Abstract
There is an emerging paradigm shift from coronary heart disease having a purely hereditary and nutritional causation to possibly having an infectious etiology. Recent epidemiological studies have shown a correlation between periodontal disease and coronary heart disease. However, to date, there is minimal information as to the possible disease mechanisms of this association. It is our hypothesis that invasion of the coronary artery cells by oral bacteria may start and/or exacerbate the inflammatory response in atherosclerosis. Since a few periodontal pathogens have been reported to invade oral epithelial tissues, we tested the ability of three putative periodontal pathogens—Eikenella corrodens, Porphyromonas gingivalis, and Prevotella intermedia—to invade human coronary artery endothelial cells and coronary artery smooth muscle cells. In this study we demonstrate by an antibiotic protection assay and electron microscopy that specific species and strains invade coronary artery cells at a significant level. Actin polymerization and eukaryotic protein synthesis in metabolically active cells were required since the corresponding inhibitors nearly abrogated invasion. Many intracellular P. gingivalis organisms were seen to be present in multimembranous vacuoles resembling autophagosomes by morphological analysis. This is the first report of oral microorganisms invading human primary cell cultures of the vasculature.
Cardiovascular disease (CVD) is the leading cause of death in the Western world. Although classical risk factors (i.e., smoking, obesity, and high blood pressure, etc.) can be indications of most coronary deaths, they cannot account for all CVD-associated deaths. For example, approximately 25% of coronary deaths in males and 15% in females occur in persons in the lowest two quintiles of the multivariate Framingham Heart Study risk scores (30). This has led many to speculate that CVD may have an infectious etiology (5, 18, 47).
Periodontal disease is an inflammatory condition caused by a chronic bacterial infection with specific gram-negative organisms. Recent epidemiological data strongly suggests that periodontitis is an important risk factor for coronary heart disease (CHD). In 1989 Mattila et al. reported an association between dental health and acute myocardial infarction in that they found worse dental health in patients with acute myocardial infarction than in a control population (32). In a separate study, DeStefano et al. monitored subjects for 13 to 16 years after a baseline dental examination (11). Of the 9,760 subjects, patients with periodontitis were found to have a 25% increased risk of CHD compared to patients with minimal or no periodontal disease. Men under 50 with periodontitis or no teeth were 70% more likely to develop CHD than men with no periodontal disease. More recently, Beck et al. evaluated periodontal disease and its variables as a risk factor for CHD and stroke (1). Those authors found that for every 20% increase in mean bone loss (the most accurate measure of periodontitis), the incidence of total CHD increased 40%. When age and other ascribed risk factors were adjusted, patients with more than 40% bone loss were 2.7 times more likely to have fatal CHD. The biological basis for this association has not yet been elucidated. However, a possible route to the circulatory system for periodontal bacteria exists, since studies have shown a transient bacteremia resulting from chewing food, flossing, and toothbrushing in persons with periodontitis (3, 41, 42).
Poryphyromonas gingivalis is strongly implicated as an etiologic agent of adult periodontitis, and Prevotella intermedia is also frequently cultured from sites of periodontitis (44, 45). Previous studies have established that these organisms are capable of invading oral epithelial tissue in vitro (13, 14, 29, 40). A recent study established that P. gingivalis was also able to invade fetal bovine heart endothelial, bovine aortic endothelial, and human umbilical vein endothelial cells (10). Additionally, preliminary studies have begun to find periodontal pathogens, including P. gingivalis and P. intermedia, within atheromatous tissues (24). Eikenella corrodens is also a putative periodontal pathogen and has been shown to be an etiologic agent of infective endocarditis (2, 16).
Atherosclerosis develops due to the inflammatory response to endothelial cell injury and dysfunction and is likely a chronic process (39). It is our hypothesis that frequent bacteremias could provide a chronic insult to the vasculature and that invasion of the cells of the arterial wall by oral bacteria could contribute to the injury that initiates and/or exacerbates atherosclerosis. Therefore, we tested the ability of these organisms to invade primary cultures of human coronary artery endothelial cells (HCAEC) and coronary artery smooth muscle cells (CASMC).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. gingivalis 381 and W50 (a gift of M. A. Curtis) were grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.5% yeast extract (Difco), 0.1% cysteine, hemin (5 μg/ml), and menadione (5 μg/ml). P. intermedia 17 and 25611 (gifts of K.-P. Leung and W. E. Nesbitt) were grown in Todd-Hewitt broth (Difco) supplemented with 0.5% yeast extract, 0.075% cysteine, hemin (5 μg/ml), and menadione (0.05 mg/ml). Both P. gingivalis and P. intermedia were grown in a Coy anaerobic chamber with an atmosphere of 5% CO2, 10% H2, and 85% N2. E. corrodens 23834 (obtained from the American Type Culture Collection, Manassas, Va.) was grown in BY broth in a humidified atmosphere containing 10% CO2. Escherichia coli MC1061 (a gift of A. S. Bleiweis) was grown in Luria-Bertani medium, consisting of Bacto Tryptone (10 g/liter; Difco), Bacto yeast extract (5 g/liter), and NaCl (10 g/liter).
Cell culture.
