Abstract
A recombinant protein comprising the maltose-binding protein (MBP) of Escherichia coli fused to amino acids 5 to 337 of the FlaA flagellin of Campylobacter coli VC167 was evaluated for immunogenicity and protective efficacy against challenge by a heterologous strain of campylobacter, Campylobacter jejuni 81-176, in two murine models. The sequence of the flaA gene of strain 81-176 revealed a predicted protein which was 98.1% similar to that of VC167 FlaA over the region expressed in the fusion protein. Mice were immunized intranasally with two doses of 3 to 50 μg of MBP-FlaA, given 8 days apart, with or without 5 μg of the mutant E. coli heat-labile enterotoxin (LTR192G) as a mucosal adjuvant. The full range of MBP-FlaA doses were effective in eliciting antigen-specific serum immunoglobulin G (IgG) responses, and these responses were enhanced by adjuvant use, except in the highest dosing group. Stimulation of FlaA-specific intestinal secretory IgA (sIgA) responses required immunization with higher doses of MBP-FlaA (≥25 μg) or coadministration of lower doses with the adjuvant. When vaccinated mice were challenged intranasally 26 days after immunization, the best protection was seen in animals given 50 μg of MBP-FlaA plus LTR192G. The protective efficacies of this dose against disease symptoms and intestinal colonization were 81.1 and 84%, respectively. When mice which had been immunized with 50 μg of MBP-FlaA plus LTR192G intranasally were challenged orally with 8 × 1010, 8 × 109, or 8 × 108 cells of strain 81-176, the protective efficacies against intestinal colonization at 7 days postinfection were 71.4, 71.4, and 100%, respectively.
Campylobacter jejuni and Campylobacter coli are among the most frequently isolated causes of bacterial diarrhea worldwide (40, 41), and C. jejuni has been recognized as an important cause of diarrhea in both travellers and deployed military personnel (11, 15, 28, 36). Moreover, C. jejuni is the infectious agent most often associated with Guillain-Barre syndrome (GBS), a postinfectious polyneuropathy (2).
There are several reports indicating that prior infection with C. jejuni can result in acquisition of immunity (8, 27). However, development of vaccines has been hampered by a lack of understanding of the basic virulence mechanisms and by the antigenic complexity of these organisms. For example, the serotyping scheme developed by Lior et al. (23) is based on heat-labile antigens and has over 100 recognized serogroups. Although the serodeterminant of this scheme was originally thought to be flagellin (44), genetic studies have indicated flagellin is not the serodeterminant in most serogroups (3). The heat-stable serotyping scheme of Penner and Hennessy (35), which is thought to be based on lipopolysaccharides (LPS), has over 70 serotypes. The LPS cores of many serotypes have been shown to contain sialic acid in structures which resemble human gangliosides (30). This molecular mimicry has been implicated in the development of autoantibodies leading to GBS, although the specific structure or structures which enable a given campylobacter strain to cause GBS are not clear.
A formalin-fixed whole-cell vaccine of C. jejuni 81-176 adjuvanted with mutant E. coli heat-labile enterotoxin (LTR192G [12]) is currently undergoing human testing (38, 42). This formulation appears to offer protection against homologous challenge in animal models (6, 7), but the ability to protect against multiple serotypes of C. jejuni remains to be determined. Moreover, given the lack of understanding about the pathogenesis of Campylobacter-associated GBS, there are concerns about use of whole-cell preparations of campylobacters as vaccines. This concern becomes more compelling if multiple strains, which are less well characterized than strain 81-176, were to be combined in order to generate broad cross-serotype-specific protection. An alternate approach would be to utilize a single campylobacter protein, either as a recombinant subunit vaccine or expressed in a carrier vaccine strain, to elicit protection against multiple Campylobacter serotypes. One candidate for inclusion among such vaccines is flagellin. Flagellin is the immunodominant antigen recognized during infection (9, 10, 32), and development of antibodies against flagellin correlates with the development of protection against disease (27). The structure of campylobacter flagellin contains both highly conserved and highly variable regions (25, 37), in addition to glycosyl posttranslational modifications (14, 17, 39). In this study we explore the use of a truncated recombinant flagellin, which includes the most highly conserved domains, as a subunit vaccine against campylobacters in two mouse models.
MATERIALS AND METHODS
Bacterial strains.
C. jejuni 81-176 (Lior 5; O:27) and C. coli VC167 T2 (Lior 8; O:untypeable) have been described previously (8, 16, 18, 26, 37). E. coli DH5α was the host for cloning experiments.
Molecular biology methods.
DNA restriction enzymes and T4 DNA ligase were purchased from New England Biolabs (NEB; Beverly, Mass.) and used as recommended by the supplier. The maltose-binding protein (MBP) fusion vector, pMal-p2, was also purchased from NEB. Plasmid DNAs were routinely isolated by use of Qiagen columns (Qiagen, Chatsworth, Calif.).
DNA sequence analysis.
Double-stranded plasmid DNAs were sequenced on an Applied Biosystems (ABI) model 373 DNA sequencer by using dideoxy terminator chemistry and Taq cycle sequencing kits (Perkin-Elmer/Applied Biosystems, Foster City, Calif.). The malE primer (5′-GGTCGTCAGACTGTCGATGAAGCC-3′) was purchased from NEB. Primers for the sequence analysis of the flaA gene of strain 81-176 were synthesized on an ABI model 392 DNA synthesizer.
