Abstract
We have previously reported the construction of an isogenic mutant defective in expression of OmpB1, the TbpB homologue, in Moraxella catarrhalis 7169. In this report, we have extended these studies by constructing and characterizing two new isogenic mutants in this clinical isolate. One mutant is defective in expression of TbpA, and the other mutant is defective in expression of both TbpA and TbpB. These isogenic mutants were confirmed by using PCR analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and sequencing. In vitro growth studies, comparing all three mutants, demonstrated that the tbpA mutant and the tbpAB mutant were severely limited in their ability to grow with human holotransferrin as the sole source of iron. In contrast, the ompB1 (tbpB) mutant was capable of utilizing iron from human transferrin, although not to the extent of the parental strain. While affinity chromatography with human holotransferrin showed that each Tbp was capable of binding independently to transferrin, solid-phase transferrin binding studies using whole cells demonstrated that the tbpA mutant exhibited binding characteristics similar to those seen with the wild-type bacteria. However, the ompB1 (tbpB) mutant exhibited a diminished capacity for binding transferrin, and no binding was detected with the double mutant. These data suggest that the M. catarrhalis TbpA is necessary for the acquisition of iron from transferrin. In contrast, TbpB is not essential but may serve as a facilitory protein that functions to optimize this process. Together these mutants are essential to provide a more thorough understanding of iron acquisition mechanisms utilized by M. catarrhalis.
Iron is a critical element essential for the growth of many organisms, and there is little free iron available in the human body (5, 11, 14, 19, 29). The sequestering of iron provides an important line of defense against pathogenic microorganisms. Most of the iron present in the body is intracellular in the ferrous form as hemoglobin, heme, ferritin, or hemosiderin. In addition, extracellular iron is complexed to the high-affinity iron-binding glycoproteins transferrin (Tf) and lactoferrin (5, 14, 19, 29). Tf-bound iron is found predominantly in serum, while it is thought that lactoferrin is the primary carrier of iron on most mucosal surfaces. To survive in and colonize the human host, many mammalian pathogens have evolved numerous mechanisms that allow them to obtain iron in vivo. Both direct iron-binding compounds and specific receptors for iron carrier proteins have been described for many of these bacteria (11, 13, 19, 28).
Previous studies have demonstrated that Moraxella catarrhalis expresses proteins that bind human Tf and lactoferrin (Lf) similar to those receptors described for the pathogenic members of the families Neisseriaceae and Pasteurellaceae (10, 15, 26, 27, 31). The Tf-binding proteins (Tbps) were identified by using affinity chromatography with human holotransferrin coupled to a Sepharose matrix. These proteins are iron repressible and have molecular sizes in the range of 80 to 84 kDa (TbpB) and 115 to 120 kDa (TbpA) (31). Our previous studies identified OmpB1 as an M. catarrhalis Tf receptor (6, 20). This outer membrane protein appears to be the homologue of TbpB, which was described by Myers et al. (for clarity, OmpB1 will be referred to as TbpB in this report) (22). tbpA and tbpB have been cloned and sequenced from various strains of M. catarrhalis, and the proteins encoded by these genes have been compared (22). Similar to the tbpB of other bacteria, the M. catarrhalis tbpB exhibits marked heterogeneity between strains while tbpA appears to be highly conserved.
Although the Tbps of M. catarrhalis specifically bind human Tf, similar to the Tbps expressed by other human mucosal pathogens, there are some differences observed in the arrangement of the genes that code for these proteins. Investigators have previously reported that the M. catarrhalis tbpA gene precedes tbpB, unlike other known tbp operons, and there is a third open reading frame (termed orf3) located between these genes (22). In addition, the identification of three potential promoter sequences located upstream of each open reading frame suggests that the genes are independently transcribed. Despite these observations, the lack of specific mutants, similar to the tbp mutants constructed for the pathogenic Neisseriaceae, have precluded studies designed to evaluate the relationship between the regulation and expression of the M. catarrhalis genes which code for the Tbps. In this report, we constructed isogenic mutants in M. catarrhalis 7169 that are deficient in the expression of either TbpB (previously described), TbpA, or both of these receptors. Comparative studies were performed to characterize each mutant for the ability to bind Tf at the bacterial cell surface, to utilize iron from Tf for in vitro growth, and for the affinity isolation of each receptor from a holotransferrin matrix. These Tbp mutants provide important tools that will be critical in studies designed to provide a more thorough understanding of the Tf system utilized by M. catarrhalis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
M. catarrhalis 7169, a clinical isolate obtained by typanocentesis from a child with otitis media, was kindly provided by Howard Faden (Children's Hospital, Buffalo, N.Y.). The Chelex-treated defined growth medium (CDM) and the culturing conditions for iron depletion of M. catarrhalis were described previously (7, 21, 30). M. catarrhalis strains were cultured at 35.5°C on GC plates (without supplements) in a 5% CO2 atmosphere. Antibiotic-resistant isogenic mutants were cultured in the presence of 20 (7169b12 and 7169tbpA35) or 40 (7169tbpAB-1) μg of kanamycin per ml. M. catarrhalis strains were made competent for electroporation by a previously described method (17). Escherichia coli XLI-Blue was cultured at 37°C on Luria-Bertani agar plates or in Luria-Bertani broth in the presence of the appropriate antibiotic (100 μg of ampicillin per ml or 20 μg of kanamycin per ml).