KB cells (derived from a human oral epidermoid carcinoma), HCAEC, and CASMC were used in this study. The KB cells (ATCC CCL-17) were maintained in Eagle's minimum essential medium (Mediatech, Herndon, Va.) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc., Logan, Utah), 200 mM l-glutamine (Sigma Chemical Co., St. Louis, Mo.), and 100 mg of penicillin-streptomycin (Sigma) per ml. The HCAEC (Clonetics, Inc., San Diego, Calif.) were maintained in microvascular endothelial growth medium-2, consisting of endothelial cell basal medium-2 supplemented with fetal bovine serum, hydrocortisone, human recombinant fibroblast growth factor, vascular endothelial growth factor, recombinant insulin growth factor-1, ascorbic acid, human recombinant epidermal growth factor, gentamicin, and amphotericin (Clonetics). The CASMC (Clonetics) were maintained in smooth muscle growth medium, consisting of smooth muscle basal medium-2 supplemented with insulin, human recombinant fibroblast growth factor, fetal bovine serum, human recombinant epidermal growth factor, gentamicin, and amphotericin (Clonetics). Cells were cultured in 75-cm2 flasks at 37°C in a humidified atmosphere of 5% CO2. Both the HCAEC and CASMC were obtained cryopreserved at the third passage and were passaged an additional two or three times before use.
Invasion assay.
For the invasion assays, the bacteria were grown in broth, centrifuged at low speed, and resuspended in antibiotic-free medium to a concentration of 107 cells/ml as determined spectrophotometrically (Shimadzu UV-1201; VWR, Marietta, Ga.). Approximately 105 human cells per well in a 24-well tissue culture plate were washed three times with phosphate-buffered saline (PBS) prior to incubation with 1.0 ml of the bacterial suspension at 37°C aerobically for 90 min. In order to more closely approximate in vivo conditions, the bacteria were not centrifuged onto the cells to promote intimate contact. The medium was removed from infected cells after 90 min, and the cells were washed three times with PBS. Medium containing gentamicin (300 μg/ml) and metronidazole (200 μg/ml) was then added to each well to kill any extracellular bacteria, and the plates were incubated for 60 min aerobically at 37°C. Finally the medium was removed, and the cells were washed three times with PBS and lysed by a 20-min incubation with 1.0 ml of sterile distilled water at 37°C under aerobic conditions. Dilutions of the lysates of cells infected with P. gingivalis, P. intermedia, and E. corrodens 23834 were plated in triplicate on tryptic soy agar (Difco) plates supplemented with 5.0% sheep blood, 0.5% yeast extract, hemin (5 μg/ml), and menadione (5 μg/ml). Plates of P. gingivalis and P. intermedia were cultured anaerobically, and those of E. corrodens 23834 was cultured in a humidified atmosphere containing 10% CO2. The dilutions of the lysates of E. coli MC1061 were cultured on Luria-Bertani plates at 37°C aerobically. CFU of invasive bacteria were then enumerated. Each assay was performed in duplicate wells and was performed independently at least three times. Viability of cells was examined by trypan blue exclusion. Controls for the antibiotic were tested by adding 107 bacteria to unseeded wells.
Temperature dependence.
KB cells were infected as described above, and the bacteria were incubated concurrently at 37 and 4°C.
Treatment with cycloheximide and cytochalasin D.
The effects of cycloheximide (Sigma) and cytochalasin D (Sigma) on invasion were also investigated. Invasion assays were performed as described above with the exception of the presence of these inhibitors. Cycloheximide (1 mg/ml in ethanol) was preincubated with the human cells for 4 h prior to addition of the bacteria and was present during the assay. Cytochalasin D (5 μg/ml in dimethyl sulfoxide) was preincubated with the human cells for 0.5 h before addition of the bacteria and was present during the assay. The inhibitors were tested at the appropriate concentration for adverse effects on the human cells by trypan blue exclusion and by examining the confluency of the monolayer. Cycloheximide, cytochalasin D, dimethyl sulfoxide, and ethanol were tested for possible toxicity to the bacteria by viable counting.
Transmission electron microscopy.
Following 90 min of incubation of eukaryotic cells with bacteria, the cells were washed two times with PBS, fixed in 2% PBS-buffered glutaraldehyde at room temperature for 1 h, centrifuged, and washed with PBS (pH 7.3). Three drops of 3% low-gelling agarose were then added to the pellet and allowed to solidify at 4°C for 10 min. The agarose-embedded pellet was then washed twice for 10 min in PBS, incubated in 1% osmium tetroxide for 1 h, and washed three times in distilled H2O. The washed cell pellets were dehydrated in a graded series of ethanol solutions and stained overnight en bloc with 2% uranyl acetate. Finally, the pellets were infiltrated and embedded in EM Bed-812 (Electron Microscopy Sciences, Ft. Washington, Pa.). Thin sections were cut, poststained with uranyl acetate and lead citrate, and examined in a Hitachi 7000 transmission electron microscope.
SEM.
