Abstract
Magnesium-based amorphous alloys have aroused broad interest in being applied in marine use due to their merits of lightweight and high strength. Yet, the poor corrosion resistance to chloride-containing seawater has hindered their practical applications. Herein, we propose a new strategy to improve the chloride corrosion resistance of amorphous Mg65Cu15Ag10Gd10 alloys by engineering atomic-to-nano scale structural homogeneity, which is implemented by heating the material to the critical temperature of the liquid–liquid transition. By using various electrochemical, microscopic, and spectroscopic characterization methods, we reveal that the liquid–liquid transition can rearrange the local structural units in the amorphous structure, slightly decreasing the alloy structure’s homogeneity, accelerate the formation of protective passivation film, and, therefore, increase the corrosion resistance. Our study has demonstrated the strong coupling between an amorphous structure and corrosion behavior, which is available for optimizing corrosion-resistant alloys.
Keywords: magnesium-based alloy, liquid–liquid phase transition, corrosion resistance, structural homogeneity
1. Introduction
Magnesium (Mg) alloys are considered one of the most promising lightweight metals to potentially replace heavier structural materials in the uses of aerospace, marine, and automobile vehicles [1,2]. However, despite the great prospect, Mg alloys still suffer from a number of inherent drawbacks, including the strength–corrosion tradeoff [3,4]. In this regard, many efforts have been devoted to employing amorphous Mg alloys for anti-corrosion uses. Here, an amorphous alloy means that all the metallic atoms in the long range are arranged randomly in the structure. It is found that amorphous magnesium-based alloys are attractive for their high mechanical properties [5,6]. In addition, amorphous alloys also exhibit improved corrosion resistance due to the chemical homogeneity with reduced crystallographic breaks such as grain boundaries, dislocations, and phase segregations, so the chemical attack on those susceptible sites can be largely avoided [7,8]. Nevertheless, for the Mg-based amorphous alloys in particular, the high reactivity of Mg still makes the material vulnerable to corrosion failure [9,10]. To further improve the corrosion resistance of the Mg-based amorphous alloys, previous efforts mainly focus on strategies of surface modifications [11,12] and on alloying corrosion-resistant elements like Nb [13]. Furthermore, forming a passivation film on the alloy surface is also an effective way to prevent corrosion [14,15]. Although various progress has been achieved, the critical barrier has not yet been overcome, which severely hinders the widespread usage of Mg-based amorphous alloys [16,17]. Therefore, more rational and artful ideas for material design down to the nanostructural or atomic level are highly desired for improving the corrosion resistance of the amorphous Mg alloys.
When heating amorphous alloys before melting, typically, several phase transition processes occur in sequence, including separation into amorphous phases with different components [18,19], liquid–liquid phase transition [20,21], and crystallization [22,23]. Among these processes, the liquid–liquid phase transition involves only rearrangement of local structural units and no component variation in the amorphous alloys without a long-range order [24,25]. Although local changes in structure and density are subtle and come with a small amount of heat release, it still makes remarkable differences in mechanical and functional properties [26,27]. Studies show that the liquid–liquid transition is closely related to the thermodynamic stability and mechanical properties of Mg-based amorphous alloys [28]. The thermodynamically-favored local order and loose structure emerge during the liquid–liquid transition, which benefits higher hardness and modulus than a completely disordered structure. In addition, Hu et al. [29] reveal that the metastable state formed during the liquid–liquid transition in (Fe0.72B0.24Nb0.04)95.5Y4.5 amorphous ribbon could significantly reduce the activation energy and reaction energy barrier of high-temperature oxidization. More importantly, by using synchrotron small-angle scattering, our group has demonstrated that the liquid–liquid phase transition can significantly impact the atomic-to-nano scale homogeneity in the medium-range structure of amorphous Mg alloys [28], which is strongly related to the corrosion resistance of the material [30,31,32,33]. This motivates us to fundamentally understand the correlation between liquid–liquid transition and corrosion, improving the corrosion resistance in amorphous Mg-based alloys.
In this work, by selecting Mg65Cu15Ag10Gd10 amorphous alloy as a model material, we reveal that the chloride corrosion resistance of Mg-based alloys could be significantly improved by rearranging the local structural unit in the liquid–liquid transition. By means of potentiodynamic polarization measurement, electrochemical impedance test, immersion test, differential scanning calorimetry, high energy X-ray diffractometry, high-resolution transmission electron microscopy, X-ray photoelectron spectroscopy, and scanning electron microscopy, it is revealed that structural ordering and heterogeneity during the liquid–liquid transition accelerate the formation of the passivation film and therefore improve the corrosion resistance of the Mg-based alloy. This study proves the strong correlation between an amorphous structure and corrosion behavior and provides a new strategy for optimizing corrosion-resistance alloys.
2. Experimental Method
2.1. Sample Preparation
The target material studied in this paper was Mg65Cu15Ag10Gd10 amorphous alloy (by atomic percentage), with the purity of raw materials being 99.95 wt.%. In order to ensure uniform mixing of each component, the total metal mass was controlled for no more than 10 g. The surface oxide of raw materials was removed by file and sandpaper, and the mass error of weighing did not exceed 0.001 g.
The alloy ingot was prepared by induction heating and then vacuum arc melting many times. The chamber vacuum was below 1 × 10−3 Pa and then filled with high-purity argon gas at −0.5 Pa as protection. Then, the quartz tube containing the alloy ingot was placed inside a copper induction coil and electrified by a current of 50 A. Due to the pressure difference, the molten alloy ingot was ejected through a 0.9 mm hole onto the rotating copper roller at a high speed of 60 rps. Eventually, the amorphous ribbons were obtained. A few of them were heated to the temperature of liquid–liquid transition (i.e., TC) (444 K) in the oven, hereafter referred to as the Tc-treated samples. The Tc is determined by the temperature at an abnormal exothermic peak in differential scanning calorimetry (DSC), which will be discussed later.
