Abstract
Recurrent vulvovaginal candidiasis, caused by Candida albicans, is a significant problem in women of childbearing age. Although cell-mediated immunity (CMI) due to T cells and cytokines is the predominant host defense mechanism against C. albicans at mucosal tissue sites, host defense mechanisms against C. albicans at the vaginal mucosa are poorly understood. Based on an estrogen-dependent murine model of vaginal candidiasis, our data suggest that systemic CMI is ineffective against C. albicans vaginal infections. Thus, we have postulated that local immune mechanisms are critical for protection against infection. In the present study, the kinetic production of chemokines normally associated with the chemotaxis of T cells, macrophages (RANTES, MIP-1α, MCP-1), and polymorphonuclear neutrophils (MIP-2) was examined following intravaginal inoculation of C. albicans in estrogen-treated or untreated mice. Results showed significant increases in MCP-1 protein and mRNA in vaginal tissue of infected mice as early as 2 and 4 days postinoculation, respectively, that continued through a 21-day observation period, irrespective of estrogen status. No significant changes were observed with RANTES, MIP-1α, or MIP-2, although relatively high constitutive levels of RANTES mRNA and MIP-2 protein were observed. Furthermore, intravaginal immunoneutralization of MCP-1 with anti-MCP-1 antibodies resulted in a significant increase in vaginal fungal burden early during infection, suggesting that MCP-1 plays some role in reducing the fungal burden during vaginal infection. However, the lack of changes in leukocyte profiles in vaginal lavage fluids collected from infected versus uninfected mice suggests that MCP-1 functions to control vaginal C. albicans titers in a manner independent of cellular chemotactic activity.
Vulvovaginal candidiasis (VVC) is an opportunistic mucosal infection that affects three out of four women at least once during the reproductive years (25, 38). The causative agent in 85 to 90% of symptomatic vaginal fungal infections is Candida albicans (8, 29). While most women experience infrequent episodes of VVC, approximately 5% of otherwise healthy women have recurrent VVC (RVVC), defined by three or more episodes per year (17). In women with RVVC, antifungal therapy is very effective but does not prevent recurrence. While several exogenous factors, such as pregnancy, oral contraceptives, uncontrolled diabetes mellitus, and antibiotics, can precipitate acute episodes of VVC, the underlying factors that contribute to RVVC are largely unknown.
Because cell-mediated immunity (CMI) due to T cells, specifically Th1-type CD4+ T cells, represents the dominant host response against mucosal C. albicans infections (2, 4, 6), it had been hypothesized that RVVC occurs in women as a result of some deficiency in Candida-specific CMI. However, current evidence suggests that RVVC occurs in the presence of normal Th1-type CMI in the peripheral circulation (12). In studies using an estrogen-dependent animal model of vaginitis, it has been shown as well that Candida-specific Th1-type CMI generated in the peripheral circulation as a result of infection or immunization does not provide protection against vaginal candidiasis (14, 15). Partial protection, however, against a secondary vaginal challenge with C. albicans can be achieved following the spontaneous resolution of a low-grade primary vaginal infection in the absence of estrogen, irrespective of the presence or absence of Th1-type Candida-specific systemic CMI (11, 16). In light of this, more recent efforts have focused on determining the importance of vagina-associated CMI. Accordingly, our first series of studies showed vaginal T cells in naive mice to be phenotypically distinct from those in the periphery (18, 43). More recently, we found that these vaginal T cells did not change in percentage or composition during a primary or secondary vaginal infection (10). The lack of local cellular changes also indicated a lack of infiltration of systemically derived T cells to the vagina, supporting insufficient protection by systemic CMI against infection. These results suggested that if local T cells were playing a role in protection against the infection, they were doing so without appreciable change in composition or percentage. While activation markers on these local T cells are currently being evaluated to more fully understand the role of local CMI during a vaginal infection, it is equally important to examine the role of soluble immune modulators (i.e., cytokines and chemokines) in the vaginal tissue.
It is widely accepted that chemokines induce both the chemotaxis and chemokinesis of leukocytes during the host response to injury, allergens, or invading microorganisms (1, 40). They are produced and released by a wide variety of leukocytic (i.e., macrophages, polymorphonuclear neutrophils [PMNs], T cells, mast cells, and NK cells) and nonleukocytic (i.e., fibroblasts, keratinocytes, epithelial cells, endothelial cells, and smooth muscle) cell types (1, 40). The release of chemokines at sites of inflammation and infection is believed to be critical for the attachment and subsequent migration of leukocytes through the vascular epithelium and into the tissues. Members of the chemokine superfamily share homologous sequences and are subdivided into four subfamilies (CXC, CC, C, and CX3C) based on the structural positions of cysteines near the amino terminus of the protein. The CXC family (i.e., MIP-2, IP-10, ENA-78, and MIG) is involved in the recruitment of mainly PMNs, while the CC family (i.e., MCP-1, RANTES, MIP-1α, and eotaxin) is chemotactic for monocytes, T cells, eosinophils, and NK cells (28). The two remaining branches include the C family (lymphotactin), which is chemotactic for T cells (27), and the CX3X family (fractalkine), which has been found to be chemotactic for T cells, monocytes, and PMNs (32).
The purpose of this study was to examine the local production of chemokines during an experimental C. albicans vaginal infection. Chemokine production associated with the chemotaxis of PMNs (MIP-2) (39), macrophages, and T cells (MIP-1α, MCP-1, and RANTES) (5) was examined at the mRNA and protein levels in vaginal tissue and in the lumbar (draining) lymph nodes as a measure of the local and systemic responses, respectively.
MATERIALS AND METHODS
Mice.
Female CBA/J (H-2k) mice, 8 to 10 weeks of age, purchased from the National Cancer Institute (Frederick, Md.) were used throughout these studies. All animals were housed and handled in accordance with institutionally recommended guidelines.
