Abstract
Sugary soft drinks modify salivary pH and favor bacterial proliferation and are associated with the development of caries. Information on the effects of consuming carbonated drinks without sucrose is limited. Methods: In this crossover clinical trial, salivary and dental biofilm pH were determined at 0, 5, 10, 15, 30, 45, and 60 min after the participants (n = 18) ingested a soft drink with sucrose, a soft drink with aspartame/acesulfame K, carbonated water, and plain water on different days. Dental biofilm cultures were conducted at 0- and 120-min. Results: Salivary pH decreased significantly after ingestion of the sucrose-containing soft drink when compared with the other types of beverages (median difference, −0.3–−0.4, p ≤ 0.05), and the greatest difference was found with mineral water. A greater bacterial proliferation (Colony Forming Units [CFU]) was observed after ingestion of the drink with sucrose (↑310 × 103 CFU, p ≤ 0.01), followed by the drink with aspartame/acesulfame K (↑160 × 103 CFU, p ≤ 0.01) and carbonated water (↑60 × 103 CFU, p ≤ 0.05). No significant changes in bacterial proliferation were observed after the consumption of natural water. Conclusions: Ingestion of sucrose-containing soft drinks favors the acidification of salivary pH and the bacterial proliferation of dental biofilm. Although to a lesser extent, soft drinks containing aspartame/acesulfame K also favor bacterial proliferation.
Keywords: pH, caries, dental biofilm, carbonated beverages, saliva, artificial sweeteners
1. Introduction
The World Health Organization defines dental caries as “a localized, post-eruptive, pathological process of external origin involving softening of the hard tooth structure and proceeding to the formation of a cavity” [1]. Caries is a dynamic, non-communicable disease, modulated by diet and mediated by dental biofilm [2]. It is the main dental problem worldwide and affects individuals starting in their first stages of life. Levels of prevalence have been described for the schoolchildren population around the world between 60% and 90% [3]. The prevalence among Mexican adolescents has been estimated at 72.7% [4].
Dental caries is considered a multifactorial disease that involves the following elements in its development: 1. risk factors (low income level, lower educational level, poor oral health behaviors, genetic factors, etc.); 2. frequent exposure to dietary sugars that increase the proliferation of specific dental biofilm (Streptococcus mutans); 3. a decrease in pH brought about by the generation of acids (lactic, propionic, and butyric acid) from bacterial metabolism (unlike dental erosion); and 4. Permanent tooth demineralization [5,6,7,8].
Tooth enamel is an acellular tissue composed of minerals (85% in volume) described as substituted calcium hydroxyapatite, water (12% by volume), and organic, usually proteins and lipids (3% by volume). The mineral component of enamel is basically calcium hydroxyapatite and the stoichiometric formula for hydroxyapatite is Ca10 (PO4)6 (OH)2 [9]. Hydroxyapatite dissolution is the chemical loss of the minerals in the dental hard tissues through the continuous decrease in pH in the oral cavity [3].
Dental biofilm is made up of billions of bacteria (the majority of which are only found in the oral cavity), growing in a mass of soluble and insoluble carbohydrates [9]. The oral bacteria Streptococcus mutans is generally recognized as a major etiological factor in dental caries, it resides primarily in dental biofilm on the tooth surfaces [10] and has different capabilities: 1. Acidogenicity: the ability to transport and metabolize carbohydrates into organic acids, 2. Aciduricity: the ability to thrive under low pH and 3. Polymers synthesis: the ability to synthesize extracellular polymers of glucan from sucrose which promotes adhesion to the teeth and assists colonization [9].
Therefore, the frequent intake of sucrose results in an optimal substrate for its metabolism and multiplication and creates favorable niches for the proliferation of other bacterial species [10].
Salivary pH at rest ranges from 6.2 to 7.6 [11]. Currently, the hydroxyapatite crystals dissolve minimally in saliva and have the capacity to reintegrate into tooth enamel. Pathogenic bacteria can produce acids and reduce the pH to ≤5.5 (the critical pH for dental health) after the ingestion of fermentable carbohydrates. This forms acid phosphate complexes that do not have the ability to re-incorporate into the tooth, giving rise to dental demineralization [9,12,13,14]. Finally, components are secreted through the saliva that favor the gradual return of the pH to the baseline level through inorganic elements such as bicarbonate, calcium, and phosphate ions that make up the buffer system, which limits the loss of minerals in the dental structure [15,16,17].
