7 ± 2.0 (−) |
| RAJ87 | 14.1 ± 1.7 | 4.7 ± 1.0 (−) | 34.1 ± 2.6 (+) |
| RAJ89 | 12.7 ± 0.5 | 43.9 ± 2.3 (+) | 24.2 ± 2.3 (+) |
| RAJ104 | 14.6 ± 1.0 | 1.8 ± 0.7 (−) | 34.4 ± 3.1 (+) |
| RAJ116 | 10.6 ± 2.1 | 10.7 ± 0.4 (−) | 8.6 ± 1.9 (−) |
| RAJ133 | 17.7 ± 0.8 | 73.7 ± 2.5 (+) | 28.8 ± 4.1 (+) |
| RAJ203 | 16.1 ± 2.4 | 7.1 ± 0.6 (−) | 7.7 ± 1.7 (−) |
| RAJ220 | 15.0 ± 1.3 | 9.4 ± 1.8 (−) | 63.4 ± 1.5 (+) |
| SH8777 | 11.1 ± 2.0 | 10.1 ± 2.7 (−) | 9.2 ± 2.1 (−) |
| DL4 | 18.0 ± 1.8 | 5.5 ± 0.3 (−) | 38.2 ± 1.4 (+) |
Fifteen P. falciparum field isolates and two cloned P. falciparum lines (Dd2 and 7G8) were inoculated into normal and enzyme-treated human erythrocytes as well as normal rhesus erythrocytes at a parasitemia of 0.1 to 0.3% in duplicate wells and cultured for 1 week. Growth in normal erythrocytes carried over with the inoculum was corrected for by subtracting the parasitemia in rhesus erythrocytes from the parasitemia in test erythrocytes. Day 7 parasitemia in rhesus erythrocytes was less than 5% of the day 7 parasitemia in normal erythrocytes in all cases. If the day 7 parasitemia in enzyme-treated erythrocytes is greater than 20% of the day 7 parasitemia in normal erythrocytes, the parasite isolate is considered positive for growth in the enzyme-treated erythrocytes. (+), the parasite can multiply in the enzyme-treated erythrocytes; (−), the parasite cannot multiply in the enzyme-treated erythrocytes.
Parasitemia on day 4.
P. falciparum field isolate RKL9 uses multiple pathways to invade erythrocytes.
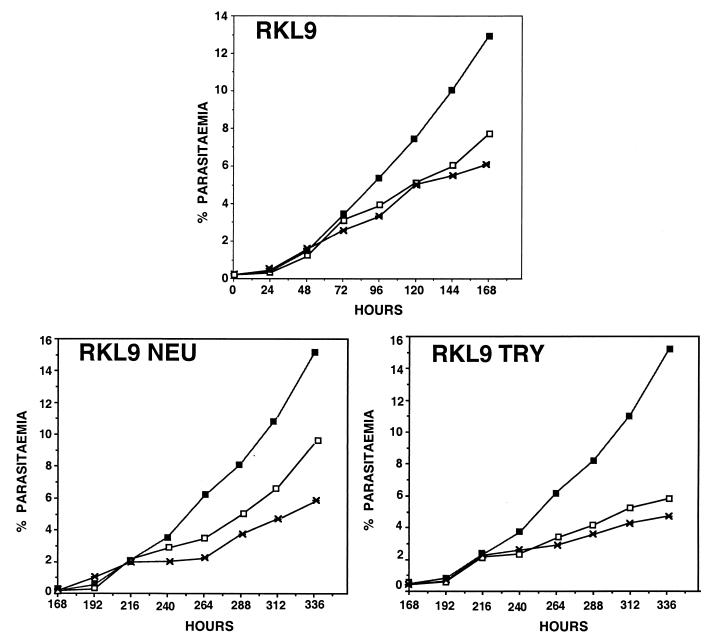
The field isolates used in this study were derived from P. falciparum malaria patients. Isolates such as RKL9, which grow in both neuraminidase-treated and trypsin-treated erythrocytes, may contain multiple P. falciparum lines, including one that invades neuraminidase-treated erythrocytes and another that invades trypsin-treated erythrocytes. Alternately, RKL9 may contain a clonal parasite line that invades both neuraminidase-treated and trypsin-treated erythrocytes. To distinguish between these possibilities, RKL9 was cultured separately in both neuraminidase-treated and trypsin-treated human erythrocytes for 7 days. Parasites cultured in neuraminidase-treated erythrocytes (RKL9Neu) were reinoculated on day 7 in normal, neuraminidase-treated, and trypsin-treated human erythrocytes at a parasitemia of 0.2%. Parasite growth was monitored at 24-h intervals for another 7 days. Similarly, parasites cultured in trypsin-treated erythrocytes (RKL9Try) were