Microorganism.
A laboratory-cultivated clinical isolate of C. albicans (3153A) was used throughout these studies. The yeast was grown to stationary phase in 1% phytone-peptone medium (Becton Dickinson, Cockeysville, Md.) supplemented with 0.1% glucose for 16 to 18 h at 25°C in a shaking water bath. The culture was then washed twice with phosphate-buffered saline (PBS) and quantified by a hemocytometer for use in infection studies.
Vaginal infection.
The vaginal infection was initiated by inoculating mice intravaginally with 5 × 104 stationary-phase blastoconidia in 20 μl of PBS as previously described (13, 14). Prior to inoculation (72 h), mice were injected subcutaneously with 0.02 mg of estradiol valerate (Sigma Chemical Co., St. Louis, Mo.) in 0.1 ml of sesame oil. Estrogen treatments were continued at weekly intervals thereafter. Mice inoculated in the absence of estrogen received sesame oil alone. Estrogen-treated control mice were treated with estrogen as described above and given PBS intravaginally. On days 2, 4, 7, 14, and 21 postinoculation, animals were sacrificed and a vaginal lavage was performed with 100 μl of PBS (11, 15). To quantify the vaginal fungal burden, the lavage fluid was serially diluted 1:10 and plated on Sabouraud dextrose agar plates supplemented with gentamicin (Sigma). After incubation at 35°C for 48 h, CFU were enumerated. A portion of the recovered lavage fluid (10 μl) was viewed microscopically on a wet-mount slide at ×400 magnification in an attempt to visualize the fungus in the infectious hyphal form. A hyphal score ranging from 0 (none) to ++++ (severe) was given to lavage fluid from each mouse as a representation of the degree of infection: +, sparse hyphae; ++, small amounts of hyphae present in several fields; +++, large amounts of hyphae in several fields; and ++++, masses of hyphae in most fields.
Tissue processing.
Following lavage, the vagina and lumbar lymph nodes were excised from each mouse and processed for enzyme-linked immunosorbent assay (ELISA) or reverse transcription (RT)-PCR. Total RNA was isolated from vaginal tissues by using the Ultraspec RNA isolation system (Biotecx, Houston, Tex.) as described previously (10). Briefly, vaginae were homogenized on ice with the Ultraspec solution (phenol, guanidine salts), incubated on ice for 5 min, and then subjected to chloroform extraction. To the resulting upper phase containing total RNA, an equal volume of isopropanol was added and the mixture was incubated on ice for 10 min. The total RNA was pelleted, washed twice with 75% ethanol, and quantified spectrophotometrically (DU Series 500; Beckman Instruments, Inc., Fullerton, Calif.) by using the Warburg-Christian equation.
To measure chemokine protein production of MCP-1, RANTES, MIP-1α, and MIP-2, tissues were snap frozen in liquid nitrogen and homogenized in 1 ml of anti-protease homogenization buffer, containing protease inhibitor tablets (Boehringer GmbH, Mannheim, Germany), PBS, and 0.05% Triton X-100 (nonionic) as described previously (21). The homogenized tissues were then centrifuged at 3,000 × g for 5 min to eliminate cellular debris, with the supernatants aliquoted and stored at −70°C until use.
Semiquantitative RT-PCR.
In preparation for PCR, 1 μg of total RNA was reverse transcribed into cDNA using an RT system in accordance with the manufacturer's (Promega, Madison, Wis.) instructions and stored at −20°C until use. From synthesized cDNA, a primer set for RANTES (22), MCP-1 (7), MIP-2 (42), MIP-1α (24), and cyclophilin (housekeeping gene) was used for semiquantitative PCR analysis (Table 1). All primer sets were synthesized by the Louisiana State University Medical Center Core Laboratories, New Orleans. Dilution analysis was employed to optimize the concentration of cDNA required in each PCR, ensuring that kinetic interpretation would not be hindered by the saturation of primers with cDNA. These studies showed that 3 μl of cDNA was optimal for all of the reactions in this study. PCRs were performed with a 50-μl reaction mixture containing a final concentration of 10× reaction buffer (50 mM KCl, 10 mM Tris-HCl, 1.5 mM MgCl2, 0.1% Triton X-100), 50 μM deoxynucleoside triphosphates, 400 ng of each primer, and 2.5 U of Taq thermostable polymerase (Promega). PCR programs included denaturation at 94°C for 45 s, annealing at 57°C for 45 s, and extension at 72°C for 1.25 min with 35 cycles in an automated thermocycler (Ericomp Corp., San Diego, Calif.).
TABLE 1.
Sequences of oligonucleotide primers used for PCRa
| Primer | Product size (bp) | Sequencesb |
|---|---|---|
| MIP-1α | 260 | F: 5′-gcc ctt gct gtt ctt ctc tgt |
| R: 5′-ggc aat cag ttc cag gtc agt | ||
| MIP-2 | 272 | F: 5′-ccg ctg ttg tgg cca gtg aac tgc g |
| R: 5′-tta gcc ttg cct ttg ttc agt at | ||
| MCP-1 | 582 | F: 5′-gga aaa atg gat cca cac ctt gc |
| R: 5′-tct ctt cct cca cca cca tgc ag | ||
| RANTES | 271 | F: 5′-gaa gat ctc tgc agc tgc cct |
| R: 5′-gct cat ctc caa ata gtt ga | ||
| Cyclophilin | 278 | F: 5′-gac agc aga aaa ctt tcg tgc |
| R: 5′-tcc agc cac tca gtc ttg g |
Annealing temperatures: Mip-1α, 58°C; Mip-2, 56°C; MCP-1, 65°C; RANTES, 58°C; cyclophilin, 57°C.