Saliva contributes to maintaining the oral microenvironment through the presence of numerous cationic peptides, immunoglobulin A, and salivary proteins in addition to regulating the pH [18,19,20,21]. However, these defense mechanisms may not be efficient in cases when there is frequent exposure to exogenous aggressors, such as the consumption of food o beverages high in sugar [9,22].
Non-caloric sweeteners (aspartame/acesulfame K, sucralose, Stevia©) have been incorporated into these products as an alternative to the use of sugar to sweeten soft drinks, and they produce a high sensation of the sweet taste with very low concentrations. Their consumption has increased in recent years [23]. Their incorporation into these beverages has been approved and they are considered to be safe substances for consumption [24]. However, there is limited information on their effects on oral health.
Drinks with sweeteners are known to produce a smaller drop in salivary pH when compared with sucrose-containing drinks [25,26]. However, the effects of other carbonated beverages with and without noncaloric sweeteners on both pH and bacterial growth have not been studied in depth. For this reason, our objective was to compare the effect of consuming soft drinks with sucrose, soft drinks with aspartame/acesulfame K, carbonated water, and natural water on oral pH and bacterial proliferation.
2. Materials and Methods
2.1. Methodology
This was a crossover randomized clinical trial (with the objective that each individual would be their own comparator), performed between January 2018 and February 2019 at Hospital Infantil de México Federico Gómez. Adolescents aged 12–18 years, of both sexes, who reported habitual consumption of soft drinks, and when evaluated by a pediatric dentist had a DMFT (Decayed, Missing, and Filled Teeth) index [27] of at least 3, were included. Sociodemographic data were collected, as well as information about the frequency of consumption of cola drinks and tooth brushing habits. The exclusion criteria were as follows: not undergoing orthodontic treatment, not having received a topical application of fluoride during the last 3 months, not having a motor disability that interfered with tooth brushing, not consuming drugs or being carriers of diseases that cause xerostomia, not being under antibiotic therapy during the study period, and not having active periodontal infections. All participants and tutors signed letters of assent and informed consent forms, respectively.
2.2. Intervention
After being selected by an investigator, participants attended four sessions with a 1-week washout interval. A standardized diet was indicated for dinner the day before each of the visits (sandwich with turkey ham and semi-skimmed milk). Subsequently, participants could ingest only plain water for up to 2 h prior to the session. They were also asked not to brush their teeth during the 48 h before the study.
All participants received 355 mL of plain water in the first session. The sequence of soft drinks for the three following appointments was randomly determined by an investigator using a computer algorithm. Then, an investigator blinded to allocation gave each participant 355 mL of one of the following types of soft drink: (1) soft drink with sucrose, (2) soft drink with aspartame/acesulfame K, and (3) carbonated water. The drinks were consumed at a temperature close to 4 °C in a maximum time of 10 min.
The decision to collect pH data at different times was based on earlier studies that reported differences caused by beverages containing caloric and non-caloric sweeteners, as well as differences in bacterial proliferation [25].
2.3. Outcomes
2.3.1. Salivary pH
Participants were asked to spit 2 mL of saliva into a wide-mouth sterile vial at baseline and at 5, 10, 15, 30, 45, and 60 min after consuming each of the beverages. pH was measured with a HANNA HI 221 potentiometer (HANNA Instruments Inc. Woonsocket-RI-USA, Romania), with previous calibration of the electrode using buffer solutions of pH 4.0 and 7.0. The electrode was washed with distilled water between each reading.
2.3.2. Dental Biofilm pH
Samples of dental biofilm were obtained using a sterile dental explorer in dental sites that represented all buccal quadrants at baseline and at 5, 10, 15, 30, 45, and 60 min after consuming each of the beverages, with a 30-s collection time. The sample was placed in 2 mL of sterile bi-distilled water and pH was determined using a potentiometer (HANNA Instruments Inc. Woonsocket, RI, USA).
2.3.3. Bacterial Growth of Dental Biofilm
Dental biofilm samples were obtained using a sterile explorer from dental sites representing all buccal quadrants at baseline and 120 min after ingestion of each of the beverages. The samples were placed in BHI (Brain Heart Infusion) culture medium. A 50-microliter aliquot was deposited in 4950 microliters of physiological saline solution and placed in a vortex until the sample was homogenized. Subsequently, 100 mL of the solution was placed in plates with Todd-Hewitt culture medium and spread evenly with a sterile glass angle. The plates were incubated at 37 °C for 24–48 h in an environment supplemented with 10% CO2. The lyophilized strain of Streptococcus mutans American Type Culture Collection (ATCC) MicroBioLogics (35668) was used as a reference for comparison.