DNA manipulations.
The restriction endonucleases, T4 ligase, DNA polymerase 1, and molecular biology reagents were purchased from New England Biolabs, Inc., Beverly, Mass., or Promega, Madison, Wis. The QIAprep spin kit was used to isolate plasmid DNA, and electrophoretically separated DNA fragments were gel purified by using the QIAquick PCR purification kit (Qiagen, Santa Clarita, Calif.). M. catarrhalis genomic DNA was isolated by standard methods, as previously described (20, 24). Chromosomal DNA was subjected to PCR amplification for 30 cycles with an annealing temperature of 55°C, using oligonucleotide primers based on sequences within tbpA (primer 61, 5′-TGGTAAGGTGGTCAAAACTG-3′ [sense]; primer 62, 5′TGTCATTAGTCCAACCCGC-3′ [antisense]) or ompB1 (primer 42, 5′-CGTCTTATTAACCGCTTGTGG-3′ [sense]; primer 43, 5′-TCGACCGCTTTCGTGTTC-3′ [antisense]), which flank the predicted insertional region of the aphA-3 cassette.
Insertional mutagenesis.
The construction of M. catarrhalis 7169b12 (tbpB::kan) has been previously described (20). To insertionally inactivate the tbpA gene, PCR primers (described above) were designed to an internal region of the M. catarrhalis Q8 tbpA sequence obtained from the GenBank database. A 1,772-bp fragment of the M. catarrhalis 7169 tbpA was amplified and cloned into the pGEM T-tailed vector (Promega Corp., Madison, Wis.). A portion of the amplified gene product was sequenced to confirm that the tbpA from M. catarrhalis 7169 had been isolated. The aphA-3 nonpolar cassette of pUC18K was subcloned into a 233-bp internal deletion of the partial tbpA gene such that an ATG codon was placed 3′ of the resistance gene and in frame with the remainder of the tbpA coding region. The disrupted tbpA::kan construct was amplified by PCR with primers 61 and 62 and electroporated into M. catarrhalis 7169, by using a previously described method (16). The double mutant was constructed essentially as described for the ompB1 mutant 7169b12, except that the pdelB1 construct was electroporated into competent M. catarrhalis 7169tbpA35 (20). Strain 7169 Tbp derivatives were selected based on resistance to kanamycin at 20 μg/ml (7169tbpA35 and 7169b12) or 40 μg/ml (7169tbpAB-1) and screened by loss of reactivity to a TbpB-specific antibody, monoclonal antibody (MAb) 11C6 (7169b12 and 7169tbpAB-1). DNA sequences of the selected clones were obtained via automated DNA sequencing (CAMBI Nucleic Acid Sequencing Facility, State University of New York at Buffalo, Buffalo) and analyzed with MacVector 6.0 and the Wisconsin Sequence Analysis Packages (Genetics Computer Group, Madison, Wis.).
Tf binding assay.
Biotinylated human Tf was purchased from Sigma Chemical Co. (St. Louis, Mo.). Bacteria were cultured in CDM broth under iron limitation, harvested, and resuspended in phosphate-buffered saline to an optical density at 600 nm of 0.3. One hundred microliters of successive 1:1 bacterial dilutions was immobilized onto nitrocellulose. After drying for 15 min at 37°C, the filters were blocked with 3% bovine serum albumin in phosphate-buffered saline and then incubated with biotinylated human Tf (250 ng/ml) for 1 h at room temperature. Horseradish peroxidase-conjugated streptavidin (Zymed Laboratories, Inc., San Francisco, Calif.) was used as the secondary reagent for colorimetric detection of Tf binding.