F, forward; R, reverse.
All PCR products were analyzed by electrophoresis on 2% agarose gels and visualized by ethidium bromide staining using the Bio-Rad Video Capture Gel Documentation System 1000 (Bio-Rad, Richmond, Calif.). The gels were analyzed by using ImageQuant software (Molecular Dynamics Corp., Sunnyvale, Calif.), with the observed pixel intensities for each gel band normalized to cyclophilin by using the following equation: normalized ratio = (pixel intensity of chemokine product)/(pixel intensity of cyclophilin product).
Protein assay.
Protein levels of each sample were determined by using a protein assay kit (Pierce, Rockford, Ill.) in accordance with the manufacturer's instructions. Briefly, dilutions of vaginal and lymph node homogenate supernatants were added in 10-μl volumes to a 96-well polystyrene flat-bottom microtiter plate (Costar, Corning, N.Y.). A standard curve was generated from serial dilutions of bovine serum albumin. Concentrations of each sample were quantified at 562 nm by using a Dynatech MR 5000 plate reader (DYNEX, Chantilly, Va.), and the protein concentration was expressed in milligrams per milliliter based on the standard curve.
ELISA.
Samples were assayed for MCP-1 by using the Mouse MCP-1 OptEIA ELISA system (PharMingen, San Diego, Calif.) in accordance with manufacturer's instructions, with baculovirus-expressed mouse MCP-1 recombinant protein used as the standard. For this, anti-mouse MCP-1 monoclonal antibodies were bound to an enzyme immunoassay-radioimmunoassay microtiter plate (Costar) and incubated at room temperature overnight. The plates were washed (PBS–0.05% Tween 20) and blocked for 2 h at room temperature by using PBS with 10% fetal bovine serum. To duplicate wells, 50 μl of each sample or standard was added and the plates were incubated at room temperature for 2 h. Following this, the plates were washed and anti-mouse MCP-1 monoclonal antibodies conjugated to horseradish peroxidase were added and the plates were incubated at room temperature for 1 h. Thereafter, Sigma Fast o-phenylenediamine dihydrochloride peroxidase substrate was added after washing and the plates were incubated for 30 min in the dark. The plates were analyzed at 450 nm by using an automated microplate reader (Ceres 900; Bio-Tek, Winooski, Vt.) with the quantitation of MCP-1 expressed in picograms per milliliter.
Assays to quantify RANTES, MIP-1α, and MIP-2 protein concentrations in the samples were performed by using an established double-ligand ELISA (23). For this, recombinant murine RANTES, MIP-1α, and MIP-2 served as standards while appropriate biotinylated polyclonal rabbit anti-chemokine antibodies were used for detection. Streptavidin-peroxidase (Bio-Rad Laboratories) served as the enzyme, and o-phenylenediamine dihydrochloride served as the substrate. The plates were read on an automated ELISA plate reader at 492 nm, and chemokine concentrations were expressed in picograms per milliliter. Data for all chemokines were normalized to total protein and expressed as picograms of chemokine per milligram of protein.
Cellular staining.
To examine the lymphocytic cellular profiles from naive, estrogen-treated infected, untreated infected, and estrogen-treated uninfected mice, 30 μl of vaginal lavage fluid was collected on days 2, 4, 7, 14, and 21 postinoculation. The lavage fluid was diluted 1:5 in PBS and cytospun onto glass slides by using a Cytospin 2 Cytocentrifuge (Shandon, Pittsburgh, Pa.) at 3,000 × g for 5 min. The slides were fixed with methanol for 1 min and stained by using a Hema-3 staining kit (Biomedical Sciences, Swedesboro, N.J.) in accordance with the manufacturer's instructions. Slides were air dried and examined under bright-field microscopy at ×400 magnification. Macrophages, PMNs, lymphocytes, and other leukocytes were identified and counted in four separate fields. The number of each cell type identified in a field was expressed as a percentage of the total number of cells.
In vivo immunoneutralization of MCP-1.
To address the role of MCP-1 in the local immune response to vaginal candidiasis, MCP-1 was neutralized in vivo through the administration of anti-MCP-1 antibodies (23) prior to and throughout a vaginal infection with C. albicans. The specificity of this serum for murine MCP-1 was tested in vitro through the immunoneutralization of a recombinant MCP-1 as detected by ELISA. For this, anti-MCP-1 rabbit serum at a titer of 105 was added to various concentrations of MCP-1. An equal volume of normal rabbit serum was also tested as a control. Results showed that MCP-1 immunoactivity was effectively inhibited by the anti-MCP-1 serum but not by the normal rabbit serum.
In passive immunization experiments, two groups of 10 mice were estrogenized as described above and randomized to receive 0.25 ml intraperitoneally and two 0.05-ml intravaginal injections of anti-MCP-1 immune serum or the same volumes of normal rabbit serum 24 h prior to intravaginal inoculation with C. albicans under pseudoestrus conditions. Antibody treatments were continued every 3 days throughout the duration of the experiment. At days 4 and 10 postinoculation, five mice from each group were sacrificed. From these mice, lavage fluid was recovered and the vaginal fungal burden was quantified as described above. Vaginae and lumbar lymph nodes were also excised and homogenized for MCP-1 protein analysis. MCP-1 was quantified in these tissue homogenates to ensure in vivo neutralization by the anti-MCP-1 antibody-containing serum.
Statistical analysis.
The unpaired Student t test was used to analyze the data from all experiments. Significant differences were defined as a confidence level at which P was <0.05.
RESULTS
Basal chemokine levels in naive mice.