3. Statistical Analysis
Variables were described with frequencies and percentages or medians and interquartile range (IQR) according to the type of variable. Salivary pH and dental biofilm at different times were compared using Friedman’s analysis with adjustment for multiple comparisons using Dunn’s correction. Changes in the bacterial proliferation of the dental biofilm at baseline and at 120 min were compared using the Wilcoxon test and the drinks were compared using the Kruskal–Wallis test. The statistical program SPSS v. 22 was used and statistical significance was considered with a p ≤ 0.05.
4. Results
4.1. Flow Diagram
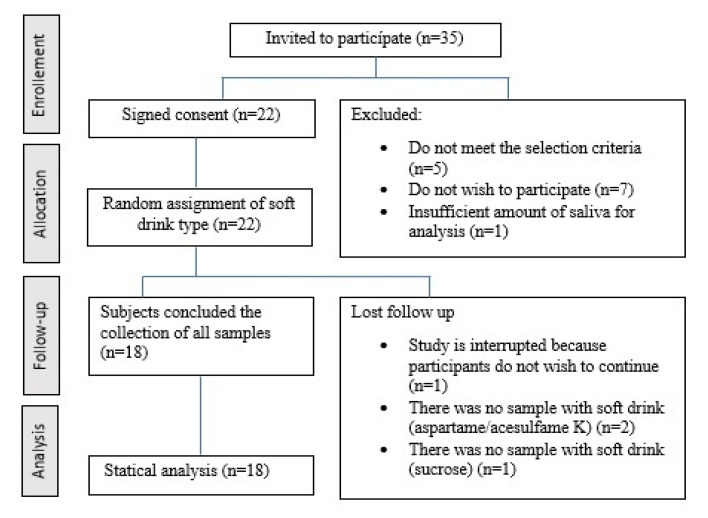
The number of participants chosen and who continued after the exclusion criteria were applied and the loss of follow-up in the study are shown in Figure 1.
Figure 1.
Diagram of subjects included in the study.
4.2. Participants’ General Characteristics
All were consumers of soft drinks, most performed oral hygiene at least twice a day, and it was determined that all had caries lesions during the oral examination (Table 1).
Table 1.
General characteristics of the participants (n = 18).
| Characteristics | n | % |
|---|---|---|
| Female | 13 | (72.2) |
| Consumption of 1–2 soft drinks per day n (%) | 18 | (100.0) |
| Tooth brushing 2 times a day n (%) | 16 | (88.9) |
| median | IQR | |
| Age (years) | 17.0 | (17.0–17.0) |
| DMFT | 3.5 | (2.0–4.0) |
IQR: interquantile range; DMFT (Decayed, Missing and Filled Teeth).
4.3. Kinetics of Salivary pH
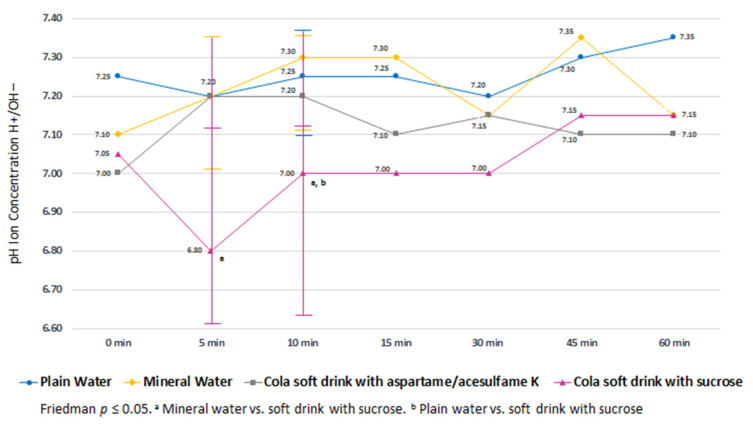
A significant reduction in pH can be seen during the first 5 min with a median pH of 6.80 (IQR, 6.35–7.10) after ingesting the soft drink with sucrose. Subsequently, there is a gradual recovery that reaches baseline pH levels up to 45 min.
The pH obtained after the consumption of the drink with sucrose at 5 and 10 min was significantly lower than that seen with the other drinks (p < 0.05). There were no significant differences in salivary pH after ingestion of soft drinks with aspartame/acesulfame K, carbonated water, and plain water, and their values remained >7.0 (Figure 2).
