Abstract
Both native and mutant forms of cholera toxin (CT) and heat-labile enterotoxin (LT) are effective adjuvants for antigens and killed whole-cell preparations. To determine whether these toxin molecules could also boost the immunogenicity and efficacy of live attenuated vaccines directed against shigellosis, the guinea pig keratoconjunctivitis model was used to evaluate the adjuvant effect of these toxin molecules on EcSf2a-3, a ΔvirG ΔaroD Escherichia coli-Shigella flexneri 2a hybrid vaccine strain that was previously found to be less protective than its parent strain in the guinea pig model. Experiments using native and mutant toxin molecules showed that both CT and LT and mutant derivatives were effective as an adjuvant for EcSf2a-3 and that the mutant toxin molecules, which were developed to retain adjuvanticity without the toxicity associated with the native molecules, were as effective as the native toxin molecules as adjuvants. Protective efficacy was enhanced for both the oral and intranasal routes of immunization. Serum antibody response to the S. flexneri 2a O antigen, the primary antigen for protective immunity, was not dependent on the addition of an adjuvant. However, enumeration of the O-antigen-specific immunoglobulin G (IgG) and IgA antibody-secreting cells in the spleen and draining lymph nodes following intranasal immunization suggested that enhancement of the local immune response by the toxin molecules may contribute to the observed increase in protective efficacy. The efficacy of heat-killed S. flexneri 2a was enhanced only by mutant LT molecules. These results suggest that the best candidates for enhancing the efficacy of both live attenuated and heat-killed Shigella vaccines with minimal reactogenicity are the mutant toxin molecules.
Shigellae are enteric pathogens that cause disease by first invading the epithelial cells of the colonic mucosa and then spreading intra- and intercellularly. This intercellular dissemination produces inflammation and ulceration, resulting in diarrhea or dysentery. The annual incidence of shigellosis is estimated at 100 to 200 million cases resulting in about 650,000 deaths (16). Mortality rates are particularly high in young children in developing countries where shigellae are endemic. Shigellosis is also a problem for immunologically naive civilian and military personnel from industrialized countries traveling to areas where the disease is endemic. Development of an efficacious vaccine directed against the most common Shigella serotypes is thus a major goal.
Mucosal immunization is thought to be the most effective route for pathogens that invade mucosal surfaces to initiate disease. Immunization by the mucosal route provides stimulation of mucosal immunity against relevant virulence antigens, making it possible to prevent the initial infection by the pathogen at the mucosal surface (2, 4, 5, 19, 24). Parenteral immunizations with inactivated bacteria or subunit vaccines rely on serum antibodies and cell-mediated immunity to protect against organisms that have a systemic phase, such as Salmonella typhi. However, parenteral vaccines do not elicit a mucosal secretory immunoglobulin A (IgA) response unless previous mucosal exposure to the immunizing antigen has occurred and thus cannot prevent infection by organisms that interact with a mucosal surface (23, 24, 26, 30). Earlier studies showed that killed Shigella whole-cell vaccines did not elicit protective immunity when administered orally and that live noninvasive strains were impractical because of the large and frequent doses required (11). Parenteral immunization with live or killed shigellae did not prevent infection in earlier experiments (11). Therefore, recent efforts have led to the development of attenuated invasive vaccine strains that can invade the colonic epithelial cells as in a natural infection, eliciting mucosal immunity against the O antigen and other virulence genes. A recurring problem with these strains has been balancing immunogenicity and protective efficacy with reactogenicity. This is exemplified by the case of EcSf2a-2, an Escherichia coli-Shigella flexneri 2a hybrid vaccine strain, which is an E. coli K-12 strain containing the invasion plasmid of S. flexneri 5a and the chromosomal O-antigen genes of S. flexneri 2a (25). Immunizing doses of EcSf2a-2 that were large enough to elicit a vigorous immune response were too reactogenic in human volunteers (18, 32). EcSf2a-3, a virG deletion derivative of EcSf2a-2 which cannot spread intra- and intercellularly in colonic epithelial cells, was constructed to provide a less reactogenic strain, but it was less protective in the guinea pig keratoconjunctivitis model than EcSf2a-2 (1). Both of these hybrid strains show variability in invasive properties (unpublished observations) which may also contribute to the decreased efficacy observed in human studies with EcSf2a-2 (11) and animal studies with both strains (unpublished observations; 1).
The effectiveness of mucosal immunization can be increased by the addition of mucosal adjuvants. Many studies have indicated that Escherichia coli heat-labile enterotoxin (LT) and cholera toxin (CT) are effective mucosal adjuvants for orally administered antigens (6, 9, 10, 33). The addition of CT or LT as an adjuvant augmented the immunogenicity and protective efficacy of killed whole-cell preparations of Helicobacter pylori and Campylobacter sp. in animal models (3, 21). To avoid using native toxin molecules as adjuvants for human vaccines, mutant toxin molecules have been developed that have retained adjuvanticity but have little or no toxicity. Mutant molecule mLT(R192G) contains an alteration in the proteolytically sensitive A subunit of LT, thus preventing trypsin activation (8), while mCT(K63) and mLT(K63) have an alteration in amino acid 63 in the crevice where NAD binding and catalysis occur (28). These mutants have retained adjuvant activity in experiments using antigens such as ovalbumin (8) and more recently using killed whole-cell preparations (4, 21).