To determine basal levels of MCP-1, RANTES, MIP-1α, and MIP-2 in vaginal tissue and lumbar lymph nodes of naive mice, tissues were excised following sacrifice and processed for mRNA and/or protein analysis. As illustrated in Table 2, mRNA transcripts for each chemokine were detected in naive vaginal tissues, with abundant constitutive expression of RANTES relative to the other chemokines tested. With respect to protein, MIP-2 was found to be present in the vagina at levels higher than those of the other chemokines measured. Interestingly, while MIP-1α mRNA expression was observed in naive vaginal tissue, MIP-1α protein was undetectable. While RANTES and MCP-1 were detected in the lumbar lymph nodes, MIP-1α and MIP-2 were largely undetectable.
TABLE 2.
Chemokine levels in naive mice
| Chemokine | Mean ratio of vaginal mRNA to cyclophilin ± SEM | Mean protein concn (pg/mg of total protein) ± SEM
|
|
|---|---|---|---|
| Vaginal tissue | Lumbar lymph nodes | ||
| MCP-1 | 0.03 ± 0.02 | 42.9 ± 19.7 | 202.8 ± 52.6 |
| RANTES | 0.547 ± 0.07 | 46.5 ± 30.7 | 1,182.8 ± 275.6 |
| MIP-2 | 0.066 ± 0.03 | 695.4 ± 128.4 | NDa |
| MIP-1α | 0.117 ± 0.04 | ND | ND |
ND, not detected.
Chemokine production during vaginal candidiasis.
To assess chemokine production during experimental vaginitis, mice were either inoculated under a state of pseudoestrus, inoculated in the absence of estrogen treatment, or treated with estrogen and given PBS intravaginally. On days 2, 4, 7, 14, and 21 postinoculation, animals were sacrificed and vaginae were lavaged. Vaginae and draining lymph nodes were excised, processed, and analyzed for chemokine mRNA and protein. Consistent with observations made in previous studies (11, 13), mice infected under pseudoestrus conditions acquired a persistent (through day 21) vaginal infection with high organism titers (>1.65 × 104). In contrast, while non-estrogen-treated infected mice had an early fungal burden similar to that of estrogen-treated mice (days 2 to 7), the infection declined rapidly by day 14 (titers, <8.9 × 100) and was undetectable by day 21. Control mice given estrogen alone did not yield any detectable yeast throughout the 21-day period. Hyphal scores obtained from mice in experimental groups correlated with the severity or lack of infection.
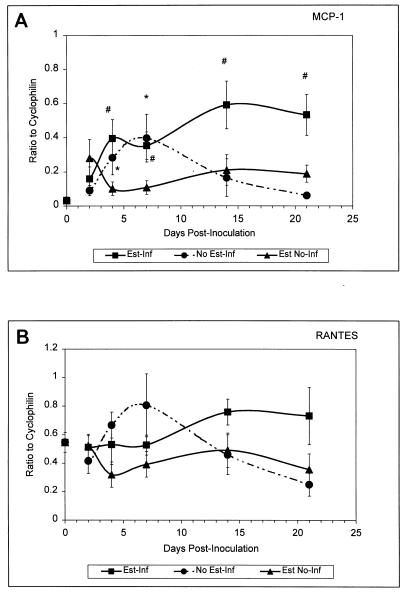
Semiquantitative RT-PCR results are shown in Fig. 1. Compared to expression in naive mice, estrogen treatment had no effect on mRNA expression for any chemokine. Compared to levels in estrogen-treated uninfected mice, significant increases in MCP-1 mRNA were observed in estrogen-treated infected mice at day 4 through day 21 postinoculation (P < 0.02). Compared to levels in naive mice, significant increases in MCP-1 mRNA were observed in untreated infected mice at days 4 (P < 0.04) and 7 (P < 0.027) postinoculation (Fig. 1A). While the level of RANTES mRNA in naive mice was higher than that of other chemokines, levels of RANTES were unaffected by infection and/or estrogen (Fig. 1B). Basal levels of both MIP-2 and MIP-1α mRNAs were relatively low and showed no significant change over time in response to infection and/or estrogen (data not shown).
FIG. 1.
Vaginal chemokine mRNA expression in mice during experimental vaginal candidiasis. Mice were sacrificed at 2, 4, 7, 14, and 21 days postinoculation. At sacrifice, vaginas were excised and total RNA was extracted and reverse transcribed into cDNA. The mRNA transcript expression measured for MCP-1 (A) and RANTES (B) are shown and expressed as the ratio to that of the housekeeping gene cyclophilin. Shown are the cumulative results of two separate experiments with three mice per group ± the standard error of the mean. Asterisks represent significant differences (P < 0.05) between (i) untreated infected or estrogen-treated uninfected mice and (ii) naive mice. The pound signs represent significant differences between estrogen-treated infected mice and estrogen-treated uninfected mice. Abbreviations: Est-Inf, estrogen-treated infected; Est No-Inf, estrogen-treated uninfected; No Est-Inf, untreated infected. The point at day zero represents the value for naive mice.
