Abstract
Among the world’s leading causes of cardiovascular disease, atherosclerosis is a chronic inflammatory disorder that affects the arteries. Both vasodilation and vasoconstriction, low levels of nitric oxide and high levels of reactive oxygen species and pro-inflammatory factors characterize dysfunctional blood vessels. Hypertension, and atherosclerosis, all start with this dysfunction. Geraniol, a compound of acyclic monoterpene alcohol, found in plants such as geranium, lemongrass and rose, is a primary constituent of essential oils. It shows a variety of pharmacological properties. This study aimed to investigate the impact of geraniol on Ox-LDL-induced stress and inflammation in human umbilical vein endothelial cells. In this study, HUVECs were treated with Ox-LDL or geraniol at different dose concentrations. MTT assay, Western blot, ROS generation and DNA fragmentation were used to evaluate geraniol’s effects on Ox-LDL-induced HUVECs inflammation. The results show that geraniol pre-incubation ameliorates Ox-LDL-mediated HUVECs cytotoxicity and DNA fragmentation. The geraniol inhibited the production of pro-inflammatory cytokines by Ox-LDL, including TNF-α, IL-6 and IL-1β. In Ox-LDL-stimulated HUVECs, geraniol suppresses the nuclear translocation and activity of NF-ᴋB as well as phosphorylation of IkBα. Moreover, geraniol activated the PI3K/AKT/NRF2 pathway in HUVECs, resulting in an increase in the expression of HO-1. Taking our data together, we can conclude that, in HUVECs, geraniol inhibits Ox-LDL-induced inflammation and oxidative stress by targeting PI3/AKT/NRF2.
Keywords: anti inflammation, Ox-LDL, geraniol, oxidative stress
1. Introduction
The leading cause of morbidity and mortality in many countries is cardiovascular disease (CVD). Heart disease is mostly caused by atherosclerosis (AS). In the past decade, AS has caused an increase in coronary heart disease morbidity [1]. Every year, almost 400 out of every 100,000 people die from the disease in Asia [1,2]. In addition, AS is considered the primary cause of non-infectious disease-related mortality worldwide. It’s widely accepted that endothelial activation and/or dysfunction are the earliest signs of atherosclerosis [3]. Ox-LDL induces the expression of pro-inflammatory genes, leading to the recruitment of monocytes into the vessel wall and vascular dysfunction [4,5]. Endothelial cells are damaged by free radicals and nitric oxide synthase gene expression and activity are impaired. Ox-LDL triggers adhesion molecules, including ICAM-1 and VCAM-1. Bifurcations and curvatures cause these adhesion molecules to be strongly expressed. By activating VCAM-1, monocytes get rolled along and adhere to the arterial wall, where they can invade the intima, causing foam cells and increasing intima-media thickness, inflammation and oxidative stress [6,7,8,9].
As adhesion molecules accumulate, inflammatory cells adhere to the endothelium, leading to AS [10]. Inflammation and oxidative stress are both fundamental mechanisms associated with various pathologies. ROS are a major target of oxidant stress and play a critical role in several diseases and disorders of the vascular system. Ox-LDL has been shown to cause intracellular ROS production in endothelial cells and alter their signaling [11]. Ox-LDL suppresses NF-ᴋB and stops endothelial cells from committing apoptosis, while other studies suggest it induces NF-ᴋB activation and the expression of pro-inflammatory genes [9,12,13].
The cytoprotective enzyme HO-1 controls inflammation and apoptosis [14]. Studies have shown that activating Nrf2 can boost HO-1 activation [15,16,17,18]. When Nrf2 is not working, HO-1 expression is inhibited, and oxidative stress is exacerbated. Due to its repressor, Keap1, Nrf2 is inactive in the cytoplasm under physiological conditions. During oxidative stress, in the nucleus, Nrf2 is released from the Keap1–Nrf2 complex. A number of studies have shown that activating the PI3K/AKT signaling pathway can facilitate nuclear translocation of Nrf2 [9,19,20]. Inflammation and induced Ox-LDL may be prevented by activating HO-1 and modulating it. The Nrf2–HO-1 pathway is activated by stimulated antioxidants. It has been found that some natural compounds trigger Nrf2 nuclear translocation and protect endothelium dysfunction [21,22,23].
Geraniol (GNL) is a monoterpene (Figure 1). There are lots of essential oils extracted from lemongrass, rose, lavender and other aromatic plants. It exerts a range of pharmacological effects, including antibacterial, anti-inflammatory, antioxidant and neuroprotective [24,25,26,27,28,29]. As an antimicrobial agent, geraniol effectively prevented dysbiosis associated with colitis in mice. Geraniol also reduced apoptotic markers and enhanced dopaminergic neurons’ ability to survive free-radical damage [28].
Figure 1.
Chemical structure of geraniol (GNL).
We hypothesize that GNL has cognitive enhancement effects on a D-gal-mediated ageing model based on our previous findings. GNL has been shown to reduce Ox-LDL-induced endothelial damage for the first time. Hopefully, our research can shed some light on GNL’s potential clinical application for Ox-LDL-related cardiovascular diseases.
2. Materials and Methods
2.1. Reagents
HUVECs were obtained from the Chinese Academy of Sciences in Shanghai. Ox-LDL was supplied by UnionBio Technology (China). 2,7-Dichlorodihydrofluorescein diacetate (DCFH2-DA) and methyl thiazolyl tetrazolium (MTT) were from Sigma, and DMEM from Gibco BRL/Invitrogen. All chemicals used were analytical grade.
2.1.1. Plant Materials
In April 2020, wild lemongrass was obtained from native farms in Al-Asha, Eastern Province, K.S.A. Taxonomists at King Saud University identified the plant. We deposited the samples in the herbarium at King Faisal University, Saudi Arabia, with receipt no. P1906.
2.1.2. Isolation of Lemongrass Essential Oil
A coarse powder was made by pulverizing the dried aerial parts of Cymbopogon commutatus (2000 g) and hydrodistilling it [30,31].
2.1.3. Lemongrass Essential Oil Analysis
According to Perveen et al., lemongrass vital oil was examined using gas chromatography–mass spectrometry (GC–MS) [30], with some modifications. Younis et al. has described the gas chromatography/flame ionization analysis (GC/FID) (2021) [31].
2.1.4. Separation of GNL from the Lemon Grass Vital Oil
A column chromatography method was used to remove GNL from the oil mixtures by Savira et al. (2019) [32], with some modification. The different isolation steps were previously described by Younis et al. (2021) [31].
2.2. Culture and Treatment
We plated HUVECs in DMEM medium and cultured them at 37 °C with 5% CO2. Serum-free medium was used to suspend GNL and Ox-LDL. Each group of cells were seeded onto 6-well plates, and then GNL, Ox-LDL, or a mixture of GNL, Ox-LDL, and a mixture of the two was added over 24 h in triplicate.