reinoculated on day 7 in normal, neuraminidase-treated, and trypsin-treated human erythrocytes at a parasitemia of 0.2% and parasite growth was monitored for another 7 days. Both RKL9Neu and RKL9Try parasites multiplied in neuraminidase-treated as well as trypsin-treated erythrocytes (Fig. 2). PCR typing data based on two polymorphic markers, MSP-1 and MSP-2, and two microsatellite markers also suggest that RKL9 contains a single parasite line (described below). It thus appears likely that RKL9 is composed of a single parasite line that invades both neuraminidase-treated and trypsin-treated erythrocytes.
FIG. 2.
Multiple erythrocyte invasion pathways used by P. falciparum field isolate RKL9. P. falciparum field isolate RKL9 was cultured in normal, neuraminidase-treated, and trypsin-treated erythrocytes for 7 days. Parasites growing in either neuraminidase-treated erythrocytes (RKL9Neu) or trypsin-treated erythrocytes (RKL9Try) were reinoculated in normal, neuraminidase-treated, and trypsin-treated erythrocytes on day 7 and cultured for another 7 days. Parasitemia of RKL9, RKL9Neu, and RKL9Try in normal (closed squares), neuraminidase-treated (open squares), and trypsin-treated (crosses) erythrocytes at 24-h intervals is shown.
Erythrocyte invasion assays confirm the invasion phenotypes of P. falciparum field isolates determined by in vitro growth assays.
Five of the 15 P. falciparum field isolates that were tested in parasite growth assays were also tested in erythrocyte invasion assays with normal and enzyme-treated erythrocytes. P. falciparum schizonts were purified and incubated with normal and enzyme-treated erythrocytes to allow invasion. The invasion rates were determined by scoring the percentage of infected erythrocytes in normal and test erythrocytes. The cloned P. falciparum lines, Dd2 and 7G8, were used as controls. The invasion rates of P. falciparum field isolates into enzyme-treated erythrocytes are shown as a percentage of invasion rates into normal erythrocytes in Table 2. The invasion rates of the P. falciparum field isolates confirm the invasion phenotypes predicted by the growth assays. The P. falciparum field isolates RKL9 and JDP8 invade both neuraminidase-treated and trypsin-treated erythrocytes. In addition, whereas RKL12 and RAJ68 invade neuraminidase-treated but not trypsin-treated erythrocytes, RAJ104 invades trypsin-treated but not neuraminidase-treated erythrocytes. None of the isolates tested invade erythrocytes treated with both neuraminidase and trypsin.
TABLE 2.