Since native and mutant toxin molecules are strong mucosal adjuvants, coadministration of these molecules with live attenuated vaccines could potentially increase the immunogenicity and protective efficacy of these strains. This is particularly relevant in the case of a vaccine strain such as EcSf2a-3, which required much larger doses to give protection equivalent to that of its parent strain in the guinea pig model. In this study, the guinea pig keratoconjunctivitis model was used to test whether native and mutant toxin molecules could enhance the efficacy of live attenuated vaccine strain EcSf2a-3, as well as heat-killed S. flexneri 2a strain 2457T, and to determine whether the mutant molecules are as effective as the native toxin molecules as adjuvants. Both serum and local immune responses to the S. flexneri 2a O antigen, which is the primary antigen in producing protective immunity against shigellosis, were measured to determine the effect of the toxin molecules on the immunogenicity of the vaccines.
MATERIALS AND METHODS
Bacterial strains and media.
EcSf2a-3 is a streptomycin-resistant E. coli-S. flexneri 2a hybrid vaccine strain with deletions in aroD and virG (1). S. flexneri 2a strain 2457T was obtained from the Walter Reed Army Institute of Research collection. EcSf2a-3 was streaked from lyophilized cultures onto Trypticase soy agar plates (TSA; Difco Laboratories, Detroit, Mich.) containing 0.01% Congo red. Congo red-binding colonies were then passed through HeLa cell monolayers as previously described (1) to select for invasive colonies. Strain 2457T was streaked from frozen cultures onto Congo red-TSA plates, and Congo red-binding colonies were selected. Cultures used for immunization or challenge were grown overnight on TSA plates at 37°C and harvested in 5 ml of phosphate-buffered saline (PBS). Heat-killed bacteria were prepared by heating harvested cultures at 60°C for 30 min.
Toxin molecules.
Native CT and LT were obtained from Berna Products Inc., Coral Gables, Fla. Recombinant mLT(R192G) was kindly provided by John D. Clements, Tulane University School of Medicine, New Orleans, La. mCT(K63) and mLT(K63) were kindly provided by Rino Rappuoli, Immunobiological Research Institute Siena, Siena, Italy.
Immunization of guinea pigs.
Hartley male guinea pigs were immunized by using three routes of immunization—oral, intranasal, and ocular. On days 0 and 14, orally immunized animals were given 1010 CFU of live or heat-killed bacteria with or without the addition of 25 μg of toxin molecules by gastric lavage 10 min after administration of 1 ml of 5% sodium bicarbonate solution. Animals were immunized intranasally on days 0 and 14 with 3 × 107 to 5 × 107 CFU of bacteria with or without the addition of 25 μg of toxin. For intranasal immunization, animals were sedated by using a mixture of 0.75 mg of xylazine hydrochloride (Rompun; Bayer Corporation, Shawnee Mission, Kans.) and 1.5 mg of ketamine hydrochloride (Ketaset; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa). Animals were immunized ocularly on days 0 and 14 with 5 × 108 CFU of bacteria per eye with 10 μg of toxin. Animals were inoculated orally and intranasally with heat-killed bacteria at the same dosage as the live vaccine strain with 25 μg of toxin.
Protective efficacy.
Ocular challenge with 4 × 108 CFU of virulent 2457T per eye was carried out 4 weeks after the last immunization. Following challenge, animals were examined for 5 days for development and severity of disease by using the following rating scale: 0, no disease or mild irritation; 1, mild conjunctivitis or late development and/or rapid clearing of disease; 2, keratoconjunctivitis with no purulence; 3, fully developed keratoconjunctivitis with purulence. Protection percentage was defined as follows: full, percentage of eyes with no disease or mild irritation (rating of 0); partial, percentage of eyes with mild disease (rating of 1); combined, sum of full and partial percentages.
Sampling of the immune response.
Blood samples for use in enzyme-linked immunosorbent assays were collected 14 days after the boosting immunization in all experiments. In experiments not studying the antibody-secreting cell (ASC) response, animals were bled by using an ear prick. A 100- to 200-μl volume of blood was collected into a Microtainer brand serum separator tube (Becton Dickinson & Co). Blood was obtained from animals used in ASC studies by cardiac puncture following sedation as described above. Animals were then euthanized. Mononuclear cells for measurement of the ASC response were isolated from the spleen and superficial ventral cervical lymph nodes (SVCLN), which drain the head region, and washed in RPMI 1640 medium with 50 μg of gentamicin per ml prior to use in the ELISPOT assay as previously described (14).
ASC.