Protein analysis.
Zwittergent-solubilized outer membrane protein preparations (OMPs) were made and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot assays were performed as previously described (6). Covalent linkage of human holotransferrin (>98% iron saturated) to CNBr-activated Sepharose 4B was carried out in accordance with the manufacturer's instructions (Sigma). Total membrane fractions of M. catarrhalis were prepared by resuspending iron-depleted bacterial cultures in 10 mM HEPES (pH 7.4) containing 1 μM phenylmethylsulfonyl fluoride. The cell suspension was frozen at −70°C, thawed in a room-temperature water bath, and then incubated for 30 min at 37°C with 5 μg of lysozyme/mg (wet weight) of bacteria. The cells were then held on ice and sonicated with four 20-s bursts with a microtip set to the microtip limit. The total membrane preparation was obtained by centrifuging the crude lysate at 100,000 × g for 1 h at 4°C. The resulting pellets were resuspended in 50 mM Tris-HCl–1 M NaCl (pH 8.0) containing 10 mM EDTA and 0.75% Sarkosyl, and any insoluble cellular debris was removed by centrifugation at 10,000 × g for 10 min. Affinity isolation experiments were performed essentially as described previously (31), with several modifications. Solubilized iron-deficient membranes were incubated with 1.5 to 2.0 ml of Tf-Sepharose slurry for 45 min at room temperature or overnight at 4°C. Tf-binding fractions, eluted with 4 M guanidine-HCl, were dialyzed overnight against 50 mM Tris (pH 8.0). Column fractions were concentrated with Centricon concentrators (Amicon, Inc., Beverly, Mass.) and analyzed by SDS-PAGE.
RESULTS
Construction of the isogenic mutants.
We have previously reported the use of the kanamycin-resistant determinant from pUC18K to construct a tbpB (ompB1) isogenic mutant in M. catarrhalis 7169 (7169b12) (20). A tbpA isogenic mutant and a tbpAB double mutant were constructed by insertional mutagenesis essentially as described for construction of the tbpB mutant (20). Linearized DNA, containing the aphA-3 gene inserted within an internal deletion of tbpA or tbpB, was electroporated into competent M. catarrhalis. Mutated clones were selected by the ability to grow on kanamycin, and tbpB mutants were screened for the loss of reactivity to MAb 11C6, a TbpB-specific MAb (20). Clones 7169tbpA35, a TbpA− mutant, and 7169tbpAB-1, a TbpA− TbpB− double mutant, were selected for further analysis.
Verification of the isogenic mutants.
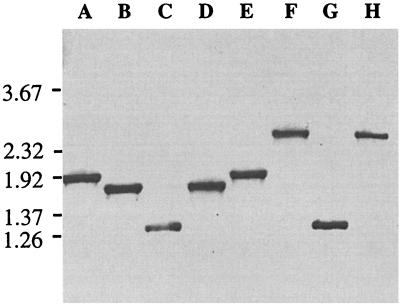
Chromosomal DNA was isolated from the three isogenic mutants and the parental strain. PCR amplifications, using primers flanking the predicted sites of mutagenesis cassette insertion, were used to verify the identity of the isogenic mutants. Agarose gel analysis of these fragments confirmed that the kanamycin resistance cassette was inserted into the tbpA and/or tbpB gene of M. catarrhalis 7169 (Fig. 1). Primers 42 and 43, recognizing sites within tbpB, amplified a 1.93-kb PCR product from the parental strain 7169 (Fig. 1, lane A) as well as from 7169tbpA35, the TbpA mutant (lane E). A 1.28-kb PCR product was obtained from both 7169b12, the TbpB mutant (lane C), and 7169tbpAB-1, the double mutant (lane G). This result is consistent with the insertion of the 850-bp aphA-3 kanamycin-resistant determinant into a 1.5-kb internal deletion of tbpB. Primers 61 and 62, specific for sites within tbpA, amplified a 1.77-kb PCR product from strain 7169 (lane B) and from 7169b12 (lane D). In contrast, a 2.4-kb fragment was obtained from PCR amplification of 7169tbpA35 (lane F) and 7169tbpAB-1 (lane H), which is consistent with the insertion of the 850-bp kanamycin cassette into a 233-bp internal deletion of tbpA. Subsequent DNA sequence analysis of these PCR products confirmed that the aphA-3 nonpolar mutagenesis cassette had been inserted into tbpB or tbpA in the predicted frame (data not shown).