Invasion rates of P. falciparum field isolates in normal and enzyme-treated erythrocytesa
| Parasite isolate | Invasion rate (avg % parasitemia ± SD) in normal erythrocytes | Invasion rate (% of normal; avg ± SD)
|
|||
|---|---|---|---|---|---|
| Neu | Try | Neu + Try | Try + Neu | ||
| Dd2 | 7.6 ± 0.7 | −1.1 ± 0.1 | 48.7 ± 0.2 | −7.1 ± 0.2 | −1.3 ± 0.4 |
| 8.0 ± 0.5 | 1.6 ± 0.2 | 52.3 ± 0.3 | −6.3 ± 0.2 | −1.5 ± 0.3 | |
| 7G8 | 6.3 ± 0.8 | 69.6 ± 0.4 | 6.3 ± 0.1 | −2.7 ± 0.1 | −0.9 ± 0.1 |
| 5.9 ± 0.5 | 65.4 ± 0.3 | 3.0 ± 0.1 | −1.9 ± 0.2 | 1.0 ± 0.1 | |
| JDP8 | 3.8 ± 0.5 | 20.4 ± 0.2 | 34.4 ± 0.1 | −4.5 ± 0.2 | −5.8 ± 0.1 |
| 3.6 ± 0.5 | 21.0 ± 0.1 | 39.2 ± 0.1 | −1.4 ± 0.1 | −3.8 ± 0.2 | |
| RKL9 | 4.7 ± 0.8 | 49.6 ± 0.7 | 37.2 ± 0.2 | 2.1 ± 0.4 | 1.3 ± 0.4 |
| 4.1 ± 0.1 | 55.2 ± 0.3 | 44.3 ± 0.5 | 6.4 ± 0.2 | 2.0 ± 0.1 | |
| RKL12 | 4.1 ± 0.5 | 33.0 ± 0.1 | 11.5 ± 0.1 | 3.7 ± 0.1 | −1.5 ± 0.1 |
| 4.2 ± 0.2 | 29.3 ± 0.2 | 8.0 ± 0.1 | 0.5 ± 0.1 | −3.3 ± 0.1 | |
| RAJ68 | 3.3 ± 0.1 | 33.6 ± 0.2 | 2.7 ± 0.3 | −3.0 ± 0.1 | −1.0 ± 0.2 |
| 3.7 ± 0.1 | 38.5 ± 0.1 | 4.0 ± 0.1 | −8.0 ± 0.1 | −3.1 ± 0.2 | |
| RAJ104 | 5.0 ± 0.3 | 1.0 ± 0.1 | 25.4 ± 0.1 | −1.2 ± 0.1 | 2.4 ± 0.1 |
| 4.1 ± 0.4 | 0.5 ± 0.1 | 29.1 ± 0.1 | 0.7 ± 0.1 | 3.2 ± 0.1 | |
Invasion rates of five P. falciparum field isolates (RKL9, RKL12, JDP8, RAJ68, and RAJ104) and two cloned parasite lines (Dd2 and 7G8) in normal and enzyme-treated human erythrocytes determined in two independent experiments are shown. Purified P. falciparum schizonts were incubated in triplicate wells with normal and enzyme-treated human erythrocytes as well as rhesus erythrocytes. Invasion into normal human erythrocytes carried over with the inoculum was accounted for by subtracting the parasitemia in rhesus erythrocytes from parasitemia in test erythrocytes. Invasion rates in rhesus erythrocytes were less than 25% of invasion rates in normal human erythrocytes in all cases. Enzymes (Neu, neuraminidase; Try, trypsin) are listed in the order they were applied to the erythrocytes.
Genotyping P. falciparum field isolates by using polymorphic markers.
P. falciparum field isolates used in this study were derived from malaria patients and may contain multiple parasite lines. PCR amplification of DNA encoding polymorphic regions of two merozoite surface proteins, MSP-1 and MSP-2, and two microsatellite markers, TA77 and TA62, was used to type field isolates and determine if they contain more than one P. falciparum genotype (12, 13, 25, 26, 27).
