Abstract
For this research article, we investigated the protective effects of enzyme-treated caviar powder extract (CV) in ultraviolet B (UVB)-irradiated hairless mice and keratinocytes by confirming moisturizing-related factors and elasticity-related factors. UVB irradiation induced wrinkle formation, dehydration, oxidative stress, and inflammation in the dorsal skin of mice; however, these were suppressed in the CV-supplemented groups in UVB-irradiated hairless mice. Furthermore, in UVB-irradiated keratinocytes, CV treatment increased the antioxidant enzyme activities and the levels of sphingomyelin and hyaluronic acid and decreased the production of pro-inflammatory cytokines and the expression of IkB-α and p65 phosphorylation. These findings indicate that CV can directly protect keratinocytes against UVB irradiation-induced oxidative stress and inflammation. Therefore, we suggest that CV can protect against UVB-induced skin photoaging. Therefore, we suggest that caviar is effective for skin health by preventing UVB-induced skin photoaging.
Keywords: caviar, UVB, oxidative stress, skin photoaging
1. Introduction
The skin is an important tissue that protects the body’s internal structures from external environmental factors, such as bacteria, chemicals, temperature, humidity, and ultraviolet (UV) radiation; however, it is susceptible to damage due to direct exposure to these factors [1,2]. Among these environmental factors, exposure to UV radiation can cause serious damage to the skin, a complex biologic process known as photoaging. Maintaining skin hydration and elasticity is the key to healthy skin. By contrast, photoaging is characterized by the formation of wrinkles and the loss of skin hydration and elasticity, leading to skin aging. Ultraviolet A (UVA; 320−400 nm) and ultraviolet B (UVB; 280−320 nm) reach the earth’s surface, and UVB can be absorbed by the epidermis, playing a major role in skin damage [3,4,5].
Keratinocytes play an essential role in skin protection and repair in the outermost layer of the skin, the epidermis. UVB irradiation induces the destruction of the antioxidant defense system of keratinocytes by overproduction of reactive oxygen species (ROS). This condition can stimulate the inflammatory response and cell damage, leading to keratinocyte function impairment [6]. Hyaluronic acid synthesized by hyaluronic acid synthase (HAS) 1, HAS2, and HAS3 in keratinocytes keeps the skin moist, whereas UVB-irradiated keratinocytes fail to maintain the moisture retention capacity of the skin [7]. Moreover, inflammatory mediators secreted from the epidermis can affect the dermis, which is mainly composed of matrix components produced by fibroblasts, such as collagen, the protein responsible for the structure, elasticity, and firmness of the skin. Collagen is the most abundant matrix component in the dermis where matrix metalloproteinases (MMPs) induced by UV irradiation are expressed and degrade collagen. The inflammatory mediators stimulate the expression and activation of MMPs via the cellular MAPK signaling pathway, exacerbating collagen degradation and wrinkle formation [8,9,10].
Caviar (lightly salted fish roe products of certain fish species, especially members of the Acipenseridae family) is known worldwide as a luxurious food. It is a health-promoting food due to its nutritional value and high content of amino acids, fatty acids, and vitamins; however, few studies have evaluated its function and mechanism because of its expense [11,12,13]. In this study, we investigated whether enzyme-treated caviar powder extract (CV) could protect the skin against UVB irradiation in hairless mice through the confirmation of moisturizing- and elasticity-related factors in the skin. We also confirmed the effect of CV treatment on UVB-irradiated HaCaT cells to demonstrate whether CV directly affects keratinocytes. This study can scientifically demonstrate the physiological effects of caviar, enhancing its value.
2. Results
2.1. CV Protected Skin against UVB Irradiation-Induced Oxidative Stress in Hairless Mice
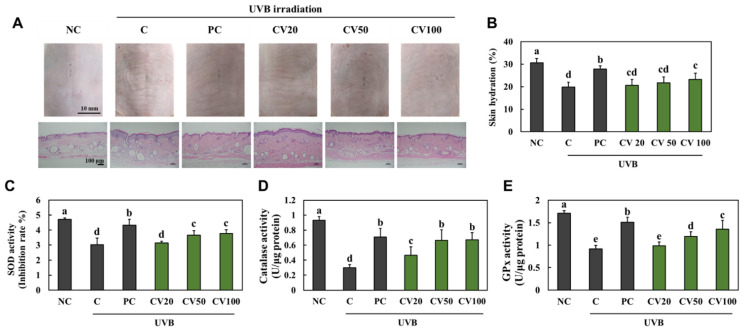
The UVB-irradiated control group (C, +UVB) showed wrinkle formation, increased epidermal thickness (Figure 1A), and decreased skin hydration (Figure 1B) compared with the normal control group (NC, −UVB). In addition, the UVB-irradiated control group had decreased activities of antioxidant enzymes, including superoxide dismutase (SOD; Figure 1C), catalase (CAT; Figure 1D), and glutathione peroxidase (GPx; Figure 1E) compared with the normal control group. However, both the group given a dietary supplement of vitamin C at 100 mg/kg (positive control, PC) and the CV-supplemented groups showed attenuation of wrinkle formation and histopathological changes induced by UVB irradiation (Figure 1A). Moreover, the vitamin C-supplemented group and the groups that received CV (especially at 100 mg/kg/body weight [bw]) had increased skin hydration and activities of antioxidant enzymes compared with the UVB-irradiated control group (p < 0.05) (Figure 1B−E). These results suggest that CV protected skin against UVB irradiation-induced oxidative stress in hairless mice.