Purification of recombinant protein.
Purification schemes were essentially as recommended by NEB. DH5α containing the flagellin-MBP fusion was grown overnight in 10 ml of rich medium (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, and 2 g of glucose/liter) supplemented with 100 μg of ampicillin per ml and used to inoculate a fresh 1-liter culture of the same medium. This culture was grown with shaking at 37°C to an optical density at 600 nm of 0.5, and IPTG (isopropyl-β-d-thiogalactoside; Gibco, Gaithersburg, Md.) was added to a final concentration of 0.3 mM. Cells were grown for an additional 2 h and harvested by centrifugation. Cells were resuspended in 10 ml of column buffer (20 mM Tris-Cl, 200 mM NaCl, 1 mM EDTA) per g (wet weight). The cells were frozen at −20°C overnight, thawed in iced water, and sonicated in an ice bath in short pulses for 2 min (Branson, Danbury, Conn.) or until the maximum amount of protein, as determined by Bio-Rad assay (Hercules, Calif.), was released. The sonicated solution was centrifuged for 30 min at 9,000 × g in a Sorvall RC5-B centrifuge, and the supernatant was diluted 1:5 with column buffer and loaded onto a 2.5- by 10-cm glass column packed with 15 ml of amylose resin (NEB) at a flow rate of 1 ml/min. The column was washed with 12 volumes of column buffer, and the fusion protein was eluted with column buffer containing 10 mM maltose. Protein-containing fractions (as determined by the Bio-Rad assay) were pooled, concentrated with a centrifugal vacuum concentrator (Jouan, Winchester, Va.), and stored in aliquots at −20°C.
Electrophoresis and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a mini-slab gel apparatus (Pharmacia, Piscataway, N.J.) by the method of Laemmli (21). Protein samples solubilized in sample buffer (21) were separated in 12.5% acrylamide (150 V) and either stained with Coomassie brilliant blue or transferred to nitrocellulose for immunological detection as previously described (22). Rabbit hyperimmune serum E288 against SDS-denatured VC167 T2 flagellin was described previously (37). Immunodetection was as described by Power et al. (37). The secondary antibody for rabbit antisera was alkaline phosphatase-tagged goat anti-rabbit immunoglobulin G (IgG; Caltag, Burlingame, Calif.) used at a final dilution of 1:5,000; the secondary antibody for ferret antisera was horseradish peroxidase-labelled goat anti-ferret IgG (Kirkegaard and Perry, Gaithersburg, Md.) used at a dilution of 1:500. Alkaline phosphatase-tagged antibodies were developed with NBT-BCIP (Nitro Blue Tetrazolium plus 5-bromo-4-chloro-3-indolylphosphate; Promega, Madison, Wis.), and peroxidase-labelled antibodies were developed with TMB (3,3′,5,5′-tetramethylbenzidine; Sigma, St. Louis, Mo.).
Purification of flagellin.
Flagellins were purified from Campylobacter spp. by the method of Power et al. (37).
Immune animal sera.
Immune ferret sera were obtained from a collection of sera at The Naval Medical Research Center-Food and Drug Administration from experiments in which ferrets were fed either VC167 T2 or 81-176 and subsequently developed diarrhea (13, 46).
Immune human sera.
Immune human sera from volunteers fed strain 81-176 were the generous gift of David Tribble of The Naval Medical Research Center.
Hyperimmune rabbit antiserum.
Antiserum against the MBP-FlaA fusion was generated in a New Zealand White rabbit by intramuscular injection of 80 μg of MBP-FlaA in 1 ml of Freund complete adjuvant, followed by a boost of the same material in Freund incomplete adjuvant 2 weeks later. The animal was exsanguinated 2 weeks after the second injection, and the resulting antiserum was designated LL1.
ELISA.
MaxiSorp 96-well immunoplates were coated with MBP-FlaA or flagellins purified from campylobacters (0.3 μg/ml, 100 μl/well). Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (6).
Mouse immunizations.
This research met the principles set forth in the 1985 edition of the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council, U.S. Department of Health and Human Services (National Institutes of Health publication 86-23). Helicobacter-free BALB/c mice (6 to 8 weeks old) were purchased from Jackson Laboratory (Bar Harbor, Maine). The animals were housed in laminar-flow cages for a minimum of 7 days before being used in experiments. During this time fecal samples were routinely cultured as described below to be certain the animals were free of campylobacter. Standard laboratory chow and water were provided ad libitum. Mice were anesthetized with methoxyflurane (Metofane; Pitman-Moore, Mundelein, Ill.) and immunized intranasally with 30 to 35 μl of fusion protein by using a micropipette. The doses used were 0, 3, 6, 12, 25, or 50 μg of fusion protein in phosphate-buffered saline (PBS), either alone or in combination with 5 μg of the genetically modified heat-labile enterotoxin of E. coli, designated LTR192G (12) as an adjuvant. A second dose was administered 8 days after the first vaccination. Intestinal lavage was collected 7 days after the second vaccination and blood was collected 21 days after the second vaccination as described earlier (7).
Challenge of immunized mice intranasally.