For scanning electron microscopy (SEM) analysis, HCAEC were incubated without bacteria and with P. gingivalis 381 for 15, 30, and 45 min. Following the infection, the cells were washed two times with PBS and fixed in 2% PBS-buffered glutaraldehyde for 30 min. After fixation, the cells were dipped in PBS, washed for 5 min in fresh PBS, and then incubated in 4% osmium tetroxide for 5 min. Following postfixation, the cells were dipped in distilled water, washed for 5 min in fresh distilled water, and then dehydrated in a graded series of ethanol solutions (50, 70, 95, 100, and 100%) for 5 min each. After dehydration, the cells were incubated twice for 5 min in hexamethyldisilizane (Sigma) and air dried for 30 min before being mounted and sputter coated with gold. The samples were then viewed with a Hitachi S-4000 field emission scanning electron microscope. Digital prints were produced with a SemAges digital imaging acquisition system (Advanced Database Systems, Boulder, Colo.).
The effect of cycloheximide preincubation was also analyzed by SEM. HCAEC were preincubated with 1 mg of cycloheximide per ml for 4 h. Following the preincubation, the HCAEC were infected for 15 min with P. gingivalis 381, after which they were washed two times with PBS and fixed in 2% PBS-buffered glutaraldehyde. These cells were then processed for SEM in the same manner as for the aforementioned samples.
RESULTS
In vitro invasion assay.
For bacterial invasion studies, primary cultures of HCAEC and CASMC were infected with the aforementioned periodontal pathogens, and invasion was quantitated by the standard antibiotic protection assay as modified for these organisms (28). The results show that certain strains of these periodontal pathogens do invade both HCAEC and CASMC (Table 1). Interestingly, the bacteria varied in their ability to invade, even among different strains of the same species. For example, P. gingivalis 381 was able to invade by 1 to 2 orders of magnitude more than P. gingivalis W50. P. intermedia 17 was found to be invasive, whereas P. intermedia 25611 was not able to invade under the same conditions. Thus, under the conditions used here, certain strains of P. gingivalis and P. intermedia were able to invade coronary artery cells.
TABLE 1.
Comparison of invasion of coronary artery cells by periodontal pathogens and a negative control
| Strain | CFU/ml recovered after antibiotic treatmenta
|
|
|---|---|---|
| HCAEC | CASMC | |
| P. gingivalis 381 | (4.6 ± 0.6) × 105 | (2.7 ± 1.4) × 105 |
| P. gingivalis W50 | (9.5 ± 0.3) × 103 | (2.0 ± 0.7) × 104 |
| P. intermedia 17 | (2.0 ± 0.3) × 103 | (3.5 ± 2.1) × 103 |
| P. intermedia 25611 | 0 | 11 ± 19 |
| E. corrodens 23834 | 446 ± 274 | 320 ± 99 |
| E. coli MC1061 | 2.2 ± 3.9 | 6.6 ± 3.5 |
Values represent the means and standard deviations for triplicate samples of lysates from the infection of 105 cells by 107 bacteria from at least three independent experiments.
E. corrodens 23834 showed a minimal ability to invade, since the number of CFU recovered per milliliter of lysate was greater than that for the negative bacterial control, E. coli MC1061, and the antibiotic control (data not shown), but the number of CFU was not of the same magnitude as that of the other species. Even increasing the number of E. corrodens 23834 organisms by 10-fold resulted in only 3.2 times greater CFU. Therefore, it is possible that certain cells phagocytosed the E. corrodens, as opposed to an active invasion by this bacterial species.
Effects of inhibitors.
The effects of temperature and metabolic inhibitors on bacterial invasion were investigated to elucidate possible mechanisms of invasion (Tables 2 and 3). Performing the incubation portion of the invasion assay at 4°C instead of 37°C produced a large reduction in the number of CFU in all cases except for E. corrodens 23834. This is further evidence that E. corrodens 23834 did not actively invade the cell lines, since metabolism nearly stops at 4°C. Additionally, the cell membranes lose their fluidity at 4°C, making invasion nearly impossible.
TABLE 2.
Effect of temperature, cycloheximide, and cytochalasin D treatment on bacterial invasion of HCAEC
| Strainb | % Inhibition of invasion of HCAECa:
|
||
|---|---|---|---|
| At 4°C | With cycloheximidec | With cytochalasin Dd | |
| P. gingivalis 381 | 97.3 | 99.9 | 99.6 |
| P. gingivalis W50 | 97.1 | 99.8 | 99.9 |
| P. intermedia 17 | 93.6 | 99.3 | 99.8 |
| E. corrodens 23834 | 60.0 | 98.5 | 97.0 |
Values represent the percentage of invasion that diminished in the presence of inhibitors compared to invasion without inhibitors present.
P. intermedia 25611 and E. coli MC1061 were not included, since there was no invasion to inhibit.
Cycloheximide (1 mg/ml) was preincubated with HCAEC for 4 h and was present during the assay.
Cytochalasin D (5 μg/ml) was preincubated with HCAEC for 0.5 h and was present during the assay.
TABLE 3.
Effect of temperature, cycloheximide, and cytochalasin D treatment on bacterial invasion of CASMC
| Strainb | % Inhibition of invasiona:
|
||
|---|---|---|---|
| At 4°C | With cycloheximidec | With cytochalasin Dd | |
| P. gingivalis 381 | 99.92 | 99.99 | 97.5 |
| P. gingivalis W50 | 97.6 | 99.7 | 99.8 |
| P. intermedia 17 | 77.8 | 99.7 | 99.7 |
| E. corrodens 23834 | 2.0 | 97.3 | 96.0 |
Values represent the percentage of invasion that diminished in the presence of inhibitors compared to invasion without inhibitors present.