2.3. MTT Assay
An MTT kit was used, as per the manufacturer’s instructions, to measure the cytotoxicity of GNL on HUVECs. In 96-well plates (5 × 103 cells per well), the 15-passage HUVECs were incubated for 24 h with different concentrations (0, 25, 50, 100 µM) of GNL and/or Ox-LDL for 24 h. We measured the absorbance of each well at 570 nm with a microplate reader (Winoosky, VT, USA). We used untreated cells to test cell viability (set to 100%).
2.4. Analysis the Level of IL-1β, TNF-α, and ICAM-1 in Culture Supernatant
We cultured HUVECs in a 12-well plate with about 6.5 × 105 cells/well. Pretreatment with GNL (0–100 µmol/mL, 2 h) followed by OxLDL treatment (100 µg/mL, 72 h), culture supernatants were collected followed by IL-1β, TNF-α, and ICAM-1 were quantified using their respective ELISA kits (Figure S1) [33,34].
2.5. Western Blot
A radioimmunoprecipitation assay was employed to isolate the whole-cell proteins from the HUVECs. Using the Nuclear and Cytoplasmic Protein Extraction Kits, we isolated the nuclear and cytoplasmic proteins. Afterwards, lysates on ice were treated with ultra-wave sonication, followed by centrifugation at 12,000× g rpm at 4 °C for 30 min. BCA protein assays were also used to measure the protein concentration. Each sample (20 µg) was separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS PAGE), and then transferred to a polyvinylidene fluoride membrane (PVDF) (Bio-Rad Laboratories, Hercules, CA, USA). Membranes were blocked for 2 h with 5% nonfat milk, and then probed with primary antibodies. Dilution is shown in Supplementary Table S1. Afterwards, PVDF membranes were hatched with secondary antibodies for 1 h, and a chemiluminescence substrate was used to develop them (Pierce Biotechnology, Rockford, IL, USA).
2.6. Measurement of ROS Generation
Through fluorescence microscopy, the cell-permeable fluorogenic test detected intracellular ROS. We performed a ROS generation assay using DCFH2-DA, as reported previously.
2.7. RT-PCR
As per the manufacturer’s instructions, we used a PrimeScript RT reagent kit (Takara Bio, Shiga, Japan) to convert the RNA to cDNA after washing the cells with PBS and applying TRIzol reagent (Invitrogen, Carlsbad, CA, USA) to isolate the HUVECs’ RNA. Supplementary Table S2 shows the primer sequences. We then used SYBR Green (Applied Biosystems, Foster City, CA, USA) and ViiA-7 Applied Biosystem (Carlsbad, CA, USA) to do real-time qPCR. The expression of mRNA was standardized to the β-actin housekeeping gene. We compared the expression of mRNA between groups by comparing the 2ΔΔ Ct values to the non-treated samples.
2.8. DNA Fragmentation
HUVECs were treated with Ox-LDL and/or GNL and DNA fragmentation was measured with the Cell Death Detection ELISA PLUS kit (Roche Applied Science, Branford, CT, USA), as per the manufacturer’s instructions.
2.9. Statistics
We performed a one-way ANOVA. Subsequently, we used Tukey’s post-hoc test, and p < 0.05 was considered statistically significant.
3. Results
3.1. GNL Ameliorated Ox-LDL-Induced Cell Death in HUVECs
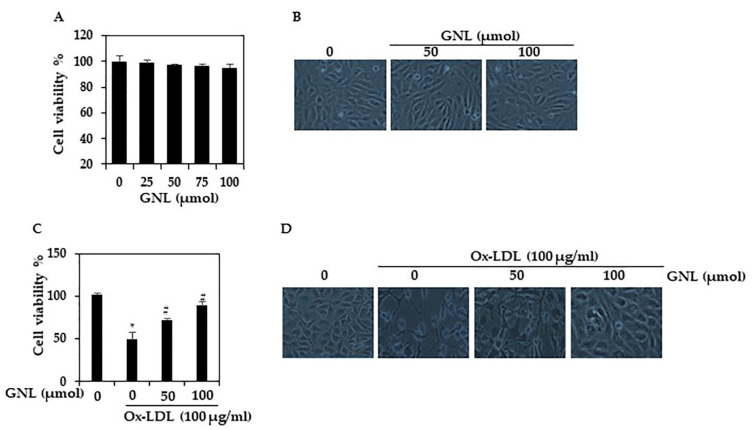
In this study, we aimed to determine the cytotoxicity and viability of HUVECs treated with GNL as well as the effect of GNL on Ox-LDL-induced cell death in HUVECs. MTT assays revealed that GNL alone did not exhibit significant cytotoxicity at a dose of 25–100 µM/mL. Besides, there is no statistical significance between the different dose concentrations (Figure 2A). Figure 2B shows the morphology appearance of HUVECs treated or not with GNL at 50 or 100 µM/mL during 24 h. Cells are normal and healthy and only a few are detached in the medium. On the other hand, Ox-LDL (100 µg/mL) significantly (p < 0.05) inhibited the proliferation of HUVECs. In contrast, GNL protected cells from Ox-LDL-induced cell death (Figure 2C) (p < 0.05). The HUVECs’ morphology was changed by Ox-LDL (100 g/mL). These cells showed the characteristics of apoptotic cells, such as shrinkage of the cell body and membrane blebbing (Figure 2D), while the GNL with Ox-LDL-treated cells had a normal architecture. At a higher concentration (100 µM/mL) of GNL, this effect was significantly and remarkably suppressed. Overall, the data above revealed that GNL might be a potent inhibitor of Ox-LDL-induced endothelial dysfunction.
Figure 2.
GNL chemical structure, its effects on Ox-LDL-induced cell viability, and the morphological changes. (A) HUVECs were added with GNL for 24 h and viability was measured by MTT. (B) GNL effect on the morphology of HUVECs. (C) In an MTT assay, GNL prevents Ox-LDL’s cytotoxicity (24 h). There are three replicates of each value, and * p < 0.05 represents a significant difference when compared to the control group. The # p < 0.05 represents significant differences between Ox-LDL alone and GNL with Ox-LDL treatment groups. (D) In Ox-LDL-induced HUVECs, GNL protects cell morphology.