FIG. 1.
PCR analysis of mutants. Chromosomal DNA from strain 7169 and the isogenic mutants 7169b12 (TbpA+ TbpB−), 7169tbpA35 (TbpA− TbpB+), and 7169tbpAB-1 (TbpA− TbpB−) was subjected to PCR analysis, and the products were separated by electrophoresis. Products in lanes A, C, E, and G were obtained with tbpB-specific primers 42 and 43 (described in Materials and Methods). Lanes B, D, F, and H were obtained with primers 61 and 63, which are specific for sites within tbpA. Both primer sets flank the predicted site of the aphA-3 cassette insertion within the mutants. Lanes: A and B, strain 7169; C and D, 7169b12; E and F, 7169tbpA35; G and H, 7169tbpAB-1. A negative image of an ethidium bromide-stained agarose gel is shown with molecular size standards, in kilobases, to the left.
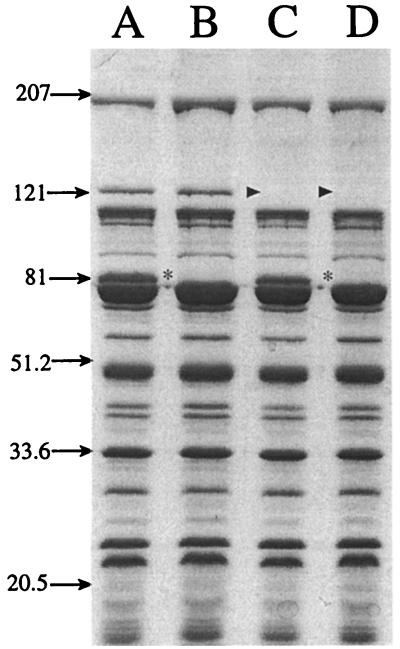
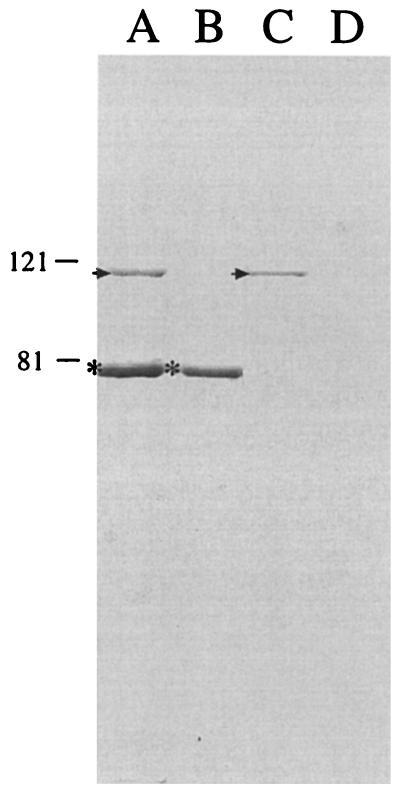
To confirm the loss of TbpA and/or TbpB expression, OMPs from wild-type strain 7169 and the isogenic mutants 7169b12, 7169tbpA35, and 7169tbpAB-1 were prepared from bacteria grown under iron limitation and analyzed by SDS-PAGE. Figure 2 illustrates that TbpB is expressed by both the wild-type strain (lane A) and the tbpA isogenic mutant 7169tbpA35 (lane C) but is absent from both the tbpB mutant 7169b12 and the double mutant 7169tbpAB-1. Both the wild-type strain (lane A) and the tbpB isogenic mutant 7169b12 (lane B) express TbpA, but this protein is not expressed by the tbpA isogenic mutant 7169tbpA35 or by the double mutant 7169tbpAB-1. Further comparison of the SDS-PAGE OMP profiles did not reveal any difference between these four strains. Western blot analysis, using MAb 11C6, also confirmed the loss of TbpB expression from 7169b12 and 7169tbpAB-1 (data not shown).
FIG. 2.