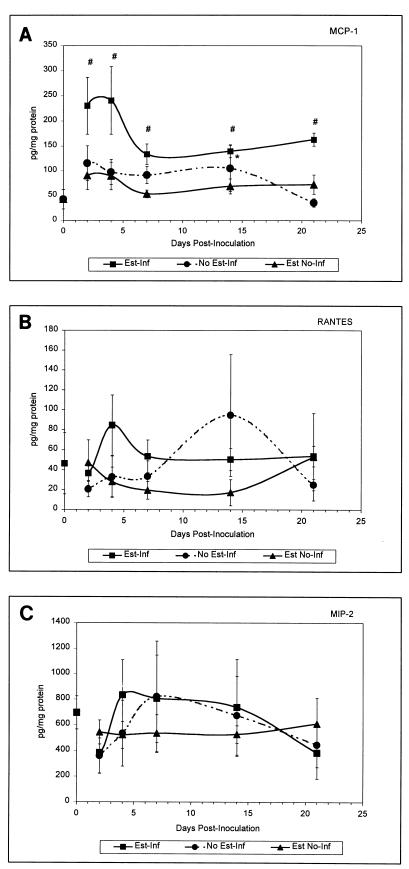
The concentrations of MCP-1, RANTES, and MIP-2 in whole vaginal tissue are shown in Fig. 2. Estrogen treatment had no effect on any of the chemokines evaluated. Compared to estrogen-treated uninfected mice, significant increases in MCP-1 in vaginal tissue were observed in estrogen-treated infected mice at days 2, 4, 7, 14, and 21 postinoculation (P < 0.039). Compared to naive mice, a significant increase in MCP-1 was observed in untreated infected mice at day 7 postinoculation (P < 0.04) (Fig. 2A). RANTES levels were low in both infected and estrogen-treated uninfected mice and did not significantly change in response to infection and/or estrogen (Fig. 2B). MIP-2 concentrations were relatively high compared to those of the other chemokines tested but did not significantly change in response to infection or estrogen treatment (Fig. 2C). MIP-1α was primarily undetectable, irrespective of infection or pseudoestrus (data not shown).
FIG. 2.
Vaginal production of chemokines in mice during experimental vaginal candidiasis. Mice were sacrificed at 2, 4, 7, 14, and 21 days postinoculation. At sacrifice, vaginas were excised, homogenized in a lysis buffer, and quantified for chemokine concentrations by ELISA. Tissue homogenates analyzed for MCP-1 (A), RANTES (B), and MIP-2 (C) are shown, with values expressed as picograms of chemokine per milligram of total protein. Shown are the cumulative results of two separate experiments with three mice per group ± the standard error of the mean. Asterisks represent significant differences (P < 0.05) between (i) untreated infected or estrogen-treated uninfected mice and (ii) naive mice. Pound signs represent significant differences between estrogen-treated infected mice and estrogen-treated uninfected mice. Abbreviations: Est-Inf, estrogen-treated infected; Est No-Inf, estrogen-treated uninfected; No Est-Inf, untreated infected. The point at day zero represents the value for naive mice.
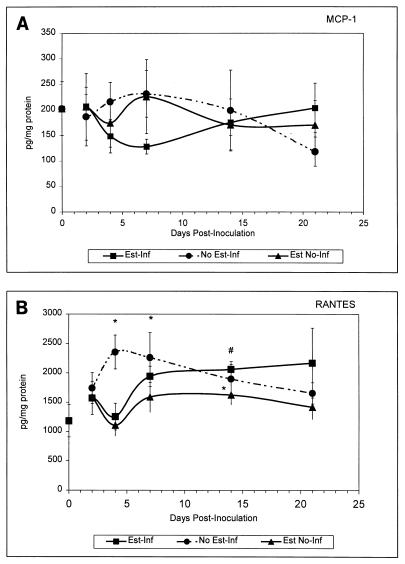
Concentrations of chemokines in the draining lumbar lymph nodes are shown in Fig. 3. As in vaginal tissue, estrogen had no effect on chemokine concentrations in the lumbar lymph nodes. Levels of MCP-1 did not significantly change in response to infection (Fig. 3A). In contrast, RANTES was significantly elevated in untreated-infected mice on days 4, 7, and 14 postinoculation (P < 0.04) and in estrogen-treated infected mice on day 14 postinoculation (P < 0.03) compared to naive mice or estrogen-treated uninfected mice, respectively (Fig. 3B). MIP-2 and MIP-1α concentrations in the draining lymph nodes were low in all experimental groups and undetectable in most cases (data not shown).
FIG. 3.
Production of chemokines in lumbar lymph nodes of mice during experimental vaginal candidiasis. Mice were sacrificed at 2, 4, 7, 14, and 21 days postinoculation. At sacrifice, lumbar lymph nodes were excised, homogenized in lysis buffer, and quantified for chemokines by ELISA. Tissue homogenates analyzed for MCP-1 (A) and RANTES (B) protein levels are shown and expressed as picograms of chemokine per milligram of total protein. Shown are the cumulative results of two separate experiments with three mice per group ± the standard error of the mean. Asterisks represent significant differences (P < 0.05) between (i) untreated infected or estrogen-treated uninfected mice and (ii) naive mice. Pound signs represent significant differences between estrogen-treated infected mice and estrogen-treated uninfected mice. Abbreviations: Est-Inf, estrogen-treated infected; Est No-Inf, estrogen-treated uninfected; No Est-Inf, untreated infected. The point at day zero represents the value for naive mice.
Leukocyte profile in vaginal lavage fluid during vaginal candidiasis.
To examine the cellular profile of leukocytes in the vaginal lumen as a result of estrogen or infection, mice were infected as previously described under pseudoestrus and nonestrus conditions. Control mice were given PBS in the presence or absence of pseudoestrus. On days 2, 4, 7, 14, and 21 postinoculation, lymphocytes, macrophages, and PMNs were identified in vaginal lavage fluid. As summarized in Table 3, macrophages, PMNs, lymphocytes, and various other leukocytes (eosinophils, basophils, mast cells, etc.) were positively identified in lavage fluids recovered from mice under all conditions. Table 3 shows representative data from day 7 postinoculation. While macrophages, lymphocytes, and various other leukocytes were present, PMNs constituted the predominant cell type (∼85%). However, the percentage of PMNs was not significantly altered in response to vaginal infection or pseudoestrus alone. Similarly, the percentages of macrophages, lymphocytes, and other leukocytes were not different from those in the control groups. Additionally, absolute numbers of leukocytes were not different between groups of animals (data not shown). Similar patterns were observed at days 2, 4, 14, and 21 postinoculation (data not shown).