3.2. GNL Reduced Ox-LDL-Induced Cytokine Production
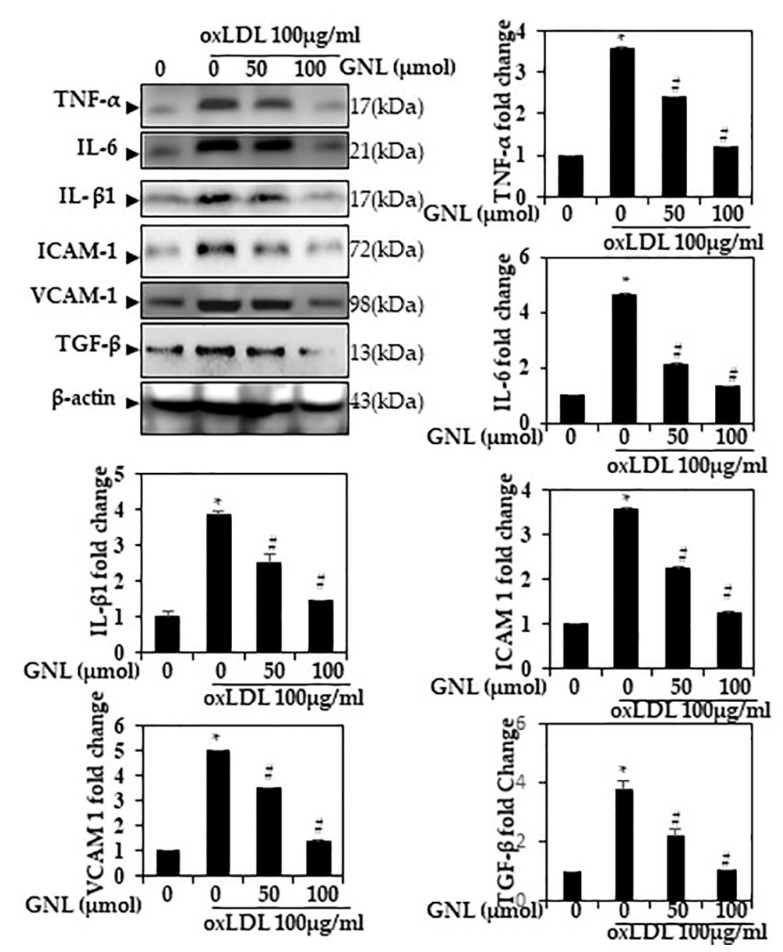
Western blot was used to measure the effect of GNL on Ox-LDL-induced inflammation in HUVECs. Ox-LDL-treated cells had a significantly elevated level of TNF-α, IL-6, IL-1β and TGF-β (p < 0.05). The pre-incubation with GNL significantly (p < 0.05) reduced the expression of pro-inflammatory cytokines TNF-α, IL-6, IL-1β and TGF-β in HUVECs compared with the Ox-LDL alone group (Figure 3). When cells were treated with 100 µM GNL, cytokine production was more inhibited than when they were treated with 50 µM. Similar changes in protein abundance IL-6, TNF- and IL-1 were found in the HUVEC culture supernatants during the same study (Supplementary File S1).
Figure 3.
GNL mitigated Ox-LDL-induced cytokine production in endothelial cells. HUVECs were treated with GNL and/or Ox-LDL for 24 h. Expression of TNF-α, IL-6, IL-1β, ICAM-1, VCAM-1 and TGF-β protein was assessed by Western blotting. There are three replicates of each value, and * p < 0.05 represents a significant difference when compared to the control group. A # represents p < 0.05; thus, significant differences between the Ox-LDL alone and GNL with Ox-LDL treatment groups.
3.3. Effect of GNL on Endothelial Dysfunction
By accelerating endothelial cell dysfunction, Ox-LDL plays a major role in GNL lesion formation. The proinflammatory vascular adhesion molecules VCAM-1 and ICAM-1 are biomarkers of endothelial dysfunction [35].
VCAM-1 and ICAM-1 were analyzed by Western blot in HUVECs. When Ox-LDL was added, VCAM-1 and ICAM-1 were increased 4.4–3.6-fold (p < 0.05), whereas pre-treatment of GNL (50 and 100 µM/mL) lowered their expression level by 2.4–1.5-fold (Figure 3). When cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM.
3.4. Effect of GNL on the Ox-LDL-Induced mRNA Level of TNF-α, IL-6 and ICAM-1
Considering the role of GNL in endothelial dysfunction, we analyzed whether GNL was involved in the inhibition inflammatory pathway activation in Ox-LDL-supplemented cells. qRT-PCR showed significant downregulation of TNF-α, ICAM-1 and IL-6 mRNA in GNL-pretreated cells (50 and 100 µM) (p < 0.05), compared to Ox-LDL-treated cells (Figure 4A–C). When cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM. Inflammatory responses to Ox-LDL were downregulated by GNL, according to these data.
Figure 4.
Effect of GNL on Ox-LDL-induced deposition of cholesterol in HUVECs. (A–C) RT-PCR quantification of pro-inflammation cytokines IL-6 and ICAM-1 and adhesion molecule ICAM-1. (D) Free cholesterol (FC); (E) Total cholesterol (TC). (F) Quantification of fatty acid-binding protein 4 (FABP4) activation by Western blot analyses. There are three replicates of each value, and * represents p < 0.05; thus, a significant difference when compared to the control group. The # represents p < 0.05; thus, significant differences between the Ox-LDL alone and GNL with Ox-LDL treatment groups.
3.5. GNL Decreases the Ox-LDL Effect on Free Cholesterol and Total Cholesterol in HUVECs
Plaque formation starts with a lesion in the endothelial layer, which allows LDL to move from the blood into the intima and become Ox-LDL [36]. The concentration of cholesterol in the blood has been recognized as an indicator for plaque development, where higher LDL and lower HDL levels indicate a higher chance of plaque formation [37]. As a next step, we checked whether or not GNL had an effect on improving the increase in free cholesterol caused by Ox-LDL in the HUVECs. Ox-LDL increased (p < 0.05) the free cholesterol level in the HUVECs, but because of the induction of 100 µM doses of GNL, the high level decreased (Figure 4D) when compared to GNL 50 µM. Treatment with GNL (50 and 100 µM) also reduced the total cholesterol (Figure 4E) despite Ox-LDL increasing the total cholesterol (p < 0.05). When cells were treated with 100 µM GNL, there was more effect than when they were treated with 50 µM.
3.6. GNL Could Inhibit FABP4 Protein Activation in Ox-LDL-Induced HUVECs
FABP4, a member of the fatty acid-binding proteins family, plays a crucial role in lipid metabolism and inflammation. FABP4 may be a useful biomarker for patients with stable peripheral vascular disease at risk of major cardiovascular problems, according to previous studies [38,39]. Further, FABP4-deficient macrophages in atherosclerosis show a significantly declined appearance of inflammatory cytokines. For this reason, next we determined the activation of FABP4 in Ox-LDL and/or GNL-treated HUVECs. An increase in FABP4 expression was seen in endothelial cells stimulated with 100 µg/mL Ox-LDL compared to the control group (Figure 4F). In contrast, GNL downregulates incremental FABP4 concentration-dependently. This result indicates that GNL could reduce the expression of the FABP4 gene in Ox-LDL-induced vascular dysfunctions.