Washed spleen and lymph node cells were counted and diluted in culture medium (RPMI 1640 medium with 2 mM glutamine, 50 μg of gentamicin per ml, and 10% fetal bovine serum) to a density of 2.5 × 106/ml. A 100-μl volume of the cell suspension was inoculated into microwells previously coated with 1 μg of S. flexneri 2a lipopolysaccharide (LPS; prepared by the method of Westphal and Jann [34]) in carbonate coating buffer, pH 9.6, or coating buffer alone. Each sample was assayed in quadruplicate. After incubation at 37°C for 4 h, plates were washed and rabbit anti-guinea pig IgG (1:1200), IgA (1:700), or IgM (1:800) (ICN Laboratories, Costa Mesa, Calif.) was added. After overnight incubation at 4°C, plates were washed and alkaline phosphatase-conjugated goat anti-rabbit serum (Sigma Chemical Co., St. Louis, Mo.) at a dilution of 1:1,200 was added. After 2 h at 37°C, plates were washed and spots were visualized by the addition of 100 μl of molten agarose containing 100 μg of 5-bromo-4-chloro-3-indolylphosphate per ml. Spot-forming cells were then counted with a stereomicroscope.
Enzyme-linked immunosorbent assay.
Alternating columns of polyvinyl microtiter wells were coated with 50 μl of LPS (10 μg/ml) in coating buffer or with coating buffer alone. Sera were serially diluted in paired columns beginning at 1:50 for IgG and 1:25 for IgA. Plates were incubated for 2 h at 37°C and washed, and bound antibodies were detected with antisera as described above for ASC. After the final washing of the plates, 100 μl of pNP substrate was added to all wells and the optical density (OD) at 405/570 nm was measured. Blank-well values were subtracted from corresponding test well values to yield the net OD. The endpoint titer was defined as the highest dilution with a net OD of ≥0.100.
RESULTS
Adjuvant effect of toxin molecules on protective efficacy of EcSf2a-3.
To determine the effect of toxin molecules as adjuvants for live vaccines, guinea pigs were immunized with EcSf2a-3 alone and in combination with native and mutant CT and LT by two different mucosal routes, the oral and intranasal routes (Table 1). Earlier experiments had shown that CT alone did not protect against a challenge with virulent shigellae in this model (13). In initial experiments with EcSf2a-3, native and mutant CT and LT from E. coli were used to ascertain the effectiveness of these molecules as adjuvants. Animals were immunized twice on days 0 and 14 and challenged 4 weeks after the last immunization (Table 1: oral immunization, experiment 1; intranasal immunization, experiments 1 and 2). In these experiments, 100% of the control animals, immunized with PBS, developed disease, giving a protection rate of 0%. Oral immunization of EcSf2a-3 with CT, LT, and mLT(R192G) significantly increased the protective efficacy of EcSf2a-3 over that obtained with the vaccine alone (P = 0.0052, P = 0.0009, and P = 0.0052, respectively, by the Wilcoxon rank-and-sum test; Table 1, oral immunization experiment 1). In initial experiments using the intranasal route of immunization, vaccine strain EcSf2a-3 was coadministered with native CT and LT and the three available mutant toxin molecules mCT(K63), mLT(K63), and mLT(R192G) (Table 1, intranasal immunization experiments 1 and 2). Statistical analysis of the sum of these two experiments using intranasal immunization showed that significant increases in protective efficacy were obtained in the groups using toxin molecules as an adjuvant compared to the groups receiving only the vaccine except for the group receiving CT as an adjuvant, and this group had a higher percentage of animals fully protected against disease (rating of 0) than did the group receiving only the vaccine [CT, P = 0.0557; LT, P = 0.0325; mLT(R192G), P = 0.0030; mCT(K63), P = 0.0007; mLT, P = 0.0041]. There was no significant difference in the adjuvant effect of the native versus the mutant molecules, nor was there any significant difference between different mutant toxin molecules in any of the experiments.
TABLE 1.
Comparative protective efficacies of EcSf2a-3 with and without addition of native or mutant toxin molecules following challenge with virulent S. flexneri 2aa
| Route of EcSf2a-3 vaccine immunization and expt no. | No adjuvant
|
CT
|
LT
|
mLT(R192G)
|
mCT(K63)
|
mLT(K63)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | C | F | P | C | F | P | C | F | P | C | F | P | C | F | P | C | |
| Oral | ||||||||||||||||||
| 1 | 0 | 0 | 0 | 0 | 50 | 50 | 0 | 75 | 75 | 0 | 50 | 50 | NDb | ND | ND | ND | ND | ND |
| 2 | 13 | 13 | 25 | ND | ND | ND | 13 | 38 | 50 | 13 | 63 | 75 | ND | ND | ND | 13 | 50 | 63 |
| Intranasal | ||||||||||||||||||
| 1 | 0 | 38 | 38 | 38 | 38 | 75 | 25 | 63 | 88 | 38 | 50 | 88 | 63 | 38 | 100 | 50 | 50 | 100 |
| 2 | 25 | 50 | 75 | 63 | 0 | 63 | 50 | 38 | 88 | 75 | 13 | 88 | 50 | 50 | 100 | 50 | 38 | 88 |
| 3 | 50 | 50 | 100 | ND | ND | ND | 88 | 13 | 100 | 88 | 13 | 100 | ND | ND | ND | ND | ND | ND |
| 4 | 0 | 50 | 50 | ND | ND | ND | 25 | 75 | 100 | 50 | 50 | 100 | ND | ND | ND | ND | ND | ND |
The percent protection for the control animals, which were immunized with PBS, was 0 to 13% for all experiments following challenge with virulent S. flexneri 2a strain 2457T. Protection percentages were defined as follows: full (F), percentage of eyes with no disease; partial (P), percentage of eyes with mild conjunctivitis; combined (C), sum of F and P.