P. intermedia 25611 was not included, since there was no invasion to inhibit.
Cycloheximide (1 mg/ml) was preincubated with CASMC for 4 h and was present during the assay.
Cytochalasin D (5 μg/ml) was preincubated with CASMC for 0.5 h and was present during the assay.
A common strategy among invasive bacteria is to trigger the host cell to undergo cytoskeletal rearrangements mediated by actin polymerization (19). Cytochalasin D, an actin polymerization inhibitor, also significantly reduced invasion in all cases. These data indicate that actin polymerization of the cytoskeleton in a metabolically active cell is needed for invasion by these bacteria. Cytochalasin D has been shown to inhibit a majority of invasive bacteria (4, 20, 21, 28, 35).
Cycloheximide, a eukaryotic protein synthesis inhibitor, also reduced the CFU recovered at 2.5 h postinfection, presumably because it prevents coronary artery cell synthesis of the proteins required during attachment and/or invasion. This data differs from that of a previous study, which reported that using 10-fold less cycloheximide than that used here did not inhibit invasion of gingival epithelial cells by P. gingivalis 33277 (28).
Transmission electron microscopy.
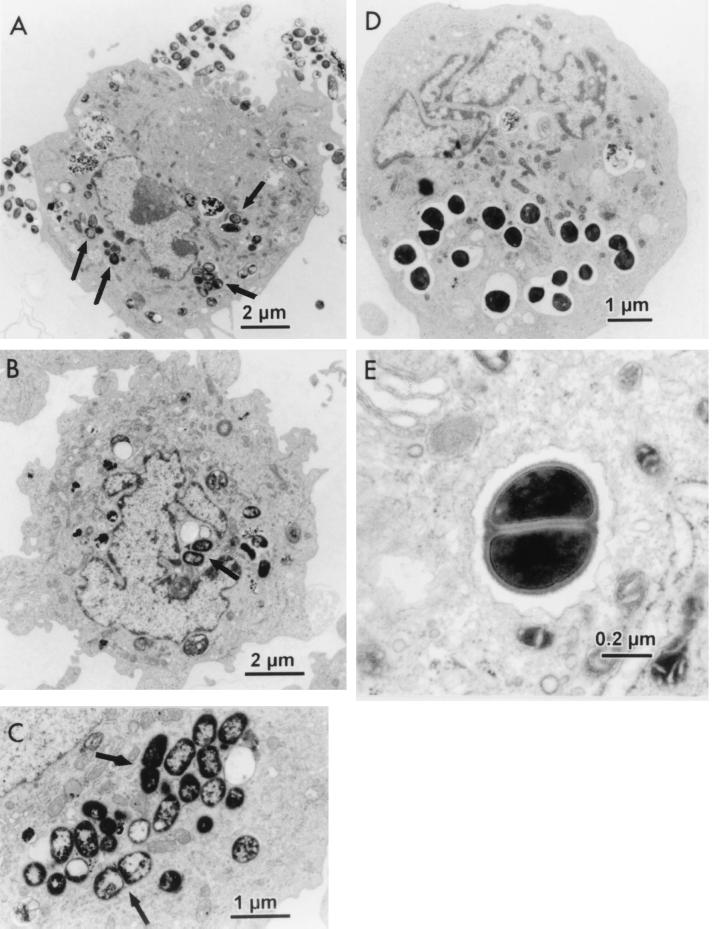
Electron microscopy of infected cells also showed evidence of invasion (Fig. 1 and 2). As demonstrated in Fig. 1A to C, numerous P. gingivalis and P. intermedia organisms were evident intracellularly. Most extracellular bacteria, especially in the case of P. gingivalis, appear to be concentrated at certain spots along the cellular membrane, while the rest of the membrane remained relatively free of bacteria (Fig. 1A). This could be due to bacterial aggregation and subsequent attachment and/or invasion by the aggregate as a whole. It could also indicate the presence of cellular receptors that are expressed only at certain areas on the surfaces of the cellular membrane. In several of the micrographs, bacteria can be seen dividing within the coronary artery cell (Fig. 1C and E). This indicates that the bacteria are metabolically active during invasion and may be able to persist in the coronary artery cells for at least short periods of time.
FIG. 1.
Transmission electron micrographs of internalized bacteria in HCAEC. (A) P. gingivalis 381 (arrows). (B) P. intermedia 17 (arrows). (C) P. intermedia 17 dividing (arrows). (D) Internalized E. corrodens 23834 after infection with 1010 organisms. (E) E. corrodens 23834 dividing within HCAEC (magnification, ×25,000).