Figure 1.
Effects of enzyme-treated caviar (CV) on morphological and histopathological changes (A), skin hydration (B), and antioxidant activities of SOD (C), CAT (D), and GPx (E) in the dorsal skin of UVB-irradiated hairless mice. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with dietary supplementation of L-ascorbic acid at 100 mg/kg/body weight [bw]), CV20 (+UVB with dietary supplementation of CV at 20 mg/kg/bw), CV50 (+UVB with dietary supplementation of CV at 50 mg/kg/bw), and CV100 (+UVB with dietary supplementation of CV at 100 mg/kg/bw). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test. SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase.
2.2. CV Suppressed UVB Irradiation-Induced Inflammation in Hairless Mice
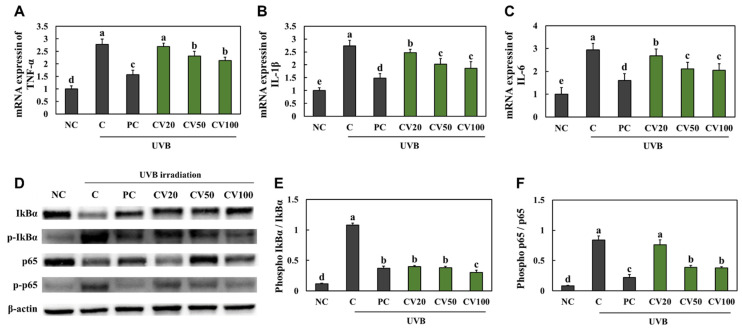
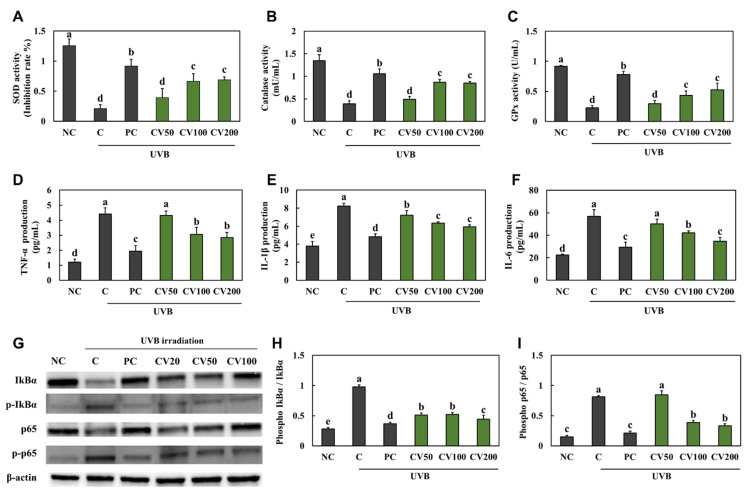
We investigated whether CV had anti-inflammatory effects on UVB irradiation-induced inflammation in hairless mice. We found that the mRNA expression levels of pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6, were significantly increased in the UVB-irradiated control group compared with the normal control group (Figure 2A−C). Moreover, the expression of IkB-α and p65 phosphorylation was significantly increased in the UVB-irradiated control group compared with the normal control group (Figure 2D−F); however, the mRNA expression levels of pro-inflammatory cytokines and expression of IkB-α and p65 phosphorylation were significantly decreased in the vitamin C-supplemented group and the CV-supplemented groups compared with the UVB-irradiated control group (p < 0.05; Figure 2A−F). Thus, our findings indicated that CV supplementation inhibited inflammation in UVB-irradiated skin.
Figure 2.
Effects of enzyme-treated caviar (CV) on mRNA expression of TNF-α (A), IL-1β (B), and IL-6 (C) and expression of IkB-α and p65 phosphorylation ((D), band image; (E), quantification of p-IkB-α/total IkB-α; (F), quantification of p-p65/total p65) in the dorsal skin of UVB irradiation-induced photoaging in mice. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with dietary supplementation of L-ascorbic acid at 100 mg/kg/body weight [bw]), CV20 (+UVB with dietary supplementation of CV at 20 mg/kg/bw), CV50 (+UVB with dietary supplementation of CV at 50 mg/kg/bw), and CV100 (+UVB with dietary supplementation of CV at 100 mg/kg/bw). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test.