ND, not done.
Since LT molecules were as effective as CT molecules as adjuvants and LT is less toxic to both humans and animals (29), subsequent experiments concentrated on the use of mutant and native LT molecules as adjuvants. A second experiment using oral immunization of EcSf2a-3 with native and mutant LT molecules also showed increased efficacy in the groups receiving an adjuvant (Table 1, oral experiment 2). The protection in the group receiving mLT(R192G) was significantly greater than that of those immunized with EcSf2a-3 alone (P = 0.0415), and the groups receiving LT and mLT(K63) had at least twice as great a percentage of combined protection as the group receiving the vaccine alone. In two additional experiments using the intranasal route of immunization, significant enhancement of efficacy [LT, P = 0.0205; mLT(R192G), P = 0.0062] was also observed when LT and mLT(R192G) were used as adjuvants (Table 1, intranasal immunization experiments 3 and 4) and no significant difference in the adjuvant effect of native versus mutant molecules was observed. For all experiments, the percent protection rate for the control animals, immunized with PBS, was 0 to 13%.
An additional mucosal immunization route, the conjunctival route, was tested by using ocular immunization. Four animals per group were immunized with EcSf2a-3 alone or with CT and LT as adjuvants. The animals receiving CT showed transient irritation of the eyes following immunization, while LT did not produce any reaction, thus confirming that LT is less reactogenic than CT. The animals receiving vaccine alone showed 38% full protection and 38% partial protection (combined protection, 75%), while animals receiving vaccine plus CT showed 25% full protection and 63% partial protection (combined protection, 88%). Only the animals receiving vaccine plus LT showed significantly greater protection than those given the vaccine alone (88% full protection, 13% partial protection, 100% combined protection, P = 0.042).
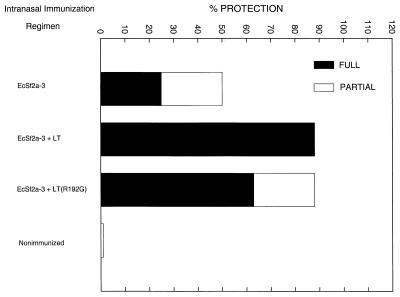
Adjuvant effect of LT and mLT on a decreased immunizing dose.
Since toxin molecules increased the protective efficacy of EcSf2a-3, it is possible that a smaller dose of the vaccine could be given when coadministered with an adjuvant, thus reducing the possibility of reactogenicity. This is important for attenuated vaccines such as EcSf2a-3, which require very large doses for adequate immunogenicity and efficacy. Four animals in each group were immunized intranasally with 3 × 106 CFU of EcSf2a-3, 1 log less than the previously used dose of 3 × 107 to 5 × 107 CFU. As is shown in Fig. 1, the addition of LT and mLT(R192G) increased the efficacy of EcSf2a-3 at the smaller dose (P = 0.010 and 0.052, respectively) and protection was comparable to that obtained with the larger dose.
FIG. 1.
Adjuvant effects of LT and mLT on a decreased immunizing dose of EcSf2a-3 using the intranasal route of immunization. Four animals in each group were immunized two times intranasally with 3 × 106 CFU, 1 log less than the usual dose of 3 × 107 to 5 × 107 CFU, and were challenged 4 weeks after the second dose with virulent S. flexneri 2a.
Adjuvant effect on serum immune response to S. flexneri 2a antigen.
The O antigen of Shigella is the dominant antigen involved in the development of protective immunity against disease. To examine the role of the serum immune response in the enhanced efficacy obtained when toxin molecules are coadministered with EcSf2a-3, the serum IgG and IgA antibody responses to the S. flexneri 2a O antigen were determined 14 days after the second immunization in all experiments. In all experiments using the three routes of immunization, no significant difference was detected in either the IgG or the IgA serum response among the groups, indicating that the magnitude of the serum antibody response to the 2a O antigen did not appear to be dependent upon the addition of toxin molecules during immunization (data not shown).
Local antibody response to S. flexneri 2a O antigen.