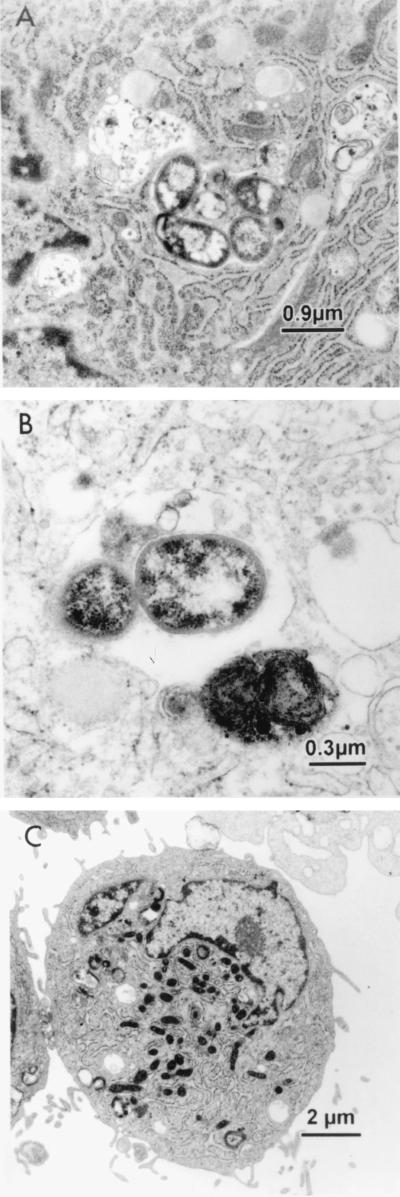
FIG. 2.
Transmission electron micrographs of bacteria internalized by CASMC. (A) P. gingivalis 381 surrounded by a large amount of rough endoplasmic reticulum. (B) P. intermedia 17. (C) No internalized bacteria after infection with E. coli MC1061.
The transmission electron micrographs also showed several interesting cellular features. Many of the micrographs showed a concentration of rough endoplasmic reticulum around internalized P. gingivalis (Fig. 2A). The internalized P. gingivalis organisms appear to be in thin membranous vacuoles, which also appeared to include cytoplasmic ground substance, surrounded by ribosomes (an association with the rough endoplasmic reticulum), indicating residence within autophagosomes (15). These bacteria thus might exploit the cell's autophagic mechanism to establish a favorable intracellular niche, similar to Legionella pneumophila in macrophages and Brucella abortus in nonphagocytic cells (36, 46).
The transmission electron microscopy study of infection of approximately 105 HCAEC by 1010 E. corrodens 23834 cells demonstrated that the majority of HCAEC did not contain any internalized bacteria. This would support the hypothesis that E. corrodens 23834 did not invade either cell line or was able to invade only a small subset of the cell population under these conditions. The HCAEC that did internalize E. corrodens 23834 contained multiple bacterial cells within the cytoplasm which appeared to be in large vacuoles (Fig. 1D), which is not the case with P. gingivalis and P. intermedia invasion (Fig. 1A to C and 2A and B).
SEM.
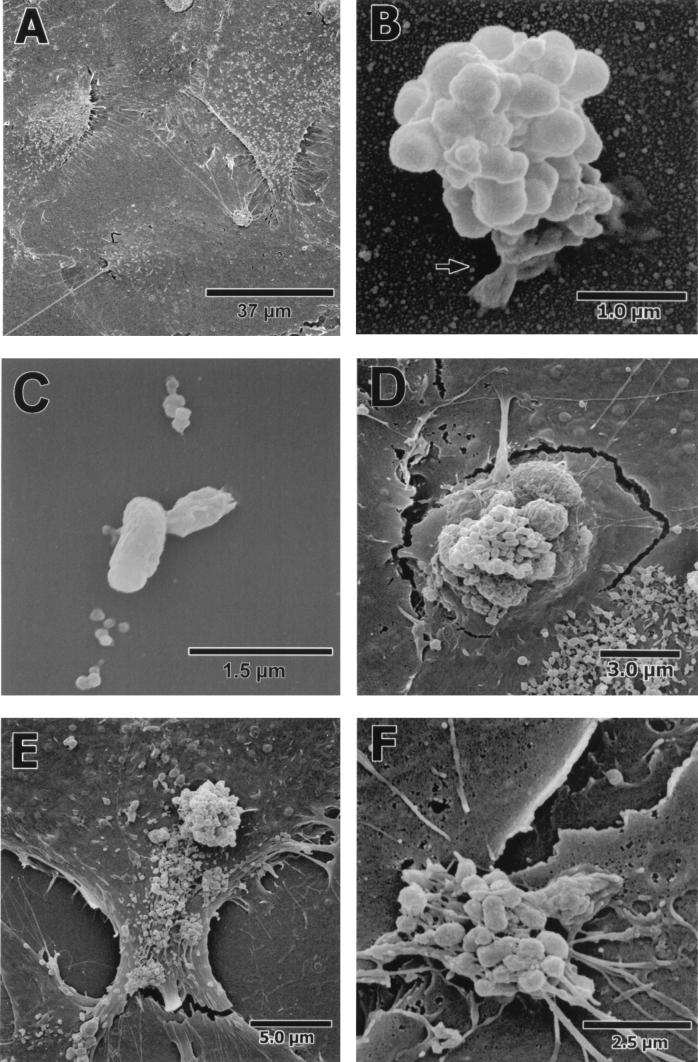
The SEMs revealed that the HCAEC monolayer was strikingly uniform, such that it was difficult to differentiate the borders between cells. The HCAEC surface surrounding the aggregate in Fig. 3B was representative of the monolayer at 15 min of infection and the monolayer with no bacteria added (Fig. 3A).