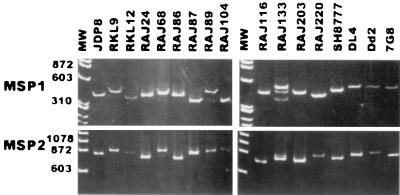
In the case of MSP-1, DNA encoding the polymorphic repeat region at the N-terminal end (block 2) was PCR amplified by using primers based on conserved sequences in the flanking regions. Each field isolate except RAJ133 yielded a single PCR product with the MSP-1 primers (Fig. 3). RAJ133 yielded three distinct PCR products with the MSP-1 primers, suggesting that it contains multiple parasite genotypes (Fig. 3). The PCR products were assessed for presence of the three MSP-1 sequence types, K1, MAD20, and R033, using type-specific, 32P-labeled oligonucleotide probes for hybridization. Two of the three PCR products from RAJ133 hybridized with the K1-type probe, and the third PCR product hybridized with the MAD20-type probe; PCR products derived from each of the other field isolates hybridized with unique, type-specific probes (Table 3). PCR typing based on block 2 of MSP-1 suggests that RAJ133 contains more than one P. falciparum genotype.
FIG. 3.
PCR typing of P. falciparum field isolates based on MSP-1 and MSP-2. DNA fragments encoding the polymorphic N-terminal region (block 2) of MSP-1 and the central, polymorphic regions (blocks 2 and 3) of MSP-2 were amplified by PCR using genomic DNA from 15 P. falciparum field isolates (JDP8, RKL9, RKL12, RAJ24, RAJ68, RAJ86, RAJ87, RAJ89, RAJ104, RAJ116, RAJ133, RAJ203, RAJ220, SH8777, and DL4) and two cloned P. falciparum lines (Dd2 and 7G8) as templates. The PCR products were separated by electrophoresis on polyacrylamide gels and visualized by staining with ethidium bromide. MW, molecular weights standards (sizes are shown in base pairs).
TABLE 3.
Typing of P. falciparum field isolates based on MSP-1 and MSP-2 allelesa
| Parasite isolate | Origin | Size(s) (bp) of PCR products
|
||||
|---|---|---|---|---|---|---|
| MSP-1
|
MSP-2
|
|||||
| K1 | MAD20 | RO33 | FC27 | CAMP | ||
| Dd2 | Lab clone | 400 | 860 | |||
| 7G8 | Lab clone | 440 | 880 | |||
| JDP8 | Madhya Pradesh | 400 | 860 | |||
| RKL9 | Orissa | 440 | 880 | |||
| RKL12 | Orissa | 390 | 880 | |||
| RAJ24 | Rajasthan | 400 | 800 | |||
| RAJ68 | Rajasthan | 440 | 880 | |||
| RAJ87 | Rajasthan | 390 | 860 | |||
| RAJ89 | Rajasthan | 480 | 870 | |||
| RAJ104 | Rajasthan | 390 | 880 | |||
| RAJ116 | Rajasthan | 440 | 700 | |||
| RAJ133 | Rajasthan | 500, 440 | 390 | 720 | 700 | |
| RAJ203 | Rajasthan | 440 | 700 | |||
| RAJ220 | Rajasthan | 400 | 720 | |||
| SH8777 | Uttar Pradesh | 440 | 700 | |||
| DL4 | Delhi | 460 | 720 | |||
DNA encoding the N-terminal, polymorphic region (block 2) of MSP-1 and the central, polymorphic regions (blocks 2 and 3) of MSP-2 were PCR amplified, separated by gel electrophoresis, and visualized by ethidium bromide staining. Radiolabeled DNA probes specific for K1-, MAD20-, and R033-type MSP-1 sequences and FC27-, and CAMP-type MSP-2 sequences were used to type the PCR products by Southern blotting.
PCR typing based on the central polymorphic regions (blocks 2 and 3) of MSP-2 confirmed the observations made with MSP-1 primers. As in case of MSP-1, all isolates except RAJ133 yielded unique PCR products with the MSP-2 primers (Fig. 3). RAJ133 yielded two distinct PCR products with the MSP-2 primers. One of the products hybridized with an FC27-specific probe, and the other hybridized to a CAMP-specific probe, confirming presence of more than one parasite genotype (Table 3). PCR products derived from each of the other field isolates hybridized with either FC27 or CAMP-specific probes, suggesting that each contains a single parasite genotype (Table 3).