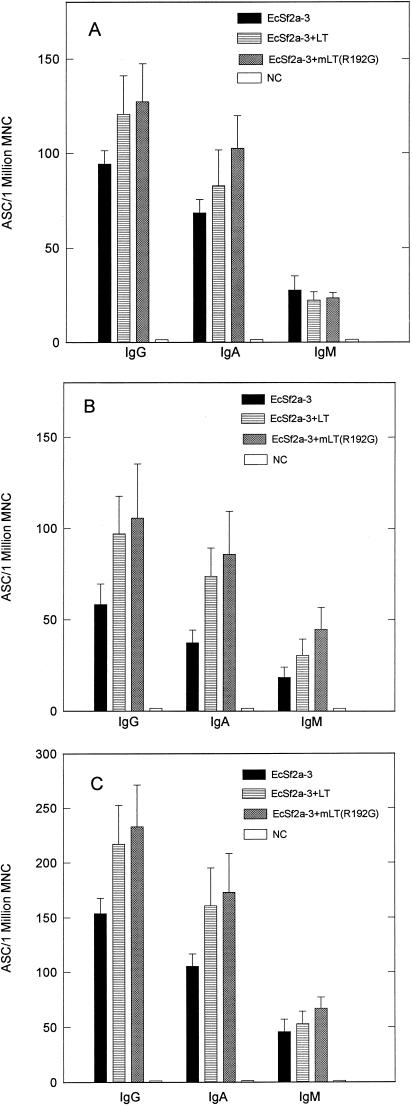
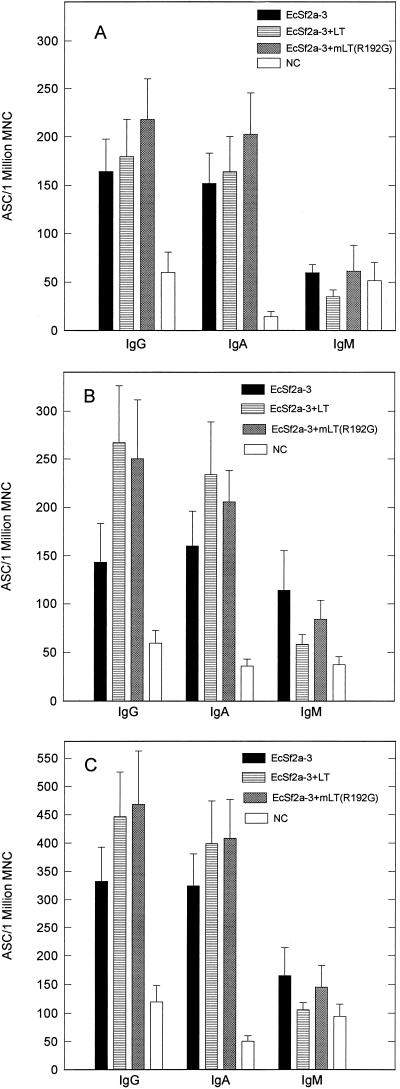
To assess the contribution of toxin molecules to the local immune response, the O-antigen-specific ASC response in the lymph nodes draining the head (SVCLN), which represent the local response to immunization, and in the spleen, which represents the migration of activated B lymphocytes to distal mucosal sites, was examined. Since LT and mLT enhanced protective efficacy as well as or better than CT and mCT, LT and mLT(R192G) were used as adjuvants in these experiments. Figures 2 and 3 show the pooled results of two identical experiments (a total of eight animals) postimmunization and postchallenge, respectively. The mean and standard error of the mean of the O-antigen-specific ASC response in the SVCLN and the spleen 7 days following the second immunization are shown in Fig. 2A and B, respectively, and the mean and standard error of the mean of the total O-antigen ASC response postimmunization are shown in Fig. 2C. Both LT and mLT(R192G) enhanced the mean O-antigen-specific IgG and IgA response over that observed in the group receiving no adjuvant. Specifically, the addition of LT as an adjuvant increased the total mean O-antigen-specific IgG ASC response by 41% and the total mean IgA ASC response by 53% over that observed in the group receiving no adjuvant. The addition of mLT(R192G) as an adjuvant increased the total mean O-antigen-specific IgG ASC response by 53% and the total mean IgA ASC response by 77%. The mLT group values were significantly higher than those observed in the EcSf2a-3-only group by the Wilcoxon rank-sum test (IgG, P = 0.029; IgA, P = 0.0052). The mean and standard error of the mean of the O-antigen-specific ASC responses in the SVCLN and the spleen 7 days postchallenge are shown in Fig. 3A and B, respectively, and the mean and standard error of the mean of the total ASC response postchallenge are shown in Fig. 3C. In the postchallenge animals, the O-antigen-specific IgG and IgA ASC responses were noticeably higher in the groups receiving LT or mLT(R192G) as an adjuvant, although the differences were not significant. Use of LT as an adjuvant increased the total mean O-antigen-specific IgG ASC response by 34% and the total mean specific IgA ASC response by 23%, while use of mLT(R192G) as an adjuvant increased the total mean specific IgG ASC response by 41% and the total mean specific IgA ASC response by 26% over the values obtained when no adjuvant was used. As observed in earlier experiments, there was no significant difference in O-antigen-specific IgG and IgA titers in serum in the three groups both postimmunization and postchallenge (data not shown).