An SDS-PAGE gel, stained with Coomassie blue, showing the OMP profile of strain 7169 (lane A) and the isogenic mutants 7169b12 (lane B), 7169tbpA35 (lane C), and 7169tbpAB-1 (lane D) grown under iron-depleted conditions. Asterisks (lanes B and D) denote the loss of TbpB expression, and the lack of TbpA expression is indicated by arrowheads (lanes C and D). Molecular size standards are shown in kilodaltons.
Characterization of the isogenic mutants. (i) Tf binding assays.
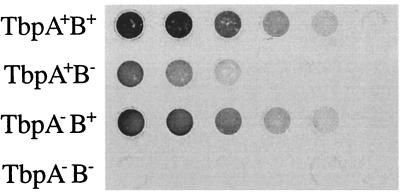
The three mutants were evaluated for their ability to bind Tf, in comparison to that of the wild type, in a whole-cell solid-phase binding assay. Aliquots of cells grown under iron limitation were immobilized onto nitrocellulose and incubated with biotinylated human Tf (Fig. 3). There was a slight observable difference in Tf binding between the parental 7169 and the TbpA mutant 7169tbpA35. In contrast, the Tf binding activity of the TbpB mutant (7169b12) was noticeably reduced but not eliminated. The inactivation of both TbpA and TbpB (7169tbpAB-1) completely eliminated Tf binding activity.
FIG. 3.
Solid-phase Tf binding assay. Equal amounts of iron-depleted whole cells from the parental and mutant Tbp derivatives were dotted onto nitrocellulose. The blots were incubated with biotinylated human holotransferrin and developed by using horseradish peroxidase-streptavidin to detect Tf binding. TbpA+B+, 7169; TbpA+B−, 7169b12; TbpA−B+, 7169tbpA35; TbpA−B−, 7169tbpAB-1.
Comparative growth studies.
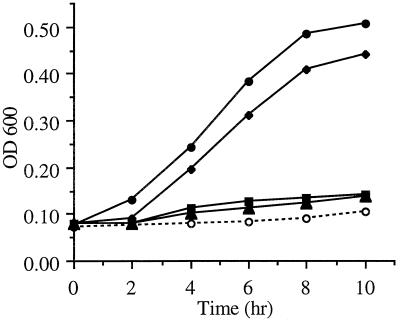
The ability of these mutants to utilize human holotransferrin as a sole source of iron for in vitro growth was analyzed. The growth rates of M. catarrhalis 7169 and the Tbp derivatives 7169b12 (TbpA+ TbpB−), 7169tbpA35 (TbpA− TbpB+), and 7169tbpAB-1 (TbpA− TbpB−) were evaluated by culturing the bacteria in iron-depleted CDM supplemented with 5.0 μM human holotransferrin (Fig. 4). In comparison to the parental strain 7169, mutants 7169tbpA35 and 7169tbpAB-1 were unable to grow with human Tf as the sole source of iron. The TbpB mutant 7169b12 exhibited growth in the presence of Tf, albeit less efficiently than the wild-type strain 7169. All of the strains grew equally well with iron-loaded lactoferrin as a sole source of iron (data not shown). These results were consistently reproducible, confirming that the observed growth differences with Tf are due to specific mutations in TbpA or TbpB.
FIG. 4.
Growth of parental strain 7169 and the isogenic mutants with Tf as the sole source of iron. Iron-depleted overnight cultures of each strain were used to inoculate fresh CDM 0 or CDM containing 5 μM human holotransferrin. All cultures contained 10 μM desferoxamine. The optical density at 600 nm (OD600) of each culture was measured in 2-h intervals for 10 h. Symbols: ●, 7169-Tf; ⧫, 7169b12-Tf; ■, 7169tbpA35-Tf; ▴, 7169tbpAB1-Tf; ○, 7169-0.
Column chromatography.
Affinity chromatography of Tbps from the isogenic mutants was performed by utilizing a human holotransferrin-Sepharose matrix. Figure 5 is a composite SDS-PAGE gel analyzing the Tf-binding fraction eluted from each column. These data demonstrate that both TbpB and TbpA were isolated from wild-type 7169 total membranes. TbpB could be isolated from 7169tbpA35, and only TbpA was isolated from 7169b12. As expected, TbpA was not isolated from 7169tbpA35 nor was TbpB purified from 7169b12, and no Tbps were purified from the double mutant 7169tbpAB-1. These results were consistent with those predicted from the PCR analysis of the isogenic mutants, confirming the insertional inactivation of the tbpB and/or tbpA genes.
FIG. 5.