2.2. Corrosion Resistance Test
Prior to the corrosion experiments, both as-cast and heated ribbon specimens were cut to 10 mm × 2 mm, fixed in epoxy resin, polished with 2000 mesh sandpaper, and then sonicated in acetone, ethanol, and deionized water, respectively. The electrochemical experiments were carried out using the CHI660E A20146 electrochemistry workstation. The reference electrode and counter electrode were saturated calomel electrode (SCE) and Pt, respectively. The potential polarization scanning was started at 150 mV below the open circuit potential (OCP) at a rate of 0.833 V·s−1 in both 1 mol·L−1 NaCl and 1 mol·L−1 NaOH solutions. Electrochemical impedance spectroscopy (EIS) was performed at the frequency ranging from 105 to 10−2 Hz with an amplitude of 5 mV. The solutions of the immersion test lasting 2 h were 0.01 mol·L−1 NaCl and 0.01 mol·L−1 NaOH.
2.3. Characterization Techniques
A total of 20 mg Mg65Cu15Ag10Gd10 ribbon was used to test the thermophysical parameters by differential scanning calorimetry (DSC, METTLER TOLEDO) at a heating rate of 10 K·min−1. The nitrogen flow rate was set to 50 mL·min−1 to isolate oxygen and prevent drastic oxidation of the sample during the heating process. High-resolution transmission electron microscopy (HRTEM, FEI TECNAL G2 20), small-angle synchrotron X-ray scattering (SAXS, Advanced Photon Source of Argonne National Laboratory), and high energy X-ray diffractometry (HEXRD) were used to probe the alloy microstructure. The thin region of the HRTEM sample was obtained by etching the amorphous ribbon with an incident angle of 8° and an energy of 8 keV at low temperature of liquid nitrogen for 25 min. The beam size of the synchrotron X-ray (λ = 0.0886 nm) was 0.1 × 0.2 mm and the SAXS data with Q range from 0.0034 to 0.364. Å−1 was calibrated and corrected by empty cell scattering, transmission, and detector response using a beamline MATLAB program package [34]. The incident wavelength of the HEXRD excited by Ag Kα1 was 0.05594 nm (22 keV), and the scattering angle (2θ) ranged from 5° to 20°. The surface morphology of the samples was characterized by scanning electron microscopy (SEM, JSM-IT500HR) with an electron beam voltage of 20 kV. The surface composition and valence state of the alloy were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific K-Alpha) with monochromatic Al Kα ray (hv = 1486.6 eV) radiation.
3. Results and Discussion
3.1. Microstructure and Thermal Analysis
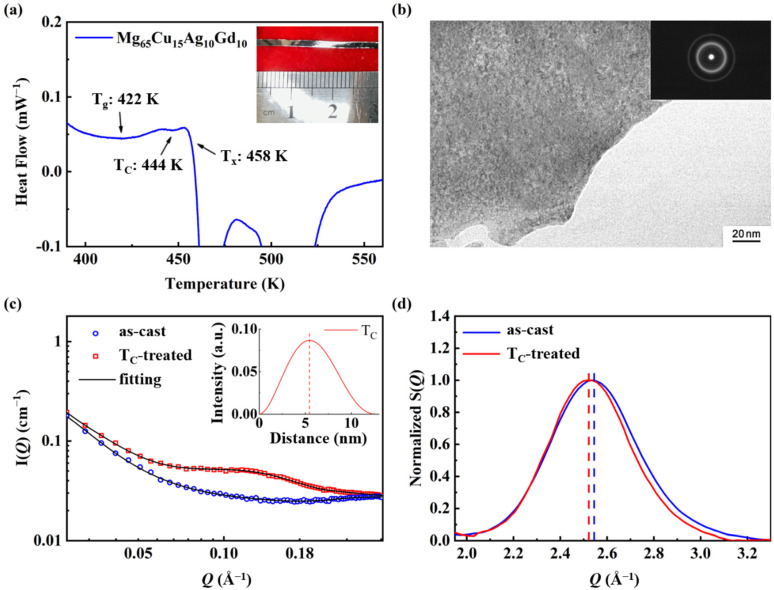
The differential scanning calorimetry (DSC) curve of Mg65Cu15Ag10Gd10 amorphous alloys (Figure 1a) shows an abnormal exothermic peak that appeared after the glass transition temperature Tg (422 K) [35]. The corresponding temperature was marked as TC (444 K), which, according to previous studies, is related to the liquid–liquid phase transition [28,36]. As the temperature continues to increase, a sharp exothermic peak starting at 458 K emerges, which can be assigned to the crystallization of the amorphous material [37]. The width and thickness of the ribbons are 2 mm and 45 μm, respectively (Figure 1a inset).
Figure 1.
(a) Differential scanning calorimetry curve of Mg65Cu15Ag10Gd10 amorphous alloy ribbon. The inset is an optical image of an as-cast sample. (b) High-resolution transmission electron microscopy of Mg65Cu15Ag10Gd10 TC-treated ribbon. The inset is the selected area electron diffraction pattern. (c) The SAXS of Mg65Cu15Ag10Gd10 as-cast and TC-treated ribbons. Reproduced with permission [28]. Copyright 2021, Springer Nature. The inset is the size distribution function based on the SAXS profiles. (d) The structure factor of Mg65Cu15Ag10Gd10 as-cast and TC-treated ribbons.
From the HRTEM image of the TC-treated sample (Figure 1b), the atoms are arranged in a maze-like disordered manner, whereas the selected area electron diffraction (SAED) pattern in the inset shows broad and dispersive rings. The results further demonstrate that the Mg65Cu15Ag10Gd10 alloys remain the amorphous feature after TC treatment.
Down to the nanoscale, an interference peak occurs in the TC-treated sample whereas it does not exist in the as-cast sample according to the results of SAXS (Figure 1c), which suggests the occurrence of nanoscale heterogeneity different from the amorphous matrix in the TC-treated sample [28,38,39,40]. Furthermore, a spheroid model with polydispersity is employed to fit the SAXS profile of the TC-treated sample, and the obtained size distribution function result is displayed in Figure 1c inset [41,42]. It is seen that the diameter of the spheroidal granular-like structure is around 5.4 nm for the TC-treated sample, verifying that the heterogeneous structure exists [43,44,45]. Further down to the atomic scale, Figure 1d compares the structure factor (S(Q)) patterns as a function of momentum transfer amplitude (Q) for both the as-cast and TC-treated alloys. The first S(Q) peak shows a lower peak position and a narrower peak width after TC treatment, which indicates that the local order is extended to the medium range and the atomic packing is less dense than the matrix. The structural correlation is enhanced from the short to the medium range (~5–10 Å) during this process, due to the recombination of the local atomic units [22,46,47]. To summarize, the nanoscale thermodynamically-favored metastable amorphous heterogeneous region with an average diameter of 5.4 nm is uniformly distributed in the amorphous alloy matrix. In each heterogeneous region, the degree of the atomic order is extended to the medium range (~5–10 Å). The heterogeneity in the atomic-to-nano scale stands for the liquid–liquid transition and the strong connection between abnormal exothermic peak at TC and liquid–liquid transition is consistent with our previous studies [28,48,49].