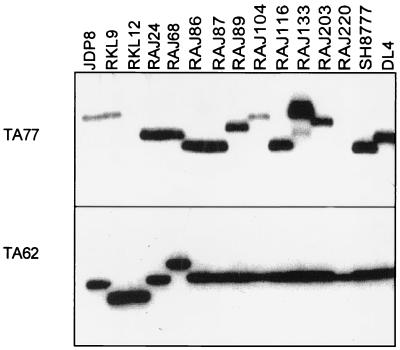
In addition to the polymorphic antigens, two microsatellite markers, TA62 and TA77, were used to type the P. falciparum field isolates. PCR amplification of the microsatellite marker TA62 yielded a single PCR product with genomic DNA from each isolate (Fig. 4). PCR amplification of the microsatellite marker TA77 did not yield any product with genomic DNA from RKL12 and RAJ220, two products with RAJ133 genomic DNA, and a unique product with each of the other P. falciparum field isolates. Data from microsatellite markers confirm the presence of multiple parasite genotypes in RAJ133 and suggest that each of the other field isolates contains a single P. falciparum genotype.
FIG. 4.
PCR typing of P. falciparum field isolates based on microsatellite markers TA62 and TA77. Two microsatellite markers, TA62 and TA77, were amplified by PCR using primers based on flanking sequences. One of the two primers used was radiolabeled with 32P. The PCR products were separated by electrophoresis on polyacrylamide gels and visualized by autoradiography.
DISCUSSION
Using both in vitro growth and invasion assays, we have characterized the invasion phenotypes of P. falciparum field isolates. Fifteen field isolates derived from P. falciparum malaria patients were tested for growth in normal, neuraminidase-treated, and trypsin-treated erythrocytes. The field isolates displayed significant diversity in growth phenotypes. Whereas five field isolates multiplied in both neuraminidase-treated and trypsin-treated erythrocytes, four multiplied in neuraminidase-treated but not in trypsin-treated erythrocytes and three multiplied in trypsin-treated but not in neuraminidase-treated erythrocytes. In all, 12 of 15 field isolates tested could grow in erythrocytes lacking either sialic acid residues or glycophorin A. Growth in neuraminidase-treated or trypsin-treated erythrocytes suggests that these isolates can invade erythrocytes using alternate invasion pathways that are independent of sialic acid residues of glycophorin A.
Growth assays do not directly measure erythrocyte invasion efficiency. Lack of growth in enzyme-treated erythrocytes could in principle result from the failure of ring stages to mature into trophozoites or schizonts and need not necessarily result from poor invasion efficiency. An erythrocyte invasion assay in which the percentage of ring-infected erythrocytes is scored following incubation of schizonts with normal and enzyme-treated erythrocytes for 16 to 20 h was used to directly measure invasion efficiencies of 5 field isolates. P. falciparum field isolates, JDP8 and RKL9, which grow in neuraminidase-treated and trypsin-treated erythrocytes, could invade these erythrocytes. In addition, RKL12 and RAJ68 could invade neuraminidase-treated erythrocytes and RAJ104 could invade trypsin-treated erythrocytes, as predicted by data from the growth assays. Correlation of invasion efficiency and growth rates suggests that data from growth assays can be used to correctly predict invasion phenotypes. The use of alternate invasion pathways that are independent of binding to sialic acid residues of glycophorin A was initially observed with cloned P. falciparum lines (7, 18, 21). Data presented here demonstrate that the use of alternate invasion pathways is not simply an artifact of long-term in vitro culture but commonly occurs in the field.
The field isolates used in this study were derived from P. falciparum malaria patients. PCR amplification of DNA encoding polymorphic regions of two merozoite surface proteins, MSP-1 and MSP-2, and two microsatellite markers was used to determine if the field isolates contain multiple parasite lines. PCR typing data based on these four markers suggest that only 1 of 15 P. falciparum field isolates, RAJ133, contains multiple parasite lines. Three of the four polymorphic markers used for typing could detect the presence of multiple parasite genotypes in RAJ133. It is unlikely that the typing methods used would fail to detect the presence of multiple genotypes in other field isolates. However, it is not possible to completely rule out the presence of more than one genotype based on these four polymorphic markers.