3.7. GNL Inhibits ROS Formation on Ox-LDL-Induced Endothelial Cells
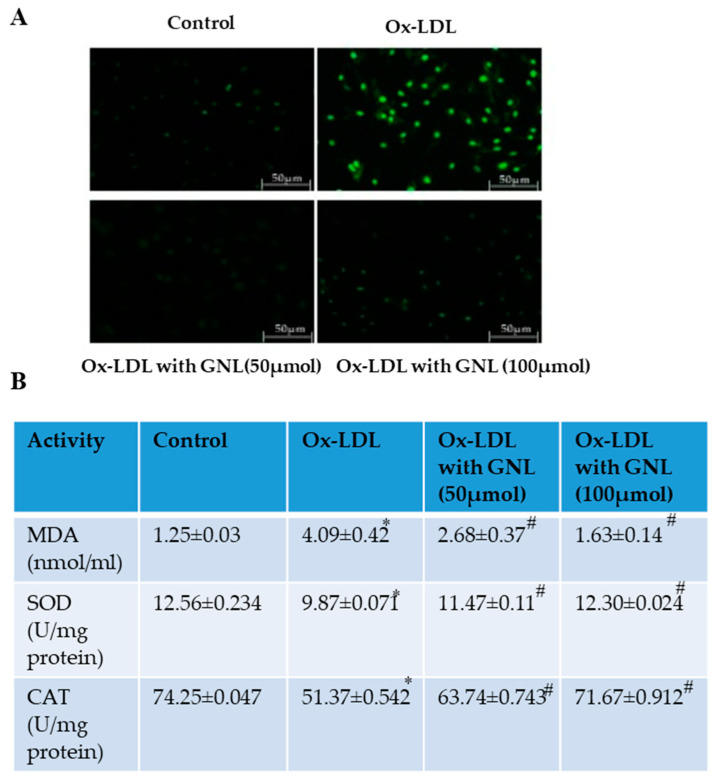
HUVECs were labeled with H2DCFDA in order to clarify the inhibitory effect of GNL on Ox-LDL-induced oxidative stress. Fluorescence microscopy was used to visualize ROS production (Figure 5A). Ox-LDL induced intracellular ROS levels significantly (p < 0.05) in Figure 5A compared to the untreated cells. On the other hand, pretreatment with GNL (50 and 100 µM) significantly reduced Ox-LDL-induced ROS levels (p < 0.05). When cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM.
Figure 5.
Effect of GNL on Ox-LDL-induced ROS production in HUVECs. (A) The HUVECs were then pretreated with GNL (0, 50 and 100 µM, for 2 h), followed by Ox-LDL (100 µg/mL) for 24 h. We measured the intracellular ROS levels using DCF fluorescence. (B) LPO, SOD and CAT. Based on the manufacturer’s instructions, we used ELISA kits. There are three replicates of each value, and * represents p < 0.05; thus, there is a significant difference when compared to the control group. The # represents p < 0.05; thus, there are significant differences between the Ox-LDL alone and GNL with Ox-LDL treatment groups.
3.8. GNL Inhibts MDA Formation in Ox-LDL-Treated Oxidative Stress
Figure 5B shows the levels of MDA in the control and experimental HUVECs. Cells that were treated with Ox-LDL had higher levels of MDA (p < 0.05) when compared to cells that were not treated. Although MDA was inhibited in GNL- (50 and 100 µM) and Ox-LDL-exposed cells (p < 0.05), when cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM GNL—alone treated cells did not show any difference from the controls. The results of this study suggest that GNL may inhibit MDA formation in HUVECs with Ox-LDL-induced vascular dysfunction.
3.9. Effect of GNL on Antioxidant Enzymes
It is well known that mammals, including endothelial cells, have developed a system that defends them by enhancing antioxidant enzymes, so oxidative stress injury can be reduced. Compared to the control group, SOD and CAT activity decreased significantly in the groups that received Ox-LDL (p < 0.05). There was an increased amount of Ox-LDL in the GNL (50 and 100 µM) when HUVECs were treated with Ox-LDL (p < 0.05) (Figure 5B). When cells were treated with 100 µM GNL, there was more activity than when they were treated with 50 µM. Based on the above results, it can be concluded that GNL plays a suppressive role in the Ox-LDL-induced oxidative stress of cells in the endothelium.
3.10. Effect of GNL on Ox-LDL-Induced NF-κB p65 Expression
Cytokines are involved in the phosphorylation of NF-ᴋB and the degradation of IᴋB-α. It is necessary for NF-ᴋB to be activated in order for p65 subunit to translocate from the cytoplasm to the nucleus, where it acts as a transcription factor for genes related to cell proliferation. Therefore, we examined the effect of GNL on NF-ᴋB activation and translocation in this study. In cells pretreated with GNL (50 and 100 µM), phosphorylation of IᴋB-α in the total extract was inhibited (p < 0.05). Ox-LDL increased NF-ᴋB p65 translocation into the nucleus, whereas GNL (50 and 100 µM) inhibited this translocation in a dose-dependent manner (Figure 6A). When cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM.
Figure 6.
NF-ᴋB p65 expression is affected by GNL. (A) p-IᴋBα and pNF-ᴋB p65 antibodies were used to detect nuclear protein extract and total protein extract on 10–12% SDS-PAGE (polyacrylamide gel electrophoresis). (B) By Western blot analysis, we measured the levels of nuclear Nrf2 and NQO-1, HO-1 and γ-GCLC. There are three replicates of each value, and * represents p < 0.05; thus, there is a significant difference when compared to the control group. The # represents p < 0.05; thus, there are significant differences between the Ox-LDL alone and GNL with Ox-LDL treatment groups.
3.11. GNL Stimulated Nrf2, HO-1 and NQO1 Expression in Ox-LDL-Treated Endothelial Cells
In earlier studies, Nrf2 played a role in anti-inflammatory processes such as inflammatory cell recruitment and gene expression regulation. In addition to being an antioxidant pathway, Keap-1/NRF2/ARE regulates anti-inflammatory gene expression, which prevents inflammation from progressing [17,40,41]. We measured the levels of several antioxidant proteins. In this study, Western blots showed that Ox-LDL-treated cells showed significantly (p < 0.05) reduced expression of antioxidants proteins, whereas the GNL (50 and 100 µM) treatment significantly increased (p < 0.05) the expression of Nrf2, HO-1, NQO-1 and γ-GCLC in the nucleus (Figure 6B). At varying dose points, we measured the fold over basal levels of the antioxidant protein expression. When cells were treated with 100 µM GNL, there was more stimulation than when they were treated with 50 µM. There was a differential but substantial effect of GNL on nuclear Nrf2, total Nrf2, HO-1, γ-GCLC and NQO-1 expression patterns.