FIG. 3.
SEMs of a time course infection of HCAEC by P. gingivalis 381. (A) Uninfected HCAEC monolayer. (B) After 15 min of incubation, an aggregate of P. gingivalis 381 can be seen attached to the HCAEC by a host-associated pseudopod (arrow). (C) Attachment of P. gingivalis 381, after 15 min of incubation, to a monolayer of HCAEC preincubated with 1 mg of cycloheximide per ml for 4 h. (D) HCAEC breaking away from the monolayer and engulfing an aggregate of P. gingivalis 381 after 30 min of incubation. (E) HCAEC tearing away from a neighboring cell after a 30-min incubation. (F) An aggregate of P. gingivalis 381 straddling a tear between neighboring HCAEC after 45 min of incubation.
The SEMs also demonstrated an interaction between aggregates of P. gingivalis 381 and the HCAEC monolayer. After 15 min of infection, aggregates of P. gingivalis 381 could be seen attached to the HCAEC monolayer (Fig. 3B). This attachment appeared to be mediated by an endothelial cell-derived structure. Therefore, cycloheximide-treated HCAEC were also analyzed by SEM. Structures as seen in Fig. 3B were not visible with any P. gingivalis 381 organisms associated with cycloheximide-treated cells. When P. gingivalis 381 could be seen attached to cycloheximide-treated HCAEC, it appeared that the attachment structure was either absent or greatly reduced in size compared with untreated HCAEC (Fig. 3C). Additionally, the protein heads on the surface of the HCAEC (Fig. 3A and B) were not nearly as abundant on the cycloheximide-treated cells. This data suggests that cycloheximide inhibits the specific attachment by P. gingivalis 381 to the monolayer by preventing the synthesis of a host-derived structure, thereby hindering the initial step in invasion.
By 30 min of infection of untreated HCAEC, gross distortions in the monolayer could be observed in conjunction with aggregates of P. gingivalis 381 (Fig. 3D and E), whereas individual bacteria observed along the monolayer did not produce any visible effects on the HCAEC (data not shown). The distortions of the cell monolayer included extensions of the cells stretching to engulf the invading bacteria (Fig. 3D). In addition, many individual cells could be seen tearing away, thus disrupting the HCAEC monolayer (Fig. 3D and E). We considered the possibility that the distortions of the monolayer were artifacts produced by the fixation process. However, the distortions were not seen at 15 min of infection or in uninfected cells. Additionally, the distortions occurred only in regions where aggregates of P. gingivalis 381 were present.
After 45 min of infection, the HCAEC monolayer had severe tears all along the monolayer with and without the presence of P. gingivalis 381 (Fig. 3F). There was a significant difference in the integrity of the monolayer in HCAEC infected for 45 and 15 min.
DISCUSSION
Invasion of the endothelial and smooth muscle cells of the arterial wall by bacterial pathogens could initiate and/or exacerbate the inflammatory response of atherosclerosis. A strong association between CHD and Chlamydia pneumoniae, a gram-negative respiratory pathogen, has also been reported (6, 27). At the molecular level, C. pneumoniae has been shown to infect and to replicate in endothelial cells, smooth muscle cells, and macrophages in vitro (23, 26). Whereas C. pneumoniae needs to be transported from the lung to the arteries via macrophages, oral organisms are introduced into the bloodstream multiple times daily in individuals with periodontitis via chewing and toothbrushing. Therefore, the oral cavity represents a potentially large reservoir of gram-negative pathogenic organisms that could interact with cardiovascular tissues.
Heart disease is the most common systemic pathology in patients with periodontal disease (34). Atherosclerosis, like periodontal disease, is mediated by the inflammatory process. Severe inflammation of the coronary arteries could even lead to acute myocardial infarction (MI). Bacteria may have a role in this process by chronically stimulating cytokines such as interleukin-1, tumor necrosis factor alpha, and/or C-reactive protein (CRP), an acute-phase reactant (47). Curiously, significantly elevated levels of CRP have been found in patients suffering from MI and periodontal disease, and these elevated CRP levels may be predictive of the first MI (8, 17, 37). Early in atherogenesis of the response-to-injury hypothesis of atherosclerosis, there is increased adherence of leukocytes (38). The T cells within atherosclerotic lesions are activated T cells, which suggests specific antigenic stimulation (31). If our hypothesis is true, this adherence of activated leukocytes may be the cell-mediated response to an intracellular pathogen.
The differences in the abilities of species and strains to invade indicate that invasion depends on specific bacterium-cell interactions and does not occur with all oral bacteria. Certain strains of bacteria likely possess adhesins and/or other characteristics that promote internalization by the coronary artery cells. The data presented here indicate that P. gingivalis 381 has one or more characteristics that would differentiate it from P. gingivalis W50 and P. intermedia 17, since it invades HCAEC and CASMC more readily. P. gingivalis W50 has a lower ability to adhere to oral epithelial cells (14). This decrease in invasion of HCAEC may thus be due to a lower percentage of strain W50 binding to the cell surface. The ability of P. gingivalis W50 to invade HCAEC and CASMC as reported here is greater than its ability to invade KB cells and an immortalized human umbilical vein endothelial cell line (12). These strains can also be differentiated from P. intermedia 25611 in that strain 25611 was not able to invade at any level.