Figure 2.
Kinetics of salivary pH after ingesting different types of beverages.
4.4. Kinetics of Dental Biofilm pH
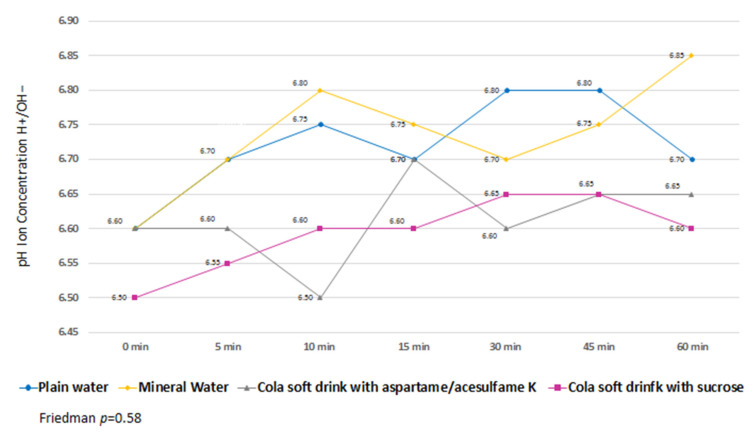
Variability was observed in the measurements of biofilm pH kinetics without finding significant differences between the effects of the different beverages (Figure 3).
Figure 3.
Kinetics of dental biofilm pH.
4.5. Bacterial Proliferation
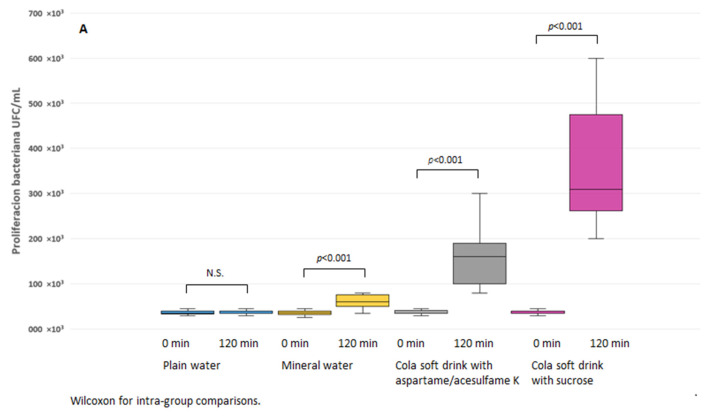
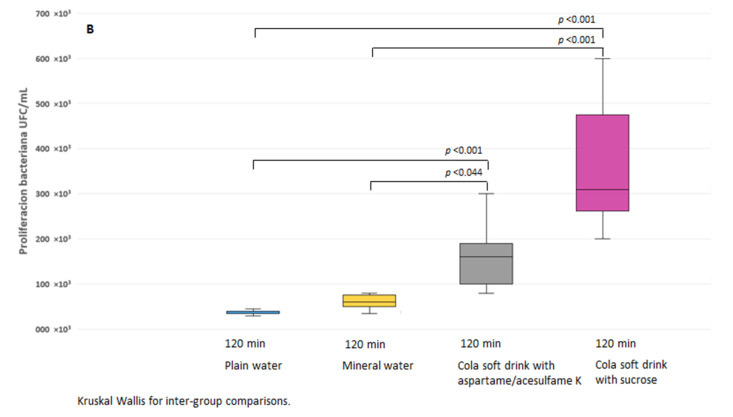
Changes in bacterial proliferation at baseline and 120 min after the ingestion of each of the drinks (Figure 4A) and the differences between them (Figure 4B) (two extreme values were removed).
Figure 4.
Bacterial proliferation of the basal dental biofilm and after the ingestion of the different types of beverages: (A) intra-group differences; (B) inter-group differences.
Greater bacterial proliferation was observed after consumption of the soft drink with sucrose (↑310 × 103 CFU, p ≤ 0.001), followed by the soft drink with aspartame/acesulfame K (↑160 × 103 CFU, p ≤ 0.001), and carbonated water (↑60 × 103 CFU, p ≤ 0.001). There were no significant changes in bacterial proliferation after consuming natural water.