C. jejuni 81-176 was grown for mouse challenge as described previously (7). Mice were intranasally challenged with 2 × 109 bacteria/mouse 26 days after the second vaccination, and the animals were monitored for sickness and death for 5 days (7). An illness index was determined by assigning a score of 0 (apparently healthy), 1 (ill as determined by a hunched back, ruffled fur, and/or lethargy), or 2 (death) for each mouse daily (7). For each observation day the total score within each group was divided by the number of mice observed to yield the daily index. Fecal excretion of C. jejuni was monitored daily for 10 to 14 days after challenge by culturing fecal homogenates (ca. 5% suspension in PBS) onto a campylobacter-selective agar (CVA; Remel, Lenexa, Kans.). Putative campylobacter colonies were confirmed by morphology and oxidase reactions.
Challenge of immunized mice orally.
Mice were challenged orally (5) with 0.5 ml of various doses of C. jejuni 81-176 grown as previously described (7). Fecal excretion was monitored as described above for 7 to 9 days.
Vaccine efficacy.
Vaccine efficacy was calculated as follows: ([rate for control mice − rate for vaccinated mice]/rate for control mice) × 100.
Statistical analysis.
Comparisons of rates were done by using the Fisher exact test, and the comparisons of means used the Student t test.
Nucleotide sequence accession number.
The DNA sequence of the strain 81-176 flaA gene has been deposited in GenBank under accession no. AF14052.
RESULTS
Construction and characterization of a flagellin-MBP fusion.
A portion of the flaA gene of VC167 T2 (16, 26) was amplified by PCR by using primers flaA-11 (5′-ACCAATATTAACACAAATGTTGCAGCA-3′) and flaA-2 (5′-TTATCTAGACTAATCTCTACCATCATTTTTAAC-3′). The first 9 bp of flaA-11 include the recognition site of SspI; upon cleavage of the PCR product with SspI the 5′ end of the PCR product corresponds to codon ATT encoding amino acid 5 of flaA (I). The 5′ end of flaA-2 allows for the addition of an XbaI site 3′ to bp 1015 of the coding region of flaA. The PCR product of this reaction was digested with SspI and XbaI, purified by agarose gel electrophoresis, and cloned into pMal-p2 which had been digested with XmnI and XbaI. The junction of the insert and vector in several appropriately sized plasmid DNAs was sequenced with the malE primer to confirm that the fusion was correct. One plasmid which contained the expected fusion to malE was termed pEB11-2 and was further characterized.
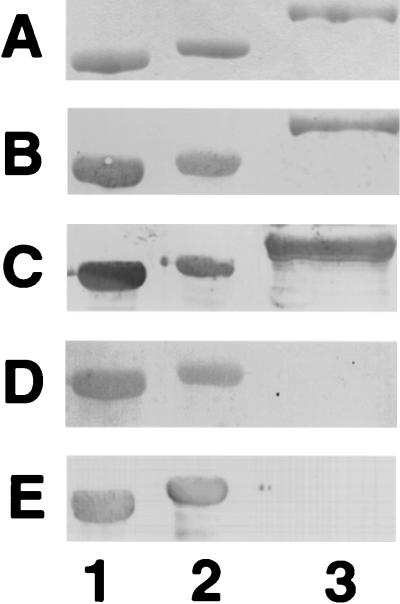
The apparent Mr of the protein produced by DH5α (pEB11-2) was approximately 80,000, as determined by SDS-PAGE (Fig. 1A, lane 3). This is consistent with the predicted Mr of 79,687 for a fusion protein which includes Mr 34,678 of FlaA and Mr 45,009 from MBP. The fusion protein was immunoreactive with the anti-flagellin antiserum E288 (Fig. 1B, lane 3) and with anti-MBP antibody (data not shown). Polyclonal hyperimmune rabbit antiserum made against the MBP-FlaA (LL1) was also reactive with the fusion protein and native flagellin from both VC167 and 81-176, as seen in Fig. 1C.
FIG. 1.
Comparison of flagellins isolated from Campylobacter spp. and MBP-FlaA isolated from E. coli. Proteins were separated by SDS-PAGE in an 8.25% gel and either stained with Coomassie blue (A) or immunodetected with various antisera (B to E): B, E288 rabbit antiserum which was generated against denatured strain VC167 T2 flagellin (37) and cross-absorbed with whole cells of E. coli DH5α at a final dilution of 1:4,000 (37); C, LL1 antiserum against MBP-FlaA at a dilution of 1:20,000; D, ferret antiserum from an animal infected with strain 81-176 at a dilution of 1:500; E, human antiserum from a human volunteer infected with 81-176 at a dilution of 1:5,000. Lane 1, VC167 T2 flagellin; lane 2, 81-176 flagellin; lane 3, MBP-FlaA. VC167 T2 flagellin migrates at an apparent Mr of 59,500 (16, 18), and 81-176 flagellin migrates at an apparent Mr of 62,000 (39).
DNA sequence analysis of the flaA gene of C. jejuni 81-176.