2.3. CV Increased the Activation of Elasticity Factors in UVB-Irradiated Hairless Mice
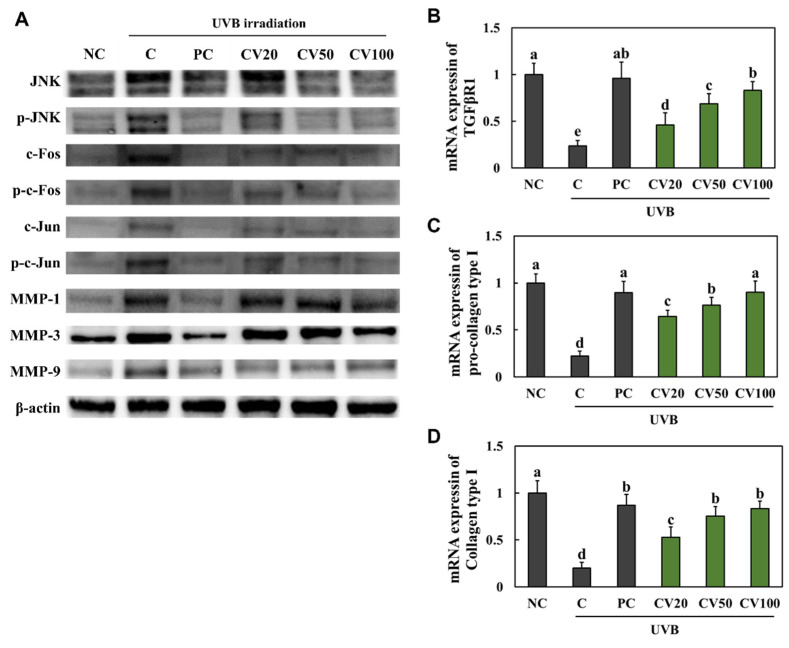
We measured the expression levels of proteins involved in the wrinkle formation pathway; c-Jun n-terminal kinase (JNK)/c-FOS/c-Jun/MMPs, and the mRNA expression levels of elasticity factors; transforming growth factor-β receptor I (TGF-β RI), pro-collagen type I, and collagen type I in the dorsal skin tissues. We found that the protein phosphorylation of JNK, c-FOS, and c-Jun and the protein expression of MMPs were increased in the UVB-irradiated control group compared with the normal control group (Figure 3A and Figure S1). The mRNA expression levels of TGF-β RI, pro-collagen type I, and collagen type I were decreased in the UVB-irradiated control group compared with the normal control group (Figure 3B−D). However, the protein phosphorylation of JNK, c-FOS, and c-Jun and the protein expression of MMPs were decreased in the vitamin C-supplemented group and the CV-supplemented groups compared with the UVB-irradiated control group (Figure 3A). Moreover, the mRNA expression levels of TGF-β RI, pro-collagen type I, and collagen type I were increased in the vitamin C-supplemented group and the CV-supplemented groups compared with the UVB-irradiated control group (Figure 3B−D).
Figure 3.
Effects of enzyme-treated caviar (CV) on protein expression of JNK/c-FOS/c-Jun/MMPs pathway (A) and mRNA expression of TGF-β RI (B), pro-collagen type I (C), and collagen type I (D) in the dorsal skin of UVB-irradiated hairless mice. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with dietary supplementation of L-ascorbic acid at 100 mg/kg/body weight [bw]), CV20 (+UVB with dietary supplementation of CV at 20 mg/kg/bw), CV50 (+UVB with dietary supplementation of CV at 50 mg/kg/bw), and CV100 (+UVB with dietary supplementation of CV at 100 mg/kg/bw). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test.
