Abstract
Cellular intoxification by exotoxin A of Pseudomonas aeruginosa (PEA) begins when PEA binds to its cellular receptor, the low-density lipoprotein receptor-related protein (LRP). This receptor is particularly abundant on macrophages. We hypothesize here that inducible changes in cellular expression levels of the LRP represent an important mechanism by which macrophage susceptibility to PEA is regulated by the host. We have examined the effect of lipopolysaccharide (LPS) on LRP expression and PEA sensitivity in the macrophage-like cell line HS-P. Using a [3H]leucine incorporation assay to measure inhibition of protein synthesis, we have demonstrated that HS-P macrophages are highly sensitive to PEA and that PEA toxicity is decreased by the LRP antagonist receptor-associated protein. LPS pretreatment decreases HS-P PEA sensitivity in a time- and dose-dependent manner. The dose of toxin required to inhibit protein synthesis by 50% increased from 11.3 ± 1.2 ng/ml in untreated cells to 25.7 ± 2.0 ng/ml in cells treated with LPS. In pulse experiments, involving brief exposure to saturating concentrations of PEA, [3H]leucine incorporation was more than threefold higher in cells pretreated with LPS than in untreated macrophages. These changes in HS-P PEA sensitivity following LPS treatment were consistently associated with a fivefold decrease in HS-P LRP mRNA expression as measured by Northern blot analysis and a three-and-a-half-fold decrease in HS-P LRP-specific ligand internalization as determined by activated α2-macroglobulin internalization studies. These data demonstrate for the first time that modulation of LRP levels by extracellular signaling molecules can alter cellular PEA sensitivity.
Pseudomonas exotoxin A (PEA) is an extracellular virulence factor produced by the opportunistic pathogen Pseudomonas aeruginosa. PEA irreversibly inhibits eukaryotic protein synthesis by ADP-ribosylating cytosolic elongation factor 2, leading to cell death (14). PEA is secreted as a 66-kDa proenzyme, which is extensively modified by target cells in order to generate and deliver the activated 37-kDa enzymatic fragment to the cytosol (28). The initial step in the intoxification process involves PEA binding to specific cell surface receptors followed by receptor-mediated endocytosis (23). A cell surface PEA binding protein was isolated from mouse fibroblasts (41) and liver cells (8) and subsequently identified as the low-density lipoprotein (LDL) receptor-related protein (LRP) (17). The isolation of LRP-deficient cells that are highly resistant to PEA confirmed the role of LRP as a cellular PEA receptor that mediates cytotoxicity (7, 45). Recently, Avramoglu et al. have restored PEA sensitivity in an LRP-deficient Chinese hamster ovary cell line by expressing functional chicken LRP (1).
The LRP is a large cell surface glycoprotein belonging to the LDL receptor gene family. The LRP is synthesized as a 600-kDa proreceptor, which is posttranslationally processed into 515- and 85-kDa chains that remain associated through noncovalent interactions (9). The heavy chain is expressed entirely on the cell surface and is capable of binding an extraordinary range of structurally and functionally diverse ligands, including lipoproteins, lipases, proteinase inhibitors, proteinase inhibitor complexes, α2-macroglobulin (α2M) growth factor complexes, pathogens, and PEA (3, 10, 11, 16–18, 20, 27, 29, 35, 39). The 39-kDa receptor-associated protein (RAP) copurifies with the LRP and acts as an antagonist for all ligands binding to the LRP, including PEA (17). It has been proposed that the LRP may play a role in such diverse physiological processes as tissue remodeling, cellular growth regulation, and the metabolism of lipoproteins and proteinases.
An important determinant of cellular PEA susceptibility is the constitutive level of functional LRP expressed on the cell surface of different target cells. Mucci et al. discovered that a positive correlation exists between LRP expression and PEA sensitivity; cells constitutively expressing low levels of the LRP are highly resistant to PEA (26). Due in part to their different LRP expression levels (25), mammalian tissues and cells display a wide range of sensitivities to PEA (13, 24, 30, 33). In particular, the observation that the liver is the most common site of damage due to systemic PEA (13, 30, 33) is largely attributable to high levels of cellular LRP expression in hepatocytes and Kupffer cells (6, 25).
Various signaling molecules such as hormones (5, 21), growth factors (4, 44), and matrix components (34) have been shown to alter LRP levels in diverse cell types. Macrophage LRP expression is subject to regulation by specific cytokines and bacterial products. We previously reported that lipopolysaccharide (LPS) and interferon-gamma markedly decreased LRP expression at the mRNA, antigen, and functional levels in the RAW 264.7 macrophage-like cell line and in bone marrow macrophages (12, 19). We hypothesize here that inducible changes in cellular expression of LRP represent an important mechanism by which cellular susceptibility to PEA is regulated by the host. This should be particularly true for decreases in LRP expression that are induced by signaling molecules expected to be present when the risk of PEA intoxification is high. In order to test this hypothesis, we have examined the effect of LPS on LRP expression and toxin susceptibility in cells of macrophage origin that are sensitive to PEA.
MATERIALS AND METHODS
Proteins and chemicals.