TABLE 3.
Murine vaginal cellular staining profile
| Groupb | Mean % of cells in vaginal lavage fluidsa ± SEM
|
|||
|---|---|---|---|---|
| Macrophages | PMN | Lymphocytes | Otherc | |
| Naive | 2.85 ± 1.4 | 86.3 ± 9.0 | 1.67 ± 1.6 | 9.15 ± 6.0 |
| No Est-Inf | 6.8 ± 4.6 | 73.5 ± 20.9 | 7.0 ± 7.0 | 12.7 ± 9.6 |
| Est No-Inf | 3.1 ± 1.3 | 93.1 ± 1.4 | 3.9 ± 3.1 | 2.0 ± 1.4 |
| Est-Inf | 8.3 ± 5.8 | 85.9 ± 9.8 | 2.0 ± 2.1 | 3.8 ± 2.1 |
Vaginal lavage fluids were collected from day 7 postinoculation, cytospun onto slides, and differentially stained with hemotoxyln and eosin. The data are for a minimum of four mice per group.
Abbreviations: No Est-Inf, untreated infected; Est No-Inf, estrogen treated not infected; Est-Inf, estrogen treated infected.
Basophils, eosinophils, mast cells, etc.
Effects of immunoneutralization of MCP-1 on experimental vaginitis.
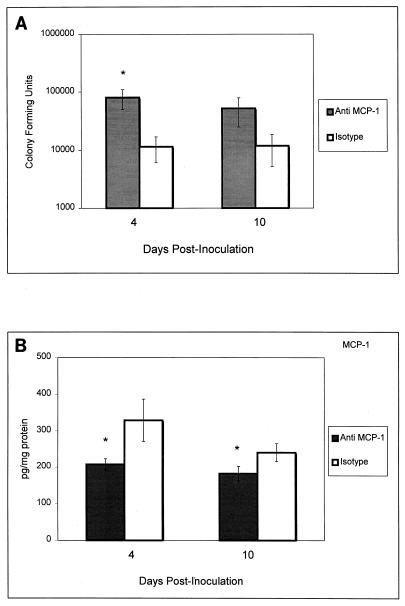
Since MCP-1 was the only chemokine significantly elevated during the vaginal infection, we investigated whether this chemokine has a role in the host response to infection. For this, MCP-1 was immunoneutralized in vivo by using rabbit serum containing anti-MCP-1 antibodies. Mice were treated with estrogen and randomized to receive either intravaginal and intraperitoneal injections of anti-MCP-1 immune serum or the same volumes of normal rabbit serum 24 h prior to intravaginal inoculation with C. albicans and every 3 days thereafter. On days 4 and 10 postinoculation, the vaginal fungal burden was monitored and vaginae were excised for MCP-1 quantitation. The results illustrated in Fig. 4A show that compared to that of control mice, the vaginal fungal burden was significantly higher in anti-MCP-1 antibody-treated mice at day 4 postinoculation (P < 0.027). On day 10, vaginal C. albicans titers were eightfold higher in anti-MCP-1-antibody-treated mice although statistical significance was not achieved. The results in Fig. 4B demonstrate that the immunoreactivity of MCP-1 was significantly reduced in the vaginal tissue of infected mice at both day 4 (P < 0.035) and day 10 (P < 0.046) postinoculation.
FIG. 4.
Effects of MCP-1 on experimental vaginal candidiasis. In passive-immunization experiments, two groups of mice were estrogenized and randomized to receive intravaginal and intraperitoneal injections of anti-MCP-1 immune serum or the same volumes of normal rabbit serum 24 h prior to intravaginal inoculation. Treatments continued every 3 days throughout the duration of the experiment. At days 4 and 10 postinoculation, the vaginal fungal burden (A) was evaluated and MCP-1 was quantified in vaginal tissue homogenates by ELISA (B). Asterisks represent significant differences (P < 0.05) between anti-MCP-1 serum-treated mice and those that received normal serum. Shown are the cumulative results of two separate experiments with five mice per group ± the standard error of the mean.
DISCUSSION
The results of this study present the first evidence of chemokine production during experimental vaginal candidiasis. Chemokines associated with the chemotaxis and activation of macrophages, PMNs, and T lymphocytes were examined at both the mRNA and protein levels. MIP-2, MCP-1, RANTES, and MIP-1α mRNA and protein were detected in whole vaginal tissue from naive mice, establishing the basal levels from which those in infected mice could be evaluated. Noteworthy were the high constitutive amounts of RANTES mRNA and MIP-2 in the vagina, as well as RANTES in the lumbar lymph nodes, relative to those of the other chemokines examined.
Semiquantitative RT-PCR for MCP-1, RANTES, MIP-2, and MIP-1α during infection showed that MCP-1 was the only chemokine for which mRNA and protein were significantly increased in the vaginal tissue of infected mice. In fact, the production of MCP-1 was dependent on the presence of the organism; vaginal MCP-1 production in mice infected in the absence of estrogen declined to basal levels as the infection resolved, while those in estrogen-treated infected mice remained elevated. Estrogen had no influence on these in vivo changes, in contrast to a previous report showing the in vitro reduction of lipopolysaccharide-stimulated macrophage MCP-1 mRNA expression in the presence of estrogen (19). Perhaps estrogen affects MCP-1 expression in vitro differently than in vivo. Interestingly, MCP-1 protein was significantly increased prior to observed increases in mRNA. This may have been due to rapid utilization and/or a high turnover of MCP-1 mRNA early in the infection. The exact source of MCP-1 remains unclear, although there are a number of potential sources, including fibroblasts, macrophages, and epithelial and endothelial cells (20, 28), all of which are present in murine vaginal tissue (31, 33). In contrast to vaginal MCP-1 production, concentrations in the draining lumbar lymph nodes remained unchanged relative to basal levels. Thus, our data suggest that MCP-1 has a role in the local vaginal mucosal response to C. albicans but not within the draining lumbar lymph nodes, where Candida-specific T cells are located. Interestingly, in a recent clinical study, vaginal lavage fluids obtained from women with RVVC were found to have lower levels of MCP-1 compared to control women (unpublished observations). These clinical data further support the importance of MCP-1 production during vaginal candidiasis.