The flaA gene of C. jejuni 81-176 has been cloned previously, and the 5′ end has been partially sequenced (45). The intact flaA gene of 81-176 as cloned in pK2-32 (45) was sequenced in order to determine the extent of similarity between the VC167 FlaA protein and that of 81-176 which would represent the challenge strain in protection experiments (see below). The results indicate that the flaA gene of 81-176 encodes a protein of 574 amino acids with a predicted Mr of 59,240. Figure 2 compares FlaA sequences from strains VC167 T2 and 81-176. Overall, the two proteins are 92% identical and 94% similar. VC167 T2 flagellin has 573 amino acids with a predicted Mr of 59,047 (16). The region of the VC167 T2 FlaA which is included in the MBP-FlaA recombinant protein is underlined (amino acids 5 to 337). This region includes those amino acids which appeared to be the most immunogenic by mimeotope analysis (37). The VC167 and 81-176 flagellins are 98.1% identical and 98.7% similar in this region. The homology is lowest between amino acids 382 and 471. In this region, which includes an additional amino acid in the 81-176 protein, the two flagellins are 73% identical and 84% similar. Comparison of the region of VC167 T2 flagellin in the MBP-FlaA fusion protein with 11 other C. jejuni flagellins (20, 29, 33) revealed a range of 82 to 90% identity and 89 to 96% similarity (data not shown).
FIG. 2.
Clustal W analysis of the FlaA flagellins from C. coli VC167 T2 (16) and C. jejuni 81-176. Asterisks indicate identical residues; conserved residues are indicated by dots. The underlining indicates residues of VC167 T2 flagellin which are in the MBP-FlaA fusion protein.
Evaluation of immunoreactivity of MBP-FlaA with sera from animals experimentally infected with campylobacters.
The ability of sera from ferrets which had been previously infected with C. jejuni 81-176 or VC167 T2 (13, 45) to recognize the MBP-FlaA fusion protein was evaluated by ELISA. The results, summarized in Table 1, indicated that all eight ferrets orally infected with 81-176 reacted with glycosylated flagellins purified from either 81-176 or VC167 T2 but that only three of these eight ferret sera reacted with MBP-FlaA. Similarly, of eight ferrets which had been infected with VC167 T2, six reacted with the homologous VC167 flagellin and seven reacted with 81-176 flagellin. However, only three of the eight reacted with the recombinant MBP-FlaA protein. This difference in response between native and recombinant truncated flagellin can also be seen by Western blot, as shown in Fig. 1D. At a dilution at which antiserum from an 81-176-infected ferret reacts strongly with both VC167 T2 (lane 1) and 81-176 (lane 2) flagellins, there is no reaction with MBP-FlaA (lane 3). Similar results are seen with antiserum from a human volunteer who had been infected with 81-176 (Fig. 1E).
TABLE 1.
Serum IgG responses as measured by ELISA of ferrets infected with Campylobacter spp. to campylobacter flagellins and MBP-FlaAa
| Infecting strain | No. (%) of animals responding to:
|
||
|---|---|---|---|
| VC167 flagellin | 81-176 flagellin | MBP-FlaA | |
| 81-176 | 8/8 (100) | 8/8 (100) | 3/8 (37.5) |
| VC167 T2 | 6/8 (75) | 7/8 (87.5) | 3/8 (37.5) |
Animals were infected with between 109 and 1010 cells of the indicated strains, and serum samples were taken 1 week after infection. An animal was considered to respond to the antigen if there was a >4-fold increase in titer compared to the preimmune sera.
Evaluation of immunogenicity and efficacy of the MBP-FlaA protein against heterologous challenge in the mouse intranasal model.
Mice were immunized intranasally with two doses of 3 to 50 μg of MBP-FlaA with or without 5 μg of LTR192G as an adjuvant. Table 2 shows the intestinal IgA and serum IgG responses to MBP-FlaA as measured by ELISA. The full range of MBP-FlaA doses elicited significant antigen-specific serum IgG responses in vaccinated animals, and these responses were enhanced by adjuvant use, with the exception of the highest dose (50 μg). In contrast, stimulation of FlaA-specific intestinal secretory IgA (sIgA) responses required immunization with higher doses of MBP-FlaA (≥25 μg) or coadministration of lower doses with adjuvant. When given with the adjuvant, as little as 3 μg of the MBP-FlaA protein was capable of stimulating a significant antigen-specific sIgA response in immunized animals. In addition, the magnitude of intestinal sIgA responses to the recombinant protein were significantly enhanced in animals receiving the adjuvanted protein compared to those given MBP-FlaA alone, with the exception of the highest dose.
TABLE 2.