5. Discussion
As far as we know, this is the first clinical trial to evaluate the effect of carbonated beverages with caloric and non-caloric sweeteners in vivo on three cariogenic mechanisms (salivary pH, dental biofilm pH, and changes in bacterial proliferation). It also allows these factors to be compared with controls such as mineral water and natural water. Furthermore, this study considers the intake of usual doses of these beverages (355 mL can), which is closer to actual consumption, unlike other authors who assess exposure through rinsing with 10–15 mL [25,26] or ingesting amounts less than 100 mL [28].
We observed acidification of salivary pH within the first few minutes after exposure to the sucrose drink, as expected. This effect is similar to that reported by Sánchez et al. [28]; however, we identified lower acidification (5.8 vs. 6.8) in our study. This may be because Sánchez et al. evaluated pH levels from the first minute after ingestion, while in this study the measurements began at the 5 min mark. The combined consumption of other acidifiers in the diet can have a synergistic effect in lowering the pH even though the critical demineralization point of pH 5.5 was not reached in either study. Moreover, we observed a prolonged return of the pH to basal levels after ingesting the drink with sucrose (45 min) in our study, which allows us to estimate the time of the cariogenic potential of these drinks.
Despite the similarity between the components of the soft drink containing sucrose and that added with aspartame/acesulfame K, their effects on salivary pH were different. Therefore, the greater acidifying capacity of a regular soft drink can be attributed more to its sucrose content and not to other compounds such as phosphoric and citric acid.
We obtained results similar to those reported by Uma for mineral water, identifying a slight alkalinization [29]. However, no statistically significant differences were observed with changes in pH after consuming plain water.
The acidification time of the dental biofilm after ingesting the soft drink with sucrose was similar to that found by Brambilla, Jawale, and Ross [25,26,30], with maximum values of acidification between 5 and 15 min. However, these authors report higher levels of acidification (pH 5.14 ± 0.5, 5.72 ± 0.2, and 5.51 ± 0.5, respectively) than those determined in our study (pH, 6.80; RIC, 6.35–7.10). This may be because these authors collected samples from interdental areas, where its buffer effect is reduced by the difficult accessibility of saliva [31]. However, Saeed who also evaluated the pH of dental biofilm taken from vestibular surfaces, as in our study, found greater acidity after ingesting the drink containing sucrose (5.86 vs. 6.55 at 5 min) [32]. This is possibly because the exposure was conducted by rinsing and not after drinking the beverages, as in our study, which could have facilitated a greater impregnation of the sugars in the biofilm.
We did not find statistically significant differences in changes in the pH of dental biofilm between the different types of beverages in this study. The above differs from that reported by Brambilla, who identified greater acidification after exposure to the drink with sucrose (pH, 5.14 ± 0.05) compared with the solution with non-caloric sweeteners, such as rebaudioside A (pH, 7.11 ± 0.03) and stevioside (pH 7.06 ± 0.03). There were also differences in the type of exposure (rinses of 1 min vs. usual intake) and the concentration of the exposure substances (10% solution vs. commercial formulas) [25], even though the differences can be attributed to the type of sweeteners assessed.
In our investigation, the greatest increase in bacterial proliferation was identified after consuming a drink containing sucrose compared with non-caloric drinks. Other authors have reported that most commercial sweeteners appear to be less cariogenic than sucrose but still retain some enamel demineralization potential [33,34]. This is also consistent with what was previously reported by Brambilla [25], who identified twice the proliferation after ingesting a drink with sucrose compared with those drinks containing rebaudioside. Bacterial proliferation was also identified in beverages containing aspartame/acesulfame K, although to a lesser degree. This suggests that other compounds apart from the sweetener, such as citric and phosphoric acid, can promote the development of acidophilic microorganisms.
The carbonated beverage with which the least bacterial proliferation occurred was mineral water, and even though an increase of 60 × 103 CFU was reported after consumption, this difference was not statistically different from that of plain water. Plain water was the only beverage that did not show an increase in bacterial proliferation after ingestion.
Within the limitations of this study, we recognize the small sample size as a limitation. This did not allow us to observe significant differences in changes in the pH of dental biofilm. Although each patient was his own control, it is necessary to include a larger sample size for further studies.
Furthermore, the first 5 min after ingestion could be of great relevance in the identification of changes in pH that was not analyzed in this investigation. However, we consider the isolated intake of each type of beverage in the present assay. However, we know that in daily life consumption may not be limited to 355 mL or may be accompanied by other foods and beverages that could enhance or neutralize the acidifying effects.