2.4. CV Increased Moisturizing Capacity in UVB-Irradiated Hairless Mice
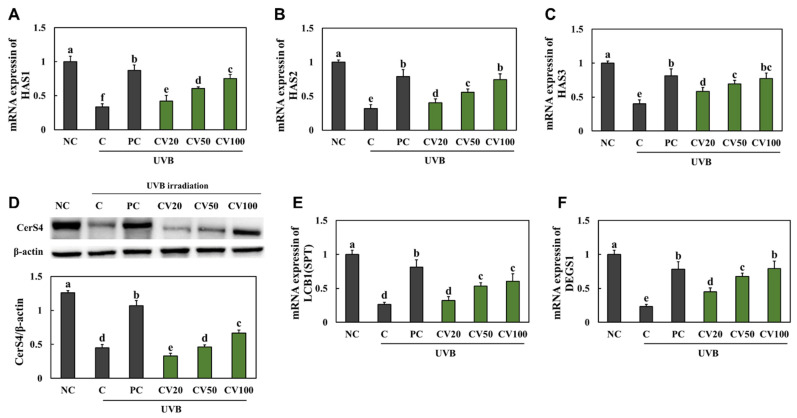
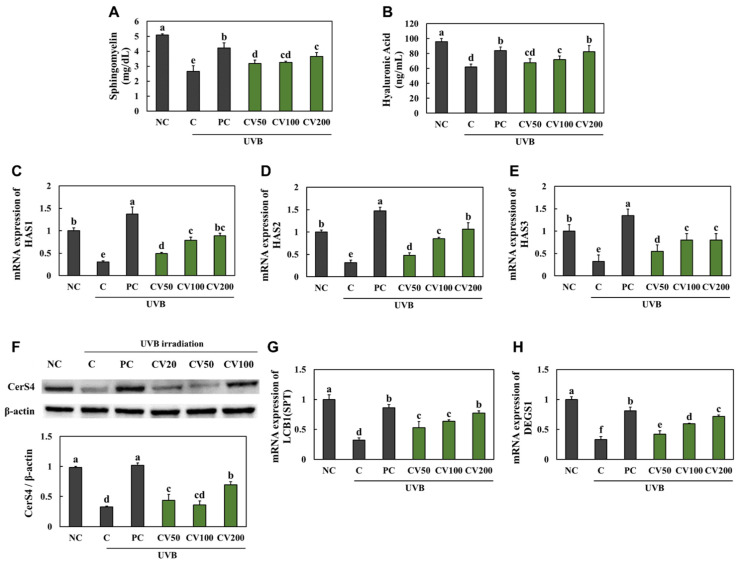
We found that the mRNA expression levels of HAS1 (Figure 4A), HAS2 (Figure 4B), HAS3 (Figure 4C), SLC35D1 (Figure 4E), and DEGS1 (Figure 4F) and the protein expression level of ceramide synthase 4 (CerS4) (Figure 4D) were significantly decreased in the UVB-irradiated control group compared with the normal control group; however, they were increased in the vitamin C-supplemented group and the CV-supplemented groups compared with the UVB-irradiated control group (p < 0.05). These data indicate that CV supplementation can suppress the degradation of collagens in UVB-irradiated skin.
Figure 4.
Effects of enzyme-treated caviar (CV) on mRNA expression of HAS1 (A), HAS2 (B), and HAS3 (C), protein expression of CerS4 (D), and mRNA expression of SLC35D1 (E) and DEGS1 (F) in the dorsal skin of UVB-irradiated hairless mice. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with dietary supplementation of L-ascorbic acid at 100 mg/kg/body weight [bw]), CV20 (+UVB with dietary supplementation of CV at 20 mg/kg/bw), CV50 (+UVB with dietary supplementation of CV at 50 mg/kg/bw), and CV100 (+UVB with dietary supplementation of CV at 100 mg/kg/bw). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test.
2.5. CV Suppressed UVB Irradiation-Induced Oxidative Stress and Inflammation in Keratinocytes
CV treatment showed no signs of cytotoxicity up to 400 μg/mL in HaCaT cells (Figure S2), we thus investigated the effect of 50, 100, and 200 μg/mL CV in HaCaT cells. In the UVB-irradiated HaCaT cells (C, +UVB), the activities of antioxidant enzymes, including SOD (Figure 5A), CAT (Figure 5B), and GPx (Figure 5C), were decreased compared with the normal HaCaT cells (NC, −UVB). The production of pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6, were significantly increased in the UVB-irradiated HaCaT cells compared with the normal HaCaT cells (Figure 5D−F). Moreover, the expression of IkB-α and p65 phosphorylation was significantly increased in the UVB-irradiated HaCaT cells compared with the normal HaCaT cells (Figure 5G−I). However, vitamin C- or CV-treated cells showed an increase in the activities of antioxidant enzymes (Figure 5A−C) and a decrease in the production of pro-inflammatory cytokines and expression of IkB-α and p65 phosphorylation (Figure 5D−F) (p < 0.05). These results suggest that CV can protect keratinocytes against UVB irradiation-induced oxidative stress and inflammation.
Figure 5.
Effects of enzyme-treated caviar (CV) on antioxidant activities of SOD (A), CAT (B), and GPx (C), production of TNF-α (D), IL-1β (E), and IL-6 (F), and phosphorylation expression of IkB-α and p65 ((G), band image; (H), quantification of p-IkB-α/total IkB-α; (I), quantification of p-p65/total p65) in UVB-irradiated HaCaT cells. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with L-ascorbic acid treatment at 100 μg/mL), CV50 (+UVB with treatment of CV at 50 μg/mL), CV100 (+UVB with treatment of CV at 100 μg/mL), and CV200 (+UVB with treatment of CV at 200 μg/mL). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test.