3.12. GNL Upregulates PI3K/AKT Phosphorylation in Ox-LDL-Induced HUVEcs
Many studies suggest ROS activate apoptosis via the PI3/AKT pathway. There is evidence that oxidative stress decreases PI3/AKT phosphorylation and inactivates various transcription factors, such as Nrf2 [42,43,44]. To determine whether Ox-LDL does or does not affect PI3/AKT phosphorylation expression, HUVECs were induced and exposed to Ox-LDL for various dose periods (Figure 7A). After Ox-LDL exposure for the indicated dose, Western blotting revealed a significant decrease in PI3/AKT expression (p < 0.05). When exposed to GNL (0–100 µM), HUVECs showed a dose-dependent increase in p-PI3K and p-AKT expression (p < 0.05). When cells were treated with 100 µM GNL, there was more phosphorylation than when they were treated with 50 µM. AKT and PI3K, however, remained relatively unchanged. As a result, the level of pPI3K and pAKT cells increased significantly after GNL treatment. GNL seems to protect against Ox-LDL-induced oxidative stress through a signaling pathway such as PI3K/AKT.
Figure 7.
GNL activates PI3K/AKT phosphorylation. (A) The cells were treated with Ox-LDL (100 µg/mL) or GNL (0, 50 and 100 μmol) for 24 h. Based on Western blots, GNL has an impact on anti-pPI3K and anti-pAKT. Th relative ratios of PI3K and AKT expression are listed in the Western blotting results. (B) Cells were pretreated for two hours with PI3K/AKT inhibitors, and then with GNL (100 mol) and/or Ox-LDL (100 g/mL) for 24 h. By using anti-pAKT and anti-Nrf2, Western blotting showed pAKT, Nrf2 levels. (C) After being exposed to SnPP (40 µM/mL) for 1 h, cells were treated with Ox-LDL and GNL for 24 h, and proteins were analyzed by Western blot. (D) DNA fragmentation. There are three replicates of each value, and * represents p < 0.05; thus, a significant difference when compared to a control group. The # represents p < 0.05; thus, significant differences between the Ox-LDL alone and GNL with Ox-LDL treatment groups.
3.13. GNLActivates PI3K/AKT Signaling to Regulate Nrf2 in Ox-LDL-Induced Endothelial Cells
In order to determine whether Nrf2 signals contributed to the upregulation of pAKT, Western blot analysis was performed. The aim of this experiment was to see if GNL and Ox-LDL activated Nrf2 signaling via PI3K/AKT. Figure 7B shows that cells treated with Ox-LDL and GNL upregulated pAKT and Nrf2. These levels were significantly downregulated by LY294002-treated cells and GNL + Ox-LDL. It was concluded that GNL may be dependent on PI3K/AKT–Nrf2 signaling in HUVECs.
3.14. GNL Upregulates HO-1 Activation through the Nrf2 Pathway
As a result of these findings, we examined the effect of GNL on Ox-LDL-induced oxidative stress when Ho-1 inhibitors were present. We studied the kind of impact Snpp had on the expression of the aforementioned protein signals in order to find out whether the GNL pathway activated by GNL played a role in ensuring HUVECs survival. Ox-LDL and/or GNL increased protein expression in comparison to Ox-LDL alone, though the Snpp result largely disputes this finding (Figure 7C). Snpp also stopped the GNL-induced rise in HO-1 protein expression.
3.15. GNL Inhibits Apoptosis in HUVECs Line
Apoptosis is characterized by DNA fragmentation. Using an ELISA kit, we tested DNA fragmentation for cell death [45]. Ox-LDL-treated cells saw a significant increase in DNA fragmentation, while GNL-treated cells saw a marked decrease in DNA fragmentation (Figure 7D) following treatment, in comparison to the cells treated with a combination of GNL and Ox-LDL. When cells were treated with 100 µM GNL, there was more inhibition than when they were treated with 50 µM. Still, GNL inhibited apoptosis in vascular endothelial cells induced by Ox-LDL-induced oxidative stress.
4. Discussion
Hypertension, hyperglycemia, hyperlipidemia, smoking and even drinking can cause AS. Vascular endothelial injury, inflammation, oxidative stress and apoptosis are believed to be the main causes of AS, despite not being fully understood [10]. LDL cholesterol accumulates under the inner membrane when endothelial cells are damaged. An oxidized and modified LDL-C can be engulfed by macrophages and form foam cells, triggering AS, thrombosis and heart attacks. Additionally, when the body is stimulated by oxidative stress factors, such as hydrogen peroxide, low-density lipoprotein (LDL) and proinflammatory factors, it produces a lot of reactive oxygen species (ROS), which can upset the oxidant–antioxidant balance [46,47,48]. Endothelial cells are overactivated by oxidative stress, which reduces their repair ability [49]. To prevent and treat AS, Ox-LDL should be reduced to prevent oxidative damage to endothelial cells. Blood vessels are kept dynamic by the proliferation and apoptosis of endothelial cells. Apoptosis of endothelial cells is a symptom of endothelial dysfunction in AS plaques [3,50,51]. An MTT assay was used to measure the viability of HUVECs, and Ox-LDL significantly decreased their viability. GNL reduced Ox-LDL-induced cell damage in HUVECs in a concentration-dependent manner. AS is mostly caused by inflammation of the vascular endothelium. Inflammatory responses in the vascular endothelium involve rolling, adhesion, migration and accumulation of white blood cells [50,52]. Various inflammatory factors and adhesion molecules can be produced when endothelial cells are stimulated by external factors. Inflammation will result from the adhesion of white blood cells to endothelial cells. Xiaozhen et al. [53] found that treatment with Ox-LDL increased IL-6, TNF-α, IL-β1 VCAM1, ICAM1 and TGF-β. Studies showed that GNL reduces inflammatory and adhesion factors in AS [54,55]. Ox-LDL significantly upregulated TNF-α, IL-6, VCAM1, ICAM1 and IL-1β expression in endothelial cells, while GNL treatment significantly (p < 0.05) downregulated these expressions, which agrees with previous studies. Ox-LDL induced an inflammatory response in HUVECs, and GNL protected them.
Oxidative stress causes abnormal apoptosis and inflammation when ROS levels exceed the body’s antioxidant ability. Dyslipidemia and metabolic syndrome increase ROS [56,57]. Antioxidases protect the body from oxidative damage. SOD and CAT expressions are downregulated by Ox-LDL, while SOD and CAT expressions are upregulated by GNL. GNL protects HUVECs from oxidative stress induced by Ox-LDL. Mechanisms may involve apoptosis-associated signal pathways, such as the PI3K/AKT pathway, and a suppression of oxidative stress via the HO-1/Nrf-2 system. GNL has also been reported to affect HO-1/Nrf-2 expression and has biological effects in different cell lines [52,58]. According to Western blot analysis, the HO-1,γ-GCLC and Nrf-2 protein expression levels of HUVECs treated with Ox-LDL were lower than those of cells treated with Ox-LDL and GNL (p < 0.05). The rate-limiting enzyme in heme catabolism is HO-1. When HO-1 is activated, it can exert cytoprotective, anti-inflammatory, anti-oxidative and inhibitory-apoptotic effects. There’s a link between modulating HO-1 and ROS production in other cell lines [59,60]. Therefore, upregulation of HO-1 via GNL could play a role in attenuating the production of intracellular ROS in GNL with Ox-LDL. GNL exposure significantly increased Nrf-2 expression (p < 0.05) compared to Ox-LDL incubation alone. These results show that GNL is involved in inhibiting oxidative stress by affecting the HO-1/Nrf-2 system.