This study shows changes in a small group of patients with an evaluation in a single exposure time. However, these effects can have a great impact at the community level as observed in the study by Hasheminejad et al. They reported that for patients who have never consumed soft beverages it is possible to observe a DMFT index of up to 39% less than those who had had a daily intake of such beverages [35].
Therefore, questions arise for future studies to evaluate the kinetics of pH and bacterial proliferation during the first minutes of consumption, the intake of different volumes, and/or the combination with other foods and beverages.
We observed that the oral pH recovery time is prolonged (up to 45 min to return to the basal state), so recurrent consumption can cause a persistent state of acidification, predisposing to cariogenic mechanisms. This can be limited by recommending a lower consumption of these drinks and tooth brushing. It has been reported that the intake of non-sweetened beverages such as water, and more tooth brushing, are associated with fewer caries in children [36]. Oral hygiene is of such relevance that it has even been related to a greater impact on oral health than a correct diet [37].
6. Conclusions
The greatest salivary acidification was observed after ingesting soft drinks containing sucrose, and a prolonged recovery time to the basal state was observed although it was not documented that the decrease in pH reached the critical demineralizing point. Moreover, the ingestion of these beverages favored bacterial proliferation, which increases their cariogenic potential. It should be noted that carbonated drinks without sucrose were not inert since they also favored bacterial proliferation, although to a lesser extent. We only evaluated a single exposure time, but we would expect that repeated exposure to these beverages could have a major deleterious impact on oral health. These findings support the recommendation to discourage the consumption of carbonated beverages, mainly those with sucrose, and to encourage the consumption of plain water in the population.
Acknowledgments
The authors are grateful to the Intestinal Bacteriology Research Laboratory of Hospital Infantil de México Federico Gómez.
Author Contributions
Conceptualization, G.C.B.-T., A.L.M.-L. and M.K.-K.; methodology, G.C.B.-T., A.L.M.-L. and J.G.-E.; investigation, M.I.F.-H., G.C.B.-T. and I.P.-O.; resources, J.G.-E. and I.P.-O.; data curation, G.C.B.-T. and M.K.-K.; writing, G.C.B.-T. and A.L.M.-L.; writing—review and editing, G.C.B.-T., A.L.M.-L., M.K.-K., J.G.-E., I.P.-O. and M.I.F.-H.; project administration, A.L.M.-L., M.K.-K. and J.G.-E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Hospital Infantil de México Federico Gómez (protocol code HIM 2017-084 SSA 1411, approved September 2017). Clinical Trials ID: NCT05437874.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Supporting reported results can be found at https://drive.google.com/drive/folders/1d_1HgpcKEtQPmhMwOtco58yui3CWh7j3?usp=sharing (accessed on 12 April 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Federal Funds HIM 2017-084 SSA 1411.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liang C.Y., Liu Y.C.G., Shieh T.Y., Tseng Y.C., Teng A.Y.T. Higher Levels of Early Childhood Caries (ECC) Is Associated with Developing Psychomotor Deficiency: The Cross- Sectional Bi-Township Analysis for The New Hypothesis. Int. J. Environ. Res. Public Health. 2019;16:3082. doi: 10.3390/ijerph16173082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machiulskiene V., Campus G., Carvalho J.C., Dige I., Ekstrand K.R., Jablonski-Momeni A., Maltz M., Manton D.J., Martignon S., Martinez-Mier E.A., et al. Terminology of Dental Caries and Dental Caries Management. Caries Res. 2020;54:7–14. doi: 10.1159/000503309. [DOI] [PubMed] [Google Scholar]