Human holotransferrin affinity purification. The Tf-binding fractions from solubilized total membranes of 7169 (lane A), 7169tbpA35 (lane B), 7169b12 (lane C), and 7169tbpAB-1 (lane D) were analyzed by SDS-PAGE and Coomassie blue staining. Asterisks are placed next to affinity-purified TbpB, and arrows denote affinity-purified TbpA. Molecular size standards, in kilodaltons, are indicated on the left.
DISCUSSION
Previous reports have identified and isolated both Tf-binding proteins from various strains of M. catarrhalis (22, 23, 31). In addition, the genes that code for each of these proteins have been cloned and sequenced (20, 22). These data, together with our earlier studies, strongly suggest that these proteins are Tf receptors (6, 20). However, these observations were based primarily on sequence homology, binding studies, and affinity chromatography. Although we have previously reported data describing the construction of an M. catarrhalis mutant defective in expression of TbpB, these studies did not analyze the growth characteristics of this mutant (20). The construction of the isogenic mutants deficient in either or both of the Tbps in M. catarrhalis 7169 and the subsequent characterization of these constructs provide conclusive proof that these proteins function as Tf receptors. In addition, these isogenic mutants provide the means to more thoroughly characterize the relationship between TbpA and TbpB as they function in the M. catarrhalis system of iron acquisition from human Tf.
The Tf binding studies using whole bacterial cells showed that the M. catarrhalis TbpA mutant (7169tbpA35) had slightly less ability to bind iron-loaded human Tf than the wild-type strain. However, the TbpB mutant (7169b12) exhibited noticeably less binding activity. This is in direct contrast with the results of the in vitro growth studies. When human holotransferrin was used as the sole source of iron, growth of 7169tbpA35 was severely limited. However, 7169b12 exhibited appreciable growth under the same conditions, although not at a rate equivalent to that of the wild type. In addition, affinity purification assays using the isogenic mutants demonstrated that both TbpB and TbpA were capable of high-affinity, independent Tf binding. Based on these results, we hypothesize that the M. catarrhalis TbpA is capable of binding Tf, removing the iron, and facilitating the transport of this element into the cell. In contrast, while the M. catarrhalis TbpB retains the ability to bind holotransferrin, the growth studies suggest that the Tf-bound iron cannot be utilized in the absence of TbpA.
It is interesting to note that the M. catarrhalis Tbps have a unique genomic arrangement, with tbpA preceding tbpB, with a third open reading frame termed orf3, in the intergenic region (22). However, the significance of this observation is currently unknown. It was reported that the cloned orf3 encodes an approximately 58-kDa recombinant protein, but the authors of that report were unable to produce antibodies to this protein and no further studies were performed (22). The function of this protein is currently unknown, and the studies described in this report do not provide any additional information related to orf3.
The mechanisms of iron acquisition from Tf have been well characterized for the pathogenic Neisseriaceae. In these organisms, TbpA has been described as a transmembrane protein with extracellular exposed loops that may act as a gated pore allowing for the transport of iron into the bacterial cell (8, 25). TbpB has been characterized as a surface-exposed lipoprotein that is anchored to the bacterial membrane via the lipid moiety (4, 15). A meningococcal mutant defective in TbpB expression was incapable of utilizing Tf for in vitro growth (18). In addition, TbpB could not be affinity isolated from a TbpA mutant, suggesting that both proteins were necessary for avid binding of the Tf molecule. In contrast, an N. gonorrhoeae mutant defective in expression of TbpB was still capable of utilizing Tf for in vitro growth, indicating that TbpA could perform all the necessary functions required to obtain iron from Tf (4). Affinity purification assays, using Sarkosyl-solubilized membrane proteins, demonstrated that both Tbps were capable of independently binding to Tf. These results are in contrast to those reported for the Tbps of the pathogenic Neisseriaceae (12, 18).
The isogenic mutants presented in this report provide unique tools that will be valuable in many aspects of future research involving colonization and infection with M. catarrhalis. First, these mutants can be used to define the regulation of expression and the relationship between each Tf receptor since these proteins function to obtain iron from human Tf. Second, recent studies have demonstrated that the Tf receptor of N. gonorrhoeae is an important virulence factor since mutants defective in expression of this receptor were avirulent in an experimental human infection model (9). Although there is currently no animal model which mimics M. catarrhalis infection in humans, our mutants will provide the most effective means of evaluating TbpA and TbpB as virulence factors when an effective model becomes available.