Pseudomonas exotoxin A and α2M were purified as previously described (15, 19). RAP–glutathione S-transferase (GST) was obtained by using the pGEX expression vector, (a kind gift from D. K. Strickland, American Red Cross, Rockville, Md.) from bacterial lysates with a GST purification module following the manufacturer's instructions (Pharmacia Biotech, Baie d'Urfe, Quebec, Canada). All chemicals were obtained from the Sigma Chemical Co., St. Louis, Mo.
Cell culture.
HS-P macrophage-like cells were cultured in T-75 flasks in RPMI medium, supplemented with 10% heat-inactivated fetal bovine serum, 50 U of penicillin per ml and 50 μg of streptomycin per ml at 5% CO2, 95% humidity, and 37°C. Media, serum, and supplements were all obtained from Gibco/BRL, Burlington, Ontario, Canada. Cells were detached with trypsin and passed every 2 to 3 days. This cell line was recently isolated from a spontaneous histocytic sarcoma from the liver of a rat (47) and was chosen for this study based on its high sensitivity to PEA and its monocyte/macrophage origins. For experiments, cells were seeded at a concentration of 4 × 104 cells/well into 96-well plates (for cytotoxicity assays) or at 2 × 106 cells/dish into 60-mm-diameter culture dishes (for Northern analysis) or at 2 × 105 cells/well into 24-well plates (for ligand internalization studies) and were incubated overnight before treatments. All tissue culture plastic was purchased from Sarstedt, Inc., St. Leonard, Quebec, Canada.
PEA cytotoxicity assay.
HS-P PEA sensitivity was determined by assaying the inhibition of protein synthesis. Following overnight incubation in 96-well plates, HS-P cells were challenged with PEA in serum-free media. Unless otherwise stated, all experiments involving PEA treatment were performed at 37°C. Cells were treated either for 24 h at various concentrations of PEA or with 50 ng of PEA per ml for various periods of time. Following challenge, toxin was removed, and cells were incubated with [3H]leucine at 3 μCi/ml (ICN, Montreal, Quebec, Canada) for 21 h. Radioactive medium was removed, and cells were detached by using a trypsin solution and harvested onto filter mats. Incorporated radioactivity was determined with a Betaplate liquid scintillation counter (LKB Wallac, Turku, Finland). Data are presented as a percentage of protein synthesis compared with that in cells that were not challenged with toxin.
RAP and LPS protection.
After overnight culture, HS-P cells were cotreated with 50 ng of PEA per ml and various concentrations of RAP-GST for 1 h. Cells were then incubated with [3H]leucine for 21 h and then harvested as described above. HS-P cells were pretreated for 24 h with various concentrations of LPS (Escherichia coli O127:B8) or with 100 ng of LPS per ml for various periods of time. Following pretreatment, cells were challenged for 2 h with 100 ng of toxin per ml and then processed as described above. HS-P cells were also pretreated for 24 h with 100 ng of LPS per ml and then challenged for 2 h with various concentrations of PEA. The 50% inhibition dose (ID50) values (the dose of PEA in nanograms per milliliter required to inhibit protein synthesis by 50% compared to that in cells receiving no toxin) were determined for both nontreated and LPS-treated HS-P cells. In short-term pulse experiments, cells were treated for 15 min with 1,000 ng of PEA per ml and then washed three times with fresh medium in order to remove unbound toxin from the cell surface. The significance of any differences in cellular PEA sensitivity was evaluated by a Student's t test or one-way analysis of variance.
RNA isolation and Northern blot analysis of cellular LRP.
HS-P cells were cultured as described above and then treated with 100 ng of LPS per ml. At specified times, cells were washed a single time in ice-cold phosphate-buffered saline, after which total cellular RNA was isolated with Trizol reagent (Gibco/BRL). RNA (20 μg) from each time point was separated by electrophoresis in 1.0% agarose gels and transferred to nylon membranes (Hybond N; Amersham International, Buckinghamshire, England). Following cross-linking, membranes were prehybridized for 1 h at 42°C in a mixture of 0.5% sodium dodecyl sulfate (SDS), 6× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) 20 μg of salmon sperm DNA per ml, 5× Denhardt's reagent, and 50% formamide. A cDNA probe specific for rat LRP (kindly provided by G. Bu, Washington University, St. Louis, Mo.) was radiolabelled with [α-32P]dCTP and the Rediprime random primer labeling kit (Amersham). Membranes were then incubated with labeled probes for 18 h in a solution identical to that used for prehybridization. Membranes were then twice subjected to a low-stringency wash for 15 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% SDS at 42°C and then to a single high-stringency wash for 20 min in 0.1× SSC–0.1% SDS at a temperature of 65°C. As a control for loading, membranes were rehybridized with a radiolabeled probe for murine 7S RNA (kindly provided by Allan Balmain, Onyx Pharmaceuticals, Richmond, Calif.) (2). A GS250 Molecular Imager (Bio-Rad, Richmond, Calif.) located in the Clarice Chalmers Molecular Imaging Facility, Department of Biomedical Sciences, University of Guelph, was used for signal detection and quantification of Northern blots.
Ligand internalization studies.