The PI3K/Akt pathway is critical in promoting cell survival in vascular cells, and there’s increasing evidence of cross-talk between the Nrf2 and PI3K/Akt pathways in response to oxidative stress [17,61,62]. We hypothesized that PI3K/AKT regulates GNL in cells treated with Ox-LDL. AKT phosphorylation was dramatically increased (p < 0.05) by GNL supplementation, suggesting that GNL stimulates Nrf2 expression by phosphorylating AKT. LY294002, an inhibitor of the PI3K/AKT pathway, as well as GNL and Ox-LDL were used to assess the PI3K/AKT pathway’s function. GNL induced PI3K/AKT and Nrf2 expression in the study. The results show that PI3K/AKT signaling plays a crucial role in GNL-induced HO-1 expression by inducing Nrf2 in oxidative stress by Ox-LDL.
5. Conclusions
In this study, GNL showed therapeutic and prophylactic efficacy against Ox-LDL-associated oxidative endothelial damage. In addition, GNL inhibits Ox-LDL-stimulated inflammation by lowering the pro-inflammatory cytokines and downregulating ICAM-1 and VACM-1 expression, which means that GNL inhibits Ox-LDL-stimulated inflammation by lowering the pro-inflammatory cytokines. Moreover, in OX-LDL-stimulated endothelium, GNL inhibited ROS-mediated inflammation activation through Nrf2 activation, by inhibiting ROS-mediated inflammation activation. Through PI3K/AKT/Nrf2/HO-1, GNL suppressed Ox-LDL-mediated ROS production, improved the cell viability and blocked cell apoptosis. Further in-depth studies will aim to establish bioactive GNL as a potential candidate for treating oxidative stress-induced AS complications.
Acknowledgments
The authors thank the Deanship of Scientific Research, King Faisal University and College of Science.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2072-6643/14/22/4817/s1, Figure S1 GNL mitigated Ox-LDL-induced cytokine production in endothelial cells; Table S1. Antibody details of the proteins used in immunoblot analysis; Table S2. Primer sequences.
Author Contributions
Conceptualization, R.B.A. and P.R.; methodology, R.B.A. and P.R.; software, S.A.A. and V.P.V.; validation, P.R., R.B.A., M.E.M., M.A. and R.K.; formal analysis, M.A.; investigation, R.B.A., M.B.I., A.K.S. and P.R.; resources, R.K. and M.E.M.; data curation, S.A.A.; writing—P.R. and M.B.I.; writing—review and editing, A.K.S., R.B.A., P.R., A.K.S. and M.A.; visualization, R.B.A. and M.B.I.; supervision, P.R.; project administration, R.B.A.; funding acquisition, R.B.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Grant 1030).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong M.C., Wang H.H. Rapid emergence of atherosclerosis in Asia: A systematic review of coronary atherosclerotic heart disease epidemiology and implications for prevention and control strategies. Curr. Opin. Lipidol. 2015;26:257–269. doi: 10.1097/MOL.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 2.Geng J., Xu H., Yu X., Xu G., Cao H., Lin G., Sui D. Rosuvastatin protects against oxidized low-density lipoprotein-induced endothelial cell injury of atherosclerosis in vitro. Mol. Med. Rep. 2019;19:432–440. doi: 10.3892/mmr.2018.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., Nishigaki I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9:1057. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santiago-Fernández C., Martín-Reyes F., Tome M., Gutierrez-Repiso C., Fernandez-Garcia D., Ocaña-Wilhelmi L., Rivas-Becerra J., Tatzber F., Pursch E., Tinahones F.J. Oxidized LDL Increase the Proinflammatory Profile of Human Visceral Adipocytes Produced by Hypoxia. Biomedicines. 2021;9:1715. doi: 10.3390/biomedicines9111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Ma G., Yao Y., Qian H., Li W., Chen X., Jiang W., Zheng R. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low density lipoprotein through downregulating expression of LOX-1. Int. J. Mol. Sci. 2012;13:1512–1523.:1512. doi: 10.3390/ijms13021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H.-p., Zheng F.-l., Zhao J.-h., Guo D.-x., Chen X.-l. Genistein inhibits ox-LDL-induced VCAM-1, ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1. Arch. Med. Res. 2013;44:13–20. doi: 10.1016/j.arcmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Yang X., Wan M., Cheng Z., Wang Z., Wu Q. Tofacitinib inhibits ox-LDL-induced adhesion of THP-1 monocytes to endothelial cells. Artif. Cells Nanomed. Biotechnol. 2019;47:2775–2782. doi: 10.1080/21691401.2019.1573740. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z.-R., Li J.-Y., Dong X.-W., Tan Z.-J., Wu W.-Z., Xie Q.-M., Yang Y.-M. Apple polyphenols decrease atherosclerosis and hepatic steatosis in ApoE−/− mice through the ROS/MAPK/NF-κB pathway. Nutrients. 2015;7:7085–7105.:5324. doi: 10.3390/nu7085324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P., Xie W., Luo Y., Lu S., Dai Z., Wang R., Sun G., Sun X. Protective effects of total saponins of Aralia elata (Miq.) on endothelial cell injury induced by TNF-α via modulation of the PI3K/Akt and NF-κB signalling pathways. Int. J. Mol. Sci. 2018;20:36. doi: 10.3390/ijms20010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao W., Wu C., Chen X. Cryptotanshinone inhibits oxidized LDL-induced adhesion molecule expression via ROS dependent NF-κB pathways. Cell Adhes. Migr. 2016;10:248–258. doi: 10.1080/19336918.2015.1119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee W.-J., Ou H.-C., Hsu W.-C., Chou M.-M., Tseng J.-J., Hsu S.-L., Tsai K.-L., Sheu W.H.-H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010;52:1290–1300. doi: 10.1016/j.jvs.2010.04.085. [DOI] [PubMed] [Google Scholar]