As anticipated from the lack of mRNA expression, MIP-1α was often undetectable in vaginal tissue. In contrast, however, the level of MIP-2 was constitutively high relative to those of the other chemokines in the virtual absence of detectable mRNA. The inability to measure changes in MIP-2 mRNA expression may have been due to either the instability of the mRNA, a long half-life of the protein, or weak affinity of the oligonucleotide primers used for MIP-2 cDNA. The latter is less likely, since concanavalin A-stimulated mouse spleen cells yielded strong MIP-2 amplification products (data not shown). Another possibility is that MIP-2 concentrations were from intracellular stores, as tissue homogenates represent both intracellular and extracellular sources of protein. The vaginal presence of MIP-2 is consistent with a report by Sonoda et al. showing that MIP-2 is required for PMN recruitment during the menstrual cycle of the mouse (every 4 days) during the metestrus-1 phase (39). Indeed PMNs are often observed in vaginal lavage fluid from infected and uninfected mice irrespective of estrogen status (Table 3). This suggests that PMNs are regulated by MIP-2 without the influence of estrogen and not in response to infection. Support for this comes from two recent studies showing the ineffectiveness of PMNs in reducing C. albicans titers during vaginal infection, despite their vaginal presence during infection (3, 10).
The RANTES level was relatively low in the vagina in the presence of estrogen and/or infection, despite high constitutive levels of mRNA. Perhaps the efficiency of translation of RANTES is low in vaginal tissue. Regardless, the low concentration of RANTES in the vagina is consistent with the lack of changes in the percentage or composition of vaginal T cells during infection (10), although the activation status of these T cells has not been evaluated. In contrast, RANTES levels were significantly increased in the draining lumbar lymph nodes during infection. Although cellular processes associated with antigen presentation of C. albicans from vaginal tissue to the draining lumbar lymph nodes is poorly understood, our laboratory has demonstrated that these lymph nodes contain Candida-specific Th1-type T cells during vaginal candidiasis (13, 14).
In any pathogen-initiated host response, local or systemic, the action of cytokines and chemokines is integral to the resulting effector function against the pathogen. In response to C. albicans at mucosal (gastrointestinal) sites, Th1- and Th2-type responses are associated with resistance and susceptibility to infection, respectively (35, 36). In response to a C. albicans vaginal infection, a Th1-type response is induced in the draining lumbar lymph nodes (9). However, this response does not provide protection against infection (11, 15). This places into question the role of this induced response or the ability of activated cells to traffic into the vaginal mucosa. There have been extensive efforts to understand processes associated with T-cell involvement or recruitment in the vaginal mucosa during a C. albicans infection. To date, there is no evidence for infiltration of systemically derived T cells into the vaginal matrix or any changes in the percentage or composition of local T cells in response to infection (10). Concordant with this observation, differential staining of vaginal cells in lavages showed no significant differences in the numbers of macrophages, lymphocytes, or PMNs in response to the vaginal presence of C. albicans. PMNs, however, were clearly the predominant leukocytes present. This was true as well for lavage fluid from anti-MCP-1 antibody-treated mice (data not shown). The infrequent presence of lymphocytes and macrophages in response to infection despite the increase in MCP-1 may be explained by the inability of MCP-1 alone to adequately provide signals necessary for chemotaxis of lymphocytes and macrophages into the vaginal tissue. In addition, the proinflammatory cytokine tumor necrosis factor alpha has been shown to be a major cofactor for chemokine and adhesion molecule expression (34). Preliminary data have shown tumor necrosis factor alpha to be produced at low and often undetectable levels in vaginal tissue during infection (unpublished observations). Alternatively, the increase in MCP-1 may not translate into chemotactic activity. For a chemokine to function chemotactically, an interaction between the chemokine and its receptor(s) is required. If the receptor for MCP-1 (CCR2) is not upregulated or sufficiently present in vaginal tissue, MCP-1-dependent chemotaxis will not occur efficiently. Recently, it was reported that transforming growth factor beta (TGF-β) inhibits the expression of various chemokine receptors, including those for RANTES (CCR3 and CCR5), MIP-1α (CCR5), and MCP-1 (CCR2) (37). Indeed, preliminary data from our laboratory have shown TGF-β to be constitutively present in vaginal tissue (unpublished observations). Therefore, it is plausible that modulation of chemokine receptor expression by TGF-β is, in part, responsible for the lack of demonstrable MCP-1-dependent leukocyte trafficking into the vagina during a vaginal C. albicans infection.