3.2. Analysis of Potentiodynamic Corrosion Behavior
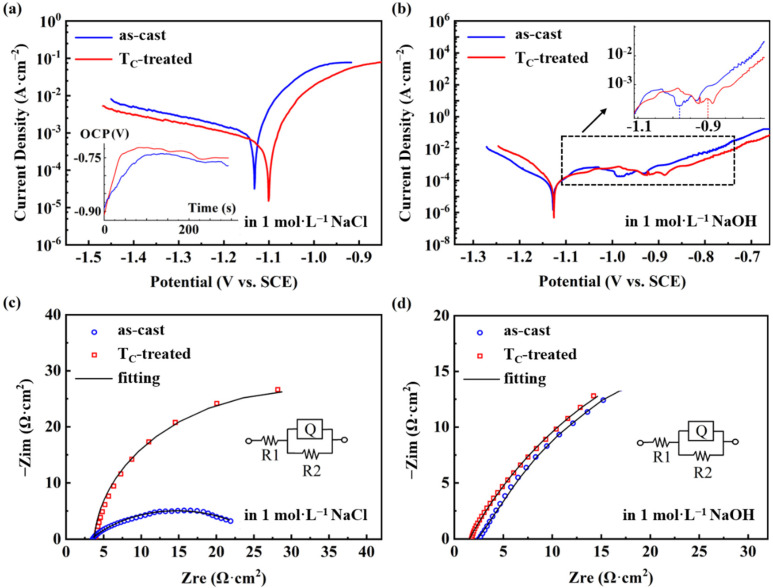
Figure 2a shows the potentiodynamic polarization curves of the Mg65Cu15Ag10Gd10 as-cast and TC-treated samples in 1 mol·L−1 NaCl solution, showing an active dissolution process through anodic polarization. By using the Tafel epitaxial method, the self-corrosion potential of the as-cast and TC-treated samples were −1.14 V and −1.11 V, respectively, and the corresponding self-corrosion current densities were 1.1 × 10−3 A·cm−2 and 5.3 × 10−4 A·cm−2, respectively. The higher corrosion potential and lower corrosion current of the TC-treated sample indicate that the chemical stability in the NaCl solution is enhanced after the TC heat treatment. Figure 2a inset shows the curve of open circuit potential of the as-cast and TC-treated samples as a function of immersion time in 1 mol·L−1 NaCl electrolyte. In the first 100 s, the OCP of the amorphous ribbons changes rapidly to positive, indicating that their stability increases due to the construction of the surface film. Subsequently, the OCP curves of these two samples gradually become stable, with a slight fluctuation in a certain potential, which is attributed to the loss and regrowth of the passivation layer on the surface. During the last 300 s immersion, the OCP of the TC-treated sample is higher than that of the as-cast sample, indicating that a better defense and stability layer is formed on the surface of the TC-treated sample.
Figure 2.
(a) Potentiodynamic polarization curves of as-cast and TC-treated samples in 1 M NaCl solution. The inset is their open circuit potential (V vs. SCE) curves dependent on the immersion time. (b) Potentiodynamic polarization curves of as-cast and TC-treated samples in 1 M NaOH solution. The inset enlarges the breakdown potential with the same coordinate units. (c) Nyquist curves of as-cast and TC-treated samples in 1 M NaCl solution. (d) Nyquist curves of as-cast and TC-treated samples in 1 M NaOH solution.
Figure 2b shows the potentiodynamic polarization curves of the as-cast and TC-treated Mg65Cu15Ag10Gd10 samples in 1 mol·L−1 NaOH solution, both of which exhibit good chemical stability with a corrosion current of about 1 × 10−5 A·cm−2 and a corrosion potential of −1.13 V. A spontaneous passivation occurs for both of the materials with a low passivation current density of about 3 × 10−4 A·cm−2. Although both the materials show a wide passivation zone (−1.10~−0.98 V for the as-cast sample vs. −1.10~−0.90 V for the TC-treated sample), the passivation film breakdown potential of the TC-treated sample is slightly higher, indicating that the passivation film of the TC-treated sample is more stable [50]. When applying the potential to above −0.90 V, the passivation films of these two samples are transpassively dissolved, and accordingly, the corrosion current density increases [51]. All the above results indicate that the Mg65Cu15Ag10Gd10 amorphous alloy system shows better corrosion resistance after TC treatment in both NaCl and NaOH solutions.
Figure 2c,d show the EIS fitting circuits in 1 mol·L−1 NaCl and 1 mol·L−1 NaOH solutions, respectively. The fitted circuit elements include solution resistance (R1), charge transfer resistance (R2), and constant phase element (Q), and the fitted results are shown in Table 1. After TC treatment, the corrosion behavior does not change compared with the as-cast one, and all the EIS curves in NaCl and NaOH solutions show a single capacitive arc with one time constant [52]. From the fitting results, the R2 values of the TC-treated samples are higher than those of the as-cast samples in both NaCl and NaOH solutions. The larger R2 is inclined towards a higher corrosion potential, lower corrosion current, and higher passivation film breakdown potential, which is consistent with the potentiodynamic polarization results (Figure 2a,b) [53].
Table 1.