Immunogenicity and efficacy of MBP-FlaA given with or without adjuvant in the mouse intranasal model
| Immunization regimen
|
Immunogenicity (geometric mean titer ± SD)a
|
Efficacy at day 7 as determined by:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Disease symptoms
|
Fecal excretion (%)
|
|||||||
| MBP-FlaA (μg) | LTR192G | n | Lavage IgA | Serum IgG | Illness index | % Efficacy | % Colonized | % Efficacy |
| None | − | 13 | 0.9 ± 0 | 5.7 ± 1.2 | 0.92 ± 0.21 | NA | 82f | NA |
| None | + | 11 | 0.9 ± 0 | 4.3 ± 1.3 | 0.86 ± 0.29 | 7.0 | 67f | 18.3 |
| 3 | − | 7 | 1.0 ± 0.3 | 9.9 ± 0.8b | 0.77 ± 0.37 | 16.6 | 100 | 0 |
| 6 | − | 7 | 1.3 ± 0.7 | 10.6 ± 1.2b | 0.77 ± 0.28 | 16.6 | 100 | 0 |
| 12 | − | 7 | 1.4 ± 0.5 | 10.2 ± 1.6b | 0.74 ± 0.43 | 18.1 | 100 | 0 |
| 25 | − | 12 | 3.5 ± 1.0b | 11.9 ± 1.9b | 0.48 ± 0.41 | 44.1 | 92 | 0 |
| 50 | − | 7 | 5.5 ± 1.1b | 14.0 ± 1.5b | 0.37 ± 0.51 | 55.3 | 43 | 47.6 |
| 3 | + | 6 | 3.5 ± 0.8bd | 13.2 ± 0.6bd | 0.79 ± 0.37 | 14.1 | 60g | 26.8 |
| 6 | + | 7 | 3.4 ± 0.8bd | 13.1 ± 0.5bd | 0.43 ± 0.38c | 53.6 | 100 | 0 |
| 12 | + | 6 | 3.4 ± 1.3ce | 12.8 ± 1.0be | 0.67 ± 0.39 | 27.8 | 83 | 0 |
| 25 | + | 12 | 5.7 ± 1.2bd | 14.1 ± 1.7be | 0.33 ± 0.44c | 64.0 | 75 | 8.5 |
| 50 | + | 8 | 6.6 ± 0.6be | 14.8 ± 0.7b | 0.17 ± 0.24b | 81.1 | 13b | 84.1 |
Geometric mean titers are expressed as natural log transformed values.
P < 0.001 compared to animals immunized with PBS.
P < 0.05 compared to animals immunized with PBS.
P < 0.001 compared to animals immunized with a comparable dose of MBP-FlaA without adjuvant.
P < 0.05 compared to animals immunized with a comparable dose of MBP-FlaA without adjuvant.
Two animals died following challenge.
One animal died following challenge.
The mice were challenged intranasally with C. jejuni 81-176 (2 × 109 bacteria/mouse) 26 days after the second immunization. The effects of the vaccine on disease symptoms and colonization on day 7 are summarized in Table 2. The mean disease indices for mice which had received no vaccine or LTR192G alone were 0.92 and 0.86, respectively. The disease index of mice receiving MBP-FlaA without adjuvant decreased as the dose of vaccine increased up to 50 μg (disease index = 0.37, reflecting 55.3% efficacy), although the results were not statistically significant. In all cases, except for the 3-μg dose, the addition of LTR192G decreased the disease index compared to the corresponding dose of MBP-FlaA without LTR192G. A dose of 50 μg of MBP-FlaA plus LTR192G achieved 81.1% efficacy in protection against disease (P < 0.001). In previous experiments, when mice which had been infected intranasally with live 81-176 were rechallenged 26 days later with the same strain, there was 71% efficacy against disease symptoms (7).
There was no effect on the numbers of mice colonized with strain 81-176 at any dose of MBP-FlaA without LTR192G except at the highest dose (50 μg), which showed 47.6% efficacy in protecting against colonization. Similarly, mice receiving the lower doses of MBP-FlaA plus LTR192G showed little to no reduction in colonization. However, a dose of 50 μg of MBP-FlaA plus LTR192G resulted in 84.1% efficacy against colonization (P < 0.05). In previous experiments with this model, infection of mice with live 81-176 resulted in 91% efficacy against colonization after a second challenge with the same strain (7).
Evaluation of the ability of MBP-FlaA to protect against colonization after oral feeding of mice.
To better examine the ability of MBP-FlaA to protect against intestinal colonization, additional mice were vaccinated with two doses each of 50 μg of MBP-FlaA with or without 5 μg of LTR192G adjuvant; the doses were given 8 days apart. Twenty-six days after the second immunization, groups of seven to eight mice were challenged orally with three different doses of 81-176: 8 × 1010, 8 × 109, or 8 × 108. Control animals immunized with either PBS or LTR192G alone were colonized throughout the course of the experiment regardless of the challenge dose. These results are shown in Fig. 3A for the high-dose challenge group only. Animals immunized with MBP-FlaA alone showed an apparent transient and insignificant reduction in total numbers colonized at days 5 and 6 (71.4% of the animals were culture positive) in the high-dose challenge group only (Fig. 3A). However, on day 7 100% of the mice immunized with MBP-FlaA alone were colonized. When animals immunized with MBP-FlaA plus LTR192G were challenged with 8 × 1010 organisms, there was a marked difference between the controls at days 5 to 7, with only 40% of the animals being colonized on days 5 and 6 (P < 0.05) and 20% being colonized on day 7 (P < 0.001; Fig. 3A). This corresponds to a 55.2% efficacy for days 5 and 6 and a 71.4% efficacy for day 7. The efficacy improved when the animals were challenged with 8 × 109 bacteria (Fig. 3B). In this case, a significant difference between MBP-FlaA plus LTR192G versus MBP-FlaA alone and control groups was apparent by day 5, with the MBP-FlaA plus LTR192G vaccine giving 55.1% efficacy (P < 0.05). By day 7 only 28.6% of the animals in this group remained (71.4% efficacy; P < 0.001). Challenge of the MBP-FlaA plus LTR192G group with 8 × 108 bacteria showed a significant reduction in colonization by day 4 (P < 0.05) and a drop in bacterial counts throughout the course of the experiment (Fig. 3C). By day 6, the MBP-FlaA plus LTR192G vaccine resulted in 78% efficacy against colonization (P = 0.001), and by day 7 no campylobacters could be detected in the stools under the sampling conditions used (P < 0.001).