Human α2M was converted to its receptor-recognized conformation with 200 nM methylamine HCl. Activated α2M (α2M*) was iodinated with 125I (Amersham) by using Iodobeads as described by the manufacturer (Pierce Chemicals Company, Rockford, Ill.). The specific activity was 1,000 to 2,000 cpm/ng. Ligand uptake studies were conducted as previously described (7). Briefly, HS-P cells were cultured as described above and then treated with 100 ng of LPS per ml for 24 h. LPS treated and nontreated cells were then washed in Earle's Balanced Salt Solution (EBSS) (Gibco/BRL) containing 10 mM HEPES, 1 mg of bovine serum albumin per ml (pH 7.4) (incubation media), and then 4 nM 125I-α2M* in incubation media was added for 2 h at 37°C. After ligand removal, cells were washed in cold EBSS containing 10 mM HEPES (pH 7.4) and then treated with a trypsin solution for 30 min at 4°C. Detached cells were subsequently collected, pelleted by centrifugation, and lysed, and radioactivity was determined with a gamma counter. Protein content was determined by the Bio-Rad protein assay. Nonspecific internalization was determined by including a 100-fold excess of unlabeled α2M*. Specific internalization was determined by subtracting nonspecific internalization from total internalization.
RESULTS
HS-P PEA sensitivity.
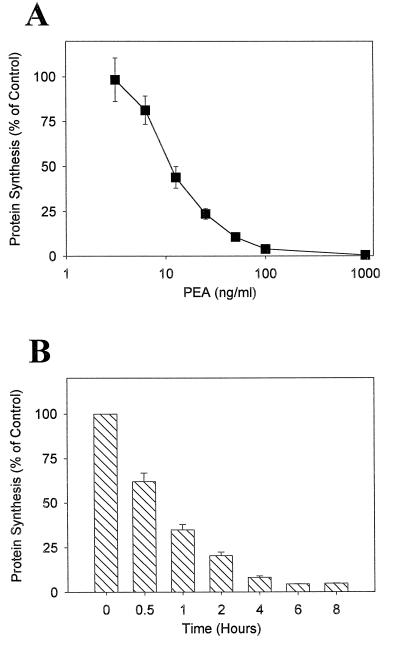
With a [3H]leucine incorporation assay to measure inhibition of protein synthesis, the cytotoxic effect of PEA on HS-P macrophage-like cells was determined. Results from experiments in which HS-P cells were treated with various concentrations of purified PEA for 2 h are displayed in Fig. 1A and indicate that HS-P cells are sensitive to PEA in a dose-dependent manner. To examine the effect duration of toxin exposure has on macrophage PEA cytotoxicity, HS-P cells were treated with 50 ng of PEA per ml for the indicated times (Fig. 1B). In both cases, HS-P cells are clearly sensitive to PEA, suggesting they possess the required cellular machinery for successful PEA intoxification, including functional cell surface LRP and are a suitable macrophage cell line with which to evaluate factors which might alter PEA susceptibility.
FIG. 1.
Cytotoxic activity of PEA on HS-P macrophage-like cells. HS-P cells were cultured overnight in 96-well plates with media containing 10% serum. Cells were incubated in serum-free media containing either various concentrations of PEA for 2 h (A) or 50 ng of PEA per ml for various time periods (B). Protein synthesis levels were determined by measuring the incorporation of [3H]leucine into cellular protein and are expressed as a percentage relative to control cells that received no toxin. Each data point represents the mean ± standard error of three separate experiments. PEA significantly (P < 0.05) inhibited protein synthesis at all concentrations greater than 10 ng/ml and at all time points.
PEA sensitivity is decreased by LPS.
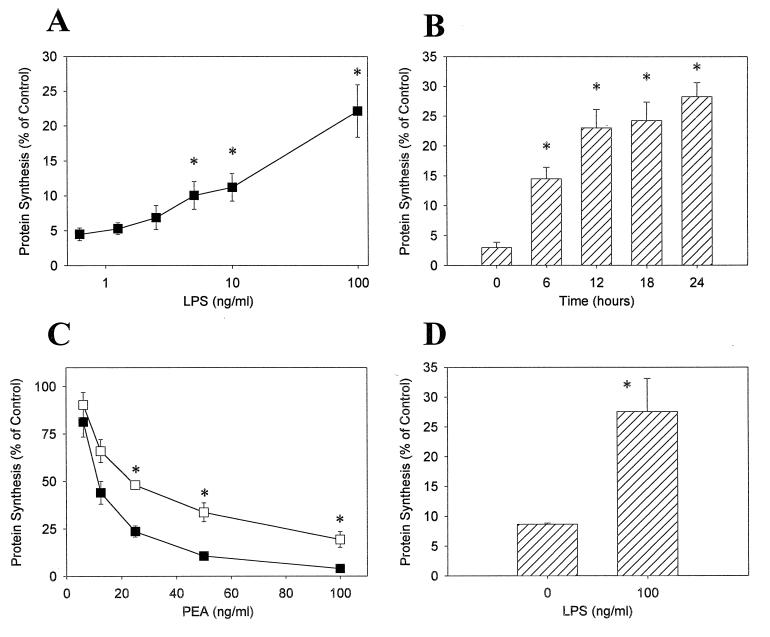
We next wished to determine if treatment of macrophages with LPS modifies their sensitivity to PEA. HS-P cells were pretreated for 24 h with LPS and then challenged with 100 ng of PEA per ml for 2 h. Increasing concentrations of LPS caused a decrease in HS-P PEA sensitivity (Fig. 2A). The duration of pretreatment also affected PEA sensitivity; cells exposed to LPS for an increased time acquired a greater resistance to PEA (Fig. 2B). These results indicate that LPS pretreatment decreases macrophage PEA sensitivity in a dose- and time-dependent fashion. To further examine this issue, the ID50 values were determined for both HS-P cells pretreated with LPS at a concentration of 100 ng/ml for 24 h and for untreated cells (Fig. 2C). The ID50 value of HS-P cells pretreated with LPS was 25.7 ± 2.0 ng/ml, compared to a value of 11.3 ± 1.2 ng/ml for untreated cells. The ID50 value for pretreated cells is significantly different (P < 0.05) from that of control cells, indicating that LPS pretreatment increases HS-P PEA resistance twofold. In order to ensure that the extent of LRP down-regulation was not offset in this assay by increased receptor turnover, we assessed PEA toxicity after brief exposure to a saturating concentration of PEA (1,000 ng/ml) in control and LPS-treated cells. As demonstrated in Fig. 2D, the results indicate [3H]leucine incorporation is threefold higher in LPS-treated cells versus untreated cells. Taken together, these results demonstrate that exposure to LPS confers partial protection from PEA-mediated toxicity in macrophages and that the protection conferred is highest when the assay conditions are designed to reflect the number of cell surface receptors at a given time.