- 3.Vieira A.R., Modesto A., Marazita M.L. Caries: Review of human genetics research. Caries Res. 2014;48:491–506. doi: 10.1159/000358333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.González-Aragón Pineda A.E., García Pérez A., García-Godoy F. Salivary parameters and oral health status amongst adolescents in Mexico. BMC Oral Health. 2020;20:190. doi: 10.1186/s12903-020-01182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krzyściak W., Pluskwa K.K., Piątkowski J., Krzyściak P., Jurczak A., Kościelniak D., Skalniak A. The usefulness of biotyping in the determination of selected pathogenicity determinants in Streptococcus mutans. BMC Microbiol. 2014;14:194. doi: 10.1186/1471-2180-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathur M.R., Tsakos G., Millett C. Socioeconomic inequalities in dental caries and their determinants in adolescents in New Delhi, India. BMJ Open. 2014;4:4–12. doi: 10.1136/bmjopen-2014-006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo H., Falsetta M.L., Klein M.I. The Exopolysaccharide Matrix: A Virulence Determinant of Cariogenic Biofilm. J. Dent. Res. 2013;92:1065–1073. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho Silva C., Gavinha S., Vilela S., Rodrigues R., Manso M.C., Severo M., Lopes C., Melo P. Dietary Patterns and Oral Health Behaviours Associated with Caries Development from 4 to 7 Years of Age. Life. 2021;11:609. doi: 10.3390/life11070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West N.X., Joiner A. Enamel mineral loss. J. Dent. 2014;42((Suppl. S1)):S2–S11. doi: 10.1016/S0300-5712(14)50002-4. [DOI] [PubMed] [Google Scholar]
- 10.Lemos J.A., Palmer S.R., Zeng L., Wen Z.T., Kajfasz J.K., Freires I.A., Abranches J., Brady L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019;7:7. doi: 10.1128/microbiolspec.GPP3-0051-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechir F., Pacurar M., Tohati A., Bataga S.M. Comparative Study of Salivary pH, Buffer Capacity, and Flow in Patients with and without Gastroesophageal Reflux Disease. Int. J. Environ. Res. Public Health. 2021;19:201. doi: 10.3390/ijerph19010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng X., Han Q., Zhou X., Chen Y., Huang X., Guo X., Peng R., Wang H., Peng X., Cheng L. Effect of pH-sensitive nanoparticles on inhibiting oral biofilms. Drug Deliv. 2022;29:561–573. doi: 10.1080/10717544.2022.2037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiuchi H., Kitasako Y., Fukuda Y., Nakashima S., Burrow M.F., Tagami J. Relationship between quantitative assessments of salivary buffering capacity and ion activity product for hydroxyapatite in relation to cariogenic potential. Aust. Dent. J. 2008;53:167–171. doi: 10.1111/j.1834-7819.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Ilie O., van Turnhout A.G., van Loosdrecht M.C., Picioreanu C. Numerical modelling of tooth enamel subsurface lesion formation induced by dental plaque. Caries Res. 2014;48:73–89. doi: 10.1159/000354123. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 16.Kaur A., Kwatra K.S., Kamboj P. Evaluation of non-microbial salivary caries activity parameters and salivary biochemical indicators in predicting dental caries. J. Indian Soc. Pedod. Prev. Dent. 2012;30:212–217. doi: 10.4103/0970-4388.105013. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadi-Motamayel F., Falsafi P., Goodarzi M.T., Poorolajal J. Comparison of Salivary pH, Buffering Capacity and Alkaline Phosphatase in Smokers and Healthy Non-Smokers: Retrospective cohort study. Sultan. Qaboos Univ. Med. J. 2016;16:317–321. doi: 10.18295/squmj.2016.16.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gornowicz A., Tokajuk G., Bielawska A., Maciorkowska E., Jabłoński R., Wójcicka A., Bielawski K. The assessment of sIgA, histatin-5, and lactoperoxidase levels in saliva of adolescents with dental caries. Med. Sci. Monit. 2014;20:1095–1100. doi: 10.12659/MSM.890468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acquier A.B., Pita A.K.D.C., Busch L., Sánchez G.A. Comparison of salivary levels of mucin and amylase and their relation with clinical parameters obtained from patients with aggressive and chronic periodontal disease. J. Appl. Oral Sci. 2015;23:288–294. doi: 10.1590/1678-775720140458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielawski K., Gabryel-Porowska H., Gornowicz A., Bielawska A., Wójcicka A., Maciorkowska E., Grabowska S.Z. Mucin levels in saliva of adolescents with dental caries. Med. Sci. Moni. 2014;20:72–77. doi: 10.12659/MSM.889718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hideaki W., Tatsuya H., Shogo M., Naruto Y., Hideaki T., Yoichi M., Yoshihiro O., Kazuo U., Hidenori T. Effect of 100 Hz electroacupuncture on salivary immunoglobulin A and the autonomic nervous system. Acupunct. Med. 2015;33:451–456. doi: 10.1136/acupmed-2015-010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moynihan P.J., Kelly S.A.M. Effect on caries of restricting sugars intake: Systematic review to inform WHO guidelines. J. Dent. Res. 2014;93:8–18. doi: 10.1177/0022034513508954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson T., Murray B., Price T., Atherton D., Hooks T. Non-Nutritive (Artificial) Sweetener Knowledge among University Students. Nutrients. 