The field isolates were cultured for two to five cycles before use in the growth assays and isolation of genomic DNA for analysis by PCR typing. Differences in growth rates could result in the survival of a single parasite line when a field isolate containing multiple parasite lines is cultured in vitro. Alternately, the absence of multiple parasite lines may be due to the fact that majority of the field isolates (12 of 15) used in this study were collected from regions of sporadic, unstable malaria transmission (e.g., Rajasthan, Delhi, and Uttar Pradesh), which are likely to have low entomological inoculation rates and limited number of variants in the parasite population. Given that the field isolates were collected in areas of low endemicity, it is possible that one or two parasite isolates were circulating and were the predominant ones sampled. Such an error in sampling could lead to an overestimation of the proportion of field isolates using alternate pathways. The results from PCR typing demonstrate that the field isolates sampled were unique and independent, allowing us to exclude this possibility.
Unlike P. falciparum, P. vivax is completely dependent on the Duffy antigen for invasion of human erythrocytes (15). The related simian malaria parasite, P. knowlesi, is also completely dependent on the Duffy antigen for invasion of human erythrocytes but can use multiple Duffy antigen independent pathways to invade rhesus erythrocytes (10, 14). Redundancy in erythrocyte invasion pathways may provide a survival advantage to P. falciparum and P. knowlesi in case of receptor heterogeneity in host populations or immune pressure (17). P. falciparum EBA-175, which binds sialic acid residues of glycophorin A, belongs to a family of erythrocyte binding proteins which also includes the P. vivax and P. knowlesi Duffy binding proteins and the P. knowlesi β and γ proteins, which are responsible for invasion of rhesus erythrocytes by Duffy antigen-independent pathways (1, 5). The functional binding domain lies in a conserved, N-terminal, cysteine-rich region, referred to as region II, that is present in each member of the erythrocyte binding protein family (5, 24). Amino acid sequence variation within region II confers diverse binding specificity to different members of the erythrocyte binding protein family. P. falciparum proteins that mediate invasion by alternate pathways have not yet been identified. EBA-175 homologues with variant binding domains (region II) may bind alternate receptors to mediate invasion by pathways that are independent of sialic acid residues of glycophorin A. Genes encoding EBA-175 homologues have been identified in P. falciparum, but their roles in erythrocyte binding and invasion remain to be determined (22).
Expression of multiple members of the erythrocyte binding protein family allows P. knowlesi to invade rhesus erythrocytes by multiple pathways (1, 5, 10). Analysis of gene expression at the single merozoite level has demonstrated that P. yoelii merozoites derived from a single schizont may express different members of a family of rhoptry proteins that bind erythrocytes to mediate invasion (11, 19, 23). Different members of the rhoptry protein family may have distinct binding specificity. Each cohort of P. yoelii merozoites released upon schizont rupture can thus invade erythrocytes by multiple pathways by using diverse receptors. As in the case of P. yoelii, P. falciparum merozoites released from a single schizont may express different members of the erythrocyte binding protein family with diverse receptor binding specificity, allowing a cohort of P. falciparum merozoites to invade by multiple pathways. Such a mechanism for differential expression of genes may provide great diversity and plasticity in erythrocyte invasion phenotypes to a clonal parasite population.
Efforts to develop a malaria vaccine based on EBA-175 attempt to elicit antibodies that will block the binding of EBA-175 to erythrocytes and inhibit erythrocyte invasion. This study has demonstrated that P. falciparum field isolates commonly use alternate invasion pathways that do not depend on sialic acid residues of glycophorin A. EBA-175 does not bind erythrocytes that lack either sialic acid residues or glycophorin A (4, 7) and therefore cannot be responsible for invasion by alternate pathways. Antibodies directed against EBA-175 should be tested for inhibition of erythrocyte invasion by P. falciparum isolates that use multiple invasion pathways. Efforts to identify the parasite ligands that mediate the alternate invasion pathways of P. falciparum are under way. It may be necessary to target these parasite ligands, in addition to EBA-175, to effectively block erythrocyte invasion by P. falciparum.