FIG. 2.
Altered HS-P PEA sensitivity following pretreatment with LPS. HS-P cells were treated for either 24 h with LPS at the indicated concentrations (A) or with 100 ng of LPS per ml for 6, 12, 18, or 24 h (B). Following LPS exposure, cells were challenged with 100 ng of PEA per ml for 2 h. (C) HS-P cells were pretreated with 100 ng of LPS per ml for 24 h (open squares) or were untreated (solid squares). Cells were then challenged with PEA for 2 h at the indicated concentrations. Following toxin exposure, cells were pulsed with media containing [3H]leucine for 21 h. (D) LPS-treated (100 ng/ml, 24 h) and nontreated cells were challenged with 1,000 ng of PEA per ml for 15 min, washed, and exposed to [3H]leucine for 12 h. Each data point represents the mean ± standard error of three separate experiments. ∗, significantly different from untreated cells (P < 0.05).
HS-P macrophages are protected from PEA by the LRP antagonist RAP.
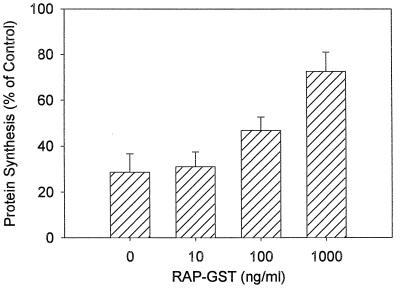
The RAP can act in vitro as a natural antagonist that prevents binding of all known ligands to the LRP. It has previously been reported that RAP prevents PEA binding to the LRP and subsequently decreases PEA cytotoxicity (17). To help determine the role of the LRP in HS-P intoxification by PEA, cells were incubated with a RAP-GST fusion protein. When HS-P cells were exposed to 50 ng of PEA per ml for 1 h, RAP-GST diminished PEA cytotoxicity in a dose-dependent manner (Fig. 3). These results indicate that HS-P cells utilize the LRP in the process of PEA intoxification and that functional antagonism of the LRP leads to reduced macrophage PEA sensitivity.
FIG. 3.
Effect of RAP-GST on PEA-induced cytotoxicity in HS-P cells. Following overnight incubation, HS-P cells were treated with both PEA (50 ng/ml) and various concentrations of RAP-GST. Protein synthesis levels were determined by measuring the incorporation of [3H]leucine into cellular protein. Each data point represents the mean ± standard error of three separate experiments. Protein synthesis in the presence of RAP-GST was significantly (P < 0.05) higher at the 1,000-ng/ml concentration.
Expression of HS-P LRP.
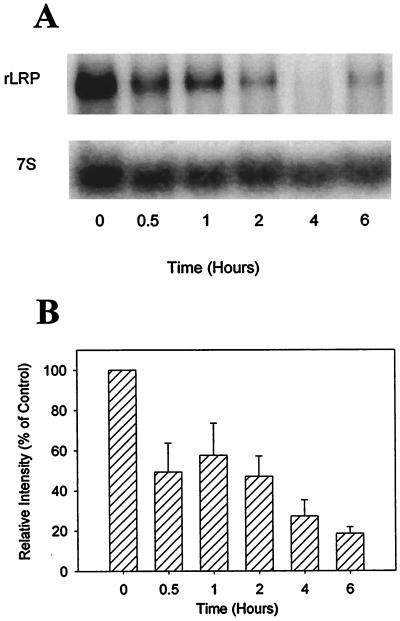
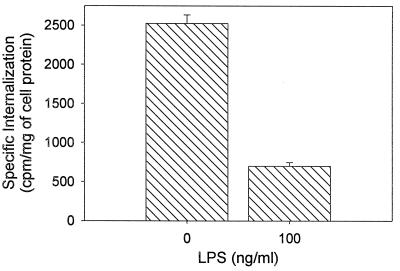
To ascertain the mechanism by which macrophage PEA susceptibility is decreased by LPS, we examined the effect of LPS treatment on the expression levels of LRP. A decrease in the number of functional receptors, resulting from a decrease in the expression levels of LRP, represents one potential mechanism by which macrophages might reduce their sensitivity to PEA. Northern blot analysis revealed that treatment of HS-P cells with 100 ng of LPS per ml rapidly and extensively decreased LRP mRNA levels (Fig. 4A). Analysis of three independent experiments revealed that the level of cellular LRP mRNA decreased to 18.5% ± 3.3% of time zero values 6 h after LPS treatment (Fig. 4B), demonstrating for the first time that, in macrophages, LPS-dependent LRP modulation occurs very rapidly, at the mRNA level, after treatment. Previous results in our laboratory, including studies with the macrophage-like cell line RAW 246.7, indicate that LRP protein and functional levels decrease concomitantly with LRP mRNA (19). To verify that LRP down regulation occurs at the functional level in HS-P cells after LPS treatment, internalization studies were conducted with the LRP-specific ligand α2M*. The results from these experiments (Fig. 5) demonstrate that treatment with 100 ng of LPS per ml for 24 h reduces HS-P α2M* internalization three-and-a-half-fold compared to nontreated HS-P cells.