2.6. CV Increased the Moisturizing Capacity in UVB-Irradiated Keratinocytes
We investigated whether CV had a direct effect on the hydration of keratinocytes. The levels of sphingomyelin (Figure 6A) and hyaluronic acid (Figure 6B), mRNA expression levels of HAS1 (Figure 6C), HAS2 (Figure 6D), HAS3 (Figure 6E), SLC35D1 (Figure 6G), and DEGS1 (Figure 6H), and protein expression level of CerS4 (Figure 6F) were significantly decreased in the UVB-irradiated HaCaT cells compared with the normal HaCaT cells. However, they were significantly increased in the vitamin C- or CV-treated cells compared with the UVB-irradiated cells (Figure 6) (p < 0.05).
Figure 6.
Effects of enzyme-treated caviar (CV) on levels of sphingomyelin (A) and hyaluronic acid (B), mRNA expression of HAS1 (C), HAS2 (D), and HAS3 (E), protein expression of CerS4 (F), and mRNA expression of SLC35D1 (G) and DEGS1 (H) in UVB-irradiated HaCaT cells. Normal control (NC, −UVB), control (C, +UVB), L-ascorbic acid (positive control, PC; +UVB with L-ascorbic acid treatment at 100 μg/mL), CV50 (+UVB with treatment of CV at 50 μg/mL), CV100 (+UVB with treatment of CV at 100 μg/mL), and CV200 (+UVB with treatment of CV at 200 μg/mL). Values are presented as mean ± SD. Different letters (a > b > c > d > e) indicate a significant difference at p < 0.05 by Duncan’s multiple range test.
3. Discussion
Assays performed by Lee et al. [13] demonstrated that caviar extracted with 70% ethanol inhibited UVB-induced skin aging by stimulation of adipocyte differentiation and adiponectin production. To identify caviar’s components inhibiting skin aging, we demonstrated the skin protective effect of CV, which excluded the lipid components after supercritical CO2 extraction. We found that the protein amount was increased by supercritical CO2 extraction than by 70% ethanol extraction (Table S1) enzyme-treated extraction had free amino acids, especially high leucine (Table S2). Murakami et al. [14] demonstrated that oral administration of combinations of branched-chain amino acid, and glutamine or proline improved skin collagen protein synthesis, but a single oral administration of the amino acid had no effect. Therefore, we expected that the enzyme-treated extraction contains various free amino acids to improve UVB irradiation-induced skin photoaging.
It is well known that UVB irradiation induces ROS overproduction, inducing skin damage. When ROS production levels exceed a certain threshold, the overproduction of ROS interferes with the cell’s antioxidant defenses, exacerbating the decline in the activity of antioxidant enzymes, a phenomenon known as oxidative stress [15]. Filip et al. [16] found that single-dose 240 mJ/cm2 UVB irradiation in the dorsal skin of mice resulted in an increase in GPx activity compared with normal control mice. However, UVB irradiation for 5 days (Ahn et al. [17]) and UVB irradiation for 10 weeks (Kong et al. [18]) induced a decrease in GPx activity in the dorsal skin. Therefore, the effect on the antioxidant system depends on the frequency and duration of UVB irradiation. In the present study, we showed that UVB irradiation for 8 weeks caused inhibition of antioxidant enzyme activities in the dorsal skin of hairless mice. However, consistent with our expectations, dietary supplementation of CV protected against UVB irradiation-induced oxidative stress in the dorsal skin. Katayama et al. [19] demonstrated the antioxidative effects of amino acids on hydrogen peroxide (H2O2)-induced oxidative stress in human intestinal epithelial cells. Therefore, we suggest that CV suppresses oxidative stress caused by UVB irradiation because CV contains free amino acids.
There is a close relationship between oxidative stress and inflammation in the pathogenesis of various skin diseases [20]. UVB-mediated oxidative stress stimulates a number of transcription factors, including active protein 1 (AP-1) and nuclear factor kappa B (NF-κB) p65, involved in the inflammatory response by ROS accumulation [5,9,20]. We found that the mRNA expression of pro-inflammatory cytokines and expression of IkB-α and p65 phosphorylation in UVB-irradiated dorsal skin and the production of pro-inflammatory cytokines and expression of IkB-α and p65 phosphorylation in UVB-irradiated keratinocytes were increased, indicating that UVB irradiation caused inflammation in the epidermis. Moreover, it was confirmed that the production of sphingomyelin and hyaluronic acid, which contribute to skin moisturizing, decreased in UVB-irradiated keratinocytes, suggesting the impairment of keratinocyte function by UVB irradiation. However, our data showed that CV suppressed the expression of inflammatory factors in both the UVB-irradiated dorsal skin of mice and UVB-irradiated keratinocytes and increased the production of sphingomyelin and hyaluronic acid in UVB-irradiated keratinocytes. These findings indicate that CV attenuated inflammation and cell damage by directly inhibiting oxidative stress in keratinocytes.