FIG. 3.
Protection against colonization of mice with different challenge doses of strain 81-176. Animals were immunized in two doses, 8 days apart, and challenged with different doses of 81-176. C. jejuni in stool samples were enumerated by plate count daily after challenge. Immunization with PBS (▵), 5 μg of LTR192G (▴), 50 μg of MBP-FlaA without adjuvant (○), or 50 μg of MBP-FlaA plus 5 μg of LTR192G (●) is as indicated. (A) Challenge dose of 8 × 1010. (B) Challenge dose of 8 × 109. (C) Challenge dose of 8 × 108.
DISCUSSION
The data presented here indicate that MBP-FlaA, when adjuvanted with LTR192G, is capable of eliciting a protective immune response against a heterologous strain of campylobacter as measured in two mouse models involving oral and nasal challenge. At the highest dose (50 μg of MBP-FlaA plus 5 μg of LTR192G) the vaccine showed 81% protective efficacy against disease and 84% efficacy against colonization of the intestine in the mouse intranasal challenge model (7). In this model, immunization with live 81-176, followed by a second infection with the same strain, resulted in 71% efficacy against disease and 91% efficacy against colonization (7). Although the mouse intranasal model uses an unnatural route of infection, it is the only mouse model for campylobacter which consistently results in disease symptoms, these being generally pneumonia and bacteremia (7). Intestinal colonization presumably occurs in this model when the mice swallow some portion of the infecting bacteria. To more directly measure the protection against colonization, we also challenged mice which had undergone the same immunization regimen (50 μg of MBP-FlaA plus LTR192G) with different oral doses of 81-176. The results showed that, when challenged with 8 × 108 bacteria, there was a reduction in colonization as early as 3 days after infection and that no campylobacters could be detected in stools by 7 days postfeeding.
Flagella are a key virulence determinant of Campylobacter spp. since motility is essential for the establishment of colonization in the mucus lining of the gastrointestinal tract (22, 31, 34, 43). Moreover, flagellin is an immunodominant antigen recognized during infection (9, 10, 27, 32), and it has been suggested that the development of antibodies against flagellin correlates with the development of protection (11, 27, 32). The observation that feeding of one strain of campylobacter protects against disease from the homologous, but not heterologous, strains (8) is consistent with the idea that the major protective antigen shows variation among strains. Although there is no flagellar serotyping scheme for campylobacters comparable to the H-antigen typing scheme of the Enterobacteriaceae, there is serological diversity among campylobacter flagellins (18, 25, 37). In Salmonella spp. and E. coli it has been demonstrated that the amino and carboxy ends of flagellins are involved in the transport of the monomer and assembly into the filament, and these regions are highly conserved among serotypes. The central region of the flagellin protein, which lacks functional constraints, is the antigenically diverse region responsible for H serospecificity and is also the region which is surface exposed in the flagellar filament. Based on comparison of DNA sequence analyses of flagellin genes from several strains of C. jejuni, including that of strain 81-176 reported here, and one strain of C. coli (16, 20, 26, 29, 32, 33), the overall structure of campylobacter flagellins appears to be similar to those of the enteric bacteria. Thus, the amino- and carboxy-terminal regions are highly conserved among campylobacter flagellins, and the central regions are more variable (19). Moreover, Power et al. (37) have shown that antibodies to the amino and carboxy regions are not surface exposed in the flagellum filaments of campylobacters. The only antibodies found in that study to be surface exposed in the filament were those which recognize a glycosyl posttranslational modification (14, 17, 39). These modifications alter the apparent Mr of flagellins on SDS-PAGE gels. For example, the masses of the flagellins of strains VC167 and 81-176 are predicted to differ by only 207, but their apparent difference on SDS-PAGE is greater (Fig. 1). Moreover, Alm et al. (4) showed that the apparent Mr of flagellin can vary when expressed in different campylobacter hosts. The presence of a carbohydrate moiety on a bacterial flagellin is highly unusual and has been shown to confer serospecificity to the flagellin (14). Thus, antisera which recognize the posttranslational modifications on the flagellar filament of VC167 (Lior 8) also react with the flagellins of other strains of Lior 8 but not with those of strains from other Lior serogroups (4). Although flagellin is not the serodeterminant of Lior 8 (i.e., nonflagellated mutants of Lior 8 strains still serotype), flagellins appear to be conserved antigenically within the serogroup. Moreover, more recent studies have suggested that glycosyl modifications on flagellin, as well as other campylobacter proteins, are immunodominant (39).