EIS fitting parameters of as-cast and TC-treated samples of all the same size in 1 M NaCl and NaOH solutions.
| Q (μs0/Ω) | α | R1 (Ω·cm2) | R2 (Ω·cm2) | |
|---|---|---|---|---|
| as-cast in 1 M NaCl | 421.1 | 0.56 | 2.77 | 24.00 |
| TC-treated in 1 M NaCl | 1000.0 | 1.00 | 3.70 | 56.06 |
| as-cast in 1 M NaOH | 545.1 | 0.65 | 2.51 | 81.72 |
| TC-treated in 1 M NaOH | 77.99 | 0.77 | 2.45 | 84.24 |
3.3. Analysis of Immersion Corrosion Behavior
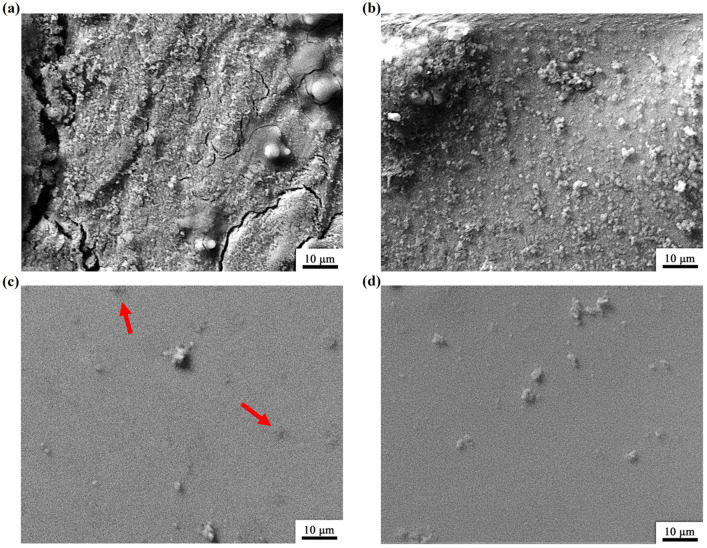
Figure 3 shows the SEM images of the as-cast and TC-treated Mg65Cu15Ag10Gd10 metal glass ribbons soaked in 0.01 mol·L−1 NaCl and 0.01 mol·L−1 NaOH solutions for 2 h. The passivation films of the as-cast ribbon in 0.01 mol·L−1 NaCl show local corrosion and cracks (arrows in Figure 3a), which are largely inhibited in the TC-treated sample (Figure 3b). The higher corrosion resistance of the TC-treated sample also verifies the experimental results of potentiodynamic polarization curves and EIS. Analogously, the corrosion resistance of the TC-treated Mg65Cu15Ag10Gd10 ribbon in 0.01 mol·L−1 NaOH solution is a little better than that of the as-cast sample, which is inferred by the disappearing pits after the TC treatment.
Figure 3.
SEM images of (a) as-cast and (b) TC-treated samples after a 2-h immersion in 0.01 M NaCl solution. SEM images of (c) as-cast and (d) TC-treated samples after a 2-h immersion in 0.01 M NaOH solution. The red arrows in Figure 3c show the corrosion pits induced by the NaOH solution.
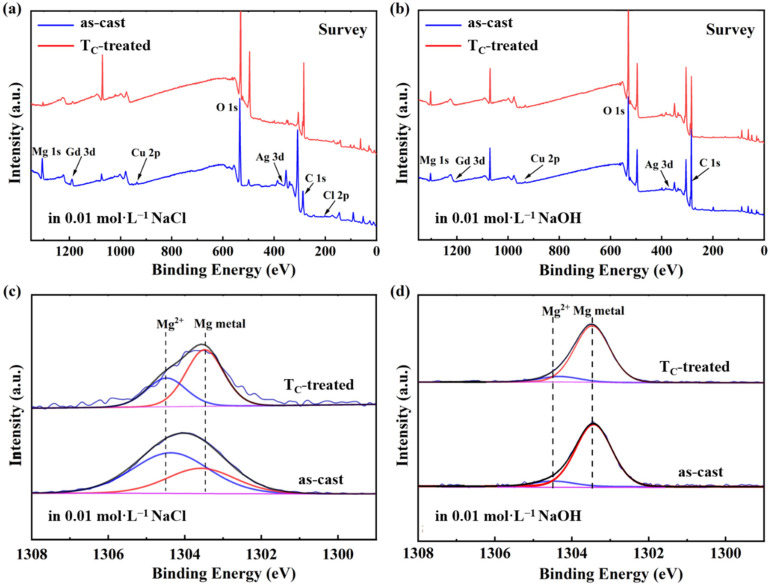
In order to better understand the chemical stability of the Mg65Cu15Ag10Gd10 amorphous alloys, XPS measurements were performed on the as-cast and TC-treated ribbons immersed in 0.01 M NaCl and 0.01 M NaOH for 2 h (Figure 4a,b). The relevant elemental content obtained from XPS is displayed in Table 2. For the as-cast samples, the Ag content is lower than the designed composition, which is related to the highest antioxidative activities of Ag [54]. Remarkably, the Mg content is much higher than the designed ratio, indicating the passivation film on the surface of the ribbon is dominated by MgO. When subjected to the TC treatment, the Mg content on the surface of the TC-treated sample relative to the as-cast sample decreases remarkably in NaCl. This phenomenon firmly validates the improved corrosion to NaCl for the TC-treated alloy. For the samples in NaOH, the Mg content of the TC-treated sample is similar to that of the as-cast sample. This is because of the higher corrosion resistance of the prepared alloy in NaOH. Chloride ions are highly corrosive because they, with a small radius and strong permeability, could penetrate the relatively loose passivation film into the matrix and act as ionic conductors to accelerate the anodic polarization corrosion of alloy [55]. Figure 4c,d show the corresponding high-resolution Mg 1 s spectra. The peak located at 1303.57 eV corresponds to Mg metal [56], whereas the peak at 1304.36 eV can be assigned to Mg2+ [57]. A lower Mg2+ content is detected in the TC-treated sample by comparing the XPS spectra of NaCl-soaked ribbons, indicating that the TC heat treatment could effectively protect Mg from oxidation corrosion.
Figure 4.
(a) Surface XPS of as-cast and TC-treated samples after a 2-h immersion in 0.01 M NaCl solution. (b) Surface XPS of as-cast and TC-treated samples after a 2-h immersion in 0.01 M NaOH solution. (c) Mg 1 s spectra of as-cast and TC-treated samples after a 2-h immersion in 0.01 M NaCl solution. (d) Mg 1 s spectra of as-cast and TC-treated samples after a 2-h immersion in 0.01 M NaOH solution.