FIG. 4.
Effects of LPS on LRP mRNA expression in HS-P cells. HS-P cells were treated with 100 ng of LPS per ml for the indicated times. (A) Northern blot analysis of total RNA (20 μg per lane) was performed with a rat LRP (rLRP) cDNA probe. The lower panel shows the results after hybridizing the blot with a 7S RNA cDNA probe, which was used as a load control. The results shown are from a representative Northern blot repeated three times. (B) Relative intensity of LRP at 0, 0.5, 1, 2, 4, and 6 h following LPS exposure normalized to 7S and expressed as a percentage of time zero. The results shown are means ± standard errors of three separate experiments. LRP was significantly decreased at all time points (P < 0.05).
FIG. 5.
Effect of LPS on HS-P α2M* internalization. HS-P cells were treated with 100 ng of LPS per ml for 24 h, washed, and incubated at 37°C for 2 h with 125I-α2M* (4 nM), in the presence or absence of excess unlabeled ligand. Cells were washed, collected, and lysed, and radioactivity was determined. The results shown are means ± standard errors of triplicate samples from three separate experiments (n = 9). Internalization was significantly decreased after LPS treatment (P < 0.05).
DISCUSSION
Macrophages constitute an important component of the host defense against bacterial pathogens such as P. aeruginosa. It is therefore not surprising that the macrophage is a target of P. aeruginosa virulence factors (37). Specifically, it has been demonstrated previously that PEA is cytotoxic to macrophages (31) and hampers their ability to carry out critical cellular processes. For example, PEA inhibits the ability of macrophages to engage in phagocytosis (31) and alters their secretion profiles of various cytokines, including interleukin-1 and tumor necrosis factor (38). Using an in vitro assay to measure the inhibition of protein synthesis, we report here that HS-P macrophage-like cells are sensitive to PEA in a time- and dose-dependent manner, similar to other cells of macrophage origin. However, macrophage cell lines appear to have marked differences in PEA sensitivity. With our assay, we have determined that the macrophage-like cell line RAW 246.7 is approximately 10-fold less sensitive to PEA (data not shown) than HS-P cells.
PEA intoxification is a complex multistep process that relies on the efficient participation of the target cell. Therefore, susceptibility to PEA should be based, at least in part, on the number of functional target cell components available for toxin entry. The observation that HS-P cells are highly sensitive to PEA, have abundant LRP mRNA levels, and are capable of internalizing the LRP-specific ligand α2M* suggests that these cells express high levels of the LRP. In addition, the ability of RAP-GST to block the cytotoxic effects of PEA confirms the LRP dependence of PEA toxicity in this cell type. Our initial hypothesis suggested that LPS exposure would act to protect macrophages from PEA through down-regulation of cell surface LRP. Northern blot analysis revealed that LRP mRNA levels dramatically and quickly decrease following LPS treatment. In addition, functional cell surface LRP levels decrease concomitantly with LRP mRNA as determined by α2M* internalization studies. These results extend our initial studies reporting that LPS treatment down-regulates the quantity of functional cell surface LRP in other macrophages (19).
LPS, a component of the outer membrane of gram-negative bacteria, is a well-recognized activating agent for macrophages, initiating a series of events which increase their ability to effectively combat invading pathogens. The primary macrophage LPS receptor is the glycosylphosphatidylinositol-anchored glycoprotein CD14 (46); however, activation can also occur via a CD14-independent pathway. The end result of LPS-induced signal transduction is an altered expression pattern for a variety of genes, including increased expression of proinflammatory cytokines and enzymes responsible for generating reactive oxygen and nitrogen species. While many of these changes in gene expression clearly enhance the ability of macrophages to destroy invading pathogens, the role of decreased cellular expression of some genes, particularly those for receptors (19, 36, 43), is far less clear. If, in fact, cell surface receptors constitute important portals of entry for pathogens or their products, then the potential advantage of actively decreasing the number of such sites is apparent.