One would expect that any protective epitopes would be surface exposed on the flagellar filament. The role of these surface-exposed posttranslational modifications on protection has been addressed in only one study with the RITARD (removable intestinal tie adult rabbit diarrhea) model. In this model, protection against colonization appeared to be limited to strains of the same Lior serotype. In other words, immunization by feeding with VC167 protected rabbits against subsequent colonization after RITARD challenge with the homologous strain, as well as challenge with two other C. jejuni strains of the Lior 8 serogroup, but not against strains of other serogroups (17). A site-specific mutant defective in a gene required for biosynthesis of the posttranslational modification in VC167 was capable of protecting against a challenge of wild-type VC167 but not the other C. jejuni Lior 8 strains, suggesting that the posttranslational modifications are responsible for this Lior 8 serospecific protection. Given this data, one would not expect that recombinant flagellin that lacked the posttranslational modifications, which are encoded by other campylobacter genes, would be protective, but the data presented here suggest otherwise. In this regard, it is interesting that antibodies generated during natural infection in ferrets by either strain 81-176 or strain VC167 appeared to react more strongly to glycosylated flagellins isolated from Campylobacter spp. than to unglycosylated, recombinant flagellins isolated from E. coli. Similar analysis with serum from a human volunteer who had been infected with 81-176 (42) also suggested a stronger immune response to native flagellin than to recombinant flagellin. Although the recombinant construction used contains a truncated FlaA, this region was selected based on its high immunogenicity in a mimeotope mapping study (37). Thus, the lack of immune response to this region with antisera from experimentally infected humans and animals was surprising and suggests that during natural gastrointestinal infection the immunodominant epitopes are those of the posttranslational modifications rather than of the primary amino acids. Immunization with the recombinant fusion protein lacking these posttranslational modifications may lead to antibody production against epitopes which are less immunogenic in the native molecule due to differences in folding and/or masking by the carbohydrate moiety but are, nonetheless, capable of eliciting a protective immune response. We are currently further evaluating this recombinant flagellin as a vaccine in a ferret diarrheal disease model (13, 46).
ACKNOWLEDGMENTS
We are indebted to J. D. Clements for providing the LTR192G adjuvant used in these studies.
This work was supported by Naval Medical Research and Development Command Work no. 61102AS13O1291 and 62787A870O1289 and by a grant to T.J.T. from the Medical Research Council of Canada.
REFERENCES
- 1.Abimiku A G, Dolby J M. Cross-protection of mice against intestinal colonization by Campylobacter jejuni: importance of heat-labile serotyping (Lior) antigens. J Med Microbiol. 1988;26:265–268. doi: 10.1099/00222615-26-4-265. [DOI] [PubMed] [Google Scholar]
- 2.Allos B M. Association between Campylobacter infection and Guillain-Barre syndrome. J Infect Dis. 1997;176(Suppl. 2):125–128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 3.Alm R A, Guerry P, Power M E, Lior H, Trust T J. Analysis of the role of flagella in the heat-labile Lior serotyping scheme of thermophilic campylobacters by mutant allele exchange. J Clin Microbiol. 1991;29:2438–2445. doi: 10.1128/jcm.29.11.2438-2445.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alm R A, Guerry P, Power M E, Trust T J. Variation in antigenicity and molecular weight of Campylobacter coli VC167 flagellin in different genetic backgrounds. J Bacteriol. 1992;174:4230–4238. doi: 10.1128/jb.174.13.4230-4238.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baqar S, Applebee L A, Bourgeois A L. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun. 1995;63:3731–3735. doi: 10.1128/iai.63.9.3731-3735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baqar S, Bourgeois A L, Schultheiss P J, Walker R I, Rollins D M, Haberberger R L, Pavlovskis O R. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13:22–28. doi: 10.1016/0264-410x(95)80006-y. [DOI] [PubMed] [Google Scholar]
- 7.Baqar S, Bourgeois A L, Applebee L A, Mourad A S, Kleinosky M T, Mohran Z, Murphy J R. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect Immun. 1996;64:4933–4939. doi: 10.1128/iai.64.12.4933-4939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 9.Blaser M J, Duncan D J. Human serum antibody response to Campylobacter jejuni infection as measured in an enzyme-linked immunoabsorbent assay. Infect Immun. 1984;44:297–298. doi: 10.1128/iai.44.2.292-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaser M J, Hopkin J A, Vasil M L. Campylobacter jejuni outer membrane proteins are antigenic for humans. Infect Immun. 1984;43:986–993. doi: 10.1128/iai.43.3.986-993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butzler J P, Glupczynsk Y, Goodson Y. Campylobacter and Helicobacter infections. Curr Opin Infect Dis. 1992;5:80–87. [Google Scholar]
- 12.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doig P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 14.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a posttranslational modification of Campylobacter flagellin: identification of a serospecific glycosyl moiety. Mol Microbiol. 1996;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 15.Echeverria P, Jackson L R, Hoge C W, Arness M K, Dunnauant G R, Larsen R R. Diarrhea in U.S. troops deployed to Thailand. J Clin Microbiol. 1993;31:3351–3352. doi: 10.1128/jcm.31.12.3351-3352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerry P, Alm R A, Power M E, Logan S M, Trust T J. Role of two flagellin genes in Campylobacter motility. J Bacteriol. 1991;173:4757–4764. doi: 10.1128/jb.173.15.4757-4764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerry P, Doig P, Alm R A, Burr D H, Kinsella N, Trust T J. Identification and characterization of genes required for posttranslational modification of Campylobacter coli VC167 flagellin. Mol Microbiol. 1996;19:369–378. doi: 10.1046/j.1365-2958.1996.369895.x. [DOI] [PubMed] [Google Scholar]