- 12.Cominacini L., Pasini A.F., Garbin U., Davoli A., Tosetti M.L., Campagnola M., Rigoni A., Pastorino A.M., Cascio V.L., Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-κB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 13.Hsuan C.-F., Hsu H.-F., Tseng W.-K., Lee T.-L., Wei Y.-F., Hsu K.-L., Wu C.-C., Houng J.-Y. Glossogyne tenuifolia extract inhibits TNF-α-induced expression of adhesion molecules in human umbilical vein endothelial cells via blocking the NF-kB signaling pathway. Molecules. 2015;20:16908–16923. doi: 10.3390/molecules200916908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang S.-K., Chen S.-E., Chang L.-C. A dual role of heme oxygenase-1 in cancer cells. Int. J. Mol. Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J., Lim J.W., Kim H. Lycopene Inhibits IL-6 Expression by Upregulating NQO1 and HO-1 via Activation of Nrf2 in Ethanol/Lipopolysaccharide-Stimulated Pancreatic Acinar Cells. Antioxidants. 2022;11:519. doi: 10.3390/antiox11030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajendran P., Alzahrani A.M., Priya Veeraraghavan V., Ahmed E.A. Anti-Apoptotic Effect of Flavokawain A on Ochratoxin-A-Induced Endothelial Cell Injury by Attenuation of Oxidative Stress via PI3K/AKT-Mediated Nrf2 Signaling Cascade. Toxins. 2021;13:745. doi: 10.3390/toxins13110745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbuZahra H.M., Rajendran P., Ismail M.B. Zerumbone Exhibit Protective Effect against Zearalenone In-Duced Toxicity via Ameliorating Inflammation and Oxidative Stress Induced Apoptosis. Antioxidants. 2021;10:1593. doi: 10.3390/antiox10101593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alzahrani A.M., Rajendran P., Veeraraghavan V.P., Hanieh H. Cardiac protective effect of kirenol against doxorubicin-induced cardiac hypertrophy in H9c2 cells through Nrf2 signaling via PI3K/AKT pathways. Int. J. Mol. Sci. 2021;22:3269. doi: 10.3390/ijms22063269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han N.-R., Ko S.-G., Park H.-J., Moon P.-D. Dexamethasone Attenuates Oncostatin M Production via Suppressing of PI3K/Akt/NF-κB Signaling in Neutrophil-like Differentiated HL-60 Cells. Molecules. 2021;27:129. doi: 10.3390/molecules27010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho B.O., Yin H.H., Park S.H., Byun E.B., Ha H.Y., Jang S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264. 7 macrophages. Biosci. Biotechnol. Biochem. 2016;80:1520–1530. doi: 10.1080/09168451.2016.1171697. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.N., Kim J.D., Park S.B., Son H.-J., Park G.H., Eo H.J., Kim H.-S., Jeong J.B. Anti-inflammatory activity of the extracts from Rodgersia podophylla leaves through activation of Nrf2/HO-1 pathway, and inhibition of NF-κB and MAPKs pathway in mouse macrophage cells. Inflamm. Res. 2020;69:233–244. doi: 10.1007/s00011-019-01311-2. [DOI] [PubMed] [Google Scholar]
- 23.Subedi L., Lee J.H., Yumnam S., Ji E., Kim S.Y. Anti-inflammatory effect of sulforaphane on LPS-activated microglia potentially through JNK/AP-1/NF-κB inhibition and Nrf2/HO-1 activation. Cells. 2019;8:194. doi: 10.3390/cells8020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavan B., Dalpiaz A., Marani L., Beggiato S., Ferraro L., Canistro D., Paolini M., Vivarelli F., Valerii M.C., Comparone A. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front. Pharmacol. 2018;9:18. doi: 10.3389/fphar.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W., Viljoen A.M. Geraniol—A review of a commercially important fragrance material. South Afr. J. Bot. 2010;76:643–651. doi: 10.1016/j.sajb.2010.05.008. [DOI] [Google Scholar]
- 26.Thapa D., Losa R., Zweifel B., Wallace R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology. 2012;158:2870–2877. doi: 10.1099/mic.0.061127-0. [DOI] [PubMed] [Google Scholar]
- 27.Khan A.Q., Khan R., Qamar W., Lateef A., Rehman M.U., Tahir M., Ali F., Hamiza O.O., Hasan S.K., Sultana S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013;94:419–429. doi: 10.1016/j.yexmp.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Rekha K.R., Selvakumar G.P., Sethupathy S., Santha K., Sivakamasundari R.I. Geraniol ameliorates the motor behavior and neurotrophic factors inadequacy in MPTP-induced mice model of Parkinson’s disease. J. Mol. Neurosci. 2013;51:851–862. doi: 10.1007/s12031-013-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajendran P., Ammar R.B., Al-Saeedi F.J., AlRamadan S.Y., Ismail M.B., Veeraraghavan V.P., Alamer S.A., Alawwad N., Moqbel M.S., Ahmed E.A. Geraniol Attenuates Oxidative Stress and Neuro-inflammation Mediated Cognitive Impairment in D-galactose-induced Mouse Aging Model. Res. Sq. 2022 doi: 10.21203/rs.3.rs-1215880/v1. [DOI] [Google Scholar]
- 30.Perveen K., Bokhari N.A., Siddique I., Al-Rashid S.A. Antifungal activity of essential oil of Commiphora molmol Oleo Gum Resin. J. Essent. Oil Bear. Plants. 2018;21:667–673. doi: 10.1080/0972060X.2018.1492975. [DOI] [Google Scholar]
- 31.Younis N.S., Elsewedy H.S., Soliman W.E., Shehata T.M., Mohamed M.E. Geraniol isolated from lemon grass to mitigate doxorubicin-induced cardiotoxicity through Nrf2 and NF-κB signaling. Chem. Biol. Interact. 2021;347:109599. doi: 10.1016/j.cbi.2021.109599. [DOI] [PubMed] [Google Scholar]
- 32.Savira A., Syahrani A., Rudyanto M. Proceedings of the BROMO Conference Symposium on Natural Product Biodiversity. BROMO; Surabaya, Indonesia: 2018. A Simple Method for Isolation of Citral Using Column Chromatography; pp. 12–20. [Google Scholar]
- 33.Yang H.-L., Yang T.-Y., Gowrisankar Y.V., Liao C.-H., Liao J.-W., Huang P.-J., Hseu Y.-C. Suppression of LPS-induced inflammation by chalcone flavokawain a through activation of Nrf2/ARE-mediated antioxidant genes and inhibition of ROS/NFκB signaling pathways in primary splenocytes. Oxidative Med. Cell. Longev. 2020;2020:3476212. doi: 10.1155/2020/3476212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo C., Wang L., Zhang J., Numazawa S., Tang H., Tang X., Han X., Li J., Yang M., Wang Z. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014;20:574–588. doi: 10.1089/ars.2012.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.R., Bae Y.H., Bae S.K., Choi K.S., Yoon K.H., Koo T.H., Jang H.O., Yun I., Kim K.W., Kwon Y.G., et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta. 2008;1783:886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Li W., Li Y., Zhao Y., Ren L. The protective effects of aloperine against ox-LDL-induced endothelial dysfunction and inflammation in HUVECs. Artif Cells Nanomed Biotechnol. 2020;48:107–115. doi: 10.1080/21691401.2019.1699816. [DOI] [PubMed] [Google Scholar]
- 37.Hao W., Friedman A. The LDL-HDL profile determines the risk of atherosclerosis: A mathematical model. PLoS ONE. 2014;9:e90497. doi: 10.1371/journal.pone.0090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiruvengadam M., Venkidasamy B., Subramanian U., Samynathan R., Ali Shariati M., Rebezov M., Girish S., Thangavel S., Dhanapal A.R., Fedoseeva N. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants. 2021;10:1859. doi: 10.3390/antiox10121859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoebaus C., Herz C.T., Pesau G., Wrba T., Koppensteiner R., Schernthaner G.H. FABP4 and cardiovascular events in peripheral arterial disease. Angiology. 2018;69:424–430. doi: 10.1177/0003319717728226. [DOI] [PubMed] [Google Scholar]