In the present study, we have identified one such potential mechanism. Pretreatment with LPS significantly decreased macrophage PEA sensitivity in a dose- and time-dependent manner. In addition, based on ID50 values, we observed that LPS pretreatment for 24 h at a concentration of 100 ng/ml decreased toxin sensitivity twofold. In order to further implicate receptor-dependent mechanisms in the observed differences in cellular toxin sensitivity, we also investigated cellular toxin susceptibility after a short duration of exposure to PEA. In this way, cellular receptors should be saturated with toxin and differences in receptor numbers may be more directly reflected by changes in cellular susceptibility than in studies utilizing longer periods of toxin exposure. Our results suggest a threefold higher susceptibility of untreated cells versus LPS-stimulated cells, further supporting our contention that receptor levels are positively correlated with toxin sensitivity. This observed decrease in toxin sensitivity correlates extremely well with the functional decrease in LRP-dependent ligand internalization in this cell type. We have also consistently observed that LPS exposure decreased RAW 264.7 PEA resistance; however, this effect was neither as reproducible nor as extensive as that reported here for HS-P cells. It is not yet clear why this is the case, but the relative resistance of RAW cells to PEA described above may play a role in masking any LPS-mediated protection. It should also be emphasized here that LPS- and cytokine-induced activation does not universally enhance cellular resistance to bacterial toxins; cellular sensitivity to Shiga and Shiga-like toxins in vascular endothelial cells is increased following LPS or cytokine treatment (22, 32, 40, 42).
Although it is not yet known whether LPS-mediated down-regulation of LRP occurs in vivo, the protective effect of LPS reported here would have obvious beneficial effects on macrophage viability during PEA challenge. In such a scenario, macrophages, which have diminished levels of the LRP, would be relatively protected from PEA because they lack an efficient route for toxin internalization. The hypothesis that inducible cellular changes in LRP expression confer relative protection against PEA is also supported by our recent studies on hepatocytes, which demonstrated that matrix-dependent changes in LRP expression correlated with PEA resistance (18a, 34). Since extracellular signaling molecules have the ability to modulate LRP levels, it is plausible that this regulatory mechanism may be a factor in determining cellular and even tissue PEA sensitivity in vivo. Indeed, the results of the present study may suggest an additional mechanism by which LPS confers enhanced resistance to PEA challenge in vivo (48). It is probable that the production of various LRP regulatory factors may be initiated in response to P. aeruginosa, thus modulating PEA cellular sensitivity during infection. It is premature to predict whether such alterations in cellular PEA sensitivity would ultimately benefit the host or the pathogen. Since it is suspected that the LRP is also utilized for cellular entry by other pathogenic organisms, such as malaria (35) and minor group cold viruses (10), the importance of LRP regulation may not be restricted to PEA susceptibility.
Although the correlation between induced changes in LRP expression and PEA sensitivity is high, it should be emphasized that the LPS-induced decrease in macrophage PEA sensitivity seen here may be a product of many changes in macrophage function that can be mediated by LPS. Changes in any of the other steps involved in the PEA intoxification pathway might readily augment or oppose the protective effect resulting from decreased LRP expression. Nevertheless, it is clear from these studies, that changes in the expression of cellular receptors which act as portals of entry for pathogenic factors constitute a strong potential mechanism of host defense during P. aeruginosa infection.
ACKNOWLEDGMENTS
We thank Thomas Ichim for critical discussions and Dudley Strickland, G. Bu, and Alan Balmain for supplying reagents used in this investigation.
This work was supported by the Medical Research Council (Canada) and the University Cooperative Research Program of the Ministry of Education, Science, Sports and Culture, Japan. J.L. is an M.R.C. (Canada) Scholar.
REFERENCES
- 1.Avramoglu R K, Nimpf J, McLeod R S, Ko K W, Wang Y, FitzGerald D J, Yao Z. Functional expression of the chicken low density lipoprotein receptor-related protein in a mutant chinese hamster ovary cell line restores toxicity of Pseudomonas exotoxin A and degradation of alpha 2-macroglobulin. J Biol Chem. 1998;273:6057–6065. doi: 10.1074/jbc.273.11.6057. [DOI] [PubMed] [Google Scholar]
- 2.Balmain A, Krumlauf R, Vass J K, Birnie G D. Cloning and characterization of the abundant cytoplasmic 7S RNA from mouse cells. Nucleic Acids Res. 1982;10:4259–4277. doi: 10.1093/nar/10.14.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bu G, Williams S, Strickland D K, Schwartz A L. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is a hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci USA. 1992;89:7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bu G, Sun Y, Schwartz A L, Holtzman D M. Nerve growth factor induces rapid increases in functional cell surface low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:13359–13365. doi: 10.1074/jbc.273.21.13359. [DOI] [PubMed] [Google Scholar]