Table 2.
Atomic ratios of elements on the surfaces of as-cast and TC-treated samples after a 2-h immersion in 0.01 M NaCl and NaOH solutions.
| Mg | Cu | Ag | Gd | O | Cl | Total | |
|---|---|---|---|---|---|---|---|
| as-cast in 0.01 M NaCl | 13.49 | 1.32 | 0.25 | 0.31 | 83.02 | 1.61 | 100 |
| TC-treated in 0.01 M NaCl | 3.49 | 0.66 | 0.33 | 0.18 | 93.77 | 1.57 | 100 |
| as-cast in 0.01 M NaOH | 8.04 | 1.54 | 0.56 | 0.31 | 89.55 | - | 100 |
| TC-treated in 0.01 M NaOH | 11.07 | 1.14 | 0.43 | 0.16 | 87.20 | - | 100 |
3.4. Discussion
Replacing Cu in the traditional ternary Mg65Cu25Gd10 amorphous alloy with Ag element to form a quaternary Mg65Cu15Ag10Gd10 one was an excellent way to improve corrosion resistance because of the higher equilibrium electrode potential of Ag, namely better chemical stability, than that of Cu [58]. Our experimental results of electrochemical and microscopic tests, as well as XPS, confirm the excellent alkali corrosion resistance of the amorphous Mg65Cu15Ag10Gd10 alloy system.
The improvement in chemical stability, i.e., corrosion performance, could be related to the thermodynamically-favored change in microstructure brought by the liquid–liquid transition. During the liquid–liquid phase transition, a medium-range ordered structure shows higher thermodynamic stability than the totally disordered structure. So, spontaneously, combinations of local atomic units occur, forming nanoscale “medium-range-ordered island” distributed heterogeneously within the amorphous matrix [59,60,61]. Driven by this structural change, the electrochemical reaction of micro galvanic cells formed by the fluctuation of surface energy is small in area and large in quantity, so the formation of passivation film is accelerated. In this micro galvanic cell, the alloy matrix acts as a cathode, whereas the amorphous heterogeneous structure acts as an anode. The area of the cathode matrix is much larger than that of the active amorphous heterogeneous structure anode, which ensures the electrochemical reaction of the passivation film generation at the early stage of corrosion. To sum up the above, the corrosion is accelerated at the beginning of the corrosion process in the TC-treated sample. As a result, at the beginning of the corrosion, the TC-treated sample could evenly form more corrosion products quickly. When the formation rate of the passivation film is higher than the dissolution rate, compact and uniform passivation film would be formed to prevent the solution from entering the matrix and causing further corrosion, meaning that the alloy possesses good chemical stability. The higher R2 value of the EIS results of the TC-treated sample clearly demonstrates that the passivation film of the TC-treated sample is not prone to be dissolved. Note that the liquid–liquid transition does not result in structural incoherence, such as grain boundary and dislocation, so the TC-treated sample is less likely to corrode by a network [62].
4. Conclusions
This paper reports a new approach to improve the corrosion resistance of a typical Mg-based amorphous alloy Mg65Cu15Ag10Gd10 of an anomalous exothermic peak by heating the material to the TC temperature of liquid–liquid transition. During this transition, the structural homogeneity slightly decreases with the rearrangement of the local structural units, which accelerates the formation of protective passivation film and therefore increases the corrosion resistance of the Mg-based alloy. Our present study has initiated a new route to optimize corrosion resistance by engineering atomic-to-nano scale structural homogeneity, which could be applied to other amorphous alloy systems with the liquid–liquid phase transition and hopefully extended to widespread corrosion-resistant alloy research.
Author Contributions
Conceptualization, S.L. (Si Lan), H.H. and X.-L.W.; methodology, K.L. and S.F.; software, Y.L., S.L. (Sinan Liu) and H.Y.; formal analysis, Y.Q., W.Z. and J.G.; investigation, Y.Q., W.Z. and J.G.; data curation, X.Z. and Y.R.; writing—original draft preparation, Y.Q. and H.Z.; writing—review and editing, H.Z. and S.L. (Si Lan); visualization, W.-D.L. and Z.W.; supervision, H.Z. and S.L. (Si Lan); funding acquisition, J.S., S.-C.W. and S.L. (Si Lan); All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the National Key R&D Program of China No. 2021YFB3802800, the National Natural Science Foundation of China (Grant Nos. 52222104, 51871120, 22275089), the Natural Science Foundation of Jiangsu Province (Grant No. BK20200019), the Shenzhen Science and Technology Innovation Commission (No. JCYJ20200109105618137), the Fundamental Research Funds for the Central Universities (No. 30922010307), and the support by Guangdong-Hong Kong-Macao Joint Laboratory for Neutron Scattering Science. The synchrotron diffraction and small-angle X-ray scattering experiments were carried out at the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhattacharjee T., Suh B.C., Sasaki T.T., Ohkubo T., Kim N.J., Hono K. High strength and formable Mg–6.2Zn–0.5Zr–0.2Ca alloy sheet processed by twin roll casting. Mater. Sci. Eng. A. 2014;609:154–160. doi: 10.1016/j.msea.2014.04.058. [DOI] [Google Scholar]