After bacterial invasion, the induction of autophagy by the host cell increases the pool of free amino acids. The autophagy exhibited by the HCAEC and CASMC could be a mechanism that is induced and exploited by the bacteria. The bacteria may induce autophagy to use the amino acids for their own metabolism or to inhibit host protein synthesis in order to increase survivability (43). A pool of free amino acids could be especially beneficial for P. gingivalis, which requires short peptides for carbon and energy (48). Further studies have been initiated to conclusively determine if P. gingivalis is localized within autophagosomes and, if so, to investigate the role of autophagy in the P. gingivalis intracellular parasitism of HCAEC. Preliminary studies indicate that P. gingivalis trafficks to autophagosomes following invasion in HCAEC (unpublished data).
The SEMs demonstrate that P. gingivalis first attaches via a host cell-derived structure. Further time points of the SEMs demonstrate a severe disruption of the endothelial monolayer after infection with P. gingivalis 381. A disruption of the endothelial layer such as that observed in the SEMs could constitute the insult to start the atherosclerotic process. Interestingly, the disruptions were observed to occur only in association with aggregates of P. gingivalis 381 and not in association with a single bacterium. The SEMs of the engulfment of the aggregates appear to be similar to those of invasomes of Bartonella henslae (9). However, the transmission electron micrographs do not show the same host cell morphology during engulfment of P. gingivalis 381 as seen with the engulfment of aggregates of B. henslae. Invasion of HCAEC by aggregates of P. gingivalis may be clinically relevant, since dental plaque is a host-associated microbial biofilm (7). Most likely, aggregates of P. gingivalis in addition to other periodontal pathogens, including P. intermedia, would be introduced to the bloodstream after mild traumas.
In summary, this study demonstrates that certain pathogenic periodontal bacterial strains invade human coronary artery cells in vitro. Bacterial invasion could constitute a chronic insult to the arterial wall. These findings raise the possibility that a chronic in vivo infection of the coronary artery wall by pathogens such as P. gingivalis and P. intermedia could be a factor in CHD. Many studies have thus far shown an association between periodontal disease and CHD (1, 11, 22, 32, 33), whereas only one study has shown no relationship (25). Whether this association is a causal relationship or a “bystander effect” has yet to be determined. This is the first of a series of studies to determine whether a molecular link between the two diseases is possible.
ACKNOWLEDGMENTS
This study was supported by a University of Florida Periodontal Disease Research Center grant and National Institute of Dental and Craniofacial Research grant DE 07496.
We thank W. E. Nesbitt, K.-P. Leung, and J. E. Beem for providing P. intermedia strains and advice; E. V. Kozarov, W. A. McArthur, and S. Sugrue for useful discussion; J. Burks and J. Whitlock for their assistance; A. Shawley for help and advice with cell culture; and R. Davis, S. Whittaker, and the University of Florida Electron Microscopy Core Laboratory of the Interdisciplinary Center for Biotechnology Research for the electron micrographs.
REFERENCES
- 1.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 2.Berbari E F, Cockerill F R R, Steckelberg J M. Infective endocarditis due to unusual or fastidious microorganisms. Mayo Clin Proc. 1997;72:532–542. doi: 10.4065/72.6.532. [DOI] [PubMed] [Google Scholar]
- 3.Carroll G C, Sebor R J. Dental flossing and its relationship to transient bacteremia. J Periodontol. 1980;51:691–692. doi: 10.1902/jop.1980.51.12.691. [DOI] [PubMed] [Google Scholar]
- 4.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coles K A, Plant A J, Rilety T A, Smith D W. Cardiovascular disease: an infectious aetiology? Rev Med Microbiol. 1998;9:17–27. [Google Scholar]
- 6.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 7.Darveau R P, Tanner A, Page R C. The microbial challenge in periodontitis. Periodontol 2000. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 8.de Beer F C, Hind C R, Fox K M, Allan R M, Maseri A, Pepys M B. Measurement of serum C-reactive protein concentration in myocardial ischaemia and infarction. Br Heart J. 1982;47:239–243. doi: 10.1136/hrt.47.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehio C, Meyer M, Berger J, Schwarz H, Lanz C. Interaction of Bartonella henselae with endothelial cells results in bacterial aggregation on the cell surface and the subsequent engulfment and internalisation of the bacterial aggregate by a unique structure, the invasome. J Cell Sci. 1997;110:2141–2154. doi: 10.1242/jcs.110.18.2141. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande R G, Khan M B, Genco C A. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeStefano F, Anda R F, Kahn H S, Williamson D F, Russell C M. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn B R, Kozarov E V, Progulske-Fox A. Presented at the 77th General Session of the International Association for Dental Research. 1999. Vancouver, Canada. [Google Scholar]