- 5.Descamps O, Bilheimer D, Herz J. Insulin stimulates receptor-mediated uptake of apoE-enriched lipoprotein and activated alpha 2-macroglobulin in adipocytes. J Biol Chem. 1993;268:974–981. [PubMed] [Google Scholar]
- 6.Feldman S R, Rosenberg M R, Ney K A, Michalopoulos G, Pizzo S V. Binding of alpha 2-macroglobulin to hepatocytes: mechanism of in vivo clearance. Biochem Biophys Res Commun. 1985;128:795–802. doi: 10.1016/0006-291x(85)90117-2. [DOI] [PubMed] [Google Scholar]
- 7.FitzGerald D J, Fryling C M, Zdanovsky A, Saelinger C B, Kounnas M, Winkles J A, Strickland D, Leppla S. Pseudomonas exotoxin-mediated selection yields cells with altered expression of low-density lipoprotein receptor-related protein. J Cell Biol. 1995;129:1533–1541. doi: 10.1083/jcb.129.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forristal J J, Thompson M R, Morris R E, Saelinger C B. Mouse liver contains a Pseudomonas exotoxin A-binding protein. Infect Immun. 1991;59:2880–2884. doi: 10.1128/iai.59.9.2880-2884.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herz J, Kowal R C, Goldstein J L, Brown M S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990;9:1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofer F, Gruenberger M, Kowalski H, Machat H, Huettinger M, Kuechler E, Blass D. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc Natl Acad Sci USA. 1994;91:1839–1842. doi: 10.1073/pnas.91.5.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain M M, Maxfield F R, Mas-Oliva J, Tabas I, Ji Z S, Innerarity T L, Mahley R W. Clearance of chylomicron remnants by the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991;266:13936–13940. [PubMed] [Google Scholar]
- 12.Hussaini I M, LaMarre J, Lysiak J J, Karns L R, VandenBerg S R, Gonias S L. Transcriptional regulation of the LDL receptor-related protein by IFN-gamma and the antagonistic activity of TGF-beta(1) in the RAW 264.7 macrophage-like cell line. J Leukoc Biol. 1996;59:733–739. doi: 10.1002/jlb.59.5.733. [DOI] [PubMed] [Google Scholar]
- 13.Iglewski B H, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin. Proc Natl Acad Sci USA. 1974;72:2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iglewski B H, Liu P V, Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin A: adenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977;15:138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinno Y, Chaudhary V K, Kondo T, Adhya S, FitzGerald D J, Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988;263:13203–13207. [PubMed] [Google Scholar]
- 16.Kounnas M Z, Moir R D, Rebeck G W, Bush A I, Argraves W S, Tanzi R E, Hyman B T, Strickland D K. LDL receptor-related protein, a multifunctional ApoE receptor, binds secreted beta-amyloid precursor protein and mediates its degradation. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 17.Kounnas M Z, Morris R E, Thompson M R, FitzGerald D J, Strickland D K, Saelinger C B. The α2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992;267:12420–12423. [PubMed] [Google Scholar]
- 18.Kristensen T, Moestrup S K, Gliemann J, Bendtsen L, Sand O, Scottrup-Jensen L. Evidence that the newly cloned low density lipoprotein receptor-related protein (LRP) is the alpha 2-macroglobulin receptor. FEBS Lett. 1990;276:151–155. doi: 10.1016/0014-5793(90)80530-v. [DOI] [PubMed] [Google Scholar]
- 18a.Laithwaite, J. E., et al. Unpublished data.
- 19.LaMarre J, Wolf B B, Kittler E L, Quesenberry P J, Gonias S L. Regulation of macrophage α2-macroglobulin receptor/low density lipoprotein receptor-related protein by lipopolysaccharide and interferon-γ. J Clin Investig. 1993;91:1219–1224. doi: 10.1172/JCI116283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMarre J, Hayes M A, Wollenberg G K, Hussaini I, Hall S W, Gonias S L. An alpha 2-macroglobulin receptor-dependent mechanism for the plasma clearance of transforming growth factor-beta 1 in mice. J Clin Investig. 1991;87:39–45. doi: 10.1172/JCI114998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Wood N, Donnelly P, Yellowlees D. Cell density and oestrogen both stimulate alpha 2-macroglobulin receptor expression in breast cancer cell T-47D. Anticancer Res. 1998;18:1197–1202. [PubMed] [Google Scholar]
- 22.Louise C B, Obrig T G. Shiga toxin-associated hemolytic-uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manhart M D, Morris R E, Bonventre P F, Leppla S, Saelinger C B. Evidence for Pseudomonas exotoxin A receptors on plasma membrane of toxin-sensitive LM fibroblasts. Infect Immun. 1984;45:596–603. doi: 10.1128/iai.45.3.596-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middlebrook J L, Dorland R B. Response of cultured mammalian cells to the exotoxins of Pseudomonas aeruginosa and Corynebacterium diphtheriae: differential cytotoxicity. Can J Microbiol. 1977;2:103–108. doi: 10.1139/m77-026. [DOI] [PubMed] [Google Scholar]
- 25.Moestrup S K, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269:375–382. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- 26.Mucci D, Forristal J, Strickland D, Morris R, FitzGerald D, Saelinger C B. Level of receptor-associated protein moderates cellular susceptibility to Pseudomonas exotoxin A. Infect Immun. 1995;63:2912–2918. doi: 10.1128/iai.63.8.2912-2918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niemuller C A, Randall K J, Web D J, Gonias S L, LaMarre J. Alpha 2-macroglobulin conformation determines binding affinity for activin A and plasma clearance of activin A/alpha 2-macroglobulin complex. Endocrinology. 1995;136:5343–5349. doi: 10.1210/endo.136.12.7588280. [DOI] [PubMed] [Google Scholar]