- 40.Kigka V.I., Potsika V., Mantzaris M., Tsakanikas V., Koncar I., Fotiadis D.I. Serum biomarkers in carotid artery disease. Diagnostics. 2021;11:2143. doi: 10.3390/diagnostics11112143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C.-Y., Deng J.-S., Huang W.-C., Jiang W.-P., Huang G.-J. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients. 2020;12:1742. doi: 10.3390/nu12061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C., You L., Yin X., Liu Y., Leng X., Wang W., Sai N., Ni J. Heterophyllin B ameliorates lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 Macrophages by Suppressing the PI3K/Akt Pathways. Molecules. 2018;23:717. doi: 10.3390/molecules23040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajendran P., Alzahrani A.M., Ahmed E.A., Veeraraghavan V.P. Kirenol inhibits B [a] P-induced oxidative stress and apoptosis in endothelial cells via modulation of the Nrf2 signaling pathway. Oxidative Med. Cell. Longev. 2021;2021:5585303. doi: 10.1155/2021/5585303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajendran P., Ammar R.B., Al-Saeedi F.J., Mohamed M.E., ElNaggar M.A., Al-Ramadan S.Y., Bekhet G.M., Soliman A.M. Kaempferol inhibits zearalenone-induced oxidative stress and apoptosis via the PI3K/Akt-mediated Nrf2 signaling pathway: In vitro and in vivo studies. Int. J. Mol. Sci. 2020;22:217. doi: 10.3390/ijms22010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabi T., Shukla S., Gupta S. Betulinic acid suppresses constitutive and TNFα-induced NF-κB activation and induces apoptosis in human prostate carcinoma PC-3 cells. Mol. Carcinog. 2008;47:964–973. doi: 10.1002/mc.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S., Yan W., Hu Y., Wu H. Shikonin Alleviates Endothelial Cell Injury Induced by ox-LDL via AMPK/Nrf2/HO-1 Signaling Pathway. Evid. Based Complement. Altern. Med. 2021;2021:5881321. doi: 10.1155/2021/5881321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou H.C., Chou W.C., Hung C.H., Chu P.M., Hsieh P.L., Chan S.H., Tsai K.L. Galectin-3 aggravates ox-LDL-induced endothelial dysfunction through LOX-1 mediated signaling pathway. Environ. Toxicol. 2019;34:825–835. doi: 10.1002/tox.22750. [DOI] [PubMed] [Google Scholar]
- 48.Li S., Sun Y., Han Z., Bu X., Yu W., Wang J. Cytoprotective effects of euxanthone against ox-LDL-induced endothelial cell injury is mediated via Nrf2. Life Sci. 2019;223:174–184. doi: 10.1016/j.lfs.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Kattoor A.J., Kanuri S.H., Mehta J.L. Role of Ox-LDL and LOX-1 in atherogenesis. Curr. Med. Chem. 2019;26:1693–1700. doi: 10.2174/0929867325666180508100950. [DOI] [PubMed] [Google Scholar]
- 50.Gimbrone Jr M.A., García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016;118:620–636. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajendran P., Chen Y.F., Chen Y.F., Chung L.C., Tamilselvi S., Shen C.Y., Day C.H., Chen R.J., Viswanadha V.P., Kuo W.W. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018;233:6458–6471. doi: 10.1002/jcp.26479. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y., Xian X., Wang Z., Bi Y., Chen Q., Han X., Tang D., Chen R. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules. 2018;8:80. doi: 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuo X., Wu Y., Yang Y., Gao L., Qiao X., Chen T. Knockdown of LSD1 meliorates Ox-LDL-stimulated NLRP3 activation and inflammation by promoting autophagy via SESN2-mesiated PI3K/Akt/mTOR signaling pathway. Life Sci. 2019;233:116696. doi: 10.1016/j.lfs.2019.116696. [DOI] [PubMed] [Google Scholar]
- 54.Jayachandran M., Chandrasekaran B., Namasivayam N. Geraniol attenuates oxidative stress by Nrf2 activation in diet-induced experimental atherosclerosis. J. Basic Clin. Physiol. Pharmacol. 2015;26:335–346. doi: 10.1515/jbcpp-2014-0057. [DOI] [PubMed] [Google Scholar]
- 55.Jayachandran M., Chandrasekaran B., Namasivayam N. Effect of geraniol, a plant derived monoterpene on lipids and lipid metabolizing enzymes in experimental hyperlipidemic hamsters. Mol. Cell. Biochem. 2015;398:39–53. doi: 10.1007/s11010-014-2203-3. [DOI] [PubMed] [Google Scholar]
- 56.Panth N., Paudel K.R., Parajuli K. Reactive oxygen species: A key hallmark of cardiovascular disease. Adv. Med. 2016;2016:9152732. doi: 10.1155/2016/9152732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dubois-Deruy E., Peugnet V., Turkieh A., Pinet F. Oxidative stress in cardiovascular diseases. Antioxidants. 2020;9:864. doi: 10.3390/antiox9090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Younis N.S., Abduldaium M.S., Mohamed M.E. Protective effect of geraniol on oxidative, inflammatory and apoptotic alterations in isoproterenol-induced cardiotoxicity: Role of the Keap1/Nrf2/HO-1 and PI3K/Akt/mTOR pathways. Antioxidants. 2020;9:977. doi: 10.3390/antiox9100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H., Liu Y.-y., Jiang Q., Li K.-r., Zhao Y.-x., Cao C., Yao J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014;69:219–228. doi: 10.1016/j.freeradbiomed.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Furfaro A., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U., Pronzato M., Nitti M. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxidative Med. Cell. Longev. 2016;2016:1958174. doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T., Liang X., Shi L., Wang L., Chen J., Kang C., Zhu J., Mi M. Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-) equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE. 2013;8:e79075. doi: 10.1371/journal.pone.0079075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang C.-S., Lin A.-H., Yang T.-C., Liu K.-L., Chen H.-W., Lii C.-K. Shikonin inhibits oxidized LDL-induced monocyte adhesion by suppressing NFκB activation via up-regulation of PI3K/Akt/Nrf2-dependent antioxidation in EA. hy926 endothelial cells. Biochem. Pharmacol. 2015;93:352–361. doi: 10.1016/j.bcp.2014.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.