- 2.Zeng Z., Nie J.F., Xu S.W., HJDavies C., Birbilis N. Super-formable pure magnesium at room temperature. Nat. Commun. 2017;8:972. doi: 10.1038/s41467-017-01330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y., Liu B., Zhao X.A., Zhang X., Miao Y., Yang N., Yang B., Zhang L., Kuang W., Li J., et al. Turning a native or corroded Mg alloy surface into an anti-corrosion coating in excited CO2. Nat. Commun. 2018;9:4058. doi: 10.1038/s41467-018-06433-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao M.C., Liu M., Song G., Atrens A. Influence of the β-phase morphology on the corrosion of the Mg alloy AZ91. Corros. Sci. 2008;50:1939–1953. doi: 10.1016/j.corsci.2008.04.010. [DOI] [Google Scholar]
- 5.Inoue A., Ohtera K., Kita K., Masumoto T. New Amorphous Mg-Ce-Ni Alloys with High Strength and Good Ductility. Jpn. J. Appl. Phys. 1988;27:L2248. doi: 10.1143/JJAP.27.L2248. [DOI] [Google Scholar]
- 6.Gonzalez S., Figueroa I.A., Todd I. Influence of minor alloying additions on the glass-forming ability of Mg-Ni-La bulk metallic glasses. J. Alloys Compd. 2009;484:612–618. doi: 10.1016/j.jallcom.2009.05.002. [DOI] [Google Scholar]
- 7.Wang C., Shuai Y., Yang Y., Zeng D., Liang X., Peng S., Shuai C. Amorphous magnesium alloy with high corrosion resistance fabricated by laser powder bed fusion. J. Alloys Compd. 2022;897:163247. doi: 10.1016/j.jallcom.2021.163247. [DOI] [Google Scholar]
- 8.Wang J., Huang S., Wei Y., Guo S., Fusheng P. Enhanced mechanical properties and corrosion resistance of a Mg-Zn-Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013;91:311–314. doi: 10.1016/j.matlet.2012.09.098. [DOI] [Google Scholar]
- 9.Romzi M.A.F., Alias J., Ramli M.I.M. Effect of zinc (Zn) on the microstructure and corrosion behaviour of magnesium (Mg) Mater. Today Proc. 2022;48:1873–1879. doi: 10.1016/j.matpr.2021.09.261. [DOI] [Google Scholar]
- 10.Matias T.B., Roche V., Nogueira R.P., Asato G.H., Kiminami C.S., Bolfarini C., Botta W.J., Jorge A.M., Jr. Mg-Zn-Ca amorphous alloys for application as temporary implant: Effect of Zn content on the mechanical and corrosion properties. Mater. Des. 2016;110:188–195. doi: 10.1016/j.matdes.2016.07.148. [DOI] [Google Scholar]
- 11.Chen S., Tu J., Hu Q., Xiong X., Wu J., Zou J., Zeng X. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids. 2017;456:125–131. doi: 10.1016/j.jnoncrysol.2016.11.011. [DOI] [Google Scholar]
- 12.Zai W., Sun S., Man H.C., Lian J., Zhang Y. Preparation and anticorrosion properties of electrodeposited calcium phosphate (CaP) coatings on Mg-Zn-Ca metallic glass. Mater. Chem. Phys. 2022;290:126532. doi: 10.1016/j.matchemphys.2022.126532. [DOI] [Google Scholar]
- 13.Guan W., Cui-Xia G., Shu-Jie P. Thermal stability, mechanical properties and corrosion behavior of a Mg–Cu–Ag–Gd metallic glass with Nb addition. Rare Met. 2017;36:183–187. [Google Scholar]
- 14.Guoqiang L., Lijing Z., Huanxi L. Corrosion behavior of the bulk amorphous Mg65Cu25Gd10 alloy. Rare Met. Mater. Eng. 2009;38:110–114. [Google Scholar]
- 15.Sun Y., Li Z., Liu J., Liang Z. Corrosion characteristics of Mg-Cu-Gd amorphous alloys. J. Nanjing Univ. Aeronaut. Astronaut. 2010;42:636–640. [Google Scholar]
- 16.Zai W., Man H.C., Su Y., Li G., Lian J. Impact of microalloying element Ga on the glass-forming ability (GFA), mechanical properties and corrosion behavior of Mg-Zn-Ca bulk metallic glass. Mater. Chem. Phys. 2020;255:123555. doi: 10.1016/j.matchemphys.2020.123555. [DOI] [Google Scholar]
- 17.Li H., Pang S., Liu Y., Sun L., Liaw P.K., Zhang T. Biodegradable Mg-Zn-Ca-Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015;67:9–19. doi: 10.1016/j.matdes.2014.10.085. [DOI] [Google Scholar]
- 18.Park E.S., Jeong E.Y., Lee J.K., Bae J.C., Kwon A.R., Gebert A., Schultz L., Chang H.J., Kim D.H. In situ formation of two glassy phases in the Nd-Zr-Al-Co alloy system. Scr. Mater. 2007;56:197–200. doi: 10.1016/j.scriptamat.2006.10.020. [DOI] [Google Scholar]
- 19.Sarlar K., Kucuk I. Phase separation and glass forming ability of (Fe0.72Mo0.04B0.24)(100-x)Gd-x (x = 4, 8) bulk metallic glasses. J. Non-Cryst. Solids. 2016;447:198–201. doi: 10.1016/j.jnoncrysol.2016.06.003. [DOI] [Google Scholar]
- 20.Lan S., Ren Y., Wei X.Y., Wang B., Gilbert E.P., Shibayama T., Watanabe S., Ohnuma M., Wang X.L. Hidden amorphous phase and reentrant supercooled liquid in Pd-Ni-P metallic glasses. Nat. Commun. 2017;8:14679. doi: 10.1038/ncomms14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge J., He H., Zhou J., Lu C., Dong W., Liu S., Lan S., Wu Z., Wang A., Wang L., et al. In-situ scattering study of a liquid-liquid phase transition in Fe-B-Nb-Y supercooled liquids and its correlation with glass-forming ability. J. Alloys Compd. 2019;787:831–839. doi: 10.1016/j.jallcom.2019.02.114. [DOI] [Google Scholar]