- 28.Ogata M, Chaudhary V K, Pastan I, FitzGerald D J. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem. 1990;265:20678–20685. [PubMed] [Google Scholar]
- 29.Orth K, Madison E L, Gething M-J, Sambrook J F, Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc Natl Acad Sci USA. 1992;89:7422–7426. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlovskis O R, Shackelford A H. Pseudomonas aeruginosa exotoxin in mice: localization and effect on protein synthesis. Infect Immun. 1974;9:540–546. doi: 10.1128/iai.9.3.540-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollack M, Anderson S E., Jr Toxicity of Pseudomonas aeruginosa exotoxin A for human macrophages. Infect Immun. 1978;19:1092–1096. doi: 10.1128/iai.19.3.1092-1096.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramegowda B, Tesh V L. Differentiation-associated toxin receptor modulation, cytokine production, and sensitivity to Shiga-like toxins in human monocytes and monocytic cell lines. Infect Immun. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saelinger C B, Snell D K, Holder I A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissue. J Infect Dis. 1977;136:555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- 34.Schmoelzl S, Benn S J, Laithwaite J E, Greenwood S J, Marshall W S, Munday N A, FitzGerald D J, LaMarre J. Expression of hepatocyte low density lipoprotein receptor-related protein is post-transcriptionally regulated by extracellular matrix. Lab Investig. 1998;78:1405–1413. [PubMed] [Google Scholar]
- 35.Shakibaei M, Frevert U. Dual interaction of the malaria circumsporozoite protein with the low density lipoprotein receptor-related protein (LRP) and heparan sulfate proteoglycans. J Exp Med. 1996;184:1699–1711. doi: 10.1084/jem.184.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shepherd V L, Abdolrasulnia R, Garrett M, Cowan H B. Down-regulation of mannose receptor activity in macrophages after treatment with lipopolysaccharide and phorbol esters. J Immunol. 1990;145:1530–1536. [PubMed] [Google Scholar]
- 37.Speert D P. Pseudomonas aeruginosa-phagocytic cell interactions. In: Campa M, Bendinelli M, Friedman H, editors. Pseudomonas aeruginosa as an opportunistic pathogen. New York, N.Y: Plenum Press; 1993. pp. 163–181. [Google Scholar]
- 38.Staugas R E M, Harvey D P, Ferrante A, Nandoskar M, Allison A C. Induction of tumor necrosis factor (TNF) and interleukin-1 (IL-1) by Pseudomonas aeruginosa and exotoxin A-induced suppression of lymphoproliferation and TNF, lymphotoxin, gamma interferon, and IL-1 production by human leukocytes. Infect Immun. 1992;60:3162–3168. doi: 10.1128/iai.60.8.3162-3168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickland D K, Ashcom J D, Williams S, Burgess W H, Migliorini M, Argraves W S. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem. 1990;265:17401–17404. [PubMed] [Google Scholar]
- 40.Tesh V L, Samuel J E, Perera L P, Sharefkin J B, O'Brien A D. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis. 1991;164:344–352. doi: 10.1093/infdis/164.2.344. [DOI] [PubMed] [Google Scholar]
- 41.Thompson M R, Forristal J, Kauffmann P, Madden T, Kozak K, Morris R E, Saelinger C B. Isolation and characterization of Pseudomonas exotoxin A binding glycoprotein from mouse LM cells. J Biol Chem. 1991;266:2390–2396. [PubMed] [Google Scholar]
- 42.van der Kar N C, Monnens L A, Karmali M A, van Hinsbergh V W M. Tumor necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the hemolytic uremic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- 43.Van Lenten B J, Fogelman A M, Seager J, Ribi E, Haberland M E, Edwards P A. Bacterial endotoxin selectively prevents the expression of scavenger-receptor activity on human monocyte-macrophages. J Immunol. 1985;134:3718–3721. [PubMed] [Google Scholar]
- 44.Weaver A M, McCabe M, Kim I, Allietta M M, Gonias S L. Epidermal growth factor and platelet-derived growth factor-BB induce a stable increase in the activity of low density lipoprotein receptor-related protein in vascular smooth muscle cells by altering receptor distribution and recycling. J Biol Chem. 1996;271:24894–24900. doi: 10.1074/jbc.271.40.24894. [DOI] [PubMed] [Google Scholar]
- 45.Willnow T E, Herz J. Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A. J Cell Sci. 1994;107:719–726. [PubMed] [Google Scholar]
- 46.Wright S D, Ramous R A, Tobias P S, Ulevitch R J, Mathison J C. CD14 serves as the cellular receptor for complexes of lipopolysaccharide with lipopolysaccharide binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 47.Yamate J, Tsujino K, Kumagai D, Nakatsuji S, Kuwammura M, Kotani T, Sakuma S. Morphological characteristics of a transplantable histiocytic sarcoma (HS-J) in F344 rats and appearance of renal tubular hyaline droplets in HS-J-bearing rats. J Comp Pathol. 1996;116:73–86. doi: 10.1016/s0021-9975(97)80045-x. [DOI] [PubMed] [Google Scholar]
- 48.Zehavi-Willner T, Barnea A, Pinto M. In vivo protective effect of lipopolysaccharide against Pseudomonas aeruginosa exotoxin A in mice. Infect Immun. 1991;59:1667–1672. doi: 10.1128/iai.59.5.1667-1672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]