FIG. 2.
S. flexneri 2a O-antigen-specific ASC responses detected 7 days after the second immunization in the SVCLN (A) and spleen (B) and the total ASC response (C) from animals immunized intranasally with EcSf2a-3 with and without toxin molecules LT and mLT(R192G). Each data point represents the mean and standard error of the mean of values from eight animals. MNC, mononuclear cells; NC, nonimmunized control animals.
FIG. 3.
S. flexneri 2a O-antigen-specific ASC responses detected 7 days postchallenge in the SVCLN (A) and spleen (B) and the total ASC response (C) from animals initially immunized intranasally with EcSf2a-3 with and without toxin molecules LT and mLT(R192G) and then challenged with strain 2457T 28 days after the second immunization. Each data point represents the mean and standard error of the mean of values from eight animals. MNC, mononuclear cells; NC, nonimmunized control animals.
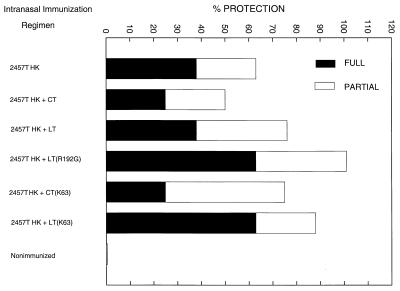
Adjuvant effect on heat-killed shigellae.
To determine whether toxin molecules can enhance the protective efficacy of inactivated whole-cell vaccines, 5 × 107 CFU of heat-killed S. flexneri 2a strain 2457T, alone and with native and mutant toxins as adjuvants, were administered intranasally to four animals per group on days 0 and 14. Four weeks after the second immunization, immunized and unimmunized animals were challenged ocularly with 2457T (Fig. 4). When either form of mLT was used as an adjuvant, the percentage of totally protected animals (rating of 0) increased over that obtained when no adjuvant was used (63% for both mLT molecules versus 38% for heat-killed bacteria alone), as did the total percentage of protection [100% for mLT(R192G) and 88% for mLT(K63) versus 63% for heat-killed bacteria alone], although the differences were not statistically significant. When animals were immunized by the oral route of administration with EcSf2a-3 alone or with LT, mLT(R192G), or mLT(K63), there was no protection when the vaccine alone was administered while 25, 63, and 25% partial protection was observed, respectively, when the adjuvants were coadministered (data not shown). Only the addition of mLT(R192G) significantly increased protection over that obtained with heat-killed bacteria alone (P = 0.0074). As was observed with the live vaccine EcSf2a-3, the serum IgG and IgA antibody responses to the O antigen were not significantly different among the groups for either immunization route (data not shown).
FIG. 4.
Protection against challenge in animals receiving two intranasal immunizations with heat-killed S. flexneri 2a strain 2457 or heat-killed 2457T plus native or mutant toxin molecules as adjuvants. Animals were challenged with virulent strain 2457T 4 weeks after the second immunization.
DISCUSSION
The use of CT and LT as mucosal adjuvants for killed whole-cell and subunit vaccines directed against bacterial and viral pathogens has been extensively examined. These studies have focused on the ability of these molecules to enhance the immunogenicity and protection afforded by killed whole-cell Campylobacter preparations (3), inactivated influenza virus (17), and subunit vaccines such as urease from Helicobacter spp. (21, 35), pneumococcal surface protein A (36), and measles virus synthetic peptides (27). These and other studies have suggested that LT and CT can be used effectively in mucosal immunization with killed cells or virulence-associated antigens of pathogens that enter the host by a mucosal route. Following the construction of mutant detoxified CT and LT molecules (8, 28), experiments have shown that these molecules are also effective as mucosal adjuvants (4, 21, 27). The effects of recombinant Salmonella typhimurium clones expressing native and mutant toxins in the absence and presence of coexpressed heterologous antigens have been examined, and the results indicate that toxins expressed by Salmonella vectors can stimulate higher levels of immune response to the toxin molecule and the heterologous antigen (7, 12). However, the effectiveness of coadministered native and mutant toxin molecules as mucosal adjuvants for protective antigens of live attenuated vaccines has not been examined.
This study shows that mutant and native toxin molecules are also effective in enhancing the protective immunity developed by a live attenuated vaccine. Significantly higher efficacy with all immunization routes was observed when toxins were administered concurrently with the vaccine strain EcSf2a-3. The mutant toxins were as effective as the native toxin molecules as adjuvants. Although there was some variability in the efficacy of EcSf2a-3 alone, probably due to the instability of the invasive properties of the strain, the addition of toxins as adjuvants consistently gave a higher percentage of full protection against disease (rating of 0) in all experiments. This increase in efficacy was also observed when animals were immunized with 1 log fewer bacteria coadministered with LT or mLT(R192G). These results suggest that coadministration of mutant toxins might allow the administration of a smaller dose of a live, reactogenic vaccine.