- 22.Liu S., Ge J., Ying H., Lu C., Ma D., Wang X.L., Zuo X., Ren Y., Feng T., Shen J., et al. In Situ Scattering Studies of Crystallization Kinetics in a Phase-Separated Zr-Cu-Fe-Al Bulk Metallic Glass. Acta Metall. Sin. Engl. Lett. 2022;35:103–114. doi: 10.1007/s40195-021-01304-3. [DOI] [Google Scholar]
- 23.Lan S., Wei X., Zhou J., Lu Z., Wu X., Feygenson M., Neuefeind J., Wang X.L. In-situ study of crystallization kinetics in ternary bulk metallic glass alloys with different glass forming abilities. Appl. Phys. Lett. 2014;105:201906. doi: 10.1063/1.4901905. [DOI] [Google Scholar]
- 24.Lan S., Dong W.X., Wang X.L. The progress of research on anomalous exothermic phenomenon and hidden amorphous phase transition in metallic glasses. Chin. J. Nat. 2017;39:327–339. [Google Scholar]
- 25.Sheng H.W., Liu H.Z., Cheng Y.Q., Wen J., Lee P.L., Luo W.K., Shastri S.D., Ma E. Polyamorphism in a metallic glass. Nat. Mater. 2007;6:192–197. doi: 10.1038/nmat1839. [DOI] [PubMed] [Google Scholar]
- 26.Liu S., Wang L., Ge J., Wu Z., Ke Y., Li Q., Sun B., Feng T., Wu Y., Wang J.T., et al. Deformation-enhanced hierarchical multiscale structure heterogeneity in a Pd-Si bulk metallic glass. Acta Mater. 2020;200:42–55. doi: 10.1016/j.actamat.2020.08.077. [DOI] [Google Scholar]
- 27.Di Y.X., Wang Y.M. Local Structure-Property Correlation of Fe-Based Amorphous Alloys: Based on Minor Alloying Research. Acta Metall. Sin. 2020;56:1558–1568. [Google Scholar]
- 28.Li K.H., Ge J.C., Liu S.N., Fu S., Yin Z.X., Zhang W.T., Chen G.X., Wei S.C., Ji H., Feng T., et al. In situ scattering study of multiscale structural evolution during liquid–liquid phase transition in Mg-based metallic glasses. Rare Met. 2021;40:3107–3116. doi: 10.1007/s12598-021-01767-4. [DOI] [Google Scholar]
- 29.Hu X., Ge J., Liu S., Fu S., Wu Z., Feng T., Liu D., Wang X., Lan S. Combustion mechanism of Fe-Nb-B-Y amorphous alloys with anomalous exothermic phenomena. Acta Metall. Sin. 2021;57:542–552. [Google Scholar]
- 30.Shin S.S., Kim H.K., Lee J.C., Park I.M. Effect of Sub-T_g Annealing on the Corrosion Resistance of the Cu–Zr Amorphous Alloys. Acta Metall. Sin. Engl. Lett. 2018;31:273–280. doi: 10.1007/s40195-017-0637-9. [DOI] [Google Scholar]
- 31.Liang D., Liu X., Zhou Y., Wei Y., Wei X., Xu G., Shen J. Effects of Annealing Below Glass Transition Temperature on the Wettability and Corrosion Performance of Fe-based Amorphous Coatings. Acta Metall. Sin. Engl. Lett. 2022;35:243–253. doi: 10.1007/s40195-021-01228-y. [DOI] [Google Scholar]
- 32.Ren Y., Babaie E., Bhaduri S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018;118:1–8. doi: 10.1016/j.porgcoat.2018.01.014. [DOI] [Google Scholar]
- 33.Eivani A.R., Mehdizade M., Chabok S., Zhou J. Applying multi-pass friction stir processing to refine the microstructure and enhance the strength, ductility and corrosion resistance of WE43 magnesium alloy. J. Mater. Res. Technol. 2021;12:1946–1957. doi: 10.1016/j.jmrt.2021.03.021. [DOI] [Google Scholar]
- 34.Qiu X., Thompson J.W., Billinge S.J. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 2004;37:678. doi: 10.1107/S0021889804011744. [DOI] [Google Scholar]
- 35.Dong W., Ge J., Ke Y., Ying H., Zhu L., He H., Liu S., Lu C., Lan S., Almer J., et al. In-situ observation of an unusual phase transformation pathway with Guinier-Preston zone-like precipitates in Zr-based bulk metallic glasses. J. Alloys Compd. 2020;819:153049. doi: 10.1016/j.jallcom.2019.153049. [DOI] [Google Scholar]
- 36.Lan S., Guo C., Zhou W., Ren Y., Almer J., Pei C., Hahn H., Liu C.T., Feng T., Wang X.L., et al. Engineering medium-range order and polyamorphism in a nanostructured amorphous alloy. Commun. Phys. 2019;2:117. doi: 10.1038/s42005-019-0222-9. [DOI] [Google Scholar]
- 37.Chen S.Q., Hui K.Z., Dong L.Z., Li Z., Zhang Q.H., Gu L., Zhao W., Lan S., Ke Y., Shao Y., et al. Excellent long-term reactivity of inhomogeneous nanoscale Fe-based metallic glass in wastewater purification. Sci. China Mater. 2020;63:453–466. doi: 10.1007/s40843-019-1205-5. [DOI] [Google Scholar]
- 38.Yang L., Miller M.K., Wang X.L., Liu C.T., Stoica A.D., Ma D., Almer J., Shi D. Metallic Glasses: Nanoscale Solute Partitioning in Bulk Metallic Glasses. Adv. Mater. 2009;21:305–308. doi: 10.1002/adma.200801183. [DOI] [Google Scholar]
- 39.Kelton K.F. A new model for nucleation in bulk metallic glasses. Philos. Mag. Lett. 1998;77:337–344. doi: 10.1080/095008398178318. [DOI] [Google Scholar]
- 40.Duparc O.H. The Preston of the Guinier-Preston Zones. Guinier. Metall. Mater. Trans. 2010;41:1873. doi: 10.1007/s11661-010-0320-5. [DOI] [Google Scholar]
- 41.Oden M., Rogström L., Knutsson A., Terner M.R., Hedström P., Almer J., Ilavsky J. In situ small-angle x-ray scattering study of nanostructure evolution during decomposition of arc evaporated TiAlN coatings. Appl. Phys. Lett. 2009;94:053114. doi: 10.1063/1.3078283. [DOI] [Google Scholar]
- 42.Ilavsky J., Jemian P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009;42:347–353. doi: 10.1107/S0021889809002222. [DOI] [Google Scholar]
- 43.Gleiter H. Nanoglasses: A New Kind of Noncrystalline Material and the Way to an Age of New Technologies? Small. 2016;12:2225–2233. doi: 10.1002/smll.201500899. [DOI] [PubMed] [Google Scholar]