2019;11:2201. doi: 10.3390/nu11092201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruyère O., Ahmed S.H., Atlan C., Belegaud J., Bortolotti M., Canivenc-Lavier M.-C., Charrière S., Girardet J.-P., Houdart S., Kalonji E., et al. Review of the nutritional benefits and risks related to intense sweeteners. Arch. Public Health. 2015;73:49. doi: 10.1186/s13690-015-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brambilla E., Cagetti M.G., Ionescu A., Campus G., Lingström P. An in vitro and in vivo comparison of the effect of Stevia rebaudiana extracts on different caries-related variables: A randomized controlled trial pilot study. Caries Res. 2014;48:19–23. doi: 10.1159/000351650. [DOI] [PubMed] [Google Scholar]
- 26.Jawale B.A., Bendgude V., Mahuli A.V., Dave B., Kulkarni H., Mittal S. Dental Plaque pH Variation with Regular Soft Drink, Diet Soft Drink and High Energy Drink: An in vivo Study. J. Contemp. Dent. Pract. 2012;13:201–204. doi: 10.5005/jp-journals-10024-1121. [DOI] [PubMed] [Google Scholar]
- 27.Moradi G., Mohamadi Bolbanabad A., Moinafshar A., Adabi H., Sharafi M., Zareie B. Evaluation of Oral Health Status Based on the Decayed, Missing and Filled Teeth (DMFT) Index. Iran J. Public Health. 2019;48:2050–2057. doi: 10.18502/ijph.v48i11.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez G.A., Fernandez De Preliasco M.V. Salivary pH changes during soft drinks consumption in children. Int. J. Paediatr. Dent. 2003;13:251–257. doi: 10.1046/j.1365-263X.2003.00469.x. [DOI] [PubMed] [Google Scholar]
- 29.Uma E., Sze Theng K., Huan Yi L.L., Hong Yun L., Varghese E., Htoo Htoo Kyaw S. Comparison of Salivary pH Changes after Consumption of Two Sweetened Malaysian Local Drinks among Individuals with Low Caries Experience: A Pilot Study. Malays. J. Med. Sci. 2018;25:100–111. doi: 10.21315/mjms2018.25.4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos E.H., Donly K.J. In vivo dental plaque pH variation with regular and diet soft drinks. Pediatr. Dent. 2002;24:350–353. [PubMed] [Google Scholar]
- 31.Llena Puy C. Saliva and Oral Health The rôle of saliva in maintaining oral health and as an aid to diagnosis. Med. Oral Patol. Oral Cir. Bucal. 2006;11:449–455. [PubMed] [Google Scholar]
- 32.Saeed S., Al-Tinawi M. Evaluation of acidity and total sugar content of children′s popular beverages and their effect on plaque pH. J. Indian Soc. Pedod. Prev. Dent. 2010;28:189. doi: 10.4103/0970-4388.73783. [DOI] [PubMed] [Google Scholar]
- 33.Giacaman R.A., Pailahual V., Díaz-Garrido N. Cariogenicity induced by commercial carbonated beverages in an experimental biofilm-caries model. Eur. J. Dent. 2018;12:27–35. doi: 10.4103/ejd.ejd_188_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giacaman R.A., Campos P., Muñoz-Sandoval C., Castro R.J. Cariogenic potential of commercial sweeteners in an experimental biofilm caries model on enamel. Arch. Oral Biol. 2013;58:1116–1122. doi: 10.1016/j.archoralbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Hasheminejad N., Malek Mohammadi T., Mahmoodi M.R., Barkam M., Shahravan A. The association between beverage consumption pattern and dental problems in Iranian adolescents: A cross sectional study. BMC Oral Health. 2020;20:74. doi: 10.1186/s12903-020-01065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall T.A., Curtis A.M., Cavanaugh J.E., Warren J.J., Levy S.M. Beverage Intakes and Toothbrushing During Childhood Are Associated with Caries at Age 17 Years. J. Acad. Nutr. Diet. 2021;121:53–60. doi: 10.1016/j.jand.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pita-Fernández S., Pombo-Sánchez A., Suárez-Quintanilla J., Novio-Mallón S., Rivas-Mundiña B., Pértega-Díaz S. Relevancia clínica del cepillado dental y su relación con la caries. Aten. Primaria. 2010;42:372–379. doi: 10.1016/j.aprim.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Supporting reported results can be found at https://drive.google.com/drive/folders/1d_1HgpcKEtQPmhMwOtco58yui3CWh7j3?usp=sharing (accessed on 12 April 2022).