A typical symptom of photoaging is the formation of deep wrinkles. The pro-inflammatory cytokines secreted from keratinocytes can stimulate fibroblasts in the dermis and activate MMPs, including MMP-1 (collagenase), MMP-3 (stromelysin-1), and MMP-9 (gelatinase), by JNK-mediated-c-Fos and c-Jun. These upregulated MMPs degrade matrix components, especially collagen, resulting in wrinkle formation [21,22,23]. Our data showed that dietary supplementation of CV suppressed wrinkle formation, phosphorylation of JNK, c-Fos, and c-Jun, and the expression of MMPs in the UVB-irradiated dorsal skin of mice. We suggest that CV treatment can suppress wrinkle formation by directly inhibiting the development of oxidative stress and inflammation in keratinocytes.
4. Materials and Methods
4.1. Preparation of CV
The caviars were obtained from a sturgeon farming company (Almascaviar, Inc., Seoul, Korea). Caviar was freeze-dried and used for the preparation. The dried caviar (5 kg) was extracted using a supercritical CO2 extraction system (Ilsin Autoclave, Inc., Daejeon, Korea). The extraction conditions were a pressure of 400 bar, a temperature of 40 °C, a flow rate of 60 mL/min, and an extraction time of 300 min to separate residue and lipids. The residue, excluding the lipid components, was extracted with enzymes in 10 volumes (w/v) of distilled water at pH 7, 50 °C for 4 h, followed by heat-induced inactivation of the enzymes at 100 °C for 1 h. The enzymes used were Prozyme1000L (Vision Biochem Co., Ltd., Seoul, Korea) and Protamex (Novozymes Co., Ltd., Bagsvaerd, Denmark). The enzyme-treated product was filtered and concentrated and then lyophilized to obtain enzyme-treated powder (1 kg).
4.2. Animals and UVB Irradiation
The Institutional Animal Care and Use Committee of Kyung Hee University approved the protocol (KHGASP-21–325) for the animal studies. The animals were cared for in accordance with the “Guidelines for Animal Experiments” established by the university. Male hairless mice (SKH-1) (5-week-old) were purchased from SaeRon Bio (Uiwang, Korea) and housed in cages under automatically controlled temperature (23 ± 2 °C), humidity (50 ± 5%), and lighting (12:12-h light−dark cycle). All animals were acclimatized for 7 days before the experiment, fed standard pellet chow, and given fresh water ad libitum. All mice were randomly divided into six groups (eight animals/group): normal control (−UVB), control (+UVB), L-ascorbic acid (positive control, PC; +UVB with dietary supplementation of L-ascorbic acid at 100 mg/kg/body weight [bw]), CV20 (+UVB with dietary supplementation of CV at 20 mg/kg/bw), CV50 (+UVB with dietary supplementation of CV at 50 mg/kg/bw), and CV100 (+UVB with dietary supplementation of CV at 100 mg/kg/bw).
Skin wrinkles were induced using a UVB lamp (Sankyo Denki Co., Yokohama, Japan) according to methods described previously [24]. The minimal erythematous dose (MED) was set at 150 mJ/cm2. Each time intensity of UVB irradiation was 1 MED (150 mJ/cm2) at week 1; 2 MED (300 mJ/cm2) at week 2; 3 MED (450 mJ/cm2) at week 3; and 4 MED (600 mJ/cm2) at week 4 to 8. At the end of the 8 weeks, all animals were sacrificed, and the dorsal skin and blood (by orbital venipuncture) were collected for analysis.
4.3. Histological Observation
Dorsal skin tissues were fixed in 10% buffered formalin and then embedded in paraffin. The paraffin blocks were sliced into 4 μm sections that were subsequently stained with hematoxylin and eosin (H&E) for histopathological observation.
4.4. Measurement of Skin Hydration
Hydration of the dorsal skin surface was measured with the Howskin device (Seoul, Korea) according to previously described methods [24], under standardized conditions of 22–24 °C and 55–60% humidity.
4.5. Measurement of Antioxidant Enzyme Activity
Dorsal skin tissues were collected from mice, and the activities of SOD, CAT, and GPx were measured using respective assay kits purchased from BioVision, Inc. (Milpitas, CA, USA); superoxide dismutase 1 (SOD1) (Mouse) ELISA Kit, Catalase Activity Colorimetric/Fluorometric Assay Kit, and Glutathione Peroxidase Activity Colorimetric Assay Kit, according to methods described previously [24].