When toxin molecules were used as an adjuvant with heat-killed whole-cell bacteria, the increase in efficacy was not as pronounced. In fact, only the mLT molecules were effective in increasing the percentage of fully protected animals following intranasal immunization and only mLT(R192G) had a significant effect on efficacy following oral immunization.
The mechanisms by which toxin molecules augment immunogenicity and efficacy are only partly understood. It has been shown that the toxin molecules must be administered concurrently with the antigen by the same route to achieve an adjuvant effect (20). Based on studies with coadministered antigens or killed whole-cell vaccines, the following basic mechanisms of adjuvanticity have been suggested for CT (see reference 10 for a good review): (i) enhanced uptake of coadministered antigen caused by changes in gut permeability or increased delivery into intestinal follicles, (ii) enhanced antigen presentation by affecting antigen-presenting cells, (iii) enhanced priming of CD4 T cells specific for the toxin and for the coadministered antigen, and (iv) effects on B-cell development, including increased switching to IgA and IgG. Although similar methods of adjuvanticity have been proposed for LT, there are some important differences between LT and CT. LT is thought to stimulate both the Th1 and Th2 responses, while CT apparently enhances only the Th2 response (22, 31). LT and its mutant derivatives differ from CT in that LT has an affinity for galactose-containing molecules, including glycoproteins and LPSs. Thus, LT has a broader receptor range than CT, which only binds to GM1 (15).
In this study, enhanced efficacy was observed when toxin molecules were coadministered with live attenuated Shigella vaccine strain EcSf2a-3. At the present time, we can only speculate on the mechanism(s) by which these molecules enhance the efficacy of a live vaccine strain. Since the vaccine strain is invasive, increased permeability of the gut may not contribute to the adjuvant effect. There did not appear to be a significant difference between CT versus LT molecules, including the mutant forms, suggesting that receptor range does not contribute to the adjuvant effect. The coadministration of toxin molecules may enhance the presentation of the relevant Shigella antigens and enhance the mucosal immune response at the site of exposure. There was no significant difference in the serum antibody response in animals that received toxin molecules during immunization with EcSf2a-3, indicating that serum immune responses did not appear to be dependent on the presence of an adjuvant, but the increase in the total mean O-antigen-specific IgG and IgA ASC response over that obtained with vaccine alone indicated an enhancing effect on the local immune response which was still evident after challenge. Further immune studies including cytokine responses and further examination of the local antibody response by measurements of secretory IgA in tears and lung lavage fluids from immunized animals are necessary to better determine the mechanism of action of these mucosal adjuvants.
The adjuvant mechanism for killed whole-cell vaccines may be different than that for live invasive organisms, and an increase in gut permeability may play a role in the action of toxin molecules coadministered with killed whole-cell bacteria. Experiments with mice orally immunized with a killed whole-cell Campylobacter vaccine showed enhancement of the mucosal response to Campylobacter-specific antigens as measured by intestinal secretory IgA responses (3). Further experiments with dosages of killed shigellae, coupled with examination of the local immune response, is necessary to determine whether a killed whole-cell vaccine coadministered with toxin molecules is a potential vaccine candidate for protection against shigellosis.
In conclusion, the data presented here indicate that enterotoxin molecules are effective as mucosal adjuvants for live attenuated vaccines in this model. Both the efficacy and ASC results suggest that the mutant molecules are at least as effective as adjuvants for live attenuated vaccines as the native molecules. In these studies, there was no significant difference in the adjuvant effects of the different mutant molecules on the live attenuated vaccine, while only the mLT molecules appeared to appreciably increase the efficacy of the killed whole-cell shigellae. The use of mLT or mCT with an efficacious but reactogenic vaccine administered at a lower dose or with multivalent vaccines to increase the immune response to several Shigella serotypes are possibilities that should be further explored. For use in human clinical trials, a dose-response curve for the mutant toxins coadministered with live vaccines should be examined.
ACKNOWLEDGMENTS
We express our appreciation to John D. Clements, Tulane University of Medicine, New Orleans, La., and to Rino Rappuoli, Immunobiological Research Institute Siena, Siena, Italy, for providing the mutant CT and/or LT that made this study possible.