ACKNOWLEDGMENTS
We thank C. Ushadevi and Sangeeta Arora for help with parasite culture, Sue Kyes for advice on using MSP-1 and MSP-2 markers, Xinzhuan Su for advice on using microsatellite markers, Sandip Basu for providing access to the primate facility at the National Institute of Immunology, New Delhi, India, Vir Chauhan and Robin Anders for reviewing the manuscript, and V. P. Sharma for encouragement and support.
J.N.O. was supported by an ICGEB postdoctoral fellowship. This work was partially funded by a grant from the Human Frontier Science Program to C.E.C.
REFERENCES
- 1.Adams J H, Sim B K L, Dolan S A, Fang X, Kaslow D C, Miller L H. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci USA. 1992;89:7085–7089. doi: 10.1073/pnas.89.15.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnwell J W, Galinski M R. Invasion of vertebrate cells: erythrocytes. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C: American Society for Microbiology; 1998. pp. 93–120. [Google Scholar]
- 3.Breuer W V, Ginsburg H, Cabantchik Z I. An assay of malaria parasite invasion into human erythrocytes. The effects of chemical and enzyme modification of erythrocyte membrane components. Biochim Biophys Acta. 1983;755:263–271. doi: 10.1016/0304-4165(83)90213-1. [DOI] [PubMed] [Google Scholar]
- 4.Camus D, Hadley T J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science. 1985;230:553–555. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 5.Chitnis C E, Miller L H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med. 1994;180:497–506. doi: 10.1084/jem.180.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolan S A, Miller L H, Wellems T E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Investig. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolan S A, Proctor J L, Alling D W, Okubo Y, Wellems T E, Miller L H. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol Biochem Parasitol. 1994;64:55–63. doi: 10.1016/0166-6851(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 8.Friedman M J, Blankenburt T, Sensabaugh G, Tenforde T S. Recognition and invasion of human erythrocytes by malarial parasites: contribution of sialoglycoproteins to attachment and host specificity. J Cell Biol. 1984;98:1682–1687. doi: 10.1083/jcb.98.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadley T J, Klotz F W, Pasvol G, Haynes J D, McGinniss M H, Okubo Y, Miller L H. Falciparum malaria parasites invade erythrocytes that lack glycophorin A and B (MkMk). Strain differences indicate receptor heterogeneity and two pathways for invasion. J Clin Investig. 1987;80:1190–1193. doi: 10.1172/JCI113178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haynes J D, Dalton J P, Klotz F W, McGinniss M H, Hadley J, Hudson D E, Miller L H. Receptor like specificity of a Plasmodium knowlesi malarial protein that binds to Duffy antigen ligands on erythrocytes. J Exp Med. 1988;167:1873–1881. doi: 10.1084/jem.167.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keen J, Sinha K A, Brown K N, Holder A A. A gene coding for a high molecular mass rhoptry protein of Plasmodium yoelii. Mol Biochem Parasitol. 1994;65:171–177. doi: 10.1016/0166-6851(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 12.Kimura E, Mattei D, di Santi S M, Scherf A. Genetic diversity in the major merozoite surface antigen of Plasmodium falciparum: high prevalence of a third polymorphic form detected in strains derived from malaria patients. Gene. 1990;91:57–62. doi: 10.1016/0378-1119(90)90162-k. [DOI] [PubMed] [Google Scholar]