4.6. Cell Culture and Treatments
HaCaT cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s minimal essential medium (Hyclone Laboratories, Logan, UT, USA) with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), 100 mg/L penicillin–streptomycin (Hyclone Laboratories, Logan, UT, USA), and 2 mmol/L glutamine (Hyclone Laboratories, Logan, UT, USA). HaCaT cells were exposed to UVB (50 mJ/cm2) using a UVB, followed by treatment with 100 µg/mL L-ascorbic acid or CV. The treated HaCaT cells were then harvested for further assays.
4.7. Total RNA Isolation and Real-Time PCR
Total RNA was extracted from dorsal skin tissues and HaCaT cells and analyzed for expression of the genes TNF-α, IL-1β, IL-6, TGF-β RI, pro-collagen type I, collagen type I, HAS1, HAS2, HAS3, SLC35D1, DEGS1, and GAPDH, by real-time PCR according to methods described previously [24].
4.8. Protein Extraction and Western Blot Analysis
Proteins were extracted from dorsal skin tissues and HaCaT cells and analyzed for the expression of IkB-α, phospho-IkB-α, p65, phospho-p65, JNK, phospho-JNK, c-FOS, phospho-c-FOS, c-Jun, phospho-c-Jun, MMP-1, MMP-3, MMP-9, CerS4, and β-actin, according to methods described previously [24].
4.9. Measurement of Pro-Inflammatory Cytokines
The levels of TNF-α, IL-1β, and IL-6 in the HaCaT cells supernatant were measured using the DuoSet ELISA Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s method.
4.10. Measurement of Sphingomyelin and Hyaluronic Acid
HaCaT cells were lysed, and the levels of sphingomyelin and hyaluronic acid were then determined using the Sphingomyelin Assay Kit (Abcam, Cambridge, UK) and the Hyaluronic Acid ELISA Kit (BioVision, Inc., Milpitas, CA, USA), respectively, according to the manufacturer protocols.
4.11. Statistical Analysis
All data are presented as mean ± standard deviation (SD). The data were statistically evaluated by Duncan’s multiple range test after one-way ANOVA using SPSS statistical procedures (SPSS PASW Statistic 23.0, SPSS, Inc., Chicago, IL, USA). Statistically significant differences were considered at p < 0.05.
5. Conclusions
In the present study, we investigated the protective effect of CV against UVB irradiation-induced skin photoaging. We demonstrated that CV supplementation suppressed UVB irradiation-induced wrinkle formation, oxidative stress, inflammation, and moisture loss. In UVB-irradiated keratinocytes treated with CV, the CV treatment reduced oxidative stress and inflammation, besides increasing the expression of moisturizing-related factors. These findings indicated that CV treatment could suppress wrinkle formation by directly inhibiting oxidative stress and inflammation in keratinocytes. This study provides scientific evidence for the beneficial effects of caviar on skin health.
Acknowledgments
Not applicable.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20110685/s1.
Author Contributions
Conceptualization, O.-K.K.; methodology, J.P. and O.-K.K.; data curation, J.P. and O.-K.K.; investigation, J.P., D.K., M.L., S.H., W.J., H.M.J., Y.K.K., G.H.N., S.H.H. and J.H.; supervision, O.-K.K.; writing—original draft, J.P. and O.-K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Institutional Animal Care and Use Committee of Kyung Hee University approved the protocol (KHGASP-21–325) for animal studies. The animals were cared for in accordance with the “Guidelines for Animal Experiments” established by the university.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chuong C.M., Nickoloff B.J., Elias P.M., Goldsmith L.A., Macher E., Maderson P.A., Sundberg J.P., Tagami H., Plonka P.M., Thestrup-Pederson K., et al. What is the ‘true’ function of skin? Exp. Dermatol. 2002;11:159–187. doi: 10.1034/j.1600-0625.2002.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boer M., Duchnik E., Maleszka R., Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy. Dermatol. Alergol. 2016;33:1–5. doi: 10.5114/pdia.2015.48037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rittié. L., Fisher G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002;1:705–720. doi: 10.1016/S1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 4.Dai G., Freudenberger T., Zipper P., Melchior A., Grether-Beck S., Rabausch B., de Groot J., Twarock S., Hanenberg H., Homey B., et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007;171:1451–1461. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 6.Lam P.L., Wong M.M., Hung L.K., Yung L.H., Tang J.C., Lam K.H., Chung P.Y., Wong W.Y., Ho Y.W., Wong R.S., et al. Miconazole and terbinafine induced reactive oxygen species accumulation and topical toxicity in human keratinocytes. Drug Chem. Toxicol. 2022;45:834–838. doi: 10.1080/01480545.2020.1778019. [DOI] [PubMed] [Google Scholar]