Finally, recent in vitro studies have shown that TbpB is capable of stimulating antibodies that elicit complement-mediated bactericidal activity against M. catarrhalis (20, 22). These data are consistent with previous reports of antibodies directed to the TbpB of the pathogenic Neisseriaceae (1–3). Although the focus of this report does not encompass vaccine studies, our isogenic mutants will also be useful in future studies designed to evaluate the effect of human antibodies to TbpA and TbpB, providing important data as to the vaccine potential of these proteins versus M. catarrhalis infection.
ACKNOWLEDGMENT
We thank Thomas Russo for helpful suggestions in the preparation of the manuscript.
REFERENCES
- 1.Ala'Aldeen D A. Transferrin receptors of Neisseria meningitidis: promising candidates for a broadly cross-protective vaccine. J Med Microbiol. 1996;44:237–243. doi: 10.1099/00222615-44-4-237. [DOI] [PubMed] [Google Scholar]
- 2.Ala'Aldeen D A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 3.Ala'Aldeen D A, Stevenson P, Griffiths E, Gorringe A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2900. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullen J J. The significance of iron in infection. Rev Infect Dis. 1981;3:1127–1138. doi: 10.1093/clinids/3.6.1127. [DOI] [PubMed] [Google Scholar]
- 6.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campagnari A A, Shanks K L, Dyer D W. Growth of Moraxella catarrhalis with human transferrin and lactoferrin: expression of iron-repressible proteins without siderophore production. Infect Immun. 1994;62:4909–4914. doi: 10.1128/iai.62.11.4909-4914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 10.Cornelissen C N, Sparling P F. Identification of receptor-mediated transferrin-iron uptake mechanism in Neisseria gonorrhoeae. Methods Enzymol. 1994;235:356–363. doi: 10.1016/0076-6879(94)35154-6. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 12.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Criado M T, Pintor M, Ferreiros C M. Iron uptake by Neisseria meningitidis. Res Microbiol. 1993;144:77–82. doi: 10.1016/0923-2508(93)90217-p. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein R A, Sciortino C V, McIntosh M A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983;5(Suppl.4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- 15.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 16.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helminen M E, Maciver I, Paris M, Latimer J L, Lumbley S L, Cope L D, McCracken G H, Jr, Hansen E J. A mutation affecting expression of a major outer membrane protein of Moraxella catarrhalis alters serum resistance and survival in vivo. J Infect Dis. 1993;168:1194–1201. doi: 10.1093/infdis/168.5.1194. [DOI] [PubMed] [Google Scholar]
- 18.Irwin S W, Averil N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 19.Litwin C M, Calderwood S B. Role of iron regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luke N R, Russo T A, Luther N, Campagnari A A. Use of an isogenic mutant constructed in Moraxella catarrhalis to identify a protective epitope of outer membrane protein B1 defined by monoclonal antibody 11C6. Infect Immun. 1999;67:681–687. doi: 10.1128/iai.67.2.681-687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morse S A, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 22.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmore S M. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Retzer M D, Yu R, Zhang Y, Gonzalez G C, Schryvers A B. Discrimination between apo and iron-loaded forms of transferrin by transferrin binding protein B and its N-terminal subfragment. Microb Pathog. 1998;25:175–180. doi: 10.1006/mpat.1998.0226. [DOI] [PubMed] [Google Scholar]
- 24.Russo T A, Guenther J E, Wenderoth S, Frannk M M. Generation of isogenic K54 capsule-deficient Escherichia coli strains through TnphoA-mediated gene disruption. Mol Microbiol. 1993;9:357–364. doi: 10.1111/j.1365-2958.1993.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 25.Rutz J M, Liu J, Lyons J A, Goranson J, Armstrong S K, McIntosh M A, Feix J B, Klebba P E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 26.Schryvers A B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol Microbiol. 1988;2:467–472. doi: 10.1111/j.1365-2958.1988.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 27.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 28.Schryvers A B, Morris L J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988;56:1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West S E, Sparling P F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985;47:388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu R H, Schryvers A B. The interaction between human transferrin and transferrin binding protein 2 from Moraxella (Branhamella) catarrhalis differs from that of other human pathogens. Microb Pathog. 1993;15:433–445. doi: 10.1006/mpat.1993.1092. [DOI] [PubMed] [Google Scholar]