- 13.Dorn B R, Leung K, Progulske-Fox A. Invasion of human oral epithelial cells by Prevotella intermedia. Infect Immun. 1998;66:6054–6057. doi: 10.1128/iai.66.12.6054-6057.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan M J, Nakao S, Skobe Z, Xie H. Interactions of Porphyromonas gingivalis with epithelial cells. Infect Immun. 1993;61:2260–2265. doi: 10.1128/iai.61.5.2260-2265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn W A., Jr Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol. 1994;4:139–143. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 16.Dzink J L, Tanner A C R, Haffajee A D, Socransky S S. Gram-negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:649–659. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 17.Ebersole J L, Machen R L, Steffen M J, Willmann D E. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–352. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis R W. Infection and coronary heart disease. J Med Microbiol. 1997;46:535–539. doi: 10.1099/00222615-46-7-535. [DOI] [PubMed] [Google Scholar]
- 19.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 20.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genco R, Chadda S, Grossi S, Dunford R, Taylor G, Knowler W, Pettitt D. Periodontal disease is a predictor of cardiovascular disease in a native American population. J Dent Res. 1997;76:409. [Google Scholar]
- 23.Godzik K L, O'Brien E R, Wang S K, Kuo C C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haraszthy V I, Zambon J J, Trevisan M, Shah R, Zeid M, Genco R J. Identification of pathogens in atheromatous plaques. J Dent Res. 1998;77:666. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 25.Joshipura K J, Rimm E B, Douglass C W, Trichopoulos D, Ascherio A, Willett W C. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 26.Kaukoranta-Tolvanen S S, Laitinen K, Saikku P, Leinonen M. Chlamydia pneumoniae multiplies in human endothelial cells in vitro. Microb Pathog. 1994;16:313–319. doi: 10.1006/mpat.1994.1032. [DOI] [PubMed] [Google Scholar]
- 27.Kuo C C, Grayston J T, Campbell L A, Goo Y A, Wissler R W, Benditt E P. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) Proc Natl Acad Sci USA. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamont R J, Oda D, Persson R E, Persson G R. Interaction of Porphyromonas gingivalis with gingival epithelial cells maintained in culture. Oral Microbiol Immunol. 1992;7:364–367. doi: 10.1111/j.1399-302x.1992.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 30.Leaverton P E, Sorlie P D, Kleinman J C, Dannenberg A L, Ingster-Moore L, Kannel W B, Cornoni-Huntley J C. Representativeness of the Framingham risk model for coronary heart disease mortality: a comparison with a national cohort study. J Chronic Dis. 1987;40:775–784. doi: 10.1016/0021-9681(87)90129-9. [DOI] [PubMed] [Google Scholar]
- 31.Libby P, Hansson G K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991;64:5–15. [PubMed] [Google Scholar]
- 32.Mattila K J, Nieminen M S, Valtonen V V, Rasi V P, Kesaniemi Y A, Syrjala S L, Jungell P S, Isoluoma M, Hietaniemi K, Jokinen M J. Association between dental health and acute myocardial infarction. Br Med J. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattila K J, Valtonen V V, Nieminen M, Huttunen J K. Dental infection and the risk of new coronary events: prospective study of patients with documented coronary artery disease. Clin Infect Dis. 1995;20:588–592. doi: 10.1093/clinids/20.3.588. [DOI] [PubMed] [Google Scholar]
- 34.Meyer D H, Fives-Taylor P M. Oral pathogens: from dental plaque to cardiac disease. Curr Opin Microbiol. 1998;1:88–95. doi: 10.1016/s1369-5274(98)80147-1. [DOI] [PubMed] [Google Scholar]
- 35.Meyer D H, Sreenivasan P K, Fives-Taylor P M. Evidence for invasion of a human oral cell line by Actinobacillus actinomycetemcomitans. Infect Immun. 1991;59:2719–2726. doi: 10.1128/iai.59.8.2719-2726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege J L, Gorvel J P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–2392. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker P M, Cushman M, Stampfer M J, Tracy R P, Hennekens C H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 38.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 39.Ross R. Mechanisms of disease: atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 40.Sandros J, Papapanou P, Dahlen G. Porphyromonas gingivalis invades oral epithelial cells in vitro. J Periodontal Res. 1993;28:219–226. doi: 10.1111/j.1600-0765.1993.tb01072.x. [DOI] [PubMed] [Google Scholar]
- 41.Sconyers J R, Crawford J J, Moriarty J D. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc. 1973;87:616–622. doi: 10.14219/jada.archive.1973.0453. [DOI] [PubMed] [Google Scholar]
- 42.Silver J G, Martin A W, McBride B C. Experimental transient bacteraemias in human subjects with varying degrees of plaque accumulation and gingival inflammation. J Clin Periodontol. 1977;4:92–99. doi: 10.1111/j.1600-051x.1977.tb01888.x. [DOI] [PubMed] [Google Scholar]
- 43.Sinai A P, Joiner K A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 44.Slots J, Listgarten M A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 45.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 46.Swanson M S, Isberg R R. Formation of the Legionella pneumophila replicative phagosome. Infect Agents Dis. 1993;2:224–226. [PubMed] [Google Scholar]
- 47.Valtonen V V. Infection as a risk factor for infarction and atherosclerosis. Ann Med. 1991;23:539–543. doi: 10.3109/07853899109150515. [DOI] [PubMed] [Google Scholar]
- 48.Wahren A, Gibbons R J. Amino acid fermentation by Bacteroides melaninogenicus. Antonie Leeuwenhoek. 1970;36:149–159. doi: 10.1007/BF02069017. [DOI] [PubMed] [Google Scholar]