REFERENCES
- 1.Alexander W A, Hartman A B, Oaks E V, Venkatesan M M. Construction and characterization of virG (icsA)-deleted Escherichia coli K12-Shigella flexneri hybrid vaccine strains. Vaccine. 1996;14:1053–1061. doi: 10.1016/0264-410x(96)00002-3. [DOI] [PubMed] [Google Scholar]
- 2.Apter F M, Michetti P, Winner II L S, Mack J A, Mekalanos J J, Neutra M M. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baqar S, Applebee L A, Bourgeois A L. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun. 1995;63:3731–3735. doi: 10.1128/iai.63.9.3731-3735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chong C, Friberg M, Clements J D. LT(R192G), a non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella spp. Vaccine. 1998;16:732–740. doi: 10.1016/s0264-410x(97)00255-7. [DOI] [PubMed] [Google Scholar]
- 5.Clemens J D, Sack D A, Harris J R, Chakraborty J, Neogy P K, Stanton B F, Huda N, Khan M U, Kay B A, Khan M R, Ansaruzzanan M, Yunus M, Rao R, Svennerholm A, Holmgren J. Cross protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J Infect Dis. 1988;158:372–377. doi: 10.1093/infdis/158.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 7.Covone M G, Brocchi M, Palla E, Dias da Silveira W, Rappuoli R, Galeotti C L. Levels of expression and immunogenicity of attenuated Salmonella enterica serovar typhimurium strains expressing Escherichia coli mutant heat-labile enterotoxin. Infect Immun. 1998;66:224–231. doi: 10.1128/iai.66.1.224-231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 73–87. [Google Scholar]
- 10.Elson C O. Cholera toxin as a mucosal adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press; 1996. pp. 59–72. [Google Scholar]
- 11.Hale T L. Shigella vaccines. In: Ala'Aldeen D A A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 179–204. [Google Scholar]
- 12.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartman A, Van De Verg L, Bendiuk N, Collins H. Abstracts of the General Meeting of the American Society for Microbiology 1993. Washington, D.C: American Society for Microbiology; 1993. Oral immunization of guinea pigs with and without cholera toxin as adjuvant protects against ocular challenge with Shigella, abstr. E-102; p. 160. [Google Scholar]
- 14.Hartman A B, Van De Verg L L, Collins H H, Jr, Tang D B, Bendiuk N O, Taylor D N, Powell C J. Local immune response and protection in the guinea pig keratoconjunctivitis model following immunization with Shigella vaccines. Infect Immun. 1994;62:412–420. doi: 10.1128/iai.62.2.412-420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren J, Lindblad H, Fredman P, Svennerholm L, Myrvold A. Comparison of receptors for cholera and Escherichia coli enterotoxin in human intestine. Gastroenterology. 1985;89:27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Medicine. New vaccine development: establishing priorities. 2. Diseases of importance in developing countries. Washington, D.C: National Academy Press; 1986. The prospect for immunizing against Shigella spp; pp. 329–337. [Google Scholar]
- 17.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 18.Kotloff K L, Herrington D A, Hale T L, Newland J W, Van De Verg L L, Cogan J P, Snoy P J, Sadoff J C, Formal S B, Levine M M. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992;60:2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamm M E, Nedrud J G, Kaetzel C S, Mazanec M B. New insights into epithelial cell function in mucosal immunity: neutralization of intracellular pathogens and excretion of antigens by IgA. In: Kagnoff M F, Kiyono H, editors. Essentials of mucosal immunology. New York, N.Y: Academic Press, Inc.; 1996. pp. 141–148. [Google Scholar]
- 20.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 21.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 22.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;15:4621–4629. [PubMed] [Google Scholar]
- 23.Mestecky J. The common mucosal system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 24.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newland J W, Hale T L, Formal S B. Geneotypic and phenotypic characterization of an aroD deletion-attenuated Escherichia coli K-12-Shigella flexneri hybrid vaccine expressing S. flexneri 2a somatic antigen. Vaccine. 1992;10:766–776. doi: 10.1016/0264-410x(92)90512-i. [DOI] [PubMed] [Google Scholar]
- 26.Ogra P L, Chiba Y, Beutner K R, Morag A. Vaccination by non-parenteral routes: characterization of immune response. Dev Biol Stand. 1976;33:19–26. [PubMed] [Google Scholar]
- 27.Partidos C D, Pizza M, Rappuoli R, Steward M W. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89:483–487. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizza M, Domenighini M, Hoi W, Gianelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 29.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svennerholm A M, Hanson L A, Holmgren J, Lindblad B S, Nilsson B, Quereshi F. Different secretory immunoglobulin A antibody responses to cholera vaccination in Swedish and Pakistani women. Infect Immun. 1980;30:427–430. doi: 10.1128/iai.30.2.427-430.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 32.Taylor D N, Philip D F, Zapor M, Trofa A, Van De Verg L, Hartman A, Bendiuk N, Newland J W, Formal S B, Sadoff J C, Hale T L. Outpatient studies of the safety and immunogenicity of an auxotrophic Escherichia coli K-12-Shigella flexneri 2a hybrid vaccine candidate, EcSf2a-2. Vaccine. 1994;12:565–568. doi: 10.1016/0264-410x(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 33.Walker R I, Clements J D. Use of heat-labile toxin of enterotoxigenic Escherichia coli to facilitate mucosal immunization. Vaccine Res. 1993;2:1–10. [Google Scholar]
- 34.Westphal O, Jann K. Bacterial lipopolysaccharide extraction with phenol:water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 35.Wetzin R, Kleanthous H, Guirakhoo F, Monath T P, Lee C K. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–376. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto M, Briles D E, Yamamoto S, Ohmura M, Kiyono H, McGhee J R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;15:4115–4121. [PubMed] [Google Scholar]