- 7.Kang W., Choi D., Park T. Dietary Suberic Acid Protects Against UVB-Induced Skin Photoaging in Hairless Mice. Nutrients. 2019;11:2948. doi: 10.3390/nu11122948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavinato M., Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017;94:78–82. doi: 10.1016/j.exger.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Chiang H.M., Chen H.C., Chiu H.H., Chen C.W., Wang S.M., Wen K.C. Neonauclea reticulata (Havil.) Merr Stimulates Skin Regeneration after UVB Exposure via ROS Scavenging and Modulation of the MAPK/MMPs/Collagen Pathway. Evid. Based Complement Alternat. Med. 2013;2013:324864. doi: 10.1155/2013/324864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y.I., Kim K.S., Ahn H.J., Kang I.H., Shin M.K. Reduced matrix metalloproteinase and collagen transcription mediated by the TGF-beta/Smad pathway in passaged normal human dermal fibroblasts. J. Cosmet. Dermatol. 2020;19:1211–1218. doi: 10.1111/jocd.13114. [DOI] [PubMed] [Google Scholar]
- 11.Xu X., Lu S., Li X., Bai F., Wang J., Zhou X., Gao R., Zeng M., Zhao Y. Effects of microbial diversity and phospholipids on flavor profile of caviar from hybrid sturgeon (Huso dauricus × Acipenser schrencki) Food Chem. 2022;377:131969. doi: 10.1016/j.foodchem.2021.131969. [DOI] [PubMed] [Google Scholar]
- 12.Vasconi M., Tirloni E., Stella S., Coppola C., Lopez A., Bellagamba F., Bernardi C., Moretti V.M. Comparison of Chemical Composition and Safety Issues in Fish Roe Products: Application of Chemometrics to Chemical Data. Foods. 2020;9:540. doi: 10.3390/foods9050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K.E., Nho Y.H., Yun S.K., Park S.M., Kang S., Yeo H. Caviar Extract and Its Constituent DHA Inhibits UVB-Irradiated Skin Aging by Inducing Adiponectin Production. Int. J. Mol. Sci. 2020;21:3383. doi: 10.3390/ijms21093383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami H., Shimbo K., Inoue Y., Takino Y., Kobayashi H. Importance of amino acid composition to improve skin collagen protein synthesis rates in UV-irradiated mice. Amino Acids. 2012;42:2481–2489. doi: 10.1007/s00726-011-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valko M., Rhodes C.J., Moncol J., Izakovic M., Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Filip A., Daicoviciu D., Clichici S., Mocan T., Muresan A., Postescu I.D. Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin. J. Med. Food. 2011;14:761–766. doi: 10.1089/jmf.2010.0142. [DOI] [PubMed] [Google Scholar]
- 17.Ahn J.H., Kim D.W., Park C.W., Kim B., Sim H., Kim H.S., Lee T.K., Lee J.C., Yang G.E., Her Y., et al. Laminarin Attenuates Ultraviolet-Induced Skin Damage by Reducing Superoxide Anion Levels and Increasing Endogenous Antioxidants in the Dorsal Skin of Mice. Mar. Drugs. 2020;18:345. doi: 10.3390/md18070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong Y.H., Xu S.P. Juglanin administration protects skin against UVB-induced injury by reducing Nrf2-dependent ROS generation. Int. J. Mol. Med. 2020;46:67–82. doi: 10.3892/ijmm.2020.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katayama S., Mine Y. Antioxidative activity of amino acids on tissue oxidative stress in human intestinal epithelial cell model. J. Agric. Food Chem. 2007;55:8458–8464. doi: 10.1021/jf070866p. [DOI] [PubMed] [Google Scholar]
- 20.Bickers D.R., Athar M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 21.Brenneisen P., Sies H., Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta J., Kar S., Liu R., Joseph J., Kalyanaraman B., Remington S.J., Chen C., Melendez J.A. Reactive oxygen species control senescence-associated matrix metalloproteinase-1 through c-Jun-N-terminal kinase. J. Cell. Physiol. 2010;225:52–62. doi: 10.1002/jcp.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romana-Souza B., Monte-Alto-Costa A. Olive oil inhibits ageing signs induced by chronic stress in ex vivo human skin via inhibition of extracellular-signal-related kinase 1/2 and c-JUN pathways. Int. J. Cosmet. Sci. 2019;41:156–163. doi: 10.1111/ics.12520. [DOI] [PubMed] [Google Scholar]
- 24.Kim O.K., Kim D., Lee M., Park S.H., Yamada W., Eun S., Lee J. Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants. 2021;10:1452. doi: 10.3390/antiox10091452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.