- 18.Harris L A, Logan S M, Guerry P, Trust T J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987;169:5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Homma M, Fujita H, Yamaguchi S, Iino T. Regions of Salmonella typhimurium flagellin essential for its polymerization and excretion. J Bacteriol. 1987;169:291–296. doi: 10.1128/jb.169.1.291-296.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khawaja R, Neote K, Bingham H L, Penner J L, Chan V L. Cloning and sequence analysis of the flagellin gene of Campylobacter jejuni TGH9011. Curr Microbiol. 1992;24:213–221. [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, O'Rourke J L, Barrington P J, Trust T J. Mucus colonization by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986;51:536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lior H, Woodward D H, Edgar J A, Laroche L J, Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982;15:761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Logan S M, Trust T J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42:675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan S M, Trust T J. Location of epitopes on Campylobacter jejuni flagella. J Bacteriol. 1986;168:739–745. doi: 10.1128/jb.168.2.739-745.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan S M, Trust T J, Guerry P. Evidence for posttranslational modification and gene duplication of Campylobacter flagellin. J Bacteriol. 1989;171:3031–3038. doi: 10.1128/jb.171.6.3031-3038.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin P M, Mathiot J, Ipero J, Kirimat M, Georges A J, Georges-Courbot M C. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect Immun. 1989;57:2542–2546. doi: 10.1128/iai.57.8.2542-2546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattila L, Siitonen A, Kyronseppa H, Simula I, Oksanen D, Stenvik M, Salo P, Peltola H. Seasonal variation in etiology of travelers' diarrhea. Finnish-Moroccan Study Group. J Infect Dis. 1992;165:385–388. doi: 10.1093/infdis/165.2.385. [DOI] [PubMed] [Google Scholar]
- 29.Meinersmann R J, Helsel L O, Fields P I, Hiett K L. Discrimination of Campylobacter jejuni isolates by fla gene sequencing. J Clin Microbiol. 1997;35:2810–2814. doi: 10.1128/jcm.35.11.2810-2814.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran A P, Appelmelk B J, Aspinall G O. Molecular mimicry of host structures by lipopolysaccharides of Campylobacter and Helicobacter spp.: implications for pathogenesis. J Endotoxin Res. 1996;3:521–531. [Google Scholar]
- 31.Morooka T, Umeda A, Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985;131:1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- 32.Nachamkin I, Hart A M. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J Clin Microbiol. 1985;21:33–38. doi: 10.1128/jcm.21.1.33-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuijten P J, van Asten F J, Gaastra W, van der Zeijst B A. Structural and functional analysis of two Campylobacter jejuni flagellin genes. J Biol Chem. 1990;265:17798–17804. [PubMed] [Google Scholar]
- 34.Pavlovskis O R, Rollins D M, Haberberger R L, Jr, Green A E, Habash L, Stroko S, Walker R I. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect Immun. 1991;59:2259–2264. doi: 10.1128/iai.59.7.2259-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penner J L, Hennessy J N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petruccelli B P, Murphy G S, Sanchez J L, Walz S, DeFraites R, Gelnett J, Haberberger R L, Echeverria P, Taylor D N. Treatment of traveler's diarrhea with ciprofloxacin and loperamide. J Infect Dis. 1992;165:557–560. doi: 10.1093/infdis/165.3.557. [DOI] [PubMed] [Google Scholar]
- 37.Power M E, Guerry P, McCubbin W D, Kay C M, Trust T J. Structural and antigenic characteristics of Campylobacter coli FlaA flagellin. J Bacteriol. 1994;176:3303–3313. doi: 10.1128/jb.176.11.3303-3313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scott D A. Vaccines against Campylobacter jejuni. J Infect Dis. 1997;176(Suppl. 2):183–188. doi: 10.1086/513791. [DOI] [PubMed] [Google Scholar]
- 39.Szymanski C M, Yao R, Ewing C P, Trust T J, Guerry P. Evidence for a system of general protein glycosylation in Campylobacter jejuni. Mol Microbiol, 1999;32:1022–1030. doi: 10.1046/j.1365-2958.1999.01415.x. [DOI] [PubMed] [Google Scholar]
- 40.Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 41.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 42.Tribble, D. Unpublished data.
- 43.Wassenaar T M, Van der Zeijst B A M, Ayling R, Newell D G. Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol. 1993;139:1171–1175. doi: 10.1099/00221287-139-6-1171. [DOI] [PubMed] [Google Scholar]
- 44.Wenman W M, Chai J, Louie T J, Goudreau C, Lior H, Newell D G, Pearson A D, Taylor D E. Antigenic analysis of Campylobacter flagellar protein and other proteins. J Clin Microbiol. 1985;21:108–112. doi: 10.1128/jcm.21.1.108-112.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao R, Burr D H, Doig P, Trust T J, Niu H, Guerry P. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni defective in invasion of eukaryotic cells: the role of flagella in invasion. Mol Microbiol. 1994;14:883–893. doi: 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 46.Yao R, Burr D H, Guerry P. CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1032. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]