- 13.Kyes S, Harding R, Black G, Craig A, Peshu N, Newbold C, Marsh K. Limited spatial clustering of individual Plasmodium falciparum alleles in field isolates from coastal Kenya. Am J Trop Med Hyg. 1997;57:205–215. doi: 10.4269/ajtmh.1997.57.205. [DOI] [PubMed] [Google Scholar]
- 14.Miller L H, Mason S J, Dvorak J A, McGinniss M H, Rothman I K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 15.Miller L H, Mason S J, Clyde D F, McGinniss M H. The resistance factor to Plasmodium vivax in Blacks: Duffy blood group genotype, FyFy. N Engl J Med. 1976;295:302–304. doi: 10.1056/NEJM197608052950602. [DOI] [PubMed] [Google Scholar]
- 16.Miller L H, Haynes J O, McAuliffe F M, Shiroishi T, Durocher J, McGinniss M H. Evidence for differences in erythrocyte surface receptors for the malaria parasites, Plasmodium falciparum and P. knowlesi. J Exp Med. 1977;146:277–281. doi: 10.1084/jem.146.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller L H. Impact of malaria on genetic polymorphism and genetic diseases in Africans and African Americans. Proc Natl Acad Sci USA. 1994;91:2415–2419. doi: 10.1073/pnas.91.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell G H, Hadley T J, McGinniss M H, Klotz F W, Miller L H. Invasion of erythrocytes by Plasmodium falciparum malaria parasites. Evidence for receptor heterogeneity and two receptors. Blood. 1986;67:1519–1521. [PubMed] [Google Scholar]
- 19.Ogun S A, Holder A A. A high molecular mass Plasmodium yoelii rhoptry protein binds to erythrocytes. Mol Biochem Parasitol. 1996;76:321–324. doi: 10.1016/0166-6851(95)02540-5. [DOI] [PubMed] [Google Scholar]
- 20.Pasvol G, Wainscoat J S, Weatherall D J. Erythrocytes deficient in glycophorin resist invasion by the malarial parasite, Plasmodium falciparum. Nature. 1982;297:64–66. doi: 10.1038/297064a0. [DOI] [PubMed] [Google Scholar]
- 21.Perkins M E, Holt E H. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol Biochem Parasitol. 1988;27:23–34. doi: 10.1016/0166-6851(88)90021-7. [DOI] [PubMed] [Google Scholar]
- 22.Peterson D S, Miller L H, Wellems T E. Multiple sequences in the Plasmodium falciparum genome encode conserved domains homologous to those in erythrocyte binding proteins. Proc Natl Acad Sc USA. 1995;92:7100–7104. doi: 10.1073/pnas.92.15.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preser P R, Jarra W, Capiod T, Snounou G. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature. 1999;398:618–622. doi: 10.1038/19309. [DOI] [PubMed] [Google Scholar]
- 24.Sim B K L, Chitnis C E, Wasniowska K, Hadley T J, Miller L H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 25.Smythe J A, Copel R L, Day K P, Martin R K, Oduola A M, Kemp D J, Anders R F. Structural diversity in the Plasmodium falciparum merozoite surface antigen 2. Proc Natl Acad Sci USA. 1991;88:1751–1755. doi: 10.1073/pnas.88.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snewin V A, Herrera M, Sanchez G, Scherf A, Langsley G. Polymorphism of the alleles of the merozoite surface antigens MSA-1 and MSA-2 in Plasmodium falciparum wild isolated from Colombia. Mol Biochem Parasitol. 1991;49:265–275. doi: 10.1016/0166-6851(91)90070-m. [DOI] [PubMed] [Google Scholar]
- 27.Su X-Z, Wellems T E. Towards a high resolution Plasmodium falciparum linkage map: polymorphic markers from hundreds of simple sequence repeats. Genomics. 1996;33:430–444. doi: 10.1006/geno.1996.0218. [DOI] [PubMed] [Google Scholar]
- 28.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 29.Ward G, Chitnis C, Miller L H. The invasion of erythrocytes by malarial merozoites. In: Russell D, editor. Strategies of intracellular survival of microbes. W. B. London, England: Saunders and Co.; 1994. pp. 155–190. [Google Scholar]
- 30.Wooden J, Kyes S, Sibley C H. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]