Despite the apparent lack of a conventional function for MCP-1, in vivo immunoneutralization or reduction of MCP-1 in vaginal tissue resulted in significantly higher vaginal fungal titers early during an infection. Although the effects might have been greater if levels of MCP-1 were reduced further (25 to 35% in the present study), MCP-1 appears to play a role in limiting population numbers of C. albicans during vaginal infection. Conceivably, MCP-1 functions in a capacity independent of chemotaxis (i.e., direct effects on C. albicans or on other innate or acquired host defenses). Indeed, MCP-1 has been reported to increase interleukin-4 production (26), as well as to enhance lymphocyte (41) and macrophage (30) effector functions. A direct effect, however, is not likely, as preliminary studies confirmed that MCP-1 has no effect on the growth of C. albicans (unpublished observations). It is interesting to speculate, based on the pleiotropic nature of MCP-1, that early production of MCP-1 during infection is protective but that its continued presence may promote chronic infection. Thus, additional studies are required to better understand the role of MCP-1 during a C. albicans vaginal infection. Elucidation of these mechanisms should have an impact on immunotheraputic strategies to treat or prevent vaginal candidiasis.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant AI32556 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 2.Balish E, Filutowicz H, Oberley T D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990;58:107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black C A, Eyers F M, Russell A, Dunkley M L, Clancy R L, Beagley K W. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun. 1998;66:1273–1275. doi: 10.1128/iai.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantorna M T, Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990;58:1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr M W, Alon R, Springer T A. The c-c chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- 6.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 7.Daigle J, Carr D J J. Androstenediol antagonizes herpes simplex virus type 1-induced encephalitis through the augmentation of type I IFN production. J Immunol. 1998;160:3060–3066. [PubMed] [Google Scholar]
- 8.Ebert E C. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989;97:1372–1381. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- 9.Fidel P, Jr, Lynch M, Sobel J., Jr Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidel P L, Jr, Luo W, Steele C, Chabain J, Baker M, Wormley F L. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67:3135–3140. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel P L, Jr, Lynch M E, Conaway D H, Tait L, Sobel J D. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–553. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel P L, Jr, Lynch M E, Redondo-Lopez V, Sobel J D, Robinson R. Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis (RVVC) J Infect Dis. 1993;168:1458–1465. doi: 10.1093/infdis/168.6.1458. [DOI] [PubMed] [Google Scholar]
- 13.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–1995. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidel P L, Jr, Lynch M E, Sobel J D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–4207. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel P L, Jr, Lynch M E, Sobel J D. Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun. 1994;62:1032–1038. doi: 10.1128/iai.62.3.1032-1038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidel P L, Jr, Lynch M E, Sobel J D. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403–2408. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidel P L, Jr, Sobel J D. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev. 1996;9:335–348. doi: 10.1128/cmr.9.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidel P L, Jr, Wolf N A, KuKuruga M A. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793–3799. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazier-Jessen M R, Kovacs E J. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995;154:1838–1845. [PubMed] [Google Scholar]
- 20.Fuentes M E, Durham S K, Swerdel M R, Lewin A C, Barton D S, Megill J R, Bravo R, Lira S A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- 21.Gunn M D, Nelken N A, Liao X, Williams L T. Monocyte chemoattractant protein-1 is sufficient for the chemotaxis of monocytes and lymphocytes in transgenic mice but requires an additional stimulus for inflammatory activation. J Immunol. 1997;158:376–383. [PubMed] [Google Scholar]
- 22.Halford W P, Gebhardt B M, Carr D J J. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- 23.Hogaboam C M, Steinhauser M L, Schock H, Lukacs N W, Strieter R M, Standiford T, Kunkel S L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect Immun. 1998;66:650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huffnagle G B, Strieter R M, McNeil L K, McDonald R A, Burdick M D, Kunkel S L, Toews G B. Macrophage inflammatory protein-1α is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J Immunol. 1997;159:318–327. [PubMed] [Google Scholar]
- 25.Hurley R, De Louvois J. Candida vaginitis. Postgrad Med J. 1979;55:645–647. doi: 10.1136/pgmj.55.647.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karpus W J, Lukacs N W, Kennedy K J, Smith W S, Hurst S D, Barrett T A. Differential cc chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 27.Kelner G, Kennedy J, Bacon K, Kleyensteuber S, Largaespada D, Jenkins N, Copeland N, Bazan J, Moore K, Schall T. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–1399. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 28.Miller M, Krangel M. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 29.Morton R S, Rashid S. Candidal vaginitis: natural history, predisposing factors and prevention. Proc R Soc Med. 1977;70:3–12. doi: 10.1177/00359157770700S402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano Y, Kasahara T, Mukaida N, Ko Y, Nakano M, Matsushima K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect Immun. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandi D, Allison J P. Characterization of neutrophils and T lymphocytes associated with the murine vaginal epithelium. Reg Immunol. 1994;5:332–338. [PubMed] [Google Scholar]
- 32.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 33.Parr M B, Parr E L. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491–498. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 34.Robbins S, Kumar V, Cotran R S. Inflammation and repair. In: Schoen F, editor. Pathologic basis of disease. 5th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 53–67. [Google Scholar]
- 35.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Romani L, Puccetti P, Bistoni F. Th1 and Th2 cells in health and disease. In: Romagnani S, editor. Biological role of Th cell subsets in candidiasis. S. Farmington, Conn: Karger; 1996. pp. 114–137. [PubMed] [Google Scholar]
- 37.Sallusto F, Lanzavecchia A, Mackay C R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 38.Sobel J D. Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci. 1988;544:547–557. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 39.Sonoda Y, Mukaida N, Wang J, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine. J Immunol. 1998;160:6159–6165. [PubMed] [Google Scholar]
- 40.Strieter R M, Standiford T J, Huffnagle G B, Colletti L M, Lukacs N W, Kunkel S L. The role of chemokines in models of human disease. J Immunol. 1996;156:3583–3586. [PubMed] [Google Scholar]
- 41.Taub D, Ortaldo J, Turcovski-Corrales S, Key M, Longo D, Murphy W. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J Leukoc Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Tessier P A, Naccache P H, Clark-Lewis I, Glaude R P, Kuldeep S N, McColl S R. Chemokine networks in vivo. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- 43.Wormley, F. L., Jr., M. Scott, W. Luo, and P. L. Fidel, Jr. Evidence for a unique expression of CD4 on murine CD4+ vaginal cells. Submitted for publication. [DOI] [PMC free article] [PubMed]