- 44.Debye P., Anderson H.R., Jr., Brumberger H. Scattering by an Inhomogeneous Solid. II. The Correlation Function and Its Application. J. Appl. Phys. 1957;28:679–683. doi: 10.1063/1.1722830. [DOI] [Google Scholar]
- 45.Fu S., Liu S., Ge J., Wang J., Ying H., Wu S., Yan M., Zhu L., Ke Y., Luan J., et al. In situ study on medium-range order evolution during the polyamorphous phase transition in a Pd-Ni-P nanostructured glass. J. Mater. Sci. Technol. 2022;125:145–156. doi: 10.1016/j.jmst.2022.01.038. [DOI] [Google Scholar]
- 46.Lan S., Blodgett M., Kelton K.F., Ma J.L., Fan J., Wang X.L. Structural crossover in a supercooled metallic liquid and the link to a liquid-to-liquid phase transition. Appl. Phys. Lett. 2016;108:211907. doi: 10.1063/1.4952724. [DOI] [Google Scholar]
- 47.Wessels V., Gangopadhyay A.K., Sahu K.K., Hyers R.W., Canepari S.M., Rogers J.R., Kramer M.J., Goldman A.I., Robinson D., Lee J.W., et al. Rapid chemical and topological ordering in supercooled liquid Cu46Zr54. Phys. Rev. B. 2011;83:094116. doi: 10.1103/PhysRevB.83.094116. [DOI] [Google Scholar]
- 48.Dong W., Wu Z., Ge J., Liu S., Lan S., Gilbert E.P., Ren Y., Ma D., Wang X.L. In situ neutron scattering studies of a liquid–liquid phase transition in the supercooled liquid of a Zr–Cu–Al–Ag glass-forming alloy. Appl. Phys. Lett. 2021;118:191901. doi: 10.1063/5.0048486. [DOI] [Google Scholar]
- 49.Lan S., Zhu L., Wu Z., Gu L., Zhang Q., Kong H., Liu J., Song R., Liu S., Sha G., et al. A medium-range structure motif linking amorphous and crystalline states. Nat. Mater. 2021;20:1347. doi: 10.1038/s41563-021-01011-5. [DOI] [PubMed] [Google Scholar]
- 50.Li Z., Yu Q., Zhang C., Liu Y., Liang J., Wang D., Zhou F. Synergistic effect of hydrophobic film and porous MAO membrane containing alkynol inhibitor for enhanced corrosion resistance of magnesium alloy. Surf. Coat. Technol. 2019;357:515–525. doi: 10.1016/j.surfcoat.2018.10.054. [DOI] [Google Scholar]
- 51.Lopes D.R., Silva C.L., Soares R.B., Pereira P.H.R., Oliveira A.C., Figueiredo R.B., Langdon T.G., Lins V.F. Cytotoxicity and Corrosion Behavior of Magnesium and Magnesium Alloys in Hank’s Solution after Processing by High-Pressure Torsion. Adv. Eng. Mater. 2019;21:1900391. doi: 10.1002/adem.201900391. [DOI] [Google Scholar]
- 52.Kaur H., Tian R., Roy A., McCrystall M., Horvath D.V., Lozano Onrubia G., Smith R., Ruether M., Griffin A., Backes C., et al. Correction to Production of Quasi-2D Platelets of Nonlayered Iron Pyrite (FeS2) by Liquid-Phase Exfoliation for High Performance Battery Electrodes. ACS Nano. 2021;14:13418–13432. doi: 10.1021/acsnano.0c05292. [DOI] [PubMed] [Google Scholar]
- 53.Vladescu A., Pruna V., Kulesza S., Braic V., Titorencu I., Bramowicz M., Gozdziejewska A., Parau A., Cotrut C.M., Pana I., et al. Influence of Ti, Zr or Nb carbide adhesion layers on the adhesion, corrosion resistance and cell proliferation of titania doped hydroxyapatite to the Ti6Al4V alloy substrate, utilizable for orthopaedic implants. Ceram. Int. 2018;45:1710–1723. doi: 10.1016/j.ceramint.2018.10.053. [DOI] [Google Scholar]
- 54.Zhu Y.B., Shen Z.C., Zhnag C.F., Huang D.P. Electrochemical Data Manual. Hunan Science & Technology Press; Changsha, China: 1985. [Google Scholar]
- 55.Zhang Y., Yan C., Wang F., Li W. Electrochemical behavior of anodized Mg alloy AZ91D in chloride containing aqueous solution. Corros. Sci. 2005;47:2816–2831. doi: 10.1016/j.corsci.2005.01.010. [DOI] [Google Scholar]
- 56.McFeely F.R., Kowalczyk S.P., Ley L., Shirley D.A. Multiplet splittings of the 4s and 5s core levels in the rare earth metals. Phys. Lett. A. 1974;49:301–302. doi: 10.1016/0375-9601(74)90821-4. [DOI] [Google Scholar]
- 57.Soma M., Seyama H. Surface compositions of powdered rock samples studied by X-ray photoelectron spectroscopy. Chem. Geol. 1986;55:97–103. doi: 10.1016/0009-2541(86)90130-0. [DOI] [Google Scholar]
- 58.Song G. Corrosion of Magnesium Alloys. Woodhead Publishing Ltd.; Sawston, UK: 2011. [Google Scholar]
- 59.Zhang Y., Fang Y., Feng S., Ma D., Zhou Y., Wang L.M. Trapping mechanism of metastable β-Ga disclosed by its lattice stability optimization and nucleation behavior exploration. Calphad. 2022;79:102475. doi: 10.1016/j.calphad.2022.102475. [DOI] [Google Scholar]
- 60.Li Q. Formation of bulk ferromagnetic nanostructured Fe 40 Ni 40 P 14 B 6 alloys by metastable liquid spinodal decomposition. Sci. China Ser. E Technol. Sci. 2009;52:1919–1922. doi: 10.1007/s11431-009-0108-2. [DOI] [Google Scholar]
- 61.Feng S., Qi L., Wang L., Pan S., Ma M., Zhang X., Li G., Liu R. Atomic structure of shear bands in Cu64Zr36 metallic glasses studied by molecular dynamics simulations. Acta Mater. 2015;95:236–243. doi: 10.1016/j.actamat.2015.05.047. [DOI] [Google Scholar]
- 62.Xiang S., Li Q., Zuo M., Cao D., Li H., Sun Y. Influence of the preparation cooling rate on crystallization kinetics of Fe 74 Mo 6 P 13 C 7 amorphous alloys. J. Non-Cryst. Solids. 2017;475:116–120. doi: 10.1016/j.jnoncrysol.2017.09.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.